Abstract
Adoptive cell therapy has revolutionized cancer treatment, especially for hematologic malignancies. T cells are the most extensively utilized cells in adoptive cell therapy. Currently, tumor-infiltrating lymphocytes, T cell receptor-transgenic T cells and chimeric antigen receptor T cells are the three main adoptive T cell therapies. Tumor-infiltrating lymphocytes kill tumors by reinfusing enlarged lymphocytes that naturally target tumor-specific antigens into the patient. T cell receptor-transgenic T cells have the ability to specifically destroy tumor cells via the precise recognition of exogenous T cell receptors with major histocompatibility complex. Chimeric antigen receptor T cells transfer genes with specific antigen recognition structural domains and T cell activation signals into T cells, allowing T cells to attack tumors without the assistance of major histocompatibility complex. Many barriers have been demonstrated to affect the clinical efficacy of adoptive T cell therapy, such as tumor heterogeneity and antigen loss, hard trafficking and infiltration, immunosuppressive tumor microenvironment and T cell exhaustion. Several strategies to improve the efficacy of adoptive T cell therapy have been explored, including multispecific chimeric antigen receptor T cell therapy, combination with immune checkpoint blockade, targeting the immunosuppressive tumor microenvironment, etc. In this review, we will summarize the current status and clinical application, followed by major bottlenecks in adoptive T cell therapy. In addition, we will discuss the promising strategies to improve adoptive T cell therapy. Adoptive T cell therapy will result in even more incredible advancements in solid tumors if the aforementioned problems can be handled.
Graphical abstract
Keywords: Immunotherapy, Adoptive cell therapy, Chimeric antigen receptor, T cell receptor, Tumor-infiltrating lymphocytes
Background
In addition to surgery, radiation and chemotherapy, immunotherapy is now playing an essential role in cancer treatment. In 2013, the world authoritative journal Science listed tumor immunotherapy as the most important scientific advance [1]. As a result of immunotherapy, cancer treatments have been transformed in the past ten years. Clinical trials have shown that blocking cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD-1) could improve overall survival (OS) in advanced melanoma, which were first respectively approved by the US Food and Drug Administration (FDA) in 2011 and 2014 [2, 3]. Despite immune checkpoint inhibitor (ICI), a novel cancer immunotherapy, has provided unprecedented results in cancer treatment, there is still potential for improvement [4].
Adoptive cell therapy (ACT) was found to be highly effective for cancer immunotherapy, which has aroused intense research. Autologous or allogeneic immune cells were cultured and modified in a lab setting to improve their capacity for targeted killing before being reinfused into the patient [5]. Upon trafficking to the tumor, these cells would destroy it. ACT enhanced the ability of tumor antigens to be recognized by effector cells which could kill tumor availably [6]. This review will primarily introduce T cell-based ACT.
Tumor infiltrating lymphocytes (TILs) isolated from patients with metastatic melanoma were infused into tumors to initiate this therapeutic strategy, showing promising antitumor properties [7]. The first attempt to use chimeric antigen receptor (CAR) T cells, targeting CD19 and leading to the complete remission of relapsed and refractory leukemia, was encouraging [8]. ACT has shown the best clinical results for CD19+ leukemias and lymphomas when CAR T cells were used [9–11]. By genetically transferring the cancer antigen specific T cell receptor (TCR) from one T cell to another [12], it is possible to produce large numbers of transgenic T cells for ACT. Autologous T cells from a patient could be transduced with cells from different patients with matching human leukocyte antigens (HLAs) [13].
Although ACT has shown promising results in some human malignancies, only a few solid tumor trials have demonstrated positive results because of numerous remaining problems [14]. These include tumor heterogeneity and antigen loss, hard trafficking and infiltration, immunosuppressive tumor microenvironment, etc. [15]. In this work, we will review recent advances and limitations in the clinical use of ACT and discuss approaches to improve its efficacy by addressing present hurdles.
Types of adoptive cell therapy
Tumor-infiltrating lymphocytes
There was evidence that immune infiltrates in the tumor microenvironment (TME) were crucial for tumor development and had a significant bearing on the clinical outcomes of those with cancers [16]. TILs in tumors were heterogeneous populations of cells that recognized a wide range of antigens [15]. By profiling TILs, we can gain insights into the mechanisms of cancer-immune evasion and develop new therapeutic strategies.
With the advent of ICIs and ACT, the role of T cells in antitumor immunity has become undeniable. T cells recognized antigens and mediated immune responses by TCRs. Immature T cells produce various cell clones carrying specific and diverse antigen TCR structures by genetic rearrangement and combination of a small number of germline gene segments, which can generate disparate TCR repertoires [17]. Positive selection endows mature T cells with the ability to recognize and bound major histocompatibility complex (MHC) or HLA, which present short peptides of tumor antigens to TCRs [18]. And then, negative selection eliminates T cells that show high affinity between their TCRs and self-MHC-peptide complexes [19]. T cells acquire functional, various TCRs and MHC restriction through these important events in development.
CD8+ T cells, also known as cytotoxic T lymphocytes (CTLs), are the critical effector cells responsible for killing tumor cells through two main mechanisms. First, activated CD8+ T cells directly and specifically destroy tumor cells by releasing granzymes (GZM) and perforin [20]. Second, they kill tumor cells indirectly by secreting cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and lymphotoxin [21]. Almost all types of cancers benefit from CD8+ CTLs. However, tumor-infiltrating CTLs rarely control tumor growth due to exhaustion or dysfunction caused by immunosuppressive TME [22–24].
Assisting CTLs in overcoming negative regulation, CD4+ helper T cells enabled CTLs priming, as well as their effector and memory activities [25], which was found in a second T cell-priming step [26]. In the lymphoid organs, CD4+ and CD8+ T cells first interacted independently with conventional dendritic cells (cDCs), which was non-synchronous [27]. And then, on the same cDC, CD4+ and CD8+ T cells recognized their respective antigens, providing cytokines and co-stimulatory signals to promote proliferation and differentiation of CD8+ T cells [25]. To support the differentiation of CD8+ T cells into effector CTLs, cDCs produced Type I IFN, interleukin (IL)-12, and IL-15 [28, 29]. Another subset of CD4+ T cells could mediate cytotoxicity in cancers by expressing GZM and perforin, or directly exerting cytotoxic effects [30].
Recent studies have also shown that tumor-infiltrating B lymphocytes (TIL-Bs), which included tumor-infiltrating B cells and plasma cells, played a crucial role in immunotherapy. Their presence has been linked to improve prognoses for different cancer types, including breast cancer, head and neck squamous cell carcinoma lung adenocarcinoma, etc. [31–35]. Meanwhile, Catalina et al. demonstrated that B cells expressing PD-L1, CD155, IL-10 and tumor growth factor (TGF)-β could prevent activated CD8+ T cells from proliferating and obtaining an effector phenotype [36]. This was due to the different B cell phenotypes presented and the antibodies they produced, as well as the various composition of the TME [37]. TIL-Bs were capable of supporting antitumor immune responses in several ways. As antigen-presenting cells, B cells boosted cellular immunity by presenting tumor-associated antigens (TAAs) to T cells and facilitating the endocytosis by dendritic cells [38, 39]. Moreover, TIL-Bs were capable of killing tumor cells directly with cytokines like IFN-γ and GZMB [40]. Previous studies indicated that it was possible for plasma cells to produce IgG1 antibodies that could cause antibody-dependent cell cytotoxicity [41, 42]. Additionally, B cells were involved in the formation of tertiary lymphoid structures (TLSs) [43]. The prognostic value of TLSs has been demonstrated among the various types of cancer [44–46].
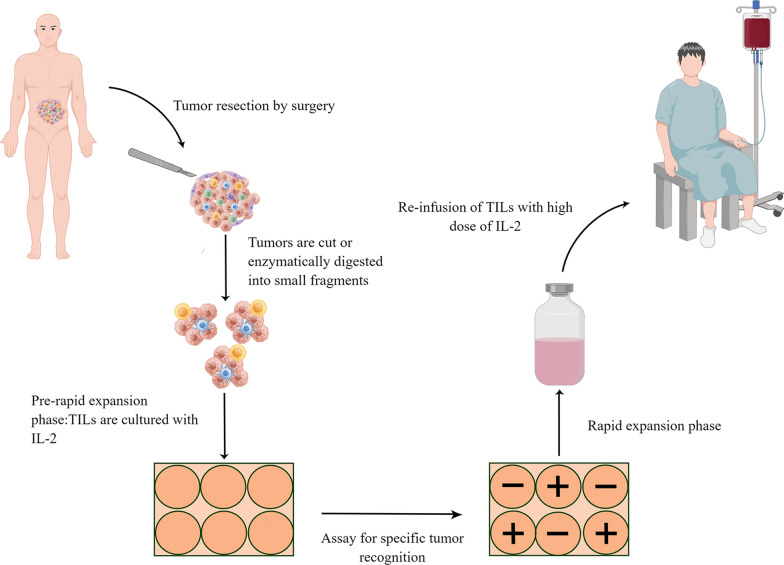
The main process of TIL therapy included isolation of TILs from tumor tissue samples, amplification ex vivo and transfusion of TILs (Fig. 1). Researchers collected TILs from freshly resected melanomas and expanded ex vivo. Twenty patients with metastatic melanoma were treated with intravenous infusion of TILs, followed by injecting IL-2, which caused objective responses of eleven patients [47]. Since then, several studies have been conducted on TILs in cancer. In a phase II clinical trial, the autologous TILs were utilized to treat 93 patients with metastatic melanoma. This study found that adoptive transfer of TILs contributed to a 56% objective response rate (ORR) and a 22% complete response rate (CRR) [48]. Thirty-one metastatic melanoma patients who depleted their lymphocytes by using cyclophosphamide and fludarabine in advance, were treated with their harvested TILs and high-dose IL-2 [49]. The ORR was 48.4% and two patients had a complete response. Most studies on TIL therapy have focused on melanoma, although new data indicated TILs may also be effective in other solid tumors. Metastatic adenocarcinomas and squamous cell carcinomas associated with human papillomavirus were treated with TILs [50]. In this study, 7 of 29 (24%) patients had objective response and two of them had complete response. A clinical trial (NCT01174121) has demonstrated that adoptive transfer of TILs could mediate regression of metastatic colorectal cancer and breast cancer [51, 52]. It seemed that IL-2, fludarabine, and cyclophosphamide lymphodepletion were crucial for adoptive transfer TILs. It was not hard to see that recent studies have deliberately sought to identify which population of cells played a major role in adoptive transfer of TILs. Laszlo et al. found that CD8+ T cells expressing B and T-lymphocyte attenuator were strongly correlated with a beneficial clinical outcome [49]. Metastatic epithelial carcinoma could be reversed by CD4+ T lymphocytes that identified an erbb2 interacting protein mutation [53]. Complete cancer remission and T cell persistence were associated with stem-like neoantigen-specific CD8+ T cells [54]. Therefore, the best course of action could be to transfer TILs specifically for various tumor mutant neoantigens.
Fig. 1.
Process of TIL treatment. The tumor was excised by surgery and prepared into a single-cell suspension by mechanically cut and digestive enzymes. Different types of TILs proliferated on cell plates with high dose of IL-2, producing billions of TILs within three weeks. Any TILs that have anti-tumor effect were left as the positive TIL populations after culturing with the tumor cells of patients. TILs were then expanded to treatment levels by rapid expansion phase. Patients underwent lymphodepletion prior to receiving TILs, and then 10–150 billion TILs and high dose of IL-2 were administered into them. By Figdraw
The role of B cells in adoptive transfer of TILs has also been described. It was reported that B cells gathered from tumor-draining lymph nodes, which were activated with LPS and anti-CD40 mAb, could inhibit spontaneous metastases of a 4T1 breast cancer model [55]. Yang et al. reported that the combination of activated B effector cells and IL-2 could kill 4T1 tumor cells by activating the CXCR4/CXCL12 and perforin pathways, as well as the Fas/FasL interaction [56]. 4-1BBL+ B cells activated with CD40 agonism and IFN-γ resulted in a powerful effect against glioblastoma cancer model [57].
