Abstract
Objective
To isolate bioactive compounds from the endophytic fungus Fusarium sporotrichioides isolated from Rauwolfia yunnanensis, and investigate their pharmacological activities.
Methods
The chemical constituents were isolated and purified by combining with ODS column chromatography, silica gel column chromatography and by performing semipreparative HPLC. Their structures were established on the basis of 1D NMR (1H-NMR and 13C-NMR) and 2D NMR (1H–1H COSY, HSQC, HMBC and NOESY), as well as HRESIMS and comparison with literature data. In addition, the absolute configuration of compound 1 was determined by calculated ECD data.
Results
One previously undescribed tetracyclic triterpenoid derivative, named as integracide L (1), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β,11β-triol (2), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-2α,3β,11β-triol (3), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-3β,11β-triol (4), and 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β,11β-triol (5) were isolated from F. sporotrichioide. Moreover, compound 1 was rare tetracyclic triterpenoid with single methyl replacement at C-4 position.
Conclusion
Compound 1 was a new tetracyclic triterpenoid isolated from the endophytic fungus F. sporotrichioides. In addition, compound 2 could inhibit the growth of three different human cancer cells significantly. Compounds 3 and 5 were found to possess better cytotoxic activities on HepG-2 cells than the other compounds, with IC50 values of (2.8 ± 0.1) and (6.3 ± 0.3) μmol/L respectively.
Keywords: antitumor; Fusarium sporotrichioides; integracide L; tetracyclic triterpenoid; 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8 14-diene-2α, 3β,11β-triol; 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-2α, 3β, 11β-triol; 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β, 11β-triol
1. Introduction
Rauwolfia yunnanensis Tsiang is a type of shrub, belongs to a genus of Apocynaceae family. There are about 135 species of Apocynaceae in the world. Among them, there are nine species in China. R. yunnanensis mainly distributed in Yunnan, Guizhou, Guangxi and other southwestern provinces of China. In China, the root of R. yunnanensis is mainly used as medicine to treat scabies (Flora of China, 1977). Studies have shown that there were a large number of monoterpene indole alkaloids and other types of alkaloids in R. yunnanensis (Cheng et al., 2009, Hu et al., 2006). The pharmacological activities of these alkaloids were mainly anti-tumor, blood pressure lowering, anti-arrhythmia, anti-inflammation, sedation and anti-pruritu (Li, Shi, Liu, & Zou, 2019).
Endophytic fungi are a kind of microbial resources with a broad prospecting application, which can produce abundant secondary metabolites with novel structures and high bioactivities. In recent years, compounds with novel structures have been isolated from endophytic fungi, which have become an important source of searching active leading compounds. Many studies have shown that endophytic fungi could not only produce the same or similar structures as host plants (Strobel et al., 1994, Amna et al., 2006), but also synthesis a number of other bioactive metabolites, indicating that endophytic fungi have great potentiality for developing (Fraga et al., 2003, Sato et al., 2018, Wang et al., 2018, Lardon et al., 2019, Wang et al., 2020, Zi et al., 2014).
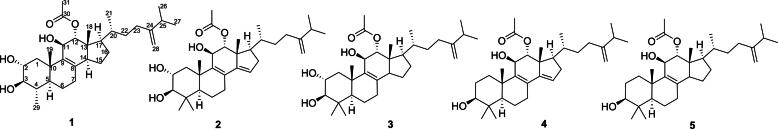
At present, there have no research report on the secondary metabolites of the endophytic fungi of R. yunnanensis at home and abroad. In order to obtain the active leading compounds with novel structures, In this paper, the secondary metabolites of endophytic fungus Fusarium sporotrichioides from R. yunnanensis were isolated and identified (Fig. 1) including one new compound. Also, the bioactivities of those compounds were evaluated as well.
Fig. 1.
Structures of isolated compounds 1–5.
