Abstract
Background
Tardive dyskinesia is a chronic and disabling abnormal movement disorder affecting the muscles of the face, neck, tongue and the limbs. It is a common side effect of long‐term antipsychotic medication use in individuals with schizophrenia and other related psychotic disorders. While there are no known effective treatments for tardive dyskinesia to date, some reports suggest that pyridoxal 5 phosphate may be effective in reducing the severity of tardive dyskinesia symptoms.
Objectives
To determine the effectiveness of pyridoxal 5 phosphate (vitamin B6 or Pyridoxine or Pyridoxal phosphate) in the treatment of neuroleptic‐induced tardive dyskinesia among people with schizophrenia and other related psychotic disorders.
Search methods
The Cochrane schizophrenia group's register of clinical trials was searched (January 2013) using the phrase: [*Pyridoxal* OR *Pyridoxine* OR *P5P* OR *PLP* OR *tardoxal* OR *Vitamin B6* O *Vitamin B 6* R in title, abstract or index terms of REFERENCE, or interventions of STUDY. References of relevant identified studies were handsearched and where necessary, the first authors of relevant studies were contacted.
Selection criteria
Studies described as randomised controlled trials comparing the effectiveness pyridoxal 5 phosphate with placebo in the treatment of neuroleptic‐induced tardive dyskinesia among patients with schizophrenia.
Data collection and analysis
The review authors independently extracted data from each selected study. For dichotomous data, we calculated risk ratios (RR) and their 95% confidence intervals (CIs) on an intention‐to‐treat basis based on a fixed‐effect model. For continuous data, we calculated mean differences (MD) with 95% CIs, again based on a fixed‐effect model. We assessed risk of bias for each included study and used GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to rate quality of evidence.
Main results
Of the 12 records retrieved by the search, three trials published in 2001, 2003 and 2007, involving 80 inpatients with schizophrenia, aged 18 to 71 years, admitted in a psychiatric facility and followed up for a period nine weeks to 26 weeks, were included. Overall, pyridoxal 5 phosphate produced a significant improvement in tardive dyskinesia symptoms when compared with placebo, assessed by a change in Extrapyramidal Symptoms Rating Scale (ESRS) scores from baseline to the end of the first phase of the included studies (2 RCTs n = 65, RR 19.97, CI 2.87 to 139.19, low quality evidence). The endpoint tardive dyskinesia score (a measure of its severity) assessed with the ESRS, was significantly lower among participants on pyridoxal 5 phosphate compared to those on placebo (2 RCTs n = 60, MD ‐4.07, CI ‐6.36 to ‐1.79, low quality evidence).
It was unclear whether pyridoxal 5 phosphate led to more side effects (n = 65, 2 RCTs, RR 3.97, CI 0.20 to 78.59, low quality evidence) or caused deterioration in tardive dyskinesia symptoms when compared to placebo (n = 65, 2 RCTs, RR 0.16, CI 0.01 to 3.14, low quality evidence). Five participants taking pyridoxal 5 phosphate withdrew from the study because they were not willing to take more medications while none of the participants taking placebo discontinued their medications (n = 65, 2 RCTs, RR 8.72, CI 0.51 to 149.75, low quality evidence).
There was no significant difference in the endpoint positive and negative psychiatric symptoms scores, measured using the Positive and Negative symptoms Scale (PANSS) between participants taking pyridoxal 5 phosphate and those taking placebo. For the positive symptoms: (n = 15, 1 RCT, MD ‐1.50, CI ‐4.80 to 1.80, low quality evidence). For negative the symptoms: (n = 15, 1 RCT, MD ‐1.10, CI ‐5.92 to 3.72, low quality evidence).
Authors' conclusions
Pyridoxal 5 phosphate may have some benefits in reducing the severity of tardive dyskinesia symptoms among individuals with schizophrenia. However, the quality of evidence supporting the effectiveness of pyridoxal 5 phosphate in treating tardive dyskinesia is low, based on few studies, short follow‐up periods, small sample sizes and inadequate adherence to standardised reporting guidelines for randomised controlled trials among the included studies.
Keywords: Adult; Aged; Female; Humans; Male; Middle Aged; Antipsychotic Agents; Antipsychotic Agents/adverse effects; Dyskinesia, Drug‐Induced; Dyskinesia, Drug‐Induced/drug therapy; Dyskinesia, Drug‐Induced/etiology; Pyridoxal Phosphate; Pyridoxal Phosphate/adverse effects; Pyridoxal Phosphate/therapeutic use; Randomized Controlled Trials as Topic; Schizophrenia; Schizophrenia/drug therapy; Vitamin B Complex; Vitamin B Complex/adverse effects; Vitamin B Complex/therapeutic use
Plain language summary
Pyridoxal 5 phosphate for neuroleptic‐induced tardive dyskinesia.
Review question.
To look at the effects of pyridoxal 5 phosphate in the treatment of the movement disorder tardive dyskinesia, which is caused by long term use of antipsychotic drugs in people with schizophrenia.
Background.
The main treatment for schizophrenia is antipsychotic drugs. However, these drugs sometimes have severe and disabling side effects. Tardive dyskinesia is a movement disorder that causes the muscles of the face, neck, tongue and limbs to twitch. It can be caused by taking antipsychotic drugs over a long period of time. It often results in stigma, low quality of life and can lead to people stopping their antipsychotic medication. While there are no known treatments for tardive dyskinesia, some reports suggest that pyridoxal 5 phosphate may reduce tardive dyskinesia.
Study characteristics.
A search for relevant randomised studies was conducted in January 2013. The review includes three studies with 80 participants. All participants had tardive dyskinesia as a result of taking antipsychotic medication and were randomised into treatment groups. One group received pyridoxal 5 phosphate, the other group received a placebo. Antipsychotic treatment continued as usual throughout the trials.
Key results.
People taking pyridoxal 5 phosphate in these studies experienced more than 40% improvement in their tardive dyskinesia compared to those on placebo, so had less severe tardive dyskinesia. Experience of side effects were similar between treatment groups with participants taking pyridoxal 5 phosphate experiencing no more or less side effects than participants in the placebo group and they did not experience greater worsening of their psychiatric symptoms than those on placebo. Evidence from the studies is weak, but suggests pyridoxal 5 phosphate may be effective in the treatment of tardive dyskinesia.
Quality of the evidence.
Evidence is weak. The number of studies and participants is few. The quality of studies is low. Better evidence could be gathered by better designed, conducted and reported trials.
Ben Gray, Senior Peer Researcher, McPin Foundation. http://mcpin.org/.
Summary of findings
Summary of findings for the main comparison. Pyridoxal 5 phosphate (vitamin B6) compared with Placebo for neuroleptic‐induced tardive dyskinesia.
| Pyridoxal 5 phosphate (vitamin B6) compared with Placebo for neuroleptic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with neuroleptic‐induced tardive dyskinesia Settings: Inpatients Intervention: Pyridoxal 5 phosphate (vitamin B6) Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pyridoxal 5 phosphate (vitamin B6) | |||||
| Clinical efficacy ‐ improvement (> 40%) in ESRS scores from baseline ESRS1 Follow‐up: mean 17.5 weeks2 | Study population | RR 19.97 (2.87 to 139.19) | 65 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Global: Other adverse effects than tardive dyskinesia Self‐report by participants Follow‐up: mean 17.5 weeks2 | Study population | RR 3.97 (0.2 to 78.59) | 65 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Global: Discontinuation of Vitamin B6 Follow‐up: mean 17.5 weeks2 | Study population | RR 8.72 (0.51 to 149.75) | 65 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Tardive dyskinesia: Deterioration in tardive dyskinesia symptoms ESRS1 Follow‐up: mean 17.5 weeks2 | Study population | RR 0.16 (0.01 to 3.14) | 65 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 69 per 1000 | 11 per 1000 (1 to 217) | |||||
| Moderate | ||||||
| 46 per 1000 | 7 per 1000 (0 to 144) | |||||
| General mental state: Positive psychiatric symptom score PANSS5. Scale from: 7 to 49. Follow‐up: 9 weeks | The mean general mental state: positive psychiatric symptom score in the control groups was 15.6 | The mean general mental state: positive psychiatric symptom score in the intervention groups was 1.50 lower (4.80 lower to 1.80 higher) | 15 (1 study) | ⊕⊕⊝⊝ low3,6 | ||
| General mental state: Negative psychiatric symptoms PANSS5. Scale from: 7 to 49. Follow‐up: 9 weeks | The mean general mental state: negative psychiatric symptoms in the control groups was 14.6 | The mean general mental state: negative psychiatric symptoms in the intervention groups was 1.10 lower (5.92 lower to 3.72 higher) | 15 (1 study) | ⊕⊕⊝⊝ low3,6 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Extrapyramidal Symptom Rating Scale (high scores = worse) 2 Study duration: 9 weeks and 26 weeks respectively for the two included studies 3 Adequate description of randomisation methods was not provided. Methods for allocation concealment and blinding were not described. 4 The included studies have small sample sizes 5 Positive and Negative Symptoms Scale (high scores = worse) 6 Study has a small sample size
Background
Description of the condition
Since 1950 when the antipsychotic properties of chlorpromazine were first discovered (Lopez‐Munoz 2005), antipsychotic medications have been used effectively in treating the symptoms of schizophrenia, such as hallucinations and delusions. However, one of the drawbacks of long‐term antipsychotic medication use is a disabling, potentially irreversible movement disorder called tardive dyskinesia. Tardive dyskinesia is a late onset, movement disorder often resulting from long‐term use of neuroleptic (antipsychotic) medications. It manifests as repetitive, involuntary movements affecting the face, jaw, tongue, extremities and the trunk. Abnormal movements in tardive dyskinesia may involve tongue darting, repetitive grimacing, chewing movements of the mouth, sucking movements or puckering of the lips and purposeless, irregular, jerking movements of the limbs. These symptoms are highly stigmatising and associated with poor quality of life in affected individuals (Ascher‐Svanum 2008; Browne 1996).
