Abstract
Monovarietal olive oils, known for their distinct aromatic profiles, face challenges in preserving their initial quality due to variations in stability and susceptibility to oxidative deterioration. This study focused on the storage stability of two Italian cultivars, ‘Biancolilla’ and ‘Cerasuola’, from Sicily, chosen for their aromatic complexity and divergent storage stability. Oils, whether filtered or unfiltered, underwent storage at two thermal regimes (18 °C and – 20 °C) over a year. The combination of filtration and low-temperature storage was employed to mitigate oxidative deterioration and hydrolytic processes, as filtration removes suspended particles and emulsified water, while low temperatures slow down enzymatic activities and oxidative reactions, thereby enhancing the overall stability and shelf life of the olive oils. Unfiltered samples at room temperature showed a significant increase in secoiridoid aglycone derivatives (Hydroxytyrosol and Tyrosol) due to hydrolytic processes, along with a decrease in secoiridoid aglycone. Filtration delayed these processes, with a more pronounced effect observed when combined with −20 °C storage. Sensory analysis identified the emergence of the “fusty” defect in the less resilient ‘Biancolilla’ cultivar after six months, a phenomenon mitigated by filtration and freezing. Consumer tests validated these findings. In summary, the synergistic approach of combining filtration with low-temperature storage emerges as a promising strategy for maintaining high-quality standards, especially for less stable monovarietal extra virgin olive oils. This strategy ensures compliance with EU regulations beyond the conventional 12-month shelf life, offering a practical solution for preserving the nutritional and sensory quality of olive oil.
Keywords: Filtration, Frozen EVOO, Tyrosol, Hydroxytyrosol, Storage
Highlights
-
•
Filtration delays hydrolytic processes, preserving polyphenols profile of olive oil.
-
•
Synergistic filtration and low-temperature storage extend shelf life of olive.
-
•
Filtration and freezing mitigated “Fusty” defect in sensitive cultivar such as ‘Biancolilla’.
-
•
Consumers test reveal significant preference for filtered samples stored at both temperature.
1. Introduction
Extra virgin olive oil (EVOO) stands out as the premier choice among olive oils due to its exceptional sensory qualities, oxidative stability, and rich chemical composition. Notably, EVOO is unique as a vegetable oil providing essential nutritional elements such as vitamins and antioxidants [1]. The composition of virgin olive oil primarily consists of triacylglycerols (97–98 %), with minor variations in free fatty acids and glyceridic compounds, including partial glycerides, phospholipids, and oxidized triacylglycerols. Notably, the high oleic acid content in olive oil plays a pivotal role in impeding the penetration of fatty acids into arterial walls, contributing to cardiovascular health [1]. Oils with higher monounsaturated fatty acids (MUFAs) and lower saturated fatty acids (SFAs) are favored due to the established positive effects of MUFAs on serum cholesterol levels. The biological properties of olive oil are also linked to minor components, such as squalene and phytosterols, as well as antioxidant compounds like tocopherols and phenols. Among natural antioxidants, phenolic compounds, α-tocopherol, and β-carotene are recognized for their key role in preventing oxidation, correlating with the storage stability of virgin olive oils (VOO) [2]. The clear, distinctive taste, aroma, and health-promoting properties as well as the oxidative stability, of EVOO stem from its remarkable and well-balanced chemical composition. However, oxidation processes can occur during storage including enzymatic oxidation, photooxidation, and autoxidation [3]. These reactions can lead to the formation of sensory defects and a decrease in the overall oil's quality.
Factors such as cultivar, oxygen, light, temperature, and storage time can influence the oxidative stability of EVOO during storage [4]. Numerous authors have extensively investigated the correlation between aging process and storage condition of oils and different quality parameters including acidity, peroxide value, and fatty acid profile [5]. For instance, after 18 months of room temperature storage EVOOs exhibited a substantial decrease in secoiridoids (nearly 50 %) [6]. Similarly, Kotsiou and Tasioula-Margari [7] observed up to a 79 % loss of α-tocopherol after 4 months of light-exposed storage, with nearly complete loss over 12–24 months. On the other hand, EVOOs conserved at low temperature (0–8 °C) for 24 months or in the dark for 21 months remained unchanged in Ref. [8]. Eposto et al. [9], demonstrated the robust association between storage condition, initial phenolic composition and VOO stability. As clear, storage alters the sensory profile and phenolic composition, resulting in diminished hedonic and health characteristics. Preserving the quality of EVOO during storage is therefore crucial for producers and consumers, as highlighted by the International Olive Council [10].
To prevent or slow down oxidation in olive oil during storage, several methods can be employed. For instance, suitable adjustment of the balance of oxygen and water is crucial in controlling the oxidation process in extra-virgin olive oil. Antioxidant compounds, can help protect the oil from oxidative degradation and maintain its quality [11] as well as processing and controlled storage conditions. Filtration stands out as a commonly employed stabilization process for EVOOs. Filtration is a recommended step to eliminate suspended particles and natural turbidity caused by dispersed microdroplets of vegetation water. These microdroplets, over time, can promote hydrolysis, oxidative rancidity, or fermentative processes, facilitating the survival of microbial colonies [12]. It is used to obtain a clearer and more stable final product with an extended shelf life. Despite the acknowledged benefits of filtration, it is infrequently adopted because consumers often perceive slight turbidity as an authenticity guarantee. Moreover, filtration can be performed using different technologies, and the choice of filtration method can influence the phenolic composition, volatile profile, and sensory characteristics of the oil.
Storage temperature has been analysed in various works, highlighting freezing as a method to maintain quality in long-term storage [13]. While freezing can lead to the precipitation of some phenolic compounds, particularly when combined with filtration, some studies indicate that maintaining low temperatures contributes to preserving EVOO quality during prolonged storage [14]. Furthermore, despite the acknowledged importance of storage conditions in maintaining the quality of extra virgin olive oil (EVOO), the impact of these conditions is inherently linked to the specific characteristics and sensitivity of each olive cultivar. However, existing literature lacks comprehensive studies that delve into the intricate relationship between cultivar sensitivity and storage quality. Therefore, this study aims to investigates the effect of filtration and different temperature storage conditions, alone or in combination, on the chemical and sensory profile of two monovarietal Italian oils over a 12-month storage period. Specifically, the study focuses on the ‘Biancolilla’ and ‘Cerasuola’ cultivars, renowned for their distinct sensory profiles and varying stability during storage. Specifically, the oil from ‘Biancolilla’, with its delicate and harmonious notes, tends to lose characteristics rapidly due to its modest natural content of antioxidant compounds. In contrast, ‘Cerasuola’, with its high natural concentrations of polyphenols, produces more stable oils characterized by an intense taste and pronounced bitter and spicy notes. By elucidating the interactions between cultivar sensitivity and storage conditions, this research aims to provide insights into optimizing olive oil preservation strategies, thereby ensuring the maintenance of high-quality standards and sensory attributes over extended storage durations.
2. Materials and methods
2.1. Olives samples and extraction process
Olives (Olea europaea L. cv ‘Biancolilla’ and ‘Cerasuola’) were grown and harvested in a commercial farm located in Xiggiari (37°55′ 11″N, 12°36′34″E), in the territory of the D.O.P. area Valli Trapanesi (Sicily, Italy), area designated for producing organic olive oil under Reg. 1151/2012. According with the regulatory disciplinary, soils are identified as brown, alluvial, red earth soils, with textures ranging from sandy to medium loam tending towards clayey. The planting density, training systems, and pruning methods must be those commonly used or, in any case, suitable for not altering the characteristics of the olives and the oil. The maximum olive production per hectare cannot exceed 8000 kg per hectare. The average ripening index at the harvest was about 1.5 for ‘Cerasuola’ and 0.95 for ‘Biancolilla’. Olives were processed separately a few hours after harvesting. Olives were washed, crushed using a disk crusher operating at 1400 rpm, and malaxed in a sealed malaxing chamber connected to a nitrogen tank with an O2 sensor for 30 min at 25 ± 2 °C. Oil separation was obtained by a continuous three-phase decanter (Alfa Laval X 32, Macchine Olearie Ferri s. r.l., Andria (BT), Italy) with water addition (1:2 v:w). The produced EVOOs were divided into two batches for each cultivar. One batch was subjected to filtration and the other remains unfiltered. Filtration was performed using a filter press (0,3 atm) with a cellulose filter (40 × 40 cm, Gurley porosity 8 μm Pa. s-1) [15]. The olive oil not used for the study was bottled and sent for sale.
