Abstract
Through investigating the combined impact of the environmental exposures experienced by an individual throughout their lifetime, exposome research provides opportunities to understand and mitigate negative health outcomes. While current exposome research is driven by epidemiological studies that identify associations between exposures and effects, new frameworks integrating more substantial population-level metadata, including electronic health and administrative records, will shed further light on characterizing environmental exposure risks. Molecular biology offers methods and concepts to study the biological and health impacts of exposomes in experimental and computational systems. Of particular importance is the growing use of omics readouts in epidemiological and clinical studies. This paper calls for the adoption of mechanistic molecular biology approaches in exposome research as an essential step in understanding the genotype and exposure interactions underlying human phenotypes. A series of recommendations are presented to make the necessary and appropriate steps to move from exposure association to causation, with a huge potential to inform precision medicine and population health. This includes establishing hypothesis-driven laboratory testing within the exposome field, supported by appropriate methods to read across from model systems research to human.
Keywords: Exposome, Molecular Biology, Toxicology, Human Health, Exposure, GxE, Environment
Short abstract
This paper presents recommendations to integrate mechanistic molecular biology approaches in exposomics by bringing together expertise from an interdisciplinary research community.
Introduction
The term “exposome” was first defined in 20051 to capture the nongenetic factors in epidemiology. The exposome concept has promoted the systematic study of the environmental exposures (biological, chemical, physical, and social) that influence health, including the cumulative burden of exposure responses over time (e.g., allostatic load).2,3 In the last 10 years, uptake of the exposome concept in molecular epidemiology has led to the wider application of omics-based methods for the large-scale determination of associations between exposures and population-level health. Yet, identifying which associations are causal is complex. The integration of mechanistic molecular biology approaches within exposome research provides an unprecedented opportunity to elucidate molecular mechanisms for exposure effects, including growing potential in defining underlying gene–environment interactions (GxE) at cellular-to-population scales.
Written from a molecular epidemiology perspective, the seminal paper by Wild1 raised the potential for biomolecular approaches (genomics, transcriptomics, and proteomics) to define exposure impacts. Such approaches are now beginning to advance our understanding of the biological responses to exposures, not only through defining biomarkers but also pathways leading to disease phenotypes.4,5 This research is pressing considering the rise in preventable noncommunicable diseases (NCDs; e.g., cancer, mental illness, neurological, cardiovascular, and autoimmune diseases), which accounted for 90% of the premature deaths recorded in the EU in 20216 and result from a complex GxE interplay. The necessity to capture molecular mechanisms underlying exposures, including GxE interactions, is widely acknowledged and the European Commission has allocated ∼€3 billion in environment and health research since 2000.7 However, the systemic adoption of molecular methods in epidemiology and exposome research is in its infancy and there is need for a roadmap to establish how biological information is integrated within the exposome field.8,9 Addressing exposome effects requires an interdisciplinary approach, and advanced molecular biology techniques have the potential to establish collaborations across data science, fundamental biology, environmental toxicology, chemistry, biotechnology, sociology, environmental science, genomics, and epidemiology to understand human health phenotypes. Building such an interdisciplinary research community requires the facilitation of knowledge exchange across fields with clear experimental guidelines, consistent terminology, and a robust, longstanding, international research infrastructure.
While the use of multiomics methods in epidemiology is now widely accepted, it constitutes only one, yet important, contribution of molecular biology to the exposome. In fact, molecular approaches can significantly aid in establishing causal, mechanistic relationships between exposures and health impacts (e.g., smoking and lung cancer10), particularly for phenotypes that result from complex and multifaceted exposures. Combining the holistic concept of the exposome with an in-depth mechanistic approach would be extremely beneficial to deciphering the underpinning processes of health trajectories. This review discusses approaches and recommendations to leverage such an interaction.
A Call for Action: Integrating Biomolecular Approaches in Exposome Research
The multifactorial nature of the exposome challenges our ability to make direct associations between exposures and phenotypic outcomes.11 As such, the exposome has generally been studied in relation to three interconnected domains: (i) internal (e.g., from ingested chemicals), (ii) specific external (e.g., occupation, educational attainment), and (iii) general external (e.g., sociodemographic factors, air pollution). Since the emergence of the field in 2005,1 technological and methodological developments are helping to characterize exposures across these domains in greater detail, supported by advanced computational approaches that leverage data capital for the improved inference of phenotype-exposure associations.12−14
Novel approaches are required to overcome emerging barriers in identifying trends across fields and diverse data sources. These challenges can be broadly summarized into five key areas: (i) incorporating social exposures, challenges in capturing social exposure effects such as sociodemographic impacts and early determinants of health;2,15(ii) analysis of multiomics data, interpretation, cohesion, and comparison of multiomics data sets both within studies and between studies;16(iii) statistical power, exposome cohorts with omics readouts are generally small scale (<1k individuals), challenging the ability to identify statistically significant trends at the biomolecular level between exposure and effect; (iv) deciphering GxE, genotype is an essential and underpinning component of exposome impact, but as the exposome is multimodal in comparison to genotype, the increased complexity, variability, and dynamics of GxE interactions and a lack of a conclusive description of the exposome is a limiting factor in enabling such analysis;16(v) defining generational impacts, difficulties in distinguishing between genetic and nongenetic inheritance and a need for intergenerational cohorts.17,18
The following sets out the current progress and future steps for addressing these challenges using biomolecular approaches.
Using Molecular Biology Techniques to Better Integrate Equity, Diversity, and Inclusion in Exposome Research
Recent research has emphasized the role of social inequalities as an overarching factor impacting health outcomes. Their influence, starting in early life, is supported by at least three streams of evidence in relation to health and disease: (a) the DOHaD theory (developmental origin of health and disease);19 (b) literature on Adverse Childhood Experiences (ACEs); (c) evidence on life-course socioeconomic disparities in health. ACEs have been associated with ischemic heart disease, obesity, perceived health, psychopathology, inflammation, mortality, health behaviors, and allostatic load later in life.
The onset of NCDs can be seen as the end point of a life-long trajectory of exposures that act via multistage mechanisms and express the biography of a person, with socioeconomic position throughout life being an overarching determinant of health.20 This highlights a pressing need to represent social inequalities in epidemiological studies as a determinant of health rather than a confounding factor (e.g., the Fundamental Cause Theory20,21). This is similarly related to the concept of “weathering” which is used to describe the consequences of structural discrimination (such as ethnicity and gender) on health.22 Establishing more accurate and specific data collection on social inequalities and social structure will enable more meaningful associations with molecular markers. Current data collection on structural social inequalities and social stratification in epidemiological studies has been very simplified. This is even more difficult in low-to-middle income countries (LMICs), where social structures tend to be more complex and difficult to capture with the tools usually applied in Western populations.
However, there are increasing opportunities to incorporate social epidemiology within exposomics, for example by connecting human cohorts with administrative records (e.g., the Administrative Data Research UK program23), geographic indicators and, more recently, historic records (e.g., air quality and deprivation indices24). In the case of social exposures, where quantitative measures cannot be obtained, consideration of errors, bias, and uncertainty, and methods such as weight-of-evidence scores are beginning to be implemented.25−27 Population and health research is biased toward Western populations, which significantly impacts the strength of findings in a global context (e.g., as highlighted in genomics28). The challenges associated with moving toward equality in health research are multifaceted and include a reduced ability to record specific external factors in LMICs due to high costs of personal monitoring and supporting infrastructures, compounded by poor public health resources. While measures of general environmental factors can be facilitated at a global scale (e.g., via satellite monitoring) and provide indicators of general exposures, the ability to understand responses experienced at the individual level by specific and internal exposures is challenging. Lessons can be learned from population-health genomics which has benefited those of European ancestry but, due to a lack of genomic diversity, fails to provide the same benefit on a global scale.29
Investigations into the intermediate mechanisms that mediate biological responses to social experiences have begun in fields such as epigenetics,30,31 through understanding mechanisms of age-acceleration (DNA-based clocks)32 and biomarker discovery for allostatic load.32−34 This mechanistic research gives a biological basis to the concept of the “embodiment” of social inequalities.35 Capturing the influence of negative early exposures on health throughout life has significant implications for informing positive interventions in public health policy and, for example, promoting investment into various forms of social, economic, and cultural capital in childhood. Capturing social exposures in a systematic way will provide opportunities for identifying mechanisms associated with social exposure effects.36
We recommend that social circumstances, including inequity, structural racism, and social exclusion, should be incorporated into exposome research as an integral component, leading to physiological and pathological changes that can also be traced through internal biomolecular changes. This concept, also expressed as the “embodiment” of social relationships, requires novel approaches to data collection and study design. The current collection of information on social circumstances in epidemiology is rudimentary, and an improvement requires strong collaboration with social scientists. A common misconception is that social inequalities (frequently and incorrectly named “SES”, socioeconomic status) are a confounder of causal relationships. The use of internal markers to investigate the propagation of social circumstances into the body is a useful but also expensive approach that is not necessarily feasible, for example, in LMICs. This calls for a refinement of other types of tools imported from the social sciences with better discriminatory and predictive power compared to questionnaires. Molecular tools—like “epigenetic clocks”37—can be used to better clarify the causal pathways, to provide biological plausibility, and to validate other types of nonmolecular evidence.
Bridging Omics in Exposome Research
The application of omics-based molecular techniques in human cohort studies is now commonplace, providing quantitative biomolecular readouts in large cohorts (e.g., from blood, saliva, and fecal samples38) through to clinical settings (e.g., tumors and tissue samples). An increasing number of exposomic research approaches rely on omics technologies to characterize responses to exposures8,9,39 which provide opportunities to capture gene (transcriptomics and epigenomics40−42) to protein-level responses (proteomics31) as well as define internal microbial community composition (metagenomics32) and metabolic perturbations.43−47 Examples include identifying associations across >100 different exposure types (social, chemical, and lifestyle) during pregnancy with DNA-methylation changes in children,5 providing opportunities to investigate adverse childhood exposures on health phenotypes in later life. These data sets can also be supported and analyzed in the context of recent frameworks defining the exposome and mixture effects acting via eight core biomolecular mechanisms as outlined by Peters et al;4 ensuring data sets for each hallmark are established in cohort studies provides opportunities to define mixture effects.
Technological advances in mass spectrometry (MS) analysis and quantitative characterization of chemical exposure agents in field and biosamples22 have been particularly beneficial to exposome research. It is now possible to track chemicals and their transformation products over time both in the environment and individuals at increasingly lower concentrations using targeted and nontargeted (NT) approaches.48−51 Nontargeted approaches hold promise for extrapolating the more comprehensive measures of chemical exposure agents and their metabolites in biosamples to mixture dosages more representative of real-world exposure. However, challenges exist in analyzing these results against existing chemical libraries with most features remaining undefined. The standardization of methods will assist in strengthening NT-MS in exposomics further, and guidance is being developed toward this aim.52
As the use of omics increases, this highlights challenges in identifying the biomolecular responses to specific exposure source(s) and parameters (e.g., exposure route(s) and time period, environmental vs endogenous effects) from cohort exposure data alone (e.g., defining impacts of air pollution from satellite data). Readouts provide a snapshot of the biological responses at tissue or cellular levels, reflective of sampling time postexposure, and are likely to be affected by confounding factors, such as external physiological cues (light, temperature, and seasonal variation53), which form part of the exposome but complicate the understanding of specific exogenous stressors.
