Abstract
Background:
Identifying the characteristics of individuals who demonstrate response to an intervention allows us to predict who is most likely to benefit from certain interventions. Prediction is challenging in rare and heterogeneous diseases, such as primary progressive aphasia (PPA), that have varying clinical manifestations. We aimed to determine the characteristics of those who will benefit most from transcranial direct current stimulation (tDCS) of the left inferior frontal gyrus (IFG) using a novel heterogeneity and group identification analysis.
Methods:
We compared the predictive ability of demographic and clinical patient characteristics (e.g., PPA variant and disease progression, baseline language performance) vs. functional connectivity alone (from resting-state fMRI) in the same cohort.
Results:
Functional connectivity alone had the highest predictive value for outcomes, explaining 62% and 75% of tDCS effect of variance in generalization (semantic fluency) and in the trained outcome of the clinical trial (written naming), contrasted with <15% predicted by clinical characteristics, including baseline language performance. Patients with higher baseline functional connectivity between the left IFG (opercularis and triangularis), and between the middle temporal pole and posterior superior temporal gyrus, were most likely to benefit from tDCS.
Conclusions:
We show the importance of a baseline 7-minute functional connectivity scan in predicting tDCS outcomes, and point towards a precision medicine approach in neuromodulation studies. The study has important implications for clinical trials and practice, providing a statistical method that addresses heterogeneity in patient populations and allowing accurate prediction and enrollment of those who will most likely benefit from specific interventions.
Keywords: prediction, heterogeneity, primary progressive aphasia, transcranial direct current stimulation, inferior frontal gyrus, semantic retrieval, verbal fluency, semantic fluency, precision medicine
1. Introduction
Precision medicine allows for specialized care, the minimization of unnecessary procedures, and a streamlined approach to treatment. Precision medicine implementation is emerging in language therapy interventions in aphasia (for both post-stroke and primary progressive aphasia), through prediction studies that show which patients will benefit from specific treatments are not yet clinically applicable. Some problems with prediction studies in aphasia include variability in aphasia profiles, the lack of area/symptom correspondence, the extent of damage / atrophy, and premorbid cognitive/language abilities. Addressing this heterogeneity is essential, because predicting whether and how well an individual with aphasia will respond to treatment is a key factor in developing individualized treatment plans. This need to address heterogeneity becomes even more important when considering new pharmacological, genetic, and neuromodulatory treatments that are becoming available for neurodegenerative conditions.
Our interest lies in primary progressive aphasia (PPA), a neurodegenerative disorder affecting language functions primarily [1,2], for which the only disease-modifying treatments are symptomatic language therapy and emerging neuromodulation approaches, especially transcranial direct current stimulation (tDCS) [3,4]. There is feasibility and efficacy of tDCS stimulation to trained items, as well as transfer of benefits to untrained items/words [4–6]. Investigation of factors that predict treatment response in PPA is at a nascent stage (see [7,8] for recent reviews) and is challenging, because PPA is a heterogenous clinical syndrome that includes at least three different clinical variants with varying patterns of decline [2]. Early prediction of treatment outcomes (before any treatment starts) is important in a neurodegenerative disorder because losing time means losing brain tissue.
Recent studies from different groups, including our own, have identified several baseline factors that predict efficacy of tDCS for a given patient or patient group [see [6–8] for reviews]. Five predictive factors have been identified: (1) baseline language and cognitive performance [9,10], (2) baseline cortical volume and thickness [11–13], (3) baseline white matter integrity [14], (4) clinical variant PPA [15], and (5) baseline sleep efficiency [16]. To our knowledge, no studies have utilized baseline functional brain connectivity as a predictor of tDCS effects in PPA. This is an important gap for tDCS, since evidence suggest that it modulates language outcomes in PPA is changes via functional brain connectivity changes [17–19]. Brain functional connectivity is correlated with general cognition as in Fronto-Temporal Dementia Clinical Rating Scale FTD-CDR, as we and others have reported. Finally, change in functional connectivity is one of the first alterations that happen in neurodegeneration including PPA [20,21]. Functional connectivity has been used as a predictor only in some recent tDCS studies research [22–24] that aimed to predict responses to tDCS in schizophrenia or healthy controls.
Recent studies in healthy controls highlight the specificity of functional connectivity (FC) changes after electrical stimulation [25]. We were among the first to demonstrate this particular mechanism (changes in FC) in PPA [17,19]. We found that tDCS downregulates the abnormally high FC in PPA in the areas stimulated. This hyperconnectivity was correlated with higher FTD-CDR, i.e., worse global cognition. Hyperconnectivity or hyperexcitability is a known hallmark of AD, and probably is involved in other neurodegenerative disorders [26,27]. Reductions in FC between the stimulated area (left inferior frontal gyrus, IFG) and other brain areas (such as the left middle temporal gyrus, MTG, with which it is structurally and functionally connected [28]), correlated with improvement in treatment outcomes.
