Abstract
BACKGROUND
Lung protective ventilation is considered standard of care in the intensive care unit. However, modifying the ventilator settings can be challenging and is time consuming. Closed loop modes of ventilation are increasingly attractive for use in critically ill patients. With closed loop ventilation, settings that are typically managed by the ICU professionals are under control of the ventilator's algorithms.
OBJECTIVES
To describe the effectiveness, safety, efficacy and workload with currently available closed loop ventilation modes.
DESIGN
Systematic review of randomised clinical trials.
DATA SOURCES
A comprehensive systematic search in PubMed, Embase and the Cochrane Central register of Controlled Trials search was performed in January 2023.
ELIGIBILITY CRITERIA
Randomised clinical trials that compared closed loop ventilation with conventional ventilation modes and reported on effectiveness, safety, efficacy or workload.
RESULTS
The search identified 51 studies that met the inclusion criteria. Closed loop ventilation, when compared with conventional ventilation, demonstrates enhanced management of crucial ventilator variables and parameters essential for lung protection across diverse patient cohorts. Adverse events were seldom reported. Several studies indicate potential improvements in patient outcomes with closed loop ventilation; however, it is worth noting that these studies might have been underpowered to conclusively demonstrate such benefits. Closed loop ventilation resulted in a reduction of various aspects associated with the workload of ICU professionals but there have been no studies that studied workload in sufficient detail.
CONCLUSIONS
Closed loop ventilation modes are at least as effective in choosing correct ventilator settings as ventilation performed by ICU professionals and have the potential to reduce the workload related to ventilation. Nevertheless, there is a lack of sufficient research to comprehensively assess the overall impact of these modes on patient outcomes, and on the workload of ICU staff.
KEY POINTS
Closed loop ventilation automates ventilator settings that are typically manually adjusted by the user during conventional ventilation.
This systematic review identified 51 studies regarding six closed loop ventilation modes.
Closed loop ventilation is at least as effective in choosing lung protective ventilator settings as ventilation performed by ICU professionals.
Closed loop ventilation has the potential to decrease ICU staff workload, and even improve patient outcomes, although these findings are limited by underpowered study designs.
Introduction
Mechanical ventilation is a key element of respiratory support in critically ill patients with respiratory failure. In the early years of critical care, the one–single goal of mechanical ventilation was to provide sufficient gas exchange, often targeting physiological levels of arterial partial pressures of oxygen (paO2) and carbon dioxide (paCO2).1 In the last decades, the goals of ventilation shifted towards lung protection, even if this jeopardised the initial ventilatory targets (e.g. by applying permissive hypercapnia, to reduce tidal volume and plateau pressure).2 While so-called lung protective ventilation has become the standard of care,3 its application in clinical practice can be challenging and time consuming; achieving the ventilatory targets requires complex titrations of ventilator settings according to the individual needs of patients, which change over time. There is clearly no ‘one–size–fits–all’, and constant individualisation and titration of ventilatory settings are required mandating the use of sometimes complex bedside calculations. Currently, lung protective ventilation includes a low tidal volume (VT), to prevent volutrauma and barotraumas; low pressures and energy, to avoid energy trauma; and restricted oxygen, to minimise chemotrauma.
Automated, or closed loop modes of ventilation, are increasingly attractive for use in the ICU.4 Ventilator settings that are typically manually adjusted by the user during conventional ventilation can, once the targets are manually set, be controlled by the software during closed loop ventilation. Closed loop ventilation has the potential to optimise ventilator settings, to increase safety of ventilation, and even to improve patient outcomes.5,6 Closed loop ventilation might also reduce ICU nursing and medical staff workload, through immediate reaction to patients’ changing demands.7 This is particularly interesting when faced with increasing challenges due to shortages in ICU nursing staff,8 and especially in extreme situations as seen in the recent coronavirus disease 2019 (COVID-19) pandemic when large numbers of patients required invasive mechanical ventilation.
We present the results of a systematic search of the literature for publications on randomised clinical trials of closed loop ventilation that focused on effectiveness in providing lung protective ventilation and settings, safety, patient outcomes related efficacy and ICU staff workloads (Table 1). We hypothesised that currently available closed loop ventilation modes are effective, well tolerated and efficacious, while reducing the ICU staff workloads.
Table 1.
Definitions used for Outcome parameters
| Outcome parameters | Definition |
| Effectiveness | The ability of the closed loop mode to institute appropriate settings as reflected by VT, ΔP, MP or FiO2, and to provide lung protective ventilation |
| Safety | Any adverse event, or discontinuation or change in a ventilator setting related to the closed loop mode under investigation because of unacceptable changes in clinical parameters |
| Efficacy | The effect of the closed loop mode on patient–related outcomes such as mortality, duration of ventilation, and ICU and hospital lengths of stay |
| Workload | The effect on staff workload such as the number of manual interventions to ventilator settings, or the number of alarms |
Materials and methods
Search details
We conducted a literature search using various combinations of keywords and MeSH terms, including ‘Interactive Ventilatory Support’, ‘Respiration, Artificial’, ‘Automation’, ‘closed loop ventilation’, ‘automated ventilation’, ‘mechanical ventilation’ and ‘explicit computerized protocols’ in PubMed, Embase and the Cochrane Central register of Controlled Trials (CENTRAL). Inclusion criteria were randomised clinical trials that studied the effect of closed loop ventilation modes on ventilator settings, patient outcomes and ICU staff workload. We used no time or language restrictions, and included publications of studies in all patient categories, including paediatric and adult ICU cohorts. The reference lists of studies and systematic reviews identified by the search were used to find additional reports that may have been missed by the original search. The search was registered at PROSPERO with registration number CRD42023446174, and a final search was performed in January 2023.
Publications identified by the search were screened for eligibility by two independent investigators (RLG and LAB-K) by reading the titles and abstracts. If a study was considered potentially eligible, the full text was obtained, and reviewed for using the predefined inclusion and exclusion criteria.
