Abstract

Pediatric pulmonary hypertension is a serious syndrome with significant morbidity and mortality. Sildenafil is widely used off-label in pediatric patients with pulmonary arterial hypertension. In this study, bile salt-stabilized nanovesicles (bilosomes) were screened for their efficacy to enhance the transdermal delivery of the phosphodiesterase type 5 inhibitor, sildenafil citrate, in an attempt to augment its therapeutic efficacy in pediatric pulmonary hypertension. A response surface methodology was implemented for fabricating and optimizing a bilosomal formulation of sildenafil (SDF-BS). The optimized SDF-BS formulation was characterized in terms of its entrapment efficiency (EE), zeta potential, vesicle size, and in vitro release profile. The optimized formula was then loaded onto hydroxypropyl methyl cellulose (HPMC) hydrogel and assessed for skin permeation, in vivo pharmacokinetics, and pharmacodynamic studies. The optimized SDF-BS showed the following characteristic features; EE of 88.7 ± 1.1%, vesicle size of 185.0 + 9.2 nm, zeta potential of −20.4 ± 1.1 mV, and efficiently sustained SDF release for 12 h. Skin permeation study revealed a remarkable improvement in SDF penetration from bilosomal gel compared to plain SDF gel. In addition, pharmacokinetic results revealed that encapsulating SDF within bilosomal vesicles significantly enhanced its systemic bioavailability (∼3 folds), compared to SDF oral suspension. In addition, pharmacodynamic investigation revealed that, compared to plain SDF gel or oral drug suspension, SDF-BS gel applied topically triggered a significant elevation (p < 0.05) in cGMP serum levels, underscoring the superior therapeutic efficacy of SDF-BS gel. Conclusively, bilosomes can be viewed as a promising nanocarrier for transdermal delivery of SDF that would grant higher therapeutic efficiency while alleviating the limitations encountered with SDF oral administration.
1. Introduction
Pulmonary arterial hypertension (PAH) is a severe condition that causes an increase in pulmonary vascular resistance, eventually resulting in right heart failure and death.1 It is a rare condition that can affect people of any age, including infants and children.2 Sildenafil citrate (SDF) is a powerful and highly selective phosphodiesterase type 5 (PDE-5) blocker that has been acknowledged for exerting an expanding role in treating pulmonary hypertension among adults.3 Generally, SDF acts via inhibiting PDE-5-mediated degradation of cyclic guanosine monophosphate (cGMP) to GMP, leading to an elevated level of intracellular cGMP, which would trigger smooth muscle cell relaxation and vasodilation.4 In addition, despite the fact that SDF is not currently approved by FDA for use in the pediatric population, it has been extensively used off-label for pediatric PAH since 2005, showing promising clinical results in pediatric PAH.5−7 Nevertheless, oral administration of SDF is limited, on the one hand, by delayed onset, short half-life, poor bioavailability, and large variability in the absorption profile8,9 and, on the other hand, the difficulty of administration by pediatric patients. As a result, there is an urgent need to create a dosage form that could improve SDF systemic bioavailability and ease pediatric drug administration, as well.
The transdermal route of administration has been identified as a viable pathway for both local and systemic medication delivery.10 Transdermal delivery for systemic action has various merits, particularly for drugs having limited oral bioavailability or when drug could exhibit certain adverse side effects after oral administration.11,12 Nevertheless, the fundamental constraint of transdermal drug administration is the barrier nature of the skin, which provides remarkable resistance to penetrating drug molecules13 and thereby might hinder the attainment of clinically efficacious plasma concentrations of drugs following transdermal administration. Consequently, various approaches have been proposed to alleviate the skin’s barrier function, including physical approaches,14−16 chemical approaches,17,18 and the use of delivery carriers, in order to boost the transdermal delivery of bioactive drugs.19,20
Vesicular systems have been introduced as a plausible means for topical/transdermal delivery of bioactive drugs.21 The reason behind their use as transdermal drug delivery systems (TDDSs) is their ability to deliver incorporated drug entities across the skin, along with their inherent ability to act as penetration enhancers.22,23 Furthermore, vesicular systems can act as a depot for extended drug delivery of active drugs.24 Many vesicular systems including liposomes have shown the potential to deliver bioactive substances like nucleic acid, proteins, and other synthetic drugs across the skin.25,26 Nevertheless, limited stability and difficulties in scaling up necessitate the development of novel vesicular systems. Bilosomes, bile salts-stabilized vesicles, are a relatively new lipid-based vesicular system that is made of a nonionic surfactant; they resemble niosomes but with the inclusion of bile salts in their structure.27,28 Bilosomes elicited a significant advance in oral vaccine delivery because they are less susceptible to degradation by gastric enzymes as well as acids.29 Furthermore, bilosomes have been recently adopted for transdermal drug delivery.30 The nanosized range of bilosomes along with the presence of negatively charged bile salts (sodium deoxycholate) significantly supports the skin permeability of the drug and enhances the stability of the formed bilosomes, respectively. In addition, bilosomes have a fluidizing effect, which could ameliorate transdermal delivery.
In this study, bilosomes was adopted as a delivery vehicle for the PDE-5 inhibitor, sildenafil citrate (SDF). Box Behnken design was implemented for fabricating and optimizing SDF-loaded bilosomes (SDF-BS). The optimized formula was then characterized in terms of particle size, entrapment effectiveness, and in vitro release. The optimized SDF-BS was then loaded onto a HPMC hydrogel and assessed for skin permeation, in vivo pharmacokinetics, and in vivo pharmacodynamic studies and compared to plain drug formulations.
2. Materials and Methods
2.1. Materials
Sildenafil citrate (SDF) was supplied from Mash Premiere for the pharmaceutical industry (Cairo, Egypt). Cholesterol, sodium deoxycholate (SDC), Span 60, soybean phosphatidylcholine (SPC), and cellulose membrane (12,000–14,000 Da MWCO) were procured from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Fabrication of SDF-Loaded Bilosomes
SDF-loaded bilosomes (SDF-BSs) were prepared by the thin film hydration method as described previously.31 In brief, a mixture of lipids, consisting of soybean phosphatidylcholine (SPC) and fixed amount of cholesterol (20 mg), and Span 60 were added to 10 mg of SDF and dissolved in 20 mL of methanol/chloroform (1:1/v) mixture. The obtained organic solution was evaporated at 60 °C by using a rotary evaporator under reduced pressure. The dry lipid film formed was then hydrated with 10 mL of an aqueous solution of sodium deoxycholate (SDC). Particle size reduction of the resultant vesicles was achieved using both probe sonication (5 min in an ice bath; 25 s on followed by 5 s off) and bath sonication for 30 min using a bath ultrasonicator to obtain the required vesicle size. The obtained SDF-BSs were stored in the refrigerator for further characterization.
