Abstract
We studied early changes in gene expression during fibroblast contraction of stressed collagen matrices. The level of c-fos mRNA increased dramatically and peaked 50 to 60 min after matrix contraction was initiated. This response did not require serum and could not be accounted for simply by disruption of the actin cytoskeleton. Increased c-fos mRNA levels required Ca2+ influx but not the cyclic AMP or extracellular signal-regulated kinase (ERK 1/2) signaling pathways, both of which are activated when fibroblasts contract stressed collagen matrices. The levels of two other immediate-early genes, fosb and c-jun, also increased transiently after fibroblast contraction, whereas the levels of fra-1, fra-2, c-myc, and the transcription factor NF-κB remained the same, indicating that fibroblast contraction caused changes in a selective group of genes. The increase in c-fos mRNA during contraction of stressed collagen matrices may reflect a unique role for c-fos in mechanoregulated events at the end of wound repair.
Mechanical force influences cell function in animal and plant tissues (6, 11, 30, 67). For instance, increased mechanical load, such as fluid flow over endothelial cells or tension applied to muscle cells, has been shown to result in cell proliferation or hypertrophy (15, 64). During wound repair, mechanical force within the wound tissue pulls the wound edges closer together, which in pathological cases results in scarring and loss of function (29, 52). Wound contraction is believed to be mediated by fibroblasts as they go through a cycle of migration, proliferation, contraction, and regression (13, 40).
Fibroblasts cultured in collagen matrices have been used as an in vitro model for wound contraction (3, 20). In this model, cells reorganize the extracellular matrix through migratory and contractile activities (3, 25), resulting in formation of a dense, tissue-like structure. The fibroblast phenotype that develops during collagen matrix reorganization differs dramatically depending upon whether collagen matrices are floating in medium or attached to a rigid support. After reorganization of attached matrices, the cells resemble proliferating fibroblasts of wound tissue under mechanical stress and are characterized by polarized morphology, prominent stress fibers, and fibronexus junctions (23, 37, 45, 62). Cells in attached collagen matrices exert force on the matrix similar to that observed in contracting skin wounds (12, 34). After reorganization of floating matrices, on the other hand, cells resemble nondividing fibroblasts of resting dermis or scar tissue in a state of mechanical equilibrium and are characterized by isometric (stellate) cell morphology and cytoskeletal meshwork (3, 4).
We have combined the attached and floating models to study the regulatory events associated with the transition of fibroblasts from mechanically stressed to mechanically equilibrated conditions (stress-relaxation) such as occurs by the end of wound repair. Initially, fibroblasts are cultured in attached collagen matrices until mechanical stress develops. Then, the stressed matrices are released and allowed to float in medium, which initiates a rapid and synchronous smooth-muscle-like contraction as mechanical stress is dissipated.
Morphological changes in the cells during contraction of stressed matrices include disruption of actin stress fibers (but not of microtubules or intermediate filaments) and collapse of fibronexus junctions (37, 45, 62). Subsequently, cells show marked changes in growth factor receptor function and biosynthetic activity (e.g., decreased synthesis of collagen) and become quiescent (39, 45, 46). During the initial moments of contraction, cells transiently open passages ∼3 nm in diameter in their plasma membranes, resulting in a burst of Ca2+ uptake from the medium (38). Ca2+ uptake activates a signal transduction pathway, resulting in increased synthesis of phosphatidic acid, arachidonic acid, diacylglycerol, and cyclic AMP and activation of protein kinase A (PKA) (26, 27).
Our study was carried out to learn if c-fos (2, 51) or other immediate-early genes are modulated when fibroblasts contract stressed collagen matrices and to learn if changes in gene expression can be attributed to the signal transduction processes previously shown to be activated during contraction. We found that the level of c-fos mRNA transcription increased dramatically and peaked 50 to 60 min after matrix contraction was initiated. Increased c-fos mRNA levels required Ca2+ influx but not the cyclic AMP or ERK 1/2 signaling pathways, both of which are activated when fibroblasts contract stressed collagen matrices. Levels of other immediately-early genes, fosB and c-jun, also increased in response to contraction. Details are reported herein.
MATERIALS AND METHODS
Materials.
Dulbecco’s modified Eagle’s medium (DMEM), restriction enzymes, reverse transcriptase (RT), and deoxynucleotides were purchased from Gibco/BRL (Gaithersburg, Md.). T4 DNA ligase used for subcloning, the MAP/ERK kinase (MEK) inhibitor PD98059, and anti-phosphoCREB and anti-CREB antibodies were acquired from New England Biolabs (Beverly, Mass.). Anti-active ERK 1/2 was obtained from Promega Corp. (Madison, Wis.). Nylon (Nytran) membranes for Northern hybridization were purchased from Schleicher and Schuell (Keene, N.H.). Fetal bovine serum (FBS) was purchased from Intergen Co. (Purchase, N.Y.). Type I collagen (Vitrogen) was purchased from Collagen Corp. (Palo Alto, Calif.). Guanadinium thiocyanate for solution D (8) was purchased from Fluka Chemical Co. (Ronkonkoma, N.Y.). All other chemicals were purchased from Sigma (St. Louis, Mo.).
Collagen matrix contraction.
Fibroblasts from human foreskin specimens (<10 passages) were maintained in Falcon 75-cm2 tissue culture flasks in DMEM supplemented with 10% FBS. Hydrated collagen matrices were prepared from Vitrogen 100 collagen (Collagen Corp.). Neutralized collagen solutions (1.5 mg/ml) contained fibroblasts in DMEM but no serum. Aliquots (0.2 ml) of the cell-collagen mixtures were prewarmed to 37°C for 3 to 4 min and then placed in Corning 24-well culture plates. Each aliquot occupied an area outlined by a 12-mm-diameter circular score within a well. Polymerization of collagen matrices required 60 min at 37°C in a humidified incubator with 5% CO2.
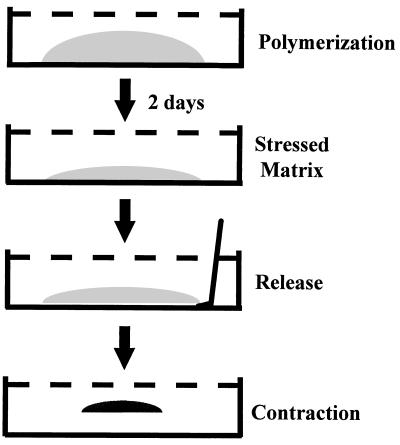
The protocol for collagen matrix contraction has been described previously (27, 38) and is shown schematically in Fig. 1. After polymerization, 1.0 ml of DMEM–10% FBS and 50 μg of ascorbic acid per ml were added to each well. Cultures were incubated for 2 days, during which stress developed. To initiate contraction, stressed matrices were gently released from the underlying culture dish with a spatula and incubated at 37°C in DMEM with serum or other constituents as indicated below. In some experiments, polymerized collagen matrices were released from the underlying culture dish immediately after polymerization and maintained as floating matrices subsequently.
