Abstract
Mosquito-borne illnesses represent a significant global health peril, resulting in approximately one million fatalities annually. West Nile, dengue, Zika, and malaria are continuously expanding their global reach, driven by factors that escalate mosquito populations and pathogen transmission. Innovative control measures are imperative to combat these catastrophic ailments. Conventional approaches, such as eliminating breeding sites and using insecticides, have been helpful, but they face challenges such as insecticide resistance and environmental harm. Given the mounting severity of mosquito-borne diseases, there is promise in exploring innovative approaches using synthetic biology to bolster mosquitoes’ resistance to pathogens, or even eliminate the mosquito vectors, as a means of control. This review outlines current strategies, future goals, and the importance of gene editing for global health defenses against mosquito-borne diseases.
Effective vector control strategies are key to managing vector-borne diseases
Mosquito-borne diseases are responsible for approximately one million deaths worldwide annually, underscoring the need to control the mosquitoes that transmit viruses and parasites such as West Nile virus, dengue virus, Zika virus, and Plasmodium spp. [1–3]. However, despite control efforts, mosquito-borne diseases have seen a recent surge, posing a significant threat to public health. The re-emergence and expansion of these diseases is linked to various factors that have made it easier for mosquitoes to multiply and transmit their pathogens [4–8]. To reduce the burden of mosquito-borne diseases on public health, a holistic approach that takes into account different factors involved in their transmission is required. These factors include urbanization, global travel, trade, climate change, and access to water sources [7–13]. Tackling mosquito-borne diseases through specific interventions, novel technologies, and community involvement is essential.
Traditionally, vector-based interventions such as habitat management, insecticides, larvicides, bed nets, and biological control have successfully reduced the impact of most mosquito-borne diseases [14,15]. However, these methods have limitations, including resistance and environmental impacts [16]. Moreover, there is a lack of quantitative evidence guiding vector control strategies against mosquito-borne diseases in tropical regions [17]. Nevertheless, new mosquito control techniques, such as approaches that utilize the principles of synthetic biology (see Glossary), offer promising solutions [18–22]. Synthetic biology involves redesigning organisms using genetic engineering to grant them new capabilities for practical applications. Synthetic biology-based technologies have emerged as a core element of the bioeconomy and present an opportunity to address global healthcare and other pressing issues [23,24]. In this review, we aim to discuss current strategies and their limitations for mosquito control and propose future goals, utilizing novel gene-editing tools. By exploring the latest advances, we can gain insights into how to better protect public health against the devastating effects of mosquito-borne diseases.
Current mosquito-control strategies
Controlling mosquito populations is an important aspect of public health, and the most widely used control method relies on the use of insecticides, which can either kill, or repel, mosquitoes, can be sprayed directly into the environment, or used in traps and baits [25]. However, insecticide-based methods may cause broad-spectrum effects on non-target species and harm to the environment (Table 1) [26–28]. In addition to insecticides, there are several genetic biocontrol methods that are currently in use for mosquito and pathogen control. For example, genetic biocontrol techniques – such as the sterile insect technique (SIT), Wolbachia-based population suppression/modification, and Oxitec OX5034 mosquitoes – have gained prominence (Table 1). SIT, Wolbachia-based population suppression, and Oxitec OX5034 mosquitoes focus on suppressing disease transmission by reducing mosquito populations. Notably, certain Wolbachia strains have demonstrated a natural ability to curtail pathogen replication within mosquitoes, thereby prompting the adoption of Wolbachia-mediated population modification as an alternative means to curtail disease transmission. The combination of these genetic biocontrol methods presents promising avenues for effectively controlling mosquito populations and reducing the risk of disease transmission, while also addressing the environmental concerns associated with traditional insecticide-based strategies. Nonetheless, it is vital to demonstrate the safety of genetically modified mosquitoes (GMMs) for both the environment and human well-being in the context of genetic biocontrol methods.
Table 1.
Advantages and concerns of current strategies for mosquito control
| Strategy | Mechanism | Advances | Challenges | Refs |
|---|---|---|---|---|
| Environmental management | Elimination of mosquito habitats | Low cost and safe for the environment Effective in reducing mosquito populations and interrupting the life cycle |
Difficult to implement in urban areas Require frequent inspections and maintenance Not enough on its own to control mosquito populations |
[1,3,47] |
| Chemical control | Using chemical products to exterminate mosquitoes | Effective in reducing mosquito populations Easy and quick to apply Can be inexpensive |
Can harm non-target organisms Can lead to pesticide resistance May have health risks with overexposure or improper application |
[1,3,16,26,47] |
| Biological control | Eliminating mosquitoes by introducing their natural predators | Environmentally friendly No health risks when used properly Can be an effective long-term solution |
May not be effective in areas without natural predators May take longer to see results Must be used in combination with other methods for best results |
[1,3,47] |
| Classical sterile insect technique (SIT) | Preventing the production of viable offspring | Environmentally friendly and harmless to unintended species Advancements in mass rearing technology have facilitated the extensive manufacturing of sterile insects intended for release. |
Radiation used for sterilization can negatively affect insect fitness and mating competitiveness. High economic costs of rearing, sterilization, and releasing |
[30,31] |
| Incompatible insect technique (IIT) | Preventing the production of viable offspring | Environmentally friendly and harmless to unintended species Eliminates the necessity for insect irradiation The Wolbachia-infected males can coexist with sterilized males used in irradiation-based SIT, enabling a synergistic approach. |
Artificially increasing Wolbachia infection levels can impose fitness costs on infected insects. An efficient sex separation system is necessary. |
[39,40] |
| Wolbachia-mediated population replacement | Decreasing in the vectorial capacity of the vector | Environmentally friendly and harmless to unintended species Sex separation system is unnecessary. Wolbachia can spread itself through insect populations after an initial release, reducing need for repeated mass rearing and release. |
Artificially increasing Wolbachia infection levels can impose fitness costs on infected insects. Viruses could potentially adapt to counter Wolbachia’s blocking activity. Specific viruses undergo amplification within Wolbachia-infected mosquitoes. Heat stress has adverse effects on Wolbachia. Complex dynamics between Wolbachia, host genetics, and environmental factors complicates predictions of spread and stability. Evidence of increasing vector competence |
[43,47,55] |
| Release of insects carrying a dominant lethal gene (RIDL) | Daughter-killing | Environmentally friendly and harmless to unintended species No health risks when used properly Effective in reducing mosquito populations |
Ecological impact Ethical considerations |
[111] |
Sterile insect technique (SIT)
SIT is a method of biological control that involves iteratively releasing irradiated sterile males in massive numbers [29]. These sterile males find, mate with, and induce sterility in females via the production of unfertilized progeny. This results in embryos that are left nonviable, leading to fewer insects in subsequent generations. Over time, this approach dramatically reduces the overall density of the targeted population [29]. SIT has demonstrated its efficacy against various pests with great significance [29]. For mosquitoes, there are 42 ongoing SIT pilot projects occurring globally, which underscores the wide acceptance of this approach [30].
Recent research has highlighted another facet of SIT: releasing high ratios of sterile males to wild females might also impact the target female population due to mating disturbances [31]. Laboratory experiments reveal that male-to-female ratios exceeding 50:1 lead to reduced longevity and feeding success among female Aedes mosquitoes. Similar effects were observed in semi-field conditions, where blood intake from an artificial host and human biting rates significantly diminished. Notably, a field trial was conducted in China, showing an 80% reduction in female mosquito biting rates. This reduction coincided with a 40% decrease in female mosquito density. These findings imply that SIT’s impact extends beyond population suppression through sterility induction; it could potentially decrease disease transmission by elevating female mortality and lowering host contact [31].
