ABSTRACT
Bovine fasciolosis is a parasitic disease with a global reach. Coprological based on egg detection in fecal samples and liver inspection to evaluate the presence of the parasite is currently the gold standard for diagnosing chronic fasciolosis in cattle. However, these techniques are labor-intensive and ineffective during the acute phase of the disease. Serodiagnosis using native and recombinant antigens has become an interesting alternative in efforts to identify cattle fasciolosis. We evaluated cattle from abattoir (n = 139) and farms (n = 500) through liver inspection and coprological examination, respectively. Our laboratory team optimized and validated enzyme-linked immunosorbent assay tests based on somatic antigen, excretory/secretory proteins, and the recombinant antigen cathepsin L-1 to detect serum antibodies against fasciolosis in cattle. For animals from abattoir, 10 were positive for fasciolosis according to liver inspection. Both FhES and FhrCL-1 presented an area under the receiver operating characteristic (AUROC) curve of 0.80, with a sensitivity of 0.80 (95% CI: 0.46–0.95) and 0.70 (95% CI: 0.38–0.90) and specificity of 0.81 (95% CI: 0.73–0.87) and 0.87 (95% CI: 0.80–0.92), respectively. For those cattle from farms, 28 were positive only for fasciolosis according to coprological examination. In this scenario, FhES gave the best performance, with an AUROC of 0.84, sensitivity of 0.79 (95% CI: 0.60–0.90), and specificity of 0.86 (95% CI: 0.82–0.89). In conclusion, our study highlights the potential of serodiagnosis for accurately screening cattle fasciolosis. The promising sensitivity and specificity values of FhES when compared to liver inspection or coprological examination enhance its importance for cattle fasciolosis diagnosis.
IMPORTANCE
The aim of this article was to identify antibodies against fasciolosis in cattle in Brazil. The methodology was reproduced in our laboratory and applied for the first time to the Brazilian cattle herd. The antigens tested can be used as a screening test and thus speed up the diagnosis of bovine fascioliasis.
KEYWORDS: fasciolosis, cattle, native antigens (FhES and FhSA), recombinant antigen (FhrCL-1), ELISA
INTRODUCTION
Fasciola hepatica, a plant-borne trematode species, is responsible for the zoonotic disease known as fasciolosis or liver fluke disease in humans and animals (1–3). The disease has traditionally been characterized as important in the veterinary context due to the substantial production and economic losses it causes in livestock (4–7). Herbivorous mammal hosts, such as cattle, goats, and sheep, are the most important disease transmission path to humans (8). Human fasciolosis is considered a neglected tropical disease by the World Health Organization, with estimates of 2.4 million infected individuals and 180 million people at risk of infection worldwide (9, 10).
This trematode has an extensive global distribution and is found on every continent except Antarctica. Human fasciolosis poses major health problems in Europe, Cuba, Oceania, and the Americas (1, 11), with a higher number of cases reported in South America (Bolivia, Peru, Chile, Ecuador, and Venezuela) than in other regions (3, 12–14). In contrast, non-Andean, lowland countries in South America have reported sporadic and isolated human cases, including Uruguay (15) and Brazil (16, 17). Among animals, studies in the Americas have demonstrated a wide prevalence in goats and a lower prevalence in cattle (18, 19). In the Brazilian state of Santa Catarina, a prevalence of 10.8% in cattle was documented in an abattoir (20). Fasciolosis causes economic losses related to cattle production and severely impacts public health (6, 20, 21). Such economic losses have been quantified at a national level in Brazil, with a 5.8% reduction in carcass weight translating to a 35 USD loss per animal in this country (22, 23).
F. hepatica is adaptable to different environmental conditions and has the ability to switch hosts (24), resulting in a broad host range (10). Its spread is also related to the geographic expansion of its original intermediate host, the Lymnaeidae snails (1). The life cycle of this disease comprises three stages, each characterized by distinct symptoms. The acute phase, initiated through ingestion of metacercariae in contaminated vegetation and water, lasts 2–4 months and manifests as abdominal pain, fever, urticaria, and gastrointestinal disturbances (2, 25). The latent phase involves newly encysted juveniles penetrating the intestinal wall and peritoneum, migrating to the liver tissue, and reaching the bile ducts over several months (26–28). In the chronic phase, mature parasites in bile ducts produce eggs, causing severe liver and bile duct damage.
The established diagnostic method for bovine fasciolosis is the identification of eggs in feces (coprological examination), which is cost-effective and the gold standard for various parasitic diseases in humans and animals (27, 29). Diagnosis throughout coprological examination often occurs during the chronic phase, when much of the liver damage has already occurred (28). However, there is a consensus that this method is not completely reliable for several reasons. A period of 8–15 weeks post-infection is required for F. hepatica eggs to appear in feces when many pathological lesions have already manifested (30, 31). Additionally, the method may not detect low-intensity or intermittent infections (27, 32). In regions where the disease is not endemic, infections with immature flukes are not detected. Furthermore, the eggs are released intermittently from the bile ducts, so stool samples from infected patients (humans and animals) may not contain eggs (27).
Postmortem worm counting in the liver can be considered a valuable diagnostic method if the livers are appropriately sliced and soaked. F. hepatica can also be identified by inspecting cattle livers for adult worms in abattoirs. Postmortem examination of the bovine liver is a key approach to assessing the severity of F. hepatica infections. This entails examining livers for juvenile worms and bile ducts for adults, along with any associated pathological changes. Different rates of bovine fasciolosis have been reported in different abattoirs globally, with Brazil, for instance, documenting a 29.51% infection rate among animals (33). However, even mild or prepatent infections can evade detection, impacting the estimated sensitivity and specificity of the test.
Serological techniques, including lateral flow assays (34) and the indirect enzyme-linked immunosorbent assay (ELISA) (35–38), have been explored for detecting specific antibodies. ELISA-based detection of serum antibodies is a widely used diagnostic tool. It is highly regarded for its sensitivity and reliability in diagnosing acute infections, and it can complement fecal analysis for diagnosing latent and chronic infections (27). The antigens traditionally employed in serological tests consist of native antigens (somatic antigens and excretory/secretory antigens) of F. hepatica (35). To enhance diagnostic specificity, several purified F. hepatica antigens and recombinant antigens (36, 37) have been used, most notably cathepsin L, a major protease involved in bovine fasciolosis. Serological tests have demonstrated high accuracy in diagnosing human, bovine, and ovine fasciolosis. The recombinant cathepsin L1 test utilizes recombinant pro-cathepsin L1 and targets antibodies against cathepsin, a cysteine protease, for diagnosing fasciolosis caused by F. hepatica (37, 39), with no reported cross-reactions. Similarly, other studies observed no cross-reactions in native antigens and cathepsin-based ELISA tests, reporting strong performance (39–44). While many serological methods have been published, only a few have been commercially adopted.
In this context, the present study aimed to assess the potential of available native antigens, both somatic (FhSA) and excretory/secretory (FhES), and the recombinant antigen cathepsin L (FhrCL-1) for serodiagnosis of cattle fasciolosis in Brazil.
MATERIALS AND METHODS
Characteristics of the cattle included in the study
Abattoir cattle sample
A total of 139 serum samples were collected from a cattle abattoir located in southern Santa Catarina, Brazil. The presence of cattle fasciolosis was determined through liver inspection. According to this approach, 10/139 (7.2%) cattle were diagnosed with F. hepatica, with no other parasites investigated during the veterinary inspection. Serum samples were processed, divided into aliquots, and stored at −30°C for subsequent ELISA testing.