FIG. 1.
Fibroblast contraction of stressed collagen matrices. See the text for details.
RNA isolation.
RNA was isolated by a technique adapted from Chomczynski and Sacchi (8). Briefly, collagen matrices (10 to 15 per sample for Northern hybridization or 1 per sample for RT-PCR) were dissolved with solution D. Total RNA was extracted with phenol-chloroform-isoamyl alcohol and precipitated with isopropanol. Subsequently, RNA was redissolved in 260 μl of diethyl pyrocarbonate treated (DEPC) water instead of in solution D and reprecipitated by addition of 40 μl of sodium acetate (pH 5) and 750 μl of 100% ethanol (55). Finally, the samples were redissolved in DEPC water and subjected to Northern hybridization or RT-PCR. RNA prepared by the above-described modification showed a more uniform electrophoretic mobility in agarose gels.
Generation of gene-specific DNA probes.
The c-fos- and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific DNA probes were generated with a Boehringer Mannheim random primed labeling kit with a subcloned PCR fragment of the c-fos gene and a partial cDNA clone derived from the full human GAPDH cDNA clone pHcGAP (63) obtained from the American Type Tissue Collection (accession no. 57091). The c-fos probe was generated with 5′ TTATCTCCAGAAGAAGAAGAGAAAAGGAGAATC 3′ and 5′ AGGGCCAGCAGCGTGGGTGAGCTGAGCGAGTCA 3′ as primers in a reaction mixture with human genomic DNA as the template. The initial PCR product generated was 860 bp, and a 491-bp ApaI fragment was subcloned into the ApaI site of pBluescript SK− (pBS) (Stratagene) and sequenced. When we generated probes for Northern hybridization, this c-fos–pBS construct was restricted with AccI and ApaI, generating a fragment 448 bp in size that contained the final 7 bp of intron 3 and all of exon 4. The GAPDH probe was generated from the 554-bp HindIII/XbaI restriction fragment of pHcGAP subcloned into pBS. Plasmids containing cloned sequences were amplified in Escherichia coli DH5α (55) and purified with a Maxiprep kit (Qiagen, Santa Clarita, Calif.) according to the manufacturer’s instructions. Insert sequences were verified by automated sequencing. Preparation of c-fos primers and sequencing analyses were performed in the Molecular Biology Core Facility of the University of Texas Southwestern Skin Diseases Research Center.
Northern hybridization.
Northern hybridization was performed according to standard procedures (55) except that RNA samples (8 to 15 matrices/sample) were initially mixed 1:3 with ethidium bromide-containing denaturing buffer (0.75 mg of ethidium bromide per ml, 10 mM MOPS [morpholinepropanesulfonic acid, pH 7.0], 4 mM sodium acetate, 0.5 mM EDTA, 13% formaldehyde, 50% formamide). Samples were subjected to electrophoresis with 1% agarose–6% formaldehyde minigels for 1 h at 100 V (55). The agarose gel was then washed twice for 10 min each time in 100 ml of DEPC water and soaked in 0.05 N NaOH in DEPC water for 15 min to improve transfer of larger RNAs. The gels were then pH neutralized with 20× SSPE (1× SSPE is 0.18 M sodium chloride, 10 mM sodium phosphate, and 1 mM EDTA [pH 7.7]) (55) buffer for 5 min. After transfer, RNA on the nylon membranes was cross-linked with a Stratalinker UV light source (Stratagene) at a setting of 1,200 μJ of radiation per cm2. Hybridizations were carried out under aqueous conditions at 65°C according to standard protocols (55).
RT-PCR.
RNA was isolated from collagen matrices (1 to 6 matrices/sample) as described above and used for a first-strand synthesis reaction with Moloney murine leukemia virus (Gibco/BRL) and oligo dT(15) primer according to the manufacturer’s protocol except for experiments to be carried out with c-fos heterogeneous nuclear RNA (hnRNA) (see below), where first-strand synthesis was carried out with p(N)d6 random primers. After incubation for 2.5 h at 37°C, Moloney murine leukemia virus RT enzyme was heat killed by incubation at 70°C for 10 min. Second-strand synthesis was then carried out with RNase H and Klenow polymerase (Gibco/BRL) by standard techniques (55). Subsequently, double-stranded cDNA was extracted with phenol-chloroform-isoamyl alcohol, precipitated with ethanol-NaCl, washed with 80% ethanol, and resuspended in 20 μl of DEPC water. PCRs were carried out with 1 μl of cDNA preparation per sample. DNA was denatured at 94°C for 4 min, followed by 20 to 25 cycles of a 45-s denaturation at 94°C, a 45-s annealing at 54°C, and a 90-s elongation at 72°C and a final 10-min elongation at 72°C. Reaction mixtures (20 μl) contained 20 mM Tris-HCl (pH 8.4), 0.1 U of Taq polymerase per μl, 50 mM KCl, 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, and 0.5 μM each primer. (In the experiments comparing c-fos mRNA with c-fos hnRNA, only 10 to 12 cycles were used [see below].) After PCR, the overlying oil from each sample was removed and 4 μl of 6× loading buffer II (55) was added. Whole reaction mixtures were loaded onto 1% agarose gels containing 1× Tris-acetate-EDTA (55) and 10 μg of ethidium bromide per ml. Samples were subjected to electrophoresis for 1 h at 100 V. DNA was visualized with a UV light source, and the image was captured with an Is-1000 Digital Imaging System (Alpha-Innotech Corp.).
RT-PCR–Southern hybridization experiments to detect c-fos hnRNA.
RT-PCR was carried out as described above except that DNA was denatured at 94°C for 4 min, followed by 10 to 12 cycles of a 30-s denaturation at 94°C, a 30-s annealing at 57°C, and a 90-s elongation at 72°C and a final 10-min elongation at 72°C (68). PCR products were detected by the method for Northern hybridization described above except that the probe for c-fos hnRNA was generated by five cycles of PCR with [32P]dCTP and a gel-purified c-fos hnRNA fragment and c-fos hnRNA primer. No PCR products were obtained in the absence of RT, showing that the samples were not contaminated with genomic DNA. c-fos mRNA and GAPDH mRNA PCR products were detected with the same probes as those used in the Northern hybridization studies (described above).
RT-PCR primers.
The sequences of each primer set (synthesized by the University of Texas Southwestern Molecular Biology Core Facility) were as follows: c-fos mRNA, the same as those used to generate Northern hybridization probes; c-fos hnRNA, 5′ ATGATGTTCTCGGGCTTCAACGCAGCAG 3′ and 5′ AACCAATTCTTACTATGGCAAGCG 3′; fosB, 5′ ACACCAGGCATGAGTGGCTACAGCAG 3′ and 5′ GGCGAACGCGGAGACCTCCGGGCAGG 3′; fra-1, 5′ ACCCCGGCCAGGAGTCATCCGGGCCC 3′ and 5′ AGGCGCCTCACAAAGCGAGGAGGGTT 3′; and fra-2, 5′ GGGCCTGGCCTCTGTCCCTGGACACA 3′ and 5′ TTGGAGCAGGATTCGGAGGGAGATGC 3′.
