ABSTRACT
Pseudomonas aeruginosa harboring Verona Integron-encoded metallo-β-lactamase enzymes (VIM-CRPA) have been associated with infection outbreaks in several parts of the world. In the US, however, VIM-CRPA remain rare. Starting in December 2018, we identified a cluster of cases in our institution. Herein, we present our epidemiological investigation and strategies to control/manage these challenging infections. This study was conducted in a large academic healthcare system in Miami, FL, between December 2018 and January 2022. Patients were prospectively identified via rapid molecular diagnostics when cultures revealed carbapenem-resistant P. aeruginosa. Alerts were received in real time by the antimicrobial stewardship program and infection prevention teams. Upon alert recognition, a series of interventions were performed as a coordinated effort. A retrospective chart review was conducted to collect patient demographics, antimicrobial therapy, and clinical outcomes. Thirty-nine VIM-CRPA isolates led to infection in 21 patients. The majority were male (76.2%); the median age was 52 years. The majority were mechanically ventilated (n = 15/21; 71.4%); 47.6% (n = 10/21) received renal replacement therapy at the time of index culture. Respiratory (n = 20/39; 51.3%) or bloodstream (n = 13/39; 33.3%) were the most common sources. Most infections (n = 23/37; 62.2%) were treated with an aztreonam–avibactam regimen. Six patients (28.6%) expired within 30 days of index VIM-CRPA infection. Fourteen isolates were selected for whole genome sequencing. Most of them belonged to ST111 (12/14), and they all carried blaVIM-2 chromosomally. This report describes the clinical experience treating serious VIM-CRPA infections with either aztreonam–ceftazidime/avibactam or cefiderocol in combination with other agents. The importance of implementing infection prevention strategies to curb VIM-CRPA outbreaks is also demonstrated.
KEYWORDS: Pseudomonas aeruginosa, carbapenemase, antimicrobial stewardship, infection prevention, Verona Integron metallo-beta-lactamase
INTRODUCTION
Pseudomonas aeruginosa (P. aeruginosa) is a ubiquitous water-borne pathogen that is frequently implicated in healthcare-associated infections (1). The CDC estimates that 28,800 cases and 2,500 deaths were associated with multi-drug-resistant (MDR) P. aeruginosa among hospitalized patients in 2020 (2). This pathogen is particularly concerning due to its ability to develop antimicrobial resistance via several mechanisms that include efflux pump and porin channel modifications, as well as antimicrobial-modifying enzymes (e.g., chromosomally mediated Pseudomonas-derived cephalosporinases and plasmid-mediated β-lactamases, as well as aminoglycoside-modifying enzymes) (1). One family of plasmid-mediated carbapenemases, the metallo-beta-lactamases (MBLs), have spread from their initial geographic foci to other parts of the world in the last 30 years. For example, carbapenem-resistant P. aeruginosa (CP-CRPA) harboring the Verona Integron-encoded metallo-β-lactamase (VIM-CRPA) were first reported in 1996 in Marseilles, France, and in 1997 in Verona, Italy (3). Subsequently, VIM-CRPA strains have been linked to outbreaks and environmental contamination in a range of countries including Japan, Switzerland, Spain, Poland, the Netherlands, Belgium, Korea, Colombia, and Mexico (4–11). In the US, VIM-CRPA remain relatively rare. According to the CDC, 892 isolates of VIM-CRPA were identified from 2017 to 2022 (1.3% of all CRPA isolates tested) (12). Clinical information on VIM-CRPA is limited to a few case reports (13–19).
VIM is globally the most frequently identified carbapenemase in P. aeruginosa (20). Infections caused by VIM-CRPA present therapeutic challenges due to the paucity of effective pharmaceutical options. Predisposing risk factors encompass prior antibiotic exposure, prolonged hospital stay, severe underlying disease or critical illness, and the presence of invasive medical devices, as well as intravenous catheters (21, 22). MBL enzymes, such as VIM, are encoded on mobile genetic elements like plasmids and integrons. These elements facilitate horizontal gene transfer among bacterial populations, conferring resistance by inactivating carbapenems and the vast majority of β-lactam antibiotics (23). Infection prevention (IP) studies have demonstrated that fomites within a patient’s room such as bedside tables, sink faucets, and aerators facilitate the transmission of bacterial pathogens, leading to nosocomial outbreaks within the intensive care unit (ICU) (24–27).
Beginning in December 2018, a cluster of cases was reported in our tertiary healthcare facility, prompting us to prospectively identify VIM-CRPA isolates using molecular rapid diagnostics. Herein, the epidemiological investigation, molecular analysis, IP interventions, microbiological testing, and the role of our antimicrobial stewardship program (ASP) in addressing these infections are presented.
MATERIALS AND METHODS
This study was conducted in a large academic healthcare system in Miami, FL, between December 2018 and January 2022. Cases were identified prospectively based on the detection of VIM-CRPA in microbiology laboratory cultures. Patients were included in our analysis whether they were infected or colonized with VIM-CRPA. Only the first isolate per episode of infection or colonization was included.
In January 2019, we formally implemented the Cepheid Xpert Carba-R assay for blaVIM detection in-house. Prior to this, VIM-CRPA isolates were identified through routine epidemiological surveillance via our state health department, led by our infection prevention department. In late 2020, the BioFire FilmArray Blood Culture Identification 2 Panel (BCID2) was implemented, allowing for direct identification of blaVIM genes in blood isolates; the Cepheid Xpert remained for surveillance and clinical cultures from sites other than the bloodstream. Species identification was done using matrix-assisted laser desorption ionization-time of flight mass spectrometry. The VITEK 2 GN 74 Automated Susceptibility (AST) card was employed for species identification and susceptibility testing against seven antimicrobial agents: amikacin, gentamicin, tobramycin, ceftazidime, cefepime, meropenem, and levofloxacin. Results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) reference methods (28). Isolates were classified as difficult-to-treat (DTR) according to previously established definitions (29).
