Abstract
The human embryonic β-like globin (ɛ-globin) gene is expressed in primitive erythroid cells of the yolk sac during the first few weeks of development. We have previously shown that developmental stage-specific expression of the ɛ-globin gene is mediated by multiple positive and negative regulatory elements upstream of the start of transcription. Of particular interest is one positive regulatory element, PRE II, that works together with other elements (PRE I and PRE V) to confer developmental stage- and/or tissue-specific expression on a minimal promoter. An ∼85- to 90-kDa PRE II binding factor (PREIIBF) was identified in the nuclei of erythroid cells and shown to bind specifically to a novel 19-bp region within PRE II; binding of this protein to PRE II resulted in bending of the target DNA and was required for promoter activation. In this report, we present the cDNA expression cloning of PREIIBF. The cDNA encodes a previously identified member of the HMG domain family of DNA binding proteins termed SSRP1. By a number of biochemical and immunological criteria, recombinant SSRP1 appears to be identical to the PREII binding factor from erythroid nuclei. A hallmark of HMG domain proteins is their ability to bend their target DNAs; therefore, as we speculated previously, DNA bending by SSRP1/PREIIBF may contribute to the mechanism by which PRE II synergizes with other regulatory elements located upstream and downstream. In contrast with reports from other investigators, we demonstrate that SSRP1 binds DNA with clear sequence specificity. Moreover, we show that SSRP1/PREIIBF lacks a classical activation domain but that binding by this protein to PRE II is required for activation of a minimal promoter in stable erythroid cell lines. These studies provide the first evidence that SSRP1 plays a role in transcriptional regulation. SSRP1/PREIIBF may serve an architectural function by helping to coordinate the assembly of a multiprotein complex required for stage-specific regulation of the human ɛ-globin gene.
Globin genes are expressed in erythroid cells during restricted stages of development and have served as an important model for studying tissue- and developmental stage-specific gene regulation (reviewed in reference 3). Sequential changes in globin gene expression during embryogenesis are referred to as hemoglobin switching (reviewed in reference 45). The first stage-specific switch in globin gene transcription involves both the α- and β-globin loci. Primitive erythroid cells formed in the blood islands of the yolk sac during the first few weeks of human gestation produce the embryonic globins ɛ (β-like) and ζ (α-like) (3, 45). Around the sixth week of gestation, the site of erythropoiesis shifts to the fetal liver, where definitive erythroid cells express the γ (β-like)- and α-globin genes (44). The second hemoglobin switching event occurs around the time of birth and involves only the β-globin locus. Coincident with a shift in the site of erythropoiesis to the bone marrow, the fetal β-like globin gene (γ) is downregulated and the adult β-globin gene is activated (3, 45). Although hemoglobin switching has been studied extensively, the molecular mechanisms underlying stage-specific expression of the globin genes are not completely understood (discussed in reference 3).
We have previously mapped several positive and negative control elements upstream of the human ɛ-globin minimal promoter (50). One of the positive regulatory elements (PRE II) can synergize with each of two other elements located upstream (PRE V) or downstream (PRE I) to confer tissue- and stage-specific expression on a linked promoter (50). Nuclear extracts from embryonic erythroid cells contain a PRE II binding factor (PREIIBF) of ∼85 to 90 kDa that specifically interacts with a novel 19-bp region within PRE II (50, 51) and whose binding is required for activation of the ɛ-globin gene promoter (50, 51). Nuclear extracts prepared from adult erythroid cells contain a PRE II binding activity that exhibits faster migration in electrophoretic mobility shift assays (EMSAs) (50, 51). A number of biochemical criteria suggest that embryonic and adult forms of the two proteins are very similar (15, 50, 51). Distinct forms of PREIIBF may result from differential posttranscriptional modifications such as alternative mRNA splicing, phosphorylation, proteolysis, and/or glycosylation.
PREIIBF introduces a bend of 54 to 63° toward the minor groove, where it binds to PRE II (15). Based on the observations that PRE II cooperates with at least two other positive regulatory elements and that PREIIBF can bend its target DNA, we have proposed that PREIIBF acts as an architectural transcription factor (reviewed in references 21 and 48) to bring together distantly bound proteins upstream of the human ɛ-globin gene (15).
In this report, we present the cDNA expression cloning of PREIIBF from human embryonic erythroid cells. This cDNA encodes SSRP1 (structure-specific recognition protein), a previously identified member of the high-mobility-group (HMG) domain family of proteins. A hallmark of this family is their ability to bend target DNAs. Based on several biochemical and immunological criteria, we demonstrate that PREIIBF and SSRP1 are identical. Like the lymphoid enhancer-binding factors LEF-1 and TCF-1α (49, 57) and the testis-determining factor Sry (23, 41), PREIIBF/SSRP1 contains a single HMG domain. In contrast with studies from other groups (8, 18), we show that PREIIBF/SSRP1 binds to DNA with modest affinity but with clear sequence specificity. These DNA binding characteristics, and the requirement for its binding to its cognate DNA element for transcriptional activation of a minimal ɛ-globin promoter (reference 51 and this report), distinguish SSRP1 from HMG-1, HMG-2, and other family members that bind DNA nonspecifically (6, 28). Although T160, the murine ortholog of SSRP1, was isolated in a cDNA expression screen using a V-(D)-J recombination signal sequence (RSS) as a probe, we find that the binding of the human protein to PRE II is significantly stronger than binding to the V-(D)-J RSS. A strongly conserved acidic region in PREIIBF/SSRP1 cannot activate transcription on its own or within larger stretches of the protein and may, like comparable regions of LEF-1 and TCF-1α, function as a “context-dependent” activation domain (12, 19). Taken together, these various observations provide strong support for the idea, first proposed on the basis of structural alignments (6), that SSRP1 defines a distinct subfamily of HMG domain proteins. While human SSRP1 was first cloned on the basis of its ability to bind to cisplatinated DNA (9), its function in vivo was unknown. The studies presented here, together with our earlier work (15, 50, 51), suggest a function for SSRP1 as an architectural transcription factor involved in activation of the human embryonic β-like globin gene (ɛ) and provide insights into the molecular basis for synergistic interactions among ɛ-globin gene upstream control elements (50). To our knowledge, this is the first report of a role for HMG domain proteins in globin gene regulation.
MATERIALS AND METHODS
Isolation of cDNA clones and DNA sequencing.
Two independent, size-selected, directionally cloned human embryonic erythroid (K562 cell) cDNA expression libraries were constructed in the phage vector λgt22A (Gibco-BRL), a derivative of λgt11. Poly(A)-selected RNA was prepared from K562 embryonic erythroid cells (13). First-strand cDNA synthesis was initiated from primer oligo dT(NotI) (Gibco-BRL). Following second-strand synthesis, a SalI restriction endonuclease half site was ligated to the cDNAs, which were then digested with NotI and SalI and size selected by gel filtration chromatography. Fractions containing cDNAs greater than 1 kb were pooled and ligated to λgt22A arms.
Based on a series of pilot experiments to optimize conditions for DNA probe binding to filter-immobilized PREIIBF protein, denaturation/renaturation conditions with a multimerized probe (55) were used for binding site screening of the library. Approximately 106 plaques were screened on duplicate nitrocellulose filters as described previously (42, 55). The filters were probed with a double-stranded multimerized PRE II oligonucleotide probe consisting of MAD32 (top strand; 5′-AATTCGAGATTCTGTTTTAACAGCTTTG-3′) and MAD33 (bottom strand; 5′-AATTCAAAGCTGTTAAAACAGAATCTCG-3′). Underlined sequences represent EcoRI half sites that facilitated multimerization and radiolabeling. The probes were labeled by filling in with the Klenow fragment of DNA polymerase in the presence of [α-32P]dATP and [α-32P]dTTP. For all hybridizations, the probes were added to a final concentration of 106 cpm/ml.
Phage plaques giving a positive hybridization signal were subjected to two rounds of purification and one round of amplification to generate a high-titer stock as described previously (37). Bacteriophage were isolated by standard methods (37), and DNA inserts were cloned into pBluescript SK+ (Stratagene). Single-stranded phagemid DNA was prepared and sequenced by the dideoxy-chain termination method (37). Sequence data were obtained from the 5′ and 3′ ends of each cDNA (approximately 200 to 300 bp each) and used for homology searches (BLAST network service, National Center for Biotechnology Information). All of the sequence data matched exactly with previously published sequences for SSRP1 (four clones) or HMG-2 (two clones).
Southwestern blotting.
Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels (2) in a Bio-Rad minigel apparatus. Following SDS-PAGE, the gel was equilibrated in transfer buffer (25 mM Tris-HCl [pH 8.3], 192 mM glycine, 20% methanol) for 1 h. Proteins were transferred to nitrocellulose at 100 V for 1 h in a Bio-Rad transblot apparatus. Following transfer, the filter was stained with Ponceau S and the positions of the molecular weight markers (New England Biolabs Broad-range protein molecular weight markers) were marked with permanent ink. Proteins bound to the filter were denatured in 6 M guanidine hydrochloride–10 mM Tris-HCl (pH 7.5)–50 mM KCl–0.5 mM dithiothreitol (DTT) for 5 min. Renaturation was carried out by performing six serial twofold dilutions of the guanidine hydrochloride solution with 10 mM Tris-HCl (pH 7.5)–50 mM KCl–0.5 mM DTT for 5 min at room temperature. Following the final dilution, the filters were washed in 10 mM Tris-HCl (pH 7.5)–50 mM KCl–0.5 mM DTT for 5 min at room temperature. Prehybridization was carried out overnight in the same buffer containing nonfat powdered milk (Carnation) at a final concentration of 50 mg/ml. For the hybridization, the concentration of milk was reduced to 2.5 mg/ml, and calf thymus DNA (Sigma) was added at a final concentration of 25 μg/ml. Radiolabeled multimerized oligonucleotide probes (see above) was added to a final concentration of 106 cpm/ml, and hybridization was carried out for 1 h at room temperature. Washes were performed in 10 mM Tris-HCl (pH 7.5)–50 mM KCl–0.5 mM DTT–2.5 mg of nonfat powdered milk per ml. Filters were then dried at room temperature and autoradiographed.
Plasmid constructs. (i) rSSRP1 expression constructs.
