Abstract
Tepsin is an established accessory protein found in Adaptor Protein 4 (AP-4) coated vesicles, but the biological role of tepsin remains unknown. AP-4 vesicles originate at the trans-Golgi network (TGN) and target the delivery of ATG9A, a scramblase required for autophagosome biogenesis, to the cell periphery. Using in silico methods, we identified a putative LC3-Interacting Region (LIR) motif in tepsin. Biochemical experiments using purified recombinant proteins indicate tepsin directly binds LC3B preferentially over other members of the mammalian ATG8 family. Calorimetry and structural modeling data indicate this interaction occurs with micromolar affinity using the established LC3B LIR docking site. Loss of tepsin in cultured cells dysregulates ATG9A export from the TGN as well as ATG9A distribution at the cell periphery. Tepsin depletion in a mRFP-GFP-LC3B HeLa reporter cell line using siRNA knockdown increases autophagosome volume and number, but does not appear to affect flux through the autophagic pathway. Reintroduction of wild-type tepsin partially rescues ATG9A cargo trafficking defects. In contrast, reintroducing tepsin with a mutated LIR motif or missing N-terminus drives diffuse ATG9A subcellular distribution. Together, these data suggest roles for tepsin in cargo export from the TGN; ensuring delivery of ATG9A-positive vesicles; and in overall maintenance of autophagosome structure.
Tepsin has been identified as an accessory or helper protein in AP-4 mediated trafficking from the trans-Golgi network (TGN), where AP-4 coated vesicles sort ATG9A, the essential scramblase required in autophagy. However, the biological role of tepsin remains unclear.
The authors report a new interaction between tepsin and LC3B using biochemical, biophysical, computational structural modelling, and cell-based approaches.
These data suggest a potential link between AP-4 trafficking and autophagosome biogenesis or maintenance.
INTRODUCTION
Membrane trafficking pathways are fundamental for diverse cellular and physiological functions. The assembly of cytosolic coat protein complexes carefully regulates vesicle and tubule transport between compartments in the secretory and post-Golgi trafficking networks (Dacks and Robinson, 2017). Mammalian Assembly Polypeptide (AP) complexes 1–5 are a family of structurally homologous heterotetramers that drive vesicle coat formation at various organelle membranes (Sanger et al., 2019). The structures, mechanisms, and functions of coat formation are well understood for clathrin-associated complexes AP-1 and AP-2 (Robinson, 2015). In contrast, nonclathrin-associated AP coats are less well understood, with some exhibiting unique compositions (AP-5) and different assembly mechanisms (Schoppe et al., 2020, 2021; Hirst et al., 2021). The AP-4 complex (ε/β4/μ4/σ4 subunits) is recruited to the trans-Golgi network (TGN) by Arf1 (Boehm et al., 2001). AP-4 does not interact with clathrin (Dell’Angelica et al., 1999; Hirst et al., 1999), and no scaffold proteins have been identified in AP-4 coated vesicles. AP-4 is ubiquitously expressed but appears particularly important in neurons and neuronal tissues. AP-4 loss in humans results in a complex neurological disorder termed AP-4-deficiency syndrome (Abou Jamra et al., 2011; Bauer et al., 2012; Abdollahpour et al., 2014; Jameel et al., 2014; Hardies et al., 2015; Tessa et al., 2016; Ebrahimi-Fakhari et al., 2018).
Biochemical approaches including proteomics have identified AP-4 coat accessory proteins: tepsin (Borner et al., 2012; Mattera et al., 2015; Frazier et al., 2016); RUSC1 and RUSC2 (Davies et al., 2018); and Hook1 and Hook2 (Mattera et al., 2020). These studies also identified several AP-4 transmembrane protein cargoes: ATG9A (Mattera et al., 2017; Davies et al., 2018; Ivankovic et al., 2020), SERINC1 and SERINC3 (Davies et al., 2018), and diacylglycerol lipase β, or DAGLB (Davies et al., 2022). ATG9A trafficking by AP-4 has been identified as a diagnostic marker of AP-4-deficiency syndrome to aid development of therapeutics (Behne et al., 2020). This discovery highlights the importance of revealing the molecular mechanisms of AP-4 biology. Loss of AP-4 in many cell types, including patient-derived cells (Davies et al., 2018; Behne et al., 2020), results in retention of ATG9A in the TGN (Mattera et al., 2017; Ivankovic et al., 2020). ATG9A is a lipid scramblase (Guardia et al., 2020; Maeda et al., 2020; Matoba et al., 2020) important in early steps of autophagosome biogenesis (Noda et al., 2000; Young et al., 2006). Aberrant autophagosome formation has been observed in cellular models of AP-4 deficiency (Mattera et al., 2017; Davies et al., 2018) as well as in the axons of neurons in AP4B1 (Matsuda et al., 2008) and AP4E1 (Ivankovic et al., 2020) knockout mouse models. The RUSC accessory proteins coordinate anterograde transport of ATG9A, SERINC1/3, and DAGLB toward the cell periphery in AP-4-derived vesicles (Davies et al., 2018, 2022). Conversely, Hook1 and Hook2 act as part of the FHF (FTS, Hook, and FHIP) complex thought to mediate retrograde trafficking of AP-4-coated and ATG9A-containing vesicles. This latter interaction is proposed to maintain a functional distribution of these proteins throughout the cytoplasm (Mattera et al., 2020). Despite being the first identified AP-4 accessory protein (Borner et al., 2012), the role of tepsin within the AP-4 coat has remained unclear.
Tepsin is a member of the epsin family of adaptor proteins (Borner et al., 2012), but structural and evolutionary evidence indicate it has functionally diverged (Archuleta et al., 2017). The tepsin unstructured C-terminus contains two conserved motifs for binding AP-4 appendage domains (Figure 1A; Mattera et al., 2015; Frazier et al., 2016). Tepsin contains two structured domains: an epsin N-terminal homology (ENTH) and VHS/ENTH-like (VHS-like) domains (Figure 1A; Frazier et al., 2016; Archuleta et al., 2017). The X-ray crystallography structures of both domains revealed they lack critical features observed in other epsins that promote either phosphoinositide binding to ENTH domains or ubiquitin binding to VHS domains (Zouhar and Sauer, 2014; Archuleta et al., 2017). Tepsin has yet to be directly implicated in phenotypes associated with deficiencies in AP-4-trafficking or autophagy.
FIGURE 1:
Tepsin directly and specifically binds LC3B in vitro. (A) Schematic diagrams of tepsin and AP-4 depicting the structural basis for the AP-4/tepsin interaction (PDB: 2MJ7); the predicted LIR motif (iLIR database; Jacomin et al., 2016) lies in the unstructured region between the tepsin ENTH (PDB: 5WF9) and VHS-like domains (PDB: 5WF2). (B) Coomassie-stained SDS–PAGE gel and Western blot (α-His; Abcam ab184607) of GST pulldowns using recombinant full-length tepsin-GST (residues 1-525) with His6x-LC3B, His6x-GABARAP (GRP), or His6x-GABARAPL2 (GPL2). Experiments show tepsin binds LC3B and weakly binds GABARAP; free GST was used as a negative control. Representative of three independent experiments.
Macroautophagy (hereafter autophagy) regulates cellular homeostasis by engulfing cytosolic material and dysfunctional organelles into autophagosomes that fuse with lysosomes for degradation and to promote macromolecule recycling within cells. Many essential autophagy genes are conserved from yeast to humans, though higher eukaryotes possess an expanded number of autophagy-related proteins. This is exemplified by a key yeast autophagy protein, ATG8 (Feng et al., 2014). Mammals contain multiple ATG8 orthologues referred to as the mammalian ATG8 (mATG8) family. These proteins are further divided into two subfamilies: the LC3 (LC3A/B/C) and GABARAP (GABARAP and GABARAPL1, and GABARAPL2; Shpilka et al., 2011) families. The independent function of each mATG8 protein is not completely understood. They appear to have distinct roles at various stages of autophagy, from phagophore expansion and recruitment of autophagosome contents to maturation and closure of the autophagosome membrane (Lee and Lee, 2016). Thus far, biochemical analysis of both yeast and mammalian ATG8 proteins reveals many protein–protein interactions are mediated through short peptide motifs, commonly termed LIR motifs (LC3 Interacting Region) in mammals (Birgisdottir et al., 2013).
In this study, we have identified a role for tepsin in regulating autophagy dynamics beyond export of ATG9A from the TGN. We demonstrate that tepsin directly interacts with LC3B in vitro and in cultured cell lines. The tepsin/LC3 interaction occurs between a LIR motif in tepsin’s first unstructured region (Figure 1A) and the LIR docking site (LDS) on the surface of LC3B. Biochemical data and AlphaFold modeling suggest how the tepsin LIR motif conveys specificity for LC3 over other mATG8 proteins. Tepsin depletion in cells drives partial accumulation of ATG9A near the TGN and promotes accumulation of ATG9A at the cell periphery. Tepsin depletion also dysregulates the morphology of autophagosomes and autolysosomes. The effect of tepsin depletion partly differs from AP-4 depletion providing evidence for a distinct function for tepsin in AP-4 coated vesicles. Finally, we tested the functional relevance of the tepsin LIR motif in ATG9A trafficking. While wild-type tepsin can partially rescue ATG9A trafficking defects, tepsin constructs lacking the LIR motif cannot rescue ATG9A subcellular distribution to the wild-type pattern. Together, these data suggest tepsin is important for ATG9A-containing AP-4 vesicle formation and the tepsin/LC3B interaction contributes to the distribution of ATG9A in cells.
RESULTS
Tepsin directly and specifically binds LC3B in vitro
The in silico iLIR database (Jacomin et al., 2016) identified a strongly predicted LIR motif in tepsin (Figure 1A). LIR motifs commonly contain the sequence [W/F/Y]xx[V/I/L], where critical hydrophobic residues are buried into corresponding hydrophobic pockets on the surface of mATG8 proteins (Popelka and Klionsky, 2015). The putative tepsin LIR motif fits the canonical sequence (GGWDEL). Two glycine residues immediately preceding the WDEL sequence in tepsin were also highlighted in the iLIR result, based on common sequences found in annotated functional LIR motifs (Jacomin et al., 2016). As is common for LIR motifs (Popelka and Klionsky, 2015), this motif lies in an unstructured region located between the tepsin ENTH- and VHS-like domains (Figure 1A). The mATG8 subfamilies are partially distinguished by varying preferences for the specific sequence of LIR motifs (Rogov et al., 2017; Wirth et al., 2019). We tested whether tepsin could bind three distinct mATG8 members. We purified full length tepsin (residues 1-525) as a Glutathione S-Transferase (GST)-fusion protein. GST pulldown assays using recombinant purified tepsin-GST with His-tagged LC3B, GABARAP, or GABARAPL2 show tepsin preferentially binds to LC3B in vitro (Figure 1B). The tepsin/LC3 interaction is faintly visible by Coomassie staining on an SDS–PAGE gel and further confirmed by probing 6x-histidine-tags on mATG8 proteins by Western blot. Tepsin-GST weakly bound GABARAP and showed no detectable binding at either Coomassie or Western blot level to GABARAPL2 in vitro.