While SIT has the potential to offer sustainable pest suppression, there are limitations. The radiation doses to render insects sterile can detrimentally impact their overall fitness and competitive mating abilities. Therefore, the determination of the ideal dosage presents a formidable challenge, making it difficult to use this approach for all species [32,33]. Furthermore, the introduction of sterile insects into the environment necessitates their competition with fertile wild insects for mating opportunities. This mandates a substantial surplus of fit, sterile insects relative to their wild counterparts. To achieve effective control, SIT demands multiple releases covering a wide geographical expanse and a sustained commitment to these releases. In SIT projects, when the ratio of overflooding is inadequate, only a partial reduction of induced sterility (IS) is observed in the target population – which is insufficient to achieve effective suppression and elimination [34]. Moreover, this approach can incur significant fitness costs due to the requirement to mass rear and perform interactive releases making it costly, difficult to scale and maintain suppression. Taken together, SIT has revealed itself as a gold standard for genetic biocontrol interventions for some species; however, the high fitness costs imposed by radiation, competitive mating capabilities, and the necessity for mass rearing can limit its effectiveness and incur costs (Table 1).
Wolbachia-based population suppression
Another method of population suppression involves the use of Wolbachia in a technique termed incompatible insect technique (IIT) [25,35–39]. Wolbachia naturally induces a form of sterility known as cytoplasmic incompatibility, causing embryo development to falter when infected males mate with uninfected females. This leads to the production of eggs that are unable to hatch, effectively curbing mosquito populations. Importantly, similar to SIT, implementation of IIT demands frequent and repeated releases of perfectly sex-sorted male mosquitoes [40]. Unfortunately, accidental release of Wolbachia-infected females within the target population pose a significant threat to the effectiveness of IIT. This is because Wolbachia-infected females are capable of reproduction with both infected and uninfected males, and will transmit Wolbachia to all of their offspring, resulting in eventual fixation in the population [41]. Unfortunately, this will render the Wolbachia strain ineffective for IIT, as compatibility develops between the infected females and the males that are released. This has indeed been observed in the field, as previous endeavors involving Wolbachia IIT trials in Singapore, which were designed to diminish Aedes aegypti populations, encountered obstacles stemming from the inadvertent establishment of the Wolbachia strain in the natural environment [42]. This establishment resulted from the unintentional release of an exceedingly small cohort of females (estimated to be as few as three). This incident highlights the rapidity with which the Wolbachia strain can become established in the field [42] and underscores the extreme difficulty of achieving perfect sex-sorting efficiency which is, unfortunately, a strict requirement for this form of IIT (Table 1) [40].
To counteract the establishment of the Wolbachia IIT strain, the Singapore trial adopted an alternative approach by combining IIT and low-dose radiation to effectively sterilize any accidentally released females [42]. A similar combined IIT–SIT approach had been previously tested in a small field trial involving Aedes albopictus [39]. This method ensures that inadvertently released Wolbachia-infected females are rendered sterile through radiation, thus preventing the transmission of Wolbachia to their offspring and maintaining the integrity of the strain. Implemented within a small field trial on two secluded riverine islands within Guangzhou, a city exhibiting the highest dengue transmission rate in China, this strategy effectively led to a decrease in the population. Taken together, Wolbachia IIT can be an effective approach – but given the difficulty of ensuring 100% male-only release, this approach requires the use of radiation (Wolbachia IIT +radiation) to sterilize released females to ensure success.
Wolbachia-mediated population modification
Numerous mosquito strains, which were infected with Wolbachia through embryo injection, have exhibited the remarkable capacity to hinder the transmission of significant human pathogens such as dengue, Zika, and chikungunya viruses [43]. Since 2011, the World Mosquito Program (WMP) has collaborated with governments and communities in many countries to spread Wolbachia-infected mosquitoes all over the world. Initial studies demonstrated that releasing mosquitoes with a strain of Wolbachia called wMelPop in Northern Australia and Vietnam did not effectively replace the mosquito population due to the strain’s high density causing fitness issues [44]. However, releasing mosquitoes carrying an alternative strain termed wMel in Cairns, Australia, led to successful population replacement. The wMel strain rapidly spread and reportedly reduced dengue cases by 96% [41]. Similar positive outcomes were seen in releases in Yogyakarta, Indonesia, and Niteroi, Brazil, with dengue incidence reductions of 77% and 69%, respectively [45,46]. Further releases are ongoing in other countries too, such as Colombia, Mexico, Fiji, and Sri Lanka. The Vector Control Advisory Group of the World Health Organization concluded in December 2020 that the introduction of wMel through introgression offers valuable public health benefits against dengue [47]. But not all releases of wMel were successful, as seen in Nha Trang City, Vietnam, where temperature fluctuations led to the loss of wMel infection, and in Cairns, Australia, where heat waves caused reductions in wMel frequencies [48,49]. Moreover, the wAlbB strain of Wolbachia was effectively used for controlling dengue in Greater Kuala Lumpur, Malaysia. The released wAlbB-infected Ae. aegypti did not cause a significant increase in population due to inherent fitness costs and cytoplasmic incompatibility between released males and wild females. wAlbB rapidly spread in the wild Ae. aegypti, exceeding 90% frequency. After releases stopped, wAlbB frequencies remained stable in most sites, with occasional drops managed by low-level releases. Having wAlbB correlated with 40–85% reduction in dengue cases compared with before, but this might be an underestimation due to people traveling outside release areas [50].
While Wolbachia-mediated population modification is exciting, there are limitations. For example, the effectiveness of this approach may be influenced by the genetic background of the host in terms of the strength of Wolbachia-mediated inhibition of arbovirus [51]. In addition, several studies have shown that the temperature at which Wolbachia-infected larvae are reared has a notable effect on the density of Wolbachia in the resulting adult population. These findings suggest that raising the temperature can lead to a significant decrease in the density of certain strains [52–55]. Another consideration with Wolbachia is that it has been shown to have the capability of promoting the evolution of viruses, enhancing the infectivity of certain pathogens within mosquitoes [56,57]. Moreover, Wolbachia cannot be engineered, so once a strain loses effectiveness through evolution, or other factors, another strain or technology will need to be employed. Taken together, these significant limitations dampen excitement and illuminate potential issues associated with the long-term sustainability of this approach (Table 1).
Synthetic biology-based strategies for mosquito control
Over the past few years, the emergence of modern biotechnology has paved the path for precise genetic modification of mosquitoes using a range of innovative techniques. Thanks to numerous genetic tools that have been developed to manipulate mosquito genomes, scientists have been able to transfer foreign genes into the mosquito germline through microinjection. As a result, the identification of markers displaying specific traits during certain developmental stages and tissues, as well as discovery of novel effector genes, has significantly enhanced the genetic tools available for transgenic mosquito research.
To create a transgenic mosquito, a genetically engineered cassette (GEC) must be inserted into the mosquito’s genome. This can be achieved by randomly integrating the GEC into the genome using transposable element (TE)-based transformation or by precisely inserting it into a specific DNA sequence using PhiC31 or homology-directed repair (HDR). Some transposon vector systems used in mosquitoes are Hermes, Minos, Mos1/mariner, piggyBac, and TN5. HDR, which plays a vital role in repairing damaged chromosomes, can be initiated using a DNA nuclease that introduces a double-stranded break (DSB) at the target gene’s location. Zinc-finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 are three types of nuclease that have been extensively used in mosquitoes [22,58,59].
