Abstract
The LAR family protein tyrosine phosphatases (PTPs), including LAR, PTPδ, and PTPς, are transmembrane proteins composed of a cell adhesion molecule-like ectodomain and two cytoplasmic catalytic domains: active D1 and inactive D2. We performed a yeast two-hybrid screen with the first catalytic domain of PTPς (PTPς-D1) as bait to identify interacting regulatory proteins. Using this screen, we identified the second catalytic domain of PTPδ (PTPδ-D2) as an interactor of PTPς-D1. Both yeast two-hybrid binding assays and coprecipitation from mammalian cells revealed strong binding between PTPς-D1 and PTPδ-D2, an association which required the presence of the wedge sequence in PTPς-D1, a sequence recently shown to mediate D1-D1 homodimerization in the phosphatase RPTPα. This interaction was not reciprocal, as PTPδ-D1 did not bind PTPς-D2. Addition of a glutathione S-transferase (GST)–PTPδ-D2 fusion protein (but not GST alone) to GST–PTPς-D1 led to ∼50% inhibition of the catalytic activity of PTPς-D1, as determined by an in vitro phosphatase assay against p-nitrophenylphosphate. A similar inhibition of PTPς-D1 activity was obtained with coimmunoprecipitated PTPδ-D2. Interestingly, the second catalytic domains of LAR (LAR-D2) and PTPς (PTPς-D2), very similar in sequence to PTPδ-D2, bound poorly to PTPς-D1. PTPδ-D1 and LAR-D1 were also able to bind PTPδ-D2, but more weakly than PTPς-D1, with a binding hierarchy of PTPς-D1>>PTPδ-D1>LAR-D1. These results suggest that association between PTPς-D1 and PTPδ-D2, possibly via receptor heterodimerization, provides a negative regulatory function and that the second catalytic domains of this and likely other receptor PTPs, which are often inactive, may function instead to regulate the activity of the first catalytic domains.
Tyrosine phosphorylation, controlled by the activity of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), plays a critical role in the regulation of many cellular processes, including cell proliferation and differentiation. PTPs, like PTKs, can be classified into cytosolic and receptor-type PTPs (11, 25). One subclass of receptor PTPs (RPTPs) is represented by the LAR family of phosphatases, which includes LAR and Drosophila DLAR (37, 39), PTPδ (20, 23, 29), PTPς (also known as LAR-PTP2, PTP-P1, CRYPα, PTP-NU3, PTP-NE3, and CPTP1 [27, 30, 34, 44, 45, 49, 52] and referred to herein as PTPς), and the three related phosphatases PTPκ, PTPμ, and PTPλ (6, 12, 16). These PTPs are characterized by an extracellular domain composed of multiple immunoglobulin (Ig)-like and fibronectin type III (FNIII) repeats, resembling cell adhesion molecules (CAM) such as N-CAM and L1 (7, 24) and several receptor PTKs. The CAM-like ectodomain can also be expressed alone, due to either alternative splicing or ectodomain shedding, thus disconnecting it from the intracellular catalytic domains (16, 26, 36). Like most RPTPs, the LAR family phosphatases contain a single transmembrane domain and two tandemly repeated catalytic domains (D1 and D2). Mutation of the highly conserved Cys in LAR-D1 abrogates PTP catalytic activity, suggesting that D2 is inactive, as also demonstrated for CD45 (28, 38). These results were also supported by direct measurements of the catalytic activity of LAR-D1 and -D2, or PTPς-D1 and -D2, against several artificial substrates (11a, 15, 44). Based on these findings and the observation that in PTPζ and RPTPγ, the highly conserved Cys in the second catalytic domain is replaced by Asp (2, 19), it has been proposed that the second catalytic domains of most RPTPs may have a regulatory rather than a catalytic function or, alternatively, that the second catalytic domains have a different substrate specificity than the first catalytic domains.
Dimerization of receptor PTKs has long been recognized as an essential step in their autophosphorylation and activation (42). Recently, homodimerization of a tyrosine phosphatase, RPTPα, was demonstrated (3). Determination of the crystal structure of RPTPα has revealed that the first catalytic domain (D1) dimerizes, to form a D1-D1 complex. This dimerization occurs by insertion of a “wedge” sequence, located at the N terminus of each D1 and conforming to a helix-turn-helix structure, into the active site of the partner D1 (3). Based on this dimeric structure, it was proposed that a D1-D1 dimeric complex would inhibit catalytic activity. Indeed, an epidermal growth factor receptor-CD45 chimera in which the ecto- and transmembrane domain of the EGF receptor was linked to the intracellular catalytic domains of CD45, was previously shown to dimerize in response to epidermal growth factor and to inhibit CD45-mediated T-cell activation, which requires intact catalytic activity of CD45-D1 (9, 10). Thus, homodimerization or, possibly, heterodimerization may provide a mechanism to regulate the function of PTPs.
In this report, we describe the isolation of the second catalytic domain of PTPδ (PTPδ-D2) in a two-hybrid screen using the first catalytic domain of PTPς (PTPς-D1) as bait. Moreover, we show strong interactions between these two domains in both two-hybrid binding assays and coprecipitations in mammalian cells, an interaction which requires the presence of the wedge region of PTPς-D1 and which leads to partial inhibition of the catalytic activity of PTPς. The first catalytic domains of PTPδ and LAR bind more weakly than PTPς-D1, to PTPδ-D2 and the binding of the D1 proteins of all three LAR family members appears to be specific to PTPδ-D2. We thus propose that the association between the first and second catalytic domains of LAR family members, particularly PTPς-D1 and PTPδ-D2, may provide a negative regulatory function and that the second catalytic domain of PTPδ and, possibly, those of other RPTPs, which are usually inactive, function instead to regulate the activity of the first catalytic domains.
MATERIALS AND METHODS
Yeast two-hybrid library screens.
A PCR fragment encompassing nucleotides (nt) 3896 to 5002 (amino acids [aa] 1238 to 1606) that encodes the first catalytic domain (D1) of PTPς (rat LAR-PTP2; accession no. L11587; reference 52) with a C→S point mutation (M) in the signature motif (C1504S) and includes a sequence encoding the HA epitope was subcloned into the LexA DNA binding domain fusion vector pBTM116 (43); such a C→S point mutation in the catalytic core of PTPs (including PTPς) abolishes catalytic activity but still allows substrate binding (40). The insert-containing plasmid [called pBTM116HAςD1(M)] was used to transform Saccharomyces cerevisiae L40 (MATa his3 LYS2:LexA-His3 URA3::LexA-lacZ) by the standard Li acetate method to give Trp prototrophs. Transformants were tested for expression of the LexA–PTPς-D1 fusion protein by immunoblotting using an anti-HA antibody (Boehringer Mannheim). The L40 cells transformed with pBTM116HAςD1(M) were cotransformed with either an adult rat lung cDNA library or an 11-day mouse embryo library (Matchmaker; Clontech) constructed in the pGAD10 (Gal4 activation domain) plasmid and selected on medium lacking Trp, Leu, and His and containing 0 to 20 mM 3-aminotriazole. Plates were incubated for 5 days and overlaid with replica filters, and cells were permeabilized by freezing filters in liquid nitrogen and then thawing them at room temperature. Filters were transferred onto Whatman 3MM paper saturated with an X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) solution and incubated at 30°C to monitor color development. β-Galactosidase (β-gal)-positive colonies were selected and streaked on medium lacking Trp and Leu for further analysis. Total DNA was extracted from these colonies, used to transform Escherichia coli HB101. The bacteria were then plated on M9 minimal plates or subjected to PCR with pGAD-specific primers, and religated into pGAD10. Unique inserts were identified by sequencing. To test for true positives, the unique inserts were transformed into L40 cells either alone, with pBTM116HAςD1(M), or with an unrelated pBTM116 construct.
