Abstract
Background
Hodgkin lymphoma (HL) is a B‐cell lymphoma accounting for 10% to 15% of all lymphoma in industrialised countries. It has a bimodal age distribution with one peak around the age of 30 years and another after the age of 60 years. Although HL accounts for fewer than 1% of all neoplasms worldwide, it is considered to be one of the most common malignancies in young adults and, with cure rates of 90%, one of the most curable cancers worldwide. Current treatment options for HL comprise more‐ or less‐intensified regimens of chemotherapy plus radiotherapy, depending on disease stage. [18F]‐fluorodeoxy‐D‐glucose (FDG)‐positron emission tomography (PET, also called PET scanning) is an imaging tool that can be used to illustrate a tumour's metabolic activity, stage and progression. Therefore, it could be used as a standard interim procedure during HL treatment, to help distinguish between individuals who are good or poor early responders to therapy. Subsequent therapy could then be de‐escalated in PET‐negative individuals (good responders) or escalated in those who are PET‐positive (poor responders). It is currently unknown whether such response‐adapted therapeutic strategies are of benefit to individuals in terms of overall and progression‐free survival, and the incidence of long‐term adverse events (AEs).
Objectives
To assess the effects of interim [18F]‐FDG‐PET imaging treatment modification in individuals with HL.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; latest issue) and MEDLINE (from 1990 to September 2014) as well as conference proceedings (American Society of Hematology; American Society of Clinical Oncology; European Hematology Association; and International Symposium on Hodgkin Lymphoma) for studies. Two review authors independently screened search results.
Selection criteria
We included randomised controlled trials (RCTs) comparing FDG‐PET‐adapted therapy with non‐adapted treatment in individuals with previously untreated HL of all stages and ages.
Data collection and analysis
Two review authors independently extracted data and assessed the quality of trials. As none of the included studies provided HRs for OS, we described risk ratios (RRs) for this outcome and did not pool the data. As an effect measure we used hazard ratios (HRs) for progression‐free survival (PFS). We described RRs for the dichotomous data on AEs. We also calculated 95% confidence intervals (CIs).
Main results
Our search strategies led to 308 potentially relevant references. From these, we included three studies involving 1999 participants. We judged the overall potential risk of bias as moderate. The studies were reported as RCTs; blinding was not reported, but given the study design it is likely that there was no blinding. One study was published in abstract form only; hence, detailed assessment of the risk of bias was not possible.
Two trials compared standard treatment (chemotherapy plus radiotherapy) with PET‐adapted therapy (chemotherapy only) in individuals with early‐stage HL and negative PET scans. The study design of the third trial was more complex. Participants with early‐stage HL were divided into those with a favourable or unfavourable prognosis. They were then randomised to receive PET‐adapted or standard treatment. Following a PET scan, participants were further divided into PET‐positive and PET‐negative groups. To date, data have been published for the PET‐negative arms only, making it possible to perform a meta‐analysis including all three trials.
Of the 1999 participants included in the three trials only 1480 were analysed. The 519 excluded participants were either PET‐positive, or were excluded because they did not match the inclusion criteria.
One study reported no deaths. The other two studies reported two deaths in participants receiving PET‐adapted therapy and two in participants receiving standard therapy (very‐low‐quality evidence). Progression‐free survival was shorter in participants with PET‐adapted therapy (without radiotherapy) than in those receiving standard treatment with radiotherapy (HR 2.38; 95% CI 1.62 to 3.50; P value < 0.0001). This difference was also apparent in comparisons of participants receiving no additional radiotherapy (PET‐adapted therapy) versus radiotherapy (standard therapy) (HR 1.86; 95% CI 1.07 to 3.23; P value = 0.03) and in those receiving chemotherapy but no radiotherapy (PET‐adapted therapy) versus standard radiotherapy (HR 3.00; 95% CI 1.75 to 5.14; P value < 0.0001) (moderate‐quality evidence). Short‐term AEs only were assessed in one trial, which showed no evidence of a difference between the treatment arms (RR 0.91; 95% CI 0.54 to 1.53; P value = 0.72) (very‐low‐quality evidence). No data on long‐term AEs were reported in any of the trials.
Authors' conclusions
To date, no robust data on OS, response rate, TRM, QoL, or short‐ and long‐term AEs are available. However, this systematic review found moderate‐quality evidence that PFS was shorter in individuals with early‐stage HL and a negative PET scan receiving chemotherapy only (PET‐adapted therapy) than in those receiving additional radiotherapy (standard therapy). More RCTs with longer follow ups may lead to more precise results for AEs, TRM and QoL, and could evaluate whether this PFS advantage will translate into an overall survival benefit.
It is still uncertain whether PET‐positive individuals benefit from PET‐based treatment adaptation and the effect of such an approach in those with advanced HL.
Keywords: Female, Humans, Male, Positron‐Emission Tomography, Positron‐Emission Tomography/methods, Antineoplastic Agents, Antineoplastic Agents/therapeutic use, Chemoradiotherapy, Chemoradiotherapy/methods, Disease‐Free Survival, Fluorodeoxyglucose F18, Hodgkin Disease, Hodgkin Disease/diagnostic imaging, Hodgkin Disease/mortality, Hodgkin Disease/pathology, Hodgkin Disease/therapy, Radiopharmaceuticals, Randomized Controlled Trials as Topic, Selection Bias
Plain language summary
Imaging‐adapted therapy for individuals with Hodgkin lymphoma
Background
Hodgkin lymphoma (HL) is a malignant disease of the lymphatic system of the body. It accounts for 10% to 15% of all lymphoma in industrialised countries and tends to show two peaks in incidence at around 30 and 60 years of age. While it is considered a relatively rare disease, it is one of the most common malignancies in young adults. With cure rates of up to 90% over 5 years, it is one of the most curable cancers worldwide.
The imaging of tumour tissue using a technique termed positron emission tomography (PET) has been shown to provide a good way of estimating the activity of a tumour. The question therefore arises of whether this technique could be used as an tool during therapy to identify individuals who are, or are not, responding to chemotherapy. This would enable further treatment to be modified, resulting in individualised therapy. Treatment could be reduced or stopped in individuals who show a good response to chemotherapy, thus reducing the risk of long‐term adverse events, or increased in those showing a poor response to chemotherapy.
Review question
In this systematic review we address the issue of whether PET‐adapted therapy in individuals with HL results in beneficial outcomes such as longer overall survival (OS) and survival without disease progression (termed progression‐free survival or PFS), higher responses to therapy and participant quality of life (QoL), or reductions in adverse events (such as second malignancies) or treatment‐related mortality.
Study characteristics
We searched important medical databases such as the Cochrane Central Register of Controlled Trials and MEDLINE. Two review authors independently screened, summarised and analysed the results. This lead to the inclusion of three randomised controlled trials (RCTs) with 1999 participants. Currently, only data for 1480 of these participants have been published and were included in this systematic review. Participants were randomised to receive either standard therapy (chemotherapy followed by radiotherapy) or PET‐adapted therapy (chemotherapy only). The median age of participants was 32 years and 52% were male.
The evidence provided is current to September 2014.
Key results
We are unable to draw conclusions about the effect of PET‐adapted therapy on OS as there was insufficient data available (4 deaths in 1480 participants). However, PFS was shorter following PET‐adapted therapy than with standard treatment. Based on our data, we can assume that of 1000 individuals receiving PET‐adapted treatment over 4 years, 222 individuals would experience disease progression or death compared with 100 of 1000 individuals receiving standard treatment. Only one trial reported on short‐term adverse events and the findings were uncertain and do not provide reliable evidence. The studies did not provide any information on the outcomes of QoL, response to therapy or treatment‐related mortality.
Quality of evidence
We judged the quality of evidence for the outcomes of OS and adverse events as very low. We considered the quality of evidence for PFS to be moderate.
Conclusion
To date, no robust data on OS are available. This systematic review shows that individuals with early‐stage HL have a shorter PFS after PET‐adapted therapy compared with those who receive standard therapy. More RCTs with longer follow ups may lead to more information on adverse events, treatment‐related mortality and QoL, and could evaluate whether the PFS advantage seen with standard therapy will translate into a benefit in terms of OS.
Summary of findings
Summary of findings for the main comparison. PET‐adapted therapy in individuals with HL.
| PET‐adapted therapy in individuals with HL | ||||||
| Population: individuals with HL Settings: Intervention: PET‐adapted therapy in individuals with HL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard/radiotherapy | PET‐adapted/without radiotherapy | |||||
| Overall survival | See comment | See comment | Not estimable | 1480 (3 studies/4 comparisons) | ⊕⊝⊝⊝ very low1 | As no events (death) happened in two of four comparisons, calculations were not possible |
|
PFS: relapse or death Follow up: median 4 years |
Moderate | HR 2.38 (1.62 to 3.50) | 1480 (3 studies/4 comparisons) | ⊕⊕⊕⊝ moderate2,3 | ||
| 100 per 1000 | 222 per 1000 (157 to 308) | |||||
| Adverse events | Study population | RR 0.91 (0.54 to 1.53) | 160 (1 study) | ⊕⊕⊝⊝ very low4,5 | ||
| 275 per 1000 | 250 per 1000 (149 to 421) | |||||
| Quality of life | not reported | not reported | not reported | |||
| Treatment related mortality | not reported | not reported | not reported | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HL: Hodkin lymphoma; HR: Hazard ratio; PET: positron emission tomography | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 HRs not reported, no events in one trial, therefore calculation not possible 2 One trial reported results in opposite direction to PFS advantage. Of the seven participants who dies, five (IFRT arm UK NRCI RAPID) did so before they received the experimental intervention (radiotherapy)
3 Moderate heterogeneity (I2 = 53%) 4 Only one trial provided data, leading to strong imprecision
5Only short‐term events were described, not the more important long‐term adverse events such as secondary malignancies and cardiotoxicity, leading to indirectness
Background
Description of the condition
Hodgkin lymphoma (HL) is a cancer of the lymphatic system of the body, involving the lymph nodes, spleen and other organs such as the liver, lung, bone or bone marrow, depending on the tumour stage (Lister 1989). It is a B‐cell lymphoma that accounts for fewer than 1% of all neoplasms throughout the world (Fraga 2007). HL is characterised by the presence of a small proportion of tumour cells known as Hodgkin‐ and Reed‐Sternberg‐cells. These malignant cells usually account for only about 1% of cells in the tumour tissue surrounded by a specific inflammatory microenvironment (Diaz 2011; Shenoy 2011).