2. Materials and methods
2.1. General experimental procedures
Optical rotations were measured on NICOLET iS5 (Thermo, Waltham, USA) spectrometer, UV spectra were recorded on Evolution 220 UV/Vis spectrometer (Thermo, Waltham, USA). IR spectra were measured on IR Tracer-100 (Shimadzu, Kyoto, Japan). 1D and 2D NMR spectra were measured on a Bruker AV-600 spectrometer (Karlsruhe, Germany). HRESIMS data were determined by Waters Vion IMS QTOf mass spectrometer (Milford, USA). ECD spectra were measured on Biologic MOS-450 circular dichrometer (Grenoble, France). Open column chromatography (CC) was performed using silica gel (200–300 mesh, Qingdao Haiyang Chemical Group Co., Qingdao, China), and ODS (50 μm, YMC, Kyoto, Japan). The samples were purified by semipreparative high-performance liquid chromatography (HPLC, Kyoto, Japan) equipped with an LC-20AR system, a UV detector, and an HPLC column (250 mm × 10 mm, 5 μm, COSMOSIL AR II-C18 column; COSMOSIL MS II-C18 column).
2.2. Strain isolation and cultivation
The fungal strain was isolated from the inner tissue of the surface-sterilized fruit of R. yunnanensis, which was collected in Yunnan Province in China in 2017. The fungus was identified as F. sporotrichioides by its rRNA gene sequence with the Genbank accession number OP630489. A voucher specien (No. luo4-fruit-2i-b) was deposited at the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing. The strain was fermented on autoclaved rice solid-substrate medium (eighty 500 mL Erlenmeyer flasks, each containing 40 g rice, 60 mL water) for 12 d at 28 °C.
2.3. Extraction and isolation
Following incubation, the rice culture was extracted three times with EtOAc and concentrated under vacuum. The concentrated extract (22.3 g) was mixed with 500 mL distilled H2O and partitioned between petroleum ether and 95% MeOH. The total 95% MeOH extract (10.2 g) was fractionated by ODS flash column chromatography (5 cm × 30 cm) eluting with 2 L of each of 20%, 40%, 60%, 80%, and 100% (volume percent) MeOH in H2O. The fraction eluted with 100% MeOH was divided into eight subfractions (G1 − G8) by using CC (silica gel 200–300 mesh, petroleum ether/acetone 1:0 to 0:1), and then, subfraction G8 was purified by semipreparative HPLC (Cosmosil AR II-C18 column, 250 mm × 10 mm i.d., 5 μm, 3 mL/min) with a gradient elution from 80% to 95% MeOH in H2O with 0.1% AcOH to afford compounds 4 (tR = 34.6 min, 5.0 mg) and 5 (tR = 38.9 min, 4.5 mg). The eighth subfraction G8 was further purified by semipreparative HPLC (Cosmosil MS II-C18 column, 250 mm × 10 mm i.d., 5 μm, 3 mL/min) with 84% ACN in H2O with 0.1% AcOH to yield compounds 2 (tR = 26.3 min, 5.5 mg), 1 (tR = 29.9 min, 2.5 mg) and 3 (tR = 41.2 min, 6.5 mg).
2.3.1. Integracide L (1)
White power; +55 (c 0.1, MeOH); UV (MeOH) λmax (log) 200 (3.62) nm, 241 (3.89) nm; IR (KBr) 3452, 1727, 1629, and 898 cm−1. CD (MeOH) 218 nm (Δε − 2.63); For 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) spectroscopic data (Table 1). HRESIMS m/z [M + H]+ 503.3732 (calcd for 503.3731).
Table 1.
1H-NMR (600 MHz) and 13C-NMR (150 MHz) spectral data for compound 1 in CD3OD.
