Abstract
Background
This is an update of a Cochrane review previously published in 2008. Smoking increases the risk of developing atherosclerosis but also acute thrombotic events. Quitting smoking is potentially the most effective secondary prevention measure and improves prognosis after a cardiac event, but more than half of the patients continue to smoke, and improved cessation aids are urgently required.
Objectives
This review aimed to examine the efficacy of psychosocial interventions for smoking cessation in patients with coronary heart disease in short‐term (6 to 12 month follow‐up) and long‐term (more than 12 months). Moderators of treatment effects (i.e. intervention types, treatment dose, methodological criteria) were used for stratification.
Search methods
The Cochrane Central Register of Controlled Trials (Issue 12, 2012), MEDLINE, EMBASE, PsycINFO and PSYNDEX were searched from the start of the database to January 2013. This is an update of the initial search in 2003. Results were supplemented by cross‐checking references, and handsearches in selected journals and systematic reviews. No language restrictions were applied.
Selection criteria
Randomised controlled trials (RCTs) in patients with CHD with a minimum follow‐up of 6 months.
Data collection and analysis
Two authors independently assessed trial eligibility and risk of bias. Abstinence rates were computed according to an intention to treat analysis if possible, or if not according to completer analysis results only. Subgroups of specific intervention strategies were analysed separately. The impact of study quality on efficacy was studied in a moderator analysis. Risk ratios (RR) were pooled using the Mantel‐Haenszel and random‐effects model with 95% confidence intervals (CI).
Main results
We found 40 RCTs meeting inclusion criteria in total (21 trials were new in this update, 5 new trials contributed to long‐term results (more than 12 months)). Interventions consist of behavioural therapeutic approaches, telephone support and self‐help material and were either focused on smoking cessation alone or addressed several risk factors (eg. obesity, inactivity and smoking). The trials mostly included older male patients with CHD, predominantly myocardial infarction (MI). After an initial selection of studies three trials with implausible large effects of RR > 5 which contributed to substantial heterogeneity were excluded. Overall there was a positive effect of interventions on abstinence after 6 to 12 months (risk ratio (RR) 1.22, 95% confidence interval (CI) 1.13 to 1.32, I² 54%; abstinence rate treatment group = 46%, abstinence rate control group 37.4%), but heterogeneity between trials was substantial. Studies with validated assessment of smoking status at follow‐up had similar efficacy (RR 1.22, 95% CI 1.07 to 1.39) to non‐validated trials (RR 1.23, 95% CI 1.12 to 1.35). Studies were stratified by intervention strategy and intensity of the intervention. Clustering reduced heterogeneity, although many trials used more than one type of intervention. The RRs for different strategies were similar (behavioural therapies RR 1.23, 95% CI 1.12 to 1.34, I² 40%; telephone support RR 1.21, 95% CI 1.12 to 1.30, I² 44%; self‐help RR 1.22, 95% CI 1.12 to 1.33, I² 40%). More intense interventions (any initial contact plus follow‐up over one month) showed increased quit rates (RR 1.28, 95% CI 1.17 to 1.40, I² 58%) whereas brief interventions (either one single initial contact lasting less than an hour with no follow‐up, one or more contacts in total over an hour with no follow‐up or any initial contact plus follow‐up of less than one months) did not appear effective (RR 1.01, 95% CI 0.91 to 1.12, I² 0%). Seven trials had long‐term follow‐up (over 12 months), and did not show any benefits. Adverse side effects were not reported in any trial. These findings are based on studies with rather low risk of selection bias but high risk of detection bias (namely unblinded or non validated assessment of smoking status).
Authors' conclusions
Psychosocial smoking cessation interventions are effective in promoting abstinence up to 1 year, provided they are of sufficient duration. After one year, the studies showed favourable effects of smoking cessation intervention, but more studies including cost‐effectiveness analyses are needed. Further studies should also analyse the additional benefit of a psychosocial intervention strategy to pharmacological therapy (e.g. nicotine replacement therapy) compared with pharmacological treatment alone and investigate economic outcomes.
Keywords: Aged, Female, Humans, Male, Middle Aged, Coronary Disease, Myocardial Infarction, Distance Counseling, Motivation, Obesity, Obesity/therapy, Randomized Controlled Trials as Topic, Risk Factors, Sedentary Behavior, Self Care, Smoking Cessation, Smoking Cessation/methods, Smoking Cessation/psychology, Telephone, Time Factors
Plain language summary
Psychosocial smoking cessation interventions help patients with heart attacks to quit.
Smoking is a risk factor for heart attacks and stopping smoking is recommended for patients after a heart attack. Psychosocial smoking cessation interventions like counseling can help such patients to stop smoking, if they are provided for over one month. Psychosocial interventions can help such patients to quit within 6 months but studies about the long term effects did not support the beneficial short‐term findings. Most trials used a mixture of different intervention strategies, therefore no single strategy showed superior efficacy.
Background
Description of the condition
Smoking is a major, and independent risk factor for coronary heart disease (CHD). Compared to non‐smokers the odds ratio (OR) for myocardial infarction is about 2.5, and for cardiovascular diseases overall the OR is about 2 (Cook 1986; Jacobs 1999; Kawachi 1993; Kawachi 1994; Keil 1998; Njolstad 1996; Nyboe 1991; Prescott 1998; Shaper 1985; Tunstall‐Pedoe 1997; Willett 1987; Woodward 1999). Furthermore, after a cardiac event smokers are twice as likely to get restenosis or to die from a cardiovascular disease (Cullen 1997; Fulton 1997; Kawachi 1993; Kawachi 1994; Kuller 1991; Luoto 1998; Tverdal 1993; Willett 1987). A systematic review in patients with CHD estimated a reduction in mortality risk of 36% in 3‐5 years after quitting smoking (Critchley 2003). Non‐fatal myocardial infarction (MI) also occurs less often in smokers who quit after their first cardiac event (OR 0.62, 95% CI 0.46 to 0.83) (Barth 2007). Compared to persistent smokers, quitters after an acute coronary syndrome have at 6 months a lower risk ratio (RR) of 0.74 for MI/stroke/death (95% CI 0.53 to 1.02; P=0.0698) (Chow 2010). However, many smokers do not quit even after a CHD diagnosis (Critchley 2003) , or resume smoking after the initial "smoking‐free" acute cardiac event hospitalisation and it is critical to summarise available evidence on the effectiveness of different intervention strategies for smoking cessation in this patient group.
Available interventions for smoking cessation
Several intervention strategies in healthy people have shown encouraging results in systematic reviews. For self‐help interventions tailored materials were more efficacious than no intervention (RR 1.31, 95% CI 1.20 to 1.42) but "standard materials" were also efficacious (RR 1.21, 95% CI 1.05 to 1.39). Tailored materials are adapted to the situation of the client and to the specific support needs. Tailored materials might potentially be more effective due to additional patient contact whilst assessing individual patient needs (Noar 2007). Other reviews of smoking cessation using telephone support have suggested that continuous personal contact might improve cessation rate. Continuous telephone counselling was more effective than less intense interventions such as educational self‐help materials only (Stead 2013a). Self‐help interventions as "add‐ons" to counselling was shown to be not efficacious (Lancaster 2009). Telephone support as a single intervention increased quit rates by 37% (RR 1.37, CI 1.26 to 1.50). (Stead 2013a)
Different treatment providers also showed beneficial effects in smoking cessation counselling. Brief advice from a physician was effective for quitting (RR 1.66, 95% CI 1.42 to 1.94) with somewhat larger efficacy in more intense interventions (RR 1.84, 95% CI 1.60 to 2.13) (Stead 2013b). Counseling by nurses was less effective but still showed positive results (RR 1.29, 95%CI 1.20 to 1.39) (Rice 2013).
Rigotti has demonstrated the efficacy of smoking cessation interventions for hospitalised patients (RR 1.37, 95% CI 1.27 to 1.48) and stressed the importance of at least one follow‐up contact to maintain abstinence (Rigotti 2012). This finding is in line with the review on nursing interventions, which pointed out the need for follow up contacts as well (Rice 2013). The incorporated treatment strategies in these reviews can be summarised as psychosocial interventions and can be differentiated from psychopharmacological or substance replacement treatment strategies (e.g. antidepressants, nicotine replacement). For cardiac patients, such psychosocial interventions to quit smoking are recommended along with nicotine replacement therapies and bupropion (ACC/AHA 2002; ACC/AHA 2004; DeBacker 2003; Ockene 1997).The most recent European guidelines (ESC 2012; ESC 2013; ESC/EACPR 2012) underline the importance of assessing smoking status and offering adequate interventions for quitting smoking in cardiac patients.
Pharmacological interventions (e.g. NRTs and medication such as bupropion) are also established medical treatment for smoking cessation, which may be used in isolation or in conjunction with a psychosocial intervention. Nicotine replacement therapy (NRT) and medication (especially Bupropion and Varenicline) are also established medical treatments for smoking cessation. NRT and Bupropion showed similar efficacy compared to placebo with an OR of 1.84 (95% CI 1.71 to 1.99) and an OR of 1.82 (95% CI 1.60 to 2.06) respectively (Cahill 2013). Varenicline improved cessation rates as well with an OR of 1.57 (95% CI 1.29 to 1.91) (Cahill 2013). No increase in adverse cardiac events for Bupropion was found (Stead 2012) but concerns about adverse cardiac events caused by Varenicline were raised (Singh 2011).
Why is it important to do this review
Our initial meta‐analysis of psychosocial smoking cessation interventions in CHD patients showed that they were effective in increasing abstinence at 6‐12 months provided the interventions were of sufficient intensity . However, studies with long‐term follow up were scarce and study quality of early studies was very limited. In an updated systematic review we incorporated more recent trials in order to increase the study pool and the robustness of our findings.
Objectives
This review aimed to evaluate psychosocial intervention strategies for smoking cessation in patients with CHD, with four specific objectives.
1. To examine the efficacy of psychosocial interventions for smoking cessation in patients with coronary heart disease in short‐term (6 to 12 month follow‐up) and long‐term (more than 12 months). 2. To examine the efficacy of different psychosocial intervention types (e.g. telephone support) to stop smoking in patients with coronary heart disease. 3. To investigate the dose‐response relationship: Are brief interventions as effective as more intense interventions? 4. To examine methodological criteria which may moderate the efficacy of smoking cessation interventions in patients with coronary heart disease (for example validation versus self‐report of abstinence).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials studying the efficacy of psychosocial interventions for smoking cessation in CHD patients with an assessment of smoking status at least 6 months after baseline assessment of smoking status.
In the control condition usual care or no specific intervention was delivered.
Types of participants
Patients with CHD ‐ myocardial infarction, coronary artery bypass surgery, percutaneous transluminal coronary angioplasty (International Classification of Diseases 9 codes 410‐414). Studies including patients with other diseases were accepted if at least 80% of patients in the sample suffered from CHD. CHD patients with any co‐morbidities were included. Patients had to be smokers at baseline. Initial smoking status was assessed either by self‐report or by a validated measure. Studies on hospital populations with mixed somatic diagnosis (for example cancer and CHD) were excluded. Trials were also excluded if there was not sufficient information available about the patient's somatic diagnoses.
Types of interventions
The psychosocial intervention could be provided in two ways; either as a separate psychosocial intervention with a main focus on smoking cessation or as a part of a more comprehensive cardiac rehabilitation programme targeting also other risk factors (e.g. obesity, inactivity). Any psychosocial intervention with the goal to change smoking behaviour in CHD patients was of interest. Psychosocial interventions use counselling, motivational support and advice, with or without provision of written educational materials about strategies for smoking cessation. We excluded studies, that used only a pharmacological treatment or nicotine replacement therapy. Other non pharmacological interventions like exercise or physiotherapy were not considered as psychosocial interventions due to the missing psychological ingredient of such interventions. Interventions could be delivered initially during hospital admission or after hospital admission to non acute patients during rehabilitation. The interventions could be provided in group or individual settings.
The psychosocial interventions were categorised according to their ingredients into five non exclusive categories (behavioural therapy, phone support, self‐help, multirisk, specific intervention). Behavioural therapy is based on learning theory and applies strategies like coping with risky situations for smoking, incentives for abstinence and motivational issues (i.e. motivational interviewing). Phone support provide encouraging support via the telephone. Self‐help provides information on how to withdraw from smoking. These three intervention strategies are often used as combination. We additionally coded if the intervention focus on smoking (specific intervention) or if other risk factors were also adressed by the intervention (multirisk intervention). These two strategies are mutually exclusive.
The control conditions were either usual care (patients were allowed to seek support for smoking cessation but a structured referal was not done) or control conditions with unspecific interventions (such as educational material on health issues).
Types of outcome measures
Abstinence by self‐report or validated (e.g. carbon monoxide) measurement at a minimum of 6 months. The outcome is dichotomous (non smoker versus smoker). We did not extract data on the number of cigarettes smoked per day, as there is little evidence that smoking reduction alters the risk of future cardiac events or mortality.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 12, 2012) on The Cochrane Library, MEDLINE (OVID, 1950 to January week 1 2013), EMBASE (OVID, 1980 to 2013 week 1), PsycINFO (OVID, 1806 to January week 2 2013), Conference Proceedings Citation Index ‐ Science (CPCI‐S, 1990 to 11 January 2013) on Web of Science (Thomson Reuters) and PSYNDEX (1977 to June 2003). This is an updated search (see Appendix 1) of the initial search which was done in 2003 (see Appendix 2). The sensitivity maximising Cochrane RCT filter has been applied to the MEDLINE search and adaptations of it to EMBASE, PsycINFO and Web of Science (Lefebvre 2011).
Additionally we searched in The Cochrane Library for reviews on smoking cessation for primary studies (Issue 4, 2012).
Searching other resources
We searched for trials included in other reviews (Lancaster 2005; Rigotti 2007; Stead 2005; Stead 2006; Wiggers 2003) and hand‐searched relevant journals from 1998 to 2003 (Annals of Internal Medicine, Archives of Internal Medicine, British Medical Journal, Psychology and Health, Health Psychology, Tobacco Control) for the initial selection of trials for the first version of this review.
Data collection and analysis
Data extraction and coding Data were independently extracted by two people (initially: JB and Corina Güthlin; update: TJ and ID). In case of differences between codings in the updated dataset a consensus was reached with a third rater (JB).
Assessment of risk of bias in included studies
We coded four quality indicators according to the risk of bias tool from each study (see Cochrane Handbook). Two reviewers independently assessed risk of bias (JB, TJ): Allocation concealment, sequence generation, completeness of outcome data, and validation of smoking status. Allocation concealment and sequence generation was coded according to the guidelines of the Cochrane Collaboration (see Cochrane Handbook). We extracted data on both an intention‐to‐treat (ITT) analysis and a completer analysis. In the first model we classified persons without information about smoking status at follow‐up as smokers (ITT analysis) (attrition bias). In the second model we included only participants with follow‐up information on smoking status (completer analysis) as presented by the authors. In ambiguous studies we classified the outcome data as completer analysis. If an ITT analysis from the study report was possible, we extracted the data from this information.
We coded biochemical validation of smoking status. If trials used cotinine levels in urine or other standardised procedures to assess smoking status we coded this as validated outcome assessment. In studies with self‐report or peer‐report outcome assessment we classified this as non‐validated outcome assessment.
Three quality indicators were not separately coded for each study. Blinding of treatment providers and patients is not possible in psychosocial interventions (performance bias). The assessment of study outcomes (i.e. validation) was used to rate the validity of smoking status (detection bias). However, an overall rating of blinding comprising both facets was not done. We were not able to rate the selective outcome reporting, since the majority of studies did not specify any primary or secondary outcomes in the publications or protocols. Therefore the category "unclear" reflects the available information.
Data about sample and intervention Data on setting, CHD diagnosis or procedure, number of subjects, sex, age, and length of follow up were extracted. Four types of intervention strategies were coded; behavioural therapeutic approaches ; phone support ; additional self‐help intervention ; multi‐risk factor interventions vs. specific interventions for smoking cessation (see Characteristics of included studies table). The number of patients is indicated by a small n and the number of trials for a specific analysis is indicated with a capitalised N. Data about treatment duration We assessed duration of treatment as in another review (Rigotti 2012) and coded this as follows:
1) Single initial contact lasting <= 1 hour, no follow‐up support; 2) One or more contacts in total > 1 hour, no follow‐up support; 3) Any initial contact plus follow‐up <=1 month; 4) Any initial contact plus follow‐up > 1 month and <= 6 month; and 5) Any initial contact plus follow‐up > 6 month.
A cut off of 3 was used to classify interventions as brief (categories 1 to 3) or as intense (4 and 5).
Measures of treatment effect
Risk ratios (RR) were calculated from abstinence rates after the intervention comparing the intervention group with the control group. A RR > 1 indicates superiority of the intervention group over the control group and vice versa.
Dealing with missing data
We used data from the conservative analysis (ITT) in preference, and only used data from the completer analysis if data from the ITT analysis was not available. As a sensitivity analysis, we performed a meta‐analysis with studies with ITT data only.
Data synthesis Risk ratios with 95% confidence intervals were calculated for the pooled estimates. A random‐effects model for pooling the studies was employed because of expected heterogeneity in the primary studies (DerSimonian and Laird method) (Deeks 1999). A forest plot presents the results of the overall analysis with all studies.
Assessment of heterogeneity
Heterogeneity was assessed by examining forest plots of trials, by calculating chi squared heterogeneity test, and I² statistics. The chi squared value tests for statistically significant heterogeneity between trials; higher I² values indicate greater variability between trials than would be expected by chance alone (range 0‐100%) (Higgins 2003).
Subgroup analysis and investigation of heterogeneity
We performed four sub‐group analyses according to risk of bias indicators:
Trials with adequate sequence generation were compared to trials with inadequate or unclear sequence generation
Trials with adequate allocation concealment were compared to trials with inadequate or unclear allocation concealment
Trials with validated smoking status were compared to non‐validated trials
Trials were grouped according to their procedure to deal with incomplete outcome data. As described above ITT analysis (adequate) and completer analysis (inadequate) were coded.
In addition to that we conducted sub‐group analysis according to intervention characteristics and length of follow‐up
Trials were grouped by type of intervention (i.e. behavioural therapy, phone support, self‐help, multirisk, specific intervention).
Trials with a treatment duration of less than 1 month (brief intervention) versus studies with an intervention of 1 month or more (intense intervention).
Intervention effects were measured in short‐term (6 to 12 months) and long‐term (more than 12 months).
Results
Description of studies
The combination of the electronic database searches and additional citations found by scanning references in relevant Cochrane Reviews, other meta‐analyses, and journals in 2006, 2009 and 2013 resulted in 2761 records (Appendix 4; see flowchart in Figure 1). After exclusions on the basis of title and abstract, 364 full‐text articles were assessed for inclusion. Of these a further 321 full‐text articles were excluded for reasons detailed in the characteristics of excluded studies table (Characteristics of excluded studies) and under http://www.juergen‐barth.de/en/wp‐content/uploads/2013/09/Reasons%20for%20excluded%20studies%20Initial%20Review%202006.pdf . Four papers are awaiting assessment as we could not access the papers and authors did not respond to PDF requests (Becker 2003; Boulay 2001; Enriquez‐Puga 2001; Puente‐Silva 1989).
1.
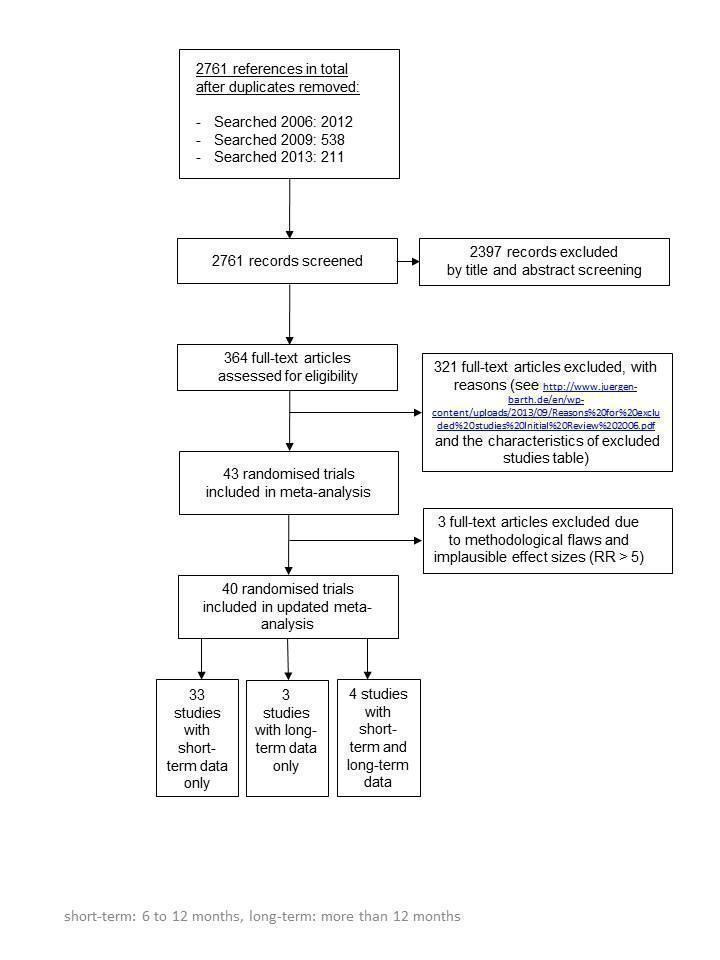
Flow chart of study selection
Forty trials (with short‐term and long‐term results) were included in the review (Allen 1996; Benner 2008; Blasco 2012; Bolman 2002a; Burt 1974; Carlsson 1997; CASIS 1992; Chan 2012; Cossette 2011; Costa e Silva 2008; DeBusk 1994; Dornelas 2000; Froelicher 2004; Gao 2011; Hajek 2002; Han 2011; Hanssen 2007; Hanssen 2009; Heller 1993; Holmes‐Rovner 2008; Jiang 2007; Kubilius 2012; Mildestvedt 2007; Mosca 2010; Naser 2008; Neubeck 2011; Ortigosa 2000; Otterstad 2003; Pedersen 2005; Quist‐Paulsen 2003; Quist‐Paulsen 2005; Quist‐Paulsen 2006a; Reid 2003; Rigotti 1994; Sivarajan 1983; Smith 2009; Taylor 1990; van Elderen (group); van Elderen (phone); Zwisler 2008). Of those 40 trials 33 reported on short‐term results, 4 trials reported both short‐ and long‐term results and 3 trials reported on long‐term results only. In summary seven trials provided data on a long‐term follow up after 12 months (CASIS 1992; Froelicher 2004; Hanssen 2009; Mildestvedt 2007; Naser 2008; Otterstad 2003; Rigotti 1994). As a result of this update we identified 21 new trials, which contributed to this review. Five new trials contributed to long‐term results.
From the studies with short‐term findings (N = 37), sixteen studies were carried out in Europe (1 Sweden, 2 United Kingdom, 3 Netherlands, 5 Norway, 2 Spain, 1 Lithuania, 2 Denmark), ten were from the USA, two from Australia, three from Canada, four from China, one from Brazil and one was a multinational study. The papers were mainly published in English, one was written in Spanish (Ortigosa 2000), one in French (Cossette 2011) and one in Danish (Pedersen 2005). All trials compared a specific smoking cessation intervention with a usual care condition, had comparable groups at study entry, had lower than 50% drop out rates and assessed smoking status before a cardiac event or procedure. 3830 patients were randomised to the usual care group and 3852 received a special psychosocial intervention. As expected, 70 to 90% of the patients were male, mean age was relatively young, 50 to 60 years.The patients suffered predominantly from myocardial infarction or had invasive interventions (bypass surgery, stent). The intervention strategies employed were behavioural therapeutic interventions (20 studies), and self‐help programmes (18 studies). Additional phone support was provided in 26 trials. Seventeen studies reported interventions aimed specifically at smoking cessation, 20 studies employed multi‐risk strategies. Behavioural therapeutic interventions were either provided in a group setting or as individual counselling. The aim was to identify cues related to smoking, or more generally stress reduction and relaxation techniques. Other components included preparation for relapse or specific motivational techniques based on the transtheoretical model (Prochaska 1986) or the strategy of motivational interviewing (Rollnick 1997). Self‐help interventions consisted of information booklets, audio‐ or videotapes. Information booklets which simply described risk factors were not considered self‐help interventions. No studies were available for the comparison of different psychosocial interventions or of psychosocial intervention with different intensity.