Our modified outcome analysis predicts individual causal effects (e.g., had they been assigned to active tDCS vs. sham tDCS, along with oral and written naming treatment in both tDCS conditions) rather than the actual responses for the assigned intervention. Such heterogeneity analysis of tDCS in PPA patients offers an opportunity to predict the additional benefit received from active tDCS compared to sham tDCS, even before actual treatment begins. Outcomes of such analysis will be valuable to clinicians, patients, and caregivers, as it assists personalized precision health decisions.
This study closes the loop between the mechanism of tDCS and the clinical prediction of who will benefit from tDCS based on its mechanism. We hypothesized that: (1) if tDCS downregulates hyperconnectivity in stimulated areas, then baseline hyperconnectivity of the stimulated area will predict treatment outcomes. Furthermore, we hypothesized that: (2) baseline hyperconnectivity will predict tDCS effects better than other demographic or clinical factors. We tested these hypotheses using special statistical methods to account for heterogeneity in a rare disease. This study has important implications for clinical trials of potential new treatments in the spirit of precision medicine, because the methods implemented herein appear to allow us to predict the effects of these treatments before they are initiated.
2. Materials and Methods
2.1. Participants
Thirty-six patients with PPA participated in this study (17 female): 14 with logopenic variant PPA (lvPPA), 13 with non-fluent variant PPA (nfvPPA), and 9 with semantic variant PPA (svPPA). All were right-handed, native English speakers, between 50 and 80 years old, and diagnosed based on clinical assessment, neuropsychological and language testing, and MRI, according to consensus criteria [2]. Informed consent was obtained from participants or their spouses, and all data were acquired in compliance with the Johns Hopkins Hospital Institutional Review Board. Figure 1 shows recruitment and randomization to the active tDCS or sham tDCS conditions. Each PPA variant group was matched for sex, age, education, years post onset of symptoms, overall FTD-CDR score and language severity measures (Tables 1A, 1B).
Figure 1.
Participants recruited and randomized to active tDCS or sham tDCS.
Table 1A.
Means and standard deviations of demographic variables and baseline semantic fluency scores grouped by first-phase condition (n=36).
| Active tDCS first | Sham tDCS first | F (1,34) | p-value | |
|---|---|---|---|---|
| Sex | 9 F, 9 M | 8 F, 10 M | * | 1.000 |
| Variant | 7 L, 6 N, 5 S | 7 L, 7 N, 4 S | * | 0.500 |
| Age (years) | 66.17 (7.49) | 69.72 (5.42) | 2.66 | 0.113 |
| Years post symptom onset | 5.17 (3.40) | 4.72 (2.55) | 0.20 | 0.660 |
| Language severity (FTD-CDR) | 1.92 (0.90) | 1.83 (0.71) | 0.10 | 0.759 |
| Total severity (FTD-CDR) | 6.89 (4.53) | 7.53 (4.66) | 0.17 | 0.679 |
| Number of sessions in phase 1 | 12.72 (2.11) | 11.06 (1.63) | 7.05 | 0.012 |
| Baseline semantic fluency (words per minute) | 14.50 (11.17) | 11.81 (7.49) | 0.72 | 0.400 |
Fisher’s exact test used. FTD-CDR, Frontotemporal Dementia Clinical Dementia Rating Scale sum of boxes [29]. F, female; M, male. L, logopenic; N, nonfluent; S semantic.
Table 1B.
Means and standard deviations of demographic variables and baseline semantic fluency scores grouped by PPA variant (n=36).
| lvPPA | nfvPPA | svPPA | F(2,33) | p-value | |
|---|---|---|---|---|---|
| Sex | 7 F, 7 M | 5 F, 8 M | 5 F, 4 M | * | 0.800 |
| First-phase condition | 7 s, 7 t | 7 s, 6 t | 4 s, 5 t | * | 1.000 |
| Age (years) | 66.29 (8.11) | 69.77 (6.00) | 67.89 (4.96) | 0.91 | 0.412 |
| Years post symptom onset | 4.82 (3.33) | 4.65 (2.66) | 5.56 (3.08) | 0.25 | 0.780 |
| Language severity (FTD-CDR) | 1.57 (0.83) | 2.04 (0.72) | 2.11 (0.78) | 1.76 | 0.188 |
| Total FTD-CDR | 6.18 (3.76) | 7.85 (4.19) | 7.89 (6.17) | 0.57 | 0.571 |
| Number of sessions in phase 1 | 11.93 (2.02) | 11.85 (1.91) | 11.89 (2.47) | 0.01 | 0.990 |
| Baseline semantic fluency (words per minute) | 17.50 (10.97) | 12.08 (8.38) | 7.94 (5.15) | 3.30 | 0.049 |
Fisher’s exact test used. FTD-CDR, Frontotemporal Dementia Clinical Rating Scale sum of boxes [29]. F, female; M, male. s, sham tDCS; t, active tDCS.