2.3. Design Optimization of SDF-Loaded Bilosomes
Box Behnken experimental designs with three factors and three levels (33 BBD) were adopted by using Design-Expert software to examine the impact of formulation variables on the fundamental properties of the prepared bilosomes and to optimize their composition. The independent variables were lipid amount (X1), the quantity of surfactant (X2) and the amount of edge activator (X3). The dependent variables were percentage entrapment efficiency (Y1) and vesicle size (Y2) (Table 1). The data from the derived formulations were incorporated into several experimental models, such as quadratic, second order (2F1), and linear models, and the best fit model was adopted to scrutinize the influence of independent formulation variables on various formulation attributes. According to the design of the experiment, a total of 15 formulations were prepared with three center points (Table 2). To explain the findings, contour and 3D response plots were established. To select the optimized formula, the point prediction method was implemented.
Table 1. Factors and Factor Levels in the 33 BBD Design.
| independent variables | coded value | ||
|---|---|---|---|
| – 1 | 0 | + 1 | |
| X1: SPC amount (mg) | 200 | 300 | 400 |
| X2: span 60 amount (mg) | 40 | 50 | 60 |
| X3: SDC amount (mg) | 10 | 20 | 30 |
| dependent variables | constrains | ||
| Y1: entrapment efficiency (%) | maximize | ||
| Y2: vesicle size (nm) | minimize | ||
Table 2. Composition of SDF-BS Formulations and the Obtained Formulation Attributesa.
| formula | independent variables | responses | |||
|---|---|---|---|---|---|
| X1: SPC (mg) | X2: span 60 (mg) | X3: SDC (mg) | Y1: entrapment efficiency (%) | Y2: vesicle size (nm) | |
| F1 | 200 | 40 | 20 | 53.6 ± 1.7 | 254.3 ± 18.7 |
| F2 | 200 | 50 | 10 | 80.5 ± 1.4 | 174.3 ± 10.1 |
| F3 | 200 | 50 | 30 | 69.3 ± 1.1 | 191.2 ± 20.1 |
| F4 | 200 | 60 | 20 | 88.0 ± 1.5 | 167.1 ± 14.3 |
| F5 | 300 | 40 | 10 | 69.6 ± 1.9 | 206.8 ± 19.6 |
| F6 | 300 | 40 | 30 | 47.3 ± 0.8 | 325.6 ± 21.3 |
| F7 | 300 | 50 | 20 | 77.6 ± 1.8 | 194.6 ± 15.4 |
| F8 | 300 | 50 | 20 | 78.7 ± 1.2 | 205.1 ± 19.5 |
| F9 | 300 | 50 | 20 | 78.1 ± 1.7 | 199.3 ± 13.5 |
| F10 | 300 | 60 | 10 | 91.6 ± 1.2 | 227.7 ± 20.1 |
| F11 | 300 | 60 | 30 | 90.3 ± 1.1 | 189.5 ± 17.6 |
| F12 | 400 | 40 | 20 | 58.6 ± 1.9 | 251.2 ± 15.6 |
| F13 | 400 | 50 | 10 | 86.6 ± 2.1 | 184.7 ± 15.4 |
| F14 | 400 | 50 | 30 | 78.1 ± 1.4 | 254.3 ± 24.1 |
| F15 | 400 | 60 | 20 | 93.9 ± 1.7 | 241.5 ± 22.5 |
Each value is the mean ± SD of the three experiments.
2.4. Characterization and Optimization of SDF-BS
2.4.1. Determination of Vesicle Size, Size Distribution, and Surface Charge
The vesicle size, polydispersity index (PDI) and zeta potential of prepared SDF-BS were estimated utilizing the Nano ZS Zetasizer (Malvern Instruments UK) at 25 ± 1.0 °C. Bilosomal dispersion was adequately diluted with deionized water prior to measurement to achieve the desired scattering intensity. For each measurement, three replicates were conducted.
2.4.2. Percentage Entrapment Efficiency
The encapsulation efficiency (EE%) of SDF was assessed as previously described.32 In brief, 2 mL of SDF-BS were centrifuged at 15,000 rpm for 1 h using a cooling centrifuge. The supernatant of the centrifuged BS sample was carefully collected and analyzed spectrophotometrically at λmax = 290 nm to determine the amount of unentrapped drug. The entrapment efficiency (% EE) was calculated according to eq 1:
| 1 |
2.4.3. Measurement of Deformability Index
The elasticity of the optimized SDF-BS was assessed by the extrusion method.33 In brief, the vesicular dispersion was properly dispersed and diluted (10-fold) before extrusion at a constant pressure of 2.5 bar through a 200 nm pore size nylon filter. The deformability index (DI) was calculated according to eq 2:
| 2 |
where J is the weight of the extruded dispersion in 10 min, rp is the pore size of the barrier (nm), and rv is the size of the bilosomes after extrusion (nm).
2.4.4. Drug-Excipient Interactions
Differential scanning calorimetry (DSC) was calibrated with 99.9% pure indium and was used to investigate the thermal properties of pure SDF, cholesterol, SPC, Span 60, and SDC, a physical mixture of pure drug with formulation excipients and the optimized bilosomes. The samples (3 mg) were placed in an aluminum pan and heated to 300 °C at a rate of 10 °C/min under nitrogen purge (30 mL/min).
2.5. In Vitro Release Studies of SDF-BS
The in vitro dissolution/release of SDF from either SDF suspension or SDF-BS was assessed using the dialysis bag diffusion method. Equal volumes (5 mL) of either SDF suspension or SDF-BS dispersion, at SDF concentration of 2 mg/mL, were filled in a dialysis tubing cellulose membrane (12,000–14,000 Da MWCO), presoaked overnight in a sufficient volume of release medium, and were suspended in 250 mL of phosphate buffer saline (PBS; pH 7.4). The release medium (PBS; pH 7.4) was kept at 37 ± 1 °C and was agitated at 100 rpm for 12 h. At predetermined intervals, 3 mL of samples was collected and replaced with 3 mL of fresh release media to ensure sink conditions. After the samples were appropriately diluted, the released amount of SDF was quantified spectrophotometry at λmax 290 nm.
2.6. Stability Study
The stability of bilosomal dispersion was assessed by keeping the optimized formula for 90 days at 4 °C. At different time intervals (0, 30, and 90 days), samples of each formulation were collected. The level of stability was determined, in terms of changes in entrapment efficiency, vesicle size, and zeta potential, by comparing the original measurements to those taken after storage.
2.7. Formulation of SDF-BS Gel
The bilosomal gel of the optimized formula was fabricated by incorporating the bilosomal dispersion in a HPMC (2% w/w) gel base. In brief, a sufficient amount of HPMC was poured in an adequate volume of deionized water, properly mixed, and kept aside for 4–6 h. The optimized SDF-BS formulation was then added to the gel base to get the SDF bilosomal gel with a final concentration of 1% w/w of SDF. Sildenafil-loaded gel was prepared in a similar manner but with replacing SDF-BS with pure SDF to obtain plain SDF gel with a final concentration of 1% w/w of SDF.
