Abstract
Background:
Contraception is important for women who are postpartum, including those who are breastfeeding. Use of combined hormonal contraceptives (CHCs) may affect breastfeeding performance and infant health outcomes.
Objective:
The objective was to identify evidence examining clinical outcomes for breastfeeding and infant health among breastfeeding women using CHCs compared to nonusers.
Search strategy:
We searched the PubMed database for all articles published from database inception through September 30, 2014.
Selection criteria:
We included primary research studies that compared breastfeeding women using CHCs with breastfeeding women using nonhormonal or no contraception, or compared breastfeeding women initiating combined hormonal contraception at early versus later times postpartum. Breastfeeding outcomes of interest included duration, rate of exclusive breastfeeding and timing of supplementation. Infant outcomes of interest included growth, health and development.
Results:
Fifteen articles describing 13 studies met inclusion criteria for this review. Studies ranged from poor to fair methodological quality and demonstrated inconsistent effects of combined oral contraceptives (COCs) on breastfeeding performance with COC initiation before or after 6 weeks postpartum; some studies demonstrated greater supplementation and decreased breastfeeding continuation among COC users compared with nonusers, and others demonstrated no effect. For infant outcomes, some studies found decreases in infant weight gain for COC users compared with nonusers when COCs were initiated at <6 weeks postpartum, while other studies found no effect. None of the studies found an effect on infant weight gain when COCs were started after 6 weeks postpartum, and no studies found an effect on other infant health outcomes regardless of time of COC initiation.
Conclusion:
Limited evidence of poor to fair quality demonstrates an inconsistent impact of COCs on breastfeeding duration and success. The evidence also demonstrated conflicting results on whether early initiation of COCs affects infant outcomes but generally found no negative impact on infant outcomes with later initiation of COCs. The body of evidence is limited by older studies using different formulations and doses of estrogen and poor methodologic quality. Given the significant limitations of this body of evidence, the importance of contraception for postpartum women and the theoretical concerns that have been raised about the use of combined hormonal contraception by women who are breastfeeding, rigorous studies examining these issues are needed. In addition, postpartum women should be counseled about the full range of safe alternative contraceptive methods, particularly during the first 6 weeks postpartum when the risk of venous thromboembolism is highest and use of estrogen may exacerbate this risk.
Keywords: Combined hormonal contraceptives, Combined oral contraceptives, Breastfeeding, Lactation, Systematic review
1. Introduction
Initiation of contraception during the postpartum period is important to prevent unintended pregnancy and short birth intervals, which can lead to negative health outcomes for mother and infant [1,2]. For women who are breastfeeding, the lactational amenorrhea method can be an effective contraceptive method but is only effective for six months, or less if menstrual bleeding resumes or supplemental feedings are introduced [3]. Therefore, use of contraception even among breastfeeding women is critical to prevent early repeat pregnancy. Combined hormonal contraceptives (CHCs) play an important role in the contraceptive method mix, as many women prefer their familiarity and ease of use, immediate return to fertility when discontinued and effectiveness [4]. However, concern has been raised over possible effects of CHCs on breastfeeding performance and infant health.
Breastfeeding has important well-established health benefits for both mother and infant, and these benefits can be maximized with at least 6 months of exclusive breastfeeding [5]. Therefore, anything that potentially interferes with breastfeeding is of concern. Two important areas of consideration for potential impact of medications include effects on breastfeeding and effects on the infant. A Cochrane systematic review that attempted to determine the effect of hormonal contraceptives on breastfeeding concluded that the existing randomized controlled trials (RCTs) do not sufficiently establish an effect of hormonal contraception on milk quality or quantity [6]. Some studies have demonstrated that levels of hormones absorbed by the infant are fairly low [7]; however, it is still unclear what effect exogenous hormones have on infant growth and development.
This systematic review examines the safety of CHC use among breastfeeding women and updates the previous review conducted for the World Health Organization (WHO), as part of the process of updating the Medical Eligibility Criteria for Contraceptive Use (MEC) [8]. The previous review concluded that the evidence was inconsistent on whether COCs negatively impacted breastfeeding duration and success and that the evidence largely did not show negative effects on infant growth and development. However, the review also concluded that the body of evidence was very limited given the poor methodologic quality. Therefore, we have updated the previous review with additional evidence in preparation for the forthcoming update of the WHO MEC [9]. Specifically, the review examines the effects of CHC use on clinical outcomes such as breastfeeding duration, frequency, initiation of supplemental feeding, weaning and infant growth, and health and development, and examines outcomes by timing of CHC initiation.
2. Materials and methods
We assessed two specific questions for this review: (a) Do CHCs initiated by breastfeeding women at <6 weeks or >6 weeks postpartum have negative effects on breastfeeding outcomes or infant outcomes compared with no contraception or nonhormonal contraception? (b) Do CHCs initiated by breastfeeding women at <6 weeks postpartum have negative effects on breastfeeding outcomes or infant outcomes compared with initiation at >6 weeks postpartum?
We conducted this systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [10].
2.1. Literature search
We searched the PubMed database for all relevant articles published from database inception through September 30, 2014, using the following search strategy:
((((((((((“Contraceptives, Oral”[Mesh]) OR “oral contraceptives”)) OR oral contracept*))) OR (“Ortho Evra”[Supplementary Concept] OR ortho evra OR “contraceptive patch” OR “transdermal patch”)) OR (“NuvaRing”[Supplementary Concept] OR nuvaring OR “vaginal ring”)) OR (((once a month OR monthly) AND inject*) AND contracept* OR cyclofem OR lunelle OR mesigyna OR cycloprovera))) AND (((“Breast Feeding” [Mesh] or breast feeding or breastfeeding)) OR (“Lactation” [Mesh] or lactation)) Filter: limit to human.
Articles in all languages were accepted. We also searched reference lists of identified articles and relevant review articles for additional citations of interest. We did not consider unpublished studies, abstracts of conference presentations or dissertations. We previously contacted the author of one study to clarify study methodology [11,12].