In subsequent text we first describe the Oxitec OX5034 mosquitoes. We then dive into the next-generation technologies that have been accelerated by the emergence of genetic manipulation techniques like CRISPR. These include novel population-suppression systems such as precision-guided SIT (pgSIT) and Ifegenia. Moving forward, these technologies hold the potential to serve as safe and scalable next-generation methods to manage wild mosquito populations, thus reducing the widespread transmission of human disease. We next dive into how GMMs can also be developed to alter mosquitoes in order to prevent the transmission of pathogens [60] and how gene drives may serve as a potential mechanism for spreading these effectors into populations. Finally, we discuss the ethical considerations of GMMs and lead into concluding remarks and future perspectives.
Oxitec’s GMMs
In terms of the utilization of GMMs in the field, Oxitec has paved the path. They have conducted field trials in various countries, such as India, Panama, and Brazil, with promising results [61]. For example, the first GMM, known as OX513A, incorporates a gene for lethality, more precisely termed the ‘tetracycline transcriptional activator varian’ (or tTAV) [62]. This tTAV gene encodes a protein that hinders the transcription of several crucial genes necessary for mosquito development. Consequently, the GMM larvae that produce the tTAV protein perish before reaching maturity. And, the authors named this approach RIDL (Release of Insects carrying a Dominant Lethal). However, in the presence of the antibiotic tetracycline, the tTAV protein’s capacity to block other gene transcription is reduced. In essence, tetracycline functions as an antidote, reversing the effects of the lethality gene. In controlled laboratory conditions, the GMM larvae are raised in water containing tetracycline, allowing them to develop into adult mosquitoes without issue. Once these adult GMMs are released into the wild and interbreed with wild, non-GMMs, their offspring inherit the lethality gene. In the absence of tetracycline in the environment to safeguard them, these offspring do not survive. The genetically engineered Oxitec OX5034 mosquito is designed to specifically express tTAV in females, which can prevent the survival of female offspring [63]. The release of OX5034 male mosquitoes into the environment allows them to mate with wild female mosquitoes, which produces more OX5034 male mosquitoes – which, over time, can effectively reduce the population of Ae. aegypti mosquitoes.
In the USA, the Environmental Protection Agency (EPA) has granted experimental use authorizations to test OX5034 GM Ae. aegypti mosquitoes in selected states like Florida and Texas to prevent disease outbreaks [63–66]. The experimental use permit (EUP) amendment is effective until 30 April 2024, and covers an area of 5360 acres in Monroe County, Florida. This is a relatively small area, but it is where previous field tests have been conducted. These tests have not reported any adverse effects. Studies have shown that the use of OX5034 mosquitoes can significantly decrease the population of Ae. aegypti mosquitoes. For example, one study observed a 95.5% decrease in Ae. aegypti compared with control sites [64,65,67,68]. This is a highly promising result, as it suggests that this approach could be very effective in reducing the spread of mosquito-borne diseases. It is important to note that OX5034 Ae. aegypti is targeted specifically at the Ae. aegypti species and does not affect other mosquito species or other insects [63,64,68].
Despite Oxitec’s success, the use of GMMs has been a topic of much debate due to the regulatory obstacles and community pushback that it has encountered. It is worth acknowledging, however, that there are numerous advantages associated with the utilization of GMMs in mosquito control. For instance, OX5034 mosquitoes have been shown to effectively target specific mosquito species, reducing the need for chemical pesticides [69]. Additionally, they could play a crucial role in controlling local mosquito populations. Moreover, mosquito-borne illnesses remain a significant public health concern in many parts of the world, where the availability of treatments, preventative measures, or vaccines is limited. In this context, the use of GMMs could potentially help in the fight against these diseases, by reducing the number of disease-transmitting mosquitoes. Notwithstanding, it is important to continue monitoring the impact of GMMs on the environment and public health, and to address any legitimate concerns that may arise (Table 1).
Next-generation confinable CRISPR-based population-suppression systems
While Oxitec has commercialized one type of GMM that is safe, effective, and controllable, it relies on the use of a leaky tetracycline repressible system that was discovered over three decades ago [70]. The elevated levels of basal expression in Tet systems are likely attributed to the specific site of chromosomal integration, which has been recognized as a critical factor in tightly regulating the Tet promoter. Furthermore, the presence of false promoters or cryptic initiation signals may also contribute to the leaky/unintended expression of the Tet system under noninduced conditions [71].
The discovery of new genetic engineering tools such as CRISPR [72] has enabled the development of next-generation technologies for population control [73]. One such technology that has been developed is pgSIT, which has been successfully employed in multiple species. This approach uses the precision of CRISPR to disrupt genes in offspring that are crucial for female survival and male fertility. In simple terms, by disrupting these genes, the offspring become sterile males and dead or flightless females (Figure 1) [74–77]. Though successful in the production of F1 sterile males, the pgSIT method involves a genetic cross between the two parental strains, necessitating the management and differentiation of two strains within a facility. To further improve pgSIT by eliminating this crossbreeding step, a next-generation temperature-inducible pgSIT (TI-pgSIT) technology has been developed [78]. Another CRISPR-based technology that has been developed for population suppression is called Inherited Female Elimination by Genetically Encoded Nucleases to Interrupt Alleles (Ifegenia) [79]. This technology was tested on Anopheles gambiae and works by killing females using CRISPR-based techniques. Ifegenia is a binary system that involves separate Cas9 and guide (g)RNA lines that, when combined, disrupt the female-essential femaless (fle) gene, resulting in the death of female offspring. Males that inherit the mutated gene and editing machinery remain viable and fertile. However, subsequent generations inheriting this mutation will also result in the death of female offspring (Figure 1). While these technologies have shown promise in laboratory settings, it remains to be shown how effective they will be in the natural world.
Figure 1. Synthetic biology-based strategies for mosquito control.