2.2. Overall Design
We used a within-subjects, double-blind, crossover design with two experimental conditions: speech-language therapy plus conventional anodal tDCS over the left IFG, and speech-language therapy plus sham tDCS. Each condition lasted approximately 12 consecutive weekday sessions; the two phases were separated by a 2-month wash-out period. Stratified randomization of stimulation condition within each variant determined whether each participant received active tDCS or sham tDCS first, according to our main clinical trial (ClinicalTrials.gov identifier: NCT02606422). Performance was assessed before, immediately after, two weeks after, and two months after each period of stimulation (active tDCS or sham tDCS). Participants, speech-language pathologists, and examiners were blinded to the experimental condition. In statistical analysis, we focused on first-phase data only in order to avoid impact of carryover effects due to possibly insufficient wash-out period as we had noticed when we analyzed the behavioral results[15].
2.3. tDCS Methods
Each daily therapy session lasted one hour. For both active tDCS and sham conditions, two 5cm x 5 cm, non-metallic, conductive, rubber electrodes covered with saline-soaked sponges were placed over the right cheek (cathodal electrode) and the left IFG centered at F7 of the EEG 10–20 electrode position (anodal electrode) [30]. The electrodes were hooked up to a Soterix 1×1 Clinical Trials device, which elicited a tingling sensation on the scalp as it ramped up within 30 seconds, to deliver current at an intensity of 2 mA (estimated current density 0.08 mA/cm2; estimated total charge 0.096 C/cm2). In the active tDCS condition, current was delivered for 20 minutes for a daily maximum of 2.4 Coulombs; in the sham tDCS condition, current ramped up to 2 mA over a 30 sec interval and immediately ramped down to elicit the same tingling sensation, a procedure that has been shown to blind participants to treatment condition [31]. Stimulation started at the beginning of each therapy session and lasted for 20 minutes; speech-language therapy continued for the full session, i.e., 25 additional minutes, for 45–50 minutes. Twice during each session, participants rated their level of pain with the Wong-Baker FACES Pain Rating Scale (www.WongBakerFACES.org).
2.4. Language intervention
In the present study we based our prediction methodology on generalization effects we previously found in semantic fluency [32] and not on the trained task, since trained tasks are always expected to show benefits and generalization of treatment to other tasks is the most desirable outcome in neurodegenerative disorders. Also, we have extensively reported on the results for the trained task, oral and written naming [4]. We replicated the prediction analysis for the trained task and reported the results in Appendix 5.
2.5. Imaging methods
Of the 36 participants, 29 had magnetic resonance imaging (MRI) scans—five were severely claustrophobic and two had pacemakers and were therefore excluded. MRI scans took place at the Kennedy Krieger Institute at Johns Hopkins University. Magnetization-prepared rapid acquisition gradient echo (MPRAGE) and resting-state functional MRI (rsfMRI) scans were acquired before treatment on a 3-Tesla Philips Achieva MRI scanner with a 32-channel head coil. See Appendix 1 for more details.
Resting-state fMRI scans were co-registered with MPRAGE scans into the same anatomical space (native space); then 78 of the ROIs were parcellated on the rsfMRI scans, according to a multi-atlas fusion label algorithm (MALF) and large deformation diffeomorphic metric mapping, LDDMM [33,34]. Average time courses for the voxels in each ROI were normalized, and correlations between ROI pairs were calculated and normalized with the Fisher z-transformation. Of the 78 ROIs, we chose the ones that comprise the language-network ROIs and are functionally or structurally connected to the left IFG, the stimulated areaz0. In total 13 ROIs from the left hemisphere were selected. In addition, we conducted sensitivity analysis with an extended network that also includes the right homologues.
2.6. Statistical analyses
Prediction of potentially heterogeneous tDCS effects
In our prediction analysis, we modeled the additional, individual-specific tDCS effect using the so called modified outcome method, which adheres to established guidelines that account for heterogeneity in treatment effects [35–39]. For each participant, the individual-specific additional tDCS effect was defined as the difference between the potential change in the outcome if the participant had been assigned to active tDCS vs. sham tDCS, YiT = 1 − YiT = 0. We modeled the conditional average treatment effects (CATE), E[YiT = 1 − YiT = 0 | Xi] = E[Y|T = 1, X] − E[Y|T = 0, X], via direct prediction modeling of a transformation Ui = 2YiTi − 2Yi(1 − Ti) given the baseline covariates, X. This method replaced unobserved individual-specific additional tDCS effects, YiT = 1 − YiT = 0, with a fully observed modified outcome, Ui, which allowed for prediction analysis of U given X. In addition, compared with the modeling analysis of E[Y|T, X], the modeling of E[U|X] avoided interaction terms between the treatment and covariates, thereby allowed valid prediction modeling with lower dimension, which was crucial given the modest sample size.
