Abstract
Background:
Low-grade systemic inflammation is implicated in the pathogenesis of various neuropsychiatric conditions affecting mood and cognition. While much of the evidence concerns depression, large-scale population studies of anxiety, affect, and cognitive function are scarce. Importantly, causality remains unclear. We used complementary non-genetic, genetic risk score (GRS), and Mendelian randomization (MR) analyses to examine whether inflammatory markers are associated with affect, depressive and anxiety disorders, and cognitive performance in the Lifelines Cohort; and whether associations are likely to be causal.
Methods:
Using data from up to 55,098 (59% female) individuals from the Dutch Lifelines cohort, we tested the cross-sectional and longitudinal associations of C-reactive protein (CRP) with (i) depressive and anxiety disorders; (ii) positive and negative affect scores, and (iii) five cognitive measures assessing attention, psychomotor speed, episodic memory, and executive functioning (figural fluency and working memory). Additionally, we examined the association between inflammatory marker GRSs (CRP, interleukin-6 [IL-6], IL-6 receptor [IL-6R and soluble IL-6R (sIL-6R)], glycoprotein acetyls [GlycA]) on these same outcomes (Nmax=57,946), followed by MR analysis examining evidence of causality of CRP on outcomes (Nmax=23,268). In genetic analyses, all GRSs and outcomes were z-transformed.
Results:
In non-genetic analyses, higher CRP was associated with diagnosis of any depressive disorder, lower positive and higher negative affect scores, and worse performance on tests of figural fluency, attention, and psychomotor speed after adjusting for potential confounders, although the magnitude of these associations was small. In genetic analyses, CRPGRS was associated with any anxiety disorder (β=0.002, p=0.037, N=57,047) whereas GlycAGRS was associated with major depressive disorder (β=0.001, p=0.036; N=57,047). Both CRPGRS (β=0.006, p=0.035, N=57,946) and GlycAGRS (β=0.006, p=0.049; N=57,946) were associated with higher negative affect score. Inflammatory marker GRSs were not associated with cognitive performance, except sIL-6RGRS which was associated with poorer memory performance (β=−0.009, p=0.018, N=36,783). Further examination of the CRP-anxiety association using MR provided some weak evidence of causality (β=0.12; p=0.054).
Conclusions:
Genetic and non-genetic analyses provide consistent evidence for an association between CRP and negative affect. Genetic analyses suggest that IL-6 signaling could be relevant for memory, and that the association between CRP and anxiety disorders could be causal. These results suggest that dysregulated immune physiology may impact a broad range of trans-diagnostic affective symptoms. However, given the small effect sizes and multiple tests conducted, future studies are required to investigate whether effects are moderated by sub-groups and whether these findings replicate in other cohorts.
Keywords: Depression, Anxiety, Affect, Cognition, Inflammation, CRP, Lifelines Cohort, Mendelian randomization
1. Introduction
Depression affects 300 million individuals worldwide at any given point in time and is the leading cause of mental health-related global disease burden (1–3). Persistent cognitive problems, such as poor memory and concentration, are reported in 11% of adults aged ≥45 years (4) and are frequently observed across a broad range of physical [cancer (35%); COVID-19 (22%); HIV (43%); hepatitis C (50%)] (5–8) and mental health conditions [depression (30%); schizophrenia (50%)] (9, 10). Existing treatments for depression are only modestly effective (11) and almost inexistent for cognitive dysfunction (12, 13). A mechanistic understanding of depression and cognitive dysfunction is urgently needed to inform the development of effective treatments and prevention approaches.
Chronic, low-grade systemic inflammation may represent one such mechanism. Indices of inflammation [e.g., circulating levels of cytokines (e.g., interleukin-6 (IL-6) and acute phase proteins (e.g., C-reactive protein (CRP)] are elevated in individuals with depression compared to controls (14) and inflammatory biomarkers have been linked to specific aspects of depression, such as anhedonia and negative affect (15–17). Further, longitudinal observational studies have found that higher levels of inflammatory biomarkers (e.g., IL-6, CRP) are prospectively associated with higher depressive symptoms (18). Observational studies have linked inflammation with impaired cognition in population-based (19–22) and in physical (5–9, 23–27) and mental health conditions (28–32). Inflammation also impacts neural circuitry relevant to affective disorder and cognitive task performance (33, 34), particularly the hippocampus (35) and striatum (36–39). To date, inflammation-cognition research has primarily relied upon observational data.
Inferring causality from observational studies is a challenge due to confounding (e.g., stress, poor sleep (27)) and reverse causality (i.e., whether inflammation impacts depression/cognition, or vice versa). Mendelian randomization (MR) is a genetic epidemiological method that can test causal relationships by using genetic variants associated with an exposure (e.g., inflammation) as proxies for the exposure (40, 41). As genetic variants are randomly inherited from parents to offspring and are fixed at conception, they are less likely to be associated with confounders and overcome issues of reverse causation (40, 41). Preliminary evidence, using MR, implicate IL-6 and its soluble IL-6 receptor (sIL-6R) in depression (42–45). To date, most MR studies examining the effect of IL-6 on health have focused on circulating IL-6 levels. However, IL-6 signals via multiple pathways (trans-signaling, classical-signaling, and trans-presentation) and there is growing evidence that IL-6 trans-signaling is primarily responsible for the pathogenic inflammatory effects of IL-6 (46, 47). Here, we include variants related to (1) circulating IL-6 levels, and (2) sIL-6R levels (relevant for IL-6 trans-signaling). Causal evidence for CRP and other proinflammatory markers [i.e., Glycoprotein Acetyls (GlycA) a composite biomarker thought to provide a more stable marker of inflammation which reflects the glycosylation of multiple acute-phase proteins (48–51)] on depression are mixed (42, 45, 52–55). Regarding cognition, few studies have examined potential causal relationships with inflammation. MR analyses using available genome-wide association studies (GWAS) report both null results of inflammatory biomarkers on emotion recognition, working memory, response inhibition (56) as well as effects of specific cytokines/chemokines (i.e., Eotaxin, IL-8, MCP1, IL-4) on fluid intelligence (57).
The current study used data from the Lifelines Cohort Study – a large population-based cohort in the Netherlands – to conduct complementary non-genetic and genetic analysis to investigative the causal relationship between inflammation and negative affect, depressive disorders, and cognitive task performance. First, we used cross-sectional and longitudinal non-genetic analysis examine the association between circulating levels of CRP and depression/cognitive performance. Second, we conducted genetic risk score (GRS) and MR analysis to test whether genetic variants regulating levels and activity of CRP, IL-6, and GlycA were causally related with depression/cognitive performance. We also conducted the above analyses on closely related constructs (e.g., anxiety, negative/positive affect), for which associations with inflammation have previously been observed (58–63) but for which considerably less empirical data has been published. We hypothesized that both circulating CRP levels and genetically predicted inflammatory biomarkers (i.e., CRP, IL-6, sIL-6R, and GlycA) would be associated with depression, cognitive task performance, affect, and anxiety.