In an initial pooling of the short‐term results from 40 included trials we found a RR of 1.24 (95% CI 1.14 to 1.35, n = 7928, N = 40) indicating the efficacy of psychosocial smoking cessation interventions in CHD patients (Analysis 1.1). However, a funnel plot showed outliers with very large effect sizes (Figure 2). These outliers contributed to a large amount of heterogeneity, since we found in the initial analysis an I² of 61%. Therefore a further three studies were excluded using the post hoc exclusion criteria of an unrealistic treatment effect of an RR larger than 5. Feeney 2001 reported a RR of 32.94. Lisspers 1999 reported a RR of 8.25. Mitsibounas 1992 found an effect of RR = 6.00. These trials all had also very substantial methodological flaws. Excluding these three studies had only a small effect on the overall effectiveness of the intervention (RR 1.22, 95% CI 1.13 to 1.32, n = 7682, N = 37) but achieved a more balanced funnel plot (Figure 3).
1.1. Analysis.
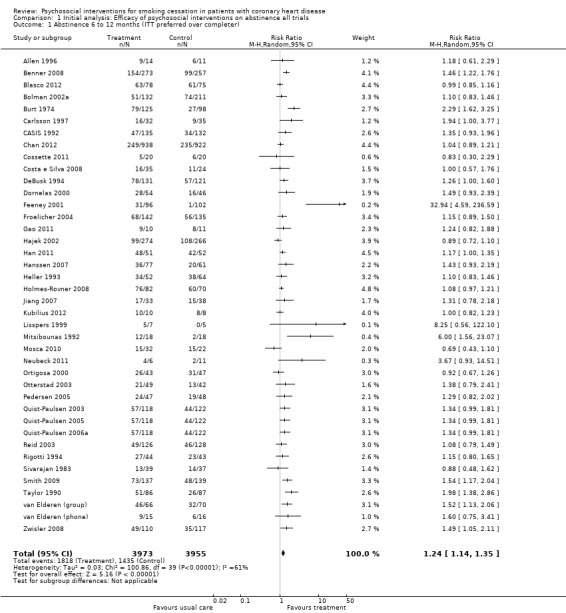
Comparison 1 Initial analysis: Efficacy of psychosocial interventions on abstinence all trials, Outcome 1 Abstinence 6 to 12 months (ITT preferred over completer).
2.
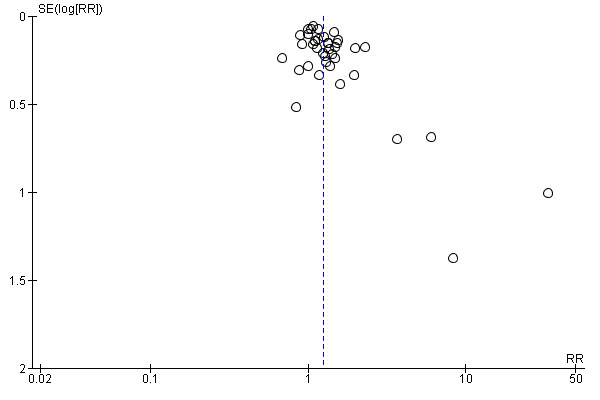
Funnel plot of comparison: 1 Initial analysis: Efficacy of psychosocial interventions on abstinence (6 to 12 months; all trials), outcome: 1.1 Abstinence 6 to 12 months (ITT preferred over completer).
3.
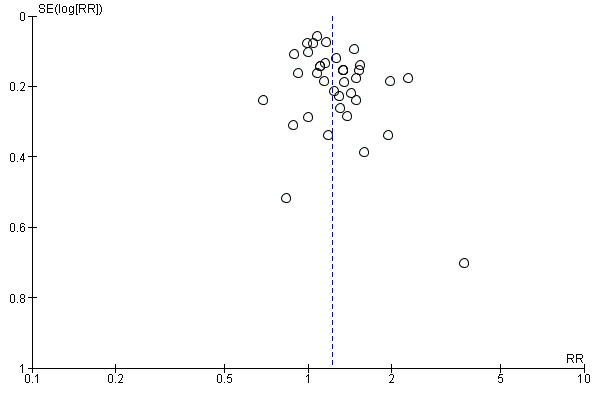
Funnel plot of comparison: 2 Efficacy of psychosocial interventions on abstinence (6 to 12 months; all trials), outcome: 2.1 Abstinence 6 to 12 months (ITT preferred over completer).
Risk of bias in included studies
Out of 37 studies, 18 reported an adequate method for random sequence generation (Allen 1996; Benner 2008; Blasco 2012; Chan 2012; Cossette 2011; Costa e Silva 2008; DeBusk 1994; Dornelas 2000; Froelicher 2004; Hajek 2002; Hanssen 2007; Jiang 2007; Mosca 2010; Neubeck 2011; Reid 2003; Smith 2009; Taylor 1990; Zwisler 2008). Seventeen studies had unclear information concerning sequence generation (Bolman 2002a; Carlsson 1997; CASIS 1992; Gao 2011; Han 2011; Holmes‐Rovner 2008; Kubilius 2012; Ortigosa 2000; Otterstad 2003; Pedersen 2005; Quist‐Paulsen 2003; Quist‐Paulsen 2005; Quist‐Paulsen 2006a; Rigotti 1994; Sivarajan 1983; van Elderen (group); van Elderen (phone)) and only Burt 1974 and Heller 1993 reported an inadequate method. Only 13 studies reported adequate allocation concealment (Benner 2008; Chan 2012; Cossette 2011; DeBusk 1994; Froelicher 2004; Hajek 2002; Hanssen 2007; Otterstad 2003; Pedersen 2005; Quist‐Paulsen 2005; Quist‐Paulsen 2006a; Reid 2003; Zwisler 2008). Sixteen studies reported unclear information (Allen 1996; Blasco 2012; Bolman 2002a; Carlsson 1997; CASIS 1992; Gao 2011; Han 2011; Jiang 2007; Kubilius 2012; Mosca 2010; Ortigosa 2000; Otterstad 2003; Rigotti 1994; Sivarajan 1983; van Elderen (group); van Elderen (phone)) and eight studies were inadequate concerning allocation concealment (Burt 1974; Costa e Silva 2008; Dornelas 2000; Heller 1993; Holmes‐Rovner 2008; Neubeck 2011; Smith 2009; Taylor 1990). Blinding was inadequate in all studies, except in Bolman 2002a where it was adequate since the personnel were not blinded but this was unlikely to introduce bias because the whole hospital was randomised with significant difference between the two groups at the process evaluation. Concerning incomplete outcome data, 26 studies reported adequate information (Blasco 2012; Bolman 2002a; CASIS 1992; Chan 2012; Cossette 2011; Costa e Silva 2008; DeBusk 1994; Dornelas 2000; Froelicher 2004; Hajek 2002; Hanssen 2007; Holmes‐Rovner 2008; Jiang 2007; Mosca 2010; Ortigosa 2000; Otterstad 2003; Quist‐Paulsen 2003; Quist‐Paulsen 2005; Quist‐Paulsen 2006a; Reid 2003; Rigotti 1994; Sivarajan 1983; Smith 2009; Taylor 1990; van Elderen (group); Zwisler 2008). Eleven studies were either unclear or reported inadequate information about incomplete outcome data (Allen 1996; Benner 2008; Burt 1974; Carlsson 1997; Gao 2011; Han 2011; Heller 1993; Kubilius 2012; Neubeck 2011; Pedersen 2005; van Elderen (phone)). In 24 studies, smoking cessation was self‐reported and 13 studies validated self‐reported smoking cessation with measurements of cotinine in urine or saliva. Summary of risk of bias are found in Figure 4; Figure 5.
4.
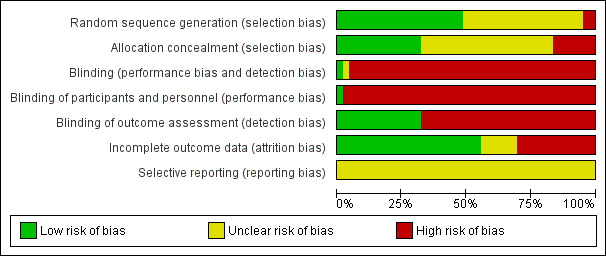
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.
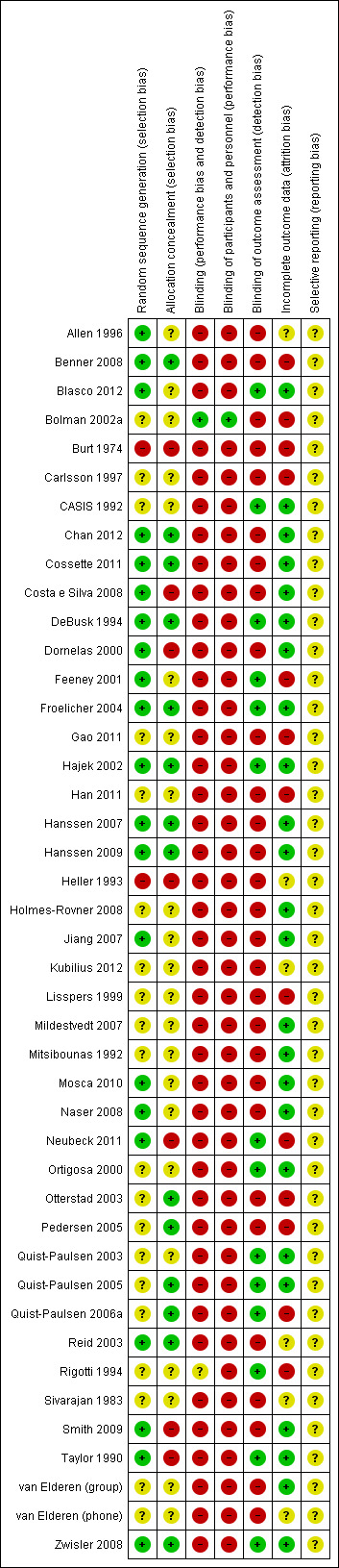
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Psychosocial smoking cessation interventions were effective in achieving smoking abstinence in CHD patients (number of patients = 3852), compared with usual care (number of patients = 3830) (see Table 1). In all trials, patients receiving the specific psychosocial intervention had more than a 20% higher chance of quitting (RR 1.22, 95% CI 1.13 to 1.32, n = 7682, N = 37). There was moderate heterogeneity between studies (chi² 77.98; df 36, P < 0.0001, I² 54%) (Analysis 2.1). Therefore the overall result should be interpreted with some caution. There were considerable differences between trials in the proportion of abstinent patients after the intervention: Kubilius 2012 achieved 100% abstinence in the intervention group, but Chan 2012 reports the lowest abstinence rate with 26.5%. These differences between trials in quit rates might also be responsible for some of the heterogeneity in findings. The pooled RR suggests that psychosocial interventions can increase the chance of quitting compared with usual care, but the heterogeneity in the results need further exploration. The quality of the trials may partly explain this heterogeneity and therefore a subgroup analysis according to risk of bias indicators was used.
1. Summary of findings short term.
| Analysis | Studies | Participants | Risk Ratio | Confidence Interval 95% | Heterogeneity I square |
| Overall analyses | |||||
| All studies (with outliers) | 40 | 7928 | 1.24 | 1.14‐1.35 | 61% |
| All studies (without outliers) | 37 | 7682 | 1.22 | 1.13‐1.32 | 54% |
| Stratified analyses by risk of bias indicators | |||||
| Sequence generation | |||||
| Adequate | 18 | 5046 | 1.21 | 1.07‐1.36 | 61% |
| Inadequate | 19 | 2636 | 1.24 | 1.12‐1.37 | 53% |
| Allocation concealment | |||||
| Adequate | 13 | 4784 | 1.21 | 1.09‐1.34 | 39% |
| Inadequate | 24 | 2898 | 1.24 | 1.11‐1.38 | 65% |
| Handling of incomplete outcome data | |||||
| Adequate | 26 | 6436 | 1.18 | 1.09‐1.28 | 46% |
| Inadequate | 11 | 1246 | 1.36 | 1.12‐1.65 | 72% |
| Validation of outcome | |||||
| Validated outcome measure | 13 | 2803 | 1.22 | 1.07‐1.39 | 60% |
| Non‐validated outcome measure | 24 | 4879 | 1.23 | 1.12‐1.35 | 52% |
2.1. Analysis.
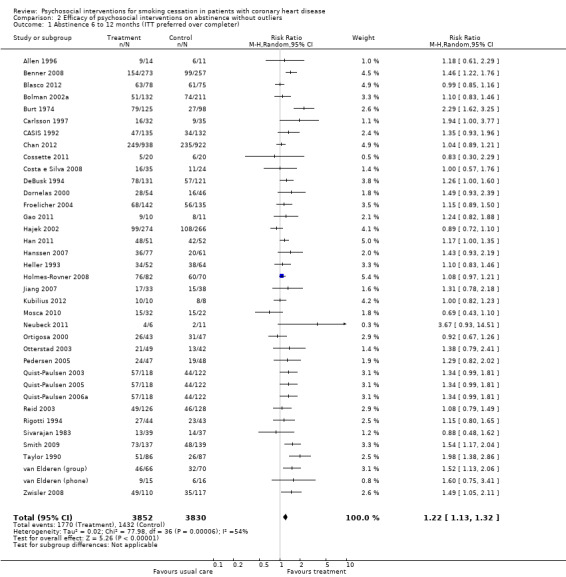
Comparison 2 Efficacy of psychosocial interventions on abstinence without outliers, Outcome 1 Abstinence 6 to 12 months (ITT preferred over completer).
Sub‐group analyses according to risk of bias indicators:
None of the quality indicators affected the effect estimates (all p values > 0.20). Trials with adequate sequence generation showed similar effects compared to trials with inadequate or unclear sequence generation. Trials with adequate sequence generation had a pooled RR of 1.21 (95% CI 1.07 to 1.36, n = 5046, N = 18); trials with inadequate or unclear sequence generation had a pooled RR of 1.24 (95% CI 1.12 to 1.37, n = 2636, N = 19) (Analysis 3.1). No reduction in heterogeneity was achieved by this stratification.
3.1. Analysis.
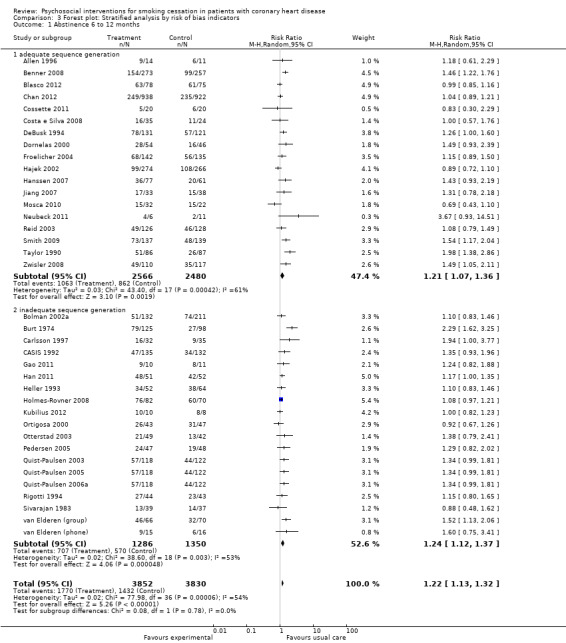
Comparison 3 Forest plot: Stratified analysis by risk of bias indicators, Outcome 1 Abstinence 6 to 12 months.
We have a similar picture when we compared trials with adequate allocation concealment versus inadequate or unclear allocation concealment. The RR was 1.24 (95% CI 1.11 to 1.38, n = 2898, N = 24) for trials with inadequate or unclear allocation concealment, and 1.21 (95% CI 1.09 to 1.34, n = 4784, N = 13) for trials with adequate allocation concealment (Analysis 3.2). Heterogeneity in the subgroup of studies with adequate allocation concealment was considerably reduced (I² 39% compared to the initial analysis with an I² of 54%)
3.2. Analysis.
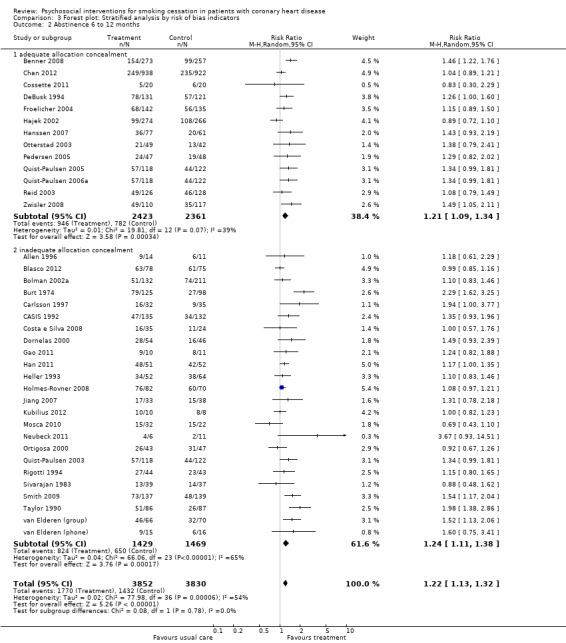
Comparison 3 Forest plot: Stratified analysis by risk of bias indicators, Outcome 2 Abstinence 6 to 12 months.
Trials with an adequate method to analyse incomplete outcome data (ITT) appeared less efficacious compared to trials with inadequate or unclear methods to analyse incomplete outcome data (completer analysis), but this difference was not statistically significant. Trials with completer analysis had a larger effect with a RR of 1.36 (95% CI 1.12 to 1.65, n = 1246, N = 11) (Analysis 3.3). Trials with ITT analysis found a reduced effect of 1.18 (95% CI 1.09 to 1.28, n = 6436, N = 26). Heterogeneity in the subgroup of studies with ITT analysis was slightly reduced (I² 46% compared to the initial analysis with an I² of 54%).
3.3. Analysis.
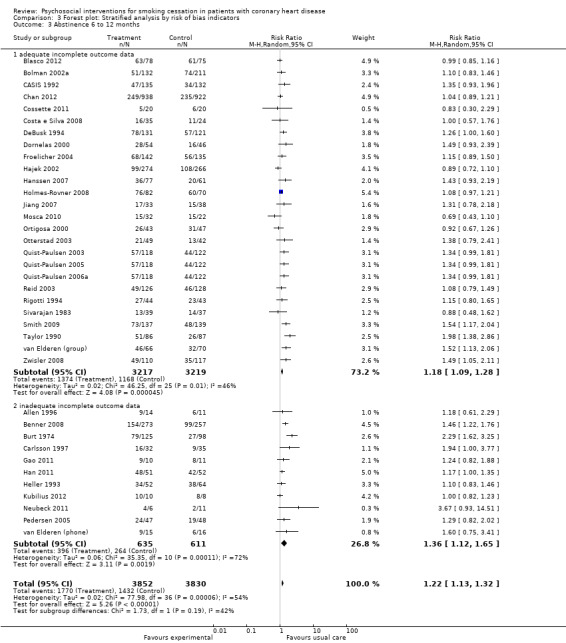
Comparison 3 Forest plot: Stratified analysis by risk of bias indicators, Outcome 3 Abstinence 6 to 12 months.
Trials which validated self‐reported smoking status showed similar efficacy to trials with non validated outcome assessment. Trials with validated abstinence reported a RR of 1.22 (95% CI 1.07 to 1.39, n = 2803, N = 13); non‐validated trials had a similar effect with a RR of 1.23 (95% CI 1.12 to 1.35, n = 4879, N = 24) (Analysis 3.4). Heterogeneity was not reduced.
3.4. Analysis.
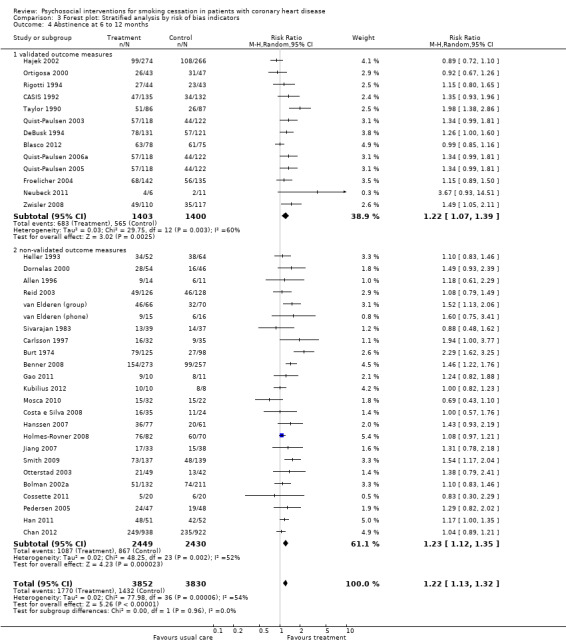
Comparison 3 Forest plot: Stratified analysis by risk of bias indicators, Outcome 4 Abstinence at 6 to 12 months.
Types of intervention We found no clear evidence that any treatment strategy was more efficacious than others, but heterogeneity was reduced slightly within the intervention cluster. Behavioral therapeutic interventions showed a significant effect on abstinence (RR 1.23, 95% CI 1.12 to 1.34, n = 5170, N = 20) with lower heterogeneity (I² 40%) (Analysis 4.1). Telephone support was also effective (RR 1.21, 95% CI 1.12 to 1.30, n = 5807, N = 26) and trials more consistent (I² 44%) (Analysis 4.2). However, as most behavioural therapy trials also used telephone support as an intervention strategy, it is difficult to separate the effects of these two types of interventions. Five trials used solely a behavioural therapeutic approach without additional phone contacts (Bolman 2002a; Otterstad 2003; Sivarajan 1983; van Elderen (group); Zwisler 2008). Nine trials used telephone support without behavioural therapeutic techniques (Benner 2008; Blasco 2012; Gao 2011; Han 2011; Hanssen 2007; Jiang 2007; Neubeck 2011; Ortigosa 2000; Quist‐Paulsen 2005). Interventions using self‐help materials showed comparable effectiveness (RR 1.22, 95% CI 1.12 to 1.33m n = 3789, N = 18) (Analysis 4.3). Stratification of trials using self‐help materials reduced heterogeneity (I² 40%). We also considered the specificity of the intervention (smoking cessation alone compared with a multi‐risk factor intervention). No meaningful difference was found between multi‐risk factor interventions (RR 1.19, 95% CI 1.08 to 1.32, n = 2337, N = 20) and specific cessation intervention (RR 1.26, 95% CI 1.11 to 1.42, n = 5345, N = 17) (Analysis 4.4). Specific intervention showed highly heterogenous effects (I² 61%); likewise multi‐risk factor intervention effects differed substantially between trials (I² 52%). Duration of the intervention We found clear evidence that brief interventions (i.e. no follow‐up contact or within 4 weeks after initial intervention) were not effective (Chan 2012; Hajek 2002; Heller 1993; Ortigosa 2000; Rigotti 1994) (RR 1.01, 95% CI 0.91 to 1.12, I² 0%, n = 2693, N = 5) (Analysis 5.1). When CHD patients were treated with interventions including follow‐up contacts after the initial period of 1 month, the chance of quitting increased substantially (RR 1.28, 95% CI 1.17 to 1.40, I² 58%, n = 4968, N = 31) (Analysis 5.1). One study did not report on the duration of the intervention (Gao 2011). Long‐term follow‐up We found preliminary evidence from seven trials for the efficacy of smoking cessation interventions in the long‐term (number of patients in the intervention group = 382; number of patients with usual care = 359) (see Table 2). Due to high drop‐out rates after 12 months, separate meta‐analyses for completer and ITT effects were done. In the completer analysis smoking cessation interventions were effective (RR 1.16, 95% CI 1.02 to 1.31, n = 741, N = 7) however, this initial finding was not confirmed in the ITT analysis (RR 1.08, 95% CI 0.92 to 1.28, n = 854, N = 5) (Analysis 6.1 and Analysis 6.2, respectively).