In western countries, HL typically shows a bimodal age distribution with a first peak around the age of 30 years and a second peak after the age of 60 years. It accounts for 10% to 15% of all lymphoma in industrialised countries, with an incidence of 2 to 3 per 100,000 inhabitants. It can therefore be regarded as a relatively rare disease, but is nevertheless one of the most common malignancies in young adults. (Thomas 2002)
Two types of HL are distinguishable according to the Revised European‐American Lymphoma/World Health Organization (REAL/WHO) classification: lymphocyte predominant HL, representing about 5% of all HL, and classic HL (representing about 95% of all HL). The two types differ in morphology, phenotype and molecular features, and therefore in clinical behaviour as well as clinical presentation (Harris 1999; Re 2005). Classic HL is further divided into four subtypes (nodular sclerosis, mixed cellularis, lymphocyte‐rich classic HL and lymphocyte‐depleted HL), all of which are treated similarly (Fraga 2007).
The disease usually develops in lymph nodes in the upper part of the body, mostly the latero‐cervical lymph nodes, and results in painless swelling of the lymphatic tissue involved. Normally HL appears within these parts of the body, with peripheral extranodal involvement being rare. As a sign of large tumour size or spreading, 25% of individuals present with B‐symptoms such as fever, drenching night sweats and a loss of more than 10% of body weight (Connors 2009; Pileri 2002).
For staging, the Ann Arbor Classification is used to distinguish between four different tumour stages. Stages one to three indicate the degree of lymph node involvement, whereas stage four is indicative of disseminated organ involvement, which can be found in 20% of cases. Factors associated with a poor prognosis include large mediastinal mass, three or more involved lymph node areas, a high erythrocyte sedimentation rate, extranodal lesion and advanced age, but these may slightly vary between different study groups. Additionally, the occurrence of bulky disease (largest tumour diameter greater than 10 cm), often referred to as the Cotswold modification (Lister 1989), is taken into consideration. Generally, HL is classified as early favourable, early unfavourable or advanced‐stage disease (Engert 2007; Klimm 2005). In Europe, the early favourable‐stage group usually comprises Ann Arbor stages I and II without risk factors. The early unfavourable‐stage includes individuals in stages I and II with risk factors. Most individuals with stage III and IV disease are included in the advanced‐stage risk group (Diehl 2001; Engert 2003). Individuals with Ann Arbor stage IIB and bulky disease may be included in trials in individuals with advanced‐stage disease.
With cure rates of up to 90%, HL is one of the most curable cancers worldwide (Engert 2010; Engert 2012; von Tresckow 2012). A combination of adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) is widely accepted as the gold‐standard chemotherapy regimen in HL (Canellos 1992; Engert 2010). Individuals with limited‐stage disease usually receive a combination of chemotherapy and involved‐field radiation (IF‐RT) (Engert 2010; von Tresckow 2012), whereas those with advanced‐stage disease usually receive an intensified regimen, such as BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone) (Bauer 2011; Borchmann 2011; Engert 2012) or ABVD. Recently, a large randomised trial showed that two cycles of ABVD followed by 20 Gy of IF‐RT is sufficient for the treatment of early favourable HL (Engert 2010), whereas four cycles of chemotherapy followed by 30 Gy IF‐RT is more suitable for individuals with early unfavourable HL. Recently, two cycles of escalated BEACOPP (BEACOPPesc) followed by two cycles of ABVD have been shown to improve progression‐free survival (PFS) in comparison to four cycles of ABVD in individuals with early unfavourable HL (von Tresckow 2012).
In individuals who relapse or whose disease is refractory to initial treatment, high‐dose chemotherapy followed by autologous haematopoietic stem cell transplantation (ASCT) is the therapy of choice (Rancea 2013).
Description of the intervention
Computed tomography, an imaging tool used for the interim staging of tumours, is associated with a high exposure to radiation. Another imaging tool that can inform about the progression or non‐progression of a tumour during therapy is [18F]‐fluorodesoxy‐D‐glucose (FDG)‐positron emission tomography (PET, also called PET scanning). FDG‐PET has become a standard procedure for numerous oncological situations. The principle of FDG‐PET is based on the fact that FDG, being a radio‐labelled glucose analogue, is therefore a good representative of the glucose metabolism of a tissue. More precisely, the 18F‐FDG molecule comprises two parts: a vector (2‐deoxy‐D‐glucose) and 18F, a positron‐emitting nuclide. When FDG is taken up by cells, preferably those with a high basic metabolic rate, it is not metabolised but is 'trapped' within the cell and can then be detected by scintigraphy. This process is used to give evidence about FDG‐avid tumours, such as HL, and their state and degree of progression (Boellaard 2010).
FDG‐PET is used not only for tumour staging, but also for the evaluation of treatment response in individuals with lymphoma, and is a widely accepted procedure (Kobe 2010a; Markova 2009; Specht 2007). Recent data demonstrate that early interim FDG‐PET is a good predictor of prognosis and could therefore help to distinguish between good (negative PET scan) and poor responders (positive PET scan) at an early point in therapy (Gallamini 2007; Kobe 2010b; Markova 2012). The ability to differentiate between responding and non‐responding individuals could lead to the use of response‐adapted therapeutic strategies, such as de‐escalation in responding individuals or escalation in non‐responding individuals. The potential for therapy adaptation is a fairly new approach to treatment, which was introduced following further exploration of the FDG‐PET procedure (Engert 2012; Kobe 2008).
How the intervention might work
As FDG‐PET imaging gives us a good insight into the metabolic activity of the tumour, treatment modification will comprise escalation in PET‐positive individuals, as well as de‐escalation in those who are PET negative. More precisely, this means therapy intensification (e.g. increases in the number of cycles or number of chemotherapeutic agents or additional immunotherapy) on the one hand, or therapy reduction (e.g. reductions in the number of cycles or number of chemotherapeutic agents, or no further treatment/no radiotherapy) on the other. The idea behind this approach is to achieve maximum efficacy in terms of overall survival (OS) and PFS, and to reduce the incidence of long‐term adverse events (AEs).
Why it is important to do this review
To our knowledge, no systematic review on the effectiveness of interim PET‐based treatment modification in individuals with HL has been performed to date. As the question of PET‐guided therapy adaptation is very important, and pivotal for decision‐making, the potential advantages and disadvantages of this approach has been evaluated in several randomised controlled trials (RCTs). The data available from these RCTs and any ongoing studies will be summarised in our review. Meta‐analysing these individual trials will lead to a more precise and reliable evaluation of the effect of this strategy. In this way we will overcome the limitations of individual studies, such as small sample sizes and a lack of statistical power. Pooling data will help identify the best available therapeutic strategy, as well as allowing us to draw conclusions about the treatment modification in question.
Objectives
To assess the effects of interim [18F]‐FDG‐PET imaging treatment modification in individuals with HL.
Methods
Criteria for considering studies for this review
Types of studies
RCTs. We included both full‐text and abstract publications if sufficient information was available on study design, characteristics of participants, interventions and outcomes. We excluded quasi‐randomised trials (e.g. assignment to treatment in alternation or by date of birth) and cross‐over trials.
Types of participants
Individuals with a newly confirmed diagnosis of HL, with no age, gender or ethnicity restriction. We considered individuals with all stages and subtypes of newly diagnosed HL. In trials involving mixed populations of individuals with haematological malignancies we used only data from those with HL. We intended to exclude trials in which fewer than 80% of participants had HL, unless subgroup data for these individuals were provided after contacting the trial authors.
Types of interventions
The main experimental intervention was PET‐adapted treatment modification and the comparison was standard therapy for HL (current at the time the study was conducted) with no modification.
If PET‐adapted treatment modification was evaluated in a randomised design, we considered the following comparisons.
-
In individuals with early‐stage, PET‐negative HL
Experimental: treatment modification (e.g. no further treatment/no radiotherapy or further chemotherapy instead of radiotherapy)
Control: standard approach (e.g. further chemotherapy plus radiotherapy or additional radiotherapy only)
-
In individuals with early‐stage, PET‐positive HL
Experimental: treatment modification (chemotherapy intensification (e.g. shift to BEACOPP) plus radiotherapy)
Control (e.g. further chemotherapy plus radiotherapy)
-
In individuals with advanced‐stage, PET‐negative HL
Experimental: treatment modification: chemotherapy reduction (e.g. number of cycles or chemotherapeutic agents)
Control: standard chemotherapy (e.g. BEACOPP or ABVD)
-
In individuals with advanced‐stage, PET‐positive HL
Experimental: treatment modification: chemotherapy intensification (e.g. number of cycles or number of chemotherapeutic agents or plus additional immunotherapy)
Control: standard chemotherapy (e.g. BEACOPP or ABVD)
The studies included in this review addressed only early‐stage HL as no data for PET‐adapted therapy in interim or advanced‐stage HL are available.
Types of outcome measures
Primary outcomes
The specific aim of this systematic review was to meta‐analyse OS as the primary endpoint, as this is the outcome of greatest clinical relevance and utmost importance to patients.
Secondary outcomes
-
PFS
The time interval from random treatment assignment onto the study to first confirmed progression, relapse or death from any cause, or to the last follow up
-
Response rate
Measured as overall response, complete response and partial response
AEs
Treatment‐related mortality (TRM)
Quality of life (QoL), if measured using reliable and valid instruments.
We did not evaluate the diagnostic value of PET.
Search methods for identification of studies
We adapted search strategies from the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We sought studies in all languages in order to limit language bias.
Electronic searches
We searched the following databases and sources.
-
Databases of medical literature:
Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 9) (for search strategy see Appendix 1);
MEDLINE (Ovid) (1990 to 22.09.2014) (for search strategy see Appendix 2).