2.8. Evaluation of SDF-BS Gel
2.8.1. Organoleptic Properties
The developed gel was visually inspected for organoleptic properties such as clarity, homogeneity, color, and phase separation.
2.8.2. pH Estimation
A digital pH meter (MW802, Milwaukee Instruments, Szeged, Hungary) was used to estimate the pH of the developed bilosomal gel formulation.
2.8.3. Spreadability
Spreadability of SDF-BS gel was tested by spreading 500 mg of gel on a 2 cm diameter circle premarked on a glass slide, and then, a second glass slide was placed above the gel. A certain weight (500 g) was applied above the upper slides for 1 min. Eventually, the circumference of the circle after the gel was dispersed was measured.34
2.8.4. Viscosity
A Brookfield viscometer (DV-II + Pro, USA) was utilized to measure the viscosity of the developed SDF-BS gel.35
2.9. Ex Vivo Skin Permeation Studies
Ex vivo skin permeation studies of the plain SDF gel and optimized SDF-BS gel through rat skin were investigated using the Franz diffusion cell. In brief, a full thickness hairless abdominal rat skin was fixed between the receptor and the donor compartments of the Franz cell. The donor compartment was supplied with a specified amount of either plain SDF gel or optimized SDF-BS gel, equivalent to 2.5 mg of SDF, while the receptor compartment was supplemented with 100 mL of phosphate buffer (PBS; pH 7.4) stirred at 100 rpm and maintained at 37 ± 0.5 °C. At predetermined time points (0.5, 1, 2, 4, 6, 8, and 12 h), 2 mL of samples were withdrawn from the receptor fluid and were replaced with fresh medium. Spectrophotometric analysis was then adopted to quantify the amount of SDF in each sample. Permeation parameters such as the enhancement ratio (ER) and the flux at 12 h (Jmax) were calculated using the following formula:
2.10. In Vivo Studies
2.10.1. Animals
Male Wistar rats (220–250 g) were utilized for in vivo studies. The animals were fed regularly, had free access to water, and were housed under standardized humidity and temperature conditions. The animals were acclimatized to their new environment over 7 days. All animal investigations were revised and approved by the animal ethics committee of Prince Sattam Bin Abdulaziz University, Al-Kharj, KSA (approval number: 048/2022).
2.10.2. Pharmacokinetic Study
Male Wistar rats were used to study the in vivo fate of SDF following transdermal application of an optimized SDF-BS gel. In brief, the dorsal hair of all animals was shaved, and the rats were randomly divided into 3 groups (n = 5). Group I was orally treated with 1% w/v SDF suspension (10 mg/kg). Group II was treated topically with 1% w/w plain SDF gel (10 mg SDF/kg). Group III was treated topically with 1% w/w optimized SDF-BS gel (10 mg SDF/kg). At scheduled time points post-treatment (0, 0.5 1, 2, 4, 6, 8, 12, and 24 h), 200 μL specimens were collected from each animal in heparinized Eppendorf tubes. Blood samples were centrifuged for 15 min at 5000 rpm, and plasma drug concentrations were determined using a reverse phase chromatography method. In brief, Shimadzu HPLC (Tokyo, Japan), supplied with a C-18 column (4.6 mm × 25 cm, 5 μm), was utilized for the evaluation of SDF. The mobile phase consisted of acetonitrile and water (35:65, pH 4.0) which was pumped at a flow rate of 1 mL/min. SDF was detected using a UV detector set at 291 nm. PKSolver 2.0 software was used to analyze the kinetic data obtained from a plasma concentration versus time curve, using a noncompartmental model. Key pharmacokinetic parameters such as Cmax, Tmax, t1/2, MRT, and AUC were also calculated.
2.10.3. Estimation of cGMP Level in Serum
Male Wistar rats were categorized into four groups (n = 5). Group I received an oral solution of phosphate buffer saline and served as a negative control. Group II was orally treated with an SDF suspension (10 mg/kg). Group III was treated topically with plain SDF gel (10 mg of SDF/kg). Group IV was treated topically with optimized SDF-BS gel (10 mg of SDF/g). At specified time intervals (2, 4, and 6 h), blood specimens (200 μL) were collected from each animal and centrifuged at 5000 rpm for 15 min to separate serum. Finally, a cGMP direct immunoassay kit (Abcam, MA, USA) was adopted to quantify serum cGMP levels according to manufacturer instructions.
3. Results and Discussion
3.1. Fabrication of Sildenafil-Loaded Bilosomes (SDF-BS)
A response surface methodology (33 BBD) was used in the current investigation for preparing and optimizing sildenafil-loaded bilosomes (SDF-BS). The variables were indicated, and their amounts were chosen based on preliminary studies to determine the most likely independent factors. By variation of three formulation variables, lipid amount (X1), surfactant concentration (X2), and edge activator percentage (X3), a total of 15 runs with three center points were achieved (Table 2). The impact of these formulation factors on two formulation characteristics, percentage entrapment efficiency (Y1) and vesicle size (Y2), was investigated.
3.2. Effect of Independent Factors on SDF-BS Characteristics
3.2.1. Influence of Independent Factors on Percentage Entrapment Efficiency (% EE; Y1)
The capability of bilosomes to entrap high quantities of drug is a key determinant that potentially supports their clinical applicability. The % EE of SDF-BS was found to be affected by different formulation variables and to fluctuate from 47.3 ± 0.8% (F6) to 93.9 ± 1.7% (F15). As shown in Figure 1A,B, it was clear that increasing lipid content (X1) triggered a substantial increment in % EE. At fixed surfactant concentration (X2) and EA concentration (X3), increasing lipid content (X1) from 200 mg (F3) to 400 mg (F14) triggered a significant rise in % EE. The %EE of F14 and F3 were 78.1 ± 1.4 and 69.3 ± 1.1%, respectively. Similar results were reported by Khafagy and his colleagues who verified a mutual increment in the entrapment of simvastatin within nanobilosomal vesicles with an increase in lipid concentration from 1 to 3%.30 On the same context, the Span 60 concentration exerted a synergistic effect on the EE% of SDF within bilosomes. At constant SPC and SDC concentrations, the percent drug entrapped within bilosomes fabricated with 60 mg of Span 60 (F4) was obviously greater than those prepared with 40 mg of Span 60 (F1). The % EE of F4 and F1 were 88.0 ± 1.5 and 53.6 ± 1.7%, respectively. This significant increase in EE% upon increasing Span concentration might be ascribed to the increased solubility of SDF, which favored drug entrapment within bilosomal vesicles. In addition, the higher transition temperature along with the long alkyl chain of Span 60 might contribute to the higher entrapment of SDF within bilosomes upon rising Span 60 concentration.36 On the other hand, increasing the edge activator amount from 10 to 30 mg was found to adversely affect %EE. The %EE of F3 fabricated with 30 mg of SDC was 69.3 ± 1.1%, which was considerably lower than that of F2 prepared with 10 mg of SDC (80.5 ± 1.4%,). It has been stated that the presence of high bile salt concentration could enhance vesicular membrane permeability via forming holes within the vesicular membrane, resulting in greater fluidity and drug leakage.37
Figure 1.