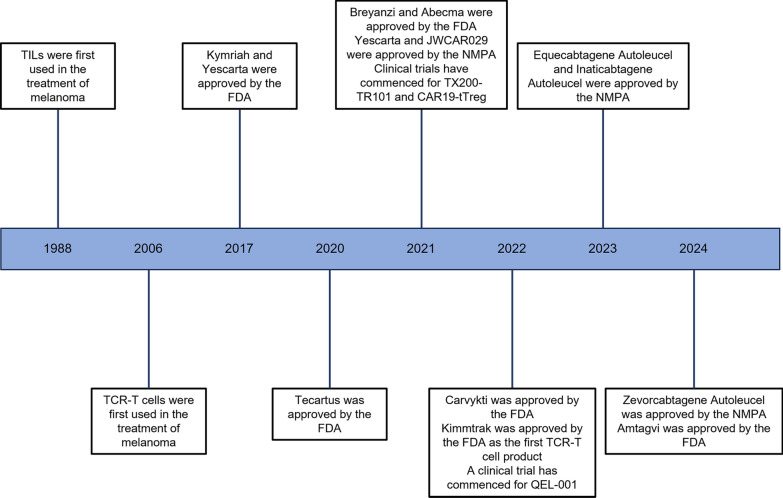
In order to improve the effect of TIL products, combination approaches were being used, including the addition of checkpoint blockade. Patients with metastatic non-small cell lung cancer (NSCLC) were treated with TILs and nivolumab in a phase I trial (NCT03215810). In this study, Patients received 4 cycles of nivolumab prior to TILs infusion. Following lymphodepletion chemotherapy, TILs and IL-2 infusions were given to 16 patients who were proven progression. After that, patients continued receiving nivolumab every 4 weeks for up to a year. Of the 13 evaluable patients, three had proved to be responsive and two of them achieved a sustained complete response [58]. However, isolating tumor-specific TILs that were not present in all patients or expanding a small number of cells for therapeutic efficacy was sometimes problematic. On February 16, 2024, Lifileucel (Amtagvi), the first TIL therapy, was approved by the FDA for the treatment of advanced melanoma that has progressed after PD-1 antibody therapy.
T cell receptor-transgenic T cells
TCRs are responsible for recognizing and binding antigens, which can activate T cells to participate in immune responses against infections and tumors. The transmission of signals to the interior of the T cell and subsequent activation occur exclusively upon the binding of the TCR to a specific MHC molecule, which is presenting a specific peptide chain. TCRs can be divided into two types which included TCRαβ and TCRγδ, and αβT cells account for the vast majority of total T cells and mediate cellular immunity. A functional receptor is formed by the complexing of TCR α/β heterodimers with CD3 ε/γ/δ/ζ subunits [59]. The CD3 cytoplasmic region is long and contains immunoreceptor tyrosine-based activation motif (ITAM) related to T cell activation signal transduction [60]. Activation of T cells and initiation of effector functions result from this process. The TCR-based therapy involves genetically modifying T cells from blood to express transgenic TCRs which are capable of recognizing tumor antigens and attacking cancer cells. Antigens produce or express abnormally by tumor cells in the course of cancerous and malignant growth are known as tumor antigens. The classes of tumor antigens that have been widely studied include TAAs and tumor-specific antigens (TSAs).
It is important to note that TAAs are expressed not only in tumor cells, but also in healthy tissues. Therefore, therapies targeting TAAs must take into consideration possible on-target toxicity caused by T cells. Moreover, proteins that are structurally similar to these antigens may also be targeted by T cells [61]. In several trials with different antigen targets, TCRs have been found to be clinically active against solid tumors (Table 1). Testes, embryos, and placentas all exhibited a subset of TAAs known as cancer testis antigens (CTAs) which started with the identification of the melanoma-associated antigen (MAGE) gene family [62]. As the cell dose-escalation phase progressed, an objective complete response occurred in a patient with metastatic cervical cancer treated with CD4+ T cells expressing TCRs that could recognize MAGE-A3 [63]. In a Phase I/II trial, HLA-DP0401/0402 restricted anti-MAGE-A3 TCR genetically modified cells and aldesleukin were administered after lymphatic clearance for metastatic malignancy expressing MAGE-A3 (NCT02111850). This resulted in partial responses in two patients and a complete response in one patient. Other clinical trials are actively underway, including TCRs targeting MAGEA1, MAGE-A3/A6, MAGEA4/8, MAGE-C2 (NCT03441100, NCT05430555, NCT03139370, NCT03247309, NCT04729543). Another CTA of high clinical significance was New York esophageal squamous cell carcinoma-1 (NY-ESO-1), which was highly expressed in various solid cancers and hematological tumors [64]. The effectiveness of transferring autologous NY-ESO-1-specific T cells was assessed in HLA-A*02 patients with synovial sarcoma (SS). This marked the first clinical study of designed TCR treatment for sarcoma, validating tumoral NY-ESO-1 expression [65]. Another group of SS patients with advanced HLA-A*02, separated into 4 groups based on tumoral NY-ESO-1 expression, were treated with the NY-ESO-1-targeting specific peptide enhanced affinity receptor (SPEAR) T cells in a pilot trial (NCT01343043). Across all cohorts, 35% (15/42) of patients experienced a full or partial response, with the remaining 57% (24/42) having stable disease [66]. The National Cancer Institute (NCI) treated 10 patients with high-dose aldesleukin and peripheral blood cells transduced with the anti-ESO murine TCR, achieving a 50% ORR and a 10% CRR (NCT01967823). Twenty-five patients with recurrent, refractory or high-risk multiple myeloma were enrolled in a phase II clinical trial group (NCT01352286). They assessed the function and safety of T cells with th e NY-ESO-1c259 TCR. At the end of the first year, 11/25 patients had responded after being infused a large number of NY-ESO-1 SPEAR T cells and no serious adverse events (AEs) and no cases of cytokine release syndrome (CRS) were recorded during this trial [67].
Table 1.
Selected engineered TCR therapy trials in solid tumor from ClinicalTrials.gov
| Target | Type of cancer | Phase | Clinical trial number |
|---|---|---|---|
| MAGEA1 | Solid tumors | I | NCT03441100 |
| MAGEA1 | Advanced solid tumors | I/II | NCT05430555 |
| MAGEA3 | Cervical cancer; Renal cancer; Urothelial cancer; Melanoma; Breast cancer | I/II | NCT02111850 |
| MAGE-A3/A6 | Solid tumors | I | NCT03139370 |
| MAGEA4/8 | Solid tumors | I | NCT03247309 |
| MAGE-C2 | Melanoma; Head and neck Cancer | I/II | NCT04729543 |
| NY-ESO-1 | Neoplasms | I | NCT01343043 |
| NY-ESO-1 | Melanoma; Meningioma; Breast Cancer; Non-Small Cell Lung Cancer; Hepatocellular Cancer | II | NCT01967823 |
| NY-ESO-1 | Multiple Myeloma | II | NCT01352286 |
| HPV-16 E7 | Metastatic or refractory/recurrent human papillomavirus (HPV)-16 + cancers | I/II | NCT02858310 |
| HBV | Hepatocellular Carcinoma | I | NCT03899415 |
| EBV | Nasopharyngeal Carcinoma | III | NCT02578641 |
| EBV | Nasopharyngeal Carcinoma | I/II | NCT04509726 |
The combination of engineered TCR therapy with genome editing offered the potential to improve the efficacy and safety of modified T cells. Edward et al. conducted a phase I human pioneer study. They targeted endogenous T cells using CRISPR-Cas9 technology to knock out TCR α chain gene, TCR β chain gene, and PD-1 gene, thereby increasing the activity and safety of NY-ESO-1 TCR-engineered T cells and demonstrating initial feasibility [68]. However, only a restricted number of patients might benefit from these medicines because of the limited cancer types that expressed these TAAs and the limitation of HLA types. Furthermore, the heterogeneous expression of TAAs on malignancies complicated this therapy [69].
Due to TSAs are expressed only on tumor cells, T cell receptor-transgenic T (TCR-T) exerts a stable anti-tumor effect without harming normal cells [70]. TSAs currently under investigation included viral antigens and neoantigens. Viral antigens were mainly referred to those antigens that were associated with tumorigenesis. Viral infections were the root cause of many human cancers. When it comes to human papillomavirus (HPV), we considered it to be primarily related to cervical cancer [71]. The involvement of HPV in the development of various malignancies, such as vaginal, vulvar, anal and oropharyngeal cancers have lately been studied [72–74]. Hepatitis B virus (HBV) is a non-cytopathic DNA virus that could induce chronic infection, eventually leading to hepatocellular cancer [75]. Epstein-Barr virus (EBV) is a ubiquitous, oncogenic virus that was linked to a variety of human cancers, including nasopharyngeal carcinoma (NPC), Hodgkin lymphomas, non-Hodgkin lymphomas (NHL), natural killer (NK)/T cell lymphomas and a subset of gastric cancers [76, 77].
In a clinical trial with a primary endpoint of maximum tolerated dose, 6 of 12 patients treated with HPV-16 E7 TCR-T cells showed objective clinical responses (NCT02858310). Cancer regression was considerable in several individuals, with some tumors regressing durably [78]. For example, after 8 months of treatment, 31% of complete tumor regression occurred in one patient with more than 80 metastatic tumors. No treatment-related damage to normal tissue or deaths were found in this study. Preliminary evidences of anticancer efficacy and safety have been obtained from an ongoing phase I clinical trial on eight patients with advanced hepatocellular carcinoma employing short-lived HBV-specific TCR-T cells [79]. For EBV, a completed phase III clinical trial combining gemcitabine and carboplatin with EBV-specific T cells for NPC resulted in a 9% CRR and a 63% ORR (NCT02578641). Moreover, LMP2-specific TCR-T cells with IL12 auto-secreting element have been used to treat NPC in a clinical trial (NCT04509726). However, cancers caused by viruses accounted for only a portion of cancers [80], and the effective application of TCR-T cells may depend heavily on the discovery of neoantigens.
Neoantigens were mutated peptides originating from somatic mutations that were absent from normal tissues and specifically recognized by TCR-T cells [81]. The TCRs had the precise specificity to recognize neoantigens generated by single point mutations in peptide sequences presented by the MHC on the surface of cancer cells. Therefore, they were ideal targets for engineered TCR therapy. For the development of cell-based cancer immunotherapies, the precise identification of antitumor TCRs posed1 a significant hurdle. Single-cell RNA sequencing, T cell receptor sequencing, whole-exome/transcriptome sequencing, mass spectrometry and NeoScreen were used to discover T cells that specifically recognized neoantigens [82–84]. Current immunotherapeutic strategies against neoantigens often target patient-specific private antigens produced by non-recurrent driver mutations or passenger mutations [85]. Identification of neoantigens will allow engineered TCR therapy to become more individualized. In this phase 1 clinical trial (NCT03970382), researchers employed whole genome sequencing to identify patient-specific tumor mutations, while RNA sequencing was utilized to discern expression levels of genes associated with these mutations. Consent was obtained from patients who contributed tumor biopsies and peripheral blood mononuclear cells for the screening of personalized neoTCR products. In order to concurrently knock out endogenous TCRs and knock in TCRs that specifically target patient-specific neoantigens using non-viral precision gene editing, several TCRs that detect patient-specific mutations in neoantigens have to be identified and generated [86]. There are still ongoing investigations using autologous T cells modified to express TCRs reactive against neoantigens in patients with refractory solid malignancies (NCT05194735, NCT04520711, NCT05349890, NCT03412877).
However, the produce of personalized TCR-T cells for each patient was time-consuming and costly, which increased the possibility of disease progression. Therefore, targeting a public neoantigen that arose from a driver hotspot mutation and was presented by a common HLA allele may circumvent many of the limitations. For instances, a single infusion of autologous T cells was given to a patient with metastatic pancreatic adenocarcinoma who had not responded to TIL therapy, causing a 72% overall partial response. To specifically target KRAS G12D, two allogeneic TCRs that were HLA-C*08:02-restricted were expressed on these T cells through genetic modification [87]. However, another patient died after 6 months of treatment, even though he had a high levels of specific T cells in his blood and no mechanisms of immunotherapy resistance were found. In a study initiated by NCI (NCT00068003), 97 patients with chemorefractory metastatic epithelial cancer were shown to contain nonsynonymous TP53 mutations [88]. Smita S et al. demonstrated that mutant PIK3CA produced an immunogenic public neoantigen shared among HLA-A*03:01+ patients [89]. However, there were few public neoantigens available for targeting, which may lead to treatment resistance due to antigen loss [90]. Due to the absence of neoantigens presented by common HLAs resulting from specific driver gene mutations, the actual cohort of patients who could benefit from public neoantigen-targeted therapies was notably smaller than the projected theoretical number. We also needed to know whether the public neoantigen was immunogenic for TCR-T cell therapy.