Upon receipt of culture and molecular diagnostic assay results from the microbiology department, both ASP and IP teams were notified in real time through an integrated surveillance software system. After receiving the alert, a series of coordinated interventions were initiated to reduce horizontal transmission and ensure effective treatment for each patient. Susceptibility testing was conducted in accordance with the institutional reflex-testing algorithm (Fig. 1). When DTR P. aeruginosa was identified, the algorithm indicated E-tests for ceftazidime–avibactam (CZA), ceftolozane–tazobactam (C/T), and imipenem–cilastatin–relebactam (I-R). Additionally, a Sensititre GNX3F microdilution panel was utilized to ascertain susceptibilities for aztreonam (ATM), colistin (CST), and polymyxin B (PMB). When non-susceptibility to both CZA and C/T was observed, the algorithm indicated the use of the Cepheid Xpert Carba-R assay. Upon detection of the blaVIM gene on the Carba-R (or BCID2), a cefiderocol (FDC) 30-µg disk diffusion test was conducted, along with antimicrobial combination tests by E-tests for CZA and ATM, as well as I-R and ATM (30). The results were interpreted as follows: a fractional inhibitory concentration index of ≤0.5 was deemed synergistic, values between >0.5 and 1 were considered additive, those from >1 to 4 were viewed as indifferent, and values >4 were categorized as antagonistic (31).
Fig 1.
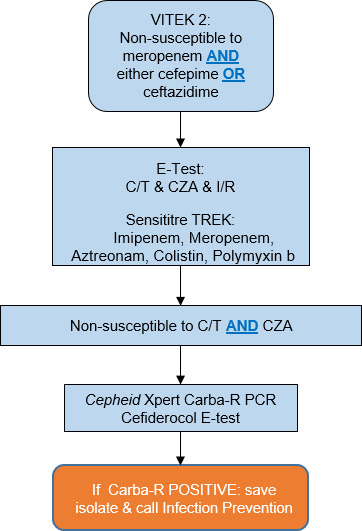
Microbiology automatic reflex testing for multi-drug resistant Pseudomonas aeruginosa. Abbreviations: C/T, ceftolozane-tazobactam; CZA, ceftazidime-avibactam; I/R, imipenem-cilastatin-relebactam.
A retrospective review of the electronic medical record (EMR) was undertaken to gather data on patient demographics, comorbidities, and clinical outcomes. Additionally, information on antimicrobial susceptibility, treatment regimens, and their durations was documented. This study was approved by the University of Miami Institutional Review Board.
Genome sequencing
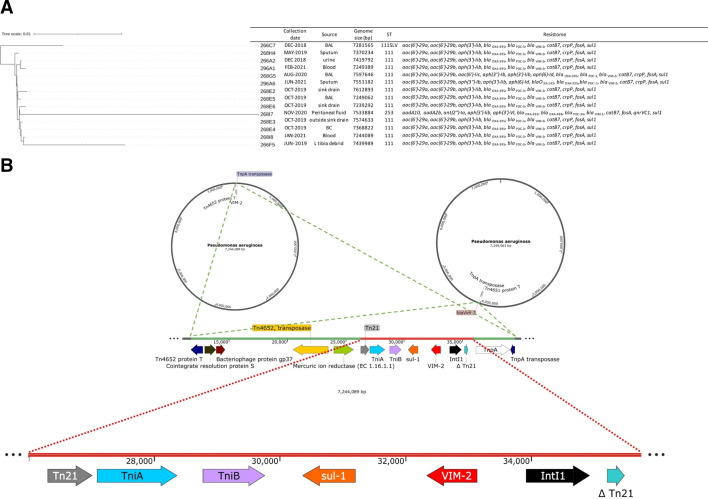
DNA was extracted using the MasterPure Gram Positive DNA purification kit following the manufacturer’s instructions (Epicentre, Madison, WI), libraries were prepared using the Oxford Nanopore Technology’s Rapid Barcoding Kit, and whole genome sequencing was performed in an ONT MinION. The genome was de novo assembled with Flye 2.9, polished with Medaka 1.6, and annotated using the Bacterial and Viral Bioinformatics Resource Center (BV-BRC) and confirmed with the Basic Local Alignment Search Tool (NCBI). Resistome was determined using ResFinder 4.1 from the Center for Genomic Epidemiology (https://www.genomicepidemiology.org/services/) using default parameters (90% threshold for ID, 60% minimum length). Relatedness among isolates was determined using the BV-BRC Bacterial Genome Tree Service (https://www.bvbrc.org/app/PhylogeneticTree) using default parameters and visualized using iTOL (Fig. 2; https://itol.embl.de).
Fig 2.
(A) Tree depicting relatedness among isolates; (B) map depicting genetic context of blaVIM-2 and its position within two representative P. aeruginosa chromosomes.
Infection prevention and control
Enhanced contact precautions were promptly initiated upon VIM-CRPA identification. This included a private room setting, dedicated patient-care equipment, special signage at the room door, environmental cleaning of the rooms with bleach twice daily (Sani-Cloth Bleach), UV light disinfection at terminal cleaning (Helios UVC Disinfection System), personal protective equipment for healthcare personnel, close monitoring of hand hygiene practices and environmental disinfection, patient/family/visitor education, staff cohorting, and tagging of the EMR for enhanced contact precaution requirements. Room door signage included an advisory for “enhanced contact precautions” stating that all persons entering the room must wash hands and wear gloves and a disposable gown. Before exiting the room, they must remove and discard gown/gloves and wash hands once again. Hand hygiene was facilitated by providing alcohol-based hand sanitizers (Purell) in strategic locations. Compliance with hand hygiene was monitored by direct observation using trained observers. In fall 2019, all aerators attached to faucets in the affected unit sinks were removed, the sink P-traps were replaced, and point-of-use sink filters were installed and replaced every 2 months. In collaboration with hospital leadership and environmental services, education regarding horizontal transmission was provided to medical teams and nursing staff.
Surveillance cultures were collected in alignment with established hospital protocols; all international patients and new admissions to critical care units underwent weekly rectal surveillance for MDR Gram-negative bacteria (32). This surveillance incorporated molecular testing for genes associated with carbapenemase production. Patients with VIM-CRPA-positive cultures collected as part of routine clinical management were deemed to be colonized if they had no symptoms of infection as determined by infectious diseases providers. In addition, each time a new hospital-onset (at least 3 days after admission) case was identified, point prevalence surveillance (PPS) for carbapenem-resistant organisms was collected from all patients in the unit hosting the new case. PPS was based on contact tracing investigation; it was collected from rectal swabs and sent for processing initially to the CDC Antimicrobial Resistance Laboratory Network. Beginning in January 2019, they were processed in-house.
Based on literature review and consultation with peers with VIM-CRPA cluster-related experience, environmental cultures from unit sinks were collected to identify if this was a possible source of carbapenemase-producing organisms (26, 27, 33, 34). Two series of environmental cultures were collected from sinks in units hosting patients with VIM-CRPA, one in May 2019 and the other in October 2019. We collected water samples, including from ice machines, as well as swab samples from sink faucets and drains. The water samples were processed by our water management testing contractor. The swab samples from faucets and drains were collected in tryptic soy broth (TSB) with two meropenem disks; the TSBs that were cloudy after 48 hr of incubation were sub-cultured to blood agar plates with a meropenem disk; resulting colonies were identified and tested for carbapenemase genes as described above. Some of these samples were submitted for genome sequencing to determine if there was a correlation with the clinical samples.