2.2. Storage conditions and samplings
Filtered and unfiltered samples were bottled into 0,5 L−1 green glass bottles, saturated with nitrogen, and stored at two different temperature: −20 ± 3 °C or 20 ± 2 °C in the dark. Samples were collected at 0, 3, 6, and 12 months of storage. At each sampling time, three bottles of each sample were taken for analysis and used as replicates. Table 1 summarized the obtained samples.
Table 1.
Samples used in the study and relative code.
| Sample | Code |
|---|---|
| Filtered Biancolilla | BF |
| Biancolilla unfiltered | B |
| Cerasuola Filtered | CF |
| Cerasuola uniltered | C |
| Frozen filtered Biancolilla | fBF |
| Frozen unfiltered Biancolilla | fB |
| Frozen filtered Cerasuola | fCF |
| Frozen unfiltered Cerasuola | fC |
2.3. EVOO quality index
All reagents, standards, and solvents, analytical or HPLC grade, were purchased from Merck (Merck KGaA, Darmstadt, Germany). Free fatty acid (FFA) expressed in % of oleic acid, and peroxide value (PV) (meq O2/kg−1) were carried out according with the analytical methods described in the International Organization for Standardization [ [16,17]].
2.4. Total phenol content (TPC)
Total phenol content was performed as described by Waterhouse [18], with minor modifications. 2 g of oil samples were mixed with 5 mL of 80 % (v/v) of methanol, the resulting mixture was stirred for 15 min in the dark in an ultrasonic ice bath (Argolab mod. DU-32, Carpi, MO, Italy) and then centrifuged (Neya 16R, Vasay, India) at 4900 g at 4 °C for 15 min. After centrifugation, the supernatant was collected and used for the analysis. 1580 μL of distilled water, 20 μL of supernatant, 100 μL of Folin-Ciocalteau reagent, and 300 μL of 20 % (w/v) sodium carbonate (Na2CO3) were used for the sample reaction; 2 mL of distilled water were used for blank. The mix was incubated for 30 min at 40 °C in a thermostatic water bath unit (Astor 800D model, Astori Tecnica, Poncarale, IT). Measurements were performed using a spectrophotometer (Lambda 25 UV–Vis, Agilent, Santa Clara, US) at 760 nm. The concentration of TPC was expressed as mg−1 gallic acid equivalent (GAE) per Kg−1 of fw, based on a calibration curve obtained with known concentrations of gallic acid.
2.5. HPLC analysis of phenolic profile
The phenolic profile was characterized according to the method described by the International Olive Council [19] with minor modifications. The separation was performed by RP-HPLC (Dionex Ultimate 3000 HPLC - Thermo Fisher Scientific, Waltham, MA, USA) equipped with a PDA detector. 20 μL of filtered extract, 0.45 μm PVDF, was injected into a Dionex Acclaim 120C18 column (150 mm × 4.6 mm, 5 μm; Thermo Fisher Scientific, Waltham, MA, USA) and eluted by a 0,8 mL min−1 constant flow. The run time for each sample was 82 min. The eluents were 0.2 % H3PO4 (v/v) (A), methanol, and acetonitrile 1:1 (v/v) (B). The initial composition was 96 % phase A, and the gradient changed, increasing the B phase to 50, 60, and 100 % in 60 min. The 100 % B eluent was maintained for 10 min, then decreased to 4 % in 2 min and held for 10 min. Absorption was measured with a UV detector at 280 nm. Individual phenols were quantified by calibration curves obtained with known concentration of specific commercial standards.
2.6. Sensory analysis
The IOC official method was used for sensory analysis using standard profile sheet [10] with minor adjustments (Table S1). The panel consisted of eight judges trained for organoleptic evaluation of EVOO [20]. The tests were conducted in a tasting room, on a 15 mL of oil, in standard blue tasting glass [21], covered with a watch glass, and conditioned in a heater electric (Panel-Test Mod.145, Ettore Pasquali srl, Italy) at 28 ± 2 °C temperature. The intensity of the different attributes was scored on a 10 cm intensity scale. The standard profile sheet contains three positive attributes (fruity, bitter, and pungent) and six negative attributes (Fusty/muddy sediment; Musty/humid/earthy; Winey/vinegary acid/sour; Frostbitten olives; Rancid; Other negative attributes). Additionally, a persistence assessment was included in the evaluation process. The research was carried out in line with the ethical standards of the Declaration of Helsinki. Written informed consent has been obtained from every participants, after the nature of the study has been explained in understandable terms and all of them participated voluntarily.
2.7. Consumer tests
The opinions of 65 olive oil consumers recruited among students and staff of Tuscia University (Viterbo, Italy) and acquaintances willing to undergo the test were collected through a hedonic test. The participants in the sensory laboratory received four EVOOs of each variety simultaneously at each sampling time. In disposable 70 mL white paper cups, 15 mL of each EVOO were rated, using a simplified form (Table S2) to indicate their overall preference using a 9-point hedonic scale, where 1 = extremely unpleasant and 9 = extremely pleasant. The research was carried out in line with the ethical standards of the Declaration of Helsinki. Written informed consent has been obtained from every participants, after the nature of the study has been explained in understandable terms and all of them participated voluntarily.
2.8. Data processing
All the data were subjected to Bartlett test and Shapiro-Wilk to verify normality and homogeneity of the variances. Once these pre-requisites were verified, two-way ANOVA and Tuckey test at p ≤ 0.05 was performed. HPLC, PV and FFA data were autoscaled and used for Hierarchical Clustering analysis with Ward method (Euclidean distance). A non-parametric Kruskall-Wallis test was carried out on the consumer test data. The XL-Stat version 1.3, 2020 ((Addi Soft, New York, NY, USA)) and GraphPad Prism (© 2024 GraphPad Software, Boston, MA, USA) were used to process the analytical data.
3. Results and discussion
3.1. PV and FFA
During storage, a significant increase in the peroxide values (PV) (Table 2) and FFA (Table 3) was observed in both cultivars and treatments. However, the levels (max 16 meq O2/kg−1, and 0.5 % oleic acid observed in B sample) was below the maximum level required for EVOO quality standards [2,[22], [23], [24]]. Interesting, the PV in untreated samples (namely ‘Biancolilla’ and ‘Cerasuola’ unfiltered and stored at room temperature, B and C), reached the highest value at 12 months of storage. The level of PV in these samples was higher than the value observed in treated samples at 6 months of storage. Thus, suggesting that without filtration and storage at room temperature, the oils have a high oxidation rate, much higher than what observed with filtration and freezing.
Table 2.
