ABSTRACT
The prevalence of obesity has increased considerably in the last few decades. Pathophysiological changes in obese patients lead to pharmacokinetic (PK) and pharmacodynamic (PD) alterations that can condition the correct exposure to antimicrobials if standard dosages are used. Inadequate dosing in obese patients can lead to toxicity or therapeutic failure. In recent years, additional antimicrobial PK/PD data, extended infusion strategies, and studies in critically ill patients have made it possible to obtain data to provide a better dosage in obese patients. Despite this, it is usually difficult to find information on drug dosing in this population, which is sometimes contradictory. This is a comprehensive review of the dosing of different types of antimicrobials (antibiotics, antifungals, antivirals, and antituberculosis drugs) in obese patients, where the literature on PK and possible dosing strategies in obese adults was critically assessed.
KEYWORDS: antimicrobial, obesity, weight, dosing, pharmacokinetics, pharmacodynamics
INTRODUCTION
Obesity, classified as a body mass index (BMI) ≥ 30 kg/m2, is now considered to be one of the most important public health problems. According to the World Health Organization (WHO), in 2016, approximately 2 billion adults were overweight, of which 650 million were affected by obesity (1). If current trends continue, it is estimated that by 2025, 2.7 billion adults will be overweight, more than 1 billion people will be affected by obesity, and 177 million adults will be severely affected by obesity (2).
Obesity is related to increased morbidity and mortality in patients with bacteremia, hospital infections, surgical site infections, skin infections, and periodontal infections (3–11). It is also associated with impaired immune function (12–14), with reports of increased risk of death during the H1N1 (15, 16) and SARS-CoV-2 (17–19) pandemics and reduced immune response to vaccines (20–25). Although several hypotheses have been proposed, the exact mechanism by which obesity determines susceptibility to infection is still unclear. First, obesity can lead to disorders of the innate and adaptive immune system, which are characterized by impaired chemotaxis, altered macrophage differentiation, imbalance of cytokine production, and dysregulated crosstalk between the immune system and fat cells (13, 14). Second, due to the respiratory anatomy and physiology related to obesity, this group of patients presents restricted pulmonary function, reduced lung volume, ventilation-perfusion mismatch, obstructive sleep apnea, and a high risk of pulmonary embolism, which translates into a greater predisposition to develop respiratory infections and their derived complications (26). Finally, due to delayed wound healing, disrupted micro- and macrocirculation, and lymphedema, obesity can also increase the risk of surgical infection (27).
The objective of all antimicrobial therapy is to reach adequate systemic antimicrobial concentrations to eradicate the infectious agent while minimizing toxicity and side effects. In obesity, especially in morbid obesity, drug pharmacodynamics (PD) may be altered due to possible changes in its pharmacokinetic (PK) characteristics (28). The management of infections is a special problem in obese patients because available data on the dosage and PK/PD in this population are still scarce and, sometimes, contradictory, especially for morbidly obese patients (BMI ≥ 40 kg/m2). This is partly because obese subjects are often excluded from clinical trials. Although data have been increasing in recent decades, more studies are still necessary to allow clinicians to establish an optimal antimicrobial dosage in this population (29). This review aims to describe the different physiological changes in obesity, provide the latest knowledge on their impact on different PK and/or PD parameters, and compile antimicrobial dosing recommendations for this population. It is the first work to compile data on all groups of antimicrobials: antibiotics, antifungals, antivirals, and antituberculosis drugs.
METHODS AND DESIGN
The EMBASE and PubMed databases were searched from inception to October 2023 using the following terms: “obesity” or “obese” and “anti-infectives” or “antimicrobials” or “pharmacokinetics” or “antibiotics” or “antifungals” or “antivirals” or “antitubercular.” All articles that provided information on the behavior of the drug in obese people or recommendations on its dosage in this population were selected. Information regarding specific antimicrobials was completed by searching for data related to each drug.
BODY SIZE DESCRIPTORS
Body composition changes in obese people, something that must be considered when dosing drugs. Normal-weight people have a total body weight (TBW) comprising lean and adipose weight in an estimated 4:1 ratio, while in obese individuals, the excess adipose weight is accompanied by a 20%–40% increase in lean body weight. This results in a lean:adipose weight ratio of approximately 3:2 (30). Several body descriptors have been used to date.
Body mass index is calculated by dividing the TBW by the square of the height (kg/m2). This is how the WHO stratifies individuals (31, 32). Its main limitation is the inability to distinguish between adipose tissue and lean muscle mass since the same BMI may not correspond to the same degree of adiposity in different individuals. For this reason, BMI has not been widely adopted as a dosing scalar (33).
Body surface area (BSA) is a function of weight and height, which correlates with cardiac output, blood volume, and renal function, calculated through the Dubois and Dubois or Mosteller formulas (Table 1) (34). However, its use is controversial in patients with extreme weights because, like BMI, it does not account for body composition. BSA is widely used in oncology to determine dosages of many anticancer agents; however, its utility in dosing obese patients is still unclear. Many clinicians assign 2 m2 when BSA exceeds this arbitrary cut-off, potentially resulting in sub-therapeutic treatment (35, 36).
TABLE 1.
Equations for body weight descriptorsa
| Body weight descriptor | Equation |
|---|---|
| Body mass index (BMI) | TBW/height (m)2 |
| Body surface area (BSA) | Dubois and Dubois = 0.007184 × TBW (kg)0.425 × height (m)0.725 Mosteller = √ [(height (m) × TBW)/3,600] |
| Ideal body weight (IBW) | Male = 49.9 + 0.89 × [height (cm) − 152.4] Female = 45.4 + 0.89 × [height (cm) − 152.4] |
| Lean body weight (LBW) | Male = (9,270 × TBW)/[6,680 + (216 × BMI)] Female = (9,270 × TBW)/[8,780 + (244 × BMI)] |
| Adjusted body weight (ABW) | IBW + [C x (TBW − IBW)] |
C, drug-specific correction factor (generally 0.3–0.6).
Total body weight refers to the actual weight of the patient (37), which is equivalent to assuming that the drug pharmacokinetics are linearly scalable from normal-weight patients to obese patients. If the dose is increased with weight, the clearance of the drug should also increase, which may not be correct. Toxicities of high doses of antimicrobials are known, such as nephrotoxicity or neurotoxicity; however, it may also be a risk to use dose reductions that lead to infra-therapeutic exposure and therapeutic failure (30, 33).
Ideal body weight (IBW) was developed for insurance purposes, not for drug dosing, and basically describes what weight a person should have to have the lowest mortality (38). It is only a function of height and sex, without regard to body composition. Using the IBW, all patients of the same height and sex will receive the same dose, which usually leads to underdosing. This parameter is calculated using the Devine equation (Table 1) (39).
Lean body weight (LBW) and fat-free mass describe body weight devoid of adipose tissue, while fat-free mass refers to certain body tissues (muscle, bone, organs, and extracellular fluid), which is usually measured by bioelectric impedance analysis or estimated by an equation. Several studies have suggested that LBW appears to be the best metric to describe the clearance of drugs in the obese population (40). The formula described by Janmahasatian et al. is the most commonly used (Table 1) (41).
Adjusted body weight (ABW) was developed to account for adipose tissue, which does not affect drug clearance. This concept attempts to overcome the limitation of IBW by adding a certain percentage of the difference between TBW and IBW to IBW for dosing purposes (33). The equation that describes ABW contains a correction factor (C), which is commonly between 0.3 and 0.6 (Table 1).
PHARMACOKINETICS IN OBESE PATIENTS
Several physiological changes happen in obese patients that may affect serum antimicrobial concentrations and should be considered when prescribing in this population. These alterations can affect the different stages a drug goes through from its administration until a therapeutic response takes place and is finally eliminated.
Absorption
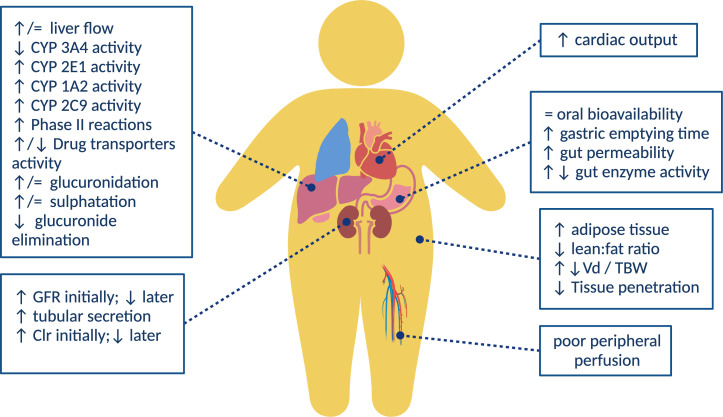
Oral drug absorption is a complex process affected by several factors, such as formulation, physiochemical properties, the physiology of the gastrointestinal tract, food intake, concomitant drugs, and environmental exposure to other xenobiotics, among others (42, 43). On the one hand, obese patients have been shown to have delayed gastric emptying, possibly because of a higher fat diet or gastric distension, which may result in a lower maximum drug concentration (Cmax) or reduced absorption (44, 45) (Fig. 1). On the other hand, intramuscular injections may inadvertently be administered deep subcutaneously, which could affect the absorption efficiency (37). The current limited data show that it is not clear what role these factors may play in the drug absorption of obese patients (40).
Fig 1.
Physiological changes in obesity and its corresponding influence on PK parameters. Image was created with BioRender.com.
Volume of distribution
The volume of distribution (Vd) is affected by the drug’s physiochemical properties and its transfer from the blood to the tissue, its ability to pass through membranes, binding within tissue and blood, and its distribution into fat (33, 42). In obese patients, an augmented Vd is commonly observed due to increased fat and lean muscle mass. Even so, if the drug does not enter the adipose tissue, the Vd may be overestimated based on the TBW, for example, with hydrophilic drugs. ABW may be more appropriate when calculating weight-based loading doses in these cases (46). On the contrary, if the absolute Vd increases, but the Vd/TBW ratio of obese patients and non-obese patients is similar, it indicates that the drug is significantly distributed in the excess body weight (mainly adipose tissue), and then TBW is more suitable for calculation (33). In short, the increase in adipose tissue may increase the Vd of lipophilic agents, while increased lean body mass (which may account for 20%–40% of an overweight individual) and increased blood volume may increase the Vd of hydrophilic drugs (47). Vd is particularly important in surgical prophylaxis where high skin/adipose concentrations are necessary for the duration of surgery, with perfusion in these tissues being low. The local blood flow into the adipose tissue is estimated to account for only 5% of the cardiac output, while the lean tissue receives about 73% of the cardiac output (48). Therefore, obese individuals may have poor peripheral perfusion, resulting in lower concentrations of antimicrobial drugs in subcutaneous adipose tissue (49) (Fig. 1).
Protein binding
Obesity does not significantly alter the albumin binding of drugs, but plasma concentrations of α1-acid glycoprotein and free fatty acids are increased in obese patients, so changes in the binding of antimicrobial drugs to proteins can increase or decrease their Vd (50–52). There is a positive correlation between the level of acid glycoprotein and the protein binding of vancomycin, but the clinical relevance is still unclear (53, 54). In addition, high concentrations of free fatty acids are associated with a significant decrease in the protein binding of cefamandole, dicloxacillin, and sulfamethoxazole, but they are also related to an increase in the protein binding of benzylpenicillin, cephalothin, and cefoxitin (51). The impact of these data on changing the dosing regimen remains unclear.
Clearance
Clearance (Cl) is the PK parameter with the greatest impact on clinical applications, with a direct implication in maintenance doses. Unlike Vd, the physical and chemical properties of the drug have almost no effect on Cl, because this parameter is mainly controlled by physiology. For any organ, Cl can be defined as the volume of plasma that completely removes the drug in a given time, depending on the blood flow to the organ and the organ’s ability to extract the drug (33).
Obesity is related to increased liver volume due to fatty infiltration but without increased metabolic capacity, leading to the risk of steatosis, hepatitis, and fibrosis, altering hepatic blood flow, and affecting the hepatic Cl. Among the cytochrome P450 enzymes related to phase I oxidative metabolism, CYP2E1 and possibly CYP1A2 and CYP2C9 levels are elevated, while CYP3A4 levels are low (29, 55). Regarding CYP2C19 and CYP2D6, there are no conclusive data (56) (Fig. 1). There is limited information about increased phase II combined metabolism involving glucuronidation and sulfation (37).
The effect of obesity on renal clearance (Clr) is unclear. The increased organ mass during obesity may increase kidney functionality and renal blood flow in obese patients, which may affect the elimination rate (k), although organ size or weight may not fully reflect its function (Fig. 1). Obesity predisposes individuals to hypertension and diabetes, making it difficult to assess the effects on glomerular filtration rate (GFR) (57). In addition, Cl estimations are affected by the weight used, and weight-normalized Cl usually has a better correlation with the modified weight, such as LBW instead of TBW (46, 47, 58). Currently, there is no single, well-validated weight descriptor to characterize drug Cl in obese individuals.
REVIEW OF SPECIFIC ANTIMICROBIAL AGENTS
According to the relationship between drug exposure, pathogen susceptibility, and clinical response to antimicrobial treatment, antimicrobial drugs can be divided into three PK/PD categories (59–61). Time-dependent antimicrobials are those whose effect is best described by the percentage of time that the free concentration of the drug remains above the minimum inhibitory concentration (MIC) throughout the dosing interval (fT > MIC). Concentration-dependent antimicrobials achieve an increasing killing effect with increased serum concentration of the drug (Cmax/MIC). These drugs are dosed to achieve maximum safe concentrations at the infection site for optimal bactericidal activity. Finally, in the case of concentration-dependent with time-dependent antimicrobials, the efficacy depends on the total drug concentration achieved over 24 h above the MIC of the microorganism [area under the plasma concentration–time curve over 24 h (AUC0-24)/MIC]. The available evidence on the dosing of antimicrobials in obese patients is detailed below by pharmacological groups and subgroups. Recommendations are summarized in Table 2.
