Abstract
This study investigated the impact of resveratrol on abnormal metabolic remodeling in atrial fibrillation (AF) and explored potential molecular mechanisms. An AF cell model was established by high-frequency electrical stimulation of HL-1 atrial muscle cells. Resveratrol concentrations were optimized using CCK-8 and flow cytometry. AF-induced increases in ROS and mitochondrial calcium, along with decreased adenosine triphosphate (ATP) and mitochondrial membrane potential, were observed. Resveratrol mitigated these changes and maintained normal mitochondrial morphology. Moreover, resveratrol acted through the SIRT3-dependent pathway, as evidenced by its ability to suppress AF-induced acetylation of key metabolic enzymes. SIRT3 overexpression controls acetylation modifications, suggesting its regulatory role. In conclusion, resveratrol's SIRT3-dependent pathway intervenes in AF-induced mitochondrial dysfunction, presenting a potential therapeutic avenue for AF-related metabolic disorders. This study sheds light on the role of resveratrol in mitigating AF-induced mitochondrial remodeling and highlights its potential as a novel treatment for AF.
Key words: atrial fibrillation, resveratrol, SIRT3, key metabolic enzyme acetylation, mitochondrial function
Introduction
Atrial fibrillation (AF) is the most common persistent arrhythmia in clinical practice, and AF is associated with an increased prevalence and increased risk of thromboembolic events and heart failure.1 Epidemiological studies have shown that the global prevalence of AF is 3,757.4 million cases (0.51% of the global population), an increase of 33% over the past 20 years.2,3 Future projections suggest that the absolute AF burden may increase by 60% by 2050.2 Current antiarrhythmic drugs primarily suppress arrhythmia attacks by preferentially blocking the heart’s ion channels. In a large proportion of patients, the application of antiarrhythmic drugs leads to harmful side effects including arrhythmia and extracardiac toxicity.4 Therefore, it is critical to find new targets and improved treatments for AF patients.
The initiation and progression of AF stems from atrial remodeling, including electrical remodeling, structural remodeling, and systolic remodeling, which has been shown to contribute to the self-perpetuating nature of AF (i.e., “AF produces AF”).5 Recently, the understanding of the pathophysiology of AF has begun to shift, and atrial energy metabolic remodeling has been implicated in the pathogenesis of AF. Remodeling of atrial energy metabolism is an adaptive physiological response designed to meet the energy demands of atrial tissues under different loads and pressures. However, long-term cardiac stress and stress stimulation may lead to abnormal remodeling of atrial energy metabolism, leading to abnormal atrial function and atrial pathological changes, ultimately leading to the deterioration of AF disease progression and resulting in adverse clinical outcomes.6-8 It is worth noting that significant changes in cardiac energy metabolism have also been recorded in clinical and basic experiments of AF.7,9 Therefore, this research intends to explore more effective treatment methods for AF from the perspective of energy metabolism remodeling.
Resveratrol, a bioactive polyphenol found in grapes and red wine, has a wide range of biological activities.10 Recent clinical trials and preclinical studies have shown that resveratrol reduces the progression of AF by regulating the signaling pathway of cardiac remodeling and the activity of ion channels that control cardiac excitability.11-13 In addition, we have also noted that resveratrol may have a protective effect on other cardiovascular diseases by regulating mitochondria-related functions.14,15 Although resveratrol has been found to improve the progression of AF, resveratrol-mediated mitochondrial function to improve the abnormal metabolic remodeling of AF and its potential molecular regulatory mechanisms have not been reported.
Mammalian sirtuins (SIRT1-7), a family of deacetylases, are mainly active through nicotinamide adenine dinucleotides (NAD), among which SIRT3 is mainly expressed in mitochondria.16,17 SIRT3 can regulate a variety of processes, such as energy homeostasis, REDOX balance, mitochondrial quality control, mitochondrial biogenesis, kinetics and mitochondrial autophagy.18,19 SIRT3 can participate in the progression of AF by restructuring mitochondrial function. For example, icariin improves atrial remodeling and mitochondrial dysfunction by activating SIRT3/AMPK signaling, thereby alleviating the occurrence of AF induced by excessive alcohol consumption.20 In addition, Liu et al. also showed that honokiol inhibits atrial metabolic remodeling of AF by the SIRT3 pathway.21 Therefore, SIRT3 was the most sought-after candidate gene in our study. Intriguingly, in other studies, resveratrol has been found to activate SIRT3 to regulate disease progression. Based on the above background, we hypothesize that resveratrol may mediate mitochondrial function through the SIRT3 pathway to improve abnormal metabolic remodeling in AF.
In this study, we proved the protective influence of resveratrol on AF by establishing an in vitro model, focusing on the substantial benefits of resveratrol in maintaining mitochondrial homeostasis and energy metabolism and further illustrating that the possible mechanism of homeostasis exerting its protective effect may depend upon the SIRT3 pathway.
Materials and Methods
Cell culture and construction of AF models
Following the Claycomb method for cultivating HL-1 cells, HL-1 cells were cultured in Claycomb with 10% fetal bovine serum in a 37°C, 5% CO2 cell incubator. When HL-1 cells were cultured for 48 h to 90% cell density, serum-free Claycomb cell culture medium was added to serum-starved HL-1 cells to synchronize their cell cycles. During the construction of the AF cell model, HL-1 cells were stimulated by high-frequency electricity using a C-Pace100TM cell electrical stimulator for 24 h with parameters set to 5 ms duration, 25 Hz square-wave pulses and a voltage of 7 V/cm. The needed cell capture efficiency for the entire stimulation period was 90% (confirmed by microscopy and shortened duration of action potentials).22
Cell grouping and administration
Group 1: HL-1 cells were randomly divided into 5 groups: 0, 0.1, 1, 10 and 50. Resveratrol (Sigma-Aldrich, St. Louis, MO, USA) at concentration gradients (0, 0.1, 1, 10, and 50 μmol/L) interfered with HL-1 cells for stimulation induction followed by pacing. The optimal dose of resveratrol was selected according to cell activity and apoptosis and was used as the experimental concentration for the following experiments. Detailed grouping is shown in Table 1.
