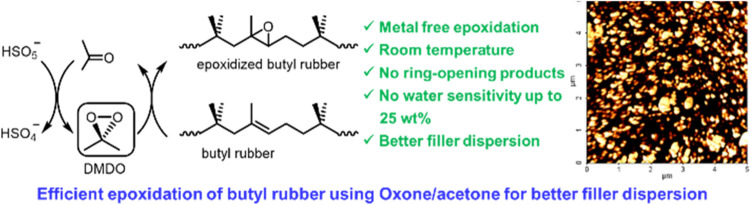
Abstract
Incorporation of a polar filler such as silica into a nonpolar rubber matrix is challenging and energy consuming due to their large difference in polarity. Epoxidation of carbon–carbon double bonds in unsaturated rubber, especially for rubber with low unsaturation such as butyl rubber, is an effective method to introduce polar functional groups to the rubber macromolecules for better filler dispersion. Although different epoxidation reagents including hydrogen peroxide (H2O2), peracid, and meta-chloroperoxybenzoic acid (mCPBA) have been previously reported, these reagents have different drawbacks. In this article, a metal-free epoxidation reagent, dimethyl dioxirane (DMDO), generated from acetone and Oxone is explored for efficient epoxidation of rubber with low unsaturation. The effects of the addition manner of the reactant Oxone and buffer sodium bicarbonate (NaHCO3) and reaction temperature on the epoxide formation are studied. Compared to peracid, a faster and more efficient epoxidation without the generation of a ring-opened product is achieved when DMDO is used as the epoxidation reagent. Furthermore, it is found that the epoxidation using DMDO is not sensitive to the water concentration in the rubber solution up to 20 wt %. The addition of quaternary ammonium salt as a phase transfer catalyst not only improves the conversion but also further increases the water tolerance to 25 wt %. The reaction conditions for preparation of epoxidized butyl rubber with different percentages of epoxide group are optimized by Design of Experiments (DoE). At the end, improved dispersion of silica in the matrix of epoxidized butyl rubber is achieved, as revealed by the rubber process analyzer (RPA) and atomic force microscopy (AFM).
1. Introduction
Butyl rubber is prepared by the cationic copolymerization of isobutylene and isoprene (0.5–2.5 mol %).1−3 The incorporation of isoprene units provides butyl rubber with unsaturation,4 which allows for vulcanization5 and further postpolymerization functionalization.6−9 Despite the low unsaturation as compared to other synthetic rubbers such as butadiene rubber (BR), butyl rubber is widely used as an innerliner in tires3 and for pharmaceutical stoppers10 owing to its exceptional impermeability to gas resulting from the densely packed polymeric chains.11,12 Meanwhile, in rubber compounding, fillers such as carbon black, clay, and silica are often added to reduce the cost and improve the physical properties of rubber compounds.13 The dispersion of fillers in a rubber compound plays an important role in the performance of a rubber product.14,15 However, because of the nonpolar nature of butyl rubber, the dispersion of fillers, especially polar fillers such as silica, into the butyl rubber matrix is difficult.16 To achieve a better filler dispersion, increasing the polarity of butyl rubber is desired,17 which can be attained by introducing polar functional monomers in the polymerization or by postpolymerization modification on the unsaturated sites. The introduction of functional monomers with polar groups such as aldehyde and anhydride into butyl rubber polymerization is challenging as they can poison the polymerization.18,19 Therefore, postpolymerization modification is a more feasible way to introduce polar moieties along the backbone of the butyl rubber.20,21
Behera et al.20 recently published a review on the postpolymerization functionalization of butyl rubber, from which we can understand that epoxidation is a relatively easy way to functionalize butyl rubber in one step as compared to other reactions that often require multiple steps. Meanwhile, the epoxy group in butyl rubber opens the possibility for further functionalization, such as the formation of allylic alcohol and exodiene. Different epoxidation reagents, including hydrogen peroxide (H2O2),22,23 and peracids, such as performic acid (PFA),24 peracetic acid (PAA),25 and meta-chloroperoxybenzoic acid (mCPBA),26−28 have been previously reported; however, these reagents have different drawbacks that limit their viability. For example, a catalyst is usually required for the epoxidation using H2O2.22,29,30 The peracid systems suffer from the potential ring opening of the epoxy group and subsequent gelation,31−33 and an elevated temperature is required to accelerate the reaction for performic acid and peracetic acid. In addition, mCPBA has a high price and the removal of the excess mCPBA and reaction side product benzoic acid from organic solvents is time-consuming.34 Therefore, a fast, efficient, and cost-effective process for the epoxidation of butyl rubber without the contamination of the ring-opening and gelation products is desired.
Oxone (KHSO5·0.5KHSO4·0.5K2SO4) is a cheap and green oxidant with K2SO4 as the only byproduct in the oxidation reaction, and it has been widely used in synthetic chemistry.35 The active component in Oxone is potassium peroxymonosulfate (KHSO5). Epoxidation of small molecules such as olefins and synthetic rubber with high unsaturation, such as styrene–butadiene-styrene (SBS) rubber, using potassium peroxymonosulfate/acetone has been previously reported. For example, Rajabi et al.36 studied the epoxidation of styrene–butadiene rubber (SBR) using in situ-generated dimethyl dioxirane (DMDO) generated from Oxone/acetone at 50–60 °C. Nikje et al.37 reported the epoxidation of ethylene propylene diene monomer (EPDM) using the in situ-generated DMDO/MoO3 complex, where the authors found that the reaction did not proceed without MoO3. However, to the best of our knowledge, there are no reports on the epoxidation of butyl rubber, which has a relatively low unsaturation as compared to other synthetic rubber using Oxone without the addition of a transition metal catalyst. In addition, for butyl rubber produced from a slurry process in industry, 6–12 wt % of water is present in the cement (cement is defined as the viscous solution of unvulcanized rubber in an organic solvent) before the postpolymerization modification such as halogenation. Therefore, it is of interest to explore an epoxidation method that is tolerant to fluctuation of water concentration.
Herein, we report a fast and efficient method for the epoxidation of butyl rubber using Oxone and acetone, which are used to in situ generate DMDO. The reaction is buffered by NaHCO3. The experimental results reveal that both the addition manner of Oxone and NaHCO3 and the reaction temperature exert great effects on the reaction. Epoxidized butyl rubber with a high epoxy content is achieved when the reaction is performed at room temperature with multiple additions of Oxone/NaHCO3. Compared to PFA epoxidation, the Oxone/acetone epoxidation system shows a faster reaction rate and no water sensitivity with water concentration up to 20 wt %. The addition of a quaternary ammonium salt as a phase transfer catalyst (PTC) further improves the degree of epoxidation by 10% and increases the water tolerance up to 25 wt %. Design of Experiments (DoE)38 is employed to optimize the conditions for the epoxidation reaction. At the end, silica dispersion in the epoxidized butyl rubber is studied using a rubber process analyzer (RPA) and atomic force microscopy (AFM) and the results indicate improved dispersion of silica in the rubber matrix as compared to the parent butyl rubber. The better dispersion of silica is beneficial to the reduction of the energy consumption during the mixing process and the improvement of the physical property of the rubber compound.
