Abstract
Chipilin (Crotalaria longirostrata) is consumed as a vegetable in the preparation of traditional dishes. As a folk medicine, Chipilin extracts are used as a hypnotic and sedative agent; however, there are few reports that support these uses. This study aimed to characterize the compounds present in Chipilin leaf extracts and to investigate their sedative effect using zebrafish as an in vivo model. Extracts were obtained by maceration with water (H2O), ethanol (EtOH), and EtOH-H2O, while oleoresin was obtained by supercritical fluid extraction (SFE). Total phenolic and flavonoid contents were quantified by colorimetric methods. Phytochemical constituents were identified by gas chromatography–mass spectrometry (GC-MS) analysis. The chronic and acute toxicities of Chipilin extracts were tested in zebrafish embryos and larvae, respectively. Chipilin sedative effect was tested by the larvae response to dark–light–dark transitions. EtOH-H2O extracts had the highest value of total phenolics (5345 ± 5.1 μg GAE/g), followed by water and oleoresin (1815 ± 5.1 and 394 ± 5.1 μg GAE/g, respectively). In water extracts were identified the alkaloid trachelanthamidine, 1,2β-epoxy- and the alkyl ketone 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione, while oleamide, α-monostearin, and erucamide were detected in all samples except in water extracts. Oleoresin extract had the lowest embryotoxicity (LC50 = 4.99 μg/mL) and the highest sedative effects. SFE is a green alternative to obtain Chipilin extracts rich in erucamide, an endocannabinoid analogue, which plays an important role in the development of the central nervous system and in modulating neurotransmitter release.
1. Introduction
Crotalaria longirostrata Hook. & Arn. (Fabaceae), known as Chipilin, is a leguminous plant native to Central America and Southern Mexico. Chipilin leaves and shoots have been used for thousands of years as vegetables in the preparation of traditional meals. Local empirical knowledge about the medicinal uses of Crotalaria sp. has been registered,1 but despite the fact that C. longirostrata is considered one of the 16 most important edible leaf species worldwide, scarce literature is reported to support its use.2
As a folk medicine, Chipilin extracts are used to treat several health conditions.1,3,4 The antimicrobial, antifungal, insecticidal, and anticancer activities of acetonic and methanolic extracts of Chipilin leaves and branches have been reported.5−8 As an ethnomedicine, Chipilin is recommended as an evening meal to treat insomnia and anxiety.1,9−11 However, its pharmacological activity as a sedative agent is not well-known; in addition, little is known about the compounds that are present in Chipilin extracts that could be related to this action. Thus, further research is needed.1,12 On the other hand, the selection of an efficient extraction method is decisive for the identification of novel phytochemicals. Indeed, the identification of new sources of bioactive compounds and their functions has a growing demand in the healthcare, pharmaceutical, food, and beverage industries.13
Traditional methods such as maceration, digestion, percolation, decoction, infusion, countercurrent extraction, and continuous hot extraction have been commonly used for the extraction of bioactive compounds. These processes have some disadvantages, such as the use of a large number of solvents, low extraction yields, and high extraction temperatures. Supercritical fluid extraction (SFE) using carbon dioxide (CO2) as a solvent is considered a new green technology classified as generally recognized as safe (GRAS), which has motivated its application for the extraction of plant bioactive compounds.14,15
For all of the above, this study aimed to characterize Chipilin leaf extracts obtained by the traditional maceration process using water, ethanol, and ethanol–water as solvents, as well as the oleoresin obtained by the SFE process. The total phenolic compound and flavonoid contents as well as the antioxidant activity of Chipilin extracts were evaluated by colorimetric methods. Phytochemicals in Chipilin extracts were identified by gas chromatography–mass spectrometry (GC-MS), and their toxicological effects were tested in zebrafish embryos, while their sedative activity was evaluated in larvae using the swimming test in a dark–light–dark transition assay.
2. Materials and Methods
2.1. Plant Material
C. longirostrata leaves were collected in September 2021 from plants grown in open fields in the municipality of Chiapa de Corzo, Chiapas, Mexico (16°39′16″N, 93°0′36″W), at an altitude of about 400 m and characterized by their humid and warm tropical climate (Figure 1). A voucher specimen of C. longirostrata was taxonomically identified and deposited in the Herbarium of the National Institute of Forestry and Agricultural Research (INIFAP-Mexico) under the number INI23000126. The leaves were cleaned and left to dry at 34 °C. Clean and dry leaves were ground in a knife mill to obtain a fine powder, which was passed through a 100 mesh to obtain a uniform particle size.
Figure 1.
Crotalaria longirostrata plants. (A) Plants growing in traditional fields; (B) C. longirostrata leaves; (C) C. longirostrata flowers.
2.2. Preparation of Extracts
2.2.1. Maceration Process
Extracts were prepared by maceration of Chipilin leaf powder in a ratio of 1:20 (w/v) in distilled water (H2O), ethanol (EtOH), and EtOH-H2O (1:1, v/v), for 24 h at room temperature under constant agitation. Extracts were centrifuged at 12,000g for 1 min, and the supernatants were filtered through 0.22 μm sterile syringe filters (Sigma-Aldrich, St. Louis, MI). The supernatants were recovered and stored in an amber flask at 4 °C until needed. All extractions were carried out in triplicates.
2.2.2. Supercritical Fluid Extraction (SFE)
Chipilin leaf powder (100 g) was subjected to a dynamic extraction using an FSC BioBotanical Extraction System (Waters Co., Milford, MA). The extraction conditions using ethanol as the cosolvent are shown in Supporting Table S1. The obtained oleoresin was stored in amber flasks at 4 °C until needed. Oleoresins were obtained in triplicate.
2.3. Phytochemical Screening
2.3.1. Total Phenolic Compound Content
Total phenolic compounds were quantified using the Folin–Ciocalteu method16 with some modifications. Briefly, the extracts (20 μL) were mixed with 780 μL of Milli-Q water and 50 μL of Folin–Ciocalteu reagent. The mixture was incubated for 10 min in the dark, and then, 150 μL of 20% Na2CO3 solution was added, mixed, and incubated for 2 h at room temperature in the dark. The absorbance of the samples was measured at 765 nm by using a microplate reader (Multiskan GO spectrophotometer, Thermo-Fisher Scientific Inc., Waltham, MA). A calibration curve was prepared using gallic acid (GA) at different concentrations (0–500 μg/mL). The total phenolic content was expressed as milligrams of GA equivalents/g of sample (GAE/g). All measurements were carried out in triplicates.
2.3.2. Total Flavonoid Content
Flavonoid quantification was carried out using 200 μL of the extract mixed with 800 μL of 2% AlCl3 ethanolic solution.17 Samples were then incubated for 10 min in the dark. Absorbance was read at 430 nm using a microplate reader (Multiskan GO, Thermo-Fisher). A calibration curve was prepared using quercetin (Q) as the standard at concentrations from 0 to 100 μg/mL. The results were expressed as Q equivalents/g sample (QE/g).18 All determinations were carried out in triplicates.