Group 2: HL-1 cells were randomly divided into 7 groups: Control group (HL-1 cell nonpacing treatment, as control group), AF group (HL-1 cell pacing treatment, as model group), AF+RES group (model group + resveratrol treatment), AF+RES+sh-NC group (in the case of resveratrol intervention, HL-1 cells were transfected with sh-NC and incubated for 1 h and then stimulated with pacing for 24 h), AF+RES+sh-SIRT3 group (HL-1 cells were transfected with short hairpin RNA (sh-SIRT3) under the intervention of resveratrol. The pacing stimulation was performed for 1 h after incubation for 24 h), AF+OE-NC group (transfected HL-1 cells with overexpressed negative control and stimulated for 24 h after incubation for 1 h), AF+OE-SIRT3 group (transfected HL-1 cells with OE-SIRT3 and stimulated for 24 h after incubation for 1 h). sh-SIRT3 targeting SIRT3, overexpressed SIRT3 plasmid (OESIRT3) and their controls (sh-NC, OE-NC) were obtained from Thermo Fisher Technologies (Waltham, MA, USA). Transfection was performed using the Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) kit according to the manufacturer’s instructions. Detailed grouping is shown in Table 2.
Table 1.
Experimental group 1.
| Groups | Model | Type of administration |
|---|---|---|
| 0 group | AF | No processing required |
| 0.1 group | AF | The HL-1 cells were incubated with 0.1 μmol/L Resveratrol and stimulated for 24 hours after incubation for 1 h |
| 1 group | AF | The HL-1 cells were incubated with 1 μmol/L Resveratrol and stimulated for 24 hours after incubation for 1 h |
| 10 group | AF | The HL-1 cells were incubated with 10 μmol/L Resveratrol and stimulated for 24 hours after incubation for 1 h |
| 50 group | AF | The HL-1 cells were incubated with 50 μmol/L Resveratrol and stimulated for 24 hours after incubation for 1 |
Cell counting Kit-8 (CCK-8) assay
CCK-8 (Beyotime, Shanghai, China) was used to detect cell proliferation; 4×103 logarithmically grown HL-1 cells were inoculated in 96-well plates and incubated with DMEM. After incubation for 24 h, 10 µL CCK-8 was appended to each well and cultivated in a dark environment for 2 h. Finally, the optical density (OD) at a wavelength of 450 nm was measured by an RT-6000 enzyme-labeled instrument (Rayto, Norcross, GA, USA).
Apoptosis assessment by flow cytometry
The apoptosis rate was detected by flow cytometry (FCM) using Annexin V-fluorescein isothiocyanate (FITC)/propyl iodide (PI) (Beyotime) staining. After incubation, Five hundred microliters of untreated and treated cells were transferred into different tubes with 5 µL Annexin V-FITC and 5 µL PI added to each tube. The tubes were incubated in darkness at room temperature for 15 min and then analyzed by FCM (Attune NxT, Waltham, MA, USA) within 1 h. Triplicates were performed for each sample to ensure accuracy and reproducibility of the results.
Determination of ATP content
The ATP concentration was detected using an ATP detection kit (Beyotime). In simple terms, HL-1 cells (1×105/well) were collected by trypsinization, followed by centrifugation and washing with PBS. The cells were mixed with RIPA lysis buffer containing protease inhibitors at 4°C for 10 min, and then centrifuged at 4°C for 5 min at 12,000 × g. Subsequently, the cell supernatant was incubated with 300 µL of kit solution for 5 min, and the ATP level in the supernatant was measured via enzyme labeling using a luminometer to detect luminescence produced by the luciferase reaction in the ATP detection kit. Triplicates were performed for each sample to ensure accuracy and reproducibility of the results.
ROS measurement
Intracellular ROS were labeled using DCFH-DA (Beyotime). DCFH-DA was diluted in serum-free medium at a ratio of 1:1000 to achieve a final concentration of 10 μmol/L. After cell treatment, the cell culture medium was removed, and 3 mL of diluted DCFH-DA was added to all the wells and incubated for 20 min. The cells were washed with PBS 3 times to fully remove the DCFH-DA that did not enter the cells. Subsequently, the supernatant was discarded after centrifugation at 1000 rpm for 5 min. Finally, intracellular ROS were detected by FCM. The cells were collected using trypsinization followed by centrifugation at 1000 rpm for 5 min. Approximately 1×106 cells were collected per sample. Triplicates were performed for each sample to ensure accuracy and reproducibility of the results.
Mitochondrial membrane potential measurement
After the cell treatment as described above, JC-1 staining solution (Beyotime) was added to the cells and incubated at 37°C for 20 min. After incubation, the supernatant was removed and washed twice with 1 mL JC-1 staining buffer (1×) per well. After adding 2 mL of cell culture medium, the cells were observed under a fluorescence microscope (EVOS™ M5000; Thermo Fisher Technologies). The red/green fluorescence ratio was measured using specialized Image J software. Regions of interest can be selected within individual cells to measure the intensity of red (aggregated JC-1, indicating high mitochondrial membrane potential - MMP) and green (monomeric JC-1, indicating low MMP) fluorescence. The ratio of red to green fluorescence intensity was calculated to assess the mitochondrial membrane potential changes in the cells. Triplicates were performed for each sample to ensure accuracy and reproducibility of the results.