Peroxide Value (meq O2/kg−1) in different olive oils stored for 3, 6 or 12 months.
| Storage time | Room temperature and dark |
(f)Frozen (−20 ± 3 °C) |
||
|---|---|---|---|---|
| BF | B | fBF | fB | |
| 0 | 6,8 ± 0.8 f | 7,3 ± 0.1 f | 6,8 ± 0.8 f | 7,3 ± 0.1 f |
| 3 | 9,8 ± 0.5 d | 10,4 ± 0.1 d | 7,1 ± 0.1 f | 8,4 ± 0.e |
| 6 | 11,8 ± 0.2 c | 14,8 ± 0.2 a | 8,8 ± 0.2 e | 10,8 ± 0.2 d |
| 12 | 12,5 ± 0.1 c | 16 ± 0.1 a | 9,4 ± 0.1 e | 14 ± 0.1 b |
| CF | C | fCF | fC | |
|---|---|---|---|---|
| 0 | 4,8 ± 0.1 g | 5,5 ± 0.2 f | 4,8 ± 0.1 g | 5,5 ± 0.2 f |
| 3 | 6,9 ± 0.4 e | 8,0 ± 0.2 d | 5,5 ± 0.1f | 7 ± 0.1 e |
| 6 | 8,9 ± 0.2 d | 11 ± 0.1 b | 6,8 ± 0.3 e | 9,8 ± 0.2 c |
| 12 | 10,8 ± 0.3 bc | 13,2 ± 0.3 a | 7,5 ± 0.2 de | 11,2 ± 0.3 b |
Values are the means of three replicates ± sd. Different letters represent significant differences between samples of each cv according to two-way ANOVA and Tuckey test (p < 0.05). Filtered Biancolilla (BF), Biancolilla unfiltered (B), Cerasuola Filtered (CF), Cerasuola unfiltered (C), Frozen filtered Biancolilla (fBF), Frozen unfiltered Biancolilla (fB), Frozen filtered Cerasuola (fCF), Frozen unfiltered Cerasuola (fC).
Table 3.
Free Fatty Acid (% oleic acid) in different olive oils stored for 3, 6 or 12 months.
| Storage time | Room temperature and dark |
(f)Frozen (−20 ± 3 °C) |
||
|---|---|---|---|---|
| BF | B | fBF | fB | |
| 0 | 0.13 ± 0.01 d | 0.14 ± 0.01 d | 0.13 ± 0.01 d | 0.14 ± 0.01d |
| 3 | 0.12 ± 0.02 d | 0.17 ± 0.03 cd | 0.11 ± 0.05 d | 0.16 ± 0.04 cd |
| 6 | 0.21 ± 0.03 bc | 0.26 ± 0.04 bc | 0.15 ± 0.02 d | 0.22 ± 0.05 bc |
| 12 | 0.28 ± 0.01 bc | 0.50 ± 0.10 a | 0.22 ± 0.01 c | 0.32b ± 0.06 b |
| CF | C | fCF | fC | |
|---|---|---|---|---|
| 0 | 0.08 ± 0.02 d | 0.10 ± 0.03 d | 0.08 ± 0.03 d | 0.10 ± 0.03 d |
| 3 | 0.11 ± 0.02 d | 0.15 ± 0.01 d | 0.10 ± 0.03 d | 0.11 ± 0.01 d |
| 6 | 0.18 ± 0.03 cd | 0.28 ± 0.02 b | 0.15 ± 0.01 d | 0.20 ± 0.03 bc |
| 12 | 0.25 ± 0.05 bc | 0.40 ± 0.06 a | 0.22 ± 0.02 c | 0.32 ± 0.03 ab |
Values are the means of three replicates ± sd. Different letters represent significant differences between samples of each cv according to two-way ANOVA and Tuckey test (p < 0.05). Filtered Biancolilla (BF), Biancolilla unfiltered (B), Cerasuola Filtered (CF), Cerasuola unfiltered (C), Frozen filtered Biancolilla (fBF), Frozen unfiltered Biancolilla (fB), Frozen filtered Cerasuola (fCF), Frozen unfiltered Cerasuola (fC).
In filtered samples, the PV increases over time, but between 6 and 12 months, the value remains unchanged. Low temperature significantly lowers peroxide formation, especially when coupled with filtration. The peroxide value is a remarkable indicator of the primary oxidative processes and tends therefore to increase over time. However, the application of low temperature and filtration significantly slow down hydrolytic and oxidation process in oils as already reported [ [25,26]] and as confirmed by the results of this study.
As far as the FFA is concerned, an increase was recorded in both varieties during storage. As observed for PV, unfiltered ‘Biancolilla’ and ‘Cerasuola’ (B and C) reached the highest value of acidity at 12 months of storage, regardless the temperature. On the other hand, filtration significantly reduce the increase of acidity especially when combined with low-temperature storage. Hence, in both fBF and fCF at 12 months of storage, the acidity value remains similar to the value observed in the other samples after 3 or 6 months of storage. Thus confirming once again the effectiveness of filtration and low-temperature storage in slowing down the oxidation process. The acidity of olive oil can be affected by storage conditions and duration. Several studies have shown that the acidity of olive oil increases with storage duration [ [[27], [28], [29]]]. The increase in acidity is more pronounced when olive oil is stored at higher temperatures. Olive oil stored at ambient temperature for two months showed a significant increase in acidity, while no significant difference was observed in olive oil stored in refrigeration [28]. It is important to note that the sensory profile of olive oil can be affected by its acidity level. Higher acidity levels may result in a more pronounced pungency and a decrease in positive fruity attributes. In the same way, PV, which indicates the level of oxidation in olive oil, can influence its sensory profile. Excessive oxidation, indicated by a high PV, can result in sensory defects such as rancidity [30]. When both acidity and PV increase, the sensory profile of olive oil may be further affected. The presence of high levels of phenolic compounds can mitigate the negative sensory effects of increased acidity, but may not have the same impact on the perception of rancidity caused by high PV [30].
3.2. TPC (total phenolic compounds)
Total polyphenols content at T0 in the two cultivars was slightly different with ‘Biancolilla’ having the lowest value (Table 4) (348 mg kg−1 versus 557 mg kg−1 of ‘Cerasuola’). This result highlights the differences in terms of storage stability of the two cultivars. Hence, the relationship between oxidation stability and initial polyphenols content has been demonstrated [2,9]. For instance, El Yamani et al. [31], showcased enhanced stability in olive oil through the incorporation of natural phenols. As previously discussed, olive oil rich in antioxidants exhibits stability over time by impeding lipid oxidation through the counteraction of free radicals and the inhibition of singlet oxygen [32]. However, as observed here, TPC underwent a natural reduction during storage [29]. The storage conditions, such as exposure to light and high temperature, can promote the degradation of polyphenols. On the other hand, storage at low temperature and darkness appears to be adequate for maintaining stability and preserving the phenolic profile of the oil [29]. The results presented here match perfectly with these statements. Hence, unfiltered ‘Biancolilla’ and ‘Cerasuola’ stored at room temperature (B and C) lose 40 and 29 % of TPC respectively. High degradation rate was observed already after 3 months of storage. On the other hand, filtration seems to reduce the degradation of polyphenols. Hence, Filtered ‘Biancolilla’ and ‘Cerasuola’ lose 18 and 16 % of TPC when stored at room temperature and just 14 and 7 % when stored at low temperature. On the other hand, the unfiltered samples stored at low temperature lose about 28 %. Thus, for the preservation of TPC, filtration seems to be more effective than temperature. Interestingly, considering the significant initial difference between the two cultivars, the reduction rate of TPC was higher in ‘Biancolilla’, confirming the more unstable characteristics of this cultivar.
Table 4.