-
Conference proceedings of the annual meetings of the following societies for abstracts (2000 to 2014, if not included in CENTRAL):
American Society of Hematology;
American Society of Clinical Oncology;
European Hematology Association;
International Symposium on Hodgkin Lymphoma.
-
Databases of ongoing trials:
meta‐register of controlled trials: http://www.controlled‐trials.com/mrct/
databases and websites of relevant institutions, agencies, organisations, societies and registries.
Searching other resources
-
Handsearching:
We checked the reference lists of all identified trials, relevant review articles and current treatment guidelines for further literature.
-
Personal contacts:
We contacted experts in the field in order to retrieve information on unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (MS, NS) independently screened the results of the search strategies for eligibility for this review by reading the abstracts. In the case of disagreement we obtained the full text publication. If no consensus could be reached, we asked a third review author for final decision (Higgins 2011a).
We documented the study selection process in a flow chart, as recommended in the PRISMA (Preferred Rporting Items for Systematic reviews and Meta‐Analysis) statement (Moher 2009), showing the total numbers of retrieved references and the numbers of included and excluded studies (see Figure 1).
1.
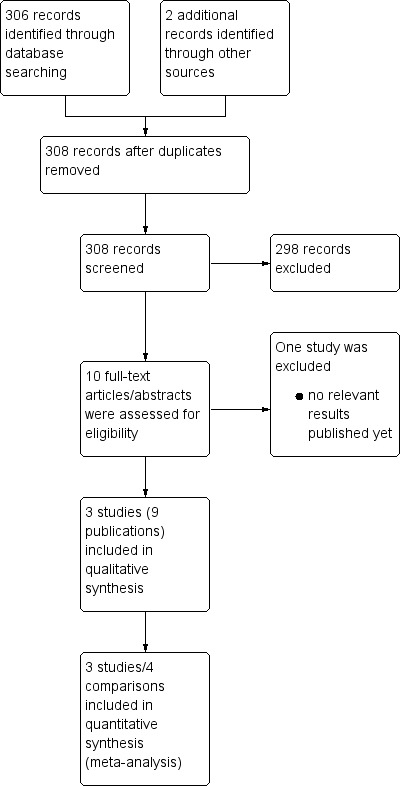
Study flow diagram.
Data extraction and management
Two review authors (M‐TS, NS) extracted data as specified in the guidelines of The Cochrane Collaboration. If required, we contacted the authors of particular studies for supplementary information (Higgins 2011b).
For the data extraction we used a standardised form containing the following items:
general information: author, title, source, publication date, country, language, duplicate publications;
quality assessment: (as specified in the 'Assessment of risk of bias in included studies' section);
study characteristics: trial design, aims, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, subgroup analysis, statistical methods, power calculations, treatment cross‐overs, compliance with assigned treatment, length of follow up, time point of randomisation;
participant characteristics: age, gender, ethnicity, number of participants recruited/allocated/evaluated, participants lost to follow up, additional diagnoses, stage of disease;
interventions: setting, PET technique, PET assessment, type of (multi‐agent) chemotherapy (intensity of regimen, number of cycles), field and dose of radiotherapy, duration of follow up;
outcomes: OS, PFS, response rate, AEs, QoL.
Assessment of risk of bias in included studies
Two review authors (MS, NS) independently assessed the risk of bias in each study using the following criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b):
sequence generation;
allocation concealment;
blinding (participants, personnel, outcome assessors);
incomplete outcome data;
selective outcome reporting;
other sources of bias.
For every criterion we made a judgement using one of three categories:
'low risk': if the criterion was adequately fulfilled in the study (i.e. the study was at a low risk of bias for the given criterion);
'high risk': if the criterion was not fulfilled in the study (i.e. the study was at high risk of bias for the given criterion);
'unclear risk': if the study report did not provide sufficient information to allow for a judgement of 'low risk' or 'high risk', or if the risk of bias was unknown for one of the criteria listed above.
Measures of treatment effect
For binary outcomes, we calculated risk ratios (RRs) with 95% confidence intervals (CIs) for each trial. For time‐to‐event outcomes, we extracted hazard ratios (HRs) from published data, according to Parmar 1998 and Tierney 2007. We intended to calculate continuous outcomes (e.g. QoL) as mean differences; however, no continuous outcomes were reported in the included studies.
Dealing with missing data
A number of potential sources for missing data are suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), which need to be taken into account: at study level, at outcome level and at summary data level. In the first instance it is of the utmost importance to differentiate between data 'missing at random' and 'not missing at random'.
If data were missing, we intended in the next step, to request this from the original investigators. If, after this, data were still missing, we would have made explicit assumptions of any methods used: for example, that the data were assumed to be missing at random or that missing values were assumed to have a particular value, such as a poor outcome.
Additionally, we intended to perform sensitivity analyses to estimate how sensitive the results were to reasonable changes in the assumptions that we had made. The potential impact of missing data on the findings of the review is addressed in the Discussion.
Assessment of heterogeneity
We assessed heterogeneity of treatment effects between trials using a Chi2 test with a significance level at P value < 0.1. We used the I2 statistic to quantify possible heterogeneity (I2 > 30% moderate heterogeneity, I2 > 75% considerable heterogeneity) (Deeks 2011). We intended to explore potential causes of heterogeneity through sensitivity and subgroup analyses.
Assessment of reporting biases
In meta‐analyses involving at least 10 trials, we intended to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test (Sterne 2011). We would have considered a P value of < 0.1 as significant for this test. However, as we included only three trials in the review, this test was not conducted (Sterne 2011).
Data synthesis
We performed analyses according to the recommendations of The Cochrane Collaboration (Deeks 2011). We used the Cochrane statistical software Review Manager (RevMan) 5 for analyses. Had the data been considered sufficiently similar to be combined, we had intended to pool the results using a fixed‐effect model, while using a random‐effects model in sensitivity analyses.
We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) profiler to create 'Summary of findings' tables, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We prioritised outcomes according to their relevance to patients. The most important outcome was OS, followed by PFS, TRM, AEs and QoL.
Subgroup analysis and investigation of heterogeneity
Due to the design of the studies, we added a subgroup analysis for PFS comparing 'radiotherapy versus no radiotherapy' with 'radiotherapy versus chemotherapy without radiotherapy'.
If there were sufficient data, we intended to perform subgroup analyses of the following characteristics:
age;
stage (early favourable/early unfavourable/advanced);
type;
intensity of chemotherapeutic regimen (e.g. aggressive therapy, such as BEACOPP, or less‐aggressive therapy, such as ABVD);
duration of follow up.
We intended to use the tests for interaction to test for differences between subgroup results.
Sensitivity analysis
We performed only one sensitivity analysis of fixed‐ versus random‐effects models. We intended to perform sensitivity analyses of the following characteristics if there were sufficient data:
quality components, including full‐text publications/abstracts;
preliminary results versus mature results.
Results
Description of studies
Results of the search
A search of the literature, via databases and by handsearching, led to the identification of 308 potentially relevant references. At the initial screening stage we excluded 298 due to a lack of conformity with our predefined inclusion criteria. We further evaluated the remaining ten publications either as full‐text publications or, if not available, as abstract publications. This led to us excluding one ongoing trial due to the fact that no relevant data had yet been published (HD 0607). Hence, three trials (H10F/H10U; Picardi; UK NCRI RAPID) reported in nine publications, involving four comparisons (one trial having two subgroups: H10F/H10U)) with a total of 1999 participants were included in this systematic review.
We document the overall numbers of references screened, identified, selected, excluded and included in a PRISMA flow diagram (Figure 1).
Included studies
See also the 'Characteristics of included studies' tables.
Three trials evaluated the efficacy and possible advantage of PET‐adapted therapy in individuals with HL. One of these trials was published only in abstract form (UK NCRI RAPID), whereas Picardi and H10F/H10U were published as full‐text articles. H10F/H10U was published as the preplanned interim analysis. Because in only abstract form, UK NCRI RAPID provided little information about study methods, design and participants. However, we obtained further data on this study from a presentation at the Ninth Hodgkin Symposium in Cologne (UK NCRI RAPID).
In the Picardi and UK NCRI RAPID trials, participants showing a good response to induction chemotherapy with negative PET scans were randomised to either the observation (PET‐adapted therapy) or the IF‐RT (standard therapy) arm. No participant showing a complete response to induction chemotherapy was randomised.
The design of the H10F/H10U trial was slightly different (for study design, see Figure 2). Here, participants were first divided into those with either favourable (F) or unfavourable (U) HL. Following this, participants were randomised to either an experimental (PET‐adapted therapy) or standard therapy arm. All participants then received induction chemotherapy and underwent a PET scan. On the basis of the findings of this scan, participants in the experimental (PET‐adapted therapy) arm were allocated to either a PET‐positive or PET‐negative group. Hence, participants received different therapy schemes, according to the result of the PET scan and their affiliation to a certain main group (F or U). Generally speaking, PET‐negative participants received chemotherapy only, whereas PET‐positive individuals received a stronger chemotherapy regimen plus involved‐node radiotherapy (IN‐RT).
2.
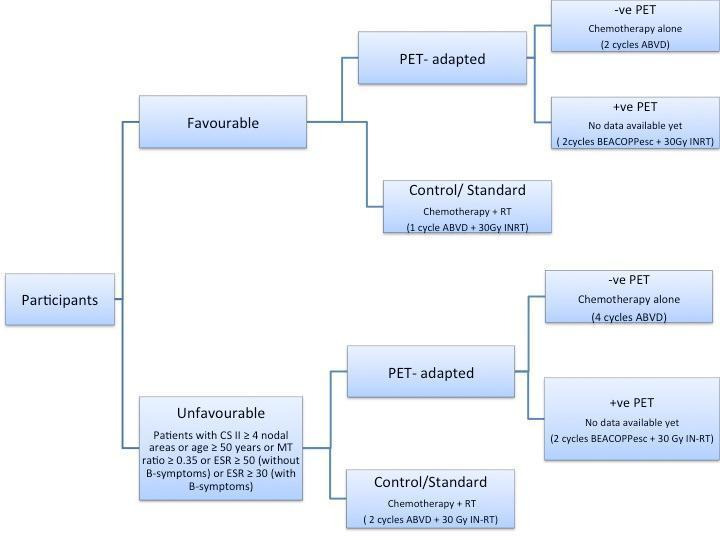
We included only the PET‐negative participants from each of the main groups of the H10F/H10U trial (F, U) in this systematic review, as the part of the study involving the PET‐positive participants is still ongoing, and no results for this population have yet been published.