Chimeric antigen receptor T cells
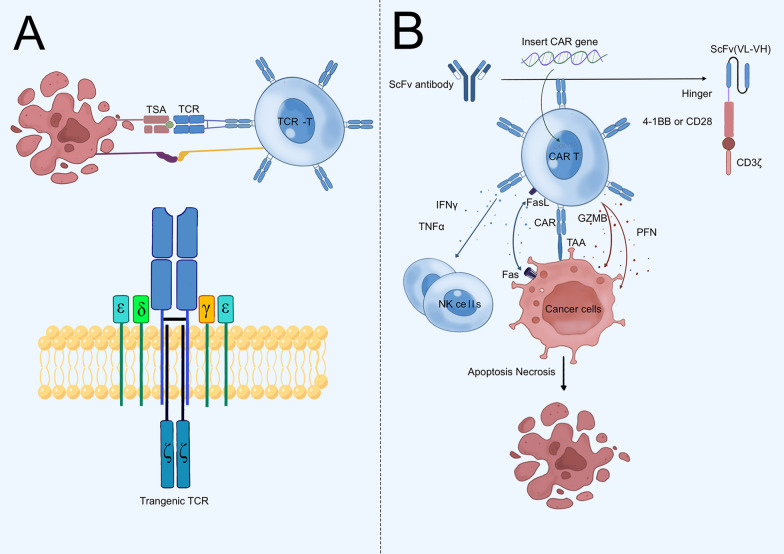
TCRs were able to recognize antigens with the restriction of MHC, therefore, T cells with particular TCR could only be utilized to treat patients with the corresponding MHC genetic background, limiting the usage of TCR-T. CAR is a modular, genetically modified synthetic antigen receptor with antibody-like characteristics and efficient TCR activation signaling [91]. In contrast to TCR-T cells, CAR T cells can kill tumor cells without the restriction of MHC (Fig. 2).
Fig. 2.
Differences between T cell receptor-transgenic T (TCR-T) cells and chimeric antigen receptor (CAR) T cells. A Transgenic TCRs were capable of forming functional TCR-CD3 complexes and did not differ from the standard TCR structure of an α/β chain heterodimer. Major histocompatibility complex (MHC)-presented intracellular peptide antigens were recognized by TCRs. The two intracellular CD3 domains triggered downstream TCR signaling upon antigen recognition. B CARs, unlike TCRs, were unable to assemble CD3 complexes, and the single-chain fragment variable (scFv) did not require MHC to recognize surface antigens. By Figdraw
The first generation of CAR T cells incorporated a single-chain fragment variable (scFv) to the CD3ζ signaling chain, which included the ITAM, endowing T cells with MHC-independent activation [92]. However, these CAR T cells have demonstrated absolutely no efficacy. To improve cell proliferation and cytotoxicity, the second generation of CAR incorporated a co-stimulatory structural domain mostly derived from CD28 or 4-1BB (CD137), located between the transmembrane and CD3 signaling regions [93]. Compared with CD28-based CARs, which induced effector-like memory cell differentiation and secreted higher levels of cytokines, CARs with 4-1BB could increase central memory T cell differentiation and persistence, as well as enhance mitochondrial biogenesis with increased fatty acid metabolism [94]. The design of the third generation of CAR combined the different advantages of the two co-stimulatory structural domains to further enhance the activity of CAR T cells. The fourth-generation CAR-T cells, also known as T cells redirect for universal cytokine killings (TRUCKs) were designed to release cytokines into tumor tissues when the CARs bound to targeted antigens. The cytokine which was overexpressed in CAR was IL-12, a highly effective substance that used NK cells to destroy tumor cells that were not identified by CARs and boosted the IFN-γ, GZMB and perforin secretion of T cells [95]. An IL-2 receptor domain between the CD3 and CD28 signaling domains as well as a STAT3-binding motif were recently added to a unique CAR that followed the framework of the second generation [96]. Until now, seven CAR T cells have been approved for marketing worldwide (Table 2).
Table 2.
CAR T therapeutic products approved for commercial use
| Trade name | Co-stimulatory domain | Target | Indication | Dose level (cells) | Clinical efficacy | Clinical Trial |
|---|---|---|---|---|---|---|
| Kymriah | 4-1BB | CD19 | R/R ALL (≤ 25 years of age) | 3.1 × 106/kg | ORR: 81% CRR: 60% | NCT02435849 |
| R/R LBCL | 3 × 108 | ORR: 52% CRR: 40% | NCT02445248 | |||
| R/R FL | 2.06 × 108 | ORR: 86% CRR: 69% | NCT03568461 | |||
| Yescarta | CD28 | CD19 | R/R LBCL | 2 × 106/kg | ORR: 82% CRR: 54% | NCT02348216 |
| R/R FL | 2 × 106/kg | ORR: 94% CRR: 79% | NCT03105336 | |||
| Tecartus | CD28 | CD19 | MCL | 2 × 106/kg | ORR: 91% CRR: 68% | NCT02601313 |
| ALL | 1 × 106/kg | ORR: NR CRR: 71% | NCT02614066 | |||
| Breyanzi | 4-1BB | CD19 | LBCL |
50 × 106 100 × 106 150 × 106 |
ORR:68% CRR:60% ORR:74% CRR:52% ORR:73% CRR:51% |
NCT02631044 |
| Abecma | 4-1BB | BCMA | R/R MM |
150 × 106 300 × 106 450 × 106 |
ORR:50% CRR:25% ORR:69% CRR:29% ORR:81% CRR:39% |
NCT03361748 |
| Carvykti | 4-1BB | BCMA | R/R MM | 0.75 × 106/kg | ORR:98% CRR:83% | NCT03548207 |
| JWCAR029 | 4-1BB | CD19 | R/R LBCL | 100 × 106 or 150 × 106 | ORR:76% CRR:52% | NCT04089215 |
R/R, relapsed/refractory; ALL, acute lymphoblastic leukemia; LBCL, large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; ORR, objective response rate; CRR, complete response rate; NR, not reported
An autologous CD19-targeted CAR T cell therapy called Tisagenlecleucel (Kymriah) was first approved for the treatment of patients under the age of 25 with relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (B-ALL) based on a global trial (NCT02435849) [97]. Tisagenlecleucel was latter authorized for the treatment of adult patients with R/R large B-cell lymphoma (LBCL) [98] Recently, Tisagenlecleucel was allowed for R/R follicular lymphoma (FL) based on the ELARA phase II global trial (NCT03568461). In this trial, Tisagenlecleucel was administered in 97 enrolled patients with R/R FL (below grade 3B). The ORR was 86% and the CRR was 69% of the 94 patients who could be evaluated for efficacy, and 46% of the patients developed Tisagenlecleucel-related AEs of grade 3 or 4 [99].
In October 2017, the FDA approved Axicabtagene ciloleucel (Yescarta) for the treatment of adult patients with R/R LBCL following two or more lines of systemic therapy. This included patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), high-grade B-cell lymphoma (HGBCL) and DLBCL arising from FL [100]. In a pivotal phase I/II trial called ZUMA-1 (NCT02348216), the ORR and CRR were 82% and 54%, respectively [101]. Grade 3 or higher CRS, as well as immune effector cell-associated neurotoxicity syndrome (ICANS), affected 13% and 28% of patients, respectively. ZUMA-5 was a single-arm and multicentre phase II trial (NCT03105336), which the overall response was achieved in 94% of the 84 FL patients, with a complete response in 79% of those patients [102], causing the approval of the indication for adult patients with R/R FL following two or more lines of systemic treatment. In an international phase III trial, ZUMA-7 (NCT03391466), Yescarta demonstrated a higher event-free survival and response rate in patients with early R/R LBCL compared with second-line standard care [103]. A retrospective study found that R/R LBCL patients administrated with Axicabtagene ciloleucel had higher toxicity but comparable non-relapse mortality and efficacy to those treated with tisagenlecleucel [104].
Brexucabtagene autoleucel (Tecartus) was the sole CAR T cell therapy recognized for the treatment of individuals with mantle cell lymphoma (MCL), which targeted CD19 [105]. After a 3-year follow-up in the key ZUMA-2 study (NCT02601313), 68 patients with R/R MCL received Brexucabtafene autoleucel infusion still showed an ORR as high as 91% (68% CRR) [106]. With a median duration of response (DOR) of 28.2 months, responses were long-lasting, and 37% of the patients who underwent treatment still had positive effects. In the retrospective historical external control study SCHOLAR-3, OS of patients with R/R B-ALL in the ZUMA-3 clinical trial (NCT02614066) was nearly 20 months longer than matched patients who received standard-of-care therapies [107].
Lisocabtagene maraleucel (Breyanzi) was a CD19-directed CAR T cell product with a 4-1BB co-stimulatory domain that consisted of purified CD8+ and CD4+ CAR T cells in a specific ratio (1:1) [108]. Based on an ORR of 73% and a CRR of 53% in 256 patients in the TRANSCEND NHL 001 trial (NCT02631044), Lisocabtagene maraleucel was approved for R/R LBCL following two or more systems of therapy, including DLBCL, HGBCL, PMBCL and FL grade 3B [109]. In TRANSFORM (NCT03575351) phase III trial, 184 patients with LBCL who were primary refractory or relapsed within 12 months of the first-line therapy were randomly assigned to one of two treatment groups: Lisocabtagene maraleucel or standard of care [110]. When compared to standard-of-care treatment, treatment with Lisocabtagene maraleucel increased median event-free survival by over 8 months and demonstrated a higher CRR (66% vs 39%) [110]. ICANS (Grade3 = 4%) and CRS (Grade3 = 1%) were occasionally reported.
Idecabtagene vicleucel (Abecma) became the first CAR T therapy approved for the treatment of adult patients with R/R multiple myeloma (MM) who have received ≥ 4 lines of prior therapies on March 26, 2021 [111]. Idecabtagene vicleucel was a genetically modified autologous CAR T cell therapy with a 4-1BB co-stimulatory domain, targeting B-cell maturation antigen (BCMA) [112]. The type III transmembrane protein BCMA, also known as CD269 or TNFRSF17, was expressed only by normal and malignant plasma cells, constituting a member of the TNF receptor superfamily. [113]. In the KarMMa trial (NCT03361748), patients with R/R MM who had undergone at least three prior treatments were administrated Idecabtagene vicleucel target doses ranging from 150 × 106 to 450 × 106 CAR T cells [114]. The best outcomes were shown in patients who received 450 × 106 dose, with 81% of them demonstrating an objective response and 39% demonstrating a complete response [114]. Compared to other CAR T products, the CRR of Abecma needed further improvement.
The second CAR T therapy to target BCMA was Ciltacabtagene autoleucel (Carvykti, LCAR-B38M, JNJ-4528), which was used to treat R/R MM in adults. This was the first Chinese CAR T cell treatment to be approved by the FDA. Two BCMA-targeting domains on the Ciltacabtagene autoleucel have been designed to increase avidity [115]. LCAR-B38M demonstrated potent efficacy in the LEGEND-2 longest follow-up (NCT03090659), with an ORR of 87.8% (73% CRR), minimal residual disease negativity rate of 67.6%, median DOR of 23 months and median progression-free survival (PFS) of 18 months in R/R MM after 4 years [116]. A phase Ib/II study called CARTITUDE-1 (NCT03548207) was conducted on Ciltacabtagene autoleucel with the goal of evaluating the drug efficacy and safety [117]. The ORR for CARTITUDE-1 was 97.9% after a 2-year follow-up, with 82.5% of patients achieving a stringent complete response [118]. The median PFS and OS were not attained. Only 4% of CRS patients had a grade 3 or higher, with grade 1 or 2 individuals making up the majority [119]. Neurotoxicity events, including ICANS and other neurotoxicities, occurred in 20 individuals following Ciltacabtagene autoleucel infusion [120].
Relmacabagene autolucel (JWCAR029), an autologous CAR-T cellular immunotherapy product that targeted CD19, was approved by the National Medicine Products Administration (NMPA) for the treatment of adult patients with R/R LBCL following the second-line or more systemic therapies. When compared to Lisocabtagene maraleucel, Relmacabagene autolucel used microbeads to provide a variety of dosages with similar product qualities without the requirement for distinct CD4 and CD8 T-cell production trains [121]. The best ORR and CRR in 58 patients with evaluable efficacy in the pivotal phase II clinical study (NCT04089215) were 75.9% and 51.7%, respectively [121]. On the safety side, the percentage of grade 3 or higher CRS and neurotoxic events in 59 treated patients were less than 5%.
In addition to its application in oncology, CAR T therapy has shown significant promise for the treatment of many types of autoimmune diseases. CAR T cell can treat many different types of autoimmune diseases by targeting and eliminating pathogenic immune cells implicated in the disease pathology. In addition, CAR regulatory T cell (Treg) therapy activates and proliferates Treg cells, which further enhances the immunosuppressive effect. Mycophenolate mofetil, an immunosuppressant, in conjunction with CD19-targeting CAR T cells had the potential to disrupt pathogenic B-cell and T-cell responses, leading to remission in patients with refractory antisynthetase syndrome [122]. Anti-CD19 CAR T cells were proven to lead to deep B-cell depletion, clinical symptoms improvement, and drug-free remission in five patients with systemic lupus erythematosus [123]. A recent study demonstrated favorable therapeutic outcomes and controllable safety of anti-CD19 CAR T cells in patients with severe systemic lupus erythematosus, idiopathic inflammatory myositis, and systemic sclerosis [124]. CAR Treg has also shown potential in preclinical studies and early clinical trials for a variety of autoimmune diseases, including graft-versus-host disease, type 1 diabetes, multiple sclerosis, etc. For instance, in immunodeficient mice that were reconstituted with human PBMCs, anti-CD19 CAR Tregs inhibited the generation of antibodies, lowering the likelihood of graft-versus-host disease [125].