2. Experimental Section
2.1. Materials
Regular butyl rubber (1.72 mol % unsaturation, Mn = 135 000 g/mol) was obtained from the ARLANXEO Canada Inc. Sarnia site. Reagent-grade acetone, ethanol, hexane, chloroform, and sodium bicarbonate were purchased from VWR and used as received. Oxone with 44.7% active oxidant potassium peroxymonosulfate (PMS), methyltrioctylammonium hydrogen sulfate (≥95%), 88 wt % formic acid, and 30 wt % hydrogen peroxide were purchased from Sigma-Aldrich. 98 wt % sulfuric acid was purchased from Jahn–Teller (JT) Baker. Silica filler HI-SIL 233 was obtained from PPG. All of the chemicals were used as received unless otherwise stated.
2.2. Characterization
2.2.1. Nuclear Magnetic Resonance (NMR) Analysis
1H and 13C NMR characterization data were obtained by using a Bruker DRX 500 MHz spectrometer (500.13 MHz). 1H NMR and 13C NMR samples were run in CDCl3 and referenced to TMS.
2.2.2. Rubber Process Analyzer (RPA) Analysis
Rubber process analyzer (RPA) analysis was performed to determine the Payne effect at 60 °C at a frequency of 0.1 Hz and strain sweep between 0.3 and 450%. This test was used as an indication of filler–filler interactions, where a lower modulus at low strain indicates less filler–filler interactions and in theory better filler dispersion.
2.2.3. Atom Force Microscopy (AFM) Analysis
Uncured butyl rubber/silica compounds were pressed at 100 °C for 5 min into 2 mm thick sheets. These sheets were sliced with a razor blade for analysis of a fresh surface by AFM. The phase separation in the samples was investigated by analyzing the surface created by cutting with a razor blade. The surface was imaged by using the dynamic force mode of a Park Systems XE-100 AFM. A silicon cantilever with a nominal spring constant of 40 N/m, a resonant frequency of 300 kHz, and a tip radius of 10 nm was used. The phase shift angle (phase difference between the piezo driver signal and the oscillation of the cantilever as detected by the photodetector) of the dynamic force mode AFM is sensitive to the tip–sample interaction. A smaller phase shift angle (i.e., darker contrast in the phase image) suggests a softer component, and a larger phase shift angle (brighter contrast) suggests a more rigid component. Images were collected from areas of 5 μm × 5 μm.
2.3. Epoxidation of Butyl Rubber Using Oxone/Acetone
A 10 wt % rubber cement was prepared by dissolving regular butyl rubber in hexanes overnight in a round-bottom flask equipped with a magnetic stir bar. For reactions that required heating, the flasks were submerged in a heated oil bath equipped with a thermocouple. Water, acetone, and, in applicable experiments, methyltrioctylammonium hydrogen sulfate were added to the reaction flask before addition of the Oxone and NaHCO3 buffering agent. Oxone and NaHCO3 were mixed in the solid state and placed in the reaction flask. The addition manner of Oxone and NaHCO3 and the reaction times were varied in the experiments to obtain optimal conversion. Aliquots were taken by pipetting out approximately 3 mL of the reaction mixture at the desired times, and the samples were coagulated into ethanol. On completion of the reaction, the final product was coagulated into ethanol, dried, and characterized by 1H NMR spectroscopy.
2.4. Preparation of 10 wt % of Performic Acid (PFA) Solution
Ten wt % performic acid solution was prepared by mixing 12.5 mL of 88 wt % formic acid, 12.5 mL of 30 wt % hydrogen peroxide, and 1.2 mL of 98 wt % of sulfuric acid in an ice bath for 2 h. The concentration of the resulting PFA solution was determined by two-step titrations.39
2.5. Procedure of Mixing of Butyl Rubber or Epoxidized Butyl Rubber with Silica
The rubber sample was added to a Brabender internal mixer (70 g capacity) at 60 °C and 60 rpm. The sample was allowed to mix alone for a short period of time (1 min) followed by the addition of the silica filler. The ram of the mixer was left up for 2 min in order to allow the silica to enter the mixer. After the ram was lowered, the compound was mixed for an additional minute (4 min total) and a sweep was performed. The compound was mixed for 5 min total. The compound was then processed on a 4 × 6″ mill operating at 30 °C and 18 rpm with a thin nip. Any loose silica from the mixer was added, and 6 three-quarter cuts and 6 endwise passes were performed. RPA was run exactly 1 h after mixing.
3. Results and Discussion
Zhu et al. demonstrated that the epoxidation of olefins is possible in an aqueous potassium peroxymonosulfate (PMS) solution without the requirement of a ketone dioxirane precursor.40 However, the 1H NMR results indicated negligible conversion for the epoxidation of butyl rubber when only Oxone was used. Subsequent epoxidation experiments were carried out using the in situ-generated dimethyl dioxirane (DMDO) intermediate as the epoxidation agent. DMDO is generated from the nucleophilic attack of potassium peroxymonosulfate on the ketone, followed by a single oxygen transfer to the alkene substrate41 (Scheme 1). The regeneration of the ketone renders this process catalytic. The mechanism for the generation of DMDO and its epoxidation on the isoprene units of butyl rubber is illustrated in Scheme 1. In this work, acetone was selected as a simple and effective precursor to dimethyl dioxirane, and NaHCO3 was selected as the buffering agent.
Scheme 1. Epoxidation of Butyl Rubber by Dimethyl Dioxirane (DMDO) Generated from Potassium Peroxymonosulfate and Acetone.
3.1. Effect of Addition Manner on the Formation of the Epoxide Group
The epoxidation was first attempted with 5.0 g (Run 1 and 2) of regular butyl rubber, which has an unsaturation of 1.72 mol %. Run 1 and 2 have the same reaction conditions except for the addition manner of Oxone and NaHCO3. Before the addition of Oxone and NaHCO3, water and acetone were first added to the round-bottom flask containing 10 wt % of rubber cement. Afterward, for Run 1, Oxone and NaHCO3 were mixed in the solid state and added at one time. In contrast, for Run 2, the Oxone and NaHCO3 solid mixture were proportionally added stepwise at every 10 min in the 1 h reaction time. In Run 3, the epoxidation was scaled up to 20.0 g with stepwise reagent addition. In Run 4, the reaction was scaled up to 500.0 g and was performed in a 20.0 L reactor, again with stepwise addition. The molar ratios of unsaturation, Oxone, NaHCO3, and acetone remain consistent for the four epoxidation reactions listed in Table 1.
Table 1. Effects of the Addition Manner of Oxone/NaHCO3 and Scaling-Up of the Reaction on the Epoxidation of Butyl Rubber.
| run | butyl rubber (g) | Oxone (g) | NaHCO3 (g) | acetone (mL) | addition manner of Oxone/NaHCO3 | percentage of epoxide (%)b |
|---|---|---|---|---|---|---|
| 1a | 5.0 | 2.35 | 1.28 | 8.0 | one addition | 70 |
| 2a | 5.0 | 2.35 | 1.28 | 8.0 | multiple addition | 95 |
| 3a | 20.0 | 9.39 | 5.13 | 32.0 | multiple addition | ∼100 |
| 4a | 500.0 | 234.71 | 128.28 | 796.0 | multiple addition | ∼100 |
The molar ratio of unsaturation: Oxone/NaHCO3/acetone = 1:5:10:71.