Farms cattle sample
Five hundred serum and fecal samples (420 from female and 80 from male cattle) were obtained from 37 farms in southern Santa Catarina, Brazil. The samples were collected from cattle ranging from 6 months to 20 years old. Fecal samples (6 g) were used for in vivo diagnostics of fasciolosis and other parasites through coprological examination based on a sedimentation protocol (32). The tests were conducted in triplicate, and the entire sediments were analyzed under a stereomicroscope (32, 45). Serum samples were processed, divided into aliquots, and stored at −30°C for subsequent ELISA testing.
FhSA and FhES
The FhSA and FhES preparations were carried out as follows: intact and live adult parasites were obtained from cattle livers at a local abattoir. Initially, the parasites underwent a series of 3–4 washes at room temperature using 0.01 M phosphate-buffered saline (PBS) with a pH of 7.2 to eliminate any traces of blood and bile.
For the FhSA preparation, the parasites were kept in a PBS solution and transported to the laboratory. Subsequently, the parasites were macerated and divided into separate portions. The protease inhibitor trans-Epoxysuccinyl-L-leucylamido(4-guanidino) butane (E-64; Sigma-Aldrich, US) was added to each sample at a concentration of 10 µM to minimize protein degradation. The antibiotics penicillin (100 U/mL) and streptomycin (0.25 mg/mL) were also incorporated to counteract bacterial activity.
For the FhES preparation, parasites were incubated in Roswell Park Memorial Institute (RPMI) 1640 medium at 37°C for 6 h. Within the laboratory setting, the parasites were subjected to five washing rounds with PBS containing antibiotics (penicillin and streptomycin). The first two washes used a volume of 10 mL PBS with antibiotics, while the subsequent three used a volume of 8 mL. Subsequently, the parasites were transferred using forceps into a 15 mL falcon tube containing RPMI 1640 medium preheated to 37°C. They were then cultured at a concentration of six parasites per 3 mL for 6 h at 37°C.
After incubation, the falcon tube was centrifuged at 14,000 × g for 30 min. The supernatant was then collected and divided into three microtubes, each containing 1 mL. E-64 was introduced to prevent protein degradation. The secretory/excretory antigens were obtained by culturing F. hepatica in RPMI medium and filtered using an Amicon Ultra-15 100 kDa centrifugal filter (Millipore, UK). During the antigen filtration process from the excretory/secretory systems, the RPMI medium was replaced with a saline buffer.
SDS-PAGE was conducted to analyze the protein content within FhSA and FhES. Quantification of both the somatic antigen and the excretory/secretory antigens was carried out using a fluorimetric method in a Qubit (Thermo Fischer Scientific, US) instrument. Following protein quantification, the supernatants of FhSA and FhES were divided into aliquots and stored at −30°C until use.
Expression and purification of FhrCL-1
The full-length cDNA of F. hepatica preprocathepsin L1 (U62288.2) was obtained commercially in the pPIC9K vector from (GenScript, US). Protein expression was conducted using the multicopy system of the Pichia pastoris GS115 strain. The recombinant sequence featured a single amino acid substitution, replacing the active site Cys25 with Gly. This alteration resulted in the loss of functional activity while preserving the enzyme’s conformation, rendering it more stable during fermentation and downstream isolation processes (39, 46, 47).
To generate the inactive enzyme, fermentation was performed in a liquid minimal medium containing yeast extract and glycerol (BMGY) to enhance yeast cell density. Cultivation in BMGY took place for 16 h at 30°C with agitation at 250 rpm. Once the yeast cell density reached an OD600 of 2–6, approximately 1 mL of the inoculum was transferred to a liquid minimal medium containing yeast extract and methanol (BMMY) to induce FhrCL1 expression. Cultivation in BMMY lasted 92 h at 30°C under agitation at 250 rpm. During this time, the medium was supplemented with 1% methanol every 24 h.
After completing the cultivation period, the culture was centrifuged at 10,000 × g for 30 min at room temperature. The resulting pellets were discarded. FhrCL-1 was isolated from the supernatant using Ni-NTA affinity chromatography, following previously described methods (39, 47, 48).
ELISA optimization and development
FhSA, FhES, and FhrCL-1
To define ELISA conditions, we performed a matrix comparison using various antigen concentrations, dilutions of the primary sera, and dilutions of secondary antibodies for FhSA, FhES, and FhrCL-1 antigens, respectively.
Optimal antigen concentrations and serum dilutions were determined by checkerboard titrations. FhSA, FhES, and FhrCL-1 antigens (0.5 µg/mL, 1.0 µg/mL, and 1.0 µg/mL, respectively) were dissolved separately in bicarbonate/carbonate coating buffer at pH 9.0 and added to each ELISA plate (Sarstedt AG & Co. KG, DE). One hundred microliters of the solution were then added to each well and incubated overnight at 4°C. The plates were washed three times with 0.05% Tween-80 in water. After coating, an additional blocking step with 100 µL 1% skimmed milk in 0.05% Tween-80 was performed for 1 h at 37°C. After a further washing procedure, 100 µL of sera-diluted pooled samples were added to each antigen (1:50, 1:100, and 1:50, respectively), and the plates were incubated for 1 h at 37°C. Following another wash, 100 µL of peroxidase-conjugated anti-bovine antibody (Sigma-Aldrich, US) for each antigen (1:10.000, 1:10.000, and 1:30.000, respectively) was added to the wells, and the plates were incubated for 30 min at 37°C. After a final washing step, bound antibodies were detected by adding 100 µL of tetramethylbenzidine (Thermo Fischer Scientific, US). After incubation at room temperature in the dark for 10–20 min, the reaction was stopped with 50 µL of 0.1 M sulfuric acid. The plates were read on an ELISA reader at 450 nm to determine absorbance values.
After developing and optimizing serological ELISA conditions, we tested serum samples from cattle collected in an abattoir and cattle farms. Negative and positive controls were used to diagnose fasciolosis in cattle by ELISA, using FhSA, FhES, and FhrCL-1 as antigens. A pool of four samples (two negative samples for the presence of fasciolosis in the visceral inspection and two negative samples for the coprological examination) was used as a negative control on each plate. As a positive control, a pool of four samples was used on each plate (two positive samples for the presence of fasciolosis in the visceral inspection and two positive samples for the coprological examination). Positive control, negative control, and plate control were used in duplicate in all experiments.
Statistical analysis
To evaluate the diagnostic performance of native (FhSA and FhES) and recombinant antigens (FhrCL-1), we used liver inspection and coprological examination as the gold standard test for cattle from abattoir and farms, respectively. Initially, the distribution of the quantitative values for the serodiagnosis tests was analyzed according to the categories (positive or negative) of the gold standard tests, aiming to explore their descriptive statistics, such as minimum, maximum, and median values, first and third quartile, mean values and SD, as well as to inspect for outliers.