RESULTS
During the study period, the microbiology laboratory reported 3,827 isolates of P. aeruginosa; of these, 1,164 (30.42%) were carbapenem-resistant. Infections caused by VIM-CRPA accounted for 37 isolates affecting 21 distinct patients, as detailed in Fig. 3. Nine patients developed multiple VIM-CRPA infections. Three patients were excluded from the analysis: one due to the identification of a VIM-CRPA infection less than 24 hr before death and the other two due to receiving antimicrobial therapy that lacked activity against the blaVIM enzymes. Among the cohort, two distinct patients each underwent two separate hospital admissions, resulting in a total of 23 hospital encounters. Furthermore, 13 VIM-CRPA isolates were detected in 12 distinct patients who were only colonized (not infected), as detailed in Table 2.
Fig 3.
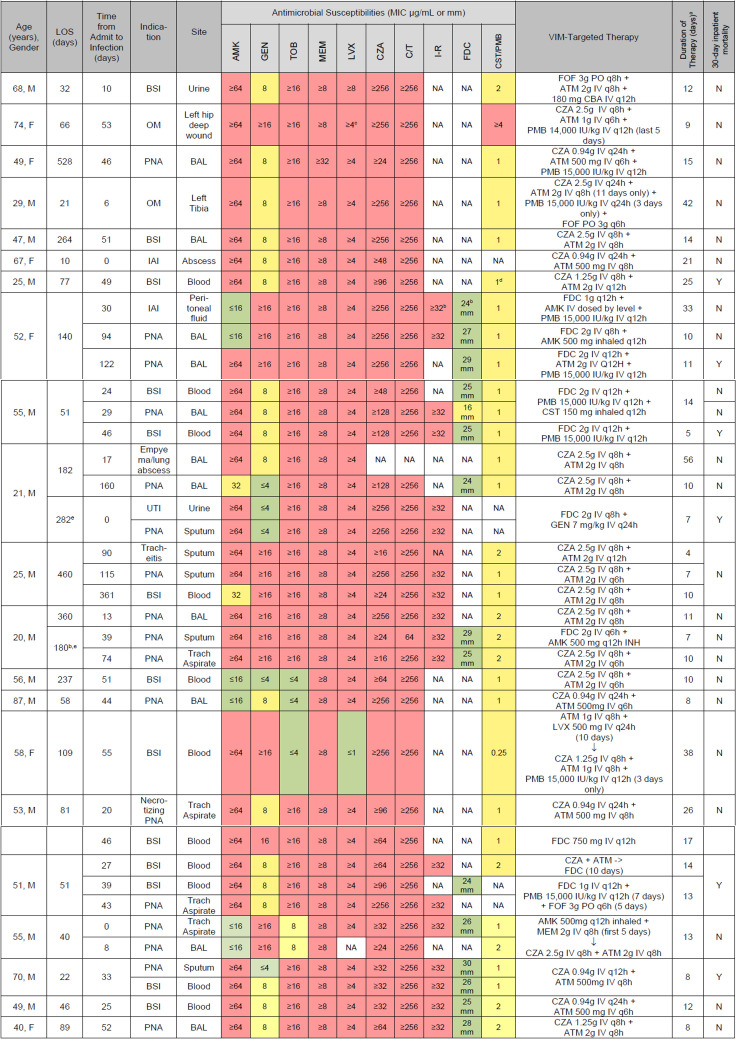
Isolate susceptibilities and targeted VIM therapies. Includes all infectious episodes treated per patient; only the first isolate per infection. For bacteremia cases, the source isolate also listed when applicable. All doses adjusted for renal impairment by our ASP pharmacists. Abbreviations: BAL, bronchoalveolar lavage; DDKT, deceased donor kidney transplant; EOT, end of therapy; ET, endotracheal; inh, inhaled; IU, international units; IV, intravenous; MIC, minimum inhibitory concentration; OM, osteomyelitis; PNA, pneumonia; PO, by mouth drugs; AMK, amikacin; GEN, gentamicin; TOB, tobramycin; MEM, meropenem; LVX, levofloxacin; FOF = fosfomycin. aTotal duration of ATM plus CZA, or FDC backbone; alternative agents used empirically or as adjunction therapy are listed with their total durations in parenthesis. bCefiderocol and imipenem–cilastatin–relebactam approved and first available at our institution. cLevofloxacin breakpoint changed for Pseudomonas. dCLSI breakpoints for polymyxin B changed for Pseudomonas beginning Jan 2020: <2, intermediate; >4, resistant; no susceptible breakpoint. eSecond hospitalization during the study period.
The majority of patients were male (76.2%), and the median age was 52 years. The most common comorbidities were renal failure (n = 9; 42.9%) and hypertension (n = 8; 38.1%). Four patients were solid organ transplant recipients (three kidneys and one multivisceral). More than half of the patients were mechanically ventilated (n = 15; 71.4%) and 47.6% (n = 10) had received renal replacement therapy at the time of infection (Table 1). The most common sites of infection were respiratory (n = 20/39; 51.3%) and bloodstream (n = 13/39; 33.3%) (Fig. 3). Thirteen patients (61.9%) were in the ICU for at least 24 hr prior to index culture collection (Table 1). The mean duration of therapy overall was 12.8 days for the 37 documented infections. Most infections (n = 23; 62.2%) were managed using ATM and CZA, based on previously published series (35). Additionally, 35.1% (n = 13) underwent treatment with a regimen that included FDC (29). Overall, adjunctive agents included polymixin b (n = 8), amikacin (n = 4), gentamicin (n = 1), fosfomycin (n = 3), and colistimethate (n = 2) (Fig. 3).
TABLE 1.