The reaction stopped at 60 min reaction time.
After the reaction, the product was collected by ethanol or steam coagulation and characterized by 1H NMR spectroscopy. The 1H NMR spectrum for regular butyl rubber and the epoxidation product from Run 1 and 2 is illustrated in Figure 1. For the regular butyl rubber, two peaks at 5.1 ppm (triplet) and 4.9 ppm (doublet) are observed, which are attributed to the isoprene units adopting the 1,4-configuration and the rearranged structure, respectively.42,43 After epoxidation, the intensity for the peak at 5.1 ppm decreases and a new peak at 2.7 ppm resulting from the epoxide group indicates the occurrence of epoxidation.
Figure 1.
1H NMR spectra for (a) regular butyl rubber; (b) epoxidized butyl rubber produced by single addition of Oxone/NaHCO3 in Run 1; and (c) stepwise addition of Oxone/NaHCO3 in Run 2.
In addition to 1H NMR spectroscopy, the epoxidized butyl rubber from Run 2 in Table 1 was also analyzed by 13C NMR (Figure 2) and Fourier transform infrared (FT-IR) spectroscopy (Figure S1). As shown in Figure 2, two resonance signals at 132.36 and 129.98 ppm attributed to the isoprene units from regular butyl rubber are observed. The chemical shift for the saturated carbons is found at high fields. After the epoxidation, these two isoprene peaks disappear, indicating that the unsaturation has been converted into the epoxide group.44
Figure 2.
13C NMR spectra for (a) regular butyl rubber and (b) epoxidized butyl rubber produced by stepwise addition of Oxone and NaHCO3 in Run 2.
The percentage of epoxide group is calculated from the 1H NMR spectroscopy results as shown in eq 1, where I is the signal intensity and the subscripts represent the values of the chemical shift in ppm.
| 1 |
According to eq 1, 70 and 95% epoxide are obtained for Run 1 and Run 2, respectively. It is apparent that when Oxone/NaHCO3 is added stepwise to the cement, a higher percentage of epoxide is produced. A kinetic study on the epoxidation of butyl rubber was performed to understand the effect of addition manner on the generation of the epoxide group. 3.0 mL of aliquots of the reaction mixture were taken at 5, 15, 30, 45, and 60 min. The sample was coagulated into ethanol and the percentage of epoxide group was calculated by 1H NMR spectroscopy using eq 1. As illustrated in Figures 3 and S2, with a single addition of Oxone and NaHCO3, 55.2% of epoxide is produced in the first 15 min of the reaction (blue curve in Figure 3). Subsequently, the reaction rate decreases and plateaus at around 45 min with 66% epoxide at the end of the reaction. In contrast, with stepwise addition of Oxone and NaHCO3, the reaction rate remains more constant throughout the 1 h reaction time (orange curve in Figure 3). Although the single-addition method allows for higher conversion within 45 min, the gradual addition of Oxone and NaHCO3 provides a more constant conversion over time and results in an overall higher epoxide group of 95% at 60 min.
Figure 3.
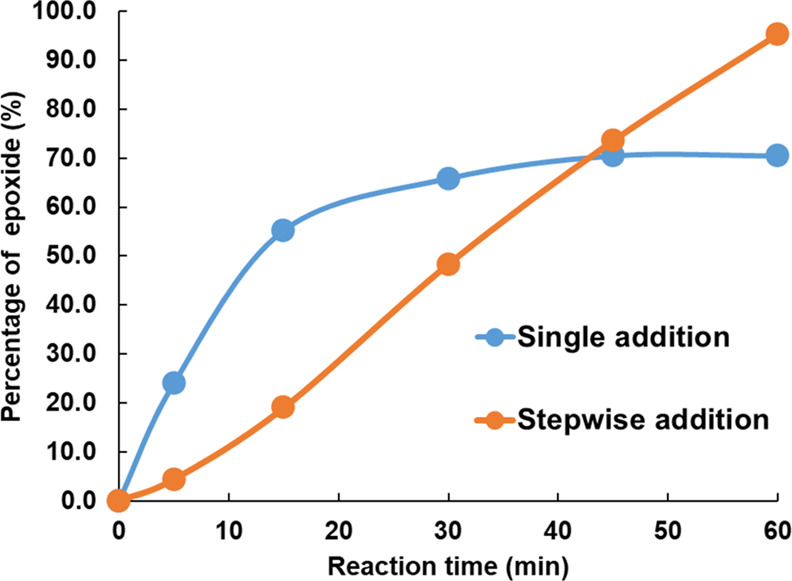
Kinetic study on the formation of an epoxide group by single addition (blue) and stepwise addition (orange) of Oxone/NaHCO3.
The above result suggests that when Oxone and NaHCO3 were added by a single addition, not all of the Oxone participated in the epoxidation reaction. In other words, there was nonproductive consumption of the Oxone that can be avoided by slower Oxone addition. Edwards et al.45 showed through isotopic 18O labeling that in an Oxone-abundant environment, dimethyl dioxirane causes the conversion of KHSO5 (potassium potassium peroxymonosulfate), which is the active component in Oxone, to oxygen and potassium hydrogen sulfate. Therefore, limiting the concentration of Oxone in the rubber cement suppresses this side reaction and a higher percentage of epoxide can be achieved at the end.
In Runs 3 and 4, the reaction was scaled up to 20.0 and 500.0 g and all of the isoprene units with 1,4 conformations were converted into epoxide as indicated by the 1H NMR spectrum in Figure S3. Therefore, the efficiency for the epoxidation was not compromised when the reaction was scaled up.
3.2. Effect of Temperature on the Formation of the Epoxide Group
The efficiency of the Oxone/acetone epoxidation of butyl rubber was also evaluated at an elevated temperature. As shown in Table 2, Run 1 and Run 5 have the same reaction conditions except that Run 5 was conducted at 45 °C while Run 1 was performed at room temperature. In both runs, Oxone and NaHCO3 were added with a single addition. Based on the 1H NMR analysis, increasing the reaction temperature to 45 °C leads to a low percentage epoxide of 20%.
Table 2. Effect of Reaction Temperature on the Epoxidation of Butyl Rubber.
| run | butyl rubber (g) | temp. (°C) | acetone (mL) | Oxone (g) | NaHCO3 (g) | percentage of epoxide (%)b |
|---|---|---|---|---|---|---|
| 1a | 5.0 | 23 | 8.0 | 2.35 | 1.28 | 70 |
| 5a | 5.0 | 45 | 8.0 | 2.35 | 1.28 | 20 |
Oxone and NaHCO3 were added by single addition, The molar ratio of unsaturation: Oxone:NaHCO3:acetone = 1:5:10:71.
The reaction stopped at 60 min reaction time.