The interpretation of static readouts therefore requires knowledge of biomolecular pathway cascades and effects across the cellular-to-whole-organism trajectory, including how responses are spatially and temporally interlinked. Integration of molecular measures and analysis of mechanisms such as gene-regulatory networks, cell-to-cell and interorgan communication (particularly through the blood) will go some way to achieve this and begin to reveal biomarkers reflective of an exposome-induced propensity to disease.2,54
Multiomics data sets from longitudinal human cohorts are beginning to provide new opportunities to establish more substantial and grounded evidence of biomolecular responses to exposures,3,5,55−58 but conducting omics profiling (metabolomics, genomics, transcriptomics, proteomics, microbiome) is limited by financial constraints. Predicting the total cost of complete biomolecular profiling per sample and the feasibility of omics at scale is dependent on multiple factors, including whether samples are analyzed in industry vs academic settings as well as the availability of commercial kits or providers. Metabolomic profiling of blood and urine provided by Nightingale Health is one example59 (with costs starting from <€30 per sample), whose clients include the UKBioBank, where nuclear resonance mass-spectrometry (NMR) has been used to profile 249 metabolomic biomarkers across all 500k UKBB samples.60 By comparison, MS-based metabolomic profiling platforms are diverse with costs varying depending on factors including sample preparation, analyte coverage, and the precision of quantification. While nontargeted screening assays cost <€100 per sample, for targeted analysis, costs can vary from €20 to €2k per sample depending on the analytes measured and approach used. The decision to choose between nontargeted and targeted screens is dependent on the scientific question. While costs will decrease as technologies continue to develop, there is a need to prioritizse readouts of interest and work collectively and across fields to define key biomarkers indicative of exposure effects that could be profiled at scale.
Currently, two clear study designs have been developed in relation to the application of omics in the exposome space: (1) to understand phenotypic responses over time and (2) to identify population-level effects. Studies with repeated, and ideally multigenerational, measures using a single omics technique are currently far more valuable than multiomics analysis at a single time point with respect to understanding phenotypic change. However, single time-point multiomics analysis provides a different understanding of associations e.g. between levels of biological organization (cellular to protein to metabolic). In both instances, there is a need to cross-reference cohorts to increase the statistical power. In (1) this can be done by comparing an individual response across time points against a continuous variable and in (2) by comparing individuals to a wider population using the population as a reference baseline.
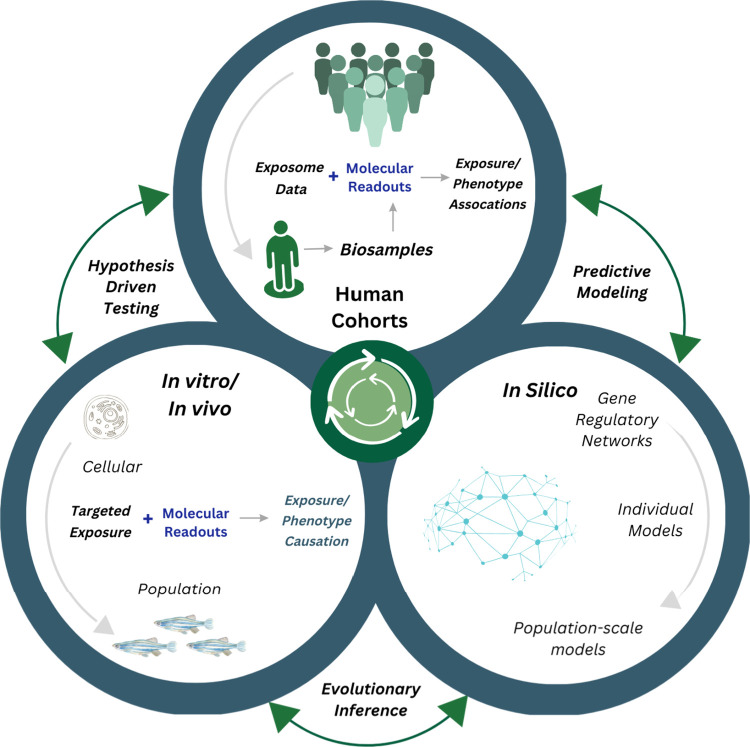
With the cost of multiomics a limiting factor, hypothesis-driven in vitro/in vivo studies based on omics associations identified in exposome cohorts are not only cost-effective but vital to providing further evidence of suitable molecular biomarkers as proxies of both exposure and affect that could be profiled at scale (Figure 1). As the field develops, there are opportunities to retrospectively leverage existing omics data from cohorts to maximize exposure-causation with the support of outputs from experimental studies providing evidence to interpret exposure outcomes in specific time frames.
Figure 1.
Integrating molecular biology approaches into exposome research. Integrating the analysis of human cohort data with molecular techniques to identify exposure causation and GxE through informing hypothesis driven secondary testing in vitro and in vivo using model systems. Wet lab methods are supported by in silico techniques, from cell to population levels (e.g., gene regulatory networks to predictive population exposure risk models). Evidence of effects is compiled in a knowledge repository (such as an Adverse Outcome Pathway model).
Opportunities to Integrate Exposome Research and Population Level Genomics
The ability to characterize genotype-specific and/or individual responses to exposures is particularly relevant to the future application of exposomics in predictive GxE models for precision medicine, population-scale monitoring, and resilient health care systems.
Whole-genome sequencing in population-scale biobanks such as Iceland (deCODE),61 FINNGEN,62 UK BioBank,38 and the Danish National Cohort Study (DANCOS)63 has led to the development of a number of techniques to identify cause–effect relationships between genetic signatures and phenotypes including genome-wide association studies (GWAS),64 phenome-wide association studies (PheWAS),65 and forward/reverse Mendelian randomization.66 For example, these methods have allowed for the characterization of rare genetic variants associated with health phenotypes such as the eye67 and cardiovascular diseases.68 In clinical cohorts, genomic data sets have been used to characterize mutational signatures underlying complex NCDs such as cancer.69 Using GWAS, polygenic risk scores (PGSs)70,71 are being adopted to predict relative likelihoods that an individual will acquire a disease based on genetic background alone. While the application of PGS in clinical settings is beginning to be realized internationally with the development of associated infrastructure resources,72 the full indication of risk and potential routes of intervention cannot be successful without an understanding of environmental exposure influences.71,73−75 This is particularly true for NCDs, where genetic variance is predicted to account for only a small share of disease risk.73
Characterizing human phenotypes based on cause–effect relationships between the exposome and genomic variability is a vital step that will benefit substantially from the integration of genomics and exposome research fields. The application of newer omics technologies to large biosample collections is providing unprecedented insights into human health, for example single-cell sequencing of blood samples in population-scale biobanks;76,77 these data sets can be advantageous to the exposome field through the immune-profiling of blood cells as an indicator of an individual’s past exposure to infectious agents. Qualitative proxies of exposures (for example, dietary self-reporting, smoking, and mental health questionnaires in the UK BioBank38) exist in such cohorts which are beginning to be leveraged to identify GxE associations for single-exposure interactions e.g. identifying the impact of childhood tobacco exposure and polygenic-risk scores on cancer incidence.78
There are growing opportunities to leverage national prescription registries and health record data to define GxE. In the absence of genetic data, longitudinal records of prescriptions alone can provide insights into optimizing drug selection in precision medicine,79,80 though this faces challenges in data interpretation.81 Current efforts to compile administrative and health records such as in the UK (ADRUK)23 have potential to be integrated with genomic data (e.g., through UKBB and genomics health records) and are a growing area of potential for exposome research. Such a focus would be beneficial, considering that medical prescription records are well-documented across Europe.
While cohort studies can provide evidence for a GxE association, in vitro studies (e.g., organoid or other) are required to address the pathways involved in the interactions (e.g., if a genotype decreases metabolism of a xenobiotic, then the latter may have larger effects82).
The Application of Hypothesis-Driven Secondary Testing in Exposomics
Due to the complexity of the exposome, it is not possible to ascertain (1) causal relationships with multiple exposures and (2) GxE relationships from the biomolecular analysis of human cohort studies alone. It is therefore necessary and appropriate to provide further evidence for environmental-molecular effect associations through defined, hypothesis-driven research in closed systems. The exposome community must learn and apply expertise from other fields to better model human health exposure risks at the molecular level.
The application of existing research evidence into a human exposome context, for example, from fundamental research into epigenetics83 or from environmental toxicology,84 could provide an additional avenue for strengthening causal determination.
In the context of GxE, the in-depth genomic profiling of health phenotypes to gain mechanistic understandings using appropriate model systems (for example, microbial exposures in humanized mice85) provide opportunities for interactions between genotype and controlled exposures of environmental relevance to be identified (Figure 1). Such research, informed by the prioritization of responses from epidemiology studies to functional biology-driven screens, will allow for the identification of exposures with the greatest detectable effects.
One such avenue of untangling GxE from cohort studies is through identifying the interaction between PGS, widely used to define genotype–phenotype relationships, and the exposome.86,87 In a toxicology context, human-induced pluripotent stem cells (iPSCs) represent a new approach methodology (NAM) to reduce animal testing in human toxicology screens (in this case, secondary laboratory testing using iPSCs and the models derived from them (e.g., organoids)). With emerging opportunities to derive primary cells from a range of biosamples (e.g., peripheral blood mononuclear cells relevant to the immune responses, hair keratinocytes, and urine88 that can subsequently be reprogrammed to differentiate into multiple cell types (e.g., urine-derived iPSCs can be reprogrammed to urinary epithelial cells, endothelial cells, nerve cells, skeletal myogenic cells, osteoblasts, adipocytes, and chondrocytes88)), this provides a promising opportunity to test GxE.
Research should focus on the specific biological pathways and processes in the cell types associated with the PGSs established for the phenotypes of interest, which can be validated through the analysis of molecular and cellular end points (e.g., gene expression and functional assays). Replicating experiments across multiple cell lines and assays will ensure robustness and reproducibility of the findings and representativeness across the population. Finally, comparing the results with omics readouts from population cohorts will establish the implications of biomolecular effects, bridging the gap between in vitro findings and alignment with population-level disease prevalence. Stem-cell-derived models (e.g., organoids) are a promising approach but requires overcoming major limitations in the reproducibility and scaling (e.g., establishing protocols for reprogramming and differentiation) as well as improving financial accessibility to allow for high-throughput testing.89
In the past decade, advances in molecular biology have led to the development of a range of tools and high-throughput methods that have allowed the adoption of molecular techniques in the laboratory analysis of environmental exposure risk. This includes structural biology, where methods have been applied to assess ligand-binding affinities of environmental pollutants and associated biological receptors as indicators of molecular mechanisms of action for subsequent downstream effects.90 Such research provides supporting evidence for safe-by-design chemicals with lower impacts on human and environmental health.
Technological advances such as expansion microscopy90 are allowing high-resolution images with increased granularity to decipher cellular processes and protein dynamics.91 When coupled with immunostaining methods targeting proteins or microbial communities, such evidence on spatial localization is establishing new insights into internal human microenvironments and exposure interactions.92 In line with this, computational methods have advanced across the sciences, enabling the deployment of artificial intelligence (AI) and machine learning (ML) to model biological systems, compounds, and complex molecule-to-phenotype pathways by incorporating evidence with increasingly complex parameters.93,94 These developments provide opportunities to understand the exposome using experimentally tractable methods relevant to humans and able to capture real-life combinational responses.
From a genomics perspective, the advent of CRISPR-based tools allows high-throughput screens of gene-function through gene editing in cell lines and model organisms. Such systems can be used to secondarily test genetic trends identified in human cohorts, for example, for obesity where rare homozygous variants identified in human cohorts have been established using Drosophila melanogaster as a model system.95 In addition, the advent of self-organizing stem-cell-based models (organoids) has allowed for patient-derived tissues of specific organs to be partially recapitulated in the laboratory to understand GxE interactions.87 Such human organoids are more physiologically relevant to humans than animal models, and the impact of environmental perturbations can be ascertained under defined experimental windows and across a variety of genetic backgrounds.
