Abstract
Amblyomma maculatum Koch (the Gulf Coast tick) is an aggressive, human-biting ixodid tick distributed throughout much of the southeastern United States and is the primary vector for Rickettsia parkeri, an emerging human pathogen. Amblyomma maculatum has diverse host preferences that include white-tailed deer, a known reservoir for Ehrlichia and Anaplasma species, including the human pathogens E. ewingii and E. chaffeensis. To examine more closely the potential role of A. maculatum in the maintenance of various pathogenic Ehrlichia and Anaplasma species, we screened DNA samples from 493 questing adult A. maculatum collected from six U.S. states using broad-range Anaplasmataceae and Ehrlichia genus-specific PCR assays. Of the samples tested, four (0.8%) were positive for DNA of Ehrlichia ewingii, one (0.2%) was positive for Anaplasma platys, and one (0.2%) was positive for a previously unreported Ehrlichia species closely related to Ehrlichia muris and an uncultivated Ehrlichia species from Haemaphysalis longicornis ticks in Japan. No ticks contained DNA of Ehrlichia chaffeensis, Ehrlichia canis, the Panola Mountain Ehrlichia, or Anaplasma phagocytophilum. This is the first identification of E. ewingii, A. platys, and the novel Ehrlichia in questing Gulf Coast ticks; nonetheless the low prevalence of these agents suggests that A. maculatum is not likely an important vector of these zoonotic pathogens.
Keywords: Amblyomma maculatum, Gulf Coast tick, Anaplasma, Ehrlichia
Amblyomma maculatum Koch (the Gulf Coast tick) is an aggressive human-biting tick found across much of the southern and mid-Atlantic region of the United States, though the recognized distribution of this medically important tick has increased markedly in the past 70 yr (Nadolny et al. 2015). In the United States A. maculatum is the primary vector of Rickettsia parkeri, a rickettsial human pathogen that causes an illness similar to Rocky Mountain spotted fever (RMSF), and is also infected variably with “Candidatus Rickettsia andeanae,” a spotted fever group Rickettsia of unknown pathogenicity (Paddock and Goddard 2015, Paddock et al. 2015). A few surveys also reveal that A. maculatum is rarely infected with Ehrlichia chaffeensis, the causative agent of human monocytic ehrlichiosis, and the Panola Mountain Ehrlichia (PME), a suspected human pathogen (Reeves et al. 2008, Lee et al. 2014, Loftis et al. 2016).
The Gulf Coast tick has a wide host range, with immature stages typically feeding on ground-nesting birds and rodents, while adults tend to feed on larger mammals, including white-tailed deer (Odocoileus virginianus) (Teel et al. 2010), which serve as reservoirs for several species of Anaplasmataceae, including several pathogenic Ehrlichia species (Lockhart et al. 1997, Yabsley et al. 2002, Varela-Stokes 2007, Yabsley et al. 2008, Teel et al. 2010, Lobanov et al. 2012, Tate et al. 2013). Herein, we describe a survey of 493 questing adult A. maculatum samples collected from six U.S. states to evaluate for infections with Ehrlichia and Anaplasma species.
Materials and Methods
Tick Collecting and Processing
Questing adult A. maculatum were collected from vegetation by using flannel cloth flags during 1999–2015. Specimens collected prior to 2015 were obtained from multiple locations in Georgia, Florida, Kansas, Mississippi, North Carolina, and Oklahoma to survey for R. parkeri and “Ca. R. andeanae” (Sumner et al. 2007, Paddock et al. 2010, Varela-Stokes et al. 2011, Paddock et al. 2015; Table 1). Ticks collected in 2015 were frozen at −80 °C or preserved in 70% ethanol at ambient temperature until the time of DNA extraction. Species identification was determined by standard taxonomic keys.
Table 1.
Collection locations for ticks used in this study
| State | Year collected | Females | Males | Total |
|---|---|---|---|---|
| Georgia | 1999 | 3 | 9 | 147 |
| 2003 | 7 | 4 | ||
| 2005 | 3 | 4 | ||
| 2015 | 66 | 51 | ||
| Florida | 2004 | 13 | 9 | 130 |
| 2005 | 16 | 10 | ||
| 2007 | 40 | 42 | ||
| Mississippi | 2003 | 7 | 3 | 77 |
| 2004 | 5 | 4 | ||
| 2007 | 34 | 24 | ||
| Kansas | 2012 | 1 | 5 | 70 |
| 2013 | 32 | 32 | ||
| North Carolina | 2009 | 23 | 18 | 41 |
| Oklahoma | 2013 | 18 | 10 | 28 |
DNA Extraction of Ticks and PCR Screening for Anaplasmataceae
DNA was extracted from ticks using a QIAamp DNA Mini kit or DNeasy extraction kit (QIAGEN, Valencia, CA) and eluted into a final volume of 200 μl. Samples collected in 2015 were stored at 4°C until PCR analyses were performed. DNA samples made during 1999–2014 were stored at −80°C until specific tests were performed.