Demographics of patients treated for VIM-Pseudomonas (PsA) infections
| Demographics, N = 21 patients | |
|---|---|
| Male, n (%) | 16 (76.2) |
| Median age, years (IQR)e | 52 (40–58) |
| Comorbidities on admission, n (%) | |
| Hypertension | 8 (38.1) |
| Diabetes | 5 (23.8) |
| Congestive heart failure | 6 (28.6) |
| Chronic respiratory failure | 5 (23.8) |
| Cardiac arrest | 6 (28.6) |
| Renal failure | 9 (42.9) |
| Solid organ transplant | 4 (19.0) |
| Coronary artery disease | 4 (19.0) |
| Anoxic/traumatic brain injury | 5 (23.8) |
| Cerebrovascular accident | 2 (9.5) |
| COVID-19 coinfection | 4 (19.0) |
| Chronic obstructive pulmonary disease | 3 (14.3) |
| Renal replacement therapy at time of culture collection, n (%) | 10 (47.6) |
| Mechanical ventilation at time of index culture, n (%) | 15 (71.4) |
| Tracheostomy at time of index culture, n (%) | 10 (47.6) |
| PEG tube at time of index culture, n (%) | 3 (14.3) |
| ICU for at least 24 hr, n (%) | 13 (61.9) |
| Medical | 4 (19.0) |
| Surgical | 6 (28.6) |
| Trauma | 3 (14.3) |
| Median total length of stay, days (IQR)a | 77 (46–237) |
| Median length of stay to positive culture, days (IQR) | 30 (17–51) |
| Onset of colonization and/or infection | |
| Hospital onsetb | 19 (90.4) |
| Communityc | 1 (4.8) |
| Hospital onset; OSHd | 1 (4.8) |
| Colonized prior to infection, n (%) | 0 (0) |
| Colonized after infection, n (%) | 8 (38.1) |
| Disposition at 30 days from index culture, n (%) | |
| Expired | 6 (28.6) |
| Hospitalized | 8 (38.1) |
| Home | 2 (9.5) |
| Long-term care center | 4 (19.0) |
| Skilled nursing facility | 1 (4.8) |
Includes two different encounters for two patients who were re-admitted and re-infected with VIM-PsA.
VIM isolates found >3 days of hospitalization.
VIM isolates found <3 days of hospitalization.
Sample taken after patient transferred from OSH (outside hospital).
IQR, interquartile range.
The distribution of 21 patients with infections caused by VIM-CRPA reveals that 18 (86%) were admitted directly from their homes or the community, 1 (5%) was from a long-term care facility, and 2 (9%) were transfers from other hospitals. For 19 patients (90.4%), VIM-CRPA isolates were obtained after a minimum of 3 days post-admission, categorizing these as hospital-onset infections (Table 1).
A higher proportion of patients received ATM–ceftazidime/avibactam (n = 14; 66.7%) compared to FDC-backbone therapy (n = 2; 9.5%). Four patients received both ATM–ceftazidime/avibactam and FDC for different infectious episodes (Fig. 3). ATM susceptibility was restored in only 2/10 (20%) of isolates tested, when combined with CZA. Two of the 14 patients (14.2%) treated with only ATM–ceftazidime/avibactam died within 30 days of infection (Fig. 3). FDC was in vitro susceptible for all but one of the isolates tested, according to CLSI breakpoints first published in 2020 (28). The single patient with an FDC-intermediate isolate had been exposed to FDC for 5 days before the index culture. Both patients treated with only FDC-backbone therapy died. The overall 30-day mortality rate was 28.6% (n = 6; Table 1). In four patients, mortality was attributable to septic shock, one resulted from an indeterminate cause of either cardiogenic or septic shock, and another was due to cardiopulmonary arrest secondary to renal failure, which was not attributable to infection. Excluding the two patients that were re-admitted, the median total hospital stay was 66 days, with a median of 30 days leading up to the first confirmed VIM-CRPA culture. Among the 11 patients with VIM-CRPA bacteremia, 10 achieved documented clearance, with one mortality prior to collection of subsequent blood cultures. The median time to microbiological clearance was 2.5 days, though two patients later exhibited recurrent VIM-CRPA bacteremia after initial clearance.
During the study period, 12 patients were colonized with VIM-CRPA but did not develop clinical infection with this organism. Nine (75%) had VIM-CRPA isolates identified after a minimum of 3 days following admission, classifying these as hospital-onset colonizations (Table 2). Positive rectal surveillance cultures were obtained from two distinct patients within the first 2 days of hospital admission.
TABLE 2.
VIM-colonized patients during the study perioda
| Age | Sex | Duration of hospitalization (days) | Time to first positive culture (days) | Source (N = 13) | Discharge disposition |
|---|---|---|---|---|---|
| 52 | M | 13 | 2 | Rectal swab | Home |
| 50 | M | 251 | 30 | Sputum | Home |
| 74 | F | 39 | 39 | Sputum | Deceased |
| 30 | M | 92 | 92 | Urine | Rehab |
| 44 | M | 241 | 110 | Urine | Home |
| 97 | 76 | Jackson–Pratt (JP) drain | Home | ||
| 63 | M | 44 | 1 | Rectal swab | LTAC |
| 38 | M | 152 | 96 | Urine | Home |
| 85 | F | 11 | 1 | Urine | Deceased |
| 31 | M | 230 | 153 | Sputum | Deceased |
| 53 | M | 289 | 62 | Bronchoalveolar lavage | Hospitalized |
| 26 | M | 77 | 74 | Bronchial wash | Deceased |
| 69 | M | 37 | 26 | Bronchial aspirate | LTAC |
Zero colonized patients were immunocompromised [defined as solid organ transplant, active malignancy, or AIDS (acquired immunodeficiency syndrome)] at time of index culture.
None of the water samples collected grew CRPA. In total, 29 swabs were collected from the sinks in the affected units: 13 in May 2019 and 16 during the follow-up in October 2019. None of the 14 swabs collected from the faucets grew CRPA (0%). Nine of fifteen (60%) drain swabs grew CRPA, and only 4/15 (26%) of them had blaVIM detected.
Fourteen isolates (11 clinical and 3 sink cultures) were selected for whole genome sequencing based on clinical discretion. Fourteen of them carried blaVIM-2 (Fig. 2A) chromosomally, flanked by Tn21, TniA, and TniB upstream and recombinase IntI and Tn-3-like transposase downstream (Fig. 2B). They were ≥98.7% similar to each other, while the remaining isolate was 98.7% similar and carried blaVIM-1. Most isolates belonged to ST111 (12/14) and carried genes encoding for several aminoglycoside-modifying enzymes, blaOXA (395/488) catB7, crpP, fosA, and sul1 (Fig. 2A).
