ABSTRACT
Nocardiosis typically requires a prolonged treatment duration of ≥6 months and initial combination therapy with 2–3 antibiotics. First-line regimens for nocardiosis are associated with considerable toxicity; therefore, alternative therapies are needed. Omadacycline is an aminomethylcycline with broad antimicrobial activity whose in vitro activity against Nocardia species has not been formally assessed. The in vitro potency of omadacycline was evaluated against 300 Nocardia clinical isolates by broth microdilution. The most common Nocardia species tested were N. cyriacigeorgica (21%), N. nova (20%), and N. farcinica (12%). The most common specimens were respiratory (178 isolates, 59%) and wound (57 isolates, 19%). Omadacycline minimum inhibitory concentrations (MICs) across all Nocardia species ranged from 0.06 µg/mL to 8 µg/mL, with an MIC50 of 2 µg/mL and MIC90 of 4 µg/mL. The lowest MICs were found among N. paucivorans (MIC50 = 0.25 µg/mL, MIC90 = 0.25 µg/mL), N. asiatica (MIC50 = 0.25 µg/mL, MIC90 = 1 µg/mL), N. abscessus complex (MIC50 = 0.5 µg/mL, MIC90 = 1 µg/mL), N. beijingensis (MIC50 = 0.5 µg/mL, MIC90 = 2 µg/mL), and N. otitidiscaviarum (MIC50 = 1 µg/mL, MIC90 = 2 µg/mL). The highest MICs were found among N. farcinica (MIC50 = 4 µg/mL, MIC90 = 8 µg/mL). In vitro potency differed by species among Nocardia clinical isolates. Further studies are warranted to evaluate the potential clinical utility of omadacycline for nocardiosis.
KEYWORDS: Nocardia, omadacycline, antimicrobial susceptibility
INTRODUCTION
Nocardia species are a diverse group of ubiquitous, aerobic, partially acid-fast, filamentous, Gram-positive bacilli (1–3). There are more than 100 recognized Nocardia species; approximately half are known human pathogens (4–6). Infections occur predominantly in patients with impaired cell-mediated immunity, typically via inhalation from the environment, and most frequently manifest as respiratory tract infections (7–10). Extrapulmonary disease is common, occurring in ~30%–40% of patients, with the central nervous system (CNS) and skin and subcutaneous tissue being the most common dissemination sites, with each occurring in ~10%–30% of patients (7–11).
Trimethoprim-sulfamethoxazole (TMP-SMX) at higher, weight-based doses for 6–12 months or longer has long been the standard of care for nocardiosis (1, 2, 5, 12). Guidelines recommend the addition of a second and occasionally a third agent for severe or disseminated nocardiosis. The selection of these regimens is highly individualized based on several factors such as the Nocardia species involved, site of infection (e.g., need for CNS penetration), side effects, and drug interaction profile of the antimicrobials (2). Oral antimicrobials are desired given their ease of administration and to prevent complications from long-term central venous catheter access. Antimicrobials frequently considered for use in combination with TMP-SMX include linezolid, ceftriaxone, imipenem-cilastatin, meropenem, amikacin, minocycline, and fluoroquinolones. Because of long treatment durations and aggressive dosing schemes, often in combination regimens, drug toxicity occurs in 17%–67% of patients (9, 13–15). Indeed, a high proportion of patients require therapy modification because of drug toxicity. Additionally, many of these first-line therapies can only be administered parenterally, which complicates outpatient management.
The tetracyclines minocycline, doxycycline, and glycylcycline tigecycline all have in vitro activity against several species of Nocardia. Omadacycline, a first-in-class aminomethylcycline derived from minocycline, displays activity against a broad range of bacteria (16, 17). It possesses in vitro and in vivo activity against rapidly growing mycobacteria (RGM), such as Mycobacteroides abscessus (18–22). Due to their phylogenetic relatedness, it is reasonable to suspect that omadacycline could also have activity against Nocardia species. Omadacycline may be a desirable option for treating nocardiosis given its oral formulation, once-daily dosing, low potential for drug-drug interactions, and favorable tolerability profile. Given the potential clinical advantages of omadacycline and the need for additional therapies for nocardiosis, this study aimed to determine the in vitro activity of omadacycline and comparator antimicrobials against a diverse collection of clinical Nocardia isolates.