Impact of independent variables on entrapment efficiency (Y1). (A) Contour plots of Y1 and (B) 3D surface plots for Y1.
The numerical data from Y1 were incorporated into several polynomial models, and regression analysis verified that the quadratic model was the best fitting model for the data (Table S1). The quadratic polynomial equation clearly verified the synergistic effect of both lipid content (X1) and Span 60 concentration (X2), and the antagonistic effect of the edge activator amount (X3), on entrapment efficiency (Y1) (Table S2).
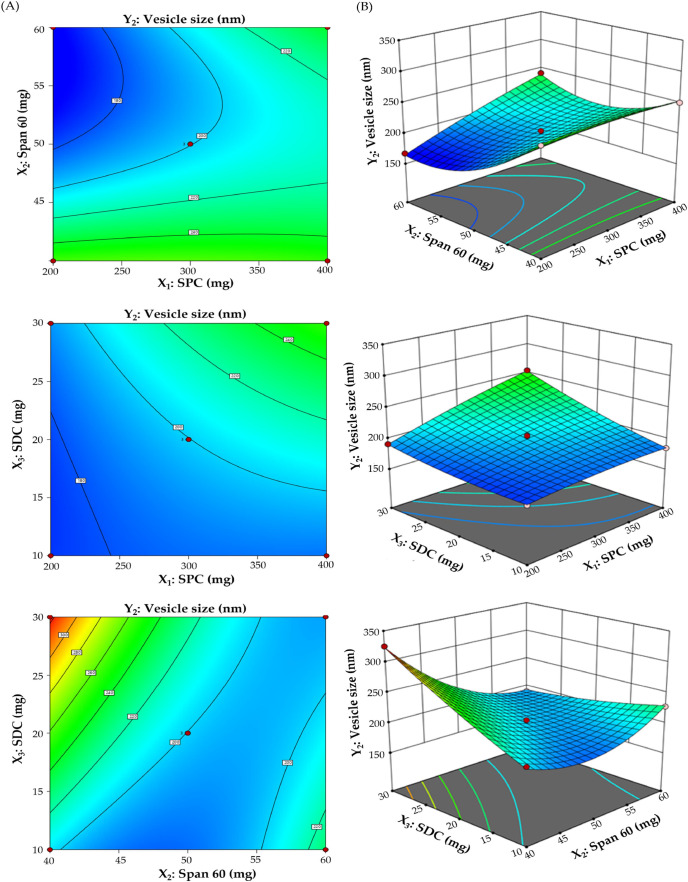
3.2.2. Effect of Independent Formulation Variables on Vesicle Size (Y2)
Vesicle size has a critical influence on transdermal permeability and therapeutic activity, as well. As summarized in Table 2, the mean vesicle size of different bilosomal formulations fluctuated from 167.1 ± 14.3 to 325.6 ± 21.3 nm and was influenced by various formulation variables. As illustrated in Figure 2A,B, there was a direct relationship between lipid content (X1) and vesicle size at a constant surfactant concentration (X2) and EA amount (X3). The mean vesicle size of bilosomes prepared with 200 mg lipid concentration (F3; 191.2 ± 20.1 nm) was significantly smaller than those prepared with 400 mg of lipid concentration (F14; 254.3 ± 24.1 nm). In a similar manner, increasing the edge activator (SDC) amount from 10 to 30 mg brought about a remarkable increase in the bilosome size. The size of F14 fabricated with 50 mg of SDC was 254.3 ± 24.1 nm, which was considerably higher than that of F13 fabricated with 10 mg of SDC (184.7 ± 15.4 nm). At higher SDC concentrations, SDC tends to form aggregates itself, triggering an increase in the aqueous core space volume and thereby resulting in a remarkable increase in vesicle size.38,39
Figure 2.
Impact of independent variables on vesicle size (Y2). (A) Contour plots of Y2 and (B) 3D surface plots for Y2.
By contrary, at fixed lipid content (X1) and fixed EA (X3), increasing the surfactant (X2, span 60) concentration resulted in a concomitant reduction in vesicles size. Bilosomes prepared with 60 mg surfactant concentration (F11) showed a smaller vesicle size (189.5 ± 17.6 nm), compared to bilosomes prepared with 40 mg of surfactant concentration (F6; 325.6 ± 21.3 nm). Such a remarkable decrease in particle size upon increasing Span 60 concentration could be attributed to the increase in vesicle curvature with elevating surfactant concentration, which in turn, resulted in decreased vesicle size.40
The numerical data from Y2 were incorporated into several polynomial models, and regression analysis verified that the quadratic model was the best fitting model for the data (Table S3). The derived polynomial equation from the BBD represented the total impact of independent factors on the vesicle size (Y2):
Analysis of variance (ANOVA) verified the synergistic effect of both lipid content and SDC amount and the antagonistic effect of Span 60 concentration on SDF-BS vesicle size (Table S4).
3.2.3. Formulation Optimization
A mathematical optimization approach, implementing a desirability function, was employed to retrieve the optimized formula that fulfills the desired characteristics of maximum EE with minimal vesicle size. The generated optimal SDF-BS formulation contained 251.5 mg of SPC, 30 mg of SDC, and 60 mg of Span 60, with a desirability close to 1. To ratify the optimization method, the suggested optimized formulation was fabricated and evaluated for entrapment efficiency and vesicle size and compared with the expected responses. The observed entrapment efficiency (%) and vesicle size were 88.7 ± 1.1% and 185.0 + 9.2 nm, respectively, which were comparable to the expected values for the optimized formulation (89.3% and 177.1 nm). These findings validated the optimization technique used to create SDF-BS based on 33 BBD.
3.3. Physicochemical Characterization and Optimization of SDF-BS
3.3.1. Vesicle Size, Zeta Potential, and Polydispersity Index of Optimized SDF-BS
The particle size of nanocarriers is a key element that dictates the in vivo fate of the drug following transdermal delivery. Generally, nanovesicles having a particle size approaching 200 nm are efficient to penetrate different skin layers and exert a systemic effect following topical application.41 Herein, the particle size of the optimized SDF-BS was 185.0 ± 9.2 nm with a PDI value of 0.113 ± 0.02 (Figure 3A). This relatively small vesicle size verifies the utility of bilosomal vesicles as an efficient nanocarrier for transdermal delivery of SDF.