Risk factors for tardive dyskinesia in patients with schizophrenia include female gender (Yassa 1992), older age (Leung 2003; Niehaus 2008), cognitive impairment or neurological deficits (Waddington 1987), diagnosis of a mood disorder especially depression (Casey 1995; Kane 1986; Sachdev 1989), worsening of psychopathology (Tenback 2007) and diabetes mellitus (Casey 1995; Woerner 1993). Tardive dyskinesia is also higher among those taking first generation (conventional) antipsychotic drugs compared with those on second generation (atypical) antipsychotic medications (Corell 2008; Nasrallah 2006). However, neuroleptic medications are not the sole cause of tardive dyskinesia, as similar abnormal motor movements have been reported among patients with schizophrenia who were never exposed to neuroleptic medications (Ayehu 2014; Fenton 2000).
The exact pathogenesis of tardive dyskinesia is unknown and several theories about its aetiology has been proposed. Foremost is the dopamine receptor supersensitivity hypothesis which posits that long‐term receptor antagonism by antipsychotic drugs results in dopamine receptor supersensitivity and the movement disorder in tardive dyskinesia (Sachdev 1989; Teo 2012). The neurotoxicity hypothesis states that tardive dyskinesia results from the neurotoxic effects of free radicals that are released during dopamine metabolism. It is believed that these free radicals cause cellular degeneration of striatal GABA‐ergic neurons, leading to the loss of their inhibitory activities and the hyperkinetic state of tardive dyskinesia (Sachdev 1989). The use of antioxidants as possible treatment for tardive dyskinesia symptoms is based on the neurotoxicity hypothesis (Zhang 2004).
There are reports that pyridoxal 5 phosphate, a metabolite of vitamin B6 (pyridoxine) helps in alleviating these symptoms (Miodownik 2008).
Description of the intervention
Pyridoxal 5 phosphate is the metabolically active form of vitamin B6, a collective term for the chemically‐ and structurally‐related substances, pyridoxine, pyridoxamine and pyridoxal. Vitamin B6 is a naturally occurring vitamin that can be obtained from both animal and plant sources. Following ingestion, pyridoxine is absorbed from the upper small intestine, where it is transported to the liver and oxidised to form pyridoxal. It is then phosphorylated by pyridoxal kinase to pyridoxal 5 phosphate. Unlike pyridoxine, which causes peripheral neuropathy when high doses are given, pyridoxal 5 phosphate is not associated with any known adverse effects, even at doses as high as 1200 mg/day (Miodownik 2008; Schaumburg 1983).
How the intervention might work
Pyridoxal 5 phosphate is a coenzyme in the decarboxylation of DOPA to dopamine and other neurotransmitters such as serotonin and gamma amino butyric acid (GABA). Although its actual mechanism of action is not clear, 9t is speculated that vitamin B6 may reduce the symptoms of tardive dyskinesia through its effects on the biogenic amines, mainly dopamine, GABA, serotonin and by scavenging free radicals (Lerner 2007).
Why it is important to do this review
Tardive dyskinesia is very disabling and disfiguring and its occurrence is associated with poor treatment adherence (Barnes 1993) and low quality of life (Ascher‐Svanum 2008). The reported prevalence of tardive dyskinesia ranged from 3% to 70% with a median of 24% among patients on long‐term use of neuroleptic medications (Yassa 1992).The annual incidence is 5.2% in those with a first episode of schizophrenia placed on neuroleptic medications, the rate increasing to about 20% at five years (Chakos 1996). Several drugs have been used in an attempt to treat tardive dyskinesia, but little evidence exists to support their efficacy. Such interventions include melatonin (Nelson 2003), cholinergic agonists (Caroff 2001), vitamin E (Adler 1998), calcium channel blockers (Fay‐McCarthy 1997) and clozapine (Bassitt 1998). While the evidence for the effectiveness for some of these interventions has been evaluated (McGrath 2001; Soares‐Weiser 2004; Tammenmaa 2002), no evaluation has taken place for pyridoxal 5 phosphate. Thus, it is uncertain if pyridoxal 5 phosphate is effective in the treatment of neuroleptic‐induced tardive dyskinesia. This review may provide evidence for its efficacy in the treatment of tardive dyskinesia among patients with schizophrenia and other psychotic disorders.
Objectives
To determine the effectiveness of pyridoxal 5 phosphate (vitamin B6, pyridoxine, pyridoxal phosphate) in the treatment of neuroleptic‐induced tardive dyskinesia among patients with schizophrenia and other related psychotic disorders.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials in this review. Quasi‐randomised studies, such as those allocating by alternate days of the week were not eligible for inclusion. We planned that if people were given additional treatments to pyridoxal 5 phosphate, we would only include the data if the adjunct treatment was evenly distributed between groups and it was only pyridoxal 5 phosphate that was randomised.
Types of participants
People with schizophrenia or other chronic mental illness, diagnosed by any criteria, irrespective of gender, age or nationality, who:
require the use of antipsychotics for more than three months;
developed tardive dyskinesia (diagnosed by any criteria at baseline and at least one other occasion) during antipsychotic treatment; and
for whom the dose of antipsychotic medication has been stable for one month or more (the same applied for those free of antipsychotics).
We included only trials where the majority of participants had a diagnosis of schizophrenia.
Types of interventions
Pyridoxal 5 phosphate (pyridoxal phosphate, pyridoxine, vitamin B6): any dose or means of administration.
Placebo or no intervention.
Types of outcome measures
Primary outcomes
1. Clinical efficacy: Clinical efficacy was defined as an improvement in the symptoms of tardive dyskinesia of more than 40%, on any peer‐reviewed scale, after at least four weeks of intervention
Secondary outcomes
1. Global Outcomes
1.1 Death due to suicide or other causes
1.2 Average endpoint dose of pyridoxal 5 phosphate or vitamin B6
1.3 Any adverse effects (other than deterioration of tardive dyskinesia symptoms or change in mental state)
1.4 Average time to discontinuation of pyridoxal 5 phosphateand reasons for discontinuation
2. Tardive dyskinesia
2.1 Deterioration in tardive dyskinesia symptoms
2.2 Average endpoint tardive dyskinesia score
3. General mental state changes
3.1 Any deterioration in psychiatric symptoms (such as delusions and hallucinations)
3.2 Average endpoint psychiatric symptoms score
4. 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADE profiler (GRADEPRO) to import data from RevMan 5 (Review Manager) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision‐making. We selected all of our listed outcomes for inclusion in the 'Summary of findings' table.
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
1. Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group’s Trials Register (January 2013) using the phrase:
[*Pyridoxal* OR *Pyridoxine* OR *P5P* OR *PLP* OR *tardoxal* OR *Vitamin B6* O *Vitamin B 6* R in title, abstract or index terms of REFERENCE, or interventions of STUDY]
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches of journals and conference proceedings (see Group Module). Incoming trials are assigned to relevant existing or new review titles.
Searching other resources
1. Reference searching
We inspected references of all identified studies for more studies.
