Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative dementia worldwide. AD is a multifactorial disease that causes a progressive decline in memory and function precipitated by toxic beta-amyloid (Aβ) proteins, a key player in AD pathology. In 2022, 6.5 million Americans lived with AD, costing the nation $321billion. The standard of care for AD treatment includes acetylcholinesterase inhibitors (AchEIs), NMDA receptor antagonists, and monoclonal antibodies (mAbs). However, these methods are either: 1) ineffective in improving cognition, 2) unable to change disease progression, 3) limited in the number of therapeutic targets, 4) prone to cause severe side effects (brain swelling, microhemorrhages with mAb, and bradycardia and syncope with AchEIs), 5) unable to effectively cross the blood-brain barrier, and 6) lack of understanding of the aging process on the disease.
mAbs are available to lower Aβ, but the difficulties of reducing the levels of the toxic Aβ proteins in the brain without triggering brain swelling or microhemorrhages associated with mAbs make the risk-benefit profile of mAbs unclear.
A novel multitarget, effective, and safe non-invasive approach utilizing Repeated Electromagnetic Field Stimulation (REMFS) lowers Aβ levels in human neurons and memory areas, prevents neuronal death, stops disease progression, and improves memory without causing brain edema or bleeds in AD mice. This REMFS treatment has not been developed for humans because current EMF devices have poor penetration depth and inhomogeneous E-field distribution in the brain. Here, we discussed the biology of these effects in neurons and the design of optimal devices to treat AD.
Keywords: Alzheimer’s treatment, Electromagnetic fields stimulation, Birdcage, Computer simulation, Human phantom
Introduction
The Physics
Electromagnetic field:
The electromagnetic field is a wave motion consisting of oscillating electric and magnetic fields. It is characterized by the wavelength λ in meter, the frequency f in Hertz (H), the photon energy U in Joule (J), and the absolute temperature Tin Kelvin (K) [1]. Among them the following relationships hold λ=cf, U=h f, T=U/k=h f/k where c (3 × 108 m/s) is the approx. speed of light, h (=6.626 × 10−34 Js) is the Planck constant, and k (1.381 × 10−23 J/K) is the Boltzmann constant. The photon energy and the temperature increase with an increase in the frequency or the decrease of the wavelength.
Electromagnetic Field (EMF) can be viewed in a classical or quantum field which is produced by electric charges in classical field theory or by quantized EMF tensors in quantum field theory. EMF is a mix of an electric field and a magnetic field. The stationary and moving charges produced the EMFs; Maxwell’s equations define its interaction. The EMF quanta of energy (photons) are integer multiples of hf where h is Plank’s constant, and f is the frequency of the radiation (Hertz) [2]. The quantum effects produced by the photon oscillation on molecules are the most likely mechanism of the EMF and biological system interaction [3]. The EMF effects on biological systems can be produced by thermal vs. non-thermal EMF stimuli [4]; here, we will discuss the non-thermal effects of radiofrequency (RF) radiation. A whole series of biological effects produced by weak static or alternating EMF action is explained only from the viewpoint of non-thermal mechanisms. The bioeffects of these exposures include changes at various levels: alterations in membrane structure and function, changes in several subcellular structures as proteins and nucleic acids, protein phosphorylation, cell proliferation, free radical formation, and ATP synthesis. Another factor in the interaction of RF fields with biological tissues is influenced by the geometry and composition of the exposed object and the frequency and configuration of the field. Also, the distance from the antenna and its configuration affects the width and strength of the incident field. In the near field, quasistatic interactions prevail. In the far field, the RF energy propagates as plane waves. Therefore, the interaction with biological systems is independent of the antenna configuration [5].
Challenges:
The main challenge to explain these effects is that the RF photon energy is low, insufficient to excite electrons (13.6 eV) [6], and is thereby considered non-ionizing. For example, the photon energy of Repeated Electromagnetic Field Stimulation (REMFS) at 50 MHz is 2.0678–7 eV, at 64 MHz it is 2.64–7 eV, and at 915 MHz it is 3.7841–6 eV; these photons produce low energy insufficient to cause chemical changes [6]. Additionally, protein conformational changes cannot occur under direct electric field magnitudes lower than 108 V/m [7], and REMFS only produce 16.22 V/m [8]. REMFS energies are incapable of directly causing the dissociation of chemical bonds such as the H-O-H covalent bond of a water molecule (H2O) because this type of reaction would require 493.4 kJ/mol or 5.1138 eV [9,10], an exponentially higher amount of energy. Thus, classical physics is unable to explain the biological responses to REMFS.
Mechanism of action
At the quantum level:
Nevertheless, quantum physics provides an explanation of how this reaction occurs. Here, we consider low-energy EMF with frequencies below the THz wavelength. Interestingly, high-energy EMF is not able to produce the biological effects of the low energy EMF [11]. In addition, Panagopoulos found that oscillating EMF with frequencies lower than 1.6 × 104 Hz produce bioeffects, even at very low intensities. Conversely, as the frequency of the EMF increases to more than 1.6 × 104 Hz a higher field intensity is required to produce biological effects [12]. This difference could be due to RF and microwave range correlating to the rotation of polyatomic molecules and higher frequency to the vibrations of flexible bonds [13].
Another possible explanation is the effect of the RF oscillation on the H-bond at the quantum level. The difference in frequencies between RF oscillation (Hz to GHz) and hydrogen bond vibration (74 THz) may cause the hydrogen bond to behave as a driven quantum oscillator under REMFS exposure [14,15]. Data indicates that REMFS amplifies hydrogen bond vibrations around negatively charged biomolecules, influencing proton tunneling by increasing both vibration amplitude and the distance between the proton and acceptor [16], consequently raising the probability of tunneling. This protonation creates tautomers in RNA or other biomolecules that produce conformational changes and affect biological functions [3].
Specifically, the oscillatory effects of the RF on the degradation of abnormal proteins as beta-amyloid (Aβ) initially affect H-bonds confined to the first layer of the interfacial water in the vicinity of the non-coding RNA Heat Shock RNA-1 (HSR1) [17]. This EMF oscillation shortens the length of the H-bond, increasing the probability of proton tunneling [18] and protonation of the nucleic acids [19], leading to the formation of tautomers [20] that produce conformational changes in HSR1 [17] to allow binding and activation of HSF1. Subsequently, HSF1 binds to DNA to express chaperones that initiate chaperone autophagy and degradation of Aβ and other abnormal proteins, such as Tau, with the consequent clinical improvement in Alzheimer’s Disease (AD).
Another important factor in the energy deposition depends on the orientation of the E-field vector or polarization with respect to the body, so underlying the importance of polarized vs. non-polarized EMF. Panagopoulos et al. [11] analyzed the role of polarization in the biological activity of Electromagnetic Fields. They found that polarized (man-made) in contrast to non-polarized (natural) have biological effects due to 1) Ability to produce constructive interference effects and amplify their intensities at many locations. 2) Ability to force polar molecules (water) within and around negatively charged biomolecules to oscillate on parallel planes and in phase with the applied polarized field. This oscillation at the quantum level produces proton tunneling and protonation of the biomolecules to produce conformational changes to change into an active structure able to activate a signaling pathway that regulates the proteostasis and AD pathology [3]. Therefore, we must consider the use of polarized EMF for human treatments because if the non-polarized EMF photons have all possible orientations forming angles between each two of them from 0° to 360° and the superposition of many such equal vectors converge on the same point in space will be the sum of vectors applied on the center of a sphere with their ends equally distributed around the surface of the sphere. The sum of an infinite number of such vectors (all applied on the center) tends to become zero energy, producing destructive interference and a lack of biological effects [21].
At the molecular level:
The protonation of nucleic acids produces tautomerism and conformational changes. In the RNA nucleic acid bases occur in several tautomeric forms due to solvent-exchangeable protons [20]. Tautomers [22] are used by multiple RNA to produce their functions [20,23]. Interestingly, Guanine and cytosine protonation affect RNA structure and function; their different structures come from the changes of single and double bonds in the ring systems of purines and pyrimidines [24]. Furthermore, it is well known that tautomeric equilibria are affected by several chemical and physical factors such as metals, temperature, pH [25] and recently electric field exposures [18,26] and can adopt various secondary structures such as double helices, stem-loops, pseudoknots, and G-quadruplexes responsible for a variety of functions during biological processes like DNA replication, packaging, and transcription [27,28]. Often, such conformational changes promote binding to activating factors that in turn affect transcription and translation of proteins.
Evidence suggests that REMFS protonates biomolecules [29], with important tautomeric interconversions and conformational changes resulting [20,30]. These data suggest that REMFS can cause tautomerism and conformational changes in biomolecules similar to the regulation of HSR by RNA thermometers [26] in bacteria [32].