2. Methods and Materials
2.1. Participants
Lifelines is a multi-disciplinary prospective population-based cohort study examining in a unique three-generation design the health and health-related behaviors of 167,729 persons living in the North of the Netherlands. It employs a broad range of investigative procedures in assessing the biomedical, socio-demographic, behavioral, physical and psychological factors which contribute to the health and disease of the general population, with a special focus on multi-morbidity and complex genetics (64). This cohort has previously been described in detail (64, 65). In brief, participants were recruited via their general practitioner (49%), participating family members (38%), and self-registration on the Lifelines website (13%). Exclusion criteria for recruitment through the general practitioner included: insufficient knowledge of Dutch language, severe psychiatric or physical illness, limited life expectancy (<5 years). Baseline data included approximately: 140,000 adults (18–65 years), 15,000 children (0–17 years), 12,000 elderly individuals (65+ years). Following baseline assessment, participants are invited to complete an in-person study visit every 5 years (2nd in person follow-up assessment just finished end of 2023). Phenotypic and genotypic data are collected by Lifelines to permit investigation on determinants of health. Data for the current study were drawn from 147,815 individuals who were aged 18+ years at baseline and who did not report a diagnosis that typically impairs cognitive function, specifically Alzheimer’s disease, other dementias, epilepsy, multiple sclerosis, Parkinson’s disease, and stroke. In the non-genetic analyses, the analytic sample is smaller as CRP was assessed in a sub-sample of individuals (N=55,098) as was baseline cognitive performance on the Ruff Figural Fluency Test (N=88,096). The analytic sample is smaller for non-genetic (N≤55,098), GRS (N≤57,946) and MR (N≤23,268) analysis as only a subset of participants who met inclusion criteria had outcome data and (i) CRP data (non-genetic analysis), (ii) genetic data (GRS), or (iii) genetic and CRP data (MR) due to time and cost constraints. Phenotypic data were drawn from both the baseline assessment and the first follow-up assessment; whether a specific measure was assessed at baseline, first follow-up or both assessments is noted for each measure.
2.2. Measures
2.2.1. Measures of Cognitive Task Performance
Ruff Figural Fluency Test (baseline in-person assessment).
The Ruff Figural Fluency Test (RFFT) is a reliable and valid measure of figural fluency, a dimension of executive function (66), measured at the baseline assessment. It is likely that performance on the RFFT also depends on other cognitive abilities, such as processing speed (67, 68). Participants were asked to draw as many unique designs as possible within 60 seconds by connecting dots in different patterns. The task is composed of five parts, with each part containing 35 identical five-dot patterns (with or without distractors). The total number of unique designs was used as the dependent variable in the analyses, consistent with previous studies (69, 70). In Lifelines, the RFFT was administered to all participants until April 2012, and subsequently in a random half of the sample. Data from participants who failed to generate a single unique design per trial (n = 181) were deemed invalid and removed.
Cogstate Test Battery (first follow-up in-person assessment).
Assessments included in the Cogstate Test Battery consisted of four tasks and took approximately 10–15 minutes to complete. Each task was designed to tap into specific cognitive domains: detection task (psychomotor speed), identification task (attention), one-back task (working memory), and one card learning task (episodic memory), although, like in the RFFT, processing speed likely plays a role in all tasks. For each task, outcomes recommended by Cogstate were selected, specifically: log10 transformed response time in millisecond (detection and identification tasks) and arcsine-transformed response accuracy (one-back and one card learning tasks). For the detection and identification tasks, higher values reflect poorer performance and for the one-back and one card learning tasks, higher values equal better performance. Data cleaning involved excluding participants with a high number of errors. The percentage of successful trials per Cogstate task was high, averaging 66% (n = 85,050; SD = .11) on the episodic memory task, 91% (n =85,053; SD = .17) on the visual attention task, 92% (n =85,053; SD = .20) on the psychomotor speed task, and 90% (n = 85,051; SD = .15) on the working memory task. A small number of participants exhibiting implausibly low accuracy rates indicative of poor effort, failure to comprehend task instructions, or technical errors were excluded from analyses. Specifically individuals with an accuracy rate less than: 25% on the episodic memory task (n = 231), 40% on the visual attention task (n = 2,878), 45% on the psychomotor speed task (n = 3,914), and 35% on the working memory task (n = 1,330). For more details on the Cogstate Test Battery, see Supplementary Methods 1.1.
2.2.2. Clinical Assessments
Anxiety and Depressive Disorders (baseline and first follow-up in-person assessments).
The Mini International Neuropsychiatric Interview – Simplified (MINI) is a reliable, valid, and brief structured interview that was designed to screen for psychiatric disorders (71–73). Lifelines used an adapted version of a Dutch translation of the MINI that was administered by trained interviewers at baseline and self-administered on location at follow-up – details on the version used in Lifelines have previously been published (74). Participants were considered to meet criteria for any depressive disorder if they met the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for Major Depressive Disorder (MDD) or dysthymia at the time of the interview. Impairment was assessed in the MINI for dysthymia but not depression and consequently, impairment was not used as a criterion for MDD. Any anxiety disorder refers to meeting current criteria for any one of the following conditions that was assessed using the MINI: panic disorder, agoraphobia, social phobia, or Generalized Anxiety Disorder (GAD). We used four diagnostic groups as outcome variables: MDD, any depressive disorder, GAD, and any anxiety disorder.
Positive and Negative Affect Schedule (baseline in-person assessment).
The Positive and Negative Affect Schedule (PANAS) is composed of two subscales which are designed to assess positive and negative affect (75). Each subscale has 10 items (examples of items include ‘excited’ on positive subscale; ‘upset’ on negative subscale). Participants are asked to rate the extent that they experienced each item during the last four weeks on a five-point scale (ranging from ‘not at all’ to ‘extremely’). The outcome is the summed score on each subscale, which ranges from 10 to 50 (higher value reflects higher positive or negative affect, respectively). The PANAS has been shown to be reliable and valid (76).
2.2.3. C-reactive Protein (baseline in-person assessment)
Participants gave blood samples before 10AM via venipuncture following an overnight fast. Complete details on blood specimen data collection have previously been reported (64, 70). Due to assay costs, CRP was assessed in approximately 35% of Lifelines participants and data were available for 55,098 individuals in the analytic sample. CRP was quantified using three separate methods over the course of baseline assessment (Method 1: 12.90% of total CRP values assessed in serum; CardioPhase hsCRP; Method 2: 84.58% of total CRP values, assessed in plasma; CardioPhase high sensitivity (hs)CRP, Siemens Healthcare Diagnostics, Marburg, Germany; Method 3: 2.52% of total CRP values; assessed in plasma; CRPL3, Roche Diagnostics, Mannheim, Germany). Assay methods 2 and 3 were identical and only differed in terms of the manufacturer. A conversion formula (new = 0.92 x old - 0.01) was derived from an internal validation using 39 samples, according to the AMC (alternative method comparison, Deming Regression) protocol in order that Method 1 could be compared with Method 2 and 3 (70). For CardioPhase hsCRP, the intra-assay coefficient of variability was 3.45% and the inter-assay coefficient of variability was 3.15%. For CRPL3, the intra-assay coefficient of variability was 4.15% and the inter-assay coefficient of variability was 5.8%.