Gene sequences were obtained from the GenBank sequence database, and primers for RT-PCR were selected based on regions of greatest differences as determined with the Clustal W Multiple Sequence Alignment Program, except primers for c-fos hnRNA, which were designed to hybridize to the first exon and second intron of c-fos (68). Primer sets for c-myc, c-jun, and GAPDH were purchased from Clontech (Palo Alto, Calif.).
SDS-PAGE and immunoblotting.
Collagen matrices to be extracted (two matrices per sample) were placed into 100 μl of ice-cold lysis buffer (0.2% Nonidet P-40, 150 mM NaCl, 3 mM KCl, 6 mM Na2HPO4 [pH 7.4], 1 mM KH2PO4, 0.5 mM MgCl2, 1 μg of leupeptin per ml, 1 μg of pepstatin A per ml, 10 mM 4-(2-aminoethyl)benzenesulfonylfluoride (AEBSF), 50 mM NaF, 1 mM Na3VO4, 1 mM Na2MoO4) and homogenized (50 strokes) with a Dounce homogenizer (pestle B; Wheaton Scientific, Millville, N.J.). Samples were clarified by centrifugation for 5 min at 16,000 × g (Eppendorf microcentrifuge, model 5415 C), and the supernatants were dissolved in 4× reducing sample buffer (250 mM Tris, 4% sodium dodecyl sulfate [SDS], 40% glycerol, 20% mercaptoethanol, 0.04% bromophenol blue) and boiled for 5 min. Equal volumes from each sample were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) with 9% acrylamide minislab gels and transferred to polyvinylidene difluoride membranes. For analysis of CREB, membranes were subjected to immunoblotting with anti-phosphoCREB (Ser133) and anti-CREB antibodies (New England Biolabs) according to the manufacturer’s instructions, except the primary antibodies were diluted 1:800. For analysis of ERK 1/2, membranes were subjected to immunoblotting with anti-active ERK 1/2 or anti-ERK 1/2 (Y691, a gift from Melanie Cobb).
Densitometry.
Autoradiographs from Northern and Western blots were scanned and stored as image files with a Personal Densitometer (Molecular Dynamics). The pixel volume of each band was measured with the ImageQuant program (Molecular Dynamics). Readings from each sample were then adjusted for background. Ratios derived from experimental samples and loading controls were plotted with CA-Cricket Graph III (Computer Associates).
RESULTS
c-fos mRNA transcription increases during fibroblast contraction of stressed collagen matrices.
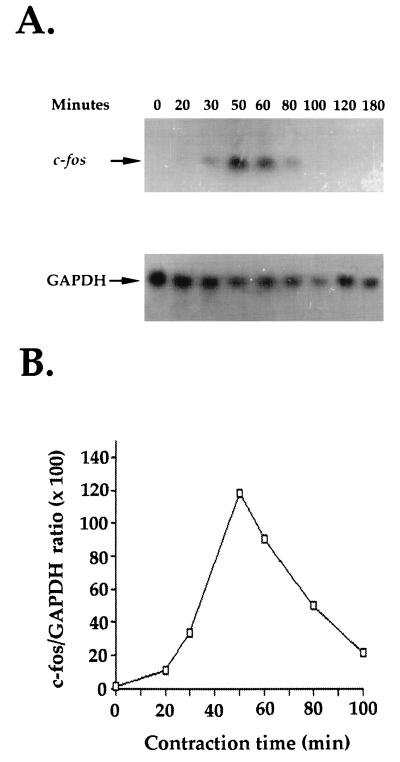
Fibroblast contraction of stressed collagen matrices was previously shown to result in activation of signaling pathways that may have activated transcription of genes important in the wound healing process (26, 27, 38, 39). To examine this possibility, fibroblasts were cultured for 2 days in attached collagen matrices, which allowed mechanical stress to develop, and then the matrices were released to initiate contraction (Fig. 1). Cells in stressed and contracting matrices were extracted, and Fig. 2A shows the results of a typical Northern hybridization analysis of c-fos mRNA levels in the cells. GAPDH mRNA levels were determined for loading controls. The results were quantified, and Fig. 2B shows a time course for contraction versus c-fos/GAPDH mRNA ratios. In stressed matrices, levels of c-fos mRNA were low or undetectable. Within 30 min after release and initiation of contraction, c-fos mRNA levels began to increase, reached maximal levels by 50 to 60 min, and then declined.
FIG. 2.
c-fos mRNA levels increase during contraction. Stressed collagen matrices were released to initiate contraction. RNA was isolated from the matrices at the times indicated. (A) Northern hybridization analysis with c-fos and GAPDH probes; (B) c-fos/GAPDH ratios based on densitometry readings.
Fibroblasts cultured 24 to 48 h in floating collagen matrices had undetectable c-fos mRNA levels. Physically manipulating these matrices by transferring them between culture dishes did not increase levels of c-fos mRNA, but tearing the matrix in half with two spatulas resulted in increased c-fos expression (data not shown) analogous to that of fibroblasts wounded in tissues (10, 41, 47).
The findings shown in Fig. 2 may have resulted from an increase in c-fos mRNA transcription or stability. Because of technical limitations of the cell-collagen matrix cultures, it was not possible to perform in vitro nuclear run-on assays to make the above-described distinction. As an alternative, two kinds of experiments were carried out. First, we used RT-PCR to compare contraction-stimulated changes in levels of c-fos mRNA and c-fos hnRNA. Levels of hnRNA have been shown to be directly proportional to the transcriptional activity of the c-fos gene (68). In addition, both RT-PCR and Southern blot analyses were carried out to determine the effects of inhibiting transcription with actinomycin D on contraction-stimulated changes in levels of c-fos mRNA and hnRNA.
Figure 3A shows that low levels of c-fos mRNA and hnRNA could be detected by RT-PCR even in stressed collagen matrices. These levels increased markedly after contraction, and the increase was inhibited by addition of 5 μg of actinomycin D per ml. In other experiments, we used Northern blot analysis to examine the effects of actinomycin D on the time course of c-fos mRNA expression when the inhibitor was added 30 min after initiating contraction. Figure 3B shows that in the absence of actinomycin D, the level of c-fos mRNA continued to increase between 30 to 60 min, consistent with the results shown in Fig. 2. In the presence of actinomycin D, however, the level of c-fos mRNA decreased between 30 to 60 min and was undetectable by 80 min. Taken together, these findings indicate that the contraction-stimulated increase in c-fos mRNA levels resulted from an increase in c-fos mRNA transcription rather than an increase in mRNA stability.
FIG. 3.