TABLE 2.
Recommended antimicrobial dosing in obese patients (assuming normal renal function)a
| Antimicrobials | Recommended setting | Dose | TDM | Remarks | References |
|---|---|---|---|---|---|
| Antibiotics | |||||
| Aminoglycosides | |||||
| Amikacin | ABW (C = 0.4) for the initial dose. TDM to guide following doses. | 15–20 mg/kg/24 h IV | Always recommended | – | (62–64) |
| Gentamicin | 5–7 mg/kg/24 h IV | (65) | |||
| Tobramycin | 5–7 mg/kg/24 h IV | (62–64) | |||
| β-lactams | |||||
| Ampicillin | Maximum dose In case of sepsis or severe infection, loading doses followed by an extended/continuous infusion are recommended to maximize clinical benefit. |
2 g/4 h IV | Recommended if possible | – | (66, 67) |
| Cloxacillin | 2 g/4 h IV | (66, 67) | |||
| Amoxicillin/clavulanic | No data available. Usual maximum dose: IV: 2 g/200 mg/8 h OR: 875 mg/125 mg/8 h |
(66–68) | |||
| Aztreonam | 2 g/6–8 h IV | (66, 67) | |||
| Cefazolin | 2 g/8 h in continuous infusion or 1.5–2 g/6 h intermittent infusion IV | Surgical prophylaxis: 2 g single dose If >120 kg use 3 g |
(66, 67, 69, 70) | ||
| Cefuroxime | 1.5 g/6 h IV | – | (66, 67) | ||
| Ceftriaxone | 2 g/12 h IV | (66, 67) | |||
| Cefotaxime | Maximum doses as indicated up to a maximum of 2 g/4 h IV |
(66, 67) | |||
| Ceftazidime | 2 g/8 h IV | (66, 67) | |||
| Cefepime | 2 g/8 h IV | (66, 67) | |||
| Ceftaroline | 600 mg/12 h IV | (66, 67, 71) | |||
| Ceftazidime/avibactam | 2 g/0.5 g every 8 h IV | (66, 67) | |||
| Ceftolozane/tazobactam | 1 g/0.5 g every 8 h IV Nosocomial pneumonia: 2 g/1 g every 8 h IV |
(66, 67) | |||
| Piperacillin/tazobactam | 4 g/0.5 g every 6 h IV | (66, 67, 72) | |||
| Cefiderocol | 2 g every 6–8 h IV | If CrCl ≥120 mL/min use 2 g/6 h | (66, 67, 73) | ||
| Ertapenem | 1 g/24 h IV | Consider 2 g/24 h if MIC >0.25–0.5 mg/mL | (66, 67, 74, 75) | ||
| Imipenem/cilastatin | 1 g/6 h IV | Meropenem is recommended for seizure risk |
(66, 67) | ||
| Meropenem | 2 g/8 h IV | – | (66, 67, 76) | ||
| Meropenem/vaborbactam | 4 g/ 8 h IV | – | (66, 67) | ||
| Cotrimoxazole | |||||
| Trimethoprim/sulfamethoxazole | ABW (C = 0.4) (limited evidence) |
8–20 mg TMP/kg/day IV divided into several doses |
Not recommended | Oral route <5 mg/kg/day suboptimal in obese |
(52, 77, 78) |
| Fluoroquinolones | |||||
| Ciprofloxacin | Maximum dose. Some authors recommend dosing according to ABW (C = 0.45) |
400 mg/8 h IV 750 mg/12 h OR |
Not recommended | – | (79–81) |
| Levofloxacin | Maximum dose. Some authors recommend dosing by IBW |
750 mg/24 h OR-IV | Up to 1,000 mg/24 h if CrCl (IBW) >110 mL/min in G infections | (82, 83) | |
| Phosphonic acid derivatives | |||||
| Fosfomycin trometamol | TBW | 3 g orally in a single dose 2 g orally every 48 h, two doses |
Not recommended | – | (84–87) |
| Disodium fosfomycin | 100–300 mg/kg/day IV divided into three to four doses (maximum dose 24 g/24 h) | Maximum dose: 8 g | |||
| Glycopeptides | |||||
| Vancomycin | TBW | Loading dose: 20–25 mg/kg (max. 3 g) IV. Maintenance: 15–20 mg/kg/8–12 h (max. 4.5 g/24 h) IV dose 10–12.5 mg/kg/12 h if BMI ≥ 40 kg/m² |
Always recommended | – | (88–91) |
| Teicoplanin | Loading dose: 12 mg/kg/12 h IV three doses Maintenance: 6–12 mg/kg/24 h IV |
In deep infections, the use of 15 mg/kg has been described |
(10, 92–94) | ||
| Lincosamides | |||||
| Clindamycin | Unknown | 900 mg/8 h IV | Not recommended | – | (95, 96) |
| Lipopeptides | |||||
| Daptomycin | If BMI ≥ 30 kg/m² dose according to ABW (C = 0.4) | 6–10 mg/kg/24 h IV | Not recommended | – | (97–99) |
| Lipoglycopeptides | |||||
| Dalbavancin | Standard dosage | 1,500 mg single dose IV | Not recommended | Alternative: 1,000 mg + 500 mg 1 week later |
(100, 101) |
| Oritavancin | 1,200 mg in 3 h single dose IV | (102) | |||
| Macrolides | |||||
| Azithromycin | Unknown | No data available. Usual maximum dose: 500 mg/24 h OR-IV |
Not recommended | – | (103) |
| Clarithromycin | Eradication of Helicobacter pylori: 500 mg/8 h OR-IV |
||||
| Erythromycin | 1 g/6 h IV | ||||
| Nitroimidazole | |||||
| Metronidazole | TBW | Loading dose: 15 mg/kg (max. 1 g) OR-IV Maintenance: 7.5 mg/kg (max. 500 mg) every 6–8 h OR-IV |
Not recommended | – | (104–106) |
| Oxazolidinone | |||||
| Linezolid | Standard dosage | 600 mg/8–12 h OR-IV | Recommended if possible | If decreasing age, eGFR > 60 mL/min/1.73 m2, MIC ≥ 2mg/L is recommended to use 600 mg c/8 h concomitant with TDM. |
(107, 108) |
| Polymyxins | |||||
| Sodium colistimethate (1 million IU = 30 mg colistin base) |
IBW | Loading dose (sepsis, severe infection): Weight > 50 kg: 9 MUI; weight ≤ 50 kg: 6 MUI IV Maintenance dose: 4.5 MUI c/12 h (2.5–5 mg colistin base/kg/24 h divided into two doses) IV |
Not recommended | Maximum dose: 360 mg colistin base /24 h | (109, 110) |
| Tetracyclines | |||||
| Doxycycline | Standard dosage | Loading dose: 200 mg OR-IV Maintenance: 100–200 mg/24 h OR-IV |
Not recommended | Max. 300 mg/24 h OR-IV | (111) |
| Tigecycline | Loading dose: 100 mg IV Maintenance: 50 mg/12 h IV |
If infection with carbapenemase- producing bacteria: Loading dose: 200 mg IV Maintenance: 100 mg IV/12 h |
(112–118) | ||
| Antifungals | |||||
| Echinocandins | |||||
| Anidulafungin | Standard dosage | Loading dose: 200 mg/24 h IV one dose Maintenance: 100 mg/24 h IV |
Not recommended | – | (119–128) |
| Caspofungin | 70 mg/24 h IV | (129–135) | |||
| Micafungin |
Candida albicans: 200 mg/24 h IV (115–185 kg) 150 mg/24 h IV (<115 kg) Candida glabrata: 200 mg/24 h IV (<115 kg) |
Max. 200 mg/24 h. Higher doses do not provide benefits. |
(136–144) | ||
| Azole derivatives | |||||
| Fluconazole | TBW | 6–12 mg/kg/24 h IV | Not recommended | Maximum dose described: 1,600 mg/day |
(145–147) |
| Isavuconazole | Standard dosage | Loading dose: isavuconazole base 200 mg/8 h six doses OR-IV Maintenance: isavuconazole base 200 mg/24 h OR-IV |
– | (148–152) | |
| Itraconazole | 200 mg/12 h OR | – | (153, 154) | ||
| Posaconazole | Maximum dose | Loading dose: 300 mg OR-IV/12 h, two doses Maintenance: 300 mg OR-IV/24 h |
If >120 kg, there may be a lower plasma exposure |
(155–177) | |
| Voriconazole | OR: Standard dosage IV: ABW (C = 0.4) |
OR: Loading dose 400 mg/12 h, two doses Maintenance: 200 mg/12 h IV: Loading dose 6 mg/kg/12 h, two doses Maintenance: 4 mg/kg/12 h |
Always recommended | – | (178–183) |
| Polyene | |||||
| Liposomal amphotericin B | TBW | 3–5 mg/kg/24 h IV | Not recommended | Maximum recommended per dose: 500 mg | (52, 153, 184–187) |
| Nucleoside analog | |||||
| Flucytosine | IBW | 25–40 mg/kg/6 h OR-IV | Not recommended | – | (52, 188) |
| Antiviral agents | |||||
| Acyclovir | ABW (C = 0.4) |
10–15 mg/kg/8 h IV | Not recommended | OR: not specified | (189–194) |
| Cidofovir | ABW (C = 0.4) No data available, extrapolation from acyclovir data |
Loading dose: 5 mg/kg/7 days IV, two doses Maintenance: 3–5 mg/kg/14 days IV |
– | (52) | |
| Foscarnet | Consult dosage according to indication | – | (52, 195) | ||
| Ganciclovir | Treatment: 5 mg/kg/12 h IV Prophylaxis: 5 mg/kg/24 h IV |
– | (196) | ||
| Valganciclovir | Standard dosage | Treatment: 900 mg/12 h OR Prophylaxis: 900 mg/24 h OR |
– | (197) | |
| Oseltamivir | 75 mg/12 h OR | – | (198–200) | ||
| Antitubercular agents | |||||
| Ethambutol | IBW | 15–25 mg/kg OR-IV/24 h (max. 2.5 g/24 h) |
Not recommended | Dosing on a TBW basis may increase toxicity | (201–204) |
| Isoniazid | 5 mg/kg OR-IV/24 h (max. 300 mg OR-IV/24 h) |
(204) | |||
| Pyrazinamide | 20–30 mg/kg/24 h (max. 2,000 mg OR/24 h) |
(204) | |||
| Rifampin | Standard dosage | 600 mg OR-IV/12 h Tuberculosis: 600 mg OR-IV/24 h |
(204, 205) | ||
C, drug-specific correction factor; IV, intravenous; OR, oral; and TDM, therapeutic drug monitoring.
Antibiotics
Aminoglycosides
Aminoglycosides are hydrophilic antimicrobials with low Vd and whose elimination is mostly renal and proportional to glomerular filtration. They are concentration-dependent agents whose PK/PD target is Cmax/MIC. In obese patients, Vd of hydrophilic agents is increased, and renal function is probably higher than in normal-weight population due to larger kidney size (206). These two factors will probably generate a decrease in the plasmatic antibiotic concentrations. However, since aminoglycoside doses are based on weight, using TBW would overestimate the dose, so the use of ABW is recommended with a 0.4 correction factor since it is estimated that 40% of the dose is distributed to adipose tissue (62, 63). The current recommendation is to use ABW for the calculation of the initial dose and guide the following doses with therapeutic drug monitoring (TDM). It is recommended to adjust the dose according to renal function, but it is not clear which equation for calculating renal clearance is the best. Some authors prioritize the use of the MDRD and CK-EPI or Salazar-Corcoran over Cockroft-Gault (CG) (62, 64, 65), but the evidence is not high, so CG continues to be widely used in clinical practice.
β-lactams
β-lactams are time-dependent antibiotics with renal elimination, and their target PK/PD is 100%ft > (MIC or 4×MIC in critically ill patients). There is much evidence of the high interindividual variability of β-lactams’ PK (207, 208). In obese patients, the increase in lean mass and the increase in Clr imply an increase in this variability, especially due to their hydrophilic nature (66). Different pharmacokinetic studies suggest a need for an increased dose of β-lactams in obese patients, mainly due to an increase in the Vd and inadequate tissue penetration, although evidence regarding decreased penetration in this population is still limited (67, 68, 72, 74, 75). However, many studies have failed to demonstrate the need for a dose increase in this population, obtaining similar results in obese and non-obese patients with the same dosing (71, 76, 209). As a general recommendation, it is suggested to start treatment with β-lactams at higher doses in obese patients with complicated infections, infections caused by microorganisms with high MICs, critically ill patients, or those whose Clr is increased [creatinine clearance (CrCl) > 100 mL/min]. It is important to consider the source of infection, with bone infections being the most difficult to treat in obese patients and requiring an increase in the dose (69).
A highly recommended way to intensify β-lactam treatment is to reduce the frequency of administration or to use extended or continuous infusions (207). In all these cases, it is recommended to perform TDM and to adjust future doses with the aim of reaching target concentrations but avoiding potentially toxic effects (208, 210).