| Position | δH (J-Hz) | δC, type | Position | δH (J-Hz) | δC, type |
|---|---|---|---|---|---|
| 1 | 1.17, m | 43.2, CH2 | 1.98–2.01, m | ||
| 2.38, dd (12.1, 4.6) | 17 | 1.58–1.63, m | 48.5, CH | ||
| 2 | 3.60, ddd (12.1, 9.6, 4.6) | 72.7, CH | 18 | 0.89, s | 12.5, CH3 |
| 3 | 2.80, t (9.6) | 82.4, CH | 19 | 1.19, s | 22.9, CH3 |
| 4 | 1.38, overlapped | 38.6, CH | 20 | 1.44–1.51, m | 36.7, CH |
| 5 | 0.99–1.01, m | 49.2, CH | 21 | 0.92, d (6.5) | 18.5, CH3 |
| 6 | 1.35, m | 22.3, CH2 | 22 | 1.09–1.13, m | 35.7, CH2 |
| 1.84, ddd (13.6, 7.9, 3.0) | 1.54–1.58, m | ||||
| 7 | 1.98–2.01, m | 28.5, CH2 | 23 | 1.89–1.96, m | 32.1, CH2 |
| 2.12–2.16, m | 2.11–2.15, m | ||||
| 8 | − | 133.2, C | 24 | − | 157.6, C |
| 9 | − | 136.1, C | 25 | 2.22–2.29, m | 34.9, CH |
| 10 | − | 38.2, C | 26 | 1.03–1.05, m | 22.5, CH3 |
| 11 | 4.08, br s | 71.4, CH | 27 | 1.03–1.05, m | 22.0, CH3 |
| 12 | 5.10, br s | 81.9, CH | 28 | 4.66, d (1.4) | 107.0, CH2 |
| 13 | − | 43.8, C | 4.74, d (1.4) | ||
| 14 | 2.50, dd (11.8, 8.2) | 47.2, CH | 29 | 1.03, m | 15.7, CH3 |
| 15 | 1.44–1.51, m | 23.9, CH2 | 30 | − | 172.3, C |
| 1.69–1.76, m | 31 | 2.06, s | 21.1, CH3 | ||
| 16 | 1.41–1.43, m | 29.1, CH2 |
Note: a Measured in CD3OD at 600 MHz for 1H-NMR and 150 MHz for 13C-NMR. Proton coupling constants (J) in Hz are given in parentheses. Assignments were based on 1H–1H COSY, HSQC, and HMBC experiments.
2.3.2. 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β,11β-triol (2)
White power; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) spectroscopic data, see Table S1.
2.3.3. 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-2α,3β,11β-triol (3)
White power; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) spectroscopic data, see Table S2.
2.3.4. 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-3β,11β-triol (4)
White power; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) spectroscopic data, see Table S3.
2.3.5. 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β,11β-triol (5)
White power; 1H-NMR (600 MHz, CD3OD) and 13C-NMR (150 MHz, CD3OD) spectroscopic data, see Table S4.
2.4. Cytotoxicity assays
Human hepatocellular carcinomas HepG-2 cells, human pulmonary epithelial A549 cells and human cervical carcinoma Hela cells, were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum. All the cultures were maintained in an incubator at 37 °C with 5% CO2 in a humidified atmosphere. Cell viability was measured by Cell Counting Kit (CCK)-8 (Dojindo, Tokyo, Japan) assay. A549, Hela and HepG-2 cells (5.0 × 103 cells per well) were seeded into 96-well plates (Corning, NY, USA) and cultured for 24 h. Cells were then incubated with fresh media containing the compound sunder study at various concentrations for 24, 48, or 72 h. After incubation, the media were removed and the wells were washed twice with PBS to remove non-adherent cells. Then, 100 μL fresh medium and 10 μL CCK-8 were added to each well at the indicated time points. The cells were further incubated at 37 °C for 60 min. The absorbance of the samples was measured at 450 nm using a Bio-Rad model 3550 micro platereader (Richmond, CA, USA).
2.5. Computational details
The conformational analyses of compound 1 were carried out by MOE 2018 using MMFF94 with an energy cutoff of 7 kcal/mol. The obtained stable conformers were optimized using Gaussian09 at b3lyp/6-31 g(d) level in gas phase. The optimized stable conformers were further subjected to ECD calculations at cam-b3lyp/6-31 + g(d) level with the PCM solvation model of methanol with the first 10 electronic excitations. The overall theoretical ECD data were weighted by Boltzmann distribution, and the ECD spectra were produced by SpecDis 1.70 software (UV correction = 14 nm, sigma = 0.3 eV).