A kinetic study of the epoxidation at 45 °C was performed and compared with that performed at 23 °C. As shown in Figures 4 and S4, 20% of epoxide was generated at 45 °C after 10 min of reaction. After that, the reaction stopped, and the percentage of epoxide remained unchanged. The low epoxide formation can be attributed to the thermal instability of dimethyl dioxirane, which decomposes into dioxyl diradical at elevated temperature.46 Therefore, to achieve a higher percentage of epoxide, a lower temperature is preferred.
Figure 4.
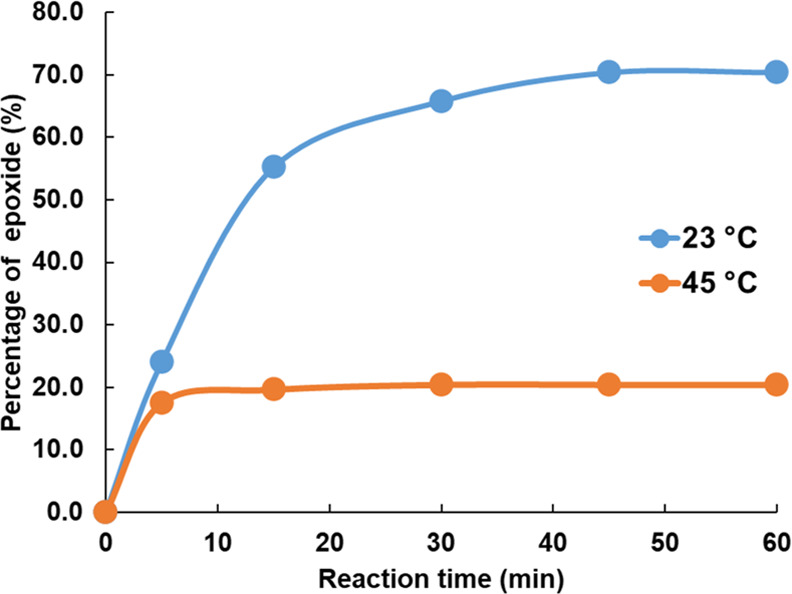
Kinetic study on the formation of the epoxide group using Oxone/acetone when epoxidation is performed at 23 °C (blue) and 45 °C (orange).
3.3. Comparison of Oxone/acetone with Performic Acid (PFA) for the Epoxidation of Butyl Rubber
Performic acid (PFA) is a common oxidant that is known to epoxidize natural rubber. An experiment was performed to compare the efficiency of using PFA to epoxidize butyl rubber with the epoxidation using Oxone/acetone. The PFA solution was prepared by mixing formic acid with H2O2 for 2 h at 0 °C (Figure S5),47,48 with the concentration determined by titration.49 During the experiments, it is observed that it is necessary to quench any remaining acid from PFA. Without this purposeful quenching, undesirable ring opening and gelation occur, as shown in Figures S6 and S7. The percentage of epoxide as a function of the reaction time for both reactions is plotted in Figure 5. The data indicate clearly that the Oxone-acetone system has a much faster conversion, allowing for greater than 20% conversion in 5 min, while the PFA system has only about 1% conversion in the same amount of time. As shown in Table 3, after 60 min, the final epoxide for the PFA system is only 36%, while the Oxone-acetone system achieves a 70% conversion. In an industrial environment, using Oxone-acetone for epoxidation shows benefit over a PFA system in both speed and overall conversion, in addition to providing a gel-free epoxidation.
Figure 5.

Kinetic study on the formation of an epoxide group when Oxone/acetone (blue) and performic acid (orange) are used as the epoxidation reagents.
Table 3. Comparison of Oxone/Acetone with Performic Acid for the Epoxidation of Butyl Rubber.
| run | butyl rubber (g) | temp. (°C) | acetone (mL) | Oxone (g) | NaHCO3 (g) | 10 wt % PFA (mL) | percentage of epoxide (%)b |
|---|---|---|---|---|---|---|---|
| 1a | 5.0 | 23 | 8.0 | 2.35 | 1.28 | 70 | |
| 6 | 5.0 | 23 | 6.3 | 36 |
Oxone and NaHCO3 was added by single addition.
The reaction stopped at 60 min reaction time.
3.4. Water Sensitivity Study
Butyl rubber prepared via a slurry process contains 6–12 wt % water in the rubber cement,50−52 which means that in practice the epoxidation of butyl rubber is a biphasic reaction. It is of interest to determine the effect of water on the epoxidation of butyl rubber as it has not been studied for known epoxidation systems including H2O2, performic acid, and peracetic acid. To investigate the water sensitivity, the epoxidation of butyl rubber was carried out at different water concentrations ranging from 0 to 25 wt % when PFA and Oxone/acetone was used as the epoxidation reagent. The experimental conditions are detailed in Tables S1 and S2.
All experiments were performed at room temperature for 1 h. The butyl rubber was first epoxidized using a performic acid system. The PFA solution with concentration of 10 wt % was freshly prepared before the reaction. After the reaction, the product was coagulated, dried, and characterized by 1H NMR spectroscopy. The conversion of unsaturation concentration was calculated according to eq 2 and is plotted as a function of water concentration in Figure 6.
| 2 |
where 1.72 represents the percentage of unsaturation by mole in regular butyl rubber, and A represents the percentage of unsaturation by mole in epoxidized regular butyl rubber.
Figure 6.

Plot of unsaturation conversion versus water concentration in the cement when performic acid (blue), Oxone/acetone (orange), and xone/acetone in the presence of the phase transfer catalyst (gray) is used as the epoxidation reagent.
As revealed in the blue curve in Figure 6, water concentration exerts a significant effect on the conversion when PFA is the epoxidation agent. An initial epoxidation reaction with 0 wt % water in the cement results in 38% conversion. As the water concentration increases to 5 and 10 wt %, the epoxide formation gradually decreases, yielding 23 and 14% conversion, respectively. When the water concentration is larger than 10 wt %, the epoxide formation is consistent at approximately 14% conversion. In contrast, when Oxone/acetone was used for the epoxidation, as indicated by the orange curve, the epoxide formation did not decrease with an increase in water concentration up to 20%. In other words, the conversion remains consistent at 70% until the water concentration is above 20%. A significant decrease is observed when the water concentration reaches 25 wt %, yielding a conversion of 48%. This demonstrates that when the water concentration is in the range of 0–20 wt %, the Oxone-acetone system is more robust than the PFA system.