Cotrimoxazole (sulfamethoxazole/trimethoprim)
Sulfamethoxazole/trimethoprim is lipophilic with a protein bound of 70% for sulfamethoxazole and 44% for trimethoprim and excreted primarily through the kidneys. The target PK/PD is not well defined, although the most used are Cmax and AUC0-24/MIC. Sulfamethoxazole/trimethoprim is usually dosed by weight with little evidence on the impact of overweight on its pharmacokinetics. The usual recommendation is to use AWB with a 0.4 correction factor (52). However, this recommendation is based on PK changes of sulfisoxazole (another sulfonamide antibiotic with similar PK properties to sulfamethoxazole) in morbidly obese patients undergoing intestinal bypass surgery (77). In this study, no differences in PK parameters were observed in obese patients, suggesting that an obese individual may require a dose like a normal-sized individual. In a more recent study, in which cotrimoxazole concentration was measured in patients with different weights, a reduction in drug concentration was observed using the same dose in obese patients, suggesting that higher doses should be used in overweight patients (78). Since no clinical data are available to support a reference dosing strategy, appropriate dosing should be determined on a case-by-case basis, monitoring for signs of clinical improvement and drug toxicity.
Fluoroquinolones
Fluoroquinolones are hydrophilic molecules, except levofloxacin, which has an intermediate lipophilic character (211). Clinical efficacy is related with AUC0-24/MIC or Cmax/MIC. There is little evidence on the dosing of fluoroquinolones such as levofloxacin or ciprofloxacin in obese patients. Data regarding the effects of obesity on Vd and Cl for ciprofloxacin are conflicting (79). However, the need to give higher doses in obese patients to ensure target tissue concentrations has been observed, suggesting dosing by ABW (using a correction factor of 0.45) (80). A recent study of PK changes of ciprofloxacin in obese patients showed no differences, recommending not to adjust routinely and assessing that higher doses may be necessary in difficult-to-access infections (81). A PK study conducted in obese patients treated with levofloxacin failed to demonstrate an increase in the Cl of levofloxacin in overweight patients, although a dose adjustment is suggested based on the CrCl estimated by the CG equation and IBW. In this work, the need to increase the dose to 1,000 mg every 24 h is recommended for patients with CrCl > 110 mL/min in Gram-negative infections (82). Some case series suggest the use of higher doses (1,000 mg every 12 h) in obese patients to achieve therapeutic objectives (83). Limited PK data for moxifloxacin suggest that adjustment for obesity is not necessary (212). Data for delafloxacin are limited to skin infections, although the data also suggest that no dose adjustment is necessary (213, 214).
Phosphonic acid derivatives
Fosfomycin is a small, hydrophilic antibiotic that has total renal elimination (84). Information on the pharmacokinetics of fosfomycin is scarce, both in obese and normal-weight patients, and the PK/PD target is still not well defined (85, 86). In a PK study conducted on obese versus non-obese patients treated with intravenous fosfomycin, a decrease in Cmax, a decrease in AUC, and an increase in Vd were observed in obese patients. In addition, a decrease in tissue penetration was observed in the group of obese patients (84). However, it has not been possible to demonstrate the need to increase the dose of fosfomycin due to the condition of obese patients, although increasing the dose is suggested in obese patients with infections caused by pathogens with high MICs or with glomerular hyperfiltration (87).
Glycopeptides
Glycopeptides are hydrophilic agents predominantly cleared by renal elimination, whose PK/PD target is AUC0-24/MIC (208). For vancomycin dosage, clinical guidelines recommend empiric dosing based on TBW and TDM at trough concentrations, which may not be optimal for obese and severely obese patients because an increase in the Vd of vancomycin with weight has been widely described (215, 216). However, the relationship between Vd and weight is not proportional. In addition, Cl is also increased in obese patients due to increased kidney size and increased blood flow. Some studies suggest a higher Cl in obese adolescent patients compared to adults, which is also higher in males than females, so higher doses could be necessary in this subgroup of patients (88). Alternative strategies were tested in some studies using ABW, obtaining promising results (89). However, the last vancomycin’s guidelines recommend using actual body weight-based loading doses of 20–25 mg/kg of body weight (using TBW), with consideration of capping doses at 3 g in obese patients with serious infections, followed by maintenance doses calculated by 15–20 mg/kg of body weight every 8–12 h (maximum 4.5 g/day) (90, 91).
Teicoplanin is dosed by weight in all indications. Generally, the use of maximum doses of teicoplanin is recommended in obese patients. Several dosing regimens have been published depending on the objective target and the type of infection (92). Currently, the most accepted dosage is three doses of 12 mg/kg of body weight every 12 h and a maintenance dose of 6–12 mg/kg of body weight per day, using TBW (10, 93). Some guidelines recommend TDM to guide maintenance dosing in obese patients (94).
Lincosamides
Clindamycin is a lipophilic molecule with mainly biliary elimination and high binding to plasma proteins (60%–90%). PK/PD target is fT > CMI (percentage of optimal time not established). There are no PK studies performed in adult obese patients. Clinical guidelines recommend the use of maximum doses in bone and skin and soft tissue infections (95). In a study conducted on obese children, dosing by weight was compared using TBW, normal fat mass , free fat mass, and LBW, supporting the dosing based on TBW for obese and nonobese children (96).
Lipopeptides
Daptomycin is a hydrophilic antibiotic with high protein binding (92%) and renal elimination (78%). The PK/PD target is AUC0-24/MIC (97). The use of TBW, lean body mass, and ABW has been evaluated without reaching a clear conclusion (98). Obese patients treated with daptomycin (TBW) were found to have higher rates of creatine phosphokinase elevations and treatment discontinuation in a multicenter study compared to published data for primarily normal-weight patients. ABW may be used in place of TBW with concerns for toxicity, especially in the setting of indications for high-dose daptomycin (99).
Lipoglycopeptides
Dalbavancin is an antibiotic with an extended elimination half-life because of its exceptionally high protein binding (≥93%) and widespread tissue distribution. The PK/PD index established for dalbavancin is the free area under the dalbavancin concentration–time curve over minimum inhibitory concentration (AUC0-24/MIC). The usual posology of dalbavancin is a once-weekly infusion (1,500 mg as a single dose or 1,000 mg on day 0 followed by 500 mg on day 7) (100). The effect of obesity was studied in a subgroup analysis of a phase III clinical trial, and it was demonstrated that no changes are needed (101). In a retrospective registry study in patients treated with oritavancin (median BMI 31.4 kg/m2), a high clinical success rate of 88.1% was achieved in skin and soft-tissue infections and complicated Gram-positive infections (102). No subgroup analysis was performed in obese patients, so different dosage recommendations cannot be made for this population.
Macrolides
Macrolides are lipophilic antibiotics whose PK/PD target is AUC0-24/MIC. Dosing strategies for macrolides such as azithromycin, clarithromycin, or erythromycin have not been studied in the obese population. However, some studies revealed good treatment results in the cohort of obese patients with treatment at standard doses (103).
Nitroimidazole
Metronidazole is a concentration-dependent antibiotic whose PK/PD target is AUC0-24/MIC. There is not much evidence on the need for dose adjustment of metronidazole in obese patients. In some studies, a decrease in plasma concentration and an increase in Vd were observed in obese patients, leading to failure to reach the PK/PD target in these patients (104). However, clinical guidelines currently state that there is insufficient data to support the use of higher doses in obese patients to date (105, 106).
Oxazolidinone
Linezolid is a time-dependent antibiotic, and the goal of effectiveness is AUC0-24/MIC or Cmin. Vd and CL could be increased in overweight and obese patients. For this reason, it seems to have a higher risk of treatment failure using the usual doses. The risk is higher with decreasing age, eGFR ≥ 60 mL/min/1.73 m2 and MIC values ≥ 2 mg/L. In these cases, higher doses of linezolid such as 600 mg every 8 h, concomitant with TDM, may be considered (107, 108).
Polymyxins
Colistin methanesulfonate is the prodrug of colistin (polymyxin E) with a rapid and concentration-dependent bactericidal effect. The PK/PD index that best describes its efficacy is AUC0–24/MIC. There are limited data comparing the pharmacokinetics of colistin in obese and normal-weight patients. However, some studies suggest that there is a significant risk of nephrotoxicity with colistin in obese patients, so dosing based on IBW may be recommended, with a maximum daily dose of 360 mg of colistin base or 12 MU of colistimethate (109, 110).
Tetracyclines
Tetracyclines are highly lipophilic antibiotics, so changes in their disposition are expected at extremely high body weights. Nevertheless, there are limited data evaluating their clinical outcomes in obese patients. The PK/PD target in this group is AUC0-24/MIC (112, 113). The recommended dose for doxycycline in obese patients does not vary from that used in patients with normal weight (111). Tigecycline has a large Vd (approximately 7–10 L/kg), resulting in widespread tissue distribution and very low plasma drug concentrations (112). In addition, tigecycline exhibits non-linear plasma protein binding over therapeutic drug concentrations, being the major route of elimination for its unchanged excretion in the feces, which accounts for 59% of the dose (114). Tigecycline is an antibiotic with bacteriostatic and post-antibiotic activity. In obese patients, standard doses are recommended (an initial loading dose of 100 mg followed by a maintenance dose of 50 mg/12 h) (115). However, for resistant Gram-negative bacteria, higher doses may be considered regardless of the patient’s weight (200 mg loading dose, followed by 100 mg every 12 h) (116–118).
Antifungals
Echinocandins
They bind strongly to proteins (97%–99%), leading to lower drug concentrations in serum and tissues (217). They are concentration-dependent antifungals whose PK/PD index is associated with their AUC0-24/MIC ratio (218). Accumulating evidence suggests that obese patients treated with echinocandins have reduced exposure due to changes in pharmacokinetics (219). A recent systematic review carried out by Alsowaida et al. (220) has collected all the studies published to date on the exposure of obese patients to echinocandins. Out of a total of 25 studies, 17 studies reported lower echinocandin exposures in overweight and obese subjects compared to normal-weight subjects based on PK/PD targets (119–124, 129–133, 136–142). In contrast, eight studies have also been published that endorse no differences in echinocandin exposure in overweight and obese subjects compared with normal-weight subjects (125–128, 134, 135, 143, 144). Therefore, and due to the lack of high-quality evidence, consensus on recommendations for echinocandins in obese patients is the use of standard doses (220).
Azole derivatives
Azole derivatives are weak bases, mainly lipophilic, with limited water solubility, apart from fluconazole, which is more hydrophilic than other antifungal azoles (145). Regarding their PK/PD index, AUC0-24/MIC correlates with effectiveness against invasive fungal infections (221). Itraconazole, voriconazole, and posaconazole require solubilizers to prepare oral solutions and intravenous dosage forms due to their lipophilicity (222–226). Fluconazole differs from these drugs by its high bioavailability, low protein binding, and minimal hepatic metabolism, resulting in dose-dependent linear pharmacokinetics (145). As obese patients are less likely to achieve the dose/MIC and AUC0-24/MIC ratios, fluconazole should be dosed based on TBW (146). Doses of up to 1,600 mg/day for 2 weeks have been described, but there are no data to support higher dosing of obese patients (147). Regarding isavuconazole, intravenous and oral formulations produce similar pharmacokinetics, although it has not been uniformly studied in obese patients (148). To date, it is not possible to recommend dose adjustment of isavuconazole in the obese population since data obtained on the effect of body weight and/or BMI on pharmacokinetics are not consistent (149–152).
Further studies are needed to determine the optimal dosing regimen of itraconazole in the obese population, so the recommendation for these patients is the standard dosage of 200 mg of itraconazole every 12 h (153, 154). The pharmacokinetics of posaconazole have not been studied directly in obese patients in any of its different dosage forms. However, contradictory results have been found in studies that sought to correlate body size descriptors (in kilograms, BMI, or BSA) as a covariate that may affect posaconazole concentrations (155–177). Therefore, it is not possible to recommend a change in posaconazole dosage based on weight. TDM studies showed that voriconazole plasma concentrations were significantly higher in patients receiving TBW doses, within the reference range when ABW is used, and below the range in IBW (178–182). No differences were found when voriconazole was dosed orally (183).
Polyenes
Amphotericin B exhibits non-linear kinetics resulting in large increases in serum concentrations relative to dose escalation (52, 184). To date, there are insufficient data from which definitive dosing recommendations for most amphotericin B formulations can be established, although for liposomal amphotericin B, ABW or TBW dose may be used depending on the severity of the infection (153, 185). Recent studies proposed the use of fixed doses of amphotericin B in obese patients since body size had no effect on clearance, showing that TBW dosing might lead to an increased risk of toxicity. Wasmann et al. (186) recommended the use of 3 or 5 mg/kg of body weight/dose, limiting the dose to a maximum weight of 100 kg, resulting in a fixed dose of 300 or 500 mg, respectively. However, Nix et al. (187) express their concern about the extrapolation of these findings resulting from the simulation of 10,000 subjects with BW ranging from 60 to 180 kg. In this sense, these authors state that further studies are needed to identify the most appropriate liposomal amphotericin B dosing strategy for obese patients.
Nucleoside analog
The pharmacokinetics of flucytosine in obesity have only been described in a case report of a morbidly obese female with extrameningeal cryptococcal disease treated with 0.3–0.5 mg/kg of body weight/day using IBW (188). In the absence of reliable clinical data, administration of flucytosine using ABW in obese patients may be prudent for initial dosing in life-threatening fungal infections, whereas for non-life-threatening infections, dosing based on IBW may be sufficient.