Selection of studies
A publication was eligible if reporting on a randomised clinical trial of closed loop ventilation; in invasively ventilated paediatric or adult ICU patients; and reporting on aspects regarding effectiveness, safety, efficacy or workload.
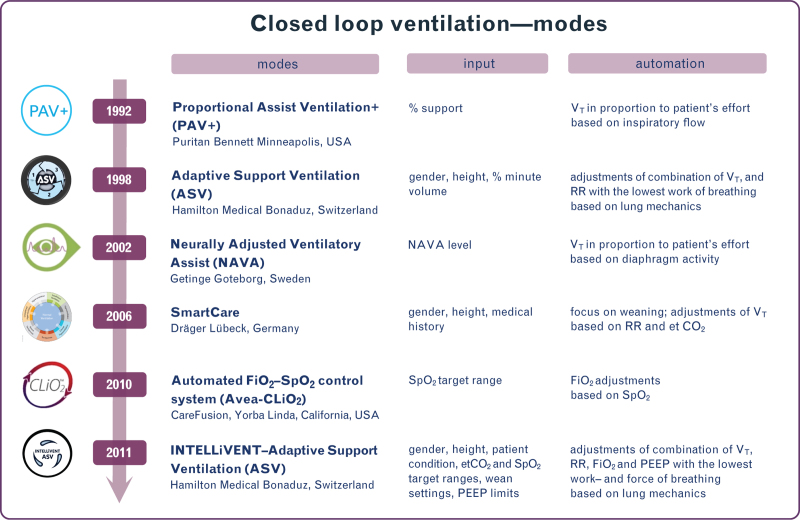
We selected studies that tested either SmartCare (Dräger, Lübeck, Germany), Adaptive Support Ventilation (ASV) or INTELLiVENT–ASV (Hamilton Medical, Bonaduz, Switzerland), Neurally Adjusted Ventilatory Assist (NAVA) (Getinge, Goteborg, Sweden), Proportional Assist Ventilation Plus (PAV+) (Puritan Bennett, Minneapolis, USA) and Avea–CLiO2 (CareFusion, Yorba Linda, California, USA). For details on these closed loop modes, see Fig. 2.
Fig. 2.
Closed loop ventilation modes.
etCO2, end-tidal carbon dioxide; FiO2, fraction of inspired oxygen; PEEP, positive end expiratory pressure; RR, respiratory rate; SpO2, pulse oximetry; VT, tidal volume.
We excluded reports on studies of noninvasive ventilation, and ventilation in another setting than the ICU, that is ventilation in an emergency department or in an operating room.
Extracted data
From each study, we collected the following data: patient characteristics, duration of ventilation or study intervention, and the investigated mode of closed loop and conventional ventilation. Data regarding effectiveness included ventilator settings and ventilation parameters such as tidal volume (VT), driving pressure (ΔP), mechanical power (MP) or fraction of inspired oxygen (FiO2). The rationale for choosing these effectiveness parameters in the light of closed loop ventilation and the current challenges in lung protective ventilation can be found in the Supplement. Data regarding safety included any adverse event, or discontinuation or change in a ventilator setting related to the closed loop mode under investigation because of unacceptable change in clinical parameters. Efficacy data included patient-related outcomes, such as duration of ventilation, length of stay in ICU or mortality rates. Data regarding workload included the number of manual interventions at the ventilator, or the number of alarms.
Risk of bias and study quality
For each study, information was collected for the assessment of the risk of bias. The Cochrane Collaboration's tool for assessing risk of bias was used to assess the risk of bias for the included studies.9
We also calculated the fragility index for studies having a statistically significant dichotomous primary outcome,10,11 and compared them with the number of patients lost to follow-up for that endpoint in order to assess the robustness of the study results; the fragility index calculates the number of patients required to lose statistical significance.12
Reporting
Data were reported as medians with interquartile ranges or means with standard deviations. For each study that reported a dichotomous primary endpoint, the fragility index and the number of patients lost to follow-up were reported. We did not perform a meta-analysis of the studies identified by the search, because the studies used various outcome measures, and had different study designs and durations.