Tick extracts were screened using an Anaplasmataceae-specific real-time PCR assay (Li et al. 2002, Allerdice et al. 2016) and 4 μl of DNA extract. Primers ECHSYBR-F and ECHSYBR-R were used to amplify a 155-bp product of the 16S ribosomal RNA gene. All PCR reactions were conducted in duplicate on a BioRad CFX 96 thermal cycler using the BioRad SsoFast EvaGreen Supermix kit (Life Science, Hercules, CA). Each set of reactions included two negative controls, and Ehrlichia muris AS145T extracted from cell culture was used consistently as a positive control. To verify DNA quality, all tick samples were screened using conserved primers T1B and T2A to amplify a 360 bp-portion of the tick mitochondrial 12S rRNA gene as previously reported (Beati and Keirans 2001).
Samples that produced amplicons with the SYBR Green real-time assay were visualized in 1.5% agarose gels containing 0.1 μg/ml ethidium bromide. Amplicons were extracted and purified using the Promega Wizard SV Gel and PCR Clean-up System (Promega, Madison, WI). Products were sequenced in both directions and assembled using Sequencher 5.1 (Gene Codes, Ann Arbor, MI). Resultant sequences were compared to GenBank data using BLASTn analysis.
12S rRNA PCR products were also visualized in agarose gels to verify that DNA quality was sufficiently high to be used for PCR. The 12S rRNA gene amplicon was sequenced as described above for those samples that were positive for an Anaplasmataceae species in order to verify species identification of the tick.
Results and Discussion
Of the 493 DNA extracts obtained from adult Gulf Coast ticks collected from six states, only six (1.2%) contained DNA of an Anaplasmataceae species. Four (0.8%) specimens, collected in Copiah County, MS, in 2004 (1), Craven County, NC, in 2010 (1), and Geary County, KS, in 2013 (2) revealed an identical Anaplasmataceae sequence showing >99% identity to the corresponding segment of the 16S rRNA gene of Ehrlichia ewingii strain Stillwater (NR_044747). A single tick collected in Franklin County, FL, in 2007 produced an amplicon with >99% identity to the corresponding segment of the 16S rRNA gene of Anaplasma platys strain Okinawa (AF536828). One tick sample collected in Neosho County, KS, in 2013 produced a 16S rRNA amplicon (KX365750) showing 95% identity to a noncultivated Ehrlichia species detected in Haemaphysalis longicornis ticks from Okinawa, Japan (HQ697589) (Matsumoto et al. 2011). No tick samples were found to contain E. chaffeensis, PME, Ehrlichia canis, or Anaplasma phagocytophilum. All ticks included in this study produced amplicons of appropriate size when screened with the 12S rRNA PCR assay. Additionally, all Anaplasmataceae-positive ticks were confirmed as A. maculatum by amplification and sequencing of a 360-bp segment of the 12S rRNA gene
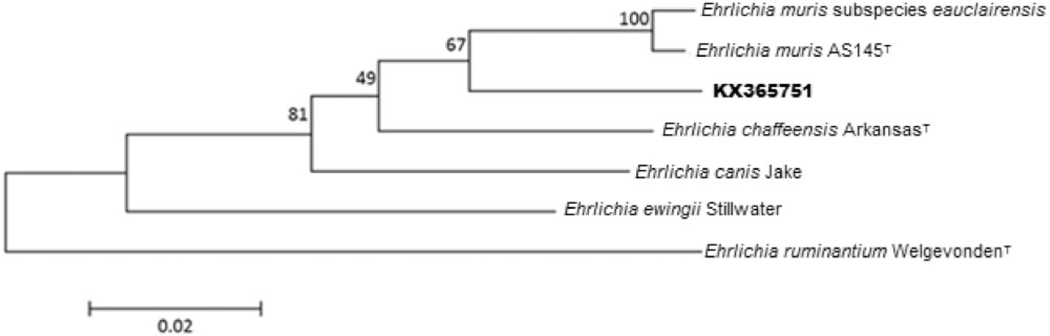
To further characterize the previously unreported Ehrlichia species detected in the tick from Kansas, analysis was performed using a nested PCR assay targeting a 595-bp segment of the heat shock operon (groEL) (Telford Iii et al. 2011). The amplified portion of the groEL gene (KX365751) exhibited 95% identity to E. muris AS145T. A maximum likelihood phylogenetic tree based on this portion of groEL indicates that this sample clusters most closely with E. muris and the recently named E. muris subspecies eauclairensis, formerly referred to as the E. muris-like agent (EMLA; Fig. 1) (Pritt et al. 2016).