Based on biochemical and cell biological data, tepsin requires AP-4 binding to become membrane-associated (Borner et al., 2012; Mattera et al., 2015; Frazier et al., 2016; Archuleta et al., 2017). Like yeast ATG8, all mATG8 proteins are conjugated to phosphatidylethanolamine through a ubiquitination-like cascade upon induction of autophagy (Shpilka et al., 2011). With this lipid modification, ATG8 proteins can be incorporated into the forming phagophore membrane to regulate autophagy. We tested whether AP-4 binding to tepsin affects the ability of tepsin to interact with LC3B using a tripartite GST pulldown assay. Tepsin-GST binding to the AP-4 β4 appendage domain does not significantly alter tepsin binding to LC3B in vitro (Supplemental Figure S1, A and B).
We next tested whether tepsin interacts with LC3B in cultured cells. Using an established HeLa cell line stably expressing tepsin-GFP (Borner et al., 2012), coimmunoprecipitation experiments show enrichment for endogenous LC3B (Supplemental Figure S1, C and D). Together, in vitro data using purified proteins suggests tepsin directly binds LC3B, while immunoprecipitations from cell lysates indicate tepsin and LC3B transiently interact in cells.
The tepsin LIR motif binds the hydrophobic pocket on LC3
The critical aromatic residues in a LIR motif interact with LC3B using two hydrophobic pockets termed the LDS. Using AlphaFold Multimer (Jumper et al., 2021, a and b), we modeled binding between LC3B and a peptide containing the tepsin LIR motif (residues 185-193). The tepsin/LIR motif interaction was superposed over an experimentally determined structure of LC3B bound to a p62 LIR motif (PDB: 2ZJD). As observed in p62, the critical tryptophan and leucine residues are positioned in the hydrophobic pockets of the LC3 LDS (Figure 2A). Additionally, the LDS region contains corresponding basic patches to accommodate the acidic aspartate and glutamate residues of the tepsin LIR motif (WDEL).
FIGURE 2:
The tepsin LIR motif binds the established binding pocket on LC3B in vitro. (A) Electrostatic surface representation of LC3B (PDB: 2ZJD) indicating the LDS (shown in inset) bound to the LIR peptide in p62. A model generated in Alphafold Multimer shows the tepsin LIR motif peptide superposed (yellow) with the Trp and Leu residues occupying established hydrophobic pockets on the LC3B surface. (B) Representative ITC experiments. Purified recombinant LC3B proteins and tepsin LIR motif peptide were used in ITC experiments to quantify binding affinities. The tepsin LIR motif peptide (residues 185-193) binds wild-type LC3B with a KD of 44.4 µM ± 0.4 µM by ITC (n = 3 independent experiments). The tepsin LIR peptide shows no detectable binding to the LC3B LDS binding mutant (F52A/L53A). KD values are represented as average ± SD. ITC data are summarized in Table 1.
TABLE 1:
ITC data summary. This table summarizes representative ITC experiments from Figure 2, including protein constructs, relevant mutations, and peptide sequences; calculated KD values; SD (s.d.) and stoichiometry (n) values. “WT” denotes wild-type sequence; n.b. denotes no detectable binding.
| LC3B | Tepsin LIR peptide | KD | s.d | n |
|---|---|---|---|---|
| residues 1-120 WT | SGGGWDELS | 44.4 μM | 0.4 μM | 0.523 |
| residues 1-120 F52A/L53A | SGGGWDELS | n.b. | n.b. | n.b. |
We used isothermal titration calorimetry (ITC) to quantify tepsin LIR motif binding to LC3B in vitro. Purified recombinant LC3B (residues 1-120) binds a synthesized tepsin LIR peptide (SGGGWDELDS, underlined residues 185-193) with micromolar affinity (KD = 44.4 μM ± 0.4 μM). Mutant LC3B containing point mutations in the LDS (F52A/L53A; Noda et al., 2008; Behrends et al., 2010; Marshall et al., 2019) exhibits no detectable binding to the tepsin LIR peptide (KD > 300 μM; Figure 2B). In the context of full length recombinant tepsin, pulldowns reveal mutation of either the tepsin LIR motif or the LC3B LDS abrogates binding at both Coomassie and Western blot detection levels (Supplemental Figure S2A). Together, these biochemical data indicate the tepsin LIR motif mediates an interaction between tepsin and LC3B in the established LIR binding site (Noda et al., 2008).
To better understand the selectivity of the tepsin LIR motif for LC3B over representative mATG8 proteins in pulldown assays (Figure 1B), we utilized AlphaFold to generate models of tepsin LIR peptides bound to LC3A, GABARAP, and GABARAPL2. These models were then superposed with experimentally determined structures of each mATG8 bound to a LIR motif. Models of LC3A show tepsin LIR residues W188 and L191 fit well into the corresponding hydrophobic pockets (Supplemental Figure S2B), as observed for LC3B models. The LC3A LDS also contains basic patches that could accommodate the acidic Asp and Glu residues in tepsin. The GABARAP model shows tepsin LIR residues W188 and L191 dock well into hydrophobic pockets (Supplemental Figure S2C). The reduced binding potential observed biochemically for GABARAP may instead be explained by two acidic residues (D189 and E190) because the region between the hydrophobic pockets has a greatly reduced electrostatic potential compared with LC3A and LC3B. Finally, the GABARAPL2 model clearly exhibits a clash with the critical tepsin LIR W188 residue (Supplemental Figure S2D). Together, AlphaFold2 computational modelling provides a molecular explanation for our biochemical data (Figure 1B) indicating the tepsin LIR motif preferentially binds LC3 over GABARAP proteins.
ATG9A accumulates near the periphery in cells acutely depleted of tepsin
We next sought to test the impact of tepsin knockdown in the context of established AP-4-deficiency phenotypes. Given the emerging links between AP-4 trafficking and autophagy (Matsuda et al., 2008; Mattera et al., 2017, 2020; Davies et al., 2018; De Pace et al., 2018; Ivankovic et al., 2020; Guardia et al., 2021), we utilized a well-established autophagy reporter line overexpressing a mRFP-GFP-LC3B fusion construct (Kimura et al., 2007; Sarkar et al., 2009). Fixation and immunostaining partially quench mRFP and GFP fluorescence such that fluorophores do not significantly contribute to immunostaining signal (Supplemental Figure S3C). This allowed us to assay ATG9A trafficking and autophagy dynamics (next section) within the same reporter cell line. We acutely depleted these cells for tepsin or AP-4 using siRNA-mediated knockdown (Supplemental Figure S3B). Whole lysate characterization by Western blot of these cells showed tepsin depletion increased ATG9A expression by 1.5-fold (Supplemental Figure S5A).
We tested whether and how the subcellular distribution of ATG9A might be altered upon tepsin knockdown by coimmunostaining for ATG9A and the trans-Golgi marker, TGN46 (Figure 3A; Supplemental Figure S4). In tepsin-depleted cells, isolated instances of ATG9A accumulation at the TGN were observed (Figure 3B.1), but ATG9A more notably redistributed to the cell periphery (Figure 3B.2). Tepsin-depleted cells exhibit decreased colocalization of ATG9A with the TGN marker as quantified by Pearson’s correlation coefficient (Figure 3C). We also observed a decrease in the volume of ATG9A overlapping with the TGN in tepsin-depleted cells (Figure 3D). Some peripheral accumulations of ATG9A were also observed upon AP-4 knockdown (Figure 3B.2). However, following AP-4 knockdown, the majority of ATG9A remains trapped in the TGN (Figure 3, C and D Supplemental Figure S5B), as observed in multiple systems (Mattera et al., 2017; Davies et al., 2018; De Pace et al., 2018; Behne et al., 2020; Ivankovic et al., 2020; Scarrott et al., 2023).
FIGURE 3:
Tepsin depletion drives ATG9A accumulation at the cell periphery. (A) Representative maximum intensity projection confocal images of mRFP-GFP-LC3B HeLa cells immunostained for ATG9A (α-ATG9A Abcam ab108338; secondary α-Rabbit-647 Thermo Fisher Scientific A32733) and TGN46 (α-TGN46 Bio-Rad AHP500GT; secondary α-Sheep-488 Thermo Fisher Scientific A11015). Cells were treated with control, nontargeting siRNA; tepsin siRNA; or AP-4 ε siRNA. Scale bar: 10 µm. (B) Enlargements showing ATG9A distribution near the trans-Golgi (B.1; yellow boxes, A) and at the cell periphery (B.2; cyan boxes, A); Scale bar: 2 µm. (C) Pearson’s correlation coefficient, analyzed for the whole cell volume, indicates less ATG9A colocalizes with TGN46 after tepsin depletion. ATG9A is enriched in the TGN in AP-4-depleted cells. Results depict individual cells from four independent siRNA experiments as data points (colored per replicate) with the overall mean ± SD. Statistical significance analyzed by Welch’s ANOVA with Games-Howell’s posthoc test; ***p ≤ 0.001, ****p ≤ 0.0001. (D) Quantification of ATG9A volume overlap with TGN46. ATG9A overlap with TGN46 is decreased in tepsin-depleted cells while increased in AP-4-depleted cells. Results are displayed and analyzed as described for C; **p ≤ 0.01, ****p ≤ 0.0001. Total number of cells analyzed for C and D was 186 (control), 170 (tepsin), and 235 (AP-4 ε).