2. Personal communication
We contacted the first author of each included study for more information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review authors AOA and OA independently inspected citations from the searches and identified relevant abstracts. A random 20% sample was independently re‐inspected by TMO to ensure reliability. Where disputes arose, the full report was acquired for more detailed scrutiny. Full reports of the abstracts meeting the review criteria were obtained and inspected by AOA and OA. Again, a random 20% of reports was re‐inspected by TMO in order to ensure reliable selection.There were no disagreements as to the inclusion or otherwise of any study among the review authors.
Data extraction and management
1. Extraction
Review authors AOA and OA extracted data from all included studies. In addition, to ensure reliability, TMO independently extracted data from a random sample of the included studies. Disagreements were discussed, decisions documented and, where necessary, the authors of studies were contacted for clarification. Data presented only in graphs and figures were extracted whenever possible, but were included only if two review authors independently had the same result. We contacted authors of studies through an open‐ended request in order to obtain missing information or for clarification where necessary.
2.1 Forms
We extracted data onto standard, simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if: a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and b) the measuring instrument has not been written or modified by one of the trialists for that particular trial.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data in our analyses.
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion: a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996);
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We presented and entered all useable change data into analyses.
2.7 Direction of graphs
We entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for pyridoxal 5 phosphate.If we had to enter data so the area to the left of the line indicated a favourable outcome for the control group, this was noted in the relevant graphs.
Assessment of risk of bias in included studies
Review authors AOA and OA independently assessed risk of bias by using criteria described in the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If inadequate details of randomisation and other characteristics of trials were provided, we contacted the authors of the studies in order to obtain further information.
The level of risk of bias was noted in both the text of the review and in the 'Table 1
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The number needed to treat to benefit/harm (NNTB/H) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Table 1, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated the mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, where scales of very considerable similarity were used, we presumed there was a small difference in measurement, and calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992), whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
We did not find any cluster trials in this search. If we had, we would have employed the methods below.
Where clustering is not accounted for in primary studies, we planned to present data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, if cluster trials are included, we will seek to contact the first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We sought statistical advice and were advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported, it will be assumed to be 0.1 (Ukoumunne 1999).
Where cluster studies have been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data from the first phase of cross‐over studies.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). For any particular outcome, where more than 50% of data was unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table by down‐rating quality. Finally, we also downgraded quality within the 'Table 1 where loss was 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome is between 0% and 50% and where these data are not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ was used for those who did not.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported, we reproduced these.
3.2 Standard deviations
When only the standard error (SE) was reported, SDs were calculated by the formula SD = SE * square root (n).
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers, others use the method of last observation carried forward (LOCF), while more recently methods such as multiple imputation or mixed effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach used. However, we prefer to use the more sophisticated approaches. E.g. MMRM or multiple‐imputation is preferred to LOCF and completer analyses would only be presented if some kind of ITT data are not available at all. Moreover, we addressed this issue in the item "incomplete outcome data" of the risk of bias tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies was investigated by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, was interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Higgins 2011). Had substantial levels of heterogeneity been found in the primary outcome, we would have explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011). We tried to locate protocols of the included randomised trials. Had protocols been available, outcomes in the protocol and in the published report would have been compared. Consequently, outcomes listed in the methods section of the trial report were compared with actually reported results.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We therefore chose to use a fixed‐effect method for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We did not undertake any subgroup analyses due to lack of data.
1.2 Clinical state, stage or problem
We undertook this review and provided an overview of the effects of pyridoxal 5 phosphate for people with neuroleptic‐induced tardive dyskinesia in general.
2. Investigation of heterogeneity
We would have reported inconsistency where it was high. First, we would have investigated whether data had been entered correctly. Second, where data were correct, the graphs would have been visually inspected and outlying studies were removed to see if homogeneity was restored.
We decided that if unanticipated clinical or methodological heterogeneity were obvious, we would simply state hypotheses regarding these for future reviews or versions of this review. We did not plan to undertake analyses relating to these.
Sensitivity analysis
1. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption/s and used data only from people who completed the study to that point. When there was a substantial difference, we reported the results and discussed them, but continued to employ our assumption.
2. Risk of bias
If we had found trials with a high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome, we would have analysed the effects of excluding trials that were judged to be at high risk of bias. If the exclusion of trials at high risk of bias does not substantially alter the direction of effect or the precision of the effect estimates, then data from these trials would have been included in the analysis.
3. Fixed and random effects
All data were synthesised using a fixed‐effect model.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
Using the search criteria, the electronic database search carried out in January 2013 yielded 12 trials No additional relevant references were identified from the reference lists of the published trials. See Figure 1.
1.
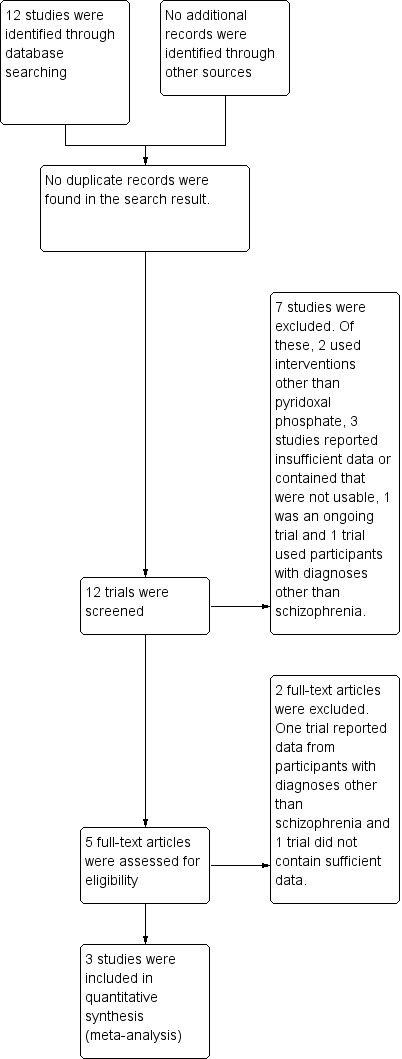
Study flow diagram.
Included studies
The review included three randomised, double blind, controlled studies published in 2001, 2003 and 2007 (Lerner 2001; Miodownik 2003; Lerner 2007). For more details, see Characteristics of included studies and the accompanying 'Risk of bias' tables.
1. Setting
The three studies included in this review were conducted at the mental health unit of Be'er Sheva Mental Health Centre in Israel.
2. Length of Trials
All three included studies had cross‐over designs. The included trials varied in duration; from nine weeks (Lerner 2001; Miodownik 2003), to 26 weeks (Lerner 2007), including a wash‐out period of either one week (Lerner 2001; Miodownik 2003) or two weeks (Lerner 2007).
3. Participants
There were a total of 80 participants in the included trials. Participants in all the studies were inpatients of a mental health facility with diagnoses of schizophrenia and schizoaffective disorders. The diagnoses were either based on DSM IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) criteria for schizophrenia or schizoaffective disorders (Lerner 2007) or ICD‐10 (International Statistical Classification of Diseases, 10th Revision) criteria (Miodownik 2003). One of the studies did not specify a diagnostic criteria (Lerner 2001).
In total there were more women (n = 44) than men (n = 36) in the included studies. Participants' ages ranged from 18 to 60 years (Miodownik 2003) to 28 to 71 years (Lerner 2001) and 20 to 66 years (Lerner 2007).
4. Trial Size
The overall trial size in the three included studies was small. The total number of participants in each trial ranged from 15 each in Lerner 2001 and Miodownik 2003 to 50 in Lerner 2007.
5. Interventions
Participants in the three included studies either received vitamin B6 or placebo, in addition to their ongoing medication regimens, which remained unchanged throughout the study durations. The maximal daily dose of vitamin B6 used in the included studies ranged from 400 mg (Lerner 2001; Miodownik 2003) to 1200 mg (Lerner 2007).
6. Outcomes
Details of scales providing useable data are described below:
6.1 Tardive dyskinesia
6.1.1. Extrapyramidal Symptom Rating Scale (ESRS) ‐ Chouinard 2005
The ESRS is a measure of the severity of movement disorders in patients with schizophrenia taking antipsychotic medications. The ESRS measures four types of drug‐induced movement disorders viz: parkinsonism, akathisia, dystonia and tardive dyskinesia. Each of these movement disorders can be scored from normal (score of zero) to extremely severe (score of six), according to the symptom severity.