4.1. Analysis.
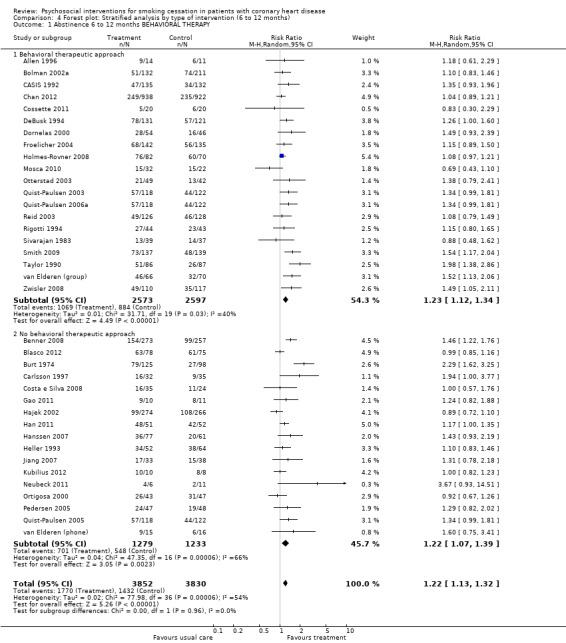
Comparison 4 Forest plot: Stratified analysis by type of intervention (6 to 12 months), Outcome 1 Abstinence 6 to 12 months BEHAVIORAL THERAPY.
4.2. Analysis.
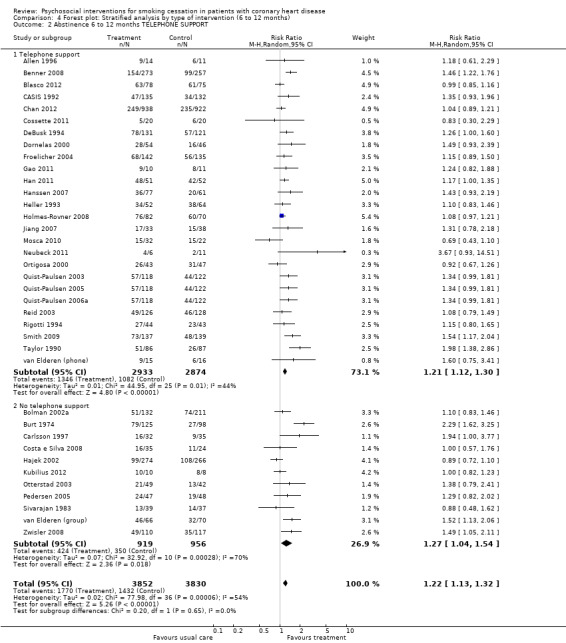
Comparison 4 Forest plot: Stratified analysis by type of intervention (6 to 12 months), Outcome 2 Abstinence 6 to 12 months TELEPHONE SUPPORT.
4.3. Analysis.
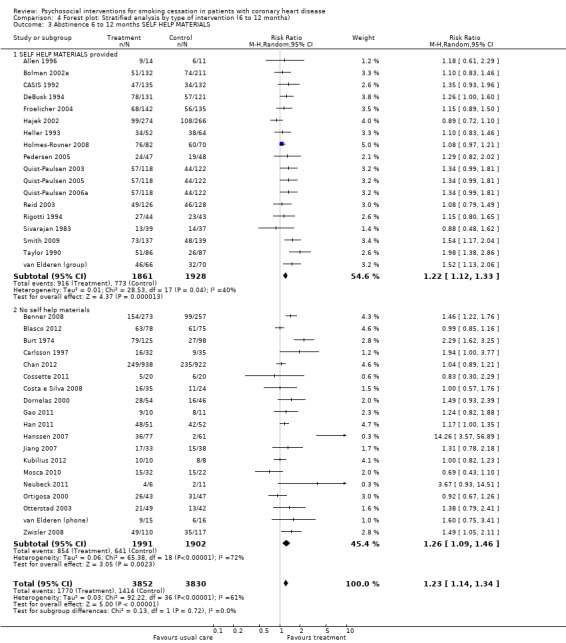
Comparison 4 Forest plot: Stratified analysis by type of intervention (6 to 12 months), Outcome 3 Abstinence 6 to 12 months SELF HELP MATERIALS.
4.4. Analysis.
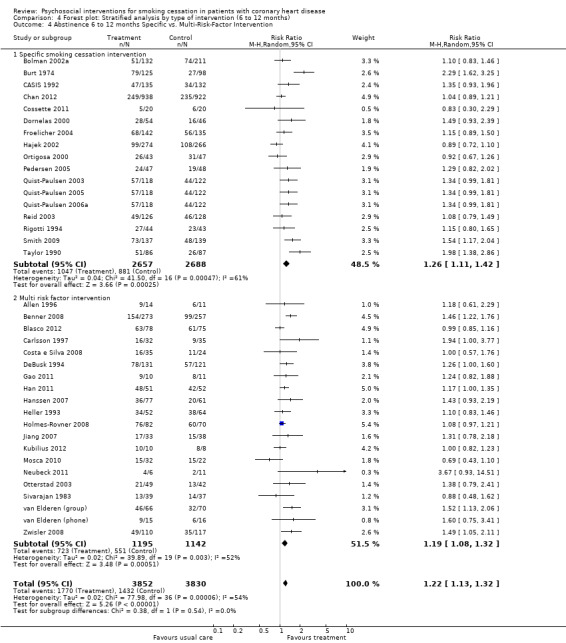
Comparison 4 Forest plot: Stratified analysis by type of intervention (6 to 12 months), Outcome 4 Abstinence 6 to 12 months Specific vs. Multi‐Risk‐Factor Intervention.
5.1. Analysis.
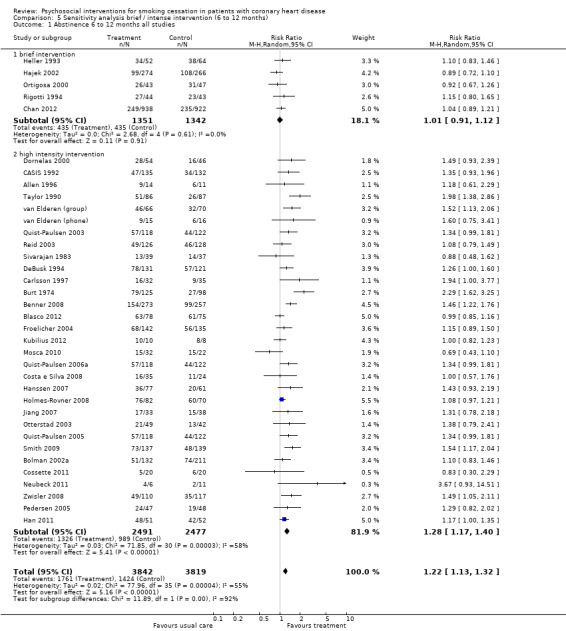
Comparison 5 Sensitivity analysis brief / intense intervention (6 to 12 months), Outcome 1 Abstinence 6 to 12 months all studies.
2. Summary of finding long term.
| Analysis | Studies | Participants | Risk Ratio | 95% Confidence Interval | Heterogeneity I square |
| Overall analysis | |||||
| All studies (completer data) | 7 | 741 | 1.16 | 1.02‐1.31 | 6% |
| Sensitivity analysis | |||||
| Studies with ITT analysis | 5 | 854 | 1.08 | 0.92‐1.28 | 0% |
6.1. Analysis.
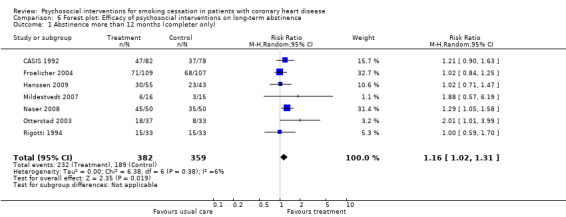
Comparison 6 Forest plot: Efficacy of psychosocial interventions on long‐term abstinence, Outcome 1 Abstinence more than 12 months (completer only).
6.2. Analysis.
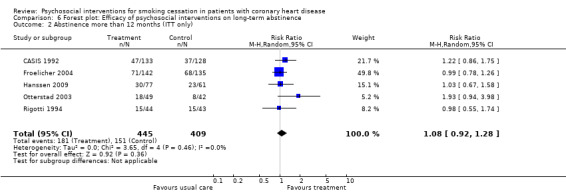
Comparison 6 Forest plot: Efficacy of psychosocial interventions on long‐term abstinence, Outcome 2 Abstinence more than 12 months (ITT only).
Out of seven studies, three reported an adequate method for random sequence generation (Froelicher 2004; Hanssen 2009; Naser 2008) and four had unclear information (CASIS 1992; Mildestvedt 2007; Otterstad 2003; Rigotti 1994). Only three studies reported adequate allocation concealment (Froelicher 2004; Hanssen 2009; Otterstad 2003) and four contained unclear information (CASIS 1992; Mildestvedt 2007; Naser 2008; Rigotti 1994). Blinding was coded as inadequate in all studies, however, blinding of treatment providers is not possible in psychosocial interventions. Concerning incomplete outcome data, five studies reported adequate information (CASIS 1992; Froelicher 2004; Hanssen 2009; Mildestvedt 2007; Naser 2008) and only two were inadequate (Otterstad 2003; Rigotti 1994).
A potential problem with all systematic reviews is publication bias. Our literature search was comprehensive, prepared and partly carried out by the Cochrane Heart Group (UK). Additionally, we investigated publication bias using a funnel plot. The results appear reasonably symmetric which is an indicator of a publication of the studies independent of the study result (Figure 5). There may be a slight tendency for larger trials to show smaller benefits; but larger studies may have interventions with shorter duration and hence smaller effect sizes.
Discussion
Summary of main results
We found support for the efficacy of smoking cessation interventions with more than 1 month duration, but brief interventions of less than 1 month without supporting contact over time were not effective. We were unable to determine the minimum number of contacts needed. There was no evidence for the efficacy in long‐term follow up studies (over 12 months) with high study quality. Only long‐term studies with completer analysis showed a beneficial effect of psychosocial smoking interventions.
Overall completeness and applicability of evidence
Detailed conclusions about effective intervention strategies are obscured by the fact that a mixture of different interventions was included in many trials. Interventions using telephone support, behavioural therapies, and self‐help were all effective. Some interventions focused only on smoking cessation, but others addressed smoking as part of a multiple risk factor intervention programme (generally a 'cardiac rehabilitation programme'). There was no difference in the chance of quitting for multiple risk factor cardiac rehabilitation programmes, compared with interventions focusing on smoking cessation only. 'Cardiac rehabilitation' programmes may vary in their components, but generally include a graded exercise programme and may also include advice and support from a range of health professionals (such as dieticians, behavioural change specialists etc.). It is difficult to distinguish between the effects of the smoking cessation component of these programmes, and the general support and encouragement of a lifestyle change. Some trials employ nicotine replacement therapy (NRT) as additional cessation strategies, which we could not control for. In one trial, more patients in the psychosocial intervention group received NRT compared to the usual care group (DeBusk 1994) which might bias findings. Other trials did not report use of NRT or other medications to assist with quitting such as bupropion.
Agreements and disagreements with other studies or reviews
Our findings confirm the magnitude of the effect of a smoking cessation intervention of about 30% as shown in other studies. However, the effect is much lower compared to an advice from a physician (about 70% increase in quit rates). This difference can be explained by the high quit rates in the control condition. In GP practices among predominantly healthy patients only about 1% of the population quit smoking without any specific intervention. The situation is completely different in patients with an acute or chronic medical condition. In the included studies of this review about two thirds of the studies reported an abstinence rate of more than 30% without any specific intervention and about half of the studies reported an abstinence rate of more than 50% in the control condition. Therefore, the number to treat statistics should be reported from psychosocial smoking cessation studies to allow health policy makers an appropriate evaluation of the effect of the intervention beyond statistical significance.
Interventions on an individual level should be accompanied by policy measures like smoke free legislation. Non‐smoking environment policy interventions were found to be associated with lower rates of myocardial infarction (Lin 2013). Web‐based interventions for smoking cessation have the advantage of good accessibility. Such interventions were also found to improve quit rates substantially in adult populations (Myung 2009; Civljak 2013) and randomised studies in cardiac patients should be done. The older age of CHD patients might limit the feasibility of web‐based interventions due to impaired vision, cognition and physical skills (Becker 2004).
Quality of the evidence
One possible threat to our results might be methodological flaws in the included primary studies which might overestimate the effectiveness of psychosocial smoking interventions in CHD patients. In the stratified analyses according to quality criteria no significant differences were found. However, the pattern showed larger effects in studies with methodological weaknesses. In particular, studies with an ITT analysis showed smaller effects compared to a "completer analysis". RCTs of smoking cessation should not be published without an ITT analysis as the primary outcome. Trial procedures and quality should be described in more detail, according to CONSORT guidelines (Schulz 2010). The overall reporting quality was very poor and the risk of bias was difficult to assess since no information was available in many cases. This leads to conclusions that many trials had a high risk of bias but this may be reflecting the limited quality of reporting rather than the intervention delivery. The validation of smoking status was not a standard procedure in the trials as only 14 out of 37 (38%) described using any measure of biochemical validation. However, there were no differences between trials with validated or self‐reported smoking status in this review. Some studies provide data to apply post hoc ITT analysis in addition to reported completer results. Since we used such an approach the number of trials with ITT data (26 of 37, 70%) is an overestimation of the quality in publications.
Authors' conclusions
Implications for practice.
After a cardiac event about 30% to 50% of smokers with CHD quit without professional help. Additional psychosocial interventions show a superior quitting rate compared to usual care in the short‐term. Long‐term follow‐up showed an attenuation in the benefit of psychosocial interventions for smoking cessation but psychosocial smoking cessation intervention are still a promising strategy. Interventions for smoking cessation in CHD patients should last for more than 1 month. Brief interventions were not effective. The overall effect of psychosocial smoking cessation interventions in CHD patients can be expressed by the number needed to treat statistics with a figure of twelve if a spontanous quitting rate of 30% is assumed. This means that about fifteen patients had to be treated for one person to be abstinent from tobacco after 1 year (NNT = 14.9, CI 11.1 to 24.3). For intense intervention the NNT is somewhat lower (NNT = 11.9, CI 9.6 to 16.7).
Implications for research.
We found that the intensity of psychosocial interventions is of crucial importance for their efficacy. Approaches with very low intensity were not promising. Also the most recent large study (published in 2012) with a brief intervention did not find a significant effect. Future trials should also compare the additional benefits of combining NRT (or other pharmacological interventions such as bupropion) and psychosocial interventions, compared with NRT or psychosocial interventions alone in CHD patients. This would allow a comprehensive evaluation, since psychosocial interventions might increase abstinence per se but might also increase the efficacy of NRT itself by an increase of motivation for treatment (Heckman 2010).
In general more details on the intensity of the intervention (total duration, number of sessions, numbers of pages in leaflets etc) and the underlying theoretical approach are needed. We did not find any difference in the efficacy of different psychosocial approaches. Treatment differences might be blurred by difficulties in classifying studies due to limited reporting of the interventions. However, this result is in line with other studies of non‐pharmacological intervention namely in psychotherapy outcome research, where different interventions strategies showed no differences in their efficacy beyond chance in a various disorders (Wampold 1997), which was also supported in a recent network meta‐analysis on depression (Barth 2013). It is impossible to decide which type of intervention is most promising since direct comparison studies of different interventions are generally unavailable and the intervention itself can often not be classified adequately.
We had not been able to incorporate studies on economic outcomes since studies did not report on that. Any future trials should include such cost‐effectiveness analyses to help decision making for health care professionals also according to this outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 2 July 2015 | New citation required but conclusions have not changed | The addition of 21 trials did not change the short‐term findings. Psychosocial interventions for smoking cessation might also be effective in long‐term, but evidence is weak. |
| 24 October 2013 | New search has been performed | Updated search in January 2013. |
History
Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format. |
| 3 October 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
Used abbreviations AMI: acute myocardial infarction AP: angina pectoris
BT: behavioral therapeutic intervention BIOSIS: database on life science and biomedical research (www.biosis.org) CABG: coronary artery bypass graft CHD: coronary heart disease CI: confidence interval
CONSORT: Consolidated Standards of Reporting Trials EMBASE: database with biomedical and pharmacological information (www.embase.com) ICD: International Classification of Diseases ITT: intention to treat analysis
I²: I square, heterogeneity from 0 to 100. Medline: database of the U.S. National Library of Medicine MI: myocardial infarction MeSH; medical subject heading
MR: multi‐risk factor intervention N: number of studies n: number of patients
NRT: nicotine replacement therapy OR: odds ratio
Ph: support by phone PsycINFO: database of the American Psychological Association PSYNDEX: database of the Center for Psychological Information and Documentation at the University of Trier, Germany PTCA: percutaneous transluminal coronary angioplasty
RR: relative risk
SH: self‐help intervention
UK: United Kingdom
Acknowledgements
We would like to thank for the support of the Cochrane Heart Group, especially Fiona Taylor, Theresa Moore, Margaret Burke and Shah Ebrahim for comments on the protocol of our review and assistance in literature search. Many thanks to Corina Güthlin for her assistance in extracting the data of the initial review. Thanks to Jürgen Bengel for his contribution on the first version of this review. Thanks also go to Anna Hirsbrunner and Petra Büchler for support in managing references and checking accuracy of data entry, as well as to Jingying Wang for the data extraction of a Chinese study.
Appendices
Appendix 1. Search strategy 2013
CENTRAL
#1 MeSH descriptor Heart Diseases explode all trees #2 MeSH descriptor Coronary Artery Bypass explode all trees #3 angina* #4 cabg #5 coronary near bypass* #6 (coronary near disease*) or chd #7 (myocard* near infarct*) or (heart near infarct*) #8 (cardiac next disease*) or (heart next disease*) #9 acs #10 ami #11 cardiac near/2 inpatient* #12 cardiac near/2 in‐patient* #13 cardiac near/2 patient* #14 heart near/2 patient* #15 heart near/2 inpatient* #16 heart near/2 in‐patient* #17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 MeSH descriptor Smoking Cessation, this term only #19 smok* near cessation #20 smok* near cease* #21 smok* near quit* #22 antismoking #23 anti‐smoking #24 smok* near giv* #25 smok* near stop* #26 (#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) #27 (#17 AND #26)
MEDLINE OVID
1. exp Heart Diseases/ 2. exp Coronary Artery Bypass/ 3. angina*.tw. 4. cabg.tw. 5. (coronary adj6 bypass*).tw. 6. (coronary adj6 disease*).tw. 7. (myocard* adj6 infarct*).tw. 8. (heart adj6 infarct*).tw. 9. chd.tw. 10. (heart adj disease*).tw. 11. (cardiac adj disease*).tw. 12. acs.tw. 13. ami.tw. 14. (cardiac adj2 inpatient*).tw. 15. (cardiac adj2 in‐patient*).tw. 16. (cardiac adj2 patient*).tw. 17. (heart adj2 patient*).tw. 18. (heart adj2 inpatient*).tw. 19. (heart adj2 in‐patient*).tw. 20. or/1‐19 21. Smoking Cessation/ 22. (smok* adj6 cessation).tw. 23. (smok* adj6 cease*).tw. 24. (smok* adj6 quit*).tw. 25. antismoking.tw. 26. anti‐smoking.tw. 27. (smok* adj6 giv*).tw. 28. (smok* adj6 stop*).tw. 29. or/21‐28 30. 20 and 29 31. randomized controlled trial.pt. 32. controlled clinical trial.pt. 33. randomized.ab. 34. placebo.ab. 35. drug therapy.fs. 36. randomly.ab. 37. trial.ab. 38. groups.ab. 39. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 40. exp animals/ not humans.sh. 41. 39 not 40 42. 30 and 41 43. (20* not (2000* or 2001* or 2002*)).ed. 4. 42 and 43
EMBASE OVID
1. heart disease/ 2. coronary artery bypass graft/ 3. angina*.tw. 4. cabg.tw. 5. (coronary adj6 bypass*).tw. 6. (coronary adj6 disease*).tw. 7. (myocard* adj6 infarct*).tw. 8. (heart adj6 infarct*).tw. 9. chd.tw. 10. (heart adj disease*).tw. 11. (cardiac adj disease*).tw. 12. acs.tw. 13. ami.tw. 14. (cardiac adj2 inpatient*).tw. 15. (cardiac adj2 patient*).tw. 16. (heart adj2 patient*).tw. 17. (heart adj2 inpatient*).tw. 18. (heart adj2 in‐patient*).tw. 19. (cardiac adj2 in‐patient*).tw. 20. or/1‐19 21. smoking cessation/ 22. (smok* adj6 cessation).tw. 23. (smok* adj6 cease*).tw. 24. (smok* adj6 quit*).tw. 25. antismoking.tw. 26. anti‐smoking.tw. 27. (smok* adj6 giv*).tw. 28. (smok* adj6 stop*).tw. 29. or/21‐28 30. 20 and 29 31. random$.tw. 32. factorial$.tw. 33. crossover$.tw. 34. cross over$.tw. 35. cross‐over$.tw. 36. placebo$.tw. 37. (doubl$ adj blind$).tw. 38. (singl$ adj blind$).tw. 39. assign$.tw. 40. allocat$.tw. 41. volunteer$.tw. 42. crossover procedure/ 43. double blind procedure/ 44. randomized controlled trial/ 45. single blind procedure/ 46. 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 47. (animal/ or nonhuman/) not human/ 48. 46 not 47 49. 30 and 48 50. (20* not (2000* or 2001* or 2002*)).em. 51. 49 and 50
PsycINFO
1. exp heart disorders/ 2. angina*.tw. 3. cabg.tw. 4. (coronary adj6 bypass*).tw. 5. (coronary adj6 disease*).tw. 6. (myocard* adj6 infarct*).tw. 7. (heart adj6 infarct*).tw. 8. chd.tw. 9. (heart adj disease*).tw. 10. (cardiac adj disease*).tw. 11. acs.tw. 12. ami.tw. 13. (cardiac adj2 inpatient*).tw. 14. (cardiac adj2 in‐patient*).tw. 15. (cardiac adj2 patient*).tw. 16. (heart adj2 patient*).tw. 17. (heart adj2 inpatient*).tw. 18. (heart adj2 in‐patient*).tw. 19. or/1‐18 20. smoking cessation/ 21. (smok* adj6 cessation).tw. 22. (smok* adj6 cease*).tw. 23. (smok* adj6 quit*).tw. 24. antismoking.tw. 25. anti‐smoking.tw. 26. (smok* adj6 giv*).tw. 27. (smok* adj6 stop*).tw. 28. or/20‐27 29. 19 and 28 30. random$.tw. 31. factorial$.tw. 32. crossover$.tw. 33. cross‐over$.tw. 34. placebo$.tw. 35. (doubl$ adj blind$).tw. 36. (singl$ adj blind$).tw. 37. assign$.tw. 38. allocat$.tw. 39. volunteer$.tw. 40. control*.tw. 41. "2000".md. 42. or/30‐41 43. 29 and 42 44. (20* not (2000* or 2001* or 2002*)).up. 45. 43 and 44
Conference Proceedings Citation Index‐ Science (CPCI‐S) on Web of Science
#29 #28 AND #27 #28 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) #27 #26 AND #18 #26 #19 or #20 or #21 or #22 or #23 or #24 or #25 #25 TS=(smok* SAME stop*) #24 TS=(smok* SAME giv*) #23 TS=anti‐smoking #22 TS=antismoking #21 TS=(smok* SAME quit*) #20 TS=(smok* SAME cease*) #19 TS=(smok* SAME cessation) #18 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #17 TS=(heart SAME in‐patient*) #16 TS=(heart SAME inpatient*) #15 TS=(heart SAME patient*) #14 TS= (cardiac SAME patient*) #13 TS=(cardiac SAME in‐patient*) #12 TS=(cardiac SAME inpatient*) #11 TS=ami #10 TS=acs #9 TS="heart disease*" #8 TS="cardiac disease*" #7 TS=chd #6 TS=(heart SAME infarct*) #5 TS=(myocard* SAME infarct*) #4 TS=(coronary SAME disease*) #3 TS=(coronary SAME bypass*) #2 TS=cabg #1 TS=angina*
Appendix 2. Search strategy 2003
CENTRAL
#1 HEART DISEASES (exp MeSH) #2 CORONARY ARTERY BYPASS (exp MeSH) #3 angina* #4 cabg #5 (coronary near bypass*) #6 (coronary near disease*) #7 MYOCARDIAL INFARCTION (exp MeSH) #8 (myocard* near infarct*) #9 (heart near infarct*) #10 chd #11 (heart next disease*) #12 (cardiac next disease*) #13 acs #14 ami #15 (cardiac next inpatient*) #16 (cardiac next patient*) #17 (heart next patient*) #18 (heart next inpatient*) #19 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #20 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18) #21 (#19 or #20) #22 SMOKING CESSATION (exp MeSH) #23 (smoking near cessation) #24 (smoking near cease*) #25 (smoking near quit*) #26 antismoking #27 (anti next smoking) #28 (smoking near giv*) #29 (smoking near stop*) #30 (#22 or #23 or #24 or #25 or #26 or #27 or #28 or #29) #31 (#21 and #30)
MEDLINE, Pre‐MEDLINE, BIOSIS and Journals@Ovid
#1 (HEART DISEASES ) in KW,MESH,PS #2 (coronary artery bypass) in KW,MESH,PS #3 angina* #4 cabg #5 coronary near bypass #6 coronary near disease #7 (myocardial infarction) in KW,MESH,PS #8 myocard* near infarct* #9 heart near infarct* #10 chd #11 heart next disease* #12 acs #13 ami #14 cardiac next inpatient* #15 cardiac next patient* #16 heart next patient* #17 heart next inpatient* #18 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 (smoking cessation) in KW,MESH,PS #20 smoking near cease* #21 smoking near cessation #22 smoking near quit #23 antismoking #24 anti next smoking #25 smoking near giv* #26 smoking near stop* #27 #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 #28 #18 and #27
EMBASE
#1 HEART DISEASES (exp MeSH) #2 CORONARY ARTERY BYPASS (exp MeSH) #3 angina* #4 cabg #5 (coronary near bypass*) #6 (coronary near disease*) #7 MYOCARDIAL INFARCTION (exp MeSH) #8 (myocard* near infarct*) #9 (heart near infarct*) #10 chd #11 (heart next disease*) #12 (cardiac next disease*) #13 acs #14 ami #15 (cardiac next inpatient*) #16 (cardiac next patient*) #17 (heart next patient*) #18 (heart next inpatient*) #19 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #20 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18) #21 (#19 or #20) #22 SMOKING CESSATION (exp MeSH) #23 (smoking near cessation) #24 (smoking near cease*) #25 (smoking near quit*) #26 antismoking #27 (anti next smoking) #28 (smoking near giv*) #29 (smoking near stop*) #30 (#22 or #23 or #24 or #25 or #26 or #27 or #28 or #29) #31 (#21 and #30)
PsycINFO
#1 TI coronary artery bypass Or AB coronary artery bypass Or MJ coronary artery bypass #2 TI angina Or AB angina OrMJ angina #3 TI cabg Or AB cabg Or MJ cabg #4 TI coronary Or AB coronary Or MJ coronary #5 TI bypass Or AB bypass Or MJ bypass #6 TI myocard Or AB myocard Or MJ myocard #7 TI myocard Or AB myocard Or MJ myocard #8 TI diseas* Or AB diseas*Or MJ diseas* #9 TI heart* Or AB heart* Or MJ heart* #10 TI chd Or AB chd OrMJ chd #11 TI acs Or AB acs Or MJ acs #12 TI ami Or AB ami Or MJ ami #13 TI cardiac Or AB cardiac Or MJ cardiac #14 TI patient* Or AB patient* OrMJ patient* #15 TI inpatient* Or AB inpatient* Or MJ inpatient* #16 TI smok* Or AB smok* Or MJ smok* #17 TI cessation Or AB cessation Or MJ cessation #18 TI cease* Or AB cease* Or MJ cease* #19 TI quit Or AB quit Or MJ quit* #20 TI anti Or AB anti Or MJ anti #21 TI giv* Or AB giv* Or MJ giv* #22 TI stop* Or AB stop* OrMJ stop* #23 (S5 And S4) #24 (S8And S4) #25 (S7 And S6) #26 (S9And S7 #27 (S14 And S13) #28 (S15 And S13) #29 (S14 And S9) #30 (S15 And S9) #31 (S30 Or S29 Or S28 Or S27 Or S26 Or S25 Or S24 Or S23 Or S15 Or S14 Or S13 Or S12 Or S11 Or S10 Or S9 Or S8 Or S7 Or S6 Or S5 Or S4 Or S3 Or S2 Or S1) #32 (S17 And S16) #33 (S18 And S16) #34 (S19 And S16) #35 (S20 And S16) #36 (S21 And S16) #37 (S22 And S16) #38 (S37 Or S36 Or S35 Or S34 Or S33 Or S32 Or S22 Or S21 Or S20 Or S19 Or S18 Or S17 Or S16) #39 (S37 Or S36 Or S35 Or S34 Or S33 Or S32) #40 (S13 Or S12 Or S11 Or S10 Or S9 Or S8 Or S7 Or S6 Or S5 Or S4 Or S3 Or S2 Or S1) #41 (S39 and S40)
PSYNDEXplus
#1 HEART DISEASES #2 CORONARY ARTERY BYPASS #3 angina* #5 coronary near bypass* #6 coronary near disease* #7 MYOCARDIAL INFARCTION #8 myocard* near infarct* #9 heart near infarct* #10 chd #11 heart next disease* #12 cardiac next disease* #13 acs #14 ami #15 cardiac next inpatient* #16 cardiac next patient* #17 heart next patient* #18 heart next inpatient* #19 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 SMOKING CESSATION #21 smoking near cessation #22 smoking near cease* #23 smoking near quit* #24 antismoking #25 anti next smoking #26 smoking near giv* #27 smoking near stop* #28 #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 #29 herz #30 herzinfarkt #31 kardiovaskulaer* #32 KHK #33 myokard* #34 koronar* #35 bypass #36 #29 or #30 or #31 or #32 or #33 or #34 or #35 #37 raucherentwoehnung #38 tabakabstinenz #39 tabak near abstinenz #4 cabg #40 rauchen near abstinenz #41 rauchen near aufhoeren #42 #37 or #38 or #39 or #40 or #41 #43 #19 or #36 #44 #28 or #42 #45 #43 and #44