Tepsin-depleted cells exhibit dysregulated autophagosomes and autolysosomes
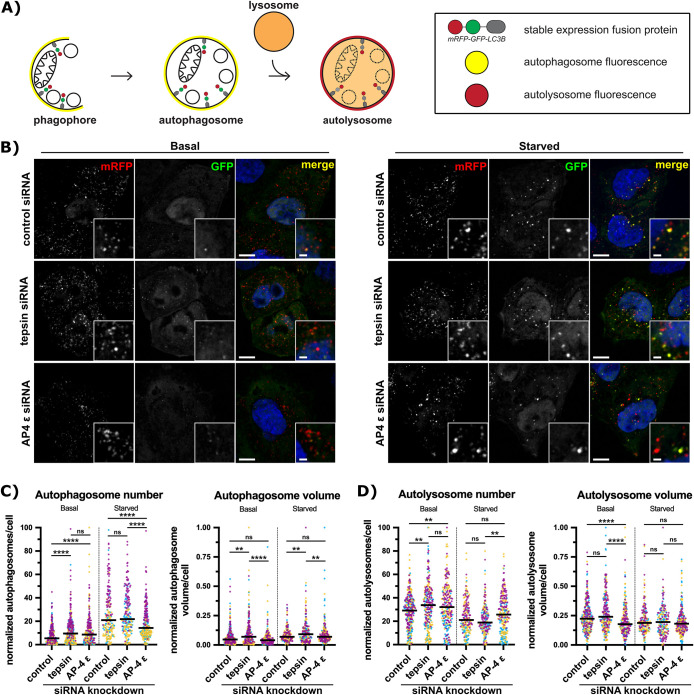
The biochemical and cellular data presented thus far suggest there are additional functions for the AP-4 coat in regulating autophagy dynamics via the accessory protein, tepsin. We next used the mRFP-GFP-LC3B cell line to assay flux through the autophagic pathway (Figure 4A; Supplemental S6). Coincident red and green structures positive for mRFP-GFP-LC3 are identified as yellow autophagosomes (or early autophagic structures). Lysosome fusion with autophagosomes quenches GFP fluorescence, so red (mRFP-only) LC3 structures are classified as autolysosomes. We visualized autophagosome and autolysosome structures in tepsin-depleted or AP-4-depleted cells under basal or starvation (amino acid- and serum-free) conditions (Figure 4B). Knockdown efficiency and autophagy induction were confirmed by Western blot (Supplemental Figure S7A).
FIGURE 4:
Acute tepsin depletion increases autophagosome number and volume under basal nutrient conditions. (A) Schematic of autophagy assay in HeLa cells stably expressing mRFP-GFP-LC3B. Coincident green and red fluorescence marks autophagosomes in yellow, while acidification of autolysosomes quenches GFP and leaves only red (RFP) signal. (B) Representative single plane confocal images of mRFP-GFP-LC3B HeLa cells cultured in either complete media (basal) or starved for 2 h in EBSS. Cells were treated with control (nontargeting) siRNA, tepsin siRNA, or AP-4 ε siRNA. Scale bar: 10 µm; inset scale bar: 2 µm. (C and D) Quantification of three independent experiments with at least 200 total cells per condition. (C) Tepsin-depleted cells under basal conditions contain more autophagosomes with greater apparent autophagosome volume (µm3) under both basal and starved conditions. AP-4 depleted cells contain more autophagosomes under basal conditions but fewer autophagosomes in starved conditions. (D) Tepsin-depleted cells under basal conditions contain more autolysosomes but no apparent difference in autolysosome volume. AP-4-depleted cells under basal and starved conditions contain increased autolysosome number but decreased apparent autolysosome volume only under basal conditions. Plots for C and D depict individual cells depict individual cells from three independent siRNA experiments as data points (colored per replicate) with the overall median (horizontal bar). Total number of cells analyzed for C and D was 305 (control basal), 258 (tepsin basal), 246 (AP-4 ε basal), 206 (control starved), 205 (tepsin starved), and 255 (AP-4 ε starved). Statistical results from Kruskal-Wallis test with Dunn’s Multiple Comparison posthoc test. All data was subject to min-max normalization; ns > 0.05, **p ≤ 0.01, ****p ≤ 0.0001.
Under basal conditions, both AP-4- and tepsin-depleted cells contain more autophagosomes (Figure 4C). However, in starvation conditions, AP-4-depleted cells contain fewer autophagosomes (Figure 4C). The apparent volume of autophagosomes in tepsin-depleted cells was increased under both basal and starvation conditions (Figure 4C). These data suggest tepsin-depletion results in the aberrant formation and growth of autophagosomes, while AP-4 acute depletion primarily affects the formation of early autophagosomes.
Tepsin or AP-4 depletion results in a greater number of autolysosomes in cells under basal conditions (Figure 4D), corresponding to the increase in autophagosome number (Figure 4C). This suggests, autophagic flux is not drastically altered by tepsin depletion. Additionally, starved cells show no significant change to the number of autolysosomes, and neither basal nor starved cells show changes to the apparent volume of autolysosomes. Apparent autolysosome volume is decreased in AP-4-depleted cells under basal conditions (Figure 4D).
We further utilized the GFP tag in this reporter line to assay for an interaction with endogenous tepsin. GFP immunoprecipitations from mRFP-GFP-LC3B cells are enriched for endogenous tepsin and the AP-4 ε subunit by Western blot (Supplemental Figure S7B). These data complement in vitro biochemical data (Figures 1B and 2B) to indicate tepsin interacts with LC3B in this cell line, and AP-4 is also present. Overall tepsin loss dysregulates autophagosome and autolysosome morphology without blocking autophagic flux, though higher resolution imaging by electron microscopy is needed to confirm the identity of these LC3B structures. The effect of acute tepsin depletion is distinct from acute AP-4 depletion, suggesting a separate, or an additional, function for tepsin in autophagosomes biogenesis.
The tepsin/LC3 interaction modulates AP-4-dependent ATG9A trafficking
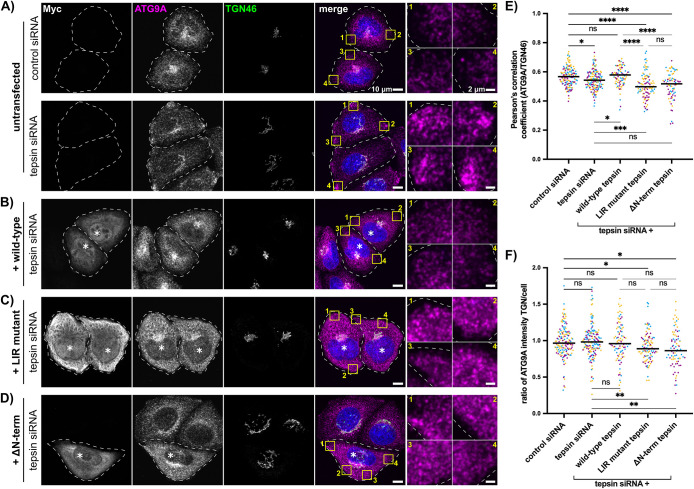
Our data indicate two things happen when tepsin is acutely depleted: ATG9A accumulates near the cell periphery, and autophagosome and autolysosome morphology is dysregulated. ATG9A peripheral accumulation when tepsin is lost suggests tepsin may target ATG9A-containing AP-4 vesicles for use in autophagy. We generated an siRNA-resistant, myc-tagged construct of wild-type tepsin to test whether ATG9A accumulation was specific to tepsin loss. Tepsin-myc expression level was similar between control and tepsin-siRNA treatments confirming siRNA resistance (Supplemental Figure S8A, WT tepsin). We monitored the colocalization of ATG9A with the TGN46 marker in the mRFP-GFP-LC3B cell line (Figure 5, A–D). Untransfected cells depleted for tepsin exhibited decreased ATG9A signal at the TGN (Figure 5, A and E). However, reintroduction of wild-type tepsin rescues peripheral ATG9A accumulations and restores ATG9A intracellular distribution (as measured by TGN46 colocalization) to levels similar to those observed in control cells (Figure 5, A, B, and E). These data indicate ATG9A mistrafficking (Figure 3) was specific to the loss of tepsin.
FIGURE 5:
Re-introduction of wild-type tepsin partially restores ATG9A cellular distribution while LIR mutant tepsin results in dispersed ATG9A in tepsin-depleted cells. (A and B) Representative maximum intensity projection confocal images of mRFP-GFP-LC3B HeLa cells immunostained for ATG9A (α-ATG9A Abcam ab108338; secondary α-Rabbit-647 Thermo Fisher Scientific A32733), TGN46 (α-TGN46 Bio-Rad AHP500GT; secondary α-Sheep-488 Thermo Fisher Scientific A11015), and myc-tag (α-myc-tag Cell Signaling Technology 2276; secondary goat α-Mouse-555 Thermo Fisher Scientific A32727). Cells were treated with nontargeting siRNA (control) or tepsin siRNA. Cells in B and D were subsequently transfected with wild-type, LIR mutant (WDEL/SSSS), or ΔN-terminus (residues 360-525; ΔΝ-term) siRNA resistant tepsin; transfected cells (anti-myc stained) are marked by asterisks (*). ATG9A accumulates at the cell periphery to a greater extent in tepsin-depleted cells (A; yellow inset boxes) compared with cells rescued with wild-type tepsin (B; yellow inset boxes). Scale bar: 10 µm; inset scale bar: 2 µm. (C and D) Re-introducing either LIR mutant tepsin or ΔΝ-term tepsin fails to restore ATG9A cellular distribution. (E) Quantification of ATG9A colocalization with TGN46. Pearson’s correlation coefficient was analyzed for the whole cell volume. Results depict individual cells from three independent siRNA experiments as data points (colored per replicate) and horizontal bar as the overall median. Statistical significance analyzed by Kruskal-Wallis test with Dunn’s multiple comparison posthoc test; ns > 0.05, *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001. (F) Quantification of the ratio of ATG9A mean fluorescence intensity between the TGN and the rest of the cell relative to the mean control siRNA value. Results are displayed and analyzed as described for E; ns > 0.05, *p ≤ 0.05, **p ≤ 0.01. Total number of cells analyzed for E and F was 150 (control siRNA), 163 (tepsin siRNA), 92 (+wild-type tepsin), 104 (+LIR mutant tepsin), and 79 (+ΔΝ-term tepsin).
Based on the in vitro characterization of the tepsin-LC3B interaction, we specifically tested the role of the tepsin LIR motif in ATG9A cargo trafficking. We generated siRNA-resistant myc-tagged constructs of LIR mutant tepsin (WDEL→SSSS) and a truncated tepsin containing only the C-terminal tail (ΔN-term tepsin; residues 360-525). Each construct transfected at similar efficiency and exhibited resistance to tepsin siRNA treatments (Supplemental Figure S8A, ΔN-term and LIR mutant). Reintroducing wild-type, LIR mutant, or ΔN-term tepsin was able to rescue ATG9A protein expression back to steady-state levels in tepsin siRNA-treated cells (Supplemental Figure S8B). Despite the rescue of ATG9A protein levels, expression of LIR mutant tepsin failed to rescue ATG9A colocalization with TGN46 in tepsin-depleted cells (Figure 5, C and E). Interestingly, instead of the TGN and peripheral accumulations previously observed, ATG9A is more diffusely distributed throughout the cell. The ratio of ATG9A mean intensity between the TGN and the rest of the cell significantly decreases, which we interpret to be dispersed ATG9A cytoplasmic distribution (Figure 5, C and F). We also tested the ΔN-term tepsin mutant, which contains only tepsin C-terminal tail harboring established AP-4 binding motifs (Mattera et al., 2015; Frazier et al., 2016). Expression of the ΔN-term tepsin mutant also fails to rescue ATG9A localization to the TGN, resulting in more dispersed cytoplasmic distribution similar to LIR mutant tepsin (Figure 5, D–F). As with LIR mutant tepsin, ATG9A cellular distribution is dysregulated following reintroduction of ΔN-term tepsin even though ATG9A returns to steady-state protein levels. These data further indicate ATG9A peripheral accumulations are directly the result of disrupted tepsin function, likely requiring the LIR motif. Furthermore, expression of either the LIR mutant or ΔN-term tepsin gives a dominant negative phenotype, in which ATG9A colocalization with the TGN46 marker is decreased in control siRNA-treated cells (Supplemental Figure S8, C and D). Expression of ΔN-term tepsin also decreases the ratio of ATG9A mean intensity in control siRNA-treated cells suggesting this construct acts more robustly as a dominant negative factor (Supplemental Figure S8E).