The optimal cutoff value for each ELISA method was based on a logistic regression model, considering as response variable the gold standard test results (positive or negative) and as predictor the log of the quantitative values for the serodiagnosis test. Briefly, we applied a logistic regression model to adjust a classifier and a leave-one-out cross-validation (CV) technique to evaluate its diagnostic performance in data not used for its adjustment. Thus, on each CV iteration, the observations were divided into training and test data; the former was used to adjust a logistic model and the latter to estimate the probability of being classified as a positive sample. After all samples were part of the training and test data, the vector of estimated probabilities was used to evaluate the diagnostic performance of the model. For this, it was necessary to choose a cutoff point for the estimated probability, aiming to classify samples as positive or negative. We chose the cutoff that maximizes the model’s sensitivity and specificity and calculated the area under the receiver operating characteristic (AUROC) curve, sensitivity (S), specificity (E), positive predictive values (PPVs), and negative predictive values (NPVs) and the respective 95% CI of all of these estimates. The analyses were performed separately for cattle from abattoir and farms on R software using caret, pROC, and CompareTests packages. The script used for the analysis is available at https://github.com/Hellengeremias/Fasciolosis.
RESULTS
Abattoir cattle sample
Table 1 shows a summary of the FhES, FhSA, and FhrCL-1 values according to the presence (positive group) or absence (negative group) of fasciolosis detected by liver inspection of cattle from the abattoir (n = 139). In general, for the three tests, the positive group (n = 10) had higher values for the first and third quartiles as well for median and mean than the negative group (n = 129).
TABLE 1.
Descriptive summary of the three tests when applied to cattle from abattoirs (n = 139)a
| Summary values | Native antigens (OD) | Recombinant antigen (OD) | |
|---|---|---|---|
| FhES | FhSA | FhrCL-1 | |
| Positive group (n = 10) | |||
| Minimum | 0.360 | 0.196 | 0.076 |
| First quartile | 0.490 | 0.270 | 0.111 |
| Median | 0.571 | 0.443 | 0.252 |
| Mean (SD) | 0.573 (0.141) | 0.439 (0.186) | 0.235 (0.126) |
| Second quartile | 0.668 | 0.568 | 0.336 |
| Maximum | 0.815 | 0.716 | 0.436 |
| Negative group (n = 129) | |||
| Minimum | 0.192 | 0.114 | 0.057 |
| First quartile | 0.298 | 0.169 | 0.078 |
| Median | 0.362 | 0.222 | 0.090 |
| Mean (SD) | 0.393 (0.133) | 0.263 (0.144) | 0.104 (0.064) |
| Second quartile | 0.452 | 0.303 | 0.107 |
| Maximum | 0.806 | 1.247 | 0.638 |
SD= Standard Deviation; OD= Optical Density.
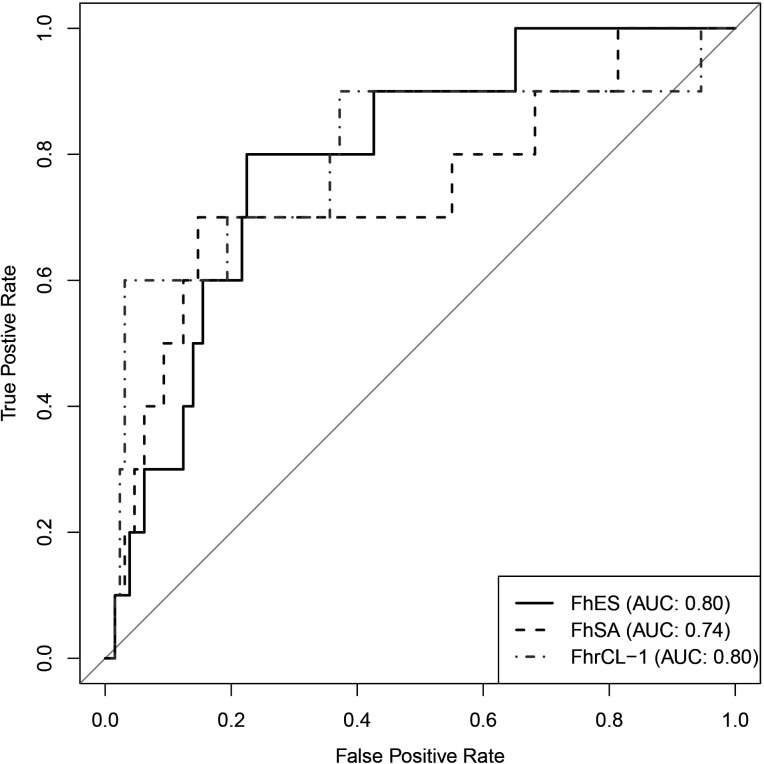
The AUROC for FhES, FhSA, and FhrCL-1 adjusted models was 0.80 (95% CI: 0.67–0.92), 0.74 (95% CI: 0.55–0.93), and 0.80 (95% CI: 0.61–0.98), respectively (Fig. 1). For each test, we chose the cutoff point that maximizes the model’s sensitivity and specificity (Table 2). The three tests had a moderately accurate performance. The chosen cutoff value for the FhES ELISA test showed higher sensitivity and NPV (0.80 and 0.98, respectively), indicating the test suitability in screening fasciolosis: only 2 of the 10 fasciolosis cases were mistakenly classified as negative (false negative result). Thus, out of 106 negative results, 104 were true negative. Nonetheless, since the fasciolosis prevalence is low (10 positive cases in 139 cattle), we observed a large number of false positives and consequently a low PPV: only 8 of the 33 positive results were true positive, suggesting the serological tests cannot be used to confirm the presence of the disease.
Fig 1.
Receiver operating characteristic curves for FhES, FhSA, and FhrCL-1 for cattle from abattoir (n = 139).
TABLE 2.
Diagnostic performance measures for the three tests on abattoir cattle considering liver inspection as the gold standard method (n = 139, 10 positive cases)a
| Performance measures | Native antigens | Recombinant antigen | |
|---|---|---|---|
|
FhES estimate (95% CI) |
FhSA estimate (95% CI) |
FhrCL-1 estimate (95% CI) |
|
| Cutoff | 0.4895 (OD) | 0.379 (OD) | 0.1050 (OD) |
| Sensitivity | 8/10 (0.80) (0.46–0.95) |
7/10 (0.70) (0.38–0.90) |
7/10 (0.70) (0.38–0.90) |
| Specificity | 104/129 (0.81) (0.73–0.87) |
113/129 (0.88) (0.81–0.92) |
112/129 (0.87) (0.80–0.92) |
| PPV | 8/33 (0.24) (0.17–0.34) |
7/23 (0.30) (0.19–0.45) |
7/24 (0.29) (0.18–0.43) |
| NPV | 104/106 (0.98) (0.94–0.99) |
113/116 (0.97) (0.94–0.99) |
112/115 (0.97) (0.94–0.99) |
CI= Confidence Intervals; PPV= Positive Predictive Values; NPV= Negative Predictive Values; OD= Optical Density.
Farms cattle sample
The coprological examination resulted in 405/500 (81%) negative and 95/500 (19%) positive results. Of the 95 positive results, 28/500 (5.6%) were positive only for F. hepatica eggs, and 10/500 (2%) for F. hepatica and other parasites: 7/500 (1.4%) also contained Strongylidae eggs, 2/500 (0.4%) Eimeria eggs, and 1/500 (0.2%) Strongylidae and Eimeria eggs. The examination also showed that 44/500 (8.8%) cattle were positive only for Strongylidae eggs and 13/500 (2.6%) for both Strongylidae and Eimeria eggs. Animals positive for other parasites than F. hepatica (n = 67) were excluded from the diagnostic performance evaluation of native and recombinant antigens described below.