Contraction-stimulated accumulation of c-fos mRNA and hnRNA detected by RT-PCR and Northern blot analysis. (A) Stressed collagen matrices were preincubated for 30 min with or without 5 μg of actinomycin D (Act D) per ml. Subsequently, the matrices were released to initiate contraction. After 60 min, RNAs were isolated from the matrices and analyzed by RT-PCR with primers for c-fos mRNA, c-fos hnRNA, and GAPDH. (B) Stressed collagen matrices were released to initiate contraction. Thirty minutes later, 5 μg of actinomycin D per ml was added to the samples indicated. RNAs were isolated from the matrices at the times shown and analyzed by Northern hybridization with c-fos mRNA and GAPDH probes.
The basal component of fibroblast contraction of stressed collagen matrices is sufficient to stimulate c-fos mRNA transcription.
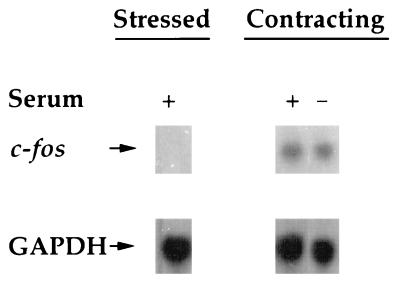
Fibroblast contraction of stressed collagen matrices exhibits basal and stimulated (by serum) components (38, 62). Basal contraction results in disruption of actin stress fibers, Ca2+ uptake, and cyclic AMP signaling (38). Stimulated contraction, on the other hand, is required for actin cytoskeletal retraction to the cellular perinuclear region (38) and growth factor receptor desensitization (39). To determine if the basal component of contraction was sufficient to stimulate c-fos transcription, c-fos mRNA levels were compared during contraction in serum-free and serum-containing DMEM. Figure 4 shows that levels of stimulation of c-fos mRNA expression during contraction were similar with or without serum in the medium. This result not only showed that the basal component of contraction was sufficient to stimulate c-fos transcription but also ruled out the possibility that increased c-fos mRNA transcription during contraction was a result of direct serum stimulation (2, 19).
FIG. 4.
The increase in c-fos mRNA levels during contraction does not require serum. Stressed collagen matrices in medium with or without 10% serum as shown were released to initiate contraction as indicated. After 50 min, RNAs were isolated from the matrices and analyzed by Northern hybridization with c-fos and GAPDH probes.
Contraction-stimulated increase in c-fos transcription is independent of actin cytoskeletal organization.
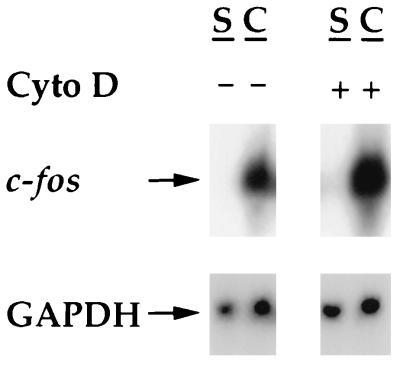
Disruption of the actin cytoskeleton has been reported to cause an increase in c-fos expression (69). Since disruption of actin stress fibers (but not microtubules or intermediate filaments) occurs during contraction-stressed matrices (37, 38, 62), it was of interest to learn whether disruption of actin stress fibers of fibroblasts in stressed collagen matrices was sufficient to induce c-fos expression. Figure 5 shows that treatment of fibroblasts in stressed collagen matrices with 10 μM cytochalasin D (which completely disrupts stress fibers of fibroblasts in stressed collagen matrices and blocks stimulated contraction [data not shown]) did not cause a significant increase in c-fos mRNA levels. Also, treatment of cells with cytochalasin D did not prevent increased c-fos mRNA levels during contraction. The latter finding is consistent with the results of experiments on serum dependence (Fig. 4), since cytochalasin D inhibits the stimulated component of contraction (38, 62) but only partially blocks the basal component of contraction, e.g., it results in reduced cyclic AMP signaling (26) and no change in Ca2+ uptake (38).
FIG. 5.
Cytochalasin D treatment does not affect the level of c-fos mRNA in stressed or contracting collagen matrices. Stressed collagen matrices were preincubated for 10 min with 10 μM cytochalasin D (Cyto D) or an equivalent amount of dimethyl sulfoxide (DMSO). Subsequently, the matrices were released to initiate contraction. After 50 min, RNAs were isolated from the matrices and analyzed by Northern hybridization with c-fos and GAPDH probes. S, stressed; C, contracting.
Increased c-fos mRNA expression requires Ca2+ uptake but not the cyclic AMP signaling pathway.
Basal contraction of stressed collagen matrices results in Ca2+ uptake and an increase in cyclic AMP (26, 27, 38), and Ca2+ and cyclic AMP have been implicated in the stimulation of c-fos transcription through the cyclic AMP response element (58). Therefore, we tested the possibility that Ca2+ and cyclic AMP are important for c-fos stimulation during contraction.
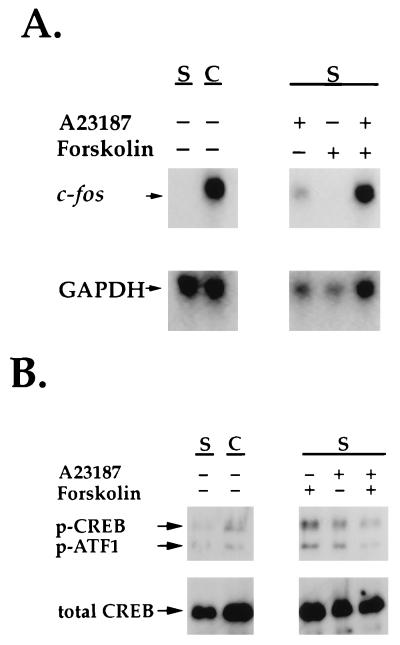
Figure 6 shows that addition of EGTA to chelate extracellular calcium inhibited increased c-fos mRNA expression in response to contraction and that inhibition was prevented by the addition of excess Ca2+ with EGTA to the medium. Therefore, extracellular Ca2+ uptake appeared to be necessary for the contraction-stimulated increase in c-fos expression. Figure 7A, on the other hand, shows that Ca2+ uptake alone was not sufficient for increased c-fos expression, since addition of calcium ionophore to fibroblasts in stressed collagen matrices caused an increase in the level of c-fos mRNA that was less than half of that observed during matrix contraction.
FIG. 6.
The increase in c-fos mRNA levels during contraction requires extracellular Ca2+. Stressed collagen matrices were preincubated for 10 min with 3 mM EGTA and/or 3 mM CaCl2 as shown. Subsequently, the matrices were released to initiate contraction. After 50 min, RNAs were isolated from the matrices and analyzed by Northern hybridization with c-fos and GAPDH probes.
FIG. 7.
Effects of calcium ionophore and forskolin on c-fos mRNA levels and CREB phosphorylation in fibroblasts in stressed collagen matrices. Stressed collagen matrices were incubated for 50 min with 50 μM A23187 and/or 30 μM forskolin or allowed to contract for 10 min as indicated. (A) RNAs were isolated from the matrices and analyzed by Northern hybridization with c-fos and GAPDH probes. (B) The matrices (two per sample) were extracted, subjected to SDS-PAGE, and immunoblotted with phosphoCREB (p-CREB) (Ser133) antibody or control CREB antibody. The phosphoCREB antibody also reacts with phosphorylated ATF-1 (p-ATF1) S, stressed; C, contracting.