2.3.3. DPPH Radical Scavenging Test
Chipilin leaf extracts were assayed for their antioxidant capacity using the DPPH (2,2-difenil-1-picrylhydrazyl) method.18 Extracts (100 μL) were mixed with 100 μL of a 400 μM DPPH ethanolic solution. The samples were incubated in the dark for 30 min, and the absorbance was measured at 517 nm. The results were expressed as the percentage of reduced DPPH using the following formula
where Am is the absorbance of the testing sample and Ao is the DPPH initial absorbance.
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Chromatography analyses were carried out using an Agilent 7820A/5977E GC-MS system (Agilent Technologies, Inc., Willington, DE) provided with an autosampler 7863 (Agilent Technologies) and a capillary column HP5-MS (30 m × 0.25 μm i.d., ×0.25 mm film thickness, Agilent Technologies); the injector temperature was settled at 220 °C. The initial oven temperature was 50 °C for 1 min, followed by 30 °C/min until 280 °C for 10 min, and then at 15 °C/min until reaching 300 °C for 4 min. Helium was used as the carrier gas at a flow rate of 1.0 mL/min. The sample (5 μL) was injected (20:1 split ratio), the ionization energy was 70 eV, and the mass range evaluated ranged from 45 to 550 m/z. The GC-MS system was calibrated automatically by the system verification Tune (detector optimization) using the Perfluoro-compound FC-43 (Thermo-Fisher) as the standard. Compounds were identified by comparing their retention times and mass spectra against the National Institute of Standards and Technology Mass spectra (NIST MS v2.2.).
2.5. Evaluation of Toxic and Sedative Effects
2.5.1. Zebrafish Maintenance
Adult zebrafish of the AB-Tu/-Wik hybrid line (>6 months of age) were maintained in tanks with a recirculating water system under standard rearing conditions (pH 7.5, conductivity 500–750 μS, 28 °C, and 14/10 h light/dark cycle). Zebrafish were fed daily with a brine shrimp culture (Brine Shrimp Direct, pellets, Zeigler Bros, Inc.). The nitrate, nitrite, and ammonia contents were monitored every week to maintain the water quality. Egg production was carried out by natural spawning in a breeding tank at a 1:2 (male/female) ratio. The experimental protocol was approved by the Animal Care and Use Committee of the Institute of Neurobiology of the National Autonomous University of Mexico (protocol number 95A).
Chipilin extracts at different concentrations were prepared daily in an E3 buffer (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, and 1% DMSO). The control group for each treatment was maintained in the E3 buffer. For the chronic toxicity test, the extract concentrations were 31.25, 62.5, 125, 250, and 750 μg/mL. For the acute toxicity test, extract concentrations tested were 0.5, 1, 2.5, 5, 7, 10, and 50 mg/mL.
2.5.2. Zebrafish Embryo Chronic Toxicity Test (FET)
The toxic effect of the Chipilin extracts was evaluated as a chronic fish embryotoxicity (FET) test under the Guidelines for the Testing of Chemicals No. 236 of the Organization for Economic Co-operation and Development.19 The embryotoxicity test evaluates lethality, sublethality, and teratogenic parameters within the first 120 h post fertilization (hpf).20
Fertilized embryos (n = 20) at 2 hpf were transferred to Petri dishes (60 mm × 15 mm) and incubated with different extract concentrations (31.25, 62.5, 125, 250, and 750 μg/mL). Embryos were exposed to the extract solutions (8 mL) since 2 hpf and continued until 5 days post fertilization (dpf), with daily replacement of the solutions (Supporting Figure S1). The plates were incubated at 28 °C with a 14/10 h light/dark cycle, and the evaluation criteria for embryos and larvae from 24 to 120 hpf were carried out.21 Egg coagulation, somite formation, and tail detachment were monitored from 24 hpf; edema formation, pigmentation, and heart rate from 48 hpf; and embryo hatching and scoliosis from 72 hpf. The mean lethal concentrations (LC50) were obtained from the percentage of accumulated mortality at different hours of evaluation, compared to the logarithm of the concentrations. All experiments were carried out in triplicate.
2.5.3. Zebrafish Larvae Acute Toxicity Test
To assess the acute toxicity of the Chipilin extracts, larvae (n = 10) of 72 hpf were individually transferred to a 96-well plate and incubated with a single dose of 200 μL of different working solutions (0.5, 1, 2.5, 5, 7, 10, and 50 mg/mL). Changes in morphology, blood circulation, and heartbeats were monitored at 0, 1, 3, 5, 7, 12, and 24 h. The plates were incubated at 28 °C with a 14/10 h light/dark cycle. The experiments were carried out in triplicate.
2.5.4. Sedative Activity of Chipilin Extracts
The response of the larvae to dark–light–dark transitions was evaluated according to a reported protocol.22 Larvae at 7 days post fertilization (n = 16) that were exposed to working solutions with daily replacement from 2 hpf were individually transferred to a 24-well plate with 1.5 mL of E3 buffer. The plate was set above a light chamber and covered with black conical plastic that held a video camera on top. The larvae were habituated to darkness for 10 min, and recording began during the last 3 min of this period, after which the light was turned on and recorded for a further 3 min. Finally, the light was turned off, and the recording continued for another 3 min.
Velocity and swimming trajectories were analyzed using EthoVisionXT software (Noldus IT, Wageningen, The Netherlands). The total locomotion of the individual larvae was summarized by three response variables: the distance traveled, speed, and latency at the moment of starting the movement. Experiments were carried out in triplicate in a fish room at 28 °C.
2.6. Statistical Analysis
Statistical analyses were carried out using GraphPad Prism 8 software (La Joya, CA). The comparison between groups was performed using one-way ANOVA followed by Tukey’s test with p < 0.05 for statistically significant differences.