DISCUSSION
The incidence of reported VIM-CRPA species in the United States (US) has been historically low. The first case described in the US was a VIM-7-producing strain isolated in a liver transplant patient in 2004 (14). At least 14 other cases have been documented since then, with the largest series originating in 2014 in northeast Ohio (17). In their study, Perez and colleagues documented a 2-year prevalence of carbapenem resistance among P. aeruginosa of 20%; seven (0.9%) isolates were VIM-producing isolates (17). In 2017, a local case series out of Orange County, Florida, was the first to report the identification of VIM-CRPA in the state. After identifying an initial colonized patient and implementing appropriate precautions, six subsequent patients were found to be colonized during a prevalence survey (33). In comparison, our average 3-year prevalence of carbapenem-resistant P. aeruginosa during the study period was 30.4%, and among these, 4.5% were VIM-CRPA (52/1,164 isolates). To our knowledge, this is the largest report to date of VIM-CRPA isolates in a US healthcare system.
The optimal treatment regimen for VIM-CRPA infections remains unknown. The development of novel antibiotics, such as ceftolozane–tazobactam, has allowed us to overcome common resistance mechanisms in DTR PsA (e.g., efflux pump and porin changes); however, there remains a gap in activity against MBLs. Apart from FDC, which has limited clinical data for VIM-CRPA, there are currently no singular treatment options for MBL organisms, leading many to rely on combination therapy (36). At the time of this study, only a single case report had reported the use of ATM–ceftazidime/avibactam for a patient with VIM-CRPA osteomyelitis (35).
We describe our clinical experience treating serious VIM-CRPA infections with either ATM–avibactam or FDC in combination with other agents. A higher proportion of patients were treated with ATM–ceftazidime/avibactam compared to FDC, despite in vitro results demonstrating ATM–ceftazidime/avibactam synergy for only a small number of isolates. Our theoretical approach was to take advantage of ATM’s activity against MBLs while protecting it against Pseudomonas-derived cephalosporinase (PDC) and other β-lactamases with a beta-lactamase inhibitor (avibactam), as has been documented for Enterobacteriales (29, 37). In addition, inhibition of different penicillin-binding proteins by ceftazidime and aztreonam may confer some benefit (29). It remains to be seen whether utilizing ATM–meropenem/vaborbactam or ATM–imipenem/relebactam leads to better outcomes for VIM-CRPA. It is unknown whether the choice of novel beta-lactam/beta-lactamase inhibitor bears any clinical impact on overcoming resistance from co-expressed extended-spectrum beta-lactamases, PDC β-lactamases, and efflux pumps/porin channel mutations in these isolates (38). We did not see excess mortality in patients receiving AZM–ceftazidime/avibactam compared to FDC, although our numbers are too small to draw meaningful conclusions. The IDSA currently recommends FDC as the preferred treatment option for MBL-producing PsA. More studies are needed to compare the efficacy of AZM–ceftazidime/avibactam versus FDC for VIM-CRPA. In addition, future studies may shed light on whether VIM-CRPA are prone to developing FDC resistance while on therapy, as has been documented for carbapenemase-producing Enterobacteriales (39, 40).
Overall, we observed a 30-day mortality rate of 28.6%. Due to the scarcity and heterogeneity of published VIM-CRPA case series, we cannot adequately compare our mortality rate to the literature. A recent case series of 24 patients with VIM-Gram-negative infections found a 30-day mortality rate of 17% (41). However, only two patients in this series had VIM-CRPA isolated; in addition, severity of illness cannot be robustly compared among the two cohorts.
Unique among our population were the four patients with solid organ transplants. One patient was a multivisceral transplant recipient 6.5 months out from transplant at the time of index culture. Other case series have documented VIM-CRPA clusters within various international transplant units (34, 42–44). Similarly to our institution, Ambrogi et al. describe a cluster within a renal transplant unit where contaminated taps were identified as a source of VIM-CRPA isolates. Interventions made included disinfecting and replacing hand wash basin taps (40). By implementing similar interventions in the fall of 2019, such as changing sink P-traps and installing point-of-use sink filters in the ICU environment, we were able to control further spread to vulnerable patients in the following quarter (Fig. 4). However, likely due to the COVID-19 pandemic, we observed an increase in cases as described elsewhere (2).
Fig 4.
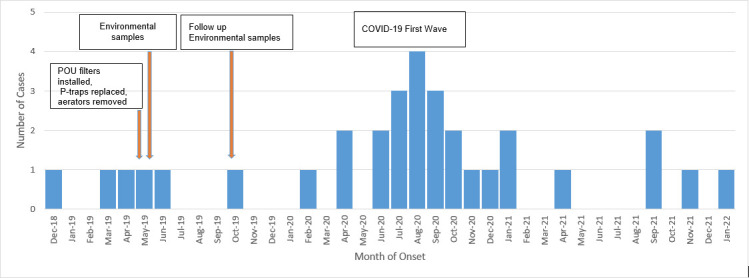
Timeline and epidemiological curve of VIM-CRPA cases by month of onset. Includes only the first isolate identified per patient; POU: point-of-use.
During our outbreak, we identified multifactorial sources including possible horizontal transmission due to breaches in infection control measures and environmental reservoirs. Our findings suggest that while environmental reservoirs were identified, the evidence did not conclusively establish these as the sole source of the outbreak. The genetic differences observed between the clinical and environmental isolates indicate multiple potential sources and transmission routes, rather than a singular point. This investigation highlights the complexity of VIM-CRPA transmission in healthcare settings and underscores the importance of comprehensive approaches that include both clinical diagnostic stewardship and environmental surveillance. Our interventions to stop the spread included provider education and hand hygiene reinforcement, bare below the elbows and removal of all jackets at bedside, and environmental cleaning and disinfection. In addition to erring on the side of caution, we proceeded with changing all the sink P-traps in the involved ICUs and operationalized weekly sink and drain bleach disinfection and placement of point-of-use sink filters in the ICU environment. Routine testing for Pseudomonas in high-risk areas was also incorporated into the water management plan until the outbreak was under control (Fig. 4).
Other risk factors identified in our case series were prolonged length of stay and ICU status. The ICU environment itself is well suited for the development of antibiotic-resistant organisms as invasive medical devices, patients with chronic comorbidities, and prior antibiotic use are but some of the many risk factors for MDR Gram-negative infections (22, 45). Prior work has identified ICU outbreaks due to the patient environment (26, 27). Not surprisingly, the median time-to-positive culture in our cohort was 30 days, and the average total length of stay was 66 days. Most of our patients were in the ICU for at least 24 hours prior to index culture collection. In addition, the most frequent source of infection was from the respiratory tract, which makes sense for our critically ill cohort in which 71.4% of patients were mechanically ventilated and 47.6% had a tracheostomy at the time of index culture. This highlights the importance of hand hygiene, respiratory therapy education, and ventilator-associated pneumonia prevention strategies. Such strategies should include minimizing sedation if possible, elevating the head of the bed 30°–45°, early exercise and mobilization, providing daily oral care with tooth brushing, early enteral rather than parenteral nutrition, and maintaining ventilator circuits clean (46). The use of waterless patient care has also been shown to prevent environmental transmission of VIM-CRPA in ICU patients (5).