2.2. Selection criteria
Articles were included in this review if they were primary reports on studies of breastfeeding women using CHCs compared with breastfeeding women using nonhormonal contraception or no contraception. Articles were also included if they compared women who initiated CHCs early with women who initiated CHCs at a later time postpartum. Study designs without a comparison group were excluded. CHCs of interest included COCs, the combined hormonal patch, the combined vaginal ring and combined injectables. We also included articles that included a comparison group of women using progestin-only contraceptives but considered this indirect evidence if there was no nonhormonal comparison group. Outcomes of interest included breastfeeding performance and infant health outcomes. We considered clinical breastfeeding performance outcomes such as duration of breastfeeding, exclusivity and timing of initiation of supplemental feedings. Studies reported a variety of breastfeeding clinical outcomes including percent fully breastfeeding at certain times postpartum, percent continuing to breastfeeding at certain times postpartum, total duration of breastfeeding (without specifying whether full or partial breastfeeding), percent using supplementation and age at infant supplementation. Articles that only investigated milk quality and composition or milk quantity, as measured by volume of pumped milk or infant weight before and after feedings, were excluded. We considered infant health outcomes such as growth (as measured by weight, length, head circumference, arm circumference or skin-fold thickness), health (as measured by illness and mortality) and development.
2.3. Study quality assessment and data synthesis
We summarized the evidence using standard abstraction forms. Two authors (N.T. and S.P.) independently assessed the quality of each piece of evidence using the system developed by the United States Preventive Services Task Force [13,14]. Summary odds ratios were not calculated given the heterogeneity of contraceptive initiation, results and nonquantifiable outcomes reported. Results were summarized and reported by timing of contraception initiation (<6 weeks postpartum and >6 weeks postpartum) and outcome (breastfeeding and infant health).
3. Results
Our search identified 925 articles, from which 15 primary research articles describing 13 studies met our inclusion criteria for this review (Fig. 1 and Table 1) [11,12,15–28]. Of these articles, 10 [11,12,15,18–20,24–27] were described in a previous review [8], 3 were newly identified for this review but originally published before 1973, and 2 were published since the last review [16,17,21–23]. All included articles reported on women using COCs. No articles were identified that reported on women using other CHCs. One article provided indirect evidence only, as the comparison group was women using progestin-only pills (POPs) [17].
Fig. 1.
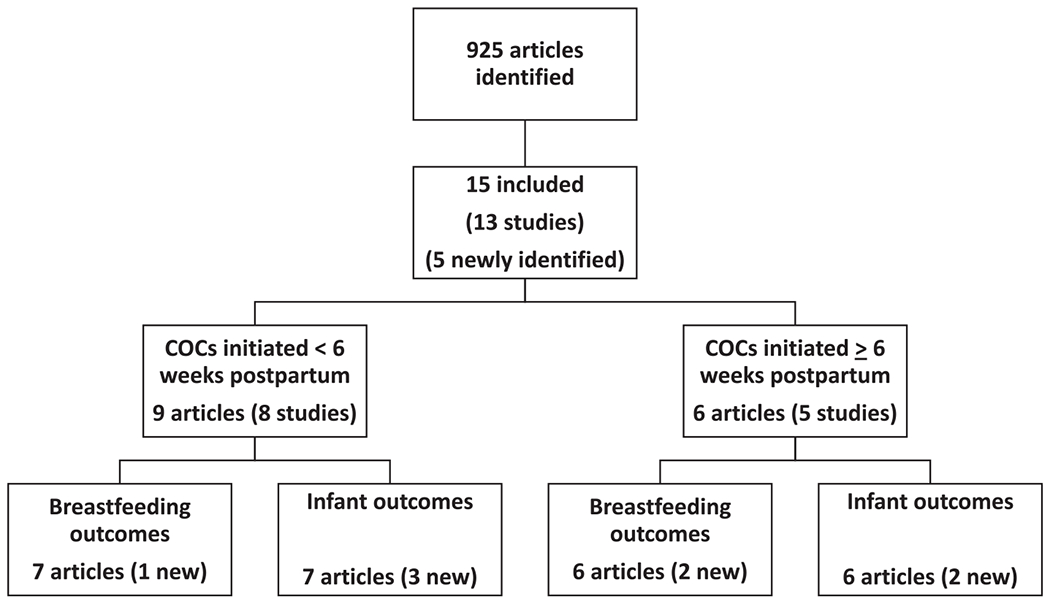
Systematic review of breastfeeding and CHCs.
Table 1.
Evidence table for studies of clinical outcomes among breastfeeding women using CHCs.
| Author, year, location, support | Study design | Population | Interventions (CHC and comparison groups only) | Outcomes | Results | Strengths/weaknesses | Quality grading |
|---|---|---|---|---|---|---|---|
| COCs initiated <6 weeks postpartum | |||||||
| Kaern [20], 1967 Denmark Source of support not stated (COCs supplied by Syntex Pharmaceuticals) | RCT | N=451 breastfeeding women after healthy delivery | COC (0.05 mg mestranol+1 mg norethisterone)=233 Placebo=218 Initiated on postpartum day 1 |
Breastfeeding performance Infant weight |
Breastfeeding performance: Supplemental feeding on day 8: COC group: 12.3% (26/212) Placebo group: 3.4% (7/206) (p<.05) Infant weight: No significant weight change between groups from days 2 to 8 |
Strengths: Randomized treatment Weaknesses: Short follow-up (8 days) Extent of blinding difficult to assess Method of randomization unclear |
Level I, poor |
| Gambrell [18], 1970 US military hospital, Germany Source of support not stated | Prospective cohort | N= 174 breastfeeding women, aged 17–44 | COC (various regimens)=83 Controls (“other method of family planning”)=91 Initiated on postpartum day 5 |
Breastfeeding duration |
Breastfeeding performance: 54% of COC users and 59% control group were breastfeeding at 6 weeks (authors state not significant) |
Weaknesses: Various COC formulations used Short follow-up (6 weeks) Control group may have included users of progestin-only methods Self-selected intervention No assessment of time of breastfeeding initiation postpartum No adjustment for potential confounders including age, parity and past lactation duration High loss to follow-up (~40%) over 6 weeks among all study women; follow-up among breastfeeding women not noted |
Level II-2, poor |
| Kamal [21], 1970 Egypt Source of support not stated (newly identified) | Nonrandomized clinical trial | N=40 breastfeeding women ages 20–37, delivered by cesarean section | COC (0.1 mg mestranol+1 mg lynestrenol)=10 Estrogen-only (0.1 mg ethinyl estradiol)=10 Placebo=10 POP=10 (results not reported here) Initiated on postpartum day 2 |
Infant growth |
Infant growth: Percent changes in infant weight higher in COC group and estrogen-only group than control group through 14 days (no p value reported) |
Strengths: Double-blinded Weaknesses: Assignment to groups not described Patient selection and inclusion/exclusion criteria not described Small numbers Short follow-up (14 days) p value for comparison of interest not reported |
Level II-1, poor |
| Miller [24], 1970 USA Ortho Research Foundation | Partially RCT (women who requested OCs randomized to COC or oral placebo) | N=100 women planning to breastfeed at least 3 months; 95 included in analyses | COC (0.08 mg mestranol+1 mg norethindrone)=24 Placebo (until 6 weeks, then COCs)=23 None=48 Initiated on postpartum day 14 or at postpartum week 6 |
Breastfeeding performance Infant weight |
Breastfeeding performance: More supplemental calories required for infants in the COC group compared to placebo group at weeks 4 and 5 (p values not reported) At 12 weeks postpartum, 73% in the nonhormonal group, 52% of COC initiators at 6 weeks and 21% of COC initiators at 2 weeks were still breastfeeding (p values not reported) Infant weight: Infants with mothers in the placebo group had significantly greater weight gain than those using COCs at weeks 4 and 5 (p values not reported) |