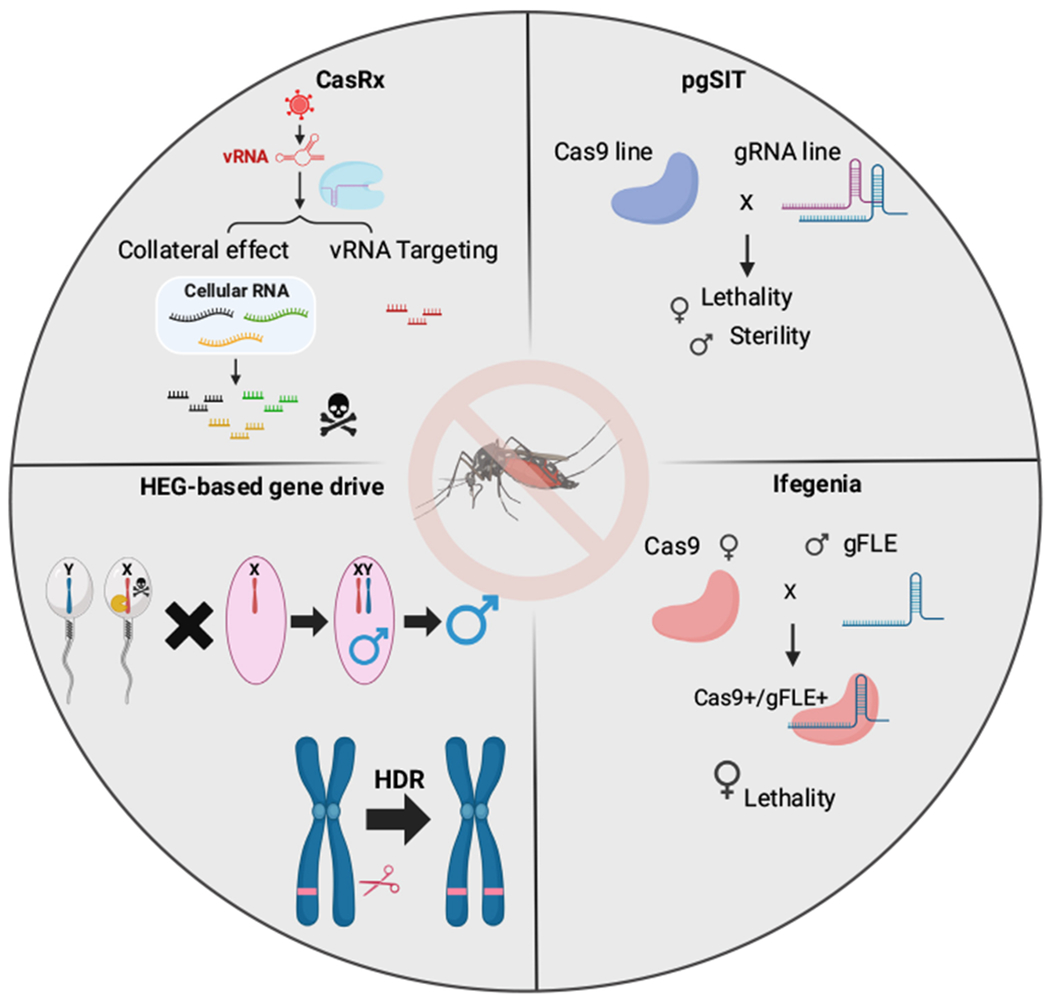
The figure summarizes synthetic mosquito-control strategies. Both pgSIT and Ifegenia use the CRISPR-Cas9 system to target essential genes in mosquitoes. pgSIT targets essential genes in female mosquitoes, aiming either to eliminate them or induce functional lethality, which leads to the removal of female mosquitoes. Also, it targets genes related to sperm development to sterilize males. Ifegenia eliminates female mosquitoes through precise gene targeting [femaless (fle) gene], resulting in populations consisting only of males, thereby leading to effective suppression of the mosquito population. Homing-endonuclease genes (HEGs) have a precise capability to cleave specific nucleotide sequences. When the targeted sequence resides on the X chromosome, and the HEG is active during spermatogenesis, potential outcomes encompass the generation of nonviable female offspring or the selective reduction of X-carrying sperm cells. Once the HEG is inserted into the potential cleavage site, the subsequent repair process, homology-directed repair (HDR), following HEG-induced cleavage can result in the duplication of the allele carrying the HEG, a phenomenon known as ‘homing’. The homing event within the germ line gives rise to an inheritance pattern that exceeds the usual Mendelian ratios, with over 50% of the offspring from an HEG heterozygote inheriting the allele that carries the HEG. REAPER (vRNA expression activates poisonous effector ribonuclease), a CasRx system that specifically targets RNA viral genomes, becomes active in the presence of the targeted arbovirus, such as chikungunya. Consequently, the REAPER approach substantially reduces viral replication, and its precise targeting (virus-induced collateral effect) could potentially result in the mortality of infected mosquitoes. Abbreviations: CRISPR, clustered regularly interspaced short palindromic repeats; gRNA, guide RNA; Ifegenia, Inherited Female Elimination by Genetically Encoded Nucleases to Interrupt Alleles; pgSIT, precision-guided sterile insect technique.; vRNA, viral RNA.
Anti-pathogen effectors within the mosquito
Scientists are also working toward engineering mosquitoes that are unable to transmit pathogens as another form of population-suppression technology. For instance, transgenic mosquitoes expressing SM1 peptide that binds to the midgut and salivary glands of Anopheles stephensi were the first generation of mosquitoes reported to be refractory to disease transmission. The expression of SM1 in female mosquitoes resulted in reduced numbers of oocysts and sporozoites of Plasmodium berghei, a rodent malaria parasite species [80]. Recently, researchers produced transgenic Ae. aegypti mosquitoes with high resistance to dengue-2 virus (DENV2) and Zika virus by expressing a virus-specific inverted-repeat RNA in a conditional and tissue-specific manner. The double-stranded RNA molecules were employed to specifically target the viral genome, initiating RNA interference and thereby diminishing the viral load [60,81–83]. Moreover, genetic modification techniques have facilitated the development of mosquitoes exhibiting a range of novel characteristics not found naturally. These features encompass enhanced innate immunity [84,85], flight defects [76,86], male sterility [76,87], hyper-hemolysis of ingested blood [88], and production of anti-pathogen antibodies [89,90]. These engineered mosquitoes have the potential to reduce the impact of mosquito-borne illnesses on public health and the economy, while also providing new insights into the genetic basis of mosquito behavior and physiology [63,91].
RNA-targeting Cas13 systems can effectively combat arboviruses, unlike traditional Cas9 technologies that target DNA in the mosquito vector. Cas13 evolved as an RNA antiviral in prokaryotes and has surfaced as a programmable RNA-targeting ribonuclease [92]. While Cas13 has demonstrated high efficiency in various organisms, collateral cleavage of bystander RNAs accompanies the high on-target activity, which provides opportunities for developing diagnostics [93,94] but limits its potential for therapeutic applications. However, this collateral cleavage could be leveraged in mosquitoes to combat viral infections, providing a basis for developing flexible antiviral technologies. For instance, an RNA-targeting CRISPR system to target chikungunya (CHIKV) viral RNA (vRNA) via a process called vRNA expression activates poisonous effector ribonuclease (REAPER) has been demonstrated. The REAPER system relies on the collateral cleavage activity of the RNA-targeting Cas13 ribonuclease, resulting in increased mosquito mortality in the presence of the virus (Figure 1) [95]. Taken together, these technologies provide tools to develop antiviral effectors for supporting population modification, and upcoming technologies are expected to push this even further.
Gene drives in mosquitoes
While anti-pathogen effectors are being developed (see earlier), if these are to be used in wild populations they will require a mechanism for spread. Gene drive is a technology that has the potential to provide this needed mechanism to efficiently transmit these effectors into populations. Essentially, gene drive allows for the transmission of genes, transgenes, or genetic traits to offspring at a rate greater than that of normal Mendelian inheritance, resulting in preferential inheritance. This is a powerful tool for genetic engineering as it allows for the rapid spread and increase in frequency of desirable traits in target organisms of wild populations [96]. One exciting example of gene drive technology tested so far is the use of homing-endonuclease genes (HEGs) to encode a DNA endonuclease that recognizes and cleaves a distinct DNA sequence [97]. This HEG can exist on a chromosome within its own recognition sequence, but when the chromosome carrying the HEG is paired with a chromosome that does not have the HEG, but only contains the intact recognition sequence, that chromosome is cleaved, resulting in a DSB in the DNA [97]. As the broken strand is repaired, the loose DNA strands are slightly trimmed and subsequently joined to the intact chromosome at regions of homology. This process, known as HDR, utilizes the intact chromosome as a template to synthesize new DNA and ’fill’ the gap. As a result, the HEG and a small amount of the surrounding sequence are duplicated on both chromosomes, leading to the ’homing’ of the HEG [98]. If homing occurs efficiently in the cells that become gametes, an individual who is heterozygous for the HEG will produce a majority of gametes with a copy of the HEG and its associated trait, resulting in more than the 50% expected through normal Mendelian segregation. This allows the HEG to rapidly increase in frequency in each generation (Figure 1).