Understanding Molecular Mechanisms Underlying Exposures in Toxicology
While the majority of exposome research aims to identify the influence of environmental exposures (chemical, biological, physical, and social) on health using human cohorts, there is an opportunity to gain a mechanistic understanding of exposures at the molecular level by building on advances in toxicology, with promise for better understanding and modeling adverse health outcomes related to the exposome96 (Figure 1). Recent publications highlight synergies between exposome and toxicology research with compelling reasons to forge collaborations by bridging relevant information across different levels of biological organization.96 This is demonstrated through the successful integration of mechanistic molecular knowledge from epidemiological approaches and experimental evidence from molecular in vitro assays in the risk assessment of endocrine disruptors.97−99
The toxicology field is already leveraging the benefits of molecular techniques to ascertain the mechanisms underlying single chemical compound exposures in human health, where a reductionist approach is taken to drive chemical risk assessment and regulation. The adoption of molecular methods in high-throughput systems has identified common signatures and biomarkers of affected molecular pathways underlying environmental exposure responses. These approaches are beginning to provide avenues to determine causal relationship between exposures and health outcomes (for example, phenol/phthalate effects in pregnancy97).
More recently, the movement toward NAMs aimed at establishing nonanimal models for human toxicology testing holds promise for exposome research.100 Access to, and the study of, precise human molecular pathways in vivo (e.g., tissue specific, dose–response relationships) is limited by ethical, financial, and technical factors. NAMs overcome these challenges by providing opportunities to understand molecular pathways through in vitro models, including stem cell lines and organoids as well as in silico approaches such as machine learning techniques, computational systems biology, and agent-based modeling.101 The movement toward NAMs could create new scientific paradigms in exposome research by characterizing the mechanistic pathways underlying observations in human cohorts97−99 across stressors by enabling (i) high-throughput testing and comparison of omics readouts in vivo and in vitro, (ii) data integration and analysis through the use of ML/AI to identify experimentally defined responses in large human cohort data sets, and (iii) establishing biomarkers of exposures to aid in the identification of potential risk factors in epidemiological studies. Importantly, testing strategies using a combination of NAMs have been shown to be better predictors of adverse effects in humans than animal models alone,101 particularly for complex end points such as brain development.102 These experimental systems are starting to form the basis of “Integrated Approaches for Testing and Assessment” (IATAs), which combine multiple sources of experimental evidence to map mechanistic effects from genotypic to population levels103 (Figure 1).
Research initiatives aimed at establishing animal-free risk assessment methods for human toxicology testing, such as the ASPIS Cluster104 of Horizon Europe projects and the PARC European Partnership,105 could provide further opportunities and frameworks for identifying exposure impacts. This includes developments in comparative toxicology (as initiated by the Horizon Europe funded PrecisionTox106), which is beginning to inform risk assessment approaches by grouping chemical toxicants based on shared outcome responses across animal phyla. Such research is highlighting not only how pathways of toxicity are known to be conserved but that many exposure agents operate via the same pathway (i.e., relatively nonspecific).107 This provides opportunities for the application of nonmammalian animal models used in fundamental biomedical research to define the molecular mechanisms of exposures (e.g., Drosophila melanogaster, Danio rerio, Caenorhabditis elegans) including for social stress108 and diet.95 Such research should help to prioritize hypothesis-driven laboratory testing from exposome cohorts and has implications for identifying exposures of concern across environmental to human health perspectives.
Developing Frameworks for Integrating Molecular Biology and Exposomics
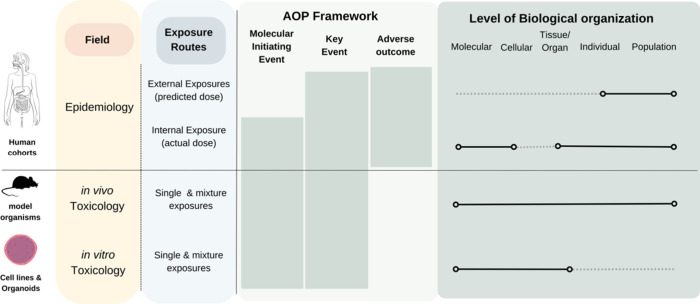
Integrating information from various disciplines to unravel how exposures affect health outcomes is complex, requiring clear frameworks to interpret experimental results across scales and different levels of biological organization5,6 (Figure 2). As such, there are opportunities to leverage established approaches in toxicology within the exposome context to facilitate the transfer of knowledge from mechanistic studies. Defined as linear cascades, adverse outcome pathways (AOPs) provide one option by organizing mechanistic knowledge on overt adverse health effects (adverse outcomes) at individual and population scales109 from molecular initiating events (MIEs) through to key events (KEs) at cellular, tissue, and organ levels. As an agnostic conceptual framework, AOPs allow for the interpretation of the sequential molecular evidence of exposure impacts across stressors (social, chemical, biological, and physical).96 This makes the framework particularly relevant to exposome science as different types/combinations of stressors can influence the same key event (KE) or AOP. The adoption of AOPs in exposome research could enhances the utility of mechanistic molecular data110 by
-
1.
Informing a mechanistic understanding of adverse effects
-
2.
Examining the relative contributions of various components of the exposome on health phenotypes
-
3.
Determining the primary risk drivers under multiple exposures.
Figure 2.
Integrating evidence across biological scales. Molecular Initiating Events (MIEs) including processes such as receptor activation/antagonization, oxidative stress, and DNA/protein and enzyme interactions can be determined in vivo and in vitro. Through measuring responses to exposures using omics and imaging techniques (e.g., histology and immunohistochemistry), Key Events (KEs) can then be described leading to the AO. In humans, longitudinal biomonitoring of selected cohorts can be used to determine responses to exposures, the mechanism of which can be supported by model-organism research.
The proposal for “life-course” AOPs to account for the allostatic load of social, physical, and chemical exposures experienced throughout life could enable a framework for networks of interactions between KEs influenced by different stressors to be defined in a time-dependent manner.20 This would allow for a holistic understanding of how adverse outcomes result from multiple exposures. However, while collaborations with toxicology and molecular biology fields are essential to establish mechanistic evidence in vitro/in vivo (Figure 2), this presents several challenges.
This includes researching molecular responses to multiple exposures in a single study. For example, studying effects of complex chemical mixtures still has many limitations such as accurate mixture reconstruction in laboratory testing (lack of standards, limited information on real-world compositions, complexity of exposure sequences,87 lack of suitable data exchange formats) and current frameworks for assessing risk, largely established in chemical and drug toxicity testing, lack additional information, for example, exposure route in pharmacokinetic models (e.g., dermal vs ingested, which impacts dose and elimination route). Coordinating, interlinking, and building on developments in toxicology (such as in ONTOX111 and PARC105), may begin to allow for characterizing complex mixture effects in detail, particularly when coupled with advances in exposome characterization as discussed above. There are also opportunities to couple AOPs with aggregate exposure pathways (AEPs) to define the impacts of mixtures on shared molecular targets.112
Overall, molecular pathways identifying how an exposure might affect a health outcome (such as AOPs) can be used to inform exposome studies and build hypotheses about toxicologically relevant chemicals and mixtures to study specific health outcomes. Single compounds and mixtures can then be studied using relevant NAMs to investigate hypotheses and assess causality. Knowledge of pathways and underlying molecular mechanisms will strengthen biological plausibility, helping with the selection of relevant biomarkers in exposome studies, informing epidemiological analyses of the exposome–health associations. Such approaches have been implemented, for example, to address bisphenol A and analogues.98
Recommendations toward a Molecular Future in Exposome Research
A clear roadmap to integrate advanced molecular techniques in exposome science is necessary to gain a better understanding of the causative mechanisms underlying the exposure effects. This must take advantage of the current strengths and opportunities in the exposome field by building on existing infrastructure frameworks and research networks. It also requires addressing challenges such as creating cohesive terminologies across disciplines and in designing and capturing human cohort data consistently to allow for interoperability across data sets. There are three main outcomes from a close collaboration between the exposome and molecular biology that are critical for the development of functional exposomics:2,8 (1) using and exploiting multiomics in epidemiological studies; (2) improving knowledge on GxE interactions; (3) using experimental and computational studies to improve the evidence on causal associations between exposure and effects.
Our vision is to develop a connected community of scientists who address exposome research questions through dynamic interdisciplinary collaborations across fields such as epidemiology, toxicology, public and environmental health, bioinformatics, and the biomedical and social sciences. The impacts would be enhanced by robust, scalable research infrastructure and funding opportunities that are responsive to the interdisciplinary, interconnected nature of exposome research. Moving away from classical single-exposure single-outcome approaches, research funding in the past decade has reflected the value of cross-cutting, integrative approaches to understand adverse or protective effects of multiple exposures over various life periods.7 These efforts build on current funding approaches headed by the European Commission, including the European Human Exposome Network (EHEN),113 a cluster of nine research and innovation action projects, and subsequent research infrastructure support through the European Strategy Forum on Research Infrastructures (ESFRI) to form the Environmental Exposure Assessment Research Infrastructure (EIRENE).114 This program of work has established the EU as a global leader in exposome research, further supported by the initiation of a Horizon Europe coordination and support action in 2024 to leverage an International Human Exposome Network (IHEN); this network will be linked with similar efforts in the US National Institutes of Health (NIH) call 2024 to promote and advance international cooperation in exposome science.
There is a need to develop approaches to better understand the spatiotemporal effects of mixed, multifaceted exposure types (e.g., combined impacts of exposures in urban environments such as physiological impacts of air pollution and psychological responses to a lack of green space) rather than single agent/factor responses in isolation. It is essential that the growing community develops strong links with risk assessment organizations and policy makers to effect change and deliver on the full potential of its discoveries.
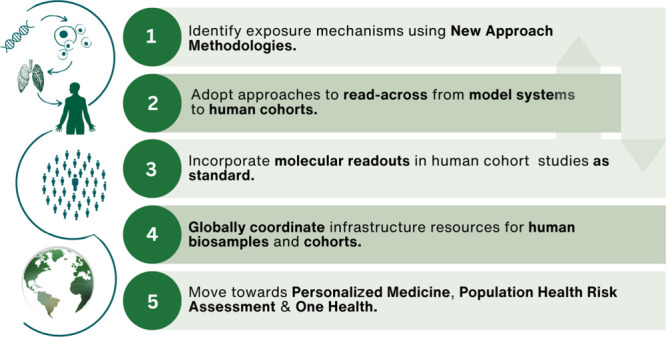
The following recommendations outline an interdisciplinary vision for exposome research whereby the identification of the molecular mechanisms underlying the responses to exposures are prioritized in a move from exposure association to causation.
Underpin Exposome Research with New Approach Methodologies to Identify Molecular Mechanisms
NAMs provide opportunities to characterize the molecular responses to exposures at a level that is inaccessible in human cohorts. Moving from identifying exposure associations to causation using these systems employed in fundamental biology and toxicology would strengthen the evidence underlying exposome research in policy and governance. This is particularly true in the context of AOPs, where if several exposures are linked to the same AO, NAMs provide an opportunity to study KE relationships allowing for (i) high-throughput testing and comparison of omics readouts, (ii) data integration and analysis including through the use of ML/AI to interpret large data sets and identify trends in epidemiology readouts, and (iii) establishing biomarkers of exposures and exposure effects to aid in the identification of potential risk factors in epidemiological studies. The use of NAMs to define the effects of the exposome will be particularly beneficial for the following.