Results
Search results and risk of bias
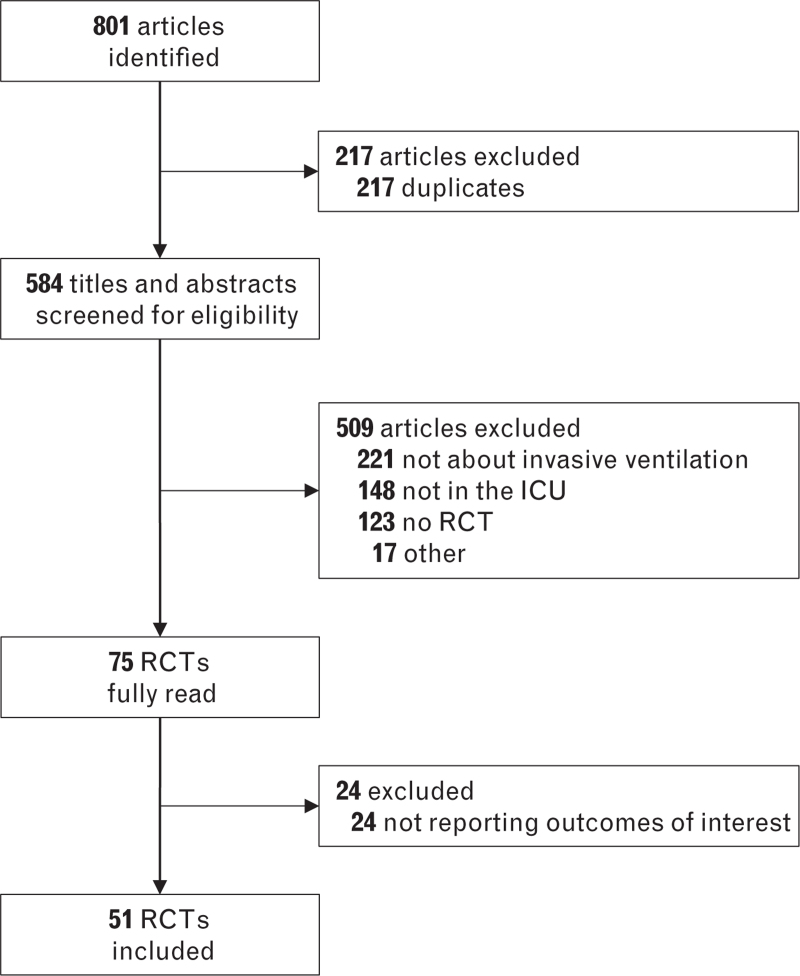
The search identified a total of 801 studies; after removal of duplicates and screening for eligibility, 45 studies in adult13–57 and six studies in paediatric patients58–63 were considered for this analysis (Fig. 1 and eTable S1). Thirty-five studies had a parallel randomised design13–19,21,23,25–31,33,34,36,38–40,43–57,62,63; 12 had a crossover randomised design.20,22,24,32,35,37,41,42,58–61 Only five studies were multicentre investigations.23,33,36,38,39 Most studies were performed in critically ill patients with respiratory failure15,16,18,22,24,26,27,29–31,33,35,36,39,41,46,47,54,62; twelve studies in patients after cardiac surgery13,17,25,28,34,48–51,57 or general surgery,20,23,43 four in difficult to wean patients,19,32,38,40,56 four in COPD patients14,21,44,45 and four in ARDS patients.37,42,53,55 Nine studies tested SmartCare,14,15,18,19,23,32,47,54,63 fourteen studies ASV,13,17,21,42,43,45,46,48–53,55 eight studies INTELLiVENT–ASV,22,24,25,27,28,30,34,35 eight studies NAVA,20,36–41,62 six studies PAV+,16,26,29,31,33,44 three studies Avea–CLiO258–60 and one closed loop FiO2 titrations during ASV.61 Observation duration differed substantially between studies (eTable S1).
Fig. 1.
Search results.
As blinding of personnel was not possible due to the nature of the intervention, risk of performance bias was high in all studies (eFigure S1). In most studies, it was unclear how detection bias was avoided. Allocation concealment was used in 24 studies to reduce the risk of selection bias. The fragility index could be calculated in five studies and varied between 0 and 18 (eTable S2,).
Effectiveness
With SmartCare, VT decreased in difficult to wean patients32 but was not affected in critically ill patients47 compared with conventional ventilation (Table 2, Fig. 3 and eTable S3). With ASV, VT increased in cardiac surgery patients17 and in ARDS patients.42 ASV decreased VT in ARDS patients,55 but did not affect VT in cardiac surgery48,51 and COPD patients.21,47 INTELLiVENT-ASV led to a lower VT22,25,34 in cardiac surgery and unselected ICU patients, but VT increased24 or was unaffected in general ICU patients.27,30,35 With NAVA, VT decreased in ARDS patients.37 NAVA did not affect VT in abdominal surgery patients.20 PAV+ did not affect VT in a general ICU population.16,29,31
Table 2.
Effectiveness, safety, efficacy and workload with closed loop ventilation
| Author | Year | Ref | Patients | Closed loop mode tested | Effectiveness | Safety | Efficacy | Workload |
| Jiang et al. | 2006 | 14 | 38 COPD patients | SmartCare | – | – | ↑ | ↑ |
| Stahl et al. | 2009 | 18 | 60 patients needed ventilation >24 h | SmartCare | – | = | ↑ | = |
| Ma et al. | 2010 | 19 | 62 difficult to wean patients | SmartCare | ↑ | – | ↑ | ↑ |
| Rose et al. | 2008 | 15 | 102 patients needed ventilation >24 h | SmartCare | – | – | = | – |
| Schädler et al. | 2012 | 23 | 300 surgical patients needed ventilation >9 h | SmartCare | – | – | = | – |
| Burns et al. | 2013 | 54 | 92 critically ill patients | SmartCare | – | = | ↑ | – |
| Jouvet et al. | 2013 | 63 | 30 unselected paediatric patients | SmartCare | – | = | ↑ | – |
| Liu et al | 2013 | 56 | 39 difficult to wean patients | SmartCare | – | = | ↑ | – |
| Taniguchi et al. | 2015 | 47 | 70 critically ill patients | SmartCare | = | = | ↓ | – |
| Grieco et al. | 2018 | 32 | 30 difficult to wean patients | SmartCare | ↑ | – | – | – |
| Sulzer et al. | 2001 | 57 | 36 cardiac surgery patients | Adaptive Support Ventilation | – | – | ↑ | – |
| Petter et al. | 2003 | 13 | 30 cardiac surgery patients | Adaptive Support Ventilation | – | = | = | ↑ |
| Dongelmans et al. | 2009 | 17 | 128 cardiac surgery patients | Adaptive Support Ventilation | = | – | = | – |
| Kirakli et al. | 2011 | 21 | 97 COPD patients | Adaptive Support Ventilation | = | – | ↑ | – |
| Agarwal et al. | 2013 | 55 | 48 ARDS patients | Adaptive Support Ventilation | ↑ | – | = | – |
| Celli et al. | 2014 | 43 | 20 abdominal surgery patients | Adaptive Support Ventilation | – | – | ↑ | ↑ |