2.2.4. Genetic Data
Genotype data were available for a subgroup of participants in Lifelines. Genotyping was conducted using three chip arrays: (i) Illumina CytoSNP-12 Bead Chip v2 array (N=17,033), (ii) Infinium Global Screening Array (GSA) Beadchip-24 v1.0 (N=38,030), (iii) FinnGen Thermo Fisher Axiom ® custom array (Affymetrix; N=29,166). For details on quality checks (QC’s) and imputation conducted by Lifelines, see Supplementary Methods 1.2. Following Lifelines QC’s, the total sample size for participants who met criteria for this study: CytoSNP (N=14,942), GSA (N=31,810) and Affymetrix (N=26,334). We applied additional QC’s which included removing: (i) one of the duplicates (individuals who were genotyped on more than one chip) and first-degree relatives between chips, (ii) non-European individuals (identified by Lifelines), and (iii) genetic outliers (identified by Lifelines); see Supplementary Figure 1 for more details. This resulted in a total of 58,713 participants with genetics data included in this study (CytoSNP N=7,632; GSA N=24,975; Affymetrix N=26,106). For more details on the genetic data in Lifelines, see Supplementary Methods 1.2.
2.2.5. Covariates
Covariates included age, sex, educational attainment, body mass index (BMI) and health status. Age, sex, and educational attainment were self-reported by participants. Educational attainment was determined using a single-item question and was categorized by Lifelines as: low [no education, primary education, lower/preparatory vocational education, lower general secondary education (leaving secondary school aged >16 years)], moderate (intermediate vocational education/apprenticeship, higher secondary education), and high (higher vocational education, university). We recoded educational attainment so that higher values represent lower educational attainment. To estimate body mass index (BMI), height was measured to the closest 0.1 cm and body weight was measured without shoes to 0.1 kg precision. For health status, a composite measure was created counting several self-reported chronic medical conditions related to increased levels of inflammation (i.e., arthritis, asthma, coeliac disease, Crohn’s disease, diabetes, and psoriasis); we then categorized participants into those with no relevant chronic medical condition, 1, 2 or 3+ conditions.
2.3. Analyses
Analyses were conducted in R version 4.1.1.(77)
2.3.1. Non-genetic Analyses
Multivariable linear and logistic regression models were estimated using base functions in R (i.e., ‘lm’, ‘glm’). CRP was transformed by natural log to impose a normal distribution.
2.3.2. Genetic Risk Scores
Genetic risk scores (GRS) were calculated to determine whether GRS for inflammatory markers (CRP, IL-6, IL-6R, sIL-6R, GlycA) were associated with depression/anxiety, affect and cognitive outcomes. To create GRS for each inflammatory marker, we identified genetic variants (single nucleotide polymorphisms [SNPs]) associated with these proteins in large available GWAS or using SNP lists from previous publications, see Table 1. For details on the GWAS used and accessing summary statistics, please see Supplementary Methods 1.3 and Supplementary Tables 1-2. The following criteria were used to identify SNPs from GWAS for each inflammatory marker: (i) p-value < 5 × 10−8, (ii) linkage disequilibrium clumping (r2=0.01, kb=1000 based on the European-clustering individuals in the 1000 genomes reference panel) using ld_clump() (78) in the ieugwasr package, (iii) minor allele frequency > 0.01. In the primary analysis, we restricted the SNP set to cis variants (SNPs +/− 1Mb from protein coding gene based on Genome Reference Consortium Human Build used in the GWAS) (79–84). The reason for restricting to cis variants in the primary analysis is because, due to their proximity to the protein coding gene, they are more likely to be valid instruments, as they are more likely to influence mRNA expression and protein levels (thus being less pleiotropic) (85). For GlycA, which does not have a single coding gene due to its composite nature, we used the largest available GWAS in our primary analysis. In our secondary analyses, we used both cis and trans variants from GWAS (i.e., we did not restrict to cis variants). Each SNP list (Table 1) was used to create a weighted GRS for each Lifelines participant. Specifically, the risk alleles were weighted by the effect size (beta) reported in the GWAS/previous study and then summed to provide a risk score. Any SNP identified in GWAS/previous study that was not available in Lifelines was replaced with a proxy (where possible) that had r2 > 0.8 (using LDproxy_batch function in EUR population in R), rsID (SNP name) available, SNP available in full summary statistic GWAS, and in Lifelines (86, 87). GRS were created in Plink v1.90 (88) and continuous phenotypes were standardized within each chip (z-scored) for direct comparison (CRP levels were log transformed but not standardized).
Table 1.
Large available genome-wide association studies and previous publications used to identify single nucleotide polymorphisms associated with systemic inflammatory markers.
| Marker | Author of GWAS/Instrument | Sample size | Outcome | SNPs Identified | SNPs available on each chip |
|---|---|---|---|---|---|
| Primary analysis | |||||
| CRP | (Said et al., 2022) | 575,531 | Circulating CRP | 19 | 16 |
| IL-6R | (Ahluwalia et al., 2021) | 52,654 | Circulating IL-6 | 2 | 2 |
| (Sarwar et al., 2012) | 27,185 | Circulating IL-6 | 1 | 1 | |
| sIL6R | (Rosa et al., 2019) | 3,301 | Circulating sIL6R | 34 | 34 |
| GlycA | (Borges, 2020) | 115,078 | Circulating GlycA | 87 | 73–74 |
| Secondary analysis | |||||
| CRP | (Said et al., 2022) | 575,531 | Circulating CRP | 485 | 445–446 |
| IL-6 | (Ahluwalia et al., 2021) | 52,654 | Circulating IL-6 | 3 | 2–3 |
CRP = C-reactive protein; IL-6 = interleukin-6, IL-6R = IL-6 receptor, sIL6R = soluble IL-6R
To adjust for relatedness within each chip, two approaches were taken. The primary approach applied the GRAMMAR method (89) and the secondary approach involved re-running analyses removing close relatives (up to first-degree, up to second-degree, and up to third-degree), see Supplementary Materials 1.4 and 1.5 for details on how we identified close relatives. We then ran regression models predicting each outcome using the standardized GRS, including top 10 genetic PCs (calculated on merged Lifelines genotype data), age, sex, and chip. Maximum sample size for analyses: no relatives within chips removed (N=58,713), up to first-degree removed (N=50,955), up to second-degree removed (N=50,255), up to third-degree removed (N=48,880). Unadjusted analyses are also reported in the Supplementary Tables 5 and 6 for comparison.