Design
Two of the included studies were two‐armed RCTs (Picardi; UK NCRI RAPID). One trial (H10F/ H10U) divided participants into two main groups (F, U) prior to randomisation, and then used a three‐armed design for each group (see Figure 2).
Sample sizes
The smallest trial involved 160 randomised participants (Picardi), UK NCRI RAPID had 420 participants and H10F/H10U, the largest trial, involved 1137 participants (however, results were available for only 900 PET‐negative participants).
Location
None of the published references reported the location of the trials. However, it is probable that Picardi was conducted in Italy, UK NCRI RAPID, which was conducted by the United Kingdom's National Cancer Research Center, was conducted in the UK, and H10F/H10U was conducted in several European countries.
Participants
The studies included in this review addressed only early‐stage HL; as soon as data for PET‐adapted therapy in advanced‐stage HL are available, it will be the task of an update to this review to evaluate these results.
Figure 3 illustrates the flow of participants included in this review. In total, the trials included 1999 male and female participants with early‐stage previously untreated HL. Of these, 1717 participants were randomised and a total of 1480 were analysed in this review. Of the 237 participants not analysed in this review, all from the H10F/H10U trial, 13 were excluded after induction chemotherapy (either because they did not complete the first 2 cycles of ABVD or they did not undergo a PET scan or no validated PET scan was available) and for the remaining 224 PET‐positive participants results have not yet been published. Two trials enrolled only PET‐negative participants, who received either chemotherapy only (PET‐adapted therapy arm) or chemotherapy plus IF‐RT (standard therapy arm) (Picardi; UK NCRI RAPID). In H10F/H10U, PET‐adapted therapy in PET‐negative as well as PET‐positive participants was compared with standard treatment.
3.
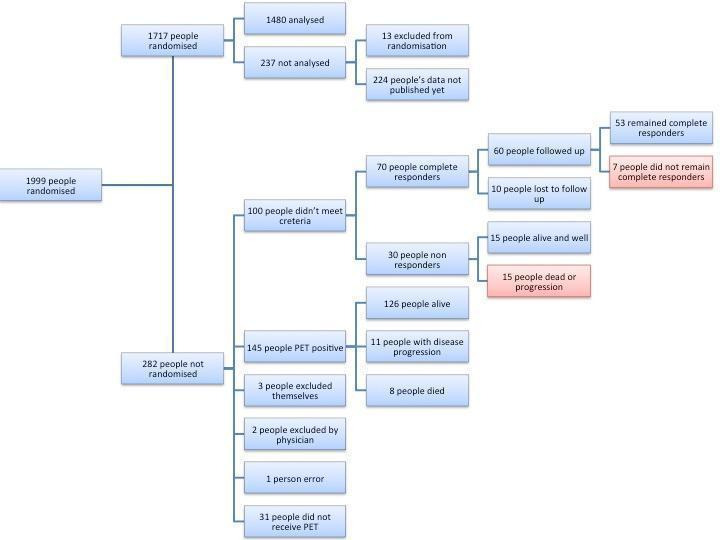
Flow diagram for included studies
Of the 1480 participants included in our analysis, 28 in UK NCRI RAPID did not receive treatment as randomised: 2 received radiotherapy in the PET‐adapted therapy arm and 26 did not receive radiotherapy in the standard therapy arm (due to participant or clinician choice in 19 cases, to death in 5 participants, and to Pneumocystis jirovecii pneumonia and withdrawn consent in 1 participant each). These participants were still included in the analysis.
Of the 282 of 1999 participants who were not randomised, 100 were PET‐negative but did not match eligibility criteria (Picardi) and 182 were excluded: 145 for PET‐positivity; 31 for non receipt of a PET scan; and 6 PET‐negative participants were excluded due to participant choice (n = 3), clinician choice (n = 2) or error (n = 1) (UK NCRI RAPID). Of the 100 participants excluded from Picardi, 70 were classified as complete responders and 30 as non‐responders. Of the 70 complete responders, 10 were lost to follow up and 53 of the 60 remaining participants stayed in sustained complete remission without receiving any further treatment. Of the 30 non‐responders, who received high‐dose chemotherapy and ASCT, 15 are alive and well. Of the 145 PET‐positive participants in UK NCRI RAPID, who received four cycles of ABVD and IF‐RT, 126 are alive and progression free, 11 progressed and 8 have died.
In the Picardi trial, the median age of participants in the observation (PET‐adapted therapy) arm was 31 years (range 15 to 70 years) and 55% were male; corresponding values in the radiation (standard therapy) arm were 30 years (range 15 to 70) and 56%. The UK NCRI RAPID trial provided data on age and gender only prior to randomisation: 321 males and 281 females with a median age of 34 years were enrolled in the trial. The H10F/H10U trial reported that participants had a median age of 31 years (age range 15 to 70 years) and that 51% were male.
Interventions
Two trials compared induction chemotherapy only (PET‐adapted therapy) with induction chemotherapy plus IF‐RT (standard) in PET‐negative participants (Picardi; UK NCRI RAPID). In the Picardi trial, six cycles of VEBEP (vinblastine, etoposide, bleomycin, epirubicin and prednisone) repeated every four weeks were performed as induction chemotherapy. This standard therapy was compared to additionally applied 32 Gy radiotherapy between five and seven weeks after chemotherapy. Induction chemotherapy in the UK NCRI RAPID trial consisted of three cycles of ABVD. In the experimental arm this was followed by IF‐RT. No information about the method or duration of radiotherapy was provided in the available abstracts of the study.
In the H10F/H10U trial, participants were allocated to one of two groups based on whether their HL was considered favourable or unfavourable, and were subsequently randomised to a standard therapy or an experimental (PET‐adapted therapy) arm (see Figure 2). All participants then received two cycles of ABVD followed by a PET scan. In the next step, both PET‐positive and PET‐negative participants in the H10F standard therapy arm received another cycle of ABVD followed by 30 Gy IN‐RT. H10F PET‐negative participants in the experimental arm were treated with two additional cycles of ABVD, without radiotherapy, whereas H10F‐PET‐positive participants in the experimental arm received two cycles of BEACOPPesc followed by 30 Gy IN‐RT. Treatment of both PET‐positive and PET‐negative participants in the H10U standard arm consisted of two additional cycles of ABVD plus 30 Gy IN‐RT. H10U PET‐negative participants in the experimental arm received a total of six cycles of ABVD, without radiotherapy, compared with H10U PET‐positive participants who received with two auxiliary cycles of BEACOPPesc followed by 30 Gy IN‐RT.
As no results have been reported for the H10F/H10U PET‐positive participants, we were not able to analyse outcomes in this experimental arm.
Outcomes
Primary outcome measure
OS was reported in each included trial. No trial reported OS as a primary outcome.
Secondary outcome measures
PFS was reported by the H10F/H10U and UK NCRI RAPID trials, whereas Picardi reported event‐free survival (EFS). As PFS and EFS are fairly similar outcomes, we summed the two as one outcome: PFS. H10U reported one death 'due to toxicity'. We therefore counted this as a death (for the outcome OS) and an AE. As well as EFS, Picardi reported toxicity. The latter we defined as 'adverse events'. Beyond this, no secondary outcomes were predefined or reported in any of the included studies.
Conflicts of interest
Both H10F/H10U and UK NCRI RAPID reported no relevant conflicts of interest. Picardi was supported by grants from the Associazione Italiana contro le Leucemie (Salerno and Benevento Sections).
Excluded studies
After the screening of abstracts we excluded 298 trials that clearly did not match our inclusion criteria. One study (HD 0607) was excluded after detailed evaluation of the retrieved abstract publication (see the 'Characteristics of excluded studies' table).
Risk of bias in included studies
Overall, we considered the quality of the included trials to be moderate. For further information, refer to the 'Risk of bias' sections in the 'Characteristics of included studies' tables and to Figure 4: this 'Risk of bias' summary figure presents all our judgements in a cross‐tabulation of study by entry.
4.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
The methods of random sequence generation and allocation concealment were reported only in the H10F/H10U trial and we considered these to be at low risk of potential bias. These methods were not reported in the other two trials; hence, we judged the risk of selection bias for both criteria in both trials to be unclear.
Blinding
Blinding was not mentioned in any of the included studies; however, given the study design it is likely that there was no blinding of either participants or physicians. It is unlikely that this will have affected the performance of the study regimen (additional radiotherapy), therefore we judged the studies to be at low risk of performance bias.
For the primary outcome OS we judged performance and detection bias to be at low risk, as death is an endpoint not susceptible to bias from the outcome assessor. For all of the secondary outcomes, blinding was relevant as the lack of blinding could have influenced the evaluation of outcomes; hence, these outcomes would not be free of bias. In the H10F/H10U trial, an additional blind PET review was carried out in all participants with an event and an equal number of randomly selected participants without an event, so as to exclude bias caused by differences in the interpretation of early PET scans. Moreover, the outcome assessors were blinded. Therefore we judged the risk of detection bias to be low for this trial and that for the other two trials to be high.
Incomplete outcome data
We judged the risk of attrition bias to be low because the analyses of the outcomes in all studies were by intention to treat and all randomised PET‐negative participants were included.
Selective reporting
We considered the risk of reporting bias in the Picardi trial to be low: the study protocol was available and all of the study's prespecified (primary and secondary) outcomes that are of interest to the review were reported as prespecified. Due to insufficient information and the unavailability of the study protocols, we judged the risk of reporting bias for both H10F/H10U and UK NCRI RAPID to be unclear.
Other potential sources of bias
Picardi and H10F/H10U appeared to be free of any other potential sources of bias. We therefore judged them to be at low risk of other sources of bias. Due to its publication in abstract form, UK NCRI RAPID did not provide sufficient information on this subject, so we assessed the risk of other potential sources of bias to be unclear in this study.