Cloning of PTPδ-D1.
The first catalytic domain of PTPδ (PTPδ-D1), including its wedge sequence, was cloned from a mouse brain cDNA library (Marathon cDNA library; Clontech) by PCR using the 5′ oligonucleotide AAAAGGAAGAGGGCAGAGTCGGACTCC and the 3′ oligonucleotide TTTTGAGCTGGCTAGACGCTTAAATTC as primers.
Constructs and yeast two-hybrid binding assays.
PCR fragments encompassing the first catalytic domain (D1) of PTPς (nt 3896 to 5002, aa 1238 to 1606), PTPδ (nt 2494 to 3582, aa 673 to 1035), and LAR (nt 3846 to 4928, aa 1276 to 1636) or the first catalytic domain of PTPς lacking its wedge sequence (nt 4138 to 5002, aa 1318 to 1606) were subcloned into the LexA DNA binding domain fusion vector pBTM116. The second catalytic domains (D2) of PTPς (nt 5003 to 5776, aa 1607 to 1863), PTPδ (nt 3577 to 4353, aa 1034 to 1291), and LAR (nt 4938 to 5717, aa 1640 to 1898) were subcloned into the pGAD10 (Gal4 activation domain) plasmid. The same D2 fragments were also FLAG tagged at their 3′ termini and subcloned into pACT2 (Gal4 activation domain); this plasmid has a stronger promoter than pGAD10, thus allowing immunodetection of the expressed proteins. L40 cells were transformed with these D2 constructs either alone or in combination with the D1 constructs and grown on medium lacking Trp for the pBTM116 transformants, lacking Leu for the pGAD10 or pACT2 transformants, or lacking Trp and Leu for the double transformants. Individual colonies were streaked onto fresh medium for filter β-gal assays as described above.
Liquid β-gal assays were performed in accordance with the manufacturer’s (Clontech) instructions. Briefly, individual yeast transformant colonies were grown in 20 ml of selective medium at 30°C until the cultures reached an optical density at 600 nm of ∼1.3. An aliquot (0.1 ml) of each culture was lysed and incubated with a 0.6-mg/ml o-nitrophenyl-β-d-galactopyranoside solution at 30°C for 10 min. The reactions were then quenched, and the absorbance of the supernatant was measured at 420 nm to quantify the release of o-nitrophenol. The same cultures used for the β-gal assays were analyzed for protein expression levels by immunoblotting using an anti-HA antibody (Boehringer Mannheim) for the LexA-D1 fusion proteins or an anti-FLAG antibody (IBI) for the FLAG-tagged Gal4-D2 fusion proteins.
Preparation of GST fusion proteins in bacteria.
All glutathione S-transferase (GST) fusion proteins were prepared by PCR amplification of the appropriate regions of PTPς or PTPδ and subcloning into pGEX-KG, pGEX-4T1, or pGEX-4T2. The insert-containing plasmids were transformed into E. coli HB101. Expression of fusion proteins was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside, and bacteria were collected and lysed in lysozyme buffer containing 33 mM Tris-HCl (pH 7.4), 2.5 mM EDTA, 10 mM β-mercaptoethanol, 1-mg/ml lysozyme, and protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 10-μg/ml aprotinin, and 10-μg/ml leupeptin) by sonication. The lysate was treated with MgCl2 and DNase I at final concentrations of 2 mM and 25 ng/ml, respectively. After 20 min of incubation at 25°C, EDTA was added to a 4 mM concentration and Triton X-100 was added to 1% and incubation proceeded for an additional 10 min at 25°C. The resulting lysate was cleared by centrifugation at 10,000 × g for 10 min (4°C), and the supernatant was incubated with glutathione-agarose beads. The pellet was treated with 1.5% (wt/vol) N-lauroylsarcosine–25 mM triethanolamine–1 mM EDTA (pH 8.0) and incubated for 15 min at 4°C. CaCl2 was added to a 1 mM final concentration, and then the solubilized pellet was cleared by centrifugation at 10,000 × g for 10 min. The supernatant was collected and pooled with the previous supernatant and incubated with glutathione-agarose beads. The proteins were then eluted with 30 mM reduced glutathione (pH 8.0). This extensive purification procedure of the GST fusion proteins was necessary because the proteins produced in bacteria were largely insoluble.
Transfections in mammalian cells and coprecipitations.
PCR-generated fragments of PTPς corresponding to D1 or to D1 missing the N-terminal wedge sequence (see above and Fig. 1A) were subcloned into the mammalian expression vector pEBG to generate GST–PTPς-D1 and the GST–PTPςD1-W construct with the wedge deleted, respectively. GST–PTPδ-D1 and GST–LAR-D1 were generated in pEBG in a similar fashion. The second catalytic domains (D2) of PTPς, PTPδ, and LAR were generated by PCR (as described above) with HA tags and subcloned into the pCMV4 mammalian expression vector. Insert-containing plasmids were transiently transfected (alone or in combination) into Cos7 cells in six-well plates by using Lipofectamine (Gibco). Transfected cells were lysed in 200 μl of lysis buffer plus protease inhibitors (50 mM HEPES [pH 7.5], 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10-μg/ml leupeptin, and 10-μg/ml aprotinin) per well; 20-μl aliquots were taken to verify the protein expression of each construct, and the remaining lysate was incubated with 25 to 30 μl of a 50% glutathione-agarose slurry for 1 h at 4°C. Beads were then washed four times with high-salt HNTG (20 mM HEPES [pH 7.5], 500 mM NaCl, 0.1% Triton X-100, 10% glycerol), and proteins were separated by sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose, and immunoblotted with either anti-GST antibodies to detect the precipitated D1 domains of PTPς, PTPδ, or LAR or anti-HA antibodies to detect the coprecipitated D2 domains of these PTPs. In parallel sets of experiments, cells transfected with GST–PTPς-D1, alone or together with HA–PTPδ-D2, were lysed and the lysate was incubated with glutathione agarose beads to precipitate GST–PTPς-D1. The precipitated PTPς-D1 was then analyzed for catalytic (phosphatase) activity.
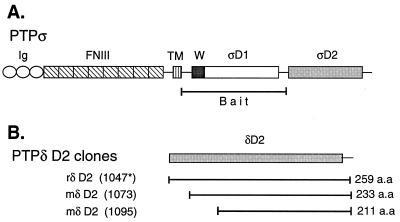
FIG. 1.
(A) Schematic representation of PTPς (LAR-PTP2; accession no. L11587; reference 52) and the bait used for the yeast two-hybrid screen corresponding to its first catalytic domain (PTPς-D1). Ig, Ig-like repeat; FNIII, FNIII-like repeat; TM, transmembrane domain; W, wedge motif; ςD1, PTPς-D1; ςD2, PTPς-D2. (B) Regions of PTPδ (all in the second catalytic domain, D2) isolated in yeast two-hybrid screens of rat (r) lung and mouse (m) embryo (day 11) libraries. The general architecture and domain arrangement of PTPς shown in panel A are also shared by PTPδ and LAR. Splice variants of PTPς are not shown. The asterisk refers to the mouse amino acid number, since the entire rat PTPδ has not been cloned.
Phosphatase assays.