Weaknesses: Small numbers Values and statistical tests not stated Method of randomization, allocation and blinding not specified No power calculation |
Level I, poor |
| Koetsawang [23], 1972 Thailand Source of support not stated (newly identified) | Prospective cohort | N=94 healthy breastfeeding women ages 20–39; results reported for 60 women who completed 16 weeks of study | COC group 1 (0.1 mg mestranol+1 mg ethinodiol-diacetate)=20 COC group 2 (0.08 mg mestranol+2 mg chlormadinone acetate)=20 Control (no hormonals)=20 Initiated within 6 weeks postpartum (implied) |
Infant growth |
Infant growth: Average weight gain per week from weeks 6 to 16 postpartum: Group 1: 147.25 g (n=16) Group 2: 179.84 g (n=13) Control: 202.24 g (n=20) (authors state significant but p value not reported) |
Weaknesses: Included only women with previous breastfeeding experience Included only male infants with birth weight 2500–3999 g Small numbers Assignment to contraceptive groups not described Exact timing of contraceptive initiation not stated 36% (34/94) lost to follow-up Infant growth not reported for all women in COC groups p value for comparison of interest not reported |
Level II-2, poor |
| Guiloff, [19], 1974 Chile Population council, Warner-Lambert Research Institute | Cohort study with historic comparison information |
N=696 Multiparous women, 16–40 Control measures of duration of lactation came from study women with past lactation history and no hormonal or mechanical contraceptive use who were still lactating at 30 days |
COC group 1 (2 mg quinestrol+5 mg quingestanol acetate)=194 COC group 2 (2 mg quinestrol+2.5 mg quingestanol acetate)=81 COC group 3 (0.05 mg ethinyl estradiol+1 mg norethindrone)=40 COC group 4 (0.08 mg mestranol+1.5 mg chlormadinone)=52 POC (various regimens)=168 (results not reported here) Historical controls=346 Initiated on postpartum day 30 |
Length of breastfeeding |
Breastfeeding performance: Mean length of lactation: Group 1: 2.5 months* Group 2: 2.5 months* Group 3: 4.6 months Group 4: 3.7 months* Controls: 5.3 months *p=.01 when compared with controls |
Weaknesses: Retrospective information from past lactation durations of current study participants was used as historical control rather than comparison between contraceptive groups Assignment or choice of contraceptive method unclear |
Level II-2, poor |
| Croxatto [11], 1983 and Diaz [12], 1983 Chile International Development Research Center of Canada, The Population Council | Partially RCT (randomized for first part of study; also women who requested OCs randomized to COC or oral placebo) | N=330 breastfeeding, healthy women with normal delivery and postpartum course | COC (0.03 mg ethinyl estradiol+0.15 mg LNG)=103 Placebo (injectable and pill)=188 After 91 days, 109 used nonhormonal methods Copper IUD= 118 Initiated on postpartum days 30–35 |
Breastfeeding performance Infant health (weight, weight gain, physical abnormalities) |
Breastfeeding performance: COC group had significantly lower rates of exclusive breastfeeding at postpartum day 91 than placebo group (80.6% vs. 92%; p<.05). Also significantly lower rates at 4–10 months postpartum (p<.025) but no difference at 12 months. Percent weaned at 6 months: COC: 16.3% Placebo: 9% Copper IUD: 4.7% (p<.01 for COC versus IUD) Percent weaned at 8 months: COC: 33.3% Placebo: 19.8% Copper IUD: 16.5% (p<.05) Percent weaned not different between groups at 2, 4, 10 and 12 months Infant health: Total infant weight increase at 6 months: COC: 4636±765 g Placebo: 4971±669 g p<.05 Average weight significantly lower for COC users, from 61 to 183 days PP, and at 366 days PP from the nonhormonal users (p<.05) Average infant weight at 366 days: COC: 9938±592 g Placebo: 10746±729 g p<.025 No physical abnormalities at 1 year of age (breasts, genitals) in COC group |
Strengths: Long follow-up Weaknesses: Although placebos used, women chose their allocation to pill or injectable No determination of reason for weaning |
Level II-1, fair |
| Espey [17], 2012 USA ACOG Contraceptive grant and University of New Mexico (newly identified) | RCT | N=127 women ages 15–45, planning to breastfeed and to use oral contraceptives | COC (0.035 mg ethinyl estradiol+1 mg norethindrone)=64 POP (0.35 mg norethindrone)=63 Initiated at 2 weeks postpartum |
Breastfeeding performance Infant growth (weight, length, head circumference) |
Breastfeeding performance: No difference in breastfeeding continuation at 8 weeks (64% in COC group, 63.5% in POP group) No difference in supplementation at 8 weeks (percents not reported) No difference in breastfeeding continuation through 6 months Infant growth: No difference in growth parameters through 8 weeks |
Strengths: Randomization procedure described Double-blinded Minimal amount of method switching among women continuing to breastfeed at 8 weeks Loss to follow-up similar between groups Weaknesses: Small numbers Short follow-up of infant outcomes (8 weeks) >20% loss to follow-up in both groups |
Level I, fair Indirect |
| COCs initiated >6 weeks postpartum | |||||||
| Kamal [22], 1969 Egypt Source of support not stated (newly identified) | Nonrandomized clinical trial | N=120 women | COC group 1 (0.075 mg mestranol+2.5 mg lynestrenol) COC group 2 (0.1 mg mestranol+1 mg lynestrenol) IUD+placebo POC (results not reported here) Deladroxate (10 mg estradiol enanthate+150 mg dihydroxyprogesterone acetophenide) (results not reported here) Numbers in each group not specified Initiated 6–10 weeks postpartum |
Breastfeeding performance Infant weight |
Breastfeeding performance: Average age of infant at supplementation: Group 1: 13.8 weeks Group 2: 11.6 weeks Placebo: 15.0 weeks (no p value reported) Infant weight: Mean infant weight at 32 weeks postpartum lower in group 1 and higher in group 2, compared with placebo. Authors state no differences in growth curves (exact numbers and p values not reported). |
Strengths: Double-blinded Weaknesses: Assignment to groups not described Numbers of women in each group and retained in study not reported p value for comparisons of interest not reported |
Level II-1, poor |
| Peralta [26], 1983 Chile International Development Research Center of Canada, The Population Council | Prospective cohort | N=141 fully breastfeeding, healthy women with healthy infants | COC (0.03 mg ethinyl estradiol+0.15 mg LNG)=59 Nonhormonal (spermicides, IUD)=82 Initiated at postpartum day 90 |