Table 3 shows the summary values of FhES, FhSA, and FhrCL-1 according to the presence (positive group) or absence (negative group) of fasciolosis detected by coprological examination in cattle from farms. The positive group (n = 28) had higher values for the first and third quartiles as well as for the median and mean for the three serological tests than the negative group (n = 405).
TABLE 3.
Descriptive summary for the three tests when applied to cattle from farms (n = 433). Cattle diagnosed with other than parasites than F. hepatica (n = 67) were excluded from the analysesa
| Summary values | Native antigens (OD) | Recombinant antigen (OD) | |
|---|---|---|---|
| FhES | FhSA | FhrCL-1 | |
| Positive group (n = 28) | |||
| Minimum | 0.213 | 0.168 | 0.058 |
| First quartile | 0.412 | 0.440 | 0.102 |
| Median | 0.529 | 0.637 | 0.141 |
| Mean (SD) | 0.560 (0.213) | 0.641 (0.299) | 0.184 (0.116) |
| Second quartile | 0.776 | 0.927 | 0.248 |
| Maximum | 0.828 | 1.312 | 0.454 |
| Negative group (n = 405) | |||
| Minimum | 0.085 | 0.078 | 0.054 |
| First quartile | 0.202 | 0.266 | 0.088 |
| Median | 0.259 | 0.382 | 0.103 |
| Mean (SD) | 0.289 (0.122) | 0.406 (0.191) | 0.113 (0.044) |
| Second quartile | 0.353 | 0.510 | 0.126 |
| Maximum | 0.898 | 1.373 | 0.410 |
SD= Standard Deviation; OD= Optical Density.
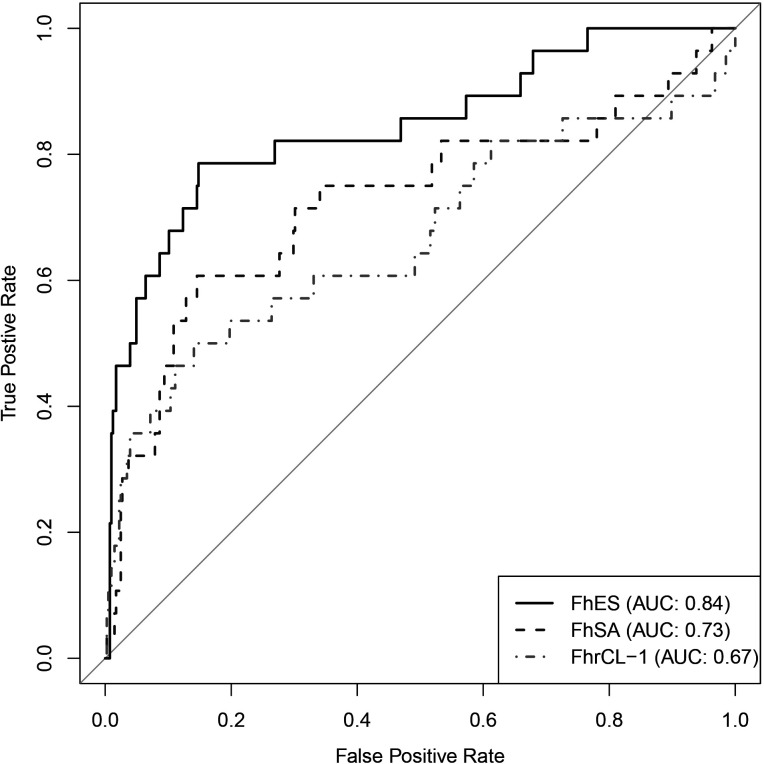
The AUROCs for FhES, FhSA, and FhrCL-1 adjusted models were 0.84 (95% CI: 0.76–0.93), 0.73 (95% CI: 0.61–0.85), and 0.67 (95% CI: 0.54–0.80), respectively (Fig. 2). For each test, we chose the cutoff point that maximizes the model’s sensitivity and specificity (Table 4). For this scenario, the FhES also presented better results, with a sensitivity of 0.79 and an NPV of 0.98. Of the 353 negative results, 347 were true negatives when using the chosen cutoff value for the FhES-adjusted model. Nonetheless, since the disease prevalence was low (28/433, 6,5%), the cutoff value for the FhES adjusted model resulted in a higher number of false positives: out of the 80 positive results, only 22 were true positives.
Fig 2.
Receiver operating characteristic curves for FhES, FhSA, and FhrCL-1 for cattle from farms (n = 433).
TABLE 4.
Diagnostic performance measures for the three tests on farms cattle considering coprological examination as the gold standard method (n = 433, 28 positive cases). Cattle diagnosed with other than parasites than F. hepatica (n = 67) were excluded from the analysesa
| Performance measures | Native antigens | Recombinant antigen | |
|---|---|---|---|
|
FhES estimate (95% CI) |
FhSA estimate (95% CI) |
FhrCL-1 estimate (95% CI) |
|
| Cutoff | 0.4105 (OD) | 0.4830 (OD) | 0.1270 (OD) |
| Sensitivity | 22/28 (0.79) (0.60–0.90) |
20/28 (0.71) (0.52–0.85) |
16/28 (0.57) (0.39–0.74) |
| Specificity | 347/405 (0.86) (0.82–0.89) |
285/405 (0.70) (0.66–0.75) |
304/405 (0.75) (0.71–0.79) |
| PPV | 22/80 (0.28) (0.22–0.34) |
20/140 (0.14) (0.11–0.18) |
16/117 (0.14) (0.10–0.19) |
| NPV | 347/353 (0.98) (0.97–0.99) |
285/293 (0.97) (0.95–0.98) |
304/316 (0.96) (0.94–0.97) |
CI= Confidence Intervals; PPV= Positive Predictive Values; NPV= Negative Predictive Values; OD= Optical Density.
Aiming to investigate the impact of cross-reactions on the diagnostic performance of the three antigens (FhES, FhSA, and FhrCL-1), we reanalyzed the data from farms also considering the 67 samples positive by coprological examination that were excluded for the analyzes presented previously. The 10 samples positive for fasciolosis and other parasites were considered in the positive group, together with the 28 samples positive only for fasciolosis, and the 57 samples positive only for other parasites were considered in the negative group, together with the 405 samples negative for fasciolosis. First, we evaluated the distribution of absorbance values of these 67 samples and those 28 positive only for fasciolosis and compared this distribution with the cutoff points obtained from the adjusted models that considered samples positive only for fasciolosis (Fig. S1). Overall, these results show that samples positive for fasciolosis presented higher absorbance values. Table S1 shows the summary values of FhES, FhSA, and FhrCL-1 according to the presence (positive group) or absence (negative group) of fasciolosis diagnosed by coprological examination, and Table S2 shows the results of the diagnostic performance of the adjusted models. The AUROC curve for the FhES, FhSA, and FhrCL-1 was similar to those that considered samples positive only for fasciolosis: 0.83 (95% CI: 0.75–0.90), 0.73 (95% CI: 0.63–0.83), and 0.68 (95% CI: 0.58–0.79), respectively (Fig. S2).