The NotI-SalI cDNA fragment was excised from each pBluescript SK+ subclone and inserted into the XhoI and NotI sites of a modified version of pRSETA (Invitrogen), pRSETANotI. Recombinant proteins expressed from these constructs were used only for the initial Southwestern analysis of the cDNA clones. Recombinant SSRP1 (rSSRP1) for all subsequent experiments was expressed from pRSETNotI(myc), a modified version of pRSETANotI encoding a Myc epitope tag. A double-stranded oligonucleotide encoding the minimal 11-amino-acid Myc epitope recognized by monoclonal antibody 9E10 was synthesized (17). Oligonucleotide sequences were as follows: myc.top, 5′-TATGGAGCAAAAGCTCATTTCTGAAGAGGACTTG-3′; and myc.bottom, 5′-GATCCAAGTCCTCTTCAGAAATGAGCTTTTGCTCCA-3′. BamHI and NdeI restriction endonuclease half sites (underlined sequences) were included in these oligonucleotides to facilitate in-frame cloning into the pRSETA vector (Invitrogen). A NotI linker (5′-GCGGCCGC-3′) was ligated into a filled-in HindIII site in the polylinker to give pRSETNotI(myc). SSRP1 sequences were PCR amplified from the pBluescript SK+ subclones by using a T7 primer and a primer that hybridized to the 3′ end of SSRP1. The SSRP1-His,NotI primer sequence was 5′-ATAGTTTAGCGGCCGCTAatgatgatgatgatgatgCTCATCGGATCCTGAC-3′. This 3′ PCR primer was engineered to contain His6 codons (lowercase) following the last SSRP1 amino acid, a stop UAG codon (boldface), and a NotI site (underlined). The PCR products were digested with SalI, filled in with the Klenow fragment of DNA polymerase I, digested with NotI, and then cloned into the PvuII and NotI sites of pRSETNotI(myc).
(ii) Generation of a full-length SSRP1 cDNA.
A full-length SSRP1 cDNA was generated by using the following PCR strategy. The 5′ primer was 5′-CACCACAGgcggccgcCACCATGGCAGAGACACTG-GAG-3′, where the lowercase sequence corresponds to a NotI restriction site and the underlined sequence corresponds to the 5′ end of the SSRP1 open reading frame (9). The 3′ PCR primer (5′-CCAATGTCAAAGGAAAGCAGCTGCCCACC-3′) spans the SSRP1 coding sequence from amino acids 115 to 124 that includes a PvuII site (underlined). These primers were used to amplify the SSRP1 sequence encoding the amino-terminal 124 residues from embryonic erythroid (K562) cell cDNA. Additional details of the construction are given in reference 14.
(iii) ɛ-Globin expression constructs used for generation of stable cell lines.
PRE II point mutations were introduced into the −2-kb upstream regulatory region of the human embryonic β-like globin gene by a PCR mutagenesis strategy (24). Briefly, PCR amplifications were performed individually with oligonucleotide primers containing specific nucleotide mutations in PRE II. Restriction fragments spanning wild-type PRE II were excised from the upstream control region by restriction endonuclease digestion and replaced with the mutated versions of PRE II. A XhoI site was introduced at +44 of the human embryonic β-like globin gene by the same strategy. The ∼6.5-kb mini-LCR (locus control region) fragment used for the constructs was described previously (47). Plasmids were linearized with SalI prior to electroporation (see below).
rSSRP1 expression and purification.
SSRP1 expression vectors were transformed into Escherichia coli BL21(DE3, pLYS) grown in LB medium containing ampicillin (200 μg/ml) and chloramphenicol (50 μg/ml) and induced with isopropyl-β-d-thiogalactopyranoside as described previously (14). A clarified cell lysate was incubated with Ni2+-nitrilotriacetic acid (NTA) agarose (Qiagen), and His6-tagged rSSRP1 was eluted from the resin by using a buffer containing a cocktail of protease inhibitors (14).
EMSAs and determination of Kd.
Binding reaction mixtures (30 μl) contained 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 10% glycerol, 0.5 mM DTT, 0.1 ng of 32P-labeled oligonucleotide, and either partially purified PREIIBF (DEAE eluate [15]) (1 to 2 μg) or rSSRP1 (100 to 200 ng). Binding reaction mixtures were incubated for 20 to 30 min at room temperature and separated on 4% nondenaturing polyacrylamide gels (29.2:0.8 acrylamide-bisacrylamide) containing 1× Tris-borate-EDTA buffer and 5% glycerol at 4°C. Gels were dried and autoradiographed overnight at room temperature. For antibody supershift experiments, 10-μl aliquots of antisera or preimmune sera were preincubated with rSSRP1 or PREIIBF for 10 min prior to addition of probe. For equilibrium binding analyses with recombinant protein, equilibrium binding constant (Kd) values were determined as described previously (15).
Methylation interference analysis.
Methylation interference analysis was performed exactly as described previously (50).
Antiody production and purification.
Peptides corresponding to the amino-terminal (NH2-CAETLEFNDVY-COOH) and carboxy-terminal (NH2-CPSSEDSASGS-COOH) residues of SSRP1 were chemically synthesized (Bio-Synthesis Inc.). Cysteine residues (boldface) were added to facilitate coupling to carrier protein keyhole limpet hemocyanin (KLH). Peptides were dissolved in oxygen-free phosphate buffer and were conjugated to KLH as described previously (4). Each KLH-peptide was injected into two rabbits at Cocalico Biologicals, Inc. Crude serum was purified on a Bio-Rad Affigel-Blue column. Saturated ammonium sulfate was then added to a final concentration of 45% (vol/vol) on ice. The resulting precipitate was collected by centrifugation and then resuspended in and dialyzed against phosphate-buffered saline (PBS).
Peptide affinity columns were generated by coupling 10 μmol to a 1-ml Pharmacia Hi-Trap column (14). For affinity purification, 1 ml of concentrated, Affigel-purified antipeptide antibody was loaded onto the peptide affinity column and incubated at room temperature for 15 min. Two 10-ml washes were performed. The first wash, with Tris-HCl (10 mM, pH 7.5), was followed by a 10-ml wash with Tris-HCl (10 mM, pH 7.5)–500 mM NaCl. Bound antibody was eluted with 2 ml of 100 mM glycine (pH 2.5) and was immediately neutralized with 0.1 volume of 1 M Tris-HCl (pH 8.0). Following dialysis against PBS, the antibodies were concentrated approximately fivefold with a Centricon-30 spin column, frozen in liquid nitrogen, and stored in aliquots at −80°C.
Immunoblot analysis.
For immunoblot analysis, proteins were separated on a standard SDS-polyacrylamide gel and transferred to nitrocellulose (14). Filters were prehybridized in TBST (10 mM Tris [pH 8.0], 150 mM NaCl, 0.1% Tween 20)–5% calf serum overnight at room temperature. Hybridization with primary antibody (affinity-purified anti-SSRP1 peptide antibodies or anti-Myc monoclonal antibody 9E10 [17]) was carried out for 2 h and was followed by three washes in TBST. Secondary alkaline phosphatase-conjugated antibody (goat anti-rabbit immunoglobulin G [IgG; Sigma] or goat anti-mouse IgG [Santa Cruz Biotechnologies]) was added in TBST–5% calf serum, incubated for 1 h, and then washed three times in TBST. Immunoblots were developed in 100 mM Tris (pH 9.5)–100 mM NaCl–5 mM MgCl2–0.33 mg of nitroblue tetrazolium (Sigma) per ml–0.16 mg of 5-bromo-4-chloro-3-indolyl phosphate (Sigma) per ml, and the reaction was terminated in 5 mM EDTA.
Generation of stable K562 cell lines.
K562 cells were maintained in RPMI–10% calf serum. To generate stable lines, 2 × 107 cells were mixed with 20 μg of ɛ-globin DNA linearized with SalI and 2 μg of pSV2neo DNA linearized with EcoRI in 0.8 ml of RPMI–10% calf serum. Electroporation was performed with a Bio-Rad gene pulser at 960 μF and 2.8 kV followed by a 10-min incubation on ice. RPMI–10% calf serum (10 ml) was added to the cells, which were transferred to 10-cm-diameter plates and incubated for 48 h at 37°C in 5% CO2. G418 (Gibco-BRL) was then added to a final concentration of 800 μg/ml (active drug), and the cells were serially diluted over several orders of magnitude into individual wells of 48-well plates. The medium was changed every 2 to 3 days until colonies became visible; individual colonies were expanded in 10-cm-diameter dishes at a G418 concentration of 400 μg/ml. Several lines from each construct were transferred to 90% fetal calf serum–10% dimethyl sulfoxide for long-term liquid nitrogen storage.
Genomic Southern blot analysis.
Chromosomal DNA for Southern blotting was prepared according to a standard method as described elsewhere (14). Restriction enzyme-digested chromosomal DNA was separated on an 0.8% agarose gel in 1× Tris-borate-EDTA, stained with ethidium bromide, photographed, and transferred to a nylon membrane (Zeta-Probe; Bio-Rad) as specified by the manufacturer. Prehybridization and hybridization steps were carried out at 65°C as described in reference 37.
The probe for Southern blot analysis was a 1.2-kb region of the human embryonic β-like globin gene from the BamHI site at +550 to the EcoRI site at +1726. It was radiolabeled by random priming (37) and purified on a NENSORB column (DuPont-NEN). Due to the XhoI site at +44 in the ɛ-globin expression constructs, the transgene was detected as a 2.0-kb band and the endogenous embryonic globin gene was detected as a 3.7-kb band on autoradiographs. The intensity of each band was quantitated with a Fuji-200 phosphorimager. Plasmid copy number in each cell line was determined by taking the ratio of the 2.0-kb band (ɛ-globin expression construct) to the 3.7-kb band (endogenous ɛ-globin).
RNase protection analysis.
RNase protection assays were carried out as described previously (5) with RNA prepared from each cell line carrying an ɛ-globin expression construct. Briefly, 10 μg of RNA was hybridized to an excess of antisense radiolabeled probe spanning −274 to +167 of the human ɛ-globin gene and its upstream region. mRNA expressed from the ɛ-globin expression constructs contains an XhoI site at +44 that is not present in mRNA encoded by the endogenous gene. The antisense (−274 to +167) probe also contains an XhoI site at +44, and therefore RNase digestion yields distinct protected fragments for endogenous (123 bp) and transgene mRNA (167 bp). An actin probe used to normalize for RNA recovery was described previously (5). The intensity of each band was quantitated with a Fuji-200 phosphorimager. Expression levels were taken as the ratio of XhoI-marked embryonic globin mRNA to actin mRNA normalized to plasmid copy number as determined by Southern blot analysis (see above).
