Abstract
Surgical neuromodulation has witnessed significant progress in recent decades. Notably, deep brain stimulation (DBS), delivered precisely within therapeutic targets, has revolutionized the treatment of medication-refractory movement disorders and is now expanding for refractory psychiatric disorders, refractory epilepsy, and post-stroke motor recovery. In parallel, the advent of incisionless treatment with focused ultrasound ablation (FUSA) can offer patients life-changing symptomatic relief. Recent research has underscored the potential to further optimize DBS and FUSA outcomes by conceptualizing the therapeutic targets as critical nodes embedded within specific brain networks instead of strictly anatomical structures. This paradigm shift was facilitated by integrating two imaging modalities used regularly in brain connectomics research: diffusion MRI (dMRI) and functional MRI (fMRI). These advanced imaging techniques have helped optimize the targeting and programming techniques of surgical neuromodulation, all while holding immense promise for investigations into treating other neurological and psychiatric conditions. This review aims to provide a fundamental background of advanced imaging for clinicians and scientists, exploring the synergy between current and future approaches to neuromodulation as they relate to dMRI and fMRI capabilities. Focused research in this area is required to optimize existing, functional neurosurgical treatments while serving to build an investigative infrastructure to unlock novel targets to alleviate the burden of other neurological and psychiatric disorders.
Keywords: Neuromodulation, Functional MRI, Diffusion MRI, Neural circuitry, Deep brain stimulation, Deep brain nuclei
Introduction
As surgical neuromodulation treatments advance, an evolving field of study aims to optimize the treatment parameters by precisely targeting the neural circuitry of interest using microstructural and functional neuroimaging [1]. Conventionally, neurosurgeons utilized structural magnetic resonance imaging (MRI) to indirectly localize the relevant anatomical targets, using atlas superimposition and indirect coordinates relative to the anterior and posterior commissures. Subsequent advances in target-specific imaging sequences improved the visualization of anatomical boundaries. For example, the fast gray matter acquisition T1 inversion recovery (FGATIR) sequence provides excellent resolution of the globus pallidus pars interna (GPi) nucleus for deep brain stimulation (DBS) to treat dystonia and Parkinson's disease (PD) [2]. Similarly, high-resolution quantitative susceptibility mapping (QSM) improved visualization of the subthalamic nucleus (STN) for presurgical targeting [3].
Despite these advances, a few limitations exist when applying standard-of-care imaging for surgical targeting. Some nuclei of therapeutic interest cannot be visualized, such as the ventral intermediate nucleus (VIM), which is indistinguishable from adjacent ventral thalamic nuclei. In other cases, optimal therapeutic targets can be embedded as distinct subregions within the known anatomical nucleus, yet the borders of those subregions cannot be seen adequately on standard sequences [4,5]. Finally, side effect minimization is crucial for optimal clinical outcomes, and the treatment-associated side effects are often derived from critical white matter tracts adjacent to the anatomical nuclei, such as the pyramidal tract or the medial lemniscus. Avoiding these critical structures becomes paramount for DBS and focused ultrasound ablation (FUSA), an incisionless FDA-approved treatment for essential tremor (ET) and PD [6,7]. Considering these limitations alongside a growing body of literature indicating the importance of patient-specific surgical targeting to optimize outcomes, current research aims to achieve precise and personalized identification of therapeutic targets that conventional imaging cannot identify. To this end, two primary imaging modalities, diffusion MRI (dMRI) and functional MRI (fMRI), have been tested and implemented in the surgical workflow [1,8,9]. Current research is underway to better understand if dMRI and fMRI can improve clinical outcomes. This review summarizes dMRI and fMRI techniques, their utility in surgical planning post-operative programming, and their role in discovering the mechanisms underlying neuromodulation.
Evolution of Targeting Techniques in Surgical Neuromodulation
The first descriptions of stereotaxy that paved the way for surgical targeting for neuromodulation began in 1906 when Horsley and Clarke created preclinical stereotactic frame and stereotactic needles [10]. Speigel and Wycis are credited with inventing the first human stereotactic frame [11]. As technology for the modulation of deep brain targets developed in the 1970s, the field adapted to stimulation, rather than lesioning, for surgical neuromodulation. The first target for neuromodulation to treat the extrapyramidal tremor of PD was VIM electrical stimulation, described in 1963 by Fessard et al. [12,13] and successfully implemented in the clinical practice to treat ET and PD tremor by Benabid et al. [12]. In 1990, Bergman et al. described the STN as a therapeutic target in an experimental model of PD [14]. In the early 1990s, STN stimulation was clinically utilized for symptom control in PD patients, followed by GPi DBS [15,16]. In the early 2000s, the indications for DBS expanded to dystonia and psychiatric disorders such as obsessive-compulsive disorder (OCD) and depression [17]. More recently, DBS was approved for treating epilepsy [18]. Ongoing trials are investigating the role of targeted neuromodulation in treating Tourette's syndrome, depression, Alzheimer's disease, and motor recovery after stroke [17,[19], [20], [21], [22], [23]]. In the last decade, FUSA has emerged as a treatment for thermal ablation of therapeutic targets. It is now FDA-approved to treat essential tremor (ET), tremor-predominant PD, and Parkinson's disease fluctuations and dyskinesia [6,7,24].
Accurately identifying the therapeutic targets is critical to clinical outcomes and treatment efficacy, and even a millimeter deviation in any planar direction can lead to sub-optimal outcomes [25,26]. There has yet to be a consensus on which sequences are optimal for target visualization. Intraoperative physiology was successfully implemented in the surgical workflow to determine patient-specific differences in functional anatomy and explore whether test stimulation provides the desired clinical benefit with enough margin before side effects appear at high stimulation thresholds [27]. Although intraoperative physiology reliably predicts postoperative outcomes [28], it is optimally performed in an awake state during surgery, which presents challenges limiting the broader adoption of DBS. Similarly, there is limited opportunity for physiological exploration during FUSA, where test stimulation can be performed using thermal neuromodulation [29].