DISCUSSION
Our study is the first to compare native and recombinant antigens for diagnosing cattle fasciolosis in Brazilian animals. Coprological and liver inspection were used as the gold standard diagnostic tests for both farms and abattoir animals. The FhES serological test was better able to discriminate positive and negative samples for both farms and abattoir animals, and it seems suitable for screening purposes. The economic and public health problems caused by cattle fasciolosis have been reported in different parts of the world, including Brazil (20, 21, 28, 49). It is important to develop and establish a reliable, simple, and rapid diagnostic tool for properly diagnosing cattle fasciolosis in Brazil, especially in endemic areas. In the present study, we evaluated the performance of three ELISA tests using FhES, FhSA, and FhrCL-1 antigens to diagnose cattle fasciolosis based on information obtained from a meta-analysis study (50).
Liver necropsy, which diagnoses fasciolosis when bile ducts are dissected, is the only conclusive diagnostic procedure for F. hepatica (7, 51). This is impractical as a herd or flock management tool, as it can only be carried out postmortem. Specific ELISA tests for liver flukes have been developed to meet these requirements and are now routinely used for cattle (30, 52). ELISAs for F. hepatica are versatile tests capable of detecting specific antibodies or antigens in fecal samples as well as pooled or individual milk and sera (36, 37, 53). One significant drawback of relying on fecal egg counts is the inability to diagnose immature migrating stages of liver flukes within the final host. Consequently, using ELISA tests with early diagnostic potential represents a notable advantage (27, 36). The most detrimental phase of this infection occurs during the migration of immature stages (36, 37). The application of ELISA techniques for F. hepatica diagnosis has consistently exhibited enhanced sensitivity compared to coprological methods (36–38). Moreover, it offers the distinct advantage of detecting pre-patent infections.
Serological diagnosis of cattle fasciolosis based on fractions of adult worm antigens has been reported in different studies worldwide (36, 43, 54). To this end, we used two cattle populations with known infection status (the presence of eggs in the feces or parasites in the liver). Our first serological panel comprised more than 100 cattle samples collected in an abattoir. A small number of articles that evaluated the diagnosis of fasciolosis in cattle used samples collected in abattoirs (36). Our second serological panel consisted of 500 samples of blood and feces from cattle collected on farms. The studies that evaluated the serological diagnosis of bovine fasciolosis used small panels with up to 100 animals (43, 54–56).
A critical point for evaluating a new immunodiagnostic test is to propose a cutoff point that properly discriminates between negative and positive samples. The absorbance values of FhES, FhSA, and FhrCL-1 antigens tested had a good ability to distinguish between positive and negative samples in abattoir samples. Only the FhES antigen performed well in differentiating positive and negative cattle fasciolosis on serum samples collected on farms. Our investigation demonstrated that the absorbance values for the FhES antigen were comparable to those reported in other studies when sera from cattle with fasciolosis were examined using coprological testing as the gold standard (55).
Our study established a cutoff value for each proposed ELISA test based on positive and negative samples using liver inspection and coprological examination as the gold standard tests. The cutoff points for FhES, FhSA, and FhrCL-1 were 0.4895, 0.379, and 0.1050, respectively, for cattle from the abattoir and 0.4105, 0.4830, and 0.1270 for those from farms. The native antigens FhES and FhSA consist of a complex mixture of proteins, potentially leading to elevated absorbance values. In contrast, the recombinant antigen FhrCL-1 is a single purified protein, which could account for the comparatively lower absorbance values observed. Different approaches are employed when developing ELISA tests for serological diagnosis of fasciolosis in cattle. The cutoff values reported by studies assessing one of these antigens vary, although they are often higher than those found in our analysis. Different methods based on the average absorbance value and the ROC curve are used in the ELISA tests created using native and recombinant antigens for the serological diagnosis of bovine fasciolosis (36–38, 55, 57).
Serology offers the advantage of earlier detection of infections in comparison to fecal egg detection. In addition, when compared to coprological methods, serological approaches, particularly the ELISA test, are very sensitive and specific. Since F. hepatica is the main cause of cattle fasciolosis, most of the studies related to the disease diagnosis focus on purified subunits from either FhSA or FhES (native antigens) of this parasite species (36, 43, 58, 59). The cattle in this study come from farms in southern Santa Catarina, where the prevalence of the disease is considered low (20). Despite the observed low prevalence of the disease, the antigen FhES showed good diagnostic performance for both samples collected in the abattoir and farms, with sensitivities of 80% and 79% and specificities of 81% and 86%, respectively. Other studies that also used native antigens reported sensitivity ranging from 80% to 100% and specificity from 50% to 100% for serological diagnosis of bovine fasciolosis (37, 60).
Serological diagnosis for cattle fasciolosis using recombinant antigens (cathepsin and saposin) has been developed in the last years. Cathepsin is an important enzyme the parasite uses to elicit a humoral response in cattle as early as 2 weeks after infection (36, 38). In our study, the antigen FhrCL-1 presented diagnostic performance as good as those observed in FhES for abattoir cattle.
Sera samples from farm cattle infected with other parasites were used to evaluate the impact of cross-reactivity in our ELISA tests. Cross-reactivity analysis is fundamental since fasciolosis is a worldwide parasitic disease that can co-occur with other cattle parasitic diseases. Furthermore, current parasitological methods depend on the worker’s expertise because F. hepatica eggs can be confused with eggs from other helminths. Therefore, a good diagnostic test needs to be able to distinguish between Fasciola and other parasitic diseases. We did not observe substantial differences between the adjusted models without and with positive samples for other parasites, which suggests that the test differentiated animals positive for fasciolosis from cattle samples with other parasites.
In our study, the cattle in the positive group had positive fecal egg counts or the presence of F. hepatica in the liver, indicating that each animal was currently infected. Diagnosis of this infection is usually based on coprological techniques. The intermittent nature of the eggs’ evacuation through the feces was the reason for the low sensitivity of the coproscopy in detecting fasciolosis in cattle (31). Moreover, a prolonged pre-patent period of 8–15 weeks after the infection is required for the eggs to be shed in the feces (27, 31). Compared to fecal egg counts, serology can detect infections 7–8 weeks earlier (36, 37) and is considered a very sensitive method (61), but it does not distinguish between current and past infections. Results indicated that indirect ELISA using FhES and FhrCL-1 antigens could be an efficient and rapid diagnostic method for cattle fasciolosis compared to coprology. Therefore, using both methods together provided excellent information about the real infection situation. Of the three antigens (FhSA, FhES, and FhrCL-1) tested for the serological diagnosis of F. hepatica in cattle, the FhES presented satisfactory results in both scenarios, when compared to liver inspection in cattle from abattoir and to coprological examination in those from farms, suggesting it may be used for the development of ELISA tests for fasciolosis screening.