Both the tepsin LIR mutant and ΔN-term tepsin constructs exhibited increased cytotoxicity (based on viability during sample preparation) and greater variability in expression level for individual cells when visualized by immunofluorescence imaging. As a result, fluorescence intensity of both constructs is higher than for wild-type tepsin in many cells (represented in Figure 5, A and B; 6, A and B). However, for each construct, exogenous expression levels by Western blot were comparable between control and tepsin-depleted cells. Increased toxicity and dominant negative effects following ΔN-term tepsin transfection could indicate the tepsin N-terminus performs several important functions (see Discussion). Though both the LIR mutant and ΔN-term tepsin constructs retain AP-4 binding motifs, they fail to rescue ATG9A cellular distribution phenotypes. Together with the in vitro data, this suggests cells need a functional tepsin N-terminus and a direct interaction between tepsin and LC3B to mediate AP-4-dependent trafficking of ATG9A vesicles for their intended function during autophagy.
FIGURE 6:
Models for tepsin function in AP-4 trafficking and autophagy. AP-4 coats containing tepsin assemble at the TGN, packaging ATG9A into vesicles. AP-4-coated and ATG9A-containing vesicles are proposed to undergo anterograde (Davies et al., 2018) and retrograde (Mattera et al., 2020) transport to maintain ATG9A distribution. Tepsin depletion leads to partial peripheral ATG9A accumulation which may suggest AP-4-coated vesicles function in ATG9A delivery. Together, published data and new data presented here lend themselves to three possible interpretations, that are not mutually exclusive. (1) Tepsin and AP-4 are required for efficient packaging of ATG9A into AP-4-coated vesicles exiting the TGN. (2) The tepsin/LC3B interaction may be required for efficient ATG9A delivery to LC3-positive (2a) autophagic membranes or (2b) late-endosome/lysosomes. Possibly contributing AP-4 derived vesicles to the ATG9A reservoir. (3) Alternatively, tepsin may bind LC3B to coordinate retrieval of ATG9A from maturing autophagosomes.
Acute tepsin depletion reduces ATG9A and LC3 colocalization in cells
Following tepsin depletion, ATG9A distribution is disrupted and autophagosome morphology is altered in mRFP-GFP-LC3 HeLa cells. Because ATG9A distribution requires a functional tepsin LIR motif, we tested whether ATG9A accumulations coincide with LC3B structures in tepsin-depleted HeLa cells coimmunostained for endogenous LC3 and ATG9A (Supplemental Figure S9, A and B). Acute tepsin depletion (Supplemental Figure S9C) results in decreased ATG9A/LC3 colocalization (Supplemental Figure S9, D and E), reflecting peripheral accumulations of ATG9A with no apparent LC3 signal (Supplemental Figure S9A inset 2). In tepsin-depleted cells we also observed anomalous LC3 ring-like structures in a limited number of cells across independent replicates which could correspond to malformed or enlarged autophagosomes (Supplemental Figure S9B). LC3-positive structures in control and tepsin-depleted cells typically coincide with ATG9A signal (Supplemental Figure S9F) suggesting some ATG9A can still form maturing autophagosomes. Also supporting data from the mRFP-GFP-LC3B HeLa reporter cell line (Figure 4), endogenous LC3 structures in basal tepsin-depleted cells are larger (Supplemental Figure S9G) than in control cells but LC3 signal intensity on these structures is not significantly altered (Supplemental Figure S9H). These data further indicate tepsin contributes to proper ATG9A cellular distribution. Peripheral ATG9A accumulations do not colocalize with LC3; however, subpopulations of ATG9A can still contribute to autophagosome formation in tepsin-depleted cells.
DISCUSSION
Summary
Published work has characterized the molecular interaction of tepsin with AP-4 (Borner et al., 2012; Mattera et al., 2015; Frazier et al., 2016) and determined X-ray structures of the tepsin ENTH and VHS-like domains (Archuleta et al., 2017). Tepsin function within AP-4 coats has remained elusive. Using in silico biochemical methods and fluorescence imaging, we identified and characterized a functional LIR motif in tepsin that binds LC3B in vitro and in cultured cells. Tepsin depletion in cells induces partial ATG9A accumulation at the TGN and enrichment at the cell periphery. Re-introduction of tepsin constructs with a mutated LIR motif or lacking the N-terminus are unable to rescue ATG9A trafficking defects. These data suggest tepsin may play roles in AP-4 vesicle formation and possibly in effective delivery or recycling of ATG9A-positive vesicles (Figure 6).
Molecular characterization of the tepsin LIR motif
Emerging models of AP-4-deficiency syndrome highlight autophagy dysregulation arising from ATG9A retention (Noda et al., 2000; Young et al., 2006; Guardia et al., 2020; Maeda et al., 2020; Matoba et al., 2020) at the TGN (Mattera et al., 2017; Davies et al., 2018; Behne et al., 2020; Ivankovic et al., 2020). Identification and characterization of a LIR motif within tepsin further links AP-4 coats to autophagy and cellular homeostasis. The tepsin LIR motif (WDEL) fits an established consensus sequence (WxxL; Noda et al., 2008). Computational structural modeling and biochemical data provide strong evidence that tepsin binds LC3B with low micromolar affinity using the LDS. LIR interaction affinities exhibit a large range (KD values from submicromolar to ∼100 μM), because mATG8 family proteins exhibit selectivity (reviewed Wesch et al., 2020). This broad affinity range reflects the diverse functional roles of mATG8/LIR interactions (Birgisdottir et al., 2013). The tepsin LIR motif shows partial specificity for LC3 over other mATG8 proteins in vitro (Figure 1B). AlphaFold modelling indicates tepsin acidic aspartate and glutamate residues (WDEL) may confer specificity by binding the basic patch located between hydrophobic pockets in the LC3 LDS (Figure 2 Supplemental Figure S2). The weak micromolar affinity, as well as weak detection by traditional immunoprecipitation methods, suggest the tepsin interaction with LC3B occurs transiently in cells.
A major question arising from identification of the tepsin LIR motif regards where tepsin and LC3 interact in cells, because where AP-4 vesicles deliver cargo remains unclear. Live cell imaging of cells stably expressing tepsin-GFP shows punctate tepsin structures in the perinuclear region that persist and move peripherally within the cell before dissipating (Borner et al., 2012; Video 1). Both tepsin and LC3 cycle between cytosolic and membrane-associated states, with tepsin primarily localized at Golgi membranes during steady-state (Borner et al., 2012) while LC3 is associated with autophagosomal membranes following lipid conjugation (Ichimura et al., 2000; Kabeya et al., 2000; Kirisako et al., 2000). LC3 also localizes to other membranes for noncanonical autophagy (Sanjuan et al., 2007; Xu et al., 2007; Florey et al., 2011; Cross et al., 2023) or lysosome biogenesis and repair (Nakamura et al., 2020; Corkery et al., 2023). It seems unlikely LC3 would be a cargo in AP-4 vesicles, so we favor the idea that tepsin transiently binds LC3B either at autophagosomal or other LC3-positive membranes.
The tepsin/LC3B interaction affects ATG9A cellular distribution
Impaired ATG9A export from the TGN is a hallmark of AP-4-deficiency syndrome (Behne et al., 2020). Tepsin knockout HAP1 cells did not accumulate ATG9A at the TGN (Mattera et al., 2017), but the role of tepsin in ATG9A trafficking was largely unexplored. Data here show how acute tepsin depletion has two distinct effects on ATG9A: it drives partial ATG9A accumulation near the TGN (Figure 3B.1) and promotes ATG9A accumulation at the cell periphery (Figure 3B.2–3D). Our quantification approach reflects a separation of function in which tepsin loss drives ATG9A peripheral accumulation while AP-4 loss traps ATG9A at the TGN. We note how AP-4-depleted cells exhibit some peripheral ATG9A accumulation (Figure 3B.2) and tepsin-depleted cells show partial accumulation near the TGN (Figure 3B.1).
ATG9A accumulation at and near the TGN may reflect abortive or malformed AP-4 coated vesicles unable to depart. Whole cell lysates exhibit increased ATG9A expression upon tepsin loss (Supplemental Figure S5A). ATG9A expression levels are also increased in AP-4-deficient patient-derived cells (Davies et al., 2018) presumably as cells attempt to compensate for failed ATG9A export. In this work, acute tepsin-depletion led to slightly higher (1.5-fold increase) ATG9A expression levels than did AP-4-depletion (1.25-fold increase). Compensatory ATG9A overexpression in tepsin-depleted cells may be more effective, because ATG9A export is not blocked as significantly. Reintroducing LIR mutant or ΔN-term tepsin was able to restore ATG9A steady-state protein levels; both constructs retain AP-4 binding motifs, providing evidence that tepsin functions to promote AP-4 vesicle formation and ATG9A export (Figure 6.1). However, LIR mutant or ΔN-term tepsin constructs do not fully restore ATG9A trafficking (Figure 5, C–F). The resulting diffuse ATG9A distribution indicates specific contributions from the tepsin LIR motif and N-terminus. These data suggest tepsin-LC3 binding facilitates effective ATG9A delivery (Figure 6.2). ATG9A colocalization with LC3-positive structures decreases following tepsin depletion (Supplemental Figure S9), further suggesting the tepsin-LC3 interaction facilitates some aspect of autophagosome maturation.