MATERIALS AND METHODS
Clinical isolates
Clinical isolates were referred to Associated Regional and University Pathologists (ARUP) Laboratories from institutions throughout the United States for identification and/or routine susceptibility testing. Isolates that were identified as Nocardia spp. between February 2018 and April 2023 were eligible for inclusion in this study. Isolates were identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry (Bruker Biotyper, Billerica, MA, USA), 16s rRNA gene sequencing, or by client laboratories (23, 24). Isolates from a range of clinical specimens including respiratory, wound, body fluid, blood, CNS, and ocular sources were included. Nocardia spp. isolates were selected for inclusion in this study based on the frequency of isolation, availability through routine laboratory testing or laboratory isolate archives, and to achieve a broad diversity of species.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing (AST) was performed on 300 Nocardia isolates. For the less common Nocardia species, archived isolates were used after sub-culturing twice onto sheep blood agar plates. AST was performed using 96-well frozen reference broth microdilution (BMD) panels in cation-adjusted Mueller-Hinton broth (CAMHB) (Thermo Fisher) according to CLSI M24, third ed. (25). Briefly, isolate suspensions were normalized to 0.5 McFarland in sterile water, diluted in CAMHB to achieve a final inoculum of 1–5 × 104 CFU/well, covered with adhesive seals and incubated for up to 5 days. Minimum inhibitory concentrations (MICs) were determined at 48 hours for imipenem and when at least 2+ growth was observed in control wells (25), according to CLSI M24S-2 guidelines (26). The antimicrobials, and range of concentrations tested, included omadacycline (0.015–32 µg/mL), tigecycline (0.015–32 µg/mL), minocycline (0.015–32 µg/mL), TMP-SMX (0.03/0.59–16/304 µg/mL), linezolid (0.12–32 µg/mL), ceftriaxone (0.25–128 µg/mL), imipenem (0.12–64 µg/mL), amikacin (0.06–32 µg/mL), and ciprofloxacin (0.06–32 µg/mL). MICs with interpretations were determined for minocycline, TMP-SMX, linezolid, ceftriaxone, imipenem, amikacin, and ciprofloxacin (26). MICs alone were determined for omadacycline and tigecycline, given the absence of breakpoints in the CLSI M24S-2 guidelines. Ten isolates representing eight different Nocardia species [N. wallacei, N. veterana, N. transvalensis, N. farcinica, N. cyriacigeorgica (two isolates), N. beijingensis (two isolates), N. nova, N. abscessus complex] were tested in triplicate to evaluate reproducibility. No significant trailing was encountered when interpreting omadacycline MICs from BMD, even with slower-growing species; therefore, MICs were read at 100% inhibition (18, 26).
Quality control
In accordance with the CLSI M24S-2 guidelines, quality control (QC) was performed using Nocardia nova ATCC BAA-2227 and Staphylococcus aureus ATCC 21213 (26). Results were included only if the QC values were within range. Omadacycline and tigecycline MICs were determined for Nocardia nova ATCC BAA-2227 for a total of 18 independent replicates; however, the CLSI M24S-2 does not include omadacycline or tigecycline QC ranges for this reference strain.
Data analysis
The MIC ranges, MIC50, and MIC90 of omadacycline, tigecycline, and minocycline were determined for each species of Nocardia, and the percentage of susceptibility was determined for comparator antimicrobials. Figures were created using the ggplot2 package in R version 4.2.2 (27).
RESULTS
AST was performed for 300 Nocardia clinical isolates, covering 28 different species. The majority of isolates (216, 72%) were identified by ARUP Laboratories with the remainder identified by client laboratories. The most common Nocardia species tested were N. cyriacigeorgica (64 isolates, 21%), N. nova (59 isolates, 20%), and N. farcinica (36 isolates, 12%). Specimen sources included respiratory (178 isolates, 59%), wound (57 isolates, 19%), body fluid (17 isolates, 6%), blood (10 isolates, 3%), CNS (5 isolates, 2%), and ocular (4 isolates, 1%), while 29 isolates (10%) were from undisclosed sources.
Omadacycline MICs across all Nocardia species ranged from 0.06 µg/mL to 8 µg/mL (Table 1). When evaluating all Nocardia isolates, omadacycline displayed an MIC50 of 2 µg/mL and MIC90 of 4 µg/mL. Omadacycline was most active against N. paucivorans (MIC50 = 0.25 µg/mL, MIC90 = 0.25 µg/mL), N. asiatica (MIC50 = 0.25 µg/mL, MIC90 = 1 µg/mL), N. abscessus complex (MIC50 = 0.5 µg/mL, MIC90 = 1 µg/mL), N. beijingensis (MIC50 = 0.5 µg/mL, MIC90 = 1 µg/mL), and N. otitidiscaviarum (MIC50 = 1 µg/mL, MIC90 = 2 µg/mL). Omadacycline was least active against N. farcinica (MIC50 = 4 µg/mL, MIC90 = 8 µg/mL).