Noncommunicable Diseases
These require the need to identify mechanisms underlying complex mixture and exposure effects. Such research will involve building on current toxicology methods that investigate chemical mixture responses and expanding to account for additional exposure parameters such as social stress, diet, temperature, and underlying genetics.
Intergenerational and Transgenerational Effects
These require analyzing impacts across multiple generations by adopting model organisms with short lifespans (e.g. Drosophila melanogaster, Caenorhabditis elegans, and Danio rerio). Measuring GxE is essential to understanding phenotypic change, yet, it is challenging to differentiate between the genetic and nongenetic inheritance of traits. The use of biomodels provides the opportunity to disentangle inherited gene regulation (e.g., epigenetics) and other nongenetic impacts (e.g., social learning of behaviors to represent cultural transmission).
GxE Interactions
These can be best studied using high-throughput screens in cell lines and stem-cell-derived models (organoids) by screening single exposures and mixture effects on models with a variety of different genomic backgrounds. Patient-derived organoid models allow exciting opportunities to identify responses to exposures in the context of genetic modifications associated with disease outcomes, particularly for tumorigenesis. The development of integrated organoid models and microfluidic devices creates additional opportunities to study factors such as interorgan communication (e.g., combining vasculature and brain organoids to model the blood–brain barrier) in human-relevant contexts.
While approaches to investigate complex mixtures are beginning to be adopted in environmental toxicology, more is needed to link such experimental frameworks with the exposome field with clear applications to human health research. This requires developing experimental exposure systems synergistic to real-world human health exposure scenarios through both dosing and route of uptake (e.g., dermal, ingestion, inhalation). There is a need to standardize experimental exposure scenarios to ensure they are comparable across research institutions. For example, the feasibility of multisite operation of a standardized GC-HRMS assay to profile chemicals in human blood is being assessed via the EIRENE analytical QA/QC working group. There is also the need to establish protocols for deriving iPSCs.89 Possibilities also exist to begin developing proxies of exposure mixtures (e.g., air pollution mixtures in urban environments from epidemiological evidence) that could be used for in vitro/in vivo mixture studies.
Incorporate Molecular Readouts in Human Cohort Studies as Standard
There is a need to build on work in coordinated biobanks (e.g BBMRI-ERIC115) to collect consistent, appropriately preserved biosamples from human cohorts (e.g., blood, saliva, fecal samples). The standardization of subsequent omics methods and analysis to community defined guidelines and in accordance with Findable, Accessible, Interoperable, Reuseable (FAIR) principles116 will begin to allow for more coordinated cross-comparison between studies and with hypothesis-driven secondary testing. Data sets would allow for the systematic analysis of omics readouts with opportunities to identify markers of both exposures (e.g., through profiling for chemical toxicants and their metabolites using mass spectrometry) and their impacts (e.g., biomarkers of molecular response pathways). However, it is important to acknowledge that such biomarkers will only provide a snapshot of the molecular responses at the time of sampling, the response to which will be dependent on multiple factors including exposure type, dose, age, genomic background, and allostatic load background of the individual.
Adopt Approaches to Read-Across from Model Systems to Human Cohorts
Given the complexity of exposures, there is a need to harness computational methods that have the potential to bridge experimental evidence across epidemiological data sets and laboratory assays. The ability to integrate data sets generated using model systems (recommendation 1) through to omics readouts from human cohorts (recommendation 2) is required to establish causation and identify conserved response pathways through biomarkers and omics techniques. In this regard, AOPs provide a useful framework, as they allow the identification of relevant exposure biomarkers using NAMs relevant to human health, thus providing an evidence-based comparison of experimental and human data. The international collaborative effort to identify and develop AOPs and AOP networks such as the AOPwiki117 and to develop guidelines that allow for the transfer of AOPs mostly developed through experimental work to human health should be increased.
Create Globally Coordinated Infrastructure Resources for Human Biosamples and Federated Cohort Data Access
Exposome research must establish relationships with other fields working in the areas of human health including biomedical and clinical communities who have established infrastructure resources at local, national, and international levels: for example, the International HundredK+ Cohorts Consortium (IHCC).118 This includes access to BioBanks and federated cohort data, including for LMICs. Infrastructures can support large-scale exposomics and omics approaches, data storage, and data analysis, and these are critical for the development of exposome research. In addition, the community must adopt and build on standards and frameworks defined by the Global Alliance for Genomics and Health (GA4GH)119 for the ethical and FAIR use of human genomic data.
Moving toward Personalized Medicine, Population Health Risk Assessment, and One Health
Better integration of molecular methods in exposome science has potential impacts on both precision medicine and environmental health. The field must engage with key stakeholders in both to fully realize the benefits of exposome science. To achieve this, clinical studies that include exposomics information, in addition to other molecular data, should be encouraged, which could include specific funding agencies (e.g., National Institute of Environmental Health Sciences, Horizon Europe). Consortia including clinicians, exposome scientists, and biologists can achieve this. Such studies will support the transfer of personalized approaches into the clinics, taking into consideration environmental and occupational exposures.
Summary
Mechanistic molecular techniques have real potential in defining the causative effects of the exposome on health. While the adoption of multiomics is beginning to become the norm across exposome cohorts, subsequent hypothesis-driven laboratory testing with support of relevant in silico approaches is not only necessary but should be prioritized to fully leverage the potential insights of exposure effects on phenotypes from the molecular scale.
Acknowledgments
The contents of this paper arose from a collaborative workshop run in 2020 on “Connecting molecular biology and big data in humans to study the exposome” funded and coordinated by the Human Ecosystems Transversal Theme (European Molecular Biology Laboratory) and Inserm with support provided by HP and GC. Thanks go to participants Ewan Birney, Remy Slama, Naomi Allen, Marc Chadeau, Andreas Kortenkamp, William Bourguet, Samuli Ripatti, Marc Chadeau-Hyam, and Xavier Basagańa. S.B. acknowledges support from the Novo Nordisk Foundation (grants NNF17OC0027594 and NNF14CC0001). E.L.S. acknowledges funding support from the Luxembourg National Research Fund (FNR) for project A18/BM/12341006. E.J.P., H.H., J.K., and K.H. acknowledge the research infrastructure RECETOX RI (LM2023069), H2020 CETOCOEN Excellence 857560, and OP RDE CZ.02.1.01/0.0/0.0/17_043/0009632. E.L.S. acknowledges funding support from the Luxembourg National Research Fund (FNR) for project A18/BM/12341006. This publication reflects only the authors’ views, and the European Commission is not responsible for any use that may be made of the information it contains.
Biography

Dr Amy Foreman is the Scientific Programme Manager for the Human Ecosystems Transversal Theme at the European Molecular Biology Laboratory (EMBL). The theme forms part of EMBL’s “Molecules to Ecosystems” Programme and aims to get a complete understanding of how genotype and environmental exposure interactions underlie human health and disease. Spanning all of EMBL’s sites and EMBL-EBI, Human Ecosystems aims to leverage and support fundamental molecular biology research and services at EMBL and externally to address some of the biggest questions in relation to human health and the exposome. The theme aims to bridge expertise across genomics, exposomics, toxicology, and epidemiology to get a molecular understanding of exposure effects. Amy completed her Ph.D. in EcoToxicology at the University of Exeter and previously held the role of Research Associate/Research Project Manager at the University of Cambridge.
Author Contributions
The manuscript was written with contributions of all authors. All authors have given approval to the final version of the manuscript.
The information and views presented in this article are those of the authors and do not reflect those of the European Commission.
The authors declare no competing financial interest.
Special Issue
Published as part of Environmental Science & Technologyvirtual special issue “The Exposome and Human Health”.
References
- Wild C. P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiology Biomarkers and Prevention 2005, 14, 1847–1850. 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Price E. J.; Vitale C. M.; Miller G. W.; David A.; Barouki R.; Audouze K.; Walker D. I.; Antignac J.-P.; Coumoul X.; Bessonneau V.; Klá Nová J. Merging the Exposome into an Integrated Framework for “‘omics’” Sciences. iScience 2022, 25, 103976. 10.1016/j.isci.2022.103976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. W.; Jones D. P. The Nature of Nurture: Refining the Definition of the Exposome. Toxicol. Sci. 2014, 137 (1), 1–2. 10.1093/toxsci/kft251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A.; Nawrot T. S.; Baccarelli A. A. Hallmarks of Environmental Insults. Cell. 2021, 184, 1455–1468. 10.1016/j.cell.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre L.; Bustamante M.; Hernández-Ferrer C.; Thiel D.; Lau C. H. E.; Siskos A. P.; Vives-Usano M.; Ruiz-Arenas C.; Pelegrí-Sisó D.; Robinson O.; Mason D.; Wright J.; Cadiou S.; Slama R.; Heude B.; Casas M.; Sunyer J.; Papadopoulou E. Z.; Gutzkow K. B.; Andrusaityte S.; Grazuleviciene R.; Vafeiadi M.; Chatzi L.; Sakhi A. K.; Thomsen C.; Tamayo I.; Nieuwenhuijsen M.; Urquiza J.; Borràs E.; Sabidó E.; Quintela I.; Carracedo Á.; Estivill X.; Coen M.; González J. R.; Keun H. C.; Vrijheid M. Multi-Omics Signatures of the Human Early Life Exposome. Nat. Commun. 2022, 13 (1), 7024. 10.1038/s41467-022-34422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . The European Health Report 2021; World Health Organisation: 2021. [Google Scholar]
- European Commission . Research and Innovation to Address the Impact of Environmental Factors on Health. ;Publications Office of the European Union: 2022. [Google Scholar]
- Vermeulen R.; Schymanski E. L.; Barabási A. L.; Miller G. W. The Exposome and Health: Where Chemistry Meets Biology. Science (1979) 2020, 367 (6476), 392–396. 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R. Invited Perspective: Inroads to Biology-Informed Exposomics. Environ. Health Perspect 2022, 130 (11), 1. 10.1289/EHP12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Chen P. L.; Li Z. H.; Zhang A.; Zhang X. R.; Zhang Y. J.; Liu D.; Mao C. Association of Smoking and Polygenic Risk with the Incidence of Lung Cancer: A Prospective Cohort Study. British Journal of Cancer 2022 126:11 2022, 126 (11), 1637–1646. 10.1038/s41416-022-01736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Carlsten C.; Chaleckis R.; Hanhineva K.; Huang M.; Isobe T.; Koistinen V. M.; Meister I.; Papazian S.; Sdougkou K.; Xie H.; Martin J. W.; Rappaport S. M.; Tsugawa H.; Walker D. I.; Woodruff T. J.; Wright R. O.; Wheelock C. E. Defining the Scope of Exposome Studies and Research Needs from a Multidisciplinary Perspective. Environmental Science and Technology Letters 2021, 8, 839–852. 10.1021/acs.estlett.1c00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanyan H.; Portengen L.; Kaplani O.; Huss A.; Hoek G.; Beulens J. W. J.; Lakerveld J.; Vermeulen R. Associations between the Urban Exposome and Type 2 Diabetes: Results from Penalised Regression by Least Absolute Shrinkage and Selection Operator and Random Forest Models. Environ. Int. 2022, 170, 107592. 10.1016/j.envint.2022.107592. [DOI] [PubMed] [Google Scholar]
- Brun-Usan M.; Zimm R.; Uller T. Beyond Genotype-Phenotype Maps: Toward a Phenotype-Centered Perspective on Evolution. BioEssays 2022, 44 (9), 2100225. 10.1002/bies.202100225. [DOI] [PubMed] [Google Scholar]
- Xue B. K.; Sartori P.; Leibler S. Environment-to-Phenotype Mapping and Adaptation Strategies in Varying Environments. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (28), 13847–13855. 10.1073/pnas.1903232116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi-Mindermann H.; White M.; Roczen J.; Riedel N.; Dreger S.; Bolte G. Integrating the Social Environment with an Equity Perspective into the Exposome Paradigm: A New Conceptual Framework of the Social Exposome. Environ. Res. 2023, 233, 116485. 10.1016/j.envres.2023.116485. [DOI] [PubMed] [Google Scholar]
- Tarazona S.; Arzalluz-Luque A.; Conesa A. Undisclosed, Unmet and Neglected Challenges in Multi-Omics Studies. Nature Computational Science 2021, 1, 395–402. 10.1038/s43588-021-00086-z. [DOI] [PubMed] [Google Scholar]
- Adrian-Kalchhauser I.; Sultan S. E.; Shama L. N. S.; Spence-Jones H.; Tiso S.; Keller Valsecchi C. I.; Weissing F. J. Understanding “Non-Genetic” Inheritance: Insights from Molecular-Evolutionary Crosstalk. Trends in Ecology and Evolution 2020, 35, 1078–1089. 10.1016/j.tree.2020.08.011. [DOI] [PubMed] [Google Scholar]
- Macartney E. L.; Drobniak S. M.; Nakagawa S.; Lagisz M. Non-Genetic Inheritance of Environmental Exposures: A Protocol for a Map of Systematic Reviews with Bibliometric Analysis. Environ. Evid 2021, 10 (1), 31. 10.1186/s13750-021-00245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A.; Poston L.; Gluckman P. D. DOHaD - The Challenge of Translating the Science to Policy. Journal of Developmental Origins of Health and Disease 2019, 10, 263–267. 10.1017/S2040174419000205. [DOI] [PubMed] [Google Scholar]
- Vineis P.; Barouki R. The Exposome as the Science of Social-to-Biological Transitions. Environ. Int. 2022, 165, 107312. 10.1016/j.envint.2022.107312. [DOI] [PubMed] [Google Scholar]
- Phelan J. C.; Link B. G.; Tehranifar P. Social Conditions as Fundamental Causes of Health Inequalities: Theory, Evidence, and Policy Implications. J. Health Soc. Behav 2010, 51, S28–S40. 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- Forde A. T.; Crookes D. M.; Suglia S. F.; Demmer R. T. Weathering Hypothesis as an Explanation for Racial Disparities in Health: A Systematic Review 2019, 33, 1. 10.1016/j.annepidem.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADR UK . ADR UK Annual Report; 2021.