Breastfeeding performance Infant weight |
Breastfeeding performance: COC group had lower rates of exclusive BF than nonhormonal group at 4, 6, 8, 10 and 12 months postpartum (p<.05) No difference between groups in rates of weaning through 12 months (p values not reported) Infant weight: No difference in mean infant weight between groups through postpartum day 366 At 4 months, COC group infants had lower mean weight increase than nonhormonal group (p<.001). No other significant differences between groups through 6 months. |
Strengths: Long follow-up Low loss to follow-up Weaknesses: Treatment chosen by participants Included only healthy infants Small numbers reported for infant outcomes by 12 months No adjustment for potential confounders |
Level II-2, fair |
| WHO [15,27], 1984 and 1988 Hungary, Thailand WHO/HRP | Partially randomized clinical trial (women who chose OCs randomized to COC or POP, other groups chose methods) | N=341 women ages 20–35, prior experience breastfeeding, parity 2–4, after healthy term delivery | COC (0.03 mg ethinyl estradiol+0.15 mg LNG)=86 Nonhormonal (barriers, sterilization, IUD)=111 POC=144 (results not reported here) Initiated at 6 weeks postpartum |
Breastfeeding performance Infant growth (weight, length, ponderal index, arm circumference, skinfold thickness, head circumference) and morbidity |
Breastfeeding performance: No differences in breastfeeding continuation between contraceptive groups (rates not reported) No difference in prevalence of supplementation through 24 weeks (p values not reported) Infant growth: No differences in mean weight or rate of growth between contraceptive groups through 24 weeks; female infants smaller in the control group, compared to COC group (16–24 weeks) at one site No differences in other infant measurements through 24 weeks No differences in episodes of infant illness between groups |
Strengths: Multiple countries Examined several infant measurements and infant illness Power calculation reported for changes in infant weight Weaknesses: Included only women with prior breastfeeding experience and with healthy infants No details of method switching/discontinuation Did not assess supplementation No adjustment for potential confounders Follow up variable among study groups and study sites; 58% follow-up at 24 weeks in COC and nonhormonal groups |
Level II-1, fair |
| Nilsson [25], 1986 Sweden Source of support not stated | Cohort, ambidirectional Sweden | N=96 women using COCs while breastfeeding and their infants, and breastfeeding, non-OC user controls and their infants | COC (most containing 0.05 mg ethinyl estradiol)=48 Non-OC users=48 Initiated at 2 months postpartum |
Breastfeeding duration Infant health |
Breastfeeding duration: Mean length of breastfeeding (from postpartum records): COC: 3.7 months Controls: 4.6 months (p<.05) Infant health: No differences in occurrence of serious illness, performance in school up to age 8 |
Strengths: Long follow-up time (8 years) Low loss to follow-up Weaknesses: Small numbers 19 used COCs for <1 month and 21 used for 1–3 months Retrospective collection of breastfeeding information Study group may have contained progestin-only OC users; control group may have included nonoral hormonal contraceptive users No adjustment for potential confounders |
Level II-2, fair |
| Bahamondes [16], 2013 Brazil FAPEP and CNPq (newly identified) | Prospective cohort | N=40 healthy parous women ages 18–44, planning to breastfeed and to use one of study contraceptives | COC (0.03 mg ethinyl estradiol+0.15 mg LNG)=10 Copper IUD=10 LNG-IUD=10 (results not reported here) ENG implant=10 (results not reported here) Initiated on postpartum day 42 |
Breastfeeding performance Infant growth (weight, height and tibia length) |
Breastfeeding performance: Mean number of breastfeeding episodes significantly higher in COC group vs Copper IUD group on 7 out of 21 days (p<.05) Duration of exclusive breastfeeding similar between groups at 6 months (duration not reported) Infant growth: No significant differences in infant weight, height and tibia length between COC and Copper IUD groups at day 63 |
Strengths: No loss to follow-up by day 63 Weaknesses: Small numbers Duration of exclusive breastfeeding at 6 months not reported Included only women with previous breastfeeding experience |
Level II-2, fair |
ACOG, American College of Obstetricians and Gynecologists; CIC, combined injectable contraceptive; CNPq, Conselho Nacional de Pesquisa; ENG, etonogestrel; FAPEP, Fundacao de Apoio a Pesquisa do Estago de Sao Paulo; LNG, levonorgestrel; OC, oral contraceptive; POC, progestin-only contraceptive.
Excluded articles most frequently reported only outcomes of milk composition or volume without including clinically relevant outcomes. Several additional articles were excluded because the type of oral contraceptive (combined or progestogen-only) was not specified, no comparison group was included, timing of initiation of contraception or measurement of outcomes was not stated, or the methods did not provide enough information to determine if inclusion criteria were met [29–37]. One article was excluded [38] because it was a duplicate of two more comprehensive English-language publications [12,26] of the same study. Another article was excluded [28] because it was a subgroup report from the WHO study, the results of which were already included in this review [15,27].
3.1. COCs initiated at <6 weeks postpartum
3.1.1. Breastfeeding performance
We identified seven articles describing six studies that examined women who initiated COCs at <6 weeks postpartum and reported on breastfeeding performance (Table 1) [11,12,17–20,24]. Studies included one RCT, four partially randomized trials or cohort studies and one RCT that provides indirect evidence. Four of the studies were conducted prior to 1973 and evaluated older formulations of COCs [18–20,24]. All of the studies were included in the previous review, with the exception of the RCT that provides indirect evidence.
A poor-quality RCT conducted in Denmark investigated the use of COCs (containing 0.05 mg mestranol) versus placebo initiated on postpartum day 1 in 451 breastfeeding mothers of healthy infants [20]. By postpartum day 8, significantly more women in the COC group initiated supplemental feeding for their infants than those in the placebo group (12.3% versus 3.4%; p<.05).