TABLE 1.
Omadacycline MICs observed against Nocardia species
| Species | Number of isolates tested | Omadacycline MIC (µg/mL) | MIC50 | MIC90 | MIC range | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |||||
| N. abscessus complex | 16 | 1 | 2 | 7 | 5 | 1 | 0.5 | 1 | 0.06–2 | |||||||
| N. asiatica | 10 | 1 | 2 | 2 | 3 | 2 | 0.25 | 1 | 0.06–1 | |||||||
| N. beijingensis | 10 | 2 | 2 | 1 | 3 | 1 | 1 | 0.5 | 2 | 0.12–4 | ||||||
| N. brasiliensis | 19 | 1 | 3 | 4 | 11 | 2 | 2 | 0.25–2 | ||||||||
| N. cyriacigeorgica | 64 | 2 | 9 | 30 | 23 | 2 | 4 | 0.5–4 | ||||||||
| N. farcinica | 36 | 1 | 27 | 8 | 4 | 8 | 2–8 | |||||||||
| N. nova | 59 | 1 | 6 | 18 | 29 | 5 | 4 | 4 | 0.5–8 | |||||||
| N. otitidiscaviarum | 11 | 1 | 3 | 3 | 4 | 1 | 2 | 0.25–2 | ||||||||
| N. paucivorans | 11 | 3 | 8 | 0.25 | 0.25 | 0.12–0.25 | ||||||||||
| N. veterana | 14 | 4 | 10 | 4 | 4 | 2–4 | ||||||||||
| N. wallacei | 12 | 1 | 1 | 1 | 8 | 1 | 4 | 4 | 0.25–8 | |||||||
| Nocardia speciesa | 38 | 1 | 1 | 3 | 4 | 2 | 15 | 11 | 1 | 2 | 4 | 0.06–8 | ||||
| Total Nocardia isolates | 300 | 3 | 8 | 20 | 25 | 34 | 86 | 109 | 15 | 2 | 4 | 0.06–8 | ||||
Nocardia species includes species with ≤6 isolates and Nocardia spp. that could not be identified to species level. This group consisted of N. vulneris (n = 6), N. asteroides (n = 4), N. transvalensis complex (n = 4), N. africana/nova (n = 3), N. pseudobrasiliensis (n = 3), N. brasiliensis/vulneris (n = 2), N. farcinica/kroppenstedtii (n = 2), N. africana (n = 1), N. amikacinitolerans (n = 1), N. araoensis (n = 1), N. araoensis/niwae (n = 1), N. carnea (n = 1), N. grenadensis (n = 1), N. rhamnosiphila (n = 1), N. sienata (n = 1), N. thailandica (n = 2), N. vinacea (n = 1), and Nocardia spp. (n = 3).
Among comparator drugs, TMP-SMX, linezolid, and amikacin displayed the best in vitro activity with 98.7%, 99.7%, and 96.7% of isolates testing susceptible, respectively (Table 2). Minocycline is the only tetracycline in this study with breakpoint interpretations in the CLSI M24S-2 (26), and minocycline susceptibility varied widely (0%–100%) depending on the Nocardia species. In general, omadacycline, tigecycline, and minocycline displayed similar activities against each Nocardia species, tracking within one twofold dilution of each other. The MIC50 and MIC90 of omadacycline against all Nocardia species were 2 µg/mL and 4 µg/mL, respectively; tigecycline: 1 µg/mL and 4 µg/mL, respectively; and minocycline: 2 µg/mL and 4 µg/mL, respectively. Regarding the five species for which omadacycline was found to have the most potent in vitro activity (N. paucivorans, N. asiatica, N. abscessus complex, N. beijingensis, and N. otitidiscaviarum), minocycline was found to have 100%, 100%, 100%, 100%, and 54.5% susceptibility, respectively.
TABLE 2.