2.3.3. Mendelian randomization
To conduct MR using individual level data and two-stage least squares regression, genetic data, exposure data, and outcome data are required. As only CRP is available within the Lifelines cohort (IL-6 and GlycA are not currently available), only this inflammatory marker could be assessed in the MR analysis. Where there was evidence of associations between CRP GRS and outcomes, we followed this up with MR to assess potential causality. Three key assumptions are necessary for valid inferences from MR: (i) genetic variants are robustly associated with the exposure, (ii) genetic variants are not associated with potential confounders, (iii) genetic variants are associated with the outcome only via the exposure. Two-stage least squares regressions were conducted using the AER package (90). Analyses were GRAMMAR adjusted for relatedness and all regression models adjusted for age, sex, and chip. To check MR assumptions, we ran linear regressions to test whether CRP GRS were associated with circulating CRP levels in participants with both genetic and CRP data available (n = 23,607) using the GRAMMAR method. We also checked whether any inflammatory marker GRS (CRP, IL-6, IL-6R, sIL-6R, GlycA) were associated with potential confounders (BMI, current smoking status, educational attainment; all models were adjusted for age, sex, and chip).
3. Results
The characteristics of the Lifelines cohort sample are described in Table 2 and Pearson correlations between study variables are presented in Table 3.
Table 2.
Lifelines Cohort Sample Characteristics at Baseline and First Follow-up Assessment
| Measures | Cohort Analyses (n = 147,815) |
Genetic Analyses (n = 58,713) |
|---|---|---|
| Baseline Assessment | ||
| Age [Mean (SD)] | 44.52 (13.12) n = 147,815 |
43.04 (13.56) n = 58,713 |
| Sex (% Female) | 59 % n = 147,815 |
60% n = 58,695 |
| Education (N %) | n = 146,050 | n = 58,112 |
| Lower | 43,750 (30%) | 16,359 |
| Moderate | 57,785 (40%) | 23,770 |
| Higher | 44,515 (30%) | 17,983 |
| Body mass index | 26.05 (4.34) n = 147,719 |
25.81 (4.27) n = 58,680 |
| RFFT (Unique designs) [Mean (SD)] | 81.50 (22.94) n = 88,096 |
82.46 (23.01) n = 36,563 |
| Any Depressive Disorder (Current Major Depression or Dysthymia) | 3.4% n = 141,045 |
2.9% n = 56,861 |
| Major Depressive Episode (Current) | 2.1% n = 141,538 |
1.8% n = 57,048 |
| Any Anxiety Disorder (panic disorder, agoraphobia, social phobia, or GAD ) | 7.8% n = 141,538 |
7.2% n = 57,048 |
| Generalized Anxiety Disorder | 4.2% n = 141,539 |
3.8% n = 57,048 |
| Negative Affect Score [Mean (SD)] | 20.71 (13.12) n = 139,217 |
20.70 (5.22) n = 57,964 |
| Positive Affect Score [Mean (SD)] | 35.37 (4.25) n = 139,217 |
35.37 (4.19) n = 57,964 |
| C-reactive protein level (mg/L), [Median (IQR), Mean (SD)] | 1.2 (.60, 2.80), 2.61 (4.76) n = 55,098 |
1.2 (2.2) 2.62 (4.60) n = 23,607 |
| First follow up | ||
| Any Depressive Disorder (Current Major Depression or Dysthymia) | 4.1% n = 77,758 |
Not used |
| Major Depressive Episode (Current) | 3.0% n = 77,758 |
Not used |
| Any Anxiety Disorder (panic disorder, agoraphobia, social phobia, or GAD ) | 8.3% n = 77,758 |
Not used |
| Generalized Anxiety Disorder | 5.9% n = 77,758 |
Not used |
| Cogstate: Episodic Memory (Accuracy), Mean (SD) | 0.96 (0.12) n = 84,819 |
0.96 (0.12) n = 36,798 |
| Cogstate: Working Memory (Accuracy), Mean (SD) | 1.31 (0.19) n = 83,721 |
1.32 (0.19) n = 36,363 |
| Cogstate: Visual Attention (Response Time), Mean (SD) | 2.69 (0.09) n = 82,175 |
2.68 (0.09) n = 35,743 |
| Cogstate: Psychomotor Speed (Response Time), Mean (SD) | 2.56 (0.16) n = 81,139 |
2.55 (0.16) n = 35,314 |
Lower = no education, primary education, lower/preparatory vocational education, lower general secondary education; Moderate = intermediate vocational education/apprenticeship, higher secondary education; Higher = higher vocational education, university; IQR = Inter Quartile Range.
Table 3.