Effects of interventions
See: Table 1
Primary outcome: OS
Participants
All studies (1480 participants) reported the numbers of participant deaths, but did not provide survival curves or HRs (H10F/H10U; Picardi; UK NCRI RAPID).
Results
As none of the studies provided HRs for OS, we described RRs for this outcome. Data were not pooled as insufficient results were available. In Picardi, no deaths were reported. In the H10F/H10U study, no participant died in either arm of H10F, whereas one participant died in the PET‐adapted therapy arm of H10U. In UK NCRI RAPID one participant died in the PET‐arm and two in the standard/radiotherapy arm (per‐protocol analysis).
Secondary outcome: PFS
Participants
Information on this outcome was provided by each of the studies for a total of 1480 participants (H10F/H10U; Picardi; UK NCRI RAPID).
Results
Overall analysis of PFS showed the statistically significant inferiority of PET‐adapted treatment (without radiotherapy) compared with standard treatment (with additional radiotherapy): HR 2.38 (95% CI 1.62 to 3.50; P value < 0.0001; Analysis 1.2). Heterogeneity between the trials was moderate, with an I2 of 53%. One reason for the moderate heterogeneity were the results from the H10F trial, which reported only one event in the radiotherapy arm and nine events in the arm without radiotherapy, leading to a HR of 9.36 (95% CI 2.45 to 35.73).
1.2. Analysis.
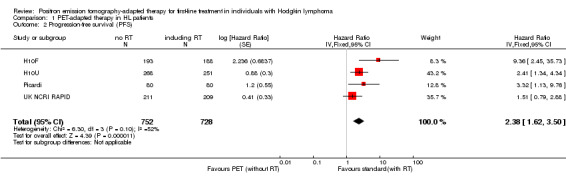
Comparison 1 PET‐adapted therapy in HL patients, Outcome 2 Progression‐free survival (PFS).
The test for differences between the subgroups radiotherapy versus no radiotherapy and radiotherapy versus no radiotherapy + chemotherapy showed no statistical differences (P value = 0.22). In both comparisons, PFS in the groups who did not receive radiotherapy (PET‐adapted therapy arm) was statistically significantly shorter than that in individuals who received standard therapy with additional radiotherapy (radiotherapy versus no radiotherapy: HR 1.86; 95% CI 1.07 to 3.23; P value = 0.03) and radiotherapy versus no radiotherapy + chemotherapy (HR 3.00; 95% CI 1.75 to 5.14: P value < 0.0001; Analysis 1.3); see also Figure 5.
1.3. Analysis.
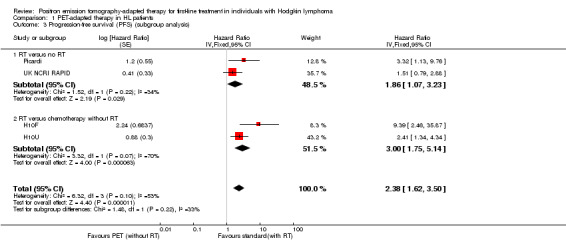
Comparison 1 PET‐adapted therapy in HL patients, Outcome 3 Progression‐free survival (PFS) (subgroup analysis).
5.
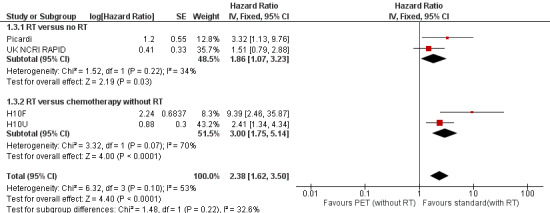
Forest plot of comparison: 1 PET‐adapted therapy in individuals with HL, outcome: 1.3 Progression‐free survival (PFS) (subgroup analysis).
This finding of a shorter PFS with PET‐adapted therapy compared with standard therapy was confirmed in sensitivity analysis using a random‐effects model (HR 2.69; 95% CI 1.46 to 4.96; P value = 0.001; Analysis 1.4).
1.4. Analysis.
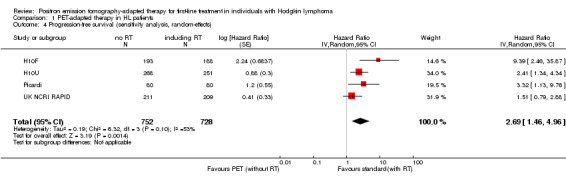
Comparison 1 PET‐adapted therapy in HL patients, Outcome 4 Progression‐free survival (sensitivity analysis, random‐effects).
Secondary outcome: AEs
Participants
One study with 160 participants addressed this outcome (Picardi), see also Table 2.
1. Adverse events.
| Name of study | Adverse event | PET‐adapted‐arm (N) | Standard arm (N) | Total number of events (N) |
| Picardi | Haematological toxicity of WHO grade ≥ 2 | 20% of 80 (16/80) | 22% of 80 (18/80) | 21% of 160 (34/160) |
Non‐haematological toxicity
|
5% of 80% (4/80) | 5% of 80 (4/80) | 5% of 160 (8/160) | |
| 42/160 |
PET: positron emission tomography; WHO: World Health Organization
Results
Overall analysis of AEs showed no statistical significant difference between PET‐adapted therapy and standard treatment: RR 0.91 (95% CI 0.54 to 1.53; P value = 0.72; Analysis 1.5); see also Figure 6.
1.5. Analysis.
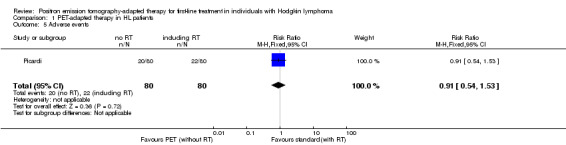
Comparison 1 PET‐adapted therapy in HL patients, Outcome 5 Adverse events.
6.
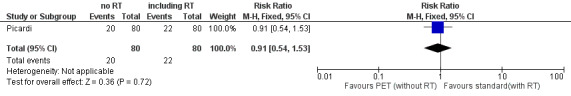
Forest plot of comparison: 1 PET‐adapted therapy in individuals with HL, outcome: 1.5 Adverse events.
Secondary outcome: response rate
This outcome was not addressed in any of the studies.
Secondary outcome: TRM
This outcome was not addressed in any of the studies.
Secondary outcome: QoL
This outcome was not addressed in any of the studies.
Discussion
Summary of main results
The following findings emerge from this Cochrane Review and meta‐analysis evaluating PET‐adapted therapy in individuals with previously untreated early‐stage HL with PET‐negative interim findings.
Based on the currently available research, we are unable to draw any conclusions with regard to OS as there are insufficient data available
PFS was significantly shorter in participants receiving chemotherapy only (PET‐adapted therapy) than in those treated with chemotherapy plus radiotherapy (standard therapy)
There were no significant statistical differences between PET‐adapted treatment and standard therapy with regard to AEs, but the quality of evidence was low for this outcome
QoL, response rate and TRM were not reported in the trials
When interpreting these results, it is important to consider that results lacking statistical significance do not necessarily translate into a lack of differentiation. This means that results with no statistical significance may still make a difference to the individual.
Overall completeness and applicability of evidence
There are three published RCTs dealing with PET‐adapted therapy to date. Each of the trials dealt with PET‐adapted therapy in previously untreated individuals with early‐stage HL and PET‐negative interim results. Two trials compared chemotherapy only (PET‐adapted therapy) with chemotherapy plus radiotherapy (standard therapy) in participants with a good response to initial chemotherapy and a negative PET scan (Picardi; UK NCRI RAPID). H10F/H10U compared PET‐adapted therapy without radiotherapy in both PET‐positive and PET‐negative participants with standard treatment (chemotherapy + radiotherapy) in those with either favourable or unfavourable early‐stage HL. As results for the PET‐negative arm have been published, we were able to pool all the included studies in a meta‐analysis. Only two of the included studies were published as full‐text articles, providing sufficient information about the design, participants, methods and outcomes (H10F/H10U; Picardi). The remaining trial was published in abstract form and therefore lacked information on relevant data. Furthermore, 28 of 420 participants included in the UK NCRI RAPID trial were excluded from randomisation but were still included in analyses.
Results for the PET‐positive participants in the H10F/H10U trial are still pending.
In addition to these published studies, we are aware of five ongoing studies (Casanovas PHRC N 2010; CRUK‐07/146; EU‐20931; HD0607; HD 18). Of these, four studies are designed to compare treatment adaptation in individuals with advanced HL (Ann Arbor stages IIB to IV(B)). The remaining study includes individuals with early‐stage HL, as dealt with in this systematic review. The publication of the results of these studies will necessitate an update to this review. The conclusions of this updated review could differ from those of the present review, and may allow a judgement to be made regarding the disputed benefit of PET‐adapted therapy in HL.
The primary endpoint of this review was OS, due to its prime clinical relevance and its importance for patients. Moreover, it is a commonly accepted direct measure of the benefit of cancer treatment, as well as an endpoint that is not subject to bias by the evaluator. None of the included trials provided survival curves for OS; moreover, no QoL, TRM and long‐term AE data were reported, which are also outcomes of the utmost importance to patients and for clinical decision making.
Quality of the evidence
As one of the trials was published in abstract form only, we could not assess the potential risk of bias for this trial in detail. All trials were reported as randomised studies, but only one of the trials reported allocation concealment. Blinding was not mentioned, but given the study design it is likely that there was no blinding of either participants or physicians. However, it is unlikely that this will have affected the performance of the study therapy (additional radiotherapy). Blinding of the outcome assessor would have made no difference to the primary outcome, OS, and therefore we judged the risk of performance and detection bias to be low for all three trials. With regard to the secondary outcomes, blinding would have increased the quality of the studies and would have been feasible. As only one study reported on blinding with reference to the secondary outcomes (H10F/H10U), we judged the risk of performance and detection bias for secondary outcomes in this study to be low and that for the two others (Picardi; UK NCRI RAPID) to be high. A study protocol was not available for two trials ((H10F/H10U; UK NCRI RAPID), therefore, we judged the risk of selective reporting bias to be unclear for these two studies, and low for Picardi. An unblinded design and unclear allocation concealment could lead to selection, performance or detection bias.