ESRS was used to assess the severity of tardive dyskinesia symptoms and clinical efficacy of the interventions by the studies included in this review (Lerner 2001; Lerner 2007, Miodownik 2003). The assessments were performed at baseline, repeated either every week (Lerner 2001; Miodownik 2003), or at least every two weeks (Lerner 2007). A 20% reduction in ESRS scores from baseline to week four (Lerner 2001; Miodownik 2003) or week 12 (Lerner 2007) was taken to represent no clinical response, 21% to 40% as minimal improvement, 41% to 60% as moderate improvement, and more than 61% as marked improvement.
6.2 General mental state changes
6.2.1 Positive And Negative Symptoms Scale (PANSS) ‐ Kay 1987
The PANSS is a 30‐item seven‐point rating scale divided into positive (seven items), negative (seven items) and general psychopathology (16 items) sub‐scales. It was designed to assess the clinical symptoms of schizophrenia by a clinician rater, using a semi‐structured interview. Each item on the PANSS is scored on a seven‐point Likert scale ranging from one to seven and the rating can generally be completed in 30 to 40 minutes. The PANSS can reliably be administered to assess symptoms improvement and exacerbations.
PANSS was used in one of the included trials (Miodownik 2003) to assess the severity of psychiatric symptoms among participants taking vitamin B6 and placebo.
Excluded studies
Overall, eight studies were excluded from the review. Of these four randomised double blind controlled studies reported insufficient data and some data obtained following communication with study authors were not usable (Lerner 1999; Lerner 2002; Lerner 2007a; Lerner 2009). Two studies did not use vitamin B6/pyridoxal phosphate (Xiao 2002, Greenberg 2003), while two studies had participants with diagnoses other than schizophrenia/schizoaffective disorders (Venegas 2006, NCT00202280).
Ongoing studies
One study was still ongoing as at July 2013 (NCT00917293).
Risk of bias in included studies
For a graphical overview please see Figure 2 and Figure 3.
2.
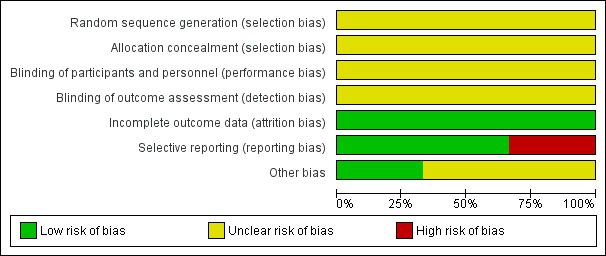
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
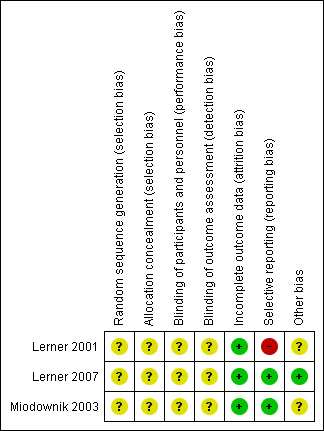
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included studies were described as randomised placebo‐controlled studies. However, none of the included studies described a specific method of randomisation or of allocation concealment, making allocation bias to be unclear.
Blinding
Although the included studies were described as double‐blind, placebo‐controlled studies, none explicitly described how this was undertaken and how the investigators, participants and outcome raters were blinded.
Incomplete outcome data
All participants randomised at the beginning of the three studies included were accounted for, making their attrition bias to be low.
Selective reporting
Two of the included studies (Lerner 2007; Miodownik 2003) accounted for all outcomes at the study completion. Lerner 2001 did not account for all outcomes with the specific number of participants who showed none, minimal, moderate or marked improvement in their tardive dyskinesia symptoms from both arms of the study not reported by this study, making the risk of bias to be high. Specific outcomes such as the number of participants who withdrew due to side effects were not accounted for by the second included study (Lerner 2001), thus making the risk of bias to be high. Also, due to inadequate reporting, it was difficult to ascertain from which arm of the study the numbers of participants who left the study early came from. For Lerner 2001, using a flow chart would have been an appropriate measure to accurately reflect these numbers.
Other potential sources of bias
1. Funding
Only one of the included studies reported financial sponsorship from a research institution (Lerner 2007). It was not clear if the other studies (Lerner 2001; Miodownik 2003) received funding from any sponsor.
2. Plasma level of pyridoxal 5 phosphate
The three studies included in this review did not mention who measured the plasma level of pyridoxal 5 phosphate and did not describe the extent to which the raters were independent.
Effects of interventions
See: Table 1
Although three studies were included in this review, in some instances the data reported by Miodownik 2003 were not useable, hence only outcomes from the other two included studies (Lerner 2001; Lerner 2007) were reported.
1. COMPARISON: Pyridoxal 5 phosphate (vitamin B6) versus placebo
1.1 Clinical efficacy (Extrapyramidal Symptom Rating Scale (ESRS), high = worse)
1.1.1 Improvement in tardive dyskinesia symptoms from baseline
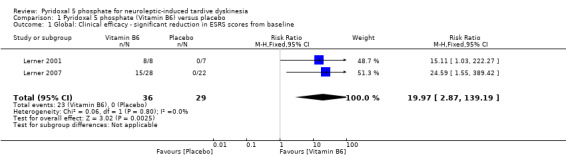
Lerner 2001 reported a 68.6% (SD 23%) reduction in ESRS symptoms from baseline to week four among participants taking vitamin B6, indicating marked improvement. In Lerner 2007, 15 (65%) of the 23 participants treated with vitamin B6 demonstrated > 40% reduction in their ESRS scores from baseline to week 12, indicating moderate to marked improvement. No clinical response to placebo was observed among participants in both studies, implying that placebo failed to produce not more than 20% reduction in ESRS scores from baseline to either week four or week 12 (Lerner 2001; Lerner 2007).The pooled summary result showed that pyridoxal 5 phosphate was clinically efficacious compared to placebo in reducing tardive dyskinesia symptoms (2 RCTs, n = 65, risk ratio (RR) 19.97, 95% confidence interval (CI) 2.87 to 139.19).
1.2 Global
1.2.1 Death due to suicide or other causes
None of the included studies reported any data on this outcome.
1.2.2 Average endpoint dose of pyridoxal 5 phosphate
Lerner 2001 and Miodownik 2003 reported an endpoint vitamin B6 dose of 400 mg daily, while Lerner 2007 reported an endpoint vitamin B6 dose of 1200 mg daily.
1.2.3 Other adverse effects than tardive dyskinesia
Adverse effects other than the deterioration of tardive dyskinesia symptoms were reported in two of the included studies (Lerner 2001; Lerner 2007). There was no significant difference in adverse effects reported by participants on pyridoxal 5 phosphate when compared with those on placebo (2 RCTs, n=65, RR 3.97, 95% CI 0.20 to 78.59).
1.2.4 Discontinuation of treatment
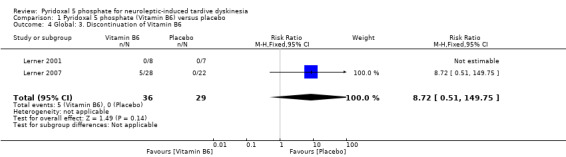
Lerner 2007 reported 5 participants on pyridoxal 5 phosphate who discontinued their medications because they were unwilling to take additional medications. None of the participants in Lerner 2001 study discontinued their treatment. The pooled summary estimate did not show any significant difference between participants on pyridoxal 5 phosphate who discontinued their medications compared to those on placebo. (2 RCTs, n= 65, RR 8.72, 95% CI 0.51 to 149.75). The time to discontinuation of pyridoxal 5 phosphate was not stated.
1.3 Tardive dyskinesia (ESRS, high = worse)
1.3.1 Deterioration in tardive dyskinesia symptoms
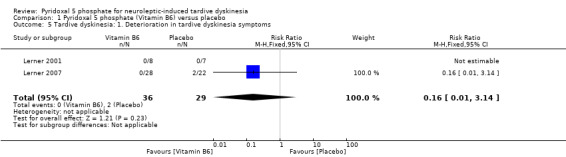
Lerner 2007 reported deterioration of tardive dyskinesia symptoms in two study participants on placebo. However, the pooled summary did not show any significant difference in deterioration of tardive dyskinesia symptoms in those on placebo compared to those on pyridoxal 5 phosphate (2 RCTS, n = 65, RR 0.16, 95% CI 0.01 to 3.14).