Antimicrobial susceptibility of omadacycline and comparators against various Nocardia speciesc
| Species | No. tested | OMCa | TGCa | MIN | SXT | LZD | AXO | IMI | AMI | CIP | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50b | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | % S | % S | % S | % S | % S | % S | % S | ||
| N. abscessus complex | 16 | 0.5 | 1 | 0.25 | 0.5 | 0.5 | 1 | 100 | 100 | 100 | 100 | 25 | 100 | 0 |
| N. asiatica | 10 | 0.25 | 1 | 0.25 | 1 | 0.25 | 0.5 | 100 | 100 | 100 | 90 | 70 | 100 | 0 |
| N. beijingensis | 10 | 0.5 | 2 | 1 | 2 | 0.25 | 1 | 100 | 100 | 100 | 100 | 90 | 100 | 0 |
| N. brasiliensis | 19 | 2 | 2 | 0.25 | 0.5 | 1 | 4 | 52.6 | 100 | 100 | 52.6 | 5.3 | 100 | 5.3 |
| N. cyriacigeorgica | 64 | 2 | 4 | 1 | 2 | 2 | 4 | 14.1 | 100 | 100 | 84.4 | 82.8 | 98.4 | 0 |
| N. farcinica | 36 | 4 | 8 | 4 | 4 | 2 | 4 | 0 | 97.2 | 100 | 2.8 | 44.4 | 100 | 47.2 |
| N. nova | 59 | 4 | 4 | 1 | 2 | 2 | 4 | 20.3 | 100 | 100 | 66.1 | 98.3 | 100 | 0 |
| N. otitidiscaviarum | 11 | 1 | 2 | 0.5 | 1 | 1 | 2 | 54.5 | 100 | 100 | 0 | 0 | 100 | 0 |
| N. paucivorans | 11 | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 | 0.25 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| N. veterana | 14 | 4 | 4 | 2 | 4 | 2 | 4 | 14.3 | 100 | 100 | 35.7 | 100 | 100 | 0 |
| N. wallacei | 12 | 4 | 4 | 2 | 4 | 2 | 2 | 33.3 | 75 | 100 | 75 | 8.3 | 41.7 | 100 |
| Nocardia speciesd | 38 | 2 | 4 | 0.5 | 4 | 2 | 4 | 39.5 | 100 | 100 | 65.8 | 52.6 | 94.7 | 26.3 |
| Total Nocardia isolates | 300 | 2 | 4 | 1 | 4 | 2 | 4 | 35 | 98.7 | 99.7 | 63 | 64.7 | 96.7 | 17 |
There are no breakpoint interpretations for omadacycline and tigecycline against Nocardia species in the CLSI M24S-2; therefore, only the MIC50 and MIC90 are reported.
MIC values are reported in units of µg/mL.
OMC: omadacycline, MIN: minocycline, TGC: tigecycline, SXT: trimethoprim-sulfamethoxazole, LZD: linezolid, CIP: ciprofloxacin, IMI: imipenem, AXO: ceftriaxone, CIP: ciprofloxacin, AMI: amikacin.
Nocardia species includes species with ≤6 isolates and Nocardia spp. that could not be identified to species level. This group consisted of N. vulneris (n = 6), N. asteroides (n = 4), N. transvalensis complex (n = 4), N. africana/nova (n = 3), N. pseudobrasiliensis (n = 3), N. brasiliensis/vulneris (n = 2), N. farcinica/kroppenstedtii (n = 2), N. africana (n = 1), N. amikacinoitolerans (n = 1), N. araoensis (n = 1), N. araoensis/niwae (n = 1), N. carnaea (n = 1), N. grenadensis (n = 1), N. rhamnosiphila (n = 1), N. sienata (n = 1), N. thailandica (n = 2), N. vinacea (n = 1), and Nocardia spp. (n = 3).
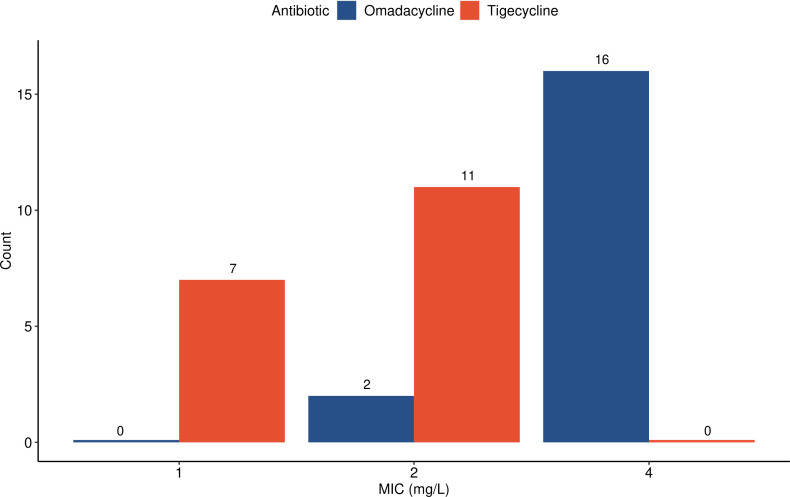
Notably, the CLSI M24S-2 guidelines do not include omadacycline or tigecycline QC ranges for the reference strain Nocardia nova ATCC BAA-2227 (26). To assess this strain’s suitability for QC of these agents, MICs for omadacycline and tigecycline were determined across 18 independent replicates. MICs for omadacycline ranged from 2 µg/mL to 4 µg/mL and MICs for tigecycline ranged from 1 µg/mL to 2 µg/mL (Fig. 1). Omadacycline BMD testing was shown to be reproducible with all MIC values within one twofold dilution across triplicate results from 10 clinical isolates representing eight species.