A poor-quality, US-based, partially randomized trial compared women who chose not to use hormonal contraception (n=50) with women who chose to initiate COCs (n=50) [24]. Randomization was partial because women choosing COCs were randomized to initiate use of COCs (containing 0.08 mg mestranol, n=25) or placebo (n= 25) at 2 weeks postpartum. At 6 weeks postpartum, women on placebo switched to COCs. Women who initiated COCs at 2 weeks had higher supplemental calories given to their infants at 4 and 5 weeks postpartum compared with the placebo group (p values not reported). This study additionally provided some information on early compared with later COC initiation. By 12 weeks, the percentages of women still breastfeeding were 73% in the nonhormonal group, 52% among those initiating COCs at 6 weeks and 21% among those initiating COCs at 2 weeks (p values not reported).
A fair-quality partially randomized trial, in which randomization was performed for the first portion of the study, examined 291 women after a normal delivery in Chile [11,12]. Thirty to 35 days postpartum, COCs (containing 0.03 mg ethinyl estradiol), placebo (until 90 days postpartum when nonhormonal methods were started) or the copper intrauterine device (IUD) was initiated in 103, 188 and 118 women, respectively, either randomly or according to the women’s preference. At postpartum day 91, the percent exclusively breastfeeding was lower in the COC group than in the placebo group (81% versus 92%; p<.05) [12]. The percent exclusively breastfeeding was also lower in the COC group than the placebo and IUD groups from 4 to 10 months postpartum (specific percents not reported, p<.025) but not at 12 months [11]. At 6 months postpartum, there was a significantly higher percent weaned in the COC group compared with the copper IUD group. At 8 months postpartum, there was a significantly higher percent weaned in the COC group compared with the placebo and copper IUD groups. These differences did not persist at 10 and 12 months [11].
A poor-quality prospective cohort study of 174 women initiating various COC regimens (n=83) or some “other method of family planning” (not further specified) (n=91) on postpartum day 5 were followed for 6 weeks [18]. There were no significant differences between the groups in the percent of women still breastfeeding at 6 weeks. Another cohort study in Chile investigated COC use among women who initiated at 30 days postpartum [19]. The COCs used contained 2 mg quinestrol (n=275), 0.05 mg ethinyl estradiol (n=40) and 0.08 mg mestranol (n=52), with various progestin components. Duration of breastfeeding was found to be significantly shorter for the COC group than the nonhormonal historical control group for preparations using mestranol or quinestrol, but not in the COC formulations with 0.05 mg of ethinyl estradiol.
One new article of fair quality was identified, which provided indirect evidence on breastfeeding performance outcomes because the comparison group was women using other hormonal contraceptives [17]. In this RCT from the United States, women were randomized to use either COCs (0.035 mg ethinyl estradiol) (n=64) or POPs (n=63) initiated at 2 weeks postpartum. At 8 weeks postpartum, there was no statistically significant difference in breastfeeding continuation or supplementation between the COC group and the POP group. Survival analysis demonstrated no difference in breastfeeding continuation at 6 months postpartum (percents not reported).
3.1.2. Infant outcomes
There were seven articles describing six studies which examined women who initiated COCs at <6 weeks postpartum and reported on infant outcomes [11,12,17,20,21,23,24]. Three of these studies were newly identified: two were older studies, and one was published since the previous review [17,21,23]. As with the breastfeeding outcomes, four of these studies were conducted before 1973 and examined older, higher-dose COC formulations [20,21,23,24].
In the RCT from Denmark described above, no significant differences in infant weight among infants exclusively breastfed were noted by postpartum day 8 between women using COCs and those using placebo [20]. In the US partially-randomized trial described above, infants in the placebo group gained more weight than infants in the COC group at 4 and 5 weeks postpartum, although p values were not reported [24]. In the partially randomized study from Chile, the average infant weight of exclusively breastfed infants was lower in the COC group than the placebo group from 61 to 183 days and at 366 days postpartum (p<.05) [11,12]. The total infant weight increase at 6 months was lower in the COC group than in the placebo group (4636 g versus 4971 g; p<.05) [11]. No physical manifestations of exogenous estrogen, such as genital or breast changes, were noted in the infants in the COC group up to 1 year postpartum [11].
One newly identified poor-quality clinical trial from Egypt provided women after cesarean delivery with oral hormonal contraceptives or placebo pills initiated on postpartum day 2 [21]. The study was double-blinded, but the authors did not specify whether women were randomized. Ten women used COCs (0.1 mg mestranol), 10 women used an estrogen-only pill (0.1 mg ethinyl estradiol), and 10 women used placebo pills. At 14 days postpartum, the percent increases in infant weight were higher in the COC and estrogen-only groups compared with the placebo group; however, exact percents and p values were not reported.
One newly identified poor-quality prospective cohort study from Thailand reported on postpartum women who initiated COCs within 6 weeks postpartum [23]. Group 1 included 20 women using a COC with 0.1 mg of mestranol, and group 2 included 20 women using a COC with 0.08 mg mestranol. The control group included 20 women using no hormonal contraceptive. The outcome of average infant weight gain per week was only reported for 16 women in group 1 and 13 women in group 2 versus all 20 women in the control group. The average infant weight gain per week from weeks 6 to 16 postpartum was lower in the COC groups (group 1=147 g; group 2=180 g) than in the control group (202 g); the authors state that this difference was significant, but no p values were reported.
In the newly published RCT described above which provided indirect evidence, there were no differences in infant growth parameters, as measured by weight, length and head circumference, at 8 weeks postpartum among those whose mothers were using COCs compared with POPs [17].
3.2. COCs initiated at >6 weeks postpartum
3.2.1. Breastfeeding performance
Six articles reporting on five studies examined women initiating COCs at >6 weeks postpartum and reported on breastfeeding performance [15,16,22,25–27]. Two of these studies were newly identified: one was an older study, and one was published since the previous review [16,22]. One study was a partially randomized trial, one was a nonrandomized trial, and three were cohort studies. Two studies evaluated higher-dose pills [22,25], while the other three studies examined 0.03-mg ethinyl estradiol pills.
WHO conducted a fair-quality partially randomized clinical trial at three centers in two countries on the effect of oral contraception, both progestin-only and combined (0.03 mg ethinyl estradiol), initiated at 6 weeks postpartum [15,27]. Women choosing oral contraceptives were randomly assigned to either progestin-only or combined pills (n=86 for COCs). Women who chose IUDs, barrier methods, sterilization or no contraception were included as nonhormonal controls (n=111). At 24 weeks postpartum, there were no significant differences in breastfeeding continuation (rates not reported) or prevalence of supplementation between groups (p values not reported) [27].