Quaternary ammonium salts have been widely used as phase transfer catalysts in biphasic reactions. Kachasakul et al.53 used tetra butyl ammonium hydrogen sulfate as a PTC when conducting a similar series of experiments investigating the epoxidation of cyclohexene in biphasic conditions. The potential to improve epoxide conversion and eliminate water sensitivity at high water concentrations was explored by utilizing methyltrioctylammonium hydrogen sulfate as a phase transfer catalyst. Reaction conditions are detailed in Table S3. As revealed in the gray curve in Figure 6, the addition of a PTC increases the conversion of the unsaturation by 13% when compared to the identical experiment without a PTC. Furthermore, with the addition of PTC, the conversion of unsaturation remains unchanged with the water concentration up to 25 wt %, which means that the presence of PTC eliminates the water sensitivity to a higher level, which will allow for the consistent high epoxide formation when the water concentration in the cement fluctuates in the range of 0–25 wt %. The improvement in the conversion of unsaturation and reduced water sensitivity can be first explained by Scheme 2. There are two routes for the formation of DMDO. In route 1, DMDO is produced in the aqueous phase through the reaction between acetone and KHSO5. In route 2, DMDO is produced in the organic phase. To be specific, PTC complexes with the HSO5– anion through ionic interaction in the aqueous phase to form an organic salt, which subsequently migrates to the organic phase. In the organic phase, the organic salt reacts with acetone to form dimethyl dioxirane and regenerates the PTC, which returns to the aqueous phase. The dioxirane then epoxidizes the unsaturation unit and regenerates the acetone. In brief, the addition of a PTC offers another way which is route 2 to form DMDO, leading to an increase in the rate of DMDO formation and a further decrease in water sensitivity.
Scheme 2. Proposed Mechanism for the Epoxidation of Butyl Rubber with PMS-Acetone System in the Presence of a Phase Transfer Catalyst.
In addition, as discussed above, DMDO and peroxymonosulfate can react to form oxygen and potassium hydrogen sulfate.45 In experiments without a PTC, all DMDO is formed in the peroxymonosulfate-abundant aqueous phase as Oxone is water-soluble but hexane-insoluble, which will result in some of the newly formed DMDO reacting with peroxymonosulfate. When a PTC is used, there is less DMDO formed in the aqueous layer as some of it is formed directly in the organic layer. Consequentially, less DMDO is consumed unproductively, ultimately resulting in greater epoxidation of the rubber. Thus, the use of a PTC results in more DMDO being used to epoxidize the rubber.
3.5. DoE Study on the Epoxidation
In the above studies, excess amounts of Oxone, acetone, and NaHCO3 were used to achieve a high degree of epoxidation. In this section, Design of Experiment (DoE)41 is employed to optimize the conditions for the epoxidation. Three factors including the equivalent of Oxone, equivalent of NaHCO3, and equivalent of acetone against the unsaturation were studied. The range for each factor is shown in Table 4. The percentages of epoxide from 15 min and 1 h reaction time were investigated as the responses.
Table 4. Factors and Responses for DoE.
| name | type | low | high |
|---|---|---|---|
| equivalent of oxone | factor 1 | 1.25 | 5 |
| equivalent of NaHCO3 | factor 2 | 2.5 | 10 |
| equivalent of acetone | factor 3 | 18 | 72 |
| % of epoxide at 15 min | response 1 | ||
| % of epoxide at 1 h | response 2 |
Based on the above three factors and the range for each factor, 20 epoxidation experiments were generated from an optimized response surface DoE in the software program Design Expert. All epoxidation reactions were performed at 23 °C with a single addition of Oxone and NaHCO3. The percentage of epoxide is calculated by using eq 1 from 1H NMR analysis. The design and results are presented in Table 5.
Table 5. Experiments Generated from the DoE and Experimental Results.
| run | factor 1 | factor 2 | factor 3 | response 1 | response 2 |
|---|---|---|---|---|---|
| 1 | 5.0 | 10.0 | 72.0 | 49 | 61 |
| 2 | 3.3 | 9.9 | 42.3 | 24 | 27 |
| 3 | 1.3 | 5.9 | 42.3 | 17 | 17 |
| 4 | 1.3 | 5.9 | 42.3 | 12 | 15 |
| 5 | 1.3 | 10.0 | 18.0 | 7 | 7 |
| 6 | 3.3 | 5.9 | 70.9 | 61 | 75 |
| 7 | 2.5 | 2.5 | 49.6 | 25 | 33 |
| 8 | 3.3 | 5.9 | 70.9 | 50 | 64 |
| 9 | 1.3 | 10.0 | 72.0 | 51 | 27 |
| 10 | 5.0 | 7.2 | 18.0 | 17 | 35 |
| 11 | 3.7 | 5.2 | 36.6 | 23 | 45 |
| 12 | 2.7 | 2.5 | 18.0 | 7 | 11 |
| 13 | 5.0 | 2.5 | 18.0 | 1 | 1 |
| 14 | 5.0 | 7.4 | 50.4 | 9 | 50 |
| 15 | 2.9 | 7.9 | 18.0 | 15 | 15 |
| 16 | 3.3 | 9.9 | 42.3 | 25 | 25 |
| 17 | 1.3 | 5.9 | 42.3 | 16 | 17 |
| 18 | 5.0 | 2.5 | 52.0 | 2 | 7 |
| 19 | 3.3 | 5.9 | 70.9 | 55 | 67 |
| 20 | 1.3 | 2.5 | 72.0 | 44 | 51 |
The modeling equations obtained from the DoE for percentage of epoxide at 15 and 60 min are shown in eqs 3 and 4 based on the DoE factors. Equation 3 shows that the percentage of epoxide at 15 min is linear to the equivalent of NaHCO3, but quadratic with respect to the equivalent of Oxone and acetone. In contrast, eq 4 indicates that the percentage of epoxide at 1 h is quadratic with respect to Oxone, acetone, and NaHCO3.
 |
3 |
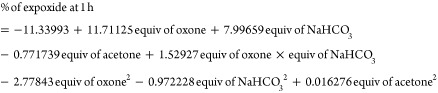 |
4 |
As shown in Figures 7 and 8, at 15 min reaction time, the percentage of epoxide is maximized when 3.1 equiv of Oxone and the highest level of acetone are added. To be specific, epoxide formation increases up to 3.1 equiv of Oxone, but when more than 3.1 equiv of Oxone is added, a decrease in the epoxide formation results. Different from Oxone, it was found that the increase in NaHCO3 and acetone always leads to the increase in epoxide formation and the slope in the graphs from the DoE confirms that an increase in acetone has a much bigger impact on the formation of epoxide than the increase in NaHCO3.
Figure 7.
One-factor graphs for the effect of amount of (a) Oxone; (b) NaHCO3; and (c) acetone on the formation of the epoxide group in butyl rubber.
Figure 8.
Two-factor graphs for the effect of amount of (a) Oxone and NaHCO3; (b) Oxone and acetone; and (c) NaHCO3 and acetone on the formation of epoxide in butyl rubber at 15 min reaction time.
Therefore, during the software optimization of the reaction, 3.1 equiv of Oxone, the minimized amount of NaHCO3, and amount of acetone in the range 18–72 equiv are generally suggested to achieve the target percentages of epoxide in the product. The optimized experimental conditions from the software with predicted % epoxide at 15 min are listed in Table 6. Furthermore, three epoxidation reactions (Runs 1, 4, and 6) are preformed experimentally to test the accuracy of the model, and the results are summarized in Table 6. Epoxidized butyl rubber with 54, 22, and 10% are prepared in Runs 1, 4, and 6, which match the prediction from DoE with a high degree of accuracy.