Data and analyses
Comparison 1. Initial analysis: Efficacy of psychosocial interventions on abstinence all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence 6 to 12 months (ITT preferred over completer) | 40 | 7928 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.14, 1.35] |
Comparison 2. Efficacy of psychosocial interventions on abstinence without outliers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence 6 to 12 months (ITT preferred over completer) | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
Comparison 3. Forest plot: Stratified analysis by risk of bias indicators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence 6 to 12 months | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 1.1 adequate sequence generation | 18 | 5046 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.07, 1.36] |
| 1.2 inadequate sequence generation | 19 | 2636 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.12, 1.37] |
| 2 Abstinence 6 to 12 months | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 2.1 adequate allocation concealment | 13 | 4784 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.09, 1.34] |
| 2.2 inadequate allocation concealment | 24 | 2898 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.11, 1.38] |
| 3 Abstinence 6 to 12 months | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 3.1 adequate incomplete outcome data | 26 | 6436 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.09, 1.28] |
| 3.2 inadequate incomplete outcome data | 11 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.12, 1.65] |
| 4 Abstinence at 6 to 12 months | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 4.1 validated outcome measures | 13 | 2803 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.39] |
| 4.2 non‐validated outcome measures | 24 | 4879 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.12, 1.35] |
Comparison 4. Forest plot: Stratified analysis by type of intervention (6 to 12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence 6 to 12 months BEHAVIORAL THERAPY | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 1.1 Behavioral therapeutic approach | 20 | 5170 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.12, 1.34] |
| 1.2 No behavioral therapeutic approach | 17 | 2512 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.39] |
| 2 Abstinence 6 to 12 months TELEPHONE SUPPORT | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 2.1 Telephone support | 26 | 5807 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.12, 1.30] |
| 2.2 No telephone support | 11 | 1875 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.04, 1.54] |
| 3 Abstinence 6 to 12 months SELF HELP MATERIALS | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.14, 1.34] |
| 3.1 SELF HELP MATERIALS provided | 18 | 3789 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.12, 1.33] |
| 3.2 No self help materials | 19 | 3893 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.09, 1.46] |
| 4 Abstinence 6 to 12 months Specific vs. Multi‐Risk‐Factor Intervention | 37 | 7682 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 4.1 Specific smoking cessation intervention | 17 | 5345 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.11, 1.42] |
| 4.2 Multi risk factor intervention | 20 | 2337 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.08, 1.32] |
Comparison 5. Sensitivity analysis brief / intense intervention (6 to 12 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence 6 to 12 months all studies | 36 | 7661 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 1.1 brief intervention | 5 | 2693 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.12] |
| 1.2 high intensity intervention | 31 | 4968 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.17, 1.40] |
Comparison 6. Forest plot: Efficacy of psychosocial interventions on long‐term abstinence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abstinence more than 12 months (completer only) | 7 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.02, 1.31] |
| 2 Abstinence more than 12 months (ITT only) | 5 | 854 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.92, 1.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 1996.
| Methods | Completer Analysis | |
| Participants | 138 women who underwent first‐time CABG at a hospital. IG: 14 smokers CG: 11 smokers | |
| Interventions | Usual care: patient education and instructions for exercise. Psychosocial intervention: nurse‐directed multimodal behavioural program based on social cognitive theory (videotape, workbook, counselling). Started with discharge from hospital, two updates 1 and 2 months later (BT, Ph, SH, MR, Intensity 4) | |
| Outcomes | Follow‐up at 12 months (abstinence self report) | |
| Notes | IG: Completer = 64.3% CG: Completer = 54.5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized schema that achieved a balanced allocation |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Benner 2008.
| Methods | Completer Analysis | |
| Participants | patients with an increased cardiovascular risk, not necessarily CHD in all cases. IG: 273 smokers CG: 257 smokers |
|
| Interventions | Usual care: predicted Framingham 10‐year risk of CHD was calculated but was not communicated to either the physician or the patient until the final visit Psychosocial intervention: patients were advised according to a CHD risk evaluation and communication programme. They received a Heart Health Report and had three follow‐up phone calls by a physician or study nurse and the patients completed a "Knowledge, Attitudes and Behavior" (KAB) questionnaire. (Ph, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 6 month (abstinence self‐report) | |
| Notes | IG: Completer = 56.4% CG: Completer = 38.5% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based algorithm |
| Allocation concealment (selection bias) | Low risk | Random permutation of 100 numbers |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Blasco 2012.
| Methods | ITT | |
| Participants | 203 acute coronary syndrome patients IG: 78 smokers CG: 75 smokers |
|
| Interventions | Usual care: written recommendations and verbal information about CVD prevention only Psychosocial intervention: written recommendations and verbal information about CVD prevention. Patients received an automatic sphygmomanometer, a glucose and lipid meter and a cellular phone. Results were sent through their mobile phones and a cardiologist then sent individualised short messages with recommendations to the patients during the 12‐month follow‐up period. (Ph, MR, Intensity 5) |
|
| Outcomes | Follow‐up at 12 month (validation by 1‐step cotinine immunoassay in urine) | |
| Notes | IG: ITT = 80.8% CG: ITT = 81.3% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two different randomisation lists |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Bolman 2002a.
| Methods | Completer Analysis, ITT | |
| Participants | Hospitalised smokers with multiple coronary disorders IG: 132 smokers CG: 211 smokers |
|
| Interventions | Usual care: no systematic attention was given to smoking Psychosocial intervention: C‐Mis which consisted of stop‐smoking advice by the cardiologist followed by 15‐30min of standardised individual counselling and the provision of self‐help material by the ward nurse and aftercare by the cardiologist. After that more in‐depth counselling, which was attuned to the patient's stage of change. (BT, SH, Specific, Intensity 4) |
|
| Outcomes | Follow‐up at 3 and 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 58.0% ITT = 38.6% CG: Completer = 45.4% ITT = 77.3% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The whole hospital was randomised with significant difference between the two groups at the process evaluation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Burt 1974.
| Methods | Completer Analysis | |
| Participants | 280 men in a coronary care unit with AMI IG: 125 smokers CG: 98 smokers | |
| Interventions | Usual care: conventional advice to stop smoking by physician. Psychosocial intervention: information about the effects of smoking by physician and nurse, reinforced by a booklet about coronary‐risk factors. Continued in follow‐up clinic and through a community nurse. (Specific, Intensity 5) | |
| Outcomes | Follow‐up at 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 63.2% CG: Completer = 27.5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation according to the day of admission |
| Allocation concealment (selection bias) | High risk | The group allocation can be foreseen from the day of admission |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Carlsson 1997.
| Methods | Completer | |
| Participants | 168 patients with AMI admitted to the coronary care unit at Malmö General Hospital IG: 32 smokers CG: 35 smokers | |
| Interventions | Usual care: two visits to general practitioner. Psychosocial intervention: nurse‐directed secondary prevention unit after the usual follow‐up schedule: education and counselling (individual and group sessions) about smoking, exercise, nutrition for about 9 hours and exercise training 2‐3 times per week. Visits to cardiologist after 2, 3, 6 months and to nurse after 3, 5, 6, 9, 12 months. (MR, Intensity 5) | |
| Outcomes | Follow‐up at 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 50% CG: Completer = 25.7% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
CASIS 1992.
| Methods | Completer, ITT | |
| Participants | 267 smokers with CAD of 3 hospitals who scheduled for coronary arteriography IG: 135 smokers CG: 132 smokers | |
| Interventions | Usual care: brief advice from physician to stop smoking. Psychosocial intervention: intervention provided by trained behaviorally oriented health educators: inpatient counselling session (30 min), outpatient counselling visits and telephone calls (at 1and 3 weeks, abstinent smokers at 3 months, relapsed smokers at 2 and 4 months), outpatient group program, self‐help materials. (BT, Ph, SH, Specific, Intensity 4) | |
| Outcomes | Follow‐up at 6, 12 and 60 months (validation by saliva samples) | |
| Notes | IG: Completer = 57.3% ITT = 34.8% CG: Completer = 47.4% ITT = 28% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Chan 2012.
| Methods | ITT | |
| Participants | 1860 Chinese cigarette smokers who attended the cardiac out‐patient clinic of 10 major hospitals in Hong Kong for routine follow‐up visits. More than 50% of the patients suffered from CHD. IG: 938 smokers CG: 922 smokers |
|
| Interventions | Usual care: a 15‐minute face‐to‐face counselling on healthy diet by the nurse counsellor at baseline visit and a one‐page A4‐sized leaflet which highlighted the importance of a healthy diet for cardiac patients. No telephone counselling. Psychosocial intervention: 30‐minute individualised face‐to‐face smoking cessation counselling matched with their stage of readiness to quit at baseline visit. The individualised stage‐matched smoking cessation counselling was developed based on the Transtheoretical Model of Change. They also received telephone calls from the nurse counsellor at 1 week and 1 month after the baseline visit. (BT, Ph, Specific, Intenstity 4) |
|
| Outcomes | Follow up at 3, 6 and 12 months (abstinence self‐report) | |
| Notes | IG: ITT = 26.5% CG: ITT = 25.4% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple random sampling procedure (without replacement) using MS Excel |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed and opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Cossette 2011.
| Methods | Completer Analysis, ITT | |
| Participants | Hospitalised smokers in a centre for cardiovascular care (CHA) with multiple coronary disorders. IG: 20 smokers CG: 20 smokers |
|
| Interventions | Usual care: phone support via "J'arrête" or CAT (= Centre d'abandon du tabagisme = Center for smoking cessation) Psychosocial intervention: Intervention by ISCT (= infirmière spécialisée en cessation tabagique = specialised nurse for smoking cessation). Information about why it is important to stop smoking according to the Stages of Change model from Prochaska and DiClimente. Motivational letters were sent to the patients until 6 months after hospitalisation. (BT, Ph, Specific, Intensity 4) |
|
| Outcomes | Follow‐up at 6 months (abstinence self‐report) | |
| Notes | IG: Completer = 38.5% ITT = 25% CG: Completer = 60% ITT = 30% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Preparation of random list by independent coordinating center |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed and opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Costa e Silva 2008.
| Methods | ITT | |
| Participants | 153 patients that were hospitalised because of their first acute myocardial infarction IG: 35 smokers CG: 24 smokers |
|
| Interventions | Usual care: conventional outpatient clinic for hear care at the Institute of Cardiology/University Foundation of Cardiology, where the patiens were seen only by the appointed cardiologist Psychosocial intervention: transciplinary care was provided at the outpatient clinic for secondary prevention of CAD by a cardiologist, endocrinologist, nurse and dietitian (MR, Intensity 4) |
|
| Outcomes | Follow‐up at 3 and 6 months (abstinence self‐report) | |
| Notes | IG: ITT = 45.7% CG: ITT = 45.8% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation Software (Random, PEPI 4.0) |
| Allocation concealment (selection bias) | High risk | "The patients and health professionals involved in outpatient treatment were not blinded as to their allocation." (Costa e Silva 2008, page 490) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
DeBusk 1994.
| Methods | Completer Analysis | |
| Participants | 585 patients with AMI in five medical centres IG: 131 smokers CG: 120 smokers | |
| Interventions | Usual care: counselling on dietary change, lipid lowering therapy, and on demand smoking cessation interventions. Psychosocial intervention: physician‐directed, nurse‐managed, home‐based case‐management system (behavioural intervention, telephone and mail contact) in addition to the usual care. Started in the hospital and finished 12 months later. (BT, Ph, SH, MR, Intensity 5) | |
| Outcomes | Follow‐up at 6 and 12 months (abstinence validated) | |
| Notes | IG: Completer = 70.2% ITT = 59.5% CG: Completer = 53.3% ITT = 47.1% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program that achieved a balanced allocation |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Dornelas 2000.
| Methods | Completer Analysis, ITT | |
| Participants | 100 smokers with AMI admitted to hospital IG: 54 smokers CG: 46 smokers | |
| Interventions | Usual care: verbal and written recommendation to watch education video of AHA. Psychosocial intervention: bedside cessation counselling (motivation for cessation, relapse prevention) delivered by a psychologist based on the transtheoretical model. Seven telephone calls at 1, 4, 8, 12, 16, 20, 26 weeks (BT, Ph, Specific, Intensity 4) | |
| Outcomes | Follow‐up at 6 and 12 months (validation by a significant other) | |
| Notes | IG: Completer = 70% ITT = 51.8% CG: Completer = 40% ITT = 34.8% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing from random numbers |
| Allocation concealment (selection bias) | High risk | Random assignment by drawing from random numbers from an envelope, but no information if sequentially numbered, opaque or sealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Feeney 2001.
| Methods | Completer Analysis, ITT | |
| Participants | 198 patients sequentially admitted to the coronary care unit (CCU) all having suffered from AMI. IG: 96 smokers CG: 102 smokers |
|
| Interventions | Usual care: Verbal and printed advice about tobacco cessation (primarily didactic). Patients watched an educational video during their stay and were reviewed by a nurse. Outpatients supportive counselling and follow‐up at 3, 6 and 12 months. All patients were advised by the attending cardiologist to stop smoking. Psychosocial intervention: Standford Heart Attack Staying Free programme: behavioral components, 2 audiotapes for home use with the programme's principal points. On hospital discharge the patients received telephone contact weekly for 4 weeks and at 2, 3, 6 and 12 months including inquiries about relapse. Additional support and advice were given if considered necessary. (BT, Ph, SH, specific, Intensity 5) |
|
| Outcomes | Follow‐up at 1, 3 and 12 months (validated) | |
| Notes | IG: Completer = 66% ITT = 32.3% CG: Completer = 4.8% ITT = 0.9% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list of odd and even numbers |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Froelicher 2004.
| Methods | Completer Analysis, ITT | |
| Participants | 277 women hospitalised with a diagnosis of CVD or peripheral vascular disease IG: 142 smokers CG: 135 smokers |
|
| Interventions | Usual care: brief counselling from a physician regarding the need for and benefits of smoking cessation, a copy of the pamphlet "Calling it quits" and a list of local smoking cessation classes and programs in the communities Psychosocial intervention: nurse‐managed smoking cessation and relapse prevention intervention. Usual care plus 30 to 45min individualised counselling session with multimedia aids that study participants were given before their discharge. During the session: videotape from the American Heart Association (AHA) together with a videotape on smoking cessation. After discharge up to 5 structured telephone calls to continue the intervention (BT, Ph, SH, Specific, Intensity 4) |
|
| Outcomes | Follow‐up at 6, 12, 24 and 30 months (validation by saliva sample) | |
| Notes | IG: Completer = 56.2% ITT = 47.9% Long‐term IG: Completer = 65.1% ITT = 50% CG: Completer = 44.8% ITT = 41.5% Long‐term CG: Completer = 63.6% ITT = 50.4% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutated blocks |
| Allocation concealment (selection bias) | Low risk | Equal chance of assignment to CG or IG |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Gao 2011.
| Methods | Completer Analysis | |
| Participants | A consecutive series of patients who accepted the PCI were recruited from a metropolitan hospital in Taiyuan IG: 10 smokers CG: 11 smokers |
|
| Interventions | Usual care: ordinary health education including discharge guidance and distribution of health education materials Psychosocial intervention: comprehensive health educating program including centralised training and telephone follow‐up. Comprehensive health education consisted of basic medical knowledge about CHD including secondary prevention of post‐PCI, information about healthy diet, correct pos‐operation rehabilitation exercise and doctors and nurses tutored patients on how to quit smoking and refrain from drinking (Ph, MR, Intensity 9) |
|
| Outcomes | Follow‐up at 6 and 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 90% CG: Completer = 72.7% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Hajek 2002.
| Methods | Completer Analysis, ITT | |
| Participants | 540 patients in hospitals in UK with MI and CABG IG: 274 CG: 266 | |
| Interventions | Usual care: verbal advice to stop smoking and booklet "Smoking and your heart". Psychosocial intervention: in 17 hospitals patients motivated to stop smoking received intervention. Education with booklet (Smoking and your Heart) from the British Heart Foundation. Short quiz on contents of the booklet; support of other cardiac patient is arranged by nurse Duration about 34min (SH, Specific, Intensity 1) | |
| Outcomes | Follow‐up at 6 weeks and 12 months (validated) | |
| Notes | IG: Completer = 39% ITT = 36% CG: Completer = 43% ITT = 41% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In batches of 20 with envelopes provided to each nurse |
| Allocation concealment (selection bias) | Low risk | Serially numbered, opaque and sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Han 2011.
| Methods | Completer Analysis | |
| Participants | Patients with acute coronary syndrome and PCI IG: 51 smokers CG: 56 smokers |
|
| Interventions | Usual care: only received 2 follow‐ups Psychosocial intervention: exercise, body weight measurement, how to relax. For smokers: follow‐up, time since last cigarette (Ph, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 6 and 12 months (not validated) | |
| Notes | IG: Completer = 94.1% CG: Completer = 80.8% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Hanssen 2007.
| Methods | Completer Analysis, ITT | |
| Participants | 413 patients with AMI admitted to the Department of Heart Disease IG: 77 smokers CG: 61 smokers |
|
| Interventions | Usual care: one visit to a physician at the outpatient clinic 6‐8 weeks after discharge and subsequent visits to the patient's general practitioner Psychosocial intervention: telephone follow‐up and an open telephone line providing patients with information, education and support on the basis of individual needs. Also provided patients with information about what are common questions after AMI and encourage further elaboration on the issues if desired (Ph, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 3 and 6 months (abstinence self‐report) | |
| Notes | IG: Completer = 60% ITT = 46.8% CG: Completer = 40.8% ITT = 32.8% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Hanssen 2009.
| Methods | Completer Analysis, ITT | |
| Participants | 413 patients with AMI admitted to the Department of Heart Disease IG: 77 smokers CG: 61 smokers |
|
| Interventions | Usual care: one visit to a physician at the outpatient clinic 6‐8 weeks after discharge and subsequent visits to the patient's general practitioner Psychosocial intervention: telephone follow‐up and an open telephone line providing patients with information, education and support on the basis of individual needs. Also provided patients with information about what are common questions after AMI and encourage further elaboration on the issues if desired (Ph, MR, Intensity 5) |
|
| Outcomes | Follow‐up at 12 and 18 months (abstinence self‐report) | |
| Notes | IG: Completer = 54.6% ITT = 39.0% CG: Completer = 53.5% ITT = 37.7% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Heller 1993.
| Methods | Completer Analysis | |
| Participants | 450 patients with AMI discharged from the hospital IG: 66 smokers CG: 73 smokers | |
| Interventions | Usual care: no specific information given Psychosocial intervention: letter to the subjects' GP, three mail‐out packages for the subjects (information about nutrition, smoking, walking programme) and monthly newsletters. (SH, MR, Intensity 1) | |
| Outcomes | Follow‐up at 6 months (abstinence self‐report) | |
| Notes | IG: Completer = 65% CG: Completer = 53% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation to an intervention or usual care group according to the name of the usual general practitioner |
| Allocation concealment (selection bias) | High risk | The group allocation can be foreseen by the name of the usual general practitioner |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Holmes‐Rovner 2008.