1.3.2 Average endpoint ESRS tardive dyskinesia score
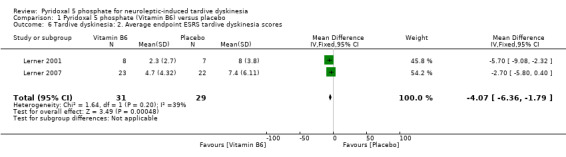
The pooled summary result from two of the included studies (Lerner 2001; Lerner 2007) showed a beneficial effect of pyridoxal 5 phosphate when compared with placebo (2 RCTs, n = 60, mean difference (MD) ‐4.07, 95% CI ‐6.36 to ‐1.79). Miodownik 2003 reported the endpoint ESRS tardive dyskinesia scores on an unscaled graph, thereby making the data unable to be used.
1.4 General mental state changes (Positive and Negative Symptoms Scale (PANSS), high = worse)
1.4.1 Deterioration in psychiatric symptoms (e.g. hallucinations and delusions)
None of the included studies reported data on this outcome
1.4.2 Average endpoint PANSS psychiatric symptoms score
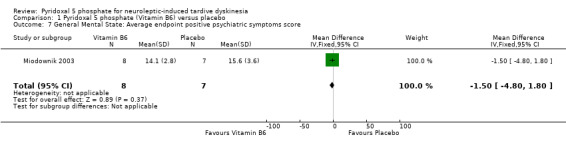
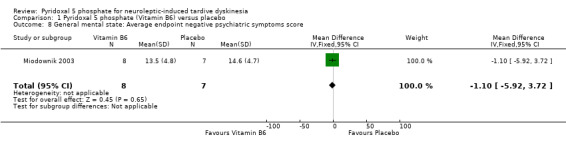
One of the included studies (Miodownik 2003) reported average endpoint psychiatric symptoms scores for participants on vitamin B6 and placebo, assessed with the PANSS. The pooled summary result for positive symptoms of schizophrenia did not show any significant differences between participants on vitamin B6 compared to those on placebo (1 RCT, n = 15, MD ‐1.50, CI ‐4.80 to 1.80). The pooled summary estimate showed no significant improvements in negative symptoms of schizophrenia (such as social withdrawal, lack of motivation) among participants on vitamin B6 compared to those on placebo (1 RCT, n = 15, MD ‐1.10, 95% CI ‐5.92 to 3.72).
Discussion
Summary of main results
The aim of this review was to determine the effectiveness of pyridoxal 5 phosphate (vitamin B6 or Pyridoxine or Pyridoxal phosphate) in the treatment of neuroleptic‐induced tardive dyskinesia among patients with schizophrenia and other related psychotic disorders. The review included all relevant randomised controlled trials obtained from the Cochrane Schizophrenia Group's Trial Register as at January 2013.
However, it should be noted that there are few studies included in this review, the size of the studies is small and all outcomes of interest were rated as 'low' quality evidence (also see Table 1).
Evidence from the three available studies in this review showed that pyridoxal 5 phosphate may be superior to placebo in reducing tardive dyskinesia symptoms among patients with schizophrenia on antipsychotic medications. The number of participants on pyridoxal 5 phosphate who had > 40% improvement in their tardive dyskinesia symptoms at the end of the study was significantly higher than those on placebo. However, its effect for other outcomes of interest are unclear. There was no significant difference in other adverse effects experienced by those on pyridoxal 5 phosphate compared to those on placebo and pyridoxal 5 phosphate did not cause significant worsening or improvement of psychiatric symptoms among study participants.
Overall completeness and applicability of evidence
The sample size of trials and the number of participants included in this review are small, furthermore the duration of follow‐up is short, thereby making generalisation of the evidence obtained on the efficacy of pyridoxal 5 phosphate compared with placebo in the treatment of tardive dyskinesia difficult and limited.
Quality of the evidence
There is a dearth of studies on the effectiveness of pyridoxal 5 phosphate in the treatment of tardive dyskinesia. The quality of evidence for the efficacy of pyridoxal 5 phosphate provided by the studies included in this review is low. This review evaluated evidence from three studies with a total of 80 participants, assessed over a period of nine to 26 weeks in a cross‐over study design. The quality of evidence from future studies could be significantly improved with larger sample sizes to increase their power, as well as longer duration of follow‐up for study participants, especially since tardive dyskinesia symptoms often run a fluctuating course (APA 1992). In addition, adherence to guidelines for reporting randomised controlled trials that emphasise the explicit description of the processes of randomisation, allocation concealment and blinding etc could help improve the quality of future studies on this subject. Such reporting guidelines include the CONSORT (Consolidated Standards of Reporting Trials) statement (Moher 2001). The evidence provided by the studies included in this review should be interpreted in the light of the limitations mentioned above.
Potential biases in the review process
No known potential biases in the review process were identified by the authors of this review.
Agreements and disagreements with other studies or reviews
A recent systematic review presented as a conference proceeding, comprising 105 randomised controlled trials which assessed the evidence for various natural medicines in reducing antipsychotics side effects in schizophrenia, reported vitamin B6 to be effective in reducing medication‐induced tardive dyskinesia (Hoenders 2014). However, no quantitative data were reported in the abstract of the systematic review and no full text of the review is available yet. To the best knowledge of the authors of this review, there are no other reviews to which the findings of this review could be compared.
Authors' conclusions
Implications for practice.
1. For individuals with tardive dyskinesia
Pyridoxal 5 phosphate (vitamin B6) may help in alleviating the symptoms of tardive dyskinesia for those people with schizophrenia. Vitamin B6 was not significantly associated with a worsening of tardive dyskinesia symptoms or increased occurrence of other adverse effects, when compared with placebo. Lerner 2007 reported that high doses of vitamin B6 (up to1200 mg/day) is safe and acts for up to eight weeks after cessation of treatment. However, available data in support of these conclusions about the efficacy of vitamin B6 are weak and require larger studies in the future for validation.
2. For clinicians
Pyridoxal 5 phosphate (vitamin B6) may be considered in the treatment of tardive dyskinesia though the evidence supporting its effectiveness is limited. The effectiveness of vitamin B6 in treating tardive dyskinesia was noticed at doses from 300 mg daily (Lerner 2001). Vitamin B6 dose as high as 1200 mg daily was given in one of the studies included in this review (Lerner 2007) with only two participants experiencing side effects (acne and light itch) after two months of treatment. Therefore, given its safety and tolerability, clinicians might consider vitamin B6 as add on to antipsychotic medications. What is not clear however, is if other side effects are likely to develop when pyridoxal phosphate is used for a duration longer than that used in the included studies.
3. For policy makers and funders of studies
While there are indications that pyridoxal 5 phosphate may be effective for the treatment of tardive dyskinesia, the available data supporting this conclusion are weak. In addition, the exact mechanism by which pyridoxal 5 phosphate reduces tardive dyskinesia symptoms is still unclear.
Implications for research.
1. General
The evidence regarding the effectiveness of pyridoxal 5 phosphate in the treatment of tardive dyskinesia could be demonstrated in the future by well designed, conducted and reported randomised controlled trials (Moher 2001). Other recommendations regarding the design of future trials are suggested in Table 2.
1. Possible design for a future study.
| Method | Allocation: randomised ‐ clearly described generation of sequence and concealment of allocation.
Blinding: double ‐ described and tested.
Duration: long term.
Setting: Inpatients and outpatients Design: Single phase, longer study duration. |
| Participants | People with schizophrenia or schizophrenia‐like disorder.
N = Sample size obtained through power calculation.
Age: any
Sex: both History: History of tardive dyskinesia, fulfilling diagnostic criteria for tardive dyskinesia, stable on antipsychotic medication for at least 3 months. |
| Intervention | 1.Pyridoxal Phosphate (vitamin B6), any dose 2. Placebo |
| Outcomes | Tardive dyskinesia scores measured using AIMS (primary outcome) Deterioration of tardive dyskinesia symptoms Any other adverse effects Discontinuation of pyridoxal phosphate (with reasons) Psychiatric symptoms score using a standardised rating scale (PANSS, BPRS) Pyridoxal phosphate dose Plasma pyridoxal phosphate level Quality of life Satisfaction with care |
AIMS: Abnormal Involuntary Movement Scale BPRS: Brief Psychiatric Rating Scale PANSS: Positve and Negative Scale of Schizophrenia
2. Specific
Use of cross‐over designs
Tardive dyskinesia is a chronic symptom with fluctuating course (APA 1992). The studies included in this review used a cross‐over study design, which is more suitable for conditions with a stable course (Fleiss 1984).
Sample size calculation
Only one of the included studies in this review used a statistical method to calculate the sample size (Lerner 2007). The power of future studies could be significantly improved by larger sample sizes.
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods sections of their reviews. This text was used as the basis of the protocol, with modifications where necessary.