Figure 3.
(A) Particle size and (B) Zeta potential of the optimized SDF-BS.
Zeta potential is another crucial parameter that measures the strength of the attraction/repulsion between adjacent particles and, thereby, offers information on the physical stability of the produced bilosomal formulations. Generally, in colloidal dispersion, a zeta potential of ±20 mV is required for ensuring electrostatic colloidal stability.30 The ZP value of the optimized formula was −20.4 ± 1.1 mV (Figure 3B), suggesting the high physical stability of the formulated bilosomes.
3.3.2. Measurement of the Elasticity of the Optimized Bilosomal Formula
The optimized SDF-BS were tested for their flexibility and compared to other formulas that do not include EA (blank bilosomes). The membrane elasticity, as measured by the deformability index (DI), was determined for both the blank and optimized bilosomes. The optimized bilosomes showed superior elasticity compared to blank bilosomes in terms of the deformability index (p = 0019). The calculated DI values were 24.26 ± 2.17 for optimized SDF-BS and 4.32 ± 0.16 for blank bilosomes. This superior elasticity of optimized bilosomes bestows vesicles with higher penetrability through skin pores that are narrower than their diameters.41
3.3.3. Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) is a thermal analytic technique used to evaluate the thermal behavior of drug samples as well as their interactions with excipients. DSC thermograms of pure SDF, Span 60, SPC, SDC, cholesterol, and optimized SDF-BS are shown in Figure 4. The DSC thermogram of pure SDF inferred a distinct endothermic spike at 199.7 °C, equivalent to its melting point. The thermograms of Span 60, cholesterol, and SDC showed endothermic peaks at 53.9, 149.1, and 215.2 °C, respectively. Additionally, the physical mixture showed all of the characteristic endothermic peaks of pure drug and formulation excipients. In contrast, the thermogram of the optimized bilosomal formula showed no distinct drug sharp peak, demonstrating the efficient entrapment of SDF into the bilosomal vesicles.
Figure 4.

DSC thermograms of pure SDF, SPC, cholesterol, SDC, Span 60, physical mixture, and optimized SDF-BS.
3.4. Stability Studies
The stability of SDF-BS was assessed following storage at 4 °C for 3 months. Upon comparing the EE %, vesicle size, and zeta potential of fresh and stored bilosomes, no significant alterations in the physicochemical properties of optimized SDF-BS were detected (p > 0.05) (Table S5). These findings provide strong evidence for the good physical stability of the prepared bilosomal dispersion.
3.5. In Vitro Drug Release
The evaluation of in vitro drug release is a critical step during the development of transdermal delivery systems. Preliminary experiments were conducted to emphasize the effect of different formulation variables (SPC amount, Span 60 amount, and SDC amount) on the drug release pattern from bilosomal vesicles. All SDF-BS formulations displayed a sustained release profile that ranged from 76.4 ± 3.3% (F15) to 93.1 ± 4.1% (F1) after 12 h. In addition, it was obvious that increasing the SDC amount had a positive effect on drug release, while both SPC and Span 60 amounts exerted a negative effect on in vitro drug release (Figure S1).
Most importantly, the in vitro release pattern of SDF from optimized SDF-BS was assessed using the dialysis bag method and was compared with that of the pure SDF suspension. Figure 5 inferred that entrapment of SDF within bilosomes remarkably augmented in vitro drug release compared to a free drug suspension. The percentage cumulative release of SDF from bilosomes was 84.9 ± 6.7% at the end of release study, which was considerably higher than that from drug suspension (38.2 ± 2.1% at 12 h). Of note, the optimized SDF-BS showed biphasic release patterns, depicted by a rapid immediate release of the drug in the first hour (28.5 ± 2.6%), followed by a continuous release profile for the next 12 h. This biphasic drug release characteristics from bilosomal formulation is anticipated to be useful in terms of therapeutic efficiency. However, the initial rapid drug release could help in inducing rapid therapeutic effect post drug administration, while the sustained release over 12 h would keep patients under sustained drug action. Of note, analysis of release kinetic data disclosed that SDF release from optimized bilosomal formulation fitted well with Higuchi release kinetics, elucidating a diffusion-controlled process.
Figure 5.

In vitro release pattern of SDF from a SDF suspension and optimized SDF-BS.
3.6. Preparation of Sildenafil-Loaded Bilosomal Gel
Sildenafil citrate-loaded bilosomal hydrogel (SDF-BS gel) was prepared by the direct dispersion method using hydroxypropyl methylcellulose (HPMC) as a gel base. Herein, adequate weight of bilosomal dispersion, containing 10 mg of SDF, was dispersed in a HPMC gel base to produce SDF-BS gel formulation with a final concentration of 1% w/w of SDF.
3.7. Characterization of SDF-BS Gel
Visual inspection of SDF-BS gel formulation revealed that the developed gel formulation was homogeneous and smooth, with no evidence of phase separation. SDF-BS gel had a pH of 6.3 ± 0.19, which is regarded suitable for topical use and would be tolerated by the skin without causing any irritation.10 Additionally, SDF-BS gel had a good spreadability of 36.2 ± 2.9 mm, indicating easy application of the gel with a low degree of shear.42 Furthermore, the viscosity of the prepared gel was found to be 5216 ± 213 cPs, which is considered appropriate for topical application.35
3.8. Skin Permeation Study
Since the quantity of drug permeated through skin dictates the amount of drug accessible for absorption into the systemic circulation, ex vivo permeation study could provide a crucial judgment regarding the in vivo activity of transdermal delivery systems. Figure 6 illustrates the time-dependent cumulative amounts of SDF permeated per unit area from different SDF formulations. When comparing skin permeability parameters, it was obvious that entrapping SDF within bilosomal vesicles greatly enhanced SDF permeation through rat skin, leading to higher transdermal flux in 12 h, compared to plain drug gel formulation (p < 0.05). The cumulative amounts of SDF permeated through the skin per unit area in 12 h were 248.5 ± 33.2 and 581.3 ± 28.9 μg/cm2, for plain SDF gel and SDF-BS gel, respectively. The permeation parameters of SDF from various SDF gel formulations were tabulated in Table 3. The relatively higher transdermal flux from bilosomal-based formulations, compared to plain SDF gel, might account for the presence of bile salts (SDC) within the bilosomal construct. Bile salts are known to act as edge activators (EA); they may increase the deformability of vesicles, which aids in their penetration through the skin’s pores. In addition, bile salts have the potential to disrupt the tightly packed subcutaneous (SC) lipids, modifying SC layer barrier characteristics,43 and thus, promote drug paracellular permeability.44 Furthermore, it has been reported that bile salts could extract membrane protein or lipids, producing reverse micelles that could enhance drug transcellular transport.45 Most notably, SDF-BS gel formulations elicited a 2.34-fold increase in the permeation enhancement ratio (ER) with respect to plain SDF gel, indicating that bilosomal SDF surpasses plain SDF in enhancing SDF permeation through the skin.