Figure 7A shows that treatment of fibroblasts in stressed collagen matrices with forskolin did not stimulate c-fos mRNA transcription even though it resulted in an increase in cyclic AMP twice as high as that observed in fibroblasts contracting collagen matrices. A c-fos mRNA response similar in magnitude to that observed during collagen matrix contraction was observed when cells in stressed matrices were subjected to combined treatment with Ca2+ ionophore and forskolin, but under these conditions there was a synergistic effect resulting in cyclic AMP levels sixfold higher than those observed during contraction. Specifically, in an experiment in which the conditions were the same as those described for Fig. 7 except that after 10 min the matrices were extracted and cyclic AMP was determined as described previously (26, 27), the cyclic AMP levels of fibroblasts (means ± standard deviations) were as follows: 1.2 × 103 cpm ± 0.2 × 103 cpm for a stressed matrix, 9.3 × 103 cpm ± 1.0 × 103 cpm for a contracting matrix, 7.2 × 103 ± 2.4 × 103 cpm for a stressed matrix with A23187, 21.0 × 103 ± 0.1 × 103 cpm for a stressed matrix with forskolin, and 61.8 × 103 ± 7.0 × 103 cpm for a stressed matrix with A23187 and forskolin.
The above-described results indicated that there was not a clear correlation between cyclic AMP levels of fibroblasts in stressed matrices and stimulation of c-fos. Nor was there a correlation between c-fos mRNA levels and phosphorylation of CREB, the cyclic AMP response element-binding protein on which Ca2+ and cyclic AMP signals converge, leading to activation of c-fos transcription (58). Indeed, as shown in Fig. 7B, the highest levels of phosphoCREB (and the related activating transcription factor 1 [ATF-1]) were observed when fibroblasts in stressed matrices were treated with forskolin, even though levels of c-fos mRNA did not increase under these conditions.
To clarify the above-described findings, additional experiments were carried out with the PKA inhibitor H89. Addition of H89 to fibroblasts contracting stressed collagen matrices only slightly inhibited stimulation of c-fos mRNA (Fig. 8A) but completely blocked phosphorylation of CREB stimulated by forskolin (Fig. 8B). Taken together, the results in Fig. 7 and 8 suggest that although increased cyclic AMP synthesis may contribute to stimulation of c-fos transcription during fibroblast contraction of stressed collagen matrices, this increase is neither necessary nor sufficient to account for the overall c-fos response.
FIG. 8.
PKA inhibitor H89 blocks contraction-stimulated CREB phosphorylation but not increased transcription of c-fos mRNA. Stressed collagen matrices were preincubated for 1 h with H89 at the concentrations indicated or an equivalent amount of DMSO. Subsequently, collagen matrices were either released to initiate contraction or treated with 30 μM forskolin to activate PKA. After 50 min, matrices were extracted. (A) RNAs were isolated and subjected to Northern hybridization with c-fos and GAPDH probes. S, stressed; C, contracting. (B) Samples were subjected to SDS-PAGE and immunoblotted with antibodies to phosphoCREB (p-CREB) or total CREB. p-ATF1, phosphorylated ATF-1.
Increased c-fos mRNA expression does not require the ERK 1/2 signaling pathway.
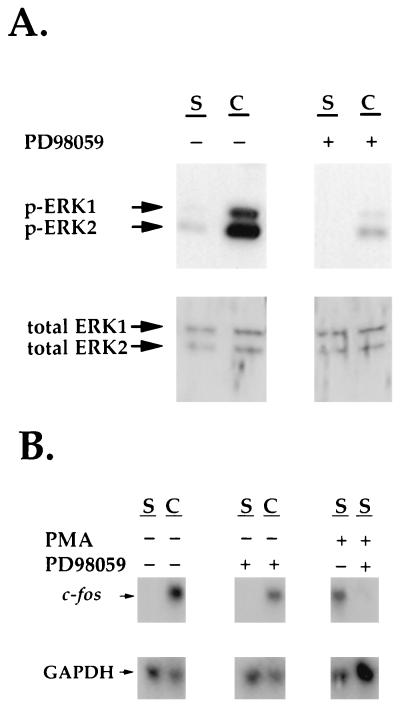
In addition to calcium influx and increased cyclic AMP production, Fig. 9A shows that fibroblast contraction of stressed collagen matrices also results in activation of the ERK 1/2 signaling pathway. This activation was completely blocked by the MEK 1/2 inhibitor PD98059 (1). Since activation of the ERK 1/2 signaling pathway can stimulate c-fos transcription through the serum-response element (SRE) of the c-fos promoter (32, 51), experiments were carried out to learn if the ERK 1/2 pathway plays a role in the contraction-stimulated increase in the level of c-fos. Figure 9B shows that PD98059 only partially inhibited stimulation of c-fos mRNA during contraction. Under the same conditions, however, PD98059 completely blocked the c-fos mRNA increase when fibroblasts in stressed collagen matrices were treated with 100 nM phorbol myristate acetate, which also stimulates c-fos mRNA transcription through the SRE (2, 18, 59). These data suggest that activation of the ERK 1/2 signaling pathway was not required for the contraction-activated increase in c-fos mRNA transcription.
FIG. 9.
The MEK 1/2 inhibitor PD98059 blocks contraction-stimulated activation of ERK 1/2 but not increased c-fos mRNA levels. (A) Stressed collagen matrices were preincubated for 1 h with 100 μM PD98059 or an equivalent amount of DMSO. Subsequently, the collagen matrices were released to initiate contraction. After 10 min, the matrices (two per sample) were extracted, subjected to SDS-PAGE, and immunoblotted with antibodies to phosphorylated ERK 1/2 or total ERK 1/2 (p-ERK). (B) Stressed collagen matrices were preincubated for 1 h with 100 μM PD98059 or an equivalent amount of DMSO as shown. Subsequently, the matrices were released to initiate contraction or treated with 100 nM phorbol myristate acetate to stimulate protein kinase C. After 50 min, RNAs were isolated from the matrices and analyzed by Northern hybridization with c-fos and GAPDH probes. S, stressed; C, contracting.
fosB and c-jun mRNA levels increase in response to fibroblast contraction of stressed collagen matrices.
Finally, to examine if increased transcription during matrix contraction was a widespread response of immediate-early genes to contraction or limited to c-fos, RT-PCR experiments were carried out to probe the levels of several other immediate-early genes as well as the transcription factor NF-κB. Figure 10 shows results from a typical experiment comparing levels of gene expression by fibroblasts in stressed matrices (lanes S), contracting matrices (lanes C), and matrices that had been floating in culture medium for 2 days (lanes F). Consistent with the results of experiments already described, c-fos was essentially undetectable in cells in stressed matrices, markedly upregulated in response to contraction, and low again in floating matrices. A second member of the fos family, fosB (both transcripts) responded similarly to c-fos, while two other fos family members, fra-1 and fra-2, showed no differences in mRNA levels detected by RT-PCR. Three other genes, c-jun, c-myc, and that encoding NF-κB, were expressed at detectable levels in fibroblasts in stressed matrices. Of these, only c-jun showed a detectable increase in response to contraction. Taken together, these results indicate that contraction of stressed collagen matrices stimulates highly selective changes in gene expression.