With a goal to offer both DBS and FUSA treatments to patients widely, it is critical to improve surgical targeting to differentiate therapeutic targets from neighboring structures that cause side effects and ultimately improve clinical outcomes. This shift in practice underscores the need to accurately delineate patient-specific white matter tracts and neural pathways while creating personalized neuromodulation treatment plans. The advent of advanced imaging, such as fMRI and dMRI, allows for imaging patient-specific connectomics, which can be implemented for personalized DBS and FUSA targeting and treatment optimization. As a result, there have been significant initiatives to implement dMRI and fMRI to advance the care of patients undergoing neuromodulation treatments.
dMRI and Tractography
Brief background
The dMRI technique quantifies the Brownian motion of water molecules using specialized sequences with diffusion gradients. These gradients are added to the MRI pulse sequence, affecting signal phases. Water molecules moving in the same direction as these gradients produce signal attenuation. Thus, the signal becomes dependent on diffusion (i.e., diffusion-weighted). The direction in which the signal weakening occurs provides information into tissue-specific microscopic structure. The methods to analyze dMRI fall into two categories: model-based and model-free approaches [9]. Model-based techniques fit data to predefined models, such as the tensor or kurtosis model, providing fiber orientations for tracking. Among all the model-based methods, diffusion tensor imaging (DTI) is the most commonly used in dMRI analyses [8]. In brief, neural tissue comprises gray matter (cell bodies and nuclei) and white matter (axons, myelin, and microtubules), with axonal projections connecting specific brain regions. DTI is based on a voxel-wise analysis of the water diffusivity to derive the anisotropy measure, also known as the fractional anisotropy (FA). Typically, anisotropy is higher in white matter than gray matter, and a higher value may indicate more compact axonal bundles or better axonal integrity. The principal determinant of this diffusion anisotropy suggests the overall orientations of the tubular structure of axonal walls lined by myelin sheaths [30]. Modeling the diffusivities reveals the preferential axonal directions, which can be further used in fiber tracking to map connections within the brain. DTI methods provide voxel-wise information about the structure (i.e., anisotropy) and trajectory (i.e., direction) of axons in three-dimensional space. Voxel-wise analysis can then be implemented to generate the maps of white matter pathways, a processing known as tractography [8,31]. The anisotropy defines the termination criterion of fiber trajectories. A lower value of anisotropy may indicate the termination of a pathway. Fiber directions are used by fiber tracking methods to guide the tracking process of a white matter pathway. The commonly used fiber tracking methods can also be categorized into probabilistic or deterministic tractography. Deterministic tractography uses predetermined directional information, resulting in deterministic reconstruction of pathways [32,33]. Deterministic tractography is commonly implemented in FDA-approved software for neurosurgical localization. In contrast, probabilistic tractography introduces a random variable into the directional information [[34], [35], [36]]. As a result, the reconstructed pathways have a probabilistic distribution [37]. The final product can be used to estimate probabilistic connectivity between brain regions in each individualized patient.
Pre-operative planning with dMRI and tractography
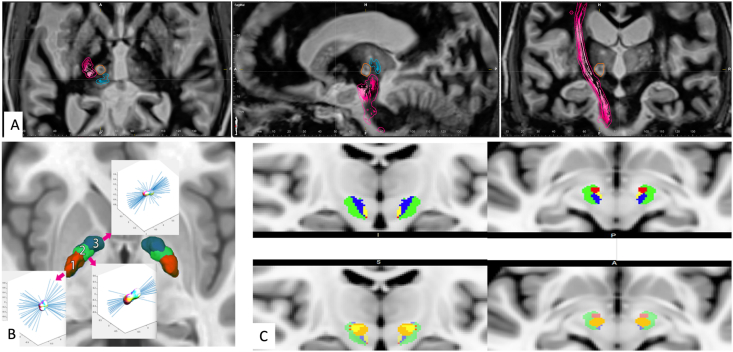
Table 1 outlines the literature highlighting the use of dMRI to delineate patient-specific brain anatomy and for target optimization in surgical neuromodulation. VIM is the most studied target in the context of DBS for treating ET. VIM DBS proves to be particularly challenging, given that this target is poorly visualized on both 1.5T and 3T MRI, and published studies of the use of tractography for tremor control optimization are promising [38,39]. One study by Coenen et al. described a series of patients who had tremors refractory to standard placement of DBS and underwent re-placement of leads, using tractography targeting the center of the dentatorubrothalamic tract (DRT). The active electrode was positioned close to the DRT, improving tremor control [40]. In another study, twenty patients with intention tremor underwent dMRI tractography for individualized DRT mapping, which was targeted with millimeter precision, significantly improving the tremor amplitude [41]. Sammartino et al. used deterministic tractography to develop a tractography-based VIM targeting and compared it with conventional targeting methods (Fig. 1A). Using tractography, the VIM was observed to be more lateral and anterior to the traditional atlas-based VIM [42], and this method proved to be accurate when verified against the intraoperative physiology [43]. The tractography-based VIM targeting was prospectively implemented in patients undergoing focused ultrasound ablation, and outcomes were determined rigorously using blinded independent raters [44]. The key findings from this investigation were a 56% tremor improvement and the frequency of patient-reported side effects, which was significantly lower than in the reported literature. A recent meta-analysis subsequently verified these findings and found a reduced risk of side effects (specifically the most common side effect, ataxia) when tractography was used to target VIM for focused ultrasound ablation [45]. The use of dMRI for tractography presurgical target planning can be standardized, as per one study, with FDA-approved software so that other institutions can utilize methods for improving target identification and reducing side effects [46].
Table 1.
Literature utilizing dMRI for neuromodulation target optimization.
| Manuscript | Target | Methods and key findings |
|---|---|---|
| Coenen et al., 2014 [41] | VIM DBS for treatment of ET | Retrospective analysis. Effective DBS contacts were located inside or in proximity to the DRT. In moderate tremor reduction, the electric field was centered on anterior DRT border. In good and excellent tremor reduction, the electric field was centered on DRT. |
| Riva-Posse et al., 2014 [51] | SCC DBS for TRD | Retrospective analysis. The white matter connections critical for successful antidepressant response to SCC DBS include forceps minor, uncinate fasciculus, and cingulum bundle. |
| Sammartino et al., 2016 [43] | VIM DBS for treatment of ET | Methodology of tractography-based VIM targeting and prospective verification with electrophysiology. The tractography-based VIM was more lateral and anterior compared to conventional targeting. The concordance between imaging and physiology was within millimeters when comparing locations of the VIM, sensory thalamic nucleus, medial lemniscus, and pyramidal tracts. |
| Chazen et al., 2018 [39] | VIM FUSA for treatment of ET | Retrospective analysis. Tractography was able to identify white matter tracts adjacent to the FUSA lesion including DRT. There was a clinical improvement in ET observed after disruption of the DRT. |
| Krishna et al., 2019 [45] | VIM FUSA for treatment of ET | Prospective tractography-based targeting. In a blinded assessment of tremor outcomes, FUSA of the tractography-based VIM target reduced tremor by >50% without significant motor or sensory side effects. |
| Sammartino et al., 2021 [49] | GPi FUSA for treatment of PD | Retrospective analysis. Quantitative diffusion MRI analysis used to distinguish the motor subregions of globus pallidus. Optimal outcomes associated with FUSA lesions overlapping with the motor subregion. |
| Wu et al., 2021 [34] | VIM FUSA for treatment of ET | Retrospective analysis. Probabilistic tractography was found to be more precise and safer in delineation of VIM compared to deterministic tractography. |
| Feltrin et al., 2022 [47] | VIM FUSA for treatment of ET | Technical overview of four tract tractography, on an FDA approved software, with a potential to standardize treatment protocol for VIM FUSA. |
Abbreviations: VIM – ventral intermediate nucleus, DBS - deep brain stimulation, ET – essential tremor, DRT – dentatorubrothalamic tract, SCC – subcallosal cingulate gyrus, TRD – treatment-resistant depression, FUSA – focused ultrasound ablation, GPi – Globus pallidus.