Also, REMFS exposures are not likely to produce protein denaturation, so the mechanism must be related to an EMF-sensitive biomolecule such as HSR1. EMF exposure also increases HSF1-heat shock element binding activity, thereby directly contributing to the activation of HSF1 and the stress-induced Hsp70 [33] transcription and translation in cells exposed to REMFS [34,35]. HSF1 is a transcriptional factor master regulator of stress gene expression (molecular chaperones) [36,37]. Recently, in addition to chaperone expression, accumulating evidence indicates multiple additional functions for HSF1 beyond chaperone production. HSF1 acts in diverse stress-induced cellular processes and molecular mechanisms, including the endoplasmic reticulum, unfolded protein response, and ubiquitin-proteasome system, multidrug resistance, autophagy, apoptosis, immune response, cell growth arrest, differentiation underlying developmental diapause, chromatin remodeling, cancer development, and aging [38]. Protein aggregation is an important factor in the progression of aging and age-related diseases such as AD [39]. Several pathways are associated with abnormal protein clearance, including molecular chaperones, the ubiquitin-proteasome system, and autophagy pathways [40]. The production of these chaperones depends on the activation of HSF1, an event attenuated by the aging process [41]. HSF1 is repressed by the Hsp90 complex and released to get activated under several cellular stresses [42]. The triggering of the HSR by stressors after REMFS treatment produces a fast and vigorous expression of chaperones (Heat shock proteins, Hsps) [43,44]. The most important protein is the Hsp70, which promotes degradation and inhibits the accumulation of toxic Aβ peptides [45–48], an APP fragment of [44–48] amino acids [49], which is a key factor in AD. Hsp70 decreases Aβ levels when given to microglia from rats [50]. GRP78 is another member of the HSP70 family with a role in AD. In a HEK cell model co-transfected with APP and GRP78binds to APP in the ER, prevents the β/γ-secretase cleavage necessary to produce Aβ, decreasing Aβ intracellular levels and toxicity [51]. In addition, the overexpression of GRP78 decreases the level of Aβ40 and Aβ42 in mutant APP (APPsw) cells [51]. Furthermore, HSF1 upregulates ATG7 and RIPK1 to promote autophagy [52,53]. HSP70 transports APP to lysosomes for Chaperone-Mediated Autophagy (CMA) or endosomal Micro-Autophagy (eMI) for degradation to reduce Aβ levels [54].
Preclinical studies
In vitro studies:
Our experiments [8,43,55] and our review of the literature [56–58] from cell culture [59–64], animal [65–80], and human [81–85] studies found that the minimal therapeutic REMFS dose for AD is ~0.4–0.9 W/kg Specific Absorption Rate (SAR) for one hour/day. This dose activated autophagy pathways [43,61,86–89] to lower Aβ levels in human brain cultures [55] and animal models [65–80]. Our initial hypothesis was that the effect of aging on the loss of the proteostasis [90] and the consequent Aβ accumulation is an early event [91] in aging and AD pathology. AD usually emerges during aging, when the proteostasis quality control and autophagy are unable to prevent the aggregation of misfolded proteins. The central role of HSF1 and autophagy on the proteostasis and aging [92] prompted us to examine REMFS at different frequencies, exposure times, input powers, SARs, and schedules to determine if these RF exposures upregulate HSF1, autophagy, and delay aging. We found that low EMF frequency (50 MHz-100 MHz), exposure time of 5, 15, 30, 60, and 120 min, power of 0.1, 0.5, 1 W, and a SAR of 0.4, 0.6, 0.9 W/kg were effective except the 5- and 15-minutes exposure confirming that these effects are time-dependent. To verify that EMF did not increase temperatures, 37°C cell cultures and distilled water were irradiated for 5, 15, 30, 80, and 120 min and monitored for temperature changes. REMFS does not alter cell culture temperature. Thus, biological effects from REMFS are unlikely due to thermal effects. We found that REMFS non-thermally activates the HSF1 (master regulator of the proteostasis [93,94] and the autophagy proteins ATG5 and ATG12 [61,87]), increasing levels of HSP70, achieving a 17% increase in lifespan potential in human lymphocytes and mouse fibroblasts compared to knockout HSF1 cells [43]. Other studies have found that REMFS activate autophagy pathways in cell cultures [59,95,96] and animal models [88,89], and decreased Aβ levels in both [65,68,80,97]. Additionally, REMFS activates multitarget pathways, including the heat shock factor 1 [43,98], autophagy-lysosome system [61], ubiquitin-proteasome system [60], oxidative stress [62,99], cytoprotection [63], inflammation [100], mitochondrial, and neuronal activity [67], to lower Aβ levels and potentially improve cognition in AD patients. Given HSF1’s central role in the process of abnormal protein autophagy that occurs during aging, this suggests that EMF interventions to push HSF1 toward its activated state are essential for the autophagy of abnormal proteins such as Aβ.
Based on the above results, we hypothesized that REMFS potentially lower Aβ levels by autophagy [101] in human neurons; this prompted our group to expose Primary Human Mixed Brain cultures (PHB) with different EMF frequencies, times of exposure, schedules, and SARs [55] to determine if REMFS was effective in human neurons. We recently utilized REMFS to lower Aβ levels in cell cultures of PHB [55]. REMFS treatment decreased Aβ-40 and Aβ-42 levels without evidence of toxicity. After 14 days of REMFS, we determined levels of Aβ40 peptide in exposed and non-exposed cells; treatment started on day 7 in vitro (DIV 7). The REMFS parameters were a frequency of 64 MHz with a SAR of 0.6 W/Kg for one hour daily; this treatment achieved a 46% reduction in Aβ40 levels (p=0.001, g=0.798) compared to the non-treated cultures [55]. The same REMFS parameters achieved a 36% decrease in Aβ42 levels. Subsequently, we demonstrated that REMFS at 64 MHz or 100 MHz with a lower SAR of 0.4 W/kg for 14 days achieved a comparable reduction in Aβ40 and Aβ42 levels. Furthermore, when we increased the exposure time from 1 to 2 hours, there was a similar reduction in the Aβ levels. Also, when we increased the frequency from 64 MHz to 100 MHz, we found a comparable difference in Aβ levels. The results of our experiments suggest that REMFS at 64 MHz with a SAR of 0.4 W/kg for 1 hour (typical of that already utilized in clinical MRI contexts) would be the minimal energy needed to produce bio-effects in human neurons, specifically a reduction in levels of toxic Aβ peptides.
In vivo studies:
Also, the efficacy and safety of REMFS have been demonstrated in Transgenic (Tg) AD mouse models in vivo. An initial REMFS study prevented or reversed memory loss in the Tg AD mouse model (AβPPsw) when a pulsed and modulated RF-EMF at 918 MHz with a SAR of 0.25–1.05 W/kg was applied over a 7 to 9-month period [68]. REMFS-exposed Tg mice preserved good cognitive function, whereas control Tg mice showed a cognitive decline. Tg mice of advanced age (21–27 months) with daily REMFS exposure for two months showed improved memory in the Y-maze task, although not in more complex tasks [65]. These older Tg controls showed high levels of Aβ aggregates, in treated mice showing a 24%-30% decrease in Aβ deposits. These data suggest a degradation of Aβ deposits with REMFS exposure. In addition, these long-term treatments were safe (daily for up to 9 months) without any toxic effects on multiple health parameters, including oxidative stress, brain histology, brain heating, damage to DNA, or cancer in peripheral tissues [102].
A higher frequency study (1950 MHz) showed decreased AD pathology in Tg-5xFAD transgenic mice, which overexpress APP, and Wild-Type (WT) mice treated with REMFS at 1950 MHz with SAR 5W/kg for 2 hours per day, five days per week [80]. This long-term exposure to REMFS decreased Aβ plaques, APP, and APP carboxyl-terminal fragments in the brain. REMFS also decreases the expression of β Beta secretase 1 (BACE1) to prevent inflammation.
Additionally, REMFS reverses cognitive decline in AD mice. REMFS treatment showed that when compared to WT mice, five genes that are all implicated in Aβ processing (Tshz2, Gm12695, St3gal1, Isx, and Tll1) are affected in Tg-5xFAD mice treated with REMFS. Specifically, WT showed the same genetic profile as non-REFMS-treated Tg mice, while REMFS-treated Tg mice demonstrated different patterns. Therefore, these data suggest that chronic REMFS treatment influences Aβ processing in AD mice but not in wild or Tg controls [80]. Additionally, Other investigators demonstrated improved cognitive function that accompanied reduction of Aβ in AD mouse models [65,68,80,102].
Taken together, the enhancement pathways involved in Aβ degradation through upregulation of the HSF1 pathway [43], the autophagy-lysosome system [61], the ubiquitin-proteasome system [60,103], and a reduction in β-secretase activity following REMFS produce a protective effect through reduction ofAβ [80]. Furthermore, REMFS also targets multiple aging [104] and cell defense pathways that are involved in AD pathology [57], including oxidative stress [62], cytoprotection [63], inflammation [105], mitochondrial enhancement, and neuronal activity [102], thereby making REMFS a potential multi-target therapeutic strategy for AD that lowers Aβ [55] in memory areas and potentially stop disease progression [68] and improve memory without brain swelling [68]. In conclusion, AD mouse studies and human brain cell studies revealed that REMFS exposures reduce Aβ. It also prevents and decreases brain Aβ aggregation without causing inflammation, as seen in passive or active immunity treatment trials [56,106]. REMFS represents a potential therapeutic strategy in the treatment of AD patients who already have large amounts of Aβ deposits.