Fig 1.
Distribution of omadacycline and tigecycline MICs against reference QC strain N. nova ATCC BAA-2227. Because QC ranges have not been established for tigecycline or omadacycline, we performed 18 independent replicate reference BMD tests using the CLSI Nocardia nova QC organism. All MICs were 1–2 µg/mL for tigecycline and 2–4 µg/mL for omadacycline.
DISCUSSION
In this susceptibility study evaluating the in vitro activity of omadacycline against a large set of clinical Nocardia isolates, omadacycline activity was shown to vary between species. Omadacycline was most active against N. paucivorans, N. asiatica, N. abscessus complex, N. beijingensis, and N. otitidiscaviarum, whereas it was least active against N. farcinica. MIC distributions were generally within one twofold dilution of minocycline and tigecycline.
There is a paucity of high-quality randomized controlled trials to support evidence-based recommendations for Nocardia infections (2). Thus, selection of initial antibiotic regimens is individualized based on clinical presentation (site and severity of infection), immunocompromised state, Nocardia species involved, drug-drug interactions, and pharmacokinetic-pharmacodynamic advantages of a selected regimen. A combination of two or three active agents is typically recommended for severe, disseminated, or life-threatening forms of nocardiosis, consisting of high-dose TMP-SMX, an oxazolidinone, and an intravenous agent such as meropenem, imipenem, or ceftriaxone. Based on disease burden, severity at presentation, and clinical response, a lengthy duration of therapy (6–12 months or longer) is usually recommended for nocardiosis in immunocompromised hosts (2). The necessity for prolonged antimicrobial therapy with multiple agents often leads to treatment-associated adverse effects and organ toxicities. Additionally, populations at risk for nocardiosis often have concomitant therapies such as immunosuppressive agents, antimicrobial prophylactic agents, and chemotherapy that place them at risk for additive toxicities, including acute kidney injury and myelosuppression. Consequently, treatment discontinuation rates for TMP-SMX-based regimens have been reported to be greater than 50% (14, 28) due to poor gastrointestinal tolerance, renal toxicity, electrolyte imbalances, and myelosuppression. Similarly, the risk for adverse events with linezolid—particularly myelosuppression and mitochondrial toxicity (including lactic acidosis and peripheral and optic neuropathy)—increases with length of therapy, frequently leading to premature discontinuation of linezolid during the treatment of Nocardia infections (29, 30). Due to the safety profile of omadacycline and other tetracyclines, their regulatory approval in pneumonia and skin-soft tissue infections, and the immunomodulatory effects of this class of antibiotics, omadacycline may constitute a feasible option for consolidation of long-term therapy either as a single agent or in combination once susceptibility testing is available. Moreover, our study results support omadacycline empirical therapy for cases involving N. paucivorans, N. asiatica, N. beijingensis, N. abscessus complex, or N. otitidiscaviarum. Lastly, for those patients whose risk for renal and hematological toxicities limits the initial use of TMP-SMX or linezolid, our study supports consideration of omadacycline as a therapeutic option in combination with other active agents, for cases where N. paucivorans, N. asiatica, N. beijingensis, N. abscessus complex, or N. otitidiscaviarum are suspected or confirmed.