With regard to ASP intervention on time to appropriate therapy, we describe our process for timely microbiological identification/susceptibility testing and antimicrobial initiation elsewhere (47). Utilizing this process, we found a significant decrease in median time to appropriate therapy for multidrug-resistant Gram-negative infections overall, over the span of a 2-year period (data not shown). In this cohort, the median time to final susceptibility results (including antimicrobial combination testing) was 140.43 hours [interquartile range (IQR) 100.83–172.3]. The median time to initiation of antimicrobial therapy was lower, at 105.1 hours (IQR 79.85–133.3). Since all antimicrobials used to treat VIM-CRPA are restricted to the ASP at our institution, this speaks to our efforts in prompt initiation of empiric therapy once AST results are available. For the 10 patients that developed bacteremia and had repeat blood cultures, the median time to clearance was 51.28 hours (36.62–73.43).
As addressed above, all isolates sequenced in our study carried the blaVIM-2 gene chromosomally, flanked by Tn21, TniA, and TniB upstream and recombinase IntI and a Tn-3-like transposase downstream. This indicates a complex genomic environment surrounding blaVIM-2, which may have implications for its mobility and transfer mechanisms. However, as all these elements are highly mobile and this particular genomic arrangement is likely the result of multiple recombination events, it is not possible to establish where the blaVIM gene originated from.
Our study is limited by the retrospective nature of chart review, as well as by the sample size. In addition, we are not able to present in vitro antimicrobial combination testing (i.e., synergy) or molecular testing for all isolates. Finally, there is currently no gold standard method for synergy testing of MBL-producing P. aeruginosa. Therefore, there may be limitations to the E-test approach used by our microbiology laboratory. Nevertheless, to the best of our knowledge, this is the largest case series of individual infections caused by VIM-CRPA and the first to describe experience with ATM–ceftazidime/avibactam and FDC as treatment options. Our case series highlights the utility of prompt identification of VIM-CRPA, as well as the subsequent interventions that were made: antimicrobial stewardship for appropriate and timely antibiotic initiation, infection control for cohorting and disinfecting properly, and actions on the hospital ward and environmental level to decrease intra-hospital spread. Antimicrobial combination testing results provided in real time facilitated antimicrobial selection. ATM–ceftazidime/avibactam and FDC combinations should be further explored and compared for VIM-CRPA infections. Our study contributes to the growing understanding of how VIM-CRPA can spread in healthcare environments; we highlight the interventions to timely diagnose and treat these infections and implement the necessary infection control measures to prevent and mitigate outbreaks.
Contributor Information
Ana D. Vega, Email: ana.vega@jhsmiami.org.
Pranita D. Tamma, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA
DATA AVAILABILITY
The sequencing data have been deposited in the GenBank database under BioProject number PRJNA1064149.
REFERENCES
- 1. Araos R, D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species, p 2686–2699. In Mandell, Douglass and Bennett’s principles and practice of infectious diseases [Google Scholar]
- 2. COVID-19: U.S. impact on antimicrobial resistance, special report 2022. 2022. National Center for Emerging and Zoonotic Infectious Diseases [Google Scholar]
- 3. Giani T, Marchese A, Coppo E, Kroumova V, Rossolini GM. 2012. VIM-1-producing Pseudomonas mosselii isolates in Italy, predating known VIM-producing index strains. Antimicrob Agents Chemother 56:2216–2217. doi: 10.1128/AAC.06005-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hishinuma T, Uchida H, Tohya M, Shimojima M, Tada T, Kirikae T. 2020. Emergence and spread of VIM-type metallo-β-lactamase-producing Pseudomonas aeruginosa clinical isolates in Japan. J Glob Antimicrob Resist 23:265–268. doi: 10.1016/j.jgar.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 5. Catho G, Martischang R, Boroli F, Chraïti MN, Martin Y, Koyluk Tomsuk Z, Renzi G, Schrenzel J, Pugin J, Nordmann P, Blanc DS, Harbarth S. 2021. Outbreak of Pseudomonas aeruginosa producing VIM carbapenemase in an intensive care unit and its termination by implementation of waterless patient care. Crit Care 25:301. doi: 10.1186/s13054-021-03726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pérez-vázquez M, Sola-campoy PJ, Zurita ÁM, Ávila A, Gómez-bertomeu F, Solís S, López-urrutia L, Gónzalez-barberá EM, Cercenado E, Bautista V, Lara N, Aracil B, Oliver A, Campos J, Oteo-iglesias J. 2020. Carbapenemase-producing Pseudomonas aeruginosa in Spain: interregional dissemination of the high-risk clones ST175 and ST244 carrying blaVIM-2, blaVIM-1, blaIMP-8, blaVIM-20 and blaKPC-2. Int J Antimicrob Agents 56:106026. doi: 10.1016/j.ijantimicag.2020.106026 [DOI] [PubMed] [Google Scholar]
- 7. Urbanowicz P, Izdebski R, Baraniak A, Żabicka D, Hryniewicz W, Gniadkowski M. 2021. Molecular and genomic epidemiology of VIM/IMP-like metallo-β-lactamase-producing Pseudomonas aeruginosa genotypes in Poland. J Antimicrob Chemother 76:2273–2284. doi: 10.1093/jac/dkab188 [DOI] [PubMed] [Google Scholar]
- 8. De Geyter D, Vanstokstraeten R, Crombé F, Tommassen J, Wybo I, Piérard D. 2021. Sink drains as reservoirs of VIM-2 metallo-β-lactamase-producing Pseudomonas aeruginosa in a Belgian intensive care unit: relation to patients investigated by whole-genome sequencing. J Hosp Infect 115:75–82. doi: 10.1016/j.jhin.2021.05.010 [DOI] [PubMed] [Google Scholar]