Clinical studies
Another group found that EMF at 915 MHz [65] stops AD progression in mice [68]. However, a human trial of Transcranial Electromagnetic Treatment (TEMT) with 8-transmitters at 915 MHz [82] did not stop AD progression due to poor penetration depth (3.9 cm) in a human head, not reaching deep brain memory areas such as the hippocampus, posterior cingulate [107,108], or locus caeruleus [109] affected early in AD. Therefore, frequency should be decreased to improve the penetration depth and SAR distribution before transposing AD mice results to human trials. Also, the position of the transmitters is at different angles that produce non-polarized EMF; it causes constructive and destructive interference, causing an erratic E-field distribution with non-treated and hot spots areas. Therefore, the EMF exposure should provide polarized EMF that and produces a homogeneous field distribution.
The rationale for how REMFS would improve memory is based on the activation of autophagy pathways [43,55,59,87–89,95,96] to degrade Aβ oligomers [110] in the hippocampus and deep subcortical memory areas [107,109], prevent neuronal dysfunction and death, and potentially stop disease progression and memory loss in AD. While these REMFS studies show promising results, this data may not be easily transposed to human treatments because the tissue characteristics, geometry, anatomy, body size, and EMF wavelength/head size ratio in mice differ substantially from humans. What remains unknown is whether REMFS technology can deliver a homogeneous therapeutic SAR to all human brain memory areas, lower Aβ, prevent neuronal death, and potentially improve memory and function in AD.
Device and dosimetry
EMF devices:
EMF devices are very important in healthcare [111]; the most common devices are based on the, direct interaction of EMF and the body 1) by induced electric currents 2) by energy converted into heat, 3) by Magnetic Resonance Imaging (MRI) to obtain diagnostic information 4) by Transcranial Magnetic Stimulation (TMS) to trigger functional responses, and 5) by introducing EMF devices into the organisms for monitoring, targeting, tracking, and navigating using electronic implants or capsular endoscopes able to inject nanoparticles into tissues.
However, in the meanwhile, evolving EMF applications for treating protein deposition diseases such as AD are broadening these devices by directly exposing the tissues to activate molecular pathways that control the fate of a protein from synthesis to degradation (proteostasis). A complex molecular network, including molecular chaperones, proteolytic systems, and transcriptional factors, guarantees the preservation of proteostasis. Nevertheless, the aging process produces a significant decline in proteostasis with the resulting accumulation of protein aggregates and age-related diseases such as AD or Parkinson’s disease. The possibilities of EMF upregulation of the proteostasis hold great promise for delaying the onset of age-related diseases and prolonging our healthy life expectancy.
Dosimetry:
Electromagnetic fields must interact with tissues, and energy must be absorbed or deposited in the tissues to activate biomolecules to produce biological effects. The dosimetric quantities commonly used include incident field, induced field, Specific Absorption Rate (SAR), and Specific Absorption (SA) in tissue media. The metric SAR (in watt per kilogram) is a derived quantity and is defined as the time derivative of the incremental energy absorbed by an incremental mass contained in a volume of a given density (NCRP 1981) [112]. SAR value commonly uses 1 g or 10 g of tissue. The metric SA (in joules per kilogram) is the total amount of energy deposited or absorbed and is given by the integral of SAR over a finite interval of time. Information on SA and SAR is significant because it can serve as a framework to transpose experimental results from cell to animal, animal to animal, and animal/cell to human exposures. SAR was accepted worldwide as the dosimetric measure in guidelines for limiting exposure to EMF devices such as MRI, cell phones, etc. The SAR levels can be transposed to human treatments since the internal fields measured in terms of deposed power (SAR), not the radiated external fields, are the ones causing the biological effect. It is necessary to perform numerical modeling, computer simulation, and practical validation experiments in realistic nonhomogeneous (multilayer) human head phantoms to find the external fields that will produce the Same Internal Fields (SAR) of the neuronal cultures and AD mice in the human brain.
The complex geometry of the head and the several concentric layers of other tissues shows in computer simulations that the effect of skin, fat, skull, dura, and cerebrospinal increases the SAR in the skin [112]. There is less energy deposition in the bone and fat. If we compare homogeneous head models to multilayer models, we can see that SAR values are several times greater than the value in the multilayer model due to the resonant coupling of plane-wave RF into the brain sphere by the outer tissue layers.
One of the main advantages of accurate measurement of the SAR in human exposures is finding the Minimum SAR with Biological Effects (MSBE), this measure would be much more valuable compared to studying high SAR exposures. An MSBE will establish frame work the EMF effects on biomolecular responses (e.g., oxidative response). In addition, it is more likely to reduce the complexity of the EMF interaction targets in cell cultures by lowering the exposure power, which at least reduces the overall rise in temperature [113]. The MSBE value might differ regarding the case under study and depends on the physical and biological conditions of the exposed tissue. Determining the MSBE for a therapeutic SAR range is significant because it provides a framework to monitor and improve future treatments.
The evidence to substantiate the therapeutic SAR range for Alzheimer’s comes from our experiments [8,43,55] and our review of the literature [55–58] from cell culture [59–64], animal [65–80], and human [81–85] studies that found lower Aβ in memory areas, prevent neuronal death and improve memory in AD rodents when the local SAR was between 0.3–5 W/g, but not in exposures longer than 3 h/d [114–120] or at high energy [121–124], suggesting a dose- and time-dependent therapeutic SAR window. In addition, many REMFS studies found that a SAR lower than 0.3 W/kg [99,125–137] or higher than 5 W/kg [97,138–150] has detrimental effects on AD. A rodent study [151] found an adverse impact in Blood Brain Barrier (BBB) leakage or neuron degeneration at a SAR of 0.26 and 13 W/kg but not at 2.6 W/kg, supporting a therapeutic SAR window. Similarly, two human studies support the therapeutic range; one study found impaired speed in cognitive tasks [152] at a SAR of 0.2 and 5 W/kg, in contrast to their previous results at a SAR of 1 W/kg where accuracy increased. All the above studies support that a local head SAR between 0.3 W/kg to 5 W/kg is the therapeutic range for AD. We adapted the upper limit SAR for this novel context based on the ICNIRP [153] and IEEE [154] safety standards of 2 W/kg local head SAR. In addition, our studies in primary human brain neurons found that the MSBE was 0.4 W/kg, providing a framework for treatments in AD. Moreover, recognizing differences in thermal physiology in the general population and the longer duration of our exposures (60 vs. 6 minutes averaged), we have chosen a SAR of 0.4 W/kg-0.9 W/kg as the minimal therapeutic range to lower Aβ and decrease the risk of thermal injury as a framework for future AD treatments.
Finding the perfect wave:
High-quality EMF exposures produce a homogeneous E and SAR Field distribution, and a high Signal-To-Noise Ratio (SNR) [111]. However, the dielectric effect causes an inhomogeneous E-field distribution, because when the E-field of an electromagnetic field interacts with tissues, it decreases the wavelength, generates electric currents, and develops wave reflection or refraction at tissue interfaces. At higher frequencies than 200 MHz and shorter wavelengths compared to the size of the body, standing wave currents might flow in opposite directions from two sides of the patient, creating a pattern with destructive interference (non-treated areas) and constructive interference (hot spots areas), the field uniformity of the large coils deteriorates at higher frequencies. The field distribution is uniform at 64 MHz and 128 MHz, respectively. From 200 MHz to 500 MHz, the field distribution begins to lose its uniformity, given the interaction between the sample’s electrical properties and the reduced wavelength of the E-field.
For example, a cell phone frequency of 915 MHz has a penetration depth of 3.9 cm, unable to reach deep brain areas. On the other hand, a frequency of 64 MHz has a penetration depth of 13.5 cm [8,155], sufficient to reach the hippocampus and other deep structures affected early in AD for better treatment. 915 MHz energy applied is ten times higher [157] than 64 MHz and has a higher risk of thermal injuries in the tissues [157]. REMFS is safer and more efficient in reaching the hippocampus posterior cingulate [107,109], or locus caeruleus [109] affected early in AD. Therefore, higher frequencies than 200 MHz need to increase the strength of the RF signal to increase the penetration depth with an increased risk of thermal injuries. To increase the strength of the transmitted RF signal, RF coils are designed to operate in Circular-Polarized (CP) mode. Such coils require a quadrature hybrid interface that combines signals from two channels with a 90° phase difference between them. Also, to obtain a homogeneous E-field distribution we should optimize the excitation current at the transmit coil by changing the amplitude and phase.