| Mohamed et al. | 2014 | 45 | 50 COPD patients | Adaptive Support Ventilation | – | = | ↑ | – |
| Kirakli et al. | 2015 | 46 | 229 critically ill patients | Adaptive Support Ventilation | – | – | = | ↑ |
| Zhu et al. | 2015 | 48 | 53 cardiac surgery patients | Adaptive Support Ventilation | = | = | ↑ | – |
| Yazdannik et al. | 2016 | 49 | 64 cardiac surgery patients | Adaptive Support Ventilation | – | – | ↑ | – |
| Moradian et al. | 2017 | 50 | 115 cardiac surgery patients | Adaptive Support Ventilation | – | ↑ | ↑ | – |
| Eremenko et al. | 2020 | 51 | 78 cardiac surgery patients | Adaptive Support Ventilation | = | = | = | ↑ |
| Baedorf Kassis et al. | 2022 | 42 | 20 ARDS patients | Adaptive Support Ventilation | = | = | – | – |
| Sehgal et al. | 2022 | 52 | 48 envenomation patients | Adaptive Support Ventilation | – | – | = | – |
| Soydan et al. | 2022 | 61 | 30 critically ill paediatric patients | Adaptive Support Ventilation with closed–loop FiO2 titration | ↑ | ↑ | – | ↑ |
| Zhang et al. | 2022 | 53 | 100 ARDS patients | Adaptive Support Ventilation | – | – | ↑ | – |
| Arnal et al. | 2012 | 22 | 50 critically ill patients | INTELLiVENT–ASV | ↑ | = | = | – |
| Clavieras et al. | 2013 | 24 | 14 critically ill patients | INTELLiVENT–ASV | = | – | = | – |
| Lellouche et al. | 2013 | 25 | 60 cardiac surgery patients | INTELLiVENT–ASV | ↑ | – | = | ↑ |
| Bialais et al. | 2016 | 27 | 80 critically ill patients | INTELLiVENT–ASV | ↑ | – | = | ↑ |
| Fot et al. | 2017 | 28 | 40 cardiac surgery patients | INTELLiVENT–ASV | = | – | = | ↑ |
| Arnal et al. | 2018 | 30 | 60 critically ill patients | INTELLiVENT–ASV | = | – | = | ↑ |
| De Bie et al. | 2020 | 34 | 220 cardiac surgery patients | INTELLiVENT–ASV | ↑ | ↑ | = | – |
| Chelly et al. | 2022 | 35 | 265 critically ill patients | INTELLiVENT–ASV | = | ↑ | = | ↑ |
| Coisel et al. | 2010 | 20 | 15 abdominal surgery patients | Neurally–adjusted Ventilatory Assist | ↑ | ↓ | – | – |
| Demoule et al. | 2016 | 36 | 128 patients with ARF | Neurally–adjusted Ventilatory Assist | – | – | = | – |
| Diniz–Silva et al. | 2020 | 37 | 20 ARDS patients | Neurally–adjusted Ventilatory Assist | = | – | – | – |
| Hadfield et al. | 2020 | 38 | 72 difficult to wean patients | Neurally–adjusted Ventilatory Assist | – | – | ↑ | – |
| Liu et al. | 2020 | 40 | 47 difficult to wean patients | Neurally–adjusted Ventilatory Assist | – | – | ↑ | – |
| Kacmarek et al. | 2020 | 39 | 306 patients with ARF | Neurally–adjusted Ventilatory Assist | – | ↑ | ↑ | – |
| Cammarota et al. | 2022 | 41 | 16 patients with AHRF | Neurally–adjusted Ventilatory Assist | = | – | – | – |
| Xirouchaki et al. | 2008 | 16 | 208 critically ill patients | Proportional Assist Ventilation+ | = | = | – | – |
| Elganady et al. | 2014 | 44 | 60 COPD patients | Proportional Assist Ventilation+ | – | – | ↑ | – |
| Kallio et al. | 2015 | 62 | 170 critically ill paediatric patients | Proportional Assist Ventilation+ | – | = | = | – |
| Teixeira et al. | 2015 | 26 | 160 patients needed controlled ventilation >24 h | Proportional Assist Ventilation+ | – | – | = | – |
| Bosma et al. | 2016 | 29 | 50 patients needed ventilation >36 h | Proportional Assist Ventilation+ | = | = | = | – |
| Botha et al. | 2018 | 31 | 50 patients needed controlled ventilation >24 h | Proportional Assist Ventilation+ | ↑ | = | ↑ | – |
| Delgado et al. | 2019 | 33 | 102 patients with ARF | Proportional Assist Ventilation+ | – | ↓ | = | – |
| Claure et al. | 2011 | 58 | 32 preterm infants | Avea–CLiO2 | ↑ | ↑ | – | ↑ |
| Lal et al. | 2015 | 59 | 27 preterm infants | Avea–CLiO2 | ↑ | ↑ | – | ↑ |
| Kaam et al. | 2015 | 60 | 80 preterm infants | Avea–CLiO2 | ↑ | ↑ | – | ↑ |
AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; ARF, acute respiratory failure; ASV, adaptive support ventilation; COPD, chronic obstructive pulmonary disease; FiO2, fraction of inspired oxygen.
↑ improved, compared with conventional ventilation;
= no difference, compared with conventional ventilation;
↓ worse, compared with conventional ventilation;
– not reported
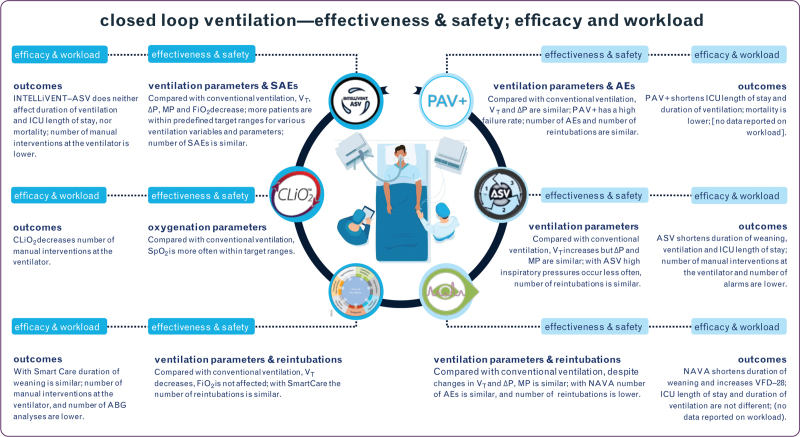
Fig. 3.