Challenges and strategies of ACT in solid tumors
Tumor heterogeneity and antigen loss
The success of ACT in hematological malignancies was attributable to the specific expression of identifiable antigens (CD19 and BCMA) on tumors. However, only a small number of antigens were tumor-specific. The majority of candidate antigens frequently co-expressed on normal and cancerous tissues, posing a significant risk of morbidities due to on-target, off-tumor toxicity [126]. Some tumor-specific neoantigens have been found thanks to the advancement of genomic and proteomic methods. Disialoganglioside (GD2) was overexpressed in some tumor types but showed limited expression in normal tissues [127]. In a phase I clinical trial (NCT04196413), 75% of patients with H3K27M-mutated diffuse intrinsic pontine glioma (DIPG) or spinal cord diffuse midline gliomas, exhibiting high GD2 expression, experienced clinical and radiographic improvements. No on-target or off-tumor toxicity was observed [128].
Despite the fact that some antigens may be overexpressed on tumor cells, solid tumors had a high degree of heterogeneity, which was a common mechanism of therapy resistance [129]. Based on a phase III trial (NCT03070392), Tebentafusp was approved by the FDA on January 25, 2022, for the treatment of people with HLA-A*02:01-positive metastatic or incurable uveal melanoma [130]. Compared to the control group, 252 patients with metastatic uveal melanoma who received tebentafusp treatment had longer OS (73% vs 59%) [131]. The immune-mobilizing monoclonal TCR against cancer, in which the anti-CD3 scFv was capable of recruiting any T cells to the tumor cell perimeter, was the foundational component of tebentafusp. This method was independent of the tumor mutation status or the presence of tumor antigen-specific T cells. High antigen density requirements were present in traditional CAR designs, but the antigen density thresholds could be tuned by optimizing the CAR designs [132]. The expression of Glypican 2 (GPC2) was higher in optic neuroblastoma tissues compared to normal pediatric tissues, making it a prime candidate for CAR T cell therapy [133]. The GPC2-CAR T cells, equipped with CD28TM/endodomains and augmented c-Jun expression, effectively lowered the threshold of GPC2-CAR antigen density. This enhancement facilitated the proficient and sustained eradication of neuroblastomas exhibiting clinically relevant GPC2 antigen densities [134]. One of the most clearly reasons for relapse following CAR T cell therapy was antigen loss. To overcome antigen loss and lower the likelihood of tumors resistance, CARs against multiple antigens have been tested. CD20 and CD22 were promising CD19-negative tumor targets for CAR T cell therapy [135]. In a phase I/II single-arm trial (NCT03097770), CD19/CD20 CAR T cells were administered to 87 patients with R/R NHL. The best ORR among 87 patients was 78%, with 70% of patients achieving a complete response [136]. The bispecific CARs were effective in seven of nine patients who had relapsed after receiving CD19-CAR T cells, and only one of sixteen patients who relapsed after treatment of CD19/CD20 CAR T cells was found to have antigen loss [136]. The effectiveness and safety of bispecific CARs, as well as their capacity to prevent antigen loss in lymphoma patients, have been confirmed by additional clinical trials [137–142]. Table 3 summarized the multi-antigen-targeting CAR T cells for B cell malignancies being studied. The similar approach has also been conducted for other cancers, including glioblastoma [143], lung cancer [144], cholangiocarcinoma [145], gastric cancer [146] and hepatocellular carcinoma [147]. These findings suggested that multispecific CAR T cell therapy could be a promising strategy for preventing relapse due to antigen loss. However, a tiny proportion of individuals had antigen loss. Furthermore, more clinical trials and longer follow-up were required to assess the safety of multispecific CAR T cell therapy, particularly for on-target, off-tumor toxicity [148].
Table 3.
Selected multi-antigen-targeting CAR T cell therapy
| Target | Type of cancer | Dose level (cells per kg) | Response | Antigen loss relapse | Clinical trial |
|---|---|---|---|---|---|
| CD19/CD20 | R/R B-NHL and CLL | 2.5 × 105–2.5 × 106 | ORR: 82% CRR: 64% | 1/13 CD20 loss | NCT03019055 |
| CD19/CD20 | R/R NHL | 0.5–8 × 106 | ORR: 78% CRR: 70% | 1/16 CD19/CD20 loss | NCT03097770 |
| CD19/CD22 | R/R aggressive B-cell lymphoma | 4.9–9.4 × 106 | ORR: 87.5% CRR: 62.5% | None | ChiCTR1800015575 |
| CD19/CD22 | R/R B-ALL and LBCL | 1–3 × 106 | ORR: 79% CRR: 55% | 9/24 CD19 loss/low | NCT03233854 |
| CD19/CD22 | R/R B-ALL and B-NHL |
CD19: 2.6 ± 1.5 × 106/5.1 ± 2.1 × 106 CD22: 2.7 ± 1.2 × 106/5.3 ± 2.4 × 106 |
ORR: 89% CRR: 77% | 1/42 CD19 loss | ChiCTR-OPN-16008526 |
| CD19/CD22 | R/R B-NHL |
CD19: 4.1 × 106 CD22: 4.0 × 106 |
ORR: 90.5% CRR: 81% | None | ChiCTR-OPN-16009847 |
| CD19/CD22 | R/R B-ALL | 1.7 × 106–3 × 106 | CRR: 100% | 1/3 CD19/CD20 loss/low | NCT03185494 |
R/R, relapsed/refractory; B-NHL, B cell non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; B-ALL, B cell acute lymphoblastic leukemia; LBCL, large B-cell lymphoma; ORR, objective response rate; CRR, complete response rate
T-cell trafficking and infiltration
Anti-tumor efficacy needs effective trafficking of effector cells to tumor tissues, which depend on the interaction between chemokines released by tumor cells and chemokine receptors on T cells [149]. Given that larger and persistent CAR T cells were associated with a higher likelihood of response in the blood of lymphoma patients, the difficulty of ACT to traffic to tumor sites due to disruption of the chemokine axes helps explain the lack of efficacy of these therapies in solid tumors to date. Tumors presumably diminished or even silenced the activity of chemokine axes involved in anti-tumor responses, while likely increasing the activity of chemokine axes engaged in pro-tumor immune cell activation [150]. In NSCLC, co-expression of tumor antigen mesothelial protein specific CAR T cells with the chemokine receptor CCR4 increased the migration of CAR T cells [151]. The infiltration of CAR T cells into glioblastoma was facilitated by CXCL11-armed oncolytic adenovirus, which also reprogramed the immunosuppressive TME to produce a significant antitumor effect and prolong survival [152]. When administered without prior lymphodepletion, CAR T cells were potentially modified to generate chemokine ligands like CCL19 to promote the recruitment of endogenous T cells and dendritic cells to tumor locations [153].
T cells encountered strong physical obstacles that could prevent their infiltration and impair their activity as they trafficked to solid tumor locations. The tumor stroma, which was made up of extracellular matrix (ECM), cancer-associated fibroblasts (CAFs), and the aberrant vasculature at the tumor site is a major hurdles [154]. To overcome these issues, a variety of techniques to increase ACT infiltration have been proposed. Mild heating has been proven to increase the infiltration of transferred cells by directly killing tumor cells and partially destroying ECM when combined with ACT [155]. Others have concentrated their efforts on matrix-degrading enzymes such as collagenases and hyaluronidases. Nearly all ECM components could be degraded by matrix metalloproteinases (MMPs), a class of calcium and zinc-dependent proteolytic enzymes [156]. MMPs could be secreted by macrophages [157]. A breast cancer study revealed that CAR-147 macrophages reduced collagen deposition in tumors and enhanced T cell infiltration into tumors, leading to a suppression of human epidermal growth factor receptor 2 (HER2)-4T1 tumor growth in mice [158]. The ECM was disrupted and more endogenous CD8+ T lymphocytes could be produced by targeting CAR T cells to fibroblast activating proteins (FAP) that were present in the stroma of most cancers [159]. Furthermore, a study found that modifying CAR-T cells to secrete heparinase enzyme could destroy the tumor matrix and improve tumor infiltration while increasing anticancer efficacy [160]. Fibronectin, an essential scaffold of the ECM, acted as a barrier between T cells and tumor cells, significantly affecting T cell infiltration [161]. Extra domain A (EDA) and extra domain B (EDB), two selectively spliced fibronectin exons, are overexpressed in most cancers but barely expressed in healthy tissues [162]. In vitro, Anti-EDA EDA CAR-T cells recognized and eliminated tumor cell lines that expressed EDA, and they exhibited antitumor effects in mice with immunocompetence [163]. In addition, when NSG mice were exposed to the human hepatocarcinoma cell line PLC, the human version of EDA CAR, which contained the human 4-1BB and CD3 endo domains, demonstrated potent antitumor activity [163]. EDB-CAR T cells had powerful anti-tumor activity in three xenograft mouse models and behaved in an antigen-dependent manner in vitro while posing minimal on-target, off-tumor toxicity [164]. Integrin αvβ3, a crucial component of tumor angiogenesis and metastasis, is typically promoted by the hypoxic TME. Therefore, it is a desirable target for ACT therapy [165]. In NSG mice, αvβ3 CAR-T cells significantly suppressed DIPG and glioblastoma and showed durable efficacy [166].
The structure and function of tumor blood vessels are markedly different from those of normal blood vessels, exhibiting unusual leakiness, high tortuosity, and inadequate pericyte coverage being some of their distinguishing features [167]. The delivery of T cells to tumors was hampered by aberrant vasculature, which also created an immune-hostile microenvironment and hypoxic tumors [168]. The initial solutions focused on anti-angiogenesis, a therapy that deprived tumors of oxygen and nutrients, resulting in delayed tumor growth [169]. However, since numerous growth factors, as well as a wide variety of cytokines and biomolecules, are involved in the process of angiogenesis, the current anti-angiogenesis therapies, which primarily target vascular endothelial growth factor (VEGF), have only a temporary effect on tumors [170]. Furthermore, destroying tumor vasculature may foster tumor metastasis with little reward to patient survival [171]. As a result, vasculature normalization was becoming more popular as an effective method. Vasculature normalization improved the efficiency of anti-tumor therapy and prevented tumor metastasis by restoring the structure and functionality of tumor vessels within a specific time window [172]. Tumor vasculature served as an effective target for CAR T cells due to its stable expression. Vascular endothelial growth factor receptor (VEGFR)-2 CAR T cells were more effective at controlling tumors when an anti-VEGF-A antibody was also administered in B16 tumor-bearing mice [173]. A recent study showed that the inhibition for phosphoglycerate dehydrogenase improved T cell infiltration and activation in tumors, pruned the aberrant vasculature, and made glioblastoma more responsive to CAR T immunotherapy [174].
Other strategies have also been used to enhance T cell infiltration. For instance, Tao et al. infused tumor-specific targeting peptides identified by phage display biopanning technology onto the T cell membranes to precisely target highly heterogeneous solid tumors while also significantly increasing CD8+ T cell infiltration [175]. Interestingly, scientists found that glucose/mannose analogue 2-deoxy-D-glucose, a medication that prevented N-glycan synthesis, eliminated the protective effect of N-glycans on tumors and made solid tumors more accessible for CAR T cells to infiltrate and eradicate [176].
Immunosuppressive tumor microenvironment
Effective tumor-specific T cell responses to cancers were severely hampered by the immunosuppressive TME. Effector cells in immunosuppressive TME could be inhibited by immunosuppressive cells, immune inhibitory ligands on the surface of tumor cells and immunosuppressive cytokines [177].