Total polyphenols content mg kg−1 in different olive oils stored for 3, 6 or 12 months.
| Storage time | Room temperature and dark |
(f)Frozen (−20 ± 3 °C) |
||
|---|---|---|---|---|
| BF | B | fBF | fB | |
| 0 | 326 ± 18 a | 348 ± 14 a | 326 ± 18 a | 348 ± 14 a |
| 3 | 316 ± 10 ab | 305 ± 15 ab | 320 ± 12 ab | 310 ± 12 ab |
| 6 | 285 ± 8.0 b | 275 ± 7.9 bc | 290 ± 8.0 b | 278 ± 11 bc |
| 12 | 265 ± 12 bc | 207 ± 10 d | 280 ± 9.8 b | 250 ± 9.5 c |
| CF | C | fCF | fC | |
|---|---|---|---|---|
| 0 | 541 ± 9.5 a | 557 ± 12 a | 541 ± 9.5 a | 557 ± 12 a |
| 3 | 523 ± 11 a | 486 ± 9.6 b | 530 ± 8.7 a | 500 ± 14 b |
| 6 | 480 ± 8.8 b | 417 ± 12 c | 510 ± 10 ab | 485 ± 7.9 b |
| 12 | 450 ± 10 c | 390 ± 9.0 d | 500 ± 12 b | 400 ± 11 d |
Values are the means of three replicates ± sd. Different letters represent significant differences between samples of each cv according to two-way ANOVA and Tuckey test (p < 0.05). Filtered Biancolilla (BF), Biancolilla unfiltered (B), Cerasuola Filtered (CF), Cerasuola unfiltered (C), Frozen filtered Biancolilla (fBF), Frozen unfiltered Biancolilla (fB), Frozen filtered Cerasuola (fCF), Frozen unfiltered Cerasuola (fC).
The effect of filtration on polyphenol content in olive oil reported in literature is not always consistent. While some studies have shown a decrease in phenolic compounds during filtration [ [23,33]], others have found an increase in secoiridoids [34].
The reduction of polyphenols after filtration has been related with the hydrophilic characteristics of the phenolic constituents present in olive fruit which undergo partial modification during the extraction process. This process triggers a sequence of enzymatic activations that facilitate the conversion of primary secoiridoids into their respective aglycones. This transformation enables a partial distribution of the phenolic fraction within the oil matrix. However, owing to their inherent hydrophilic nature, minute quantities of vegetable water dispersed in unfiltered oils harbour fluctuating concentrations of phenols, which are subsequently eliminated through filtration processes [35]. Overall, the impact of filtration on polyphenols in olive oil is complex and depends on various factors, including the filtration system used and the specific phenolic compounds being considered. While filtration can lead to a decrease in certain phenolic compounds, such as hydroxytyrosol and 3,4-DHPEA-EA, it can also result in an increase in other phenolic compounds, particularly secoiridoids [35].
3.3. HPLC phenol profile
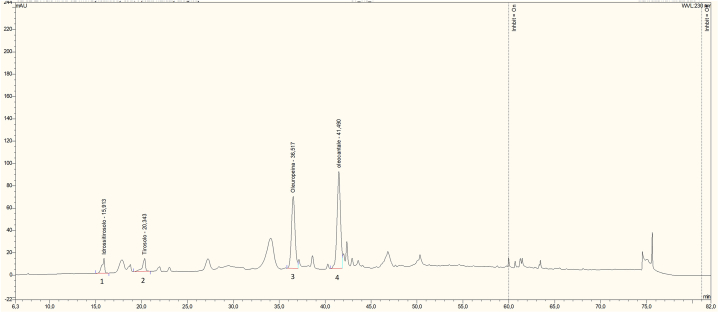
In Fig. 1 a representative chromatogram of the phenolic profile of EVOO is shown. Four compounds were selected as the best representatives of the olive oil degradative evolution as already reported by previous studies [ [33,36]]. The phenolic profile of the different EVOO confirms, as stated above, that different phenolics has different trend during storage.
Fig. 1.
HPLC chromatogram of the selected phenolics. Signal was recorded at 280 nm. Peak assignments: 1 hydroxytyrosol (3,4-DHPEA); 2 Tyrosol, 3 3,4-DHPEA-EA Oleuropein aglycon; 4 p-HPEA-EDA Oleocanthal.
As shown in Table 5, hydroxytyrosol increases in both cultivars and in all the samples during storage. As is known, the content of hydroxytyrosol can increase in aged oils, and it is therefore used as a marker of oil storage [37]. More specifically, in unfiltered samples stored at room temperature (B and C) the content of hydroxytyrosol increase of 598 and 284 % respectively. As discussed for TPC, the higher increase observed in ‘Biancolilla’ highlights the more unstable profile of this cultivar due to its lower initial polyphenol content. Freezing unfiltered samples (fB and fC), result in a modest increment of hydroxytyrosol, slightly lower than B and C. On the other hand, filtration seems to be effective in reducing the production of hydroxytyrosol reaching an increment of 305 and 222 % for ‘Biancolilla’ (BF) and ‘Cerasuola’ (CF) respectively. The effect of filtration on slowing down the oxidative processes is significantly greater when combined with low-temperature storage (increment of 236 and 165 % for fBF and fCF respectively). The same trend is observed for Tyrosol. Hence, in unfiltered samples stored at room temperature (B and C), the tyrosol content reached the highest value (Table 5). In ‘Cerasuola’ oils, the increase compared to the initial value is more contained, probably due to a greater capacity to counteract oxidative processes, typical of this variety. In freezing unfiltered samples (fB and fC) and filtered samples (FB and FC) a significant and similar increment of tyrosol was observed at 12 months of storage. Conversely, in filtered oils stored at −20 °C, the lowest tyrosol value was observed. As previously reported, storage temperature and filtration can play a significant role in the stability of EVOO during storage. For instance, higher temperatures, such as ambient temperature, can lead to a significant increase in tyrosol levels, while refrigeration helps maintain the quality properties of the oil during storage [27].
Table 5.
Polyphenols identified through HPLC and expressed as mg kg−1 oil in different olive oils stored for 3, 6 or 12 months.
| 3,4-DHPEA-Hydroxytyrosol | Room temperature and dark |
(f)Frozen (−20 ± 3 °C) |
||
|---|---|---|---|---|
| BF | B | fBF | fB | |
| 0 | 0.55 ± 0.01 f | 0.65 ± 0.03 f | 0.55 ± 0.01 f | 0.65 ± 0.03 f |
| 3 | 0.95 ± 0.02 ef | 1.55 ± 0.06 d | 0.75 ± 0.06 f | 1.36 ± 0.05 d |
| 6 | 1.64 ± 0.05 d | 3.12 ± 0.09 b | 1.05 ± 0.03 e | 2.03 ± 0.03 c |
| 12 | 2.23 ± 0.08 c | 4.54 ± 0.02 a | 1.85 ± 0.08 cd | 3.15 ± 0.09 b |
| CF | C | fCF | fC | |
|---|---|---|---|---|
| 0 | 1.20 ± 0.09 h | 1.55 ± 0.12 h | 1.20 ± 0.09 h | 1.55 ± 0.12 h |
| 3 | 2.39 ± 0.18 fg | 3.82 ± 0.24 c | 2.25 ± 0.18 g | 3.02 ± 0.29 ef |
| 6 | 3.19 ± 0.13 d | 4.76 ± 0.21 b | 2.77 ± 0.32 f | 4.05 ± 0.21 b |
| 12 | 3.87 ± 0.18 c | 5.96 ± 0.29 a | 3.19 ± 0.24 d | 4.88 ± 0.33 b |
| p-HPEA (Tyrosol) | BF | B | fBF | fB |
|---|---|---|---|---|
| 0 | 1.00 ± 0.27 e | 1.22 ± 0.38 e | 1.00 ± 0.27 e | 1.22 ± 0.38 e |
| 3 | 1.41 ± 0.06 d | 2.11 ± 0.15 cd | 1.39 ± 0.06 | 1.64 ± 0.06 d |
| 6 | 2.56 ± 0.14 c | 4.38 ± 0.09 b | 1.85 ± 0.10 d | 2.28 ± 0.08 c |
| 12 | 4.19 ± 0.62 b | 5.65 ± 0.25 a | 2.86 ± 0.59 c | 4.16 ± 0.15 b |
| CF | C | fCF | fC | |
|---|---|---|---|---|
| 0 | 4.55 ± 0.33 f | 5.01 ± 0.62 ef | 4.55 ± 0.33 f | 5.01 ± 0.62 ef |