Fig. 1.
A. The implementation of deterministic tractography for personalized targeting for tremor surgery. Preoperative dMRI was acquired to precisely target the ventral intermediate nucleus and the adjacent white matter tracts using FDA-approved software (Brainlab Elements, Brainlab Inc., Munich, Germany). Shown here are the pyramidal tract (in red) and medial lemniscus (in blue) relative to the FUSA lesion (shown in orange) in axial, sagittal, and coronal projections. B. The use of dMRI to distinguish subregions of therapeutic interest within anatomically defined nuclei (adapted Sammartino et al. JNS 2021). Quantitative analysis of diffusion parameters revealed three distinct subregions within the globus pallidus (posterior, central, and anterior). The posterior and central subregion corresponded with the motor part of the globus pallidus. C. The functional segmentation of the subthalamic region is shown in a dMRI investigation (adapted from Sammartino et al. Brain Imaging and Behavior 2021). The stimulation volumes were modeled, and distinct stimulation-induced clinical effects were analyzed to reveal the symptom-specific tracts. The tracts associated with motor improvement (green) were lateral while those associated with non-motor side effects were medial with an anterior-to-posterior organization such that the tracts associated with stimulation-induced changes in mood (red) were organized most anteriorly. In contrast, tracts associated with dizziness (yellow) and sweating (blue) were the most posterior.
The white matter connections with the motor, premotor, supplementary motor, and frontal eye field regions are closer to STN and GPi than VIM, making investigations with deterministic tractography particularly challenging [47]. Therefore, investigators implemented alternative approaches. In one study, dMRI was employed to detect specific patterns of microstructural organization within the GPi [48]. Three distinct anatomical subregions (anterior, central, and posterior) were detected within GPi, and these sub-regions were found to have functionally distinct connections to regions involved in motor and non-motor processing (Fig. 1B). Furthermore, distinct hot spots of improvement in dyskinesias and motor impairment were localized within the posterior subregion, highlighting its significance as a therapeutic hot spot for PD patients undergoing DBS and FUSA.
dMRI also plays a critical role in DBS targets without singular anatomical structures of interest, such as those targeted in psychiatric disorders. dMRI has been used to assess the structural connectivity of white matter tracts associated with optimal outcomes following subcallosal cingulate DBS to treat depression [49,50]. The investigators found that the best response was mediated by three white matter bundles: the uncinate fasciculus, forceps minor, and the cingulum bundle. Another study utilized deterministic tractography to identify the medial forebrain bundle (MFB), which cannot be seen on conventional MRI, to successfully treat treatment-resistant depression [51].
Post-operative programming and understanding neuromodulation mechanisms
dMRI tractography has the potential for postoperative use in DBS patients to localize the white matter tracts that need to be modulated for therapeutic effect [52]. In one study, Gonzalez-Escamilla et al. demonstrated that successful STN DBS in PD patients was associated with fiber pathways from STN to M1 and the supplemental motor association (SMA). What remained unknown was whether maximizing the overlap between therapeutic fiber tracts with the stimulation volumes using the latest beyond-DTI applications can improve DBS therapeutic effects [53]. Furthermore, dMRI tractography has recently been used to study long-term connectome changes in patients treated with DBS. One study analyzed patients who had undergone STN DBS for PD and found elevated fractional anisotropy in white matter tracts, offering the possibility that long-term stimulation can enhance connectivity over time [54]. While considering the use of dMRI to optimize DBS programming, it is critical to consider that the unintended stimulation of certain neighboring white matter tracts can cause side effects limiting the therapeutic potential of DBS. Therefore, recent dMRI research aimed to distinguish fiber tracts associated with specific side effects from those involved in the therapeutic efficacy (Fig. 1C). For example, fiber tracts associated with non-motor side effects are organized medial to those associated with improvement in Parkinsonian symptoms [55] (Fig. 1). Interestingly, the symptom-specific tracts have an anterior-to-posterior organization such that the tracts associated with stimulation-induced changes in mood were organized most anteriorly. In contrast, tracts associated with sweating were the most posterior. By incorporating the extent of fiber tractography of different DBS targets, one can attempt to optimize the STN DBS stimulation parameters to maximize therapeutic potential while minimizing side effects [53]. Finally, the most novel is the neuro-restorative use of DBS. Evolving DBS research aims to target subcortical regions to improve function in patients with stroke and traumatic brain injury deficits. One study used tractography to identify cortico-spinal tracts injured after diffuse axonal injury in traumatic brain injury (TBI) and targeted the optimal motor thalamus nuclei to restore motor function [19].
fMRI and Functional Connectivity
Brief background
Functional MRI (fMRI) is a modality of imaging blood-oxygen-level-dependent (BOLD) brain regions, of which 4-dimensional imaging is created by changes in local ratios of paramagnetic deoxyhemoglobin to diamagnetic oxyhemoglobin [56,57]. As a result, local tissue oxygenation levels can serve as a surrogate marker for detecting neuronal activity and connections. Pulse sequences are acquired during a designated repetition time (TR) with millimeter voxel resolution. The TR ranges from fractions of seconds to a few seconds per entire brain volume and is generally acquired through interleaved slices to minimize error [56,58]. Resting-state fMRI (rs-fMRI), wherein the patient in the scanner can be awake or asleep, without a specific motor or cognitive task, can be used to localize connectivity derangements in overlapping BOLD sources [59]. Rs-fMRI has proven helpful in pathological network characterization in many neurological disorders, as shown in several meta-analyses. In PD, rs-fMRI research revealed that cognitive decline was associated with changes in functional connectivity and abnormal BOLD alterations in the bilateral supplementary motor areas, left putamen, left premotor cortex, left inferior parietal gyrus, and right inferior parietal gyrus, corresponding to motor deficits and compensation in PD [60,61]. A meta-analysis of major depression demonstrated the value of rs-fMRI in identifying targets for neuromodulation [62] and treatment response prediction value [63]. Finally, the localization of DBS targets by rs-fMRI in Alzheimer's and other dementias is reproducible by meta-analysis [64].