Mitochondrial Ca2+ levels
A calcium indicator X-Rhod-1 staining assay kit (X14210; Thermo Fisher Technologies) was used to stain mitochondrial Ca2+ in HL-1 cells. The treated cells were incubated in medium with 2.5 µM X-Rhod-1/AM for 30 min at 5% CO2 and 37°C. Next, 1 mL of CoCl2 tyrode solution was added and incubated for 10 min. The cells were then stained with Hoechst 33342 (Sigma-Aldrich) for 10 min at a concentration of 1 μg/mL in phosphate-buffered saline (PBS). Finally, the cells were observed with a fluorescence microscope and photographed (EVOS™ M5000; Thermo Fisher Technologies). The fluorescence intensity was measured using specialized Image J software. Triplicates were performed for each sample to ensure accuracy and reproducibility of the results.
Transmission electron microscopy
Cells were collected and fixed with 2.5% glutaraldehyde for 2-4 h and 1% OsO4 for 2 h. The sample was then rewashed, dehydrated with graded alcohol, and encased in Epon-Araldite resin (Spi-Chem, West Chester, PA, USA). Ultrathin sections were stained with 3% uranyl acetate water for 8 min and reverse stained with 2.7% lead citrate for 8 min. Finally, the sections were observed on a transmission electron microscope (HITACHI, HT7700, Tokyo, Japan).
Table 2.
Experimental group 2.
| Groups | Model | Type of administration |
|---|---|---|
| Control group | AF | HL-1 cells were treated with nonpacing |
| AF group | AF | HL-1 cells were treated with pacing stimulation for 24 h |
| AF+RES group | AF | HL-1 cells were incubated with 10 μmol/L Resveratrol and stimulated for 24 h after incubation for 1 h |
| AF+RES+sh-NC group | AF | HL-1 cells were transfected with sh-NC under the intervention of 10 μmol/L resveratrol, and after incubation for 1 h, pacing stimulation was performed for 24 h |
| AF+RES+sh-SIRT3 gr | oupAF | Under the intervention of 10 μmol/L resveratrol, HL-1 cells were transfected with sh-SIRT3, and the pacing stimulation was performed for 24 h after incubation for 1 h |
| AF+OE-NC group | AF | HL-1 cells were transfected with OE-NC, incubated for 1 hour and then stimulated for 24 h |
| AF+OE-SIRT3 group | AF | HL-1 cells were transfected with OE-SIRT3, incubated for 1 hour and stimulated for 24 h after pacing |
Quantitative real-time PCR
Total RNA was extracted from cells using TRIzol (Vazyme, Nanjing, China). cDNA was synthesized using a HiScript II firststrand cDNA synthesis kit (Vazyme). Gene expression was then detected by real-time PCR analysis of SIRT3 on a real-time fluorescent quantitative PCR apparatus (CFX96 Touch 1855195). Beta-actin and U6 served as internal controls. All primers used in this study are listed in Table 3. Normalize gene expression using Method 2-∆∆Ct. Triplicates were performed for each sample to ensure accuracy and reproducibility of the results.
Western blotting assay
Proteins were extracted from cells and tissues using RIPA lysis buffer (Biosharp, Shanghai, China). Protein concentrations were determined by a BCA protein assay kit (NCM Biotech, Suzhou, China). All cell lysates containing 40 μg of protein were subjected to SDS-PAGE and electrophoretically imprinted on PVDF membranes. The membrane was blocked with Tween-Tris buffered brine (TTBS) containing 5% skim milk at room temperature for 2 h and then incubated with the following primary antibodies: SIRT3 (C73E3) rabbit mAb (2627, Cell Signaling Technology, Danvers, MA, USA), rabbit anti-LCAD antibody (2980, Cell Signaling Technology), rabbit anti-GDH antibody (12793, Cell Signaling Technology), rabbit anti-ACE2 antibody (92485, Cell Signaling Technology), and GAPDH (4970, Cell Signaling Technology). The dilution of primary antibodies was 1:500. After that, the membrane containing protein bands was incubated with HRP polymerized secondary antibody (1:500, bs-0296G-HRP) at room temperature for 2 h. The bands were visualized by an ECL chemiluminescence detection system, and the protein expression was analyzed by ImageJ software for optical density values. Triplicates were performed for each sample to ensure accuracy and reproducibility of the results.
Statistical analysis
Data were analyzed and plotted using GraphPad Prism 9 (Version 9.5.0, La Jolla, CA, USA). AI was used to collate the graph. All plots are represented by the means ± SD, and the significant difference between groups was tested by one-way test. A p-value less than 0.05 was considered a significant difference (*p<0.05, **p<0.01, ***p<0.001).