Adegoke Oloruntoba Adelufosi was awarded a Reviews for Africa (Nigeria) Programme Fellowship funded by a grant from the UK Department for International Development (DFID) through the Effective Healthcare Research Consortium at the Liverpool School of tropical Medicine. This review was developed in part during the Reviews for Africa Programme protocol development course organised by the Nigerian Branch of South African Cochrane Centre, April 2013.
The search terms were developed by the Trials Search Co‐ordinator of the Cochrane Schizophrenia Group, Samantha Roberts.
We would like to thank Michael Wilson for peer reviewing this version of our review, his comments were most helpful.
We would also like to acknowledge and thank our copy editor, Heather Maxwell.
Data and analyses
Comparison 1. Pyridoxal 5 phosphate (Vitamin B6) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.97 [2.87, 139.19] |
| 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate | Other data | No numeric data | ||
| 3 Global: 2. Other adverse effects than tardive dyskinesia | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.20, 78.59] |
| 4 Global: 3. Discontinuation of Vitamin B6 | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.72 [0.51, 149.75] |
| 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.14] |
| 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐6.36, ‐1.79] |
| 7 General Mental State: Average endpoint positive psychiatric symptoms score | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐4.80, 1.80] |
| 8 General mental state: Average endpoint negative psychiatric symptoms score | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐5.92, 3.72] |
1.1. Analysis.
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 1 Global: Clinical efficacy ‐ significant reduction in ESRS scores from baseline.
1.2. Analysis.
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 2 Global 1. Average endpoint dose of pyridoxal 5 phosphate.
| Global 1. Average endpoint dose of pyridoxal 5 phosphate | |
|---|---|
| Study | |
| Lerner 2001 | 400 mg daily |
| Lerner 2007 | 1200 mg daily |
| Miodownik 2003 | 400 mg daily |
1.3. Analysis.

Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 3 Global: 2. Other adverse effects than tardive dyskinesia.
1.4. Analysis.
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 4 Global: 3. Discontinuation of Vitamin B6.
1.5. Analysis.
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 5 Tardive dyskinesia: 1. Deterioration in tardive dyskinesia symptoms.
1.6. Analysis.
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 6 Tardive dyskinesia: 2. Average endpoint ESRS tardive dyskinesia scores.
1.7. Analysis.
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 7 General Mental State: Average endpoint positive psychiatric symptoms score.
1.8. Analysis.
Comparison 1 Pyridoxal 5 phosphate (Vitamin B6) versus placebo, Outcome 8 General mental state: Average endpoint negative psychiatric symptoms score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lerner 2001.
| Methods | Allocation: Randomised Blinding: Double Duration: 9 weeks Setting: Inpatients in Be'er Sheva Mental Health Centre, Israel. Design: Cross‐over study, divided into two phases of 4 weeks each, with 1 week wash‐out period. |
|
| Participants | Diagnosis: Schizophrenia or schizoaffective disorder. N = 15. Age: 28 ‐ 71 years. Sex: 4M,11F. History: Mean chlorpromazine equivalent of 490 mg/day. Included were patients who fulfilled diagnostic criteria for tardive dyskinesia; stable on antipsychotic medication for at least one month; Excluded were patients on vitamin treatment, concurrent medical/neurological disorder and those with substance or alcohol abuse. |
|
| Interventions | 1. Vitamin B6, increased by 100 mg/week from 100 mg/day to 400 mg/day in twice daily divided doses (n = 8). 2. Placebo (n = 7). |
|
| Outcomes | Global: Clinical efficacy: reduction in ESRS scores from baseline by 4 weeks Adverse effects other than tardive dyskinesia ‐ by 4 weeks Average time to discontinuation of P5P: in days ‐ by 4 weeks Average endpoint dose of P5P: in mg ‐ by 4 weeks Deterioration in tardive dyskinesia symptoms: ESRS ‐ by 4 weeks Average endpoint tardive dyskinesia scores: ESRS ‐ by 4 weeks |
|
| Notes | The authors did not mention the specific diagnostic instrument used in confirming the diagnoses of participants included in the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details were provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described by authors. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The study design was double blind, with crossover and placebo control." No further details were provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The raters were kept blind to the results". The specific method by which blinding was achieved was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition among participants was reported. |
| Selective reporting (reporting bias) | High risk | Not all outcomes were accounted for e.g. the specific number of participants who showed none, minimal, moderate or marked improvement in their tardive dyskinesia symptoms from both arms of the study were not reported. |
| Other bias | Unclear risk | The authors did not give details as to what extent raters in the study were independent. It was not mentioned if any funding was received for the study. The specific diagnostic criteria used for inclusion of participants in the study was not mentioned. |
Lerner 2007.
| Methods | Allocation: Randomised Blinding: Double Setting: Inpatients at Be'er Sheva Mental Health centre, Israel. Duration: 26 weeks Design: Cross‐over study, divided into two phases of 12 weeks each with 2 weeks wash‐out period. |
|
| Participants | Diagnosis: All participants met DSM‐IV criteria for Schizophrenia (n = 34) or Schizoaffective disorder (n =16). N = 50 Age: Mean ± SD = 47 ± 11 years, Range = 20 ‐ 66 years Sex: 28 Males, 22 Females History: Diagnosis of tardive dyskinesia; exposure to neuroleptics; stable psychotropic regimen for at least 1 month; duration of symptoms of at least 1 year; mean antipsychotic dose = 396.7 ± 280.4 mg/day in Chlorpromazine equivalents. Excluded: Concurrent medical/neurologic illness; pregnant/lactating mothers; patients on any vitamin supplements; substance/alcohol abuse. |
|
| Interventions | 1. Vitamin B6 (n = 28), 600 mg twice daily (Total ‐ 1200 mg/day) 2. Placebo (n = 22), two tablets twice daily |
|
| Outcomes | Clinical efficacy: ESRS ‐ reduction in ESRS score by 12 weeks Adverse effects other than tardive dyskinesia Average endpoint dose of P5P: in mg ‐ by 12 weeks Average time to discontinuation of P5P: in days ‐ by 12 weeks. Deterioration in tardive dyskinesia symptoms: ESRS ‐ by 12 weeks Average endpoint tardive dyskinesia score: ESRS ‐ by 12 weeks. |
|
| Notes | The study was supported by a clinical trials grant from the Stanley Medical Research Institute, Bethesda, Md. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further descriptions. |
| Allocation concealment (selection bias) | Unclear risk | Specific method of allocation concealment not described. " After breaking the code following database lock......" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, no further details was provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The plasma levels of Vitamin B6 were not reported to the raters, in order to keep them 'blind' to the patients' drug assignment." The specific method by which blinding was achieved was not described. The extent to which the raters were independent was not mentioned by the authors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised at the beginning of the study were accounted for. |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting in the study. |
| Other bias | Low risk | Funded by Stanley Medical Research Institute. |
Miodownik 2003.