Rs-fMRI-derived seizure onset zone networks (SzNET) and more typical resting state networks (RSN) are connectivity patterns that can be derived from a data-driven solution, which detects differentiating oscillating signals over time and conform to established spatial and temporal network pattern criteria [[65], [66], [67]]. Hence, abnormal rs-fMRI connectivity has the potential to not only differentiate epilepsy from healthy but can classify epilepsy sub-types [68,69]. In summary, rs-fMRI is highly sensitive to seizure network (SzNET) activity in deep brain regions in epilepsy [70]. Finally, compared to rs-MRI, task-based fMRI can capture functional connectivity during tasks or stimuli and neuronal connectivity at rest [71]. However, a limitation of fMRI is the complexity of neural systems in the brain, as many tasks include several different areas of activation. From a surgical perspective, interpreting task-based fMRI data remains challenging, as it is hard to differentiate activated areas critical for the function from those activated but as supplemental activity [58].
Pre-operative planning with fMRI
fMRI has limited utility in pre-operative planning to determine therapeutic targets and has been studied mainly for the treatment of drug-resistant epilepsy (DRE). In neuromodulation for epilepsy, fMRI and Rs-fMRI could be used to find neuromodulation targets that provide therapeutic antiseizure effects [72]. The most common clinically used targets in DRE include the anterior nucleus of the thalamus (ANT), the centromedian nucleus of the thalamus (CMT), and the hippocampus (HC) [72,73]. Rs-fMRI has also helped define the connectivity of these neuromodulation targets to the medial and dorsal thalamus and dorsal and ventral mesencephalon, postulating an underlying brain network that is being stimulated [72]. Another study demonstrated the effect within the rs-fMRI networks of the default mold (DMN) and limbic systems in patients with neuromodulation of ANT correlating with excellent therapeutic efficacy [74].
Post-operative programming and understanding neuromodulation mechanism
fMRI was shown to detect real-time changes in functional connectivity during DBS ON and OFF states [75] and, more recently, distinguish efficacious and non-efficacious stimulation settings. Table 2 summarizes literature describing the utility of fMRI to optimize DBS therapeutic settings and understand the neuromodulation mechanisms. Conventionally, patients have stimulation settings adjusted and titrated over many appointments over several months. One could argue that this trial-and-error approach is resource-intensive for patients and providers [76]. fMRI can potentially detect neural circuity changes in real-time, making the process of stimulation adjustment more efficient.
Table 2.
Literature utilizing fMRI in neuromodulation.
| Manuscript | Disease/Target | Key findings |
|---|---|---|
| Knight et al., 2015 [77] | STN DBS for PD | Treatment efficacy associated with BOLD activation of primary and premotor circuity and limbic circuitry. |
| Gibson et al., 2016 [79] | VIM DBS for ET | Long-term outcomes associated with stimulation-evoked activation on of tremor circuit, as well as brainstem, supplemental motor area, and contralateral cerebellum. |
| Boutet et al., 2021 [76] | STN DBS for PD | Clinically optimal stimulation produced a characteristic fMRI brain response pattern marked by preferential engagement of the motor circuit, which can be used as a biomarker for stimulation programming in PD patients. |
| Sarica et al., 2021 [73] | ANT DBS for DRE | Specific BOLD changes associated with ANT DBS in the anterior thalamus, putamen, precuneus and cingulate, which could be used as biomarker for optimal stimulation for ANT based epileptic neural networks. |
| Gibson et al., 2021 [78] | STN DBS for DBS | Signature BOLD activation with STN stimulation with a potential to serve as biomarker for therapeutic DBS. |
Abbreviations: STN – Subthalamic nucleus, DBS - deep brain stimulation, PD – Parkinson's disease, VIM – ventral intermediate nucleus, ET – essential tremor, ANT – Anterior thalamic nucleus, DRE – Drug-resistant epilepsy.
Most published fMRI DBS studies for movement disorder involve PD patients who underwent STN DBS, and stimulation was associated with significant BOLD changes in the cortico-basal ganglia-thalamo-cortical loop and cerebellum [57,77]. Boutet et al. described the largest cohort in an observational study of sixty-seven PD patients undergoing stimulation setting changes while using fMRI to capture optimal neural network responses, finding deactivation of M1 and cerebellum, and activation of the thalamus to be optimal [76]. Another study analyzed which neural networks impacted each specific PD symptom, showing contralateral tremor improved with activation of the thalamic, brainstem, and cerebellar regions. In contrast, bradykinesia improved with the activation of the primary motor cortex [78]. Side effects were noted with activating the striatum and sensorimotor tracts [78].
fMRI has been studied with other DBS targets, such as VIM for ET. One study reported significant BOLD activation in the contralateral cerebellar cortex and deep cerebellar nuclei in the DBS ON state when treating ET [79]. Targeting neural networks for treating psychiatric and cognitive disorders has been influenced by fMRI, especially the activation of the dorsal anterior cingulate cortex [80]. In summary, fMRI is a promising advancement, only starting to be used in DBS. The field of neuromodulation has only scratched the surface of the potential uses of fMRI. This imaging modality could enhance understanding of the network dysfunction specific to neurological disorders [81]. A recent systematic review observed that the concurrent fMRI and DBS literature has rapidly developed in the last five years, specifically emphasizing the study of functional connectivity in ON and OFF DBS states [57]. Further investigations are required to define disease-specific network dysfunction to distinguish whether any disease subtypes exist within the diagnoses currently described by shared symptomatology. It will be interesting to test whether neuromodulation treatments to address disease-specific network dysfunction are clinically effective and whether network dynamics can be developed as proximal real-time feedback to optimize the treatment parameters in situations where a significant delay in symptom improvement is observed after instituting DBS or FUSA.