We judged the quality of evidence for OS as very low, because of the low number of events (strong imprecision) and the heterogeneity of the data. The quality of the evidence for PFS we considered as moderate, due to heterogeneity. That for AEs was very low, because of strong imprecision ‐ only one trial provided data and it reported only short‐term AEs rather than the more important long‐term AEs (indirectness) (see 'Table 1').
Potential biases in the review process
To prevent bias within the review, we considered only RCTs. In addition, we tried to avoid bias by carrying out all relevant processes (searching, data collection, analysis) in duplicate. We performed a sensitive search strategy and searched all relevant data of international HL congresses by hand, so as to minimise potential publication bias. In addition, two authors of this review are very experienced in clinical studies on HL (AE and PB are the heads of the German Hodgkin Study Group). We therefore assume to have identified all relevant RCTs relevant to the review question. We are not aware of any obvious flaws in our review process. The number of included trials, three, was too low to generate a funnel plot to explore potential publication bias.
However, results for the PET‐positive participants of the H10F/H10U trial are still pending.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first comprehensive systematic review with meta‐analysis focusing on PET‐adapted therapy for individuals with HL.
Authors' conclusions
Implications for practice.
To date, no robust data on OS, response rate, TRM, QoL or short‐ and long‐term AEs are available. This meta‐analysis found that PFS was significantly shorter in individuals with early‐stage HL and a negative interim PET scan receiving chemotherapy only (PET‐adapted therapy) than in individuals receiving additional radiotherapy (standard therapy). So far, in terms of PFS, this indicates that individuals with early‐stage HL and a negative PET scan do not benefit from PET‐adapted omission of radiotherapy. Currently, data are too sparse to make a clear statement about whether PET‐adapted therapy, omitting radiotherapy, is harmful in terms of OS.
In the meantime it is still uncertain whether PET‐positive individuals benefit from PET‐adapted treatment modification and the effect of this approach in individuals with advanced HL.
With regard to these results, it is important to consider that a shorter PFS resulting from the omission of radiotherapy may still be of benefit for individuals with HL. PET‐adapted therapy could spare them second malignancies or organ toxicities, while relapses could be treated with high‐dose chemotherapy and ASCT. To determine which group of individuals (receiving PET‐adapted therapy or not) profits most in terms of OS and organ toxicities a much longer follow‐up is needed.
Implications for research.
More RCTs with OS, PFS, AEs, TRM and QoL as outcomes are needed to assess whether individuals with HL benefit from PET‐adapted therapy or not. As there are five ongoing trials dealing with the matter in question, and as results are still pending for the PET‐positive participants of one included trial, it is probable that an update of this review will be published in the near future. It might well be that this update will show different results in meta‐analysis than those published in this systematic review.
Acknowledgements
We thank the following members of the Cochrane Haematological Malignancies Group (CHMG) for their comments and improving the review: Ambuj Kumar and Lena Specht (Editors), Céline Fournier (Consumer Editor) and Michaela Rancea (Editorial base).
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor Lymphoma explode all trees |
| #2 | MeSH descriptor Hodgkin Disease explode all trees |
| #3 | Germinoblastom* |
| #4 | Reticulolymphosarcom* |
| #5 | (hodgkin* or hogkin* or hodkin* or hodgin*):ti,ab,kw |
| #6 | (malignan* NEAR/2 (lymphogranulom* or granulom*)) |
| #7 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6) |
| #8 | MeSH descriptor Positron‐Emission Tomography explode all trees |
| #9 | MeSH descriptor Tomography, Emission‐Computed explode all trees |
| #10 | (pet* or petscan* or (Positron* and emission*) or (Positron* and tomography*)) |
| #11 | (pet* and (deoxy* or fluor* or 18fluor* or fdg* or 18fdg* or fludeoxy*)) |
| #12 | (pet* or petscan*) |
| #13 | (tomograph* or tomographs* or tomographic* or tomography* or tomographies*) |
| #14 | emission* |
| #15 | (#13 AND #14) |
| #16 | (#8 OR #9 OR #10 OR #11 OR #12 OR #15) |
| #17 | (#7 AND #16) |
| #18 | "accession number" near pubmed |
| #19 | (#17 AND NOT #18) |
Appendix 2. MEDLINE search strategy
| 1 | Lymphoma/ |
| 2 | exp Hodgkin Disease/ |
| 3 | Germinoblastom$.tw,kf,ot. |
| 4 | Reticulolymphosarcom$.tw,kf,ot. |
| 5 | Hodgkin$.tw,kf,ot. |
| 6 | (malignan$ adj2 (lymphogranulom$ or granulom$)).tw,kf,ot. |
| 7 | or/1‐6 |
| 8 | exp Positron‐Emission Tomography/ |
| 9 | (pet$ or petscan$ or (Positron$ and emission$) or (Positron$ and tomography$)).tw,kf,ot. |
| 10 | (pet$ and (deoxy$ or fluor$ or 18fluor$ or fdg$ or 18fdg$ or fludeoxy$)).tw,kf,ot. |
| 11 | exp Tomography, Emission‐Computed/ |
| 12 | (pet$ or petscan$).tw,kf,ot. |
| 13 | (tomograph$ or tomographs$ or tomographic$ or tomography$ or tomographies$).tw,kf,ot. |
| 14 | emission$.tw,kf,ot. |
| 15 | 13 and 14 |
| 16 | 8 or 9 or 10 or 11 or 12 or 15 |
| 17 | 7 and 16 |
| 18 | randomized controlled trial.pt. |
| 19 | controlled clinical trial.pt. |
| 20 | randomi?ed.ab. |
| 21 | placebo.ab. |
| 22 | clinical trials as topic.sh. |
| 23 | randomly.ab. |
| 24 | trial.ti. |
| 25 | or/18‐24 |
| 26 | humans.sh. |
| 27 | 25 and 26 |
| 28 | 17 and 27 |
Data and analyses
Comparison 1. PET‐adapted therapy in HL patients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival (OS); per protocol | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Progression‐free survival (PFS) | 4 | 1480 | Hazard Ratio (Fixed, 95% CI) | 2.38 [1.62, 3.50] |
| 3 Progression‐free survival (PFS) (subgroup analysis) | 4 | Hazard Ratio (Fixed, 95% CI) | 2.38 [1.62, 3.50] | |
| 3.1 RT versus no RT | 2 | Hazard Ratio (Fixed, 95% CI) | 1.86 [1.07, 3.23] | |
| 3.2 RT versus chemotherapy without RT | 2 | Hazard Ratio (Fixed, 95% CI) | 3.00 [1.75, 5.14] | |
| 4 Progression‐free survival (sensitivity analysis, random‐effects) | 4 | 1480 | Hazard Ratio (Random, 95% CI) | 2.69 [1.46, 4.96] |
| 5 Adverse events | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.53] |
1.1. Analysis.
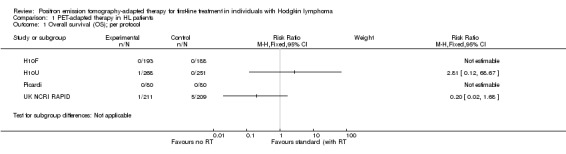
Comparison 1 PET‐adapted therapy in HL patients, Outcome 1 Overall survival (OS); per protocol.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
H10F.
| Methods | Randomised controlled trial of stage I and II, supra‐diaphragmatic classic HL, with two main groups, each with two subgroups, one consisting of two arms, the other of one arm. Comparison of three treatment models in total
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Participants randomised (N = 1137)
Mean age
Gender
Country
|
|
| Interventions | Experimental therapy
Standard therapy
FDG‐PET scans
|
|
| Outcomes | Primary outcomes
Secondary outcomes
QoL and AE were not reported |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Minimization technique was used..." |
| Allocation concealment (selection bias) | Low risk | "Centrally randomly assigned to receive either..." |
| Blinding (performance bias) | Low risk | Although the study is likely not to be blinded, it is unlikely that this affects the performance of the study (additional radiotherapy) |
| Blinding (performance bias and detection bias) Primary outcome (OS) | Low risk | Although the study is likely not to be blinded, this does not affect the outcome OS |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | The study did not address blinding of participants or physicians. Regarding the study design it is likely that there was no blinding. However, the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available, therefore insufficient information to judge |
| Other bias | Low risk | The study appears to be free of other sources of bias |
H10U.
| Methods | see H10F | |
| Participants | see H10F | |
| Interventions | see H10F | |
| Outcomes | see H10F | |
| Notes | see H10F | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Minimization technique was used..." |
| Allocation concealment (selection bias) | Low risk | "Centrally randomly assigned to receive either..." |
| Blinding (performance bias) | Low risk | Although the study is likely not to be blinded, it is unlikely that this affects the performance of the study (additional radiotherapy) |
| Blinding (performance bias and detection bias) Primary outcome (OS) | Low risk | Although the study is likely not to be blinded, this does not affect the outcome OS |
| Blinding (performance bias and detection bias) Secondary outcomes | Low risk | The study did not address blinding of participants or physicians. Regarding the study design it is likely that there was no blinding. However, the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available, therefore insufficient information to judge |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Picardi.
| Methods | Randomised controlled trial with two arms
Recruitment period
Median follow‐up time
Information about non‐randomised participants provided |
|
| Participants | Eligibility criteria
Participants randomised (N = 160)
Participants excluded from randomisation (N = 100)
Mean age of randomised participants only (range)
Gender of randomised participants only
Country
|
|
| Interventions | Induction chemotherapy
FDG‐PET scans
Radiotherapy
|
|
| Outcomes | Primary outcomes
Events being considered as failure
QoL was not reported |
|
| Notes | Supported by grants from Associazione Italiana contro le Leucemie (Salerno and benevento Sections) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation progress. ("Patients with eligibility criteria were randomly assigned to the two different study‐arms") |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information of the method of concealment |
| Blinding (performance bias) | Low risk | Although the study is likely not to be blinded, it is unlikely that this affects the performance of the study (additional radiotherapy) |
| Blinding (performance bias and detection bias) Primary outcome (OS) | Low risk | Although the study is likely not to be blinded, this does not affect the outcome OS |
| Blinding (performance bias and detection bias) Secondary outcomes | High risk | The study did not address blinding of participants or physicians. Regarding the study design it is likely that there was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol was available and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | The study seems to be free of other sources of bias |
UK NCRI RAPID.