- Aurich D.; Miles O.; Schymanski E. L. Historical Exposomics and High Resolution Mass Spectrometry. Exposome 2021, 1, 1. 10.1093/exposome/osab007. [DOI] [Google Scholar]
- Linkov I.; Loney D.; Cormier S.; Satterstrom F. K.; Bridges T. Weight-of-Evidence Evaluation in Environmental Assessment: Review of Qualitative and Quantitative Approaches. Science of The Total Environment 2009, 407 (19), 5199–5205. 10.1016/j.scitotenv.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Hoffman F. O.; Apostoaei A. I.; Kwon D.; Thomas B. A.; Glass R.; Zablotska L. B. Methods to Account for Uncertainties in Exposure Assessment in Studies of Environmental Exposures. Environ. Health 2019, 18 (1), 1–15. 10.1186/s12940-019-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Cathain A.; Murphy E.; Nicholl J. Three Techniques for Integrating Data in Mixed Methods Studies. BMJ. 2010, 341, c4587. 10.1136/BMJ.C4587. [DOI] [PubMed] [Google Scholar]
- Ding Y.; Hou K.; Xu Z.; Pimplaskar A.; Petter E.; Boulier K.; Privé F.; Vilhjálmsson B. J.; Olde Loohuis L. M.; Pasaniuc B. Polygenic Scoring Accuracy Varies across the Genetic Ancestry Continuum. Nature 2023, 618 (7966), 774–781. 10.1038/s41586-023-06079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. P.; Wang M.; Ruan Y.; Koyama S.; Clarke S. L.; Yang X.; Tcheandjieu C.; Agrawal S.; Fahed A. C.; Ellinor P. T.; Tsao P. S.; Sun Y. V.; Cho K.; Wilson P. W. F.; Assimes T. L.; van Heel D. A.; Butterworth A. S.; Aragam K. G.; Natarajan P.; Khera A. V. A Multi-Ancestry Polygenic Risk Score Improves Risk Prediction for Coronary Artery Disease. Nature Medicine 2023 29:7 2023, 29 (7), 1793–1803. 10.1038/s41591-023-02429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné R.; Ménard S.; Delpierre C. The Epigenome as a Biological Candidate to Incorporate the Social Environment over the Life Course and Generations. Epigenomics 2023, 15 (1), 5–10. 10.2217/epi-2022-0457. [DOI] [PubMed] [Google Scholar]
- Barouki R.; Melén E.; Herceg Z.; Beckers J.; Chen J.; Karagas M.; Puga A.; Xia Y.; Chadwick L.; Yan W.; Audouze K.; Slama R.; Heindel J.; Grandjean P.; Kawamoto T.; Nohara K. Epigenetics as a Mechanism Linking Developmental Exposures to Long-Term Toxicity. Environ. Int. 2018, 114, 77–86. 10.1016/j.envint.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory C.; Fiorito G.; Ni Cheallaigh C.; Polidoro S.; Karisola P.; Alenius H.; Layte R.; Seeman T.; Vineis P.; Kenny R. A. How Does Socio-Economic Position (SEP) Get Biologically Embedded? A Comparison of Allostatic Load and the Epigenetic Clock(s). Psychoneuroendocrinology 2019, 104, 64–73. 10.1016/j.psyneuen.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Chadeau-Hyam M.; Bodinier B.; Vermeulen R.; Karimi M.; Zuber V.; Castagné R.; Elliott J.; Muller D.; Petrovic D.; Whitaker M.; Stringhini S.; Tzoulaki I.; Kivimäki M.; Vineis P.; Elliott P.; Kelly-Irving M.; Delpierre C. Education, Biological Ageing, All-Cause and Cause-Specific Mortality and Morbidity: UK Biobank Cohort Study. EClinicalMedicine 2020, 29–30, 100658. 10.1016/j.eclinm.2020.100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G.; McCrory C.; Robinson O.; Carmeli C.; Rosales C. O.; Zhang Y.; Colicino E.; Dugue P.-A.; Artaud F.; McKay G. J; Jeong A.; Mishra P. P; Nøst T. H; Krogh V.; Panico S.; Sacerdote C.; Tumino R.; Palli D.; Matullo G.; Guarrera S.; Gandini M.; Bochud M.; Dermitzakis E.; Muka T.; Schwartz J.; Vokonas P. S; Just A.; Hodge A. M; Giles G. G; Southey M. C; Hurme M. A; Young I.; McKnight A. J.; Kunze S.; Waldenberger M.; Peters A.; Schwettmann L.; Lund E.; Baccarelli A.; Milne R. L; Kenny R. A; Elbaz A.; Brenner H.; Kee F.; Voortman T.; Probst-Hensch N.; Lehtimaki T.; Elliot P.; Stringhini S.; Vineis P.; Polidoro S. Socioeconimic Position, Lifestyle Habits and Biomarkers of Epigenetic Aging: A Multi-Cohort Analysis. Aging 2019, 11 (7), 2045. 10.18632/aging.101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravlee C. C. How Race Becomes Biology: Embodiment of Social Inequality. Am. J. Phys. Anthropol 2009, 139 (1), 47–57. 10.1002/ajpa.20983. [DOI] [PubMed] [Google Scholar]
- Neufcourt L.; Castagné R.; Mabile L.; Khalatbari-Soltani S.; Delpierre C.; Kelly-Irving M. Assessing How Social Exposures Are Integrated in Exposome Research: A Scoping Review. Environ. Health Perspect 2022, 130 (11), 116001. 10.1289/EHP11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory C.; Fiorito G.; McLoughlin S.; Polidoro S.; Cheallaigh C. N.; Bourke N.; Karisola P.; Alenius H.; Vineis P.; Layte R.; Kenny R. A. Epigenetic Clocks and Allostatic Load Reveal Potential Sex-Specific Drivers of Biological Aging. Journals of Gerontology - Series A Biological Sciences and Medical Sciences 2019, 75 (3), 495–503. 10.1093/gerona/glz241. [DOI] [PubMed] [Google Scholar]
- UK Biobank . UK BioBank Releases. www.ukbiobank.ac.uk.