One newly identified poor quality nonrandomized clinical trial from Egypt divided women into five groups of hormonal and nonhormonal contraceptives initiated at 6–10 weeks postpartum [22]. Two of the groups used COCs: group 1 used a COC with 0.075 mg mestranol, and group 2 used a COC with 0.1 mg mestranol. The comparison group was women using an IUD (type not specified) plus placebo. The average age of the infant at supplementation was lower in the COC groups (group 1=13.8 weeks; group 2=11.6 weeks) than in the placebo group (15 weeks); however, p values were not reported.
One newly published fair-quality prospective cohort study from Brazil examined 10 postpartum women who initiated COCs (0.03 mg ethinyl estradiol) at 42 days postpartum [16]. Compared with 10 women using copper IUDs, women using COCs had a higher mean number of breastfeeding episodes on 7 out of 21 days from postpartum days 42–63 (p<.05); breastfeeding episodes were not different on the remaining days. The duration of exclusive breastfeeding was similar between groups at 6 months, although the exact duration and p values were not reported.
In a fair-quality prospective cohort study from Chile, postpartum women exclusively breastfeeding chose to initiate either COCs (0.03 mg ethinyl estradiol, n=59) or nonhormonal contraception (n=82) at 90 days postpartum [26]. The COC group had lower rates of exclusive breastfeeding than the nonhormonal group at 4, 6, 8, 10 and 12 months (p<.05). The COC group also had higher proportions initiating supplementation at the same time points. The authors state that there were no differences in the percent weaning at 6, 8, 10 and 12 months, although p values were not reported.
One fair-quality cohort study in Sweden examined 48 women who initiated OCs (most used COCs with 0.05 mg ethinyl estradiol) at 2 months postpartum compared with 48 controls who did not use OCs [25]. The mean length of breastfeeding was shorter in the COC group than in the control group, 3.7 months versus 4.6 months (p<.05).
3.2.2. Infant outcomes
Six articles reporting on five studies examined women initiating COCs at >6 weeks postpartum and reported on infant outcomes [15,16,22,25–27]. Two of these studies were newly identified: one was an older study, and one was published since the last review [16,22].
In the newly identified study from Egypt described above, there were no differences between groups in infant growth curves at 32 weeks postpartum (stated by authors, but exact numbers and p values not reported) [22]. In the newly published study from Brazil, there were no significant differences in infant growth, as measured by weight, height and tibia length, at 63 days postpartum [16].
The remaining studies were included in the previous review and also did not demonstrate any effects on infant outcomes. In the study from Chile described above, mean infant weight did not significantly differ between COC and nonhormonal groups through postpartum day 366. At 4 months of age, mean weight increase in the COC group was lower than the nonhormonal group (p<.001); however, there were no differences at any other time points through 6 months [26]. The WHO study described above found no differences between the COC and nonhormonal groups in infant growth (including weight, length, ponderal index, arm circumference, triceps skinfold thickness and head circumference), infant illness episodes or number of days of sickness through 24 weeks [15,27]. In the study with longest child follow-up, there were no differences in weight gain, height increase, occurrence of serious illness or school performance between the COC and control groups through 8 years of follow-up [25].
4. Discussion
Studies addressing possible effects of COC use on breastfeeding success and corresponding infant health and growth include 13 studies, published in 15 articles, 5 of which are newly identified for this updated review. In general, results from the new studies added to this review are consistent with previous findings on breastfeeding performance and infant outcomes among CHC users compared with nonusers.
Among studies examining COCs initiated at <6 weeks postpartum, results were inconsistent regarding breastfeeding performance. Of the previously identified studies, three poor-quality studies and one fair-quality study found some diminished breastfeeding outcomes among COC users, including increased proportions using supplementation and decreased proportions continuing to breastfeed [11,12,19,20,24], while one poor-quality study found no effect on breastfeeding continuation at 6 weeks [18]. One newly identified, indirect study of fair quality found no effect on supplementation or breastfeeding continuation when compared with POPs [17]. Among studies examining COCs initiated at <6 weeks postpartum, results were also inconsistent on infant outcomes. Of the previously identified studies, one fair-quality study and one poor-quality study found less weight gain in infants of COC users compared with nonusers [11,12,24], and one poor-quality study found no effect on weight gain [20]. Of the newly identified studies, one poor-quality study found some effect on weight gain [23], but one poor-quality study and one fair-quality, indirect study found no effect [21,17].
Among studies examining COCs initiated at >6 weeks postpartum, results were inconsistent on breastfeeding performance. Of the previously identified articles, two fair-quality studies showed some diminished breastfeeding performance among COC users [25,26], and two fair-quality studies showed no effect [15,27]. Of the newly identified articles, one poor-quality study showed some diminished breastfeeding among COC users [22], and one fair-quality study did not [16]. Among studies examining COCs initiated at >6 weeks postpartum, results were consistent with regard to infant outcomes, with no articles finding differences in either infant growth or health. Newly identified articles reporting infant outcomes [16,22] were consistent with those previously identified [15,25–27].
There are several limitations to this body of evidence. There were only two direct-evidence randomized or partially randomized trials, both of poor quality, and neither described randomization procedures [20,24]. Most of the observational studies were of poor quality and included small numbers of women, had short follow-up times (less than 6 weeks) or had high loss to follow-up. Several poor-quality studies included only women with previous breastfeeding experience, and others did not control for previous breastfeeding experience. Many of the studies did not conduct statistical tests for comparisons of interest and did not control for other potential confounders. Studies used a variety of outcomes to define “successful” breastfeeding, therefore making comparison between studies difficult. The vast majority of articles were published in the 1960s–1980s using higher doses and different formulations of estrogen than currently available, limiting the generalizablity of this body of evidence to current formulations and delivery systems of modern CHCs.
Overall, the evidence identified by this systematic review found inconsistent effects on clinical breastfeeding measures. The physiology of breastfeeding is mediated by several hormones, including estrogen, progesterone, prolactin, insulin, thyroxin, growth hormone and cortisol [39]. Lactation is triggered by progesterone withdrawal after delivery of the placenta, which leads to prolactin secretion [39,40]. While this drop in progesterone appears to be the key trigger, estrogen withdrawal also accompanies secretory activation. Some studies have found that estrogen alone is effective in suppressing lactation; however, these studies involved administration of different types of estrogen at different doses and time frames than those given for contraceptive purposes [41]. Paradoxically, although a drop in estrogen correlates with lactation initiation, estrogen actually stimulates prolactin release [42]. The mechanism through which estrogen may inhibit lactation is not well understood but may involve direct suppression in breast tissue [42].