Fig. 1.
Molecular phylogenetic analysis was inferred by using the Maximum Likelihood method based on the Tamura-Nei model (Tamura and Nei 1993). The tree with the highest log likelihood (−1631.2822) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved seven nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 594 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 (Tamura et al. 2011).
Ehrlichia ewingii causes granulocytic ehrlichiosis in humans and dogs (Buller et al. 1999, Goodman et al. 2003, Little et al. 2010, Harris et al. 2016, Nichols Heitman et al. 2016) and is transmitted predominantly by Amblyomma americanum (Harmon et al. 2015, Sayler et al. 2016). Infection rates in this species range from 0.0–7.6% dependent on location (Wolf et al. 2000, Steiert and Gilfoy 2002, Long et al. 2004, Mixson et al. 2006, Cohen et al. 2010, Gleim et al. 2016, Maegli et al. 2016, Pompo et al. 2016). Anaplasma platys is a recognized cause of disease in dogs and less commonly in humans (Arraga-Alvarado et al. 2014, Breitschwerdt et al. 2014) and is believed to be transmitted primarily by ticks in the Rhipicephalus sanguineus group (Ramos et al. 2014).
DNA of E. ewingii has been detected previously in partially engorged adult A. maculatum collected from white-tailed deer (Mays et al. 2016); however, to our knowledge, this is the first report of E. ewingii and A. platys detected in questing adult A. maculatum. Additionally, a novel Ehrlichia species was detected in an adult tick collected in Kansas. While the pathogenic EMLA identified in the upper Midwestern United States has recently been described as a subspecies of E. muris (Pritt et al. 2016), it could be possible that other E. muris-like agents exist in the United States outside of EMLA’s limited range of Wisconsin and Minnesota.
The low percentages of A. maculatum adults infected by Ehrlichia and Anaplasma species are not unexpected based on previous reports and are consonant with the ecology and life history of the Gulf Coast tick. Unlike pathogenic tick-borne SFG Rickettsia species, Ehrlichia and Anaplasma species are not transmitted transovarially (Stich et al. 1989, Long et al. 2003). However, these bacteria are transmitted transstadially between the feeding stages of their tick vectors. This aspect of their ecology suggests that transmission of Anaplasmataceae from A. maculatum to humans can occur only when larval or nymphal stage ticks acquire the infection from a bacteremic reservoir host. The immature stages of A. maculatum feed most commonly on various species of ground-nesting birds and cotton rats (Sigmodon hispidus) (Teel et al. 2010, Paddock and Goddard 2015), none of which are recognized as reservoirs or amplifying hosts for Ehrlichia or Anaplasma species. In this context the risk of transmission of pathogenic Anaplasmataceae to humans by this tick is likely to be low.
Acknowledgments
We would like to acknowledge Lindsay Killmaster, Lauren Schumacher, Alyssa Snellgrove, Tracy Lantaff, Jerome Goddard, Lance Durden, Marcee Tolliver, Michael Dryden, and Susan Little for their assistance in collecting several of the ticks evaluated in this study. We would also like to thank Susan Little and Lindsay Starkey (Oklahoma State University) as well as Ed Breitschwerdt and Barbara Qurollo (North Carolina State University College of Veterinary Medicine) for providing A. platys DNA used as a positive control. The research reported here was supported in part by an appointment of J. Hecht to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the CDC. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References Cited
- Allerdice ME, Pritt BS, Sloan LM, Paddock CD, and Karpathy SE. 2016. A real-time PCR assay for detection of the Ehrlichia muris-like agent, a newly recognized pathogen of humans in the upper Midwestern United States. Ticks Tick Borne Dis. 7: 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arraga-Alvarado CM, Qurollo BA, Parra OC, Berrueta MA, Hegarty BC, and Breitschwerdt EB. 2014. Case report: molecular evidence of Anaplasma platys infection in two women from Venezuela. Am. J. Trop. Med. Hyg. 91: 1161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beati L, and Keirans JE. 2001. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J. Parasitol. 87: 32–48. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Qurollo BA, Saito TB, Maggi RG, Blanton LS, and Bouyer DH. 2014. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasites Vectors 7: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, et al. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341: 148–155. [DOI] [PubMed] [Google Scholar]