The design and development of RF coils are based on the physics of MR signal generation, where the RF coil transmits the Electromagnetic (EM) field into the tissues. Most RF coil designs comprise Perfect Electrical Conductor (PEC) geometries and complex samples where the E-field is measured. Therefore, an important part of RF coil engineering is the so-called realistic human head phantom models in EM simulation (Figure 1). Computational Electromagnetics (CEM) includes several techniques to compute approximations to Maxwell’s equations; CEM enables the modeling of these complex electrodynamic systems. The numerical methods that use differential-equation solvers include the Finite-Difference Time-Domain (FDTD) method and the Finite-Element Method (FEM). The most common EM simulation software applications used in RF coil design and applications are High-Frequency Structure Simulators Ansys (HFSS), Sim4Life, and Comsol COMSOL Multiphysics. FDTD solves the electric field before the magnetic field in two offset rectilinear grids at a specific time, and the calculation progresses across the problem space. When combined with volume-meshing techniques, which use voxels along a non-uniform rectilinear mesh these factors, enable FDTD to effectively simulate the behavior of complex systems, such as those comprising nonhomogeneous materials.
Figure 1:
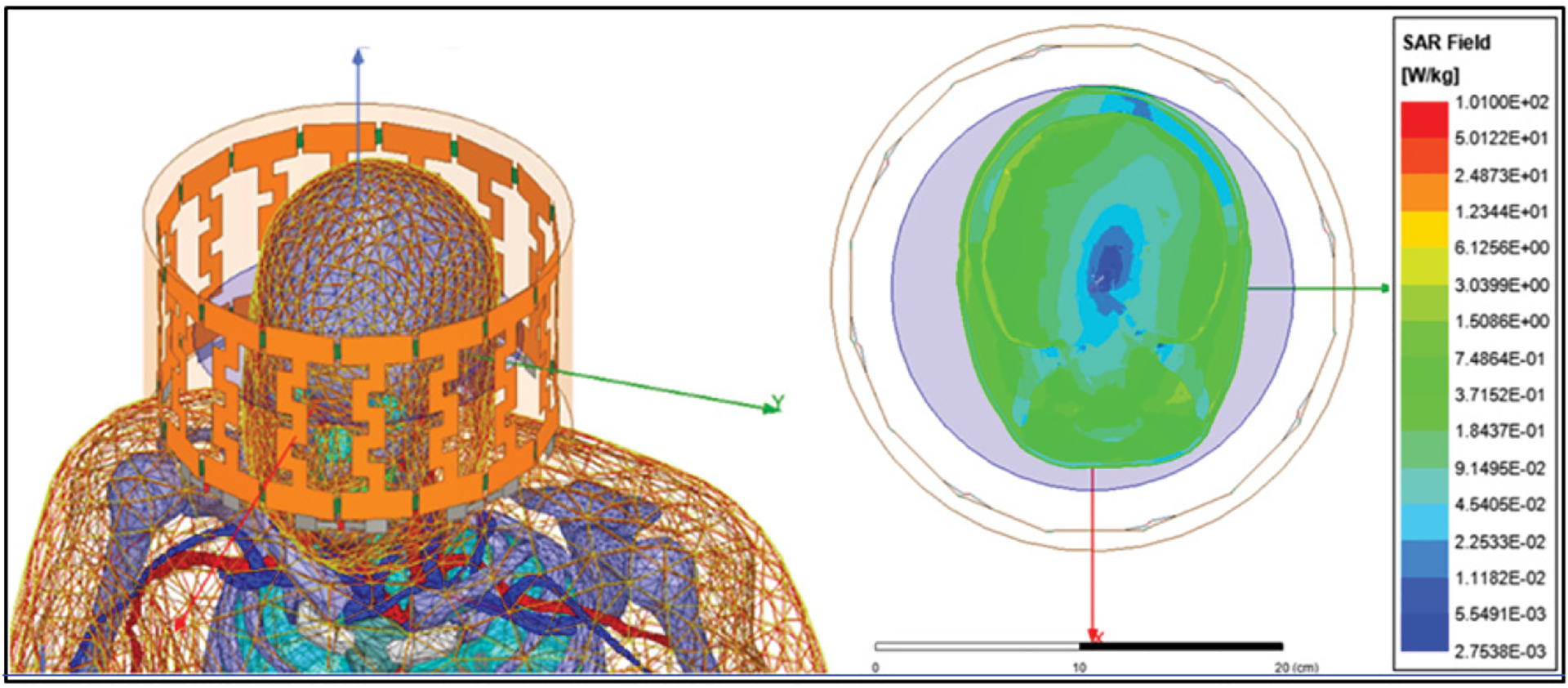
A. Realistic human phantom with all tissue layers and simulated birdcage antenna. B. SAR field distribution on simulated human brain, note the homogeneous distribution in brain mass (green) with optimal SAR values from 0.4 W/kg to 0.9 W/kg at 64 MHz. Also, cerebrospinal fluid SAR (blue) with desire lower SAR values to avoid unnecessary increase in temperature.
References
- 1.Kato M, Shigemitsu T, Miyakoshi J. Electromagnetics in biology: Springer; 2006. [Google Scholar]
- 2.Pimpale A, Razavy M. Electromagnetic coupling in charged damped systems: Classical and quantum considerations. Phys Rev. 1987;36(6):2739. [DOI] [PubMed] [Google Scholar]
- 3.Perez FP, Bandeira JP, Perez Chumbiauca CN, Lahiri DK, Morisaki J, Rizkalla M. Multidimensional insights into the repeated electromagnetic field stimulation and biosystems interaction in aging and age-related diseases. J Biomed Sci. 2022;29(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellossi A, Dubost G. Thermal and non thermal effects of electromagnetic fields in bio-systems. 2012.
- 5.Perez FP, Bandeira JP, Perez Chumbiauca CN, Lahiri DK, Morisaki J, Rizkalla M. Multidimensional insights into the repeated electromagnetic field stimulation and biosystems interaction in aging and age-related diseases. J Biomed Sci. 2022;29(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challis LJ. Mechanisms for interaction between RF fields and biological tissue. Bioelectromagnetics. 2005;Suppl 7:S98–S106. [DOI] [PubMed] [Google Scholar]
- 7.Pompa P, Bramanti A, Maruccio G, Cingolani R, De Rienzo F, Corni S, et al. Retention of nativelike conformation by proteins embedded in high external electric fields. AIP. 2005;122(18):181102. [DOI] [PubMed] [Google Scholar]
- 8.Perez F, Millholland G, Peddinti SV, Thella AK, Rizkalla J, Salama P, et al. Electromagnetic and Thermal Simulations of Human Neurons for SAR Applications. J Biomed Sci Eng. 2016;9(9):437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson DL, Cox MM, Lehninger AL. Principles of biochemistry. WH Freeman and Company, New York, fourth edition edition. 2005;1(1.1):2. [Google Scholar]
- 10.Goss DJ, Petrucci RH. General Chemistry Principles & Modern Applications, Petrucci, Harwood, Herring, Madura: Study Guide: Pearson/Prentice Hall; 2007. [Google Scholar]
- 11.Panagopoulos DJ, Johansson O, Carlo GL. Polarization: A Key Difference between Man-made and Natural Electromagnetic Fields, in regard to Biological Activity. Sci Rep. 2015;5:14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panagopoulos DJ, Messini N, Karabarbounis A, Philippetis AL, Margaritis LH. A mechanism for action of oscillating electric fields on cells. Biochem Biophys Res Commun. 2000;272(3):634–40. [DOI] [PubMed] [Google Scholar]
- 13.Wilmink GJ, Rivest BD, Ibey BL, Roth CL, Bernhard J, Roach WP, editors. Quantitative investigation of the bioeffects associated with terahertz radiation. Optical Interactions with Tissues and Cells XXI; 2010: SPIE. [Google Scholar]
- 14.Hänggi P. Driven quantum systems. Quantum transport and dissipation. 1998:250. [Google Scholar]
- 15.Piilo J, Maniscalco S. Driven harmonic oscillator as a quantum simulator for open systems. Physical review A. 2006;74(3):032303. [Google Scholar]
- 16.Roston D, Cheatum CM, Kohen A. Hydrogen donor–acceptor fluctuations from kinetic isotope effects: A phenomenological model. Biochemistry. 2012;51(34):6860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamovsky I, Nudler E. New insights into the mechanism of heat shock response activation. Cell Mol Life Sci. 2008;65(6):855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerón-Carrasco JP, Jacquemin D. Electric field induced DNA damage: an open door for selective mutations. Chem Commun. 2013;49(69):7578–80. [DOI] [PubMed] [Google Scholar]
- 19.Goryainov S. A model of phase transitions in double-well Morse potential: Application to hydrogen bond. Physica B: Condensed Matter. 2012;407(21):4233–7. [Google Scholar]
- 20.Singh V, Fedeles BI, Essigmann JM. Role of tautomerism in RNA biochemistry. RNA. 2015;21(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins CM, Wang Z. Calculation of radiofrequency electromagnetic fields and their effects in MRI of human subjects. Magn Reson Med. 2011;65(5):1470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonov L. Tautomerism: Concepts and Applications in Science and Technology: John Wiley & Sons; 2016. [Google Scholar]
- 23.Cochrane JC, Strobel SA. Riboswitch effectors as protein enzyme cofactors. RNA. 2008;14(6):993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goh GB, Knight JL, Brooks CL. Constant pH molecular dynamics simulations of nucleic acids in explicit solvent. J Chem Theory Comput. 2011;8(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh V, Peng CS, Li D, Mitra K, Silvestre KJ, Tokmakoff A, et al. Direct observation of multiple tautomers of oxythiamine and their recognition by the thiamine pyrophosphate riboswitch. ACS Chem Biol. 2013;9(1):227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felicíssimo VC, da Rocha Martins J, Boldt IS, Chacham H. Molecular switches activated by electromagnetic pulses: First-principles calculations. Phy Rev A. 2009;80(6):063410. [Google Scholar]
- 27.Peng CS, Jones KC, Tokmakoff A. Anharmonic vibrational modes of nucleic acid bases revealed by 2D IR spectroscopy. J Ame Chem Soc. 2011;133(39):15650–60. [DOI] [PubMed] [Google Scholar]
- 28.Abou-Zied OK, Jimenez R, Romesberg FE. Tautomerization dynamics of a model base pair in DNA. J Am Chem Soc. 2001;123(19):4613–4. [DOI] [PubMed] [Google Scholar]
- 29.De Ninno A, Congiu Castellano A. Deprotonation of glutamic acid induced by weak magnetic field: an FTIR-ATR study. Bioelectromagnetics. 2011;32(3):218–25. [DOI] [PubMed] [Google Scholar]
- 30.Yerkin A, Valentina Y, Tussupbayev Nessipbay ST, Zhadyra Y, Maxat B, Evgeniy B. Explanation of Tautomerism and Isomerization in Terms of the Magnetic Field. J Chem Chem Eng. 2016;10:96–8. [Google Scholar]
- 31.Giudice ED, Tedeschi A. Water and autocatalysis in living matter. Electromagn Biol Med. 2009;28(1):46–52. [DOI] [PubMed] [Google Scholar]
- 32.Cimdins A, Klinkert B, Aschke-Sonnenborn U, Kaiser FM, Kortmann J, Narberhaus F. Translational control of small heat shock genes in mesophilic and thermophilic cyanobacteria by RNA thermometers. RNA Biol. 2014;11(5):594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman R, Blank M, Lin H, Dai R, Khorkova O, Soo L, et al. Increased levels of hsp70 transcripts induced when cells are exposed to low frequency electromagnetic fields. Bioelectrochem Bioenerg. 1994;33(2):115–20. [Google Scholar]
- 34.Mannerling A-C, Simkó M, Mild KH, Mattsson M-O. Effects of 50-Hz magnetic field exposure on superoxide radical anion formation and HSP70 induction in human K562 cells. Radiat Environ Biophys. 2010;49(4):731–41. [DOI] [PubMed] [Google Scholar]