3. Results and Discussion
3.1. Phytochemical Screening and Antioxidant Activity
The highest total phenolic compound contents (5345 ± 5.1 μg GAE/g) were observed in ethanol–water extracts (EtOH-H2O), followed by water extract (1815 ± 5.1 μg GAE/g), oleoresin (394 ± 5.1 μg GAE/g), and EtOH extracts (153 μg ± 2.7 μg GAE/g) (Table 1). The Chipilin water extract showed a similar total phenolic content to the values reported in the literature.4 In relation to flavonoid content, the EtOH extract showed the highest content (4841 ± 3.5 μg QE/g) followed by oleoresin and EtOH-H2O extract (1870 ± 5.1 and 1630 ± 5.7 μg QE/g, respectively). The lowest flavonoid content was obtained with water (234 ± 4.3 μg QE/g) (Table 1). Supercritical carbon dioxide is a nonpolar solvent where low-polarity compounds are easily dissolved (isoflavones, flavanones), but the addition of a cosolvent, such as ethanol, can enhance the extraction of polar compounds.14
Table 1. Total Phenolic, Flavonoids, and Antioxidant Capacities of Different Extracts Obtained from C. longirostrata (Chipilin) Leavesa.
| extract | total phenolicb | flavonoidsc | antioxidantd capacity |
|---|---|---|---|
| water | 1815 ± 4.7b | 234.5 ± 4.3d | 21.3 ± 1.1c |
| ethanol | 153.0 ± 2.7d | 4841.3 ± 3.5a | 48.3 ± 1.4b |
| ethanol–water | 5345 ± 5.1a | 1630 ± 57c | 64.3 ± 2.0a |
| SFE | 393.9 ± 5.1c | 1870 ± 5.1b | 24.3 ± 2.1c |
Data shows the mean ± standard deviation (n = 3).
Reported as μg gallic acid equivalents/g of sample on dry matter.
Reported as μg of quercetin equivalents/g of sample on dry matter.
Reported as % of DPPH radical scavenging activity. Different superscript letters indicate significant differences among means at p ≤ 0.05. SFE, supercritical fluid extraction.
The EtOH-H2O extract showed the highest antioxidant activity with 64.3% inhibition of DPPH, followed by the EtOH extract, oleoresin, and water extract (48.3, 24.3, and 21.3%, respectively) (Table 1). The antioxidant activity was in relation to the amounts of total phenolic compounds and flavonoid content.
3.2. GC-MS Analysis
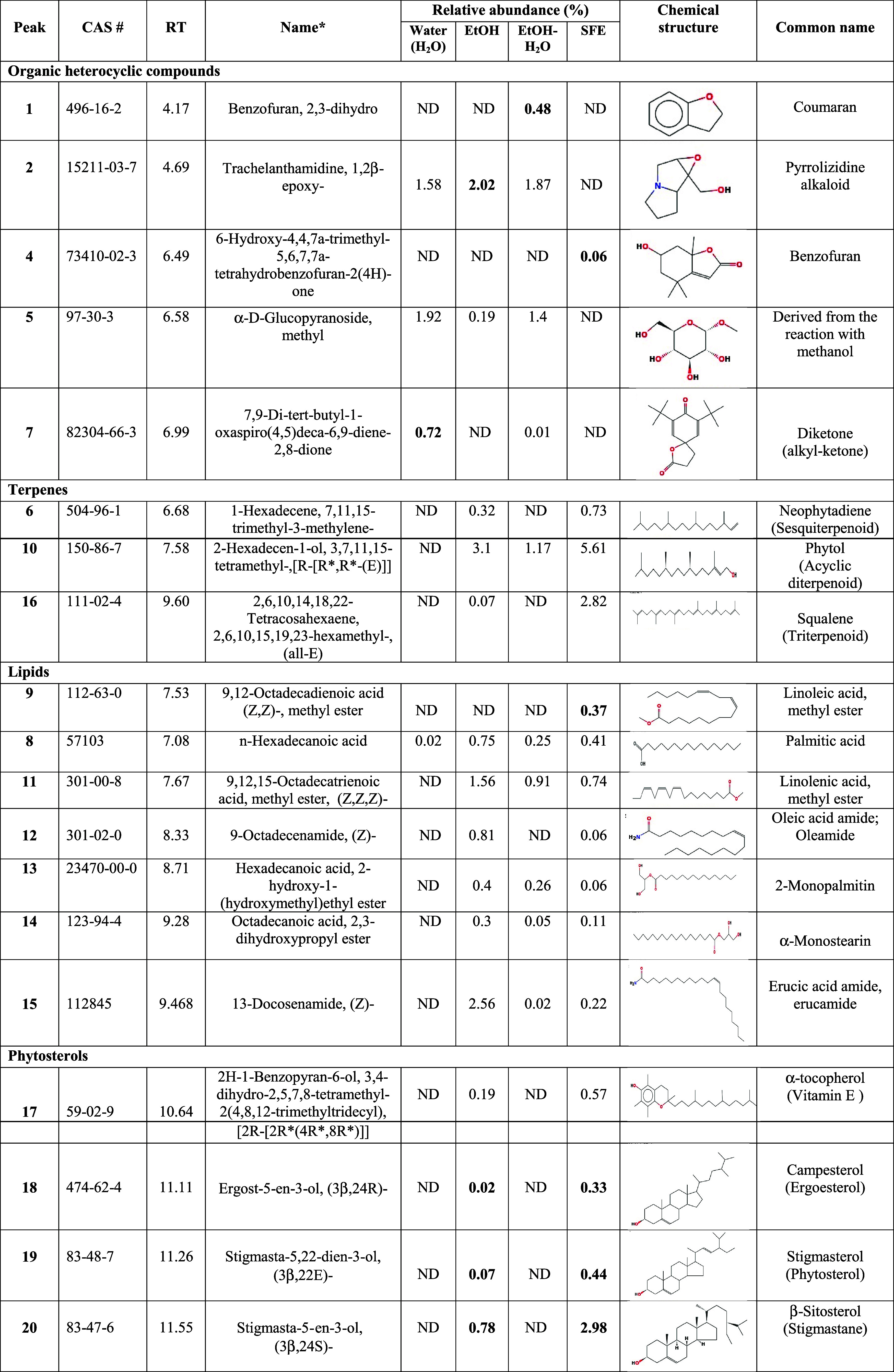
Chipilin extracts were analyzed by GC-MS, and representative chromatograms are shown in Supporting Figure S2. The relative abundance of the extracted compounds among processes varies qualitatively and quantitatively. Four compounds were identified in the water extract (peaks 2, 7, 5, and 8), 15 in the EtOH extract (peaks 2, 5, 6, 8 10–20), 10 in the EtOH-H2O extract (peaks 1, 2, 5, 7, 8, 10, 11, and 13–15), and 16 compounds in oleoresin (peaks 3, 4, 6, 8–20).