- Cohen SB, Yabsley MJ, Freye JD, Dunlap BG, Rowland ME, Huang J, Dunn JR, Jones TF, and Moncayo AC. 2010. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in ticks from Tennessee. Vector Borne Zoonotic Dis. 10: 435–440. [DOI] [PubMed] [Google Scholar]
- Gleim ER, Garrison LE, Vello MS, Savage MY, Lopez G, Berghaus RD, and Yabsley MJ. 2016. Factors associated with tick bites and pathogen prevalence in ticks parasitizing humans in Georgia, USA. Parasites Vectors 9: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RA, Hawkins EC, Olby NJ, Grindem CB, Hegarty B, and Breitschwerdt EB.2003. Molecular identification of Ehrlichia ewingii infection in dogs: 15 cases (1997–2001).Journal of the American Veterinary Medical Association. 222: 1102–1107. [DOI] [PubMed] [Google Scholar]
- Harmon JR, Scott MC, Baker EM, Jones CJ, and Hickling GJ. 2015. Molecular identification of Ehrlichia species and host bloodmeal source in Amblyomma americanum L. from two locations in Tennessee, United States. Ticks Tick-Borne Dis. 6: 246–252. [DOI] [PubMed] [Google Scholar]
- Harris RM, Couturier BA, Sample SC, Coulter KS, Casey KK, and Schlaberg R. 2016. Expanded geographic distribution and clinical characteristics of Ehrlichia ewingii infections, United States. Emerg. Infect. Dis. 22: 862–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kakumanu ML, Ponnusamy L, Vaughn M, Funkhouser S, Thornton H, Meshnick SR, and Apperson CS. 2014. Prevalence of Rickettsiales in ticks removed from the skin of outdoor workers in North Carolina. Parasites Vectors 7: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. S y., Chu F, Reilly A, and Winslow GM. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169: 1419–1425. [DOI] [PubMed] [Google Scholar]
- Little SE, O’Connor TP, Hempstead J, Saucier J, Reichard MV, Meinkoth K, Meinkoth JH, Andrews B, Ullom S, Ewing SA, et al. 2010. Ehrlichia ewingii infection and exposure rates in dogs from the southcentral United States. Vet. Parasitol. 172: 355–360. [DOI] [PubMed] [Google Scholar]
- Lobanov VA, Gajadhar AA, Al-Adhami B, and Schwantje HM. 2012. Molecular study of free-ranging mule deer and white-tailed deer from British Columbia, Canada, for evidence of Anaplasma spp. and Ehrlichia spp. Transbound. Emerg. Dis. 59: 233–243. [DOI] [PubMed] [Google Scholar]
- Lockhart JM, Davidson WR, Stallknecht DE, Dawson JE, and Little SE. 1997. Natural history of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) in the piedmont physiographic province of Georgia. J. Parasitol. 83: 887–894. [PubMed] [Google Scholar]
- Loftis AD, Kelly PJ, Paddock CD, Blount K, Johnson JW, Gleim ER, Yabsley MJ, Levin ML, and Beati L. 2016. Panola Mountain Ehrlichia in Amblyomma maculatum from the United States and Amblyomma variegatum (Acari: Ixodidae) from the Caribbean and Africa. J. Med. Entomol. 53(3): 696–698. [DOI] [PubMed] [Google Scholar]
- Long SW, Pound JM, and Yu XJ. 2004. Ehrlichia prevalence in Amblyomma americanum, Central Texas. Emerg. Infect. Dis. 10: 1342–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SW, Zhang X, Zhang J, Ruble RP, Teel P, and Yu XJ. 2003. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 40: 1000–1004. [DOI] [PubMed] [Google Scholar]
- Maegli A, Loy JD, and Cortinas R. 2016. Note on Ehrlichia chaffeensis, Ehrlichia ewingii, and “Borrelia lonestari” infection in lone star ticks (Acari: Ixodidae), Nebraska, USA. Ticks Tick-Borne Dis. 7: 154–158. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Takeuchi TN, Yokoyama Y, Katagiri M, Ooshiro S, Zakimi F, Gaowa F. Kawamori, Ohashi N, and Inokuma H. 2011. Detection of the new Ehrlichia species closely related to Ehrlichia ewingii from Haemaphysalis longicornis in Yonaguni Island, Okinawa, Japan. J. Vet. Med. Sci. 73: 1485–1488. [DOI] [PubMed] [Google Scholar]
- Mays SE, Houston AE, and Trout Fryxell RT. 2016. Specifying pathogen associations of Amblyomma maculatum (Acari: Ixodidae) in Western Tennessee. J. Med. Entomol. 53: 435–440. [DOI] [PubMed] [Google Scholar]
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, and Dasch GA. 2006. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 43: 1261–1268. [DOI] [PubMed] [Google Scholar]