- 35.Goodman R, Henderson AS. Exposure of salivary gland cells to low-frequency electromagnetic fields alters polypeptide synthesis. Proc Natl Acad Sci U S A. 1988;85(11):3928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang BJ, Guerrero ME, Prince TL, Okusha Y, Bonorino C, Calderwood SK. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch Toxicol. 2021;95(6):1943–70. [DOI] [PubMed] [Google Scholar]
- 37.Perez FP, Bose D, Maloney B, Nho K, Shah K, Lahiri DK. Late-onset Alzheimer’s disease, heating up and foxed by several proteins: pathomolecular effects of the aging process. J Alzheimers Dis. 2014;40(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barna J, Csermely P, Vellai T. Roles of heat shock factor 1 beyond the heat shock response. Cell Mol Life Sci. 2018;75(16):2897–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15(2):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nixon RA. The role of autophagy in neurodegenerative disease. Nature medicine. 2013;19(8):983–97. [DOI] [PubMed] [Google Scholar]
- 41.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–115. [DOI] [PubMed] [Google Scholar]
- 42.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94(4):471–80. [DOI] [PubMed] [Google Scholar]
- 43.Perez FP, Zhou X, Morisaki J, Jurivich D. Electromagnetic field therapy delays cellular senescence and death by enhancement of the heat shock response. Exp Gerontol. 2008;43(4):307–16. [DOI] [PubMed] [Google Scholar]
- 44.Lindquist S, Craig E. The heat-shock proteins. Annu Rev Genet. 1988;22(1):631–77. [DOI] [PubMed] [Google Scholar]
- 45.Dul JL, Davis DP, Williamson EK, Stevens FJ, Argon Y. Hsp70 and antifibrillogenic peptides promote degradation and inhibit intracellular aggregation of amyloidogenic light chains. J Cell Biol. 2001;152(4):705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78(3):365–72. [DOI] [PubMed] [Google Scholar]
- 47.Georgopoulos C, Welch W. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9(1):601–34. [DOI] [PubMed] [Google Scholar]
- 48.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–9. [DOI] [PubMed] [Google Scholar]
- 49.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kakimura J-I, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, et al. Microglial activation and amyloid-β clearance induced by exogenous heat-shock proteins. FASEB J. 2002;16(6):601–3. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Turner RS, Gaut JR. The chaperone BiP/GRP78 binds to amyloid precursor protein and decreases Aβ40 and Aβ42 secretion. J Biol Chem. 1998;273(40):25552–5. [DOI] [PubMed] [Google Scholar]
- 52.Luan Q, Jin L, Jiang CC, Tay KH, Lai F, Liu XY, et al. RIPK1 regulates survival of human melanoma cells upon endoplasmic reticulum stress through autophagy. Autophagy. 2015;11(7):975–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desai S, Liu Z, Yao J, Patel N, Chen J, Wu Y, et al. Heat shock factor 1 (HSF1) controls chemoresistance and autophagy through transcriptional regulation of autophagy-related protein 7 (ATG7). Journal of Biological Chemistry. 2013;288(13):9165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park J-S, Kim D-H, Yoon S-Y. Regulation of amyloid precursor protein processing by its KFERQ motif. BMB Rep. 2016;49(6):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez FP, Maloney B, Chopra N, Morisaki JJ, Lahiri DK. Repeated electromagnetic field stimulation lowers amyloid-β peptide levels in primary human mixed brain tissue cultures. Sci Rep. 2021;11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmad RH, Fakhoury M, Lawand N. Electromagnetic Field in Alzheimer’s Disease: A Literature Review of Recent Preclinical and Clinical Studies. Curr Alzheimer Res. 2020;17(11):1001–12. [DOI] [PubMed] [Google Scholar]
- 57.Shirbandi K, Khalafi M, Bevelacqua JJ, Sadeghian N, Adiban S, Zarandi FB, et al. Exposure to low levels of radiofrequency electromagnetic fields emitted from cell-phones as a promising treatment of Alzheimer’s Disease: A scoping review study. J Biomed Phys Eng. 2023;13(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.BioInitiative 2012: A Rationale for Biologically-based Exposure Standards for Low-Intensity Electromagnetic Radiation. 2022 Updated Research Summaries 2012. [Google Scholar]
- 59.Tsoy A, Saliev T, Abzhanova E, Turgambayeva A, Kaiyrlykyzy A, Akishev M, et al. The effects of mobile phone radiofrequency electromagnetic fields on β-amyloid-induced oxidative stress in human and rat primary astrocytes. Neuroscience. 2019;408:46–57. [DOI] [PubMed] [Google Scholar]
- 60.Hirai T, Taniura H, Goto Y, Ogura M, Sng JC, Yoneda Y. Stimulation of ubiquitin-proteasome pathway through the expression of amidohydrolase for N-terminal asparagine (Ntan1) in cultured rat hippocampal neurons exposed to static magnetism. J Neurochem. 2006;96(6):1519–30. [DOI] [PubMed] [Google Scholar]
- 61.Marchesi N, Osera C, Fassina L, Amadio M, Angeletti F, Morini M, et al. Autophagy is modulated in human neuroblastoma cells through direct exposition to low frequency electromagnetic fields. J Cell Physiol. 2014;229(11):1776–86. [DOI] [PubMed] [Google Scholar]
- 62.Osera C, Amadio M, Falone S, Fassina L, Magenes G, Amicarelli F, et al. Pre-exposure of neuroblastoma cell line to pulsed electromagnetic field prevents H2 O2 -induced ROS production by increasing MnSOD activity. Bioelectromagnetics. 2015;36(3):219–32. [DOI] [PubMed] [Google Scholar]
- 63.Osera C, Fassina L, Amadio M, Venturini L, Buoso E, Magenes G, et al. Cytoprotective response induced by electromagnetic stimulation on SH-SY5Y human neuroblastoma cell line. Tissue Eng Part A. 2011;17(19–20):2573–82. [DOI] [PubMed] [Google Scholar]
- 64.Leszczynski D, Joenväärä S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer-and blood-brain barrier-related effects. Differentiation. 2002;70(2–3):120–9. [DOI] [PubMed] [Google Scholar]
- 65.Arendash GW, Mori T, Dorsey M, Gonzalez R, Tajiri N, Borlongan C. Electromagnetic treatment to old Alzheimer’s mice reverses beta-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS One. 2012;7(4):e35751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arendash GW. Transcranial electromagnetic treatment against Alzheimer’s disease: why it has the potential to trump Alzheimer’s disease drug development. J Alzheimers Dis. 2012;32(2):243–66. [DOI] [PubMed] [Google Scholar]
- 67.Dragicevic N, Bradshaw P, Mamcarz M, Lin X, Wang L, Cao C, et al. Long-term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer’s transgenic mice and normal mice: a mechanism for electromagnetic field-induced cognitive benefit? Neuroscience. 2011;185:135–49. [DOI] [PubMed] [Google Scholar]
- 68.Arendash GW, Sanchez-Ramos J, Mori T, Mamcarz M, Lin X, Runfeldt M, et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer’s disease mice. J Alzheimers Dis. 2010;19(1):191–210. [DOI] [PubMed] [Google Scholar]
- 69.Banaceur S, Banasr S, Sakly M, Abdelmelek H. Whole body exposure to 2.4 GHz WIFI signals: effects on cognitive impairment in adult triple transgenic mouse models of Alzheimer’s disease (3xTg-AD). Behavioural brain research. 2013;240:197–201. [DOI] [PubMed] [Google Scholar]
- 70.Jeong YJ, Son Y, Choi HD, Kim N, Lee YS, Ko YG, et al. Behavioral changes and gene profile alterations after chronic 1,950-MHz radiofrequency exposure: An observation in C57BL/6 mice. Brain Behav. 2020;10(11):e01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumlin T, Iivonen H, Miettinen P, Juvonen A, van Groen T, Puranen L, et al. Mobile phone radiation and the developing brain: behavioral and morphological effects in juvenile rats. Radiat Res. 2007;168(4):471–9. [DOI] [PubMed] [Google Scholar]
- 72.Wang K, Lu J-M, Xing Z-H, Zhao Q-R, Hu L-Q, Xue L, et al. Effect of 1.8 GHz radiofrequency electromagnetic radiation on novel object associative recognition memory in mice. Sci Rep. 2017;7:44521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Son Y, Kim JS, Jeong YJ, Jeong YK, Kwon JH, Choi H-D, et al. Long-term RF exposure on behavior and cerebral glucose metabolism in 5xFAD mice. Neuroscience lett. 2018;666:64–9. [DOI] [PubMed] [Google Scholar]