Figure 6.

Skin permeation profile of SDF from plain SDF gel and SDF-BS gel.
Table 3. Ex Vivo Permeation Parameters of SDF from Various Gel Formulationsa.
| parameter | plain SDF gel | SDF-BS gel |
|---|---|---|
| Q12 (μg/cm2) | 248.5 ± 33.2 | 581.3 ± 28.9 |
| Jmax (μg/cm2.h) | 20.7 ± 2.8 | 48.4 ± 2.2 |
| Jss (μg/cm2.h) | 5.6 ± 0.48 | 25.9 ± 1.7 |
| enhancement ratio (ER) | 2.34 |
Q12: cumulative amount of SDF permeated per unit area in 12 h, Jss: steady state flux, and Jmax: maximum average drug flux after 12 h per unit area per hour. Data are the mean ± SD.
3.9. In Vivo Studies
3.9.1. In Vivo Pharmacokinetic Study
The in vivo pharmacokinetics of both plain SDF gel and optimized SDF-BS gel (10 mg of SDF/kg) were investigated in male Wistar rats and compared with those of oral SDF suspension (10 mg/kg). Figure 7 depicts the SDF mean plasma concentration versus time plot following treatment with different SDF formulations. As depicted in Figure 7, transdermal SDF-BS gel induced a higher plasma level for up to 24 h post administration, compared to orally administered plain drug suspension or topically applied plain SDF gel. The maximum plasma concentrations (Cmax) of SDF were 219.8 ± 23.1, 177.2 ± 19.1, and 323.1 ± 29.8 ng/mL post-treatment with SDF oral suspension, SDF gel, and SDF-BS gel, respectively. The comparatively lower peak concentration of SDF oral suspension with regards to SDF-BS gel might be ascribed to the inadequate aqueous solubility of the drug, which might hinder proper drug absorption. On the contrary, the comparatively higher plasma levels of SDF following topical application of SDF-BS gel, with respect to SDF gel, could be owed to the superior skin permeation of SDF-BS, which would promote efficient systemic delivery of the drug from bilosomal gel formulation. Similar results were reported by Khafagy et al.30 who highlighted the enhancing role of bilosomes for the transdermal delivery of simvastatin for the treatment of inflammation.
Figure 7.

Mean SDF plasma concentrations following the oral administration of SDF suspension and the transdermal application of plain SDF gel and SDF-BS gel. Data are represented as mean ± SD (n = 5).
The key pharmacokinetic parameters of various SDF formulations are listed in Table 4. It was evident that optimized SDF-BS gel showed a comparatively higher AUC0–24h (3606.25 ± 211.4 ng.h/mL) than both plain SDF gel and oral drug suspension (AUC0–24h = 1752.22 ± 117.6 and 1311.21 ± 98.7 ng h/mL, respectively). In addition, the SDF-BS gel substantially prolonged the SDF residence time in blood circulation. SDF-BS gel had an MRT of 14.86 ± 1.0 h, which was much longer than those of SDF oral suspension (MRT 8.53 ± 0.4 h) or plain SDF gel (MRT 12.78 ± 1.3 h). Intriguingly, the relative bioavailability of the SDF-BS gel was 2.06- and 2.75-fold greater than that of plain SDF gel or oral SDF suspension, respectively. Such higher relative bioavailability of SDF-BS gel might be accounted, on the one hand, for the evasion of hepatic first-pass metabolism observed with oral administration of SDF,8 and on the other hand, to the presence of bile salts, which might have made it easier for bilosomes to efficiently penetrate skin layers, leading to the delivery of a high amount of entrapped drug directly into systemic circulation, compared to plain gel formulation. To sum up, bilosomes could stand as an effective way to deliver SDF through the skin, avoiding problems encountered with its oral administration.
Table 4. Pharmacokinetic Parameters of Different SDF Formulationsa.
| pharmacokinetic parameter | oral SDF suspension | SDF gel | SDF-BS gel |
|---|---|---|---|
| Cmax (ng/mL) | 219.8 ± 23.1 | 177.2 ± 19.1 | 323.1 ± 29.8 |
| Tmax (h) | 1 | 4 | 4 |
| T1/2 (h) | 7.33 ± 0.6 | 8.56 ± 0.8 | 9.01 ± 1.1 |
| AUC0–24 (ng/mL h) | 1311.21 ± 98.7 | 1752.22 ± 117.6 | 3606.25 ± 211.4 |
| MRT (h) | 8.53 ± 0.4 | 12.78 ± 1.3 | 14.86 ± 1.0 |
Data presented as mean ± SD (n = 5).
3.9.2. In Vivo Pharmacodynamic Study
Cyclic guanosine monophosphate (cGMP) has recently emerged as a plausible drug target for the management of pulmonary arterial hypertension (PAH).46 cGMP regulates many cellular functions, particularly, pulmonary arterial vasodilatation.47 The plasma concentration of cGMP is restrained by cGMP-degrading phosphodiesterases.31 Consequently, increasing intracellular cGMP through inhibition of PDEs is a viable therapeutic approach for the treatment of PAH. Herein, in order to confirm the feasibility of bilosomes as an efficient delivery vehicle for the PDE-5 blocker, SDF and serum cGMP levels were estimated following topical application of either plain SDF gel or SDF-BS gel and compared with that of an oral suspension of SDF. As demonstrated in Figure 8, serum cGMP levels were significantly greater in all treated animals compared to normal rats, regardless of the route of drug administration. Nonetheless, among all treated groups, serum cGMP levels were significantly (p < 0.05) greater in rats treated with SDF-BS gel. There was ∼5-fold rise in serum cGMP levels, at 6 h, in rats topically treated with SF-BS gel, compared to control non-treated rats. This considerable elevation in cGMP serum levels might be associated with the higher AUC0–24h of SDF-BS gel, which in turn reflects the superior efficacy of bilosomal vesicles for enhancing the transdermal permeation of SDF through rat skin with the achievement of higher plasma levels. Collectively, these data support the superior applicability of bilosomes as a plausible delivery vehicle for the topical administration of SDF over oral administration of plain drug.
Figure 8.

Serum cGMP levels following the oral administration of SDF suspension and the transdermal application of plain SDF gel and SDF-BS gel. Data are represented as mean ± SD (n = 5). *p < 0.05 vs control #p < 0.05 vs SDF oral suspension; and $p < 0.05 vs plain SDF gel.