PTP activity of GST–PTPς-D1 precipitated from Cos7 cells, or generated in bacteria, was assayed by using p-nitrophenylphosphate (PNPP) as a substrate. Assays were performed at room temperature in 50 to 100 μl of reaction buffer containing 100 mM PNPP, 100 mM 2-(N-morpholino)ethanesulfonic acid (MES) at pH 5.5, 10 mM dithiothreitol, 150 mM NaCl, and 2 mM EDTA. For assays performed on GST fusion proteins generated in bacteria, 200 to 500 ng of GST–PTPς-D1 alone (soluble or immobilized on agarose beads) or together with soluble GST–PTPδ-D2 or with GST (control) was added to the reaction mixture, and the reaction was allowed to proceed for 2 to 5 min. The reaction was then stopped with 900 μl of 1.0 M NaOH, and the absorbance of p-nitrophenolate at 450 nm was determined and compared against a standard curve. For assays performed with GST–PTPς-D1 precipitated from Cos7 cells, the activity against PNPP of the precipitated GST–PTPς-D1, alone or when coprecipitated with HA–PTPδ-D2, was analyzed exactly as described above. Our unpublished work shows no difference in the amount of substrate (PNPP) metabolized after 5 min in the presence of 100, 200, or 300 mM PNPP, suggesting that the substrate was not limiting in our assays. Moreover, preincubation of the PNPP-containing reaction mixture with the D2 of PTPδ or PTPς did not have a significant effect on the amount of PNPP metabolized by PTPς-D1, suggesting that substrate sequestration by the (inactive) D2 domains is not significant.
RESULTS
Identification of PTPδ-D2 in two-hybrid screens using PTPς-D1 as bait.
We have been studying the role of PTPς in mammalian development. To gain insight into the possible regulation of this phosphatase, we performed a yeast two-hybrid screen with the first catalytic domain of rat PTPς (PTPς-D1, fused to the LexA DNA binding domain) as bait (Fig. 1A) to identify interacting, possibly regulatory, proteins. The bait sequence used (aa 1238 to 1606; reference 52) also included the wedge region of PTPς (aa 1285 to 1317) and contained a Cys-to-Ser mutation at the highly conserved (V/I)HCxAGxxR(T/S)G signature motif. Our screens of either a rat lung library or a mouse embryonic (11-day) library resulted in the isolation of several strong positive clones corresponding to the second catalytic domain of PTPδ (Fig. 1B). The clones isolated from the mouse embryonic library were 26 and 48 aa shorter at the N terminus than the rat clone (the shortest clone was missing most of the highly conserved DYINAS sequence [Fig. 1B and 2]), suggesting that these N-terminal amino acids in PTPδ-D2 are not necessary for binding. Comparison of the second catalytic domain of rat PTPδ to that of the previously cloned mouse PTPδ (23) reveals 97% sequence identity at the amino acid level (Fig. 2).
FIG. 2.
Sequence alignment of the second catalytic domains (D2) of rat (r) and mouse (m) PTPδ, rat PTPς, and rat LAR. The signature motif is in bold letters. The arrowhead represents the starting amino acid of the smallest clone isolated, located within the conserved DYINAS sequence. The previously published frameshift in the mouse PTPδ-D2 sequence (23) has been corrected based on our own sequencing.
Binding of PTPδ-D2, but not LAR-D2 or PTPς-D2, to PTPς-D1 in a yeast two-hybrid binding assay.
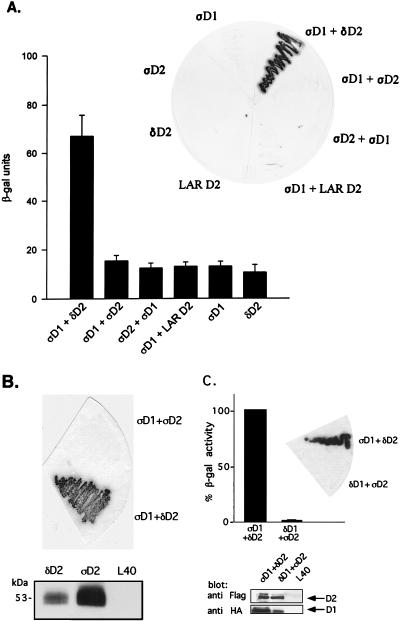
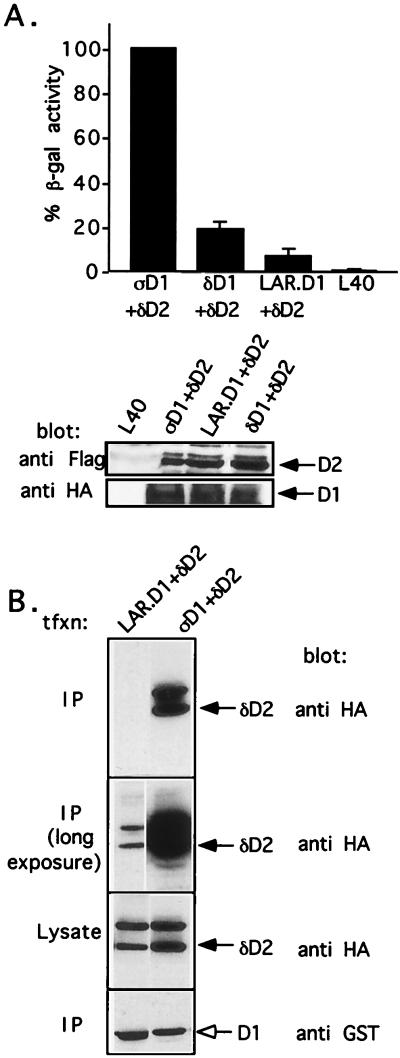
The second catalytic domains of PTPδ (PTPδ-D2), LAR (LAR-D2), and PTPς (PTPς-D2) share a high degree of sequence similarity (Fig. 2). We therefore wanted to investigate whether LAR-D2 and PTPς-D2 can also bind to PTPς-D1 by using yeast two-hybrid binding assays. As shown in Fig. 3A, cotransformation in yeast of PTPς-D1 (fused to the DNA binding domain) together with PTPδ-D2 (fused to the transactivation domain) caused strong expression of β-gal, a marker enzyme indicating an interaction between the two proteins. Only basal levels of β-gal activity were detected when either clone was transformed alone (Fig. 3A) or when PTPς-D1 was cotransformed with an unrelated protein (Nedd4; data not shown). In contrast to the PTPς-D1–PTPδ-D2 interaction, cotransformation of PTPς-D1 with LAR-D2 or with PTPς-D2 resulted in very weak β-gal expression, similar to that of the negative control (PTPς-D1 alone, LAR-D2 alone, or PTPς-D2 alone) (Fig. 3A). This lack of interaction was also apparent upon cotransformation of PTPς-D1 and PTPς-D2 expressed in the reciprocal vectors, i.e., PTPς-D2 fused to the LexA DNA binding domain and PTPς-D1 fused to the transactivation domain (Fig. 3A). To ensure that this lack of interaction was not caused by too low or inappropriate expression of PTPς-D2 relative to PTPδ-D2 in yeast cells, we epitope tagged both D2 domains with a FLAG tag and expressed them in the pACT vector, which leads to higher levels of protein expression. As can be seen in Fig. 3B, both proteins were highly expressed in yeast cells, yet only PTPδ-D2, and not PTPς-D2, was able to interact with PTPς-D1. Moreover, unlike the reported homodimerization of the D1 domain of RPTPα (3), there was no detectable PTPς-D1–PTPς-D1 association when the domain was expressed on both the DNA binding and the transactivation domains (data not shown). These results demonstrate that PTPς-D1 preferentially associates with PTPδ-D2 and not with LAR-D2 or with PTPς-D2, despite close sequence similarity between the second catalytic domains of all three PTPs. They also suggest a D1-D2 heterodimerization rather than a D1-D1 homodimerization type of interaction between these domains.