- Ayeni K. I.; Berry D.; Wisgrill L.; Warth B.; Ezekiel C. N. Early-Life Chemical Exposome and Gut Microbiome Development: African Research Perspectives within a Global Environmental Health Context. Trends in Microbiology 2022, 1084–1100. 10.1016/j.tim.2022.05.008. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Fang M. Nutritional and Environmental Contaminant Exposure: A Tale of Two Co-Existing Factors for Disease Risks. Environ. Sci. Technol. 2020, 54 (23), 14793–14796. 10.1021/acs.est.0c05658. [DOI] [PubMed] [Google Scholar]
- Van Breda S. G. J.; Wilms L. C.; Gaj S.; Jennen D. G. J.; Briedé J. J.; Kleinjans J. C. S.; De Kok T. M. C. M. The Exposome Concept in a Human Nutrigenomics Study: Evaluating the Impact of Exposure to a Complex Mixture of Phytochemicals Using Transcriptomics Signatures. Mutagenesis 2015, 30 (6), 723–731. 10.1093/mutage/gev008. [DOI] [PubMed] [Google Scholar]
- Noerman S.; Kokla M.; Koistinen V. M.; Lehtonen M.; Tuomainen T. P.; Brunius C.; Virtanen J. K.; Hanhineva K. Associations of the Serum Metabolite Profile with a Healthy Nordic Diet and Risk of Coronary Artery Disease. Clin Nutr 2021, 40 (5), 3250–3262. 10.1016/j.clnu.2020.10.051. [DOI] [PubMed] [Google Scholar]
- Noerman S.; Kokla M.; Koistinen V. M.; Lehtonen M.; Tuomainen T. P.; Brunius C.; Virtanen J. K.; Hanhineva K. Associations of the Serum Metabolite Profile with a Healthy Nordic Diet and Risk of Coronary Artery Disease. Clin Nutr 2021, 40 (5), 3250–3262. 10.1016/j.clnu.2020.10.051. [DOI] [PubMed] [Google Scholar]
- Gil A. M.; Duarte D.; Pinto J.; Barros A. S. Assessing Exposome Effects on Pregnancy through Urine Metabolomics of a Portuguese (Estarreja) Cohort. J. Proteome Res. 2018, 17 (3), 1278–1289. 10.1021/acs.jproteome.7b00878. [DOI] [PubMed] [Google Scholar]
- González-Domínguez R.; Jáuregui O.; Queipo-Ortuño M. I.; Andrés-Lacueva C. Characterization of the Human Exposome by a Comprehensive and Quantitative Large-Scale Multianalyte Metabolomics Platform. Anal. Chem. 2020, 92 (20), 13767–13775. 10.1021/acs.analchem.0c02008. [DOI] [PubMed] [Google Scholar]
- Petrick L. M.; Uppal K.; Funk W. E. Metabolomics and Adductomics of Newborn Bloodspots to Retrospectively Assess the Early-Life Exposome. Curr. Opin Pediatr 2020, 32 (2), 300. 10.1097/MOP.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth B.; Spangler S.; Fang M.; Johnson C. H.; Forsberg E. M.; Granados A.; Martin R. L.; Domingo-Almenara X.; Huan T.; Rinehart D.; Montenegro-Burke J. R.; Hilmers B.; Aisporna A.; Hoang L. T.; Uritboonthai W.; Benton H. P.; Richardson S. D.; Williams A. J.; Siuzdak G. Exposome-Scale Investigations Guided by Global Metabolomics, Pathway Analysis, and Cognitive Computing. Anal. Chem. 2017, 89 (21), 11505–11513. 10.1021/acs.analchem.7b02759. [DOI] [PubMed] [Google Scholar]
- Flasch M.; Fitz V.; Rampler E.; Ezekiel C. N.; Koellensperger G.; Warth B. Integrated Exposomics/Metabolomics for Rapid Exposure and Effect Analyses. JACS Au 2022, 2 (11), 2548–2560. 10.1021/jacsau.2c00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur D. M.; Detenchuk E. A.; Sosnova A. A.; Artaev V. B.; Lebedev A. T. GC-HRMS with Complimentary Ionization Techniques for Target and Non-Target Screening for Chemical Exposure: Expanding the Insights of the Air Pollution Markers in Moscow Snow. Sci. Total Environ. 2021, 144506. 10.1016/j.scitotenv.2020.144506. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Stapleton H. M.; Schymanski E. L. Tracking Complex Mixtures of Chemicals in Our Changing Environment. Science 2020, 367 (6476), 388. 10.1126/science.aay6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnik T.; Flasch M.; Braun D.; Fareed Y.; Wasinger D.; Seki D.; Berry D.; Berger A.; Wisgrill L.; Warth B. Next-Generation Biomonitoring of the Early-Life Chemical Exposome in Neonatal and Infant Development. Nature Communications 2022 13:1 2022, 13 (1), 1–14. 10.1038/s41467-022-30204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollender J.; Schymanski E. L.; Singer H. P.; Ferguson P. L. Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go?. Environ. Sci. Technol. 2017, 51 (20), 11505–11512. 10.1021/acs.est.7b02184. [DOI] [PubMed] [Google Scholar]
- Boyce W. T.; Sokolowski M. B.; Robinson G. E. Genes and Environments, Development and Time. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (38), 23235–23241. 10.1073/pnas.2016710117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsvold B. S.; Harder A. V. E.; Ran C.; Chalmer M. A.; Dalmasso M. C.; Ferkingstad E.; Tripathi K. P.; Bacchelli E.; Børte S.; Fourier C.; Petersen A. S.; Vijfhuizen L. S.; Magnusson S. H.; O’Connor E.; Bjornsdottir G.; Häppölä P.; Wang Y. F.; Callesen I.; Kelderman T.; Gallardo V. J.; de Boer I.; Olofsgård F. J.; Heinze K.; Lund N.; Thomas L. F.; Hsu C. L.; Pirinen M.; Hautakangas H.; Ribasés M.; Guerzoni S.; Sivakumar P.; Yip J.; Heinze A.; Küçükali F.; Ostrowski S. R.; Pedersen O. B.; Kristoffersen E. S.; Martinsen A. E.; Artigas M. S.; Lagrata S.; Cainazzo M. M.; Adebimpe J.; Quinn O.; Göbel C.; Cirkel A.; Volk A. E.; Heilmann-Heimbach S.; Skogholt A. H.; Gabrielsen M. E.; Wilbrink L. A.; Danno D.; Mehta D.; Guobjartsson D. F.; Rosendaal F. R.; Willems van Dijk K.; Fronczek R.; Wagner M.; Scherer M.; Göbel H.; Sleegers K.; Sveinsson O. A.; Pani L.; Zoli M.; Ramos-Quiroga J. A.; Dardiotis E.; Steinberg A.; Riedel-Heller S.; Sjöstrand C.; Thorgeirsson T. E.; Stefansson H.; Southgate L.; Trembath R. C.; Vandrovcova J.; Noordam R.; Paemeleire K.; Stefansson K.; Fann C. S. J.; Waldenlind E.; Tronvik E.; Jensen R. H.; Chen S. P.; Houlden H.; Terwindt G. M.; Kubisch C.; Maestrini E.; Vikelis M.; Pozo-Rosich P.; Belin A. C.; Matharu M.; van den Maagdenberg A. M. J. M.; Hansen T. F.; Ramirez A.; Zwart J. A. Cluster Headache Genomewide Association Study and Meta-Analysis Identifies Eight Loci and Implicates Smoking as Causal Risk Factor. Ann. Neurol. 2023, 94 (4), 713–726. 10.1002/ana.26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C.; Wang X.; Li X.; Inlora J.; Wang T.; Liu Q.; Snyder M. Dynamic Human Environmental Exposome Revealed by Longitudinal Personal Monitoring. Cell 2018, 175 (1), 277–291.e31. 10.1016/J.CELL.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou C.; Vineis P. Biomarkers and Omics of Health Effects Associated with Traffic-Related Air Pollution. Traffic-Related Air Pollution 2020, 281–309. 10.1016/B978-0-12-818122-5.00011-9. [DOI] [Google Scholar]
- Dennis K. K.; Auerbach S. S.; Balshaw D. M.; Cui Y.; Fallin M. D.; Smith M. T.; Spira A.; Sumner S.; Miller G. W. The Importance of the Biological Impact of Exposure to the Concept of the Exposome. Environ. Health Perspect 2016, 124 (10), 1504. 10.1289/EHP140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allesøe R. L.; Lundgaard A. T.; Hernández Medina R.; Aguayo-Orozco A.; Johansen J.; Nissen J. N.; Brorsson C.; Mazzoni G.; Niu L.; Biel J. H.; Brasas V.; Webel H.; Benros M. E.; Pedersen A. G.; Chmura P. J.; Jacobsen U. P.; Mari A.; Koivula R.; Mahajan A.; Vinuela A.; Tajes J. F.; Sharma S.; Haid M.; Hong M. G.; Musholt P. B.; De Masi F.; Vogt J.; Pedersen H. K.; Gudmundsdottir V.; Jones A.; Kennedy G.; Bell J.; Thomas E. L.; Frost G.; Thomsen H.; Hansen E.; Hansen T. H.; Vestergaard H.; Muilwijk M.; Blom M. T.; ‘t Hart L. M.; Pattou F.; Raverdy V.; Brage S.; Kokkola T.; Heggie A.; McEvoy D.; Mourby M.; Kaye J.; Hattersley A.; McDonald T.; Ridderstråle M.; Walker M.; Forgie I.; Giordano G. N.; Pavo I.; Ruetten H.; Pedersen O.; Hansen T.; Dermitzakis E.; Franks P. W.; Schwenk J. M.; Adamski J.; McCarthy M. I.; Pearson E.; Banasik K.; Rasmussen S.; Brunak S.; Froguel P.; Thomas C. E.; Haussler R.; Beulens J.; Rutters F.; Nijpels G.; van Oort S.; Groeneveld L.; Elders P.; Giorgino T.; Rodriquez M.; Nice R.; Perry M.; Bianzano S.; Graefe-Mody U.; Hennige A.; Grempler R.; Baum P.; Stærfeldt H. H.; Shah N.; Teare H.; Ehrhardt B.; Tillner J.; Dings C.; Lehr T.; Scherer N.; Sihinevich I.; Cabrelli L.; Loftus H.; Bizzotto R.; Tura A.; Dekkers K.; van Leeuwen N.; Groop L.; Slieker R.; Ramisch A.; Jennison C.; McVittie I.; Frau F.; Steckel-Hamann B.; Adragni K.; Thomas M.; Pasdar N. A.; Fitipaldi H.; Kurbasic A.; Mutie P.; Pomares-Millan H.; Bonnefond A.; Canouil M.; Caiazzo R.; Verkindt H.; Holl R.; Kuulasmaa T.; Deshmukh H.; Cederberg H.; Laakso M.; Vangipurapu J.; Dale M.; Thorand B.; Nicolay C.; Fritsche A.; Hill A.; Hudson M.; Thorne C.; Allin K.; Arumugam M.; Jonsson A.; Engelbrechtsen L.; Forman A.; Dutta A.; Sondertoft N.; Fan Y.; Gough S.; Robertson N.; McRobert N.; Wesolowska-Andersen A.; Brown A.; Davtian D.; Dawed A.; Donnelly L.; Palmer C.; White M.; Ferrer J.; Whitcher B.; Artati A.; Prehn C.; Adam J.; Grallert H.; Gupta R.; Sackett P. W.; Nilsson B.; Tsirigos K.; Eriksen R.; Jablonka B.; Uhlen M.; Gassenhuber J.; Baltauss T.; de Preville N.; Klintenberg M.; Abdalla M. Discovery of Drug–Omics Associations in Type 2 Diabetes with Generative Deep-Learning Models. Nat. Biotechnol. 2023, 41 (3), 399–408. 10.1038/s41587-022-01520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale Health, Our Services. https://research.nightingalehealth.com/services.
- Julkunen H.; Cichońska A.; Tiainen M.; Koskela H.; Nybo K.; Mäkelä V.; Nokso-Koivisto J.; Kristiansson K.; Perola M.; Salomaa V.; Jousilahti P.; Lundqvist A.; Kangas A. J.; Soininen P.; Barrett J. C.; Würtz P. Atlas of Plasma NMR Biomarkers for Health and Disease in 118,461 Individuals from the UK Biobank. Nat. Commun. 2023, 14 (1), 604. 10.1038/s41467-023-36231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCode genetics. https://www.decode.com/.
- Univesity of Helsinki . FINNGEN. https://www.finngen.fi/fi.