Among heterocyclic compounds, benzofuran, 2,3-dihydrobenzofuran (peak 1) was detected only in the EtOH-water extract (Table 2). Benzofurans and their derivatives can be used to design and develop novel therapeutics.23 Trachelanthamidine, 1,2β-epoxy- (peak 2) was identified in all samples except in oleoresin, and 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (peak 7) was identified in water and EtOH-H2O extracts (Table 2). Trachelanthamidine is a pyrrolizidine alkaloid (PA) that has been detected in Boraginaceae, Fabaceae, Asteraceae, and Heliotropium transalpinum. PAs from plants are toxic compounds that can cause mutations in DNA in herbivores and humans.24 The identification of one alkaloid belonging to the iminosugar group was reported and related to Chipilin’s insecticidal action.7 The oxaspiro compound, reported as a diketone or akyl-ketone, has been detected by GC-MS in Brasiliorchis schunkeana and Ficus religiosa seeds.25,26 Several activities such as steroidal antimineralocorticoid, antiandrogen, and glucocorticoid have been reported for this compound.27 Oleoresin obtained by SFE was characterized by the presence of 6-hydroxy-4,4,7α-trimethyl-5,6,7,7α-tetrahydrobenzofuran-2(4H)-one (peak 4), a volatile compound isolated from Sargassum horneri with tested anti-inflammatory activity showing antitumor, antibacterial, antioxidative, and antiviral activities.28
Table 2. Identification of Volatile and Semi-volatile Metabolites by GC-MS from C. longirostrata (Chipilin) Extractsa.
RT, retention time (min); SFE, supercritical fluid extraction; ND, not detected. *, name according to report at NIST Chemistry WebBook.
Neophytadiene (peak 6), a sesquiterpene, was detected only in EtOH and oleoresin extracts, whereas phytol (a diterpene) was detected in all samples, except in the water extracts (Table 2). Neophytadiene is known for its anti-inflammatory properties,29 and antimicrobial activity against Salmonella typhimurium and Staphylococcus aureus has been reported.30 Phytol is an acyclic diterpene alcohol with antioxidant properties, and its anti-inflammatory activity was demonstrated by a reduction in IL-1ß and TNF-α levels. Phytol is also associated with the prevention of metabolic diseases, and its anticonvulsant activity has been reported.31,32
The only lipid detected in water extracts was palmitic acid, which was also detected in all samples (Table 2). Linoleic acid methyl ester (9,12-octadecadienoic acid (Z,Z)-, methyl ester), a fatty acid methyl ester of linoleic acid, was detected only in oleoresin. This compound acts as a key regulator of gut homeostasis by suppressing NF-κB-mediated pro-inflammatory responses.33
The presence of oleamide, α-monostearin (an emulsifier), and erucamide was detected in all samples, except in water extracts (Table 2). It has been reported that oleamide, the sleep hormone, has hypnotic properties, which have been demonstrated in rats by reduction of distance traveled.34,35 In mice, oleamide significantly reduced sleep latency and wake time while increasing nonrapid eye movement and total sleep time.35 Erucamide is an endocannabinoid analogue, a compound that plays important roles in central nervous system development and several physiological processes such as modulation of neurotransmitter liberation, regulation of pain perception, and cardiovascular, gastrointestinal, and hepatic functions.36,37
Squalene (peak 16), a natural triterpene, is a metabolite intermediate of the sterol biosynthetic pathway that possesses antioxidant properties.38 Some sterols, α-tocopherol (vitamin E), campesterol, stigmasterol, and γ-sitosterol, were detected only in EtOH and oleoresins; however, higher abundances of these compounds were observed in oleoresins (Table 2).
3.3. Evaluation of Chronic and Acute Toxicity in Zebrafish Embryos
Zebrafish (Danio rerio) provides multiple advantages as a model organism39,40 because it easily absorbs small molecules; its use has increased as a model to evaluate toxicological studies and behavioral analysis such as stress, fear, and anxiety.40 In addition, zebrafish shares similarities with the structure and function of the human nervous system and provides a good platform for the efficient detection of psychoactive and sleep-modulating molecules.41−44 Chipilin extracts and oleoresin were tested in fish embryos, and their toxicity was evaluated by continuous exposure to different extract concentrations from 2 hpf to 5 dpf, with daily replacement of the solutions and constant monitoring. Toxicity at concentrations of 31.25, 62.5, and 125 μg/mL was not significantly different from that of the control (E3 buffer). However, abnormalities in zebrafish development were observed at concentrations of 250 μg/mL and above (Supporting Table S2). The highest toxicity of Chipilin extracts, measured by egg coagulation or mortality, was observed in the water extract (56%), followed by EtOH-water (32%) and EtOH (10%), whereas oleoresin showed no significant toxicity compared to the control (Supporting Table S2). The water and EtOH extracts induced some malformations in embryonic development after 48 hpf such as observed by delayed tail detachment, edema formation, decreased pigmentation, and decreased heart frequency. The water extract causes scoliosis (skeletal deformities), and the EtOH-water extract delayed the hatching of the embryo (Supporting Table S3).
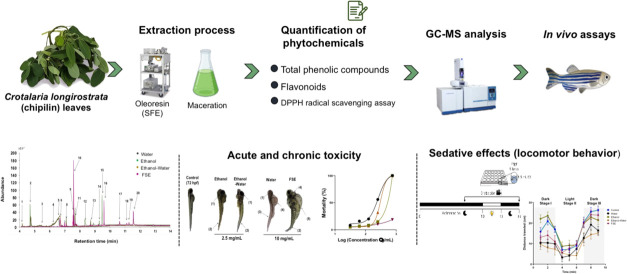
According to the dose–response curves for mortality of zebrafish embryos accumulated up to 120 hpf, the water extract showed the highest degree of toxicity with an LC50 of 2.41 μg/mL, followed by the EtOH-water extract with an LC50 of 2.49 μg/mL, and the EtOH extract with an LC50 of 3.41 μg/mL, and oleoresin presented the lowest degree of toxicity with an LC5o of 4.99 μg/mL (Figure 2A). At a maximum concentration evaluated of 750 μg/mL, most of the extracts caused 100% mortality within the first 72 hpf, highlighting again that oleoresins had the lowest mortality, 18.8% (Figure 2B).
Figure 2.
(A) Accumulated mortality of zebrafish embryos at 120 h post fertilization (hpf) in the presence of Chipilin extracts. Concentrations of Chipilin extracts were 31.25, 62.5, 125, 250, and 750 μg/mL. Each point represents the medium (n = 20), and asterisk means a mean with significant difference at p < 0.05. (B) Accumulated toxicity on zebrafish embryos of Chipilin extracts at 120 hpf. Concentration of Chipilin tested ranged from 0 to 800 μg/mL. SFE, supercritical fluid extraction.