FIG. 3.
Binding of PTPς-D1 to PTPδ-D2, but not of PTPδ-D1 to PTPς-D2, analyzed by yeast two-hybrid binding assays. (A) Quantitative liquid β-gal assays (histograms) or filter β-gal assays (inset) performed with the bait PTPς-D1 (ςD1) fused to the LexA DNA binding domain and the prey PTPδ-D2 (δD2), PTPς-D2 (ςD2), or LAR D2 fused to the Gal4 transactivation domain. Interactions between D1 and D2 of PTPς were also analyzed in the opposite configuration (LexA-ςD2 and Gal4-ςD1, third bar from the left and third yeast streak clockwise from the top in the inset). The quantitative β-gal assays represent means ± standard errors of six determinations. (B) Protein expression of FLAG-tagged D2 domains of PTPς (ςD2) and PTPδ (δD2) in the pACT vector, demonstrating strong expression of both proteins in yeast L40 cells (bottom) but an association of ςD1 with δD2 only and not with ςD2 upon coexpression in L40 cells (top). (C) Lack of binding of PTPδ-D1 to PTPς-D2. Filter β-gal assays (top, inset) or liquid β-gal (top) assays were performed with the bait LexA–PTPδ-D1 and the prey Gal4–PTPς-D2 cotransformed into yeast L40 cells or with the positive control LexA–PTPς-D1 plus Gal4–PTPδ-D2. Bars represent means ± standard errors (n = 8). The actual β-gal activities were 445.7 ± 27.6 U for ςD1 plus δD2 and 6.4 ± 1.2 U for δD1 plus ςD2. For analysis of protein expression, L40 cells untransformed or transformed with δD1 plus ςD2 or with ςD1 plus δD2 were lysed and proteins were separated by SDS–10% PAGE and immunoblotted with anti-FLAG (against D2) or anti-HA (against D1) antibodies (bottom).
Since our results demonstrated PTPς-D1–PTPδ-D2 binding, we wanted to test whether the association is reciprocal, i.e., if PTPδ-D1 can bind PTPς-D2. We therefore isolated PTPδ-D1 by PCR cloning, generated LexA–PTPδ-D1 and Gal4–PTPς-D2 constructs, and analyzed the binding of these fusion proteins in yeast two-hybrid binding assays. Our results show no interaction between these domains, despite the expression of both proteins in yeast L40 cells (Fig. 3C), suggesting that the two phosphatases interact in a unidirectional manner, by association of PTPς-D1 with PTPδ-D2, and not vice versa.
Coprecipitation of PTPς-D1 with PTPδ-D2 expressed in mammalian cells.
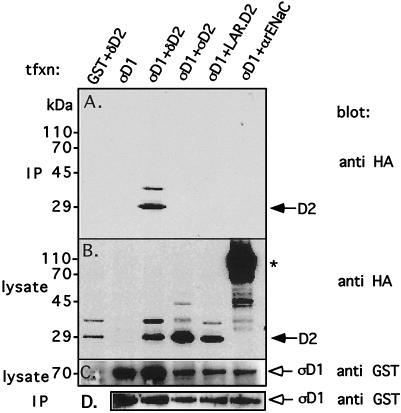
To test whether the interaction between PTPς-D1 and PTPδ-D2 is not just an anomaly associated with the yeast two-hybrid binding assay, we transfected the above-described PTP catalytic domains into mammalian cells and tested their interactions by coprecipitation. Thus, GST-tagged PTPς-D1 (in vector pEBG) was cotransfected with HA-tagged PTPδ-D2 (in vector pCMV4) into Cos7 cells. Transfected cells were lysed, and the lysate was incubated with glutathione agarose beads to precipitate the GST-tagged (PTPς-D1) proteins. The proteins were then separated by SDS-PAGE and immunoblotted with anti-HA antibodies to detect the coprecipitation of HA-tagged PTPδ-D2. As shown in Fig. 4A, precipitation of PTPς-D1 resulted in coprecipitation of PTPδ-D2 with it, confirming our above-described yeast two-hybrid binding results. That this association is not a result of nonspecific binding is evident from the observation that coexpression of an unrelated protein (HA-tagged αrENaC) together with GST–PTPς-D1 did not lead to coprecipitation of these proteins (Fig. 4A). The GST–PTPς-D1—HA–PTPδ-D2 interaction was equally strong when PTPς-D1 contained a Cys→Ser mutation in the signature motif (data not shown). In contrast to the strong association between GST–PTPς-D1 and HA–PTPδ-D2, cotransfection of GST–PTPς-D1 together with either HA–LAR-D2 or HA–PTPς-D2 did not yield significant binding between PTPς-D1 and these D2 domains (Fig. 4A), despite similar levels of protein expression in all transfected cells (Fig. 4B to D). Upon greater overexpression of the proteins, weak binding of PTPς-D1 to PTPς-D2 or LAR-D2 was also observed (data not shown). Collectively, these results are in agreement with the results of our yeast two-hybrid binding assays described above, as well as with those of our preliminary in vitro binding assays (data not shown), and confirm the selectivity for PTPς-D1–PTPδ-D2 interactions.
FIG. 4.
Coprecipitation of PTPς-D1 and PTPδ-D2 in mammalian cells. PTPς-D1 expressed as a GST fusion protein (in the mammalian expression vector pEBG) was transiently cotransfected with either HA-tagged PTPδ-D2, PTPς-D2, or LAR-D2 (in pCMV4) into Cos7 cells. Transfected cells were lysed, the lysate was incubated with glutathione agarose beads to precipitate GST–PTPς-D1 and associated proteins, and the proteins were separated on by SDS–10% PAGE and immunoblotted with anti-HA antibodies to detect coprecipitated HA-tagged D2 domains (panel A, IP). Aliquots of the lysate were also analyzed for levels of expression of either the HA-tagged D2 domains by using anti-HA antibodies (panel B, lysate) or GST–PTPς-D1 by using anti-GST antibodies (panel C, lysate). The blot in panel A was then stripped and reprobed with anti-GST antibodies to determine the amount of GST–PTPς-D1 precipitated from the cell lysates (panel D, IP). The reason for the appearance of a slower-migrating band (∼35 kDa, recognized by both anti-HA and anti-D2 antibodies) in lanes representing the D2 domains of PTPς, PTPδ, and LAR (B) is not known, but these bands do not represent phosphorylated forms of the proteins (data not shown). The asterisk marks the HA-tagged α subunit of the rat epithelial Na+ channel (αrENaC), which was used as a negative control for these experiments. tfxn, transfection.
Inhibition of PTPς-D1 catalytic activity by PTPδ-D2.
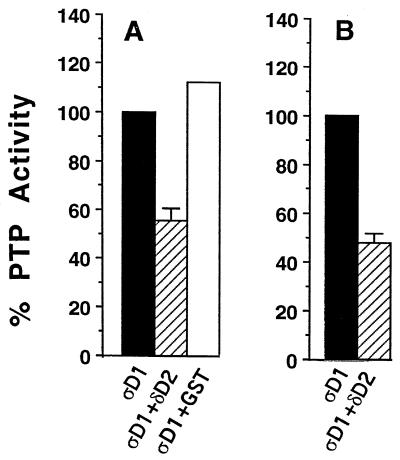
In the LAR family PTPs studied to date (including LAR and PTPς), the first, but not the second, catalytic domains are active (11a, 38, 44). To test whether the association between PTPς-D1 and PTPδ-D2 had any effect on the catalytic activity of PTPς-D1, we initially performed a series of phosphatase assays using PNPP as a substrate for PTPς-D1 and added PTPδ-D2 to the reaction mixture. For these experiments, PTPς-D1 and PTPδ-D2 were expressed in bacteria as GST fusion proteins. Figure 5A shows that upon addition of soluble GST–PTPδ-D2 to the reaction mixture, dephosphorylation of PNPP by soluble or immobilized GST–PTPς-D1 (Fig. 5A) was reduced by ∼50%, an inhibition not seen with GST alone (Fig. 5A). PTPδ-D2 was equally effective in inhibiting the catalytic activity of the full intracellular domain (which includes both D1 and D2) of PTPς (data not shown). Moreover, PTPς-D1 expressed in Cos7 cells and then immunoprecipitated was catalytically active against PNPP and its activity was inhibited by ∼50% in cells cotransfected with PTPδ-D2 (Fig. 5B), suggesting that the coprecipitated PTPδ-D2 was partially blocking PTPς-D1 activity. These results, therefore, demonstrate that the association of PTPδ-D2 with PTPς-D1 leads to partial inhibition of the catalytic activity of the latter.