Minocycline is the only tetracycline antibiotic in this study with clinical breakpoints against Nocardia species available in the CLSI M24S-2 (26). Thus far, there are no breakpoints for omadacycline against Nocardia species because, heretofore, there has been a dearth of MIC data to establish epidemiologic cutoff values as well as a lack of published clinical experience treating nocardiosis with omadacycline. Like other tetracyclines, omadacycline has demonstrated an area-under-the-curve (AUC)-dependent killing effect (31–33). Based on hollow fiber model studies, the exposures associated with the standard oral dose of omadacycline of 300 mg daily have been suggested to be effective for pulmonary infections due to non-tuberculous mycobacteria with omadacycline MICs between 1 µg/mL and 4 µg/mL (31, 32, 34). While no head-to-head pharmacokinetic studies are available comparing omadacycline to minocycline, free plasma AUCs are approximately similar between the two agents at their respective standard dosing schemes (35–37), although variable, concentration-dependent protein binding with minocycline makes comparison of free drug exposures between agents challenging (38). Even though omadacycline and tigecycline were shown to have comparable MIC patterns across the different Nocardia species, pharmacokinetic differences between the two drugs may favor omadacycline when MICs are identical. Omadacycline was found to have approximately threefold higher concentrations than tigecycline in plasma, epithelial lining fluid, and alveolar cells
Trailing endpoints are a well-known phenomenon when reading MICs for TMP-SMX and linezolid, making the determination of MICs problematic (25). Brown-Elliott and Wallace performed in vitro susceptibility testing of omadacycline against both RGM and slowly growing mycobacteria (SGM) and found considerable trailing for omadacycline against RGM, but not for SGM (18). In our study, no significant trailing was identified when reading MICs. All QC isolates tested with omadacycline and comparator agents were within the CLSI M24S-2 acceptable ranges. Although there is no QC reference range for omadacycline against N. nova ATCC BAA-2227, the MICs for omadacycline against 18 replicates were all within one twofold dilution (2–4 µg/mL). Additionally, when 10 isolates across eight species were tested in triplicate, the MIC values were all within one twofold dilution. Altogether, the above results demonstrate the reproducibility of omadacycline MIC determinations with frozen reference BMD.
One limitation of our study is the potential degradation of omadacycline throughout the process of susceptibility testing. For instance, Shankar et al. discovered that intact omadacycline concentrations declined by approximately 50% in 24 hours, and this degradation can lead to falsely elevated MICs against SGM species that require prolonged AST incubation times (39). However, the impact on observed MICs in M. abscessus, an RGM similar to Nocardia in its doubling times, was minimal. Most of our MIC interpretations were read at 72 hours per CLSI M24S-2 guidelines, and it was rarely necessary to incubate longer. Thus, we expect that drug degradation did not significantly affect the omadacycline MIC results in this study.
Based on our results and its favorable pharmacologic properties, omadacycline may be a desirable therapeutic option for nocardiosis caused by certain Nocardia species. Further studies, including clinical trials, are needed to evaluate the potential clinical utility and role of omadacycline for the treatment of nocardiosis.
ACKNOWLEDGMENTS
We would like to thank our co-workers at ARUP Laboratories for performing isolate identification and assisting with susceptibility testing.
This research was supported by an investigator-initiated research grant from Paratek Pharmaceuticals, Inc. The sponsor reviewed the study design and final manuscript draft, but final decisions on the design and content of the manuscript were the responsibility of the authors.
Contributor Information
Russell J. Benefield, Email: russell.benefield@hsc.utah.edu.
Ryan K. Shields, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
REFERENCES
- 1. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ. 2006. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev 19:259–282. doi: 10.1128/CMR.19.2.259-282.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Restrepo A, Clark NM, Infectious Diseases Community of Practice of the American Society of Transplantation . 2019. Nocardia infections in solid organ transplantation: guidelines from the infectious diseases community of practice of the American society of transplantation. Clin Transplant 33:e13509. doi: 10.1111/ctr.13509 [DOI] [PubMed] [Google Scholar]