Conclusion
We have developed three ELISAs utilizing two native antigens and one recombinant antigen for detecting F. hepatica antibodies. We validated these ELISAs using cattle serum samples collected from abattoir and farms, considering the liver inspection and coprological examination as gold standard tests, respectively. The ELISA test using FhES as an antigen had good diagnostic performance in the two scenarios (abattoir and farms) for screening fasciolosis. Notably, the results were promising even in the face of the relatively low prevalence of cattle fasciolosis. The proposed ELISA test has the potential to be used in situations where it is more challenging to do a coprological investigation or examine the liver of cattle. These assays constitute a vital component of the immunodiagnostic toolkit that our laboratory is developing to improve the serodiagnosis of fasciolosis in Brazilian cattle. Recognizing that positive outcomes in antibody detection tests may not necessarily indicate ongoing infections but a history of exposure, we are actively exploring alternatives, such as an antigen detection ELISA using monoclonal antibodies. As a prospect, it is important to apply the test to more positive samples and also to explore cross-infection. Furthermore, ongoing research efforts are focused on adapting our in-house ELISA methods into more streamlined and dependable formats, such as immunochromatography or dot ELISA. This adaptation aims to facilitate potential commercialization and validation within Brazilian regions where the disease is endemic.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the Fundação Oswaldo Cruz (FIOCRUZ-PR), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Pesquisa (CNPq) for the possibility of developing this research. We also thank the FIOCRUZ Network of Technological Platforms for providing access to the Integrative Structural Biology facility at the Carlos Chagas Institute, FIOCRUZ-PR. We thank Wagner Nagib for providing the figure design and Jaqueline de Oliveira Rosa for the plasmid design. The authors would like to thank the Centro Universitário Barriga Verde (UNIBAVE).
This study was supported by Fundação Oswaldo Cruz (FIOCRUZ).
G.D., L.G.M., and F.B.F.: Study conception and design. G.D.: Conceptualization, methodology, manuscript writing, and drafting the manuscript. H.G. dos S.: Data curation, statistical analysis, and manuscript reviewing. M.M. da G.P.: Manuscript reviewing. L.G.M.: Manuscript reviewing. F.B.F.: Manuscript writing, reviewing, and editing. L.G.M. and F.B.F. supervised the study. All authors contributed to the article and approved the submitted version.
Contributor Information
Guilherme Drescher, Email: guidrescher@yahoo.com.br.
Fabiano Borges Figueiredo, Email: fabiano.figueiredo@fiocruz.br.
Artem S. Rogovskyy, Texas A&M University, College Station, Texas, USA
Kobra Mokhtarian, Department of Parasitology and Mycology, School of Medicine, Iran University of Medical Science, Tehran, Iran.
ETHICS APPROVAL
The protocols and methods used were approved by the Ethics Committee of the Centro Universitário Barriga Verde (UNIBAVE; protocol CAAE: 82403017.4.0000.5598).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00095-24.
Distribution of absorbance values.
ROC curves.
Diagnostic performance measures for the three tests on farms cattle (n = 500, 38 positive cases).
An accounting of the reviewer comments and feedback.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Mas-Coma MS, Valero MA, Bargues MD. 2009. Chapter 2 Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasit 69:41–146. doi: 10.1016/S0065-308X(09)69002-3 [DOI] [PubMed] [Google Scholar]
- 2. Mas-Coma MS, Esteban JG, Bargues MD. 1999. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organ 77:340–346. [PMC free article] [PubMed] [Google Scholar]
- 3. Mas-Coma MS, Bargues MD, Valero MA. 2005. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol 35:1255–1278. doi: 10.1016/j.ijpara.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 4. Abdel-Fatah OR, Arafa WM, Wahba AA, El-Dakhly KM. 2022. Economic losses, morpho-molecular identification, and identity of Fasciola species recovered from Egypt. J Parasit Dis 46:1036–1046. doi: 10.1007/s12639-022-01526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arias-Pacheco C, Lucas JR, Rodríguez A, Córdoba D, Lux-Hoppe EG. 2020. Economic impact of the liver condemnation of cattle infected with Fasciola hepatica in the Peruvian Andes. Trop Anim Health Prod 52:1927–1932. doi: 10.1007/s11250-020-02211-y [DOI] [PubMed] [Google Scholar]
- 6. Hayward AD, Skuce PJ, McNeilly TN. 2021. The influence of liver fluke infection on production in sheep and cattle: a meta-analysis. Int J Parasitol 51:913–924. doi: 10.1016/j.ijpara.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 7. Mathewos M, Endale H, Kebamo M. 2023. Coprological and postmortem assessment and economic significance of bovine fasciolosis in cattle slaughtered at Tarcha municipal Abattoir, Southern Ethiopia. Parasite Epidemiol Control 22:e00316. doi: 10.1016/j.parepi.2023.e00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorny P, Praet N, Deckers N, Gabriel S. 2009. Emerging food-borne parasites. Vet Parasitol 163:196–206. doi: 10.1016/j.vetpar.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 9. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. 2008. Helminth infections: the great neglected tropical diseases. J Clin Invest 118:1311–1321. doi: 10.1172/JCI34261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mas-Coma MS. 2005. Epidemiology of fascioliasis in human endemic areas. J Helminthol 79:207–216. doi: 10.1079/joh2005296 [DOI] [PubMed] [Google Scholar]
- 11. Opio LG, Abdelfattah EM, Terry J, Odongo S, Okello E. 2021. Prevalence of fascioliasis and associated economic losses in cattle slaughtered at Lira municipality Abattoir in Northern Uganda. Animals 11:681. doi: 10.3390/ani11030681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liba JW, Atsanda NN, Francis MI. 2017. Economic loss from liver condemnation due to fasciolosis in slaughtered ruminants in Maiduguri Abattoir, Borno state, Nigeria. J Adv Vet Anim Res 4:1. doi: 10.5455/javar.2017.d192 [DOI] [Google Scholar]
- 13. Ngcamphalala PI, Malatji MP, Mukaratirwa S. 2022. Geography and ecology of invasive Pseudosuccinea columella (Gastropoda: Lymnaeidae) and implications in the transmission of Fasciola species (Digenea: Fasciolidae) – a review. J Helminthol 96:1–18. doi: 10.1017/S0022149X21000717 [DOI] [PubMed] [Google Scholar]