FIG. 5.
Inhibition of catalytic activity of PTPς-D1 by PTPδ-D2. (A) Bacterially expressed GST fusion protein of PTPς-D1 (ςD1), either soluble or immobilized on glutathione agarose beads, was incubated with 100 mM PNPP either alone, with 500 ng of bacterially expressed soluble PTPδ-D2 (δD2), or with GST (control). The reaction was stopped with 0.9 ml of 1 M NaOH, and the optical density at 450 nm of the p-nitrophenolate product was measured. These data are means ± standard errors of four independent experiments. GST–PTPδ-D2 alone had no catalytic activity (data not shown). (B) Inhibition of the catalytic activity of PTPς-D1 (ςD1) by PTPδ-D2 (δD2) coprecipitated from Cos7 cells. The activity of ςD1 precipitated from Cos7 cells, transfected with either ςD1 alone or cotransfected with ςD1 plus δD2, was analyzed as described for panel A. The data are the means ± standard errors of nine independent experiments.
Association between PTPς-D1 and PTPδ-D2 requires the wedge sequence.
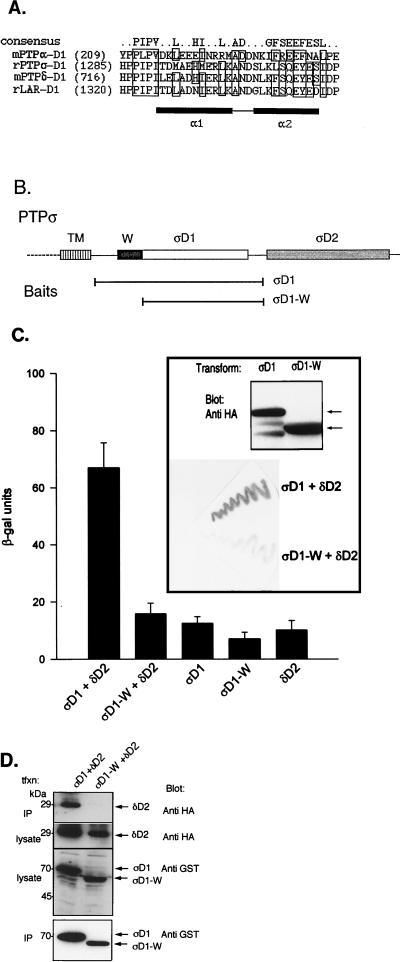
A recent report describing the dimerization of the first catalytic domains (D1) of RPTPα identified the wedge sequence (a helix-turn-helix motif) located in the N terminus of the domain as the sequence responsible for binding to the active site of the dimer partner (3). Because the wedge sequence is also conserved in LAR family members (3; Fig. 6A), we tested whether this sequence may be responsible or necessary for the association of PTPς-D1 with PTPδ-D2. We therefore repeated our yeast two-hybrid binding assays and coprecipitation experiments with mammalian cells by using a wedge-deleted PTPς-D1 (PTPςD1-W) instead of wild-type PTPς-D1 (Fig. 6B). Our results show that removal of the wedge sequence from PTPς-D1 drastically reduced binding to PTPδ-D2, as determined by yeast two-hybrid binding assays (Fig. 6C) and by coprecipitation in mammalian cells (Fig. 6D). This effect was seen despite similar levels of protein expression of PTPς-D1 and PTPςD1-W in both yeast (Fig. 6C, inset) and mammalian (Fig. 6D, two bottom panels) cells. Thus, the wedge region, previously shown to mediate RPTPα D1-D1 homodimerization (3), is also involved in the D1-D2 heterodimerization of PTPς and PTPδ.
FIG. 6.
Requirement of the wedge sequence of PTPς-D1 for interaction with PTPδ-D2. (A) Alignment of the wedge (W) sequences of the first catalytic domains of several RPTPs, including mouse (m) RPTPα, rat (r) PTPς, mouse PTPδ, and rat LAR. A more detailed alignment is provided elsewhere (3). Boxed residues represent identical or conserved amino acids conforming to the consensus of the wedge sequence. The α1 and α2 helices are represented by black boxes. (B) Schematic representation of the two-hybrid baits for panel C, including full-length PTPς-D1 (ςD1) or the N-terminally truncated, wedge-deleted (ςD1-W) regions. Both proteins were expressed as fusion proteins with the LexA DNA binding domain. (C) Yeast two-hybrid binding assays of L40 cells cotrans- formed with ςD1 (bait) plus δD2 (prey), with ςD1-W (bait) plus δD2 (prey), or with each construct alone (ςD1, ςD1-W, or δD2). Quantitative β-gal assay results (histograms) are the means ± the standard errors of six determinations. Filter β-gal assay results are shown in the inset (bottom). Levels of protein expression of the PTPς-D1 and PTPςD1-W baits (HA tagged) in these experiments were similar, as determined by immunoblotting with anti-HA antibodies (top of inset, arrows). (D) Lack of coprecipitation of PTPδ-D2 with PTPςD1-W. Cos7 cells were cotransfected with HA-tagged PTPδ-D2 (δD2) together with either GST–PTPς-D1 (ςD1) or the wedge-deleted GST–PTPςD1-W (ςD1-W) construct. Cells were then lysed, and the lysate was incubated with glutathione agarose beads to precipitate GST–PTPς-D1 or GST–PTPςD1-W and associated proteins. Proteins bound to the beads were separated by SDS–10% PAGE and immunoblotted with anti-HA antibodies to test for coprecipitation of PTPδ-D2 (top panel, IP). Aliquots of the lysate of the transfected cells were analyzed for levels of expression of PTPδ-D2 by using anti-HA antibodies (second panel) or GST–PTPς-D1 and GST–PTPςD1-W by using anti-GST antibodies (third panel). The blot in the top panel was then stripped and reprobed with anti-GST antibodies to determine the amount of GST–PTPς-D1 or GST–PTPςD1-W precipitated from the transfected cell lysate (IP, bottom panel). tfxn, transfection.
Weak binding of the D1 domains of other LAR family members to PTPδ-D2.