- Osler M.; Andersen A. M. N.; Lund R.; Batty G. D.; Hougaard C. Ø.; Damsgaard M. T.; Due P.; Holstein B. E. Revitalising the Metropolit 1953 Danish Male Birth Cohort: Background, Aims and Design. Paediatr Perinat Epidemiol 2004, 18 (5), 385–394. 10.1111/j.1365-3016.2004.00584.x. [DOI] [PubMed] [Google Scholar]
- Uffelmann E.; Huang Q. Q.; Munung N. S.; de Vries J.; Okada Y.; Martin A. R.; Martin H. C.; Lappalainen T.; Posthuma D. Genome-Wide Association Studies. Nature Reviews Methods Primers 2021, 59. 10.1038/s43586-021-00056-9. [DOI] [Google Scholar]
- Diogo D.; Tian C.; Franklin C. S.; Alanne-Kinnunen M.; March M.; Spencer C. C. A.; Vangjeli C.; Weale M. E.; Mattsson H.; Kilpeläinen E.; Sleiman P. M. A.; Reilly D. F.; McElwee J.; Maranville J. C.; Chatterjee A. K.; Bhandari A.; Nguyen K. D. H.; Estrada K.; Reeve M. P.; Hutz J.; Bing N.; John S.; MacArthur D. G.; Salomaa V.; Ripatti S.; Hakonarson H.; Daly M. J.; Palotie A.; Hinds D. A.; Donnelly P.; Fox C. S.; Day-Williams A. G.; Plenge R. M.; Runz H. Phenome-Wide Association Studies across Large Population Cohorts Support Drug Target Validation. Nat. Commun. 2018, 9 (1), 4285. 10.1038/s41467-018-06540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. Y.; Yang Y. X.; Chen S. D.; Li H. Q.; Zhang X. Q.; Kuo K.; Tan L.; Feng L.; Dong Q.; Zhang C.; Yu J. T. Investigating Causal Relationships between Exposome and Human Longevity: A Mendelian Randomization Analysis. BMC Med. 2021, 19 (1), 1–16. 10.1186/s12916-021-02030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currant H.; Fitzgerald T. W.; Patel P. J.; Khawaja A. P.; Webster A. R.; Mahroo O. A.; Birney E. Sub-Cellular Level Resolution of Common Genetic Variation in the Photoreceptor Layer Identifies Continuum between Rare Disease and Common Variation. PLoS Genet 2023, 19 (2), e1010587. 10.1371/journal.pgen.1010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk K. A.; Zhang X.; Theotokis P.; Thomson K.; Buchan R. J.; Mazaika E.; Ormondroyd E.; Macaya D.; Jian Pua C.; Funke B.; MacArthur D. G.; Prasad S.; Cook S. A.; Allouba M.; Aguib Y.; O D. P.; R Barton P. J.; Watkins H.; Ware J. S. The Penetrance of Rare Variants in Cardiomyopathy-Associated Genes: 1 a Cross-Sectional Approach to Estimate Penetrance for Secondary Findings 2. American Journal of Human Genetics 2023, 110, 1482. 10.1016/j.ajhg.2023.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov L. B.; Kim J.; Haradhvala N. J.; Huang M. N.; Wei A.; Ng T.; Wu Y.; Boot A.; Covington K. R.; Gordenin D. A.; Bergstrom E. N.; Islam S. M. A.; Lopez-Bigas N.; Klimczak L. J.; Mcpherson J. R. The Repertoire of Mutational Signatures in Human Cancer. PCAWG Mutational Signatures Working Group 1943, 578, 15. [Google Scholar]
- Lambert J. T.; Su-Feher L.; Cichewicz K.; Warren T. L.; Zdilar I.; Wang Y.; Lim K. J.; Haigh J. L.; Morse S. J.; Canales C. P.; Stradleigh T. W.; Palacios E. C.; Haghani V.; Moss S. D.; Parolini H.; Quintero D.; Shrestha D.; Vogt D.; Byrne L. C.; Nord A. S. Parallel Functional Testing Identifies Enhancers Active in Early Postnatal Mouse Brain. eLife 2021, 10, 69479. 10.7554/eLife.69479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Namba S.; Lopera E.; Kerminen S.; Tsuo K.; Läll K.; Kanai M.; Zhou W.; Wu K. H.; Favé M. J.; Bhatta L.; Awadalla P.; Brumpton B.; Deelen P.; Hveem K.; Lo Faro V.; Mägi R.; Murakami Y.; Sanna S.; Smoller J. W.; Uzunovic J.; Wolford B. N.; Wu K. H. H.; Rasheed H.; Hirbo J. B.; Bhattacharya A.; Zhao H.; Surakka I.; Lopera-Maya E. A.; Chapman S. B.; Karjalainen J.; Kurki M.; Mutaamba M.; Partanen J. J.; Chavan S.; Chen T. T.; Daya M.; Ding Y.; Feng Y. C. A.; Gignoux C. R.; Graham S. E.; Hornsby W. E.; Ingold N.; Johnson R.; Laisk T.; Lin K.; Lv J.; Millwood I. Y.; Palta P.; Pandit A.; Preuss M. H.; Thorsteinsdottir U.; Zawistowski M.; Zhong X.; Campbell A.; Crooks K.; de Bock G. H.; Douville N. J.; Finer S.; Fritsche L. G.; Griffiths C. J.; Guo Y.; Hunt K. A.; Konuma T.; Marioni R. E.; Nomdo J.; Patil S.; Rafaels N.; Richmond A.; Shortt J. A.; Straub P.; Tao R.; Vanderwerff B.; Barnes K. C.; Boezen M.; Chen Z.; Chen C. Y.; Cho J.; Smith G. D.; Finucane H. K.; Franke L.; Gamazon E. R.; Ganna A.; Gaunt T. R.; Ge T.; Huang H.; Huffman J.; Koskela J. T.; Lajonchere C.; Law M. H.; Li L.; Lindgren C. M.; Loos R. J. F.; MacGregor S.; Matsuda K.; Olsen C. M.; Porteous D. J.; Shavit J. A.; Snieder H.; Trembath R. C.; Vonk J. M.; Whiteman D.; Wicks S. J.; Wijmenga C.; Wright J.; Zheng J.; Zhou X.; Boehnke M.; Cox N. J.; Geschwind D. H.; Hayward C.; Kenny E. E.; Lin Y. F.; Martin H. C.; Medland S. E.; Okada Y.; Palotie A. V.; Pasaniuc B.; Stefansson K.; van Heel D. A.; Walters R. G.; Zöllner S.; Martin A. R.; Willer C. J.; Daly M. J.; Neale B. M.; Willer C.; Hirbo J. Global Biobank Analyses Provide Lessons for Developing Polygenic Risk Scores across Diverse Cohorts. Cell Genomics 2023, 3 (1), 100241. 10.1016/j.xgen.2022.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INTERVENE Project Consortium . INTERVENE https://www.interveneproject.eu/.
- Sud A.; Horton R. H.; Hingorani A. D.; Tzoulaki I.; Turnbull C.; Houlston R. S.; Lucassen A. Realistic Expectations Are Key to Realising the Benefits of Polygenic Scores. BMJ. 2023, 380, e073149. 10.1136/bmj-2022-073149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo D. S.; Wheeler H. E. Genetic and Environmental Variation Impact Transferability of Polygenic Risk Scores. Cell Reports Medicine 2022, 3, 100687. 10.1016/j.xcrm.2022.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald N. J.; Old R. The Illusion of Polygenic Disease Risk Prediction. Genetics in Medicine 2019, 21 (8), 1705–1707. 10.1038/s41436. [DOI] [PubMed] [Google Scholar]
- Kang J. B.; Raveane A.; Nathan A.; Soranzo N.; Raychaudhuri S. Methods and Insights from Single-Cell Expression Quantitative Trait Loci. Annu. Rev. Genom. Hum. Genet. 2023, 24, 277. 10.1146/annurev-genom-101422-100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland B.; Venkatesh S.; Tardaguila M.; Wen J.; Rosen J. D.; Tapia A. L.; Sun Q.; Graff M.; Vuckovic D.; Lettre G.; Sankaran V. G.; Voloudakis G.; Roussos P.; Huffman J. E.; Reiner A. P.; Soranzo N.; Raffield L. M.; Li Y. Transcriptome-Wide Association Study in UK Biobank Europeans Identifies Associations with Blood Cell Traits. Hum. Mol. Genet. 2022, 31, 2333–2347. 10.1093/hmg/ddac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H.; He M. M.; Wang H.; Qiu W.; Liu L.; Long L.; Shen Q.; Zhang S.; Qin S.; Lu Z.; Cai Y.; Zhang M.; Niu S.; Li J.; Shen N.; Zhu Y.; Tian J.; Chang J.; Miao X.; Zhong R. In Utero and Childhood/Adolescence Exposure to Tobacco Smoke, Genetic Risk, and Lung Cancer Incidence and Mortality in Adulthood. Am. J. Respir Crit Care Med. 2023, 207 (2), 173–182. 10.1164/rccm.202112-2758OC. [DOI] [PubMed] [Google Scholar]
- Kocsmár É.; Buzás G. M.; Szirtes I.; Kocsmár I.; Kramer Z.; Szijártó A.; Fadgyas-Freyler P.; Szénás K.; Rugge M.; Fassan M.; Kiss A.; Schaff Z.; Röst G.; Lotz G. Primary and Secondary Clarithromycin Resistance in Helicobacter Pylori and Mathematical Modeling of the Role of Macrolides. Nat. Commun. 2021, 12 (1), 2255. 10.1038/s41467-021-22557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo-Orozco A.; Haue A. D.; Jørgensen I. F.; Westergaard D.; Moseley P. L.; Mortensen L. H.; Brunak S. Optimizing Drug Selection from a Prescription Trajectory of One Patient. NPJ. Digit Med. 2021, 4 (1), 150. 10.1038/s41746-021-00522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D. M.; Gislason G.; Gerds T. Matters Arising: Immortal Time Bias in the Analysis of Drug Prescription Trajectories. NPJ. Digit Med. 2022, 5, 190. 10.1038/s41746-021-00522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier R.; Bruning T.; Roos P. H.; Rihs H.-P.; Golka K.; Ko Y.; Bolt H. M. Markers of Genetic Susceptibility in Human Environmental Hygiene and Toxicology: The Role of Selected CYP, NAT and GST Genes. Int. J. Hyg. Environ. Health 2003, 206, 149–171. 10.1078/1438-4639-00209. [DOI] [PubMed] [Google Scholar]
- Carlini V.; Policarpi C.; Hackett J. A. Epigenetic Inheritance Is Gated by Naïve Pluripotency and Dppa2. EMBO J. 2022, 41 (7), e108677. 10.15252/embj.2021108677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. L.; Winter M. J.; Norton W. H. J.; Tyler C. R. The Potential for Adverse Effects in Fish Exposed to Antidepressants in the Aquatic Environment. Environ. Sci. Technol. 2021, 55 (24), 16299–16312. 10.1021/acs.est.1c04724. [DOI] [PubMed] [Google Scholar]
- Xue X.; Wang R. P.; Fu J. Modeling of Human Neurulation Using Bioengineered Pluripotent Stem Cell Culture. Current Opinion in Biomedical Engineering 2020, 127–133. 10.1016/j.cobme.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio M.; Khan M.; Mao S.; Chong M.; Judge C.; Pathan N.; Perrot N.; Nelson W.; Lali R.; Di S.; Morton R.; Petch J.; Paré G. A Versatile, Fast and Unbiased Method for Estimation of Gene-by-Environment Interaction Effects on Biobank-Scale Datasets. Nat. Commun. 2023, 14 (1), 5196. 10.1038/s41467-023-40913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimova E. T.; Breen R.; Brazel D. M.; Mills M. C. Gene-Environment Dependencies Lead to Collider Bias in Models with Polygenic Scores. Sci. Rep 2021, 11 (1), 9457. 10.1038/s41598-021-89020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P.; Li Y.; Nasser M. I.; Guo H.; Huang H.; Zhao M.; Zhu P. Urine-Derived Induced Pluripotent Stem Cells in Cardiovascular Disease. Cardiology Research and Practice 2020, 2020, 1. 10.1155/2020/3563519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S. C.; Menendez L.; Friedrich Ben-Nun I. Overcoming the Variability of IPSCs in the Manufacturing of Cell-Based Therapies. Int. J. Mol. Sci. 2023, 24 (23), 16929. 10.3390/ijms242316929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Barron M. G. Structure-Based Understanding of Binding Affinity and Mode of Estrogen Receptor α Agonists and Antagonists. PLoS One 2017, 12 (1), e0169607 10.1371/journal.pone.0169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassie A. T.; Zhao Y.; Boyden E. S. Expansion Microscopy: Principles and Uses in Biological Research. Nature Methods 2019, 33–41. 10.1038/s41592-018-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Li A.; Wang Y.; Zhang Y. Intratumoral Microbiota: Roles in Cancer Initiation, Development and Therapeutic Efficacy. Signal Transduction and Targeted Therapy 2023, 8, 35. 10.1038/s41392-022-01304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud A.; Casale F. P.; Barkley-Levenson A. M.; Farhadi N.; Montillot C.; Yalcin B.; Nicod J.; Palmer A. A.; Stegle O. Dissecting Indirect Genetic Effects from Peers in Laboratory Mice. Genome Biol. 2021, 22 (1), 216. 10.1186/s13059-021-02415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu K.; Tardaguila M.; Mann A. L.; Watt S.; Ponstingl H.; Vasquez L.; Von Schiller D.; Morrell N. W.; Stegle O.; Pastinen T.; Sawcer S. J.; Anderson C. A.; Walter K.; Soranzo N. Genetic Associations at Regulatory Phenotypes Improve Fine-Mapping of Causal Variants for 12 Immune-Mediated Diseases. Nat. Genet. 2022, 54 (3), 251–262. 10.1038/s41588-022-01025-y. [DOI] [PubMed] [Google Scholar]