Summary of primary and secondary endpoints with significant results, reported for effectiveness, safety, efficacy and workload with closed loop ventilation. See text for details.
Clockwise the icons represent the following modes: PAV+, proportional assist ventilation; ASV, adaptive support ventilation; NAVA, neurally adjusted ventilatory assist; SmartCare; Avea–CLiO2; INTELLiVENT–ASV, INTELLiVENT adaptive support ventilation. Abbreviations: DP, driving pressure; (S)AE, (serious) adverse event; ABG, arterial blood gas analysis; MP, mechanical power; SpO2, peripheral oxygen saturation; VFD, ventilator-free days; VT, tidal volume.
ASV did not affect ΔP and MP in ARDS patients,42 but with INTELLiVENT–ASV, ΔP and MP were lower in cardiac surgery patients,.34 With NAVA, ΔP increased but MP decreased in hypoxemic respiratory failure patients.41 PAV+ did not affect ΔP in general ICU patients.29
Smartcare, ASV and NAVA did not affect FiO2 in critically ill patients,47 cardiac surgery patients51 and paediatric patients,62 respectively. INTELLiVENT–ASV reduced FiO2 in cardiac surgery patients25,34 and in critically ill patients,22,24,27,30 Avea–CLiO2 and a closed loop FiO2 controller available for use with ASV also increased time spent in preferred SpO2 ranges in paediatric patients.58–61
Safety
SmartCare, ASV and INTELLiVENT–ASV resulted in a similar number of reported ‘adverse events’18,35,42,45,47,48,51 (Table 2, Fig. 3 and eTable S4). ASV was associated with lower peak pressures47 and a lower incidence of atelectasis.50 INTELLiVENT–ASV was associated with less hypoxemic events,34,35 and increased time spent in preferred SpO2 ranges in medical ICU patients.35 NAVA was associated with less extubation failure.39 PAV+ did not affect reintubation rates31 but had to be discontinued in a large proportion of patients.33 Avea–CLiO2 as well as a closed loop FiO2 controller available for use with ASV were associated with less hypoxemic events.58–61
Efficacy
SmartCare was associated with shorter54,56,63 or similar weaning duration in various patient categories14,15,18,23 (Table 2, Fig. 3 and eTable S5). ASV21,43,45,46,48,49,57 and NAVA40 were also associated with shorter duration of weaning and shorter duration of ventilation, and NAVA with more ventilator free days.38,39 PAV+ was associated with shorter duration of ventilation, ICU and hospital length of stay29,44 and improved survival.31
Workload
SmartCare,18,19 ASV,43,46,51 INTELLIVENT–ASV25,27,28,30 and Avea–CLiO2 were associated with fewer manual interventions at the ventilator58–61 (Table 2, Fig. 3 and eTable S6). SmartCare was associated with a lower number of blood gas analyses.14 ASV was associated with fewer alarms13 and less time spent at or approaching the ventilator.51
Discussion
The findings of this systematic review can be summarised as follows: there are currently six commercially available closed loop ventilation modes for use in critically ill patients; their effectiveness (in terms of ventilator settings) and efficacy (in terms of patient outcomes), have been studied in various cohorts of patients; and safety (in terms of adverse events) has seldom been reported. In addition, the effect of these closed loop modes on workload of ICU staff has not yet been sufficiently researched.
Our analysis has several strengths. We conducted a comprehensive and unrestricted search. By reviewing the reference lists of the identified articles, we searched for additional studies that may not have been identified by the search. We applied clear inclusion and exclusion criteria for the selection of articles of interest. We checked the robustness of the study findings by comparing the fragility index with the number of patients lost to follow-up for binary clinical endpoints.
To our knowledge, this systematic review is the first to focus on all commercially available closed loop ventilation modes for use in the ICU, addressing several aspects of care and outcomes related to ventilation. The findings extend those of a previous review that focused only on INTELLiVENT–ASV.5 Although effectiveness is important in our assessment, we believe that from the four endpoints investigated, efficacy and safety, and in particular ICU staff workload, should always be considered when evaluating the advantages and disadvantages of any mode of closed loop ventilation.
Each closed loop ventilation mode seemed to be effective with regard to one or more aspects of lung protective ventilation, and some were even associated with a higher efficacy. Unfortunately, however, each study used different effectiveness endpoints, therefore an interaction with other ventilation parameters in potentially nonlung protective ranges could not be determined. Moreover, this hampered meta-analysis of the studies. Some of the included articles also reported opposite results related to ventilation parameters. This is possibly due to the different patient groups included in the study, to the study design or to the local use of the reported ventilation modes. Software changes did not occur for the different ventilation modes in a way that algorithms changed ventilation strategies leading to opposite ventilation variables. It is important to mention that most, if not all, studies were performed in centres with experience in invasive ventilation, meaning that standard ventilation care was most likely at a high level. Even while this may reduce the chance of showing superiority of the tested closed loop mode, with regard to effectiveness, most studies found the closed loop mode to be superior or at least as effective. On the contrary, with regard to efficacy endpoints, only some studies reported superiority. Herein, it should also be realised that most studies were small, and probably too small to have sufficient power to demonstrate superiority with respect to clinical outcomes.
Safety endpoints varied from adverse events that were predefined as a clinical endpoint, such as reintubation, to proportions of time spend outside of ‘safe’ zones of ventilation. In addition to effectiveness and efficacy, each study used other clinical safety endpoints. Severe adverse events, or adverse events, were never reported. Very probably, these were either not collected systematically, or simply not reported. The high failure rate of PAV+ (discontinuation of the automated mode) in one study was attributed to excessive sedation, high respiratory rate and high respiratory effort.33 Whereas this is an unfavourable event, it did not hamper patient safety. Future studies are needed to determine safety of closed loop ventilation, particularly in centres with less experience of invasive ventilation, and outside a research setting. In order to clinically interpret safety endpoints, details such as the reason for discontinuation, should be given.