Results
Effect of resveratrol on HL-1 cell viability and apoptosis
We first established the HL-1 atrial muscle cells by exposing to rapid electrical stimulation to construct a cellular AF model, according to previous published study.22 The CCK-8 results demonstrated that cell viability remained stable for 24 h in the control group, whereas it gradually decreased in the AF group (p<0.05) (Figure 1A). To initially explore the effect of resveratrol on the AF cell model, we used resveratrol at different concentration gradients (0, 0.1, 1, 10, and 50 μmol/L) to treat HL-1 cells for stimulation induction and then pacemaker treatment. The CCK-8 results manifested that resveratrol accelerated the activity of spaceinduced HL-1 cells in a concentration-dependent manner (Figure 1B). The FCM results showed that resveratrol pretreatment (0, 0.1, 1, 10, and 50 μmol/L) significantly reduced the pace-induced apoptosis of HL-1 cells in a concentration-dependent manner (Figure 1C). Since the most effective concentration was 10 μM, we selected 10 μmol/L resveratrol for the following study.
Table 3.
Primer sequences.
| Genes | Primer sequences (5’-3’) |
|---|---|
| SIRT3 | F:5'-TCCTCCTTCC TAGCATCACA-3' |
| R:5'-ATCATAACACC GCACTCCA-3' | |
| GAPDH | F:5'-ACCACAGTCCATGCCATCAC-3' |
| R:5'-TCACCACCCTGTTGCTGTA -3' |
Study on mitochondrial energy metabolism and calcium homeostasis induced by atrial fibrillation by resveratrol
To further explore the influences of resveratrol on AF-induced mitochondrial energy metabolism, ROS and calcium homeostasis, AT P content in HL-1 cells was first detected. The results are shown in Figure 2A. The ATP level in the AF group was lower than that in the control group (p<0.001). However, the ATP level in the AF+RES group was elevated vs that in the AF group (p<0.01). Intracellular ROS were subsequently labeled with a DCFH-DA probe and quantified by FCM. As shown in Figure 2B, ROS levels in the AF group were significantly upregulated vs those in the control group (p<0.001). However, RSV treatment significantly restrained cardiac function. MMP is a major indicator of mitochondrial health, and the loss of mitochondria is often associated with mitochondrial dysfunction and leads to cell death. We labeled MMP using a JC-1 probe and measured the ratio by FCM. The results demonstrated in Figure 2C show that compared with the control group, under the same fluorescence intensity in the AF group, the red fluorescence intensity representing high mitochondrial membrane potential was weakened, while the green fluorescence intensity representing low mitochondrial membrane potential was enhanced, indicating that AF caused MMP defects (p<0.001). However, the MMP defect caused by AF could be reversed after resveratrol treatment. The calcium indicator XRhod-1 was applied to evaluate mitochondrial Ca2+ levels. As exhibited in Figure 2D, AF group significantly elevated mitochondrial Ca2+ vs control group. However, compared with AF group, mitochondrial Ca2+ was significantly diminished in AF+RES group. Subsequently, we used transmission electron microscopy to observe the morphology of mitochondria in HL-1 cells. The results are shown in Figure 2E. Mitochondria in HL-1 cells of the control group were filamentous or tubular, with uniform size and structure within the normal range. The mitochondria in the AF group were larger, mostly fragmentary or spherical, and their ultrastructure was obviously damaged. The mitochondria of the AF+RES group were slightly swollen, and their ultrastructure was slightly damaged. Together, these results manifest that RES can ameliorate AFinduced mitochondrial functional metabolism and calcium homeostasis in HL-1 cells and attenuate the oxidative stress response.
Resveratrol regulates the acetylation of metabolic enzymes through SIRT3-dependent pathway during AF
To determine whether the effect of resveratrol on improving AF depends on SIRT3 stimulation, we first detected the effect of resveratrol on SIRT3. As shown in Figure 3 A-C, the expression of SIRT3 enhanced in AF-induced HL-1 cells vs the control group (p<0.01), while resveratrol increased SIRT3 expression. Subsequently, SIRT3 expression was upregulated with a SIRT3overexpressing plasmid in HL-1 cells, which were then stimulated by pacing for 24 h. Transfection efficiency was determined by RT‒qPCR and Western blotting (Figure 3 A-C). Subsequently, we examined the protein acetylation levels of acetyl-CoA synthetase 2 (AceCS2), glutamate dehydrogenase (GDH), and LCAD in fatty acid oxidation, key enzymes involved in mitochondrial metabolism. Western blot results revealed that compared with those in the control group, the acetylation levels of AceCS2, GDH and LCAD proteins in the AF group were significantly upregulated (p<0.001), while resveratrol inhibited these changes (Figure 3 D-G). In addition, compared with the AF+OE-NC group, the acetylation levels of AceCS2, GDH and LCAD proteins in the AF+OE-SIRT3 group were lowered (p<0.01), indicating that SIRT3 is a key gene that improves metabolic capacity.
Resveratrol mediated mitochondrial function through the SIRT3 pathway to improve AF-induced metabolic remodeling
To explore the effect of resveratrol on AF through the SIRT3 pathway, we conducted functional experiments. The CCK-8 results revealed that the AF+RES+sh-SIRT3 group depressed the vigour of HL-1 cells vs the AF+RES+sh-NC group (p<0.001) (Figure 4A). The FCM results revealed that the AF+RES+sh-SIRT3 group promoted the apoptosis rate of HL-1 cells vs the AF+RES+sh-NC group (p<0.001) (Figure 4B). ATP detection kit results showed that the AF+RES+sh-SIRT3 group had a lower level of ATP in HL-1 cells than the AF+RES+sh-NC group (p<0.05) (Figure 4C). The results of ROS, mitochondrial MMP and calcium levels in cells revealed that compared with the AF+RES+SH-NC group, mitochondrial MMP levels in HL-1 cells were significantly weaken in the AF+Res+sh-SIRT3 group, but ROS and mitochondrial mitochondrial Ca2+ levels were boosted (p<0.001) (Figure 4D-F). Finally, transmission electron microscopy (TEM) results revealed that mitochondria in the AF+RES+sh-NC group were slightly swollen and slightly damaged in ultrastructure, while mitochondria in the AF+RES+sh-SIRT3 group were larger, mostly fragmented or spherical, and significantly damaged in ultrastructure (Figure 4G). This suggests that resveratrol can mediate mitochondrial function through the sirtuin 3 pathway to improve AF-induced metabolic remodeling.