Limitations of dMRI and fMRI
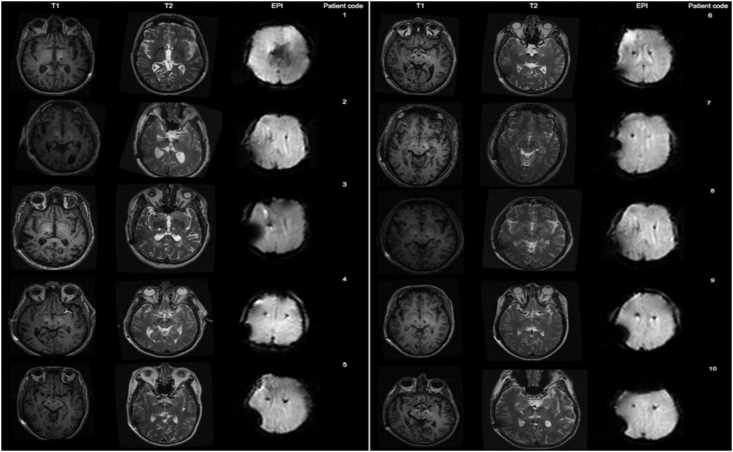
Although advanced imaging modalities are promising, it is important to recognize their current limitations in surgical neuromodulation. One major limitation is a lack of harmonization across software tools used to carry out dMRI and fMRI analysis, which can result in considerable variability. There are multiple software for dMRI analysis that can be used in functional neurosurgery [4,[82], [83], [84]]. These software offer tools for interactive visualization of tracts with DBS electrodes and stimulation volumes based on patient-specific parameters. However, software with state-of-the-art tracking algorithms are currently not approved by the FDA. FDA-approved software with advanced algorithms are required for neurosurgeons to visualize the DBS electrode and stimulation volume locations relative to patient-specific tracts. Moreover, MRI acquisition—intra-operatively or post-operatively—with DBS systems in place was initially explored cautiously to avoid adverse events such as heating at the electrode tips or aberrant current development that could cause device malfunction. With time and adequate investigation, DBS hardware manufacturers (i.e., Medtronic, Boston Scientific, and Abbott) have provided guidelines to prevent such adverse events and introduced device models [85] that carry conditional approvals under specific scanning conditions [19]. However, DBS imaging is still a lengthy process as it requires inspection of safety and the involvement of radiology and MRI technologists to ensure the safety of the procedure. There are still difficulties in interpreting fMRI at the individual patient level, and postoperative fMRI or dMRI imaging can cause signal degradation due to artifacts from implanted hardware [86,87](Fig. 2). In summary, although these imaging modalities have great potential in neuromodulation, significant evolution is still required to improve accuracy and clinical utility.
Fig. 2.
3 Tesla MRI acquired in subjects with implanted DBS leads demonstrates the range of artifacts that can occur. Adapted from Sammartino et al. 2017: J Neurosurg 127:892–898.
In conclusion, integrating dMRI and fMRI has promising utility for personalizing presurgical targeting and parameter optimization of surgical neuromodulation. However, these techniques must be rigorously tested in large clinical trials to generate robust clinical evidence and enable wider adoption. Moreover, the expedited testing and FDA approval of software equipped with cutting-edge algorithms are imperative to overcome challenges encountered during postoperative imaging, such as motion artifact correction and signal degradation. Addressing these aspects will enhance the accuracy and clinical utility of advanced neuroimaging to optimize surgical neuromodulation techniques for current and investigational indications.
Author contributions
NAS – manuscript preparation, critical review.
JBM – manuscript preparation, critical review.
FCY – manuscript preparation, critical review.
MH – manuscript preparation, critical review.
DR – manuscript preparation, critical review.
VLB – manuscript preparation, critical review.
VK – manuscript preparation, critical review.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Vibhor Krishna reports financial support was provided by National Institutes of Health. Vibhor Krishna reports a relationship with Medtronic Inc that includes: board membership and consulting or advisory. N/A If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Tohyama S., Walker M.R., Sammartino F., Krishna V., Hodaie M. The utility of diffusion tensor imaging in neuromodulation: moving beyond conventional magnetic resonance imaging. Neuromodulation. 2020;23(4):427–435. doi: 10.1111/ner.13107. [DOI] [PubMed] [Google Scholar]
- 2.Sudhyadhom A., Haq I.U., Foote K.D., Okun M.S., Bova F.J. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR) Neuroimage. 2009;47(Suppl 2):T44–T52. doi: 10.1016/j.neuroimage.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Spincemaille P., Liu Z., Dimov A., Deh K., Li J., et al. Clinical quantitative susceptibility mapping (QSM): biometal imaging and its emerging roles in patient care. J Magn Reson Imag. 2017;46(4):951–971. doi: 10.1002/jmri.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horn A., Li N., Dembek T.A., Kappel A., Boulay C., Ewert S., et al. Lead-DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage. 2019;184:293–316. doi: 10.1016/j.neuroimage.2018.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn A., Reich M., Vorwerk J., Li N., Wenzel G., Fang Q., et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82(1):67–78. doi: 10.1002/ana.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias W.J., Lipsman N., Ondo W.G., Ghanouni P., Kim Y.G., Lee W., et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 7.Krishna V., Fishman P.S., Eisenberg H.M., Kaplitt M., Baltuch G., Chang J.W., et al. Trial of globus pallidus focused ultrasound ablation in Parkinson's disease. N Engl J Med. 2023;388(8):683–693. doi: 10.1056/NEJMoa2202721. [DOI] [PubMed] [Google Scholar]
- 8.Basser P.J., Pajevic S., Pierpaoli C., Duda J., Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44(4):625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Yeh F.C., Irimia A., Bastos D.C.A., Golby A.J. Tractography methods and findings in brain tumors and traumatic brain injury. Neuroimage. 2021;245 doi: 10.1016/j.neuroimage.2021.118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffrey A., Brown J.G.P., Schulder Michael. Thieme; 2020. Functional neurosurgery: the essentials. [Google Scholar]
- 11.Spiegel E.A., Wycis H.T., Marks M., Lee A.J. Stereotaxic apparatus for operations on the human brain. Science. 1947;106(2754):349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 12.Benabid A.L., Pollak P., Louveau A., Henry S., de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50(1-6):344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 13.Albe Fessard D., Arfel G., Guiot G., Derome P., Dela H., Korn H., et al. Characteristic electric activities of some cerebral structures in man. Ann Chir. 1963;17:1185–1214. [PubMed] [Google Scholar]
- 14.Mitchell I.J., Clarke C.E., Boyce S., Robertson R.G., Peggs D., Sambrook M.A., et al. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 1989;32(1):213–226. doi: 10.1016/0306-4522(89)90120-6. [DOI] [PubMed] [Google Scholar]