Our results described above demonstrate an association between PTPς-D1 and PTPδ-D2. To test whether the D1 domains of PTPδ and LAR are also able to bind PTPδ-D2, we expressed either of these domains as a LexA fusion protein and cotransformed yeast L40 cells with this construct together with Gal4–PTPδ-D2. Our results show that PTPδ-D1 was also able to bind PTPδ-D2, but the interaction was weaker (∼20%) than that seen with PTPς-D1; the interaction of LAR-D1 with PTPδ-D2 was even weaker (∼7%) (Fig. 7A). Accordingly, cotransfection of LAR-D1 and PTPδ-D2 revealed poor binding between the two proteins, unlike the strong association observed between PTPς-D1 and PTPδ-D2 (Fig. 7B). The interaction between PTPδ-D1 and PTPδ-D2 could not be assessed in Cos7 cells due to the instability of the former protein in these cells. As observed above with PTPς-D1, neither PTPδ-D1 nor LAR-D1 was able to bind the D2 domain of PTPς or LAR, as determined by yeast two-hybrid binding assays or by coprecipitation experiments with mammalian cells (data not shown). Thus, these result suggest that the first catalytic domain of LAR family members, especially PTPς, are able to bind the second catalytic domain of PTPδ (but not other D2 domains) and that the hierarchy of interactions is PTPς-D1>>PTPδ-D1>LAR-D1.
FIG. 7.
Weak association of the D1 of other LAR family members (PTPδ and LAR) with PTPδ-D2. (A) Liquid β-gal assays performed with the bait PTPς-D1 (ςD1), PTPδ-D1 (δD1), or LAR-D1 fused to the LexA DNA binding domain either alone or together with the prey PTPδ-D2 (δD2) fused to the Gal4 transactivation domain. L40 represents the β-gal activity of untransformed yeast cells. The data are percentages of the β-gal activity of ςD1 plus δD2 and represent the means ± the standard errors of 8 to 24 determinations. One hundred percent β-gal activity of ςD1 plus δD2 corresponds to 423 ± 40 U. There was no autoactivation of each domain alone. For analysis of protein expression, L40 cells untransformed or transformed with ςD1 plus δD2, δD1 plus δD2, LAR-D1 plus δD2, or the domains alone were lysed, and the proteins were separated by SDS–10% PAGE and immunoblotted with anti-FLAG (against D2) or anti-HA (against D1) antibodies. (B) Poor association of LAR-D1 with δD2 in mammalian cells. Cos7 cells were cotransfected with GST–LAR-D1 (LAR-D1) or GST-ςD1 (ςD1) (used as a control) together with HA-tagged PTPδ-D2 (δD2). Cells were lysed, the lysate was incubated with glutathione agarose beads to precipitate the GST-tagged D1s and their associated proteins, and the proteins were separated by SDS–10% PAGE and immunoblotted with either anti-GST antibodies to detect the D1 proteins (lowest panel) or anti-HA antibodies to detect coprecipitated δD2 (upper two panels, showing short- and long-exposure autoradiograms). Equal expression of δD2 in all transfections was confirmed by immunoblotting the lysate of transfected cells with anti-HA antibodies (third panel from the top). tfxn, transfection.
DISCUSSION
In this report, we demonstrate that the first catalytic domain of PTPς binds to the second catalytic domain of PTPδ, an interaction which requires the presence of the wedge sequence of PTPς and which leads to partial inhibition of the catalytic activity of PTPς. The first catalytic domains of other LAR family members can also bind PTPδ-D2, although more weakly, and none of the LAR family D1 domains is able to bind D2 domains other than that of PTPδ.
A recent determination of the tertiary structure of RPTPα-D1 revealed that the domain crystallizes as a homodimer. This D1-D1 dimerization is mediated by an ∼30-aa helix-turn-helix (wedge) sequence located at the N terminus of RPTPα-D1 which is tucked into the active site of the opposing partner of the dimer (3). Based on this structure, it was predicted that such dimerization would inhibit catalytic activity, because the active site is occupied by the wedge sequence. Our PTPς-D1–PTPδ-D2 heterodimerization results provide a variation on this theme, but with a fundamental difference; we believe that the wedge sequence of PTPς-D1 indeed binds to the “pseudoactive” site of PTPδ-D2 (i.e., homologous to the active site of D1), which, like other LAR family D2 domains, is catalytically inactive (11a). The D2 domains of LAR family RPTPs (and other RPTPs) do not possess an N-terminal wedge sequence, and moreover, all of the PTPδ-D2 sequences that we isolated in the yeast two-hybrid screens did not contain their N termini. Thus, the observed inhibition of PTPς-D1 catalytic activity suggests either the existence of a downstream inhibitory region(s) in the D2 domain of PTPδ which may bind to and inhibits the active site of PTPς-D1 or that binding of PTPδ-D2 to the wedge sequence of PTPς-D1 somehow distorts the active site of PTPς-D1 or, alternatively, interferes with substrate accessibility. Determination of the tertiary structure of D2 domains alone or in complex with D1 domains, not yet available, should help in the identification of the exact mode of D1-D2 interactions and D2-mediated inhibition of D1 catalytic activity. Whatever the mechanism(s) of binding, the observation of partial inhibition of the PTP activity of a D1 domain by a D2 domain could have important biological implications (see below). More importantly, it may provide an explanation for the long-standing observation that the D2 domains of many RPTPs are inactive; our work suggests that the role of these D2 domains is to regulate the activity of the D1 domains. In LAR family members, this regulation is likely mediated by intermolecular interactions between these closely related phosphatases, although we cannot preclude the possibility of a weak intermolecular association between the two catalytic domains of PTPδ. Based on our lack of PTPς D1-D1 binding, we believe that the observed D1-D1 homodimerization of RPTPα (3) may represent a different mode of regulation of that phosphatase; indeed, unlike most receptor PTPs, both catalytic domains of RPTPα are catalytically active (47).
An alternative possibility to explain our data, although less likely, is that the PTPς-D1–PTPδ-D2 association somehow induces PTPς D1-D1 dimerization which was undetected by our yeast two-hybrid binding assays.
The second catalytic domains of LAR, PTPδ, and PTPς are very similar in sequence, with only minor substitutions, mostly in nonconserved amino acids (Fig. 2). It is therefore difficult to explain the vast difference between PTPδ-D2 and the D2 domain of PTPς or LAR in the ability to bind to PTPς-D1 (or other LAR family D1 domains), demonstrated here by yeast two-hybrid binding assays and coprecipitations from mammalian cells. Detailed mutation analysis is required to sort out the source of this specificity.
The ectodomains of LAR, PTPδ, and PTPς are composed of Ig and FNIII repeats, resembling the cell adhesion molecules N-CAM, fasciclin, and L1. CAMs such as N-CAM or Ng-CAM have been demonstrated to aggregate through homophilic interactions (14). Indeed, recent studies have demonstrated that PTPκ, PTPμ, and PTPλ, a subfamily of phosphatases closely resembling LAR, can each aggregate via its extracellular domain in a homophilic, but not heterophilic, manner (4–6, 13, 31, 53); such interactions, however, have no effect on the catalytic activity of these PTPs (5, 31). So far, homophilic interactions of LAR, PTPδ, and PTPς have not been demonstrated, raising the possibility that the ectodomains of these PTPs interact either with other extracellular components (e.g., extracellular matrix proteins) or, possibly, with each other in a heterophilic manner. Our results described here demonstrate that these LAR family PTPs can form heterocomplexes via their intracellular domains. Moreover, such putative heterodimerization is likely to inhibit the catalytic activity of at least one of the binding partners. Although it is not known whether these LAR family members are coexpressed in the same cells, this is likely, since recent reports have demonstrated expression of these PTPs in the same types of neuronal and epithelial tissues or cells. For example, both PTPς and PTPδ have been shown to be expressed in the hippocampus, especially in the pyramidal cell layer and granular layer of dentate gyrus (23, 45, 46, 49), and we and others have found that LAR, PTPς, and PTPδ are expressed in fetal alveolar epithelial cells (11a, 17, 18). In addition, a recent report has demonstrated colocalization of PTPς and LAR in adhesion plaques of A431 cells (1).