AP-4 trafficking is thought to help maintain an ATG9A vesicle pool for autophagy nucleation (Noda et al., 2000; Young et al., 2006; Yamamoto et al., 2012; Davies et al., 2018; Sawa-Makarska et al., 2020). AP-4 or tepsin depletion differentially affected formation and morphology of autophagosome and autolysosome structures, suggesting separation of function between tepsin and AP-4. AP-4 knockout systems exhibit an increased number of enlarged LC3 structures (Matsuda et al., 2008; Mattera et al., 2017; Davies et al., 2018; Ivankovic et al., 2020). AP-4 depleted cells in this study form more autophagosomes under basal conditions but have fewer autophagosomes following autophagy induction (Figure 4C). The mRFP-GFP-LC3B reporter assay (Sarkar et al., 2009) cannot distinguish between early phagophore structures and maturing autophagosomes, so AP-4 loss may hinder formation of early autophagic structures. Conversely, tepsin depletion primarily increases autophagosome number and apparent size in fed conditions without hindering autophagosome formation in starved cells (Figure 4C). Published data show depleting AP-4 accessory proteins caused dispersed ATG9A without significantly impacting autophagy, indicating cells more easily compensate for improperly dispersed ATG9A than ATG9A trapped at the TGN (Mattera et al., 2020; Guardia et al., 2021).
ATG9A vesicles serve as seed membranes for autophagosomes before recruitment of additional autophagy machinery (Broadbent et al., 2023; Olivas et al., 2023). How and where ATG9A vesicles are coordinated for delivery remains unclear. AP-4-derived ATG9A vesicles accumulate near autophagosomes when RUSC2 is overexpressed and drive anterograde transport (Davies et al., 2018). Our data suggest the tepsin LIR motif either helps coordinate ATG9A trafficking to LC3-positive phagophore membranes (Figure 6.2a) or dampens autophagy, perhaps by facilitating ATG9A delivery to LC3-positive late endolysosomal membranes (Figure 6.2b). It is also possible tepsin facilitates retrograde transport of ATG9A mediated by the recycler complex (Zhou et al., 2022) or AP-4 (Mattera et al., 2020) from late autophagosome/autolysosome membranes to the TGN (Figure 6.3). This interpretation would not be consistent with our current data showing a reduction in ATG9A/LC3 overlap in tepsin-depleted cells.
To distinguish among these models, it will be important to establish whether AP-4 vesicles remain coated after budding from the TGN to help mediate ATG9A delivery to the right cellular membrane, analogous to COPI coated vesicles (Bykov et al., 2017; Travis et al., 2019). Furthermore, other AP-4 coat components may interact with autophagy machinery; for example, the iLIR database (Jacomin et al., 2016) identifies multiple LIR motifs in RUSC2 (Davies et al., 2018). In future, it will be interesting to explore whether other putative LIR motifs affect ATG9A trafficking and can partially compensate for tepsin loss. Finally, tepsin depletion impacts autophagosome maturation (Figure 4), which is known to coincide with LC3 protein density on membranes (Kabeya et al., 2000; Nath et al., 2014). It may therefore be interesting to explore how avidity affects the tepsin-LC3B interaction on membranes.
MATERIALS AND METHODS
Request a protocol through Bio-protocol.
Reagents
Unless otherwise noted, all chemicals were purchased from Fisher Scientific.
The following antibodies were used in this study: rabbit anti-AP-4 epsilon 1:400 for Western blots (612019; BD Transduction Labs); rabbit anti-ATG9A 1:200 for immunofluorescence and 1:1000 for Western blots (ab108338; Abcam); rabbit anti-LC3B 1:3000 for Western blots (ab48394; Abcam); mouse anti-LC3 1:200 for immunofluorescence (M152-3; MBL); HRP-conjugated anti-GFP 1:2000 for Western blots (ab6663; Abcam); rabbit anti-tepsin 1:500 (in-house; Genscript) and 1:1000 (Robinson Lab, Cambridge) for Western blots; sheep anti-TGN46 1:1000 for immunofluorescence (AHP500GT; Bio-Rad); mouse anti-alpha tubulin 1:3000 for Western blots (66031; Proteintech); mouse anti-Myc-tag 1:8000 for immunofluorescence and 1:6000 for Western blots (2276; Cell Signaling Technology); HRP-conjugated anti-6X His tag 1:8000 for Western blots (ab184607; Abcam); HRP-conjugated secondaries for Western blots, 1:5000: Pierce goat anti-rabbit IgG (31460; Thermo Fisher Scientific); Pierce goat anti-Mouse IgG (31430; Thermo Fisher Scientific); Fluorescent secondary antibodies for immunofluorescence, 1:500: goat anti-Rabbit 647 (A32733; Thermo Fisher Scientific), goat anti-Mouse 555 (A32727; Thermo Fisher Scientific), goat anti-Mouse 488 (A32723; Thermo Fisher Scientific), donkey anti-Sheep 488 (A11015; Thermo Fisher Scientific).
Molecular biology and cloning
For biochemical analysis, full-length tepsin (Borner et al., 2012) was subcloned using NdeI/BamHI sites into in-house vector pMW172 (Owen and Evans, 1998) modified to incorporate a C-terminal, thrombin cleavable GST tag. Full-length constructs of LC3B (residues 1-125), GABARAP (residues 1-117), and GABARAPL2 (residues 1-117) were subcloned by Genscript into pGEX-6P-1 using BamHI/SalI sites to generate N-terminal GST-fusion protein; an additional His6x-tag was added to the N-terminus of each protein sequence. The GST-fusion AP-4 β4 appendage (residues 615-739) was cloned previously (Frazier et al., 2016). A two-stage mutagenesis protocol (Frazier et al., 2016) was used to truncate full-length LC3B into the mature isoform (residues 1-120) and make the following mutations to tepsin and LC3B constructs: LC3B LDS mutant (F52A/L53A); LIR mutant tepsin (W188S, D189S, E190S, and L191S); and tepsin siRNA target site silent mutations (nucleotides G1329A, A1335G, T1338C, A1341G, and G1344A).
To generate constructs for tepsin rescue experiments, full-length tepsin was subcloned into pcDNA3.1 vector (Thermo Fisher Scientific) using BamHI/XhoI sites. A C-terminal myc-tag (EQKLISEEDL) was included in the 3′ primer to generate tepsin-myc constructs. This construct was subjected to mutagenesis as described above to confer siRNA resistance and mutate the tepsin LIR motif. A truncated construct of the tepsin C-terminal tail (residues 360-525 with myc-tag) was subcloned from the siRNA-resistant tepsin-myc using BamHI/SalI sites into the pcDNA3.1 backbone. The sequences of all constructs described above were verified by Sanger DNA sequencing (Azenta). Oligonucleotides used in this study may be found in Table 2.
TABLE 2:
Oligonucleotides used in this study.
| Protein | Sequence (5′-3′) | Restriction site | Primer use |
|---|---|---|---|
| tepsin(aa1-525) | GGAGCACATATGGCTGCCGCGCCGCCG | NdeI | 5′ human tepsin, starts aa1 |
| tepsin(aa1-525) | TGCCTCGTCGACTCAGGCGTTCAGGAACGCGAA | SalI | 3′ human tepsin, ends aa525 |
| tepsin LIR mutant (WDEL/SSSS) | GCCGGAGGGGGCTCTAGCTCAAGTGACAGCGGCCCCAGCTC | N/A | 5′ mutagenesis primer, WDEL/SSSS |
| tepsin LIR mutant (WDEL/SSSS) | GAGCTGGGGCCGCTGTCACTTGAGCTAGAGCCCCCTCCGGC | N/A | 3′ mutagenesis primer, WDEL/SSSS |
| tepsin siRNA resistant | GCAGGCGATGGAGGCCCGAGCGAATCCCAGGGGGCACG | N/A | 5′ mutagenesis primer for silent mutation at siRNA binding site |
| tepsin siRNA resistant | CGTGCCCCCTGGGATTCGCTCGGGCCTCCATCGCCTGC | N/A | 3′ mutagenesis primer for silent mutation at siRNA binding site |
| tepsin(aa1-525)-Myc | GGAGCAGGATCCATGGCTGCCGCGCCGCCG | BamHI | 5′ human tepsin, starts aa1 |
| tepsin(aa1-525)-Myc | TGCTCCCTCGAGTCACAGATCCTCTTCTGAGATGAGTTTTTGTTCGGCGTTCAGGAACGC | XhoI | 3′ human tepsin, adds C-terminal Myc tag after aa525 |
| tepsin c-term (aa360-525)-Myc | GGAGCAGGATCCATGGGCACCTCAG | BamHI | 5′ human tepsin, starts at aa360 |
| tepsin c-term (aa360-525)-Myc | TGCTCCCTCGAGTCACAGATCCTCTTC | XhoI | 3′ human tepsin-myc, aa525 and Myc tag |
| LC3B(aa1-120) | CAGGAGACGTTCGGGTAAGTCGACTCGAGC | N/A | 5′ mutagenesis primer to remove aa121-125 |
| LC3B(aa1-120) | GCTCGAGTCGACTTACCCGAACGTCTCCTG | N/A | 3′ mutagenesis primer to remove aa121-125 |
| LC3B LDS mutant (F52A, L53A) | CAGCTTCCTGTTCTGGATAAAACAAAGGCCGCTGTACCTGACCATGTCAACAT | N/A | 5′ mutagenesis primer, F52A, L53A |
| LC3B LDS mutant (F52A, L53A) | ATGTTGACATGGTCAGGTACAGCGGCCTTTGTTTTATCCAGAACAGGAAGCTG | N/A | 3′ mutagenesis primer, F52A, L53A |
Protein expression and purification
Constructs were expressed in BL21(DE3)pLysS cells (Invitrogen) for 16–20 h at 22°C after induction with 0.4 mM Isopropyl β-d-1-thiogalactopyranoside (IPTG). All tepsin constructs were purified in 20 mM Tris (pH 8.5), 200 mM NaCl, and 2 mM βME. Wild-type β4 appendage domain and all mammalian ATG8 proteins (mATG8; LC3B, GABARAP, and GABARAPL2) were purified in 20 mM HEPES (pH 7.5), 200 mM NaCl and 2 mM βME. Cells were lysed by a disruptor (Constant Systems Limited) and proteins were affinity purified using glutathione sepharose (GE Healthcare) in purification buffer. GST-tagged mATG8 constructs were cleaved overnight with recombinant 3C protease at 4°C and eluted in batch. All proteins were further purified by gel filtration on preparative Superdex HiLoad 26/600 or analytical (Superdex 200 10/300) columns (GE Healthcare).