- 74.Hu Y, Lai J, Wan B, Liu X, Zhang Y, Zhang J, et al. Long-term exposure to ELF-MF ameliorates cognitive deficits and attenuates tau hyperphosphorylation in 3xTg AD mice. Neurotoxicology. 2016;53:290–300. [DOI] [PubMed] [Google Scholar]
- 75.Liu X, Zuo H, Wang D, Peng R, Song T, Wang S, et al. Improvement of spatial memory disorder and hippocampal damage by exposure to electromagnetic fields in an Alzheimer’s disease rat model. PLoS One. 2015;10(5):e0126963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akbarnejad Z, Esmaeilpour K, Shabani M, Asadi-Shekaari M, Saeedi Goraghani M, Ahmadi-Zeidabadi M. Spatial memory recovery in Alzheimer’s rat model by electromagnetic field exposure. Int J Neurosci. 2018;128(8):691–6. [DOI] [PubMed] [Google Scholar]
- 77.de Pomerai D, Daniells C, David H, Allan J, Duce I, Mutwakil M, et al. Non-thermal heat-shock response to microwaves. Nature. 2000;405(6785):417–8. [DOI] [PubMed] [Google Scholar]
- 78.Shallom JM, Di Carlo AL, Ko D, Penafiel LM, Nakai A, Litovitz TA. Microwave exposure induces Hsp70 and confers protection against hypoxia in chick embryos. J Cell Biochem. 2002;86(3):490–6. [DOI] [PubMed] [Google Scholar]
- 79.Weisbrot D, Lin H, Ye L, Blank M, Goodman R. Effects of mobile phone radiation on reproduction and development in Drosophila melanogaster. J Cell Biochem. 2003;89(1):48–55. [DOI] [PubMed] [Google Scholar]
- 80.Jeong YJ, Kang GY, Kwon JH, Choi HD, Pack JK, Kim N, et al. 1950 MHz Electromagnetic Fields Ameliorate Abeta Pathology in Alzheimer’s Disease Mice. Curr Alzheimer Res. 2015;12(5):481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arendash G, Cao C, Abulaban H, Baranowski R, Wisniewski G, Becerra L, et al. A Clinical Trial of Transcranial Electromagnetic Treatment in Alzheimer’s Disease: Cognitive Enhancement and Associated Changes in Cerebrospinal Fluid, Blood, and Brain Imaging. J Alzheimers Dis. 2019;71(1):57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arendash G, Abulaban H, Steen S, Andel R, Wang Y, Bai Y, et al. Transcranial Electromagnetic Treatment Stops Alzheimer’s Disease Cognitive Decline over a 2½-Year Period: A Pilot Study. Medicines (Basel). 2022;9(8):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao C, Abulaban H, Baranowski R, Wang Y, Bai Y, Lin X, et al. Transcranial Electromagnetic Treatment “rebalances” blood and brain cytokine levels in Alzheimer’s patients: A new mechanism for reversal of their cognitive impairment. Front Aging Neurosci. 2022;14:829049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Söderqvist F, Hardell L, Carlberg M, Mild KH. Radiofrequency fields, transthyretin, and Alzheimer’s disease. J Alzheimers Dis. 2010;20(2):599–606. [DOI] [PubMed] [Google Scholar]
- 85.Sandyk R. Alzheimer’s disease: improvement of visual memory and visuoconstructive performance by treatment with picotesla range magnetic fields. Int J Neurosci. 1994;76(3–4):185–225. [DOI] [PubMed] [Google Scholar]
- 86.Amirkavei M, Plastino F, Kvanta A, Kaarniranta K, André H, Koskelainen A. Hormetic heat shock enhances autophagy through HSF1 in retinal pigment epithelium cells. Cells. 2022;11(11):1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui T, Wang Y, Song P, Yi X, Chen J, Yang Y, et al. HSF1-dependent autophagy activation contributes to the survival of melanocytes under oxidative stress in vitiligo. J Invest Dermatol. 2022;142(6):1659–69.e4. [DOI] [PubMed] [Google Scholar]
- 88.Kim JH, Yu D-H, Kim H-J, Huh YH, Cho S-W, Lee J-K, et al. Exposure to 835 MHz radiofrequency electromagnetic field induces autophagy in hippocampus but not in brain stem of mice. Toxicol Ind Health. 2018;34(1):23–35. [DOI] [PubMed] [Google Scholar]
- 89.Kim JH, Sohn UD, Kim H-G, Kim HR. Exposure to 835 MHz RF-EMF decreases the expression of calcium channels, inhibits apoptosis, but induces autophagy in the mouse hippocampus. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological Society and the Korean J Physiol Pharmacol. 2018;22(3):277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243–278. [DOI] [PubMed] [Google Scholar]
- 91.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 2009;106(35):14914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shemesh N, Ben-Zvi A. HSF1 regulation in aging and its role in longevity. Heat shock factor: Springer; 2016;93–113. [Google Scholar]
- 93.Kovács D, Sigmond T, Hotzi B, Bohár B, Fazekas D, Deák V, et al. HSF1Base: a comprehensive database of HSF1 (heat shock factor 1) target genes. Int J Mol Sci. 2019;20(22):5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Newton TM, Duce JA, Bayle ED. The proteostasis network provides targets for neurodegeneration. Br J Pharmacol. 2019;176(18):3508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manai F, Fassina L, Venturini L, Angeletti F, Osera C, Marchesi N, et al. A Low-Frequency Electromagnetic (LF-EMF) Exposure Scheme Induces Autophagy Activation to Counteract in Vitro Aβ-Amyloid Neurotoxicity. Atti del.7. 2014. [Google Scholar]
- 96.Zielinski J, Ducray AD, Moeller AM, Murbach M, Kuster N, Mevissen M. Effects of pulse-modulated radiofrequency magnetic field (RF-EMF) exposure on apoptosis, autophagy, oxidative stress and electron chain transport function in human neuroblastoma and murine microglial cells. Toxicology in vitro. 2020;68:104963. [DOI] [PubMed] [Google Scholar]
- 97.Park J, Kwon JH, Kim N, Song K. Effects of 1950 MHz radiofrequency electromagnetic fields on Aβ processing in human neuroblastoma and mouse hippocampal neuronal cells. J Radiat Res. 2018;59(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin H, Opler M, Head M, Blank M, Goodman R. Electromagnetic field exposure induces rapid, transitory heat shock factor activation in human cells. J Cell Biochem. 1997;66(4):482–8. [DOI] [PubMed] [Google Scholar]
- 99.Maaroufi K, Had-Aissouni L, Melon C, Sakly M, Abdelmelek H, Poucet B, et al. Spatial learning, monoamines and oxidative stress in rats exposed to 900 MHz electromagnetic field in combination with iron overload. Behav Brain Res. 2014;258:80–9. [DOI] [PubMed] [Google Scholar]
- 100.Pena-Philippides JC, Yang Y, Bragina O, Hagberg S, Nemoto E, Roitbak T. Effect of pulsed electromagnetic field (PEMF) on infarct size and inflammation after cerebral ischemia in mice. Transl Stroke Res. 2014;5(4):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang Z, Yang X, Song Y-Q, Tu J. Autophagy in Alzheimer’s disease pathogenesis: Therapeutic potential and future perspectives. Ageing Res Rev. 2021;72:101464. [DOI] [PubMed] [Google Scholar]
- 102.Arendash GW. Transcranial electromagnetic treatment against Alzheimer’s disease: why it has the potential to trump Alzheimer’s disease drug development. J Alzheimers Dis. 2012;32(2):243–66. [DOI] [PubMed] [Google Scholar]
- 103.Eleuteri AM, Amici M, Bonfili L, Cecarini V, Cuccioloni M, Grimaldi S, et al. 50 Hz extremely low frequency electromagnetic fields enhance protein carbonyl groups content in cancer cells: effects on proteasomal systems. J Biomed Biotechnol. 2009;2009:834239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Novoselova EG, Novikov VV, Lunin SM, Glushkova OV, Novoselova TV, Parfenyuk SB, et al. Effects of low-level combined static and weak low-frequency alternating magnetic fields on cytokine production and tumor development in mice. Electromagn Biol Med. 2019;38(1):74–83. [DOI] [PubMed] [Google Scholar]
- 105.Kubat NJ, Moffett J, Fray LM. Effect of pulsed electromagnetic field treatment on programmed resolution of inflammation pathway markers in human cells in culture. J Inflamm Res. 2015;8:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giacobini E, Gold G. Alzheimer disease therapy-moving from amyloid-β to tau. Nature Reviews Neurology. 2013;9(12):677–86. [DOI] [PubMed] [Google Scholar]
- 107.Palmqvist S, Schöll M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H, et al. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun. 2017;8(1):1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fuller JT. Modeling Alzheimer’s Disease: a Statistical Approach to Understanding Pathogenesis Across Brain Regions. 2016.