Bivariate Correlations of Study Variables for 147,815 Participants
| Measure | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | 20. | 21. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. T1 CRP (log-transformed) | −0.03 | −0.03 | 0.04 | 0.03 | −0.07 | 0.04 | 0.03 | 0.03 | −0.05 | 0.03 | 0.14 | 0.11 | 0.36 | 0.10 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 |
| 2. T2 Episodic MemoryB | - | 0.31 | −0.16 | −0.16 | 0.21 | −0.02 | −0.03 | −0.05 | −0.02 | −0.19 | 0.01 | 0.22 | −0.08 | −0.04 | −0.02 | −0.01 | −0.03 | −0.03 | −0.01 | −0.01 |
| 3. T2 Working MemoryB | - | −0.17 | −0.17 | 0.20 | −0.02 | −0.02 | −0.04 | .00a | −0.20 | −.01a | −0.2 | −0.07 | −0.04 | −0.02 | 0 | −0.03 | −0.02 | −0.01 | −0.01 | |
| 4. T2 Psychomotor SpeedC | - | 0.63 | −0.30 | .01a | .00a | 0.04 | .00a | 0.39 | .00a | 0.21 | 0.08 | 0.04 | 0.01 | −0.02 | 0.01 | 0 | −0.01 | −0.02 | ||
| 5. T2 Visual AttentionC | - | −0.34 | 0.01 | .00a | 0.05 | −0.02 | 0.43 | −0.01 | 0.20 | 0.09 | 0.05 | 0.02 | −0.01 | 0.02 | 0.01 | 0.01 | −0.01 | |||
| 6. T1 RFFT | - | −0.04 | −0.02 | −0.07 | −0.07 | −0.32 | 0.03 | −0.35 | −0.11 | −0.08 | −0.04 | −0.01 | −0.04 | −0.02 | −0.02 | 0.01 | ||||
| 7. T1 Depression | - | 0.25 | 0.27 | −0.2 | −0.02 | 0.05 | 0.07 | 0.04 | 0.07 | 0.35 | 0.19 | 0.8 | 0.21 | 0.40 | 0.18 | |||||
| 8. T2 Depression | - | 0.21 | −0.13 | −0.05 | 0.03 | 0.05 | 0.03 | 0.05 | 0.20 | 0.41 | 0.22 | 0.83 | 0.21 | 0.42 | ||||||
| 9. T1 Negative Affect | - | −0.21 | −0.05 | 0.17 | 0.08 | −0.01 | 0.06 | 0.32 | 0.26 | 0.25 | 0.17 | 0.30 | 0.22 | |||||||
| 10. T1 Positive Affect | - | −0.02 | −0.01 | −0.1 | −0.02 | −0.02 | −0.17 | −0.13 | −0.18 | −0.1 | −0.18 | −0.11 | ||||||||
| 11. T1 Age | - | −0.04 | 0.23 | 0.19 | 0.09 | −0.01 | −0.06 | −0.01 | −0.04 | −0.04 | −0.06 | |||||||||
| 12. T1 Female | - | 0.02 | −0.06 | 0.03 | 0.07 | 0.07 | 0.04 | 0.03 | 0.05 | 0.06 | ||||||||||
| 13. T1 Education | - | 0.17 | 0.08 | 0.06 | 0.03 | 0.07 | 0.05 | 0.04 | 0.02 | |||||||||||
| 14. T1 Body mass index | - | 0.13 | 0.03 | 0.01 | 0.04 | 0.03 | 0.02 | 0.01 | ||||||||||||
| 15. T1 Health Status | - | 0.07 | 0.04 | 0.06 | 0.05 | 0.05 | 0.04 | |||||||||||||
| 16. T1 ANX | - | 0.28 | 0.31 | 0.17 | 0.72 | 0.2 | ||||||||||||||
| 17. T2 ANX | - | 0.17 | 0.37 | 0.22 | 0.84 | |||||||||||||||
| 18. T1 MDE | - | 0.20 | 0.38 | 0.16 | ||||||||||||||||
| 19. T2 MDE | - | 0.17 | 0.40 | |||||||||||||||||
| 20. T1 GAD | - | 0.21 | ||||||||||||||||||
| 21. T2 GAD | - |
Probability
= P >.05;
= higher values equal better performance;
= higher values equal poorer performance; T1 = Time 1 (Baseline); T2 = Time 2 (First Follow-up); RFFT = Ruff Figural Fluency Test; Health Status. = Number of Medical Conditions Reported; for values ≤ .001 and ≥−.001, values were rounded to 0.
3.1. Association of CRP with affect, depressive and anxiety disorders, and cognitive task performance
The association of (log-transformed) CRP with: (i) clinical outcomes (i.e., MDD, any depressive disorder, GAD, any anxiety disorder), (ii) positive and negative affect, and (iii) five cognitive measures [RFFT (executive functioning), detection task (psychomotor speed), identification task (attention), one-back task (working memory), and one card learning task (episodic memory)] are illustrated in Table 4, both unadjusted and adjusted for covariates. Notably, CRP was associated with a greater likelihood of meeting criteria for a range of clinical outcomes, with a numerically greater likelihood consistently reported for depression as compared to anxiety at baseline and first follow-up assessment. However, these associations were attenuated after controlling for confounding by age, sex, education, health status, and BMI. Higher CRP was also associated with higher negative affect, lower positive affect, and worse cognitive task performance, although the magnitude of associations was generally very small and negligible after controlling for covariates.
Table 4. Associations of CRP levels with affect, depressive and anxiety disorders, and cognitive task performance in the Lifelines cohort.
Please note: point estimates do not include 95% confidence intervals (and N, p-value) as we do not currently have access to the Lifelines Cohort Workspace, the Cohort will allow us access to data for response to reviewer comments, we will add these (p-value, 95% CI, N) as necessary during review.
| Baseline | Follow-up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | MDD | Any DEP | GAD | Any ANX | Negative Affect | Positive Affect | RFFT | MDD | Any DEP | GAD | Any ANX | Psycho motor Speed | Attention | Episodic Memory | Working Memory |
| Odd’s Ratio | Standardized regression coefficient | Odd’s Ratio | Standardized regression coefficient | ||||||||||||
| Model 1 (Unadjusted analysis) | |||||||||||||||
| CRPa | 1.59 | 1.57 | 1.24 | 1.29 | .03 | −.05 | −.07 | 1.43 | 1.42 | 1.26 | 1.22 | .03 | .03 | −.03 | −.03 |
| Model 2 (Adjusted for age, sex, education, health status, and BMI) | |||||||||||||||
| CRPa | 1.02† | 1.22 | 1.01† | 1.06† | .01 | −.04 | −.03 | 1.06† | 1.07† | 1.05† | 1.03† | .01 | .01 | 0† | −.01† |
| Age | .98 | .98 | .98 | .99 | −.06 | 0† | −.24 | .97 | .97 | .97 | .98 | .38 | .38 | −.14 | −.17 |
| Female | 1.53 | 1.59 | 1.56 | 1.66 | .17 | 0† | .04 | 1.28 | 1.33 | 1.58 | 1.6 | .01† | .01† | 0† | −.01 |
| Education: High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Education: Moderate | 1.62 | 1.76 | 1.3 | 1.28 | .04 | −.04 | −.19 | 1.39 | 1.31 | 1.1† | 1.12 | .07 | .07 | −.13 | −.08 |
| Education: Low | 2.94 | 3.02 | 1.64 | 1.72 | .11 | −.11 | −.35 | 2.16 | 1.84 | 1.26 | 1.3 | .16 | .16 | −.22 | −.18 |
| Health Status: O Dx | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Health Status: 1 Dx | 1.83 | 1.64 | 1.55 | 1.43 | .04 | −.01 | −.02 | 1.5 | 1.5 | 1.36 | 1.29 | 0† | 0† | 0† | −.01† |
| Health Status: 2 Dx | 2.97 | 2.63 | 2.26 | 1.82 | .04 | 0† | −.02 | 2.5 | 2.34 | 1.67 | 1.47 | 0† | 0† | −.01 | −.02 |
| Health Status: 3+ Dx | 4.24 | 3.76 | 2.82 | 2.65 | .02 | −.01 | −.01 | 3.71 | 3.28 | 2 | 1.67 | 0† | 0† | −.01 | 0† |
| BMI | 1.04 | 1.03 | 1.01 | 1.01 | 0† | 0† | 0† | 1.05 | 1.04 | 1.02 | 1.02 | 0† | 0† | −.03 | −.01† |
CRP = C-reactive Protein; MDD = Major Depressive Disorder; Any DEP = MDD or Dysthymia; GAD = Generalized Anxiety Disorder; Any ANX = panic disorder, agoraphobia, social phobia, or GAD; RFFT = Ruff Figural Fluency Test;
= log-transformed variable; BMI = Body Mass Index; Ref = Reference Category for categorical variables;
= p >.05; Odds ratios are reported in logistic regression predicting binary outcomes and standardized beta coefficients are reported for linear regression; For values ≤ .001 and ≥−.001, values were rounded to 0.