Recent advances in endonuclease technology, such as CRISPR-Cas9, have made it easier to reprogram recognition of a specific sequence, replicating the function of a HEG [96]. These systems enable prokaryotes to develop an adaptive immune response based on memory, which selectively destroys foreign nucleic acids that have been encountered previously. Various Cas endonucleases have been identified and studied, and have led to the development of a genome engineering technology that won the Nobel Prize [72]. Today, Cas9-based technologies are the primary techniques used for genome engineering and are applicable to a wide range of organisms. A CRISPR-based gene drive targeting doublesex in the malaria vector An. gambiae can effectively suppress populations with an age structure when raised in an environment that simulates certain conditions found in nature and induces certain mosquito behaviors seen in the field [99]. This gene drive was shown to spread efficiently through populations of An. gambiae mosquitoes of the wild type maintained in small cages or larger cages that were initiated with a low or medium frequency of the gene drive, resulting in complete population suppression [100]. Given the invasive nature of gene drives, thus far they have only been tested in the laboratory. In the future, gene-drive technology may be used to address a wide range of issues, from disease control to environmental conservation, and it will be exciting to see what new innovations emerge in this field.
Ethical and regulatory considerations
Synthetic biology, a rapidly growing field, offers a wide range of promising strategies for reducing mosquito-borne diseases. However, concerns must be addressed first to ensure the safe and effective implementation of synthetic biology-based mosquito-control strategies [63,101]. For example, effective population suppression approaches for a single species may open an ecological niche allowing for other species to come in and take over, resulting in new issues. Also, for population modification approaches it is possible that the effectors that are designed to reduce pathogen transmission become ineffective, or force pathogen selection/evolution which could accentuate the problem [102]. To ensure the safe and effective implementation of synthetic biology-based mosquito-control strategies, various international, regional, and national frameworks have been established [63,103]. For instance, the Cartagena Protocol sets the standards for the safe handling and transfer of living modified organisms (LMOs) on a global scale. At the regional level, the European Academies Science Advisory Council (EASAC) concluded that the current regulatory frameworks are sufficient for synthetic biology [103,104]. Additionally, at an expert workshop held by the Foundation for the National Institutes of Health (FNIH) and the International Life Sciences Institute Research Foundation (ILSIRF) to discuss the application of gene drive in mosquitoes, several concerns regarding human health were reviewed, and agreements were made. Two of the agreements aimed to mitigate existing concerns. First, it was concluded that exposure to GMMs through inhalation or ingestion is unlikely to result in harmful exposure. Second, it was determined that horizontal gene flow from GMMs to humans is biologically unfeasible [105]. Overall, synthetic biology-based mosquito-control strategies offer promising solutions to reducing mosquito-borne diseases. However, to ensure their safe and effective implementation, safety concerns, social and cultural considerations, and the need for public acceptance must be addressed. By doing so, we can work towards a future where mosquito-borne diseases are no longer a significant public health concern.
Concluding remarks
In recent years, high-throughput sequencing technologies have advanced, allowing for the acquisition of biological data with greater speed and accuracy, providing a stronger foundation for synthetic biology research. Furthermore, the development of gene-editing technologies has accelerated, simplifying the modification and design of biological systems and increasing our comprehension of the fundamental nature of biology. These technological breakthroughs have enabled synthetic biology to expand its range of applications in various domains, such as medicine, environment, and food. By leveraging synthetic biology techniques, researchers can precisely regulate and manipulate biological systems, enabling a diverse range of applications.
However, while the use of GMMs has shown promise, such approaches face obstacles from regulatory hurdles and community pushback. Critics argue that the insertion of foreign DNA into the genome is risky and could have catastrophic outcomes. Assuming regulatory challenges can be overcome, safe and controllable transgene-based approaches offer a well-defined pathway for future mosquito control. To alleviate these concerns and reduce genome instability during mosquito production, transgene-free genome editing presents an alternative solution (see Outstanding questions). With this method, the genome of the mosquito can be modified without introducing foreign DNA, making it highly desirable since it avoids regulatory issues associated with GMMs and decreases the chance of unintended consequences on the mosquito genome. This approach can be utilized to create functional mosquitoes that aid in preventing disease transmission and reducing mosquito populations (Table 2). For instance, mosquitoes with immune hyperactivation could be engineered to reduce their vectorial capacity [106]. Additionally, this approach could be used to create a regulatable female-killing system or a female with functional deficiency to suppress mosquito populations. Additionally, certain research has revealed that microbiome engineering may offer a resolution to express an effector gene in a mosquito without altering its genetic information, a concept referred to as paratransgenesis. This approach involves the introduction of a symbiotic bacterium that expresses the desired gene(s) in the mosquito gut. The bacterium then produces effector proteins that affect the mosquito’s physiology or behavior. This method has been successful in reducing the mosquito’s ability to transmit pathogens, such as the malaria parasite, and could potentially be used in conjunction with other mosquito-control strategies [107–109].
Outstanding questions.
How can we create highly efficient techniques for segregating mosquito sexes?
How can a strategy be formulated for the extended preservation of embryos?
How can an effective approach be formulated for the identification of transgenic mosquitoes in the wild?
What does interspecific mating affect in transgenic mosquitoes?
How does horizontal gene transfer influence transgenic mosquitoes?
Table 2.
Potentially endogenous functional module for mosquito control
| Effector | Transgene | Endogenous | Strengths | Challenges | Refs |
|---|---|---|---|---|---|
| Selection marker | Fluorescence, drug resistance | Eye-color, body-color | Achievable gene editing without the introduction of foreign genes | A thorough grasp of the gene’s endogenous function that we aim to modify Potentially elevate the fitness costs Require an efficient selection system | [58,112] |
| Anti-pathogen | miRNA, antibody, CRISPR, REAPER | Immune hyperactivation, receptor for pathogen infection, host factors | [81,89,95,113,114] | ||
| Population suppression | RIDL, HEG-based gene drive, pgSIT, TI-pgSIT, Ifegenia | Sexual disorders | [74,76–79,99,115–120] |
As only female mosquitoes can bite and transmit diseases, while male mosquitoes can mate with multiple females, the release of male mosquitoes alone for genetic biocontrol methods in mosquito control is critical. For Wolbachia-based IIT, the precise release of exclusively male individuals is of paramount importance [40]. Developing accurate techniques to distinguish between male and female mosquitoes is crucial for implementing genetic control strategies to combat vector-borne diseases (see Outstanding questions). Conventional methods for mosquito sorting rely on labor-intensive and time-consuming manual sorting based on morphological differences, which are also prone to inaccuracies. Therefore, there is a demand for automated or semi-automated methods that can quickly and accurately sort male and female mosquitoes on a large scale.
Furthermore, establishing a long-term preservation plan for transgenic mosquito embryos is essential for the successful implementation of genetic control measures against mosquito-borne diseases (see Outstanding questions). Transgenic mosquitoes are genetically engineered to carry mutations that hinder pathogen transmission or reduce their population size. However, achieving the desired outcomes requires mass production and release in significant numbers. Hence, a reliable and efficient approach for preserving and transporting mosquito embryos is critical to the success of this strategy.
Transgenic mosquitoes are genetically altered insects with the potential to diminish the transmission of diseases. Nonetheless, a significant challenge in executing this strategy is to monitor the dissemination and behavior of these modified mosquitoes in natural populations (see Outstanding questions). This necessitates dependable and economical methods for detecting and measuring the number of transgenic mosquitoes and their interactions with other organisms.