| Methods | Allocation: Randomised. Blinding: Double blind Setting: Inpatients at the Mental Health centre in Beer Sheva, Israel. Duration: 9 weeks Design: Cross‐over study, divided into two phases of 4 weeks each, with 1 week wash‐out period. |
|
| Participants | Diagnosis: Schizophrenia, schizoaffective disorder or Schizophreniform disorder (diagnosis based on ICD‐10) N: 15 Age: Range = 28 ‐ 71 years, Mean ± SD = 50.0 ± 14.2 years,. Gender: 4 males, 11 females. History: History of tardive dyskinesia for at least 1 year; Duratio of illness ± SD = 18.6 ± 13.13 years with a range of 2 to 42 years. No change in the pharmacotherapeutic treatment in the month prior to inclusion; have no other significant organic diseases on physical examination. Excluded: patients with psychotic disorders caused by psychoactive drugs or by other organic disorders; patients with known lack of vitamins, eating disorders, malabsorption disorders, or known hypersensitivity to vitamin B6; Pregnant or lactating women and patients being treated with penicillamine, isoniazid and combined oral contraceptives. |
|
| Interventions | 1. Vitamin B6: n = 8, dose = maximum of 400 mg/day 2. Placebo: n = 7 |
|
| Outcomes | Average endpoint dose of P5P: in mg ‐ by 4 weeks Average endpoint psychiatric symptoms score: PANSS ‐ by 4 weeks. |
|
| Notes | The original study was written in Hebrew and then translated into English. Other data such as endpoint tardive dyskinesia scores were reported on an unscaled graph and were therefore not usable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description. |
| Allocation concealment (selection bias) | Unclear risk | No specific method of allocation was described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, no further details were provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "patients and investigators were blinded to the results of pyridoxal phosphate assessment". The specific method by which this was achieved was not stated. The level to which raters were independent is unknown. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were accounted for at the end of the trial. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting was found. |
| Other bias | Unclear risk | The authors did not state if there was any source of funding. |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition ICD‐10: International Statistical Classification of Diseases and Related Health Problems, 10th Revision ESRS: Extrapyramidal Symptom Rating Scale mg: milligrams PANSS: Positive and Negative Symptoms Scale P5P: Pyridoxal 5 Phosphate SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Greenberg 2003 | Allocation: randomised Participants: schizophrenia Intervention: folic acid, vitamin B12 and Pyridoxine. Outcomes: unusable ‐ measured outcomes were the effects of interventions on homocysteine level, cognitive deficits and psychopathology, not tardive dyskinesia. |
| Lerner 1999 | Allocation: randomised Participants: schizophrenia/schizoaffective disorders Intervention: vitamin B6 versus placebo Outcomes: unusable ‐ incomplete study data (report is an abstract presented in a conference). Full text not available from authors. |
| Lerner 2002 | Allocation: randomised Participants: patients with tardive dyskinesia. Specific diagnoses not stated. Intervention: vitamin B6 versus placebo Outcomes: unusable data ‐ incomplete study data. Full text not available from authors when requested. |
| Lerner 2007a | Allocation: randomised Participants: schizophrenia/schizoaffective disorders Intervention: Vitamin B6 versus placebo Outcome: unusable data were reported. Study was published in a book chapter. Full text of study not obtainable from the authors. |
| Lerner 2009 | Allocation: randomised Participants: diagnoses not specified Intervention: vitamin B6 versus other drugs Outcome: No useable data from this report as the study data were incomplete (only the proportion of participants in the treatment arm who experienced a significant reduction in scores for the measured outcomes were reported). Full text of report not obtainable from the authors. |
| NCT00202280 | Allocation: randomised Participants: first episode psychosis, not specific diagnosis of schizophrenia or schizoaffective disorder. |
| Venegas 2006 | Allocation: randomised Participants: Mixed diagnosis e.g. mood disorder, schizophrenia, epilepsy, dementia etc. Although patients with schizophrenia comprised 53.7% of the total diagnoses, the specific number of patients with schizophrenia allocated to each arm of treatment is unknown, making the study data unusable. |
| Xiao 2002 | Allocation: randomised Participants: patients with organophosphorous insecticide poisoning, not schizophrenia. |
Characteristics of ongoing studies [ordered by study ID]
NCT00917293.
| Trial name or title | Safety and efficacy of avastrem (Pyridoxal 5' ‐phosphate) in the treatment of tardive dyskinesia |
| Methods | Randomised controlled, double blind trial |
| Participants | Not stated |
| Interventions | Vitamin B6 versus placebo |
| Outcomes | Tardive dyskinesia |
| Starting date | May 2009 |
| Contact information | http://www.clinicaltrials.gov |
| Notes |
Differences between protocol and review
There are two major differences between the protocol of this review and the final review viz;
1. Since the studies included in this review were generally of short duration, the classification of outcomes into short term, medium term and long term outcomes was eliminated. For the same reason, assessment of clinical improvement in tardive dyskinesia symptoms was changed from "at least six weeks of treatment" to "at least four weeks".
2. The definition of clinical efficacy for pyridoxal 5 phosphate was changed to > 40% improvement in tardive dyskinesia symptoms after at least four weeks of treatment. This was necessary for interpretation of results and to reflect the definitions of moderate to marked clinical improvement in tardive dyskinesia symptoms used in the included studies.
3. Due to the small sample sizes of studies included in this review and the possible bias that can be introduced by using a random‐effects model for data synthesis, we decided to use a fixed‐effect model for all our data analysis.
4. As at the time of writing this review, one pharmaceutical company was still recruiting participants for a trial on the safety and efficacy of pyridoxal 5 phosphate on treatment of tardive dyskinesia. Since the trial was still ongoing, we did not contact the pharmaceutical company as stated in the protocol of this review.
5. We did not conduct a sensitivity analyses as stated in the protocol due to the similarities in inclusion criteria, characteristics of intervention/comparator and study design among the included studies.
Contributions of authors
Adegoke Oloruntoba Adelufosi ‐ Data collection and interpretation, statistical analysis, writing the review.
Olukayode Abayomi ‐ Data collection and interpretation, assisted in writing the review.
Tunde Massey‐Ferguson Ojo ‐ Data collection, assisted in writing the review.
Sources of support
Internal sources
Reviews for Africa (Nigeria) Programme Fellowship, Nigeria.
External sources
UK Department for International Development (DFID) through the Effective Healthcare Research Consortium at the Liverpool School of tropical Medicine., UK.
Declarations of interest
The authors received no financial consideration from any parties for the preparation of this review.
New
References
References to studies included in this review
Lerner 2001 {published data only}
- Lerner V, Miodownik C, Kaptsan A, Cohen H, Matar M, Loewenthal U, et al. Vitamin B6 in the treatment of tardive dyskinesia: a double‐blind, placebo‐controlled, crossover study. American Journal of Psychiatry 2001;158(9):1511‐4. [DOI] [PubMed] [Google Scholar]
Lerner 2007 {published data only}
- Lerner V, Miodownik C, Kapstan A, Bersudsky Y, Libov I, Sela B‐M, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double‐blind, placebo‐controlled, crossover study. Journal of Clinical Psychiatry 2007;68(11):1648‐54. [DOI] [PubMed] [Google Scholar]
Miodownik 2003 {published data only}
- Miodownik C, Cohen H, Kotler M, Lerner V. Vitamin B6 ad‐on therapy in treatment of schizophrenic patients with psychotic symptoms and movement disorders. Harefuah 2003;142(8‐9):592‐6, 647. [PubMed] [Google Scholar]
References to studies excluded from this review
Greenberg 2003 {unpublished data only}
- Greenberg WM. A 12‐week, double‐blind, augmentation study of the effects of folic acid, B‐12, and pyridoxine in lowering homocysteine levels and treating psychopathology and cognitive deficits in schizophrenia. Stanley Foundation Research Programs 2003.
Lerner 1999 {published data only}
- Lerner V, Miodownik C, Kaptsan A, Cohen H, Loewental U, Kotler M. Double‐blind evaluation of Vitamin B6 vs Placebo in treatment of tardive dyskinesia. European Neuropsychopharmacology. 1999; Vol. 9:S369.
Lerner 2002 {unpublished data only}
- Lerner V, Cohen H. A double‐blind, randomised, placebo‐controlled, crossover study of the effects of vitamin B6 in 50 in‐patients with tardive dyskinesia. Stanley Foundation Research Institute 2002.
Lerner 2007a {published data only}
- Lerner V. Vitamin B‐sub‐6. The experience in treating psychotic symptoms and psychotropic drug‐indced movement disorders. In: Pletson JE editor(s). Psychology and Schizophrenia. Hauppauge, NY, US: Nova Science Publishers, 2007:105‐38. [Google Scholar]
Lerner 2009 {unpublished data only}
- Lerner V. Vitamin B6 for tardive movement disorder. Stanley Foundation Research Programs 2009.
NCT00202280 {unpublished data only}
- NCT00202280. Efficacy of treating first episode psychosis with folic acid, vitamin B12 and B6 in addition to antipsychotic medication. http://www.clinicaltrials.gov./ct2/show/NCT00202280?term=NCT00202280&rank=1.
Venegas 2006 {published data only}
- Venegas F, Sinning O, Millan A, Miranda C, Robles G, Astudillo A, et al. Pyridoxine for drug induced dyskinesia. A placebo‐ controlled randomised cross‐over trial [Piridoxina en el manejo de Disquinesias Tardías. Un estudio placebo controlado, randomizado,doble ciego y cruzado]. Revista Chilena de Neuropsiquiatrica 2006;44(1):9‐14. [Google Scholar]
Xiao 2002 {published data only}
- Xiao G, Jin Y, Luo X. The effect of huangqi injection in the treatment of tardive neuropathy caused by acute organophosphorus insecticide poisoning. Herald of Medicine 2002;21(9):558‐60. [Google Scholar]
References to ongoing studies
NCT00917293 {unpublished data only}
- NCT00917293. Safety and efficacy of avastrem (pyridoxal 5' ‐phosphate) in the treatment of tardive dyskinesia. http://www.clinicaltrials.gov 2009.
Additional references
Adler 1998
- Adler LA, Edson R, Lavori P, Peselow E, Duncan E, Rosenthal M, et al. Long‐term treatment effects of vitamin E for tardive dyskinesia. Biological Psychiatry 1998;43:868‐72. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1992
- American Psychiatric Association. Tardive dyskinesia: a task force report of the American Psychiatric Association. Washington DC 1992.