| Methods | Randomised controlled trial with two arms
Recruitment period
Median follow‐up time
Information about non‐randomised participants provided |
|
| Participants | Eligibility criteria
Participants randomised (N = 420)
Participants not receiving therapy as randomised (N = 28 of 420):
Participants not randomised (N = 182)
Mean age of all 602 participants registered into the RAPID trial:
Gender of all 602 participants registered into the RAPID trial
Country
|
|
| Interventions | Induction chemotherapy (all participants)
FDG‐PET scans
Radiotherapy (experimental arm)
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation progress. (" 420 PET negative patients were randomised to receive IFRT (N=209) or NFT (N=211)") |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information of the method of concealment |
| Blinding (performance bias) | Low risk | Although the study is likely not to be blinded, it is unlikely that this affects the performance of the study (additional radiotherapy) |
| Blinding (performance bias and detection bias) Primary outcome (OS) | Low risk | Although the study is likely not to be blinded, this does not affect the outcome OS |
| Blinding (performance bias and detection bias) Secondary outcomes | High risk | The study did not address blinding of participants or physicians. Regarding the study design it is likely that there was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. No study‐protocol available |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
ABVD: adriamycin, bleomycin, vinblastine, and dacarbazine; AE: adverse events; BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; CS: clinical stage; CT: computed tomography; esc: escalated; ESR: erythrocyte sedimentation rate; FDG: fluorodeoxy‐D‐glucose; HL: Hodgkin lymphoma; IF‐RT: Involved‐field radiotherapy; IN‐RT: involved‐node radiotherapy; IV: intravenous; IGEV: Ifosfamide, gemcitabine, vinorelbine; MT: magnetisation transfer; NFT: no further treatment; OS: overall survival; PBSC: peripheral blood stem cel; PET: positron emission tomography; PFS: progression‐ free survival; QoL: quality of life; VEBEP: vinblastine, etoposide, bleomycin, epirubicin, prednisone; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| HD 0607 | Ongoing study, no data relevant for this review published yet |
Characteristics of ongoing studies [ordered by study ID]
Casanovas PHRC N 2010.
| Trial name or title | Randomised phase III study of a treatment driven by early PET response compared with a treatment not monitored by early PET in individuals with Ann Arbor stage III‐IV or high‐risk IIB HL |
| Methods | There are two different types of induction therapy. FDG‐PET is performed after the second and fourth cycle of chemotherapy Induction therapy
Consolidation treatment
|
| Participants |
Age
Gender
Inclusion criteria
Country
|
| Interventions |
Chemotherapy
FDG‐PET |
| Outcomes | Progression‐free survival (5 years) |
| Starting date | May 2011 |
| Contact information | Stephanie Picard Tel: 4 72 66 93 33 ext.: 33 stephanie.picard@gelarc.org |
| Notes | Estimated enrolment: not provided Estimated completion date: not provided Study status according to ClinicalTrials.gov: this study is currently recruiting participants |
CRUK‐07/146.
| Trial name or title | A randomised phase III trial to assess response‐adapted therapy using FDG‐PET imaging in individuals with newly diagnosed, advanced HL |
| Methods |
Study design Participants undergo FDG‐PET/CT imaging at baseline, then receive ABVD chemotherapy, and between days 22 and 25 of course 2, they undergo a second FDG‐PET/CT scan to assess response. Subsequent therapy is based on FDG‐PET/CT scan results PET2 (after 2 cycles)‐negative
PET2‐positive
After completion of BEACOPP chemotherapy, participants undergo a third FDG‐PET/CT scan to assess response PET3 (after 3 cycles)‐negative
PET3‐positive
After completion of study therapy, participants are followed every 3 months for 1 year, every 4 months for 2 years, every 6 months for 2 years, and then annually thereafter |
| Participants |
Age
Gender
Eligibility DISEASE CHARACTERISTICS
Participant characteristics
Country
|
| Interventions | No prior chemotherapy, radiotherapy or other investigational drug for HL Chemotherapy
FDG‐PET scan |
| Outcomes | 3‐year progression‐free survival Overall survival Acute and chronic toxicity as assessed by NCI CTCAE v3.0 |
| Starting date | August 2008 |
| Contact information | Peter Johnson, MD Tel: 44‐2380‐796‐186 johnsonp@soton.ac.uk Southampton General Hospital Southampton England SO16 6YD |
| Notes | Estimated enrolment: 1200 Estimated completion date: September 2012 Study status according to ClinicalTrials.gov: recruitment status currently unknown |
EU‐20931.
| Trial name or title | A randomised phase III trial to determine the role of FDG‐PET imaging in clinical stages IA/IIA Hodgkin's disease |
| Methods |
Study design All participants receive 3 cycles of ABVD. On day 15 of the third course of chemotherapy, participants undergo a CT scan of the neck, thorax, abdomen and pelvis.
PET‐negative
PET‐positive
After completion of study therapy, participants are followed up every 3 months for 1 year, every 4 months for 1 year, every 6 months for 1 year, and then annually thereafter |
| Participants |
Age
Gender
Eligibility
Participant characteristics
Country
|
| Interventions | No prior treatment for HL Chemotherapy
Radiotherapy
CT FDG‐PET |
| Outcomes | Progression‐free survival Incidence of FDG‐PET scan positivity/negativity after 3 courses of chemotherapy Survival and cause of death Incidence and type of second cancers |
| Starting date | July 2003 |
| Contact information | John Radford, MD Christie Hospital Manchester England M20 4BX Tel: 44‐161‐446‐3753 |
| Notes | Enrolment: 602 Estimated completion date: December 2015 Study status according to ClinicalTrials.gov: this study is currently active, but not recruiting |
HD 18.
| Trial name or title | HD18 for advanced stages in HL |
| Methods |
Study design: All participants receive two cycles of BEACOPPesc and an additional PET scan PET‐negative
PET‐positive
|
| Participants |
Age
Gender
Inclusion criteria
Country
|
| Interventions |
Chemotherapy
Rituximab FDG‐PET |
| Outcomes | Progression‐free survival (5 years) Overall survival (5 years) Acute toxicity (5 years) Late toxicity (5 years) Complete remission rate (5 years) |
| Starting date | May 2008 |
| Contact information | Michael Fuchs GHSG@uk‐koeln.de |
| Notes | Estimated enrolment: 1500 Estimated primary completion date: May 2012 Study status according to ClinicalTrials.gov: this study is currently recruiting participants |
HD0607.
| Trial name or title | PET‐adapted chemotherapy in advanced HL |
| Methods |
Study design Initial treatment for 2 cycles ABVD (cycle repeats every 28 days) followed by FDG PET PET‐negative
Re‐evaluation with PET at the end of chemotherapy
PET‐positive
|
| Participants |
Age
Gender
Inclusion criteria
Country
|
| Interventions |
Chemotherapy
Rituximab
Radiotherapy
FDG‐PET |
| Outcomes | Progression‐free survival Event‐free survival |
| Starting date | June 2008 |
| Contact information | Andrea Gallamini, MD Tel: +39 0171 642414 gallamini.a@ospedale.cuneo.it |
| Notes | Estimated enrolment: 450 Estimated completion date: July 2015 Study status according to ClinicalTrials.gov: this study is currently recruiting participants |
ABVD: adriamycin, bleomycin, vinblastine, and dacarbazine; ANC: absolute neutrophil count; BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; CNS: central nervous system; CS: clinical staging; CT: computed tomography; ECHO: Echocardiogram; ECOG: Eastern Cooperative Oncology Group; esc: escalated; FDG: fluorodeoxy‐D‐glucose; G‐CSF: granulocyte colony‐stimulating factor; HIV: human immunodeficiency virus; HL: Hodgkin lymphoma; IPS: IV: intravenous; LVEF: left ventricular ejection fraction; N/A: not available; NCI CTCAE v 3.0: National Cancer Institute, Common Terminology Criteria for Adverse Events Version 3.0; PET: positron emission tomography; PFS: progression‐free survival; SC: subcutaneous; WHO: World Health Organization
Differences between protocol and review
We planned to evaluate the role of PET‐adapted therapy for the first‐line treatment of individuals with all stages of HL, and in both PET‐positive and PET‐negative individuals. As only data for PET‐negative individuals in early‐stage HL were available, we were unable to evaluate the role of PET‐adapted therapy in individuals with advanced‐stage or PET‐positive disease.
We planed to report the HR for OS but as we were unable to pool the data, we reported the RR. Furthermore, we were unable to analyse QoL, TRM and response rates, as these outcomes were not reported in the included trials. Due to lack of data, we were also unable to perform subgroup analyses for age, stage of disease, type and intensity of chemotherapeutic regimen, and duration of follow up, or sensitivity analyses for quality components and preliminary results versus mature results.
Due to the design of the studies, we added a subgroup analysis for PFS comparing 'radiotherapy versus no radiotherapy' with 'radiotherapy versus chemotherapy without radiotherapy'.
Contributions of authors
Marie‐Therese Sickinger: study screening, data extraction, data analysis and interpretation, development and writing of protocol and review
Bastian von Tresckow: clinical expertise
Carsten Kobe: clinical expertise
Andreas Engert: clinical expertise, content input
Peter Borchmann: clinical expertise
Nicole Skoetz: study screening, data extraction, data analysis and interpretation, proofreading of the protocol and review, statistical and methodological advice, content input
Sources of support
Internal sources
Cochrane Haematological Malignancies Group, Department of Internal Medicine, University Hospital of Cologne, Germany.
External sources
No sources of support supplied
Declarations of interest
None
New
References
References to studies included in this review
H10F {published data only}
- Andre MPE. An update on the EORTC/LYSA/FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma [abstract]. Blood. 2012; Vol. 120:549.
- Raemaekers JM, Andre MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. [PUBMED: 24637998] [DOI] [PubMed] [Google Scholar]
H10U {published data only}
- Andre MPE. An update on the EORTC/LYSA/FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma [abstract]. Blood. 2012; Vol. 120:549.