Targeting immunosuppressive cells
Tregs, tumor-associated macrophages (TAM), and myeloid-derived suppressor cells (MDSC) are major immunosuppressive cells [178]. Immunosuppressive cells diminish the efficacy of ACT through multiple mechanisms. For instance, Treg suppressed or killed effector T cells by secreting cytotoxic substances like GZMB and immunosuppressive cytokines like TGF-β and IL-10 [179]. Treg also depleted the T-cell growth factor IL-2 by expressing a high level of CD25, a subunit of the IL-2 receptor, thereby limiting T-cell activation and proliferation [180]. Another strategy used by Treg to suppress T cells was the upregulation of immune checkpoint molecules such as lymphocyte activation gene 3 (LAG-3), CTLA-4, PD-1, and inducible co-stimulatory factor (ICOS) [181]. Basic researches have utilized a number of strategies to target immunosuppressive cells in order to improve the capacity of CAR T cells to eradicate tumors. When administered intratumorally, IL-12 has been demonstrated to improve CAR T cells cytotoxicity targeting epidermal growth factor receptor variant III and decreased the number of Tregs in a mouse model of orthotopic glioblastoma multiforme [182]. When the tumor necrosis factor–related apoptosis-inducing ligand receptor 2 and 4-1BB receptor were co-expressed on CAR T cells, the CAR T cell responses against tumor-associated mucin 1 or HER2 on breast cancer were improved. This resulted in TME remodeling and enhanced T cell proliferation at the tumor site [183]. CAR T cells that targeted folate receptor β, a characteristic of immunosuppressive M2 TAM cells, increased CD8+ T cell infiltration and enhanced the efficacy of CAR T cells in ovarian cancer, colon cancer, and melanoma mouse models [184]. Together, these developments potentially ushered in a new era for CAR T cell treatment by converting immunosuppressive cells to a more advantageous niche.
Targeting immune checkpoints
Immune checkpoint receptors and ligands, including PD-1, PD-L1, CTLA-4, LAG-3, T cell immunoglobulin and mucin-domain containing-3, B7-H3 and V-domain Ig suppressor of T cell activation, have been shown to impair the efficacy of ACT and produce anergy in TME [185]. Therapies targeting PD-1 have been utilized in conjunction with ACT to improve the lifespan and effectiveness of CAR T cells. Disruption of PD-1 by CRISPR-Cas9 increased cytokine output and cytotoxicity of CAR T cells against PD-L1+ cancer cells without reducing proliferation [186]. Researchers discovered that co-expression of a PD-1 decoy receptor may be able to bypass the inhibitory signaling of B7-H1/PD-1 in the TME of solid tumors and dramatically increase the therapeutic efficacy of B7-H3 specific CAR T cells [187]. Others have developed CAR T cells to release bispecific trap proteins that specifically target PD-1 and TGF-β, attenuating suppressive T cell signaling, boosting T cell persistence and proliferation, and promoting effector function and resistance to exhaustion [188]. Although this targeting technique has not been thoroughly researched, similar strategies could be utilized against other immune checkpoints. B7-H3, also known as CD276, has been implicated in tumor growth, metastasis, and treatment resistance, all of which contribute to a bad prognosis for patients by assisting cancer cells in evading the surveillance by cytotoxic T cells and NK cells [189]. In a first-in-human phase I trial (NCT04185038), B7-H3 CAR T cells were administered to children with R/R central nervous system malignancies and DIPG, which demonstrated correlated evidence of local immune activation and persistent cerebrospinal fluid B7-H3 CAR T cells [190]. In fact, checkpoint blockade could effectively fuel CARs and enhance the efficacy of CAR T cells.
Targeting cytokines
Although activated T cells secreted a variety of cytokines, including IFN-γ and IL-2, these were obviously insufficient to maintain the prolonged effects of T cells against tumors. In preclinical and clinical trials, many cytokines have been employed in conjunction with CAR T cells to improve the efficacy of ACT [191]. In a disseminated, syngeneic RM9-hSTEAP1 tumor model in hSTEAP1-KI mice, STEAP1 CAR T cells combined with a collagen binding domain IL-12 enhanced OS, cytokine production, tumor antigen presentation, and dissemination with epitopes to prevent STEAP1 antigen escape [192]. As a cytokine involved in T cell activation, maintenance, and proliferation, IL-7 enhanced CAR T cell effectiveness in vivo and facilitated tumor cell killing [193]. In addition to increasing the number of CAR T cells, recombinant human IL-7 fused with hybrid Fc (rhIL-7-hyFc) significantly enhanced T cells cytotoxicity and lowered exhaustion in lymphoma and leukemia models [194]. P1A tumor antigen-specific TCR-T cells which produced IL7/CCL19, showed noticeably increased anticancer effects and produced long-term memory responses by enhancing the infiltration of dendritic cells and T cells in tumor tissues, including both endogenous T cells and transplanted P1A T cells [195]. GD2-specific CAR T cells were modified to release IL-15 to improve the destruction of lung cancer [196] and glioblastoma [197]. Recently, IL-15-secreting CAR T cells have been investigated to target MDSC in glioblastoma, making CAR T cells more beneficial [198]. These IL-15-secreting CAR T cells were also used in clinical studies for hepatocellular carcinoma treatment (NCT05103631, NCT04377932). In mouse models of small cell lung cancer, IL-18-secreting CAR T cells targeting Delta-like protein 3 induced a persistent response and decreased T cell exhaustion [199]. In a trial at the University of Pennsylvania, IL-18-releasing CAR T cells were investigated for the treatment of CLL, NHL, and ALL (NCT04684563) [200].
T cell exhaustion in the immunosuppressive TME
The mouse model of chronic lymphocytic choriomeningitis virus infection was the initial experiment to demonstrate T cell exhaustion, in which virus-specific CD8 T cells exposed to ongoing antigen stimulation exhibited diminished effector function and poor proliferative capacity in comparison to functional memory CD8 T cells [201]. Later researches revealed exhausted T (Tex) cells to be a distinct heterogeneous population of immune cells that played a crucial role in the development of cancer, autoimmune diseases, and chronic infections. Effector activities were gradually lost in Tex cells, which also exhibited strong and persistent inhibitory receptor expression, metabolic dysregulation, poor memory recall and homeostatic self-renewal [202]. The effectiveness of ACT in solid tumors was significantly hampered by T cell exhaustion. Decreasing exhaustion to maintain T cell efficiency and durability was a key problem.
Immune checkpoint blockade is an essential method for preventing T cell exhaustion. Currently, drugs and technologies targeting immune checkpoints have been combined with CAR T cells, showing promising results [203–205]. Immune checkpoint blockage temporarily activates Tex cells, but it has no lasting impact on their epigenetic structure. Therefore, a variety of epigenetic markers have been found to contribute to exhaustion when overexpressed or knocked down. This has rapidly become a more general direction to alleviate T cell exhaustion. For instances, knockdown of PR domain zinc finger protein 1 (PRDM1) in CD19-targeting CAR T cells resulted in the production of better-quality T cells, increasing T cell persistence and decreasing T cell exhaustion [206]. Knockout of PRDM1 and NR4A3 improved the anti-tumor response by increasing the generation of long-lived memory cells, counteracting exhaustion in tumor-infiltrating CAR T cells, and enhancing the overall anti-tumor response [207]. The extensive application of CRISPR/Cas9 and bioinformatics-based technologies has provided researchers with the chance to identify the important parameters that could regulate T cell exhaustion. The proliferation of TCR-T cells targeting NY-ESO-1 and M5 CAR T cells targeting mesothelin was increased by the double knockout of Regnase-1 and Roquin-1, which also improved anti-tumor efficacy and T cell lifespan [208]. The constant interaction between CARs and antigens was one cause of CAR T cell exhaustion. With antigens being unregulated, attention has been focused on making CARs controllable [209]. Inducing rest by downregulating CAR proteins with a drug-regulatable system or employing the tyrosine kinase inhibitor dasatinib could improve CAR-T cell effectiveness by preventing or reversing exhaustion [210]. Similar controllable strategies, such as ligand [211] and light [212] controlled systems, have been utilized to regulate CAR expression, but the effect on CAR T cell exhaustion needed to be studied further. Another approach was to select a more appropriate co-stimulatory domain. In comparison to CD28 CAR T cells, 4-1BB CAR T cells proliferated better, showed less exhaustion, had longer effectiveness, and exhibited better in vivo anti-tumor capacity [213].
Conclusion and perspective
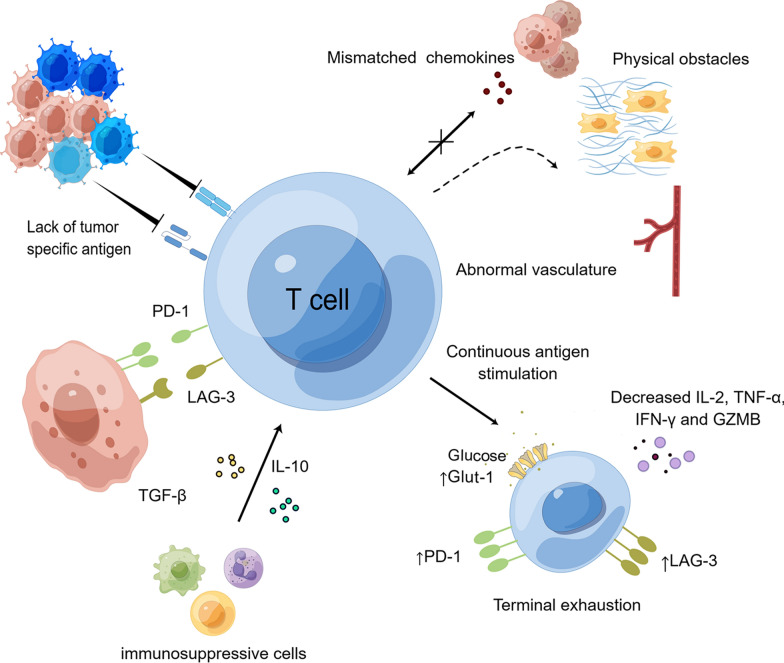
ACT has shown considerable promise in treating many hematologic cancers, but it has not matched expectations in solid tumors due to a number of constraints, such as antigen loss, poor infiltration in tumors, immunosuppressive TME and so on (Fig. 3). Numerous attempts have been undertaken by scientists to get over these restrictions, and the results have been encouraging. Using bioinformatics and sequencing technology, researchers will be able to identify more specific targets in their work in the future. The infiltration and survivability of effector cells were impacted by the extraordinarily complicated and hazardous internal and external environments of solid tumors. Meanwhile, T cells also became more anergy and exhausted as a result of the aggressive TME and continuous antigen stimulation, which resulted in the failure of ACT. Better understanding the metabolic program and epigenetic states of T cell dysregulation will enhance the efficacy of ACT in treating solid tumors. Despite the remaining obstacles, we believed that ACT would eventually play a significant role in the treatment of solid tumors.
Fig. 3.
Summary of major barriers of Adoptive cell therapy (ACT) in solid tumors. Some of the most difficult barriers to the development of ACT in solid tumors included tumor heterogeneity, antigen loss, hard trafficking and infiltration, an immunosuppressive tumor microenvironment, and T cell exhaustion. By Figdraw
Acknowledgements
This work was supported by National Key Clinical Specialties Construction Program.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte antigen 4
- PD-1
Programmed cell death 1
- OS
Overall survival
- FDA
The US Food and Drug Administration
- ICI
Immune checkpoint inhibitor
- ACT
Adoptive cell therapy
- TIL
Tumor infiltrating lymphocytes
- CAR
Chimeric antigen receptor
- TCR
T cell receptor
- HLAs
Human leukocyte antigens
- TME
Tumor microenvironment
- MHC
Major histocompatibility
- CTL
Cytotoxic T lymphocyte
- GZM
Granzymes
- IFN-γ
Interferon-γ
- TNF-α
Tumor necrosis factor-α
- cDC
Conventional dendritic cell
- IL
Interleukin
- TIL-Bs
Tumor-infiltrating B lymphocytes
- TGF
Tumor growth factor
- TLSs
Tertiary lymphoid structures
- ORR
Objective response rate
- CRR
Complete response rate
- NSCLC
Non-small cell lung cancer
- ITAM
Immunoreceptor tyrosine-based activation motif
- TAAs
Tumor-associated antigens
- TSAs
Tumor-specific antigens
- CTAs
Cancer testis antigens
- MAGE
Melanoma-associated antigen
- NY-ESO-1
New York esophageal squamous cell carcinoma-1
- SS
Synovial sarcoma
- SPEAR
Specific peptide enhanced affinity receptor
- NCI
The National Cancer Institute
- AEs
Adverse events
- TCR-T
T cell receptor-transgenic T
- HPV
Human papillomavirus
- HBV
Hepatitis B virus
- EBV
Epstein-Barr virus
- NPC
Nasopharyngeal carcinoma
- NHL
Non-Hodgkin lymphomas
- NK
Natural killer
- PBMC
Peripheral blood mononuclear cells
- scFv
Single-chain fragment variable
- TRUCKs
T cells redirect for universal cytokine killings
- R/R
Relapsed/refractory
- B-ALL
B-cell precursor acute lymphoblastic leukemia
- LBCL
Large B-cell lymphoma
- FL
Follicular lymphoma
- DLBCL
Diffuse large B-cell lymphoma
- PMBCL
Primary mediastinal large B-cell lymphoma
- HGBCL
High-grade B-cell lymphoma
- CRS
Cytokine release syndrome
- ICANS
Immune effector cell-associated neurotoxicity syndrome
- MCL
Mantle cell lymphoma
- DOR
Duration of response
- MM
Multiple myeloma
- BCMA
B-cell maturation antigen
- PFS
Progression-free survival
- NMPA
National Medicine Products Administration
- DIPG
Diffuse intrinsic pontine glioma
- GPC2
Glypican 2
- ECM
Extracellular matrix
- CAFs
Cancer-associated fibroblasts
- MMPs
Matrix metalloproteinases
- HER2
Human epidermal growth factor receptor 2
- FAP
Fibroblast activating protein
- EDA
Extra domain A
- EDB
Extra domain B
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- Treg
Regulatory T cells
- TAM
Tumor-associated macrophages
- MDSC
Myeloid-derived suppressor cells
- LAG-3
Lymphocyte activation gene 3
- ICOS
Inducible co-stimulatory factor
- rhIL-7-hyFc
Recombinant human IL-7 fused with hybrid Fc
- Tex
Exhausted T
- PRDM1
PR domain zinc finger protein 1
- GD2
Disialoganglioside
Author contributions
YL conducted information investigation, wrote the original draft, and drew pictures. TL, YZ, and CL reviewed and edited the original draft. G.S. and Z.H. conceived and designed the framework for this review, reviewed and edited the original draft, and provided valuable suggestions for this study. All authors read and approved the final manuscript.