- 3. Traxler RM, Bell ME, Lasker B, Headd B, Shieh WJ, McQuiston JR. 2022. Updated review on Nocardia species: 2006-2021. Clin Microbiol Rev 35:e0002721. doi: 10.1128/cmr.00027-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conville PS, Brown-Elliott BA, Smith T, Zelazny AM. 2018. The complexities of Nocardia taxonomy and identification. J Clin Microbiol 56:e01419-17. doi: 10.1128/JCM.01419-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coussement J, Lebeaux D, Rouzaud C, Lortholary O. 2017. Nocardia infections in solid organ and hematopoietic stem cell transplant recipients. Curr Opin Infect Dis 30:545–551. doi: 10.1097/QCO.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 6. Brown-Elliott BA, Zelazny AM, Conville PS. 2023. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic Actinomycetes. In Carroll KC, Pfaller MA (ed), Manual of clinical Microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 7. Coussement J, Lebeaux D, van Delden C, Guillot H, Freund R, Marbus S, Melica G, Van Wijngaerden E, Douvry B, Van Laecke S, Vuotto F, Tricot L, Fernández-Ruiz M, Dantal J, Hirzel C, Jais J-P, Rodriguez-Nava V, Lortholary O, Jacobs F, European Study Group for Nocardia in Solid Organ Transplantation . 2016. Nocardia infection in solid organ transplant recipients: a multicenter European case-control study. Clin Infect Dis 63:338–345. doi: 10.1093/cid/ciw241 [DOI] [PubMed] [Google Scholar]
- 8. Steinbrink J, Leavens J, Kauffman CA, Miceli MH. 2018. Manifestations and outcomes of Nocardia infections: comparison of immunocompromised and nonimmunocompromised adult patients. Medicine (Baltimore) 97:e12436. doi: 10.1097/MD.0000000000012436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cattaneo C, Antoniazzi F, Caira M, Castagnola C, Delia M, Tumbarello M, Rossi G, Pagano L. 2013. Nocardia spp infections among hematological patients: results of a retrospective multicenter study. Int J Infect Dis 17:e610–4. doi: 10.1016/j.ijid.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 10. Kim YK, Sung H, Jung J, Yu SN, Lee JY, Kim S-H, Choi S-H, Kim YS, Woo JH, Lee S-O, Chong YP. 2016. Impact of immune status on the clinical characteristics and treatment outcomes of nocardiosis. Diagn Microbiol Infect Dis 85:482–487. doi: 10.1016/j.diagmicrobio.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 11. Haussaire D, Fournier P-E, Djiguiba K, Moal V, Legris T, Purgus R, Bismuth J, Elharrar X, Reynaud-Gaubert M, Vacher-Coponat H. 2017. Nocardiosis in the south of France over a 10-years period, 2004-2014. Int J Infect Dis 57:13–20. doi: 10.1016/j.ijid.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 12. Lebeaux D, Morelon E, Suarez F, Lanternier F, Scemla A, Frange P, Mainardi J-L, Lecuit M, Lortholary O. 2014. Nocardiosis in transplant recipients. Eur J Clin Microbiol Infect Dis 33:689–702. doi: 10.1007/s10096-013-2015-5 [DOI] [PubMed] [Google Scholar]
- 13. De La Cruz O, Minces LR, Silveira FP. 2015. Experience with Linezolid for the treatment of nocardiosis in organ transplant recipients. J Infect 70:44–51. doi: 10.1016/j.jinf.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 14. Hardak E, Yigla M, Berger G, Sprecher H, Oren I. 2012. Clinical spectrum and outcome of Nocardia infection: experience of 15-year period from a single tertiary medical center. Am J Med Sci 343:286–290. doi: 10.1097/MAJ.0b013e31822cb5dc [DOI] [PubMed] [Google Scholar]
- 15. Smego RA, Moeller MB, Gallis HA. 1983. Trimethoprim-sulfamethoxazole therapy for Nocardia infections. Arch Intern Med 143:711–718. [PubMed] [Google Scholar]
- 16. Tanaka SK, Steenbergen J, Villano S. 2016. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bio Medi Chem 24:6409–6419. doi: 10.1016/j.bmc.2016.07.029 [DOI] [PubMed] [Google Scholar]
- 17. Kim DY, McElvania E. 2023. Review of novel third-generation tetracyclines: eravacycline, omadacycline, and sarecycline. Clin Microb News 45:61–67. doi: 10.1016/j.clinmicnews.2023.04.001 [DOI] [Google Scholar]
- 18. Brown-Elliott BA, Wallace RJ. 2021. In vitro susceptibility testing of omadacycline against nontuberculous Mycobacteria. Antimicrob Agents Chemother 65:e01947-20. doi: 10.1128/AAC.01947-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li A, He S, Li J, Zhang Z, Li B, Chu H. 2023. Omadacycline, eravacycline, and tigecycline express anti-Mycobacterium abscessus activity in vitro Microbiol Spectr 11:e0071823. doi: 10.1128/spectrum.00718-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrisette T, Alosaimy S, Philley JV, Wadle C, Howard C, Webb AJ. 2021. Preliminary, real-world, multicenter experience with omadacycline for Mycobacterium abscessus infections. Open Forum Infect Dis 8:ofab002. doi: 10.1093/ofid/ofab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pearson JC, Dionne B, Richterman A, Vidal SJ, Weiss Z, Velásquez GE, Marty FM, Sax PE, Yawetz S. 2020. Omadacycline for the