FIG. 10.
RT-PCR analysis of mRNA levels of c-fos and related genes in stressed, contracting, and floating collagen matrices. Collagen matrices were stressed (lanes S), contracting for 50 min (lanes C), or floating for 2 days (lanes F). RNAs were isolated from the matrices. RT-PCR was performed with probes for c-fos, fosB, fra-1, fra-2, c-jun, c-myc, and NF-κB as indicated. The 100- to 125-bp band observed in some lanes results from nonspecific primer dimerization.
DISCUSSION
c-fos mRNA levels increase when fibroblasts contract stressed collagen matrices.
The goal of our study was to determine if c-fos or other immediate-early genes are modulated in response to fibroblast contraction of stressed collagen matrices and to learn whether changes in gene expression can be attributed to the signal transduction processes activated by contraction. We found that c-fos mRNA—initially undetectable in fibroblasts in stressed collagen—increased dramatically and peaked 50 to 60 min after matrix contraction was initiated. The level of c-fos hnRNA also increased after contraction, and both c-fos mRNA and hnRNA responses to contraction were prevented by actinomycin D. These results indicated that the increase in c-fos mRNA during stressed matrix contraction resulted from an increase in mRNA synthesis rather than an increase in mRNA stability. Levels of two other immediate-early genes, fosb and c-jun, also increased transiently after fibroblast contraction, but those of fra-1, fra-2, c-myc, and the transcription factor NF-κB did not appear to change. Therefore, contraction appeared to activate a select group of genes.
The basal component of collagen matrix contraction is sufficient to account for the increase in c-fos mRNA levels.
Fibroblast contraction of stressed collagen matrices exhibits basal and stimulated (by serum) components (38, 62). The mechanism of basal contraction is unknown but probably includes an elastic element, since it is only partially inhibited by cytochalasin D. Since levels of stimulation of c-fos mRNA expression during contraction were similar with or without serum in the medium and in the presence and absence of cytochalasin D, it seems likely that the basal component of contraction is sufficient to stimulate c-fos transcription.
Contraction-stimulated c-fos mRNA transcription requires Ca2+ uptake but not the cyclic AMP signaling pathway or CREB phosphorylation.
The basal component of fibroblast contraction results in stimulation of several signaling events, including Ca2+ uptake and increased production of cyclic AMP (26, 27, 38), which might have stimulated c-fos transcription through the Ca2+-responsive transcription factor CREB (58). Our studies show that Ca2+ influx is necessary but not sufficient for contraction-stimulated c-fos expression. That is, addition of EGTA to the medium blocked the contraction-stimulated increase in c-fos mRNA expression completely but addition of Ca2+ ionophore to fibroblasts in stressed matrices resulted in only a partial increase in c-fos mRNA levels compared to those stimulated by contraction.
Also, the contraction-stimulated increase in cyclic AMP did not appear to be necessary or sufficient for the c-fos mRNA response, judging from the experiments with forskolin and H89. Nevertheless, Ca2+ ionophore and forskolin added together produced a synergistic rise in cyclic AMP levels and an elevation of c-fos mRNA levels comparable to those observed during contraction of stressed matrices. Since fibroblast contraction results in simultaneous increases in Ca2+ and cyclic AMP levels (26, 27, 38), it is possible that the mechanism by which contraction stimulates c-fos mRNA transcription involves a synergistic interaction between Ca2+ and cyclic AMP. Such synergistic effects have been described previously, but the underlying mechanism is not clearly understood (44).
The mechanism by which Ca2+ and cyclic AMP influence c-fos levels of fibroblasts in collagen matrices remains puzzling, since we did not find a correlation between c-fos mRNA levels and CREB activation (cf. reference 28). Indeed, the highest levels of phosphoCREB were observed after forskolin treatment of fibroblasts in stressed matrices, which did not cause a change in c-fos expression. On the other hand, matrix contraction or combined addition of Ca2+ ionophore and forskolin to fibroblasts in stressed matrices resulted in markedly increased c-fos mRNA expression but only a modest increase in phosphoCREB expression. Also, addition of the PKA inhibitor H89 blocked contraction-stimulated CREB phosphorylation but did not increase c-fos mRNA transcription. Although these findings suggest that CREB phosphorylation and c-fos transcription are not linked in human fibroblasts in collagen matrices, we cannot exclude the possibility that rapid turnover of CREB phosphorylation occurred as a result of Ca2+ uptake and activation of calcineurin (or some other phosphatase) that dephosphorylates CREB (5, 16, 22).
Contraction-stimulated c-fos mRNA transcription does not require the ERK 1/2 kinase signaling pathway.
Depending on the mechanism of entry into cells and on the spatial location of increased concentrations of Ca2+, Ca2+ can also can initiate signals leading to activation of the c-fos SRE (17, 24). Moreover, contraction of stressed collagen matrices by fibroblasts was found to stimulate activation of the ERK 1/2 signaling pathway, which also might have led to activation of c-fos transcription through the SRE (32, 50).
If the c-fos SRE promoter region does play a role in contraction-stimulated c-fos transcription, then the mechanism involved probably does not require ERK 1/2 signaling. The MEK 1/2 inhibitor PD98059 completely blocked contraction-stimulated activation of the ERK 1/2 pathway and increased c-fos transcription stimulated by phorbol ester but only partly reduced the increase in c-fos mRNA transcription caused by contraction. There are, however, other mitogen-activated protein kinase pathways besides that of ERK 1/2 that can result in activation of the c-fos SRE, such as those of c-Jun N-terminal kinase (JNK) and p38 (31, 32). In preliminary studies, we have found that the JNK and p38 mitogen-activated protein kinase signaling pathways also are activated during fibroblast contraction (36a). Future studies will be required to determine the possible role of these pathways in contraction-stimulated changes in gene expression.
Fibroblast contraction in relationship to mechanoregulation of cell function.
As mentioned in the introduction, fibroblast contraction of stressed collagen matrices provides a model to study the transition of fibroblasts from mechanically stressed to mechanically relaxed conditions (stress-relaxation), a transition that occurs at the end of wound repair in vivo (although over a longer period in an asynchronous fashion). Stress-relaxation is believed to be an important determinant of cell quiescence and regression (20).
The signal transduction pathways and changes in gene expression that account for mechanoregulation of cell function are just beginning to be understood. Most previous experimental studies have focused on increased mechanical force as a determinant of cell proliferation in animal and plant tissues (see, e.g., references 6, 11, 30, and 67), and c-fos transcription has been shown to increase in response to elevated fluid shear or mechanical loading (36, 49, 53). Our results show that increased transcription of c-fos and other immediate-early genes also accompanies stress-relaxation.