| Methods | ITT | |
| Participants | 719 participants with a diagnosis of ACS and a documented serum troponin I level greater than the upper limits of normal were recruited from hospitals that participated in the American College of Cardiology Guidlines Applied to Practice (GAP) QI program IG: 82 smokers CG: 70 smokers |
|
| Interventions | Usual care: GAP QI‐only: written discharge contract listing recommended outpatient medications, cardiac rehabilitation recommendations and health behaviour changes, as well as numerical values for ejection fraction and cholesterol Psychosocial intervention: HARP QI‐Plus telephone coaching: Six‐session health behaviour change telephone counselling program during the first three months after discharge. Primary behaviour goals included: reduction or elimination of smoking, increasing physical activity and eating a healthier diet. Behaviour change strategies included behavioral staging, motivational interviewing, goal setting, relapse prevention and obtaining social support. Each patient received an information booklet and goal worksheets (BT, Ph, SH, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 3 and 8 months (abstinence self‐report) | |
| Notes | IG: ITT = 92.7% CG: ITT = 85.7% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Jiang 2007.
| Methods | ITT | |
| Participants | 167 (83+84) patients with CHD (angina pectoris or myocardial infarction) in Chengdu, China IG: 33 smokers CG: 38 smokers |
|
| Interventions | Usual care: routine care Psychosocial intervention: 12‐week home‐based multifaceted cardiac rehabilitation intervention with two phases: (1) hospital‐based patient/family education, (2) home‐based rehabilitation care (Ph, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 3 and 6 months (abstinence self‐report) | |
| Notes | IG: ITT = 51.5% CG: ITT = 39.5% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generalized random table |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Kubilius 2012.
| Methods | Completer Analysis | |
| Participants | 140 patients with angiographically confirmed coronary heart disease and NYHA functional class II‐IV ischemic heart failure IG: 10 smokers CG: 8 smokers |
|
| Interventions | Usual care: drug treatment only without any rehabilitation program Psychosocial intervention: 3‐stage rehabilitation: (1) bed and floor exercises and motivation for risk factor correction. (2) 5 lectures, individual aerobic exercise training and recommendations for dietary changes and smoking cessation. 5 lectures comprising cardiovascular anatomy and pathophysiology, risk factors for ischemic heart disease and their management. Recommendations for smoking cessation included the period of smoking cessation and NRT for 3 months. (3) Indivudal programs at home. Patients were asked to exercise 30min per day and to follow dietary and smoking cessation recommendations (5 months). Healthy lifestyle counselling was performed once a month and the patients were suggested to use symptomatic and cardioprotective drugs every day (MR, Intensity 4) |
|
| Outcomes | Follow‐up at 3 and 6 months (abstinence self‐report) | |
| Notes | IG: Completer = 100% CG: Completer = 100% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Lisspers 1999.
| Methods | Completer Analysis | |
| Participants | 93 patients recruited among referrals to the Department of Cardiology, Karolinska Hospital, for PTCA. IG: 7 smokers CG: 5 smokers |
|
| Interventions | Usual care: Patients were asked to keep contact with their own physician. they were neither encouraged nor discouraged to undertake any further rehabilitative efforts. Psychosocial intervention: Health education and behavior‐change activities, including lectures and discussions but focusing mainly on practical skills training and habit rehearsal directed toward stress management and diet, exercise and smoking habits. (BT, MR, Intensity 5) |
|
| Outcomes | Follow‐up at 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 71% CG: Completer = 0% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Mildestvedt 2007.
| Methods | Completer Analysis | |
| Participants | 266 patients attending a four‐week CR programme at Krokeide Rehabilitation Centre IG: 16 smokers CG: 15 smokers |
|
| Interventions | Usual care: standard rehabilitation treatment which included dietary and smoking cessation counselling in a group setting Psychosocial intervention: standard treatment plus additional individualised self‐efficacy and autonomy‐supportive intervention. Key features of the intervention: goal setting and selecting personalised strategies to overcome barriers. Two individual sessions during the rehabilitation stay and two follow‐up telephone calls at 6 and 24 months focusing on the personally selected goals. Cognitive intervention. Counselling aimed at strengthening the motivational adherence to these lifestyle changes through autonomy‐supportive dialogues (BT, Ph, MR, Intensity 5) |
|
| Outcomes | Follow‐up at 6 and 24 months (abstinence self‐report) | |
| Notes | IG: Completer = 37.5% CG: Completer = 20.0% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Mitsibounas 1992.
| Methods | Completer Analysis, ITT | |
| Participants | 43 Athenian residents with acute myocardial infarction and no further complications. IG: 18 smokers CG: 18 smokers |
|
| Interventions | Usual care: The patients had a clinical follow‐up and electrocardiogram once a month, a treadmill exercise test and 24‐hour Holter monitoring twice a year, and a coronary arteriography once a year. Psychosocial intervention: Patients attended group meetings. The aim of these group meetings was to help patients realise that at least one of the alternative solutions to their conflict was socially (by the group) acceptable. Besides group meetings, the patients had a clinical follow‐up and electrocardiogram test every 2 weeks. Within 1 year, a treadmill exercise test and a 24‐hour Holter monitoring were performed twice, and a coronary arteriogram once. (MR, Intensity 5) |
|
| Outcomes | Follow‐up at 3, 6 and 12 months (not validated) | |
| Notes | IG: Completer = 72% ITT = 67% CG: Completer = 11% ITT = 10% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Mosca 2010.
| Methods | ITT | |
| Participants | 304 women with CHD admitted to the New York‐Presbyterian Hospital (Columbia and Weill Cornell Campuses) or the University of North Carolina Health System IG: 32 smokers CG: 22 smokers |
|
| Interventions | Usual care: encouraged to attend cardiac rehabilitation and to exercise a minimum of 3‐5 days/week Psychosocial intervention: education and counselling by a prevention facilitator/educator during hospitalisation and during phone visits at 2, 4 and 12 weeks and at 6 weeks postdischarge. 1 hour of structured counselling before discharge that reviewed smoking, exercise, nutrition, weight and blood pressure and cholesterol goals for secondary prevention. Advised on recommendations for behavior change personalised to their specific needs (BT, Ph, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 6 weeks and 6 months (abstinence self‐report) | |
| Notes | IG: ITT = 46.9% CG: ITT = 68.2% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked design using a central dial‐in web‐based system |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Naser 2008.
| Methods | Completer Analysis | |
| Participants | Patients after first MI in Iran. IG: 15 smokers CG: 5 smokers |
|
| Interventions | Usual care: did not receive any kind of intervention Psychosocial intervention: intensive multi‐factorial lifestyle modification (IMLM): 30min hospital based consultation along with written guidelines for prevention of common established risk factors. Patients were then scheduled for a two year post‐hospital intervention program consisting of CRP exercise sessions, lifestyle counselling and telephone follow‐ups (BT, Ph, MR, Intensity 5) |
|
| Outcomes | Follow‐up at 12 and 24 months (abstinence self‐report) | |
| Notes | IG: Completer = 90% CG: Completer = 70% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated variable block program |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Neubeck 2011.
| Methods | Completer Analysis | |
| Participants | Acute coronary syndrome patients IG: 6 smokers CG: 11 smokers |
|
| Interventions | Usual care: ongoing conventional health care, managing their cardiovascular health in consultation with their GP and cardiologist Psychosocial intervention: CHOICE (= Choice of Health Options In prevention of Cardiovascular Events): ongoing conventional health care and a 3‐months modular patient‐centered program. The CHOICE‐program included a 1h initial consultation and multiple telephone calls over 3 months. The program was designed to have an individualised, structured case management approach and is conducted in consultation with the patient's GP. (Ph, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 48 months (validated) | |
| Notes | IG: Completer = 66.7% CG: Completer = 18.2% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | High risk | Consecutive numbered envelopes, but no information if sealed or opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Ortigosa 2000.
| Methods | Completer Analysis, ITT | |
| Participants | 90 patients with myocardial infarction in hospital in Spain IG: 43 smokers CG: 47 smokers | |
| Interventions | Usual care: advice only. Psychosocial intervention: advice by doctor immediately after admission to hospital (10 minutes). Additional enhancement of motivation by nurses and phone contacts (after 2, 3, 4 weeks). (Ph, Specific, Intensity 3) | |
| Outcomes | Follow‐up at 1, 3 and 12 months (validated) | |
| Notes | IG: Completer = 62% ITT = 61% CG: Completer = 69% ITT = 66% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Otterstad 2003.
| Methods | Completer Analysis, ITT | |
| Participants | 197 patients after an acute myocardial infarction, hospitalisation for unstable angina pectoris, percutaneous coronary intervention or coronary artery bypass grafting IG: 49 smokers CG: 42 smokers |
|
| Interventions | Usual care: standardised, nurse‐based information on CHG in general and lifestyle measures Psychosocial intervention: lifestyle intervention: 6‐week period of "heart school"‐ Physical exercise plus group meetings. Dietary advice, smoking cessation, physical activity counselling, risk factor management, psychosocial management and health education related to cardiovascular disease, medication, reduction of mental stress, relaxation and psychosocial factors (BT, MR, Intensity 5) |
|
| Outcomes | Follow‐up at 6 and 24 months (abstinence self‐report) | |
| Notes | IG: Completer = 56.8% ITT = 42.9% Long‐term IG: Completer = 48.7% ITT = 36.7% CG: Completer = 39.4% ITT = 31.0% Long‐term CG: Completer = 24.2% ITT = 19.1% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Pre‐prepared sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Pedersen 2005.
| Methods | Completer Analysis | |
| Participants | Patients with multiple cardiovascular disorders IG: 54 smokers CG: 51 smokers |
|
| Interventions | Usual care Psychosocial intervention: According to STOP concept including information on impact of smoking on heart disease as well as on nicotine replacement alternatives, nicotine dependence test and 5x30min individual consultation (personal or via phone) in first 5 weeks. (SH, Specific, Intensity 4) |
|
| Outcomes | Follow‐up at 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 51.1% CG: Completer = 39.6% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Patients selected an envelope with the treatment condition from a pool of envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Quist‐Paulsen 2003.
| Methods | Completer Analysis, ITT | |
| Participants | Patients in hospital (reasons myocardial infarction, angina pectoris, coronary artery bypass surgery) IG: 118 smokers CG: 122 smokers | |
| Interventions | Usual care: no specific intervention. Psychosocial intervention: group intervention with nurses (twice a week). Booklet with emphasis on health benefits from smoking cessation. Also fear arousing message about death rates of persistent smokers. Advice not to smoke during hospital stay and motivation for NRT if needed additional phone contacts (5 times) in 5 months after hospital stay. (BT, Ph, SH, Specific, Intensity 4) | |
| Outcomes | Follow‐up 12 months (validated) | |
| Notes | IG: Completer = 57% ITT = 48% CG: Completer = 37% ITT = 36% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Quist‐Paulsen 2005.
| Methods | Completer Analysis, ITT | |
| Participants | 240 patients 2 to 4 days after admission for coronary heart disease (acute myocardial infarction, unstable angina or recent coronary bypass) IG: 118 smokers CG: 122 smokers |
|
| Interventions | Usual care: patients were offered group sessions in the ward twice a week, where the importance of smoking cessation was mentioned. During these sessions a video was shown and a booklet handed out that contained general information on coronary heart disease and advice on quitting smoking. They received no further specific instructions on how to stop smoking Psychosocial intervention: after discharge nurses contacted participants by telephone at 2 days, 1 week, 3 weeks, 3 months and 5 months. Those with special needs were telephoned monthly thereafter. The intervention was based on a 17‐page booklet which emphasised the health benefits of quitting smoking after a coronary event. The booklet also contained information on how to prevent relapse, how to stop smoking and how to use nicotine replacements. How to identify and cope with high‐risk situations for relapse was also explained. Spouses who smoked were also asked to give up (Ph, Sh, Specific, Intensity 4) |
|
| Outcomes | Follow‐up at 12 months (nicotine‐validated) | |
| Notes | IG: Completer = 57% ITT = 48.3% CG: Completer = 37.3% ITT = 36.1% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Doctor’s not involved = concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Quist‐Paulsen 2006a.
| Methods | Completer Analysis, ITT | |
| Participants | 240 daily smokers, motivate to quit smoking and admitted for acute myocardial infarction, unstable angina or recent coronary bypass IG: 118 smokers CG: 112 smokers |
|
| Interventions | Usual care: firm and unequivocal advice to stop smoking, but no further instructions on how to stop smoking Psychosocial intervention: smoking cessation program initiated at the hospital and delivered by cardiac nurses. It was based on a booklet produced for the study and focuses on fear arousal and prevention of relapse. The patients were contacted regularly for several months after discharge (BT, Ph, SH, Specific, Intensity 4) |
|
| Outcomes | Follow‐up at 12 months (validated) | |
| Notes | IG: Completer = 57% ITT = 48.3% CG: Completer = 37.3% ITT = 36.1% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Low risk | Nurses were given a serially numbered sealed envelope from a secretary who was otherwise uninvolved in the study. No information about opaque, but unnecessary since nurse was not involved |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Reid 2003.
| Methods | ITT | |
| Participants | Patients in hospital in Canada (reasons: angiography, PTCA, Myocardial infarction, coronary artery bypass surgery. IG: 126 smokers CG: 128 smokers | |
| Interventions | Usual care: no additional intervention after discharge. Psychosocial intervention: assessment of smoking status after 4 weeks. Abstinent patients were reinforced. Those still smoking were offered support by nurse (3 sessions with 20minutes in 8 weeks) and additionally nicotine patch therapy (BT, Ph, SH, Specific, Intensity 4) | |
| Outcomes | Follow‐up at 3 and 12 months (not validated self‐report) | |
| Notes | IG: ITT = 39% CG: ITT = 36% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Blocks of six, research staff was unaware of treatment allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Rigotti 1994.
| Methods | ITT | |
| Participants | 93 patients (smokers) scheduled for CABS in the postoperative cardiac surgery unit at Massachusetts General Hospital IG: 44 smokers CG: 43 smokers | |
| Interventions | Usual care: Brief advice not to smoke within a group session.
Psychosocial intervention: Nurse‐based smoking cessation and relapse prevention programme adapted from the American Lung Association's "In Control" programme: three‐counselling sessions, videotape, family members were included. Phone call 1 week after discharge by nurse. (BT, Ph, SH, Specific, Intensity 3) |
|
| Outcomes | Follow‐up at 2, 4, 8, 12 months and 5 years (validation by saliva cotinine) | |
| Notes | IG: ITT = 43% CG: ITT = 44% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data on ITT available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Sivarajan 1983.
| Methods | Completer Analysis, ITT | |
| Participants | 258 patients hospitalised with AMI IG1: 43 smokers IG2: 39 smokers CG: 37 smokers | |
| Interventions | Usual care: conventional medical and nursing management. Psychosocial intervention: two intervention groups ‐ EG1: exercise only (for 12 weeks, weekly clinic visits); EG2: exercise, teaching and counselling ( 8 group sessions about risk factors, diet, exercise, stress management etc, individual counselling if needed). Data only of EG 2 used for analysis. (BT, SH, MR, Intensity 4) | |
| Outcomes | Follow‐up at 3 and 6 months (abstinence self‐report) | |
| Notes | IG: Completer = 48% ITT = 33% CG: Completer = 58% ITT = 37% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Smith 2009.
| Methods | Completer Analysis, ITT | |
| Participants | CHD patients after myocardial infarction. All received bypass surgery. Canada. IG: 137 smokers CG: 139 smokers |
|
| Interventions | Usual care: minimal intervention: research nurses advised patients to quit smoking by personalizing messages to each patient's medical conditions. The nurse reviewed 2 pamphlets (how to quit and where to find help quitting) with the patient and she put a note in each patient's chart to ask the attending physician to deliver a scripted nonsmoking message at the bedside during the patient's hospital stay. Pharmacotherapy was introduced as an aid to cessation was available Psychosocial intervention: intensive intervention: minimal intervention plus 45‐60min of bedside education and counselling, take‐home materials (video, workbook, audiotape) and 7 telephone counselling sessions initiated by the research nurse (2, 7, 14, 21, 30, 45 and 60 days after discharge) (BT, Ph, SH, Specific, Intensity 4) |
|
| Outcomes | Follow‐up at 3, 6 and 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 59.4% ITT = 53.3% CG: Completer = 38.7% ITT = 34.5% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | High risk | The research nurse opened the randomisation envelopes and informed the patients of intervention assignment, but no information if envelopes were sequentially numbered, sealed and opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Taylor 1990.
| Methods | Completer Analysis, ITT | |
| Participants | 173 patients (smokers) admitted to hospital with AMI IG: 72 smokers CG: 58 smokers | |
| Interventions | Usual care: no specific instruction to stop smoking. 10% participated in non smoking classes. Psychosocial intervention: nurse‐managed intervention based on social learning theory: manual "Staying Free", 2 audio tapes for relaxation. Telephone contact after discharge (6 times), counselling after relapse. (BT, Ph, SH, Specific, Intensity 4) | |
| Outcomes | Follow‐up at 6 and 12 months (biochemical validation) | |
| Notes | IG: Completer = 70% ITT = 59% CG: Completer = 44% ITT = 30% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list of odd and even numbers |
| Allocation concealment (selection bias) | High risk | A sequence of numbers sealed in envelopes was created, but no information if envelopes were sequentially numbered and opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
van Elderen (group).
| Methods | Completer Analysis, ITT | |
| Participants | Of 477 patients with CHD after discharge from hospital 258 were included. Diagnosis: MI (144), CABS (42), PTCA (10), other (21) IG: 66 smokers CG: 70 smokers | |
| Interventions | Usual care: standard rehabilitation with medical care and physical training. Psychosocial intervention: health education programme "Heart and Health" based on Ellis' Rational Emotive Therapy (ABCDE model): information about heart disease, risks, diet, exercise, identification and modification of irrational beliefs. 8 weekly group sessions (2h) for the patients and their partners, one follow‐up session at 2 months. (BT, MR, Intensity 4) | |
| Outcomes | Follow‐up at 3 and 12 months (abstinence self‐report) | |
| Notes | IG: Completer = 72% ITT = 69% CG: Completer = 50% ITT = 46% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis possible |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
van Elderen (phone).
| Methods | Completer Analysis | |
| Participants | 60 patients admitted to hospital with AMI IG: 15 smokers CG: 16 smokers | |
| Interventions | Usual care: standard medical care. Psychosocial intervention: health education and counselling programme: two nurse‐based counselling sessions, two group health education sessions (medication, aetiology of MI, risk factors, anxiety, depression etc). Weekly telephone contacts by nurse after discharge for 6 weeks. (Ph, SH, MR, Intensity 4) | |
| Outcomes | Follow‐up after intervention, at 8 weeks and 1 year (abstinence self‐report) | |
| Notes | IG: Completer = 60% CG: Completer = 37% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information available |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
Zwisler 2008.
| Methods | Completer Analysis, ITT | |
| Participants | Patients with CHF, IHD or HR who were admitted to the Department of Cardiology of Bispebjerg Hospital IG: 110 smokers CG: 117 smokers |
|
| Interventions | Usual care: the discharging physician in the outpatient clinic or a general practitioner offered the UC patients follow‐up. The UC patients were informed that they would be contacted after 12 months to assess outcomes. Psychosocial intervention: hospital‐based CCR: standardised cardiac rehabilitation program. 6 week intensive CCR‐program with patient education, 12 exercise training sessions, dietary counselling, smoking cessation, psychosocial support, risk factor management and clinical assessment. Follow‐up visits at 3, 6 and 12 months. (BT, MR, Intensity 4) |
|
| Outcomes | Follow‐up at 12 months (validated) | |
| Notes | IG: Completer = 51% ITT = 44.5% CG: Completer = 37.6% ITT = 29.9% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, random‐permutated multiblock within‐stratum method |
| Allocation concealment (selection bias) | Low risk | Within each risk stratum the block size, unknown to the investigators, alternated between 6 and 8 patients (see secondary literature: Zwisler 2005) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in general not possible in psychosocial interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Always rated as unclear, since no protocol information was available in any study |
ITT = intention to treat analysis; CABG = coronary artery bypass surgery; CAD = coronary artery disease; AMI = acute myocardial infarction; CG = control group; IG = experimental intervention group; GP = general practitioner Duration of treatment is coded as follows (intensity): coding 1: single initial contact lasting <= 1 hour, no follow‐up support coding 2: one or more contacts in total > 1 hour, no follow‐up support coding 3: any initial contact plus follow‐up <=1 month coding 4: any initial contact plus follow‐up > 1 month and <= 6 month coding 5: any initial contact plus follow‐up > 6 month.