Staff workload is difficult to capture, and thus far, there have been no studies of ICU staff workload related to ventilation. Our search identified only a small number of studies that reported on manual interventions, alarms, and the need for blood gas analysis. While these studies all showed a reduction of these three aspects, it remains uncertain if this truly reflects a reduction in ICU staff workload, during different phases of mechanical ventilation: for example, the weaning phase in particular, is seen as a labour-intensive phase of mechanical ventilation.64 We need better studies in the future that, for instance, capture nursing activities scores with metrics that encompass the majority of tasks of an ICU nurse, including those related to invasive ventilation.65,66
Closed loop ventilation can facilitate rapid and precise adjustments to ventilator settings. In ICU subpopulations, such as traumatic brain injury patients, strict and precise titration of paCO2 and paO2 values are fundamental in order to optimise intracerebral physiology.67–69 In practice, it is difficult and time consuming for the ICU staff to achieve this. Closed loop ventilation could help to achieve strict and precise titration, while considerably reducing workload.
This systematic review has limitations. In coherence with the articles included, this review displays a large variety in the endpoints, effectiveness and efficacy, hampering meta–analysis and with that, limiting conclusions on effectiveness, safety, efficacy and workload. In particular, safety reporting was scarce, albeit we expected this to be one of the most important endpoints to report in studying available closed loop ventilation modes. We did not reach out to the researchers in order to collect individual patient data on seldom reported endpoints such as safety and workload. Moreover, the fragility index as a measure of robustness of study results was only applicable for the minority of included studies and, where assessed, high fragility was found.
Conclusion
The current commercially available closed loop ventilation modes are at least as effective compared with conventional ventilation. Safety is rarely reported, and efficacy has mostly been shown in small studies. The effect of closed loop ventilation on workload of ICU staff has not yet been sufficiently researched.
Supplementary Material
Acknowledgements relating to this article
Assistance with the article: we thank Shiqi Zang for assistance in reading the Chinese manuscripts.
Financial support and sponsorship: we did not receive any financial support.
Conflicts of interest: LBK received fees from Hamilton Medical for lecturing. CR is a speaker for Masimo and Edwards Lifescience. MJS was a team leader of Research and New Technologies at Hamilton Medical from January 2022 till January 2023. The other authors declare no conflicts of interest.
Availability of materials: no materials were used for this review.
This manuscript was handled by Nicolas Bruder.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.ejanaesthesiology.com).
References
- 1.MacIntyre N, Rackley C, Khusid F. Fifty years of mechanical ventilation-1970 s to 2020. Crit Care Med 2021; 49:558–574. [DOI] [PubMed] [Google Scholar]
- 2.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–2136. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800. [DOI] [PubMed] [Google Scholar]
- 4.Buiteman-Kruizinga LA, Serpa Neto A, Schultz MJ. Automation to improve lung protection. Intensive Care Med 2022; 48:943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botta M, Wenstedt EFE, Tsonas AM, et al. Effectiveness, safety and efficacy of INTELLiVENT-adaptive support ventilation, a closed-loop ventilation mode for use in ICU patients - a systematic review. Expert Rev Respir Med 2021; 15:1403–1413. [DOI] [PubMed] [Google Scholar]
- 6.Kampolis CF, Mermiri M, Mavrovounis G, et al. Comparison of advanced closed-loop ventilation modes with pressure support ventilation for weaning from mechanical ventilation in adults: a systematic review and meta-analysis. J Crit Care 2022; 68:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Beijers AJ, Roos AN, Bindels AJ. Fully automated closed-loop ventilation is safe and effective in postcardiac surgery patients. Intensive Care Med 2014; 40:752–753. [DOI] [PubMed] [Google Scholar]
- 8.The L. The future of nursing: lessons from a pandemic. Lancet 2023; 401:1545. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demarquette A, Perrault T, Alapetite T, et al. Spin and fragility in randomised controlled trials in the anaesthesia literature: a systematic review. Br J Anaesth 2023; 130:528–535. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein AR. The unit fragility index: an additional appraisal of “statistical significance” for a contrast of two proportions. J Clin Epidemiol 1990; 43:201–209. [DOI] [PubMed] [Google Scholar]
- 12.Baer BR, Fremes SE, Gaudino M, et al. On clinical trial fragility due to patients lost to follow up. BMC Med Res Methodol 2021; 21:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petter AH, Chioléro RL, Cassina T, et al. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg 2003; 97:1743–1750. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Yu SY, Wang LW. [Comparison of SmartCare and spontaneous breathing trials for weaning old patients with chronic obstructive pulmonary diseases]. Zhonghua Jie He He Hu Xi Za Zhi 2006; 29:545–548. [PubMed] [Google Scholar]
- 15.Rose L, Presneill JJ, Johnston L, Cade JF. A randomised, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare/PS. Intensive Care Med 2008; 34:1788–1795. [DOI] [PubMed] [Google Scholar]
- 16.Xirouchaki N, Kondili E, Vaporidi K, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med 2008; 34:2026–2034. [DOI] [PubMed] [Google Scholar]
- 17.Dongelmans DA, Veelo DP, Paulus F, et al. Weaning automation with adaptive support ventilation: a randomized controlled trial in cardiothoracic surgery patients. Anesth Analg 2009; 108:565–571. [DOI] [PubMed] [Google Scholar]