Funding
This work was supported by the National Science Fund for Excellent Young Scholars (No. 32122052) and the 1.3.5 project for disciplines of excellence—clinical research incubation project, West China Hospital, Sichuan University (No. 2021HXFH064).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yinqi Li and Yeteng Zheng have contributed equally to this work.
Contributor Information
Guobo Shen, Email: shenguobo@126.com.
Zhiyao He, Email: heyaode@163.com.
References
- 1.McNutt M. Cancer immunotherapy. Science. 2013;342(6165):1417. doi: 10.1126/science.1249481. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morotti M, Albukhari A, Alsaadi A, Artibani M, Brenton JD, Curbishley SM, et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br J Cancer. 2021;124(11):1759–1776. doi: 10.1038/s41416-021-01353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guedan S, Ruella M, June CH. Emerging cellular therapies for cancer. Annu Rev Immunol. 2019;37:145–171. doi: 10.1146/annurev-immunol-042718-041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30(6):507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, et al. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov. 2013;3(4):418–429. doi: 10.1158/2159-8290.CD-12-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribas A. T cells as the future of cancer therapy. Cancer Discov. 2021;11(4):798–800. doi: 10.1158/2159-8290.CD-21-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrea AE, Chiron A, Mallah S, Bessoles S, Sarrabayrouse G, Hacein-Bey-Abina S. Advances in CAR-T cell genetic engineering strategies to overcome hurdles in solid tumors treatment. Front Immunol. 2022;13:830292. doi: 10.3389/fimmu.2022.830292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirtane K, Elmariah H, Chung CH, Abate-Daga D. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J Immunother Cancer. 2021;9(7):e002723. doi: 10.1136/jitc-2021-002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clambey ET, Davenport B, Kappler JW, Marrack P, Homann D. Molecules in medicine mini review: the alphabeta T cell receptor. J Mol Med (Berl) 2014;92(7):735–741. doi: 10.1007/s00109-014-1145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gascoigne NR, Rybakin V, Acuto O, Brzostek J. TCR signal strength and T cell development. Annu Rev Cell Dev Biol. 2016;32:327–348. doi: 10.1146/annurev-cellbio-111315-125324. [DOI] [PubMed] [Google Scholar]
- 19.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see) Nat Rev Immunol. 2014;14(6):377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier JL, Weiss SA, Pauken KE, Sen DR, Sharpe AH. Not-so-opposite ends of the spectrum: CD8(+) T cell dysfunction across chronic infection, cancer and autoimmunity. Nat Immunol. 2021;22(7):809–819. doi: 10.1038/s41590-021-00949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 22.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356 e1316. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 23.van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20(4):218–232. doi: 10.1038/s41568-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2022;22(4):209–223. doi: 10.1038/s41577-021-00574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 26.Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18(4):842–859. doi: 10.1038/s41423-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hor JL, Whitney PG, Zaid A, Brooks AG, Heath WR, Mueller SN. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity. 2015;43(3):554–565. doi: 10.1016/j.immuni.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183(3):1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedoui S, Heath WR, Mueller SN. CD4(+) T-cell help amplifies innate signals for primary CD8(+) T-cell immunity. Immunol Rev. 2016;272(1):52–64. doi: 10.1111/imr.12426. [DOI] [PubMed] [Google Scholar]
- 30.Oh DY, Fong L. Cytotoxic CD4(+) T cells in cancer: expanding the immune effector toolbox. Immunity. 2021;54(12):2701–2711. doi: 10.1016/j.immuni.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griss J, Bauer W, Wagner C, Simon M, Chen M, Grabmeier-Pfistershammer K, et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun. 2019;10(1):4186. doi: 10.1038/s41467-019-12160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruffin AT, Cillo AR, Tabib T, Liu A, Onkar S, Kunning SR, et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat Commun. 2021;12(1):3349. doi: 10.1038/s41467-021-23355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SS, Shen S, Miyauchi S, Sanders PD, Franiak-Pietryga I, Mell L, et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin Cancer Res. 2020;26(13):3345–3359. doi: 10.1158/1078-0432.CCR-19-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell. 2019;179(5):1191–1206 e1121. doi: 10.1016/j.cell.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee-Chang C, Rashidi A, Miska J, Zhang P, Pituch KC, Hou D, et al. Myeloid-derived suppressive cells promote B cell-mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunol Res. 2019;7(12):1928–1943. doi: 10.1158/2326-6066.CIR-19-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294–307. doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 38.Rossetti RAM, Lorenzi NPC, Yokochi K, Rosa M, Benevides L, Margarido PFR, et al. B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumor responses. PLoS ONE. 2018;13(7):e0199034. doi: 10.1371/journal.pone.0199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015;521(7550):99–104. doi: 10.1038/nature14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19(21):5994–6005. doi: 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- 41.Hu X, Zhang J, Wang J, Fu J, Li T, Zheng X, et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat Genet. 2019;51(3):560–567. doi: 10.1038/s41588-018-0339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolotin DA, Poslavsky S, Davydov AN, Frenkel FE, Fanchi L, Zolotareva OI, et al. Antigen receptor repertoire profiling from RNA-seq data. Nat Biotechnol. 2017;35(10):908–911. doi: 10.1038/nbt.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375(6576):eabf9419. doi: 10.1126/science.abf9419. [DOI] [PubMed] [Google Scholar]
- 44.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 46.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma—a preliminary-report. N Engl J Med. 1988;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18(24):6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevanovic S, Helman SR, Wunderlich JR, Langhan MM, Doran SL, Kwong MLM, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human Papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25(5):1486–1493. doi: 10.1158/1078-0432.CCR-18-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375(23):2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24(6):724–730. doi: 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishna S, Lowery FJ, Copeland AR, Bahadiroglu E, Mukherjee R, Jia L, et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science. 2020;370(6522):1328–1334. doi: 10.1126/science.abb9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, et al. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17(15):4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia Y, Tao HM, Hu YY, Chen QN, Chen X, Xia LM, et al. IL-2 augments the therapeutic efficacy of adoptively transferred B cells which directly kill tumor cells via the CXCR4/CXCL12 and perforin pathways. Oncotarget. 2016;7(37):60461–60474. doi: 10.18632/oncotarget.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee-Chang C, Miska J, Hou D, Rashidi A, Zhang P, Burga RA, et al. Activation of 4–1BBL+ B cells with CD40 agonism and IFNgamma elicits potent immunity against glioblastoma. J Exp Med. 2021;218(1):e20200913. doi: 10.1084/jem.20200913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27(8):1410–1418. doi: 10.1038/s41591-021-01462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2011;11(1):47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 60.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leko V, Rosenberg SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 2020;38(4):454–472. doi: 10.1016/j.ccell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei R, Dean DC, Thanindratarn P, Hornicek FJ, Guo W, Duan Z. Cancer testis antigens in sarcoma: expression, function and immunotherapeutic application. Cancer Lett. 2020;479:54–60. doi: 10.1016/j.canlet.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 63.Lu YC, Parker LL, Lu T, Zheng Z, Toomey MA, White DE, et al. Treatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol. 2017;35(29):3322–3329. doi: 10.1200/JCO.2017.74.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafer P, Kelly LM, Hoyos V. Cancer therapy with TCR-engineered T cells: current strategies, challenges, and prospects. Front Immunol. 2022;13:835762. doi: 10.3389/fimmu.2022.835762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banks LB, D'Angelo SP. The role of immunotherapy in the management of soft tissue sarcomas: current landscape and future outlook. J Natl Compr Canc Netw. 2022;20(7):834–844. doi: 10.6004/jnccn.2022.7027. [DOI] [PubMed] [Google Scholar]
- 66.Ramachandran I, Lowther DE, Dryer-Minnerly R, Wang R, Fayngerts S, Nunez D, et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J Immunother Cancer. 2019;7(1):276. doi: 10.1186/s40425-019-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stadtmauer EA, Faitg TH, Lowther DE, Badros AZ, Chagin K, Dengel K, et al. Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv. 2019;3(13):2022–2034. doi: 10.1182/bloodadvances.2019000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365. doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ecsedi M, McAfee MS, Chapuis AG. The anticancer potential of T cell receptor-engineered T cells. Trends Cancer. 2021;7(1):48–56. doi: 10.1016/j.trecan.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He J, Xiong X, Yang H, Li D, Liu X, Li S, et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response. Cell Res. 2022;32(6):530–542. doi: 10.1038/s41422-022-00627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giannone G, Giuliano AR, Bandini M, Marandino L, Raggi D, Earle W, et al. HPV vaccination and HPV-related malignancies: impact, strategies and optimizations toward global immunization coverage. Cancer Treat Rev. 2022;111:102467. doi: 10.1016/j.ctrv.2022.102467. [DOI] [PubMed] [Google Scholar]
- 72.Albuquerque A, Nathan M, Cappello C, Dinis-Ribeiro M. Anal cancer and precancerous lesions: a call for improvement. Lancet Gastroenterol Hepatol. 2021;6(4):327–334. doi: 10.1016/S2468-1253(20)30304-6. [DOI] [PubMed] [Google Scholar]
- 73.McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol. 2022;20(2):95–108. doi: 10.1038/s41579-021-00617-5. [DOI] [PubMed] [Google Scholar]
- 74.Lechner M, Liu J, Masterson L, Fenton TR. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. 2022;19(5):306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22(1):19–32. doi: 10.1038/s41577-021-00549-4. [DOI] [PubMed] [Google Scholar]
- 76.Damania B, Kenney SC, Raab-Traub N. Epstein-Barr virus: Biology and clinical disease. Cell. 2022;185(20):3652–3670. doi: 10.1016/j.cell.2022.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2022;21(1):51–64. doi: 10.1038/s41579-022-00770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagarsheth NB, Norberg SM, Sinkoe AL, Adhikary S, Meyer TJ, Lack JB, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. 2021;27(3):419–425. doi: 10.1038/s41591-020-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, et al. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int. 2021;15(6):1402–1412. doi: 10.1007/s12072-021-10250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maomao C, He L, Dianqin S, Siyi H, Xinxin Y, Fan Y, et al. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 2022;19(8):1121–1138. doi: 10.20892/j.issn.2095-3941.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Mattos-Arruda L, Vazquez M, Finotello F, Lepore R, Porta E, Hundal J, et al. Neoantigen prediction and computational perspectives towards clinical benefit: recommendations from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(8):978–990. doi: 10.1016/j.annonc.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lowery FJ, Krishna S, Yossef R, Parikh NB, Chatani PD, Zacharakis N, et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022;375(6583):877–884. doi: 10.1126/science.abl5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18(4):215–229. doi: 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnaud M, Chiffelle J, Genolet R, Navarro Rodrigo B, Perez MAS, Huber F, et al. Sensitive identification of neoantigens and cognate TCRs in human solid tumors. Nat Biotechnol. 2022;40(5):656–660. doi: 10.1038/s41587-021-01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levy PL, Gros A. Fast track to personalized TCR T cell therapies. Cancer Cell. 2022;40(5):447–449. doi: 10.1016/j.ccell.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Foy SP, Jacoby K, Bota DA, Hunter T, Pan Z, Stawiski E, et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature. 2022;615(7953):687–696. doi: 10.1038/s41586-022-05531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leidner R, Sanjuan Silva N, Huang H, Sprott D, Zheng C, Shih YP, et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N Engl J Med. 2022;386(22):2112–2119. doi: 10.1056/NEJMoa2119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kingwell K. T cell receptor therapeutics hit the immuno-oncology stage. Nat Rev Drug Discov. 2022;21(5):321–323. doi: 10.1038/d41573-022-00073-7. [DOI] [PubMed] [Google Scholar]