Since increased c-fos transcription was found to require Ca2+ uptake and Ca2+ uptake occurs through plasma membrane passages ∼3 nm in diameter that transiently open during the basal component of cell contraction (38), the formation of these plasma membrane passages may be a critical feature of c-fos regulation. Ca2+ uptake often accompanies cell wounding (14, 56, 61), and a variety of studies have shown that increased c-fos mRNA levels occur after wounding of cells in monolayer culture in vitro (49, 60, 65) or in tissue in vivo (10, 41, 47). Typically, this upregulation has been interpreted in terms of a role for c-fos and other immediate-early genes in the wound-induced onset of cell motility and proliferation (2, 50). From the perspective of our study, however, one can also understand the experimental wound as a means of eliciting a mechanical response from cells, regardless of whether the outcome is cell proliferation or cell quiescence. Indeed, with stress-relaxation, the c-fos signal may be important for cell survival (57) or some other mechanically regulated differentiation function.
The linkage between mechanostimulation and cell wound responses has recently begun to receive increased attention (43). Significantly, mechanostimulated changes in gene expression and other cell functions often depend on autocrine signaling mechanisms (21, 36, 50, 54, 66), and cell wounding can lead to release of molecules that mediate these responses (7, 9, 33, 42). Whether the increase in c-fos mRNA levels in response to contraction of stressed collagen matrices also depends on an autocrine mechanism remains to be studied.
ACKNOWLEDGMENTS
We are indebted to William Snell and Evan Simpson for their helpful comments and suggestions.
These studies were supported by NIH grant GM31321 and by the University of Texas Southwestern Skin Diseases Research Center (grant AR41940).
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD098059 is a specific inhibitor of the activation of mitogen activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Angel P, Karin M. The role of jun, fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 3.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellows C G, Melcher A H, Aubin J E. Contraction and organization of collagen gels by cell cultured from periodontal ligament, gingiva, and bone suggest functional differences between cell types. J Cell Sci. 1981;50:299–314. doi: 10.1242/jcs.50.1.299. [DOI] [PubMed] [Google Scholar]
- 5.Bito H, Diesseroth K, Tsien R W. CREB phosphorylation and dephosphorylation: a CA(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 6.Braam J, Davis R W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G C, Libby P, Grodzinsky A J, Lee R T. Induction of DNA synthesis by a single transient mechanical stimulus of human vascular smooth muscle cells. Role of fibroblast growth factor-2. Circulation. 1996;93:99–105. doi: 10.1161/01.cir.93.1.99. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Clarke M S F, Caldwell R W, Chiao H, Miyake K, McNeil P L. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995;76:927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- 10.Dash P K, Moore A N, Dixon C E. Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J Neurosci. 1995;15:2030–2039. doi: 10.1523/JNEUROSCI.15-03-02030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies P F, Barber K A, Volin M V, Robotewskyj A, Chen J, Joseph L, Griem M L, Wernick M N, Jacobs E, Polacek D C, DePaola N, Barakat A I. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol. 1997;59:527–549. doi: 10.1146/annurev.physiol.59.1.527. [DOI] [PubMed] [Google Scholar]
- 12.Delvoye P, Wiliquet P, Leveque J-L, Nusgens B V, Lapiere C M. Measurement of mechanical forces generated by skin fibroblasts embedded in the three-dimensional collagen gel. J Investig Dermatol. 1991;97:898–902. doi: 10.1111/1523-1747.ep12491651. [DOI] [PubMed] [Google Scholar]
- 13.Desmoulière A, Gabbiani G. The role of the myofibroblast in wound healing and fibrocontractive disease. In: Clark R A F, editor. The molecular and cellular biology of wound repair. 2nd ed. New York, N.Y: Plenum Press; 1996. pp. 391–423. [Google Scholar]
- 14.Drumheller P D, Hubbell J A. Local modulation of intracellular calcium levels near a single-cell wound in human endothelial monolayers. Arterioscler Thromb. 1991;11:1258–1265. doi: 10.1161/01.atv.11.5.1258. [DOI] [PubMed] [Google Scholar]
- 15.Erdos T, Butler-Browne G S, Rappaport L. Mechanogenetic regulation of transcription. Biochemie. 1991;73:1219–1231. doi: 10.1016/0300-9084(91)90007-n. [DOI] [PubMed] [Google Scholar]
- 16.Florio T, Perrino B A, Stork P J. Cyclic 3,5 adenosine monophosphate and cyclosporin A inhibit cellular proliferation and serine/threonine protein phosphatase activity in pituitary cells. Endocrinology. 1996;137:4409–4418. doi: 10.1210/endo.137.10.8828502. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh A, Ginty D D, Bading H, Greenberg M E. Calcium regulation of gene expression in neuronal cells. J Neurobiol. 1993;25:294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- 18.Gilman M Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988;2:394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg M E, Ziff E B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 20.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grygorczyk R, Hanrahan J W. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenolikar S, Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- 23.Halliday N L, Tomasek J J. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res. 1995;217:109–117. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- 24.Hardingham G E, Chawla S, Johnson C M, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 25.Harris A K, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 26.He Y, Grinnell F. Stress relaxation of fibroblasts activates a cyclic AMP signaling pathway. J Cell Biol. 1994;126:457–464. doi: 10.1083/jcb.126.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Grinnell F. Role of phospholipase D in the cAMP signal transduction pathway activated during fibroblast contraction of collagen matrices. J Cell Biol. 1995;130:1197–1205. doi: 10.1083/jcb.130.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldin N E, Paulsson Y, Forsberg K, Heldin C H, Westermark B. Induction of cyclic AMP synthesis by forskolin is followed by a reduction in the expression of c-myc messenger RNA and inhibition of 3H-thymidine incorporation in human fibroblasts. J Cell Physiol. 1989;138:17–23. doi: 10.1002/jcp.1041380104. [DOI] [PubMed] [Google Scholar]
- 29.Hunt T K, Dunphy J E. Fundamentals of wound management. New York, N.Y: Appleton-Century-Crofts; 1979. [Google Scholar]
- 30.Ingber D E. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 31.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 32.Karin M, Liu Z-G, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 33.Kaye D, Pimental D, Prasad S, Maki T, Berger H J, McNeil P L, Kelly R A. Role of transiently altered sarcolemmal membrane permeability and basic fibroblast growth factor release in the hypertrophic response of adult rat ventricular myocytes to increased mechanical activity in vitro. J Clin Invest. 1996;97:281–291. doi: 10.1172/JCI118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodney M S, Wysolmerski R B. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komuro I, Yazaki Y. Control of cardiac gene expression by mechanical stress. Annu Rev Physiol. 1993;55:55–75. doi: 10.1146/annurev.ph.55.030193.000415. [DOI] [PubMed] [Google Scholar]
- 36.Lean J M, Mackay A G, Chow J W, Chambers T J. Osteocytic expression of mRNA for c-fos and IGF-I: an immediate early gene response to an osteogenic stimulus. Am J Physiol. 1996;270:E937–E945. doi: 10.1152/ajpendo.1996.270.6.E937. [DOI] [PubMed] [Google Scholar]
- 36a.Lee, D. J., and F. Grinnell. Unpublished observations.