GAL4 fusion constructs.
The expression construct pBXGmycHIS was generated from pBXG1 [a pECE72-based expression vector (16) which codes for GAL4(1–147), the DNA binding domain of GAL4; this construct was a gift from M. Ptashne] by insertion of a synthetic double-stranded oligonucleotide (mycHIS) into the PstI and XbaI sites. The mycHIS oligonucleotide encodes a Myc epitope tag and a His6 tag, separated by a SpeI and a NotI site (underlined). The sequences of the sense and antisense oligonucleotides were as follows: mycHIS (sense), 5′-GAT GGA GCA AAA GCT CAT TTC TGA AGA GGA CTT GAC TAG TGC GGC CGC ACA TCA TCA TCA TCA TCA TTA AT-3′; and mycHIS (antisense), 5′-CTA GAT TAA TGA TGA TGA TGA TGA TGT GCG GCC GCA CTA GTC AAG TCC TCT TCA GAA ATG AGC TTT TGC TCC ATC TGC A-3′. SSRP1 fragments (see Results) were PCR amplified with appropriate primers, digested with SpeI and NotI, and inserted into the SpeI and NotI sites of pBXGmycHIS. The resulting expression constructs contained one of a set of partially overlapping 150-amino-acid peptides spanning the entire SSRP1 protein; or the acid region, the C-terminal region of mixed charge, the amino-terminal 70% of the protein (including the acid region), the amino-terminal portion of the protein extending up to but not including the HMG domain, the amino-terminal 50% of the protein, or the C-terminal region of the protein containing the HMG domain flanked by both basic regions and followed by the region of mixed charge. Two expression constructs, pSGB17 and pSG236, were used as weak activator controls. pSGB17 encodes the DNA binding domain GAL4(1–147) fused to a fragment of the E. coli genome that functions as a weak activator (36), in a pECE vector backbone (16). pSG236 is a pECE72-based expression vector and codes for GAL(1–147+768–881) (31). pBXGalVP was used to express a strong transcriptional activator; it encodes GAL4(1–147) fused to the activation domain of VP16, again in a pECE72 backbone. pSG236 and pBXGalVP were gifts from M. Ptashne. The chloramphenicol acetyltransferase (CAT) reporter gene used for the transcriptional activation assays was G6(SP1)(−31)HIVLTRΔTAR (43).
CHO cell transfection.
DNA (1 μg of activator expression plasmid, 1 μg of reporter plasmid, and 0.5 μg of pCMVβgal, used as a transfection control) was mixed with 0.5 ml of 1 M Tris-HCl (pH 7.3). DEAE-dextran (4.5 ml of 0.25 mg/ml; molecular weight, 5 × 105; Pharmacia) in alpha minimal essential medium (α-MEM) containing 1% NaHCO3, penicillin (100 U/ml), and streptomycin (0.1 μg/ml) was then added to the DNA mixture. CHO cells plated on 10-cm-diameter dishes were washed with PBS, covered with the DNA–DEAE-dextran mixture, and incubated at 37°C in 5% CO2 for 14 h. The cells were then washed with PBS and covered with 3 ml of PBS containing 10% (vol/vol) dimethyl sulfoxide. After 3 min, the PBS was removed and replaced with 5 ml of α-MEM containing 10% fetal calf serum and 0.1 mM chloroquine. The cells were incubated for 2.5 h at 37°C in 5% CO2, washed twice with PBS, and covered with 7 ml of α-MEM–10% fetal calf serum. After 24 h, the medium was changed once. Cells were harvested after an additional 24 h by washing three times with PBS, adding 1 ml of PBS–2 mM EDTA, and then scraping and collecting the cells by centrifugation. Cells were lysed in 0.25 M Tris-HCl (pH 7.5), and CAT and β-galactosidase assays were carried out as described previously (50).
RESULTS
cDNA expression cloning of PREIIBF identifies an HMG domain protein with sequence-specific DNA binding properties.
Purification of PREIIBF had earlier been undertaken to characterize some of the biochemical properties of the protein (15). An understanding of the mechanism by which PREIIBF mediates synergistic interactions between PRE II and other regulatory elements will require molecular cloning of the cDNA, and we therefore initiated DNA recognition site expression cloning.
Recognition site screening of an expression library (42, 55) will succeed only if the protein of interest can bind DNA as a single polypeptide, which seems to be the case for PREIIBF (15). Two independent size-selected, directionally cloned human embryonic erythroid (K562 cell) cDNA expression libraries were constructed in the phage vector λgt22A. A multimerized form of the binding site was used to improve the chances of isolating cDNA clones encoding PREIIBF (55). Specificity for PRE II binding was confirmed by hybridizing duplicate filters with specific (PRE II) and nonspecific (octamer) probes. All six phage clones showed specific hybridization to the PRE II probe. The cDNA inserts from these six clones were subcloned into pBluescript SK+, sequenced, and found to encode the human HMG domain proteins SSRP1 (four clones) (9) and HMG-2 (two clones) (32).
The predicted size for SSRP1 (81 kDa [9]) is consistent with the previous size estimates for PREIIBF (∼85 to 90 kDa) (15, 51). HMG-2 (predicted size, 28 kDa [32]) is too small to account for the apparent size of PREIIBF. To determine whether the isolation of HMG-2 in this screen might be an artifact of using a multimerized probe, Southwestern blots were performed with a monomeric PRE II binding site probe. cDNAs encoding SSRP1 (clone 66 [Fig. 1A]) and HMG-2 (clone 10 [see Materials and Methods]) were subcloned into pRSETANotI (see Materials and Methods). Recombinant proteins expressed from this vector in E. coli contain an amino-terminal His6 tag for Ni2+-NTA agarose purification. Approximately equivalent amounts (as determined by Coomassie blue and Ponceau S staining) of purified rSSRP1 and HMG-2 were analyzed by Southwestern blotting using a radiolabeled monomeric PRE II probe. As shown in Fig. 1B, only rSSRP1 could bind to the specific PRE II monomer (specific probe) in this assay. Neither of the recombinant proteins recognized the unrelated (nonspecific) octamer binding motif. The ability of SSRP1 to bind to the monomeric PRE II probe suggests that its DNA binding specificity and affinity for PRE II are significantly greater than those of HMG-2. Taken together, the Southwestern blotting results and the size estimates suggest that the SSRP1 cDNAs and not HMG-2 cDNAs encode PREIIBF. The HMG-2 cDNAs were not examined further in this work.
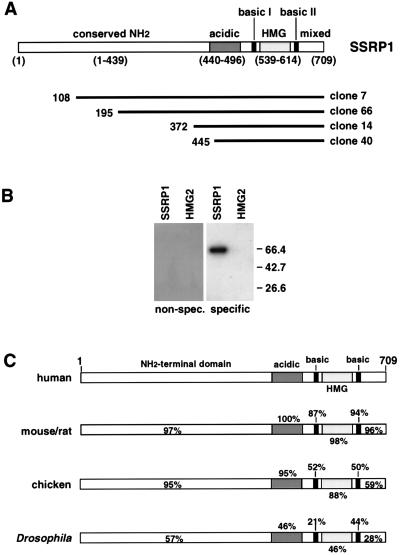
FIG. 1.
cDNA expression cloning of SSRP1 and HMG-2. (A) Schematic diagrams of four independently isolated SSRP1 cDNA clones. Numbers in parentheses below the diagram of SSRP1 indicate amino acid positions of protein domains within the translated sequence. The amino-terminal 70% of the protein (containing an acidic region) is highly conserved, but its function is unknown (see below). The HMG domain is the DNA binding domain. It is flanked by two short basic regions (22 and 17 amino acids, respectively) that may affect the specificity and/or stability of DNA binding. A region of mixed charge comprises the C-terminal portion of the protein; its function is unknown. Below the diagram of the SSRP1 protein and its domains, the four SSRP1 cDNA clones isolated by expression cloning are represented by solid lines of different lengths. The amino acid position corresponding to the 5′ end of the cDNA is shown to the left of each solid line. (B) Southwestern blot with HMG-2 and SSRP1, using a monomeric PRE II probe. Bacterially expressed HMG-2 (from clone 10 [Materials and Methods]) and SSRP1 from clone 66 (C) were purified by using Ni2+-NTA agarose. Recombinant proteins were then separated by SDS-PAGE and transferred to nitrocellulose for Southwestern blot analysis. The specific probe in this experiment is a monomeric PRE II binding site, and the nonspecific probe (non-spec.) is a monomeric octamer binding site. The expected size for SSRP1 is ∼66 kDa, and that for HMG-2 is ∼28 kDa. Equal amounts of protein were loaded as determined by Ponceau S staining of the filter prior to hybridization. Sizes are indicated in kilodaltons at the right. (C) Structures and sequence conservation of SSRP1 proteins from several species. Schematic representations of SSRP1 proteins from different species (see text) are shown. The DNA binding domain (HMG domain) is largely conserved across species. In general, the large amino-terminal region, which comprises more than two-thirds of the protein and contains an acidic domain, is even more highly conserved than the HMG domain. The sequence of this region does not resemble those of other known protein domains, and its function remains unknown. SSRP1 cDNA clones isolated from two plant species that exhibited ∼40% overall sequence identity with human SSRP1 were not included in this figure.
Human SSRP1 was originally cloned by expression screening with a cisplatinated DNA probe in a search for proteins that interact with DNA lesions induced by this chemotherapeutic agent (9). Orthologs of human SSRP1 have been cloned from metazoan species ranging from plants to both invertebrates and vertebrates (see, for example, references 8, 25, 26, 40, 56, and 59). Drosophila and chicken SSRP1 were cloned by homology to SSRP1 genes from other species (8, 56), while the murine, rat, and Arabidopsis SSRP1 genes were cloned by screening expression libraries with specific DNA sequences (40, 56, 59). In part because of the large sizes of these putative SSRP1 binding sites, we cannot yet establish a consensus sequence for DNA binding (see Discussion and reference 15).
A schematic species comparison is shown for SSRP1 from four species in Fig. 1C and highlights several important features of the protein. As expected, the DNA binding domain (HMG domain) is evolutionarily conserved across several species. One of the most striking features of the alignment is the sequence conservation within the amino-terminal domain: this region, which encompasses the first 60% of the protein (amino acids 1 to 439), is even more highly conserved than the HMG domain (8). Such a high degree of conservation suggests that the amino-terminal portion of SSRP1 is required for its function in vivo. This region of the protein bears no obvious resemblance to other well-characterized protein domains, and its function is at present unknown. Just carboxy terminal to the extended amino-terminal region is a well-conserved acidic region (amino acids 440 to 496, discussed below). Finally, the HMG domain is flanked by two short basic regions that may contribute to DNA binding, as demonstrated for the single basic region found in LEF-1 (30).