treatment of Mycobacterium abscessus disease: a case series. Open Forum Infect Dis 7:faa415. doi: 10.1093/ofid/ofaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siddiqa A, Khan S, Rodriguez GD, Urban C, Segal-Maurer S, Turett G. 2023. Omadacycline for the treatment of Mycobacterium abscessus infections: case series and review of the literature. IDCases 31:e01703. doi: 10.1016/j.idcr.2023.e01703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khot PD, Bird BA, Durrant RJ, Fisher MA. 2015. Identification of Nocardia species by matrix-assisted laser desorption Ionization-time of flight mass spectrometry. J Clin Microbiol 53:3366–3369. doi: 10.1128/JCM.00780-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schlaberg R, Fisher MA, Hanson KE. 2014. Susceptibility profiles of Nocardia isolates based on current taxonomy. Antimicrob Agents Chemother 58:795–800. doi: 10.1128/AAC.01531-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. CLSI . 2018. Susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes. In CLSI standard M24. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 26. CLSI . 2023. Performance standards for susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes. In CLSI supplement M24S, 2nd ed [Google Scholar]
- 27. Wickham H. 2016. Ggplot2: elegant graphics for data analysis. Springer-Verlag, New York. [Google Scholar]
- 28. Rosman Y, Grossman E, Keller N, Thaler M, Eviatar T, Hoffman C, Apter S. 2013. Nocardiosis: a 15-year experience in a tertiary medical center in Israel. Eur J Intern Med 24:552–557. doi: 10.1016/j.ejim.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 29. Burdette SD, Trotman R. 2015. Tedizolid: the first once-daily oxazolidinone class antibiotic. Clin Infect Dis 61:1315–1321. doi: 10.1093/cid/civ501 [DOI] [PubMed] [Google Scholar]
- 30. Jodlowski TZ, Melnychuk I, Conry J. 2007. Linezolid for the treatment of Nocardia spp. infections. Ann Pharmacother 41:1694–1699. doi: 10.1345/aph.1K196 [DOI] [PubMed] [Google Scholar]
- 31. Singh S, Gumbo T, Boorgula GD, Shankar P, Heysell SK, Srivastava S. 2022. Omadacycline pharmacokinetics/pharmacodynamics in the hollow fiber system model and potential combination regimen for short course treatment of Mycobacterium kansasii pulmonary disease. Antimicrob Agents Chemother 66:e0068722. doi: 10.1128/aac.00687-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh S, Wang J-Y, Heysell SK, McShane PJ, Wadle C, Shankar P, Huang H-L, Pasipanodya J, Boorgula GD, Philley JV, Gumbo T, Srivastava S. 2023. Omadacycline pharmacokinetics/pharmacodynamics in the hollow fiber model and clinical validation of efficacy to treat pulmonary Mycobacterium abscessus disease. Int J Antimicrob Agents 62:106847. doi: 10.1016/j.ijantimicag.2023.106847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodvold KA, Burgos RM, Tan X, Pai MP. 2020. Omadacycline: a review of the clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 59:409–425. doi: 10.1007/s40262-019-00843-4 [DOI] [PubMed] [Google Scholar]
- 34. Chapagain M, Pasipanodya JG, Athale S, Bernal C, Trammell R, Howe D, Gumbo T. 2022. Omadacycline efficacy in the hollow fibre system model of pulmonary Mycobacterium avium complex and potency at clinically attainable doses. J Antimicrob Chemother 77:1694–1705. doi: 10.1093/jac/dkac068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bidell MR, Lodise TP. 2021. Use of oral tetracyclines in the treatment of adult outpatients with skin and skin structure infections: focus on doxycycline, minocycline, and omadacycline. Pharmacotherapy 41:915–931. doi: 10.1002/phar.2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin W, Flarakos J, Du Y, Hu W, He H, Mangold J, Tanaka SK, Villano S. 2017. Pharmacokinetics, distribution, metabolism, and excretion of omadacycline following a single intravenous or oral dose of 14C-omadacycline in rats. Antimicrob Agents Chemother 61:e01784-16. doi: 10.1128/AAC.01784-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhanel GG, Esquivel J, Zelenitsky S, Lawrence CK, Adam HJ, Golden A, Hink R, Berry L, Schweizer F, Zhanel MA, Bay D, Lagacé-Wiens PRS, Walkty AJ, Lynch JP, Karlowsky JA. 2020. Omadacycline: a novel oral and intravenous aminomethylcycline antibiotic agent. Drugs 80:285–313. doi: 10.1007/s40265-020-01257-4 [DOI] [PubMed] [Google Scholar]
- 38. Zhou J, Tran BT, Tam VH. 2017. The complexity of minocycline serum protein binding. J Antimicrob Chemother 72:1632–1634. doi: 10.1093/jac/dkx039 [DOI] [PubMed] [Google Scholar]
- 39. Shankar P, Singh S, Boorgula GD, Gumbo T, Heysell SK, Srivastava S. 2022. Challenges and a potential solution to perform drug susceptibility testing of omadacycline against nontuberculous Mycobacteria. Tuberculosis (Edinb) 137:102269. doi: 10.1016/j.tube.2022.102269 [DOI] [PMC free article] [PubMed] [Google Scholar]