Ascher‐Svanum 2008
- Ascher‐Svanum H, Zhu B, Faries D, Peng X, Kinon BJ, Tohen M. Tardive dyskinesia and the 3‐year course of schizophrenia: results from a large, prospective, naturalistic study. Journal of Clinical Psychiatry 2008;69(10):1580‐8. [DOI] [PubMed] [Google Scholar]
Ayehu 2014
- Ayehu M, Shibre T, Milkias B, Fekadu A. Movement disorders in neuroleptic‐naïve patients with schizophrenia spectrum disorders. BMC Psychiatry 2014;14:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 1993
- Barnes TRE, Edwards JG. The side‐effects of antipsychotic drugs. In: Barnes TRE editor(s). Antipsychotic Drugs and their Side‐effects. CNS and Neuromuscular Effects. Vol. I, London: Harcourt Brace & Company, 1993. [Google Scholar]
Bassitt 1998
- Bassitt DP, Louzã Neto MR. Clozapine efficacy in tardive dyskinesia in schizophrenic patients. European Archives Psychiatry and Clinical Neuroscience 1998;248(4):209‐11. [DOI] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Browne 1996
- Browne S, Roe M, Lane A, Gervin M, Morris M, Kinsella A, et al. Quality of life in schizophrenia: relationship to sociodemographic factors, symptomatology and tardive dyskinesia. Acta Psychiatrica Scandinavica 1996;94:118‐24. [DOI] [PubMed] [Google Scholar]
Caroff 2001
- Caroff SN, Campbell EC, Havey J, Sullivan KA, Mann SC, Gallop R. Treatment of tardive dyskinesia with donepezil: A pilot study. Journal of Clinical Psychiatry 2001;62(10):772‐5. [DOI] [PubMed] [Google Scholar]
Casey 1995
- Casey DE. Neuroleptic‐induced extrapyramidal syndromes and tardive dyskinesia. In: Hirsch S, Weinberger DR editor(s). Schizophrenia. Oxford: Blackwell, 1995:546–65. [Google Scholar]
Chakos 1996
- Chakos MH, Alvir JMJ, Woerner MG, Koreen A, Geisler S, Mayerhoff D, et al. Incidence and correlates of tardive dyskinesia in first episode of schizophrenia. Archives of General Psychiatry 1996;53:313–9. [DOI] [PubMed] [Google Scholar]
Chouinard 2005
- Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophrenia Research 2005;76(2‐3):247‐65. [DOI] [PubMed] [Google Scholar]
Corell 2008
- Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Current Opinion in Psychiatry 2008;21(12):151‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fay‐McCarthy 1997
- Fay‐McCarthy M, Kendrick KA, Rosse RB, Schwartz BL, Peace T, Wyatt RJ, et al. The effect of nifedipine on tardive dyskinesia: a double blind study in eighteen patients. Schizophrenia Research 1997;24(1‐2):271. [Google Scholar]
Fenton 2000
- Fenton WS. Prevalence of spontaneous dyskinesia in schizophrenia. Journal of Clinical Psychiatry 2000;61(Suppl 4):10‐4. [PubMed] [Google Scholar]
Fleiss 1984
- Fleiss JL. The crossover study. The design and analysis of clinical experiments. Chichester: John Wiley & Sons 1984.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoenders 2014
- Hoenders R, Bartels‐Velthuis A, Vollbehr N, Bruggeman R, Knechtering R, Jong J. Natural medicines in schizophrenia: A systematic review. Journal of Alternative and Complementary Medicine. 2014; Vol. 20, issue 5:A79‐80.
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Kane 1986
- Kane J, Woerner M, Borenstein M. Integrating incidence and prevalence of tardive dyskinesia. Psychopharmacology Bulletin 1986;22:254–8. [PubMed] [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) forschizophrenia. Schizophrenia Bulletin 1987;13:261‐76. [DOI] [PubMed] [Google Scholar]
Leung 2003
- Leung SK, Ungvari GS, Ng FS, Cheung HK, Leung T. Tardive dyskinesia in Chinese inpatients with chronic schizophrenia. Progress in Neuropharmacology and Biological Psychiatry 2003;27(6):1029‐35. [DOI] [PubMed] [Google Scholar]
Lopez‐Munoz 2005
- Lopez‐Munoz, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G. History of the discovery and clinical introduction of chlorpromazine. Annals of Clinical Psychiatry 2005;17(3):113‐35. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McGrath 2001
- McGrath J, Soares‐Weiser K. Vitamin E for neuroleptic‐induced tardive dyskinesia. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000209] [DOI] [PubMed] [Google Scholar]
Miodownik 2008
- Miodownik C, Meoded A, Libov I, Bersudsky Y, Sela B, Lerner V. Pyridoxal plasma level in schizophrenic and schizoaffective patients with and without tardive dyskinesia. Clinical Neuropharmacology 2008;31:197‐203. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D, CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Nasrallah 2006
- Nasrallah HA. Focus on lower risk of tardive dyskinesia with atypical antipsychotics. Annals of Clinical Psychiatry 2006;18(1):57‐62. [DOI] [PubMed] [Google Scholar]
Nelson 2003
- Nelson, LA, McGuire JM, Hausafus SN. Melatonin for the treatment of tardive dyskinesia. Annals of Pharmacotherapy 2003;37:1128‐31. [DOI] [PubMed] [Google Scholar]
Niehaus 2008
- Niehaus DJH, Du Plessis SA, Koen L, Lategan BH, Steyn J, Oosthuizen PP, et al. Predictors of abnormal involuntary movement in an African schizophrenia population. Journal of Neuropsychiatry and Clinical Neurosciences 2008;20:317‐26. [DOI] [PubMed] [Google Scholar]
Sachdev 1989
- Sachdev PS. Depression‐dependent exacerbation of tardive dyskinesia. British Journal of Psychiatry 1989;155:253‐5. [DOI] [PubMed] [Google Scholar]
Schaumburg 1983
- Schaumburg H, Kaplan WA, Vick N, Rasmus S, Pleasure D, Brown MJ. Sensory neuropathy from pyridoxine abuse ‐ A new megavitamin syndrome. New England Journal of Medicine 1983;309:445‐8. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Soares‐Weiser 2004
- Soares‐Weiser K, Rathbone J. Calcium channel blockers for neuroleptic‐induced tardive dyskinesia. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000206.pub2] [DOI] [PubMed] [Google Scholar]
Tammenmaa 2002
- Tammenmaa I, McGrath J, Sailas EES, Soares‐Weiser K. Cholinergic medication for neuroleptic‐induced tardive dyskinesia. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD000207] [DOI] [PubMed] [Google Scholar]
Tenback 2007
- Tenback DE, Harten PN, Slooff CJ, Os J, SOHO Study Group. Worsening of psychosis in schizophrenia is longitudinally associated with tardive dyskinesia in the European Schizophrenia Outpatient Health Outcomes study. Comprehensive Psychiatry 2007;48(5):436‐40. [DOI] [PubMed] [Google Scholar]
Teo 2012
- Teo JT, Edwards MJ, Bhatia K. Tardive dyskinesia is caused by maladaptive synaptic plasticity: a hypothesis. Movement Disorders 2012;27:1205‐15. [DOI] [PubMed] [Google Scholar]
Waddington 1987
- Waddington JL, Youssef HA, Dolphin C, Kinsella A. Cognitive dysfunction, negative symptoms, and tardive dyskinesia in schizophrenia. Their association in relation to topography of involuntary movements and criterion of their abnormality. Archives of General Psychiatry 1987;44(10):907‐12. [DOI] [PubMed] [Google Scholar]
Woerner 1993
- Woerner MG, Saltz BL, Kane JM, Lieberman JA, Alvir JMJ. Diabetes and development of tardive dyskinesia. American Journal of Psychiatry 1993;150:966‐8. [DOI] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Yassa 1992
- Yassa R, Jeste DV. Gender differences in tardive dyskinesia: a critical review of the literature. Schizophrenia Bulletin 1992;18:701‐15. [DOI] [PubMed] [Google Scholar]
Zhang 2004
- Zhang XY, Zhou DF, Cao LY, Xu CQ, Chen DC, Wu GY. The effect of vitamin E treatment on tardive dyskinesia and blood superoxide dismutase: a double‐blind placebo‐controlled trial. Journal of Clinical Psychopharmacology 2004;24:83‐6. [DOI] [PubMed] [Google Scholar]