3.2. Associations of GRSs for inflammatory markers with affect, depressive and anxiety disorders
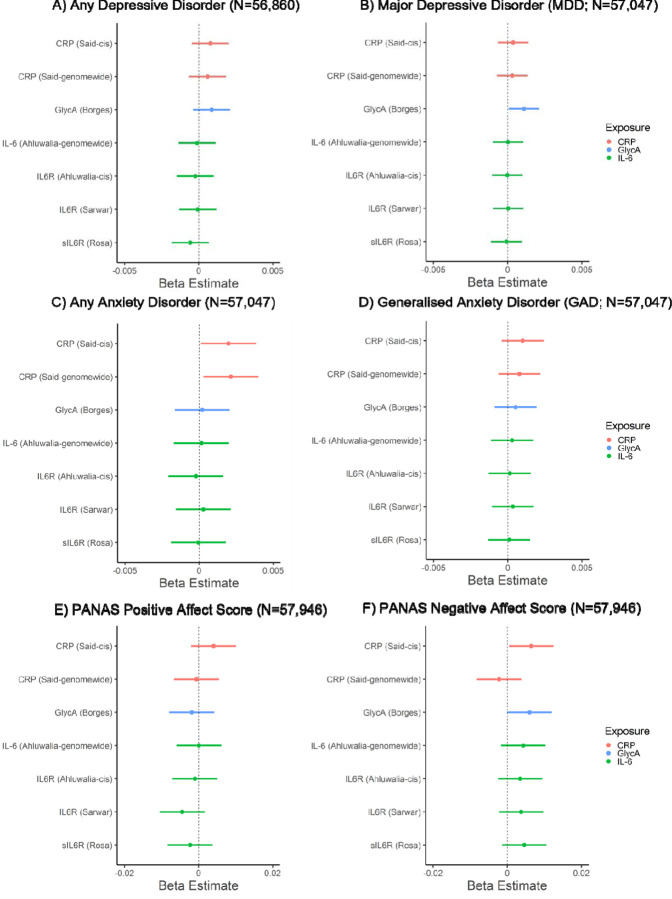
In the primary analysis, CRPGRS (cis) was associated with a higher negative affect score (beta: 0.006; 95% CI: 0.0005 to 0.012, p=0.035, N=57,946) and increased risk of any anxiety disorder (beta: 0.002, 95% CI: 0.0001 to 0.004, p=0.037, N=57,047). GlycAGRS was associated with higher negative affect score (beta: 0.006, 95% CI: 0.00002 to 0.012, p=0.049; N=57,946) and increased risk of MDD (beta: 0.001, 95% CI: 0.0001 to 0.002; p=0.036; N=57,047). Other inflammatory marker GRSs were not associated with depressive/anxiety disorders or affect scores (ps ≥ 0.15). In the secondary analysis, there was evidence that CRPGRS (genome-wide) was associated with increased risk of any anxiety disorders (beta: 0.002, 95% CI: 0.0003 to 0.004, p=0.023, N=57,047). There was little evidence that other inflammatory marker GRSs were associated with depressive/anxiety disorders or affect (ps ≥ 0.16). For all results, see Figure 1 and Supplementary Table 4. All sensitivity analyses removing differing degrees of related individuals (up to 1st-degree, up to 2nd-degree, up to 3rd-degree) within chips (non-GRAMMAR method) did not substantially alter results, see Supplementary Tables 7-12. Although, there was slightly stronger evidence for associations between GlycAGRS and negative affect score (ps ≤ 0.015) and between CRPGRS (cis) and negative affect score (ps ≤ 0.033).
Figure 1.
Associations of genetic risk scores for inflammatory markers with mood, anxiety disorders and positive and negative affect scores.
3.3. Association of GRS for inflammatory markers and cognitive task performance
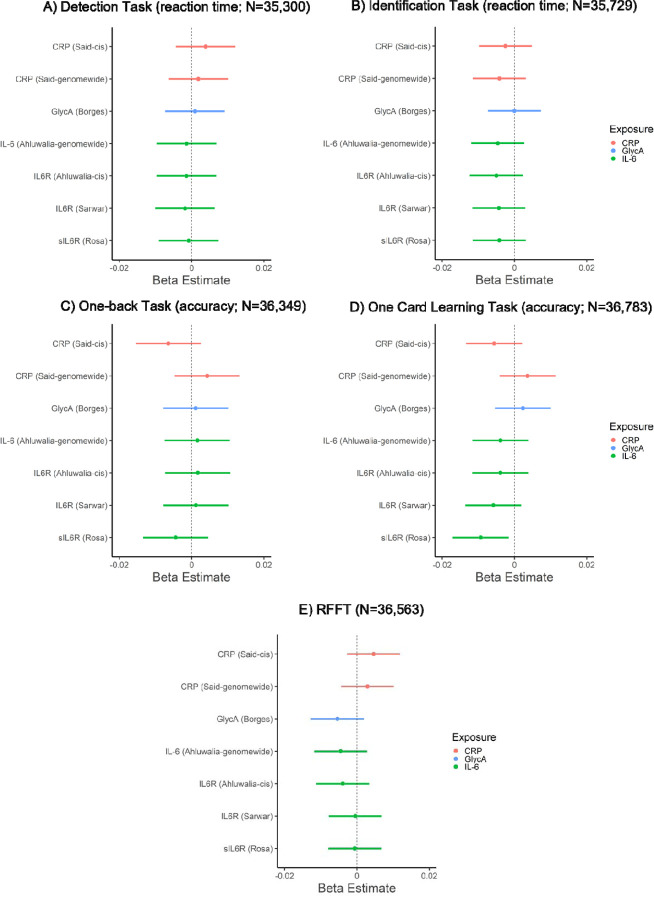
In primary analyses, inflammatory marker GRSs were not associated with performance on cognitive tasks (ps ≥ 0.14), except for sIL-6RGRS which was negatively associated with episodic memory performance (one card learning task accuracy; beta: −0.009, 95% CI: −0.017 to −0.002, p=0.018, N=36,783), see Figure 2 and Supplementary Table 4. In secondary analyses, inflammatory markers GRSs (genome-wide) were not associated with performance on cognitive tasks (ps ≥ 0.22). For all results, see Figure 2 and Supplementary Table 4. Sensitivity analyses after removing related individuals within chips (non-GRAMMAR method) did not alter the results, see Supplementary Tables 10-12.
Figure 2.
Associations of genetic risk scores for inflammatory markers with cognitive task performance.