| 3 | 5.58 ± 0.25 d | 5.99 ± 0.12 d | 5.41 ± 0.18 de | 5.29 ± 0.15 de |
| 6 | 6.49 ± 0,28 c | 7.62 ± 0.55 b | 5.90 ± 0.19 d | 6.42 ± 0.19 c |
| 12 | 7.24 ± 0.55 b | 8.50 ± 0.40 a | 6.12 ± 0.36 c | 7.42 ± 0.41 b |
| 3,4-DHPEA-EA Oleuropein aglycon | BF | B | fBF | fB |
|---|---|---|---|---|
| 0 | 18.58 ± 1.02 a | 20.50 ± 1.34 a | 18.58 ± 1.42 a | 20.50 ± 1.34 a |
| 3 | 16.26 ± 1.16 bc | 17.21 ± 1.15 b | 17.30 ± 1.86 b | 16.36 ± 2.02 bc |
| 6 | 13.24 ± 0.70 d | 11.18 ± 0.49 d | 16.38 ± 1.30 bc | 15.29 ± 0.71 c |
| 12 | 11.46 ± 0.49 d | 9.85 ± 0.80 e | 13.83 ± 0.69 d | 10.94 ± 0.52 de |
| CF | C | fCF | fC | |
|---|---|---|---|---|
| 0 | 75.43 ± 1.53 b | 82.02 ± 1.20 a | 75.43 ± 1.53 b | 82.02 ± 1.20 a |
| 3 | 66.50 ± 2.21 c | 60.13 ± 1.60 cd | 69.93 ± 2.27 c | 63.66 ± 1.14 c |
| 6 | 58.29 ± 1.85 d | 52.55 ± 2.22 d | 64.34 ± 1.30 c | 60.24 ± 1.25 cd |
| 12 | 53.78 ± 2.40 d | 48.68 ± 1.38 e | 61.73 ± 1.72 d | 55.83 ± 1.31 d |
| p-HPEA-EDA Oleocanthal | BF | B | fBF | fB |
|---|---|---|---|---|
| 0 | 19.42 ± 1.08 a | 22.34 ± 1.24 a | 19.42 ± 1.08 a | 22.34 ± 1.24 a |
| 3 | 18.79 ± 1.22 a | 14.42 ± 1.02 bc | 18.84 ± 1.22 a | 18.07 ± 0.85 a |
| 6 | 13.10 ± 1.08 c | 10.55 ± 1.03 d | 15.46 ± 0.88 b | 13.94 ± 1.21c |
| 12 | 9.01 ± 0.79 de | 8.32 ± 0.89 e | 13.95 ± 1.08 c | 10.62 ± 1.01 d |
| CF | C | fCF | fC | |
|---|---|---|---|---|
| 0 | 42.67 ± 1.52 ab | 45.09 ± 2.02 a | 42.67 ± 1.52 ab | 45.09 ± 2.02 ab |
| 3 | 36.69 ± 1.19 cd | 33.55 ± 1.35 d | 41.63 ± 2.04 b | 40.82 ± 1.64 b |
| 6 | 31.30 ± 1.88 d | 22.79 ± 2.02 e | 38.21 ± 2.50 bc | 35.91 ± 1.89 cd |
| 12 | 24.07 ± 1.89 e | 15.99 ± 1.98 f | 32.65 ± 2.12 c | 27.05 ± 2.05 e |
Values are the means of three replicates ± sd. Different letters represent significant differences between samples of each cv according to two-way ANOVA and Tuckey test (p < 0.05). Filtered Biancolilla (BF), Biancolilla unfiltered (B), Cerasuola Filtered (CF), Cerasuola unfiltered (C), Frozen filtered Biancolilla (fBF), Frozen unfiltered Biancolilla (fB), Frozen filtered Cerasuola (fCF), Frozen unfiltered Cerasuola (fC).
The evolution of the secoiridoids 3,4-DHPEA-EA (Oleuropein aglycone), p-HPEA-EDA oleocanthal is different from what observed for the phenolic alcohols (Tyrosol, and Hydroxytyrosol) undergoing an important decrease (Table 5).
Secoiridoids are a prominent chemical class of polar constituents in olive oil, known for their significant biological properties. They are prone to oxidation due to their chemical structure. Oxidized derivatives of secoiridoids have been reported in VOO during production and storage [36]. The concentration of secoiridoids in olive oil can vary depending on factors such as olive cultivars, place of cultivation, olive oil processing methods and storage conditions. Activities that can preserve the secoiridoids content in EVOO over time are desirable. The data confirms what observed for the other polyphenols: combining filtration and freezing significantly reduces secoiridoids loss and retards oxidation processes. For instance, in unfiltered olive oil stored at room temperature, the loss is more than 60 %, while in filtered and frozen oils, it remains less than 30 % in both cultivars during a year of storage. Notably, when examining varying storage durations, it is noteworthy that the combination of refrigeration and filtration effectively delays oxidative processes of approximately three months or more. This is evident in the recorded values of frozen and filtered ‘Biancolilla’ and ‘Cerasuola’ samples (fBF and fCF) at three months, which are essentially equivalent to the values observed at six months in the other samples, particularly in samples B and C. Furthermore, for certain polyphenols, the concentration at 12-months of fBF and fCF remains equal than the concentration observed in untreated samples at three month of storage.
The correlation between the phenolic profile and the conservation state of the oils is notably evident, establishing itself as a robust indicator independent of the olive variety, as demonstrated in our study. However, it is essential to acknowledge the influence of additional factors, including the extraction system, climatic conditions, and various others factors, as confirmed by previous research [ [38][9,39]]. Notably, the ‘Biancolilla’ variety exhibits inherently low values across all components associated with the phenolic fraction from the outset. However, the temporal evolution of these components remains comparable between the two cultivars.
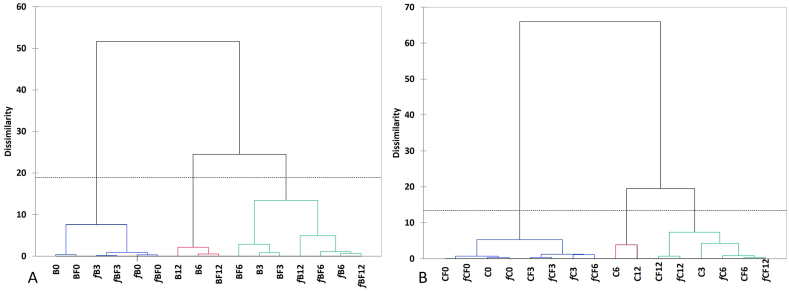
3.4. Sample clustering
In order to better visualize the temporal variations among EVOO samples during the storage period, a Hierarchical Cluster Analysis (HCA) was conducted on a dataset that included autoscaled Free Fatty Acids (FFA), Peroxide Value (PV), and phenolic profile data (Fig. 2). As depicted in Fig. 2A, the analysis resulted in the formation of three primary clusters for the ‘Biancolilla’ cultivar. The first cluster is closely associated with samples at the beginning (0) or at three months of storage, which are still characterized by freshness and pleasantness (namely B0, BF0, fB3, fBF3, fB0 and fBF0). The inclusion of fB3 and fBF3 in the cluster with oil at time 0 confirms that filtration and freezing contribute to maintaining high levels of freshness for up to 3 months. The second cluster characterizes oils that, while losing some of their freshness characteristics, still maintain favourable features in terms of phenolic composition, acidity and peroxides. Intriguingly, this group includes filtered oils at 3 and 6 months of storage (BF6 and BF3), as well as frozen oils at 6 and 12 months of storage (fB6, fB12), and both filtered and frozen oils (fBF6 and fBF12) which are maintained up to twelve months of storage. The third cluster amalgamates unfiltered oils at 6 and 12 months of storage, along with the filtered oil at 12 months stored at room temperature. This findings highlights the benefits of filtration, emphasizing its positive impact on preserving the freshness of oils. However, it also highlights the importance of controlling storage temperature, particularly for the less resistant cultivars. Overall, HCA offers a detailed insight into the temporal evolution of Biancolilla EVOO samples, illustrating how filtration, freezing, and storage duration influence freshness and phenolic composition.