- 9. Nguyen LP, Park CS, Pinto NA, Lee H, Seo HS, Vu TN, Mai H, Pham AHT, Jang E, Cho YL, Goglin K, Nguyen K, White R, D’Souza R, Fouts DE, Yong D. 2021. In vitro activity of a novel siderophore-cephalosporin LCB10-0200 (GT-1), and LCB10-0200/avibactam, against carbapenem-resistant Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa strains at a tertiary hospital in Korea. Pharmaceuticals (Basel) 14:370. doi: 10.3390/ph14040370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rada AM, De La Cadena E, Agudelo CA, Pallares C, Restrepo E, Correa A, Villegas MV, Capataz C. 2021. Genetic diversity of multidrug-resistant Pseudomonas aeruginosa isolates carrying bla VIM-2 and bla KPC-2 genes that spread on different genetic environment in Colombia. Front Microbiol 12:663020. doi: 10.3389/fmicb.2021.663020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quinones-Falconi F, Galicia-Velasco M, Marchiaro P, Mussi MA, Ballerini V, Vila AJ, Viale AM, Bermejo-Morales K, Limansky AS. 2010. Emergence of Pseudomonas aeruginosa strains producing metallo-beta-lactamases of the IMP-15 and VIM-2 types in Mexico. Clin Microbiol Infect 16:126–131. doi: 10.1111/j.1469-0691.2009.02780.x [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Carbapenem-resistant Pseudomonas aeruginosa. Available from: https://arpsp.cdc.gov/profile/arln/crpa
- 13. Lolans K, Queenan AM, Bush K, Sahud A, Quinn JP. 2005. First nosocomial outbreak of Pseudomonas aeruginosa producing an integron-borne metallo-beta-lactamase (VIM-2) in the United States. Antimicrob Agents Chemother 49:3538–3540. doi: 10.1128/AAC.49.8.3538-3540.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toleman MA, Rolston K, Jones RN, Walsh TR. 2004. blaVIM-7, an evolutionarily distinct metallo-beta-lactamase gene in a Pseudomonas aeruginosa isolate from the United States. Antimicrob Agents Chemother 48:329–332. doi: 10.1128/AAC.48.1.329-332.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li H, Toleman MA, Bennett PM, Jones RN, Walsh TR. 2008. Complete sequence of p07-406, a 24,179-base-pair plasmid harboring the blaVIM-7 metallo-β-lactamase gene in a Pseudomonas aeruginosa isolate from the United States. Antimicrob Agents Chemother 52:3099–3105. doi: 10.1128/AAC.01093-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aboufaycal H, Sader HS, Rolston K, Deshpande LM, Toleman M, Bodey G, Raad I, Jones RN. 2007. blaVIM-2 and blaVIM-7 carbapenemase-producing Pseudomonas aeruginosa isolates detected in a tertiary care medical center in the United States: report from the MYSTIC program. J Clin Microbiol 45:614–615. doi: 10.1128/JCM.01351-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perez F, Hujer AM, Marshall SH, Ray AJ, Rather PN, Suwantarat N, Dumford D, O’Shea P, Domitrovic TNJ, Salata RA, Chavda KD, Chen L, Kreiswirth BN, Vila AJ, Haussler S, Jacobs MR, Bonomo RA. 2014. Extensively drug-resistant Pseudomonas aeruginosa isolates containing blaVIM-2 and elements of Salmonella genomic island 2: a new genetic resistance determinant in Northeast Ohio. Antimicrob Agents Chemother 58:5929–5935. doi: 10.1128/AAC.02372-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tam VH, Chang K-T, Abdelraouf K, Brioso CG, Ameka M, McCaskey LA, Weston JS, Caeiro J-P, Garey KW. 2010. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 54:1160–1164. doi: 10.1128/AAC.01446-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies TA, Marie Queenan A, Morrow BJ, Shang W, Amsler K, He W, Lynch AS, Pillar C, Flamm RK. 2011. Longitudinal survey of carbapenem resistance and resistance mechanisms in Enterobacteriaceae and non-fermenters from the USA in 2007-09. J Antimicrob Chemother 66:2298–2307. doi: 10.1093/jac/dkr290 [DOI] [PubMed] [Google Scholar]
- 20. Diene SM, Rolain JM. 2014. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin Microbiol Infect 20:831–838. doi: 10.1111/1469-0691.12655 [DOI] [PubMed] [Google Scholar]
- 21. Dias VC, Resende JA, Bastos AN, De Andrade Bastos LQ, De Andrade Bastos VQ, Bastos RV, Diniz CG, Da Silva VL. 2017. Epidemiological, physiological, and molecular characteristics of a Brazilian collection of carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Microb Drug Resist 23:852–863. doi: 10.1089/mdr.2016.0219 [DOI] [PubMed] [Google Scholar]
- 22. Safdar N, Maki DG. 2002. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, Gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 136:834–844. doi: 10.7326/0003-4819-136-11-200206040-00013 [DOI] [PubMed] [Google Scholar]
- 23. Jeong JH, Shin KS, Lee JW, Park EJ, Son SY. 2009. Analysis of a novel class 1 integron containing metallo-beta-lactamase gene VIM-2 in Pseudomonas aeruginosa. J Microbiol 47:753–759. doi: 10.1007/s12275-008-0272-2 [DOI] [PubMed] [Google Scholar]
- 24. Kanamori H, Rutala WA, Weber DJ. 2017. The role of patient care items as a fomite in healthcare-associated outbreaks and infection prevention. Clin Infect Dis 65:1412–1419. doi: 10.1093/cid/cix462 [DOI] [PubMed] [Google Scholar]
- 25. Haun N, Hooper-Lane C, Safdar N. 2016. Healthcare personnel attire and devices as fomites: a systematic review. Infect Control Hosp Epidemiol 37:1367–1373. doi: 10.1017/ice.2016.192 [DOI] [PubMed] [Google Scholar]
- 26. Salm F, Deja M, Gastmeier P, Kola A, Hansen S, Behnke M, Gruhl D, Leistner R. 2016. Prolonged outbreak of clonal MDR Pseudomonas aeruginosa on an intensive care unit: contaminated sinks and contamination of ultra-filtrate bags as possible route of transmission? Antimicrob Resist Infect Control 5:53. doi: 10.1186/s13756-016-0157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knoester M, de Boer MGJ, Maarleveld JJ, Claas ECJ, Bernards AT, de Jonge E, van Dissel JT, Veldkamp KE. 2014. An integrated approach to control a prolonged outbreak of multidrug-resistant Pseudomonas aeruginosa in an intensive care unit. Clin Microbiol Infect 20:207–215. doi: 10.1111/1469-0691.12372 [DOI] [PubMed] [Google Scholar]
- 28. 2022. CLSI M100 ED32:2022 — performance standards for antimicrobial susceptibility testing. 32nd ed [Google Scholar]