Tepsin antibody production
The tepsin ENTH domain was expressed and purified as described previously (Archuleta et al., 2017). Protein was provided to Genscript to raise an antibody in New Zealand rabbits through three rounds of immunization followed by affinity purification. This tepsin antibody was tested on lysates generated from commercially available HAP1 AP-4 ε (gene: AP4E1) and tepsin (gene: ENTHD2) KO cell lines (Horizon Discovery) and compared with a published tepsin antibody (Supplemental Figure S3A) provided by the Robinson lab (Borner et al., 2012).
iLIR
Putative LIR motifs discussed in this paper were identified using the iLIR in silico resource (https://ilir.warwick.ac.uk/kwresult.php; Jacomin et al., 2016). The putative tepsin LIR motif is found in the list of identified putative LIR motif-containing proteins found searching by gene name (ENTHD2). It is also predicted when given the fasta sequence of full-length tepsin.
Structure representation and modeling
Models of tepsin LIR motif interactions with mammalian ATG8 proteins were generated using AlphaFold 2.2 Multimer (Jumper et al., 2021, a and b). Models were validated by visualization of peptide residues docked into hydrophobic pockets on the modeled mATG8 surfaces. Each model docked critical LIR motif residues (Trp188 and Leu191) into hydrophobic pockets without major clashes. The models were compared with experimental mATG8-LIR structures by superposition. ChimeraX MatchMaker was used to superpose Alphafold2 models with experimental structures deposited in the Protein Data Bank. All structural figures were generated in UCSF ChimeraX (Goddard et al., 2018; Pettersen et al., 2021).
GST-pulldown assays
GST or tepsin-GST fusion proteins (50 µg) and indicated prey proteins at a 5X molar excess to tepsin (mATG8 and/or AP-4 β4 appendage) were incubated with glutathione sepharose resin (GE Healthcare) for 2 h at 4°C in 20 mM HEPES (pH 7.5), 100 mM NaCl, 0.5% NP-40 and 2 mM dithiothreitol (DTT). Resin was washed three times with the same buffer. Proteins were eluted from the resin using the wash buffer plus 30 mM reduced glutathione. Elution buffer was incubated with resin for 20 min on ice with gentle agitation every 2 min. Gel samples were prepared from the supernatant following elution with glutathione, and the assay was analyzed by Coomassie staining and Western blotting of SDS–PAGE gels. When further analyzed by Western blot, His-tagged prey protein were detected using anti-His6-HRP conjugated primary antibody (Abcam, ab184607). Uncropped gels and films are displayed in Supplemental Figure S10.
Isothermal titration calorimetry
ITC experiments were conducted on a NanoITC instrument (TA Instruments) at 25°C. Molar peptide concentration in the syringe was at least 6.5 times that of protein in the cell; 0.13 mM LC3B was placed in the cell and 0.845 mM tepsin LIR peptide was placed in the syringe. The tepsin LIR peptide was synthesized (Genscript) with the native tepsin sequence (residues 185-193; SGGGWDELS) and an additional serine added for solubility (underlined). All experiments were carried out in 20 mM HEPES (pH 7.5), 100 mM NaCl and 0.5 mM TCEP, filtered and degassed. Incremental titrations were performed with an initial baseline of 120 s and injection intervals of 200 s. Titration data were analyzed in NANOANALYZE (TA Instruments) to obtain a fit and values for stoichiometry (n) and equilibrium association (Ka). KD values were calculated from the association constant.
Tissue culture
HAP1 cell lines (Horizon Discovery; AP4E1 KO: HZGHC000768c003; ENTHD2 (tepsin) KO: HZGHC000845c002; Parental HAP1: C631) were maintained in IMDM (Life Technologies) supplemented with 10% vol/vol fetal bovine serum (FBS; R&D Systems). Stable tepsin-GFP (Borner et al., 2012) and mRFP-GFP-LC3B (Sarkar et al., 2009) HeLa cell lines were obtained as gifts from the Robinson and Rubinsztein labs (Cambridge Institute for Medical Research), respectively. Wild-type HeLa cells (ATCC) and stable HeLa cell lines were maintained in MEM-alpha (Life Technologies), supplemented with 10% vol/vol fetal bovine serum at 37°C in a 5% CO2 atmosphere. Media for stable cell lines was additionally supplemented with 600 µg/ml G418 (Corning). Cell lines were routinely monitored for mycoplasma contamination using DAPI to stain DNA. For starvation during autophagy assays, cells were washed twice with Earle’s balanced salt solution (EBSS; Life Technologies) and incubated with EBSS for 2 h. Basal cells were concurrently given fresh complete media for the same duration. Where indicated, cells were treated with 100 nM Bafilomycin A1 (Millipore-Sigma) in either EBSS or complete cell media for 2 h.
Western blotting
Cells were resuspended and lysed in NP-40 lysis buffer (10 mM HEPES [pH 7.5], 150 mM NaCl, 0.5 mM EDTA (EDTA), 1% NP-40, and 1 cOmplete Mini EDTA-free Protease Inhibitor Cocktail (Roche). Cell slurry was vortexed briefly then incubated on ice for 30 min. The cell slurry was then centrifuged at 20,500 × RCF for 30 min. The soluble fraction (lysate) was reserved, and total protein concentration was measured using Precision Red (Cytoskeleton). Normalized samples were denatured with sodium dodecyl sulfate (SDS) loading buffer (250 mM Tris [pH 6.8], 50 mM DTT, 10% vol/vol SDS, 20% vol/vol glycerol, 0.5% wt/vol bromophenol blue) and boiled for 1 min at 95°C. Samples were subjected to SDS–PAGE using 4–20% gels (Bio-Rad) and transferred to PVDF membranes (Immobilon-P; Millipore). Blots were incubated with indicated primary and HRP-conjugated secondary antibodies then detected using Amersham ECL Western blotting reagents (GE Healthcare) or for more sensitive detection SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Scientific). All uncropped blots are shown in Supplemental Figures S, 10 and 11. Where indicated, Western blot results were quantified using the ImageStudio lite software (LI-COR). Statistical significance was analyzed using GraphPad Prism 10 (GraphPad Software) by one-way ANOVA with posthoc testing or unpaired t test as appropriate.
GFP immunoprecipitation assays
For transient transfections, wild-type HeLa cells were seeded on six well plates and used the following day. pEGFP-N1 was transfected using Fugene 4K (Promega) at 1.5:1 Fugene:DNA ratio following manufacturer protocol. Cells were allowed to incubate for 24 h before use in immunoprecipitation assays.
Cell lysates from tepsin-GFP, pEGFP-N1 transiently-transfected, or mRFP-GFP-LC3B HeLa cells were prepared as described above. Lysate was incubated with unconjugated agarose (control) resin (Chromotek) for 30 min at 4°C to reduce background binding. Tepsin-GFP lysate and pEGFP-N1 transfected precleared lysate added to GFP-trap resin (Chromotek) at a ratio normalized by GFP transfection efficiency. For mRFP-GFP-LC3B, precleared lysate was divided equally among GFP-trap resin (Chromotek) and fresh control resin for coimmunoprecipitation (IP) experiments. Dilution buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 0.5 mM EDTA, 1 cOmplete Mini EDTA-free Protease Inhibitor Cocktail tablet per 20 ml) was added to bring each sample to an equal volume, then IPs were incubated 1 h at 4°C followed by three washes with dilution buffer. IPs were eluted by adding SDS loading buffer to the washed resin pellet and boiling for 5 min at 95°C.
siRNA knockdown and DNA transfection
Cells were seeded on six well plates and used in knockdown assays the following day. AP-4 knockdown was achieved using ON-TARGETplus AP4E1 siRNA (J-021474-05; Dharmacon) and tepsin knockdown was achieved using ON-TARGETplus C17orf56 siRNA (J-015821-17; Dharmacon). Control cells were treated with ON-TARGETplus nontargeting siRNA (D-001810-01; Dharmacon). Transfections of siRNA were carried out with Oligofectamine (Thermo Fisher Scientific) with a final siRNA concentration of 7.5 nM (for each AP-4, tepsin, and control siRNA treatment) in complete culture media and assayed 48 h after transfection. Cells were reseeded 24 h after siRNA treatment to a lower cell density on six well plates (with or without coverslips) for Western blotting and immunofluorescence assays. For rescue experiments, cells were subjected to an additional transfection step. Transfection with siRNA was conducted as described above, but cells were seeded into and maintained throughout the experiment in antibiotic-free media (MEM-alpha with 10% FBS, no G418). Four hours after incubation with siRNA treatment, wild-type and mutant tepsin-myc constructs were transfected using Fugene 4K (Promega) at 1.5:1 Fugene:DNA ratio following manufacturer protocol. Replating and a total time course of 48 h from initial siRNA knockdown were maintained as described above.
Fluorescence microscopy
Cells were seeded onto 12 mm #1.5 glass coverslips (Fisher Scientific) coated with Histogrip (Invitrogen). Coverslips were imaged with a Nikon Spinning Disk confocal microscope equipped with a Photometrics Prime 95B sCMOS monochrome camera; Plan Apo Lambda Oil 60 × 1.40 NA WD 0.13 mm objective; 405, 488, 561, and 647 nm excitation lasers. Image analysis was conducted using Nikon’s NIS Elements AR Analysis software for automated batch image analysis with the custom General Analysis 3 pipelines described below.
For autophagic flux assays: mRFP-GFP-LC3B HeLa cells were fixed in 3% paraformaldehyde (Electron Microscopy Sciences) in PBS-CM (1X PBS with 0.1 mM CaCl2, 1 mM MgCl2) at room temperature for 20 min. Residual paraformaldehyde was quenched by incubating with 50 mM NH4Cl for 10 min. Coverslips were washed three times in PBS-CM with a final wash in Milli-Q H2O (Millipore Sigma). To preserve fluorophore signal, coverslips were kept in a dark box during all incubations. Coverslips were mounted in Prolong Diamond with DAPI (Invitrogen). Quantification of autophagic flux was performed using spinning disk confocal z-stack images (Supplemental Figure S6). Individual cell regions of interest (ROIs) were generated using the “grow objects” function to enlarge the masked DAPI [excitation: 405 nm] signal and segment cells within each image. Signal background in mRFP [excitation: 561 nm] and GFP [excitation: 488 nm] channels was subtracted using the Rolling Ball algorithm (1μm radius). Punctate mRFP or GFP structures corresponding to autophagosomes (defined as having mRFP and GFP signal) or autolysosomes (mRFP signal only) were segmented using a set intensity threshold for each channel, applied uniformly across images. Isolated autophagosome or autolysosome objects were then subjected to three-dimensional analysis functions to obtain object counts and measure object volume. Data from each replicate was transformed using min-max normalization and individual data points from all replicates were combined for statistical analysis and data visualization. Representative images (Figure 4) show a single z-plane to best show discrete LC3B objects without collapsing three-dimensional space.