3. Results and discussion
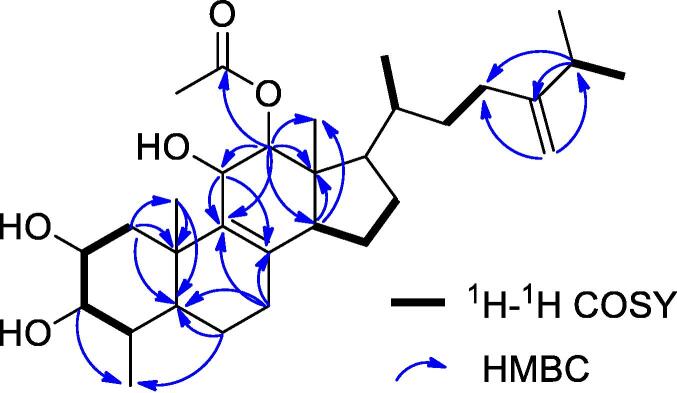
Compound 1 was obtained as white amorphous powder. The molecular formula of 1 was established as C31H50O5 based on HR-ESI-MS m/z 503.3732 [M + H]+ (calcd for C31H51O5, 503.3731), which indicated seven degrees of unsaturation. Its IR spectrum showed absorption bands at 3 452, 1 727, 1 629, and 898 cm−1, suggested the presence of hydroxyl, ester carbonyl, and a terminal methylene group, respectively. The 13C-NMR and HSQC spectra displayed 31 carbon resonances: seven methyls, eight methylenes, ten methines, four of them for oxygenated methine carbons, and six quaternary carbons, including one carbonyls and three olefinic carbons. The 1H and 13C-NMR data of 1 were closely related to those of co-isolated12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-2α,3β,11β-triol (3), displaying identical signals for rings B, C, and D, and the side chain (Table 1). Differences were recognized in the ring A: The loss of one methyl group in 1, and one quartery carbon (δC 39.5) in 3 replaced by one methine group (δH 1.38; δC 38.6). The methyl linkage was established based on the key HMBC correlation (Fig. 2) between the methyl group at H-3 (δH 2.80) and C-29 (δC 15.7), which indicated that the methyl group linkage was at the C-4 position.
Fig. 2.
Key 1H–1H COSY and HMBC correlations of compound 1.
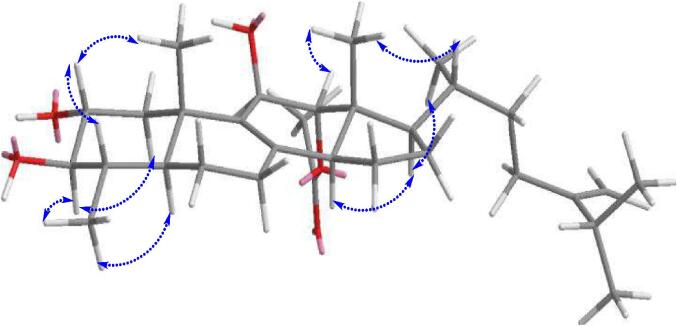
The relative configuration of compound 1 was established by NOESY (Fig. 3) and comparison of coupling constants. The configuration of H-2 deduced from the big proton coupling constant between H-2 and the axial proton of H-3 (J = 9.6 Hz). The orientation of the methyl group at C-29 position was assigned to be α-oriented according to the NOESY spectrum, which showed correlations of H-3 (δH 2.80)/Me-29 (δH 1.03). The observed NOESY correlations of H-2 (δH 3.60)/Me-19 (δH 1.19)/H-4 (δH 1.38) indicated β-configuration. In addition, the NOESY correlation of H-14 (δH 2.50)/H-17 (δH 1.60), and of the both protons with H2-15 (δH 1.72) led the assignment of an α-orientation for H-14 and H-17. Furthermore, in the NOESY spectrum of 1, observation of the NOE correlations between Me-18 (δH 0.89) with both H-20 (δH 1.44) and H-12 (δH 5.10), suggested that H-20, Me-18, and H-12 are β-oriented. Additionally, NOESY correlations between H-17 (δH 1.60) with both Me-21 (δH 0.92) and H-14 (δH 2.50) suggested that Me-21, H-17, and H-14 are all α-oriented. According to the overall analysis, the structure of compound 1 was identified as 12α-acetoxy-4α-methyl-24-methylene-5α-cholesta-8-momoene-2α,3β,11β-triol. The ECD spectrum of 1 showed a negative Cotton effect at 218 nm and it was then submitted for theoretical ECD calculation, and the calculated curve matched with that of the experimental one. Thus, the absolute configuration of 1 was thus assigned as 2R, 3R, 4S, 5S, 10S, 11R, 12R, 13R, 14S, 17R and 20R.