SSRP1 and PREIIBF bind DNA with identical sequence specificity.
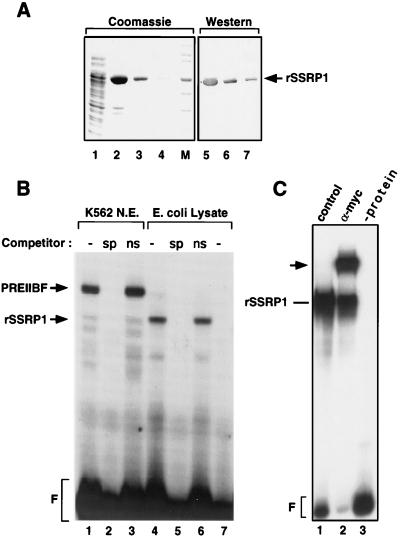
To determine whether the DNA binding characteristics of SSRP1 are identical to those of PREIIBF, bacterially expressed SSRP1 was purified and characterized. Each of the amino-truncated SSRP1 cDNAs (Fig. 1A), as well as a full-length cDNA, was ligated into pRSETNotI(myc) (see Materials and Methods). Recombinant proteins expressed from these constructs contain a carboxy-terminal His6 tag and an amino-terminal Myc epitope tag for immunological detection. All of the SSRP1 cDNA constructs expressed proteins of the expected size, as determined by immunoblotting with the anti-Myc monoclonal antibody 9E10 (Fig. 2A and data not shown) (17). However, only one of the cDNAs (clone 66 [Fig. 1A]) was expressed at high levels (Fig. 2A) and was not toxic to the bacteria. This protein was used for all subsequent PRE II binding studies and will hereafter be referred to as rSSRP1.
FIG. 2.
rSSRP1 binds specifically to PRE II. (A) Purification of a truncated (66-kDa) form of SSRP1 from an E. coli lysate. A 5′ truncated cDNA fragment was cloned into the vector pRSET(myc)NotI, which contains both a His6 tag and a Myc epitope tag (Fig. 1B). The lysate from an E. coli strain carrying this expression vector was incubated with Ni2+-NTA agarose beads, and rSSRP1 was eluted with 500 mM imidazole. SSRP1-depleted crude lysate is shown in lane 1; three successive eluates are shown in lanes 2, 3, and 4. To confirm that the 66-kDa protein purified from the E. coli lysate is rSSRP1, the same fractions were analyzed on a Western blot hybridized with the anti-Myc monoclonal antibody 9E10. The sizes of the molecular weight markers (M) are 158, 116, 97.2, 66.4, 55.6, 42.7, and 36.5 kDa. (B) rSSRP1 binds specifically to PRE II. Purified rSSRP1 was incubated with a radiolabeled PRE II binding site and analyzed by EMSA. A protein-DNA complex was observed (lane 4) that is not present when a control E. coli lysate was incubated with the same probe (lane 7). The protein-DNA complex observed in lane 4 is competed by a 20-fold excess of cold specific competitor (sp; lane 5) but not by a nonspecific competitor (ns; lane 6). Partially purified PREIIBF from K562 nuclear extracts (N. E.) was used in lanes 1 to 3. F, free probe. (C) Anti-Myc antibody 9E10 recognizes the rSSRP1-PRE II complex. Monoclonal antibody 9E10 and a control serum were incubated with rSSRP1 and a radiolabeled PRE II oligonucleotide probe. The supershifted band observed for antibody 9E10 (arrow) confirms that the protein-DNA complex contains Myc-tagged rSSRP1 bound to PRE II.
Binding reaction mixtures containing rSSRP1 and a radiolabeled PRE II binding site oligonucleotide were subjected to EMSAs. A protein-DNA complex was readily observed when purified rSSRP1 was incubated with the PRE II oligonucleotide probe (Fig. 2B, lane 4). PRE II binding activity was not detected with purified lysate from an E. coli strain carrying the expression vector alone (Fig. 2B, lane 7). Specificity of binding was established by adding an excess of cold oligonucleotide competitor corresponding to specific (PRE II) or nonspecific (octamer) sequences. As shown in Fig. 2B (lanes 5 and 6), only the specific competitor disrupted the observed protein-DNA complex. For comparison, analogous competition binding reactions for partially purified PREIIBF K562 nuclear extracts are displayed in lanes 2 and 3 of Fig. 2B. The mobility of the complex formed by protein from the bacterial lysate (containing rSSRP1) is consistent with the absence of 196 amino acids from its amino terminus (Fig. 2B; compare lanes 1 and 3 with 4 and 6).
As additional confirmation that the protein-DNA complex observed in Fig. 2B contains rSSRP1, anti-Myc monoclonal antibody 9E10 (17) was added to the binding reaction. This was expected to result in the formation of a ternary complex containing rSSRP1, antibody 9E10, and the PRE II binding site. In EMSAs, such ternary complexes are detected as additional supershifted bands with much lower electrophoretic mobilities. Antibody 9E10 (Fig. 2C, lane 2), but not a control hybridoma cell culture supernatant (lane 1) or other irrelevant antisera (not shown), gave rise to a supershifted complex when incubated with rSSRP1 and PRE II. Recombinant SSRP1 protein lacking the Myc epitope was not supershifted by antibody 9E10 (data not shown). Taken together, the oligonucleotide competition experiments and the antibody supershift experiments demonstrate that rSSRP1 can bind to PRE II in a sequence-specific manner.
To extend these observations, we determined the Kds for binding by rSSRP1 to wild-type and mutated versions of PRE II (Table 1) as described previously for PREIIBF (15). In addition, oligonucleotides containing unrelated protein binding motifs were examined. This analysis shows that rSSRP1 binds to the wild-type PRE II binding site with nearly the same affinity (2.3 nM [Table 1]) as PREIIBF (14.6 nM [15]). A subset of point mutations previously tested for the ability to interfere with PREIIBF binding (15) was tested for binding by rSSRP1. As shown in Table 1, mutations (−422, −425, and a cluster of six point mutations) that disrupted binding by PREIIBF (15, 51) also disrupted binding by rSSRP1. Mutations (−435 and −433) that had less effect on DNA binding by PREIIBF also had a more modest effect on DNA binding by rSSRP1 (Table 1). Unrelated DNA sequences that correspond to another protein binding site (GATA) or an irrelevant sequence (MAD 7,8 [50]) exhibited very little protein binding activity. The affinity of rSSRP1 for PRE II is ∼200-fold greater than its affinity for these unrelated DNA sequences (Table 1), indicating that the protein binds to PRE II in a DNA sequence-specific manner. Especially noteworthy is the finding that binding to the mouse V-(D)-J RSS is ∼14-fold lower than to PRE II, because the murine homolog of SSRP1 was cloned from an expression library by using an RSS probe (40).
TABLE 1.
DNA binding affinities of rSSRP1
| Oligonucleotide | Kd (nM)e | DNA binding specificityf | Binding site sequenceg |
|---|---|---|---|
| Wild type | 2.3 | 1.0 | GATTCTGTTTTAACAGCTTT |
| −422 mutation | >240 | <0.010 | GATTCTGTTTTAACAGATTT |
| −425 mutation | >100 | <0.023 | GATTCTGTTTTAAAAGCTTT |
| Clustered mutationsa | >140 | <0.016 | CATTATCTTTAAAGAACTTT |
| −435 mutation | 12.4 | 0.185 | GATGCTGTTTTAACAGCTTT |
| −433 mutation | 38 | 0.061 | GATTCGGTTTTAACAGCTTT |
| V-(D)-J signalb | 32 | 0.072 | GATCCCACAGTGCTCCAGGGCTGAACAAAAACCGAATT |
| GATAc | >500 | <0.005 | GGGCAACTGATAAGGATTCC |
| MAD 7,8d | >500 | <0.005 | GAAACTAAGGTACAGAAGTT |
This oligonucleotide contains a cluster of six point mutations within PRE II (51).
T160, the murine ortholog of SSRP1, was isolated from a cDNA expression library by using an oligonucleotide containing a murine V-(D)-J RSS (40).
GATA binding site of the mouse α1-globin promoter (52).
An irrelevant DNA sequence upstream of the PRE II DNA recognition element of the ɛ-globin gene (50).
Individual point mutations were characterized previously for PREIIBF binding (15). A subset of these was used to calculate Kds for rSSRP1.
Defined as the ratio of the wild-type Kd to test sequence Kd.
Mutated nucleotides are underlined.
Like other known HMG domain protein binding sites, the SSRP1/PREIIBF recognition sequence is AT rich (Table 1). Neither the V-(D)-J RSS (Table 1) nor other sequences to which SSRP1 is alleged to bind (56, 59) are notably AT rich, but the significance of SSRP1 binding to these sequences has not been established. Whether an AT-rich sequence composition is a critical feature of SSRP1 binding sites is therefore not clear.
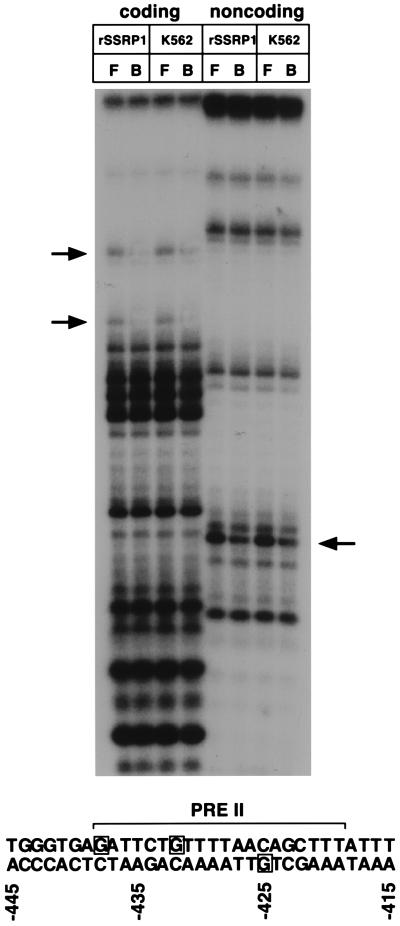
rSSRP1 and PREIIBF make identical guanosine contacts within PRE II.