- 109.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960–9. [DOI] [PubMed] [Google Scholar]
- 110.Rocchi A, Yamamoto S, Ting T, Fan Y, Sadleir K, Wang Y, et al. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. PLoS Genet. 2017;13(8):e1006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernandez D, Kim K-N. A review on the RF coil designs and trends for ultra high field magnetic resonance imaging. Investigative Magnetic Resonance Imaging. 2020;24(3):95–122. [Google Scholar]
- 112.Lin JC. Electromagnetic fields in biological systems: Taylor & Francis; 2012. [Google Scholar]
- 113.Sefidbakht Y, Moosavi-Movahedi AA, Hosseinkhani S, Khodagholi F, Torkzadeh-Mahani M, Foolad F, et al. Effects of 940 MHz EMF on bioluminescence and oxidative response of stable luciferase producing HEK cells. Photochemical & Photobiological Sciences. 2014;13(7):1082–92. [DOI] [PubMed] [Google Scholar]
- 114.Del Vecchio G, Giuliani A, Fernandez M, Mesirca P, Bersani F, Pinto R, et al. Continuous exposure to 900 MHz GSM-modulated EMF alters morphological maturation of neural cells. Neurosci lett. 2009;455(3):173–7. [DOI] [PubMed] [Google Scholar]
- 115.Haghani M, Shabani M, Moazzami K. Maternal mobile phone exposure adversely affects the electrophysiological properties of Purkinje neurons in rat offspring. Neuroscience. 2013;250:588–98. [DOI] [PubMed] [Google Scholar]
- 116.Kim JH, Yu D-H, Huh YH, Lee EH, Kim H-G, Kim HR. Long-term exposure to 835 MHz RF-EMF induces hyperactivity, autophagy and demyelination in the cortical neurons of mice. Sci Rep. 2017;7(1):41129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saikhedkar N, Bhatnagar M, Jain A, Sukhwal P, Sharma C, Jaiswal N. Effects of mobile phone radiation (900 MHz radiofrequency) on structure and functions of rat brain. Neurol Res. 2014;36(12):1072–9. [DOI] [PubMed] [Google Scholar]
- 118.Tong J, Chen S, Liu X-M, Hao D-M. Effect of electromagnetic radiation on discharge activity of neurons in the hippocampus CA1 in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013;29(5):423–7. [PubMed] [Google Scholar]
- 119.Del Giudice E, Facchinetti F, Nofrate V, Boccaccio P, Minelli T, Dam M, et al. Fifty Hertz electromagnetic field exposure stimulates secretion of β-amyloid peptide in cultured human neuroglioma. Neurosci lett. 2007;418(1):9–12. [DOI] [PubMed] [Google Scholar]
- 120.Lu Y, He M, Zhang Y, Xu S, Zhang L, He Y, et al. Differential pro-inflammatory responses of astrocytes and microglia involve STAT3 activation in response to 1800 MHz radiofrequency fields. PloS One. 2014;9(10):e108318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Delen K, Sırav B, Oruç S, Seymen CM, Kuzay D, Yeğin K, et al. Effects of 2600 MHz radiofrequency radiation in brain tissue of male Wistar rats and neuroprotective effects of melatonin. Bioelectromagnetics. 2021;42(2):159–72. [DOI] [PubMed] [Google Scholar]
- 122.Gökçek-Saraç Ç, Akçay G, Karakurt S, Ateş K, Özen Ş, Derin N. Possible effects of different doses of 2.1 GHz electromagnetic radiation on learning, and hippocampal levels of cholinergic biomarkers in Wistar rats. Electromagn Biol Med. 2021;40(1):179–90. [DOI] [PubMed] [Google Scholar]
- 123.Hassanshahi A, Shafeie SA, Fatemi I, Hassanshahi E, Allahtavakoli M, Shabani M, et al. The effect of Wi-Fi electromagnetic waves in unimodal and multimodal object recognition tasks in male rats. Neurol Sci. 2017;38(6):1069–76. [DOI] [PubMed] [Google Scholar]
- 124.Lin Y, Gao P, Guo Y, Chen Q, Lang H, Guo Q, et al. Effects of long-term exposure to l-band high-power microwave on the brain function of male mice. BioMed Res Int. 2021;2021:7932432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nittby H, Widegren B, Krogh M, Grafström G, Berlin H, Rehn G, et al. Exposure to radiation from global system for mobile communications at 1,800 MHz significantly changes gene expression in rat hippocampus and cortex. The Environmentalist. 2008;28(4):458–65. [Google Scholar]
- 126.Ntzouni M, Stamatakis A, Stylianopoulou F, Margaritis L. Short-term memory in mice is affected by mobile phone radiation. Pathophysiology. 2011;18(3):193–9. [DOI] [PubMed] [Google Scholar]
- 127.Ntzouni MP, Skouroliakou A, Kostomitsopoulos N, Margaritis LH. Transient and cumulative memory impairments induced by GSM 1.8 GHz cell phone signal in a mouse model. Electromagn Biol Med. 2013;32(1):95–120. [DOI] [PubMed] [Google Scholar]
- 128.Daniels WM, Pitout IL, Afullo TJ, Mabandla MV. The effect of electromagnetic radiation in the mobile phone range on the behaviour of the rat. Metab Brain Dis. 2009;24(4):629–41. [DOI] [PubMed] [Google Scholar]
- 129.Dasdag S, Akdag MZ, Kizil G, Kizil M, Cakir DU, Yokus B. Effect of 900 MHz radio frequency radiation on beta amyloid protein, protein carbonyl, and malondialdehyde in the brain. Electromagn Biol Med. 2012;31(1):67–74. [DOI] [PubMed] [Google Scholar]
- 130.Deshmukh PS, Banerjee BD, Abegaonkar MP, Megha K, Ahmed RS, Tripathi AK, et al. Effect of low level microwave radiation exposure on cognitive function and oxidative stress in rats. Indian J Biochem Biophys. 2013;50(2):114–9. [PubMed] [Google Scholar]
- 131.Gupta SK, Mesharam MK, Krishnamurthy S. Electromagnetic radiation 2450 MHz exposure causes cognition deficit with mitochondrial dysfunction and activation of intrinsic pathway of apoptosis in rats. J Biosci. 2018;43(2):263–76. [PubMed] [Google Scholar]
- 132.Megha K, Deshmukh PS, Ravi AK, Tripathi AK, Abegaonkar MP, Banerjee BD. Effect of low-intensity microwave radiation on monoamine neurotransmitters and their key regulating enzymes in rat brain. Cell Biochem Biophys. 2015;73(1):93–100. [DOI] [PubMed] [Google Scholar]
- 133.Megha K, Deshmukh PS, Banerjee BD, Tripathi AK, Abegaonkar MP. Microwave radiation induced oxidative stress, cognitive impairment and inflammation in brain of Fischer rats. Indian J Exp Biol . 2012. Dec;50(12):889–96. [PubMed] [Google Scholar]
- 134.Tang J, Zhang Y, Yang L, Chen Q, Tan L, Zuo S, et al. Exposure to 900 MHz electromagnetic fields activates the mkp-1/ERK pathway and causes blood-brain barrier damage and cognitive impairment in rats. Brain research. 2015;1601:92–101. [DOI] [PubMed] [Google Scholar]
- 135.Kim J-Y, Kim H-J, Kim N, Kwon JH, Park M-J. Effects of radiofrequency field exposure on glutamate-induced oxidative stress in mouse hippocampal HT22 cells. Int J Radiat Biol. 2017;93(2):249–56. [DOI] [PubMed] [Google Scholar]