Number in notes section show abstinence rates in percent
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albu 2006 | Smoking cessation was not the aim of the study |
| Andersen 2002 | Patients with other disease, or healthy subjects |
| Andersson 2010 | No data reported for smoking cessation as outcome |
| Anonymous 2003 | Review |
| Anonymous 2006 | No empirical study |
| Armstrong 2011 | No paper |
| Avanzini 2011 | Not randomized |
| Bastian 2011 | No extra results for CHD |
| Baughman 1982 | No intervention specified or no specific smoking cessation intervention |
| Belson 2002 | Heterogeneity in diagnosis included / no assessment of smoking status |
| Bock 2008 | No CVD population |
| Bolman 2002b | Follow up too short |
| Brenner 1989 | No intervention specified or no specific smoking cessation intervention / no assessment of smoking status |
| Brown 2004 | Summary of Quist‐Paulsen |
| Byfield 2001 | Patients with other disease, or healthy subjects / no assessment of smoking status |
| Campbell 1996 | Heterogeneity in diagnosis included |
| Campbell 1998a | No clear diagnosis of CHD |
| Campbell 1998b | No clear diagnosis of CHD |
| Campbell 2004 | No empirical study |
| Chan 2005 | No RCT / conference paper |
| Cho 2012 | No data on abstinence |
| Chow 2010 | No intervention specified or no specific smoking cessation intervention |
| Circo 1985 | Follow up too short |
| Connett 1984 | No information about diagnosis included |
| Cook 1989 | No data reported |
| Cupples 1999 | Patients with other heart disease |
| Davidoff 2001 | No psycosocial intervention |
| Eaker 1982 | Patients with other disease, or healthy subjects |
| Einecke 2004 | No psychosocial intervention |
| Ellingsen 2003 | Only data analysis / no primary study / outcome mortality |
| Engblom 1992 | No intervention specified or no specific smoking cessation intervention |
| Erdman 1983 | No intervention specified or no specific smoking cessation intervention |
| Espinosa 2004 | No RCT / only data analysis / wrong population |
| Finnegan 1985 | Cross sectional analysis, not sufficient data / no intervention specified or no specific smoking cessation intervention |
| Fletcher 1987 | No intervention specified or no specific smoking cessation intervention / no comparison of smoking status feasible |
| Fonarow 2007 | No RCT / Comparison of medication |
| Fortmann 1994 | No intervention specified or no specific smoking cessation intervention / no comparison of usual care and specific psychosocial intervention |
| Fox 2002 | Not randomized |
| Frasure‐Smith 1997 | No comparison of smoking status feasible |
| Fredrickson 1995 | Patients with other disease, or healthy subjects / no intervention specified or no specific smoking cessation intervention / follow up too short |
| Giallauria 2005 | No RCT / retrospective study |
| Gohlke 2004 | Review, no study |
| Goldenberg 2003 | No RCT / no intervention study / retrospective analysis of previous data |
| Guthrie 2001 | No intervention specified or no specific smoking cessation intervention |
| Hajek 2010 | No smoking cessation reported |
| Hall 1983 | Heterogeneity in diagnosis included |
| Haskell 1994 | No possibility to include data in meta‐analysis |
| Hilleman 2004 | No RCT |
| Hjermann 1986 | Heterogeneity in diagnosis included |
| Holme 2006 | Smoking intervention not an outcome of interest |
| Horlick 1984 | No comparison of smoking status feasible |
| Hosokawa 2008 | Smoking intervention not an outcome of interest |
| Houston 2005 | No RCT / observational study / outcome mortality |
| Houston‐Miller 1997 | Heterogeneity in diagnosis included |
| Jacobsen 2008 | No empirical study / no RCT |
| Jain 2003 | No RCT |
| Jami 2007 | Retrospective medical chart review |
| Jatuporn 2003 | Medication vs. lifestyle modification / Smoking is not an outcome of interest |
| Jolly 2003 | Target group consists of mixed patients (smokers / nonsmokers) / study protocol |
| Jones 1996 | No intervention specified or no specific smoking cessation intervention / no assessment of smoking status |
| Joseph 1996 | No intervention specified or no specific smoking cessation intervention |
| Joseph 2005 | No abstinence rates |
| Kallio 1981 | Follow up too short |
| Knutsen 1991 | Patients with other disease, or healthy subjects |
| Kornitzer 1980 | Patients with other disease, or healthy subjects |
| Kornitzer 1989 | Patients with other disease, or healthy subjects / no comparison of smoking status feasible |
| Kotowycz 2010 | Follow up too short |
| Kristeller 1993 | No comparison of smoking status feasible |
| Kuller 1991 | Heterogeneity in diagnosis included / no intervention specified or no specific smoking cessation intervention |
| La Rosa 2006 | Smoking cessation not goal of intervention / comparison of medication |
| Lancaster 1999 | Patients with other disease, or healthy subjects |
| Lear 2002 | Only 5 smokers and no results |
| Leung 2008 | No RCT / no intervention |
| Maddison 2011 | Study protocol |
| Marra 1985 | No intervention specified or no specific smoking cessation intervention |
| Matsui 2005 | Only text for methods |
| Mayou 2002 | No intervention specified or no specific smoking cessation intervention / no comparison of smoking status feasible |
| McGee 2006 | No psychosocial intervention |
| McHugh 2001 | No assessment of smoking status |
| Meland 1999 | Cross sectional analysis, not sufficient data |
| Murchie 2003 | No clear diagnosis of CHD |
| Murray 2007 | Smoking is not an outcome of interest |
| Muscari 2005 | Smoking cessation not goal of intervention |
| Nisbeth 2000 | Patients with other disease, or healthy subjects / no results for CG and smoking |
| O'Malley 2003 | No psychosocial intervention |
| O'Neil 2011 | Study protocol |
| Oldridge 1997 | No RCT/ only cost effectiveness |
| Ong 2005 | No RCT/ no comparison of CG and IG |
| Ornish 1990 | No comparison of smoking status feasible / no assessment of smoking status |
| Patel 1985 | No information about diagnosis included / no comparison of smoking status feasible |
| Perk 2000 | No RCT |
| Piestrzeniewicz 2004 | Study not available |
| Plans‐Rubio 2004 | No RCT / only data analysis / no comparison with CG |
| Powers 2011 | Follow up too short |
| Prieme 1998 | Patients with other disease, or healthy subjects / cross sectional analysis, not sufficient data /no intervention specified or no specific smoking cessation intervention / no assessment of smoking status |
| Puura 2003 | No CVD / wrong outcome |
| Quist‐Paulsen 2006b | Cost effectiveness |
| Redfern 2009 | Relative Risk is larger than 5, RR = 20.09 |
| Reid 2005 | Smoking is not an outcome / unclear if smoking cessation is part of the intervention |
| Reid 2007 | No psychosocial intervention / NRT vs. NRT + phone |
| Rice 1994 | Heterogeneity in diagnosis included |
| Rigotti 2006 | Comparison of medication / no psychosocial intervention |
| Rigotti 2011 | Follow up too short |
| Risser 1990 | Patients with other disease, or healthy subjects |
| Rollins 2004 | Outcome guideline compliance |
| Rose 1978 | Patients with other disease, or healthy subjects |
| Rose 1982 | Patients with other disease, or healthy subjects |
| Rose 1992 | Patients with other disease, or healthy subjects |
| Sanders 1989 | No information about diagnosis included |
| Scherr 2010 | Not randomized |
| Schimmer 2006 | No smoking cessation / no random allocation |
| Schmitz 1999 | No comparison of usual care and specific psychosocial intervention |
| Schoenenberger 2010 | No psychosocial intervention |
| Schumacher 2006 | Smoking is not an outcome of the study |
| Simon 2003 | Heterogeneity in diagnosis included |
| Sippel 1999 | Heterogeneity in diagnosis included |
| Smith 1998 | No study |
| Sondergaard 2006 | Mixed population |
| Steptoe 1999 | Other heart diseases / patients with other disease, or healthy subjects |
| Steptoe 2001 | Other heart diseases / patients with other disease, or healthy subjects |
| Stewart 1999 | Other heart diseases / no intervention specified or no specific smoking cessation intervention |
| Strandberg 2001 | Target group consists of mixed patients (smokers / nonsmokers) / no cessation data from the control group |
| Suurkula 1996 | Heterogeneity in diagnosis included |
| Taylor 1988 | No intervention specified or no specific smoking cessation intervention |
| Taylor 1997 | No assessment of smoking status |
| Thomas 2007 | No RCT / no empirical study |
| Thompson 2009 | Summary of Jolly (2003) |
| Tiffany 1986 | Patients with other disease, or healthy subjects |
| Tonnesen 1999 | Other heart diseases |
| Tonstad 2003 | Heterogeneity in diagnosis included / no intervention specified or no specific smoking cessation intervention |
| Toobert 1998 | No comparison of smoking status feasible |
| Toobert 2000 | No comparison of smoking status feasible |
| Trockel 2008 | No psychosocial intervention aiming at smoking cessation |
| Tzivoni 1998 | Cross sectional analysis, not sufficient data /no intervention specified or no specific smoking cessation intervention |
| Uysal 2012 | Follow up too short |
| Vasiliauskas 2009 | Unclear information / translation not available |
| Vedin 1976 | No intervention specified or no specific smoking cessation intervention |
| Wallner 1999 | No intervention specified or no specific smoking cessation intervention / no assessment of smoking status |
| Waters 1996 | No intervention specified or no specific smoking cessation intervention |
| Wewers 1994 | Heterogeneity in diagnosis included |
| Whitlock 1997 | Heterogeneity in diagnosis included / no information about diagnosis included |
| Wiggers 2006 | Pharmacological criteria as comparator |
| Wister 2007 | Target group consists of mixed patients (smokers / nonsmokers) |
| Woollard 2003 | Smoking is not an outcome of interest |
| Xian 2010 | No RCT / comparison of hospitals |
| Yorio 2008 | Smoking is not an outcome of the study |
| Zwisler 2005 | Smoking is not an outcome of the study |
Characteristics of studies awaiting assessment [ordered by study ID]
Becker 2003.
| Methods | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Participants | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Interventions | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Outcomes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Notes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
Boulay 2001.
| Methods | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Participants | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Interventions | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Outcomes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Notes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
Enriquez‐Puga 2001.
| Methods | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Participants | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Interventions | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Outcomes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Notes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
Puente‐Silva 1989.
| Methods | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Participants | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Interventions | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Outcomes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
| Notes | Was not extracted since we could not access the paper and authors did not respond to PDF requests |
Contributions of authors
JB performed initial searches for studies, assessed studies for inclusion, carried out data extraction. He wrote the initial draft, supervised the entire project and edited the review. TJ assessed studies for inclusion, extracted data, analysed data, and edited the review. ID extracted data and edited the review. JC gave advice on statistical procedures, and edited the review.
Sources of support
Internal sources
Department of Rehabilitation Psychology, Germany.
Wissenschaftliche Gesellschaft University of Freiburg, Germany.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Allen 1996 {published data only}
- Allen JK. Coronary risk factor modification in women after coronary artery bypass surgery. Nursing Research 1996;45(5):260‐5. [DOI] [PubMed] [Google Scholar]
Benner 2008 {published data only}
- Benner JS, Erhardt L, Flammer M, Moller RA, Rajicic N, Changela K, et al. A novel programme to evaluate and communicate 10‐year risk of CHD reduces predicted risk and improves patients' modifiable risk factor profile. International Journal of Clinical Practice 2008;62(10):1484‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blasco 2012 {published data only}
- Blasco A, Carmona M, Fernandez‐Lozano I, Salvador CH, Pascual M, Sagredo PG. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(1):25‐31. [DOI] [PubMed] [Google Scholar]
Bolman 2002a {published data only}
- Bolman Catherine, Vries Hein, Breukelen Gerard. A minimal‐contact intervention for cardiac inpatients: long‐term effects on smoking cessation. Preventive Medicine 2002;35:181‐92. [DOI] [PubMed] [Google Scholar]
Burt 1974 {published data only}
- Burt A, Thornley R, Illingworth D, White P, Shaw TR, Turner R. Stopping smoking after myocardial infarction. Lancet 1974;1(7852):304‐6. [DOI] [PubMed] [Google Scholar]
Carlsson 1997 {published data only}
- Carlsson R, Lindberg G, Westin L, Israelsson B. Influence of coronary nursing managment follow up on lifestyle after acute myocardial infarction. Heart 1997;77(3):256‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
CASIS 1992 {published data only}
- Ockene J, Kristeller JL, Goldberg R, Ockene I, Merriam P, Barrett S, et al. Smoking cessation and severity of disease: the Coronary Artery Smoking Intervention Study. Health Psychology 1992;11(2):119‐26. [DOI] [PubMed] [Google Scholar]
- Rosal MC, Ockene JK, Ma Y, Hebert JR, Ockene IS, Merriam P, Hurley TG. Coronary artery smoking intervention study (CASIS): 5‐year follow up. Health Psychology 1998;17(5):476‐8. [DOI] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan SS, Leung DY, Wong DC, Lau CP, Wong VT, Lam TH. A randomized controlled trial of stage‐matched intervention for smoking cessation in cardiac out‐patients. Addiction 2012;107:829‐37. [DOI] [PubMed] [Google Scholar]
Cossette 2011 {published data only}
- Cossette S, Frasure‐Smith N, Robert M, Chouinard MC, Juneau M, Guertin MC, et al. A pre assessment for nursing intervention to support tobacco cessation in patients hospitalized for cardiac problems: a pilot study (So‐Live). Recherche en Soins Infirmiers 2011;105:60‐75. [PubMed] [Google Scholar]
Costa e Silva 2008 {published data only}
- Costa e Silva R, Pellanda L, Portal V, Maciel P, Furquim A, Schaan B. Transdisciplinary approach to the follow‐up of patients after myocardial infarction. Clinics 2008;63(4):489‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeBusk 1994 {published data only}
- DeBusk R, Miller NH, Superko R, Dennis CA, Thomas RJ, et al. A case‐management system for coronary risk factor modification after acute myocardial infarction. Annals of Internal Medicine 1994;120(9):721‐9. [DOI] [PubMed] [Google Scholar]
Dornelas 2000 {published data only}
- Dornelas EA, Sampson RA, Gray JF, Waters D, Thompson PD. A randomized controlled trial of smoking cessation counseling after myocardial infarction. Preventive Medicine 2000;30(4):261‐8. [DOI] [PubMed] [Google Scholar]
Feeney 2001 {published data only}
- Feeney GFX, McPherson A, Connor JP, McAlister A, Young RM. Randomized controlled trial of two cigarette quit programmes in coronary care patients after acute myocardial infarction. Internal Medicine Journal 2001;31(8):470‐5. [DOI] [PubMed] [Google Scholar]
Froelicher 2004 {published data only}
- Sivarajan Froelicher ES, Miller NH, Christopherson DJ, Martin K, Parker KM, Amonetti M, et al. High rates of sustained smoking cessation in women hospitalized with cardiovascular disease: the women's initiative for nonsmoking (WINS). Circulation 2004;109:587‐93. [DOI] [PubMed] [Google Scholar]
Gao 2011 {published data only}
- Gao Y, Li Y, Zheng J, Wang R, Meng H, Zhang L, et al. The effects of a comprehensive health education program in Chinese patients after percutaneous coronary intervention. IIOAB Journal 2011;2(7):23‐30. [Google Scholar]
Hajek 2002 {published data only}
- Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: randomised controlled trial. BMJ 2002;324:87‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2011 {published data only}
- Han WZ, Zhang M, Wang J, Sun YM, Fang WY. Effects of standardized secondary prevention on lifestyle of patients with acute coronary syndrome. [Chinese]. Journal of Shanghai Jiaotong University (Medical Science) 2011;31:302‐4. [Google Scholar]
Hanssen 2007 {published data only}
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Improving outcomes after myocardial infarction: a randomized controlled trial evaluating effects of a telephone follow‐up intervention. European Journal of Cardiovascular Prevention & Rehabilitation 2007;14(3):429‐37. [DOI] [PubMed] [Google Scholar]
Hanssen 2009 {published data only}
- Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Does a telephone follow‐up intervention for patients discharged with acute myocardial infarction have long‐term effects on health‐related quality of life? A randomised controlled trial. Journal of Clinical Nursing 2009;18(9):1334‐45. [DOI] [PubMed] [Google Scholar]
Heller 1993 {published data only}
- Heller RF, Knapp JC, Valenti LA, Dobson AJ. Secondary prevention after acute myocardial infarction. American Journal of Cardiology 1993;72(11):759‐62. [DOI] [PubMed] [Google Scholar]
Holmes‐Rovner 2008 {published data only}
- Holmes‐Rovner M, Stommel M, Corser WD, Olomu A, Holtrop JS, Siddiqi A, et al. Does outpatient telephone coaching add to hospital quality improvement following hospitalization for acute coronary syndrome?. Journal of General Internal Medicine 2008;23(9):1464‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2007 {published data only}
- Jiang X, Sit JW, Wong TK. A nurse‐led cardiac rehabilitation programme improves health behaviours and cardiac physiological risk parameters: evidence from Chengdu, China. Journal of Clinical Nursing 2007;16(10):1886‐97. [DOI] [PubMed] [Google Scholar]
Kubilius 2012 {published data only}
- Kubilius R, Jasiukeviciene L, Grizas V, Kubiliene L, Jakubseviciene E, Vasiliauskas D. The impact of complex cardiac rehabilitation on manifestation of risk factors in patients with coronary heart disease. Medicina (Kaunas) 2012;48(3):166‐73. [PubMed] [Google Scholar]
Lisspers 1999 {published data only}
- Lisspers J, Sundin Ö, Hofman‐Bang C, Nordlander R, Nygren A, Ryden L, et al. Behavioral effects of a comprehensive, multifactorial program for lifestyle change after percutaneous transluminal coronary angioplasty: a prospective, randomized, controlled study. Journal of Psychosomatic Research 1999;46(2):143‐54. [DOI] [PubMed] [Google Scholar]
Mildestvedt 2007 {published data only}
- Mildestvedt T, Meland E, Eide GE. No difference in lifestyle changes by adding individual counselling to group‐based rehabilitation RCT among coronary heart disease patients. Scandinavian Journal of Public Health 2007;35(6):591‐8. [DOI] [PubMed] [Google Scholar]
Mitsibounas 1992 {published data only}
- Mitsibounas DN, Tsouna‐Hadjis ED, Rotas VR, Sideris DA. Effects of group psychosocial intervention on coronary risk factors. Psychotherapy & Psychosomatics 1992;58(2):97‐102. [DOI] [PubMed] [Google Scholar]
Mosca 2010 {published data only}
- Mosca L, Christian AH, Mochari‐Greenberger H, Kligfield P, Smith SC, Jr. MD. A randomized clinical trial of secondary prevention among women hospitalized with coronary heart disease. Journal of Women's Health 2010;19(2):195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naser 2008 {published data only}
- Naser A, Shahamfar J, Kumar GV, Daga MK, Hadi HS, Saeed D, et al. Cardiac risk factor changes through an intensive multifactorial life style modification program in CHD patients: results from a two year follow up. Journal of Biological Sciences 2008;8(2):248‐57. [Google Scholar]
Neubeck 2011 {published data only}
- Neubeck L, Freedman SB, Briffa T, Bauman A, Redfern J. Four‐year follow‐up of the Choice of Health Options In prevention of Cardiovascular Events randomized controlled trial. European Journal of Cardiovascular Prevention & Rehabilitation 2011;18(2):278‐86. [DOI] [PubMed] [Google Scholar]
Ortigosa 2000 {published data only}
- Moreno Ortigosa A, Ochoa Gomez FJ, Ramalle‐Gomara E, Saralegui Reta I, Fernandez Esteban MV, Quintana Diaz M. Efficacy of a smoking cessation intervention for patients with myocardial infarction [Eficacia de una intervencion para dejar de fumar en pacientes con infarto de miocardio]. Medicina Clinica 2000;114:209‐10. [DOI] [PubMed] [Google Scholar]
Otterstad 2003 {published data only}
- Otterstad JE. Influence on lifestyle measures and five‐year coronary risk by a comprehensive lifestyle intervention programme in patients with coronary heart disease. European Journal of Cardiovascular Prevention & Rehabilitation 2003;10(6):429‐37. [DOI] [PubMed] [Google Scholar]
Pedersen 2005 {published data only}
- Pedersen L, Johansen S, Eksten L. Smoking cessation among patients with acute heart disease. A randomised intervention project. [Danish]. Ugeskrift for Laeger 2005;167(33):3044‐7. [PubMed] [Google Scholar]
Quist‐Paulsen 2003 {published data only}
- Quist‐Paulsen P, Gallefoss F. Randomised controlled trial of smoking cessation intervention after admission for coronary heart disease. BMJ 2003;327(7426):1254‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quist‐Paulsen 2005 {published data only}
- Quist‐Paulsen P, Bakke PS, Gallefoss F. Predictors of smoking cessation in patients admitted for acute coronary heart disease. European Journal of Cardiovascular Prevention & Rehabilitation 2005;12(5):472‐7. [DOI] [PubMed] [Google Scholar]
Quist‐Paulsen 2006a {published data only}
- Quist‐Paulsen P, Bakke PS, Gallefoss F. Does smoking cessation improve Quality of Life in patients with coronary heart disease?. Scandinavian Cardiovascular Journal 2006;40:11‐6. [DOI] [PubMed] [Google Scholar]
Reid 2003 {published data only}
- Reid R, Pipe A, Higginson LK, D'Angelo MS, Cooke D, Dafoe W. Stepped care approach to smoking cessation in patients hospitalized for coronary artery disease. Journal of Cardiopulmonary Rehabilitation 2003;23(3):176‐82. [DOI] [PubMed] [Google Scholar]
Rigotti 1994 {published data only}
- Rigotti NA, McKool KM, Shiffmann S. Predictors of smoking cessation after coronary artery bypass graft surgery. Annals of Internal Medicine 1994;120(4):287‐93. [DOI] [PubMed] [Google Scholar]
Sivarajan 1983 {published data only}
- Sivarajan ES, Newton KM, Almes MJ, Kempf TM, Mansfield LW, Bruce RA. Limited effects of outpatient teaching and counseling after myocardial infarction: a controlled study. Heart & Lung 1983;12(1):65‐73. [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith PM, Burgess E. Smoking cessation initiated during hospital stay for patients with coronary artery disease: a randomized controlled trial. CMAJ Canadian Medical Association Journal 2009;180(13):1297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 1990 {published data only}
- Taylor CB, Houston‐Miller N, Killen JD, DeBusk RF. Smoking cessation after acute myocardial infarction: effects of a nurse‐managed intervention. Annals of Internal Medicine 1990;113(2):118‐23. [DOI] [PubMed] [Google Scholar]
van Elderen (group) {published data only}
- Elderen T, Maes S, Seegers G. Effects of a group health education program for coronary heart patients after the hospitalization phase. Tijdschrift voor Psychologie & Gezondheid 1991;19(3):129‐44. [Google Scholar]
- Elderen T, Maes S, Seegers G. Effects of a post‐hospitalization group health education programme for patients with coronary heart disease. Psychology and Health 1994;9(4):317‐30. [DOI] [PubMed] [Google Scholar]
van Elderen (phone) {published data only}
- Elderen‐van Kemenade T, Maes S, Broek Y. Effects of a health education programme with telephone follow‐up during cardiac rehabilitation. British Journal of Clinical Psychology 1994;33(Pt 3):367‐78. [DOI] [PubMed] [Google Scholar]
Zwisler 2008 {published data only}
- Zwisler AD, Boas Soja AM, Rasmussen S, Frederiksen M, Abadini S, Appel J, et al. Hospital‐based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12‐month results of a randomized clinical trial. American Heart Journal 2008;155(6):1107‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albu 2006 {published data only}
- Albu J, Gottlieb SH, August P, Nesto RW, Orchard TJ, Bypass ARI. Modifications of coronary risk factors. American Journal of Cardiology 2006;97(12A):41G‐52G. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 2002 {published data only}
- Andersen LB, Klausen K, Nisbeth O. One‐year effect of health counseling on life style and risk factors of heart disease [Et ars effekt af sundhedsvejledning pa livsstil og risikofaktorer for hjertesygdom]. Ugeskrift for Laeger 2002;164(13):1814‐8. [PubMed] [Google Scholar]
Andersson 2010 {published data only}
- Andersson A, Sundel KL, Unden AL, Schenck‐Gustafsson K, Eriksson I. A five‐year rehabilitation programme for younger women after a coronary event reduces the need for hospital care. Scandinavian Journal of Public Health 2010;38:566‐573. [DOI] [PubMed] [Google Scholar]
Anonymous 2003 {published data only}
- Anonymous. Aspirin and cardiovascular disease. South African Medical Journal 2003;93(12 I):892. [Google Scholar]
Anonymous 2006 {published data only}
- Anonymous. CV interventions in those aged >=75 years are not useful. Journal of Family Practice 2006;55(12):1037. [PubMed] [Google Scholar]
Armstrong 2011 {published data only}
- Armstrong A, Reid RD, McDonnell L, Aitken DA, Pipe AL. Impact of acute versus stable coronary heart disease on smoking cessation success. Journal of Cardiopulmonary Rehabilitation and Prevention 2011;31(5):E6. [Google Scholar]
Avanzini 2011 {published data only}
- Avanzini F, Giulio P, Amodeo R, Baldo S, Bergna ML, Busi G, Carlino L, Colombo F, Cotza R, Ponti A, Rocco E, Marigliani C, Negri E, Roncaglioni MC, Saltarel I, Sorbara L, Tavani A, Martini M. Effectiveness of a nurse‐led educational intervention for patients admitted for acute coronary syndrome. Assistenza Infermieristica e Ricerca: Air 2011;30:16‐23. [PubMed] [Google Scholar]
Bastian 2011 {published data only}
- Bastian LA, Fish LJ, Gierisch JM, Rohrer L, Stechuchak KM, Grambow S. Comparative effectiveness trial of family‐supported smoking cessation intervention versus standard telephone counseling for chronically ill veterans. Journal of General Internal Medicine 2011;26:S297. [Google Scholar]
Baughman 1982 {published data only}
- Baughman Kl, Hutter AMJ, DeSanctis RW, Kallman CH. Early discharge following acute myocardial infarction. Long‐term follow‐up of randomized patients. Archives of Internal Medicine 1982;142(5):875‐8. [PubMed] [Google Scholar]
Belson 2002 {published data only}
- Belson MG, Kelley TR. Bupropion exposures: Clinical manifestations and medical outcome. Journal of Emergency Medicine 2002;23(3):223‐30. [DOI] [PubMed] [Google Scholar]
Bock 2008 {published data only}
- Bock BC, Becker BM, Niaura RS, Partridge R, Fava JL, Trask P. Smoking cessation among patients in an emergency chest pain observation unit: outcomes of the Chest Pain Smoking Study (CPSS). Nicotine & Tobacco Research 2008;10(10):1523‐31. [DOI] [PubMed] [Google Scholar]
Bolman 2002b {published data only}
- Bolman C, Vries H, Breukelen G. Evaluation of a nurse‐managed minimal‐contact smoking cessation intervention for cardiac inpatients. Health Education Research 2002;17(1):99‐116. [DOI] [PubMed] [Google Scholar]
Brenner 1989 {published data only}
- Brenner H. Sekundärprävention bei Herzinfarktpatienten. Report Psychologie 1989;14(9):26‐34. [Google Scholar]
Brown 2004 {published data only}
- Brown DW. Nurse‐led intervention increases smoking cessation among people with coronary heart disease. Evidence Based Healthcare 2004;8(3):128‐30. [Google Scholar]
Byfield 2001 {published data only}
- Byfield CL. Development and evaluation of a lifestyle physical activity intervention for obese sedentary women. Colorado State University PhD Thesis 2001.