Table 6. Optimized Reaction Conditions from DoE to Prepare Epoxidized Butyl Rubber at 15 Min Reaction Time.
| run | equiv of Oxone | equiv of NaHCO3 | equiv of acetone | % of epoxide at 15 min from DoE | % of epoxide at 15 min from exp |
|---|---|---|---|---|---|
| 1 | 3.10 | 5.3 | 72.0 | 56 | 54 |
| 2 | 2.90 | 2.5 | 70.3 | 50 | |
| 3 | 3.1 | 2.7 | 63.9 | 40 | |
| 4 | 3.1 | 2.5 | 56.4 | 30 | 22 |
| 5 | 3.1 | 2.6 | 46.0 | 20 | |
| 6 | 3.1 | 2.5 | 25.7 | 12 | 10 |
The modeling for the formation of epoxide at 1 h is more complicated than at 15 min as it involves the AB interaction as well as quadratic dependence of both the factors Oxone and NaHCO3. Due to the quadratic dependency, maximum levels of these two factors are determined from Figure 9. It is found that the highest percentage of epoxide is produced for 3.8 equiv of Oxone and 6.5 equiv of NaHCO3. As the epoxide formation is more strongly affected by NaHCO3, during the optimization, the NaHCO3 is set at 6.5, the amount of Oxone is minimized, and acetone is set in the range of 18–72 equiv to produce the target percentage of epoxide.
Figure 9.
One-factor graphs for the effect of amount of (a) Oxone; (b) NaHCO3; and (c) acetone on the formation of epoxide at 1 h reaction time.
Table 7 shows the predicted experimental conditions generated from the DoE model to produce epoxidized butyl rubber with the epoxide percentage ranging from 14 to 59%. Three epoxidations using the conditions from Runs 1, 3, and 6 were performed, and the percentages of epoxide by 1H NMR are all close to DoE’s prediction, confirming the model’s accuracy.
Table 7. Optimized Reaction Conditions from DoE to Prepare Epoxidized Butyl Rubber at 1 h Reaction Rime.
| run | equiv of Oxone | equiv of NaHCO3 | equiv of acetone | % of epoxide at 60 min | % of epoxide at 60 min |
|---|---|---|---|---|---|
| 1 | 1.25 | 6.5 | 18.0 | 14 | 10 |
| 2 | 1.25 | 6.5 | 47.9 | 23 | |
| 3 | 1.25 | 6.5 | 56.2 | 30 | 29 |
| 4 | 1.25 | 6.5 | 64.3 | 40 | |
| 5 | 1.25 | 6.5 | 71.7 | 50 | |
| 6 | 1.88 | 6.5 | 72.0 | 59 | 55 |
3.6. Improved Interaction between Epoxidized Butyl Rubber and Silica
Incorporating silica into a butyl rubber matrix is challenging due to the large difference in polarity.54 It is not possible to thoroughly mix the two by mechanical force alone, because the silica tends to agglomerate as a result of hydrogen bonding.55 To know whether the epoxidized butyl rubber shows better silica dispersion than its parent regular butyl, regular butyl rubber and epoxidized butyl rubber with 1.13 and 1.49 mol % of epoxy group were mixed with 50 phr of high surface area-precipitated silica. The filler–filler interactions are first measured using RPA (rubber process analyzer), which is known as the Payne effect test (strain sweep). As shown in Figure 10, the regular butyl rubber sample had a very high modulus at low strain, indicating high filler–filler interaction and implying agglomerates or low dispersion within the rubber.55 In contrast, the modulus is decreased for the two epoxidized butyl rubbers at low strain, indicating the polar epoxy groups assist in dispersion of the silica.
Figure 10.
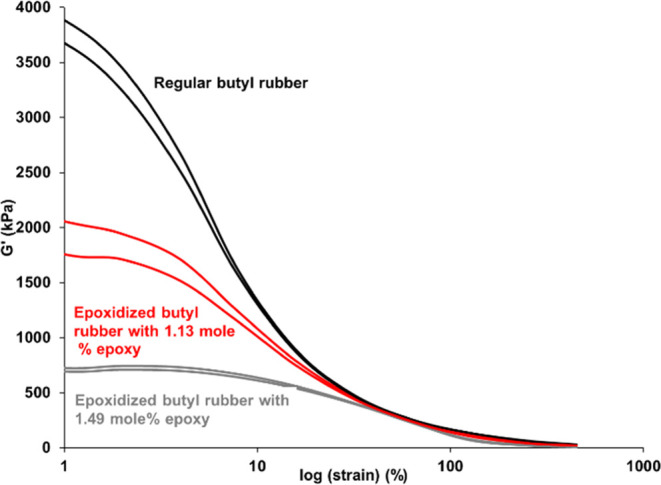
Payne effect plot of uncured butyl/silica and epoxidized butyl rubber/silica compound.
Atomic force microcopy (AFM) imaging on the same compounds to determine “hard” and “soft” areas by response to a tapping cantilever was also performed.56 The analysis in Figure 11 clearly demonstrates domains of rubber and silica agglomerates in regular butyl rubber but a more dispersed system in the two epoxidized samples.
Figure 11.
AFM images of the uncured regular butyl/silica and epoxidized butyl rubber/silica compound.
4. Conclusions
In summary, dimethyl dioxirane (DMDO) generated from Oxone/acetone is explored for the epoxidation of synthetic rubber with low unsaturation such as butyl rubber. Compared to H2O2 and peracid, a faster and more efficient epoxidation process without the formation of ring-opening and gelation products is achieved at room temperature when Oxone/acetone is used. In addition, the usage of Oxone/acetone allows for epoxidation without water sensitivity with water concentration up to 20 wt % in the cement, or further to 25 wt % water with the assistance of methyltrioctylammonium hydrogen sulfate acting as a phase transfer catalyst. The reaction conditions were modeled and optimized by DoE. Improved dispersion of silica in the rubber phase as compared to the parent butyl rubber is achieved.
Acknowledgments
The authors thank Dr. Sarah David (ARLANXEO GmbH) for the technical discussion during the preparation of the manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c01360.