The possibility of interspecific mating when releasing mosquitoes raises concerns about the stability and safety of using genetic alterations intended to modify and persist in a population [110] (see Outstanding questions). Alternatively, these interspecific matings may offer opportunities for population-suppression technologies and the potential to control multiple species. Therefore, understanding the effects of interspecific hybridization on transgenic mosquitoes is crucial.
Horizontal gene transfer (HGT) is the movement of genetic material between organisms that are not related by vertical descent, and it can occur through various mechanisms, such as TEs, hybridization, viral infection, or symbiosis. TEs are mobile DNA sequences that can insert themselves into different locations in the genome and affect the structure and function of mosquito genomes, as well as their interactions with pathogens and symbionts. Transgenic mosquitoes often carry TEs as part of their genetic constructs, which may also be subject to HGT and spread into wild mosquito populations or other organisms (see Outstanding questions). This could have unintended consequences for the ecology and epidemiology of mosquito-borne diseases, such as altering the vector competence of mosquitoes, affecting their fitness and behavior, or creating new genetic combinations that could facilitate the emergence of novel pathogens or resistance mechanisms. Therefore, the impact of HGT on transgenic mosquitoes is an important issue that needs to be carefully evaluated before releasing them into the environment, and more research is needed to understand the frequency and consequences of HGT in transgenic mosquitoes and to develop strategies to minimize its risks and maximize its benefits for disease control.
In conclusion, the recent advances in high-throughput sequencing and the gene-editing toolkit have broadened the possibilities within the field of synthetic biology. However, the use of GMMs has its limitations, and transgene-free genome editing presents a promising solution. By utilizing this approach, researchers can create functional mosquitoes that aid in disease prevention and mosquito population control. However, to achieve these objectives, it is crucial to have a comprehensive understanding of the gene’s endogenous function that we intend to edit. The more we know about these genes, the easier it becomes to manipulate their function and develop more efficient gene-editing systems. Therefore, high-quality genome sequences and annotation for all vector mosquitoes are of the utmost importance.
Highlights.
There has been a rise in mosquito-borne illnesses, creating a significant public health burden.
Conventional insecticide-based approaches have limitations, notably resistance and adverse effects on the environment.
Synthetic biology shows promise in reducing mosquito-borne diseases, but safety, societal factors, and public acceptance are key for success.
Assuming that regulatory hurdles can be overcome, the use of safe and controllable transgene-based methods offers a straightforward approach to mosquito control in the future.
Effective mosquito control requires collaboration among government, communities, and stakeholders, emphasizing public awareness and participation.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants (RO1AI148300, RO1AI175152, RO1GM132825, DP2AI152071, RO1AI151004) awarded to O.S.A. The views, opinions, and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the US government. Figures were created using BioRender.
Glossary
- Gene drive
a genetic engineering method that alters genes to disrupt their usual patterns of inheritance. Gene drives significantly increase the probability of a specific set of genes being transmitted to the next generation, enabling these genes to swiftly propagate within a population and supersede the effects of natural selection.
- Genetic biocontrol
a method of using genetically engineered living organisms to manage pests and disease-causing pathogens.
- Introgression
the transfer of genetic material between species through hybridization and backcrossing to paternal species.
- Paratransgenesis
a technique of genetically altering an organism’s symbiotic microorganisms to provide particular functions that reduce the organism’s ability to transmit pathogens.
- Synthetic biology
a discipline that involves the application of genetic engineering to biology.
- Transgene-free genome editing
the process of altering the genome without inserting foreign DNA into the target organism.
- Transgenesis
a process of introducing a foreign gene into the genome of an organism.
- Vector-borne diseases
illnesses caused by parasites, viruses, and bacteria that are transmitted by vectors such as mosquitoes, ticks, and sandflies.
- Wolbachia
a genus of intracellular bacteria that infects mainly arthropods and is passed from one generation to the next through an insect’s eggs.
Footnotes
Declaration of interests
O.S.A. is a founder of Agragene, Inc. and Synvect, Inc. with equity interest. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies. All other authors declare no competing interests.
References
- 1.Takken W et al. (2021) Global vector control response – supporting the pillars. In Ecology and Control of Vector-borne Diseases Vol 6, Innovative Strategies for Vector Control, Wageningen Academic Publishers. Published online February 18, 2021. 10.3920/978-90-8686-895-7_13 [DOI] [Google Scholar]
- 2.Boäte C (2021) Outbreaks of arboviruses, biotechnological innovations and vector control: facing the unexpected. In Ecology and Control of Vector-borne Diseases Vol 6, Innovative Strategies for Vector Control, Wageningen Academic Publishers. Published online February 18, 2021. 10.3920/978-90-8686-895-7_12 [DOI] [Google Scholar]
- 3.Velayudhan R (2021) Brief overview of the World Health Organization ‘Vector Control Global Response 2017–2030’ and ‘Vector Control Advisory Group’ activities. In Area-Wide Integrated Pest Management (Hendrichs J et al. , eds), pp. 633–644, CRC Press [Google Scholar]
- 4.World Health Organization (2023) Global report on neglected tropical diseases 2023, WHO; Published online January 29, 2023. https://www.who.int/teams/control-of-neglected-tropical-diseases/global-report-on-neglected-tropical-diseases-2023 [Google Scholar]
- 5.World Health Organization (2023) A Compendium of indicators for monitoring and evaluating progress of the road map for neglected tropical diseases 2021–2030, WHO; Published online January 6, 2023. https://www.who.int/publications-detail-redirect/9789240062863 [Google Scholar]
- 6.Claborn D et al. , eds (2020) Vector-Borne Diseases: Recent Developments in Epidemiology and Control, IntechOpen [Google Scholar]
- 7.Messina JP et al. (2019) The current and future global distribution and population at risk of dengue. Nat. Microbiol 4, 1508–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan SJ et al. (2019) Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis 13, e0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colón-González FJ et al. (2021) Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet. Health 5, e404–e414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobusch LC and Grobusch MP (2022) A hot topic at the environment–health nexus: investigating the impact of climate change on infectious diseases. Int. J. Infect. Dis 116, 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz C et al. (2020) Remote sensing for risk mapping of Aedes aegypti infestations: is this a practical task? Acta Trop. 205, 105398. [DOI] [PubMed] [Google Scholar]
- 12.Ducheyne E et al. (2018) Current and future distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in WHO Eastern Mediterranean Region. Int. J. Health Geogr 17, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanescu LM et al. (2023) The risk of emerging of dengue fever in Romania, in the context of global warming. Trop. Med. Infect. Dis 8, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lees RS et al. (2023) Insecticides for mosquito control: improving and validating methods to strengthen the evidence base. Insects 14, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinschmidt I and Rowland M (2021) Insecticides and malaria. In Ecology and Control of Vector-borne Diseases Vol 6, Innovative strategies for vector control, Wageningen Academic Publishers. Published online February 18, 2021. 10.3920/978-90-8686-895-7_2 [DOI] [Google Scholar]
- 16.World Health Organization (2022) Manual for monitoring insecticide resistance in mosquito vectors and selecting appropriate interventions, WHO; Published online June 22, 2022. https://www.who.int/publications/i/item/9789240051089 [Google Scholar]
- 17.Ferguson NM (2018) Challenges and opportunities in controlling mosquito-borne infections. Nature 559, 490–497 [DOI] [PubMed] [Google Scholar]