Campbell 1996 {published data only}
- Campbell IA, Prescott RJ, Tjeder‐Burton SM. Transdermal nicotine plus support in patients attending hospital with smoking‐related diseases: a placebo‐controlled study. Respiratory Medicine 1996;90(1):47‐51. [DOI] [PubMed] [Google Scholar]
Campbell 1998a {published data only}
- Campbell NC, Ritchie LD, Thain J, Deans HG, Rawles JM, Squair JL. Secondary prevention in coronary heart disease: A randomised trial of nurse led clinics in primary care. Heart 1998;80(5):447‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1998b {published data only}
- Campbell NC, Thain J, Deans HG, Ritchie LD, Rawles JM, Squair JL. Secondary prevention clinics for coronary heart disease: randomised trial of effect on health. BMJ 1998;316:1434‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2004 {published data only}
- Campbell NC. Secondary prevention clinics: Improving qualify of life and outcome. Heart 2004;90:iv29‐iv32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2005 {published data only}
- Chan SSC, Lam TH, Lau CP. The effectiveness of a nurse‐delivered smoking cessation intervention for cardiac patients: a randomised controlled trial. Nicotine & Tobacco Research 2005;7(4):692. [Google Scholar]
Cho 2012 {published data only}
- Cho SH. Effects of a smoking cessation education on smoking cessation, endothelial function, and serum carboxyhemoglobin in male patients with variant angina. Journal of Korean Academy of Nursing 2012;42:190‐198. [DOI] [PubMed] [Google Scholar]
Chow 2010 {published data only}
- Chow CK, Jolly S, Rao‐Melacini P, Fox KAA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010;121(6):750‐758. [DOI] [PubMed] [Google Scholar]
Circo 1985 {published data only}
- Circo A, Tosto A, Raciti S. First results of an anti‐smoke outpatient unit: Comparison among three methods [Primi risultati di un ambulatorio anti‐fumo. Confronto fra tre metodiche]. Rivista di Cardiologia Preventiva e Riabilitativa 1985;3:147‐51. [Google Scholar]
Connett 1984 {published data only}
- Connett JE, Stamler J. Responses of black and white males to the special intervention program of the Multiple Risk Factor Intervention Trial. American Heart Journal 1984;108(3 Pt 2):839‐48. [DOI] [PubMed] [Google Scholar]
Cook 1989 {published data only}
- Cook DG, Shaper AG. Stopping smoking and risk of ischaemic heart disease. Lancet 1989;1(8643):895. [DOI] [PubMed] [Google Scholar]
Cupples 1999 {published data only}
- Cupples ME, McKnight A. Five year follow up of patients at high cardiovascular risk who took part in randomised controlled trial of health promotion. BMJ 1999;319:687‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidoff 2001 {published data only}
- Davidoff R, McTiernan A, Constantine G, Davis KD, Balady GJ, Mendes LA, Rudolph RE, Bowen DJ. Echocardiographic examination of women previously treated with fenfluramine ‐ Long‐term follow‐up of a randomized, double‐blind, placebo‐controlled trial. Archives of Internal Medicine 2001;161(11):1429‐36. [PUBMED: medication & smoking cessation] [DOI] [PubMed] [Google Scholar]
Eaker 1982 {published data only}
- Eaker ED, Benfari RC, Reed RB. Coronary risk factor intervention: characteristics associated with change. Journal of Clinical Psychology 1982;38(4):703‐17. [PubMed] [Google Scholar]
Einecke 2004 {published data only}
- Einecke D. A new pill for metabolic syndrome. Successful control of lipids, kilos and cigarettes. MMW Fortschritte der Medizin 2004;146(13):10‐1. [PubMed] [Google Scholar]
Ellingsen 2003 {published data only}
- Ellingsen I, Hjermann I, Abdelnoor M, Hjerkinn EM, Tonstad S. Dietary and antismoking advice and ischemic heart disease mortality in men with normal or high fasting triacylglycerol concentrations: a 23‐y follow‐up study. American Journal of Clinical Nutrition 2003;78(5):935‐40. [DOI] [PubMed] [Google Scholar]
Engblom 1992 {published data only}
- Engblom E, Rönnemaa T, Hämäläinen H, Kallio V, Vänttinen E, Knuts LR. Coronary heart disease risk factors before and after bypass surgery: results of a controlled trial on multifactorial rehabilitation. European Heart Journal 1992;13:232‐7. [DOI] [PubMed] [Google Scholar]
Erdman 1983 {published data only}
- Erdman RA, Duivenvoorden HJ. Psychologic evaluation of a cardiac rehabilitation program: A randomized clinical trial in patients with myocardial infarction. Journal of Cardiac Rehabilitation 1983;3:696‐701. [Google Scholar]
Espinosa 2004 {published data only}
- Espinosa Caliani S, Bravo Navas JC, Gomez‐Doblas JJ, Collantes Rivera R, Gonzalez Jimenez B, Martinez Lao M, Teresa Galvan E. Postmyocardial infarction cardiac rehabilitation in low risk patients. Results with a coordinated program of cardiological and primary care. Revista Espanola de Cardiologia 2004;57(1):53‐59. [PubMed] [Google Scholar]
Finnegan 1985 {published data only}
- Finnegan DL, Suler JR. Psychological factors associated with maintenance of improved health behaviors in postcoronary patients. Journal of Psychology 1985;119(1):87‐94. [DOI] [PubMed] [Google Scholar]
Fletcher 1987 {published data only}
- Fletcher V. An individualized teaching programme following primary uncomplicated myocardial infarction. Journal of Advanced Nursing 1987;12(2):195‐200. [DOI] [PubMed] [Google Scholar]
Fonarow 2007 {published data only}
- Fonarow GC, Abraham WT, Albert NM, Gattis SW, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB. Influence of a performance‐improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE‐HF). Archives of Internal Medicine 2007;167(14):1493‐502. [DOI] [PubMed] [Google Scholar]
Fortmann 1994 {published data only}
- Fortmann SP. Nicotine replacement therapy for patients with coronary artery disease. Working Group for the Study of Transdermal Nicotine in Patients with Coronary artery disease. Archives of Internal Medicine 1994;154:989‐95. [PubMed] [Google Scholar]
Fox 2002 {published data only}
- Fox KF, Wood DA, Wright M, Bond S, Nuttall M, Arora B, Dawson E, Devane P, Sutcliffe SJ, Brown K. Evaluation of a cardiac prevention and rehabilitation programme for all patients at first presentation with coronary artery disease. Journal of Cardiovascular Risk 2002;9(6):355‐9. [DOI] [PubMed] [Google Scholar]
Frasure‐Smith 1997 {published data only}
- Frasure‐Smith N, Lesperance F, Prince RH, Verrier P, Garber RA, Juneau M, et al. Randomised trial of home‐based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997;350(9076):473‐9. [DOI] [PubMed] [Google Scholar]
Fredrickson 1995 {published data only}
- Fredrickson PA, Hurt RD, Lee GM, Wingender L, Croghan IT, Lauger G, et al. High dose transdermal nicotine therapy for heavy smokers: safety, tolerability and measurement of nicotine and cotinine levels. Psychopharmacology 1995;122(3):215‐22. [DOI] [PubMed] [Google Scholar]
Giallauria 2005 {published data only}
- Giallauria F, Paragliola T, Pilerci F, Del FD, De LA, Manakos A, Lucci R, Psaroudaki M, D'Agostino M, Vigorito C. Role of smokers in the household and of cardiac rehabilitation in smoking behaviour after acute myocardial infarction. Monaldi Archives for Chest Disease 2005;64(2):110‐5. [DOI] [PubMed] [Google Scholar]
Gohlke 2004 {published data only}
- Gohlke H. Lifestyle modification ‐ is it worth it?. Herz 2004;29(1):139‐144. [DOI] [PubMed] [Google Scholar]
Goldenberg 2003 {published data only}
- Goldenberg I, Jonas M, Tenenbaum A, Boyko V, Matetzky S, Shotan A, Behar S, Reicher‐Reiss H, Bezafibrate Infarction Prevention Study Group. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Archives of Internal Medicine 2003;163(19):2301‐5. [DOI] [PubMed] [Google Scholar]
Guthrie 2001 {published data only}
- Guthrie RM. The effects of postal and telephone reminders on compliance with pravastatin therapy in a national registry: Results of the first myocardial infarction risk reduction program. Clinical Therapeutics 2001;23:970‐980. [DOI] [PubMed] [Google Scholar]
Hajek 2010 {published data only}
- Hajek Peter, Taylor Tamara, McRobbie Hayden. The effect of stopping smoking on perceived stress levels. Addiction 2010;105:1466‐1471. [DOI] [PubMed] [Google Scholar]
Hall 1983 {published data only}
- Hall SM, Bachman J, Henderson JB, Barstow R, Jones RT. Smoking cessation in patients with cardiopulmonary disease: An initial study. Addictive Behaviors 1983;8(1):33‐42. [DOI] [PubMed] [Google Scholar]
Haskell 1994 {published data only}
- Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation 1994;89(3):975‐90. [DOI] [PubMed] [Google Scholar]
Hilleman 2004 {published data only}
- Hilleman DE, Mohiuddin SM, Packard KA. Comparison of conservative and aggressive smoking cessation treatment strategies following coronary artery bypass graft surgery. Chest 2004;125(2):435‐8. [DOI] [PubMed] [Google Scholar]
Hjermann 1986 {published data only}
- Hjermann I, Holme I, Leren P. Oslo Study Diet and Antismoking Trial. Results after 102 months. American Journal of Medicine 1986;80:7‐11. [DOI] [PubMed] [Google Scholar]
Holme 2006 {published data only}
- Holme I, Haaheim LL, Tonstad S, Hjermann I. Effect of dietary and antismoking advice on the incidence of myocardial infarction: a 16‐year follow‐up of the Oslo Diet and Antismoking Study after its close. Nutrition Metabolism & Cardiovascular Diseases 2006;16(5):330‐8. [PUBMED: risk assessment] [DOI] [PubMed] [Google Scholar]
Horlick 1984 {published data only}
- Horlick L, Cameron R, Firor W, Bhalerao U, Baltzan R. The effects of education and group discussion in the post myocardial infarction patient. Journal of Psychosomatic Research 1984;28(6):485‐92. [DOI] [PubMed] [Google Scholar]
Hosokawa 2008 {published data only}
- Hosokawa S, Hiasa Y, Miyazaki S, Ogura R, Miyajima H, Ohara Y, Yuba K, Suzuki N, Takahashi T, Kishi K, Ohtani R. Effects of smoking cessation on coronary endothelial function in patients with recent myocardial infarction. International journal of cardiology 2008;128(1):48‐52. [DOI] [PubMed] [Google Scholar]
Houston 2005 {published data only}
- Houston TK, Allison JJ, Person S, Kovac S, Williams OD, Kiefe CI. Post‐myocardial infarction smoking cessation counseling: Associations with immediate and late mortality in older Medicare patients. American Journal of Medicine 2005;118(3):269‐275. [DOI] [PubMed] [Google Scholar]
Houston‐Miller 1997 {published data only}
- Miller NH, Smith PM, DeBusk RF, Sobel DS, Taylor CB. Smoking cessation in hospitalized patients: Results of a randomized trial.. Archives of International Medcine 1997;157:409‐15. [PubMed] [Google Scholar]
Jacobsen 2008 {published data only}
- Jacobsen D, Sevin C. Improved care for patients with congestive heart failure. Joint Commission Journal on Quality & Patient Safety 2008;34(1):13‐19. [DOI] [PubMed] [Google Scholar]
Jain 2003 {published data only}
- Jain MK, Butler J, Mirvis DM. Heart failure: do we deliver quality care?. Tennessee Medicine 2003;96(2):81‐4. [PUBMED: ordered] [PubMed] [Google Scholar]
Jami 2007 {published data only}
- Jami P, Smith P, Moningi S, Moningi V, Martin SA, Rosencrance G, Reyes BJ. Compliance with Joint National Committee 7 guidelines in hypertension management in a teaching institution. American Journal of Medical Quality 2007;22(4):251‐258. [DOI] [PubMed] [Google Scholar]
Jatuporn 2003 {published data only}
- Jatuporn S, Sangwatanaroj S, Saengsiri AO, Rattanapruks S, Srimahachota S, Uthayachalerm W, Kuanoon W, Panpakdee O, Tangkijvanich P, Tosukhowong P. Short‐term effects of an intensive lifestyle modification program on lipid peroxidation and antioxidant systems in patients with coronary artery disease. Clinical Hemorheology & Microcirculation 2003;29(3‐4):429‐36. [PubMed] [Google Scholar]
Jolly 2003 {published data only}
- Jolly K, Lip GY, Sandercock J, Greenfield SM, Raftery JP, Mant J, Taylor R, Lane D, Lee KW, Stevens AJ. Home‐based versus hospital‐based cardiac rehabilitation after myocardial infarction or revascularisation: design and rationale of the Birmingham Rehabilitation Uptake Maximisation Study (BRUM): a randomised controlled trial [ISRCTN72884263]. BMC cardiovascular disorders 2003;3:10. [PUBMED: design and rationale of Jolly 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1996 {published data only}
- Jones DA, West RR. Psychological rehabilitation after myocardial infarction multicentre randomised controlled trial. BMJ 1996;313(7071):1517‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 1996 {published data only}
- Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. New England Journal of Medicine 1996;335(24):1792‐8. [DOI] [PubMed] [Google Scholar]
Joseph 2005 {published data only}
- Joseph AM, Bliss RL, Zhao F, Lando H. Predictors of smoking reduction without formal intervention. Nicotine & Tobacco Research 2005;7(2):277‐82. [DOI] [PubMed] [Google Scholar]
Kallio 1981 {published data only}
- Kallio V, Hämäläinen H, Arstila M, Luurila OJ, Hakkila J. Systematic treatment after acute myocardial infarction. Suomen Lääkärilehti 1981;36:1975‐82. [Google Scholar]
Knutsen 1991 {published data only}
- Knutsen SF, Knutsen R. The Tromso Survey: the Family Intervention study‐‐the effect of intervention on some coronary risk factors and dietary habits, a 6‐year follow‐up. Preventive Medicine 1991;20(2):197‐212. [DOI] [PubMed] [Google Scholar]
Kornitzer 1980 {published data only}
- Kornitzer M, Dramaix M, Kittel F, Backer G. The Belgian Heart disease Prevention Project: Changes in smoking habits after two years of intervention. Preventive Medicine 1980;9(4):496‐503. [DOI] [PubMed] [Google Scholar]
Kornitzer 1989 {published data only}
- Kornitzer M. The Belgian project for the prevention of cardiovascular diseases: a model of multifactorial prevention [Le projet belge de prevention des affections cardiovasculaires: un modele de prevention multifactorielle]. Bulletin et Memoires de l Academie Royale de Medecine de Belgique 1989;144(1‐2):101‐9. [PubMed] [Google Scholar]
Kotowycz 2010 {published data only}
- Kotowycz MA, Cosman TL, Tartaglia C, Afzal R, Syal RP, Natarajan MK. Safety and feasibility of early hospital discharge in ST‐segment elevation myocardial infarction‐‐a prospective and randomized trial in low‐risk primary percutaneous coronary intervention patients (the Safe‐Depart Trial). American heart journal 2010;159(1):117‐6. [DOI] [PubMed] [Google Scholar]
Kristeller 1993 {published data only}
- Kristeller JL, Merriam PA, Ockene JK, Ockene IS, Goldberg RJ. Smoking intervention for cardiac patients: in search of more effective strategies. Cardiology 1993;82(5):317‐24. [DOI] [PubMed] [Google Scholar]
Kuller 1991 {published data only}
- Kuller LH, Ockene JK, Meilahn E, Wentworth DN, Svendsen KH, Neaton JD. Cigarette smoking and mortality. MRFIT Research Group. Preventive Medicine 1991;20(5):638‐54. [DOI] [PubMed] [Google Scholar]
Lancaster 1999 {published data only}
- Lancaster T, Dobbie W, Vos K, Yudkin P, Murphy M, Fowler G. Randomized trial of nurse‐assisted strategies for smoking cessation in primary care. British Journal of General Practice 1999;49(440):191‐94. [PMC free article] [PubMed] [Google Scholar]
La Rosa 2006 {published data only}
- Rosa JC, Conti CR. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. ACC Cardiosource Review Journal 2006;15(5):97‐99. [Google Scholar]
Lear 2002 {published data only}
- Lear SA, Ignaszewski A, Linden W, Brozic A, Kiess M, Spinelli JJ, Pritchard PH, Frohlich JJ. A randomized controlled trial of an extensive lifestyle management intervention (ELMI) following cardiac rehabilitation: Study design and baseline data. Current Controlled Trials in Cardiovascular Medicine 2002;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2008 {published data only}
- Leung S, Gallup D, Mahaffey KW, Cohen M, Antman EM, Goodman SG, Harrington RA, Langer A, Aylward P, Ferguson JJ, Califf RM, Synergy Trial I. Smoking status and antithrombin therapy in patients with non‐ST‐segment elevation acute coronary syndrome. American Heart Journal 2008;156(1):177‐184. [DOI] [PubMed] [Google Scholar]
Maddison 2011 {published data only}
- Maddison R, Whittaker R, Stewart R, Kerr A, Jiang Y, Kira G, Carter KH, Pfaeffli L. HEART: heart exercise and remote technologies: a randomized controlled trial study protocol. BMC Cardiovascular Disorders 2011;11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marra 1985 {published data only}
- Marra S, Paolillo V, Spadaccini F, Angelino PF. Long‐term follow‐up after a controlled randomized post‐myocardial infarction rehabilitation programme: effects on morbidity and mortality. European Heart Journal 1985;6(8):656‐63. [DOI] [PubMed] [Google Scholar]
Matsui 2005 {published data only}
- Matsui S. Stratified analysis in randomized trials with noncompliance. Biometrics 2005;61(3):816‐23. [PUBMED: not primary data?, treatment refusal] [DOI] [PubMed] [Google Scholar]
Mayou 2002 {published data only}
- Mayou RA, Thompson DR, Clements A, Davies CH, Goodwin SJ, Normington K, Hicks N, Price J. Guideline‐based early rehabilitation after myocardial infarction. A pragmatic randomised controlled trial. Journal of Psychosomatic Research 2002;52(2):89‐95. [DOI] [PubMed] [Google Scholar]
McGee 2006 {published data only}
- McGee HM, Doyle F, Conroy RM, Harpe D, Shelley E. Impact of briefly‐assessed depression on secondary prevention outcomes after acute coronary syndrome: a one‐year longitudinal survey. BMC Health Services Research 2006;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McHugh 2001 {published data only}
- McHugh F, Lindsay GM, Hanlon P, Hutton I, Brown MR, Morrison C, et al. Nurse led shared care for patients on the waiting list for coronary artery bypass surgery: A randomised controlled trial. Heart 2001;86(3):317‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meland 1999 {published data only}
- Meland E, Maeland JG, Laerum E. The importance of self‐efficacy in cardiovascular risk factor change. Scandinavian Journal of Public Health 1999;27(1):11‐7. [DOI] [PubMed] [Google Scholar]
Murchie 2003 {published data only}
- Murchie P, Campbell NC, Ritchie LD, Simpson JA, Thain J. Secondary prevention clinics for coronary heart disease: Four year follow up of a randomised controlled trial in primary care. BMJ 2003;326(7380):84‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2007 {published data only}
- Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, Stroupe KT, Wu J, Clark D, Smith F, Gradus‐Pizlo I, Weinberger M, Brater DC. Pharmacist intervention to improve medication adherence in heart failure: A randomized trial. Annals of Internal Medicine 2007;146(10):714‐25. [DOI] [PubMed] [Google Scholar]
Muscari 2005 {published data only}
- Muscari A, Sbano D, Bastagli L, Poggiopollini G, Tomassetti V, Forti P, Boni P, Ravaglia G, Zoli M, Puddu P. Effects of weight loss and risk factor treatment in subjects with elevated serum C3, an inflammatory predictor of myocardial infarction. International journal of cardiology 2005;100(2):217‐23. [DOI] [PubMed] [Google Scholar]
Nisbeth 2000 {published data only}
- Nisbeth O, Klausen K, Andersen LB. Effectiveness of counselling over 1 year on changes in lifestyle and coronary heart disease risk factors. Patient Education & Counseling 2000;40(2):121‐31. [DOI] [PubMed] [Google Scholar]
O'Malley 2003 {published data only}
- O'Malley PG, Feuerstein IM, Taylor AJ. Impact of electron beam tomography, with or without case management, on motivation, behavioral change, and cardiovascular risk profile ‐ A randomized controlled trial. Jama‐Journal of the American Medical Association 2003;289(17):2215‐23. [DOI] [PubMed] [Google Scholar]
O'Neil 2011 {published data only}
- O'Neil A, Hawkes AL, Chan B, Sanderson K, Forbes A, Hollingsworth B, Atherton J, Hare DL, Jelinek M, Eadie K, Taylor CB, Oldenburg B. A randomised, feasibility trial of a tele‐health intervention for acute coronary syndrome patients with depression ('MoodCare'): study protocol. BMC Cardiovascular Disorders 2011;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldridge 1997 {published data only}
- Oldridge NB. Cardiac rehabilitation and risk factor management after myocardial infarction ‐ Clinical and economic evaluation. Wiener Klinische Wochenschrift 1997;109:6‐16. [PubMed] [Google Scholar]
Ong 2005 {published data only}
- Ong KC, Cheong GN, Prabhakaran L, Earnest A. Predictors of success in smoking cessation among hospitalized patients. Respirology 2005;10(1):63‐9. [DOI] [PubMed] [Google Scholar]
Ornish 1990 {published data only}
- Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 1990;336(8708):129‐33. [DOI] [PubMed] [Google Scholar]
Patel 1985 {published data only}
- Patel C, Marmot MG, Terry DJ, Carruthers M, Hunt B, Patel M. Trial of relaxation in reducing coronary risk: four year follow up. British Medical Journal Clinical Research Ed 1985;290(6475):1103‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perk 2000 {published data only}
- Perk J, Veress G. Cardiac rehabilitation: applying exercise physiology in clinical practice. European Journal of Applied Physiology 2000;83(4‐5):457‐62. [DOI] [PubMed] [Google Scholar]
Piestrzeniewicz 2004 {published data only}
- Piestrzeniewicz K, Navarro‐Kuczborska N, Bolinska H, Jegier A, Maciejewski M. The impact of comprehensive cardiac rehabilitation in patients up to 55 years old after acute myocardial infarction treated with primary coronary intervention. Polskie Archiwum Medycyny Wewnetrznej 2004;111(3):309‐17. [PUBMED: not available in English] [PubMed] [Google Scholar]
Plans‐Rubio 2004 {published data only}
- Plans‐Rubio P. Allocation of resources between smoking cessation methods and lovastatin treatment of hypercholesterolaemia: based on cost effectiveness and the social welfare function. Pharmacoeconomics 2004;22(1):55‐69. [DOI] [PubMed] [Google Scholar]
Powers 2011 {published data only}
- Powers BJ, Danus S, Grubber JM, Olsen MK, Oddone EZ, Bosworth HB. The effectiveness of personalized coronary heart disease and stroke risk communication. American Heart Journal 2011;161:673‐680. [DOI] [PubMed] [Google Scholar]
Prieme 1998 {published data only}
- Prieme H, Nyyssonen K, Gronbaek K, Klarlund M, Loft S, Tonnesen P, Salonen JT, et al. Randomized controlled smoking cessation study: Transient increase in plasma high density lipoprotein but no change in lipoprotein oxidation resistance. Scandinavian Journal of Clinical and Laboratory Investigation 1998;58(1):11‐18. [DOI] [PubMed] [Google Scholar]
Puura 2003 {published data only}
- Puura A. Transdermal nicotine increases heart rate after endotracheal intubation. Methods and Findings in Experimental and Clinical Pharmacology 2003;25(5):383‐5. [DOI] [PubMed] [Google Scholar]
Quist‐Paulsen 2006b {published data only}
- Quist‐Paulsen P, Lydersen S, Bakke PS, Gallefoss F. Cost effectiveness of a smoking cessation program in patients admitted for coronary heart disease. European Journal of Cardiovascular Prevention & Rehabilitation 2006;13(2):274‐80. [PUBMED: cost effectiveness] [DOI] [PubMed] [Google Scholar]
Redfern 2009 {published data only}
- Redfern J, Briffa T, Ellis E, Freedman SB. Choice of secondary prevention improves risk factors after acute coronary syndrome: 1‐year follow‐up of the CHOICE (Choice of Health Options In prevention of Cardiovascular Events) randomised controlled trial. Heart 2009;95:468‐475. [DOI] [PubMed] [Google Scholar]
Reid 2005 {published data only}
- Reid RD, Dafoe WA, Morrin L, Mayhew A, Papadakis S, Beaton L, Oldridge NB, Coyle D, Wells GA. Impact of program duration and contact frequency on efficacy and cost of cardiac rehabilitation: Results of a randomized trial. American heart journal 2005;149(5):862‐8. [DOI] [PubMed] [Google Scholar]
Reid 2007 {published data only}
- Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: a pilot study. Patient Education & Counseling 2007;66(3):319‐26. [DOI] [PubMed] [Google Scholar]
Rice 1994 {published data only}
- Rice VH, Fox DH, Lepczyk M, Sieggreen M, Mullin M, Jarosz P, et al. A comparison of nursing interventions for smoking cessation in adults with cardiovascular health problems. Heart & Lung 1994;23(6):473‐86. [PubMed] [Google Scholar]