FT-IR spectra of butyl rubber and epoxidized butyl rubber, 1H NMR spectra of epoxidized butyl rubber produced at different reaction time, 1H NMR spectra of epoxidized butyl rubber from scaling-up reaction, formation of 10% performic acid, water sensitivity study, DoE results (PDF)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Webb R. N.; Shaffer T. D.; Tsou A. H.. Butyl Rubber Encyclopedia of Polymer Science and Technology., 10.1002/0471440264.pst036. [DOI]
- Sharma R. K.; Mohanty S.; Gupta V. Advances in butyl rubber synthesis via cationic polymerization: an overview. Polym. Int. 2021, 70 (9), 1165–1175. 10.1002/pi.6180. [DOI] [Google Scholar]
- van Duin M.; Dikland H.; Früh T.; Groß T.; Haßmann C.; Sary N.; Schmidt R.. Handbook of Synthetic Rubber; ARLANXEO Deutschland GmbH, 2020. [Google Scholar]
- Vitiello R.; Tesser R.; Turco R.; Santacesaria E.; Compagnone G.; Di Serio M. A critical review on analytical methods and characterization of butyl and bromobutyl rubber. Int. J. Polym. Anal. Charact. 2017, 22 (4), 348–360. 10.1080/1023666X.2017.1297887. [DOI] [Google Scholar]
- Kruželák J.; Sýkora R.; Hudec I. Sulphur and peroxide vulcanisation of rubber compounds -overview. Chem. Pap. 2016, 70 (12), 1533–1555. 10.1515/chempap-2016-0093. [DOI] [Google Scholar]
- McEachran M. J.; Trant J. F.; Sran I.; de Bruyn J. R.; Gillies E. R. Carboxylic Acid-Functionalized Butyl Rubber: Synthesis, Characterization, and Physical Properties. Ind. Eng. Chem. Res. 2015, 54 (17), 4763–4772. 10.1021/acs.iecr.5b00421. [DOI] [Google Scholar]
- Deepak V. D.; Mahmud I.; Gauthier M. Synthesis of carboxylated derivatives of poly(isobutylene-co-isoprene) by azide–alkyne “click” chemistry. Polym. J. 2019, 51 (3), 327–335. 10.1038/s41428-018-0130-y. [DOI] [Google Scholar]
- Deepak V. D.; Gungör E.; Gauthier M. Facile synthesis of poly(isobutylene-co-isoprene) (IIR) carboxylated derivatives by thiol–ene click chemistry. Polym. J. 2021, 53 (2), 323–330. 10.1038/s41428-020-00425-3. [DOI] [Google Scholar]
- Sun Y.; Fan C.; Wei B.; Wesdemiotis C.; Jia L. Activated Isobutylene-Isoprene Rubber—Synthesis, Peroxide Cure, and Mechanical Properties. ACS Appl. Polym. Mater. 2020, 2 (11), 5163–5172. 10.1021/acsapm.0c00906. [DOI] [Google Scholar]
- Masuyama Y.; Nakano H.. Medical Rubber Stopper and Method for Producing Medical Rubber Stopper. US Patent US10285905B2, 2019.
- Cai F.; You G.; Zhao X.; Hu H.; Wu S. The Relationship between Specific Structure and Gas Permeability of Bromobutyl Rubber: A Combination of Experiments and Molecular Simulations. Macromol. Theory Simul. 2019, 28 (6), 1900025 10.1002/mats.201900025. [DOI] [Google Scholar]
- McDonald M. F. Jr; Shaffer T. D.; Tsou A. H. Commercial Isobutylene Polymers. Kirk-Othmer Encycl. Chem. Technol. 2016, 1–25. 10.1002/0471238961.0221202511180519.a01.pub3. [DOI] [Google Scholar]
- Fu G.; Chang X.; Mao J.; Shi X. Insights into the Reinforcement of Butyl Rubber by Carbon Black and Silica with the Aid of Their Dynamic Properties. J. Macromol. Sci., Part B: Phys. 2016, 55 (9), 925–936. 10.1080/00222348.2016.1217760. [DOI] [Google Scholar]
- Wagner M.; Sellers J. Kinetics of Filler-Polymer Interaction between Fine Particle Silica and SBR or Butyl Rubber. Ind. Eng. Chem. Res. 1959, 51 (8), 961–966. 10.1021/ie50596a044. [DOI] [Google Scholar]
- Dick J. S.; Pawlowski H.. 8 - Filler Effects on Rubber Compound Processability. In Practical Rubber Rheology and Dynamic Properties; Dick J. S.; Pawlowski H., Eds.; Hanser, 2023; pp 229–265. [Google Scholar]
- Sengloyluan K.; Sahakaro K.; Dierkes W. K.; Noordermeer J. W. M. Silica-reinforced tire tread compounds compatibilized by using epoxidized natural rubber. Eur. Polym. J. 2014, 51, 69–79. 10.1016/j.eurpolymj.2013.12.010. [DOI] [Google Scholar]
- Nuinu P.; Sirisinha C.; Suchiva K.; Daniel P.; Phinyocheep P. Improvement of mechanical and dynamic properties of high silica filled epoxide functionalized natural rubber. J. Mater. Res. Technol. 2023, 24, 2155–2168. 10.1016/j.jmrt.2023.03.101. [DOI] [Google Scholar]
- Kubisa P.; Vogl O. Cationic polymerization of chloral. Polym. Sci. 1975, 17 (4), 1072–1081. 10.1016/0032-3950(75)90286-5. [DOI] [Google Scholar]
- Matyjaszewski K.Cationic Polymerizations: Mechanisms, Synthesis & Applications; CRC Press, 1996. [Google Scholar]
- Behera P. K.; Kumar A.; Mohanty S.; Gupta V. K. Overview on Post-Polymerization Functionalization of Butyl Rubber and Properties. Ind. Eng. Chem. Res. 2022, 61 (46), 16910–16923. 10.1021/acs.iecr.2c03103. [DOI] [Google Scholar]
- Dilcher J.-P.; Jürgens H.; Luinstra G. A.. Sequential Post-modifications of Polybutadiene for Industrial Applications. In Multi-Component and Sequential Reactions in Polymer Synthesis; Theato P., Ed.; Springer International Publishing, 2015; pp 163–201. [Google Scholar]
- Jian X.; Hay A. S. Epoxidation of unsaturated polymers with hydrogen peroxide. J. Polym. Sci., Part C: Polym. Lett. 1990, 28 (9), 285–288. 10.1002/pol.1990.140280903. [DOI] [Google Scholar]
- Nguyen P.; Davidson G. J. E.; Steevensz R.. Processes for Preparing Epoxidized Polymers. US Patent EP2931800B1, 2020.
- Tanrattanakul V.; Wattanathai B.; Tiangjunya A.; Muhamud P. In situ epoxidized natural rubber: Improved oil resistance of natural rubber. J. Appl. Polym. Sci. 2003, 90 (1), 261–269. 10.1002/app.12706. [DOI] [Google Scholar]
- Sakaki T.; Tarachiwin L.; Charungchitaree K.; Kum-Ourm H.. Method for Producing Epoxidized Natural Rubber, Rubber Composition for Tires, and Pneumatic Tire. US Patent EP2808347A1, 2014.
- Puskas J. E.; Wilds C. Kinetics of the Epoxidation of Butyl Rubber; Development of a High Precision Analytical Method for Unsaturation Measurement. Rubber Chem. Technol. 1994, 67 (2), 329–341. 10.5254/1.3538678. [DOI] [Google Scholar]
- Binder B.; Elliott S.; Davidson G. J. E.; Guo S.. Process for Epoxidation of Unsaturated Polymer. US Patent US10774158B2, 2020.
- Stojcevic G.; Arsenault G.; Gillies E. R.; Bonduelle C. V.; McEachran M. J.. Functionalized Copolymers of Isoolefins and Diolefins and their Use as Compatibilizers. US Patent US10806832B2, 2020.
- Liu L.; Liu S.; Zhang L.; Wu Q.. Preparation Method of Epoxidized Rubber, Epoxidized Rubber and Application. US Patent CN116135887A, 2023.