- 18.Stahl C, Dahmen G, Ziegler A, Muhl E. Comparison of automated protocol-based versus nonprotocol-based physician-directed weaning from mechanical ventilation. Intensiv Notfallmedizin 2009; 46:441–446. [Google Scholar]
- 19.Ma YJ, Yang XJ, Cao XY, Ma XG. [Comparison of computer-driven weaning and physician-directed weaning from mechanical ventilation: a randomized prospective study]. Zhonghua Jie He He Hu Xi Za Zhi 2010; 33:174–178. [PubMed] [Google Scholar]
- 20.Coisel Y, Chanques G, Jung B, et al. Neurally adjusted ventilatory assist in critically ill postoperative patients: a crossover randomized study. Anesthesiology 2010; 113:925–935. [DOI] [PubMed] [Google Scholar]
- 21.Kirakli C, Ozdemir I, Ucar ZZ, et al. Adaptive support ventilation for faster weaning in COPD: a randomised controlled trial. Eur Respir J 2011; 38:774–780. [DOI] [PubMed] [Google Scholar]
- 22.Arnal JM, Wysocki M, Novotni D, et al. Safety and efficacy of a fully closed-loop control ventilation (IntelliVent-ASV®) in sedated ICU patients with acute respiratory failure: a prospective randomized crossover study. Intensive Care Med 2012; 38:781–787. [DOI] [PubMed] [Google Scholar]
- 23.Schädler D, Engel C, Elke G, et al. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. Am J Respir Crit Care Med 2012; 185:637–644. [DOI] [PubMed] [Google Scholar]
- 24.Clavieras N, Wysocki M, Coisel Y, et al. Prospective randomized crossover study of a new closed-loop control system versus pressure support during weaning from mechanical ventilation. Anesthesiology 2013; 119:631–641. [DOI] [PubMed] [Google Scholar]
- 25.Lellouche F, Bouchard PA, Simard S, et al. Evaluation of fully automated ventilation: a randomized controlled study in postcardiac surgery patients. Intensive Care Med 2013; 39:463–471. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira SN, Osaku EF, Costa CR, et al. Comparison of proportional assist ventilation plus, T-tube ventilation, and pressure support ventilation as spontaneous breathing trials for extubation: a randomized study. Respir Care 2015; 60:1527–1535. [DOI] [PubMed] [Google Scholar]
- 27.Bialais E, Wittebole X, Vignaux L, et al. Closed-loop ventilation mode (IntelliVent®-ASV) in intensive care unit: a randomized trial. Minerva Anestesiol 2016; 82:657–668. [PubMed] [Google Scholar]
- 28.Fot EV, Izotova NN, Yudina AS, et al. Automated weaning from mechanical ventilation after off-pump coronary artery bypass grafting. Front Med (Lausanne) 2017; 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosma KJ, Read BA, Bahrgard Nikoo MJ, et al. A pilot randomized trial comparing weaning from mechanical ventilation on pressure support versus proportional assist ventilation. Crit Care Med 2016; 44:1098–1108. [DOI] [PubMed] [Google Scholar]
- 30.Arnal JM, Garnero A, Novotni D, et al. Closed loop ventilation mode in intensive care unit: a randomized controlled clinical trial comparing the numbers of manual ventilator setting changes. Minerva Anestesiol 2018; 84:58–67. [DOI] [PubMed] [Google Scholar]
- 31.Botha J, Green C, Carney I, et al. Proportional assist ventilation versus pressure support ventilation in weaning ventilation: a pilot randomised controlled trial. Crit Care Resusc 2018; 20:33–40. [PubMed] [Google Scholar]
- 32.Grieco DL, Bitondo MM, Aguirre-Bermeo H, et al. Patient-ventilator interaction with conventional and automated management of pressure support during difficult weaning from mechanical ventilation. J Crit Care 2018; 48:203–210. [DOI] [PubMed] [Google Scholar]
- 33.Delgado M, Subirá C, Hermosa C, et al. Proportional assist ventilation feasibility in the early stage of respiratory failure: a prospective randomized multicenter trial. Minerva Anestesiol 2019; 85:862–870. [DOI] [PubMed] [Google Scholar]
- 34.De Bie AJR, Neto AS, van Meenen DM, et al. Fully automated postoperative ventilation in cardiac surgery patients: a randomised clinical trial. Br J Anaesth 2020; 125:739–749. [DOI] [PubMed] [Google Scholar]
- 35.Chelly J, Mazerand S, Jochmans S, et al. Automated vs. conventional ventilation in the ICU: a randomized controlled crossover trial comparing blood oxygen saturation during daily nursing procedures (I-NURSING). Crit Care 2020; 24:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demoule A, Clavel M, Rolland-Debord C, et al. Neurally adjusted ventilatory assist as an alternative to pressure support ventilation in adults: a French multicentre randomized trial. Intensive Care Med 2016; 42:1723–1732. [DOI] [PubMed] [Google Scholar]
- 37.Diniz-Silva F, Moriya HT, Alencar AM, et al. Neurally adjusted ventilatory assist vs. pressure support to deliver protective mechanical ventilation in patients with acute respiratory distress syndrome: a randomized crossover trial. Ann Intensive Care 2020; 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadfield DJ, Rose L, Reid F, et al. Neurally adjusted ventilatory assist versus pressure support ventilation: a randomized controlled feasibility trial performed in patients at risk of prolonged mechanical ventilation. Crit Care 2020; 24:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kacmarek RM, Villar J, Parrilla D, et al. Neurally adjusted ventilatory assist in acute respiratory failure: a randomized controlled trial. Intensive Care Med 2020; 46:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Xu X, Sun Q, et al. Neurally adjusted ventilatory assist versus pressure support ventilation in difficult weaning: a randomized trial. Anesthesiology 2020; 132:1482–1493. [DOI] [PubMed] [Google Scholar]
- 41.Cammarota G, Verdina F, De Vita N, et al. Effects of varying levels of inspiratory assistance with pressure support ventilation and neurally adjusted ventilatory assist on driving pressure in patients recovering from hypoxemic respiratory failure. J Clin Monit Comput 2022; 36:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baedorf Kassis EN, Bastos AB, Schaefer MS, et al. Adaptive support ventilation and lung-protective ventilation in ARDS. Respir Care 2022; 67:1542–1550. [DOI] [PubMed] [Google Scholar]