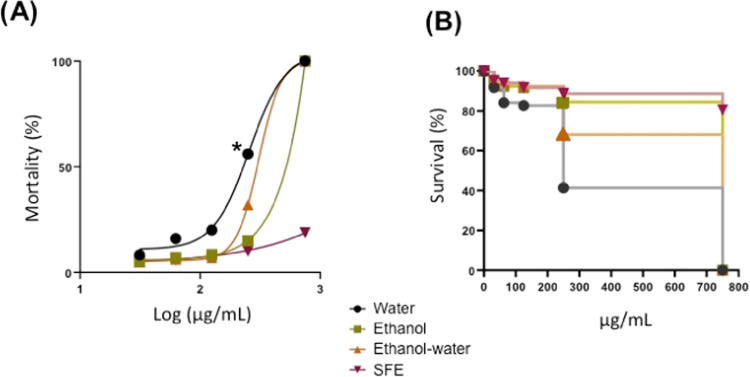
The acute toxicity or exposure to a single dose of Chipilin extracts, at different concentrations (0.5–50 mg/mL), was evaluated in 72 hpf larvae for 24 h. Results showed that the maximum concentration tolerated by larvae of water and EtOH extracts indicated by a 100% survival was 0.75 mg/mL. A mortality of 100% was observed with water extract (at 50 mg/mL) within the first 12 h of exposure, while 10 mg/mL EtOH extract was sufficient to obtain 100% mortality in 2 h (Figure 3A,B). For the EtOH-water extract, the maximum concentration tolerated by larvae was 1 mg/mL, and 100% mortality was achieved with 50 mg/mL after 2 h of exposure (Figure 3C). The highest concentration of oleoresin tolerated by larvae with 100% survival was 7 mg/mL, which was 10 times higher than that of the other extracts; however, 100% mortality was also obtained at 50 mg/mL within 2 h (Figure 3D). Some of the alterations observed in the larvae when exposed at concentrations of 2.5 mg/mL for EtOH and EtOH-water extracts and 10 mg/mL for water and oleoresin are shown in Supporting Figure S3.
Figure 3.
Survival of zebrafish larvae at 72 hpf (hours post fertilization) in the presence of different Chipilin extracts extracted with different solvents: (A) water (0.75, 10, and 50 mg/mL); (B) ethanol (0.75, 2.5, 5, and 10 mg/mL); (C) ethanol–water (10, 2.5, and 50 mg/mL); and (D) SFE (supercritical fluid extraction) at 7, 10, and 50 mg/mL.
The toxicity of the extracts was dose-dependent, and the water extract showed higher toxic effects at lower concentrations. This could be attributed to the synergistic effect of metabolites present in this sample, where the alkaloid trachelanthamidine was the most abundant, and this has been attributed to carcinogenic and hepatotoxic effects;24 however, it is also considered a harmless metabolite.45
3.4. Evaluation of Sedative Effects in Zebrafish Larvae
Zebrafish is an important model for psychoactive substance research because its brain structure, neurochemistry, and sleep/wake pattern are similar to those of humans.42 Then, behavioral tests with zebrafish larvae exposed to light–dark transitions were proposed to assess the effect of psychoactive molecules. During the light–dark transition, the locomotor activity increases and is attributed to an increased stress/anxiety level, while the dark–light transition decreases locomotion.46 To evaluate the sedative properties of Chipilin, the larvae locomotor behavior was evaluated in dark–light–dark transitions in the presence of Chipilin extracts (125 μg/mL) from 2 hpf to 7 dpf (Figure 4A). Zebrafish larvae respond to changes in lighting, and their motor activity decreases in the presence of light and increases during periods of dark. Behavioral parameters that allow the assessment of fish motor activity include swimming speed, distance traveled, angular velocity, thigmotaxis preference, and feeding performance. Variations in these criteria are related to external stress, anxiety level, and neurotoxicity.47
Figure 4.
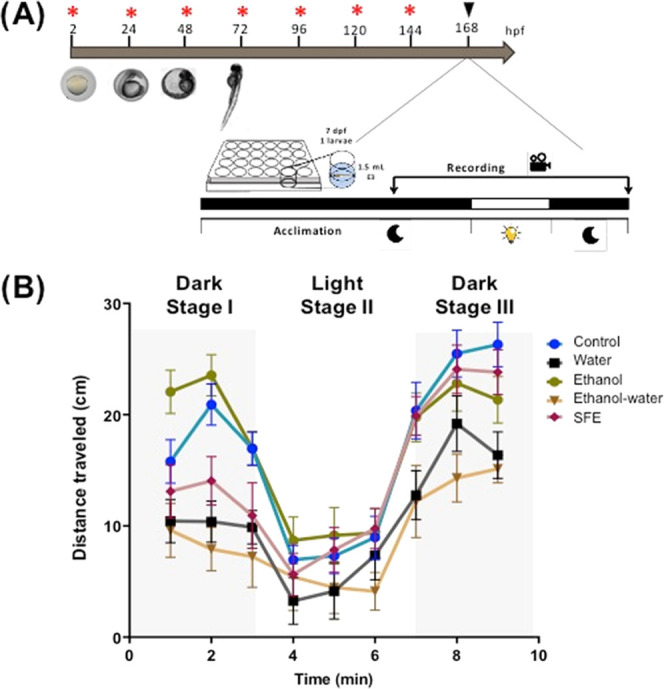
(A) Design for the assay for D. rerio larvae locomotion. In each well were added 1.5 mL of E3 buffer and one larva of 7 dpf (days post fertilization). Red asterisks indicate the time of addition of Chipilin extracts. Black triangle indicates the time for recording the fish movement. (B) Distance traveled by zebrafish embryos over time on the dark–light–dark transitions. SFE, supercritical fluid extract.
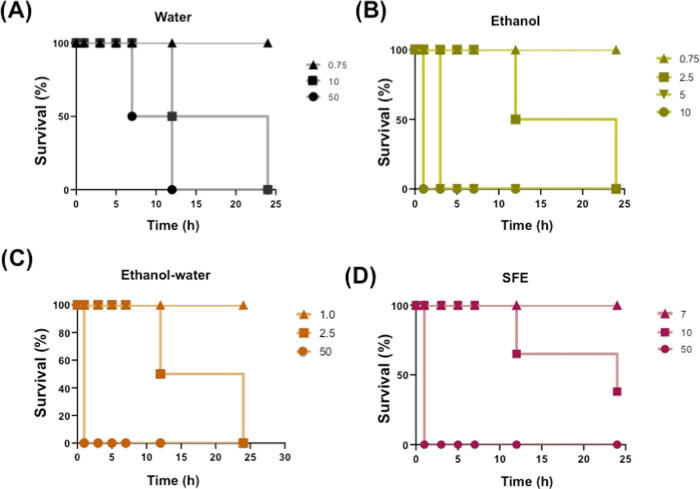
Under control conditions, zebrafish showed a normal pattern of behavior with a higher distance traveled during the dark (stages I and III) and lesser movement during illumination (stage II). When Chipilin extracts were added, all treatments induced a decrease in movement in stage I, except for the EtOH extract, which seemed to accelerate the zebrafish movement. In stage II, when light was turned on (phase III), zebrafish treated with oleoresin showed no significant differences from the control, EtOH showed a significant decrease in movement compared to stage I, but zebrafish treated with water and EtOH-water extracts showed a significant decrease in movement (Figure 4B).