Studies have generally found that very low levels of hormones transfer to the infant during breastfeeding [7]. While evidence is limited, studies have demonstrated that low levels of estrogen and progestins are present in breast milk [7,43,44]. However, there is theoretical concern that hormone levels may be higher in the infant because the immature liver may not metabolize effectively, the kidneys may be inefficient at excretion and plasma-binding capacity may be low [7]. Nonetheless, evidence identified by this systematic review generally did not support negative clinical consequences for infants exposed to CHCs.
Given the significant limitations of this body of evidence, the importance of contraception for postpartum women and the theoretical concerns that have been raised about the use of combined hormonal contraception by women who are breastfeeding, rigorous studies examining these issues are needed. Studies should be undertaken among breastfeeding women using modern low-dose COCs as well as the combined hormonal patch, combined vaginal ring and combined injectables. However, consensus is needed among researchers on several critical issues for the design and interpretation of new studies, including study design (i.e., which questions are best suited for observational studies and which might only be able to be answered with RCTs), breastfeeding and infant outcomes (i.e., which are most important to guide recommendations), and development of standard definitions and measurements. Study design should include careful consideration of intervention and comparison groups, reporting of exact timing of contraceptive initiation and control for important factors such as prior breastfeeding experience. Attempt should be made to maximize generalizability of results by considering characteristics of women who participate in such studies and by inclusion of ill or preterm infants. Studies should follow women for at least the first few months postpartum to truly assess any impact on breastfeeding performance. In addition, longer-term follow-up of infants exposed to hormones through breast milk is needed in order to more fully understand any impacts on child development.
When considering choice of contraceptive methods, it is important to consider the full context of the risks and benefits and alternatives. For breastfeeding women, in addition to potential impacts on breastfeeding and infant health, there are additional considerations due to their postpartum status. The increased risk of venous thromboembolism (VTE) among postpartum women particularly in the first 6 weeks, coupled with the increased risk of VTE with use of CHCs, suggests that estrogen-containing contraceptive methods may increase the risk of VTE in postpartum women to an unacceptable level [45–47]. Alternative methods of contraception, including more effective methods such as IUDs and implants, are safe for postpartum women, and women should be counseled about the full range of contraceptive options [9].
In conclusion, fair- to poor-quality evidence showed conflicting results on whether use of COCs affects breastfeeding performance. The evidence also demonstrated conflicting results on whether early initiation of COCs affects infant outcomes but generally no negative impact on infant outcomes with later initiation of COCs. The body of evidence is limited by older studies using different formulations and doses of estrogen and poor methodologic quality. The information in this review was presented to an expert review panel in March 2014 at a meeting convened by WHO. The findings of this systematic review will be incorporated into the forthcoming update of the WHO MEC.
Acknowledgements
The authors would like to acknowledge the contributions of Suneeta Mittal, Faysel El-Kak, Roger Chou and the other members of the WHO Guidelines Development Group for the Medical Eligibility for Contraceptive Use. This review was supported by resources from the Department of Reproductive Health and Research at the World Health Organization, the Centers for Disease Control and Prevention, the US Agency for International Development, and the National Institute of Child Health and Human Development.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the World Health Organization or US Centers for Disease Control and Prevention.
Disclosure: Dr. Kapp is currently employed by HRA Pharma which makes emergency contraception.
References
- [1].World Health Organization. Report of a WHO technical consultation on birth spacing; 2006. [Available at: http://www.who.int/maternal_child_adolescent/documents/birth_spacing.pdf?ua=1. Accessed March 18, 2015].
- [2].Gipson JD, Koenig MA, Hindin MJ. The effects of unintended pregnancy on infant, child, and parental health: a review of the literature. Stud Fam Plann 2008;39:18–38. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization Department of Reproductive Health and Research (WHO/RHR). Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP). Family planning: a global handbook for providers. Baltimore and Geneva: CCP and WHO; 2007. [Google Scholar]
- [4].Erwin PC. To use or not use combined hormonal oral contraceptives during lactation. Fam Plann Perspect 1994;26:26–30. [PubMed] [Google Scholar]
- [5].Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 2012;8:CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Truitt ST, Fraser AB, Grimes DA, Gallo MF, Schulz KF. Hormonal contraception during lactation. systematic review of randomized controlled trials. Contraception 2003;68:233–8. [DOI] [PubMed] [Google Scholar]
- [7].Halderman LD, Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol 2002;186:1250–6 [discussion 1256–8]. [DOI] [PubMed] [Google Scholar]
- [8].Kapp N, Curtis K. Combined oral contraceptive use among breastfeeding women: a systematic review. Contraception 2010;82:10–6. [DOI] [PubMed] [Google Scholar]
- [9].World Health Organization. Medical eligibility criteria for contraceptive use. 4th ed. Geneva: WHO; 2009. [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Croxatto HB, Diaz S, Peralta O, Juez G, Herreros C, Casado ME, et al. Fertility regulation in nursing women: IV. Long-term influence of a low-dose combined oral contraceptive initiated at day 30 postpartum upon lactation and infant growth. Contraception 1983;27:13–25. [DOI] [PubMed] [Google Scholar]
- [12].Diaz S, Peralta O, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: III. Short-term influence of a low-dose combined oral contraceptive upon lactation and infant growth. Contraception 1983;27:1–1. [DOI] [PubMed] [Google Scholar]