Rigotti 2006 {published data only}
- Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y, Swartz S, Torres‐Finnerty N, Emmons KM, Singer DE. Bupropion for smokers hospitalized with acute cardiovascular disease. American Journal of Medicine 2006;119(12):1080‐7. [DOI] [PubMed] [Google Scholar]
Rigotti 2011 {published data only}
- Rigotti NA, Bitton A, Kelley JK, Hoeppner BB, Levy DE, Mort E. Offering population‐based tobacco treatment in a healthcare setting: a randomized controlled trial. American Journal of Preventive Medicine 2011;41:498‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Risser 1990 {published data only}
- Risser NL, Belcher DW. Adding spirometry, carbon monoxide and pulmonary symptom results to smoking cessation couseling: a randomized trial. Journal of General Internal Medicine 1990;5(1):16‐22. [DOI] [PubMed] [Google Scholar]
Rollins 2004 {published data only}
- Rollins G. Web‐based patient management tool and collaborative quality improvement boost heart disease guideline compliance. Report on Medical Guidelines & Outcomes Research 2004;15(4):7‐9. [PUBMED: paper not available] [PubMed] [Google Scholar]
Rose 1978 {published data only}
- Rose G, Hamilton PJ. A randomised controlled trial of the effect on middle‐aged men of advice to stop smoking. Journal of Epidemiology & Community Health 1978;32(4):275‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 1982 {published data only}
- Rose G, Hamilton PJ, Colwell L, Shipley MJ. A randomised controlled trial of anti‐smoking advice: 10‐year results. Journal of Epidemiology & Community Health 1982;36:102‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 1992 {published data only}
- Rose G, Colwell L. Randomised controlled trial of anti‐smoking advice: final (20 year) results. Journal of Epidemiology & Community Health 1992;46(1):75‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanders 1989 {published data only}
- Sanders D, Fowler G, Mant D, Fuller A, Jones L, Marzillier J. Randomized controlled trial of anti‐smoking advice by nurses in general practice. Journal of the Royal College of Physicians of London 1989;39(324):273‐6. [PMC free article] [PubMed] [Google Scholar]
Scherr 2010 {published data only}
- Scherr C, Cunha AB, Magalhaes CK, Abitibol RA, Barros M, Cordovil I. Life‐habit intervention in a public institution. Arquivos Brasileiros de Cardiologia 2010;94:730‐737. [DOI] [PubMed] [Google Scholar]
Schimmer 2006 {published data only}
- Schimmer C, Krannich JH, Brauchle‐Hopp U, Elert O. Development of cardiovascular risk factors in patients after coronary artery bypass grafting with an in‐hospital rehabilitation programme (WHO Stage I of Rehabilitation): 1‐year follow‐up. Rehabilitation 2006;45(2):95‐101. [DOI] [PubMed] [Google Scholar]
Schmitz 1999 {published data only}
- Schmitz JM, Spiga R, Rhoades HM, Fuentes F, Grabowski J. Smoking cessation in women with cardiac risk: a comparative study of two theoretically based therapies. Nicotine & Tobacco Research 1999;1(1):87‐94. [DOI] [PubMed] [Google Scholar]
Schoenenberger 2010 {published data only}
- Schoenenberger AW, Jamshidi P, Kobza R, Zuber M, Stuck AE, Pfisterer M, Erne P. Progression of coronary artery disease during long‐term follow‐up of the Swiss Interventional Study on Silent Ischemia Type II (SWISSI II). Clinical Cardiology 2010;33(5):289‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2006 {published data only}
- Schumacher A, Peersen K, Sommervoll L, Seljeflot I, Arnesen H, Otterstad JE. Physical performance is associated with markers of vascular inflammation in patients with coronary heart disease. European Journal of Cardiovascular Prevention & Rehabilitation 2006;13(3):356‐62. [DOI] [PubMed] [Google Scholar]
Simon 2003 {published data only}
- Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J. Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: A randomized trial. American Journal of Medicine 2003;114(7):555‐62. [DOI] [PubMed] [Google Scholar]
Sippel 1999 {published data only}
- Sippel JM, Osborne ML, Bjorson W, Goldberg B, Buist AS. Smoking cessation in primary care clinics. Journal of General Internal Medicine 1999;14(11):670‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1998 {published data only}
- Smith SC. Need for a paradigm shift: The importance of risk factor reduction therapy in treating patients with cardiovascular disease. American Journal of Cardiology 1998;82(10B):10T‐3T. [DOI] [PubMed] [Google Scholar]
Sondergaard 2006 {published data only}
- Sondergaard J, Hansen DG, Aarslev P, Munck AP. A multifaceted intervention according to the Audit Project Odense method improved secondary prevention of ischemic heart disease: A randomised controlled trial. Family Practice 2006;23(2):198‐202. [DOI] [PubMed] [Google Scholar]
Steptoe 1999 {published data only}
- Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: Randomised trial. BMJ 1999;319(7215):943‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steptoe 2001 {published data only}
- Steptoe A, Kerry S, Rink E, Hilton S. The impact of behavioral counseling on stage of change in fat intake, physical activity, and cigarette smoking in adults at increased risk of coronary heart disease. American Journal of Public Health 2001;91(2):265‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 1999 {published data only}
- Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home‐based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet 1999;354(9184):1077‐83. [DOI] [PubMed] [Google Scholar]
Strandberg 2001 {published data only}
- Strandberg TE, Pitkala K, Berglind S, Nieminen MS, Tilvis RS. Multifactorial cardiovascular disease prevention in patients aged 75 years and older: A randomized controlled trial ‐ Drugs and evidence based medicine in the elderly (DEBATE) study. American Heart Journal 2001;142(6):945‐951. [DOI] [PubMed] [Google Scholar]
Suurkula 1996 {published data only}
- Suurkula M, Agewall S, Fagerberg B, Wendelhag I, Wikstrand J. Multiple risk intervention in high‐risk hypertensive patients. A 3‐year ultrasound study of intima‐media thickness and plaques in the carotid artery. Risk Intervention Study (RIS) Group. Arteriosclerosis, Thrombosis & Vascular Biology 1996;16(3):462‐70. [DOI] [PubMed] [Google Scholar]
Taylor 1988 {published data only}
- Taylor CB, Houston‐Miller N, Haskell WL, Debusk RF. Smoking cessation after acute myocardial infarction: The effects of exercise training. Addictive Behaviors 1988;13(4):331‐5. [DOI] [PubMed] [Google Scholar]
Taylor 1997 {published data only}
- Taylor CB, Houston‐Miller N, Smith PM, DeBusk RF. The effects of home‐based, case‐managed, multifactorial risk‐reduction program on reducing psychological distress in patients with cardiovascular disease. Journal of Cardiopulmonary Rehabilitation 1997;17:157‐62. [DOI] [PubMed] [Google Scholar]
Thomas 2007 {published data only}
- Thomas D. The care of smokers after acute coronary syndromes: an overlooked emergency. Archives des Maladies du Coeur et des Vaisseaux 2007;100(6‐7):509‐11. [PubMed] [Google Scholar]
Thompson 2009 {published data only}
- Thompson DR. Home‐based cardiac rehab was as effective as hospital‐based rehab in improving cardiac risk factors. Evidence Based Nursing 2009;12(3):83‐. [DOI] [PubMed] [Google Scholar]
Tiffany 1986 {published data only}
- Tiffany ST, Martin EM, Baker TB. Treatments for cigarette smoking: An evaluation of the contributions of aversion and counseling procedures. Behaviour Research & Therapy 1986;24(4):437‐52. [DOI] [PubMed] [Google Scholar]
Tonnesen 1999 {published data only}
- Tonnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, et al. Higher dosage nicotine patches increase one‐year smoking cessation rates: Results from the European CEASE trial. European Respiratory Journal 1999;13(2):238‐46. [DOI] [PubMed] [Google Scholar]
Tonstad 2003 {published data only}
- Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicenter randomised study. European Heart Journal 2003;24:946‐55. [DOI] [PubMed] [Google Scholar]
Toobert 1998 {published data only}
- Toobert DJ, Glasgow RE, Nettekoven LA, Brown JE. Behavioral and psychosocial effects of intensive lifestyle management for women with coronary heart disease. Patient Education & Counseling 1998;35(3):177‐88. [DOI] [PubMed] [Google Scholar]
Toobert 2000 {published data only}
- Toobert DJ, Glasgow RE, Radcliffe JL. Physiologic and related behavioral outcomes from the Women's Lifestyle Heart Trial. Annals of Behavioral Medicine 2000;22(1):1‐9. [DOI] [PubMed] [Google Scholar]
Trockel 2008 {published data only}
- Trockel M, Burg M, Jaffe A, Barbour K, Taylor CB. Smoking behavior postmyocardial infarction among ENRICHD trial participants: cognitive behavior therapy intervention for depression and low perceived social support compared with care as usual. Psychosomatic medicine 2008;70(8):875‐82. [DOI] [PubMed] [Google Scholar]
Tzivoni 1998 {published data only}
- Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovascular Drugs & Therapy 1998;12(3):239‐44. [DOI] [PubMed] [Google Scholar]
Uysal 2012 {published data only}
- Uysal Hilal, Ozcan Seyda. The effect of individual training and counselling programme for patients with myocardial infarction over patients' quality of life. International Journal of Nursing Practice 2012;18:445‐453. [DOI] [PubMed] [Google Scholar]
Vasiliauskas 2009 {published data only}
- Vasiliauskas D, Jasiukeviciene L, Kubilius R, Arbaciauskaite R, Dovidaitiene D, Kubiliene L. The effectiveness of long‐term rehabilitation in patients with cardiovascular diseases. Medicina (Kaunas) 2009;45(9):673‐682. [PubMed] [Google Scholar]
Vedin 1976 {published data only}
- Vedin A, Wilhelmsson C, Tibblin G, Wilhelmsen L. The postinfarction clinic in Goteborg, Sweden. A controlled trial of a therapeutic organization. Acta Medica Scandinavica 1976;200(6):453‐6. [DOI] [PubMed] [Google Scholar]
Wallner 1999 {published data only}
- Wallner S, Watzinger N, Lindschinger M, Smolle KH, Toplak H, Eber B, et al. Effects of intensified lifestyle modification on the need for further revascularization after coronary angioplasty. European Journal of Clinical Investigation 1999;29(5):372‐9. [DOI] [PubMed] [Google Scholar]
Waters 1996 {published data only}
- Waters D, Lesperance J, Gladstone P, Boccuzzi SJ, Cook T, Hudgin R, et al. Effects of cigarette smoking on the angiographic evolution of coronary atherosclerosis: A Canadian Coronary Atherosclerosis Intervention trial (CCAIT) substudy. Circulation 1996;94:614‐21. [DOI] [PubMed] [Google Scholar]
Wewers 1994 {published data only}
- Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse‐delivered smoking cessation intervention among hospitalized postoperative patients‐influence of a smoking‐related diagnosis: A pilot study. Heart & Lung, 1994. p. Heart & Lung 1994;23(2):151‐6. [PubMed] [Google Scholar]
Whitlock 1997 {published data only}
- Whitlock EP, Vogt TM, Hollis JF, Lichtenstein E. Does gender affect response to a brief clinic‐based smoking intervention? American Journal of Preventive Medicine. American Journal of Preventive Medicine 1997;13(3):159‐66. [PubMed] [Google Scholar]
Wiggers 2006 {published data only}
- Wiggers LC, Oort FJ, Peters RJ, Legemate DA, Haes HC, Smets EM. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine & Tobacco Research 2006;8(4):581‐9. [DOI] [PubMed] [Google Scholar]
Wister 2007 {published data only}
- Wister A, Loewen N, Kennedy‐Symonds H, McGowan B, McCoy B, Singer J. One‐year follow‐up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. CMAJ Canadian Medical Association Journal 2007;177(8):859‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woollard 2003 {published data only}
- Woollard J, Burke V, Beilin LJ. Effects of general practice‐based nurse‐counselling on ambulatory blood pressure and antihypertensive drug prescription in patients at increased risk of cardiovascular disease. Journal of Human Hypertension 2003;17(10):689‐95. [DOI] [PubMed] [Google Scholar]
Xian 2010 {published data only}
- Xian Y, Pan W, Peterson ED, Heidenreich PA, Cannon CP, Hernandez AF, Friedman B, Holloway RG, Fonarow GC. Are quality improvements associated with the Get With the Guidelines‐Coronary Artery Disease (GWTG‐CAD) program sustained over time? A longitudinal comparison of GWTG‐CAD hospitals versus non‐GWTG‐CAD hospitals. American heart journal 2010;159(2):207‐14. [DOI] [PubMed] [Google Scholar]
Yorio 2008 {published data only}
- Yorio J, Viswanathan S, See R, Uchal L, McWhorter JA, Spencer N, Murphy S, Khera A, Lemos JA, McGuire DK. The effect of a disease management algorithm and dedicated postacute coronary syndrome clinic on achievement of guideline compliance: results from the parkland acute coronary event treatment study. Journal of Investigative Medicine 2008;56(1):15‐25. [DOI] [PubMed] [Google Scholar]
Zwisler 2005 {published data only}
- Zwisler AD, Schou L, Soja AM, Bronnum‐Hansen H, Gluud C, Iversen L, Sigurd B, Madsen M, Fischer‐Hansen J, Danrehab group. A randomized clinical trial of hospital‐based, comprehensive cardiac rehabilitation versus usual care for patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease (the DANREHAB trial)‐‐design, intervention, and population. American heart journal 2005;150(5):899. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Becker 2003 {published data only}
- Becker BM, Bock BC, Partridge RA. Smoking cessation interventions in the emergency department for smokers with chest pain. Acadademic Emergency Medicine 2003;10(5):507. [Google Scholar]
Boulay 2001 {published data only}
- Boulay P, Prud'Homme D. Risk factor management after short‐term versus long‐term cardiac rehabilitation program. Coronary Health Care 2001;5(3):133‐40. [Google Scholar]
Enriquez‐Puga 2001 {published data only}
- Enriquez‐Puga A, Khunti K. Secondary prevention of coronary heart disease in primary care. Journal of Clinical Governance 2001;9(4):211‐22. [Google Scholar]
Puente‐Silva 1989 {published data only}
- Puente‐Silva FG, Cicero R, Gonzalez E, Ocampo A. Comparative study of four programs for smoking cessation among chronic cardiorespiratory patients [Estudio comparativo de cuatro programas para el abandono del tabaquismo en pacientes cronicos cardiorespiratorios]. Revista Intercontinental de Psicologia y Educacion 1989;2(3):113‐31. [Google Scholar]
Additional references
ACC/AHA 2002
- Braunwald A. ACC/AHA guideline update for the management of patient with unstable angina and non‐ST‐segment elevation myocardial infarction. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina). Available at: http://www.americanheart.org/downloadable/heart/1016214837537UANSTEMI2002Web.pdf [accessed 01/01/2007] Vol. 2002. [DOI] [PubMed]
ACC/AHA 2004
- Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation 2004;110(14):e340. [PubMed] [Google Scholar]
Barth 2007
- Barth J, Bengel J. Smoking cessation in patients with coronary heart disease: Risk reduction and an evaluation of the efficacy of interventions. In: Jordan J, Bardé B, Zeiher A editor(s). Contributions toward evidence‐based psychocardiology : a systematic review of the literature. Washington DC; London: American Psychological Association, 2007:83‐105. [Google Scholar]
Barth 2013
- Barth J, Munder T, Gerger H, Nüesch E, Trelle S, Znoj H, et al. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: a network meta‐analysis. PLoS Medicine 2013;10(5):e1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2004
- Becker SA. A study of web usability for older adults seeking online health resources. ACM Transactions on Computer‐Human Interaction (TOCHI) 2004;11(4):387‐406. [Google Scholar]
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chow 2010
- Chow CK, Jolly S, Rao‐Melacini P, Fox KA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010;121:750–8. [DOI] [PubMed] [Google Scholar]
Civljak 2013
- Civljak M, Stead LF, Hartmann‐Boyce J, Sheikh A, Car J. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD007078.pub4] [DOI] [PubMed] [Google Scholar]
Cook 1986
- Cook DG, Shaper AG, Pocock SJ, Kussick SJ. Giving up smoking and the risk of heart attacks. A report from The British Regional Study. Lancet 1986;2(8520):1376‐80. [DOI] [PubMed] [Google Scholar]
Critchley 2003
- Critchley JA, Capewell S. Mortality risk reduction associated with coronary heart disease: a systematic review. JAMA 2003;290:86‐97. [DOI] [PubMed] [Google Scholar]
Cullen 1997
- Cullen P, Schulte H, Assmann G. The Münster Heart Study (PROCAM): total mortality in middle‐aged men is increased at low total and LDL cholesterol concentrations in smokers but not in nonsmokers. Circulation 1997;96(7):2128‐36. [DOI] [PubMed] [Google Scholar]
DeBacker 2003
- Backer G, Ambrosioni E, Borch‐Johnson K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice. European Heart Journal 2003;24:1601‐10. [DOI] [PubMed] [Google Scholar]
Deeks 1999
- Deeks J, on Behalf of the Statistical Methods Working Group of the Cochrane Collaboration. Statistical methods programmed in meta view. Version 4. Available at: http://www.cochrane.org/docs/statisticalmethods.pdf [accessed 01/01/2007] 1999.
ESC 2012
- Steg G, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. European Heart Journal 2012;33:2569–619. [DOI] [PubMed] [Google Scholar]
ESC 2013
- Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease. European Heart Journal 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
ESC/EACPR 2012
- Perk J, Backer G, Gohlke H, Graham I, Reiner ZE, Verschuren WMM, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). European Heart Journal 2012;33:1635–701. [DOI] [PubMed] [Google Scholar]
Fulton 1997
- Fulton JE, Shekelle RB. Cigarette smoking, weight gain, and coronary mortality: results from the Chicago Western Electric Study. Circulation 1997;96(5):1438‐44. [DOI] [PubMed] [Google Scholar]
Heckman 2010
- Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta‐analysis.. Tobacco Control 2013;19(5):410‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 1999
- Jacobs DR, Adachi H, Mulder I, Kromhout D, Menotti A, Nissinen A, et al. Cigarette smoking and mortality risk. Twenty‐five‐year follow‐up of the Seven Country Study. Archives of Family Medicine 1999;159:733‐40. [DOI] [PubMed] [Google Scholar]
Kawachi 1993
- Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation in relation to total mortality rates in women. A prospective cohort study. Annals of Internal Medicine 1993;119(10):992‐1000. [DOI] [PubMed] [Google Scholar]
Kawachi 1994
- Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation and time course of decreased risk of coronary heart disease in middle‐aged women. Archives of Internal Medicine 1994;154(2):169‐75. [PubMed] [Google Scholar]
Keil 1998
- Keil U, Liese AD, Hense HW, Filipiak B, Döring A, Stieber J, et al. Classical risk factors and their impact on incident non‐fatal and fatal myocardial infarction and all‐cause mortality in southern Germany. European Heart Journal 1998;19:1197‐207. [DOI] [PubMed] [Google Scholar]
Lancaster 2005
- Lancaster T, Stead L. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]
Lancaster 2009
- Lancaster T, Stead LF. Self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001118.pub2] [DOI] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Lin 2013
- Lin H, Wang H, Wu W, Lang L, Wang Q, Tian L. The effects of smoke‐free legislation on acute myocardial infarction: a systematic review and meta‐analysis. BMC public health 2013;13(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luoto 1998
- Luoto R, Prättäla R, Uutela A, Puska P. Impact of unhealthy behaviors on cardiovascular mortality in Finland, 1978‐1993. Preventive Medicine 1998;27:93‐100. [DOI] [PubMed] [Google Scholar]
Myung 2009
- Myung SK, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM. Effects of Web‐and computer‐based smoking cessation programs: meta‐analysis of randomized controlled trials. Archives of Internal Medicin 2009;169(10):929. [DOI] [PubMed] [Google Scholar]
Njolstad 1996
- Njolstad I, Arnesen E, Lund‐Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12‐year follow‐up of the Finnmark Study. Circulation 1996;93(3):450‐6. [DOI] [PubMed] [Google Scholar]
Noar 2007
- Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta‐analytic review of tailored print health behavior change interventions. Psychological Bulletin 2007;133(4):673‐93. [DOI] [PubMed] [Google Scholar]
Nyboe 1991
- Nyboe J, Jensen G, Appleyard M, Schnohr P. Smoking and the risk of first acute myocardial infarction. American Heart Journal 1991;122(2):438‐47. [DOI] [PubMed] [Google Scholar]
Ockene 1997
- Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. Circulation 1997;96(9):3243‐7. [DOI] [PubMed] [Google Scholar]
Prescott 1998
- Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ 1998;316:1043‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 1986
- Prochaska JO, DiClemente CC. The transtheoretical approach. In: Norcross JC editor(s). Casebook of eclectic psychotherapy. New York: Brunner & Mazel, 1987:Brunner & Mazel. [Google Scholar]
Rice 2013
- Rice VH, Hartmann‐Boyce J, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001188.pub4] [DOI] [PubMed] [Google Scholar]
Rigotti 2007
- Rigotti NA, Munafo MR, Murphy MFG, Stead L. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001837.pub2] [DOI] [PubMed] [Google Scholar]
Rigotti 2012
- Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rollnick 1997
- Rollnick S, Butler CC, Stott N. Helping smokers make decisions: the enhancement of brief intervention for general medical practice. Patient Education and Counseling 1997;31(3):191‐203. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med 2010;7(3):e1000251. [DOI: 10.1371/journal.pmed.1000251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaper 1985
- Shaper AG, Pocock SJ, Walker M, Philips AN, Whitehead TP, MacFarlane PW. Risk factors for ischemic heart disease: the prospective phase of British Regional Heart Study. Journal of Epidemiology and Community Health 1985;39(3):197‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2011
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta‐analysis. Canadian Medical Association Journal 2011;183(12):1359‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2005
- Stead L, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2006
- Stead L, Lancaster T, Perera R. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002850.pub2] [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Stead 2013a
- Stead LF, Hartmann‐Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD002850.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2013b
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunstall‐Pedoe 1997
- Tunstall‐Pedoe H, Woodward M, Tavendale R, A´Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ 1997;315(7110):722‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tverdal 1993
- Tverdal A, Thelle D, Stensvold I, Leren P, Bjartveit K. Mortality in relation to smoking history: 13 years follow‐up of 68,000 Norwegian men and women 35‐49 years. Journal of Clinical Epidemiology 1993;46(5):475‐87. [DOI] [PubMed] [Google Scholar]
Wampold 1997
- Wampold, B. E, Mondin, G. W, Moody, M, Stich, F, Benson, K, & Ahn, H. N. A meta‐analysis of outcome studies comparing bona fide psychotherapies: Empiricially," all must have prizes.".. Psychological bulletin 1997;122(3):203. [Google Scholar]
Wiggers 2003
- Wiggers LC, Smets EM, Haes JC, Peters RJ, Legemate DA. Smoking cessation interventions in cardiovascular patients. European Journal of Vascular & Endovascular Surgery 2003;26:467‐75. [DOI] [PubMed] [Google Scholar]
Willett 1987
- Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. New England Journal of Medicine 1987;317(21):1303‐9. [DOI] [PubMed] [Google Scholar]
Woodward 1999
- Woodward M, Moohan M, Tunstall‐Pedoe H. Self‐reported smoking, cigarette yields and inhalation biochemistry related to the incidence of coronary heart disease: results from the Scottish Heart Health Study. Journal of Epidemiology & Biostatistics 1999;4(4):285‐95. [PubMed] [Google Scholar]