- 15.Pollak P., Benabid A.L., Gross C., Gao D.M., Laurent A., Benazzouz A., et al. Effects of the stimulation of the subthalamic nucleus in Parkinson disease. Rev Neurol (Paris) 1993;149(3):175–176. [PubMed] [Google Scholar]
- 16.Siegfried J., Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35(6):1126–1129. doi: 10.1227/00006123-199412000-00016. discussion 9-30. [DOI] [PubMed] [Google Scholar]
- 17.Pycroft L., Stein J., Aziz T. Deep brain stimulation: an overview of history, methods, and future developments. Brain Neurosci Adv. 2018;2 doi: 10.1177/2398212818816017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher R., Salanova V., Witt T., Worth R., Henry T., Gross R., et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 19.Ho J.C., Grigsby E.M., Damiani A., Liang L., Balaguer J.M., Kallakuri S., et al. Targeted deep brain stimulation of the motor thalamus facilitates voluntary motor control after cortico-spinal tract lesions. medRxiv. 2023 doi: 10.1101/2023.03.08.23286720. [DOI] [Google Scholar]
- 20.Paro M.R., Dyrda M., Ramanan S., Wadman G., Burke S.A., Cipollone I., et al. Deep brain stimulation for movement disorders after stroke: a systematic review of the literature. J Neurosurg. 2022:1–14. doi: 10.3171/2022.8.JNS221334. [DOI] [PubMed] [Google Scholar]
- 21.Coenen V.A., Bewernick B.H., Kayser S., Kilian H., Bostrom J., Greschus S., et al. Superolateral medial forebrain bundle deep brain stimulation in major depression: a gateway trial. Neuropsychopharmacology. 2019;44(7):1224–1232. doi: 10.1038/s41386-019-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker K.B., Plow E.B., Nagel S., Rosenfeldt A.B., Gopalakrishnan R., Clark C., et al. Cerebellar deep brain stimulation for chronic post-stroke motor rehabilitation: a phase I trial. Nat Med. 2023;29(9):2366–2374. doi: 10.1038/s41591-023-02507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Ramirez D., Jimenez-Shahed J., Leckman J.F., Porta M., Servello D., Meng F.G., et al. Efficacy and safety of deep brain stimulation in tourette syndrome: the international tourette syndrome deep brain stimulation public database and registry. JAMA Neurol. 2018;75(3):353–359. doi: 10.1001/jamaneurol.2017.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bond A.E., Shah B.B., Huss D.S., Dallapiazza R.F., Warren A., Harrison M.B., et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74(12):1412–1418. doi: 10.1001/jamaneurol.2017.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papavassiliou E., Rau G., Heath S., Abosch A., Barbaro N.M., Larson P.S., et al. Thalamic deep brain stimulation for essential tremor: relation of lead location to outcome. Neurosurgery. 2004;54(5):1120–1129. doi: 10.1227/01.neu.0000119329.66931.9e. discussion 9-30. [DOI] [PubMed] [Google Scholar]
- 26.Sammartino F., Krishna V., King N.K., Bruno V., Kalia S., Hodaie M., et al. Sequence of electrode implantation and outcome of deep brain stimulation for Parkinson's disease. J Neurol Neurosurg Psychiatry. 2016;87(8):859–863. doi: 10.1136/jnnp-2015-311426. [DOI] [PubMed] [Google Scholar]
- 27.Gross R.E., Krack P., Rodriguez-Oroz M.C., Rezai A.R., Benabid A.L. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson's disease and tremor. Mov Disord. 2006;21(Suppl 14):S259–S283. doi: 10.1002/mds.20960. [DOI] [PubMed] [Google Scholar]
- 28.Sammartino F., Rege R., Krishna V. Reliability of intraoperative testing during deep brain stimulation surgery. Neuromodulation. 2020;23(4):525–529. doi: 10.1111/ner.13081. [DOI] [PubMed] [Google Scholar]
- 29.Sammartino F., Snell J., Eames M., Krishna V. Thermal neuromodulation with focused ultrasound: implications for the technique of subthreshold testing. Neurosurgery. 2021;89(4):610–616. doi: 10.1093/neuros/nyab238. [DOI] [PubMed] [Google Scholar]
- 30.Vettel J.M.C., Nicole Garcia, Javier O., Yeh Fang-Cheng, Verstynen T.D. eLS John Wiley & Sons, Ltd.; 2017. White matter tractography and diffusion-weighted imaging. [Google Scholar]
- 31.Mori S., van Zijl P.C. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7-8):468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 32.Mori S., Crain B.J., Chacko V.P., van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Yeh F.C., Verstynen T.D., Wang Y., Fernandez-Miranda J.C., Tseng W.Y. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jbabdi S., Woolrich M.W., Andersson J.L., Behrens T.E. A Bayesian framework for global tractography. Neuroimage. 2007;37(1):116–129. doi: 10.1016/j.neuroimage.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Dyrby T.B., Sogaard L.V., Parker G.J., Alexander D.C., Lind N.M., Baare W.F., et al. Validation of in vitro probabilistic tractography. Neuroimage. 2007;37(4):1267–1277. doi: 10.1016/j.neuroimage.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Johansen-Berg H., Behrens T.E.J. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol. 2006;19(4):379. doi: 10.1097/01.wco.0000236618.82086.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chazen J.L., Sarva H., Stieg P.E., Min R.J., Ballon D.J., Pryor K.O., et al. Clinical improvement associated with targeted interruption of the cerebellothalamic tract following MR-guided focused ultrasound for essential tremor. J Neurosurg. 2018;129(2):315–323. doi: 10.3171/2017.4.JNS162803. [DOI] [PubMed] [Google Scholar]
- 39.Muller J., Alizadeh M., Matias C.M., Thalheimer S., Romo V., Martello J., et al. Use of probabilistic tractography to provide reliable distinction of the motor and sensory thalamus for prospective targeting during asleep deep brain stimulation. J Neurosurg. 2022;136(5):1371–1380. doi: 10.3171/2021.5.JNS21552. [DOI] [PubMed] [Google Scholar]
- 40.Coenen V.A., Allert N., Paus S., Kronenburger M., Urbach H., Madler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery. 2014;75(6):657–669. doi: 10.1227/NEU.0000000000000540. discussion 69-70. [DOI] [PubMed] [Google Scholar]
- 41.Fenoy A.J., Schiess M.C. Deep brain stimulation of the dentato-rubro-thalamic tract: outcomes of direct targeting for tremor. Neuromodulation. 2017;20(5):429–436. doi: 10.1111/ner.12585. [DOI] [PubMed] [Google Scholar]