- 136.Jorge-Mora T, Folgueiras MA, Leiro-Vidal JM, Jorge-Barreiro F, Ares-Pena F, Lopez-Martin ME. Exposure to 2.45 GHz microwave radiation provokes cerebral changes in induction of HSP-90 α/β heat shock protein in rat. PIER. 2010;100:351–79. [Google Scholar]
- 137.Stefi AL, Margaritis LH, Skouroliakou AS, Vassilacopoulou D. Mobile phone electromagnetic radiation affects Amyloid Precursor Protein and α-synuclein metabolism in SH-SY5Y cells. Pathophysiology. 2019;26(3–4):203–12. [DOI] [PubMed] [Google Scholar]
- 138.He G-L, Luo Z, Shen T-T, Li P, Yang J, Luo X, et al. Inhibition of STAT3-and MAPK-dependent PGE 2 synthesis ameliorates phagocytosis of fibrillar β-amyloid peptide (1–42) via EP2 receptor in EMF-stimulated N9 microglial cells. J Neuroinflammation. 2016;13(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barthélémy A, Mouchard A, Bouji M, Blazy K, Puigsegur R, Villégier AS. Glial markers and emotional memory in rats following cerebral radiofrequency exposures. Environ Sci Pollut Res Int. 2016;23(24):25343–55. [DOI] [PubMed] [Google Scholar]
- 140.Rao RR, Halper J, Kisaalita WS. Effects of 60 Hz electromagnetic field exposure on APP695 transcription levels in differentiating human neuroblastoma cells. Bioelectrochemistry. 2002;57(1):9–15. [DOI] [PubMed] [Google Scholar]
- 141.Jiang D-p, Li J, Zhang J, Xu S-l, Kuang F, Lang H-y, et al. Electromagnetic pulse exposure induces overexpression of beta amyloid protein in rats. Arch Med Res. 2013;44(3):178–84. [DOI] [PubMed] [Google Scholar]
- 142.Jiang D-P, Li J-h, Zhang J, Xu S-L, Kuang F, Lang H-Y, et al. Long-term electromagnetic pulse exposure induces Abeta deposition and cognitive dysfunction through oxidative stress and overexpression of APP and BACE1. Brain res. 2016;1642:10–9. [DOI] [PubMed] [Google Scholar]
- 143.Qiao S, Peng R, Yan H, Gao Y, Wang C, Wang S, et al. Reduction of phosphorylated synapsin I (ser-553) leads to spatial memory impairment by attenuating GABA release after microwave exposure in Wistar rats. PloS one. 2014;9(4):e95503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prochnow N, Gebing T, Ladage K, Krause-Finkeldey D, El Ouardi A, Bitz A, et al. Electromagnetic field effect or simply stress? Effects of UMTS exposure on hippocampal longterm plasticity in the context of procedure related hormone release. PloS one. 2011;6(5):e19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang H, Peng R, Zhou H, Wang S, Gao Y, Wang L, et al. Impairment of long-term potentiation induction is essential for the disruption of spatial memory after microwave exposure. Int J Radiat Biol. 2013;89(12):1100–7. [DOI] [PubMed] [Google Scholar]
- 146.Wang H, Peng R, Zhao L, Wang S, Gao Y, Wang L, et al. The relationship between NMDA receptors and microwave-induced learning and memory impairment: a long-term observation on Wistar rats. Int J Radiat Biol. 2015;91(3):262–9. [DOI] [PubMed] [Google Scholar]
- 147.Wang H, Tan S, Xu X, Zhao L, Zhang J, Yao B, et al. Long term impairment of cognitive functions and alterations of NMDAR subunits after continuous microwave exposure. Physiol Behav. 2017;181:1–9. [DOI] [PubMed] [Google Scholar]
- 148.Foroozandeh E, Naeini MS, Ahadi H, Foroozandeh J. Effects of 90min Exposure to 8mT Electromagnetic Fields on Memory in Mice. Journal of American Science. 2011;7(7). [Google Scholar]
- 149.Yang X, He G, Hao Y, Chen C, Li M, Wang Y, et al. The role of the JAK2-STAT3 pathway in pro-inflammatory responses of EMF-stimulated N9 microglial cells. J Neuroinflammation. 2010;7(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cleary SF, Cao G, Liu LM, Egle PM, Shelton KR. Stress proteins are not induced in mammalian cells exposed to radiofrequency or microwave radiation. Bioelectromagnetics. 1997;18(7):499–505. [DOI] [PubMed] [Google Scholar]
- 151.Poulletier de Gannes F, Masuda H, Billaudel B, Poque-Haro E, Hurtier A, Lévêque P, et al. Effects of GSM and UMTS mobile telephony signals on neuron degeneration and blood-brain barrier permeation in the rat brain. Scientific rep. 2017;7(1):15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Regel SJ, Tinguely G, Schuderer J, Adam M, Kuster N, LANDOLT HP, et al. Pulsed radio-frequency electromagnetic fields: dose-dependent effects on sleep, the sleep EEG and cognitive performance. J Sleep Res. 2007;16(3):253–8. [DOI] [PubMed] [Google Scholar]
- 153.Protection ICoN-IR. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health physics. 2020;118(5):483–524. [DOI] [PubMed] [Google Scholar]
- 154.C95.1:2019 IS. Safety Levels with Respect to Human Exposure to Electric, Magnetic, and Electromagnetic Fields, 0 Hz to 300 GHz, (2019). Revision of IEEE Std C951:2005/Incorporates IEEE Std C951:2019/Cor 1–20192019. [Google Scholar]
- 155.Leroy Y, Bocquet B, Mamouni A. Non-invasive microwave radiometry thermometry. Physiol Meas. 1998;19(2):127. [DOI] [PubMed] [Google Scholar]
- 156.Honsberg C, Bowden S. Photovoltaic education network. 2016.
- 157.van den Bergen B, van den Berg CA, Klomp DW, Lagendijk JJ. SAR and power implications of different RF shimming strategies in the pelvis for 7T MRI. Journal of Magnetic Resonance Imaging: An Official J Magn Reson Imaging. 2009;30(1):194–202. [DOI] [PubMed] [Google Scholar]
- 158.Phillips JL. Effects of electromagnetic field exposure on gene transcription. J Cell Biochem. 1993;51(4):381–6. [DOI] [PubMed] [Google Scholar]
- 159.Lupke M, Frahm J, Lantow M, Maercker C, Remondini D, Bersani F, et al. Gene expression analysis of ELF-MF exposed human monocytes indicating the involvement of the alternative activation pathway. Biochim Biophys Acta. 2006;1763(4):402–12. [DOI] [PubMed] [Google Scholar]
- 160.Frahm J, Mattsson M-O, Simkó M. Exposure to ELF magnetic fields modulate redox related protein expression in mouse macrophages. Toxicol lett. 2010;192(3):330–6. [DOI] [PubMed] [Google Scholar]
- 161.Fassina L, Saino E, Visai L, Silvani G, Cusella De Angelis MG, Mazzini G, et al. Electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto titanium fiber-mesh scaffolds. J Biomed Mat Res Part A. 2008;87A(3):750–9. [DOI] [PubMed] [Google Scholar]
- 162.Perez FP, Bandeira JP, Morisaki JJ, Peddinti SVK, Salama P, Rizkalla J, et al. Antenna Design and SAR Analysis on Human Head Phantom Simulation for Future Clinical Applications. Journal of biomedical science and engineering. 2017;10(9):421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perez FP, Zhou X, Morisaki J, Ilie J, James T, Jurivich DA. Engineered repeated electromagnetic field shock therapy for cellular senescence and age-related diseases. Rejuvenation Res. 2008;11(6):1049–57. [DOI] [PubMed] [Google Scholar]