Previous methylation interference experiments identified three essential guanosine residues within PRE II that are contacted by PREIIBF (−438, −432, and −425) (50). A similar experiment was performed for rSSRP1 (Fig. 3; essential guanosine contacts are indicated in the lower panel). For comparison, the methylation interference pattern for PREIIBF biochemically purified from K562 cells (Fig. 3, K562) is shown next to that of rSSRP1. This experiment demonstrates that rSSRP1 and PREIIBF make identical guanosine contacts with PRE II. These methylation interference data further establish that rSSRP1 binding to PRE II is sequence specific and indistinguishable from that of PREIIBF.
FIG. 3.
PREIIBF and rSSRP1 make identical guanosine contacts within PRE II. rSSRP1 and purified fractions of PREIIBF from K562 cell nuclear extracts were incubated with a methylated PRE II oligonucleotide probe. Following EMSA, the free (F) and bound (B) probes were isolated and cleaved with piperidine. Cleavage products were separated on a 15% denaturing gel. Arrows indicate guanosine residues that are underrepresented in bound fractions in assays using both rSSRP1 and purified fractions of PREIIBF. A summary of these results is presented at the bottom. The minimal PRE II protein binding site is denoted by a horizontal bracket. Essential guanosine contacts are boxed.
Anti-SSRP1 peptide antibodies recognize PREIIBF.
To more directly demonstrate that PREIIBF is encoded by the SSRP1 cDNA, antibodies were raised against peptides corresponding to the amino (NH2)- and carboxy (COOH)-terminal regions of SSRP1 (hereafter referred to as anti-SSRP1NH2 and anti-SSRP1COOH). Sera raised against each peptide were tested for the ability to recognize full-length rSSRP1 in immunoblotting experiments. Although all antisera interacted strongly with the synthetic peptides used as immunogen (by enzyme-linked immunosorbent assay [ELISA]), only the anti-SSRP1NH2 antisera were found to recognize full-length rSSRP1 by a variety of methods (data not shown). Affinity-purified anti-SSRP1NH2 antibodies were used for subsequent experiments.
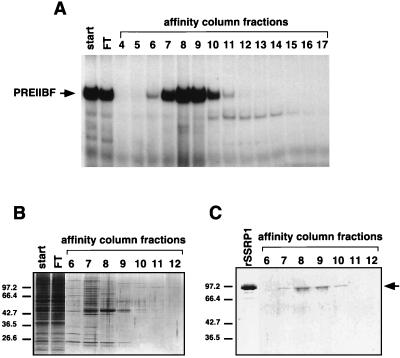
We had previously developed a purification protocol for isolating PREIIBF from human embryonic erythroid cells (K562). This protocol relies largely on ion-exchange and site-specific DNA affinity chromatography (15). To determine whether rSSRP1 copurifies with PREIIBF, fractions of affinity-purified PREIIBF (Fig. 4A and B) were tested by immunoblot analysis with the anti-SSRP1NH2 antibodies. A single polypeptide of ∼90 to 95 kDa (Fig. 4C) was detected in the fractions that contained the majority of the PREIIBF activity (Fig. 4A, fractions 7 to 10). A second round of site-specific DNA affinity chromatography resulted in even greater purification of PREIIBF (>20,000-fold) (15). An ∼90- to 95-kDa polypeptide coeluted with the majority of PREIIBF activity (data not shown). Based on its elution profile, size, and the previous immunoblotting experiments with first-round DNA affinity chromatography fractions, we conclude that this polypeptide corresponds to SSRP1.
FIG. 4.
Antibodies against SSRP1 recognize a protein of ∼95 kDa in affinity-purified fractions of PREIIBF. (A) EMSA analysis of PREIIBF fractions from DNA affinity column. Purification of PREIIBF was carried out as described previously (15). First-round site-specific DNA affinity chromatography fractions were incubated with a radiolabeled PRE II probe and analyzed by EMSA. Most of the PREIIBF activity eluted between 375 and 525 mM KCl (fractions 7 to 10). (B) Silver-stained SDS-gel after electrophoretic separation of proteins from DNA affinity column fractions of PREIIBF. A portion (20 μl) of each DNA affinity chromatography fraction (1 ml) assayed by EMSA (A) was electrophoresed through an SDS–10% polyacrylamide gel and then stained with silver nitrate (33). Fractions 7 through 10, containing most of the PREIIBF activity, contain several polypeptides. (C) Anti-SSRP1 antibody recognizes a 95-kDa polypeptide in purified fractions of PREIIBF. Portions (0.5 ml) of each 1.0-ml fraction of purified PREIIBF were concentrated by Centricon-30 (Amicon) centrifugation and were then separated by SDS-PAGE and transferred to nitrocellulose. Immunoblot analysis was performed with the purified anti-SSRP1NH2 antibodies. A 95-kDa polypeptide coeluted with PREIIBF activity (fractions 7 through 10) and comigrated with full-length rSSRP1 (arrow).
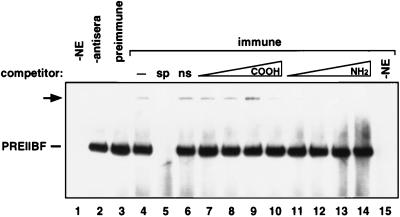
The anti-SSRP1 antibodies were next used to examine the immunological similarity between PREIIBF and SSRP1. Partially purified PREIIBF from embryonic erythroid (K562) cells was incubated with the anti-SSRP1NH2 antibodies and a radiolabeled PRE II oligonucleotide probe. Binding reactions were then analyzed by nondenaturing gel electrophoresis (Fig. 5). Although the antibody did not block complex formation between PREIIBF and PRE II, it did recognize PREIIBF. Formation of a ternary complex between the antibody, PREIIBF, and PRE II was detected as a supershifted band of lower mobility than the characteristic binary complex formed between PREIIBF and PRE II (arrow, Fig. 5, lane 4). This complex was not observed when crude or purified preimmune serum was added to the binding reaction (Fig. 5, lane 3, and data not shown). Purified anti-SSRP1NH2 antibody fractions had no effect on the protein-DNA complex formed between an unrelated DNA binding protein (Oct-1) and its cognate binding motif (data not shown). An oligonucleotide competitor that disrupted the binary complex between PREIIBF and PRE II also disrupted the supershifted complex (Fig. 5, lane 5), while a nonspecific oligonucleotide competitor had no effect on either complex (lane 6). To test the specificity of the antibody-PREIIBF interaction, peptide competitors were added to the binding reactions. Increasing amounts of the NH2-SSRP1 peptide specifically disrupted the ternary complex (Fig. 5, lanes 11 to 14), while identical molar amounts of the COOH-SSRP1 peptide had very little effect on ternary complex formation (lanes 7 to 10).
FIG. 5.
Specificity of interaction of anti-SSRP1NH2 peptide antibody with PREIIBF. Portions of DEAE-purified PREIIBF (15) were incubated with a radiolabeled PRE II probe and affinity-purified anti-SSRP1NH2 antibody. EMSA analysis revealed a supershifted complex (arrow), most likely representing a ternary complex between PRE II, PREIIBF, and anti-SSRP1NH2 antibody. To confirm the specificity of interaction between the antipeptide antibody and PREIIBF, a competition analysis was performed with the NH2-terminal SSRP1 peptide used to immunize rabbits and with an irrelevant COOH-terminal peptide (this peptide elicited an antigenic response by ELISA, but the antiserum did not recognize PREIIBF or rSSRP1 by any of the methods that we tested). Addition of a 20-fold molar excess of specific (PRE II) competitor oligonucleotide abolished both the PREIIBF-DNA complex and the supershifted complex (lane 5, sp). Increasing amounts (0.005, 0.05, 0.5, and 5 μg; lanes 7 to 10 [COOH peptide] and lanes 11 to 14 [NH2 peptide]) of synthetic peptide were added to these binding reactions (20 μl) to establish antibody specificity. The NH2 peptide competed successfully with PREIIBF for interaction with the anti-SSRP1NH2 antibody in a dose-dependent manner (lanes 11 to 14). Similar amounts of the COOH peptide did not compete for binding and had no effect on the supershifted complex (lanes 7 to 10). No supershifted complexes were observed in the absence of antiserum (lane 2) or when preimmune serum was added to the binding reaction (lane 3). −NE (lanes 1 and 15), no nuclear extract added to binding reaction.
In summary, purified anti-SSRP1NH2 antibodies can specifically bind to a ∼95-kDa protein that comigrates with full-length rSSRP1 in SDS-polyacrylamide gels and copurifies with PREIIBF activity from K562 nuclear extracts. Moreover, these antibodies specifically interact with the protein-DNA complex formed between purified fractions of PREIIBF and PRE II. These experiments provide direct evidence that PREIIBF is encoded by the SSRP1 cDNA.
DNA binding and transcriptional activation by PREIIBF/SSRP1 in a chromosomal context.
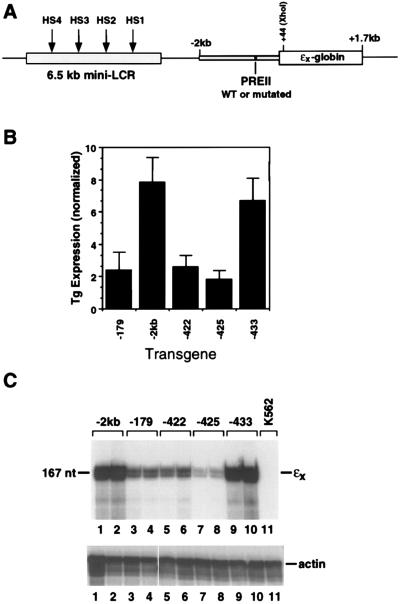
We had previously shown, using artificial constructs containing PRE II and PRE V linked to a minimal ɛ-globin gene promoter, that point mutations that disrupt PREIIBF binding also abolish promoter activation in transiently transfected K562 cells. With the demonstration of DNA bending by PREIIBF (15) and the identification of PREIIBF as an HMG domain protein, it became important to determine whether the same mutations would abolish promoter activation within a chromosomal context and within the context of the intact upstream regulatory region extending to −2 kb (Fig. 6A). We therefore generated pools of stably transformed K562 cells. Individual point mutations within PRE II were generated by a PCR strategy. Linearized reporter constructs carrying PRE II point mutations were electroporated into K562 cells together with a construct (pSV2neo) that confers G418 resistance. Multiple independent pools of G418-resistant cells were obtained for each ɛ-globin reporter construct. Chromosomal DNA was isolated, and Southern blot analysis was performed to determine the relative copy number (ɛ-globin reporter/endogenous ɛ-globin gene) of the reporter construct. To determine the effects of individual PRE II point mutations on ɛ-globin reporter gene expression, RNase protection analyses were performed. Actin was used as an internal control for variations in RNA levels. Expression levels corrected for transgene copy number and normalized to actin RNA are presented as a composite in Fig. 6B; a representative RNase protection experiment for RNAs from two pools per construct is shown in Fig. 6C. Single point mutations (−422 and −425) within PRE II that disrupted DNA binding by PREIIBF/rSSRP1 (Table 1) also reduced ɛ-globin reporter gene expression. Moreover, a PRE II mutation (−433) that had a more modest effect on DNA binding had little effect on ɛ-globin reporter gene expression. This analysis demonstrates that in a chromosomal context in which the DNA binding element (PRE II) is present in its natural position within the upstream regulatory region, binding by PREIIBF/SSRP1 is required for activation of the minimal ɛ-globin gene promoter.