The physiological substrates for most PTPs, including LAR family members, are not known. Several proteins that interact with LAR family members have been described recently, but unlike the PTPδ-D2 described here, none seem to affect PTP activity. A coiled-coil phosphoserine called LAR-interacting protein was shown to bind to the second catalytic domains of LAR, PTPδ, and PTPς and appears to localize LAR to focal adhesions (29, 32). Recently, it was demonstrated that LAR family members can associate with the β-catenin–cadherin complex and can dephosphorylate β-catenin in vitro (1, 22). The cadherin–α-, β-, or γ-catenin complex is associated with the cytoskeleton and is found in regions of cell-cell contact. The presence of these phosphatases in such regions suggests that the interactions may regulate tyrosine dephosphorylation of β-catenin, thus affecting the integrity of the cadherin–α-, β-, or γ-catenin complex and therefore that of cell adhesion. This could potentially have major implications for tissue development, particularly for events associated with neurite outgrowth and epithelial differentiation. Several drosophila receptor PTPs, including DLAR, have been shown to be expressed in a subset of the developing axons and pioneer neurons in the central nervous system (41, 50) and were recently demonstrated to be necessary for motor axon guidance in the Drosophila embryo (8, 21). This suggests that LAR or its other family members may have a parallel role in vertebrates as well. Indeed, PTPς, PTPδ, and LAR were previously shown to be strongly expressed during development in selected regions within the central nervous system and the peripheral nervous system, as well as in other epithelial and neuroepithelial cells (17, 18, 23, 34, 35, 45, 46, 49), and a recent gene knockout of LAR has demonstrated a reduction in the size of cholinergic neurons and defects in hippocampal cholinergic innervation (51).
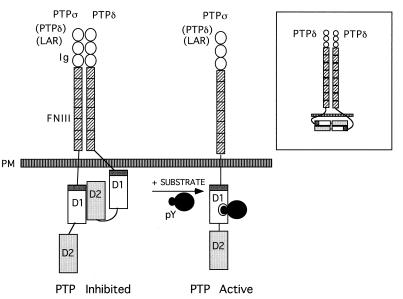
The biological meaning of our observed association between LAR family members, especially between PTPς-D1 and PTPδ-D2, and the resultant inhibition of PTP activity, is not known. It is possible, however, that such an association keeps one or both binding partners in an inactive state, perhaps analogous to the intramolecular interactions recently identified in src family members which keep the kinase domain inactive (33, 48). We speculate that upon arrival of the appropriate (high-affinity) tyrosine-phosphorylated substrate, the D1-D2 intermolecular complex is likely to dissociate, allowing substrate dephosphorylation (Fig. 8). The identification of a biological substrate(s) for PTPς, the role that this PTP and other LAR family members play in neuronal and epithelial morphogenesis and development, and the possible inhibitory role of PTPδ in these processes, are important questions that now need to be addressed.
FIG. 8.
Model of D1-D2 heterodimerization of LAR family PTPs. Under resting conditions, the first catalytic domain (D1) of PTPς (or possibly other LAR family PTPs [in parentheses]) is associated with the second catalytic domain (D2) of PTPδ, an association requiring the wedge sequence of PTPς (dark grey). Such intermolecular dimerization inhibits catalytic activity, thus keeping the phosphatase in a partially inactive state. Our current data do not support the association of PTPς-D1 with PTPς-D2 or LAR-D1 with LAR-D2 but cannot preclude the possibility of a weak inter- or intramolecular interaction of PTPδ-D1 with PTPδ-D2 (inset). We speculate that upon presentation of the as-yet-unidentified tyrosine-phosphorylated substrate (likely of high affinity), the D1-D2 heterocomplex is likely to dissociate, thus allowing substrate dephosphorylation. PM, plasma membrane.
ACKNOWLEDGMENTS
M.J.W. and C.F. contributed equally to this work.
We thank Barry Goldstein for LAR and LAR-PTP2 (PTPς) cDNA.
This work was supported by a Group Grant on Lung Development from the Medical Research Council (MRC) of Canada, an Operating Grant from the Canadian MRC, the Canadian CF Foundation, and the International Human Frontier Science Program (to D.R.). D.R. was a recipient of a Scholarship from the Canadian MRC. J.B. and M.J.W. are recipients of a Fellowship from the Canadian Lung Association/Canadian MRC.
REFERENCES
- 1.Aicher B, Lerch M M, Müller T, Schilling J, Ullrich A. Cellular redistribution of protein tyrosine phosphatases LAR and PTPς by inducible proteolytic processing. J Cell Biol. 1997;138:681–696. doi: 10.1083/jcb.138.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnea G, Silvennoinen O, Shaanan B, Honegger A M, Canoll P D, D’Eustachio P, Morse B, Levy J B, LaForgia S, Huebner K, Musacchio J M, Sap J, Schlessinger J. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTPγ defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993;13:1497–1506. doi: 10.1128/mcb.13.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilwes A M, den Hertog J, Hunter T, Noel J P. Structural basis for inhibition of receptor protein-tyrosine-α phosphatase by dimerization. Nature. 1996;382:555–559. doi: 10.1038/382555a0. [DOI] [PubMed] [Google Scholar]
- 4.Brady-Kalnay S M, Flint A J, Tonks N K. Homophilic binding of PTPμ, a receptor type protein tyrosine phosphatase, can mediate cell-cell aggregation. J Cell Biol. 1993;122:961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady-Kalnay S M, Tonks N K. Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTPμ. J Biol Chem. 1994;269:28472–28477. [PubMed] [Google Scholar]
- 6.Cheng J, Wu K, Armanini M, O’Rourke N, Dowbenko D, Lasky L A. A novel protein-tyrosine phosphatase related to the homotypically adhering κ and μ receptors. J Biol Chem. 1997;272:7264–7277. doi: 10.1074/jbc.272.11.7264. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham B A, Hemperly J J, Murray B A, Prediger E A, Brackenbury R, Edelman G M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236:799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- 8.Desai C J, Gindhart J G, Jr, Goldstein L S B, Zinn K. Receptor tyrosine phosphatases are required for motor axon guidance in the Drosophila embryo. Cell. 1996;84:599–609. doi: 10.1016/s0092-8674(00)81035-1. [DOI] [PubMed] [Google Scholar]
- 9.Desai D M, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73:541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 10.Desai D M, Sap J, Silvennoinen O, Schlessinger J, Weiss A. The catalytic activity of the CD45 membrane-proximal phosphatase domain is required for TCR signalling and regulation. EMBO J. 1994;13:4002–4010. doi: 10.1002/j.1460-2075.1994.tb06716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer E H, Charbonneau H, Tonks N K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253:401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- 11a.Fladd, C., and D. Rotin. Unpublished data.