- 37.Lee T-L, Lin Y-C, Mochitate K, Grinnell F. Stress-relaxation of fibroblasts in collagen matrices triggers ectocytosis of plasma membrane vesicles containing actin, annexins II and VI, and b1 integrin receptors. J Cell Sci. 1993;105:167–177. doi: 10.1242/jcs.105.1.167. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y C, Ho C-H, Grinnell F. Fibroblasts contracting collagen matrices form transient plasma membrane passages through which the cells take up fluorescein isothiocyanate-dextran and Ca2+ Mol Biol Cell. 1997;8:59–71. doi: 10.1091/mbc.8.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Y-C, Grinnell F. Fibroblasts in mechanically-relaxed collagen matrices show decreased levels of PDGF-stimulated receptor autophosphorylation. J Cell Biol. 1993;122:663–672. doi: 10.1083/jcb.122.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 41.Martin P, Nobes C D. An early molecular component of the wound healing response in rat embryos—induction of c-fos protein in cells at the epidermal wound margin. Mech Dev. 1992;38:209–216. doi: 10.1016/0925-4773(92)90054-n. [DOI] [PubMed] [Google Scholar]
- 42.McNeil P L, Muthukrishnan L, Warder E, D’Amore P A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989;109:811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNeil P L, Steinhardt R A. Loss, restoration, and maintenance of plasma membrane integrity. J Cell Biol. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehmet H, Morris C, Rozengurt E. Multiple synergistic signal transduction pathways regulate c-fos expression in Swiss 3T3 cells: the role of cyclic AMP. Cell Growth Differ. 1990;1:293–298. [PubMed] [Google Scholar]
- 45.Mochitate K, Pawelek P, Grinnell F. Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down regulation of DNA and protein synthesis. Exp Cell Res. 1991;193:198–207. doi: 10.1016/0014-4827(91)90556-a. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S, Pawelek P, Grinnell F. Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res. 1989;182:572–582. doi: 10.1016/0014-4827(89)90260-7. [DOI] [PubMed] [Google Scholar]
- 47.Okada Y, Saika S, Hashizume N, Kobata S, Yamanaka O, Ohnishi Y, Senba E. Expression of fos family and jun family proto-oncogenes during corneal epithelial wound healing. Curr Eye Res. 1996;15:824–832. doi: 10.3109/02713689609017623. [DOI] [PubMed] [Google Scholar]
- 48.Patrick C W, Jr, McIntire L V. Shear stress and cyclic strain modulation of gene expression in vascular endothelial cells. Blood Purif. 1995;13:112–124. doi: 10.1159/000170194. [DOI] [PubMed] [Google Scholar]
- 49.Pawar S, Kartha S, Toback F G. Differential gene expression in migrating renal epithelial cells after wounding. J Cell Physiol. 1995;165:556–565. doi: 10.1002/jcp.1041650314. [DOI] [PubMed] [Google Scholar]
- 50.Perrone C E, Fenwick-Smith D, Vandenburgh H H. Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. J Biol Chem. 1995;270:2099–2106. doi: 10.1074/jbc.270.5.2099. [DOI] [PubMed] [Google Scholar]
- 51.Piechaczyk M, Blanchard J-M. c-fos proto-oncogene regulation and function. Crit Rev Oncol-Hematol. 1994;17:93–131. doi: 10.1016/1040-8428(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 52.Rudolph R, Berg J V, Ehrlich H P. Wound contraction and scar contracture. In: Cohen I K, Diegelmann R F, Lindblad W J, editors. Wound healing: biochemical and clinical aspects. W. B. Philadelphia, Pa: Saunders; 1992. pp. 96–114. [Google Scholar]
- 53.Sadoshima J-I, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadoshima J-I, Xu Y, Slayter H S, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 56.Sammak J P, Hinman L, Tran P O T, Sjaastad M D, Machen T E. How do injured cells communicate with the surviving cell monolayer? J Cell Sci. 1997;110:465–475. doi: 10.1242/jcs.110.4.465. [DOI] [PubMed] [Google Scholar]
- 57.Schreiber M, Baumann B, Cotton M, Angel P, Wanger E F. Fos is an essential component of the mammalian UV response. EMBO J. 1995;14:5338–5349. doi: 10.1002/j.1460-2075.1995.tb00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheng M, McFadden G, Greenberg M E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 59.Siegfried Z, Ziff E B. Transcription activation by serum, PDGF, and TPA through the c-fos DSE: cell type specific requirements for induction. Oncogene. 1989;4:3–11. [PubMed] [Google Scholar]
- 60.Sosnowski R G, Feldman S, Feramisco J R. Interference with endogenous ras function inhibits cellular responses to wounding. J Cell Biol. 1993;121:113–119. doi: 10.1083/jcb.121.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanisstreet M. Calcium and wound healing in Xenopus early embryos. J Embryol Exp Morphol. 1982;67:195–205. [PubMed] [Google Scholar]
- 62.Tomasek J J, Haaksma C J, Eddy R J, Vaughan M B. Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec. 1992;232:359–368. doi: 10.1002/ar.1092320305. [DOI] [PubMed] [Google Scholar]
- 63.Tso J Y, Sun X H, Kao T H, Reece K S, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNA’s: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vandenburgh H H. Mechanical forces and their second messengers in stimulating cell growth in vitro. Am J Physiol. 1992;262:R350–R355. doi: 10.1152/ajpregu.1992.262.3.R350. [DOI] [PubMed] [Google Scholar]
- 65.Verrier B, Muller D, Bravo R, Muller R. Wounding a fibroblast monolayer results in the rapid induction of the c-fos proto-oncogene. EMBO J. 1986;5:913–917. doi: 10.1002/j.1460-2075.1986.tb04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson E, Mai Q, Sudhir K, Weiss R H, Ives H E. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993;123:741–747. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamazaki T, Komuro I, Yazaki Y. Molecular mechanism of cardiac cellular hypertrophy by mechanical stress. J Mol Cell Cardiol. 1995;27:133–140. doi: 10.1016/s0022-2828(08)80013-2. [DOI] [PubMed] [Google Scholar]
- 68.Yang M, Kurkinen M. Different mechanisms of regulation of stromelysin and collagenase genes. Eur J Biochem. 1994;222:651–658. doi: 10.1111/j.1432-1033.1994.tb18909.x. [DOI] [PubMed] [Google Scholar]
- 69.Zambetti G, Ramsey-Ewing A, Bortel R, Stein G, Stein J. Disruption of the cytoskeleton with cytochalasin D induces c-fos gene expression. Exp Cell Res. 1991;192:93–101. doi: 10.1016/0014-4827(91)90162-n. [DOI] [PubMed] [Google Scholar]