- 18.Caragata EP et al. (2020) Prospects and pitfalls: next-generation tools to control mosquito-transmitted disease. Annu. Rev. Microbiol 74, 455–475 [DOI] [PubMed] [Google Scholar]
- 19.Shaw WR and Catteruccia F (2019) Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat. Microbiol 4, 20–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G-H et al. (2021) Combating mosquito-borne diseases using genetic control technologies. Nat. Commun 12, 4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raban R et al. (2022) A perspective on the expansion of the genetic technologies to support the control of neglected vector-borne diseases and conservation. Front. Trop. Dis 3, 999273 [Google Scholar]
- 22.Benedict MQ and Scott MJ (2022) Transgenic Insects: Techniques and Applications (2nd edn), CABI [Google Scholar]
- 23.Voigt CA (2020) Synthetic biology 2020–2030: six commercially-available products that are changing our world. Nat. Commun 11, 6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samal SK and Preetam S (2022) Synthetic biology: refining human health. Microb. Eng. Ther 57–70 [Google Scholar]
- 25.Tyagi BK (2022) Genetically Modified and other Innovative Vector Control Technologies: Eco-bio-social Considerations for Safe Application, Springer Nature [Google Scholar]
- 26.World Health Organization (2022) Technical consultation on the use of economics in insecticide resistance management for malaria vector control: report of a virtual meeting, 14–16 September 2021, WHO [Google Scholar]
- 27.Rasli R et al. (2021) Insecticide resistance in dengue vectors from hotspots in Selangor, Malaysia. PLoS Negl. Trop. Dis 15, e0009205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemingway J et al. (2002) An overview of insecticide resistance. Science 29, 896–897 [DOI] [PubMed] [Google Scholar]
- 29.Dyck VA et al. , eds (2006) Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, Springer Science & Business Media [Google Scholar]
- 30.Bouyer J et al. (2020) Phased conditional approach for mosquito management using sterile insect technique. Trends Parasitol. 36, 325–336 [DOI] [PubMed] [Google Scholar]
- 31.Bouyer J et al. (2023) Mating harassment may boost the effectiveness of the sterile insect technique for Aedes mosquitoes. Res. Square Published online August 10, 2023. 10.21203/rs.3.rs-3128571/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreasen MH and Curtis CF (2005) Optimal life stage for radiation sterilization of Anopheles males and their fitness for release. Med. Vet. Entomol 19, 238–244 [DOI] [PubMed] [Google Scholar]
- 33.Maïga H et al. (2014) Mating competitiveness of sterile male Anopheles coluzzii in large cages. Malar. J 13, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouyer J (2023) When less is more: accounting for overcompensation in mosquito SIT projects. Trends Parasitol. 39, 235–237 [DOI] [PubMed] [Google Scholar]
- 35.Martíin-Park A et al. (2022) Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl. Trop. Dis 16, e0010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kittayapong P et al. (2018) Combined sterile insect technique and incompatible insect technique: sex separation and quality of sterile Aedes aegypti male mosquitoes released in a pilot population suppression trial in Thailand. Parasit. Vectors 11, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kittayapong P (2021) Combined sterile insect technique and incompatible insect technique: concept, study design, experience and lessons learned from a pilot suppression trial in Thailand. In Area-Wide Integrated Pest Management (Hendrichs J et al. , eds), pp. 405–432, CRC Press [Google Scholar]
- 38.Baton LA et al. (2021) Combining the incompatible and sterile insect techniques for pest and vector control. In Area-Wide Integrated Pest Management (Hendrichs J et al. , eds), pp. 367–404, CRC Press [Google Scholar]
- 39.Zheng X et al. (2019) Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61 [DOI] [PubMed] [Google Scholar]
- 40.Crawford JE et al. (2020) Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol 38, 482–492 [DOI] [PubMed] [Google Scholar]
- 41.Ryan PA et al. (2019) Establishment of Mel in mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 3, 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The Project Wolbachia – Singapore ConsortiumChing, N.L. (2021) Wolbachia-mediated sterility suppresses Aedes aegypti populations in the urban tropics. medRxiv Published online June 17, 2021. 10.1101/2021.06.16.21257922 [DOI] [Google Scholar]
- 43.Ant TH et al. (2023) Virus interactions and arbovirus control through population replacement in mosquitoes. Pathog. Glob. Health 117, 245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen TH et al. (2015) Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit. Vectors 8, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinto SB et al. (2021) Effectiveness of Wolbacha-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: a quasi-experimental study. PLoS Negl. Trop. Dis 15, e0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utarini A et al. (2021) Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med 384, 2177–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization (2021) Thirteenth meeting of the WHO vector control advisory group. Published online March 8, 2021. https://www.who.int/publications/i/item/9789240021792
- 48.Hien NT et al. (2021) Environmental factors influence the local establishment of in mosquitoes in two small communities in central Vietnam. Gates Open Res. 5, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross PA et al. (2020) Heatwaves cause fluctuations in wMel Wolbachia densities and frequencies in Aedes aegypti. PLoS Negl. Trop. Dis 14, e0007958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nazni WA et al. (2019) Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol 29, 4241–4248.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang X et al. (2022) AlbB remains stable in over 15 years but exhibits genetic background-dependent variation in virus blocking. PNAS Nexus 1, gac203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross PA et al. (2017) Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 13, e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulrich JN et al. (2016) Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl. Trop. Dis 10, e0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunasekaran K et al. (2022) Sensitivity of wMel and wAlbB Wolbachia infections in Aedes aegypti Puducherry (Indian) strains to heat stress during larval development. Parasit. Vectors 15, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vásquez VN et al. (2023) Mel replacement of dengue-competent mosquitoes is robust to near-term change. Nat. Clim. Chang 13, 848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thi Hue Kien D et al. (2023) Genome evolution of dengue virus serotype 1 under selection by Wolbachia pipientis in Aedes aegypti mosquitoes. Virus Evol. 9, vead016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dodson BL et al. (2023) Variable effects of on alphavirus infection in Aedes aegypti. bioRxiv Published online January 21, 2023. 10.1101/2023.01.20.524939 [DOI] [Google Scholar]
- 58.Handler AM and James AA (2000) Insect Transgenesis: Methods and Applications, CRC Press [Google Scholar]
- 59.Wimmer EA (2005) Insect transgenesis by site-specific recombination. Nat. Methods 2, 580–582 [DOI] [PubMed] [Google Scholar]
- 60.Franz AWE et al. (2006) Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A 103, 4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sabet A and Goddard J (2022) Promise or peril: using genetically modified mosquitoes in the fight against vector-borne disease. Am. J. Med 135, 281–283 [DOI] [PubMed] [Google Scholar]
- 62.Phuc HK et al. (2007) Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization (2021) Guidance Framework for Testing Genetically Modified Mosquitoes: Second Edition, WHO; Published online. https://iris.who.int/bitstream/handle/10665/341370/9789240025233-eng.pdf?sequence=1 [Google Scholar]
- 64.Burki T (2020) Genetically modified mosquitoes. Lancet Infect. Dis 20, 1375–1376 [DOI] [PubMed] [Google Scholar]
- 65.Waltz E (2022) Biotech firm announces results from first US trial of genetically modified mosquitoes. Nature 604, 608–609 [DOI] [PubMed] [Google Scholar]
- 66.Waltz E (2021) First genetically modified mosquitoes released in the United States. Nature 593,175–176 [DOI] [PubMed] [Google Scholar]
- 67.Makoni M (2021) 2021 WHO guidelines on genetically modified mosquitoes. Lancet Microbe 2, e353. [DOI] [PubMed] [Google Scholar]
- 68.Carvalho DO et al. (2015) Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis 9, e0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldstein C et al. (2019) Benefits and limitations of emerging techniques for mosquito vector control. C. R. Biol 342, 70–272 [Google Scholar]