4. Conclusions
In this investigation, bilosomes were challenged for the transdermal delivery of sildenafil citrate, a potent PDE-5 inhibitor. A response surface methodology (33 BBD) was adopted to formulate and optimize SDF-loaded bilosomes (SDF-BS). The optimized formula had a high drug entrapment efficiency and minimal vesicle size. In addition, bilosomes sustained drug release for 12 h. Furthermore, when compared with plain SDF gel, the incorporated SDF-BS within the HPMC hydrogel significantly enhanced skin permeability. Intriguingly, in vivo animal experiments revealed that the SDF-BS gel had superior pharmacokinetic parameters, in terms of higher relative bioavailability, compared to either orally administered drug suspension or plain drug gel. Such a remarkable increase in bioavailability of SDF-BS gel formulation triggered a remarkable elevation in cGMP serum levels, which grants superior therapeutic activity of the SDF-BS gel. To sum up, these findings suggest that bilosomes might be viewed as a viable vehicle for transdermal delivery of SDF, augmenting the therapeutic efficacy of the drug, while eliminating the limitations encountered with its oral administration.
Acknowledgments
This study is supported via funding from Prince Sattam Bin Abdulaziz University project number (PSAU/2024/R/1445). The authors also extend their appreciation to the Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, for supporting this work under the researcher supporting project number (PNURSP2024R205).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c01133.
Impact of formulation variables (A) SPC amount; (B) Span 60 amount; and (C) SDC amount on the in vitro SDF release from various bilosomal formulations; regression analysis of Y1 according to different polynomial models; results of statistical analysis for Y1 response according to the quadratic model; regression analysis of Y2 according to different polynomial models; results of statistical analysis for Y2 response according to the quadratic model; and stability study results of SDF-BS stored at 4 °C for 90 days (PDF)
Author Contributions
B.K.A.: methodology, data curation, and investigation. E.-S.K.: methodology and data curation. M.F.A.: investigation and software. H.F.A.: data curation and validation. A.A.: methodology, observation, and writing–original draft. A.S.A.L.: conceptualization, writing—review, and editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Abu Lila A. S.; Gomaa E.; Ghazy F. E. S.; Hasan A. A. Treatment of pulmonary arterial hypertension by vardenafil-solid dispersion lozenges as a potential alternative drug delivery system. Journal of Drug Delivery Science and Technology 2020, 55, 101444 10.1016/j.jddst.2019.101444. [DOI] [Google Scholar]
- Hatzimouratidis K. Sildenafil in the treatment of erectile dysfunction: an overview of the clinical evidence. Clin. Interv. Aging 2006, 1, 403–414. 10.2147/ciia.2006.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal S.; Khraisha O.; Al Madani M.; Treece J.; Baumrucker S. J.; Paul T. K. Sildenafil for Pulmonary Arterial Hypertension. Am. J. Ther 2019, 26, e520–e526. 10.1097/MJT.0000000000000766. [DOI] [PubMed] [Google Scholar]
- Morcos S. K. Can selective inhibitors of cyclic guanosine monophosphate (cGMP)-specific phosphadiesterase type 5 (PDE 5) offer protection against contrast induced nephropathy?. Quant. Imaging Med. Surg. 2014, 4, 214–215. 10.3978/j.issn.2223-4292.2014.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhariwal A. K.; Bavdekar S. B. Sildenafil in pediatric pulmonary arterial hypertension. J. Postgrad Med. 2015, 61, 181–192. 10.4103/0022-3859.159421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghetti M.; Rudzinski A.; Zhang M. Efficacy and safety of oral sildenafil in children with Down syndrome and pulmonary hypertension. BMC Cardiovasc Disord 2017, 17, 177. 10.1186/s12872-017-0569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai K.; Di Lorenzo M.; Zuckerman W. A.; Emeruwa E.; Krishnan U. S. Safety and Efficacy of Sildenafil for Group 2 Pulmonary Hypertension in Left Heart Failure. Children 2023, 10, 270. 10.3390/children10020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. J.; Muirhead G. J.; Harness J. A. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol. 2002, 53 (Suppl 1), 5S–12S. 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosny K. M.; Alhakamy N. A.; Almodhwahi M. A.; Kurakula M.; Almehmady A. M.; Elgebaly S. S. Self-Nanoemulsifying System Loaded with Sildenafil Citrate and Incorporated within Oral Lyophilized Flash Tablets: Preparation, Optimization, and In Vivo Evaluation. Pharmaceutics 2020, 12, 1124. 10.3390/pharmaceutics12111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah M. H.; Abu Lila A. S.; Unissa R.; Elsewedy H. S.; Elghamry H. A.; Soliman M. S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf., B 2021, 205, 111868 10.1016/j.colsurfb.2021.111868. [DOI] [PubMed] [Google Scholar]
- Paudel K. S.; Milewski M.; Swadley C. L.; Brogden N. K.; Ghosh P.; Stinchcomb A. L. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv 2010, 1, 109–131. 10.4155/tde.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner T.; Marks R. Delivering drugs by the transdermal route: review and comment. Skin Research and Technology 2008, 14, 249–260. 10.1111/j.1600-0846.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- Andrews S. N.; Jeong E.; Prausnitz M. R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. 10.1007/s11095-012-0946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhote V.; Bhatnagar P.; Mishra P. K.; Mahajan S. C.; Mishra D. K. Iontophoresis: a potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012, 80, 1–28. 10.3797/scipharm.1108-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denet A. R.; Vanbever R.; Préat V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv Rev. 2004, 56, 659–674. 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Ogura M.; Paliwal S.; Mitragotri S. Low-frequency sonophoresis: current status and future prospects. Adv. Drug Deliv Rev. 2008, 60, 1218–1223. 10.1016/j.addr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Sloan K. B.; Wasdo S. Designing for topical delivery: prodrugs can make the difference. Med. Res. Rev. 2003, 23, 763–793. 10.1002/med.10048. [DOI] [PubMed] [Google Scholar]
- Barry B. W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. 10.1016/S0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Jain S.; Jain P.; Umamaheshwari R. B.; Jain N. K. Transfersomes--a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. 10.1081/DDC-120025458. [DOI] [PubMed] [Google Scholar]