- [13].Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001;20:21–35. [DOI] [PubMed] [Google Scholar]
- [14].Mohllajee AP, Curtis KM, Flanagan RG, Rinehart W, Gaffield ML, Peterson HB. Keeping up with evidence a new system for WHO’s evidence-based family planning guidance. Am J Prev Med 2005;28:483–90. [DOI] [PubMed] [Google Scholar]
- [15].Effects of hormonal contraceptives on breast milk composition and infant growth. World Health Organization (WHO) Task Force on Oral Contraceptives. Stud Fam Plann 1988;19:361–9. [PubMed] [Google Scholar]
- [16].Bahamondes L, Bahamondes MV, Modesto W, Tilley IB, Magalhaes A, Pinto e Silva JL, et al. Effect of hormonal contraceptives during breastfeeding on infant’s milk ingestion and growth. Fertil Steril 2013;100:445–50. [DOI] [PubMed] [Google Scholar]
- [17].Espey E, Ogburn T, Leeman L, Singh R, Ostrom K, Schrader R. Effect of progestin compared with combined oral contraceptive pills on lactation: a randomized controlled trial. Obstet Gynecol 2012;119:5–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gambrell RD Jr. Immediate postpartum oral contraception. Obstet Gynecol 1970;36:101–6. [PubMed] [Google Scholar]
- [19].Guiloff E, Ibarra-Polo A, Zanartu J, Toscanini C, Mischler TW, Gomez-Rogers C. Effect of contraception on lactation. Am J Obstet Gynecol 1974;118:42–5. [DOI] [PubMed] [Google Scholar]
- [20].Kaern T. Effect of an oral contraceptive immediately post partum on initiation of lactation. Br Med J 1967;3:644–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kamal I, Hefnawi F, Ghoneim M, Abdallah M, Abdel Razek S. Clinical, biochemical, and experimental studies on lactation. V. Clinical effects of steroids on the initiation of lactation. Am J Obstet Gynecol 1970;108:655–8. [DOI] [PubMed] [Google Scholar]
- [22].Kamal I, Hefnawi F, Ghoneim M, Talaat M, Younis N, Tagui A, et al. Clinical, biochemical, and experimental studies on lactation. II. Clinical effects of gestagens on lactation. Am J Obstet Gynecol 1969;105:324–34. [DOI] [PubMed] [Google Scholar]
- [23].Koetsawang S, Bhiraleus P, Chiemprajert T. Effects of oral contraceptives on lactation. Fertil Steril 1972;23:24–8. [DOI] [PubMed] [Google Scholar]
- [24].Miller GH, Hughes LR. Lactation and genital involution effects of a new low-dose oral contraceptive on breast-feeding mothers and their infants. Obstet Gynecol 1970;35:44–50. [PubMed] [Google Scholar]
- [25].Nilsson S, Mellbin T, Hofvander Y, Sundelin C, Valentin J, Nygren KG. Long-term follow-up of children breast-fed by mothers using oral contraceptives. Contraception 1986;34:443–57. [DOI] [PubMed] [Google Scholar]
- [26].Peralta O, Diaz S, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: V. Long-term influence of a low-dose combined oral contraceptive initiated at day 90 postpartum upon lactation and infant growth. Contraception 1983;27:27–38. [DOI] [PubMed] [Google Scholar]
- [27].Tankeyoon M, Dusitsin N, Chalapati S, Koetsawang S, Saibiang S, Sas M, et al. Effects of hormonal contraceptives on milk volume and infant growth. WHO Special Programme of Research, Development and Research Training in Human Reproduction Task Force on Oral Contraceptives. Contraception 1984;30:505–22. [DOI] [PubMed] [Google Scholar]
- [28].Gellen J, Mihaly S. Breast feeding and contraception. The effect of low-dose oral contraceptives on the growth of the infant during breast feeding. Orv Hetil 1984;125:193–6. [PubMed] [Google Scholar]
- [29].Coy JF, Mair CH, Ratkowsky DA. Breastfeeding and oral contraceptives: Tasmanian survey. Aust Paediatr 1983;19:168–71. [DOI] [PubMed] [Google Scholar]
- [30].Gupta AN, Mathur VS, Garg SK. Effect of oral contraceptives on quantity and quality of milk secretion in human beings. Indian J Med Res 1974;62:964–70. [PubMed] [Google Scholar]
- [31].Briend A, Fauveau V, Chakraborty J. Contraceptive use and breastfeeding duration in rural Bangladesh. Eur J Clin Nutr 1991;45:341–6. [PubMed] [Google Scholar]
- [32].Laukaran VH, Winikoff B. Contraceptive use, amenorrhea, and breastfeeding in postpartum women. Stud Fam Plann 1985;16:293–301. [PubMed] [Google Scholar]
- [33].Borglin NE, Sandholm LE. Effect of oral contraceptives on lactation. Fertil Steril 1971;22:39–41. [DOI] [PubMed] [Google Scholar]
- [34].Frank R, Alpern WM, Eshbaugh DE. Oral contraception started early in the puerperium. A clinical study. Am J Obstet Gynecol 1969;103:112–20. [DOI] [PubMed] [Google Scholar]
- [35].Kora SJ. Effect of oral contraceptives on lactation. Fertil Steril 1969;20:419–23. [DOI] [PubMed] [Google Scholar]
- [36].Ibrahim A, el-Tawil NZ. The effect of a new low-dosage oral contraceptive pill on lactation. Int Surg 1968;49:561–5. [PubMed] [Google Scholar]
- [37].Shaaban AH, el-Minawi MF. Clinical studies in United Arabic Republic with SH 850 (Eugynon), a low dose oral contraceptive, with special reference to its effect on lactation. Med Welt 1970;40:1730–4. [PubMed] [Google Scholar]
- [38].Peralta O, Diaz S, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Effect of a combined oral contraceptive on lactation and growth of the infant. Rev Chil Obstet Ginecol 1983;48:372–80. [PubMed] [Google Scholar]
- [39].Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia 2007;12:211–21. [DOI] [PubMed] [Google Scholar]
- [40].Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr 2001;131:3005S–8S. [DOI] [PubMed] [Google Scholar]
- [41].Oladapo OT, Fawole B. Treatments for suppression of lactation. Cochrane Database Syst Rev 2012;9:CD005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kochenour NK. Lactation suppression. Clin Obstet Gynecol 1980;23:1045–59. [DOI] [PubMed] [Google Scholar]
- [43].Nilsson S, Nygren KG, Johansson ED. Ethinyl estradiol in human milk and plasma after oral administration. Contraception 1978;17:131–9. [DOI] [PubMed] [Google Scholar]
- [44].Betrabet SS, Shikary ZK, Toddywalla VS, Patel D, Vaidya P, Saxena BN. ICMR Task Force Study on hormonal contraception. Biological activity of ethinyl estradiol present in the breast milk. Contraception 1986;34:169–75. [DOI] [PubMed] [Google Scholar]
- [45].Jackson E, Curtis K, Gaffield M. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol 2011;117:691–703. [DOI] [PubMed] [Google Scholar]
- [46].Peragallo Urrutia R, Coeytaux RR, McBroom AJ, Gierisch JM, Havrilesky LJ, Moorman PG, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol 2013;122:380–9. [DOI] [PubMed] [Google Scholar]
- [47].Tepper NK, Boulet SL, Whiteman MK, Monsour M, Marchbanks PA, Hooper WC, et al. Postpartum venous thromboembolism: incidence and risk factors. Obstet Gynecol 2014;123:987–96. [DOI] [PubMed] [Google Scholar]