We conducted stepwise predictor selection based on cross-validation for the prediction of the modified outcome (see details in Appendix 2). Baseline covariates, i.e., the candidate predictors, were divided into multiple groups for further comparison across prediction models: 1) Demographic and clinical factors, including baseline semantic fluency, PPA variant, number of treatment sessions, sex, age, years post onset of symptoms, and total FTD-CDR severity and language severity measures. 2) Imaging factors, which consisted of correlations between the hypothesis-selected 13 language ROIs of the baseline rsfMRI; thus 78 ROI pairs (13 choose 2) in total. For sensitivity analysis, we also tested the functional and volumetric imaging factors within an extended network that involves right hemisphere areas as well (26 ROIs and thus 325 ROI pairs). The predictive R-squared (R2) and the root mean squared error (RMSE) are reported. Linear coefficients in the final prediction model are reported in Appendix 3 for diagnostic purposes.
3. Results
3.1. Prediction of potentially heterogeneous tDCS effects: clinical and demographic predictors
We tested the following non-imaging factors for predicting the additional tDCS effect on semantic fluency: baseline semantic fluency, PPA variant, number of treatment sessions, sex, age, years post onset of symptoms, and dementia and language severity (FTD-CDR overall sum and language measure, respectively) [29]. None of the non-imaging factors predicted the individual tDCS effect with R-squared increment thresholded at 0.1 (10%). Only dementia severity (overall FTD-CDR sum) marginally predicted the additional tDCS effect in semantic fluency with 4.4% increase in predictive R-squared, although RMSE did not improve after adding dementia severity into the model (Table 2). Even if one allows an additional round, the results remains similar: having lvPPA (or nfvPPA) provides only an additional 8.6% (or 5.4%) R2 increase, which leads to 13.0% (or 9.8%) accumulated R2 and RMSE 8.021 (or 8.166) with both overall FTD-CDR and having lvPPA (or nfvPPA) in the model. Therefore, neither lvPPA or nfvPPA would be selected in either the first or the second round. This result shows that PPA variant and severity of cognitive/language impairment cannot accurately predict individual tDCS effects.
Table 2.
Non-imaging factors for individual tDCS effect prediction.
| Accumulated R2 | R2 increase | RMSE | |
|---|---|---|---|
| Null Model | 0 | 0 | 8.360 |
| Overall FTD-CDR | 0.044 | 0.044 | 8.407 |
3.2. Prediction of potentially heterogeneous tDCS effects: functional connectivity predictors
Since we did not find any demographic or clinical factor to predict the additional tDCS effect above the R-squared minimum threshold of 10%, we did not enter any other factors in the regression model to save degrees of freedom However, key baseline covariates via the literature and previous studies were included for completeness, including behavioral baseline performance.
The comparison between the final models from the non-imaging and imaging predictors (Tables 3 and 4) in terms of the accumulated predictive R2 (0.044 vs 0.498) and the RMSE (8.407 vs 6.287) indicated that the imaging predictors outweighed the non-imaging ones in predictive value.
Table 3.
Imaging factors from the language network in the left hemisphere that predicted the individual tDCS effect. Predictiveness was evaluated by the LOOCV (predictive) R2.
| Accumulated R2 | R2 increase | RMSE | |
|---|---|---|---|
| Null Model | 0 | 0 | 8.496 |
| Left STG: Left MTG pole | 0.307 | 0.307 | 7.389 |
| Left IFG opercularis: Left IFG triangularis | 0.416 | 0.109 | 6.782 |
| Left IFG triangularis: Left AG | 0.498 | 0.082 | 6.287 |
Table 4.
Imaging factors from the language network in both hemispheres that predicted the individual tDCS effect. Predictiveness was evaluated by the LOOCV (predictive) R2.