- 89.Chandran SS, Ma J, Klatt MG, Dundar F, Bandlamudi C, Razavi P, et al. Immunogenicity and therapeutic targeting of a public neoantigen derived from mutated PIK3CA. Nat Med. 2022;28(5):946–957. doi: 10.1038/s41591-022-01786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pearlman AH, Hwang MS, Konig MF, Hsiue EH, Douglass J, DiNapoli SR, et al. Targeting public neoantigens for cancer immunotherapy. Nat Cancer. 2021;2(5):487–497. doi: 10.1038/s43018-021-00210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X, Ma C, Rodriguez Labrada R, Qin Z, Xu T, He Z, et al. Recent advances in lentiviral vectors for gene therapy. Sci China Life Sci. 2021;64(11):1842–1857. doi: 10.1007/s11427-021-1952-5. [DOI] [PubMed] [Google Scholar]
- 92.Denlinger N, Bond D, Jaglowski S. CAR T-cell therapy for B-cell lymphoma. Curr Probl Cancer. 2022;46(1):100826. doi: 10.1016/j.currproblcancer.2021.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jogalekar MP, Rajendran RL, Khan F, Dmello C, Gangadaran P, Ahn BC. CAR T-Cell-based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol. 2022;13:925985. doi: 10.3389/fimmu.2022.925985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cappell KM, Kochenderfer JN. A comparison of chimeric antigen receptors containing CD28 versus 4–1BB costimulatory domains. Nat Rev Clin Oncol. 2021;18(11):715–727. doi: 10.1038/s41571-021-00530-z. [DOI] [PubMed] [Google Scholar]
- 95.Ligon JA, Wessel KM, Shah NN, Glod J. Adoptive cell therapy in pediatric and young adult solid tumors: current status and future directions. Front Immunol. 2022;13:846346. doi: 10.3389/fimmu.2022.846346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu J, Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol Cancer. 2022;21(1):194. doi: 10.1186/s12943-022-01663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iacoboni G, Villacampa G, Martinez-Cibrian N, Bailen R, Lopez Corral L, Sanchez JM, et al. Real-world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B-cell lymphoma. Cancer Med. 2021;10(10):3214–3223. doi: 10.1002/cam4.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325–332. doi: 10.1038/s41591-021-01622-0. [DOI] [PubMed] [Google Scholar]
- 100.Pan K, Farrukh H, Chittepu V, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41(1):119. doi: 10.1186/s13046-022-02327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103. doi: 10.1016/S1470-2045(21)00591-X. [DOI] [PubMed] [Google Scholar]
- 103.Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 104.Kwon M, Iacoboni G, Reguera JL, Corral LL, Morales RH, Ortiz-Maldonado V, et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica. 2023;108(1):110–121. doi: 10.3324/haematol.2022.280805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang VC, Hao D, Jain P, Li Y, Cai Q, Yao Y, et al. TIGIT is the central player in T-cell suppression associated with CAR T-cell relapse in mantle cell lymphoma. Mol Cancer. 2022;21(1):185. doi: 10.1186/s12943-022-01655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2023;41(3):555–567. doi: 10.1200/JCO.21.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol. 2022;15(1):170. doi: 10.1186/s13045-022-01379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogasawara K, Dodds M, Mack T, Lymp J, Dell'Aringa J, Smith J. Population cellular kinetics of lisocabtagene maraleucel, an autologous CD19-directed chimeric antigen receptor T-cell product, in patients with relapsed/refractory large B-cell lymphoma. Clin Pharmacokinet. 2021;60(12):1621–1633. doi: 10.1007/s40262-021-01039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 110.Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 111.Sharma P, Kanapuru B, George B, Lin X, Xu Z, Bryan WW, et al. FDA approval summary: idecabtagene vicleucel for relapsed or refractory multiple myeloma. Clin Cancer Res. 2022;28(9):1759–1764. doi: 10.1158/1078-0432.CCR-21-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manier S, Ingegnere T, Escure G, Prodhomme C, Nudel M, Mitra S, et al. Current state and next-generation CAR-T cells in multiple myeloma. Blood Rev. 2022;54:100929. doi: 10.1016/j.blre.2022.100929. [DOI] [PubMed] [Google Scholar]
- 113.Yang J, Zhou W, Li D, Niu T, Wang W. BCMA-targeting chimeric antigen receptor T-cell therapy for multiple myeloma. Cancer Lett. 2023;553:215949. doi: 10.1016/j.canlet.2022.215949. [DOI] [PubMed] [Google Scholar]
- 114.Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 115.Parikh RH, Lonial S. Chimeric antigen receptor T-cell therapy in multiple myeloma: a comprehensive review of current data and implications for clinical practice. CA Cancer J Clin. 2023;73(3):275. doi: 10.3322/caac.21771. [DOI] [PubMed] [Google Scholar]
- 116.Zhao WH, Wang BY, Chen LJ, Fu WJ, Xu J, Liu J, et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: a phase 1, single-arm, open-label, multicenter study in China (LEGEND-2) J Hematol Oncol. 2022;15(1):86. doi: 10.1186/s13045-022-01301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 118.Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2022;41(6):1265–1274. doi: 10.1200/JCO.22.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chekol Abebe E, Yibeltal Shiferaw M, Tadele Admasu F, Asmamaw DT. Ciltacabtagene autoleucel: the second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. 2022;13:991092. doi: 10.3389/fimmu.2022.991092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cohen AD, Parekh S, Santomasso BD, Gállego Pérez-Larraya J, van de Donk NWCJ, Arnulf B, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12(2):32. doi: 10.1038/s41408-022-00629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ying Z, Yang H, Guo Y, Li W, Zou D, Zhou D, et al. Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China. Cancer Med. 2021;10(3):999–1011. doi: 10.1002/cam4.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pecher AC, Hensen L, Klein R, Schairer R, Lutz K, Atar D, et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA. 2023;329(24):2154–2162. doi: 10.1001/jama.2023.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mackensen A, Müller F, Mougiakakos D, Böltz S, Wilhelm A, Aigner M, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med. 2022;28(10):2124–2132. doi: 10.1038/s41591-022-02017-5. [DOI] [PubMed] [Google Scholar]
- 124.Müller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Völkl S, et al. CD19 CAR T-cell therapy in autoimmune disease—a case series with follow-up. N Engl J Med. 2024;390(8):687–700. doi: 10.1056/NEJMoa2308917. [DOI] [PubMed] [Google Scholar]
- 125.Imura Y, Ando M, Kondo T, Ito M, Yoshimura A. CD19-targeted CAR regulatory T cells suppress B cell pathology without GvHD. JCI Insight. 2020;5(14):e136185. doi: 10.1172/jci.insight.136185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang K, Chen H, Li F, Huang S, Chen F, Li Y. Bright future or blind alley? CAR-T cell therapy for solid tumors. Front Immunol. 2023;14:1045024. doi: 10.3389/fimmu.2023.1045024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nazha B, Inal C, Owonikoko TK. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front Oncol. 2020;10:1000. doi: 10.3389/fonc.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–941. doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carvajal RD, Sacco JJ, Jager MJ, Eschelman DJ, Olofsson Bagge R, Harbour JW, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol. 2023;20(2):99–115. doi: 10.1038/s41571-022-00714-1. [DOI] [PubMed] [Google Scholar]
- 130.Dhillon S. Tebentafusp: first approval. Drugs. 2022;82(6):703–710. doi: 10.1007/s40265-022-01704-4. [DOI] [PubMed] [Google Scholar]
- 131.Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–1206. doi: 10.1056/NEJMoa2103485. [DOI] [PubMed] [Google Scholar]
- 132.Hotblack A, Straathof K. Fine-tuning CARs for best performance. Cancer Cell. 2022;40(1):11–13. doi: 10.1016/j.ccell.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 133.Chen G, Luo D, Zhong N, Li D, Zheng J, Liao H, et al. GPC2 Is a potential diagnostic, immunological, and prognostic biomarker in pan-cancer. Front Immunol. 2022;13:857308. doi: 10.3389/fimmu.2022.857308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heitzeneder S, Bosse KR, Zhu Z, Zhelev D, Majzner RG, Radosevich MT, et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell. 2022;40(1):53–69 e59. doi: 10.1016/j.ccell.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Furqan F, Shah NN. Multispecific CAR T cells deprive lymphomas of escape via antigen loss. Annu Rev Med. 2023;74(1):279–291. doi: 10.1146/annurev-med-042921-024719. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Y, Wang Y, Liu Y, Tong C, Wang C, Guo Y, et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1–2 trial. Leukemia. 2022;36(1):189–196. doi: 10.1038/s41375-021-01345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–1575. doi: 10.1038/s41591-020-1081-3. [DOI] [PubMed] [Google Scholar]
- 138.Wei G, Zhang Y, Zhao H, Wang Y, Liu Y, Liang B, et al. CD19/CD22 dual-targeted CAR T-cell therapy for relapsed/refractory aggressive B-cell lymphoma: a safety and efficacy study. Cancer Immunol Res. 2021;9(9):1061–1070. doi: 10.1158/2326-6066.CIR-20-0675. [DOI] [PubMed] [Google Scholar]
- 139.Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang N, Hu XL, Cao WY, Li CR, Xiao Y, Cao Y, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135(1):17–27. doi: 10.1182/blood.2019000017. [DOI] [PubMed] [Google Scholar]
- 141.Cao Y, Xiao Y, Wang N, Wang G, Huang L, Hong Z, et al. CD19/CD22 chimeric antigen receptor T cell cocktail therapy following autologous transplantation in patients with relapsed/refractory aggressive B cell lymphomas. Transplant Cell Ther. 2021;27(11):910.e1–910.e11. doi: 10.1016/j.jtct.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 142.Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):30. doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schmidts A, Srivastava AA, Ramapriyan R, Bailey SR, Bouffard AA, Cahill DP, et al. Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Ralpha2 are effective against heterogeneous glioblastoma. Neurooncol Adv. 2023;5(1):vdac185. doi: 10.1093/noajnl/vdac185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang M, Tang X, Zhang Z, Gu L, Wei H, Zhao S, et al. Tandem CAR-T cells targeting CD70 and B7–H3 exhibit potent preclinical activity against multiple solid tumors. Theranostics. 2020;10(17):7622–7634. doi: 10.7150/thno.43991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv HY, et al. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J Hematol Oncol. 2017;10(1):4. doi: 10.1186/s13045-016-0378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang Y, McCloskey JE, Yang H, Puc J, Alcaina Y, Vedvyas Y, et al. Bispecific CAR T Cells against EpCAM and inducible ICAM-1 overcome antigen heterogeneity and generate superior antitumor responses. Cancer Immunol Res. 2021;9(10):1158–1174. doi: 10.1158/2326-6066.CIR-21-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang W, Li T, Guo J, Wang J, Jia L, Shi X, et al. Bispecific c-Met/PD-L1 CAR-T cells have enhanced therapeutic effects on hepatocellular carcinoma. Front Oncol. 2021;11:546586. doi: 10.3389/fonc.2021.546586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol. 2023;20(1):49–62. doi: 10.1038/s41571-022-00704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bule P, Aguiar SI, Aires-Da-Silva F, Dias JNR. Chemokine-directed tumor microenvironment modulation in cancer immunotherapy. Int J Mol Sci. 2021;22(18):9804. doi: 10.3390/ijms22189804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54(5):859–874. doi: 10.1016/j.immuni.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y, Wang J, Yang X, Yang J, Lu P, Zhao L, et al. Chemokine receptor CCR2b enhanced anti-tumor function of chimeric antigen receptor T cells targeting mesothelin in a non-small-cell lung carcinoma model. Front Immunol. 2021;12:628906. doi: 10.3389/fimmu.2021.628906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang G, Zhang Z, Zhong K, Wang Z, Yang N, Tang X, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther. 2023;31(1):134–153. doi: 10.1016/j.ymthe.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36(4):346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- 154.Milone MC, Xu J, Chen S-J, Collins MA, Zhou J, Powell DJ, et al. Engineering-enhanced CAR T cells for improved cancer therapy. Nature Cancer. 2021;2(8):780–793. doi: 10.1038/s43018-021-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31(23):e1900192. doi: 10.1002/adma.201900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dobra G, Gyukity-Sebestyen E, Bukva M, Harmati M, Nagy V, Szabo Z, et al. MMP-9 as prognostic marker for brain tumours: a comparative study on serum-derived small extracellular vesicles. Cancers (Basel). 