In addition to the distance traveled, the swimming speed of the larvae was also analyzed (Figure 5). In stage I, it was observed that the swimming speed decreased significantly in the presence of water and EtOH-water extracts as well as oleoresin, but no effect on the speed of swimming was observed when the EtOH extract was administered. This behavior was similar in stage II, but in stage III, larvae treated with water and EtOH-water extracts showed a significant decrease in swimming speed compared to the control, and the EtOH extract showed a tendency to increase the speed of swimming (Figure 5A). In the case of oleoresin, the swimming speed during stage III was similar to that of the control, which could indicate recovery from sedation. In contrast, for the water and EtOH-water extracts, the sedative effect persisted despite the deprivation of the light stimulus. The movement pattern of the larvae (Figure 5B) also showed that the control and oleoresin have a similar pattern of swimming, and zebrafish subjected to water extract induces a lower exploration.
Figure 5.
(A) Swim velocity and (B) swim trajectory of zebrafish larvae at 7 dpf (days post fertilization) exposed to 125 μg/mL Chipilin extract during the dark–light–dark transitions. C, control; SFE, supercritical fluid extract.
The present results showed that the Chipilin oleoresin has a sedative effect without causing toxic effects since after exposure to the light stimulus (stage III) its behavior was similar to that of the control. The total sedative or toxic phenotype of the aqueous and EtOH-water extracts remained throughout the entire experiment, where there was a decrease in motor activity and swimming speed. The anticonvulsant activities of numerous phyto-constituents identified in plant extracts have been described, such as phytol, oleamide, erucamide, phenolic acids, and p-coumaric acid,48 which selectively bind to the GABAA receptor and are likely involved in the sedative effect of the extracts. Zebrafish have many conserved neurotransmitter pathways compared with humans, where GABA receptors are the most abundant and, therefore, the most studied against bioactive compounds. However, we are not unaware that other neuroreceptors may be involved in the total sedative effect observed.49,50 These metabolites can act as anticonvulsants because of their high lipophilicity, which facilitates their penetration through the blood–brain barrier.51 However, despite the fact that our results coincide with the ethnopharmacological use of this medicinal plant in the popular tradition, studies must be carried out to elucidate the mechanisms of action of this property. In addition, it is necessary to establish whether the synergy between the phytochemicals could be, in part, responsible for the sedative effect or if it is attributed to a particular compound.
4. Conclusions
Chipilin leaves have been used as a vegetable to prepare traditional dishes, and in folk medicine, Chipilin leaf extracts are used as a hypnotic and sedative agent; however, there is scarce literature to support these uses. The present results showed that the maceration process with water, EtOH, and EtOH-H2O showed the highest content of total phenolic and flavonoid compounds, and the oleoresin obtained by SFE contained more diversity of compounds identified by GC-MS. SFE also had the lowest toxicity for zebrafish embryos. The locomotion assay of zebrafish larvae exposed to oleoresin and the aqueous and ethanolic extracts showed a sedation phenotype; however, the SFE oleoresin showed a complete sedation phenotype with high recovery as the control after a dark–light–dark transition test. The present results represent a new perspective on the Chipilin crop as a source for the production of high-value products with novel uses such as a sedative or hypnotic agent.
Acknowledgments
A.H.-R. thanks CONAHCYT for fellow No. 1080081. The authors thank A. Barrera-Pacheco and V.E. Balderas-Hernández for their technical support. L.R.R.-O. thanks the Investigadores por México-CONAHCyT program (Project No. 254).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c08290.
Dynamic conditions for oleoresin extraction from C. longirostrata (Chipilin) leaves using CO2-supercritical fluids (Table S1); lethal and sublethal end points of the chronic embryotoxicity test in zebrafish (D. rerio) (Table S2); cumulative embryotoxicity up to 120 hpf of C. longirostrata (Chipilin) extracts in zebrafish (D. rerio) (Table S3); measurement of D. rerio embryotoxicity by chronic and acute administration of Chipilin extracts (Figure S1); representative GC-MS chromatogram profiles of Chipilin leaf extracts obtained with water, ethanol, ethanol–water, and supercritical fluid extraction (Figure S2); and zebrafish morphological alterations at 12 h after larvae exposition with Chipilin extracts (Figure S3) (PDF)
Author Contributions
A.H.-R.: Investigation, methodology, writing. A.D.L.-R.: GC-MS analysis. V.M.R.-V. and J.M.G.-A.: Chipilin leaf recollection and leaf drying, writing—review. J.M.G.-A.: Author of Chipilin photos in Figure 1. L.R.R.-O.: Supervision, resources, and writing—review. A.P.B.d.l.R.: Funding acquisition, conceptualization, resources, supervision, writing—review and editing.
The authors thank CONAHCYT-INFRA-2015–01 (Project no. 251792).
The authors declare no competing financial interest.
Supplementary Material
References
- Hitziger M.; Heinrich M.; Edwards P.; Pöll E.; Lopez M.; Krütli P. Maya phytomedicine in Guatemala – Can cooperative research change ethnopharmacological paradigms?. J. Ethnopharmacol. 2016, 186, 61–72. 10.1016/j.jep.2016.03.040. [DOI] [PubMed] [Google Scholar]
- Arias L.; Losada H.; Rendón A.; Grande D.; Vieyra J.; Soriano R.; Rivera J.; Cortés J.. Evaluation of Chipilín (Crotalaria longirostrata) as a Forage Resource for Ruminant Feeding in the Tropical Areas of Mexico, 2003. http://www.lrrd.org/lrrd15/4/aria154.htm. Livest. Res. Rural Dev.15, Article 33. Retrieve August 2023.