FIG. 6.
Point mutations that disrupt PREIIBF binding in vitro also abolish expression of an ɛ-globin reporter gene in stably transfected cell lines. (A) ɛx-globin reporter gene construct for analysis of PRE II point mutations. The point mutations shown in Table 1 were introduced into an EcoRI fragment containing the entire human ɛ-globin gene extending from −2 to +1746 kb. The ɛ-globin gene in this construct is marked by insertion of an XhoI site at +44 and is therefore termed ɛx. A 6.5-kb β-LCR fragment was included to confer high-level, position-independent and copy number-dependent expression (47) of the linked globin gene. WT, wild type. (B) Analysis of PRE II point mutations in stably transfected K562 cells. For each transgene analyzed, three to five independent pools of stably transfected K562 cells were expanded. Total cellular RNA was prepared and subjected to RNase protection, and the results were quantitated on a phosphorimager. Transgene (Tg) RNA levels were normalized for actin RNA and T transgene copy number (estimated by Southern blotting). Pools of cells carrying the minimal promoter (−179) construct were included as a negative control. Point mutations (−422 and −425) that disrupted protein binding to PRE II abolished activation of the minimal promoter in the context of the intact upstream regulatory region (“−2kb” indicates unmutated upstream region containing minimal promoter and sequences extending to −2 kb). A point mutation (−433) that had no effect on protein binding had no significant effect on promoter activation. Error bars represent the standard deviation for three to five independent pools of stably transfected K562 cells. (C) Representative RNase protection analysis of RNA from pools of K562 cells carrying the ɛ-globin reporter constructs. An antisense riboprobe that hybridizes specifically to the XhoI-marked ɛx-globin transgene was used for quantitative RNA analysis of the stable K562 cell pools. This probe distinguishes between the marked transgene (167 nucleotides [nt]) and unmarked endogenous ɛ-globin RNA, which yields a smaller protected fragment (123 nt). The unprotected probe is 441 nt. RNA from two independent pools of stably transfected K562 cells is shown for each construct. Samples were normalized for actin (lower panel) to facilitate direct comparison among PRE II mutations. Mutations (−422 and −425) that disrupted PREIIBF/SSRP1 binding also disrupted ɛ-globin gene activation. A mutation (−433) within PRE II that did not affect protein binding had no effect on transgene expression. Thus, point mutations that disrupt PREIIBF/SSRP1 binding in vitro also abolish activation of the ɛ-globin promoter in the context of the intact upstream regulatory region. RNA from untransfected K562 cells was analyzed in lane 11 (K562).
PREIIBF/SSRP1 does not contain a classical transcriptional activation domain.
The finding that binding of PREIIBF/SSRP1 to PRE II is required for transcriptional activation of a linked minimal promoter in the context of the full upstream region to −2 kb (this work) or in the context of smaller upstream regions (50, 51), together with the discovery that binding by PREIIBF introduces a bend into its target DNA and that the protein is a member of the HMG domain family, suggests that PREIIBF/SSRP1 functions as an architectural transcription factor. It was therefore of interest to determine whether the protein contains a classical activation domain—that is, whether it can activate transcription on its own or whether it requires interactions with other proteins.
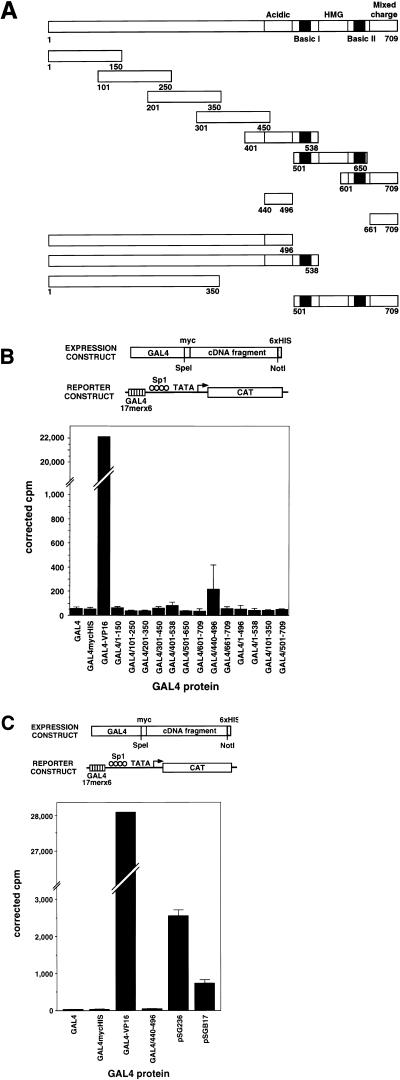
We generated a series of fusion genes encoding PREIIBF/SSRP1 polypeptides of different lengths and GAL4(1–147) (31) and examined their ability to activate a CAT reporter gene containing six GAL4 DNA binding sites upstream from a minimal promoter (43) when cotransfected into CHO cells. The regions of PREIIBF/SSRP1 tested for the presence of an activation domain (Fig. 7A) encoded a set of partially overlapping 150-amino-acid peptides spanning the entire protein; the acid region and the C-terminal region of mixed charge; the amino-terminal 70% of the protein (including the acidic region); the amino-terminal portion of the protein extending up to but not including the HMG domain; the amino-terminal 50% of the protein; and the C-terminal region of the protein containing the HMG domain flanked by both basic regions and followed by the region of mixed charge. As shown in Fig. 7B, none of the GAL4-PREIIBF/SSRP1 fusion genes showed significant activation of the target reporter construct, by comparison with the strong VP16-GAL4 activator. To determine whether the acidic region might function as a weak activator whose activity could be masked by comparison with the very potent activator VP16, we carried out a second set of experiments using two known weak activator expression constructs as our basis for comparison: pSGB17 (encoding a portion of the E. coli genome fused to the GAL4 DNA binding domain [31]) and pSG236 [encoding GAL4(768–881) fused to the GAL4 DNA binding domain]. Even by comparison with these weak activators, the GAL4-PREIIBF/SSRP1 acidic region chimera failed to activate the reporter gene (Fig. 7C), although the proteins were expressed in these cells (data not shown). Similar results were obtained for COS cells, using expression vectors containing simian virus 40 sequences to permit extrachromosomal replication (23a).
FIG. 7.
PREIIBF/SSRP1 does not contain a classical transcriptional activation domain. (A) Expression and target constructs. A set of partially overlapping fragments of PREIIBF/SSRP1, each encoding a 150-amino-acid peptide, was generated to span the entire protein. These fragments were subcloned into a version of pBXG1 modified to express a protein with the general structure GAL(1–147)-Myc-PREIIBF/SSRP1 fragment-His6. Additional expression constructs contained the acidic region and the C-terminal region of mixed charge, the amino-terminal 70% of the protein (including the acid region), the amino-terminal portion of the protein extending up to but not including the HMG domain, the amino-terminal 50% of the protein, and the C-terminal region of the protein containing the HMG domain flanked by both basic regions and followed by the region of mixed charge. The CAT reporter gene G6(SP1)(−31)HIVLTRΔTAR contained six GAL4 binding sites linked to a modified human immunodeficiency virus long terminal repeat (43). (B) By comparison with the potent activator VP16, SSRP1 polypeptides of various lengths fail to activate a reporter construct in transfected CHO cells. A mixture of DNA containing 1 μg of activator expression plasmid (A), 1 μg of the CAT reporter plasmid G6(SP1)(−31)HIVLTRΔTAR, and 0.5 μg of pCMVβgal (which served as a transfection control) was used to transfect CHO cells as described in Materials and Methods. Cell lysates were assayed for CAT and β-galactosidase activity as described previously (50). Values plotted on the vertical axis represent CAT activity corrected for β-galactosidase expression from the internal control. GAL4 indicates the DNA binding domain, GAL4(1–147). Chimeric proteins are named based on the amino acid residues from SSRP1 fused to GAL4(1–147); for example, GAL4/1–150 contains amino acids 1 to 150 of PREIIBF/SSRP1. Each activator construct was tested in duplicate; error bars represent standard deviations. (C) The acidic region of SSRP1 does not activate a reporter construct, even by comparison with two weak activators. CHO cells were transfected as for panel B with an expression construct carrying the acidic region (amino acids 440 to 496) of SSRP1 fused to the GAL4 DNA binding domain, the CAT reporter construct, and the internal control plasmid pCMVβgal. The constructs pSGB17 and pSG236 encode weak activators (see Material and Methods). Expression constructs encoding the GAL4 DNA binding domain alone or GAL4-mycHIS were used as negative controls. The GAL4-VP16 chimera pBXGalVP served as a strong activator. Even by comparison with two weak activators, the acidic region of PREIIBF/SSRP1 failed to activate the reporter construct. Transfections were carried out in triplicate; error bars represent standard deviations.
DISCUSSION
The transcriptional regulator PREIIBF is the HMG domain protein SSRP1.