For ATG9A/TGN46 immunofluorescence assays: mRFP-GFP-LC3 HeLa cells were either fixed and permeabilized in ice-cold methanol for 10 min at –20°C (tepsin-myc rescue experiments) or fixed in 3% paraformaldehyde PBS-CM followed by 5 min permeabilization with ice cold methanol at –20°C. Coverslips were washed three times with PBS-CM then blocked for 1 h in 1% bovine serum albumin (BSA) in PBS-CM. Primary antibody incubations were conducted overnight at 4°C. The next day, coverslips were washed three times (10 min each) with BSA blocking buffer. Fluorescent secondaries were diluted in BSA blocking buffer and incubated for 2 h at room temperature protected from light. Coverslips were then washed two times with BSA block, once with PBS-CM, and once with dH2O before being mounted in Prolong Diamond with DAPI. Fixation effectively quenched exogenous mRFP-GFP-LC3B fluorophore signal allowing for specific detection of TGN46 [excitation: 488 nm] signal and confirmed by comparing secondary antibody-only control cells to immuno-stained cells (Supplemental Figure S3C). Quantification of ATG9A cellular distribution was performed using spinning disk confocal z-stack images and reported as ATG9A/TGN46 colocalization, ATG9A/TGN volume overlap, and the ratio of ATG9A mean intensity TGN/cell (Supplemental Figure S4). For these analyses, individual cell ROIs were generated using the three-dimensional segmentation “OR” function to combine two ROIs (nuclear: ROI-A; cell boundary: ROI-B) resulting in more precise cell segmentation. ROI-A was defined by the DAPI [excitation: 405 nm] signal expanded by the “grow objects” function and ROI-B was defined by the ATG9A channel [excitation: 647 nm] blurred by a Gaussian filter (sigma = 5.5). Combined ROIs were subjected to the “separate objects” function to divide closely grouped cells into final individual cell ROIs (Cell mask; Supplemental Figure S4). ATG9A and TGN46 channels were background subtracted using the “detect regional maxima” top-hat transformation function. ATG9A/TGN46 colocalization was measured on background subtracted images by Pearson’s correlation coefficient for individual cell mask volumes. ATG9A or TGN46 [excitation: 488 nm] signal was isolated using Otsu’s auto-threshold method. For volume overlap, the ATG9A ROI that overlaps the TGN46 ROI was isolated using the ‘And’ binary operation (ATG9A/TGN overlap; Supplemental Figure S4). ATG9A/TGN overlap ROI volume and total ATG9A ROI volume were measured using the three-dimensional measurement “object volume” function. ATG9A/TGN volume overlap metric was calculated as the volume of ATG9A overlapping TGN46 relative to the total ATG9A volume; the raw percent of volume overlap was set relative to the control siRNA mean to normalize across replicates. For the ratio of ATG9A mean intensity TGN/cell, the TGN46 ROI was subtracted from the whole cell mask (Subtracted cell mask; Supplemental Figure S4). Mean ATG9A fluorescence intensity was measured on raw images within the TGN46 ROI and subtracted cell mask ROI using three-dimensional measurement “object mean intensity.” The ratio of ATG9A mean intensity between the TGN and the rest of the cell was set relative to the control siRNA mean value. For tepsin-myc rescue experiments, the mean intensity of the myc channel in each cell area was measured and used to subset the dataset to specifically analyze transfected cells. Individual cell data points from all replicates were combined for statistical analysis and data visualization. Representative images (Figures 3 and 5; Supplemental S8) are displayed as maximum intensity projections because TGN and peripheral ATG9A populations often occupy distinct spaces within the two-dimensional and three-dimensional cellular planes.
For ATG9A/LC3 immunofluorescence assays: HeLa cells were fixed in 3% paraformaldehyde PBS-CM followed by 10 min permeabilization with ice cold methanol at –20°C. Quantification of ATG9A and LC3 colocalization by Manders’ analysis (as signal cooccurrence may not have proportional intensity) used the general image processing workflow described for ATG9A/TGN46 above with the following changes: All z-stack images were denoised using the denoise.ai feature in Nikon Elements software then used to generate maximum intensity projections. Additionally, ROI-B for generating individual cell ROIs was defined by the LC3 channel [excitation: 488 nm] subjected to the Gaussian filter (sigma = 10). For analysis of LC3 structures, LC3 objects were segmented using the “threshold” function then subjected to three-dimensional analysis functions to measure object volume and object max fluorescence intensity. Individual cell data points from all replicates were combined for statistical analysis and data visualization.
All statistical significance was analyzed using GraphPad Prism 10. Data sets were assessed for normality and homoscedasticity in GraphPad Prism 10. Data displaying a normal distribution and homoscedasticity were assessed by ordinary one-way ANOVA with posthoc testing. When data did not display a normal distribution, nonparametric analysis of variance was assessed by the Kruskal-Wallis test and significant results were followed by a Dunn test with Bonferroni correction. When appropriate, two independent samples were compared using the Mann-Whitney U test. Welch’s ANOVA with posthoc correction was used when data did not display homoscedasticity. Data were graphed using GraphPad Prism 10. Individual data points are colored by replicate and with either the mean ± SD or median depicted, depending on whether the data display a normal distribution.
Supplementary Material
Acknowledgments
We sincerely thank Margaret Robinson (Cambridge Institute for Medical Research) for providing the tepsin-GFP HeLa cell line and David Rubinsztein (Cambridge Institute for Medical Research) for providing the mRFP-GFP-LC3B HeLa cell line. We also thank members of the Jackson lab for helpful discussion and critical reading of the manuscript. N.S.W., J.E.G., C.I.C., and L.P.J. are supported by National Institutes of Health R35GM119525. L.P.J. is a Pew Scholar in the Biomedical Sciences, supported by the Pew Charitable Trusts. N.S.W. and C.I.C. were partly supported by the Molecular Biophysics Training Grant National Institutes of Health 5T32GM008320. J.E.G. was partly supported by the Postdoctoral Program in Functional Neurogenomics National Institutes of Health 5T32MH065215. Imaging and image analysis was performed in part using the Vanderbilt Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637, and EY08126).
Abbreviations used:
- AP
assembly polypeptide
- AP-4
adapter protein 4
- ENTH
epsin N-terminal homology
- FHF
FTS, Hook, and FHIP
- GFP
green fluorescent protein
- GST
Glutathione S-transferase
- ITC
isothermal titration calorimetry
- LDS
LIR docking site
- LIR
LC3 interacting region
- mATG8
mammalian ATG8
- mRFP
monomeric red fluorescent protein
- PBS-CM
phosphate buffered saline with calcium and magnesium
- ROI
Region of interest
- TGN
trans-Golgi Network
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E23-09-0359-T) on February 21, 2024.
REFERENCES
- Abdollahpour H, Alawi M, Kortüm F, Beckstette M, Seemanova E, Komárek V, Rosenberger G, Kutsche K (2014). An AP4B1 frameshift mutation in siblings with intellectual disability and spastic tetraplegia further delineates the AP-4 deficiency syndrome. Eur J Hum Genet 2015 23:2 23, 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Jamra R, Philippe O, Raas-Rothschild A, Eck SH, Graf E, Buchert R, Borck G, Ekici, A, Brockschmidt FF, Nöthen MM, et al. (2011). Adaptor protein complex 4 deficiency causes severe autosomal-recessive intellectual disability, progressive spastic paraplegia, shy character, and short stature. Am J Hum Genet 88, 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archuleta TL, Frazier MN, Monken AE, Kendall AK, Harp J, McCoy AJ, Creanza N, Jackson LP (2017). Structure and evolution of ENTH and VHS/ENTH-like domains in tepsin. Traffic 18, 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P, Leshinsky-Silver E, Blumkin L, Schlipf N, Schröder C, Schicks J, Lev D, Riess O, Lerman-Sagie T, Schöls L (2012). Mutation in the AP4B1 gene cause hereditary spastic paraplegia type 47 (SPG47). Neurogenetics 13, 73–76. [DOI] [PubMed] [Google Scholar]
- Behne R, Teinert J, Wimmer M, D’Amore A, Davies AK, Scarrott JM, Eberhardt K, Brechmann B, Chen IPF, Buttermore ED, et al. (2020). Adaptor protein complex 4 deficiency: a paradigm of childhood-onset hereditary spastic paraplegia caused by defective protein trafficking. Hum Mol Genet 29, 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends C, Sowa ME, Gygi SP, Harper JW (2010). Network organization of the human autophagy system. Nature 466, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgisdottir ÅB, Lamark T, Johansen T (2013). The LIR motif - Crucial for selective autophagy. J Cell Sci 126, 3237–3247. [DOI] [PubMed] [Google Scholar]
- Boehm M, Aguilar RC, Bonifacino JS (2001). Functional and physical interactions of the adaptor protein complex AP-4 with ADP-ribosylation factors (ARFs). EMBO J 20, 6265–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Antrobus R, Hirst J, Bhumbra GS, Kozik P, Jackson LP, Sahlender DA, Robinson MS (2012). Multivariate proteomic profiling identifies novel accessory proteins of coated vesicles. J Cell Biol 197, 141–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DG, Barnaba C, Perez GI, Schmidt JC (2023). Quantitative analysis of autophagy reveals the role of ATG9 and ATG2 in autophagosome formation. J Cell Biol 222, e202210078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykov YS, Schaffer M, Dodonova SO, Albert S, Plitzko JM, Baumeister W, Engel BD, Briggs JA (2017). The structure of the COPI coat determined within the cell. eLife 6, e32493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkery DP, Castro-Gonzalez S, Knyazeva A, Herzog LK, Wu Y (2023). An ATG12-ATG5-TECPR1 E3-like complex regulates unconventional LC3 lipidation at damaged lysosomes. EMBO Rep 24, e56841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross J, Durgan J, McEwan DG, Tayler M, Ryan KM, Florey O (2023). Lysosome damage triggers direct ATG8 conjugation and ATG2 engagement via non-canonical autophagy. J Cell Biol 222, e202303078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks JB, Robinson MS (2017). Outerwear through the ages: evolutionary cell biology of vesicle coats. Curr Opin Cell Biol 47, 108–116. [DOI] [PubMed] [Google Scholar]