Figure 1.
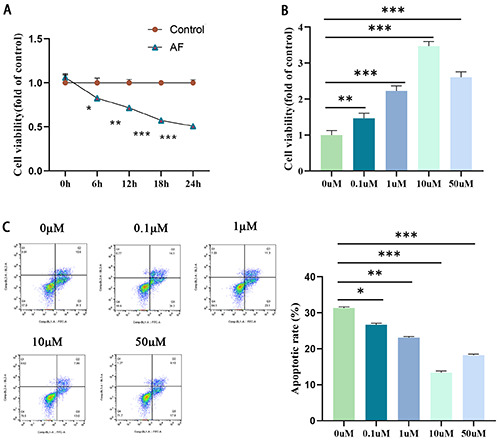
Effect of resveratrol on HL-1 cell viability and apoptosis. A) The CCK-8 method was used to evaluate the effects of the control group and AF group on HL-1 cell viability. B) The CCK-8 method was used to evaluate the effects of resveratrol intervention with different concentration gradients (0, 0.1, 1, 10, 50 μmol/L) on the proliferation of HL-1 cells. C) FCM was used to detect the effect of resveratrol intervention with different concentration gradients (0, 0.1, 1, 10, 50 μmol/L) on the apoptosis of HL-1 cells; *p<0.05, **p<0.01, ***p<0.001; n=3.
Discussion
Recently, an increasing body of research has pinpointed specific alterations in metabolic function as the earliest changes associated with AF, which subsequently lead to ongoing functional changes and structural remodeling in the heart.23 In addition, clinical and basic studies have shown that the pathogenesis of AF is related to an imbalance in energy supply and consumption as well as changes in mitochondrial morphology and function.24 Resveratrol has been found to improve the progression of AF, but resveratrol-mediated mitochondrial function to improve the abnormal metabolic remodeling of AF and its potential molecular regulatory mechanisms have not been reported. Therefore, this study aims to explore the mechanism of resveratrol metabolic remodeling during AF from the perspective of mitochondrial function to clarify the correlation between resveratrol and AF and atrial metabolic remodeling and provide a new theoretical foundation for the pharmacological effects of resveratrol on atrial metabolic remodeling in AF.-
Figure 2.
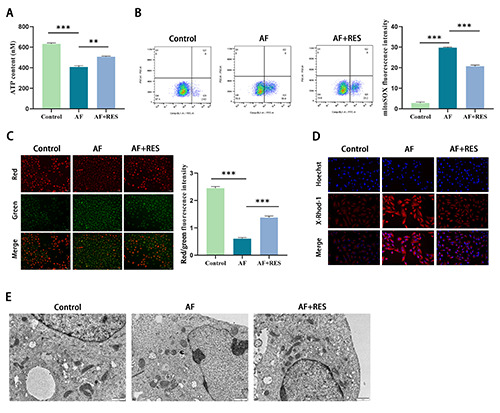
Effects of resveratrol on HL-1 cell viability and apoptosis. A) ATP assay kit evaluated ATP content in HL-1 cells. B) DCFH-DA probe labeled intracellular ROS, and flow cytometry was used to detect the representative image and quantification of fluorescence intensity. C) The MMP level was detected by JC-1 staining, and the red/green fluorescence ratio reflected the change in MMP in HL-1 cells. D) The mitochondrial Ca2+ level and fluorescence intensity were quantitatively evaluated by the calcium indicator X-Rhod-1. E) Morphological changes in mitochondria in HL-1 cells were observed by transmission electron microscopy; *p<0.05, **p<0.01, ***p<0.001; n=3.
Resveratrol is a polyphenol antioxidant that has a variety of beneficial effects, including cardioprotective, anti-inflammatory, antiproliferation, mitochondrial metabolism and immunomodulatory properties. Multiple studies have shown that resveratrol can reduce the progression of AF by regulating cardiac remodeling signaling pathways and ion channel activities that control cardiac excitability.11,12,25 Rapid electrical stimulation has been widely used to build AF models.26 Therefore, in this study, we successfully constructed AF cell models by exposing HL-1 atrial myocytes to rapid electrical stimulation. It was found that electrical stimulation decreased the viability of HL-1 cells in a time-dependent manner, while resveratrol significantly enhanced the viability of HL-1 cells and inhibited apoptosis. These results suggest that the viability and apoptosis of atrial myocytes are related to AF and that resveratrol can improve AF-induced myocardial injury by enhancing HL-1 cell viability and preventing cell apoptosis.