Antivirals
Acyclovir is poorly bound to plasma proteins (~15%) and widely penetrates tissues and body fluids, including cerebrospinal fluid. Excretion occurs primarily by glomerular filtration and tubular secretion. Acyclovir dosing is conventionally weight based, which poses a risk of overdosage in obese people if TBW is used, leading to the development of crystalluria and an acyclovir-induced acute kidney injury (189–192). The evidence available so far recommends the dosing of acyclovir in obese patients by ABW (193, 194).
The pharmacokinetics of cidofovir in obesity have not been studied, so there is currently no published literature. PK values in non-obese patients suggest limited distribution in adipose tissue; therefore, ABW appears to be the most appropriate strategy for cidofovir dosing in obese patients (52).
Dosing recommendation for foscarnet in obese patients is using ABW (52, 195). Ganciclovir hardly binds to plasma proteins and its elimination is mostly renal. Ganciclovir dosage is based on body weight, which results in an increased risk of toxicity when subsequent high doses are administered in overweight or obese patients. Using ABW dose could help reduce this risk, although a recent study has concluded that ganciclovir ABW dosing did not result in a decrease in neutropenia or treatment efficacy compared to TBW dosing (196). On the other hand, for its prodrug valganciclovir, fixed doses of 900 mg/24 h are used for prophylaxis and 900 mg/12 h for treatment (197). In the case of oseltamivir, different studies concluded that a dose modification is likely to be unnecessary in obese patients (198–200).
Antitubercular agents
Little information is available on the dosing of antitubercular agents in obese patients, although it is known that the toxicity of these drugs can be increased if they are dosed according to TBW (227). Optimal microbiocidal efficacy of anti-tuberculosis drugs is related to AUC/MIC and, in the case of ethambutol, the PK/PD target is Cmax/MIC, with drug resistance linked to fT > MIC (228, 229). Data published to date suggest that ethambutol, isoniazid, and pyrazinamide should be dosed by IBW in obese patients (201–204), while rifampin is recommended to be dosed at a flat dose of 600 mg/12 h (204, 205).
CONCLUSIONS
The present work compiles the information currently available on the dosage of antibiotics, antifungals, antivirals, and antituberculosis drugs in this population. There is currently scant and sometimes contradictory information in scientific literature on the dosing of antimicrobials in obese patients. More studies in obese patients are still needed to establish antimicrobial dosing better adapted to their characteristics, allowing therapeutic objectives to be achieved with a lower risk of adverse effects.
ACKNOWLEDGMENTS
This work was supported by Instituto de Salud Carlos (ISCIII), with funding from NextGenerationEU (ICI21/00043 and PI22/00038), and by Xunta de Galicia (IN607A 2023/004).
A.C.-B., I.V.-R., and C.M.-G. are grateful to the ISCIII for financing their personnel contracts: CM21/00114, CM22/00055, and JR20/00026.
Biographies

Teresa Rodríguez-Jato is an established researcher, who completed her studies in Pharmacy at the University of Santiago de Compostela in 2001. Subsequently, she accessed the Specialized Health Training at the Hospital Clínico de Santiago de Compostela, completing this training in 2007. Since 2013, she develops her research and care work in the area of critical patients, abdominal transplant unit and pharmaceutical care in infectious diseases at the Hospital de Santiago de Compostela. In addition, she is part of the antimicrobial stewardship program of the aforementioned hospital, playing an important role in the optimization of anti-infective therapy in the center.

Anxo Fernández-Ferreiro is an established researcher who completed his studies in Pharmacy at the University of Santiago de Compostela in 2008. Subsequently, he accessed the Specialized Health Training at the Hospital Clínico de Santiago de Compostela, completing this training in 2014. He received his Doctor of Pharmacy degree in 2015, focused on the development of anti-infective ophthalmic formulations. Since that time, he has developed an important research and care work, through the research contracts Río Hortega (2016-2018) and subsequently Juan Rodés (2019-present) at the Health Research Institute of Santiago de Compostela. His research focuses on the field of personalized medicine, with special interest in the individualized dosing of anti-infectives in the last 5 years.
Contributor Information
Teresa Rodríguez-Jato, Email: maria.teresa.rodriguez.jato@sergas.es.
Anxo Fernández-Ferreiro, Email: anxordes@gmail.com.
James E. Leggett, Providence Portland Medical Center, Portland, Oregon, USA
REFERENCES
- 1. Obesity and overweight. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Retrieved 17 Oct 2021.
- 2. Prevalence of Obesity . World obesity federation. Available from: https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity. Retrieved 17 Oct 2021.
- 3. Huttunen R, Laine J, Lumio J, Vuento R, Syrjänen J. 2007. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC Infect Dis 7:13. doi: 10.1186/1471-2334-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huttunen R, Karppelin M, Syrjänen J. 2013. Obesity and nosocomial infections. J Hosp Infect 85:8–16. doi: 10.1016/j.jhin.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 5. Huttunen R, Syrjänen J. 2013. Obesity and the risk and outcome of infection. Int J Obes (Lond) 37:333–340. doi: 10.1038/ijo.2012.62 [DOI] [PubMed] [Google Scholar]
- 6. Harrop JS, Styliaras JC, Ooi YC, Radcliff KE, Vaccaro AR, Wu C. 2012. Contributing factors to surgical site infections. J Am Acad Orthop Surg 20:94–101. doi: 10.5435/JAAOS-20-02-094 [DOI] [PubMed] [Google Scholar]
- 7. Wakefield H, Vaughan-Sarrazin M, Cullen JJ. 2012. Influence of obesity on complications and costs after intestinal surgery. Am J Surg 204:434–440. doi: 10.1016/j.amjsurg.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 8. Schuster JM, Rechtine G, Norvell DC, Dettori JR. 2010. The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine (Phila Pa 1976) 35:S125–S137. doi: 10.1097/BRS.0b013e3181d8342c [DOI] [PubMed] [Google Scholar]
- 9. Genoni G, Prodam F, Marolda A, Giglione E, Demarchi I, Bellone S, Bona G. 2014. Obesity and infection: two sides of one coin. Eur J Pediatr 173:25–32. doi: 10.1007/s00431-013-2178-1 [DOI] [PubMed] [Google Scholar]
- 10. Grupper M, Nicolau DP. 2017. Obesity and skin and soft tissue infections: how to optimize antimicrobial usage for prevention and treatment? Curr Opin Infect Dis 30:180–191. doi: 10.1097/QCO.0000000000000356 [DOI] [PubMed] [Google Scholar]
- 11. Katakam A, Melnic CM, Bedair HS. 2020. Morbid obesity is a risk factor for infection recurrence following debridement, antibiotics, and implant retention for periprosthetic joint infection. J Arthroplasty 35:3710–3715. doi: 10.1016/j.arth.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 12. Milner JJ, Beck MA. 2012. The impact of obesity on the immune response to infection. Proc Nutr Soc 71:298–306. doi: 10.1017/S0029665112000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martí A, Marcos A, Martínez JA. 2001. Obesity and immune function relationships. Obes Rev 2:131–140. doi: 10.1046/j.1467-789x.2001.00025.x [DOI] [PubMed] [Google Scholar]
- 14. Muscogiuri G, Pugliese G, Laudisio D, Castellucci B, Barrea L, Savastano S, Colao A. 2021. The impact of obesity on immune response to infection: plausible mechanisms and outcomes. Obes Rev 22:e13216. doi: 10.1111/obr.13216 [DOI] [PubMed] [Google Scholar]
- 15. Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT, California Pandemic (H1N1) Working Group . 2011. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 52:301–312. doi: 10.1093/cid/ciq152 [DOI] [PubMed] [Google Scholar]
- 16. Van Kerkhove MD, Vandemaele KAH, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, Carlino LO, Owen R, Paterson B, Pelletier L, et al. 2011. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 8:e1001053. doi: 10.1371/journal.pmed.1001053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura T, Namkoong H. 2020. Susceptibility of the obese population to COVID-19. Int J Infect Dis 101:380–381. doi: 10.1016/j.ijid.2020.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. 2020. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc 95:1445–1453. doi: 10.1016/j.mayocp.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, Kouretas D, Spandidos DA, Tsatsakis A. 2020. Obesity ‑ a risk factor for increased COVID‑19 prevalence, severity and lethality (Review). Mol Med Rep 22:9–19. doi: 10.3892/mmr.2020.11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. 1985. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA 254:3187–3189. [PubMed] [Google Scholar]
- 21. Weber DJ, Rutala WA, Samsa GP, Bradshaw SE, Lemon SM. 1986. Impaired immunogenicity of hepatitis B vaccine in obese persons. N Engl J Med 314:1393. doi: 10.1056/NEJM198605223142120 [DOI] [PubMed] [Google Scholar]
- 22. Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM. 2006. Reduced tetanus antibody titers in overweight children. Autoimmunity 39:137–141. doi: 10.1080/08916930600597326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Honce R, Schultz-Cherry S. 2019. Influenza in obese travellers: increased risk and complications, decreased vaccine effectiveness. J Travel Med 26:taz020. doi: 10.1093/jtm/taz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu F, Guo Z, Dong C. 2017. Influences of obesity on the immunogenicity of hepatitis B vaccine. Hum Vaccin Immunother 13:1014–1017. doi: 10.1080/21645515.2016.1274475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tagliabue C, Principi N, Giavoli C, Esposito S. 2016. Obesity: impact of infections and response to vaccines. Eur J Clin Microbiol Infect Dis 35:325–331. doi: 10.1007/s10096-015-2558-8 [DOI] [PubMed] [Google Scholar]
- 26. Ashburn DD, DeAntonio A, Reed MJ. 2010. Pulmonary system and obesity. Crit Care Clin 26:597–602. doi: 10.1016/j.ccc.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 27. Yosipovitch G, DeVore A, Dawn A. 2007. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 56:901–916. doi: 10.1016/j.jaad.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 28. Knibbe CAJ, Brill MJE, van Rongen A, Diepstraten J, van der Graaf PH, Danhof M. 2015. Drug disposition in obesity: toward evidence-based dosing. Annu Rev Pharmacol Toxicol 55:149–167. doi: 10.1146/annurev-pharmtox-010814-124354 [DOI] [PubMed] [Google Scholar]
- 29. Smit C, De Hoogd S, Brüggemann RJM, Knibbe CAJ. 2018. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol 14:275–285. doi: 10.1080/17425255.2018.1440287 [DOI] [PubMed] [Google Scholar]
- 30. Barras M, Legg A. 2017. Drug dosing in obese adults. Aust Prescr 40:189–193. doi: 10.18773/austprescr.2017.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iqbal A, Rehman A. 2023. Obesity brain gut adipocyte InteractionStatPearls. StatPearls Publishing, Treasure Island (FL). [PubMed] [Google Scholar]
- 32. Obesity. Available from: https://www.who.int/health-topics/obesity. Retrieved 06 Oct 2023.