Fig. 2.
Cluster plot of A. Biancolilla and B. Cerasuola olive oils stored for 3, 6 or 12 months. Filtered Biancolilla (BF), Biancolilla unfiltered (B), Frozen filtered Biancolilla (fBF), Frozen unfiltered Biancolilla (fB), Cerasuola Filtered (CF), Cerasuola unfiltered (C), Frozen filtered Cerasuola (fCF), Frozen unfiltered Cerasuola (fC).
The results for ‘Cerasuola’ (Fig. 2B) closely resemble those observed for ‘Biancolilla’, reinforcing the consistency of our findings. Similar to ‘Biancolilla’, three primary clusters are discernible, each shedding light on the varying preservation states of the olive oil samples over the storage period. The first cluster amalgamates the best-preserved samples, noteworthy for not only including the fresh samples (C0, CF0, fC0, and fCF0) but also encompassing filtered and unfiltered frozen samples for up to three months (CF3 and fC3), along with samples that are both filtered and frozen at 3 and 6 months of storage (fCF6). This observation supports the hypothesis that a combination of freezing and filtering significantly contributes to maintaining a high-quality level, as evident from the clustering with samples at time 0.
The second cluster comprises oils of medium quality, comprising the unfiltered sample stored at room temperature for three months (C3), filtered oils (CF6, CF12), and filtered frozen oils (fC6, fC12) at 6 and 12 months of storage. Additionally, it includes samples that are both filtered and frozen at 12 months (fCF12). The third cluster exclusively consists of unfiltered oils stored at room temperature for 6 and 12 months (C6 and C12). These clustering patterns offer valuable insights into the preservation dynamics of ‘Cerasuola’ and ‘Biancolilla’ EVOO, emphasizing the impact of storage conditions, filtration, and freezing on the quality attributes of the oils over time.
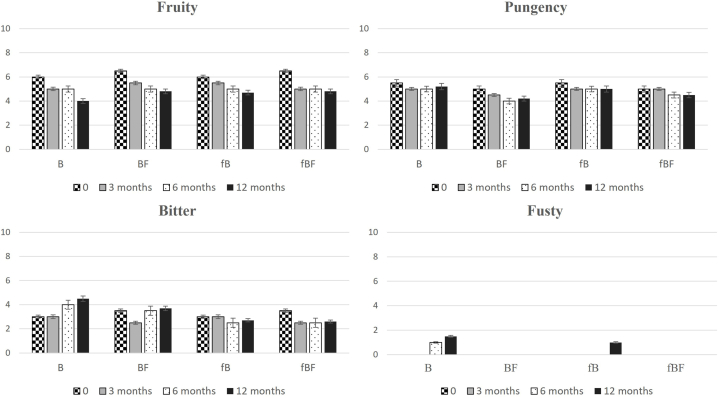
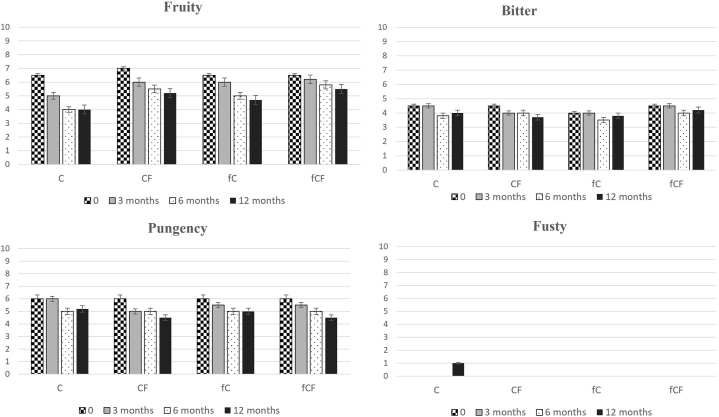
3.5. Sensory profile
The primary sensory attributes assessed for the quality of the evaluated oil were categorized as positive, including fruity, bitter, and pungency, and negative, such as fusty (Fig. 3, Fig. 4).
Fig. 3.
Evaluation of flavor attributes in Biancolilla olive oils during storage stored for 3, 6 or 12 months using descriptive sensory analysis. Filtered Biancolilla (BF), Biancolilla unfiltered (B), Frozen filtered Biancolilla (fBF), Frozen unfiltered Biancolilla (fB).
Fig. 4.
Evaluation of flavor attributes in Cerasuola olive oils d stored for 3, 6 or 12 months. Cerasuola Filtered (CF), Cerasuola unfiltered (C), (fB), Frozen filtered Cerasuola (fCF), Frozen unfiltered Cerasuola (fC).
Over a one-year storage period, a consistent decline in fruity notes was observed in all oil samples. Filtration led to a slight reduction in initial fruitiness, aligning with findings from prior studies [ [23,39,40]].
For the ‘Biancolilla’ cultivar (Fig. 3), the median of fruity attributes decreased from 5.5 to around 5, while in the ‘Cerasuola’ cultivar (Fig. 4), the fruity median decreased from 6.8 to 6.3 at the initial time point. After one year of storage, the reduction was more pronounced and occurred at varying rates among samples. Noteworthy is the evolution of bitter and spicy attributes associated with the studied phenolic components. As reported, secoiridoid aglycones were identified as major contributors to bitterness [40], while oleocanthal showed correlation with spiciness perception [41]. Despite initial varietal differences, indicating the distinct sensory intensity of ‘Cerasuola’ compared to ‘Biancolilla’, significant variations emerged in all samples during storage.
It is evident that modifications in phenolic compounds responsible for gustatory and trigeminal perception lead to a decrease in bitter and pungent notes during storage, particularly in oils stored at room temperature and left unfiltered. Additionally, room temperature storage, compared to freezing, accelerated the aging process, resulting in reduced fruitiness, bitterness, and spiciness.
At different time points for both cultivars, the “Fusty” defect appeared in unfiltered oils during storage. In ‘Biancolilla’, the median of the “Fusty” defect exceeded zero after six months for room temperature-stored unfiltered sample (B), downgrading it to the virgin category (VOO). At 12 months, the fB sample (unfiltered and frozen) also exhibited this defect, while the filtered and frozen oil maintained the quality requirements for EVOO. In the ‘Cerasuola’ variety, the “Fusty” defect was perceived only in the room temperature-stored unfiltered sample (C) after 12 months of storage, resulting in oil downgraded to the VOO category due to defect presence. The findings confirm previous observations [ [33,42]] and underscore the varying effectiveness of treatments based on the characteristics of the olive cultivar: removing microparticles retaining small droplets of vegetation water preserves olive oil quality in more stable varieties but is less effective for less resilient cultivars, where combining filtration with low storage temperatures proves beneficial for extending shelf life reasonably.