- 42.Sammartino F., Krishna V., King N.K., Lozano A.M., Schwartz M.L., Huang Y., et al. Tractography-based ventral intermediate nucleus targeting: novel methodology and intraoperative validation. Mov Disord. 2016;31(8):1217–1225. doi: 10.1002/mds.26633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King N.K.K., Krishna V., Basha D., Elias G., Sammartino F., Hodaie M., et al. Microelectrode recording findings within the tractography-defined ventral intermediate nucleus. J Neurosurg. 2017;126(5):1669–1675. doi: 10.3171/2016.3.JNS151992. [DOI] [PubMed] [Google Scholar]
- 44.Krishna V., Sammartino F., Agrawal P., Changizi B.K., Bourekas E., Knopp M.V., et al. Prospective tractography-based targeting for improved safety of focused ultrasound thalamotomy. Neurosurgery. 2019;84(1):160–168. doi: 10.1093/neuros/nyy020. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal M., Garg K., Samala R., Rajan R., Naik V., Singh M. Outcome and complications of MR guided focused ultrasound for essential tremor: a systematic review and meta-analysis. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.654711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feltrin F.S., Chopra R., Pouratian N., Elkurd M., El-Nazer R., Lanford L., et al. Focused ultrasound using a novel targeting method four-tract tractography for magnetic resonance-guided high-intensity focused ultrasound targeting. Brain Commun. 2022;4(6) doi: 10.1093/braincomms/fcac273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barkhoudarian G., Klochkov T., Sedrak M., Frew A., Gorgulho A., Behnke E., et al. A role of diffusion tensor imaging in movement disorder surgery. Acta Neurochir. 2010;152(12):2089–2095. doi: 10.1007/s00701-010-0742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sammartino F., Marsh R., Yeh F.C., Sondergaard A., Changizi B.K., Krishna V. Radiological identification of the globus pallidus motor subregion in Parkinson's disease. J Neurosurg. 2021:1–9. doi: 10.3171/2021.7.JNS21858. [DOI] [PubMed] [Google Scholar]
- 49.Choi K.S., Riva-Posse P., Gross R.E., Mayberg H.S. Mapping the "depression switch" during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 2015;72(11):1252–1260. doi: 10.1001/jamaneurol.2015.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riva-Posse P., Choi K.S., Holtzheimer P.E., McIntyre C.C., Gross R.E., Chaturvedi A., et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatr. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlaepfer T.E., Bewernick B.H., Kayser S., Madler B., Coenen V.A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatr. 2013;73(12):1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Escamilla G., Koirala N., Bange M., Glaser M., Pintea B., Dresel C., et al. Deciphering the network effects of deep brain stimulation in Parkinson's disease. Neurol Ther. 2022;11(1):265–282. doi: 10.1007/s40120-021-00318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishna V., Sammartino F., Rabbani Q., Changizi B., Agrawal P., Deogaonkar M., et al. Connectivity-based selection of optimal deep brain stimulation contacts: a feasibility study. Ann Clin Transl Neurol. 2019;6(7):1142–1150. doi: 10.1002/acn3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arevalo-Saenz A., Lopez-Manzanares L., Navas-Garcia M., Pastor J., Vega-Zelaya L., Torres C.V. Deep brain stimulation in Parkinson's disease: analysis of brain fractional anisotropy differences in operated patients. Rev Neurol. 2022;74(4):125–134. doi: 10.33588/rn.7404.2021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sammartino F., Marsh R., Rezai A., Krishna V. Non-motor effects of subthalamic nucleus stimulation in Parkinson patients. Brain Imaging Behav. 2022;16(1):161–168. doi: 10.1007/s11682-021-00487-8. [DOI] [PubMed] [Google Scholar]
- 56.Wu C., Ferreira F., Fox M., Harel N., Hattangadi-Gluth J., Horn A., et al. Clinical applications of magnetic resonance imaging based functional and structural connectivity. Neuroimage. 2021;244 doi: 10.1016/j.neuroimage.2021.118649. [DOI] [PubMed] [Google Scholar]
- 57.Loh A., Gwun D., Chow C.T., Boutet A., Tasserie J., Germann J., et al. Probing responses to deep brain stimulation with functional magnetic resonance imaging. Brain Stimul. 2022;15(3):683–694. doi: 10.1016/j.brs.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Rutten G.J., Ramsey N.F. The role of functional magnetic resonance imaging in brain surgery. Neurosurg Focus. 2010;28(2):E4. doi: 10.3171/2009.12.FOCUS09251. [DOI] [PubMed] [Google Scholar]
- 59.Boerwinkle V.L., Wilfong A.A., Curry D.J. Resting-state functional connectivity by independent component analysis-based markers corresponds to areas of initial seizure propagation established by prior modalities from the hypothalamus. Brain Connect. 2016;6(8):642–651. doi: 10.1089/brain.2015.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolters A.F., van de Weijer S.C.F., Leentjens A.F.G., Duits A.A., Jacobs H.I.L., Kuijf M.L. Resting-state fMRI in Parkinson's disease patients with cognitive impairment: a meta-analysis. Parkinsonism Relat Disorders. 2019;62:16–27. doi: 10.1016/j.parkreldis.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 61.Li K., Su W., Chen M., Li C.M., Ma X.X., Wang R., et al. Abnormal spontaneous brain activity in left-onset Parkinson disease: a resting-state functional MRI study. Front Neurol. 2020;11:727. doi: 10.3389/fneur.2020.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang B., Liu J., Bao T., Wilson G., Park J., Zhao B., et al. Locations for noninvasive brain stimulation in treating depressive disorders: a combination of meta-analysis and resting-state functional connectivity analysis. Aust N Z J Psychiatr. 2020;54(6):582–590. doi: 10.1177/0004867420920372. [DOI] [PubMed] [Google Scholar]
- 63.Long Z., Du L., Zhao J., Wu S., Zheng Q., Lei X. Prediction on treatment improvement in depression with resting state connectivity: a coordinate-based meta-analysis. J Affect Disord. 2020;276:62–68. doi: 10.1016/j.jad.2020.06.072. [DOI] [PubMed] [Google Scholar]
- 64.Pievani M., Pini L., Ferrari C., Pizzini F.B., Boscolo Galazzo I., Cobelli C., et al. Coordinate-based meta-analysis of the default mode and salience network for target identification in non-invasive brain stimulation of Alzheimer's disease and behavioral variant frontotemporal dementia networks. J Alzheimers Dis. 2017;57(3):825–843. doi: 10.3233/JAD-161105. [DOI] [PubMed] [Google Scholar]