We report here the cDNA expression cloning of the transcriptional regulator PREIIBF from a human erythroid cell line. Based on a number of biochemical and immunological criteria, we conclude that SSRP1, the HMG domain protein encoded by four independently isolated cDNAs, is identical to the biochemically characterized PREIIBF. First, the sizes of SSRP1 predicted from the cDNA sequence (this work and reference 9) and observed for the recombinant protein expressed in vitro (this work) are in agreement with size estimates for PREIIBF obtained by a variety of biochemical methods (15, 51). Second, rSSRP1 binds to PRE II with DNA sequence specificity identical to that of the biochemically characterized PREIIBF. In fact, we were unable to detect any significant difference in PRE II binding between the two proteins. Third, affinity-purified anti-SSRP1NH2 peptide antibodies cross-reacted with a protein that comigrates with full-length rSSRP1 and coelutes exactly with PREIIBF activity from a PRE II DNA affinity column. Furthermore, a specific ternary complex was formed between a purified anti-SSRP1 peptide antibody, PREIIBF, and PRE II, as measured in EMSAs. These immunological studies, combined with the biochemical studies and PRE II binding experiments, strongly suggest that PREIIBF is encoded by the SSRP1 cDNA.
The identification of PREIIBF as the HMG domain protein SSRP1 is of interest for a number of reasons. SSRP1 is phylogenetically conserved in species from plants to flies to humans (Fig. 1C and reference 14). It is encoded by a single-copy gene (23a) belonging to a family of genes that play roles in a wide variety of developmental processes, from mesoderm specification in worms (29) to pattern formation in flies (10) to organogenesis in mammals (11, 27, 35, 38, 53, 61). The present studies show for the first time that SSRP1 is capable of binding to DNA in a sequence-specific manner and point to a role for this protein in gene regulation. In addition, our studies on PREIIBF/SSRP1 provide the first evidence for a functional association between DNA binding by an HMG domain protein and regulation of globin gene expression.
Structural, biochemical, and functional properties of SSRP1/PREIIBF place it within a distinct subfamily of HMG domain proteins.
Proteins that contain an ∼80-amino-acid HMG DNA binding domain can be subdivided into two groups: (i) sequence-tolerant HMG domain proteins, which contain multiple HMG domains and bind DNA nonspecifically; and (ii) sequence-specific HMG domain proteins, which contain a single HMG domain and bind specific recognition motifs (reviewed in references 14, 22, and 28). The differences between these two classes of HMG domain proteins are believed to reflect differences in their functions in vivo. Both groups of HMG domain proteins can, however, recognize a variety of DNA distortions (discussed in references 14, 22, and 28). This shared affinity for distorted DNA is believed to reflect common structural features within the HMG domain, as demonstrated by the “boomerang” α-helical tertiary structure observed for these proteins by nuclear magnetic resonance spectroscopy (reviewed in reference 58). Therefore, the original cloning of SSRP1 by expression library screening with a distorted, cisplatin-modified DNA probe (9) does not in itself suggest a unique property for SSRP1. Moreover, the DNA of normal cells is not naturally cisplatinated, leaving unresolved the function of SSRP1 in vivo (see below).
Experiments presented here demonstrate that like other HMG domain proteins containing a single DNA binding domain, such as the lymphoid enhancer-binding factors LEF-1 and TCF-1α (49, 57) and the testis-determining factor Sry and related Sox genes (11, 23, 41), SSRP1/PREIIBF recognizes DNA in a sequence-specific manner. Furthermore, the correlation between SSRP1/PREIIBF binding and ɛ-globin reporter gene expression in stable cell lines provides evidence that this HMG domain protein participates in gene regulation in vivo.
A classification of 121 HMG domains based on structural alignments (6) has placed the SSRP1 proteins from different species in their own subgroup, clustered away from both the DNA sequence-tolerant HMG domain proteins such as HMG-1 and HMG-2 and the sequence-specific proteins such as LEF-1/TCF-1α and Sry. We have shown that an important difference between SSRP1/PREIIBF and HMG-1/-2 lies in the DNA sequence-specific binding of SSRP1/PREIIBF and the requirement for its binding to PRE II for ɛ-globin promoter activation. SSRP1/PREIIBF has four features in common with HMG domain proteins such as LEF-1/TCF-1α and Sry: it contains a single HMG domain; it binds DNA in a sequence-specific manner; its binding is required for transcriptional activation; and it does not contain a classical activation domain (see below). However, the sequence of its HMG domain more closely resembles those of HMG-1/-2, and based on structural alignments (6), its HMG domain fits most naturally into a subfamily containing SSRP1-related proteins. Our functional studies strongly support the placement of SSRP1/PREIIBF into a distinct subfamily (6) of HMG domain proteins.
Potential role for SSRP1/PREIIBF in other processes.
Our studies may provide the first clear indication of a normal function for SSRP1 in vivo. SSRP1 homologs have been isolated from other species on the basis of binding to DNA. However, such binding has not rigorously been shown to depend on DNA sequence, and indeed, others have concluded that DNA binding by SSRP1 is not sequence specific (9, 18). No functional analyses which link DNA binding by SSRP1 to another naturally occurring biological activity have been published previously.
The isolation of other SSRP1 homologs on the basis of binding to sequences within the rat collagen II promoter (56) and the promoters of two Arabidopsis desiccation response genes (59) provide circumstantial evidence in support of our argument for a role for SSRP1 in the regulation of gene expression. However, such a function has not been established for these other promoters, and there are no striking sequence similarities between any of these binding sites and PRE II of the human ɛ-globin upstream region. More detailed analyses of these promoters and the identification of other putative targets of SSRP1/PREIIBF regulation may help to clarify this question. We have not formally ruled out the possibility that SSRP1/PREIIBF recognizes a DNA structure that is disrupted or altered by introduction of the point mutations shown in Table 1 or in reference 15, though we consider this interpretation of our data unlikely.
The weak binding of SSRP1/PREIIBF to the Ig gene V-(D)-J RSS is noteworthy, because T160, the murine ortholog of SSRP1, was cloned by screening of a cDNA expression library with an RSS probe (40). Although Southwestern blotting was used to provide evidence for sequence-specific DNA binding (40), protein binding to the RSS could not be detected by EMSA or DNase I footprinting (18, 40), and more recently it has been concluded that T160 does not bind DNA with sequence specificity (18). No direct evidence for a relationship between DNA binding and recombination activity has been demonstrated for this protein. It is clear from the data in Table 1 that on its own, rSSRP1 binds more strongly (∼14-fold) to the PRE II element of the ɛ-globin gene than to the Ig gene recombinant element. However, this does not necessarily rule out a role for SSRP1 in V-(D)-J recombination. Recently it has been shown that the related HMG domain proteins HMG-1 and HMG-2 stimulate RAG protein-mediated V-(D)-J recombination in vitro (1, 54). Our observations raise the possibility (discussed below) that the functions of SSRP1 in vivo require interactions with other proteins. SSRP1/T160 mRNA is expressed in embryonic erythroid cells (23a) as well as in spleen and other tissues (9, 23a, 40), consistent with roles both in embryonic globin gene activation in primitive erythroblasts and in V-(D)-J recombination during B-cell development. More detailed analysis of sectioned embryonic and adult tissues may provide insights into other potential functions for this protein.
SSRP1/PREIIBF may play an architectural role in regulating ɛ-globin gene expression.
We previously identified multiple regulatory elements spanning several hundred base pairs upstream of the human ɛ-globin gene and demonstrated synergistic interactions among three of these elements, PRE II and PRE V or PRE I (50, 51). With the identification and characterization of a PRE II binding factor that introduces a minor groove-directed bend, we proposed that the mechanism of synergistic interactions between PRE II and PRE V (or PRE I) may involve looping out of the intervening DNA (15). Identification of the cDNA that encodes PREIIBF is in accord with this possibility, as SSRP1 belongs to the HMG domain family of proteins. Other members of this family are believed to regulate gene expression by coordinating the assembly of multiprotein complexes (reviewed in references 20, 22, and 30). Moreover, the HMG domains of HMG1 have been shown by electron microscopy to mediate DNA looping (46).
We have shown here, by criteria widely accepted for the delineation of transcriptional activation domains (Fig. 7), that SSRP1/PREIIBF lacks a classical activation domain. Therefore, at least in the context of nonerythroid cells, SSRP1/PREIIBF does not function as a classical activator and does not contain an activation domain that can function on its own. In earlier work, we found that activation by the combination PRE II plus PRE V was dependent on the orientation of the juxtaposed elements with respect to the promoter (50) and possibly on the spacing between them (49a, 50). Promoter activation could not occur directly from a single or multimerized PRE II binding sites but required additional control elements (50). These properties are strongly reminiscent of the context-dependent activation properties of the related proteins LEF-1 and TCF-1α (12, 19, 49, 57). More detailed analysis of the function of SSRP1/PREIIBF protein regions outside the HMG domain, in an experimental setting where the target ɛ-globin gene is chromosomally integrated, may reveal that SSRP1/PREIIBF also contains a context-dependent activation domain (12, 19). Perhaps, like LEF-1 (39), SSRP1/PREIIBF facilitates nucleosomal derepression.
A number of HMG domain proteins have been shown to interact functionally with other transcription factors (20, 60, 62) or (as in the case of LEF-1) with other proteins which act as coactivators (7). It seems likely that SSRP1/PREIIBF functions at least in part through interactions with other proteins, perhaps through recruitment of proteins that contain activation domains (which might function in an erythroid-specific manner) or by promoting higher-order assemblies of proteins (see discussions in references 7 and 39), thereby promoting interactions with the basic transcriptional machinery (34).
In summary, in part through interactions with SSRP1/PREIIBF bound at PRE II, proteins bound at other sites within the upstream regulatory region of the human ɛ-globin gene (50, 51) may form a multiprotein complex to activate ɛ-globin transcription in embryonic erythroid cells. Disruption or inactivation of this complex (50, 51) may result in downregulation of the ɛ-globin gene at later stages of development.
ACKNOWLEDGMENTS
We are grateful to Humphrey Wattanga for screening the cDNA expression libraries and for assisting with DNA sequencing. We thank Bill Forrester, Bob Kingston, Tom Maniatis, and Ranjan Sen for helpful discussions and Mark Ptashne for DNA constructs. Brian Dynlacht, Bill Forrester, Jun Ma, and Ranjan Sen provided thoughtful comments on the manuscript.
This work was supported by grants to M.H.B. from the National Institutes of Health (RO1 GM42413) and the Lucille P. Markey Charitable Trust (87-24). M.A.D. and P.J.H. were supported in part by NIH predoctoral training grant GM 07598. During the initial stages of this work, M.H.B. was a Lucille P. Markey Scholar in Biomedical Science.