- Singh M. R.; Singh D. K.; Saraf S. Development and in vitro evaluation of polar lipid based lipospheres for oral delivery of peptide drugs. Int. J. Drug Deliv. 2009, 1, 15–26. 10.5138/ijdd.2009.0975.0215.01002. [DOI] [Google Scholar]
- Abdallah M. H.; Sabry S. A.; Hasan A. A. Enhancing Transdermal Delivery of Glimepiride Via Entrapment in Proniosomal Gel. Journal of Young Pharmacists 2016, 8, 335–340. 10.5530/jyp.2016.4.8. [DOI] [Google Scholar]
- Richard C.; Cassel S.; Blanzat M. Vesicular systems for dermal and transdermal drug delivery. RSC Adv. 2021, 11, 442–451. 10.1039/D0RA09561C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D.; Pradhan M.; Nag M.; Singh M. R. Vesicular system: Versatile carrier for transdermal delivery of bioactives. Artificial Cells, Nanomedicine, and Biotechnology 2015, 43, 282–290. 10.3109/21691401.2014.883401. [DOI] [PubMed] [Google Scholar]
- Prodduturi S.; Sadrieh N.; Wokovich A. M.; Doub W. H.; Westenberger B. J.; Buhse L. Transdermal delivery of fentanyl from matrix and reservoir systems: effect of heat and compromised skin. J. Pharm. Sci. 2010, 99, 2357–2366. 10.1002/jps.22004. [DOI] [PubMed] [Google Scholar]
- Elsayed M. M.; Abdallah O. Y.; Naggar V. F.; Khalafallah N. M. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int. J. Pharm. 2006, 322, 60–66. 10.1016/j.ijpharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- El-Ridy M. S.; Yehia S. A.; Mohsen A. M.; El-Awdan S. A.; Darwish A. B. Formulation of Niosomal Gel for Enhanced Transdermal Lornoxicam Delivery: In-Vitro and In-Vivo Evaluation. Curr. Drug Deliv 2018, 15, 122–133. 10.2174/1567201814666170224141548. [DOI] [PubMed] [Google Scholar]
- Khafagy E. S.; Abu Lila A. S.; Sallam N. M.; Sanad R. A.; Ahmed M. M.; Ghorab M. M.; Alotaibi H. F.; Alalaiwe A.; Aldawsari M. F.; Alshahrani S. M.; et al. Preparation and Characterization of a Novel Mucoadhesive Carvedilol Nanosponge: A Promising Platform for Buccal Anti-Hypertensive Delivery. Gels 2022, 8, 235. 10.3390/gels8040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.; Kassem M. A.; Sayed S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomedicine 2020, 15, 9783–9798. 10.2147/IJN.S278688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A.; Mishra V.; Kesharwani P. Bilosomes in the context of oral immunization: development, challenges and opportunities. Drug Discov Today 2016, 21, 888–899. 10.1016/j.drudis.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Khafagy E. S.; Almutairy B. K.; Abu Lila A. S. Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation. Polymers (Basel) 2023, 15, 677. 10.3390/polym15030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldawsari M. F.; Khafagy E. S.; Alotaibi H. F.; Abu Lila A. S. Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation. Polymers (Basel) 2022, 14, 4184. 10.3390/polym14194184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar H. O.; Mohamed M. I.; Tadros M. I.; Fouly A. A. Transdermal Delivery of Ondansetron Hydrochloride via Bilosomal Systems: In Vitro, Ex Vivo, and In Vivo Characterization Studies. AAPS PharmSciTech 2018, 19, 2276–2287. 10.1208/s12249-018-1019-y. [DOI] [PubMed] [Google Scholar]
- van den Bergh B. A.; Wertz P. W.; Junginger H. E.; Bouwstra J. A. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements. Int. J. Pharm. 2001, 217, 13–24. 10.1016/S0378-5173(01)00576-2. [DOI] [PubMed] [Google Scholar]
- Abdallah M. H.; Lila A. S. A.; Unissa R.; Elsewedy H. S.; Elghamry H. A.; Soliman M. S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. 10.1208/s12249-021-02113-8. [DOI] [PubMed] [Google Scholar]
- Al-Mahallawi A. M.; Abdelbary A. A.; Aburahma M. H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. 10.1016/j.ijpharm.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Bnyan R.; Khan I.; Ehtezazi T.; Saleem I.; Gordon S.; O’Neill F.; Roberts M. Surfactant Effects on Lipid-Based Vesicles Properties. J. Pharm. Sci. 2018, 107, 1237–1246. 10.1016/j.xphs.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Abdelbary A. A.; Abd-Elsalam W. H.; Al-Mahallawi A. M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. 10.1016/j.ijpharm.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Mahmoud T. M.; Nafady M. M.; Farouk H. O.; Mahmoud D. M.; Ahmed Y. M.; Zaki R. M.; Hamad D. S. Novel Bile Salt Stabilized Vesicles-Mediated Effective Topical Delivery of Diclofenac Sodium: A New Therapeutic Approach for Pain and Inflammation. In Pharmaceuticals 2022, 15, 1106. 10.3390/ph15091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar A.; Alruwaili N. K.; Imam S. S.; Hadal Alotaibi N.; Alharbi K. S.; Afzal M.; Ali R.; Alshehri S.; Alzarea S. I.; Elmowafy M.; et al. Bioactive Apigenin loaded oral nano bilosomes: Formulation optimization to preclinical assessment. Saudi Pharm. J. 2021, 29, 269–279. 10.1016/j.jsps.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangjit S.; Pamornpathomkul B.; Opanasopit P.; Rojanarata T.; Obata Y.; Takayama K.; Ngawhirunpat T. Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int. J. Nanomedicine 2014, 9, 2005–2017. 10.2147/IJN.S60674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta M.; Peira E.; Debernardi F.; Gallarate M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int. J. Pharm. 2002, 241, 319–327. 10.1016/S0378-5173(02)00266-1. [DOI] [PubMed] [Google Scholar]
- Salem H. F.; Nafady M. M.; Kharshoum R. M.; Abd El-Ghafar O. A.; Farouk H. O. Novel Enhanced Therapeutic Efficacy of Dapoxetine HCl by Nano-Vesicle Transdermal Gel for Treatment of Carrageenan-Induced Rat Paw Edema. AAPS PharmSciTech 2020, 21, 113. 10.1208/s12249-020-01656-6. [DOI] [PubMed] [Google Scholar]
- Walve J. R.; Bakliwal S.; Rane B. R.; Pawar S. P. Transferosomes: A surrogated carrier for transdermal drug delivery system. Int. J. Appl. Biol. Pharm. Tech. 2011, 2, 204–213. [Google Scholar]
- Lin H.; Gebhardt M.; Bian S.; Kwon K. A.; Shim C. K.; Chung S. J.; Kim D. D. Enhancing effect of surfactants on fexofenadine.HCl transport across the human nasal epithelial cell monolayer. Int. J. Pharm. 2007, 330, 23–31. 10.1016/j.ijpharm.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Moghimipour E.; Ameri A.; Handali S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. 10.3390/molecules200814451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. N.; Watson G.; Zhao L. Cyclic guanosine monophosphate signalling pathway in pulmonary arterial hypertension. Vascul Pharmacol 2013, 58, 211–218. 10.1016/j.vph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Watanabe H. Treatment Selection in Pulmonary Arterial Hypertension: Phosphodiesterase Type 5 Inhibitors versus Soluble Guanylate Cyclase Stimulator. Eur. Cardiol 2018, 13, 35–37. 10.15420/ecr.2017:22:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.