Mitochondria play a key role in supporting heart function and metabolism by providing a continuous energy supply to atrial myocytes.27 Recent studies have shown that energy metabolism disorders, oxidative stress (ROS) and increased mitochondrial damage exist in the mitochondria of atrial cells in AF patients, implying that mitochondria-related changes may be involved in the pathogenesis and maintenance of AF.28-31 Studies have pointed out that reduced ATP levels, loss of mitochondrial membrane potential and fragmentation of the mitochondrial network can lead to contractile dysfunction and progression of AF in clinical and experimental studies.22 Other studies have also shown that Ca2+ overload is closely related to mitochondrial function. Mitochondria are one of the main storage sites of calcium ions in cells. Electrical remodeling occurs in the atria of AF patients, and Ca2+ overload leads to a decrease in L-type-Ca2+ channel density. Meanwhile, Ca2+ overload will open the mitochondrial permeability transition pore (mPTP), and some macromolecular substances will spread from the cytoplasm to mitochondria, causing swelling and mitochondrial membrane potential destruction. Dysfunction, such as a damaged respiratory chain, increased oxygen free radicals, and elevated oxidative stress levels, damages mitochondria and leads to impaired ATP synthesis.32 Since Ca2+ channels are ATP dependent, impaired synthesis of ATP aggravates Ca2+ overload, which in turn worsens mitochondrial dysfunction. Mitochondrial dysfunction leads to cell necrosis, apoptosis and myocardial fibrosis, which further promotes the progression of AF.33 These findings indicate that mitochondria may be potential upstream targets for anti-AF therapy, and elucidating these mechanisms is critical for the development of AF drugs targeting mitochondrial function and for the development of effective new strategies for AF treatment.
Figure 3.
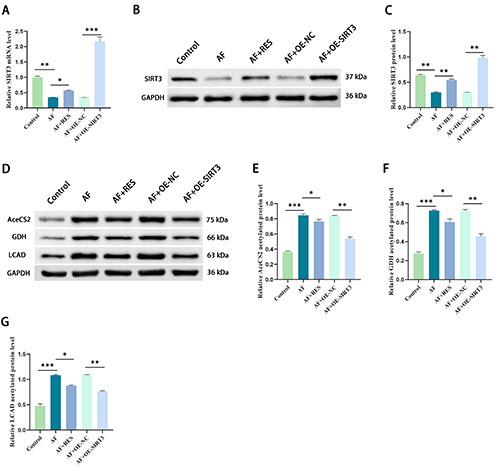
Resveratrol regulates the acetylation of metabolic enzymes through a SIRT3-dependent pathway during AF. A) The mRNA expression level of SIRT3 in HL-1 cells was detected by RT-qPCR. B,C) Western blot analysis was used to detect the protein expression level of SIRT3 in HL-1 cells and analyze its gray value. D,G) Western blotting was used to detect the acetylated protein level and gray value analysis of key metabolic enzymes (LCAD, GDH, AceCS2) in HL-1 cells; *p<0.05, **p<0.01, ***p<0.001; n=3.
For the past few years, resveratrol has been shown to impact the progression of other cardiovascular diseases by modulating mitochondrial function. For example, Tong et al. reported that VDAC1 deacetylation was involved in the protective effect of resveratrol on mitochondria-mediated apoptosis of cardiomyocytes damaged by hypoxia/reoxidation.34 Jeong et al. found that HS1793, a resveratrol analog, protects rat hearts from hypoxia/reoxidation damage by alleviating mitochondrial damage.35 However, it is unclear whether resveratrol can be involved in the progression of AF by regulating mitochondrial function. Therefore, further investigation is needed. In this study, we found that increased ROS and chondriosome calcium levels and depressed ATP and MMP levels in AF cell models resulted in altered mitochondrial morphology; however, resveratrol reversed these changes. These results suggest that resveratrol can ameliorate AF-induced metabolic disorders and mitochondrial dysfunction.
Figure 4.
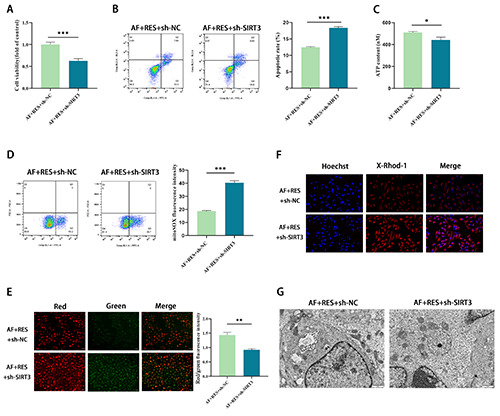
Resveratrol mediates mitochondrial function through the sirtuin 3 pathway to improve AF-induced metabolic remodeling. A) The proliferation of HL-1 cells was evaluated by the CCK-8 method. B) FCM was used to detect HL-1 cell apoptosis. C) ATP detection kit to evaluate ATP content in HL-1 cells. D) DCFH-DA probe-labeled intracellular ROS, and flow cytometry was used to detect the representative image and quantification of fluorescence intensity. E) The MMP level was detected by JC-1 staining, and the red/green fluorescence ratio reflected the change in MMP in HL-1 cells. F) The mitochondrial Ca2+ level and fluorescence intensity were quantitatively evaluated by the calcium indicator X-Rhod-1. G) Morphological changes in mitochondria in HL-1 cells were observed by transmission electron microscopy; *p<0.05, **p<0.01, ***p<0.001; n=3.