- Raemaekers JM, Andre MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. [PUBMED: 24637998] [DOI] [PubMed] [Google Scholar]
Picardi {published data only}
- Picardi M, Renzo A, Pane F, Nicolai E, Pacelli R, Salvatore M, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin's lymphoma with post‐chemotherapy negative positron emission tomography scans. Leukemia & Lymphoma 2007;48:1721‐7. [DOI] [PubMed] [Google Scholar]
UK NCRI RAPID {published data only}
- Radford J. Update on the NCRI RAPID trial. 9th International Symposium on Hodgkin Lymphoma, Cologne, Germany 2013.
- Radford J, Barrington S, Counsell N, Pettengell R, Johnson P, Wimperis J, et al. Involved field radiotherapy versus no further treatment in patients with clinical stages IA and IIA Hodgkin lymphoma and a 'negative' PET scan after 3 cycles ABVD. Results of the UK NCRI RAPID trial [abstract]. Blood. 2012; Vol. 120:547.
- Radford J, O'Doherty M, Barrington S, Qian W, Patrick P, Coltart S, et al. Results of the 2nd planned interim analysis of the rapid trial (involved field radiotherapy versus no further treatment) in patients with clinical stages 1a and 2a Hodgkin lymphoma and a 'negative' FDG‐PET scan after 3 cycles ABVD [abstract 369]. Blood. 2009; Vol. 112:143‐4.
- Radford J, O'Doherty M, Barrington S, Qian W, Popova B, Pettengell R, et al. Results of the 3rd planned interim analysis of the UK NCRI rapid trial (involved field radiotherapy versus no further treatment) in patients with clinical stages IA/IIA Hodgkin lymphoma and a 'negative' 18 FDG‐PET scan after 3 cycles ABVD [Abstract P059]. Haematologica 2010;95:16‐7. [Google Scholar]
- Radford JA, Barrington SF, O'Doherty MJ, Qian W, Mouncey P, Pettengell R, et al. Interim results of a UK NCRI randomized trial comparing involved field radiotherapy with no further treatment after 3 cycles ABVD and a negative pet scan in clinical stages IA/IIA Hodgkin lymphoma [abstract C023]. Haematologica. 2007; Vol. 92:32.
References to studies excluded from this review
HD 0607 {published data only}
- Gallamini A, Rossi A, Patti C, Picardi M, Raimondo F, Cantonetti M, et al. Early treatment intensification in advanced‐stage high‐risk Hodgkin lymphoma (HL) patients, with a positive FDG‐PET scan after two ABVD courses ‐ first interim analysis of the GITIL/FIL HD0607 clinical trial [Abstract 550]. Blood (ASH Annual Meeting Abstracts) 2012;120:550. [Google Scholar]
References to ongoing studies
Casanovas PHRC N 2010 {published data only}
- Randomised phase III study of a treatment driven by early PET response compared with a treatment not monitored by early PET in individuals with Ann Arbor stage III‐IV or high‐risk IIB HL. Ongoing study May 2011.
CRUK‐07/146 {published data only}
- A randomised phase III trial to assess response‐adapted therapy using FDG‐PET imaging in individuals with newly diagnosed, advanced HL. Ongoing study August 2008.
EU‐20931 {published data only}
- A randomised phase III trial to determine the role of FDG‐PET imaging in clinical stages IA/IIA Hodgkin's disease. Ongoing study July 2003.
HD0607 {published data only}
- PET‐adapted chemotherapy in advanced HL. Ongoing study June 2008.
HD 18 {published data only}
- HD18 for advanced stages in HL. Ongoing study May 2008.
Additional references
Bauer 2011
- Bauer K, Skoetz N, Monsef I, Engert A, Brillant C. Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD007941.pub2] [DOI] [PubMed] [Google Scholar]
Boellaard 2010
- Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. European Journal of Nuclear Medicine and Molecular Imaging 2010;37(1):181‐200. [PUBMED: 19915839] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borchmann 2011
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, et al. Eight cycles of escalated‐dose BEACOPP compared with four cycles of escalated‐dose BEACOPP followed by four cycles of baseline‐dose BEACOPP with or without radiotherapy in patients with advanced‐stage Hodgkin's lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. Journal of Clinical Oncology 2011;29(32):4234‐42. [PUBMED: 21990399] [DOI] [PubMed] [Google Scholar]
Canellos 1992
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. The New England Journal of Medicine 1992;327(21):1478‐84. [PUBMED: 1383821] [DOI] [PubMed] [Google Scholar]
Connors 2009
- Connors JM. Clinical manifestations and natural history of Hodgkin's lymphoma . Cancer Journal 2009;15(2):124‐8. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Diaz 2011
- Diaz T, Navarro A, Ferrer G, Gel B, Gaya A, Artells R, et al. Lestaurtinib inhibition of the Jak/STAT signalling pathway in Hodgkin lymphoma inhibits proliferation and induces apoptosis. PloS One 2011;6(4):e18856. [PUBMED: 21533094] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diehl 2001
- Diehl V, Mauch P, Harris NL. Hodgkin's disease. In: Vita V editor(s). Cancer Principles and Practice of Oncology. Philadelphia: Lippincott, Williams and Wilkins, 2001:2339‐87. [Google Scholar]
Engert 2003
- Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, et al. Involved‐field radiotherapy is equally effective and less toxic compared with extended‐field radiotherapy after four cycles of chemotherapy in patients with early‐stage unfavorable Hodgkin's lymphoma: results of the HD8 trial of the German Hodgkin's Lymphoma Study Group. Journal of Clinical Oncology 2003;21(19):3601‐8. [DOI] [PubMed] [Google Scholar]
Engert 2007
- Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology 2007;25(23):3495‐502. [DOI] [PubMed] [Google Scholar]
Engert 2010
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early‐stage Hodgkin's lymphoma. The New England Journal of Medicine 2010;363(7):640‐52. [PUBMED: 20818855] [DOI] [PubMed] [Google Scholar]
Engert 2012
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced‐intensity chemotherapy and PET‐guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open‐label, phase 3 non‐inferiority trial. Lancet 2012;379(9828):1791‐9. [PUBMED: 22480758] [DOI] [PubMed] [Google Scholar]
Fraga 2007
- Fraga M, Forteza J. Diagnosis of Hodgkin's disease: an update on histopathological and immunophenotypical features. Histology and Histopathology 2007;22(8):923‐35. [PUBMED: 17503349] [DOI] [PubMed] [Google Scholar]
Gallamini 2007
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography is prognostically superior to international prognostic score in advanced‐stage Hodgkin's lymphoma: a report from a joint Italian‐Danish study. Journal of Clinical Oncology 2007;25(24):3746‐52. [PUBMED: 17646666] [DOI] [PubMed] [Google Scholar]
Harris 1999
- Harris NL. Hodgkin's lymphomas: classification, diagnosis, and grading. Seminars in Hematology 1999;36(3):220‐32. [PUBMED: 10462322] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Klimm 2005
- Klimm B, Engert A, Diehl V. First‐line treatment of Hodgkin's lymphoma. Current Hematology Reports 2005;4(1):15‐22. [PubMed] [Google Scholar]
Kobe 2008
- Kobe C, Dietlein M, Franklin J, Markova J, Lohri A, Amthauer H, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first‐line chemotherapy in advanced‐stage Hodgkin lymphoma. Blood 2008;112(10):3989‐94. [PUBMED: 18757777] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobe 2010a
- Kobe C, Dietlein M, Kriz J, Furth C, Fuchs M, Borchmann P, et al. The role of PET in Hodgkin's lymphoma and its impact on radiation oncology. Expert Review of Anticancer Therapy 2010;10(9):1419‐28. [PUBMED: 20836677] [DOI] [PubMed] [Google Scholar]
Kobe 2010b
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lister 1989
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. Journal of Clinical Oncology 1989;7(11):1630‐6. [DOI] [PubMed] [Google Scholar]
Markova 2009
- Markova J, Kobe C, Skopalova M, Klaskova K, Dedeckova K, Plutschow A, et al. FDG‐PET for assessment of early treatment response after four cycles of chemotherapy in patients with advanced‐stage Hodgkin's lymphoma has a high negative predictive value. Annals of Oncology 2009;20(7):1270‐4. [PUBMED: 19228806] [DOI] [PubMed] [Google Scholar]
Markova 2012
- Markova J, Kahraman D, Kobe C, Skopalova M, Mocikova H, Klaskova K, et al. Role of [18F]‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leukemia & Lymphoma 2012;53(1):64‐70. [PUBMED: 21740300] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] [DOI] [PubMed] [Google Scholar]
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5(6):649‐55. [PUBMED: 7165009] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Pileri 2002
- Pileri SA, Ascani S, Leoncini L, Sabattini E, Zinzani PL, Piccaluga PP, et al. Hodgkin's lymphoma: the pathologist's viewpoint. Journal of Clinical Pathology 2002;55(3):162‐76. [PUBMED: 11896065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rancea 2013
- Rancea M, Monsef I, Tresckow B, Engert A, Skoetz N. High‐dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. The Cochrane Database of Systematic Reviews 2013;6:CD009411. [PUBMED: 23784872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Re 2005
- Re D, Thomas RK, Behringer K, Diehl V. From Hodgkin disease to Hodgkin lymphoma: biologic insights and therapeutic potential. Blood 2005;105(12):4553‐60. [PUBMED: 15728122] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shenoy 2011
- Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence patterns and outcomes for Hodgkin lymphoma patients in the United States. Advances in Hematology 2011;725219. [DOI: 10.1155/2011/725219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Specht 2007
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Thomas 2002
- Thomas RK, Re D, Zander T, Wolf J, Diehl V. Epidemiology and etiology of Hodgkin's lymphoma. Annals of Oncology 2002;13 Suppl 4:147‐52. [PUBMED: 12401681] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
von Tresckow 2012
- Tresckow B, Plutschow A, Fuchs M, Klimm B, Markova J, Lohri A, et al. Dose‐intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin study group HD14 trial. Journal of Clinical Oncology 2012;30(9):907‐13. [PUBMED: 22271480] [DOI] [PubMed] [Google Scholar]