| Accumulated R2 | R2 increase | RMSE | |
|---|---|---|---|
| Null Model | 0 | 0 | 8.496 |
| Right AG : Right MTG | 0.380 | 0.380 | 6.989 |
| Left IFG triangularis : Left AG | 0.526 | 0.146 | 6.112 |
| Left IFG opercularis : Left IFG triangularis | 0.621 | 0.095 | 5.463 |
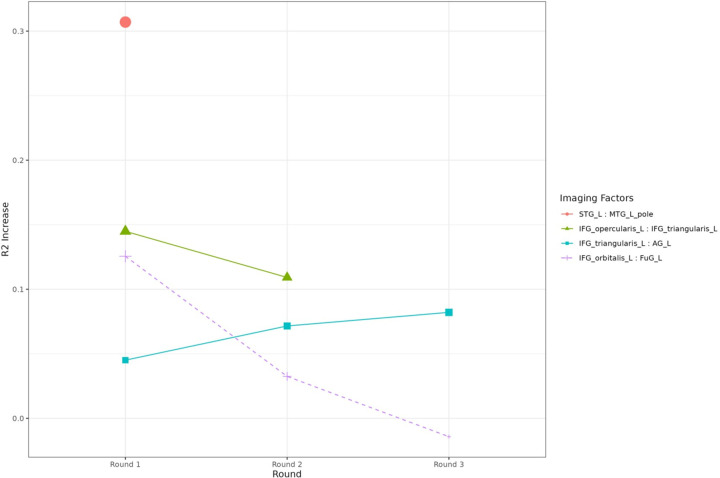
Consider specifically the left hemisphere, given that PPA is considered a LH syndrome. Two baseline resting-state functional connectivity ROI pairs predicted the additional tDCS effect on semantic fluency above 10% of R-squared increase (0.1 threshold): (1) the left superior temporal gyrus (STG)-to-left medial temporal gyrus (MTG) pole, and (2) left IFG opercularis-to-left IFG triangularis (Table 4 & Figure 2). Variable selection stopped at the last round with left IFG triangularis-to-left angular gyrus (AG) and just below 10% R-squared increase. The cumulative predictive R-squared of the final model was 49.8% (RMSE=6.29). The associated coefficients of the final model are reported in the supplementary materials (Appendix 3). Higher baseline connectivity in the first two pairs (most predictive, above 10% R2 increase) was associated with higher additional tDCS effect in the final model, whereas the trend was reversed in the last pair (8.2% R2 increase). In addition, we monitored all the pairs with above 10% R2 increases in each round of variable selection (see Figure 3).
Figure 2.
Visualization of the selected predictive imaging pairs for the additional tDCS effects. The positions of the nodes represent the average centers of each ROI from the cohort, rather than actual spatial distance. ROI pairs are plotted and connected if predictiveness of the baseline connectivity is confirmed by providing >10% R2 increase and being selected in the stepwise procedure. Thickness of the edge represents contribution to the accumulated R2 in the final prediction model.
Figure 3.
Functional connectivity pairs predicting the individual tDCS effects. Solid lines and points represent the selected factors in each round of the regression, whereas dotted lines represent the factors that were not selected but also provided over 0.1 increase of predictive R-squared in the first round. The 3 out of 4 pairs are the same as in Table 4. Note that in the first round another imaging predictor, Left IFG orbitalis: Left FuG provided R-squared increases greater than 0.1, but they were not selected because the Left STG: Left MTG pole had been selected for providing a larger R-squared increase.
In the sensitivity analysis that predicts individual treatment effects using the expanded connectivity network of 325 ROI pairs in both the LH and RH, three pairs were selected in each round, two overlapping with the main analysis (right AG-to-right MTG, left IFG triangularis-to-left AG, and left STG-to-left MTG pole, each providing R2 increases of 38.0%, 14.6%, and 9.5%; see Table 4 & Supplementary Table 3). The final prediction model showed improved prediction performance (RMSE 5.463, accumulated R2 62.1%) compared to the LH-only prediction (RMSE 6.287, accumulated R2 49.8%), as expected. In comparison, the volumetric predictors from the expanded network of 26 ROIs provided an accumulated R2 less than 10% (Appendix 4).
4. Discussion
The present study used a modified outcome prediction method that accounted for heterogeneity in PPA, to evaluate baseline clinical, demographic, behavioral (language) and imaging (rsFC and volumetric) predictors of tDCS effect on semantic fluency. Using the modified outcome addressed the problem of heterogeneity encountered in assessing treatment effects in this scenario and we conjecture that it would be similarly useful in any other heterogeneous neurodegenerative disorder. It was found that: (a) all clinical, demographic and behavioral baseline performance predictors together, accounted for 10% of the tDCS effect; (b) baseline volumes of these areas accounted only for 10% of the effect; (c) rsFC from a 7-min rsfMRI task, between temporal areas (left MTG pole to left STG) and frontal areas (left IFG pars opercularis to pars triangularis) predicted 50% of the tDCS effect on semantic fluency, when considering the LH alone, and, when considering both hemispheres, prediction improved for a total 62% of the tDCS effect on semantic fluency.
As far as we know, this is the first study that predicted a neuromodulatory effect (tDCS) in a neurodegenerative disorder and compared - in the same cohort, with the same method - other predictors of tDCS effect: clinical, demographic behavioral, and imaging (volumetric and rsFC) predictors. The present study has important implications for treatment prediction, advocating for rsFC as a biomarker for probable tDCS efficacy in PPA, and possibly for other neurodegenerative disorders, such as AD or MCI, with heterogeneous populations. We emphasize this important role that rsFC may play as a biomarker for patient capacity for treatment efficacy, rather than its typical role as an estimate of disease sequelae.