- 33. Hanley MJ, Abernethy DR, Greenblatt DJ. 2010. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 49:71–87. doi: 10.2165/11318100-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 34. Mosteller RD. 1987. Simplified calculation of body-surface area. N Engl J Med 317:1098. doi: 10.1056/NEJM198710223171717 [DOI] [PubMed] [Google Scholar]
- 35. Ingrande J, Lemmens HJM. 2010. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth 105:i16–i23. doi: 10.1093/bja/aeq312 [DOI] [PubMed] [Google Scholar]
- 36. Field KM, Kosmider S, Jefford M, Michael M, Jennens R, Green M, Gibbs P. 2008. Chemotherapy dosing strategies in the obese, elderly, and thin patient: results of a nationwide survey. J Oncol Pract 4:108–113. doi: 10.1200/JOP.0832001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Janson B, Thursky K. 2012. Dosing of antibiotics in obesity. Curr Opin Infect Dis 25:634–649. doi: 10.1097/QCO.0b013e328359a4c1 [DOI] [PubMed] [Google Scholar]
- 38. Pai MP, Paloucek FP. 2000. The origin of the “ideal” body weight equations. Ann Pharmacother 34:1066–1069. doi: 10.1345/aph.19381 [DOI] [PubMed] [Google Scholar]
- 39. Devine BJ. 1974. Gentamicin therapy
- 40. Morrish GA, Pai MP, Green B. 2011. The effects of obesity on drug pharmacokinetics in humans. Expert Opin Drug Metab Toxicol 7:697–706. doi: 10.1517/17425255.2011.570331 [DOI] [PubMed] [Google Scholar]
- 41. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. 2005. Quantification of lean body weight. Clin Pharmacokinet 44:1051–1065. doi: 10.2165/00003088-200544100-00004 [DOI] [PubMed] [Google Scholar]
- 42. Deng J, Zhu X, Chen Z, Fan CH, Kwan HS, Wong CH, Shek KY, Zuo Z, Lam TN. 2017. A review of food-drug interactions on oral drug absorption. Drugs 77:1833–1855. doi: 10.1007/s40265-017-0832-z [DOI] [PubMed] [Google Scholar]
- 43. Martinez MN, Amidon GL. 2002. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol 42:620–643. doi: 10.1177/00970002042006005 [DOI] [PubMed] [Google Scholar]
- 44. Maddox A, Horowitz M, Wishart J, Collins P. 1989. Gastric and oesophageal emptying in obesity. Scand J Gastroenterol 24:593–598. doi: 10.3109/00365528909093095 [DOI] [PubMed] [Google Scholar]
- 45. Jackson SJ, Leahy FE, McGowan AA, Bluck LJC, Coward WA, Jebb SA. 2004. Delayed gastric emptying in the obese: an assessment using the non-invasive (13)C-octanoic acid breath test. Diabetes Obes Metab 6:264–270. doi: 10.1111/j.1462-8902.2004.0344.x [DOI] [PubMed] [Google Scholar]
- 46. Meng L, Mui E, Holubar MK, Deresinski SC. 2017. Comprehensive guidance for antibiotic dosing in obese adults. Pharmacotherapy 37:1415–1431. doi: 10.1002/phar.2023 [DOI] [PubMed] [Google Scholar]
- 47. Alobaid AS, Hites M, Lipman J, Taccone FS, Roberts JA. 2016. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents 47:259–268. doi: 10.1016/j.ijantimicag.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 48. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. 2006. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Circulation 113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 49. Rossi M, Nannipieri M, Anselmino M, Guarino D, Franzoni F, Pesce M. 2012. Subcutaneous adipose tissue blood flow and vasomotion in morbidly obese patients: long term effect of gastric bypass surgery. Clin Hemorheol Microcirc 51:159–167. doi: 10.3233/CH-2011-1517 [DOI] [PubMed] [Google Scholar]
- 50. Benedek IH, Blouin RA, McNamara PJ. 1984. Serum protein binding and the role of increased alpha 1-acid glycoprotein in moderately obese male subjects. Br J Clin Pharmacol 18:941–946. doi: 10.1111/j.1365-2125.1984.tb02567.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suh B, Craig WA, England AC, Elliott RL. 1981. Effect of free fatty acids on protein binding of antimicrobial agents. J Infect Dis 143:609–616. doi: 10.1093/infdis/143.4.609 [DOI] [PubMed] [Google Scholar]
- 52. Polso AK, Lassiter JL, Nagel JL. 2014. Impact of hospital guideline for weight-based antimicrobial dosing in morbidly obese adults and comprehensive literature review. J Clin Pharm Ther 39:584–608. doi: 10.1111/jcpt.12200 [DOI] [PubMed] [Google Scholar]
- 53. Morita K, Yamaji A. 1995. Changes in the serum protein binding of vancomycin in patients with methicillin-resistant Staphylococcus aureus infection: the role of serum Α: 1: -acid glycoprotein levels. Ther Drug Monit 17:107–112. doi: 10.1097/00007691-199504000-00001 [DOI] [PubMed] [Google Scholar]
- 54. Zokufa HZ, Solem LD, Rodvold KA, Crossley KB, Fischer JH, Rotschafer JC. 1989. The influence of serum albumin and alpha 1-acid glycoprotein on vancomycin protein binding in patients with burn injuries. J Burn Care Rehabil 10:425–428. doi: 10.1097/00004630-198909000-00010 [DOI] [PubMed] [Google Scholar]
- 55. Brill MJE, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CAJ. 2012. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet 51:277–304. doi: 10.2165/11599410-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 56. Kotlyar M, Carson SW. 1999. Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther 37:8–19. [PubMed] [Google Scholar]
- 57. Griffin KA, Kramer H, Bidani AK. 2008. Adverse renal consequences of obesity. Am J Physiol Renal Physiol 294:F685–96. doi: 10.1152/ajprenal.00324.2007 [DOI] [PubMed] [Google Scholar]
- 58. Lemmens HJM, Ingrande J. 2013. Pharmacology and obesity. Int Anesthesiol Clin 51:52–66. doi: 10.1097/AIA.0b013e31829a4d56 [DOI] [PubMed] [Google Scholar]
- 59. Roberts JA, Lipman J. 2006. Antibacterial dosing in intensive care. Clinical Pharmacokinetics 45:755–773. doi: 10.2165/00003088-200645080-00001 [DOI] [PubMed] [Google Scholar]
- 60. Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug”. Nat Rev Microbiol 2:289–300. doi: 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 61. Rodríguez-Gascón A, Solinís MÁ, Isla A. 2021. The role of PK/PD analysis in the development and evaluation of antimicrobials. Pharmaceutics 13:833. doi: 10.3390/pharmaceutics13060833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Velissaris D, Karamouzos V, Marangos M, Pierrakos C, Karanikolas M. 2014. Pharmacokinetic changes and dosing modification of aminoglycosides in critically ill obese patients: a literature review. J Clin Med Res 6:227–233. doi: 10.14740/jocmr1858w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Traynor AM, Nafziger AN, Bertino JS. 1995. Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrob Agents Chemother 39:545–548. doi: 10.1128/AAC.39.2.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pai MP, Nafziger AN, Bertino JS. 2011. Simplified estimation of aminoglycoside pharmacokinetics in underweight and obese adult patients. Antimicrob Agents Chemother 55:4006–4011. doi: 10.1128/AAC.00174-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smit C, van Schip AM, van Dongen EPA, Brüggemann RJM, Becker ML, Knibbe CAJ. 2020. Dose recommendations for gentamicin in the real-world obese population with varying body weight and renal (dys)function. J Antimicrob Chemother 75:3286–3292. doi: 10.1093/jac/dkaa312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bakdach D, Elajez R, Bakdach AR, Awaisu A, De Pascale G, Ait Hssain A. 2022. Pharmacokinetics, pharmacodynamics, and dosing considerations of novel β-lactams and β-lactam/β-lactamase inhibitors in critically ill adult patients: focus on obesity augmented renal clearance, renal replacement therapies, and extracorporeal membrane oxygenation. J Clin Med 11:6898. doi: 10.3390/jcm11236898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hites M, Taccone FS, Wolff F, Maillart E, Beumier M, Surin R, Cotton F, Jacobs F. 2014. Broad-spectrum β-lactams in obese non-critically ill patients. Nutr Diabetes 4:e119. doi: 10.1038/nutd.2014.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Soares ALPPDP, Montanha MC, Alcantara CDS, Silva SRB, Kuroda CM, Yamada SS, Nicacio AE, Maldaner L, Visentainer JV, Simões CF, Locatelli JC, Lopes WA, Mazucheli J, Diniz A, Paixão PJPA, Kimura E. 2021. Pharmacokinetics of amoxicillin in obese and nonobese subjects. Br J Clin Pharmacol 87:3227–3233. doi: 10.1111/bcp.14739 [DOI] [PubMed] [Google Scholar]
- 69. Zeller V, Durand F, Kitzis M-D, Lhotellier L, Ziza J-M, Mamoudy P, Desplaces N. 2009. Continuous cefazolin infusion to treat bone and joint infections: clinical efficacy, feasibility, safety, and serum and bone concentrations. Antimicrob Agents Chemother 53:883–887. doi: 10.1128/AAC.00389-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ryan RL, Jackson D, Hopkins G, Eley V, Christensen R, Van Zundert AAJ, Wallis SC, Lipman J, Parker SL, Roberts JA. 2022. Plasma and interstitial fluid pharmacokinetics of prophylactic cefazolin in elective bariatric surgery patients. Antimicrob Agents Chemother 66:e0041922. doi: 10.1128/aac.00419-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Justo JA, Mayer SM, Pai MP, Soriano MM, Danziger LH, Novak RM, Rodvold KA. 2015. Pharmacokinetics of ceftaroline in normal body weight and obese (classes I, II, and III) healthy adult subjects. Antimicrob Agents Chemother 59:3956–3965. doi: 10.1128/AAC.00498-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Veillette JJ, Winans SA, Maskiewicz VK, Truong J, Jones RN, Forland SC. 2021. Pharmacokinetics and pharmacodynamics of high-dose piperacillin-tazobactam in obese patients. Eur J Drug Metab Pharmacokinet 46:385–394. doi: 10.1007/s13318-021-00677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. CIMA . Ficha Técnica Fetcroja 1 g polvo para concentrado para solución para perfusión. Available from: https://cima.aemps.es/cima/dochtml/ft/1201434/FT_1201434.html. Retrieved 12 Sep 2023.
- 74. Housman ST, McWhorter PB, Barie PS, Nicolau DP. 2022. Ertapenem concentrations in obese patients undergoing surgery. Surg Infect (Larchmt) 23:545–549. doi: 10.1089/sur.2022.005 [DOI] [PubMed] [Google Scholar]
- 75. Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS. 2006. Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob Agents Chemother 50:1222–1227. doi: 10.1128/AAC.50.4.1222-1227.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chung EK, Cheatham SC, Fleming MR, Healy DP, Kays MB. 2017. Population pharmacokinetics and pharmacodynamics of meropenem in nonobese, obese, and morbidly obese patients. J Clin Pharmacol 57:356–368. doi: 10.1002/jcph.812 [DOI] [PubMed] [Google Scholar]
- 77. Garrett ER, Süverkrup RS, Eberst K, Yost RL, O’Leary JP. 1981. Surgically affected sulfisoxazole pharmacokinetics in the morbidly obese. Biopharm Drug Dispos 2:329–365. doi: 10.1002/bdd.2510020405 [DOI] [PubMed] [Google Scholar]
- 78. Hall RG, Pasipanodya JG, Meek C, Leff RD, Swancutt M, Gumbo T. 2016. Fractal geometry‐based decrease in trimethoprim‐sulfamethoxazole concentrations in overweight and obese people. CPT Pharmacometrics Syst Pharmacol 5:674–681. doi: 10.1002/psp4.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Allard S, Kinzig M, Boivin G, Sörgel F, LeBel M. 1993. Intravenous ciprofloxacin disposition in obesity. Clin Pharmacol Ther 54:368–373. doi: 10.1038/clpt.1993.162 [DOI] [PubMed] [Google Scholar]
- 80. Hollenstein UM, Brunner M, Schmid R, Müller M. 2001. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int J Obes Relat Metab Disord 25:354–358. doi: 10.1038/sj.ijo.0801555 [DOI] [PubMed] [Google Scholar]
- 81. van Rhee KP, Smit C, Wasmann RE, van der Linden PD, Wiezer R, Van Dongen EPA, Krekels EHJ, Brüggemann RJM, Knibbe CAJ. 2022. Ciprofloxacin pharmacokinetics after oral and intravenous administration in (morbidly) obese and non-obese individuals: a prospective clinical study. Clin Pharmacokinet 61:1167–1175. doi: 10.1007/s40262-022-01130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pai MP, Cojutti P, Pea F. 2014. Levofloxacin dosing regimen in severely morbidly obese patients (BMI ≥40 kg/m(2)) should be guided by creatinine clearance estimates based on ideal body weight and optimized by therapeutic drug monitoring. Clin Pharmacokinet 53:753–762. doi: 10.1007/s40262-014-0154-1 [DOI] [PubMed] [Google Scholar]
- 83. Hanretty AM, Moore WS, Chopra A, Cies JJ. 2020. Therapeutic drug monitoring of levoffoxacin in an obese adolescent: a case report. J Pediatr Pharmacol Ther 25:261–265. doi: 10.5863/1551-6776-25.3.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dorn C, Petroff D, Neumann N, Kratzer A, El-Najjar N, Dietrich A, Kloft C, Zeitlinger M, Kees MG, Kees F, Wrigge H, Simon P. 2019. Plasma and tissue pharmacokinetics of fosfomycin in morbidly obese and non-obese surgical patients: a controlled clinical trial. J Antimicrob Chemother 74:2335–2340. doi: 10.1093/jac/dkz203 [DOI] [PubMed] [Google Scholar]
- 85. Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Docobo-Pérez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martín V, Ballestero-Tellez M, Rodriguez-Martinez JM, Conejo MC, van Guilder M, Rodríguez-Baño J, Pascual A, Hope WW. 2015. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 59:5602–5610. doi: 10.1128/AAC.00752-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Busse D, Simon P, Petroff D, El-Najjar N, Schmitt L, Bindellini D, Dietrich A, Zeitlinger M, Huisinga W, Michelet R, Wrigge H, Kloft C. 2022. High-dosage fosfomycin results in adequate plasma and target-site exposure in morbidly obese and nonobese nonhyperfiltration patients. Antimicrob Agents Chemother 66:e0230221. doi: 10.1128/aac.02302-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang T, Smit C, Sherwin CMT, Knibbe CAJ, Krekels EHJ. 2023. Vancomycin clearance in obese adults is not predictive of clearance in obese adolescents. Clin Pharmacokinet 62:749–759. doi: 10.1007/s40262-023-01227-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wright L, Childress DT, Brown ML, Wilkerson W, Maldonado R, Durham SH. 2023. Which “weigh” to go? Alternative vancomycin dosing strategies in obese patients. J Pharm Pract 36:870–874. doi: 10.1177/08971900221087122 [DOI] [PubMed] [Google Scholar]
- 90. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, Rodvold KA, Maples HD, Lomaestro BM. 2020. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of America, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. Am J Health Syst Pharm 77:835–864. doi: 10.1093/ajhp/zxaa036 [DOI] [PubMed] [Google Scholar]