- Jian X.; Hay A. S. Catalytic epoxidation of polyisobutylene-co-isoprene with hydrogen peroxide. J. Polym. Sci., Part A: Polym. Chem. 1991, 29 (4), 547–553. 10.1002/pola.1991.080290411. [DOI] [Google Scholar]
- Gelling I. R. Modification of Natural Rubber Latex with Peracetic Acid. Rubber Chem. Technol. 1985, 58 (1), 86–96. 10.5254/1.3536060. [DOI] [Google Scholar]
- Hsiue G.-H.; Yang J.-M. Epoxidation of styrene–butadiene–styrene block copolymer and use for gas permeation. J. Polym. Sci., Part A: Polym. Chem. 1990, 28 (13), 3761–3773. 10.1002/pola.1990.080281319. [DOI] [Google Scholar]
- Roy S.; Gupta B. R.; Maiti B. R. Effect of acid concentration and other reaction parameters on epoxidation of natural rubber latex. Ind. Eng. Chem. Res. 1991, 30 (12), 2573–2576. 10.1021/ie00060a010. [DOI] [Google Scholar]
- Elliott S.; Davidson G. J. E.; Guo S.. Butyl Rubber Containing Allylic Alcohol. US Patent US2018362683A1, 2018.
- Hussain H.; Green I. R.; Ahmed I. Journey Describing Applications of Oxone in Synthetic Chemistry. Chem. Rev. 2013, 113 (5), 3329–3371. 10.1021/cr3004373. [DOI] [PubMed] [Google Scholar]
- Rajabi F. H.; Nikje M. M. A.; Taslimipour T. Epoxidation of Styrene–Butadiene Rubber (SBR) Using In Situ Generated Dimethyldioxirane (DMD): Characterization and Kinetic Study. Des. Monomers Polym. 2010, 13 (6), 535–546. 10.1163/138577210X530648. [DOI] [Google Scholar]
- Nikje M. M. A.; Motahari S.; Haghshenas M.; Sanami R. K. Epoxidation of Ethylene Propylene Diene Monomer (EPDM) Rubber by Using In-Situ Generated Dimethyldioxirane (DMD) and MoO3. J. Macromol. Sci., Part A: Pure Appl. Chem. 2006, 43 (8), 1205–1214. 10.1080/10601320600737369. [DOI] [Google Scholar]
- Hill A.The design of experiments in the rubber industry: a European viewpoint Rubber World 1991, 204 (1), .
- Sode F. Analytical methods for peroxo acids – a review. Anal. Methods 2019, 11 (27), 3372–3380. 10.1039/C9AY00860H. [DOI] [Google Scholar]
- Zhu W.; Ford W. T. Oxidation of alkenes with aqueous potassium peroxymonosulfate and no organic solvent. J. Org. Chem. 1991, 56 (25), 7022–7026. 10.1021/jo00025a014. [DOI] [Google Scholar]
- Mikula H.; Svatunek D.; Lumpi D.; Glöcklhofer F.; Hametner C.; Fröhlich J. Practical and Efficient Large-Scale Preparation of Dimethyldioxirane. Org. Process Res. Dev. 2013, 17 (2), 313–316. 10.1021/op300338q. [DOI] [Google Scholar]
- Makhiyanov N. 1H NMR spectra (600 MHz) and structure of an industrial isobutylene-isoprene copolymer (butyl rubber). Polym. Sci., Ser. A 2014, 56 (3), 241–255. 10.1134/S0965545X14030109. [DOI] [Google Scholar]
- Chu C. Y.; Vukov R. Determination of the structure of butyl rubber by NMR spectroscopy. Macromolecules 1985, 18 (7), 1423–1430. 10.1021/ma00149a012. [DOI] [Google Scholar]
- Gemmer R. V.; Golub M. A. 13C NMR spectroscopic study of epoxidized 1,4-polyisoprene and 1,4-polybutadiene. J. Polym. Sci., Polym. Chem. Ed. 1978, 16 (11), 2985–2990. 10.1002/pol.1978.170161123. [DOI] [Google Scholar]
- Edwards J. O.; Pater R. H.; Curclf R.; Furia F. D. On the formation and reactivity of dioxirane intermediates in the reaction of peroxoanions with organic substrates*. Photochem. Photobiol. 1979, 30 (1), 63–70. 10.1111/j.1751-1097.1979.tb07116.x. [DOI] [Google Scholar]
- Singh M.; Murray R. W. Chemistry of dioxiranes. 21. Thermal reactions of dioxiranes. J. Org. Chem. 1992, 57 (15), 4263–4270. 10.1021/jo00041a036. [DOI] [Google Scholar]
- Mattila T.; Aksela R.. Method for the Preparation of Aqueous Solutions Containing Performic Acid as Well as their Use. US Patent US6049002A, 2000.
- Jolhe P. D.; Bhanvase B. A.; Patil V. S.; Sonawane S. H.; Potoroko I. Ultrasound assisted synthesis of performic acid in a continuous flow microstructured reactor. Ultrason. Sonochem. 2017, 39, 153–159. 10.1016/j.ultsonch.2017.03.059. [DOI] [PubMed] [Google Scholar]
- Heinonen-Tanski H.; Miettinen H. Performic acid as a potential disinfectant at low temperature. J. Food Process Eng. 2010, 33 (6), 1159–1172. 10.1111/j.1745-4530.2008.00332.x. [DOI] [Google Scholar]
- Murray A. H.Halogen Recovery in a Process for Halogenating Unsaturated Isoolefin Copolymer. US Patent WO2020124223A1, 2020.
- Cao K.; Davidson G. J. E.. Halogen Recovery with Oxidant and Phase Transfer Catalyst in a Process for Halogenating Unsaturated Isoolefin Copolymer. US Patent WO2023108259A1, 2023.
- Cao K.; Davidson G. J. E.. Halogen Recovery with K+-Containing Oxidant in a Process for Halogenating Unsaturated Isoolefin Copolymer. WO2022120489A1, 2022.
- Kachasakul P.; Assabumrungrat S.; Praserthdam P.; Pancharoen U. Extractive reaction for epoxidation of cyclohexene to cyclohexene oxide using dioxirane in ketone/Oxone system. Chem. Eng. J. 2003, 92 (1), 131–139. 10.1016/S1385-8947(02)00127-4. [DOI] [Google Scholar]
- Liu S.; Liu L.; Wu Q.; Zhang L. Silica reinforced epoxidized solution-polymerized styrene butadiene rubber and epoxidized polybutadiene rubber nanocomposite as green tire tread. Polymer 2023, 281, 126082 10.1016/j.polymer.2023.126082. [DOI] [Google Scholar]
- Fröhlich J.; Niedermeier W.; Luginsland H. D. The effect of filler–filler and filler–elastomer interaction on rubber reinforcement. Composites, Part A 2005, 36 (4), 449–460. 10.1016/j.compositesa.2004.10.004. [DOI] [Google Scholar]
- Natchimuthu N. AFM Studies on Silica Dispersion in EPDM Rubber. Rubber Chem. Technol. 2010, 83 (2), 123–132. 10.5254/1.3548270. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.