- 14. Keiser J, Utzinger J. 2005. Emerging foodborne trematodiasis. Emerg Infect Dis 11:1507–1514. doi: 10.3201/eid1110.050614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bargues MD, Gayo V, Sanchis J, Artigas P, Khoubbane M, Birriel S, Mas-Coma S. 2017. DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis. PLoS Negl Trop Dis 11:e0005352. doi: 10.1371/journal.pntd.0005352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Igreja RP, Barreto MGM, Soares M da S. 2004. Fascioliasis: report of two cases from rural areas of Rio de Janeiro. Rev Soc Bras Med Trop 37:416–417. doi: 10.1590/S0037-86822004000500010 [DOI] [PubMed] [Google Scholar]
- 17. Pritsch IC, Garcia RL, Douat D, Schwendler RR, Buttendorf MRB, Molento MB. 2019. First reported case of clinical fascioliasis in Santa Catarina, Brazil. Rev Soc Bras Med Trop 52:e20190070. doi: 10.1590/0037-8682-0070-2019 [DOI] [PubMed] [Google Scholar]
- 18. Mas-Coma MS, Buchon P, Funatsu IR, Angles R, Artigas P, Valero MA, Bargues MD. 2020. Sheep and cattle reservoirs in the highest human fascioliasis hyperendemic area: experimental transmission capacity, field epidemiology, and control within a one health initiative in Bolivia. Front Vet Sci 7:583204. doi: 10.3389/fvets.2020.583204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diaz-Quevedo C, Frias H, Cahuana GM, Tapia-Limonchi R, Chenet SM, Tejedo JR. 2021. High prevalence and risk factors of fascioliasis in cattle in Amazonas, Peru. Parasitol Int 85:102428. doi: 10.1016/j.parint.2021.102428 [DOI] [PubMed] [Google Scholar]
- 20. da Fe Albuquerque R, Pereira SA, de Melo S, Belo VS, de Arruda M, Mazetto D, Figueiredo FB, Weiblen R. 2022. Spatial distribution analysis of bovine fascioliasis cases recorded in an Abattoir in the state of Santa Catarina, Brazil. Cienc Rural 52:1–8. doi: 10.1590/0103-8478cr20210030 [DOI] [Google Scholar]
- 21. Américo L, Padilha MAC, Arruda PM, Drescher G, de Moura AB, Chryssafidis AL. 2022. Epidemiological survey and confirmation of autochthonous cases of bovine fasciolosis in the Serrana Mesoregion of Santa Catarina, Brazil. Front Vet Sci 9:933462. doi: 10.3389/fvets.2022.933462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehmood K, Zhang H, Sabir AJ, Abbas RZ, Ijaz M, Durrani AZ, Saleem MH, Ur Rehman M, Iqbal MK, Wang Y, Ahmad HI, Abbas T, Hussain R, Ghori MT, Ali S, Khan AU, Li J. 2017. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb Pathog 109:253–262. doi: 10.1016/j.micpath.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 23. Cristine Pritsch I, Beltrão Molento M. 2018. Recount of reported cases of human fascioliasis in Brazil over the last 60 years. Rev Patol Trop 47:75. doi: 10.5216/rpt.v47i2.53636 [DOI] [Google Scholar]
- 24. Robinson MW, Dalton JP. 2009. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philos Trans R Soc Lond B Biol Sci 364:2763–2776. doi: 10.1098/rstb.2009.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saba R, Korkmaz M, Inan D, Mamikoğlu L, Turhan O, Günseren F, Cevikol C, Kabaalioğlu A. 2004. Human fascioliasis. Clin Microbiol Infect 10:385–387. doi: 10.1111/j.1469-0691.2004.00820.x [DOI] [PubMed] [Google Scholar]
- 26. Fica A, Dabanch J, Farias C, Castro M, Jercic MI, Weitzel T. 2012. Acute fascioliasis--clinical and epidemiological features of four patients in Chile. Clin Microbiol Infect 18:91–96. doi: 10.1111/j.1469-0691.2011.03575.x [DOI] [PubMed] [Google Scholar]
- 27. Mas-Coma MS, Bargues MD, Valero MA. 2014. Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology 141:1918–1946. doi: 10.1017/S0031182014000869 [DOI] [PubMed] [Google Scholar]
- 28. Mas-Coma MS, Valero MA, Bargues MD. 2014. Fascioliasis. Adv Exp Med Biol 766:77–114. doi: 10.1007/978-1-4939-0915-5 [DOI] [PubMed] [Google Scholar]
- 29. Zárate-Rendón DA, Vlaminck J, Levecke B, Briones-Montero A, Geldhof P. 2019. Comparison of Kato-Katz thick smear, mini-FLOTAC, and Flukefinder for the detection and quantification of Fasciola hepatica eggs in artificially spiked human stool. Am J Trop Med Hyg 101:59–61. doi: 10.4269/ajtmh.18-0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adamu M, Wossene A, Tilahun G, Basu AK. 2019. Comparative diagnostic techniques in ruminant fasciolosis: fecal sedimentation, indirect ELISA, liver inspection and serum enzyme activities. Ethiop Vet J 23:42. doi: 10.4314/evj.v23i1.4 [DOI] [Google Scholar]
- 31. Alvarez Rojas CA, Jex AR, Gasser RB, Scheerlinck J-P. 2014. Techniques for the diagnosis of Fasciola infections in animals: room for improvement. Adv Parasitol 85:65–107. doi: 10.1016/B978-0-12-800182-0.00002-7 [DOI] [PubMed] [Google Scholar]
- 32. Hoffman WA, Pons JA, Janer JJ. 1934. The sedimentation concentration method in Schistosomiasis mansoni. PR J Public Health Trop Med 9:281–298. [Google Scholar]
- 33. Dutra LH, Molento MB, Naumann CRC, Biondo AW, Fortes FS, Savio D, Malone JB. 2010. Mapping risk of bovine fasciolosis in the south of Brazil using geographic information systems. Vet Parasitol 169:76–81. doi: 10.1016/j.vetpar.2009.12.015 [DOI] [PubMed] [Google Scholar]
- 34. Martínez-Sernández V, Muiño L, Perteguer MJ, Gárate T, Mezo M, González-Warleta M, Muro A, Correia da Costa JM, Romarís F, Ubeira FM. 2011. Development and evaluation of a new lateral flow immunoassay for serodiagnosis of human fasciolosis. PLoS Negl Trop Dis 5:e1376. doi: 10.1371/journal.pntd.0001376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arias M, Morrondo P, Hillyer GV, Sánchez-Andrade R, Suárez JL, Lomba C, Pedreira J, Díaz P, Díez-Baños P, Paz-Silva A. 2007. Immunodiagnosis of current fasciolosis in sheep naturally exposed to Fasciola hepatica by using a 2.9 kDa recombinant protein. Vet Parasitol 146:46–49. doi: 10.1016/j.vetpar.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 36. Kuerpick B, Schnieder T, Strube C. 2013. Evaluation of a recombinant cathepsin L1 ELISA and comparison with the Pourquier and ES ELISA for the detection of antibodies against Fasciola hepatica. Vet Parasitol 193:206–213. doi: 10.1016/j.vetpar.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 37. Martínez-Sernández V, Perteguer MJ, Hernández-González A, Mezo M, González-Warleta M, Orbegozo-Medina RA, Romarís F, Paniagua E, Gárate T, Ubeira FM. 2018. Comparison of recombinant cathepsins L1, L2, and L5 as ELISA targets for serodiagnosis of bovine and ovine fascioliasis. Parasitol Res 117:1521–1534. doi: 10.1007/s00436-018-5809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cornelissen JB, Gaasenbeek CP, Borgsteede FH, Holland WG, Harmsen MM, Boersma WJ. 2001. Early immunodiagnosis of fasciolosis in ruminants using recombinant Fasciola hepatica cathepsin L-like protease. Int J Parasitol 31:728–737. doi: 10.1016/s0020-7519(01)00175-8 [DOI] [PubMed] [Google Scholar]