- 29. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2023. Infectious diseases society of america antimicrobial-resistant treatment guidance: Gram-negative bacterial infections. Clin Infect Dis 18:ciad428. doi: 10.1093/cid/ciad428 [DOI] [PubMed] [Google Scholar]
- 30. Khan A, Erickson SG, Pettaway C, Arias CA, Miller WR, Bhatti MM. 2021. Evaluation of susceptibility testing methods for aztreonam and ceftazidime-avibactam combination therapy on extensively drug-resistant Gram-negative organisms. Antimicrob Agents Chemother 65:e0084621. doi: 10.1128/AAC.00846-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doern CD. 2014. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 52:4124–4128. doi: 10.1128/JCM.01121-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiménez A, Castro JG, Munoz-Price LS, de Pascale D, Shimose L, Mustapha MM, Spychala CN, Mettus RT, Cooper VS, Doi Y. 2017. Outbreak of Klebsiella pneumoniae carbapenemase-producing Citrobacter freundii at a tertiary acute care facility in Miami, Florida. Infect Control Hosp Epidemiol 38:320–326. doi: 10.1017/ice.2016.273 [DOI] [PubMed] [Google Scholar]
- 33. Rankin D, Caicedo L, Dotson N, Gable P, Chu A. 2018. Notes from the field: verona integron-encoded metallo-beta-lactamase-producing Pseudomonas aeruginosa outbreak in a long-term acute care hospital - Orange County, Florida, 2017. MMWR Morb Mortal Wkly Rep 67:611–612. doi: 10.15585/mmwr.mm6721a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ambrogi V, Cavalié L, Mantion B, Ghiglia M-J, Cointault O, Dubois D, Prère M-F, Levitzki N, Kamar N, Malavaud S. 2016. Transmission of metallo-β-lactamase-producing Pseudomonas aeruginosa in a nephrology-transplant intensive care unit with potential link to the environment. J Hosp Infect 92:27–29. doi: 10.1016/j.jhin.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 35. Mularoni A, Mezzatesta ML, Pilato M, Medaglia AA, Cervo A, Bongiorno D, Aprile A, Luca A, Stefani S, Grossi P. 2021. Combination of aztreonam, ceftazidime-avibactam and amikacin in the treatment of VIM-1 Pseudomonas aeruginosa ST235 osteomyelitis. Int J Infect Dis 108:510–512. doi: 10.1016/j.ijid.2021.05.085 [DOI] [PubMed] [Google Scholar]
- 36. Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, Lodise TP, Naas T, Niki Y, Paterson DL, Portsmouth S, Torre-Cisneros J, Toyoizumi K, Wunderink RG, Nagata TD. 2021. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 21:226–240. doi: 10.1016/S1473-3099(20)30796-9 [DOI] [PubMed] [Google Scholar]
- 37. Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Emeraud C, Escaut L, Boucly A, Fortineau N, Bonnin RA, Naas T, Dortet L. 2019. Aztreonam plus clavulanate, tazobactam, or avibactam for treatment of infections caused by metallo-β-lactamase-producing Gram-negative bacteria. Antimicrob Agents Chemother 63:e00010-19. doi: 10.1128/AAC.00010-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simner PJ, Mostafa HH, Bergman Y, Ante M, Tekle T, Adebayo A, Beisken S, Dzintars K, Tamma PD. 2022. Progressive development of cefiderocol resistance in Escherichia coli during therapy is associated with an increase in blaNDM-5 copy number and gene expression. Clin Infect Dis 75:47–54. doi: 10.1093/cid/ciab888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klein S, Boutin S, Kocer K, Fiedler MO, Störzinger D, Weigand MA, Tan B, Richter D, Rupp C, Mieth M, Mehrabi A, Hackert T, Zimmermann S, Heeg K, Nurjadi D. 2022. Rapid development of cefiderocol resistance in carbapenem-resistant enterobacter cloacae during therapy is associated with heterogeneous mutations in the catecholate siderophore receptor cirA. Clin Infect Dis 74:905–908. doi: 10.1093/cid/ciab511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sempere A, Viñado B, Los-Arcos I, Campany D, Larrosa N, Fernández-Hidalgo N, Rodríguez-Pardo D, González-López JJ, Nuvials X, Almirante B, Escolà-Vergé L. 2022. Ceftazidime-avibactam plus aztreonam for the treatment of infections by VIM-type-producing Gram-negative bacteria. Antimicrob Agents Chemother 66:e0075122. doi: 10.1128/aac.00751-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mlynarczyk G, Sawicka-Grzelak A, Szymanek K, Buczkowska T, Pazik J, Durlik M, Pacholczyk M, Lagiewska B, Chmura A, Paczek L, Mlynarczyk A. 2009. Resistance to carbapenems among Pseudomonas aeruginosa isolated from patients of transplant wards. Transplant Proc 41:3258–3260. doi: 10.1016/j.transproceed.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 43. Hammami S, Boutiba-Ben Boubaker I, Ghozzi R, Saidani M, Amine S, Ben Redjeb S. 2011. Nosocomial outbreak of imipenem-resistant Pseudomonas aeruginosa producing VIM-2 metallo-β-lactamase in a kidney transplantation unit. Diagn Pathol 6:106. doi: 10.1186/1746-1596-6-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kosykowska E, Szymanek-Majchrzak K, Walter de Walthoffen S, Izdebski R, Mlynarczyk A, Ciszek M, Chmura A, Durlik M, Paczek L, Deborska-Materkowska D, Sawicka-Grzelak A, Mlynarczyk G. 2014. Molecular analysis of carbapenem-resistant strains of Pseudomonas aeruginosa isolated from patients hospitalized in various transplantation wards between 2008 and 2011. Transplant Proc 46:2576–2578. doi: 10.1016/j.transproceed.2014.08.027 [DOI] [PubMed] [Google Scholar]
- 45. van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klompas M, Branson R, Cawcutt K, Crist M, Eichenwald EC, Greene LR, Lee G, Maragakis LL, Powell K, Priebe GP, Speck K, Yokoe DS, Berenholtz SM. 2022. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol 43:687–713. doi: 10.1017/ice.2022.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vega AD, Jimenez A, Rosello G, Claeys KC, Martinez OV, De Pascale B, Perez-Cardona A, Abbo L. 2020. Implementing carbapenem-resistance testing algorithms for Enterobacteriales and Pseudomonas aeruginosa: diagnostic and antimicrobial stewardship with timely infection prevention. Diagn Microbiol Infect Dis 97:115069. doi: 10.1016/j.diagmicrobio.2020.115069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing data have been deposited in the GenBank database under BioProject number PRJNA1064149.