3.4. Testing potential causality between CRP, negative affect, and anxiety disorders using Mendelian randomization with individual level data
CRP genetic instruments had F-statistics > 10 (158 for cis GRS, 1045 for genome-wide GRS), indicating adequate instrument strength (91). For tests on the MR assumptions, see Supplementary Results 2.2. There was weak evidence that genetically-proxied CRP (cis) causally increased the risk of any anxiety disorders (beta: 0.12, p=0.054, N=22,154), and little evidence on negative affect (beta: 0.27, p=0.16; N=23,268). Sensitivity analysis removing related individuals did not alter overall conclusions.
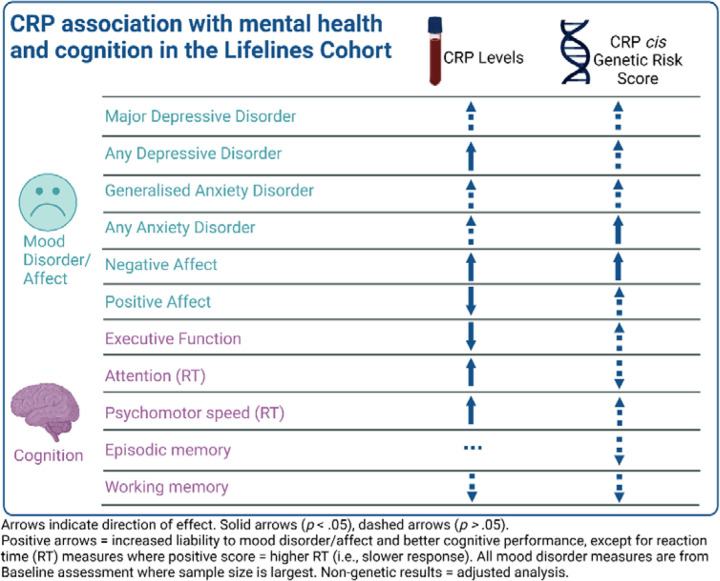
The overall pattern of results for the non-genetic and genetic analyses are visualized in Figure 3.
Figure 3.
Visualisation of the overall pattern of results for CRP in the cohort and genetic analyses.
4. Discussion
We conducted complementary non-genetic and genetic analyses to interrogate the relationship between inflammatory markers and affect, depressive and anxiety disorders, and cognitive task performance using data from the Lifelines cohort. In non-genetic analyses, higher CRP was associated with diagnosis of any depressive disorder, positive and negative affect scores, figural fluency, attention, and psychomotor speed after adjusting for potential confounders, although the magnitude of these associations was generally small. In genetic analyses, genetic risk scores for CRP (CRPGRS) and GlycAGRS were both associated with higher negative affect score. CRPGRS was associated with any anxiety disorder whereas GlycAGRS was associated with major depressive disorder. Inflammatory marker GRSs were not associated with cognitive task performance, except for soluble IL-6RGRS which was associated with poorer memory performance. Individual level MR provided weak evidence for a causal effect of CRP on any anxiety disorder. Genetic and non-genetic analyses provided consistent evidence for an association, albeit small, of CRP on negative affect. Genetic analyses suggest that IL-6 signaling could be relevant for memory, and that the association between CRP and anxiety disorders could be potentially causal.
4.1. Affect
Prior studies have generally found inflammation to be associated with higher levels of negative affect and lower levels of positive affect, although findings are primarily based on medical populations (92–94) and small community samples (15, 95, 96). To our knowledge, this is the first large, population-based study to find small but consistent associations of higher CRP with higher negative affect and lower positive affect, both unadjusted and adjusted for age, sex, education, health status and BMI. Interestingly, both CRP and GlycA genetic risk scores were associated with higher levels of negative affect, but not positive affect. This consistent association across non-genetic and genetic analyses may reflect the effect of inflammation on a range of emotional states beyond the cardinal features of depression (i.e., sadness/anhedonia), which aligns with prior research linking inflammation with fear and irritability (63, 97). Prior work has shown that inflammation is differentially associated with a specific clinical presentation characterized by anhedonia and somatic/neurovegetative symptoms (e.g., fatigue, altered sleep and appetite changes) and further work is needed that more accurately characterize an inflammatory phenotype in depression (98, 99).
4.2. Depression
These data add to a growing body of evidence evaluating the role of inflammation as measured by circulating CRP levels in the etiology of depression. The results of non-genetic analyses broadly aligns with results from the UK Biobank cohort in terms of (i) prevalence estimates of depression and anxiety, (ii) robust univariate associations between CRP and depression and anxiety, which were generally no longer statistically significant when controlling for covariates, and (iii) stronger univariate associations for CRP and depression when compared to anxiety (45). It has long been noted that variables being conceptualized as confounds that require statistical adjustment (e.g., BMI, medical illness) may, in fact, be key mechanisms in the pathophysiology of inflammatory depression (100, 101). As such, attenuation of associations following adjustment for covariates would not, by itself, indicate a non-causal relationship. Indeed, inflammation may increase risk for depression via increasing the risk of inflammation-related physical multimorbidity (e.g., cardiovascular disease) (42) – a hypothesis that requires further investigation.
In genetic analyses, there was also little evidence of an association between CRPGRS and depression outcomes, although there was evidence suggesting GlycAGRS increases liability to MDD. The null CRP findings are consistent with previous MR studies showing no evidence of effect in MDD (54, 55, 102). However, the MR literature of CRP on depression is mixed with some studies reporting CRP to decrease (45) or increase (42) risk for depression. It is unclear what accounts for these mixed findings, but potential factors may include CRP SNP selection, definition and/or measurement of depression, statistical power, and selection bias (see Supplementary Discussion). In contrast, MR studies have shown more consistent findings for the potential causal role of IL-6 on depression (43–45, 103). This is similar to MR findings for coronary heart disease, where IL-6 but not CRP have been shown to play a potential causal role (104, 105). Consequently, studies on a broader range of immune markers (e.g. cytokines, immune cells) and specific immune pathways would be more useful to understand the role of inflammation in depression, rather than CRP which is a non-specific marker of systemic immune activation (106).
4.3. Cognitive Task Performance
We observed relatively small effects of CRP on cognitive task performance, and in genetic analysis only sIL-6RGRS was associated with poor memory performance out of all inflammatory markers and cognitive tasks examined. Our findings contribute to inconsistent findings across population-based cohorts assessing circulating inflammatory biomarkers and cognitive performance where associations observed in population-based studies (22, 30, 107) often are not large in magnitude or consistently observed (56). Few MR studies have been conducted on the role of inflammation on cognition. Consistent with results presented here, our previous MR study examining the role of the same inflammatory markers (i.e., CRP, IL-6, IL-6R, sIL-6R, GlycA) on specific executive functions within the ALSPAC cohort (e.g., emotion recognition, working memory, response inhibition (56)) found little evidence of a potential causal effect. However, Pagoni et al. recently reported that other cytokines and chemokines (i.e., Eotaxin, IL-8, MCP1) may be causally related to lower fluid intelligence (and IL-4 with higher fluid intelligence) (57). The finding regarding sIL-6R and memory performance is novel and would align with convergent evidence that trans-signaling – in which sIL-6R plays a critical role – may be responsible for the deleterious effect of IL-6 on cognitive functioning (47, 108).