- Bautista-Cruz A.; Arnaud-Viñas M. R.; Martínez-Gutiérrez A.; Sánchez-Medina P. S.; Pérez-Pacheco A. The traditional medicinal and food uses of four plants in Oaxaca, Mexico. J. Med. Plants Res. 2011, 5 (15), 3404–3411. [Google Scholar]
- Jiménez-Aguilar D. M.; Grusak M. A. Evaluation of minerals, phytochemical compounds and antioxidant activity of Mexican, Central American, and African green leafy vegetables. Plant Foods Hum. Nutr. 2015, 70 (4), 357–364. 10.1007/s11130-015-0512-7. [DOI] [PubMed] [Google Scholar]
- Cruz-Rodríguez R. I.; Meza-Gordillo R.; Rodríguez-Mendiola M. A.; Arias-Castro C.; Mancilla-Margalli N. A.; Ávila-Miranda M. E.; et al. Antifungal activity of Crotalaria longirostrata Hook. & Arn. extracts against phytopathogen fungi from maize. Gayana Bot. 2017, 74 (1), 167–175. 10.4067/s0717-66432017005000102. [DOI] [Google Scholar]
- Miranda-Granados J.; Chacón C.; Ruiz-Lau N.; Vargas-Díaz M. E.; Zepeda L. G.; Alvarez-Gutiérrez P.; Meza-Gordillo R.; Lagunas-Rivera S. Alternative use of extracts of chipilín leaves (Crotalaria longirostrata Hook. & Arn) as antimicrobial. Sustainability 2018, 10, 883 10.3390/su10030883. [DOI] [Google Scholar]
- López H. L.; Beache M. B.; Fuentes Y. M. O.; del Ángel E. C.; Chávez E. C.; Ortiz J. C. D. Methanolic extract of Crotalaria longirostrata: Identification of secondary metabolites and insecticidal effect. Sci. Agropecu. 2022, 13 (1), 71–78. 10.17268/SCI.AGROPECU.2022.007. [DOI] [Google Scholar]
- Rex G. C.; Thomposon A.; Brabazan H.; McDonald S.; Lawerence M.; Wiliams S.; et al. Activities of Guatemalan medicinal plants against cancer cell lines and selected microbes: Evidence for their conservation. J. Med. Plants Res. 2014, 8 (33), 1040–1050. 10.5897/jmpr2014.5488. [DOI] [Google Scholar]
- Morton J. F. Pito (Erythrina berteroana) and chipilin (Crotalaria longirostrata), (Fabaceae), two soporific vegetables of Central America. Econ. Bot. 1994, 48 (2), 130–138. 10.1007/BF02908199. [DOI] [Google Scholar]
- Kufer J.; Förther H.; Pöll E.; Heinrich M. Historical and modern medicinal plant uses – the example of the Ch′orti′ Maya and Ladinos in Eastern Guatemala. J. Pharm. Pharmacol. 2010, 57, 1127–1152. 10.1211/jpp.57.9.0008. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Campos F.; Ramos-Torres J.; Vargas L.; Olguín C.; Ayala F.; Adriano M.; Figueroa M. Efecto del extracto acuoso del chipilín (Crotalaria longirostrata Hook & Arnot) en el sueño de la rata. Quehacer Científico Chiapas 1998, 1 (2), 47–58. [Google Scholar]
- Castañeda R.; Cáceres A.; Velasquez D.; Rodríguez C.; Morales D.; Castillo A. Medicinal plants used in traditional Mayan medicine for the treatment of central nervous system disorders: An overview. J. Ethnopharmacol. 2022, 283, 114746 10.1016/j.jep.2021.114746. [DOI] [PubMed] [Google Scholar]
- Future Market Insights Spice Oils and Oleoresins Market by Product Type, Distribution Channel, Nature, Application & Region, 2023. https://www.futuremarketinsights.com/reports/spice-oils-and-oleoresins-market. Forecast 2023 to 2033. (Reviewed July 2023).
- Rodrigues V. H.; Portugal I.; Silva C. M. Economic analysis of the supercritical fluid extraction of lupine-triterpenoids from Acacia dealbata Link bark. Ind. Crop Prod. 2023, 200, 116838 10.1016/j.indcrop.2023.116838. [DOI] [Google Scholar]
- Uwineza P. A.; Waškiewicz A. Recent advances in supercritical fluid extraction of natural bioactive compounds form natural plant materials. Molecules 2020, 25 (17), 3847 10.3390/molecules25173847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamuela-Raventós R. M.Folin-Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak R.; Capanoglu E.; Shahidi F., Eds.; John Wiley & Sons LTd, 2018; pp 107–115. [Google Scholar]
- Ramos R. T. M.; Bezerra I. C. F.; Ferreira M. R. A.; Lira-Soares L. A. Spectrophotometric quantification of flavonoids in herbal, crude extract, and fractions from leaves of Eugenia uniflora Linn. Pharmacogn. Res. 2017, 9, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev A.; Badani H.; Kapulnik Y.; Shomer I.; Oren-Shamir M.; Galili S. Determination of polyphenols, flavonoids, and antioxidant capacity in colored chickpea (Cicer arietinum L.). J. Food Sci. 2010, 75 (2), S115–S119. 10.1111/j.1750-3841.2009.01477.x. [DOI] [PubMed] [Google Scholar]
- OECD Guideline for the Testing of Chemicals 2006, 2006, 1–11..
- von Hellfeld R.; Brotzmann K.; Baumann L.; Strecker R.; Braunbeck T. Adverse effects in the fish embryo acute toxicity (FET) test: a catalogue of unspecific morphological changes versus more specific effects in zebrafish (Danio rerio) embryos. Environ. Sci. Eur. 2020, 32, 122 10.1186/s12302-020-00398-3. [DOI] [Google Scholar]
- Selderslaghs I. W. T.; van Rompay A. R.; De Coen W.; Witters H. E. Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod. Toxicol. 2009, 28 (3), 308–320. 10.1016/j.reprotox.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ortiz R.; Matínez-Torres A. Mutants of the zebrafish K+ channel Hcn2b exhibit epileptic-like behaviors. Int. J. Mol. Sci. 2021, 22 (21), 11471 10.3390/ijms222111471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y.-h.; Hu Y.-h.; Yang J.; Liu T.; Sun J.; Wang X.-j. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. 10.1039/C9RA04917G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shazly A.; Wink M. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity 2014, 6, 188–282. 10.3390/d6020188. [DOI] [Google Scholar]
- Lipińska M. M.; Golębiowski M.; Szlachetko D. L.; Kowalkowska A. K. Floral attractants in the black orchid Brasiliorchis schunkeana (Orchidaceae, Maxillariinae): clues for presumend spromyphily and potential antimicrobial activity. BMC Plant Biol. 2022, 22, 575 10.1186/s12870-022-03944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinipay F.; Rokkam R.; Botcha S.; Tamanam R. R. Evaluating the anti-urolithiasis potential of Ficus religiosa seed GC MS evaluated phytoconstituents based on their in-vitro antioxidant properties and in-silico ADMET and molecular docking studies. Clin. Phytosci. 2023, 9, 7 10.1186/s40816-023-00359-2. [DOI] [Google Scholar]
- Rukhsana K.; Varghese V.; Akhilesh V. P.; Krishnan E. K. J.; Bhaskaran K. P. P.; Bindu P. U.; Sebastian C. D. GC-MS determination of chemical components in the bioactive secretion of Anopiodesmus saussurii (Humbert, 1865). Int. J. Pharm. Sci. Rev. 2015, 6 (4), 756–776. [Google Scholar]; El-Shazly A.; Wink M. Diversity of Pyrrolizidine Alkaloids in the Boraginaceae Structures, Distribution, and Biological Properties. Diversity 2014, 6 (2), 188–282. 10.3390/d6020188. [DOI] [Google Scholar]