- 70.Gossen M and Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U. S. A 89, 5547–5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ingole KD et al. (2022) Tetracycline-controlled (TetON) gene expression system for the smut fungus. Front. Fungal Biol 3, 1029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jinek M et al. (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raban R et al. (2023) Manipulating the destiny of wild populations using CRISPR. Annu. Rev. Genet Published online September 18, 2023. 10.1146/annurev-genet-031623-105059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kandul NP et al. (2019) Transforming insect population control with precision guided sterile males with demonstration in flies. Nat. Commun 10, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M et al. (2023) Targeting sex determination to suppress mosquito populations. bioRxiv Published online November 15, 2023. 10.1101/2023.04.18.537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li M et al. (2021) Suppressing mosquito populations with precision guided sterile males. Nat. Commun 12, 5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smidler AL et al. (2023) Eliminating malaria vectors with precision guided sterile males. bioRxiv Published online July, 21, 2023. 10.1101/2023.07.20.549947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kandul NP et al. (2021) Temperature-inducible precision-guided sterile insect technique. CRISPR J. 4, 822–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smidler AL et al. (2023) A confinable female-lethal population suppression system in the malaria vector. Sci. Adv 9, eade8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ito J et al. (2002) Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417, 452–455 [DOI] [PubMed] [Google Scholar]
- 81.Buchman A et al. (2019) Engineered resistance to Zika virus in transgenic expressing a polycistronic cluster of synthetic small RNAs. Proc. Natl. Acad. Sci. U. S. A 116, 3656–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yen P-S et al. (2018) Synthetic miRNAs induce dual arboviral-resistance phenotypes in the vector mosquito. Commun. Biol 1, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu W-L et al. (2021) Transgenic refractory Aedes aegypti lines are resistant to multiple serotypes of dengue virus. Sci. Rep 11, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jupatanakul N et al. (2017) Engineered Aedes aegypti JAK/STAT pathway-mediated immunity to dengue virus. PLoS Negl. Trop. Dis 11, e0005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong Y et al. (2011) Engineered anopheles immunity to Plasmodium infection. PLoS Pathog. 7, e1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Leary S and Adelman ZN (2020) CRISPR/Cas9 knockout of female-biased genes AeAct-4 or myo-fem in Ae. aegypti results in a flightless phenotype in female, but not male mosquitoes. PLoS Negl. Trop. Dis 14, e0008971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J et al. (2021) Suppression of female fertility in with a CRISPR-targeted male-sterile mutation. Proc. Natl. Acad. Sci. U. S. A 118, e2105075118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshida S et al. (2007) Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development. PLoS Pathog. 3, e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buchman A et al. (2020) Broad dengue neutralization in mosquitoes expressing an engineered antibody. PLoS Pathog. 16, e1008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Isaacs AT et al. (2011) Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathog. 7, e1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adelman ZN (2015) Genetic Control of Malaria and Dengue, Academic Press [Google Scholar]
- 92.Abudayyeh OO et al. (2017) RNA targeting with CRISPR-Cas13. Nature 550, 280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brogan DJ and Akbari OS (2022) CRISPR diagnostics: advances toward the point of care. Biochemistry Published online March 24, 2022. 10.1021/acs.biochem.2c00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brogan DJ et al. (2021) Development of a rapid and sensitive CasRx-Based diagnostic assay for SARS-CoV-2. ACS Sens. 6, 3957–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dalla Benetta E et al. (2023) Engineered antiviral sensor targets infected mosquitoes. bioRxiv Published online January 27, 2023. 10.1101/2023.01.27.525922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Champer J et al. (2016) Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat. Rev. Genet 17, 146–159 [DOI] [PubMed] [Google Scholar]
- 97.Windbichler N et al. (2011) A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nolan T (2021) Control of malaria-transmitting mosquitoes using gene drives. Philos. Trans. R. Soc. Lond. B Biol. Sci 376, 20190803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kyrou K et al. (2018) A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol 36, 1062–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hammond A et al. (2021) Gene-drive suppression of mosquito populations in large cages as a bridge between lab and field. Nat. Commun 12, 4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Famakinde DO (2020) Public health concerns over gene-drive mosquitoes: will future use of gene-drive snails for schistosomiasis control gain increased level of community acceptance? Pathog. Glob. Health 114, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marshall JM et al. (2019) Winning the tug-of-war between effector gene design and pathogen evolution in vector population replacement strategies. Front. Genet 10, 1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keiper F and Atanassova A (2020) Regulation of synthetic biology: developments under the convention on biological diversity and its protocols. Front. Bioeng. Biotechnol 8, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deutsche Akademie der Naturforscher Leopoldina (2010) Realising European potential in synthetic biology: scientific opportunities and good governance [Google Scholar]
- 105.Roberts A et al. (2017) Results from the workshop ‘Problem Formulation for the Use of Gene Drive in Mosquitoes’. Am. J. Trop. Med. Hyg 96, 530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma E et al. (2021) Interaction of viruses with the insect intestine. Annu. Rev. Virol 8, 115–131 [DOI] [PubMed] [Google Scholar]
- 107.Wang S et al. (2017) Driving mosquito refractoriness to with engineered symbiotic bacteria. Science 357, 1399–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pike A et al. (2017) Changes in the microbiota cause genetically modified to spread in a population. Science 357, 1396–1399 [DOI] [PubMed] [Google Scholar]
- 109.Huang W et al. (2023) TC1 symbiont suppresses malaria transmission by anopheline mosquitoes. Science 381, 533–540 [DOI] [PubMed] [Google Scholar]
- 110.Zhou J et al. (2022) Interspecific mating bias may drive displacement of during its range expansion. PNAS Nexus 1, gac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beerntsen BT et al. (2000) Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev 64, 115–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li M et al. (2017) Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector. Proc. Natl. Acad. Sci. U. S. A 114, E10540–E10549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weng S-C et al. (2021) A thioester-containing protein controls dengue virus infection in through modulating immune response. Front. Immunol 12, 670122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merkling SH et al. (2023) Multifaceted contributions of Dicer2 to arbovirus transmission by Aedes aegypti. Cell Rep. 42, 112977. [DOI] [PubMed] [Google Scholar]
- 115.Hall AB et al. (2015) SEX DETERMINATION. A male-determining factor in the mosquito Aedes aegypti. Science 348, 1268–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krzywinska E et al. (2016) A maleness gene in the malaria mosquito Anopheles gambiae. Science 353, 67–69 [DOI] [PubMed] [Google Scholar]
- 117.Hall AB et al. (2016) Radical remodeling of the Y chromosome in a recent radiation of malaria mosquitoes. Proc. Natl. Acad. Sci. U. S. A 113, E2114–E2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Criscione F et al. (2016) GUY1 confers complete female lethality and is a strong candidate for a male-determining factor in Anopheles stephensi. eLife 5, e19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krzywinska E et al. (2021) Femaleless controls sex determination and dosage compensation pathways in females of Anopheles mosquitoes. Curr. Biol 31, 1084–1091.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thomas DD et al. (2000) Insect population control using a dominant, repressible, lethal genetic system. Science 287, 2474–2476 [DOI] [PubMed] [Google Scholar]