- 91. Elrggal ME, Haseeb A, AlGethamy M, Ahsan U, Saleem Z, Althaqafi AS, Alshuail SS, Alsiddiqi ZA, Iqbal MS, Alzahrani AF, AlQarni A, Radwan RM, Qul AKS, Mahrous AJ, Alsharif JM, Alqurashi MK, Faidah HS, Aldurdunji M. 2023. Dose optimization of vancomycin in obese patients: a systematic review. Front Pharmacol 14:965284. doi: 10.3389/fphar.2023.965284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yamada T, Nonaka T, Yano T, Kubota T, Egashira N, Kawashiri T, Oishi R. 2012. Simplified dosing regimens of teicoplanin for patient groups stratified by renal function and weight using Monte Carlo simulation. Int J Antimicrob Agents 40:344–348. doi: 10.1016/j.ijantimicag.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 93. Soy D, López E, Ribas J. 2006. Teicoplanin population pharmacokinetic analysis in hospitalized patients. Ther Drug Monit 28:737–743. doi: 10.1097/01.ftd.0000249942.14145.ff [DOI] [PubMed] [Google Scholar]
- 94. Hanai Y, Takahashi Y, Niwa T, Mayumi T, Hamada Y, Kimura T, Matsumoto K, Fujii S, Takesue Y. 2022. Clinical practice guidelines for therapeutic drug monitoring of teicoplanin: a consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Antimicrob Chemother 77:869–879. doi: 10.1093/jac/dkab499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC. 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:147–159. doi: 10.1093/cid/ciu296 [DOI] [PubMed] [Google Scholar]
- 96. Smith MJ, Gonzalez D, Goldman JL, Yogev R, Sullivan JE, Reed MD, Anand R, Martz K, Berezny K, Benjamin DK, Smith PB, Cohen-Wolkowiez M, Watt K. 2017. Best pharmaceuticals for children act—pediatric trials network steering committee. Antimicrob Agents Chemother 61:e02014. doi: 10.1128/AAC.02014-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gregoire N, Chauzy A, Buyck J, Rammaert B, Couet W, Marchand S. 2021. Clinical pharmacokinetics of daptomycin. Clin Pharmacokinet 60:271–281. doi: 10.1007/s40262-020-00968-x [DOI] [PubMed] [Google Scholar]
- 98. Jones TW, Jun AH, Michal JL, Olney WJ. 2021. High-dose daptomycin and clinical applications. Ann Pharmacother 55:1363–1378. doi: 10.1177/1060028021991943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bookstaver PB, Bland CM, Qureshi ZP, Faulkner-Fennell CM, Sheldon MA, Caulder CR, Hartis C, SERGE-45 Investigators . 2013. Safety and effectiveness of daptomycin across a hospitalized obese population: results of a multicenter investigation in the southeastern United States. Pharmacotherapy 33:1322–1330. doi: 10.1002/phar.1298 [DOI] [PubMed] [Google Scholar]
- 100. Molina KC, Miller MA, Mueller SW, Van Matre ET, Krsak M, Kiser TH. 2022. Clinical pharmacokinetics and pharmacodynamics of dalbavancin. Clin Pharmacokinet 61:363–374. doi: 10.1007/s40262-021-01088-w [DOI] [PubMed] [Google Scholar]
- 101. Riccobene T, Lock J, Lyles RD, Georgiades B, Nowak M, Gonzalez PL, Park J, Rappo U. 2023. Dalbavancin for the treatment of acute bacterial skin and skin structure infection in patients with obesity or diabetes: a subgroup analysis of pooled phase 3 clinical trials. Open Forum Infect Dis 10:ofad256. doi: 10.1093/ofid/ofad256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Redell M, Sierra-Hoffman M, Assi M, Bochan M, Chansolme D, Gandhi A, Sheridan K, Soosaipillai I, Walsh T, Massey J. 2019. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of Gram-positive infections. Open Forum Infect Dis 6:ofz479. doi: 10.1093/ofid/ofz479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hopkins MK, Tewari S, Yao M, DeAngelo L, Buckley L, Rogness V, Kollikonda S, Goje O. 2023. Standard-dose azithromycin in class III obese patients undergoing unscheduled cesarean delivery. Am J Perinatol. doi: 10.1055/a-2135-7084 [DOI] [PubMed] [Google Scholar]
- 104. Dorn C, Petroff D, Stoelzel M, Kees MG, Kratzer A, Dietrich A, Kloft C, Zeitlinger M, Kees F, Wrigge H, Simon P. 2021. Perioperative administration of cefazolin and metronidazole in obese and non-obese patients: a pharmacokinetic study in plasma and interstitial fluid. J Antimicrob Chemother 76:2114–2120. doi: 10.1093/jac/dkab143 [DOI] [PubMed] [Google Scholar]
- 105. Soule AF, Green SB, Blanchette LM. 2018. Clinical efficacy of 12-h metronidazole dosing regimens in patients with anaerobic or mixed anaerobic infections. Ther Adv Infect Dis 5:57–62. doi: 10.1177/2049936118766462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Martin C, Auboyer C, Boisson M, Dupont H, Gauzit R, Kitzis M, Leone M, Lepape A, Mimoz O, Montravers P, Pourriat JL, Steering committee of the French Society of Anaesthesia and Intensive Care Medicine (SFAR) responsible for the establishment of the guidelines . 2019. Antibioprophylaxis in surgery and interventional medicine (adult patients). Update 2017. Anaesth Crit Care Pain Med 38:549–562. doi: 10.1016/j.accpm.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 107. Bandín-Vilar E, García-Quintanilla L, Castro-Balado A, Zarra-Ferro I, González-Barcia M, Campos-Toimil M, Mangas-Sanjuan V, Mondelo-García C, Fernández-Ferreiro A. 2022. A review of population pharmacokinetic analyses of linezolid. Clin Pharmacokinet 61:789–817. doi: 10.1007/s40262-022-01125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Heidari S, Khalili H. 2023. Linezolid pharmacokinetics: a systematic review for the best clinical practice. Eur J Clin Pharmacol 79:195–206. doi: 10.1007/s00228-022-03446-4 [DOI] [PubMed] [Google Scholar]
- 109. Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, Li J, Silveira FP. 2017. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 64:565–571. doi: 10.1093/cid/ciw839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. 2019. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacotherapy 39:10–39. doi: 10.1002/phar.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Peyriere H, Makinson A, Marchandin H, Reynes J. 2018. Doxycycline in the management of sexually transmitted infections. J Antimicrob Chemother 73:553–563. doi: 10.1093/jac/dkx420 [DOI] [PubMed] [Google Scholar]
- 112. Narayanan N, Adams CD, Kubiak DW, Cheng S, Stoianovici R, Kagan L, Brunetti L. 2019. Evaluation of treatment options for methicillin-resistant Staphylococcus aureus infections in the obese patient. Infect Drug Resist 12:877–891. doi: 10.2147/IDR.S196264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Leng B, Yan G, Wang C, Shen C, Zhang W, Wang W. 2021. Dose optimisation based on pharmacokinetic/pharmacodynamic target of tigecycline. J Glob Antimicrob Resist 25:315–322. doi: 10.1016/j.jgar.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 114. Estes KS, Derendorf H. 2010. Comparison of the pharmacokinetic properties of vancomycin, linezolid, tigecyclin, and daptomycin. Eur J Med Res 15:533–543. doi: 10.1186/2047-783x-15-12-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pai MP. 2014. Serum and urine pharmacokinetics of tigecycline in obese class III and normal weight adults. J Antimicrob Chemother 69:190–199. doi: 10.1093/jac/dkt299 [DOI] [PubMed] [Google Scholar]
- 116. Dorn C, Petroff D, Kratzer A, Kees F, Kloft C, Zeitlinger M, Wrigge H, Simon P. 2022. Tigecycline soft tissue penetration in obese and non-obese surgical patients determined by using in vivo microdialysis. Eur J Drug Metab Pharmacokinet 47:749–755. doi: 10.1007/s13318-022-00789-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2023. Infectious diseases society of America 2023 guidance on the treatment of antimicrobial resistant Gram-negative infections. Clin Infect Dis:ciad428. doi: 10.1093/cid/ciad428 [DOI] [PubMed] [Google Scholar]
- 118. Ibrahim MM, Abuelmatty AM, Mohamed GH, Nasr MA, Hussein AK, Ebaed MED, Sarhan HA. 2018. Best tigecycline dosing for treatment of infections caused by multidrug-resistant pathogens in critically ill patients with different body weights. Drug Des Devel Ther 12:4171–4179. doi: 10.2147/DDDT.S181834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hall RG, Liu S, Putnam WC, Kallem R, Gumbo T, Pai MP. 2023. Optimizing anidulafungin exposure across a wide adult body size range. Antimicrob Agents Chemother 67:e0082023. doi: 10.1128/aac.00820-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu P. 2013. Population pharmacokinetic-pharmacodynamic analysis of anidulafungin in adult patients with fungal infections. Antimicrob Agents Chemother 57:466–474. doi: 10.1128/AAC.01473-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Liu P, Ruhnke M, Meersseman W, Paiva JA, Kantecki M, Damle B. 2013. Pharmacokinetics of anidulafungin in critically ill patients with candidemia/invasive candidiasis. Antimicrob Agents Chemother 57:1672–1676. doi: 10.1128/AAC.02139-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lempers VJ, van Rongen A, van Dongen EP, van Ramshorst B, Burger DM, Aarnoutse RE, Knibbe CA, Brüggemann RJ. 2016. Does weight impact anidulafungin pharmacokinetics? Clin Pharmacokinet 55:1289–1294. doi: 10.1007/s40262-016-0401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wasmann RE, Ter Heine R, van Dongen EP, Burger DM, Lempers VJ, Knibbe CA, Brüggemann RJ. 2018. Pharmacokinetics of anidulafungin in obese and normal-weight adults. Antimicrob Agents Chemother 62:e00063-18. doi: 10.1128/AAC.00063-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Luque S, Hope W, Campillo N, Muñoz-Bermúdez R, Sorli L, Barceló-Vidal J, González-Colominas E, Alvarez-Lerma F, Masclans JR, Montero M, Horcajada JP, Grau S. 2019. Population pharmacokinetics of anidulafungin in critically ill patients. Antimicrob Agents Chemother 63:e00378-19. doi: 10.1128/AAC.00378-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dowell JA, Knebel W, Ludden T, Stogniew M, Krause D, Henkel T. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J Clin Pharmacol 44:590–598. doi: 10.1177/0091270004265644 [DOI] [PubMed] [Google Scholar]
- 126. Liu P, Mould DR. 2014. Population pharmacokinetic-pharmacodynamic analysis of voriconazole and anidulafungin in adult patients with invasive aspergillosis. Antimicrob Agents Chemother 58:4727–4736. doi: 10.1128/AAC.02809-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hutton M, Kenney RM, Vazquez JA, Davis SL. 2022. Influence of body weight category on outcomes in candidemia patients treated with anidulafungin. J Pharm Pract 35:20–25. doi: 10.1177/0897190020938219 [DOI] [PubMed] [Google Scholar]
- 128. Brüggemann RJM, Middel-Baars V, de Lange DW, Colbers A, Girbes ARJ, Pickkers P, Swart EL. 2017. Pharmacokinetics of anidulafungin in critically ill intensive care unit patients with suspected or proven invasive fungal infections. Antimicrob Agents Chemother 61:e01894-16. doi: 10.1128/AAC.01894-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hall RG, Swancutt MA, Meek C, Leff R, Gumbo T. 2013. Weight drives caspofungin pharmacokinetic variability in overweight and obese people: fractal power signatures beyond two-thirds or three-fourths. Antimicrob Agents Chemother 57:2259–2264. doi: 10.1128/AAC.01490-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, Weigand MA. 2007. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother 60:100–106. doi: 10.1093/jac/dkm125 [DOI] [PubMed] [Google Scholar]
- 131. Würthwein G, Cornely OA, Trame MN, Vehreschild JJ, Vehreschild MJGT, Farowski F, Müller C, Boos J, Hempel G, Hallek M, Groll AH. 2013. Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis. Antimicrob Agents Chemother 57:1664–1671. doi: 10.1128/AAC.01912-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. van der Elst KCM, Veringa A, Zijlstra JG, Beishuizen A, Klont R, Brummelhuis-Visser P, Uges DRA, Touw DJ, Kosterink JGW, van der Werf TS, Alffenaar J-W. 2017. Low caspofungin exposure in patients in intensive care units. Antimicrob Agents Chemother 61:e01582-16. doi: 10.1128/AAC.01582-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Märtson A-G, van der Elst KCM, Veringa A, Zijlstra JG, Beishuizen A, van der Werf TS, Kosterink JGW, Neely M, Alffenaar J-W. 2020. Caspofungin weight-based dosing supported by a population pharmacokinetic model in critically ill patients. Antimicrob Agents Chemother 64:e00905-20. doi: 10.1128/AAC.00905-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ryan DM, Lupinacci RJ, Kartsonis NA. 2011. Efficacy and safety of caspofungin in obese patients. Med Mycol 49:748–754. doi: 10.3109/13693786.2011.571293 [DOI] [PubMed] [Google Scholar]
- 135. Muilwijk EW, Schouten JA, van Leeuwen HJ, van Zanten ARH, de Lange DW, Colbers A, Verweij PE, Burger DM, Pickkers P, Brüggemann RJM. 2014. Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother 69:3294–3299. doi: 10.1093/jac/dku313 [DOI] [PubMed] [Google Scholar]
- 136. Tabata K, Katashima M, Kawamura A, Kaibara A, Tanigawara Y. 2006. Population pharmacokinetic analysis of micafungin in Japanese patients with fungal infections. Drug Metab Pharmacokinet 21:324–331. doi: 10.2133/dmpk.21.324 [DOI] [PubMed] [Google Scholar]
- 137. Gumbo T, Hiemenz J, Ma L, Keirns JJ, Buell DN, Drusano GL. 2008. Population pharmacokinetics of micafungin in adult patients. Diagn Microbiol Infect Dis 60:329–331. doi: 10.1016/j.diagmicrobio.2007.09.018 [DOI] [PubMed] [Google Scholar]
- 138. Hall RG, Swancutt MA, Gumbo T. 2011. Fractal geometry and the pharmacometrics of micafungin in overweight, obese, and extremely obese people. Antimicrob Agents Chemother 55:5107–5112. doi: 10.1128/AAC.05193-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Boonstra JM, van der Elst KC, Veringa A, Jongedijk EM, Brüggemann RJ, Koster RA, Kampinga GA, Kosterink JG, van der Werf TS, Zijlstra JG, Touw DJ, Alffenaar JWC. 2017. Pharmacokinetic properties of micafungin in critically ill patients diagnosed with invasive candidiasis. Antimicrob Agents Chemother 61:e01398-17. doi: 10.1128/AAC.01398-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jullien V, Azoulay E, Schwebel C, Le Saux T, Charles PE, Cornet M, Souweine B, Klouche K, Jaber S, Trouillet J-L, et al. 2017. Population pharmacokinetics of micafungin in ICU patients with sepsis and mechanical ventilation. J Antimicrob Chemother 72:181–189. doi: 10.1093/jac/dkw352 [DOI] [PubMed] [Google Scholar]
- 141. Maseda E, Grau S, Luque S, Castillo-Mafla M-P, Suárez-de-la-Rica A, Montero-Feijoo A, Salgado P, Gimenez M-J, García-Bernedo CA, Gilsanz F, Roberts JA. 2018. Population pharmacokinetics/pharmacodynamics of micafungin against Candida species in obese, critically ill, and morbidly obese critically ill patients. Crit Care 22:94. doi: 10.1186/s13054-018-2019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wasmann RE, Smit C, Ter Heine R, Koele SE, van Dongen EPH, Wiezer RMJ, Burger DM, Knibbe CAJ, Brüggemann RJM. 2019. Pharmacokinetics and probability of target attainment for micafungin in normal-weight and morbidly obese adults. J Antimicrob Chemother 74:978–985. doi: 10.1093/jac/dky554 [DOI] [PubMed] [Google Scholar]