- 65.Griffanti L., Douaud G., Bijsterbosch J., Evangelisti S., Alfaro-Almagro F., Glasser M.F., et al. Hand classification of fMRI ICA noise components. Neuroimage. 2017;154:188–205. doi: 10.1016/j.neuroimage.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee A., Kamboj P., Wyckoff S.N., Sussman B.L., Gupta S.K.S., Boerwinkle V.L. Automated seizure onset zone locator from resting-state functional MRI in drug-resistant epilepsy. Front Neuroimaging. 2023;1 doi: 10.3389/fnimg.2022.1007668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boerwinkle V.L., Mohanty D., Foldes S.T., Guffey D., Minard C.G., Vedantam A., et al. Correlating resting-state functional magnetic resonance imaging connectivity by independent component analysis-based epileptogenic zones with intracranial electroencephalogram localized seizure onset zones and surgical outcomes in prospective pediatric intractable epilepsy study. Brain Connect. 2017;7(7):424–442. doi: 10.1089/brain.2016.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang S., Li H., Liu L., Yao D., Luo C. Voxel-wise functional connectivity of the default mode network in epilepsies: a systematic review and meta-analysis. Curr Neuropharmacol. 2022;20(1):254–266. doi: 10.2174/1570159X19666210325130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McSweeney M., Reuber M., Levita L. Neuroimaging studies in patients with psychogenic non-epileptic seizures: a systematic meta-review. Neuroimage Clin. 2017;16:210–221. doi: 10.1016/j.nicl.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chakraborty A.R., Almeida N.C., Prather K.Y., O’Neal C.M., Wells A.A., Chen S., et al. Resting-state functional magnetic resonance imaging with independent component analysis for presurgical seizure onset zone localization: a systematic review and meta-analysis. Epilepsia. 2020 Sep;61(9):1958–1968. doi: 10.1111/epi.16637. Epub 2020 Aug 8. PMID: 32770853. [DOI] [PubMed] [Google Scholar]
- 71.Sussman B.L., Wyckoff S.N., Heim J., Wilfong A.A., Adelson P.D., Kruer M.C., et al. Is resting state functional MRI effective connectivity in movement disorders helpful? A focused review across lifespan and disease. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.847834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vetkas A., Germann J., Elias G., Loh A., Boutet A., Yamamoto K., et al. Identifying the neural network for neuromodulation in epilepsy through connectomics and graphs. Brain Commun. 2022;4(3) doi: 10.1093/braincomms/fcac092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarica C., Yamamoto K., Loh A., Elias G.J.B., Boutet A., Madhavan R., et al. Blood oxygen level-dependent (BOLD) response patterns with thalamic deep brain stimulation in patients with medically refractory epilepsy. Epilepsy Behav. 2021;122 doi: 10.1016/j.yebeh.2021.108153. [DOI] [PubMed] [Google Scholar]
- 74.Middlebrooks E.H., Lin C., Okromelidze L., Lu C.Q., Tatum W.O., Wharen R.E., Jr., et al. Functional activation patterns of deep brain stimulation of the anterior nucleus of the thalamus. World Neurosurg. 2020;136:357–363 e2. doi: 10.1016/j.wneu.2020.01.108. [DOI] [PubMed] [Google Scholar]
- 75.Rezai A.R., Lozano A.M., Crawley A.P., Joy M.L., Davis K.D., Kwan C.L., et al. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg. 1999;90(3):583–590. doi: 10.3171/jns.1999.90.3.0583. [DOI] [PubMed] [Google Scholar]
- 76.Boutet A., Madhavan R., Elias G.J.B., Joel S.E., Gramer R., Ranjan M., et al. Predicting optimal deep brain stimulation parameters for Parkinson's disease using functional MRI and machine learning. Nat Commun. 2021;12(1):3043. doi: 10.1038/s41467-021-23311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knight E.J., Testini P., Min H.K., Gibson W.S., Gorny K.R., Favazza C.P., et al. Motor and nonmotor circuitry activation induced by subthalamic nucleus deep brain stimulation in patients with Parkinson disease: intraoperative functional magnetic resonance imaging for deep brain stimulation. Mayo Clin Proc. 2015;90(6):773–785. doi: 10.1016/j.mayocp.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gibson W.S., Rusheen A.E., Oh Y., In M.H., Gorny K.R., Felmlee J.P., et al. Symptom-specific differential motor network modulation by deep brain stimulation in Parkinson's disease. J Neurosurg. 2021;135(6):1771–1779. doi: 10.3171/2020.10.JNS202277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibson W.S., Jo H.J., Testini P., Cho S., Felmlee J.P., Welker K.M., et al. Functional correlates of the therapeutic and adverse effects evoked by thalamic stimulation for essential tremor. Brain. 2016;139(Pt 8):2198–2210. doi: 10.1093/brain/aww145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elias G.J.B., Germann J., Boutet A., Loh A., Li B., Pancholi A., et al. 3T MRI of rapid brain activity changes driven by subcallosal cingulate deep brain stimulation. Brain. 2022;145(6):2214–2226. doi: 10.1093/brain/awab447. [DOI] [PubMed] [Google Scholar]
- 81.Fox M.D. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379(23):2237–2245. doi: 10.1056/NEJMra1706158. [DOI] [PubMed] [Google Scholar]
- 82.Noecker A.M., Choi K.S., Riva-Posse P., Gross R.E., Mayberg H.S., McIntyre C.C. StimVision software: examples and applications in subcallosal cingulate deep brain stimulation for depression. Neuromodulation. 2018;21(2):191–196. doi: 10.1111/ner.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neudorfer C., Butenko K., Oxenford S., Rajamani N., Achtzehn J., Goede L., et al. Lead-DBS v3.0: mapping deep brain stimulation effects to local anatomy and global networks. Neuroimage. 2023;268 doi: 10.1016/j.neuroimage.2023.119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horn A., Kuhn A.A. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–135. doi: 10.1016/j.neuroimage.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Boutet A., Chow C.T., Narang K., Elias G.J.B., Neudorfer C., Germann J., et al. Improving safety of MRI in patients with deep brain stimulation devices. Radiology. 2020;296(2):250–262. doi: 10.1148/radiol.2020192291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sammartino F., Krishna V., Sankar T., Fisico J., Kalia S.K., Hodaie M., et al. 3-Tesla MRI in patients with fully implanted deep brain stimulation devices: a preliminary study in 10 patients. J Neurosurg. 2017;127(4):892–898. doi: 10.3171/2016.9.JNS16908. [DOI] [PubMed] [Google Scholar]
- 87.Sammartino F., Taylor P., Chen G., Reynolds R.C., Glen D., Krishna V. Functional neuroimaging during asleep DBS surgery: a proof of concept study. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.659002. [DOI] [PMC free article] [PubMed] [Google Scholar]