SIRT3 is a mitochondrion-targeted deacetylase that controls mitochondrial metabolism under physiological and pathological conditions.36 AceCS2 and GDH are key enzymes involved in tricarboxylic acid cycle metabolism, and LCAD is a key enzyme involved in fatty acid oxidation. As a regulating and controlling mechanism of chondriosome metabolism, acetylation can inhibit the liveness of metabolic enzymes, thereby affecting cardiac functional metabolism.37 SIRT3 has been shown to take part in all kinds of cardiac diseases by deacetylating a variety of enzymes in mitochondrial metabolism.38 Sirt3 downregulation in AF leads to metabolic disruptions like altered long-chain acyl-CoA dehydrogenase, AceCS2, and glutamate dehydrogenase, reducing ATP levels and causing atrial metabolic changes. Boosting Sirt3 expression can reverse these effects, highlighting its role in AF progression through metabolic regulation.39 In our research, we first found that SIRT3 expression is downregulated in AF cell models, whereas resveratrol can act as a SIRT3 agonist to increase SIRT3 expression. In addition, we discovered that the protein acetylation levels of AceCS2, GDH, and LCAD were increased during AF, but this phenomenon was reduced after resveratrol intervention or SIRT3 upregulation. To further determine whether the effect of resveratrol on AF depends on SIRT3 activation, we performed a functional salvage experiment. We found that in the case of resveratrol intervention, downregulating SIRT3 expression reversed the ameliorative effects of resveratrol on metabolic disorders and mitochondrial dysfunction during AF.
Overall, our findings provide a novel mechanism by which resveratrol improves the metabolic remodeling of AF by regulating mitochondrial function in HL-1 cells through a SIRT3-dependent pathway. Studying the mechanisms by which resveratrol improves metabolic remodeling in AF may provide a theoretical foundation for the development of novel therapeutic approaches, leading to the development of more effective treatment strategies. Investigating resveratrol’s role in AF metabolic remodeling has crucial implications for guiding clinical practice, optimizing treatment approaches, and advancing personalized medicine, potentially improving outcomes and management strategies for AF patients. However, there are some limitations to this study. First, our findings are based on cell model studies and need to be validated in an AF animal model. In addition, further studies are needed to determine which signaling pathways SIRT3 regulates affect the progression of AF. Therefore, more investigations are needed in the future to further elucidate this issue.
Funding Statement
Funding: this work was supported by the Tianjin Medical Key Discipline (Specialty) Construction Project (No. TJYXZDXK-058B).
References
- 1.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for atrial fibrillation: US Preventive Services Task Force Recommendation Statement. JAMA 2022;327:360-7. [DOI] [PubMed] [Google Scholar]
- 2.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014;11:639-54. [DOI] [PubMed] [Google Scholar]
- 3.Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke 2021;16:217-21. [DOI] [PubMed] [Google Scholar]
- 4.Dan GA, Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int J Cardiol Heart Vasc 2018;21:11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijesurendra RS, Casadei B. Mechanisms of atrial fibrillation. Heart 2019;105:1860-7. [DOI] [PubMed] [Google Scholar]
- 6.Harada M, Melka J, Sobue Y, Nattel S. Metabolic considerations in atrial fibrillation - mechanistic insights and therapeutic opportunities. Circ J 2017;81:1749-57. [DOI] [PubMed] [Google Scholar]
- 7.Jie QQ, Li G, Duan JB, Li XB, Yang W, Chu YP, et al. Remodeling of myocardial energy and metabolic homeostasis in a sheep model of persistent atrial fibrillation. Biochem Biophys Res Commun 2019;517:8-14. [DOI] [PubMed] [Google Scholar]
- 8.Ozcan C, Li Z, Kim G, Jeevanandam V, Uriel N. Molecular mechanism of the association between atrial fibrillation and heart failure includes energy metabolic dysregulation due to mitochondrial dysfunction. J Card Fail 2019;25:911-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu T, Qin F, Bai F, Xiao Y, Ma Y, Li B, et al. Quantitative acetylated proteomics on left atrial appendage tissues revealed atrial energy metabolism and contraction status in patients with valvular heart disease with atrial fibrillation. Front Cardiovasc Med 2022;9:962036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkaki A, Rashidi M, Ranjbaran M, Asareh ZDA, Shabaninejad Z, Behzad E, et al. Therapeutic effects of resveratrol on ischemia-reperfusion injury in the nervous system. Neurochem Res 2021;46:3085-102. [DOI] [PubMed] [Google Scholar]
- 11.Chong E, Chang SL, Hsiao YW, Singhal R, Liu SH, Leha T, et al. Resveratrol, a red wine antioxidant, reduces atrial fibrillation susceptibility in the failing heart by PI3K/AKT/eNOS signaling pathway activation. Heart Rhythm 2015;12:1046-56. [DOI] [PubMed] [Google Scholar]
- 12.Baczko I, Light PE. Resveratrol and derivatives for the treatment of atrial fibrillation. Ann NY Acad Sci 2015;1348:68-74. [DOI] [PubMed] [Google Scholar]
- 13.Frommeyer G, Wolfes J, Ellermann C, Kochhauser S, Dechering DG, Eckardt L. Acute electrophysiologic effects of the polyphenols resveratrol and piceatannol in rabbit atria. Clin Exp Pharmacol Physiol 2019;46:94-8. [DOI] [PubMed] [Google Scholar]
- 14.Arinno A, Apaijai N, Chattipakorn SC, Chattipakorn N. The roles of resveratrol on cardiac mitochondrial function in cardiac diseases. Eur J Nutr 2021;60:29-44. [DOI] [PubMed] [Google Scholar]