- 143. Lempers VJ, Schouten JA, Hunfeld NG, Colbers A, van Leeuwen HJ, Burger DM, Verweij PE, Pickkers P, Brüggemann RJ. 2015. Altered micafungin pharmacokinetics in intensive care unit patients. Antimicrob Agents Chemother 59:4403–4409. doi: 10.1128/AAC.00623-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. García-de-Lorenzo A, Luque S, Grau S, Agrifoglio A, Cachafeiro L, Herrero E, Asensio MJ, Sánchez SM, Roberts JA. 2016. Comparative population plasma and tissue pharmacokinetics of micafungin in critically ill patients with severe burn injuries and patients with complicated intra-abdominal infection. Antimicrob Agents Chemother 60:5914–5921. doi: 10.1128/AAC.00727-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gubbins PO. 2011. Triazole antifungal agents drug-drug interactions involving hepatic cytochrome P450. Expert Opin Drug Metab Toxicol 7:1411–1429. doi: 10.1517/17425255.2011.627854 [DOI] [PubMed] [Google Scholar]
- 146. Pai MP, Turpin RS, Garey KW. 2007. Association of fluconazole area under the concentration-time curve/MIC and dose/MIC ratios with mortality in nonneutropenic patients with candidemia. Antimicrob Agents Chemother 51:35–39. doi: 10.1128/AAC.00474-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Anaissie EJ, Kontoyiannis DP, Huls C, Vartivarian SE, Karl C, Prince RA, Bosso J, Bodey GP. 1995. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis 172:599–602. doi: 10.1093/infdis/172.2.599 [DOI] [PubMed] [Google Scholar]
- 148. Slain D, Cleary JD. 2015. Isavuconazonium sulfate: a novel antifungal agent. Curr Fungal Infect Rep 9:302–313. doi: 10.1007/s12281-015-0241-2 [DOI] [Google Scholar]
- 149. Desai A, Schmitt-Hoffmann A-H, Mujais S, Townsend R. 2016. Population pharmacokinetics of isavuconazole in subjects with mild or moderate hepatic impairment. Antimicrob Agents Chemother 60:3025–3031. doi: 10.1128/AAC.02942-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kovanda LL, Desai AV, Lu Q, Townsend RW, Akhtar S, Bonate P, Hope WW. 2016. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the VITAL study). Antimicrob Agents Chemother 60:4568–4576. doi: 10.1128/AAC.00514-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wu X, Clancy CJ, Rivosecchi RM, Zhao W, Shields RK, Marini RV, Venkataramanan R, Nguyen MH. 2018. Pharmacokinetics of intravenous isavuconazole in solid-organ transplant recipients. Antimicrob Agents Chemother 62:e01643-18. doi: 10.1128/AAC.01643-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cornely OA, Böhme A, Schmitt-Hoffmann A, Ullmann AJ. 2015. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother 59:2078–2085. doi: 10.1128/AAC.04569-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Payne KD, Hall RG. 2016. Dosing of antifungal agents in obese people. Expert Rev Anti Infect Ther 14:257–267. doi: 10.1586/14787210.2016.1128822 [DOI] [PubMed] [Google Scholar]
- 154. Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 53:24–34. doi: 10.1128/AAC.00705-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dolton MJ, Ray JE, Chen SC-A, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother 56:5503–5510. doi: 10.1128/AAC.00802-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shields RK, Clancy CJ, Vadnerkar A, Kwak EJ, Silveira FP, Massih RCA, Pilewski JM, Crespo M, Toyoda Y, Bhama JK, Bermudez C, Nguyen MH. 2011. Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob Agents Chemother 55:1308–1311. doi: 10.1128/AAC.01325-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jiménez JL, Candoni A, Raad I, Laverdiere M, Langston A, Kartsonis N, Van Iersel M, Connelly N, Waskin H. 2016. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother 71:718–726. doi: 10.1093/jac/dkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pham AN, Bubalo JS, Lewis JS. 2016. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses 59:226–233. doi: 10.1111/myc.12452 [DOI] [PubMed] [Google Scholar]
- 159. Chin A, Pergam SA, Fredricks DN, Hoofnagle AN, Baker KK, Jain R. 2017. Evaluation of posaconazole serum concentrations from delayed-release tablets in patients at high risk for fungal infections. Antimicrob Agents Chemother 61:e00569-17. doi: 10.1128/AAC.00569-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Belling M, Kanate AS, Shillingburg A, Lu X, Wen S, Shah N, Craig M, Cumpston A. 2017. Evaluation of serum posaconazole concentrations in patients with hematological malignancies receiving posaconazole suspension compared to the delayed-release tablet formulation. Leuk Res Treatment 2017:3460892. doi: 10.1155/2017/3460892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Petitcollin A, Boglione-Kerrien C, Tron C, Nimubona S, Lalanne S, Lemaitre F, Bellissant E, Verdier M-C. 2017. Population pharmacokinetics of posaconazole tablets and Monte Carlo simulations to determine whether all patients should receive the same dose. Antimicrob Agents Chemother 61:e01166-17. doi: 10.1128/AAC.01166-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tverdek FP, Heo ST, Aitken SL, Granwehr B, Kontoyiannis DP. 2017. Real-life assessment of the safety and effectiveness of the new tablet and intravenous formulations of posaconazole in the prophylaxis of invasive fungal infections via analysis of 343 courses. Antimicrob Agents Chemother 61:e00188-17. doi: 10.1128/AAC.00188-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. van Iersel MLPS, Rossenu S, de Greef R, Waskin H. 2018. A population pharmacokinetic model for a solid oral tablet formulation of posaconazole. Antimicrob Agents Chemother 62:e02465-17. doi: 10.1128/AAC.02465-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sime FB, Stuart J, Butler J, Starr T, Wallis SC, Pandey S, Lipman J, Roberts JA. 2018. Pharmacokinetics of intravenous posaconazole in critically ill patients. Antimicrob Agents Chemother 62. doi: 10.1128/AAC.00242-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Stelzer D, Weber A, Ihle F, Matthes S, Ceelen F, Zimmermann G, Kneidinger N, Schramm R, Winter H, Zoller M, Vogeser M, Behr J, Neurohr C. 2018. Posaconazole liquid vs tablet formulation in lung transplant recipients. Mycoses 61:186–194. doi: 10.1111/myc.12724 [DOI] [PubMed] [Google Scholar]
- 166. Cojutti PG, Candoni A, Lazzarotto D, Rabassi N, Fanin R, Hope W, Pea F. 2018. Co-administration of proton pump inhibitors and/or of steroids may be a risk factor for low trough concentrations of posaconazole delayed-released tablets in adult patients with haematological malignancies. Br J Clin Pharmacol 84:2544–2550. doi: 10.1111/bcp.13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Vehreschild JJ, Müller C, Farowski F, Vehreschild M, Cornely OA, Fuhr U, Kreuzer K-A, Hallek M, Kohl V. 2012. Factors influencing the pharmacokinetics of prophylactic posaconazole oral suspension in patients with acute myeloid leukemia or myelodysplastic syndrome. Eur J Clin Pharmacol 68:987–995. doi: 10.1007/s00228-012-1212-y [DOI] [PubMed] [Google Scholar]
- 168. Boglione-Kerrien C, Picard S, Tron C, Nimubona S, Gangneux J-P, Lalanne S, Lemaitre F, Bellissant E, Verdier M-C, Petitcollin A. 2018. Safety study and therapeutic drug monitoring of the oral tablet formulation of posaconazole in patients with haematological malignancies. J Cancer Res Clin Oncol 144:127–134. doi: 10.1007/s00432-017-2523-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Allegra S, Fatiguso G, De Francia S, Favata F, Pirro E, Carcieri C, De Nicolò A, Cusato J, Di Perri G, D’Avolio A. 2017. Evaluation of posaconazole pharmacokinetics in adult patients with invasive fungal infection. Biomedicines 5:66. doi: 10.3390/biomedicines5040066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hoenigl M, Raggam RB, Salzer HJF, Valentin T, Valentin A, Zollner-Schwetz I, Strohmeier AT, Seeber K, Wölfler A, Sill H, Krause R. 2012. Posaconazole plasma concentrations and invasive mould infections in patients with haematological malignancies. Int J Antimicrob Agents 39:510–513. doi: 10.1016/j.ijantimicag.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 171. Eiden C, Meniane JC, Peyrière H, Eymard-Duvernay S, Le Falher G, Ceballos P, Fegueux N, Cociglio M, Reynes J, Hillaire-Buys D. 2012. Therapeutic drug monitoring of posaconazole in hematology adults under posaconazole prophylaxis: influence of food intake. Eur J Clin Microbiol Infect Dis 31:161–167. doi: 10.1007/s10096-011-1288-9 [DOI] [PubMed] [Google Scholar]
- 172. Gross BN, Ihorst G, Jung M, Wäsch R, Engelhardt M. 2013. Posaconazole therapeutic drug monitoring in the real-life setting: a single-center experience and review of the literature. Pharmacotherapy 33:1117–1125. doi: 10.1002/phar.1328 [DOI] [PubMed] [Google Scholar]
- 173. Dolton MJ, Brüggemann RJM, Burger DM, McLachlan AJ. 2014. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob Agents Chemother 58:6879–6885. doi: 10.1128/AAC.03777-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. 2015. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses 58:432–436. doi: 10.1111/myc.12339 [DOI] [PubMed] [Google Scholar]
- 175. Mattiuzzi G, Yilmaz M, Kantarjian H, Borthakur G, Konopleva M, Jabbour E, Brown Y, Pierce S, Cortes J. 2015. Pharmacokinetics of posaconazole prophylaxis of patients with acute myeloid leukemia. J Infect Chemother 21:663–667. doi: 10.1016/j.jiac.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gesquiere I, Hens B, Van der Schueren B, Mols R, de Hoon J, Lannoo M, Matthys C, Foulon V, Augustijns P. 2016. Drug disposition before and after gastric bypass: fenofibrate and posaconazole. Br J Clin Pharmacol 82:1325–1332. doi: 10.1111/bcp.13054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother 53:958–966. doi: 10.1128/AAC.01034-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Koselke E, Kraft S, Smith J, Nagel J. 2012. Evaluation of the effect of obesity on voriconazole serum concentrations. J Antimicrob Chemother 67:2957–2962. doi: 10.1093/jac/dks312 [DOI] [PubMed] [Google Scholar]
- 179. Sebaaly JC, MacVane SH, Hassig TB. 2016. Voriconazole concentration monitoring at an academic medical center. Am J Health Syst Pharm 73:S14–21. doi: 10.2146/ajhp150372 [DOI] [PubMed] [Google Scholar]
- 180. Richards PG, Dang KM, Kauffman CA, Stalker KL, Sudekum D, Kerr L, Brinker-Bodley M, Cheriyan B, West N, Collins CD, Polega S, Malani AN. 2017. Therapeutic drug monitoring and use of an adjusted body weight strategy for high-dose voriconazole therapy. J Antimicrob Chemother 72:1178–1183. doi: 10.1093/jac/dkw550 [DOI] [PubMed] [Google Scholar]
- 181. Pascual A, Csajka C, Buclin T, Bolay S, Bille J, Calandra T, Marchetti O. 2012. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics–based analysis of adult patients with invasive fungal infections. Clin Infect Dis 55:381–390. doi: 10.1093/cid/cis437 [DOI] [PubMed] [Google Scholar]
- 182. Diller E, Krekel T, Spec A, Klaus J. 2021. Evaluation of total body weight versus adjusted body weight voriconazole dosing in obese patients. Antimicrob Agents Chemother 65:e0246020. doi: 10.1128/AAC.02460-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Davies-Vorbrodt S, Ito JI, Tegtmeier BR, Dadwal SS, Kriengkauykiat J. 2013. Voriconazole serum concentrations in obese and overweight immunocompromised patients: a retrospective review. Pharmacotherapy 33:22–30. doi: 10.1002/phar.1156 [DOI] [PubMed] [Google Scholar]
- 184. Hope WW, Goodwin J, Felton TW, Ellis M, Stevens DA. 2012. Population pharmacokinetics of conventional and intermittent dosing of liposomal amphotericin B in adults: a first critical step for rational design of innovative regimens. Antimicrob Agents Chemother 56:5303–5308. doi: 10.1128/AAC.00933-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ting MH, Spec A, Micek ST, Ritchie DJ, Krekel T. 2021. Evaluation of total body weight versus adjusted body weight liposomal amphotericin B dosing in obese patients. Antimicrob Agents Chemother 65:e0236620. doi: 10.1128/AAC.02366-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wasmann RE, Smit C, van Dongen EPH, Wiezer RMJ, Adler-Moore J, de Beer YM, Burger DM, Knibbe CAJ, Brüggemann RJM. 2020. Fixed dosing of liposomal amphotericin B in morbidly obese individuals. Clin Infect Dis 70:2213–2215. doi: 10.1093/cid/ciz885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Nix DE, Hayes JF, Al Obaidi M, Zangeneh T. 2021. Fixed dosing of amphotericin B in morbidly obese individuals. Clin Infect Dis 72:e431. doi: 10.1093/cid/ciaa1076 [DOI] [PubMed] [Google Scholar]
- 188. Gillum JG, Johnson M, Lavoie S, Venitz J. 1995. Flucytosine dosing in an obese patient with extrameningeal cryptococcal infection. Pharmacotherapy 15:251–253. [PubMed] [Google Scholar]
- 189. Richelsen RKB, Jensen SB, Nielsen H. 2018. Incidence and predictors of intravenous acyclovir-induced nephrotoxicity. Eur J Clin Microbiol Infect Dis 37:1965–1971. doi: 10.1007/s10096-018-3332-5 [DOI] [PubMed] [Google Scholar]
- 190. Barber KE, Wagner JL, Stover KR. 2019. Impact of obesity on acyclovir-induced nephrotoxicity. Open Forum Infect Dis 6:ofz121. doi: 10.1093/ofid/ofz121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Ryan L, Heed A, Foster J, Valappil M, Schmid ML, Duncan CJA. 2018. Acute kidney injury (AKI) associated with intravenous aciclovir in adults: incidence and risk factors in clinical practice. Int J Infect Dis 74:97–99. doi: 10.1016/j.ijid.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 192. Lee EJ, Jang HN, Cho HS, Bae E, Lee TW, Chang S-H, Park DJ. 2018. The incidence, risk factors, and clinical outcomes of acute kidney injury (staged using the RIFLE classification) associated with intravenous acyclovir administration. Ren Fail 40:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Turner RB, Cumpston A, Sweet M, Briggs F, Slain D, Wen S, Craig M, Hamadani M, Petros W. 2016. Prospective, controlled study of acyclovir pharmacokinetics in obese patients. Antimicrob Agents Chemother 60:1830–1833. doi: 10.1128/AAC.02010-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wong A, Pickering AJ, Potoski BA. 2017. Dosing practices of intravenous acyclovir for herpes encephalitis in obesity: results of a pharmacist survey. J Pharm Pract 30:324–328. doi: 10.1177/0897190016642689 [DOI] [PubMed] [Google Scholar]
- 195. UCSF Medical Center: Foscarnet Dosing and Monitoring . Infectious diseases management program at UCSF. Available from: https://idmp.ucsf.edu/sites/g/files/tkssra4251/f/wysiwyg/UCSF%20Medical%20Center_%20Foscarnet%20Dosing%20and%20Monitoring.pdf. Retrieved 20 Oct 2023.