4.1. Semantic processing
An important finding in our study is that the strength of functional connectivity between temporal areas (MTG and STG), and between frontal areas (IFG opercularis and triangularis), predicted the magnitude of tDCS effect on semantic fluency. Lesion studies have shown that the left temporal cortex stores information about semantic categories, and frontal areas are important in accessing this information [40]. We have previously shown that the left ITG is responsible for storage of lexical characteristics of nouns and verbs in PPA [41,42], as well as with semantic fluency [43]. Furthermore, atrophy in the anterior and inferior left temporal regions, as well as in frontal regions, was associated with semantic fluency deficits [44]. The present study showed that baseline functional connectivity within the neural substrates of semantic fluency (semantic storage and control areas) predicts tDCS effects on semantic fluency.
4.2. Atrophy and FC considerations
The results of the present study seem counterintuitive given the atrophy in frontal areas in nfvPPA, who showed the largest tDCS effect [4]. Nevertheless, we [20] and others [45] have not found any correlation between functional connectivity and atrophy. Furthermore, we and others have found that active tDCS improves lower baseline function of atrophied regions by reducing the abnormally elevated connectivity (hyperconnectivity), to improve the efficiency of language processing [17–19]. This hyperconnectivity may be due to compensatory or disease-progression reasons, as previously proposed for the hippocampus [26,27]. It follows that active tDCS over hyperconnected areas may be beneficial. Unsurprisingly, in our previous clinical trial, patients with nfvPPA with the largest amount of atrophy in left IFG showed the largest benefits from IFG stimulation [32]. Therefore, active tDCS may be beneficial on compromised tissue (atrophied but still viable) when other network or compensatory brain areas remain intact. Finally, the present findings that hyperconnectivity between two semantic areas in the RH predict the tDCS effect, highlight the importance of RH semantic network areas, and their potential as tDCS targets.
4.3. Structural connectivity considerations
In the present study, we found that two of the most significant functional connectivity pairs that predicted the tDCS effect on semantic fluency, (Left IFG triangularis – Left IFG opercularis and Left MTG pole – Left STG) correspond to the end points of structurally connected areas because they are at the edges of the extreme capsule fasciculus, i.e., a white-matter bundle connecting the left IFG triangularis to temporal areas as part of the ventral language stream [46]. Several studies have shown that the extreme capsule is important for semantic processing and comprehension [46–48]. This bundle is sometimes hard to detect and often is considered as part of the uncinate fasciculus, although the latter has a more medial trajectory. We recently identified the white-matter integrity of this bundle as a significant predictor of tDCS effects but not language therapy alone (sham condition) for trained words during written naming [14]. In that study the contribution of the structural connectivity to the tDCS effect was significant, although smaller than in the present study (predicting only 12% of variance in outcome).
4.4. Limitations and implications
Certain limitations of this work should be noted. The most important one is the small sample size (36 patients with PPA), which, although not small for a neuromodulation randomized control trial in a rare neurodegenerative disorder such as PPA, is relatively small for robust conclusions in a prediction study. To circumvent the sample size limitation, we used methods such as conservative stepwise linear predictor selection that aimed to discover the most predictive variables based on R2 increases of cross-validation predictiveness measures, rather than the multiple testing of hundreds of variables where multiplicity would be a primary concern. The second limitation is our lack of power to perform an analysis by PPA variants and thus there is a need to replicate these results in bigger samples. The novel heterogeneity analysis has helped alleviate these issues and provide a tool for prediction analyses in heterogeneous disorders. Finally, we have not considered any cerebellar regions, which could be interesting for future investigation.
5. Conclusion
The present study provides evidence that functional connectivity between areas of semantic processing (storage, control and working memory) better predict generalization of tDCS effects in semantic fluency than any other clinical, demographic, or imaging (volumetric and structural connectivity) factors in PPA. Functional connectivity was also found to be a better predictor for the main outcome than clinical, demographic, and volumetric predictors as shown in Appendix 5. These results have significant clinical implications. For example, baseline functional connectivity can serve as biomarker for selecting patients who will benefit from tDCS. These results demonstrate that a 7-minute rsfMRI scan of baseline resting-state connectivity is adequate to reliably predict which patient with PPA will benefit from neuromodulatory tDCS treatment.
Supplementary Material
Acknowledgements
We would like to thank our participants and referring physicians for their dedication and interest in our study.
Funding:
This work was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders through award R01 DC014475 and National Institute of Aging through awards R01 AG068881, R01 AG075111 and R01 AG075404) to KT. AH was supported by NIH (NIDCD) through awards R01 DC05375, R01 DC011317 and P50 DC014664.