- 15.Zheng M, Bai Y, Sun X, Fu R, Liu L, Liu M, et al. Resveratrol reestablishes mitochondrial quality control in myocardial ischemia/reperfusion injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α pathway. Molecules 2022;27:5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Huang Y, Zhang K, Chen F, Nie T, Zhao Y, et al. Changes of energy metabolism in failing heart and its regulation by SIRT3. Heart Fail Rev 2023;28:977-992. [DOI] [PubMed] [Google Scholar]
- 17.Mao H, Zhang Y, Xiong Y, Zhu Z, Wang L, Liu X. Mitochondria-targeted antioxidant mitoquinone maintains mitochondrial homeostasis through the Sirt3-dependent pathway to mitigate oxidative damage caused by renal ischemia/reperfusion. Oxid Med Cell Longev 2022;2022: 2213503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra Y, Kaundal RK. Role of SIRT3 in mitochondrial biology and its therapeutic implications in neurodegenerative disorders. Drug Discov Today 2023;28:103583. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Zhang Y, Pan Y, Sun C, Liu Z, Liu N, et al. The protective effect of 1,25(OH)(2)D(3) on myocardial function is mediated via sirtuin 3-regulated fatty acid metabolism. Front Cell Dev Biol 2021;9:627135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu LM, Dong X, Xu YL, Zhou ZJ, Huang YT, Zhao JK, et al. Icariin attenuates excessive alcohol consumption-induced susceptibility to atrial fibrillation through SIRT3 signaling. Biochim Biophys Acta Mol Basis Dis 2022;1868:166483. [DOI] [PubMed] [Google Scholar]
- 21.Liu GZ, Xu W, Zang YX, Lou Q, Hang PZ, Gao Q, et al. Honokiol inhibits atrial metabolic remodeling in atrial fibrillation through Sirt3 pathway. Front Pharmacol 2022;13:813272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko TH, Jeong D, Yu B, Song JE, Le QA, Woo SH, Choi JI. Inhibition of late sodium current via PI3K/Akt signaling prevents cellular remodeling in tachypacing-induced HL-1 atrial myocytes. Pflugers Arch. 2023;475:217-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiersma M, van Marion DMS, Wüst RCI, Houtkooper RH, Zhang D, Groot NMS, et al. Mitochondrial dysfunction underlies cardiomyocyte remodeling in experimental and clinical atrial fibrillation. Cells 2019;8:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pool L, Wijdeveld LFJM, de Groot NMS, Brundel BJJM. The role of mitochondrial dysfunction in atrial fibrillation: translation to druggable target and biomarker discovery. Int J Mol Sci 2021;22:8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephan LS, Almeida ED, Markoski MM, Garavaglia J, Marcadenti A. Red wine, resveratrol and atrial fibrillation. Nutrients 2017;9:1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Xu W, Dong Z, Zhou M, Lin C, Jin H, et al. TPEN prevents rapid pacing-induced calcium overload and nitration stress in HL-1 myocytes. Cardiovasc Ther 2015;33:200-8. [DOI] [PubMed] [Google Scholar]
- 27.Emelyanova L, Ashary Z, Cosic M, Negmadjanov U, Ross G, Rizvi F, et al. Selective downregulation of mitochondrial electron transport chain activity and increased oxidative stress in human atrial fibrillation. Am J Physiol Heart Circ Physiol 2016;311:H54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GN, Kitsis RN, et al. Mitochondrial function, biology, and role in disease: a scientific statement from the American Heart Association. Circ Res 2016;118:1960-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukowska A, Schild L, Keilhoff G, Hirte D, Neumann M, Gardemann A, et al. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Exp Biol Med 2008;233:558-74. [DOI] [PubMed] [Google Scholar]
- 30.Xie W, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX, et al. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep 2015;5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu T, Zhou S, Liu Z, Li X, Liu Q. Quantitative proteomics of changes in energy metabolism-related proteins in atrial tissue from valvular disease patients with permanent atrial fibrillation. Circ J 2014;78:993-1001. [DOI] [PubMed] [Google Scholar]
- 32.Mason FE, Pronto J, Alhussini K, Maack C, Voigt N. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res Cardiol 2020;115:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisner V, Picard M, Hajnoczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol 2018;20:755-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong Z, Xie Y, He M, Ma W, Zhou Y, Lai S, et al. VDAC1 deacetylation is involved in the protective effects of resveratrol against mitochondria-mediated apoptosis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed Pharmacother 2017;95:77-83. [DOI] [PubMed] [Google Scholar]
- 35.Jeong SH, Hanh TM, Kim HK, Lee SR, Song IS, Noh SJ, et al. HS-1793, a recently developed resveratrol analogue protects rat heart against hypoxia/reoxygenation injury via attenuating mitochondrial damage. Bioorg Med Chem Lett 2013;23:4225-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Xu H, Tan B, Yi Q, Sun Y, Xiang H, et al. SIRT3 promotes metabolic maturation of human iPSC-derived cardiomyocytes via OPA1-controlled mitochondrial dynamics. Free Radical Bio Med 2023;195:270-82. [DOI] [PubMed] [Google Scholar]
- 37.Tu T, Zhou S, Liu Q. Acetylation: a potential "regulating valve" of cardiac energy metabolism during atrial fibrillation. Int J Cardiol 2014;177:71-2. [DOI] [PubMed] [Google Scholar]
- 38.Afzaal A, Rehman K, Kamal S, Akash M. Versatile role of sirtuins in metabolic disorders: From modulation of mitochondrial function to therapeutic interventions. J Biochem Mol Toxicol 2022;36:e23047. [DOI] [PubMed] [Google Scholar]
- 39.Liu GZ, Xu W, Zang YX, Lou Q, Hang PZ, Gao Q, et al. Honokiol inhibits atrial metabolic remodeling in atrial fibrillation through Sirt3 pathway. Front Pharmacol. 2022;13:813272. [DOI] [PMC free article] [PubMed] [Google Scholar]