- 39. Gonzales Santana B, Dalton JP, Vasquez Camargo F, Parkinson M, Ndao M. 2013. The diagnosis of human fascioliasis by enzyme-linked immunosorbent assay (ELISA) using recombinant cathepsin L protease. PLoS Negl Trop Dis 7:e2414. doi: 10.1371/journal.pntd.0002414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Espinoza JR, Maco V, Marcos L, Saez S, Neyra V, Terashima A, Samalvides F, Gotuzzo E, Chavarry E, Huaman MC, Bargues MD, Valero MA, Mas-Coma S. 2007. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg 76:977–982. doi: 10.4269/ajtmh.2007.76.977 [DOI] [PubMed] [Google Scholar]
- 41. Rokni MB, Massoud J, O’Neill SM, Parkinson M, Dalton JP. 2002. Diagnosis of human fasciolosis in the Gilan province of northern Iran: application of cathepsin L-ELISA. Diagn Microbiol Infect Dis 44:175–179. doi: 10.1016/s0732-8893(02)00431-5 [DOI] [PubMed] [Google Scholar]
- 42. Aguayo V, Valdes B, Espino AM. 2018. Assessment of Fasciola hepatica glutathione S-transferase as an antigen for serodiagnosis of human chronic fascioliasis. Acta Trop 186:41–49. doi: 10.1016/j.actatropica.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mufti S, Afshan K, Khan IA, Irum S, Qureshi IZ, Rizvi SSR, Mukhtar M, Mushtaq M, Iqbal Z, Qayyum M. 2015. Serological and coprological studies of bovine fasciolosis in the Pothwar region, Pakistan. Pak Vet J 35:178–182. [Google Scholar]
- 44. Gottstein B, Schneeberger M, Boubaker G, Merkle B, Huber C, Spiliotis M, Müller N, Garate T, Doherr MG. 2014. Comparative assessment of ELISAs using recombinant saposin-like protein 2 and recombinant cathepsin L-1 from Fasciola hepatica for the serodiagnosis of human fasciolosis. PLoS Negl Trop Dis 8:e2860. doi: 10.1371/journal.pntd.0002860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Charlier J, De Meulemeester L, Claerebout E, Williams D, Vercruysse J. 2008. Qualitative and quantitative evaluation of coprological and serological techniques for the diagnosis of fasciolosis in cattle. Vet Parasitol 153:44–51. doi: 10.1016/j.vetpar.2008.01.035 [DOI] [PubMed] [Google Scholar]
- 46. Collins PR, Stack CM, O’Neill SM, Doyle S, Ryan T, Brennan GP, Mousley A, Stewart M, Maule AG, Dalton JP, Donnelly S. 2004. Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: propetide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. J Biol Chem 279:17038–17046. doi: 10.1074/jbc.M308831200 [DOI] [PubMed] [Google Scholar]
- 47. O’Neill SM, Parkinson M, Strauss W, Angles R, Dalton JP. 1998. Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am J Trop Med Hyg 58:417–423. doi: 10.4269/ajtmh.1998.58.417 [DOI] [PubMed] [Google Scholar]
- 48. O’Neill SM, Parkinson M, Dowd AJ, Strauss W, Angles R, Dalton JP. 1999. Short report: immunodiagnosis of human fascioliasis using recombinant Fasciola hepatica cathepsin L1 cysteine proteinase. Am J Trop Med Hyg 60:749–751. doi: 10.4269/ajtmh.1999.60.749 [DOI] [PubMed] [Google Scholar]
- 49. Barbosa R, Pinto C, Garcia P, Rodrigues A. 2019. Prevalence of fasciolosis in slaughtered dairy cattle from São Miguel Island, Azores, Portugal. Vet Parasitol Reg Stud Rep 17:100319. doi: 10.1016/j.vprsr.2019.100319 [DOI] [PubMed] [Google Scholar]
- 50. Drescher G, de Vasconcelos TCB, Belo VS, Pinto M da G, Rosa J de O, Morello LG, Figueiredo FB. 2023. Serological diagnosis of fasciolosis (Fasciola hepatica) in humans, cattle, and sheep: a meta-analysis. Front Vet Sci 10:1252454. doi: 10.3389/fvets.2023.1252454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Owen H, Jones E, Kowald C, Hand S, McGowan M, Cobbold R, Barnes TS, Gibson JS, Ranjbar S, Palmieri C, Allavena R. 2023. Development and application of a new liver pathology recording system for use in cattle abattoirs. Res Vet Sci 158:164–184. doi: 10.1016/j.rvsc.2023.03.002 [DOI] [PubMed] [Google Scholar]
- 52. Walsh TR, Ainsworth S, Armstrong S, Hodgkinson J, Williams D. 2021. Differences in the antibody response to adult Fasciola hepatica excretory/secretory products in experimentally and naturally infected cattle and sheep. Vet Parasitol 289:109321. doi: 10.1016/j.vetpar.2020.109321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Munita MP, Rea R, Martinez-Ibeas AM, Byrne N, Kennedy A, Sekiya M, Mulcahy G, Sayers R. 2019. Comparison of four commercially available ELISA kits for diagnosis of Fasciola hepatica in Irish cattle. BMC Vet Res 15:414. doi: 10.1186/s12917-019-2160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Şimşek S, Köroǧlu E, Ütük AE, Altay K. 2006. Use of indirect Excretory/Secretory enzyme-linked immunosorbent assay (ES-ELISA) for the diagnosis of natural Fasciola Hepatica infection in eosinophilic and non-eosinophilic cattle from Eastern Turkey. Turk J Vet Anim Sci 30:411–415. https://journals.tubitak.gov.tr/veterinary/vol30/iss4/10 [Google Scholar]
- 55. Hillyer GV, Soler de Galanes M, Buchón P, Bjorland J. 1996. Herd evaluation by enzyme-linked immunosorbent assay for the determination of Fasciola hepatica infection in sheep and cattle from the Altiplano of Bolivia. Vet Parasitol 61:211–220. doi: 10.1016/0304-4017(95)00831-4 [DOI] [PubMed] [Google Scholar]
- 56. Salimi-Bejestani MR, Cripps P, Williams DJL. 2008. Evaluation of an ELISA to assess the intensity of Fasciola hepatica infection in cattle. Vet Rec 162:109–111. doi: 10.1136/vr.162.4.109 [DOI] [PubMed] [Google Scholar]
- 57. Cornelissen JB, Gaasenbeek CP, Boersma W, Borgsteede FH, van Milligen FJ. 1999. Use of a pre-selected epitope of cathepsin-L1 in a highly specific peptide-based immunoassay for the diagnosis of Fasciola hepatica infections in cattle. Int J Parasitol 29:685–696. doi: 10.1016/s0020-7519(99)00017-x [DOI] [PubMed] [Google Scholar]
- 58. Kooshan M, Hashemi T, Naghibi A. 2010. Use of somatic and excretory-secretory antigens of Fasciola hepatica in diagnosis of sheep by ELISA. Am-Eurasian J Agric Environ Sci 7:170–175. [Google Scholar]
- 59. Heidari H, Zahiri H, Gharekhani J, Hosseini A, Aeineh S. 2015. Comparison of dot-ELISA and ELISA techniques for detection of Fasciola hepatica in sheep using excretory-secretory antigens. Istanbul Univ Vet Fak Derg 41:21–25. doi: 10.16988/iuvfd.2015.14154 [DOI] [Google Scholar]
- 60. Mezo M, González-Warleta M, Ubeira FM. 2003. Optimized serodiagnosis of sheep fascioliasis by fast-D protein liquid chromatography fractionation of Fasciola hepatica excretory-secretory antigens. J Parasitol 89:843–849. doi: 10.1645/GE-74RI.1 [DOI] [PubMed] [Google Scholar]
- 61. Charlier J, Vercruysse J, Morgan E, van Dijk J, Williams DJL. 2014. Recent advances in the diagnosis, impact on production and prediction of Fasciola hepatica in cattle. Parasitology 141:326–335. doi: 10.1017/S0031182013001662 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of absorbance values.
ROC curves.
Diagnostic performance measures for the three tests on farms cattle (n = 500, 38 positive cases).
An accounting of the reviewer comments and feedback.