REFERENCES
- 1.Agrawal A, Schatz D G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. doi: 10.1016/s0092-8674(00)80181-6. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1990. [Google Scholar]
- 3.Baron M H. Transcriptional control of globin gene switching during vertebrate development. Biochim Biophys Acta. 1997;1351:51–72. doi: 10.1016/s0167-4781(96)00195-9. [DOI] [PubMed] [Google Scholar]
- 4.Baron M H, Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982;28:395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- 5.Baron M H, Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986;46:591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- 6.Baxevanis A D, Landsman D. The HMG-1 box protein family: classification and functional relationships. Nucleic Acids Res. 1995;23:1604–1613. doi: 10.1093/nar/23.9.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruhn L, Munnerlyn A, Grosschedl R. ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRα enhancer function. Genes Dev. 1997;11:640–653. doi: 10.1101/gad.11.5.640. [DOI] [PubMed] [Google Scholar]
- 8.Bruhn S L, Housman D E, Lippard S J. Isolation and characterization of cDNA clones encoding the Drosophila homolog of the HMG-box SSRP family that recognizes specific DNA structures. Nucleic Acids Res. 1993;21:1643–1646. doi: 10.1093/nar/21.7.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruhn S L, Pil P M, Essigman J M, Housman D E, Lippard S J. Isolation and characterization of human cDNA clones encoding a high mobility group box protein that recognizes structural distortions to DNA caused by binding of the anticancer agent cisplatin. Proc Natl Acad Sci USA. 1992;89:2307–2311. doi: 10.1073/pnas.89.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner R, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 11.Capel B. New bedfellows in the mammalian sex-determination affair. Trends Genet. 1995;11:161–163. doi: 10.1016/S0168-9525(00)89031-9. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson P, Waterman M, Jones K. The hLEF/TCF-1α HMG protein contains a context-dependent transcriptional activation domain that induces the TCRα enhancer in T cells. Genes Dev. 1993;7:2418–2430. doi: 10.1101/gad.7.12a.2418. [DOI] [PubMed] [Google Scholar]
- 13.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 14.Dyer M A. Identification, characterization and expression cloning of a putative regulator of the human embryonic β-globin gene. Ph.D. thesis. Cambridge, Mass: Harvard University; 1997. [Google Scholar]
- 15.Dyer M A, Naidoo R, Hayes P, Larson C J, Verdine G L, Baron M H. A DNA bending protein interacts with an essential upstream regulatory element of the human embryonic β-like globin gene. Mol Cell Biol. 1996;16:829–838. doi: 10.1128/mcb.16.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis L, Clauser E, Morgan D O, Edery M, Roth R A, Rutter W J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- 17.Evan G, Lewis G, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gariglio M, Ying G G, Hertel L, Gaboli M, Clerc R G, Landolfo S. The high-mobility group protein T160 binds to both linear and cruciform DNA and mediates DNA bending as determined by ring closure. Exp Cell Res. 1997;236:472–481. doi: 10.1006/excr.1997.3742. [DOI] [PubMed] [Google Scholar]
- 19.Giese K, Grosschedl R. LEF-1 contains an activation domain that stimulates transcription only in a specific context of factor-binding sites. EMBO J. 1993;12:4667–4676. doi: 10.1002/j.1460-2075.1993.tb06155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giese K, Kingsley C, Kirshner J, Grosschedl R. Assembly and function of a TCRα enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein-protein interactions. Genes Dev. 1995;9:995–1008. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- 21.Grosschedl R. Higher-order nucleoprotein complexes in transcription: analogies with site-specific recombination. Curr Opin Cell Biol. 1995;16:932–942. doi: 10.1016/0955-0674(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 22.Grosschedl R, Giese K, Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 23.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Goodfellow N V P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 23a.Hayes, P. J., and M. H. Baron. Unpublished data.
- 24.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 25.Hotze M, Lurz G, Schroder J. A cDNA encoding a plant homologue to animal HMG box proteins involved in structure-specific recognition of DNA (SSRP family) Gene. 1995;161:295–296. doi: 10.1016/0378-1119(95)00266-9. [DOI] [PubMed] [Google Scholar]
- 26.Hsu T, King D L, LaBonne C, Kafatos F C. A Drosophila single-strand DNA/RNA-binding factor contains a high-mobility-group box and is enriched in the nucleolus. Proc Natl Acad Sci USA. 1993;90:6488–6492. doi: 10.1073/pnas.90.14.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kratochwil K, Dull M, Fariñas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- 28.Landsman D, Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioessays. 1993;15:539–546. doi: 10.1002/bies.950150807. [DOI] [PubMed] [Google Scholar]
- 29.Lin R, Thompson S, Priess J R. pop-1 encodes an HMG box protein required for the specification of a mesoderm precursor in early C. elegans embryos. Cell. 1995;83:599–609. doi: 10.1016/0092-8674(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 30.Love J J, Li X, Case D, Giese K, Grosschedl R, Wright P. Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature. 1995;376:791–795. doi: 10.1038/376791a0. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987;48:847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- 32.Majumdar A, Brown D, Kerby S, Rudzinski I, Polte T, Randhawa Z, Seidman M M. Sequence of human HMG2 cDNA. Nucleic Acids Res. 1991;19:6643. doi: 10.1093/nar/19.23.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaffrey P, Luo C, Kerppola T, Jain J, Badalian T, Ho A, Burgeon E, Lane W, Lambert J, Curran T, Verdine G, Rao A, Hogan P. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 34.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 35.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S-C, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 36.Sadowski I, Ma J, Triezenberg S, Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988;335:563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schilham M, Oosterwegel M, Moerer P, Ya J, de Boer P, van de Wetering M, Verbeek S, Lamers W, Kruisbeek A, Cumano A, Clevers H. Defects in cardiac outflow tract formation and pro-B-lymphocyte expansion in mice lacking Sox-4. Nature. 1996;380:711–714. doi: 10.1038/380711a0. [DOI] [PubMed] [Google Scholar]
- 39.Sheridan P L, Sheline C, Cannon K, Voz M, Pazin M, Kadonaga J, Jones K. Activation of the HIV-1 enhancer by the LEF-1 HMG protein on nucleosome-assembled DNA in vitro. Genes Dev. 1995;9:2090–2104. doi: 10.1101/gad.9.17.2090. [DOI] [PubMed] [Google Scholar]
- 40.Shirakata M, Huppi K, Usuda S, Okazaki K, Yoshida K, Sakano H. HMG1-related DNA-binding protein isolated with V-(D)-J recombination signal probes. Mol Cell Biol. 1991;11:4528–4536. doi: 10.1128/mcb.11.9.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinclair A H, Berta P, Palmer M S, Hawkins J R, Griffiths B L, Smith M J, Foster J W, Frischauf A-M, Lovell-Badge R, Goodfellow P N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 42.Singh H, LeBowitz J H, Baldwin A S, Sharp P A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988;52:415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- 43.Southgate C D, Green M R. The HIV-1 Tat protein activates transcription from an upstream DNA-binding site: implications for Tat function. Genes Dev. 1991;5:2496–2507. doi: 10.1101/gad.5.12b.2496. [DOI] [PubMed] [Google Scholar]
- 44.Stamatoyannopoulos G. Human hemoglobin switching. Science. 1991;252:383. doi: 10.1126/science.2017679. [DOI] [PubMed] [Google Scholar]
- 45.Stamatoyannopoulos G, Nienhuis A. Hemoglobin switching. In: Stamatoyannopoulos G, Nienhuis A W, Leder P, Majerus P W, editors. The molecular basis of blood diseases. W. B. Philadelphia, Pa: Saunders Co.; 1994. pp. 107–155. [Google Scholar]
- 46.Stros M, Stokrova J, Thomas J O. DNA looping by the HMG-box domains of HMG1 and modulation of DNA binding by the acidic C-terminal domain. Nucleic Acids Res. 1994;22:1044–1051. doi: 10.1093/nar/22.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talbot D, Collis P, Antoniou M, Vidal M, Grosveld F, Greaves D. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989;338:352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- 48.Tjian R, Maniatis T. Transcriptional activation: a complex puzzle with a few easy pieces. Cell. 1994;77:5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 49.Travis A, Amsterdam A, Belanger C, Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor a enhancer function. Genes Dev. 1991;5:880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- 49a.Trepicchio, W. L., and M. A. Dyer. Unpublished data.
- 50.Trepicchio W L, Dyer M A, Baron M H. Developmental regulation of the human embryonic β-globin gene is mediated by synergistic interactions among multiple tissue- and stage-specific elements. Mol Cell Biol. 1993;13:7457–7468. doi: 10.1128/mcb.13.12.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trepicchio W L, Dyer M A, Baron M H. A novel developmental regulatory motif required for stage-specific ɛ-globin gene activation and nuclear factor binding in embryonic erythroid cells. Mol Cell Biol. 1994;14:3763–3771. doi: 10.1128/mcb.14.6.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai S-F, Martin D I K, Zon L I, D’Andrea A D, Wong G G, Orkin S H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339:446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- 53.van Genderen C, Okamura R M, Fariñas I, Quo R-G, Parslow T G, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 54.van Gent D C, Hiom K, Paull T T, Gellert M. Stimulation of V(D)J cleavage by high mobility group proteins. EMBO J. 1997;16:2665–2670. doi: 10.1093/emboj/16.10.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinson C R, LaMarco K L, Johnson P F, Landschulz W H, McKnight S L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988;2:801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Precht P, Balakir R, Horton W E. Rat and chick cDNA clones encoding HMG-like proteins. Nucleic Acids Res. 1993;21:1493. doi: 10.1093/nar/21.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waterman M L, Fischer W H, Jones K A. A thymus-specific member of the HMG protein family regulates the human T cell receptor Cα enhancer. Genes Dev. 1991;5:656–669. doi: 10.1101/gad.5.4.656. [DOI] [PubMed] [Google Scholar]
- 58.Werner M H, Burley S K. Architectural transcription factors: proteins that remodel DNA. Cell. 1997;88:733–736. doi: 10.1016/s0092-8674(00)81917-0. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi-Shinozaki K, Shinozaki K. A novel Arabidopsis DNA binding protein contains the conserved motif of HMG-box proteins. Nucleic Acids Res. 1992;20:6737. doi: 10.1093/nar/20.24.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
- 61.Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:570–583. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]
- 62.Zwilling S, Konig H, Wirth T. High mobility group protein 2 functionally interacts with the POU domains of octamer transcription factors. EMBO J. 1995;14:1198–1208. doi: 10.1002/j.1460-2075.1995.tb07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]