- Nadolny R, Gaff H, Carlsson J, and Gauthier D. 2015. Comparative population genetics of two invading ticks: evidence of the ecological mechanisms underlying tick range expansions. Infect. Genet. Evol. 35: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, and Behravesh CB. 2016. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am. J. Trop. Med. Hyg. 94: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, and Goddard J. 2015. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). J. Med. Entomol. 52: 230–252. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, Loftis AD, and Varela-Stokes A. 2010. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl. Environ. Microbiol. 76: 2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Denison AM, Dryden MW, Noden BH, Lash RR, Abdelghani SS, Evans AE, Kelly AR, Hecht JA, Karpathy SE, et al. 2015. High prevalence of “Candidatus Rickettsia andeanae” and apparent exclusion of Rickettsia parkeri in adult Amblyomma maculatum (Acari: Ixodidae) from Kansas and Oklahoma. Ticks Tick Borne Dis. 6: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompo K, Mays S, Wesselman C, Paulsen DJ, and Fryxell RT. 2016. Survey of ticks collected from Tennessee cattle and their pastures for Anaplasma and Ehrlichia species. J. Parasitol. 102: 54–59. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Allerdice ME, Sloan LM, Paddock CD, Munderloh UG, Rikihisa Y, Tajima T, Paskewitz SM, Neitzel DF, Johnson DK, et al. 2016. Proposal to rename Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans in the upper mid-western United States. Int. J. Syst. Evol. Microbiol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RA, Latrofa MS, Giannelli A, Lacasella V, Campbell BE, Dantas-Torres F, and Otranto D. 2014. Detection of Anaplasma platys in dogs and Rhipicephalus sanguineus group ticks by a quantitative real-time PCR. Vet. Parasitol. 205: 285–288. [DOI] [PubMed] [Google Scholar]
- Reeves WK, Loftis AD, Nicholson WL, and Czarkowski AG. 2008. The first report of human illness associated with the Panola Mountain Ehrlichia species: a case report. J. Med. Case Rep. 2: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler KA, Loftis AD, Beatty SK, Boyce CL, Garrison E, Clemons B, Cunningham M, Alleman AR, and Barbet AF.2016. Prevalence of tick-borne pathogens in host-seeking Amblyomma americanum (Acari: Ixodidae) and Odocoileus virginianus (Artiodactyla: Cervidae) in Florida.Journal of Medical Entomology. [DOI] [PubMed] [Google Scholar]
- Steiert JG, and Gilfoy F. 2002. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne Zoonotic Dis. 2: 53–60. [DOI] [PubMed] [Google Scholar]
- Stich RW, Kocan KM, Palmer GH, Ewing SA, Hair JA, and Barron SJ. 1989. Transstadial and attempted transovarial transmission of Anaplasma marginale by Dermacentor variabilis. Am. J. Vet. Res. 50: 1377–1380. [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, and Paddock CD. 2007. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 13: 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, and Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular biology and evolution. 10: 512–526. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, and Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution. 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate CM, Howerth EW, Mead DG, Dugan VG, Luttrell MP, Sahora AI, Munderloh UG, Davidson WR, and Yabsley MJ. 2013. Anaplasma odocoilei sp. nov. (family Anaplasmataceae) from white-tailed deer (Odocoileus virginianus). Ticks Tick-Borne Dis. 4: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, and Strey OF. 2010. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 47: 707–722. [DOI] [PubMed] [Google Scholar]
- Telford Iii SR, Goethert HK, and Cunningham JA. 2011. Prevalence of Ehrlichia muris in Wisconsin deer ticks collected during the mid 1990s. Open Microbiol. J. 5: 18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Stokes AS 2007. Transmission of bacterial agents from lone star ticks to white-tailed deer. J. Med. Entomol. 44: 478–483. [DOI] [PubMed] [Google Scholar]
- Varela-Stokes AS, Paddock CD, Engber B, and Toliver M. 2011. Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg. Infect. Dis. 17: 2350–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf L, McPherson T, Harrison B, Engber B, Anderson A, and Whitt P. 2000. Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J. Clin. Microbiol. 38: 2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley MJ, Loftis AD, and Little SE. 2008. Natural and experimental infection of white-tailed deer (Odocoileus virginianus) from the United States with an Ehrlichia sp. closely related to Ehrlichia ruminantium. J. Wildl. Dis. 44: 381–387. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Varela AS, Tate CM, Dugan VG, Stallknecht DE, Little SE, and Davidson WR. 2002. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus). Emerg. Infect. Dis. 8: 668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]