Fig. 3.
Key NOESY correlations of compound 1.
The structures of the four known compounds were identified as 12α-acetoxy-4, 4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β,11β-triol (2) (Singh, Ondeyka, Schleif, Felock, & Hazuda, 2003), 12α-acetoxy-4, 4-dimethyl-24-methylene-5α-cholesta-8-momoene-2α,3β,11β-triol (3) (Singh, Ondeyka, Schleif, Felock & Hazuda, 2003), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-3β,11β-triol(4) (Zheng et al., 2014), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β,11β-triol (5) (Singh et al., 2003). Their structures were elucidated by comparing the spectroscopic data with reported values.
As shown in Table 2, compound 2 could inhibit the growth of three different human cancer cells, and compounds 3 and 5 were found to possess better cytotoxic activity on HepG-2 cells than the other compounds, with IC50 values of (2.8 ± 0.1) μmol/L and (6.3 ± 0.3) μmol/L respectively.
Table 2.
Cytotoxic activities of compounds 1–5.
| Compounds | IC50a (μmol/L) |
||
|---|---|---|---|
| A549 | HepG-2 | HeLa | |
| 1 | 19.9 ± 1.1 | 22.4 ± 2.2 | 32.9 ± 4.1 |
| 2 | 2.6 ± 0.4 | 4.1 ± 0.3 | 3.5 ± 0.9 |
| 3 | 14.9 ± 0.4 | 2.8 ± 0.1 | 20.0 ± 1.6 |
| 4 | 21.6 ± 1.2 | 12.9 ± 3.1 | 20.6 ± 4.0 |
| 5 | 14.8 ± 1.8 | 6.3 ± 0.3 | 15.5 ± 0.3 |
| HCTP b | 1.6 ± 0.2 | 0.5 ± 0.1 | 0.2 ± 0.1 |
Note: a IC50 value of compounds against A549, HeLa, and HepG-2 cells, which was defined as concentration (μmol/L) that caused 50% inhibition of cell growth in vitro;b HydroCxycamptothecin (HCTP) was used as a positive control.
4. Conclusion
In this study, a novel tetracyclic triterpenoid, named integracide L (1), together with four known compounds were isolated from endophytic fungus F. sporotrichioides. The structures of these compounds were elucidated based on HR-ESI-MS and spectroscopic analysis (NMR, ECD), then their cytotoxicity activities were evaluated. Compared to the others, compound 2 showed the most significant inhibiting activities against A549, HeLa, and HepG-2 cells. Compounds 3 and 5 exhibited significantly cytotoxic activities against HepG-2 cells. In addition, Compound 5 exhibited moderate cytotoxic activities against A549 and HeLa cells. Compound 3 also showed mild cytotoxic activity against A549. These results provided evidences for tetracyclic triterpenoids as potential anti-tumor drug candidates.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by Outstanding Youth Foundation of Heilongjiang province (NO. YQ2021H009).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2022.10.007.
Contributor Information
Chang Li, Email: lichang661@126.com.