- 196. Miller W, January S, Klaus J, Neuner E, Pande A, Krekel T. 2023. Safety and efficacy of weight-based ganciclovir dosing strategies in overweight/obese patients. Transpl Infect Dis 25:e14134. doi: 10.1111/tid.14134 [DOI] [PubMed] [Google Scholar]
- 197. Misaki Y, Kimura S-I, Kawamura M, Kawamura S, Takeshita J, Yoshino N, Yoshimura K, Gomyo A, Matsumi S, Akahoshi Y, Tamaki M, Kusuda M, Kameda K, Wada H, Kawamura K, Sato M, Terasako-Saito K, Tanihara A, Nakasone H, Kako S, Kanda Y. 2020. Impact of the patient’s body weight on the efficacy and adverse events of valganciclovir for cytomegalovirus reactivation after hematopoietic stem cell transplantation. Transpl Infect Dis 22:e13270. doi: 10.1111/tid.13270 [DOI] [PubMed] [Google Scholar]
- 198. Pai MP, Lodise TP. 2011. Oseltamivir and oseltamivir carboxylate pharmacokinetics in obese adults: dose modification for weight is not necessary ▿. Antimicrob Agents Chemother 55:5640–5645. doi: 10.1128/AAC.00422-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Jittamala P, Pukrittayakamee S, Tarning J, Lindegardh N, Hanpithakpong W, Taylor WRJ, Lawpoolsri S, Charunwattana P, Panapipat S, White NJ, Day NPJ. 2014. Pharmacokinetics of orally administered oseltamivir in healthy obese and nonobese Thai subjects. Antimicrob Agents Chemother 58:1615–1621. doi: 10.1128/AAC.01786-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Thorne-Humphrey LM, Goralski KB, Slayter KL, Hatchette TF, Johnston BL, McNeil SA, 2009 OPTIMO Study Group . 2011. Oseltamivir pharmacokinetics in morbid obesity (OPTIMO trial). J Antimicrob Chemother 66:2083–2091. doi: 10.1093/jac/dkr257 [DOI] [PubMed] [Google Scholar]
- 201. Alffenaar J-W, van der Werf T. 2010. Dosing ethambutol in obese patients. Antimicrob Agents Chemother 54:4044; doi: 10.1128/AAC.00412-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hall RG, Swancutt MA, Meek C, Leff RD, Gumbo T. 2012. Ethambutol pharmacokinetic variability is linked to body mass in overweight, obese, and extremely obese people. Antimicrob Agents Chemother 56:1502–1507. doi: 10.1128/AAC.05623-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. 2010. Efflux-pump derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and ethambutol pharmacokinetics-pharmacodynamics. J Infect Dis 201:1225–1231. doi: 10.1086/651377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Geiseler PJ, Manis RD, Maddux MS. 1985. Dosage of antituberculous drugs in obese patients. Am Rev Respir Dis 131:944–946. doi: 10.1164/arrd.1985.131.6.944 [DOI] [PubMed] [Google Scholar]
- 205. Susanto BO, Svensson RJ, Svensson EM, Aarnoutse R, Boeree MJ, Simonsson USH. 2020. Rifampicin can be given as flat-dosing instead of weight-band dosing. Clin Infect Dis 71:3055–3060. doi: 10.1093/cid/ciz1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Meng L, Mui E, Ha DR, Stave C, Deresinski SC, Holubar M. 2023. Comprehensive guidance for antibiotic dosing in obese adults: 2022 update. Pharmacotherapy 43:226–246. doi: 10.1002/phar.2769 [DOI] [PubMed] [Google Scholar]
- 207. Roberts JA, Croom K, Adomakoh N. 2023. Continuous infusion of beta-lactam antibiotics: narrative review of systematic reviews, and implications for outpatient parenteral antibiotic therapy. Expert Rev Anti Infect Ther 21:375–385. doi: 10.1080/14787210.2023.2184347 [DOI] [PubMed] [Google Scholar]
- 208. Abdul-Aziz MH, Alffenaar J-WC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, Neely MN, Paiva J-A, Pea F, Sjovall F, Timsit JF, Udy AA, Wicha SG, Zeitlinger M, De Waele JJ, Roberts JA, Infection Section of European Society of Intensive Care Medicine (ESICM), Pharmacokinetic/pharmacodynamic and Critically Ill Patient Study Groups of European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Group of International Association of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT), Infections in the ICU and Sepsis Working Group of International Society of Antimicrobial Chemotherapy (ISAC) . 2020. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 46:1127–1153. doi: 10.1007/s00134-020-06050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Santibañez M, Bunnell K, Harrington A, Bleasdale S, Wenzler E. 2019. Association between estimated pharmacokinetic/pharmacodynamic predictions of efficacy and observed clinical outcomes in obese and nonobese patients with Enterobacteriaceae bloodstream infections. Open Forum Infect Dis 6:ofz400. doi: 10.1093/ofid/ofz400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, Goutelle S, Lefeuvre S, Mongardon N, Roger C, Scala-Bertola J, Lemaitre F, Garnier M. 2019. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care 23:104. doi: 10.1186/s13054-019-2378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Lambert A, Regnouf-de-Vains J-B, Ruiz-López MF. 2007. Structure of levofloxacin in hydrophilic and hydrophobic media: relationship to its antibacterial properties. Chemical Physics Letters 442:281–284. doi: 10.1016/j.cplett.2007.05.077 [DOI] [Google Scholar]
- 212. Pai MP, Wilcox M, Chitra S, McGovern P. 2021. Safety and efficacy of omadacycline by body mass index in patients with community-acquired bacterial pneumonia: subanalysis from a randomized controlled trial. Respir Med 184:106442. doi: 10.1016/j.rmed.2021.106442 [DOI] [PubMed] [Google Scholar]
- 213. Kingsley J, Mehra P, Lawrence LE, Henry E, Duffy E, Cammarata SK, Pullman J. 2016. A randomized, double-blind, phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother 71:821–829. doi: 10.1093/jac/dkv411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pullman J, Gardovskis J, Farley B, Sun E, Quintas M, Lawrence L, Ling R, Cammarata S, PROCEED Study Group . 2017. Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a phase 3, double-blind, randomized study. J Antimicrob Chemother 72:3471–3480. doi: 10.1093/jac/dkx329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Crass RL, Dunn R, Hong J, Krop LC, Pai MP. 2018. Dosing vancomycin in the super obese: less is more. J Antimicrob Chemother 73:3081–3086. doi: 10.1093/jac/dky310 [DOI] [PubMed] [Google Scholar]
- 216. Monteiro JF, Hahn SR, Gonçalves J, Fresco P. 2018. Vancomycin therapeutic drug monitoring and population pharmacokinetic models in special patient subpopulations. Pharmacol Res Perspect 6:e00420. doi: 10.1002/prp2.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Szymański M, Chmielewska S, Czyżewska U, Malinowska M, Tylicki A. 2022. Echinocandins – structure, mechanism of action and use in antifungal therapy. J Enzyme Inhib Med Chem 37:876–894. doi: 10.1080/14756366.2022.2050224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Theuretzbacher U. 2004. Pharmacokinetics/pharmacodynamics of echinocandins. Eur J Clin Microbiol Infect Dis 23:805–812. doi: 10.1007/s10096-004-1228-z [DOI] [PubMed] [Google Scholar]
- 219. Muilwijk EW, Lempers VJC, Burger DM, Warris A, Pickkers P, Aarnoutse RE, Brüggemann RJM. 2015. Impact of special patient populations on the pharmacokinetics of echinocandins. Expert Rev Anti Infect Ther 13:799–815. doi: 10.1586/14787210.2015.1028366 [DOI] [PubMed] [Google Scholar]
- 220. Alsowaida YS, Alamer A, Thabit AK, Almulhim AS, Aleissa MM, Kalbasi A, Eljaaly K, Almangour TA, Erstad BL. 2023. Echinocandin exposures in obese patients: a scoping review and clinical perspectives. Am J Health Syst Pharm 80:503–517. doi: 10.1093/ajhp/zxad021 [DOI] [PubMed] [Google Scholar]
- 221. Lepak AJ, Andes DR. 2014. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 5:a019653. doi: 10.1101/cshperspect.a019653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Barone JA, Moskovitz BL, Guarnieri J, Hassell AE, Colaizzi JL, Bierman RH, Jessen L. 1998. Enhanced bioavailability of itraconazole in hydroxypropyl-beta-cyclodextrin solution versus capsules in healthy volunteers. Antimicrob Agents Chemother 42:1862–1865. doi: 10.1128/AAC.42.7.1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Poirier JM, Cheymol G. 1998. Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 35:461–473. doi: 10.2165/00003088-199835060-00004 [DOI] [PubMed] [Google Scholar]
- 224. Theuretzbacher U, Ihle F, Derendorf H. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet 45:649–663. doi: 10.2165/00003088-200645070-00002 [DOI] [PubMed] [Google Scholar]
- 225. Guarascio AJ, Slain D. 2015. Review of the new delayed-release oral tablet and intravenous dosage forms of posaconazole. Pharmacotherapy 35:208–219. doi: 10.1002/phar.1533 [DOI] [PubMed] [Google Scholar]
- 226. Rybak JM, Marx KR, Nishimoto AT, Rogers PD. 2015. Isavuconazole: pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy 35:1037–1051. doi: 10.1002/phar.1652 [DOI] [PubMed] [Google Scholar]
- 227. World Health Organization . 2010. Treatment of tuberculosis: guidelines. 4th ed [PubMed] [Google Scholar]
- 228. Alffenaar J-W, de Steenwinkel JEM, Diacon AH, Simonsson USH, Srivastava S, Wicha SG. 2022. Pharmacokinetics and pharmacodynamics of anti-tuberculosis drugs: an evaluation of in vitro, in vivo methodologies and human studies. Front Pharmacol 13:1063453. doi: 10.3389/fphar.2022.1063453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. 2015. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 211 Suppl 3:S96–S106. doi: 10.1093/infdis/jiu610 [DOI] [PubMed] [Google Scholar]