Funding Statement
This work was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders through award R01 DC014475 and National Institute of Aging through awards R01 AG068881, R01 AG075111 and R01 AG075404) to KT. AH was supported by NIH (NIDCD) through awards R01 DC05375, R01 DC011317 and P50 DC014664.
REFERENCES
- [1].Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–8. [DOI] [PubMed] [Google Scholar]
- [2].Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–14. 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimers Dis JAD 2014;39:799–808. [DOI] [PubMed] [Google Scholar]
- [4].Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, et al. Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimers Dement Transl Res Clin Interv 2018;4:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gervits F, Ash S, Coslett HB, Rascovsky K, Grossman M, Hamilton R. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang 2016;162:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cotelli M, Manenti R, Ferrari C, Gobbi E, Macis A, Cappa SF. Effectiveness of language training and non-invasive brain stimulation on oral and written naming performance in Primary Progressive Aphasia: A meta-analysis and systematic review. Neurosci Biobehav Rev 2020;108:498–525. 10.1016/j.neubiorev.2019.12.003. [DOI] [PubMed] [Google Scholar]
- [7].Nissim NR, Moberg PJ, Hamilton RH. Efficacy of Noninvasive Brain Stimulation (tDCS or TMS) Paired with Language Therapy in the Treatment of Primary Progressive Aphasia: An Exploratory Meta-Analysis. Brain Sci 2020;10:597. 10.3390/brainsci10090597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coemans S, Struys E, Vandenborre D, Wilssens I, Engelborghs S, Paquier P, et al. A Systematic Review of Transcranial Direct Current Stimulation in Primary Progressive Aphasia: Methodological Considerations. Front Aging Neurosci 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McConathey EM, White NC, Gervits F, Ash S, Coslett H, Grossman M, et al. Baseline performance predicts tdcs-mediated improvements in language symptoms in primary progressive aphasia. Front Hum Neurosci 2017;11:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Aguiar V, Zhao Y, Ficek BN, Webster K, Rofes A, Wendt H, et al. Cognitive and language performance predicts effects of spelling intervention and tDCS in Primary Progressive Aphasia. Cortex 2020;124:66–84. 10.1016/j.cortex.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cotelli M, Manenti R, Paternicò D, Cosseddu M, Brambilla M, Petesi M, et al. Grey Matter Density Predicts the Improvement of Naming Abilities After tDCS Intervention in Agrammatic Variant of Primary Progressive Aphasia. Brain Topogr 2016;29:738–51. 10.1007/s10548-016-0494-2. [DOI] [PubMed] [Google Scholar]
- [12].de Aguiar V, Zhao Y, Faria A, Ficek B, Webster KT, Wendt H, et al. Brain volumes as predictors of tDCS effects in primary progressive aphasia. Brain Lang 2020;200:104707. 10.1016/j.bandl.2019.104707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nissim NR, Harvey DY, Haslam C, Friedman L, Bharne P, Litz G, et al. Through Thick and Thin: Baseline Cortical Volume and Thickness Predict Performance and Response to Transcranial Direct Current Stimulation in Primary Progressive Aphasia. Front Hum Neurosci 2022;16:907425. 10.3389/fnhum.2022.907425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao Y, Ficek B, Webster K, Frangakis C, Caffo B, Hillis AE, et al. White Matter Integrity Predicts Electrical Stimulation (tDCS) and Language Therapy Effects in Primary Progressive Aphasia. Neurorehabil Neural Repair 2021;35:44–57. 10.1177/1545968320971741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, et al. Electrical brain stimulation in different variants of primary progressive aphasia: a randomized clinical trial. Alzheimers Dement Transl Res Clin Interv 2018;4:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Herrmann O, Ficek B, Webster KT, Frangakis C, Spira AP, Tsapkini K. Sleep as a predictor of tDCS and language therapy outcomes. Sleep 2021:zsab275. 10.1093/sleep/zsab275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tao Y, Ficek B, Wang Z, Rapp B, Tsapkini K. Selective Functional Network Changes Following tDCS-Augmented Language Treatment in Primary Progressive Aphasia. Front Aging Neurosci 2021;13:378. 10.3389/fnagi.2021.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meinzer M, Lindenberg R, Phan MT, Ulm L, Volk C, Flöel A. Transcranial direct current stimulation in mild cognitive impairment: behavioral effects and neural mechanisms. Alzheimers Dement 2015;11:1032–40. [DOI] [PubMed] [Google Scholar]
- [19].Ficek BN, Wang Z, Zhao Y, Webster KT, Desmond JE, Hillis AE, et al. The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage Clin 2018. 10.1016/j.nicl.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tao Y, Ficek B, Rapp B, Tsapkini K. Different patterns of functional network re-organization across the variants of primary progressive aphasia: A graph theoretic analysis. Neurobiol Aging 2020;96:184–96. 10.1016/j.neurobiolaging.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ranasinghe KG, Hinkley LB, Beagle AJ, Mizuiri D, Honma SM, Welch AE, et al. Distinct spatiotemporal patterns of neuronal functional connectivity in primary progressive aphasia variants. Brain 2017;140:2737–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rosso C, Valabregue R, Arbizu C, Ferrieux S, Vargas P, Humbert F, et al. Connectivity between right inferior frontal gyrus and supplementary motor area predicts after-effects of right frontal cathodal tDCS on picture naming speed. Brain Stimulat 2014;7:122–9. [DOI] [PubMed] [Google Scholar]
- [23].Antonenko D, Hayek D, Netzband J, Grittner U, Flöel A. tDCS-induced episodic memory enhancement and its association with functional network coupling in older adults. Sci Rep 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Paul AK, Bose A, Kalmady SV, Shivakumar V, Sreeraj VS, Parlikar R, et al. Superior temporal gyrus functional connectivity predicts transcranial direct current stimulation response in Schizophrenia: A machine learning study. Front Psychiatry 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grover S, Wen W, Viswanathan V, Gill CT, Reinhart RMG. Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation. Nat Neurosci 2022;25:1237–46. 10.1038/s41593-022-01132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012;74:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005;65:404–11. 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Petrides M. Broca’s area in the human and the non-human primate brain. Broca’s Reg 2006:31–46.