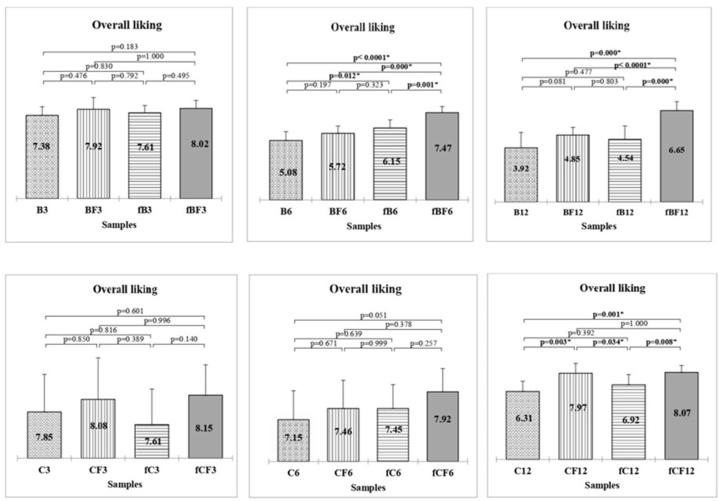
3.6. Consumer evaluation
The qualitative analytical parameters of olive oils play a crucial role in their marketing, yet the consumer's opinion remains the primary determinant of the product's commercial success. Analysis of consumer test results (Fig. 5) has revealed two key findings. Firstly, consumers, even without specific training, possess the ability to discern different quality levels of oils through direct comparison. Secondly, the ‘Biancolilla’ variety's poor stability inevitably leads to defects, which consumers can detect as early as six months of storage. Moreover, from six months of storage onward, noteworthy differences in overall liking were observed between samples stored at room temperature, unfiltered (B) and filtered (BF), and both frozen samples (fB and fBF). Additionally, a significant preference for the filtered and frozen sample (fBF) over all others was evident. In the case of ‘Cerasuola’ EVOO, significant differences in preference only surfaced between samples stored for 12 months, with no notable distinctions in liking observed in earlier samplings. Indeed, in the final sampling, the advantage of filtration in preserving oil quality over time was affirmed, with a significant preference for filtered samples stored both at room temperature (fC12) and frozen (fCF12).
Fig. 5:
Consumer-expressed overall liking for different olive oils stored for 3, 6 or 12 months. Filtered Biancolilla (BF), Biancolilla unfiltered (B), Cerasuola Filtered (CF), Cerasuola unfiltered (C), Frozen filtered Biancolilla (fBF), Frozen unfiltered Biancolilla (fB), Frozen filtered Cerasuola (fCF), Frozen unfiltered Cerasuola (fC). P value represent statistical differences based on non-parametric Kruskall-Wallis test.
4. Conclusions
By maintaining controlled storage conditions, including low temperatures, darkness, and the absence of oxygen, it becomes feasible to decelerate the oxidative processes affecting EVOO. However, the intricate and multifactorial nature of EVOO quality complicates the generalization of results in olive oil research. As various authors have confirmed, phenomena during storage impacting shelf life are closely tied to enzymatic activities influenced by factors such as variety, environment, processing methods, and, notably, storage conditions. Monovarietal oils, more prone to oxidative processes, exemplified by ‘Biancolilla’ in our research, exhibit signs of the degenerative process after approximately six months of storage, manifesting sensory defects that relegate the product to the VOO category. Filtration, promotes the removal of suspended particles and the minimal amount of emulsified vegetation water in oil, effectively slowing down hydrolytic activity over time despite slightly reducing the initial concentration of certain phenolic components. Our study's findings validate that filtration serves to retard degradation processes during extended storage periods. Combining filtration with lower temperatures, even freezing, proves effective in counteracting the degenerative process, ensuring good quality for at least a year of storage. In the case of ‘Cerasuola’, known for its inherent stability, filtration alone proves to be a suitable treatment for extending product stability beyond one year of storage. Our study suggests that for less stable cultivars, the optimal strategy for prolonging the shelf life of EVOO may involve combining filtration with freezing, providing insights into potential advancements in olive oil preservation strategies.
Data availability
Data will be made available on request to the corresponding author.
Ethic statements
The sensory and consumers tests were carried out in accordance with established ethical guidelines of the Declaration of Helsinki, accordingly, written informed consent has been obtained from every participants, after the nature of the study has been explained in understandable terms and all of them participated voluntarily. Participants were provided with a detailed explanation of the study's purpose, procedures, and use of the data, and they voluntarily agreed to participate via the statement “I am aware that my responses are confidential, and I agree to participate in this survey”. An affirmative reply was required to enter the survey. The questionnaires used in the study were provided to participants ensuring clarity and comprehensibility. The study was conducted with the utmost respect for participants' rights and confidentiality. Any identifiable information collected from participants was anonymized to protect their privacy. The methodology employed in this study was designed to minimize potential harm or discomfort to participants while maximizing the validity and reliability of the results. Sensory panelist and consumers were able to withdraw from the survey at any time without giving a reason. The products tested were safe for consumption.
Funding statement
No funding
CRediT authorship contribution statement
Diana De Santis: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization. Serena Ferri: Methodology, Formal analysis. Giorgio Milana: Formal analysis. Giovanni Turchetti: Writing – review & editing, Writing – original draft, Methodology. Margherita Modesti: Writing – review & editing, Writing – original draft, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29833.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Jimenez-Lopez C., Carpena M., Lourenço-Lopes C., Gallardo-Gomez M., Lorenzo J.M., Barba F.J., Prieto M.A., Simal-Gandara J. Bioactive compounds and quality of extra virgin olive oil. Foods. 2020;9(8):1014. doi: 10.3390/foods9081014. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagour J., Hallouch O., Asbbane A., Bijla L., Laknifli A., Lee L.-H., Zengin G., Bouyahya A., Sakar E.H., Gharby S. A review of recent progresses on olive oil chemical profiling, extraction technology, shelf-life, and quality control. Chem. Biodiversity. 2024;2024 doi: 10.1002/cbdv.202301697. [DOI] [PubMed] [Google Scholar]
- 3.Zanoni B. The Extra-Virgin Olive Oil Handbook . Editor Claudio Peri Online. 2014. The role of oxygen and water in the extra-virgin olive oil process. 9781118460412. [DOI] [Google Scholar]
- 4.Masella P., Angeloni G., Guerrini L., et al. Pumping contribution to dissolved oxygen in virgin olive oil during processing. Chemical Engineering Transactions. 2021;87 doi: 10.3303/CET2187052. [DOI] [Google Scholar]
- 5.Kalogianni E.P., Georgiou D., Hasanov J.H. Olive oil processing: current knowledge, literature gaps, and future perspectives. J. Am. Oil Chem. Soc. 2019;96(5):481–507. doi: 10.1002/aocs.12207. [DOI] [Google Scholar]
- 6.Romani A., Lapucci C., Cantini C., Ieri F., Mulinacci N., Visioli F. Evolution of minor polar compounds and antioxidant capacity during storage of bottled extra virgin olive oil. J. Agric. Food Chem. 2007;55(7):1315–1320. doi: 10.1021/jf062335r. [DOI] [PubMed] [Google Scholar]
- 7.Kotsiou K., Tasioula-Margari M. Monitoring the phenolic compounds of Greek extra-virgin olive oils during storage This work is in memory of Maria Tasioula-Margari, who was the thesis supervisor. Food Chem. 2016;200:255–262. doi: 10.1016/j.foodchem.2015.12.090. [DOI] [PubMed] [Google Scholar]
- 8.Deiana P., Molinu M.G., Dore A., Culeddu N., Dettori S., Santona M. Evolution of monovarietal virgin olive oils as a function of chemical composition and oxidation status. Nat. Prod. Res. 2022:1–5. doi: 10.1080/14786419.2022.2042813. [DOI] [PubMed] [Google Scholar]
- 9.Esposto S., Selvaggini R., Taticchi A., et al. Characterisation of Sicilian virgin olive oils: phenolic and volatile compounds as markers. La Riv. Ital. delle Sostanze grasse. 2013;40:31–41. [Google Scholar]