2023;15(3):712. doi: 10.3390/cancers15030712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He L, Kang Q, Chan KI, Zhang Y, Zhong Z, Tan W. The immunomodulatory role of matrix metalloproteinases in colitis-associated cancer. Front Immunol. 2022;13:1093990. doi: 10.3389/fimmu.2022.1093990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121(10):837–845. doi: 10.1038/s41416-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu L, Qu Y, Cheng L, Yoon CW, He P, Monther A, et al. Engineering chimeric antigen receptor T cells for solid tumour therapy. Clin Transl Med. 2022;12(12):e1141. doi: 10.1002/ctm2.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21(5):524–529. doi: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Voidazan ST, Dianzani C, Husariu MA, Gered B, Turdean SG, Uzun CC, et al. The Role of p16/Ki-67 immunostaining, hTERC amplification and fibronectin in predicting cervical cancer progression: a systematic review. Biology (Basel). 2022;11(7):956. doi: 10.3390/biology11070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hall RC, Vaidya AM, Schiemann WP, Pan Q, Lu ZR. RNA-seq analysis of extradomain A and extradomain B fibronectin as extracellular matrix markers for cancer. Cells. 2023;12(5):685. doi: 10.3390/cells12050685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Martin-Otal C, Lasarte-Cia A, Serrano D, Casares N, Conde E, Navarro F, et al. Targeting the extra domain A of fibronectin for cancer therapy with CAR-T cells. J Immunother Cancer. 2022;10(8):e004479. doi: 10.1136/jitc-2021-004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wagner J, Wickman E, Shaw TI, Anido AA, Langfitt D, Zhang J, et al. Antitumor effects of CAR T cells redirected to the EDB splice variant of fibronectin. Cancer Immunol Res. 2021;9(3):279–290. doi: 10.1158/2326-6066.CIR-20-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Casali BC, Gozzer LT, Baptista MP, Altei WF, Selistre-de-Araujo HS. The effects of alphavbeta3 integrin blockage in breast tumor and endothelial cells under hypoxia in vitro. Int J Mol Sci. 2022;23(3):1745. doi: 10.3390/ijms23031745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cobb DA, de Rossi J, Liu L, An E, Lee DW. Targeting of the alpha(v) beta(3) integrin complex by CAR-T cells leads to rapid regression of diffuse intrinsic pontine glioma and glioblastoma. J Immunother Cancer. 2022;10(2):e003816. doi: 10.1136/jitc-2021-003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ayoub NM, Jaradat SK, Al-Shami KM, Alkhalifa AE. Targeting angiogenesis in breast cancer: current evidence and future perspectives of novel anti-angiogenic approaches. Front Pharmacol. 2022;13:838133. doi: 10.3389/fphar.2022.838133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zheng W, Qian C, Tang Y, Yang C, Zhou Y, Shen P, et al. Manipulation of the crosstalk between tumor angiogenesis and immunosuppression in the tumor microenvironment: Insight into the combination therapy of anti-angiogenesis and immune checkpoint blockade. Front Immunol. 2022;13:1035323. doi: 10.3389/fimmu.2022.1035323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu Z, Zhao Q, Zheng Z, Liu S, Meng L, Dong L, et al. Vascular normalization in immunotherapy: a promising mechanisms combined with radiotherapy. Biomed Pharmacother. 2021;139:111607. doi: 10.1016/j.biopha.2021.111607. [DOI] [PubMed] [Google Scholar]
- 170.Magnussen AL, Mills IG. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br J Cancer. 2021;125(3):324–336. doi: 10.1038/s41416-021-01330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shen R, Peng L, Zhou W, Wang D, Jiang Q, Ji J, et al. Anti-angiogenic nano-delivery system promotes tumor vascular normalizing and micro-environment reprogramming in solid tumor. J Control Release. 2022;349:550–564. doi: 10.1016/j.jconrel.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 172.Tzeng HT, Huang YJ. Tumor vasculature as an emerging pharmacological target to promote anti-tumor immunity. Int J Mol Sci. 2023;24(5):4422. doi: 10.3390/ijms24054422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lanitis E, Kosti P, Ronet C, Cribioli E, Rota G, Spill A, et al. VEGFR-2 redirected CAR-T cells are functionally impaired by soluble VEGF-A competition for receptor binding. J Immunother Cancer. 2021;9(8):e002151. doi: 10.1136/jitc-2020-002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang D, Li AM, Hu G, Huang M, Yang F, Zhang L, et al. PHGDH-mediated endothelial metabolism drives glioblastoma resistance to chimeric antigen receptor T cell immunotherapy. Cell Metab. 2023;35(3):517–534 e518. doi: 10.1016/j.cmet.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen T, Xue Y, Wang S, Lu J, Zhou H, Zhang W, et al. Enhancement of T cell infiltration via tumor-targeted Th9 cell delivery improves the efficacy of antitumor immunotherapy of solid tumors. Bioact Mater. 2023;23:508–523. doi: 10.1016/j.bioactmat.2022.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Greco B, Malacarne V, De Girardi F, Scotti GM, Manfredi F, Angelino E, et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies. Sci Transl Med. 2022;14(628):eabg3072. doi: 10.1126/scitranslmed.abg3072. [DOI] [PubMed] [Google Scholar]
- 177.Liu Z, Zhou Z, Dang Q, Xu H, Lv J, Li H, et al. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics. 2022;12(14):6273–6290. doi: 10.7150/thno.76854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen C, Wang Z, Ding Y, Qin Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. 2023;14:1133308. doi: 10.3389/fimmu.2023.1133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yan Y, Huang L, Liu Y, Yi M, Chu Q, Jiao D, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J Hematol Oncol. 2022;15(1):104. doi: 10.1186/s13045-022-01322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Glasner A, Plitas G. Tumor resident regulatory T cells. Semin Immunol. 2021;52:101476. doi: 10.1016/j.smim.2021.101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 182.Agliardi G, Liuzzi AR, Hotblack A, De Feo D, Nunez N, Stowe CL, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021;12(1):444. doi: 10.1038/s41467-020-20599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nalawade SA, Shafer P, Bajgain P, McKenna MK, Ali A, Kelly L, et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J Immunother Cancer. 2021;9(11):e003237. doi: 10.1136/jitc-2021-003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O'Connor RS, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12(1):877. doi: 10.1038/s41467-021-20893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–5337. doi: 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, et al. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019;68(3):365–377. doi: 10.1007/s00262-018-2281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang B, Luo L, Wang J, He B, Feng R, Xian N, et al. B7–H3 specific T cells with chimeric antigen receptor and decoy PD-1 receptors eradicate established solid human tumors in mouse models. Oncoimmunology. 2020;9(1):1684127. doi: 10.1080/2162402X.2019.1684127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen X, Yang S, Li S, Qu Y, Wang HY, Liu J, et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-beta trap enhances antitumor efficacy of CAR-T cell therapy. Mol Ther Oncolytics. 2021;21:144–157. doi: 10.1016/j.omto.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Getu AA, Tigabu A, Zhou M, Lu J, Fodstad O, Tan M. New frontiers in immune checkpoint B7–H3 (CD276) research and drug development. Mol Cancer. 2023;22(1):43. doi: 10.1186/s12943-023-01751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vitanza NA, Wilson AL, Huang W, Seidel K, Brown C, Gustafson JA, et al. Intraventricular B7–H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2023;13(1):114–131. doi: 10.1158/2159-8290.CD-22-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saxton RA, Glassman CR, Garcia KC. Emerging principles of cytokine pharmacology and therapeutics. Nat Rev Drug Discov. 2023;22(1):21–37. doi: 10.1038/s41573-022-00557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bhatia V, Kamat NV, Pariva TE, Wu LT, Tsao A, Sasaki K, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun. 2023;14(1):2041. doi: 10.1038/s41467-023-37874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Swan SL, Mehta N, Ilich E, Shen SH, Wilkinson DS, Anderson AR, et al. IL7 and IL7 Flt3L co-expressing CAR T cells improve therapeutic efficacy in mouse EGFRvIII heterogeneous glioblastoma. Front Immunol. 2023;14:1085547. doi: 10.3389/fimmu.2023.1085547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim MY, Jayasinghe R, Devenport JM, Ritchey JK, Rettig MP, O'Neal J, et al. A long-acting interleukin-7, rhIL-7-hyFc, enhances CAR T cell expansion, persistence, and anti-tumor activity. Nat Commun. 2022;13(1):3296. doi: 10.1038/s41467-022-30860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tokunaga Y, Sasaki T, Goto S, Adachi K, Sakoda Y, Tamada K. Enhanced antitumor responses of tumor antigen-specific TCR T cells genetically engineered to produce IL7 and CCL19. Mol Cancer Ther. 2022;21(1):138–148. doi: 10.1158/1535-7163.MCT-21-0400. [DOI] [PubMed] [Google Scholar]
- 196.Reppel L, Tsahouridis O, Akulian J, Davis IJ, Lee H, Fuca G, et al. Targeting disialoganglioside GD2 with chimeric antigen receptor-redirected T cells in lung cancer. J Immunother Cancer. 2022;10(1):e003897. doi: 10.1136/jitc-2021-003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gargett T, Ebert LM, Truong NTH, Kollis PM, Sedivakova K, Yu W, et al. GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. J Immunother Cancer. 2022;10(9):e005187. doi: 10.1136/jitc-2022-005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zannikou M, Duffy JT, Levine RN, Seblani M, Liu Q, Presser A, et al. IL15 modification enables CAR T cells to act as a dual targeting agent against tumor cells and myeloid-derived suppressor cells in GBM. J Immunother Cancer. 2023;11(2):e006239. doi: 10.1136/jitc-2022-006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jaspers JE, Khan JF, Godfrey WD, Lopez AV, Ciampricotti M, Rudin CM, et al. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J Clin Invest. 2023;133(9):e166028. doi: 10.1172/JCI166028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thomas S, Abken H. CAR T cell therapy becomes CHIC: “cytokine help intensified CAR” T cells. Front Immunol. 2022;13:1090959. doi: 10.3389/fimmu.2022.1090959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. 2018;69:301–318. doi: 10.1146/annurev-med-012017-043208. [DOI] [PubMed] [Google Scholar]
- 202.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 203.Adusumilli PS, Zauderer MG, Riviere I, Solomon SB, Rusch VW, O'Cearbhaill RE, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 2021;11(11):2748–2763. doi: 10.1158/2159-8290.CD-21-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cheng K, Feng X, Chai Z, Wang Z, Liu Z, Yan Z, et al. 4–1BB-based CAR T cells effectively reverse exhaustion and enhance the anti-tumor immune response through autocrine PD-L1 scFv antibody. Int J Mol Sci. 2023;24(4):4197. doi: 10.3390/ijms24044197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hu B, Zou Y, Zhang L, Tang J, Niedermann G, Firat E, et al. Nucleofection with plasmid DNA for CRISPR/Cas9-mediated inactivation of programmed cell death protein 1 in CD133-specific CAR T cells. Hum Gene Ther. 2019;30(4):446–458. doi: 10.1089/hum.2017.234. [DOI] [PubMed] [Google Scholar]
- 206.Yoshikawa T, Wu ZW, Inoue S, Kasuya H, Matsushita H, Takahashi Y, et al. Genetic ablation of PRDM1 in antitumor T cells enhances therapeutic efficacy of adoptive immunotherapy. Blood. 2022;139(14):2156–2172. doi: 10.1182/blood.2021012714. [DOI] [PubMed] [Google Scholar]
- 207.Jung IY, Narayan V, McDonald S, Rech AJ, Bartoszek R, Hong G, et al. BLIMP1 and NR4A3 transcription factors reciprocally regulate antitumor CAR T cell stemness and exhaustion. Sci Transl Med. 2022;14(670):eabn7336. doi: 10.1126/scitranslmed.abn7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mai D, Johnson O, Reff J, Fan TJ, Scholler J, Sheppard NC, et al. Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells. Proc Natl Acad Sci USA. 2023;120(12):e2218632120. doi: 10.1073/pnas.2218632120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gumber D, Wang LD. Improving CAR-T immunotherapy: Overcoming the challenges of T cell exhaustion. EBioMedicine. 2022;77:103941. doi: 10.1016/j.ebiom.2022.103941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372(6537):eaba1786. doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Richman SA, Wang LC, Moon EK, Khire UR, Albelda SM, Milone MC. Ligand-induced degradation of a CAR permits reversible remote control of CAR T cell activity in vitro and in vivo. Mol Ther. 2020;28(7):1600–1613. doi: 10.1016/j.ymthe.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang ZL, Wu YQ, Allen ME, Pan YJ, Kyriakakis P, Lu SY, et al. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci Adv. 2020;6(8):9209. doi: 10.1126/sciadv.aay9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yin X, He L, Guo Z. T-cell exhaustion in CAR-T-cell therapy and strategies to overcome it. Immunology. 2023;169(4):400–411. doi: 10.1111/imm.13642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.