- Davies AK, Alecu JE, Ziegler M, Vasilopoulou CG, Merciai F, Jumo H, Afshar-Saber W, Sahin M, Ebrahimi-Fakhari D, Borner GHH (2022). AP-4-mediated axonal transport controls endocannabinoid production in neurons. Nat Commun 13, 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AK, Itzhak DN, Edgar JR, Archuleta TL, Hirst J, Jackson LP, Robinson MS, Borner GHH (2018). AP-4 vesicles contribute to spatial control of autophagy via RUSC-dependent peripheral delivery of ATG9A. Nat Commun 9, 3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica EC, Mullins C, Bonifacino JS (1999). AP-4, a novel protein complex related to clathrin adaptors. J Biol Chem 274, 7278–7285. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Behne R, Davies AK, Hirst J (2018). AP-4-Associated hereditary spastic paraplegia. In: GeneReviews®, eds. Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Seattle, WA: University of Washington. [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ (2014). The machinery of macroautophagy. Cell Res 24, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M (2011). Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biology 2011 13:11 13, 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier MN, Davies AK, Voehler M, Kendall AK, Borner GHH, Chazin WJ, Robinson MS, Jackson LP (2016). Molecular Basis for the Interaction Between AP4 β4 and its Accessory Protein. Tepsin. Traffic 17, 400–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, Ferrin TE (2018). UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia CM, Jain A, Mattera R, Friefeld A, Li Y, Bonifacino JS (2021). RUSC2 and WDR47 oppositely regulate kinesin- 1-dependent distribution of ATG9A to the cell periphery. Mol Biol Cell 32, ar25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia CM, Tan X-F, Lian T, Rana MS, Zhou W, Christenson ET, Lowry AJ, Faraldo-Gómez JD, Bonifacino JS, Jiang J, et al. (2020). Structure of human ATG9A, the only transmembrane protein of the core autophagy machinery. Cell Rep 31, 107837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardies K, May P, Djémié T, Tarta-Arsene O, Deconinck T, Craiu D, Helbig I, Suls A, Balling R, Weckhuysen S, et al. (2015). Recessive loss-of-function mutations in AP4S1 cause mild fever-sensitive seizures, developmental delay and spastic paraplegia through loss of AP-4 complex assembly. Hum Mol Genet 24, 2218–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Bright NA, Rous B, Robinson MS (1999). Characterization of a fourth adaptor-related protein complex. Mol Biol Cell 10, 2787–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Hesketh GG, Gingras AC, Robinson MS (2021). Rag GTPases and phosphatidylinositol 3-phosphate mediate recruitment of the AP-5/SPG11/SPG15 complex. J Cell Biol 220, e202002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, et al. (2000). A ubiquitin-like system mediates protein lipidation. Nature 408, 488–492. [DOI] [PubMed] [Google Scholar]
- Ivankovic D, Drew J, Lesept F, White IJ, López Doménech G, Tooze SA, Kittler JT (2020). Axonal autophagosome maturation defect through failure of ATG9A sorting underpins pathology in AP-4 deficiency syndrome. Autophagy 16, 391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomin A-C, Samavedam S, Promponas V, Nezis IP (2016). iLIR database: A web resource for LIR motif-containing proteins in eukaryotes. Autophagy 12, 1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameel M, Klar J, Tariq M, Moawia A, Altaf Malik N, Seema Waseem S, Abdullah U, Naeem Khan T, Raininko R, Baig SM, et al. (2014). A novel AP4M1 mutation in autosomal recessive cerebral palsy syndrome and clinical expansion of AP-4 deficiency. BMC Med Genet 15, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. (2021a). Applying and improving AlphaFold at CASP14. Proteins 89, 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. (2021b). Highly accurate protein structure prediction with AlphaFold. Nature 2021 596:7873 596, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19, 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Noda T, Yoshimori T (2007). Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460. [DOI] [PubMed] [Google Scholar]
- Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y (2000). The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol 151, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Lee JA (2016). Role of the mammalian ATG8/LC3 family in autophagy: Differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Rep 49, 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Yamamoto H, Kinch LN, Garza CM, Takahashi S, Otomo C, Grishin NV, Forli S, Mizushima N, Otomo T, et al. (2020). Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat Struct Mol Biol 27, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, Hua Z, Mali S, McLoughlin F, Vierstra RD (2019). ATG8-binding UIM proteins define a new class of autophagy adaptors and receptors. Cell 177, 766–781. e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba K, Kotani T, Tsutsumi A, Tsuji T, Mori T, Noshiro D, Sugita Y, Nomura N, Iwata S, Ohsumi Y, et al. (2020). Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. Nat Struct Mol Biol 27, 1185–1193. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Miura E, Matsuda K, Kakegawa W, Kohda K, Watanabe M, Yuzaki M (2008). Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron 57, 730–745. [DOI] [PubMed] [Google Scholar]
- Mattera R, Guardia CM, Sidhu SS, Bonifacino JS (2015). Bivalent motif-ear interactions mediate the association of the accessory protein tepsin with the AP-4 adaptor complex. J Biol Chem 290, 30736–30749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Park SY, De Pace R, Guardia CM, Bonifacino JS (2017). AP-4 mediates export of ATG9A from the trans-Golgi network to promote autophagosome formation. Proc Natl Acad Sci USA 114, E10697–E10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Williamson CD, Ren X, Bonifacinoa JS (2020). The FTS-Hook-FHIP (FHF) complex interacts with AP-4 to mediate perinuclear distribution of AP-4 and its cargo ATG9A. Mol Biol Cell 31, 963–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Shigeyama S, Minami S, Shima T, Akayama S, Matsuda T, Esposito A, Napolitano G, Kuma A, Namba-Hamano T, et al. (2020). LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Nat Cell Biology 2020 22:10 22, 1252–1263. [DOI] [PubMed] [Google Scholar]
- Nath S, Dancourt J, Shteyn V, Puente G, Fong WM, Nag S, Bewersdorf J, Yamamoto A, Antonny B, Melia TJ (2014). Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat Cell Biol 16, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda NN, Kumeta H, Nakatogawa H, Satoo K, Adachi W, Ishii J, Fujioka Y, Ohsumi Y, Inagaki F (2008). Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes to Cells 13, 1211–1218. [DOI] [PubMed] [Google Scholar]
- Noda T, Kim J, Huang WP, Baba M, Tokunaga C, Ohsumi Y, Klionsky DJ (2000). Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J Cell Biol 148, 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivas TJ, Wu Y, Yu S, Luan L, Choi P, Guinn ED, Nag S, De Camilli PV, Gupta K, Melia TJ (2023). ATG9 vesicles comprise the seed membrane of mammalian autophagosomes. J Cell Biol 222, e202208088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Evans PR (1998). A structural explanation for the recognition of tyrosine-based endocytotic signals. Science (1979) 282, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pace R, Skirzewski M, Damme M, Mattera R, Mercurio J, Foster AM, Cuitino L, Jarnik M, Hoffmann V, Morris HD, et al. (2018). Altered distribution of ATG9A and accumulation of axonal aggregates in neurons from a mouse model of AP-4 deficiency syndrome. PLoS Genet 14, e1007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, Ferrin TE (2021). UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci 30, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popelka H, Klionsky DJ (2015). Analysis of the native conformation of the LIR/AIM motif in the Atg8/LC3/GABARAP-binding proteins. Autophagy 11, 2153–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS (2015). Forty years of clathrin-coated vesicles. Traffic 16, 1210–1238. [DOI] [PubMed] [Google Scholar]
- Rogov VV, Stolz A, Ravichandran AC, Rios-Szwed DO, Suzuki H, Kniss A, Löhr F, Wakatsuki S, Dötsch V, Dikic I, et al. (2017). Structural and functional analysis of the GABARAP interaction motif (GIM). EMBO Rep 18, 1382–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger A, Hirst J, Davies AK, Robinson MS (2019). Adaptor protein complexes and disease at a glance. J Cell Sci 132, jcs222992. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SWG, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. (2007). Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007 450:7173 450, 1253–1257. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Korolchuk V, Renna M, Winslow A, Rubinsztein DC (2009). Methodological considerations for assessing autophagy modulators: A study with calcium phosphate precipitates. Autophagy 5, 307–313. [DOI] [PubMed] [Google Scholar]
- Sawa-Makarska J, Baumann V, Coudevylle N, von Bülow S, Nogellova V, Abert C, Schuschnig M, Graef M, Hummer G, Martens S (2020). Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation. Science 369, eaaz7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarrott JM, Alves-Cruzeiro J, Marchi PM, Webster CP, Yang Z-L, Karyka E, Marroccella R, Coldicott I, Thomas H, Azzouz M (2023). Ap4b1-knockout mouse model of hereditary spastic paraplegia type 47 displays motor dysfunction, aberrant brain morphology and ATG9A mislocalization. Brain Commun 5, fcac335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppe J, Mari M, Yavavli E, Auffarth K, Cabrera M, Walter S, Fröhlich F, Ungermann C (2020). AP-3 vesicle uncoating occurs after HOPS-dependent vacuole tethering. EMBO J 39, e105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppe J, Schubert E, Apelbaum A, Yavavli E, Birkholz O, Stephanowitz H, Han Y, Perz A, Hofnagel O, Liu F, et al. (2021). Flexible open conformation of the AP-3 complex explains its role in cargo recruitment at the Golgi. J Biol Chem 297, 101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka T, Weidberg H, Pietrokovski S, Elazar Z (2011). Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol 12, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessa A, Battini R, Rubegni A, Storti E, Marini C, Galatolo D, Pasquariello R, Santorelli FM (2016). Identification of mutations in AP4S1/SPG52 through next generation sequencing in three families. Eur J Neurol 23, 1580–1587. [DOI] [PubMed] [Google Scholar]
- Travis SM, Kokona B, Fairman R, Hughson FM (2019). Roles of singleton tryptophan motifs in COPI coat stability and vesicle tethering. Proc Natl Acad SciUSA 116, 24031–24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesch N, Kirkin V, Rogov VV (2020). Atg8-family proteins—Structural features and molecular interactions in autophagy and beyond. Cells 9, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Zhang W, Razi M, Nyoni L, Joshi D, O’Reilly N, Johansen T, Tooze SA, Mouilleron S (2019). Molecular determinants regulating selective binding of autophagy adapters and receptors to ATG8 proteins. Nat Commun 2019 10:1 10, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jagannath C, De Liu X, Sharafkhaneh A, Kolodziejska KE, Eissa NT (2007). Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 27, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, Ichikawa R, Kinjo M, Ohsumi Y (2012). Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol 198, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ARJ, Chan EYW, Hu XW, Köchl R, Crawshaw SG, High S, Halley DW, Lippincott-Schwartz J, Tooze SA (2006). Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci 119, 3888–3900. [DOI] [PubMed] [Google Scholar]
- Zhou C, Wu Z, Du W, Que H, Wang Y, Ouyang Q, Jian F, Yuan W, Zhao Y, Tian R, et al. (2022). Recycling of autophagosomal components from autolysosomes by the recycler complex. Nat Cell Biol 24, 497–512. [DOI] [PubMed] [Google Scholar]
- Zouhar J, Sauer M (2014). Helping hands for budding prospects: ENTH/ANTH/VHS accessory proteins in endocytosis, vacuolar transport, and secretion. Plant Cell 26, 4232–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]