- [29].Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008;131:2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Homan RW. The 10–20 Electrode System and Cerebral Location. Am J EEG Technol 1988;28:269–79. 10.1080/00029238.1988.11080272. [DOI] [Google Scholar]
- [31].Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 2006;117:845–50. [DOI] [PubMed] [Google Scholar]
- [32].Wang Z, Ficek BN, Webster KT, Herrmann O, Frangakis CE, Desmond JE, et al. Specificity in Generalization Effects of Transcranial Direct Current Stimulation Over the Left Inferior Frontal Gyrus in Primary Progressive Aphasia. Neuromodulation Technol Neural Interface 2022. 10.1016/j.neurom.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ceritoglu C, Tang X, Chow M, Hadjiabadi D, Shah D, Brown T, et al. Computational analysis of LDDMM for brain mapping. Front Neurosci 2013;7. 10.3389/fnins.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tang X, Oishi K, Faria AV, Hillis AE, Albert MS, Mori S, et al. Bayesian Parameter Estimation and Segmentation in the Multi-Atlas Random Orbit Model. PloS One 2013;8:e65591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lipkovich I, Svensson D, Ratitch B, Dmitrienko A. Overview of modern approaches for identifying and evaluating heterogeneous treatment effects from clinical data. Clin Trials 2023;20:380–93. 10.1177/17407745231174544. [DOI] [PubMed] [Google Scholar]
- [36].Kennedy EH. Towards optimal doubly robust estimation of heterogeneous causal effects 2023. 10.48550/arXiv.2004.14497. [DOI] [Google Scholar]
- [37].Li R, Wang H, Tu W. Robust estimation of heterogeneous treatment effects using electronic health record data. Stat Med 2021;40:2713–52. 10.1002/sim.8926. [DOI] [PubMed] [Google Scholar]
- [38].Tian L, Alizadeh AA, Gentles AJ, Tibshirani R. A Simple Method for Estimating Interactions between a Treatment and a Large Number of Covariates. J Am Stat Assoc 2014;109:1517–32. 10.1080/01621459.2014.951443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Künzel SR, Walter SJ, Sekhon JS. Causaltoolbox—estimator stability for heterogeneous treatment effects. Obs Stud 2019;5:105–17. [Google Scholar]
- [40].Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc 2006;12:896–900. [DOI] [PubMed] [Google Scholar]
- [41].Race DS, Tsapkini K, Crinion J, Newhart M, Davis C, Gomez Y, et al. An area essential for linking word meanings to word forms: evidence from primary progressive aphasia. Brain Lang 2013;127:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Riello M, Faria AV, Ficek B, Webster K, Onyike CU, Desmond J, et al. The Role of Language Severity and Education in Explaining Performance on Object and Action Naming in Primary Progressive Aphasia. Front Aging Neurosci 2018;10. 10.3389/fnagi.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Libon DJ, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, et al. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology 2009;73:535–42. 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Riello M, Frangakis CE, Ficek B, Webster KT, Desmond JE, Faria AV, et al. Neural correlates of letter and semantic fluency in primary progressive aphasia. Brain Sci 2021;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mandelli ML, Vilaplana E, Brown JA, Hubbard HI, Binney RJ, Attygalle S, et al. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain 2016;139:2778–91. 10.1093/brain/aww195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci 2008;105:18035–40. 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci 2009;13:175–81. [DOI] [PubMed] [Google Scholar]
- [48].Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci Off J Soc Neurosci 2011;31:16949–57. 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.