- 10.International Olive Council Sensory analysis of olive oil. Method for the organoleptic assessment of virgin olive oil. Int. Olive Counc. 2018;10 [Google Scholar]
- 11.Lobo-Prieto A., Tena N., Aparicio-Ruiz R., et al. Gradual changes of the protective effect of phenols in virgin olive oils subjected to storage and controlled stress by mesh cell incubation. J. Agric. Food Chem. 2023;71(42):15732–15744. doi: 10.1021/acs.jafc.3c04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zullo B.A., Ciafardini G. Changes in physicochemical and microbiological parameters of short and long-lived veiled virgin olive oil upon storage in the dark. Eur. J. Lipid Sci. Technol. 2018;120(1):1–8. doi: 10.1002/ejlt.201700309. [DOI] [Google Scholar]
- 13.Mulinacci N., Ieri F., Ignesti G., et al. The freezing process helps to preserve the quality of extra virgin olive oil over time: a case study up to 18months. Food Res. Int. 2013;54(2):2008–2015. doi: 10.1016/j.foodres.2013.03.052. [DOI] [Google Scholar]
- 14.Díez-Betriu J., Bustamante J., Romero A., et al. Effect of the storage conditions and freezing speed on the color and chlorophyll profile of premium extra virgin olive oils. Foods. 2023;12(1):1–11. doi: 10.3390/foods12010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ISO [International Organization for Standardization] 2013. 5636-5:2013 Paper and Board — Determination of Air Permeance [medium Range] — Part 5: Gurley Method. [Google Scholar]
- 16.ISO [International Organization for Standardization] 2017. 3960:2017 Animal and Vegetable Fats and Oils — Determination of Peroxide Value - Iodometric [Visual] Endpoint. [Google Scholar]
- 17.ISO [International Organization for Standardization] 2020. 660:2020 Animal and Vegetable Fats and Oils - Determination of Acid Value and Acidity. [Google Scholar]
- 18.Waterhouse L. Determination of total phenolics. Curr. Protoc. food Anal. Chem. 2002;6(1) doi: 10.1002/0471142913.fai0101s06. [DOI] [Google Scholar]
- 19.International Olive Council Determination of biophenols in olive oils by hplc. Int. Olive Counc. 2017:29. [Google Scholar]
- 20.International Olive Council Sensory analysis of olive oil. Standard guide for the selection, training and quality control of virgin olive oil tasters-qualifications of tasters, panel leaders and trainers. Int. Olive Counc. 2021:14. [Google Scholar]
- 21.International Olive Council Sensory analysis of olive oil. Standard glass for oil tasting. Int. Olive Counc. 2020:5. [Google Scholar]
- 22.Commission implementing Regulation [EU] 2019/1604 . 27 September 2019. Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. [Google Scholar]
- 23.Kalogeropoulos N., Tsimidou M.Z. Antioxidants in Greek virgin olive oils. Antioxidants. 2014 13;3(2):387–413. doi: 10.3390/antiox3020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skiada V., Tsarouhas P., Varzakas T. Preliminary study and observation of "kalamata PDO" extra virgin olive oil, in the messinia region, southwest of peloponnese (Greece) Foods. 2019 Nov 23;8(12):610. doi: 10.3390/foods8120610. PMID: 31771213; PMCID: PMC6963909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fregapane G., Lavelli V., León S., Kapuralin J., Salvador M.D. Effect of filtration on virgin olive oil stability during storage. Eur. J. Lipid Sci. Technol. 2006;108(2):134–142. doi: 10.1002/ejlt.200501175. [DOI] [Google Scholar]
- 26.El Yamani M., Sakar E.H., Boussakouran A., Rharrabti Y. Effect of storage time and conditions on the quality characteristics of 'Moroccan Picholine' olive oil. Biocatal. Agric. Biotechnol. 2022;39 doi: 10.1016/j.bcab.2021.102244. [DOI] [Google Scholar]
- 27.Alipour Gaskari V., Nasrollahzadeh A. Investigating the effect of conditions and storage time on the quality characteristics of virgin and extra virgin olive oil. FSCT. 2023;19(132):365–375. 2023. 1001.1.20088787.1401.19.132.30.3. [Google Scholar]
- 28.Alvarruiz A., Pardo J.E., Copete M.E., et al. Evolution of virgin olive oil during long-term storage. J. Oleo Sci. 2020;69(8):809–814. doi: 10.5650/jos.ess19258. 6. [DOI] [PubMed] [Google Scholar]
- 29.Rotondi A., Morrone L., Bertazza G., Neri L. Effect of duration of olive storage on chemical and sensory quality of extra virgin olive oils. Foods. 2021;10(10):2296. doi: 10.3390/foods10102296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genovese A., Mondola F., Paduano A., Sacchi R. Biophenolic compounds influence the in-mouth perceived intensity of virgin olive oil flavours and off-flavours. Molecules. 2020;25(8):1969. doi: 10.3390/molecules25081969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Yamani M., Sakar E.H., Boussakouran A., Rharrabti Y. Activity of two natural additives in improving the stability of virgin olive oil quality during storage. OCL - Oilseeds and fats, Crops and Lipids. 2020;27(44):1–10. doi: 10.1051/ocl/2020039. [DOI] [Google Scholar]
- 32.Di Stefano V. In: Olives and Olive Oil in Health and Disease Prevention. Preedy Victor R., Watson Ronald Ross., editors. Academic Press; 2010. 2010, ISBN 9780123744203. [DOI] [Google Scholar]
- 33.Lozano-Sánchez J., Segura-Carretero A., Fernández-Gutiérrez A. Characterisation of the phenolic compounds retained in different organic and inorganic filter aids used for filtration of extra virgin olive oil. Food Chem. 2011;24(3):1146–1150. doi: 10.1016/j.foodchem.2010.07.026. [DOI] [Google Scholar]
- 34.Jabeur H., Zribi A., Bouaziz M. Changes in chemical and sensory characteristics of Chemlali extra-virgin olive oil as depending on filtration. Eur. J. Lipid Sci. Technol. 2017;119(1):1–10. doi: 10.1002/ejlt.201500602. [DOI] [Google Scholar]
- 35.Bakhouche A., Lozano-Sánchez J., Ballus C.A., et al. Monitoring the moisture reduction and status of bioactive compounds in extra-virgin olive oil over the industrial filtration process. Food Control. 2014;40:292–299. doi: 10.1016/j.foodcont.2013.12.012. [DOI] [Google Scholar]
- 36.Krichene D., Salvador M.D., Fregapane G. Stability of virgin olive oil phenolic compounds during long-term storage [18 Months] at temperatures of 5-50°C. J. Agric. Food Chem. 2015;63(30):6779–6786. doi: 10.1021/acs.jafc.5b02187. [DOI] [PubMed] [Google Scholar]
- 37.Medina S., Auñón D., Lehoux J., et al. Hydroxytyrosol fatty acid esters as new candidate markers for detecting olive oil inadequate storage conditions by UHPLC-QqQ-MS/MS. Microchem. J. 2022;181 doi: 10.1016/j.microc.2022.107656. [DOI] [Google Scholar]
- 38.Abbattista R., Losito I., Castellaneta A., et al. Insight into the storage-related oxidative/hydrolytic degradation of olive oil secoiridoids by liquid chromatography and high-resolution fourier transform mass spectrometry. J. Agric. Food Chem. 2020;68(44):12310–12325. doi: 10.1021/acs.jafc.0c049257. 2020. [DOI] [PubMed] [Google Scholar]
- 39.Migliorini M., Cherubini C., Cecchi L., Zanoni B. Degradation of phenolic compounds during extra virgin olive oil shelf-life. La Riv. Ital. delle sostanze grasse. 2013;40:71–80. [Google Scholar]
- 40.Cecchi L., Parenti A., Bellumori M., Migliorini M., Mulinacci N., Guerrini L. Clustering monovarietal extra virgin olive oil according to sensory profile, volatile compounds, and k-mean algorithm. Eur. J. Lipid Sci. Technol. 2022;124(11):1–10. doi: 10.1002/ejlt.202200038. [DOI] [Google Scholar]
- 41.Pang K.L., Chin K.Y. The biological activities of oleocanthal from a molecular perspective. Nutrients. 2018;10(5):1–22. doi: 10.3390/nu10050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacchi R., Caporaso N., Paduano A., Genovese A. Industrial-scale filtration affects volatile compounds in extra virgin olive oil cv. Ravece. Eur. J. Lipid Sci. Technol. 2015;117(12):2007–2014. doi: 10.1002/ejlt.201400456. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request to the corresponding author.