- 43.Celli P, Privato E, Ianni S, et al. Adaptive support ventilation versus synchronized intermittent mandatory ventilation with pressure support in weaning patients after orthotopic liver transplantation. Transplant Proc 2014; 46:2272–2278. [DOI] [PubMed] [Google Scholar]
- 44.Elganady A, Beshey B, Abdelaziz A. Proportional assist ventilation versus pressure support ventilation in the weaning of patients with acute exacerbation of chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc 2014; 63:643–650. [Google Scholar]
- 45.Mohamed K, El Maraghi S. Role of adaptive support ventilation in weaning of COPD patients. Egypt J Chest Dis Tuberc 2014; 63:449–454. [Google Scholar]
- 46.Kirakli C, Naz I, Ediboglu O, et al. A randomized controlled trial comparing the ventilation duration between adaptive support ventilation and pressure assist/control ventilation in medical patients in the ICU. Chest 2015; 147:1503–1509. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi C, Victor ES, Pieri T, et al. Smart Care™ versus respiratory physiotherapy-driven manual weaning for critically ill adult patients: a randomized controlled trial. Crit Care 2015; 19:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu F, Gomersall CD, Ng SK, et al. A randomized controlled trial of adaptive support ventilation mode to wean patients after fast-track cardiac valvular surgery. Anesthesiology 2015; 122:832–840. [DOI] [PubMed] [Google Scholar]
- 49.Yazdannik A, Zarei H, Massoumi G. Comparing the effects of adaptive support ventilation and synchronized intermittent mandatory ventilation on intubation duration and hospital stay after coronary artery bypass graft surgery. Iran J Nurs Midwifery Res 2016; 21:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moradian ST, Saeid Y, Ebadi A, et al. Adaptive support ventilation reduces the incidence of atelectasis in patients undergoing coronary artery bypass grafting: a randomized clinical trial. Anesth Pain Med 2017; 7:e44619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eremenko A, Komnov R. Smart mode of mechanical lung ventilation during early activation of cardiosurgical patients. Gen Reanimatol 2020; 16:4–15. [Google Scholar]
- 52.Sehgal IS, Gandra RR, Dhooria S, et al. A randomised trial of adaptive support ventilation in patients with neuroparalytic snake envenomation. Br J Anaesth 2022; 128:e232–e234. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Yang Z, Chen K, et al. Efficacy of adaptive ventilation support combined with lung recruitment maneuvering for acute respiratory distress syndrome. Am J Transl Res 2022; 14:2109–2116. [PMC free article] [PubMed] [Google Scholar]
- 54.Burns KE, Meade MO, Lessard MR, et al. Wean earlier and automatically with new technology (the WEAN study). A multicenter, pilot randomized controlled trial. Am J Respir Crit Care Med 2013; 187:1203–1211. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology 2013; 18:1108–1115. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Xu XT, Yang Y, et al. Computer-driven automated weaning reduces weaning duration in difficult-to-wean patients. Chin Med J (Engl) 2013; 126:1814–1818. [PubMed] [Google Scholar]
- 57.Sulzer CF, Chioléro R, Chassot PG, et al. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001; 95:1339–1345. [DOI] [PubMed] [Google Scholar]
- 58.Claure N, Bancalari E, D’Ugard C, et al. Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics 2011; 127:e76–e83. [DOI] [PubMed] [Google Scholar]
- 59.Lal M, Tin W, Sinha S. Automated control of inspired oxygen in ventilated preterm infants: crossover physiological study. Acta Paediatr 2015; 104:1084–1089. [DOI] [PubMed] [Google Scholar]
- 60.van Kaam AH, Hummler HD, Wilinska M, et al. Automated versus manual oxygen control with different saturation targets and modes of respiratory support in preterm infants. J Pediatr 2015; 167:545–550.e1-2. [DOI] [PubMed] [Google Scholar]
- 61.Soydan E, Ceylan G, Topal S, et al. Automated closed-loop FiO(2) titration increases the percentage of time spent in optimal zones of oxygen saturation in pediatric patients: a randomized crossover clinical trial. Front Med (Lausanne) 2022; 9:969218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kallio M, Peltoniemi O, Anttila E, et al. Neurally adjusted ventilatory assist (NAVA) in pediatric intensive care--a randomized controlled trial. Pediatr Pulmonol 2015; 50:55–62. [DOI] [PubMed] [Google Scholar]
- 63.Jouvet PA, Payen V, Gauvin F, et al. Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med 2013; 39:919–925. [DOI] [PubMed] [Google Scholar]
- 64.Rose L, Schultz MJ, Cardwell CR, et al. Automated versus nonautomated weaning for reducing the duration of mechanical ventilation for critically ill adults and children: a cochrane systematic review and meta-analysis. Crit Care 2015; 19:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucchini A, Villa M, Del Sorbo A, et al. Determinants of increased nursing workload in the COVID-era: a retrospective analysis of prospectively collected data. Nurs Crit Care 2023; 29:196–207. [DOI] [PubMed] [Google Scholar]
- 66.Bruyneel A, Gallani MC, Tack J, et al. Impact of COVID-19 on nursing time in intensive care units in Belgium. Intensive Crit Care Nurs 2021; 62:102967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoiland RL, Fisher JA, Ainslie PN. Regulation of the cerebral circulation by arterial carbon dioxide. Compr Physiol 2019; 9:1101–1154. [DOI] [PubMed] [Google Scholar]
- 68.Gouvea Bogossian E, Peluso L, Creteur J, Taccone FS. Hyperventilation in adult TBI patients: how to approach it? Front Neurol 2020; 11:580859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med 2019; 45:1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.