- 12.Gebbink M F B G, Van Etten F I, Hateboer G, Suijkerbuijk R, Beijersbergen R L, van Kessel A G, Moolenaar W H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991;290:123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- 13.Gebbink M F B G, Zondag G C M, Wubbolts R W, Beijersbergen R L, van Etten I, Moolenaar W H. Cell-cell adhesion mediated by a receptor-like protein tyrosine phosphatase. J Biol Chem. 1993;268:16101–16104. [PubMed] [Google Scholar]
- 14.Hoffman S, Edelman G M. Kinetics of homophilic bindings by E and A forms of the neural cell adhesion molecule. Proc Natl Acad Sci USA. 1983;80:5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh M, Streuli M, Krueger N X, Saito H. Purification and characterization of the catalytic domains of the human receptor-linked protein tyrosine phosphatases HPTPβ, leukocyte common antigen (LCA), and leukocyte common antigen-related molecule (LAR) J Biol Chem. 1992;267:12356–12363. [PubMed] [Google Scholar]
- 16.Jiang Y-P, Wang H, D’Eustachio P, Musacchio J M, Schlessinger J, Sap J. Cloning and characterization of R-PTP-κ, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region. Mol Cell Biol. 1993;13:2942–2951. doi: 10.1128/mcb.13.5.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsura H, Williams M C, Brody J S, Yu Q. Two closely related receptor type tyrosine phosphatases are differentially expressed during rat lung development. Dev Dyn. 1995;204:89–97. doi: 10.1002/aja.1002040111. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Yeger H, Han R, Wallace M, Goldstein B, Rotin D. Expression of LAR-PTP2 in rat lung is confined to proliferating epithelia lining the airways and air sacs. Am J Physiol. 1996;14:L566–L576. doi: 10.1152/ajplung.1996.270.4.L566. [DOI] [PubMed] [Google Scholar]
- 19.Krueger N X, Saito H. A human transmembrane protein-tyrosine phosphatase, PTPζ, is expressed in brain and has an N terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci USA. 1992;89:7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krueger N X, Streuli M, Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990;9:3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krueger N X, Van Vactor D, Wan H I, Gelbart W M, Goodman C S, Saito H. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell. 1996;84:611–622. doi: 10.1016/s0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- 22.Kypta R M, Su H, Reichardt L F. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno K, Hasegawa K, Katagiri T, Ogimoto M, Ichikawa T, Yakura H. MPTPδ, a putative murine homolog of HPTPδ, is expressed in specialized regions of the brain and in the B-cell lineage. Mol Cell Biol. 1993;13:5513–5523. doi: 10.1128/mcb.13.9.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moos M, Tacke R, Schere H, Teplow D, Fruh K, Schachner M. Neural cell adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;33:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- 25.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 26.O’Grady P, Krueger N X, Streuli M, Saito H. Genomic organization of the human LAR protein tyrosine phosphatase gene and alternative splicing in the extracellular fibronectin type-III domains. J Biol Chem. 1994;269:25193–25199. [PubMed] [Google Scholar]
- 27.Pan M-G, Rim C, Lu K P, Florio T, Stork P J S. Cloning and expression of two structurally distinct receptor-linked protein-tyrosine phosphatases generated by RNA processing from a single gene. J Biol Chem. 1993;268:19284–19291. [PubMed] [Google Scholar]
- 28.Pot D A, Woodford T A, Remboutsika E, Haun R S, Dixon J E. Cloning, bacterial expression, purification, and characterization of the cytoplasmic domain of rat LAR, a receptor-like protein tyrosine phosphatase. J Biol Chem. 1991;266:19688–19696. [PubMed] [Google Scholar]
- 29.Pulido R, Serra-Pagès C, Tang M, Streuli M. The LAR/PTPδ/PTPς subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTPδ, and PTPς isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc Natl Acad Sci USA. 1995;92:11686–11690. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin M, Dowling J J, Hockfield S. Seven protein tyrosine phosphatases are differentially expressed in the developing rat brain. J Comp Neurol. 1995;351:617–631. doi: 10.1002/cne.903510410. [DOI] [PubMed] [Google Scholar]
- 31.Sap J, Jiang Y-P, Friedlander D, Grumet M, Schlessinger J. Receptor tyrosine phosphatase R-PTP-κ mediates homophilic binding. Mol Cell Biol. 1994;14:1–9. doi: 10.1128/mcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serra-Pagès C, Kedersha N L, Fazikas L, Medley Q, Debant A, Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 1995;14:2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 34.Stoker A W. Isoforms of a novel cell adhesion molecule-like protein tyrosine phosphatase are implicated in neural development. Mech Dev. 1994;46:201–217. doi: 10.1016/0925-4773(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 35.Stoker A W, Gehring B, Haj F, Bay B-H. Axonal localisation of the CAM-like tyrosine phosphatase CRYPα: a signalling molecule of embryonic growth cones. Development. 1995;121:1833–1844. doi: 10.1242/dev.121.6.1833. [DOI] [PubMed] [Google Scholar]
- 36.Streuli M, Krueger N X, Ariniello P D, Tang M, Munro J M, Blattler W A, Adler D A, Disteche C M, Saito H. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992;11:897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Streuli M, Krueger N X, Hall L R, Schlossman S F, Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988;168:1553–1562. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streuli M, Krueger N X, Thai T, Tang M, Saito H. Distinct functional roles of the two intracellular phosphatase-like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990;9:2399–2407. doi: 10.1002/j.1460-2075.1990.tb07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streuli M, Krueger N X, Tsai A Y M, Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci USA. 1989;86:8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun H, Charles C H, Lau L F, Tonks N K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 41.Tian S S, Tsoulfas P, Zinn K. Three-receptor linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991;67:675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- 42.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 43.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 44.Wagner J, Boerboom D, Tremblay M L. Molecular cloning and tissue-specific RNA processing of a murine receptor-type protein tyrosine phosphatase. Eur J Biochem. 1994;226:773–782. doi: 10.1111/j.1432-1033.1994.00773.x. [DOI] [PubMed] [Google Scholar]
- 45.Walton K M, Martell K J, Kwak S P, Dixon J E, Largent B L. A novel receptor-type protein tyrosine phosphatase is expressed during neurogenesis in the olfactory neuroepithelium. Neuron. 1993;11:387–400. doi: 10.1016/0896-6273(93)90193-u. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Yan H, Canoll P D, Silvennoinen O, Schlessinger J, Musacchio J M. Expression of receptor protein tyrosine phosphatase-ς (RPTP-ς) in the nervous system of the developing and adult rat. J Neurosci Res. 1995;41:297–310. doi: 10.1002/jnr.490410303. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Pallen C J. The receptor-like tyrosine phosphatase HPTPα has two catalytic domains with distinct substrate specificities. EMBO J. 1991;10:3231–3237. doi: 10.1002/j.1460-2075.1991.tb04886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu W, Harrison S C, Eck M J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 49.Yan H, Grossman A, Wang H, D’Eustachio P, Mossie K, Musacchio J M, Silvennoinen O, Schlessinger J. A novel receptor tyrosine phosphatase-ς that is highly expressed in the nervous system. J Biol Chem. 1993;268:24880–24886. [PubMed] [Google Scholar]
- 50.Yang X, Seow K T, Bahri S M, Oon S H, Chia W. Two Drosophila receptor-like tyrosine phosphatase genes are expressed in a subset of developing axons and pioneer neurons in the embryonic CNS. Cell. 1991;67:661–673. doi: 10.1016/0092-8674(91)90062-4. [DOI] [PubMed] [Google Scholar]
- 51.Yeo T T, Yang T, Massa S M, Zhang J S, Honkaniemi J, Butcher L L, Longo F M. Deficient LAR expression decreases basal forebrain cholinergic neuronal size and hippocampal cholinergic innervation. J Neurosci Res. 1997;47:348–360. doi: 10.1002/(sici)1097-4547(19970201)47:3<348::aid-jnr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W-R, Hashimoto N, Ahmad F, Ding W, Goldstein B J. Molecular cloning and expression of a unique receptor-like protein tyrosine phosphatase in the leukocyte-common-antigen-related phosphatase family. Biochem J. 1994;302:39–47. doi: 10.1042/bj3020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zondag G C M, Koningstein G M, Jiang Y-P, Sap J, Moolenaar W H, Gebbink M F B G. Homophilic interactions mediated by receptor tyrosine phosphatases μ and κ. J Biol Chem. 1995;270:14247–14250. doi: 10.1074/jbc.270.24.14247. [DOI] [PubMed] [Google Scholar]