- Agrawal N.; Lawler K.; Davidson C. M.; Keogh J. M.; Legg R.; Barroso I.; Farooqi I. S.; Brand A. H. Predicting Novel Candidate Human Obesity Genes and Their Site of Action by Systematic Functional Screening in Drosophila. PLoS Biol. 2021, 19 (11), e3001255 10.1371/journal.pbio.3001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R.; Audouze K.; Becker C.; Blaha L.; Coumoul X.; Karakitsios S.; Klanova J.; Miller G. W.; Price E. J.; Sarigiannis D. The Exposome and Toxicology: A Win-Win Collaboration. Toxicol. Sci. 2022, 186, 1–11. 10.1093/toxsci/kfab149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakiwala D.; Noyes P. D.; Faure P.; Chovelon B.; Corne C.; Gauchez A. S.; Guergour D.; Lyon-Caen S.; Sakhi A. K.; Sabaredzovic A.; Thomsen C.; Pin I.; Slama R.; Philippat C. Phenol and Phthalate Effects on Thyroid Hormone Levels during Pregnancy: Relying on In Vitro Assays and Adverse Outcome Pathways to Inform an Epidemiological Analysis. Environ. Health Perspect 2022, 130 (11), 1. 10.1289/EHP10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustieles V.; D’Cruz S. C.; Couderq S.; Rodríguez-Carrillo A.; Fini J. B.; Hofer T.; Steffensen I. L.; Dirven H.; Barouki R.; Olea N.; Fernández M. F.; David A. Bisphenol A and Its Analogues: A Comprehensive Review to Identify and Prioritize Effect Biomarkers for Human Biomonitoring. Environment International 2020, 144, 105811. 10.1016/j.envint.2020.105811. [DOI] [PubMed] [Google Scholar]
- Caporale N.; Leemans M.; Birgersson L.; Germain P. L.; Cheroni C.; Borbély G.; Engdahl E.; Lindh C.; Bressan R. B.; Cavallo F.; Chorev N. E.; D’Agostino G. A.; Pollard S. M.; Rigoli M. T.; Tenderini E.; Tobon A. L.; Trattaro S.; Troglio F.; Zanella M.; Bergman Å.; Damdimopoulou P.; Jönsson M.; Kiess W.; Kitraki E.; Kiviranta H.; Nånberg E.; Öberg M.; Rantakokko P.; Rudén C.; Söder O.; Bornehag C. G.; Demeneix B.; Fini J. B.; Gennings C.; Rüegg J.; Sturve J.; Testa G. From Cohorts to Molecules: Adverse Impacts of Endocrine Disrupting Mixtures. Science (1979) 2022, 375 (6582), 1. 10.1126/science.abe8244. [DOI] [PubMed] [Google Scholar]
- Ball N.; Bars R.; Botham P. A.; Cuciureanu A.; Cronin M. T. D.; Doe J. E.; Dudzina T.; Gant T. W.; Leist M.; van Ravenzwaay B. A Framework for Chemical Safety Assessment Incorporating New Approach Methodologies within REACH. Arch. Toxicol. 2022, 96 (3), 743–766. 10.1007/s00204-021-03215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M.; Benfenati E.; Busquet F.; Castell J.; Clevert D.-A.; De Kok T. M.; Dirven H.; Fritsche E.; Geris L.; Gozalbes R.; Hartung T.; Jennen D.; Jover R.; Kandarova H.; Kramer N.; Krul C.; Luechtefeld T.; Masereeuw R.; Roggen E.; Schaller S.; Vanhaecke T.; Yang C.; Piersma A. H. Safer Chemicals Using Less Animals: Kick-off of the European ONTOX Project. Toxicology 2021, 458, 152846 10.1016/j.tox.2021.152846. [DOI] [PubMed] [Google Scholar]
- Blum J.; Masjosthusmann S.; Bartmann K.; Bendt F.; Dolde X.; Dönmez A.; Förster N.; Holzer A. K.; Hübenthal U.; Keßel H. E.; Kilic S.; Klose J.; Pahl M.; Stürzl L. C.; Mangas I.; Terron A.; Crofton K. M.; Scholze M.; Mosig A.; Leist M.; Fritsche E. Establishment of a Human Cell-Based in Vitro Battery to Assess Developmental Neurotoxicity Hazard of Chemicals. Chemosphere 2023, 311, 137035. 10.1016/j.chemosphere.2022.137035. [DOI] [PubMed] [Google Scholar]
- Bajard L.; Adamovsky O.; Audouze K.; Baken K.; Barouki R.; Beltman J. B.; Beronius A.; Bonefeld-Jørgensen E. C.; Cano-Sancho G.; de Baat M. L.; Di Tillio F.; Fernández M. F.; FitzGerald R. E.; Gundacker C.; Hernández A. F.; Hilscherova K.; Karakitsios S.; Kuchovska E.; Long M.; Luijten M.; Majid S.; Marx-Stoelting P.; Mustieles V.; Negi C. K.; Sarigiannis D.; Scholz S.; Sovadinova I.; Stierum R.; Tanabe S.; Tollefsen K. E.; van den Brand A. D.; Vogs C.; Wielsøe M.; Wittwehr C.; Blaha L. Application of AOPs to Assist Regulatory Assessment of Chemical Risks – Case Studies, Needs and Recommendations. Environmental Research 2023, 217, 114650. 10.1016/j.envres.2022.114650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspis Cluster . ASPIS Cluster. https://aspis-cluster.eu/ (accessed 2023–05–28).
- Marx-Stoelting P.; Rivière G.; Luijten M.; Aiello-Holden K.; Bandow N.; Baken K.; Cañas A.; Castano A.; Denys S.; Fillol C.; Herzler M.; Iavicoli I.; Karakitsios S.; Klanova J.; Kolossa-Gehring M.; Koutsodimou A.; Vicente J. L.; Lynch I.; Namorado S.; Norager S.; Pittman A.; Rotter S.; Sarigiannis D.; Silva M. J.; Theunis J.; Tralau T.; Uhl M.; van Klaveren J.; Wendt-Rasch L.; Westerholm E.; Rousselle C.; Sanders P. A Walk in the PARC: Developing and Implementing 21st Century Chemical Risk Assessment in Europe. Arch. Toxicol. 2023, 97 (3), 893–908. 10.1007/s00204-022-03435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Precision Toxicology Initiative. Toxicol. Lett. 2023, 383, 33–42. 10.1016/J.TOXLET.2023.05.004. [DOI] [PubMed] [Google Scholar]
- Colbourne J. K.; Shaw J. R.; Sostare E.; Rivetti C.; Derelle R.; Barnett R.; Campos B.; LaLone C.; Viant M. R.; Hodges G. Toxicity by Descent: A Comparative Approach for Chemical Hazard Assessment. Environmental Advances 2022, 9, 100287 10.1016/j.envadv.2022.100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan A.; Gliksberg M.; Anbalagan S.; Wigoda N.; Levkowitz G. Stress Resilience Is Established during Development and Is Regulated by Complement Factors. Cell Rep 2023, 42 (1), 111973. 10.1016/j.celrep.2022.111973. [DOI] [PubMed] [Google Scholar]
- Vinken M.; Knapen D.; Vergauwen L.; Hengstler J. G.; Angrish M.; Whelan M. Adverse Outcome Pathways: A Concise Introduction for Toxicologists. Arch. Toxicol. 2017, 3697–3707. 10.1007/s00204-017-2020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher B. I.; Hackermüller J.; Polte T.; Scholz S.; Aigner A.; Altenburger R.; Böhme A.; Bopp S. K.; Brack W.; Busch W.; Chadeau-Hyam M.; Covaci A.; Eisenträger A.; Galligan J. J.; Garcia-Reyero N.; Hartung T.; Hein M.; Herberth G.; Jahnke A.; Kleinjans J.; Klüver N.; Krauss M.; Lamoree M.; Lehmann I.; Luckenbach T.; Miller G. W.; Müller A.; Phillips D. H.; Reemtsma T.; Rolle-Kampczyk U.; Schüürmann G.; Schwikowski B.; Tan Y. M.; Trump S.; Walter-Rohde S.; Wambaugh J. F. From the Exposome to Mechanistic Understanding of Chemical-Induced Adverse Effects. Environ. Int. 2017, 99, 97–106. 10.1016/j.envint.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M.; Benfenati E.; Busquet F.; Castell J.; Clevert D. A.; de Kok T. M.; Dirven H.; Fritsche E.; Geris L.; Gozalbes R.; Hartung T.; Jennen D.; Jover R.; Kandarova H.; Kramer N.; Krul C.; Luechtefeld T.; Masereeuw R.; Roggen E.; Schaller S.; Vanhaecke T.; Yang C.; Piersma A. H. Safer Chemicals Using Less Animals: Kick-off of the European ONTOX Project. Toxicology 2021, 458, 152846. 10.1016/j.tox.2021.152846. [DOI] [PubMed] [Google Scholar]
- Tan Y.-M.; Leonard J. A.; Edwards S.; Teeguarden J.; Paini A.; Egeghy P.; Opin C.; Author T. Aggregate Exposure Pathways in Support of Risk Assessment HHS Public Access Author Manuscript. Curr. Opin Toxicol 2018, 9, 8–13. 10.1016/j.cotox.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Human Exposome Network. https://www.humanexposome.eu/.
- EIRENE Research Infrastructure. https://www.eirene-ri.eu/.
- BBMRI-ERIC. https://www.bbmri-eric.eu/. https://www.bbmri-eric.eu/ (accessed 2024–01–17).
- Wilkinson M. D.; Dumontier M.; Aalbersberg Ij. J.; Appleton G.; Axton M.; Baak A.; Blomberg N.; Boiten J. W.; da Silva Santos L. B.; Bourne P. E.; Bouwman J.; Brookes A. J.; Clark T.; Crosas M.; Dillo I.; Dumon O.; Edmunds S.; Evelo C. T.; Finkers R.; Gonzalez-Beltran A.; Gray A. J. G.; Groth P.; Goble C.; Grethe J. S.; Heringa J.; t Hoen P. A. C.; Hooft R.; Kuhn T.; Kok R.; Kok J.; Lusher S. J.; Martone M. E.; Mons A.; Packer A. L.; Persson B.; Rocca-Serra P.; Roos M.; van Schaik R.; Sansone S. A.; Schultes E.; Sengstag T.; Slater T.; Strawn G.; Swertz M. A.; Thompson M.; Van Der Lei J.; Van Mulligen E.; Velterop J.; Waagmeester A.; Wittenburg P.; Wolstencroft K.; Zhao J.; Mons B. Comment: The FAIR Guiding Principles for Scientific Data Management and Stewardship. Sci. Data 2016, 3, 160018. 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. E.; Edwards S. W.; Ives C.; Mortensen H. M. AOP-DB: A Database Resource for the Exploration of Adverse Outcome Pathways through Integrated Association Networks. Toxicol. Appl. Pharmacol. 2018, 343, 71–83. 10.1016/j.taap.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Genomic Medicine Collaborative . International HundredK+ Cohorts Consortium (IHCC).
- Global Alliance for Genomics and Health . Framework for Responsible Sharing of Genomic and Health-Related Data; 2019.