Yuehu Pei, Email: peiyueh@vip.163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Amna T., Puri S.C., Verma V., Sharma J.P., Khajuria R.K., Musarrat J.…Qazi G.N. Bioreactor studies on the endophytic fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin. Canadian Journal of Microbiology. 2006;52(3):189–196. doi: 10.1139/w05-122. [DOI] [PubMed] [Google Scholar]
- Cheng B.H., Chen J.C., Qiu M.H. A new monoterpenoid indole alkaloid from Rauolfia yunnanensis. Chinese Chemical Letter. 2009;20(10):1221–1223. [Google Scholar]
- Fraga B.M., Alvarez L., Suárez S. Biotransformation of the diterpenes epicandicandiol and candicandiol by Mucor plumbeus. Journal of Natural Products. 2003;66(3):327–331. doi: 10.1021/np020420x. [DOI] [PubMed] [Google Scholar]
- Hu X.J., He H.P., Zhou H., Di Y.T., Yang X.W., Hao X.J., Kong L.Y. New indole alkaloids from Rauvolfia yunnanensis. Helvetica Chimica Acta. 2006;89(7):1344–1350. [Google Scholar]
- Lardon N., Liffert R., Linden A., Gademann K. The furan shuffling hypothesis: A biogenetic proposal for eremophilane sesquiterpenoids. Angewandte Chemie International Edition. 2019;58(21):7004–7007. doi: 10.1002/anie.201901898. [DOI] [PubMed] [Google Scholar]
- Li L.M., Shi S.D., Liu Y., Zou Q. Bioactivity-guided isolation and identification of new and immunosuppressive monoterpenoid indole alkaloids from Rauvolfa yunnanensis Tsiang. Molecules. 2019;24(24):4574. doi: 10.3390/molecules24244574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Mitsuhashi T., Yamazaki M., Abe I., Uchiyama M. Computational studies on biosynthetic carbocation rearrangements leading to quiannulatene: Initial conformation regulates biosynthetic route, stereochemistry, and type of skeleton. Angewandte Chemie International Edition. 2018;57(45):14752–14757. doi: 10.1002/anie.201807139. [DOI] [PubMed] [Google Scholar]
- Singh S.B., Ondeyka J.G., Schleif W.A., Felock P., Hazuda D.J. Chemistry and structure-activity relationship of HIV-1 integrase inhibitor integracide B and related natural products. Journal of Natural Products. 2003;66(10):1338–1344. doi: 10.1021/np030211s. [DOI] [PubMed] [Google Scholar]
- Singh S.B., Zink D.L., Dombrowski A.W., Polishook J.D., Ondeyka J.G., Hirshfield J.…Hazuda D.J. Integracides: Tetracyclic triterpenoid inhibitors of HIV-1 integrase produced by Fusarium sp. Bioorganic & Medicinal Chemistry. 2003;11(7):1577–1582. doi: 10.1016/s0968-0896(02)00529-1. [DOI] [PubMed] [Google Scholar]
- Strobel G., Stierle A., Stierle D., Hess W.M. Taxomyces andreanae, a proposed new taxon for a bulbilliferous hvphomvcete associated with Pacific yew (Taxus Brevifolia) Mycotaxon. 1994;47(71):71–80. [Google Scholar]
- Wang G.Q., Chen G.D., Qin S.Y., Hu D., Awakawa T., Li S.Y.…Gao H. Biosynthetic pathway for furanosteroid demethoxyviridin and identification of an unusual pregnane side-chain cleavage. Nature Communications. 2018;9(1):1838. doi: 10.1038/s41467-018-04298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen B., He X., Gui J. Bioinspired synthesis of nortriterpenoid propindilactone G. Journal of the American Chemical Society. 2020;142(11):5007–5012. doi: 10.1021/jacs.0c00363. [DOI] [PubMed] [Google Scholar]
- Zheng C.J., Huang G.L., Tang X.Z., Wang D.N., Gong X.L., Zhang Q., Song X.P., Chen G.Y. Secondary metabolites and antibacterial activities of a bruguiera sexangula var. Rhynchopetala-derived fungus Penicillium sp. (J41221) Chinese Journal of Organic Chemistry. 2014;34(6):1172–1176. [Google Scholar]
- Zi J., Matsuba Y., Hong Y.J., Jackson A.J., Tantillo D.J., Pichersky E., Peters R.J. Biosynthesis of lycosantalonol, a cis-prenyl derived diterpenoid. Journal of the American Chemical Society. 2014;136(49):16951–16953. doi: 10.1021/ja508477e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.