- Jayawardena T. U.; Kim H. S.; Sanjeewa K. A.; Kim S. Y.; Rho J. R.; Jee Y.; Ahn G.; Jeon Y. J. Sargassum horneri and isolated 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one(HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res. 2019, 40, 101513 10.1016/j.algal.2019.101513. [DOI] [Google Scholar]
- Bhardwaj M.; Sali V. K.; Mani S.; Vasanthi H. R. Neophytadiene from Turbinaria ornate suppresses LPS-induced inflammatory response in RAW 264.7 macrophages and Sprague Dawley rats. Inflammation 2020, 43 (3), 937–950. 10.1007/s10753-020-01179-z. [DOI] [PubMed] [Google Scholar]
- Ceyhan-Güvensen N.; Keskin D. Chemical content and antimicrobial properties of three different extracts of Mentha pulegium leaves from Mugla Region, Turkey. J. Environ. Biol. 2016, 37 (6), 1341–1346. [PubMed] [Google Scholar]
- Silva R. O.; Sousa F. B. M.; Damasceno S. R. B.; Carvahlo N. S.; Silva V. G.; Oliveira F. R. M. A.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28 (4), 455–464. 10.1111/fcp.12049. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Sun L.; Kong C. H.; Mei W. L.; Dai H. F.; Xu F. Q.; Huang S. Z. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574 10.1016/j.jep.2022.115574. [DOI] [PubMed] [Google Scholar]
- Basson A. R.; Chen C.; Sagl F.; Trotter A.; Bederman I.; Gomez-Nguyen A.; et al. Regulation of intestinal inflammation by dietary fats. Front. Immunol. 2021, 11, 604989 10.3389/fimmu.2020.604989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova I.; Hashimoto A.; Fecik R. A.; Hedrick M. P.; Hanus L. O.; Boger D. L.; et al. Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J. Pharmacol. Exp. Ther. 2001, 299 (1), 332–343. [PubMed] [Google Scholar]
- Coyne L.; Lees G.; Nicholson R. A.; Zheng J.; Neufield K. D. The sleep hormone oleamide modulates inhibitory ionotropic receptors in mammalian CNS in vitro. Br. J. Pharmacol. 2002, 135, 1977–1987. 10.1038/sj.bjp.0704651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposky A. D.; Homanics G. E.; Basile A.; Mendelson W. B. Deletion of the GABAA receptor β3 subunit eliminates the hypnotic actions of oleamide in mice. Neuroreport 2001, 12 (18), 4143–4147. 10.1097/00001756-200112210-00056. [DOI] [PubMed] [Google Scholar]
- Lu H.-C.; Mackie K. Review of the endocannabinoid system. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging 2021, 6 (6), 607–615. 10.1016/j.bpsc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micera M.; Botto A.; Geddo F.; Antoniotti S.; Bertea C. M.; Levi R.; Galo M. P.; Querio G. Squalene: more than a step toward sterols. Antioxidants 2020, 9, 688 10.3390/antiox9080688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K.; Clark M. D.; Torroja C. F.; Torrance J.; Berthelot C.; Muffato M.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496 (7446), 498–503. 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe C. D.; Jayawardena U. A. Toxicity assessment of herbal medicine using zebrafish embryos: A systematic review. Evidence-Based Complementary Altern. Med. 2019, 2019, 7272808 10.1155/2019/7272808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeta R. E.; Jesubatham P. D.; Grace V. M. B.; Viswanathan S.; Srividya S. Non-toxic and non teratogenic extract of Thuja orientalis L. inhibited angiogenesis in zebra fish and suppressed the growth of human lung cancer cell line. Biomed. Pharmacother. 2018, 106, 699–706. 10.1016/j.biopha.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Licitra R.; Martinelli M.; Jasinski L. P.; Marchese M.; Kiferle C.; Fronte B. In vivo evaluation of Cannabis sativa full extract on zebrafish larvae development, locomotion behavior and gene expression. Pharmaceuticals 2021, 14 (12), 1224 10.3390/ph14121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa T.; Marin W.; Faraco J.; Pézeron G.; Appelbaum L.; Zhang J.; Rosa F.; Mourrain P.; Mignot E. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007, 5 (10), e277 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahardehi A. M.; Arsad H.; Lim V. Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants 2020, 9 (10), 1345 10.3390/plants9101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ovalle J. D.; Moscoso-Gama J. M.; Torres-Garcia O. A. Systematic review of the phytochemical compounds use of Heliotropium indicum taking advantage of its advantage in modern medicine. J. Appl. Biotechnol. Bioeng. 2022, 9 (5), 132–136. 10.15406/jabb.2022.09.00300. [DOI] [Google Scholar]
- Cueto-Escobedo J.; German-Ponciano L. J.; Guillén-Ruiz G.; Soria-Fregozo C.; Herrera-Huerta E. V. Zebrafish as a useful tool in the research of natural products with potential anxiolytic effects. Front. Behav. Neurosci. 2022, 15, 795285 10.3389/fnbeh.2021.795285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanto M. E.; Yang C.-C.; Audira G.; Vasquez R. D.; Roldan M. J. M.; Ger T.-R.; Hsiao C.-D. Evaluation of locomotion complexity in zebrafish after exposure to twenty antibiotics by fractal dimension and entropy analysis. Antibiotics 2022, 11 (8), 1059 10.3390/antibiotics11081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelski J.; Luca S. V.; Skiba A.; Maciąg M.; Budzyńska B.; Marcourt L.; et al. Coumarins from seseli devenyense simonk.: Isolation by liquid–liquid chromatography and potential anxiolytic activity using an in vivo zebrafish larvae model. Int. J. Mol. Sci. 2021, 22 (4), 1829 10.3390/ijms22041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll M. N.; Gendelev L.; Kinser R.; Taylor J.; Bruni G.; Myers-Turnbull D.; Helsell C.; Carbajal A.; Rinaldi C.; Kang H. J.; Gong J. H.; Sello J. K.; Tomita S.; Peterson R. T.; Keiser M. J.; Kokel D. Zebrafish behavioural profiling identifies GABA and serotonin receptor ligands related to sedation and paradoxical excitation. Nat. Commun. 2019, 10, 4078 10.1038/s41467-019-11936-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadamitsu K.; Shigemitsu L.; Suzuki M.; Ito D.; Kashima M.; Hirata H. Characterization of zebrafish GABAA receptor subunits. Sci. Rep. 2021, 11, 6242 10.1038/s41598-021-84646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke A. C. M.; Miyoshi E.; Marques J. A.; Pereira R. P. Anxiolytic properties of Cymbopogon citratus (DC.) stapf extract, essential oil and its constituents in zebrafish (Danio rerio). J. Ethnopharmacol. 2020, 260, 113036 10.1016/j.jep.2020.113036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.