Interpreting the relationship between inflammation and cognitive task performance in population-based studies is difficult for several reasons. First, there is considerable heterogeneity in the type and breadth of cognitive abilities assessed across studies and there is a need for future studies to more uniformly include well-validated measures assessing individual differences [rather than detecting pathological states (e.g., dementia, epilepsy)] that characterize a broad range of cognitive functions (109). There is a similar need to measure and conceptualize the impact that inflammation has on other psychological functions that impact cognition (e.g., reward process, aversive process) – there is strong theoretical work and empirical data to support an indirect effect of inflammation on cognition via, for instance, dysregulated reward circuitry that impact performance on cognitive tasks via decreased motivation or increased fatigue (110). Moreover, there are a range of sociodemographic factors that may moderate the association between inflammation and cognition – prior work has found that inflammation and cognition may differ based on age and sex (111, 112). It is reasonable to assume, for instance, that modest increases in inflammation may exert a cumulative effect across the lifespan, and thus may only be detected later in life and/or in specific domains of cognition.
4.4. Anxiety
In the non-genetic analyses, circulating CRP levels was associated with a modestly increased likelihood of meeting criteria for anxiety disorders, although this association was substantially attenuated following adjustments for covariates. Prior research in population-based cohorts have found CRP to associated with an increased risk for anxiety disorders (113, 114), although results are inconsistent and other studies indicate that anxiety prospectively predicts an increase in circulating CRP levels (115). The MR analysis suggests a potential causal role of CRP on any anxiety disorder (which covers a broad range of anxiety-related conditions including panic disorder, social phobia, agoraphobia, GAD). Prior theory has primarily focused on anxiety as a cause of inflammation [see O’Donovan et al. for an excellent review (116)]; however, alternative theories suggest that inflammatory physiology is implicated in both sickness behaviors (e.g., anhedonia, social withdrawal) and anxiety arousal and alarm (117), which would align with the results presented here.
4.5. Limitations
In this study we used a large and broadly representative population-based sample, and we employed a triangulation of methods (non-genetic and genetic analyses) which increases confidence in the inferences drawn. Nevertheless, results should be considered in the context of the limitations of the study. First, although broadly representative, like other cohort studies (e.g., UK Biobank), the Lifelines cohort predominantly includes individuals of European descent and is less representative of individuals from low socioeconomic status (118), which consequentially limits the generalizability of findings. Second, analyses were not corrected for multiple comparisons. To check effects are not due to Type 1 errors, there is a need to replicate these findings in other cohorts. Moreover, as effect sizes reported are small and reflect associations in the general population, there is a need for studies to investigate whether there are sub-groups for whom these associations may be larger (e.g., older age, clinical populations). Third, in the genetic analysis the CRP GRS explained 1–4% of the variance in CRP [a level of variance consistent with similar analyses in the ALSPAC cohort (56)] and few cases of depression were observed in Lifelines [although the point prevalence of approximately 4% is consistent with reported population point prevalence estimates (119)]. It is possible that this limited our capacity to detect potential causal effects, were they small in magnitude or non-linear. Fourth, the CogState tasks used in the current study may not be optimal for detecting individual differences in healthy individuals, or even in some conditions such as depression; multiple studies have shown that the CogState tasks used in this study do not improve in successful antidepressant trials, even when improvement in other cognitive measures are observed (120–122). Fifth, although we include multiple instruments related to IL-6 (i.e., genetic variants related to IL-6 and sIL-6R levels), most instruments contain few genetic variants (≤ 3 SNPs) and genetic variants for IL-6 and sIL-6R overlap. While sIL-6R is involved in IL-6 trans-signaling, the overlap of SNPs makes it challenging to interpret the effect of these genetic variants on different immune phenotypes specifically (i.e., IL-6 levels vs IL-6 signaling). Future studies are needed to (1) better understand the biological role of these genetic variants and (2) develop instruments proxying specific IL-6 signaling pathways including IL-6 trans-signaling. Finally, it is worth considering that some instruments in the genetic analyses were associated with potential confounds, although it is unlikely that the small effects observed for some confounds substantially bias parameter estimates.
4.6. Conclusions
Genetic and non-genetic analyses provide consistent evidence for a modest effect of CRP on negative affect. Genetic analyses suggest that IL-6 signaling could be relevant for memory, and that the association between CRP and anxiety disorders could be causal. Overall, these results suggest that inflammation may affect a range of emotional states beyond the cardinal features of depression. However, given the small effect sizes and multiple tests conducted, future studies are required to investigate whether effects are moderated by sub-groups and whether these findings replicate in other cohorts.
Supplementary Material
Acknowledgments
The Lifelines initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG), Groningen University and the Provinces in the North of the Netherlands (Drenthe, Friesland, Groningen). The generation and management of GWAS genotype data for the Lifelines Cohort Study is supported by the UMCG Genetics Lifelines Initiative (UGLI). UGLI is partly supported by a Spinoza Grant from NWO, awarded to Cisca Wijmenga. The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centers delivering data to Lifelines, and all the study participants.
Funding
This work was supported by Harvard University’s Mind Brain Behavior Interfaculty Initiative and National Institute of Mental Health grant K23MH132893 (Dr. Mac Giollabhui), and a UK Medical Research Council (MRC) grant to GMK (MC_UU_00032/06) which forms part of the Integrative Epidemiology Unit (IEU) at the University of Bristol. The MRC grant also supports CS and EMF. GMK acknowledges additional funding support from the Wellcome Trust (201486/Z/16/Z and 201486/B/16/Z), MRC (MR/W014416/1; MR/S037675/1; and MR/Z50354X/1), and the UK National Institute of Health and Care Research (NIHR) Bristol Biomedical Research Centre (NIHR 203315). GH and GDS are supported by the MRC (MC_UU_00032/01), and the NIHR Bristol Biomedical Research Centre (NIHR 203315). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care, UK.
Funding Statement
This work was supported by Harvard University’s Mind Brain Behavior Interfaculty Initiative and National Institute of Mental Health grant K23MH132893 (Dr. Mac Giollabhui), and a UK Medical Research Council (MRC) grant to GMK (MC_UU_00032/06) which forms part of the Integrative Epidemiology Unit (IEU) at the University of Bristol. The MRC grant also supports CS and EMF. GMK acknowledges additional funding support from the Wellcome Trust (201486/Z/16/Z and 201486/B/16/Z), MRC (MR/W014416/1; MR/S037675/1; and MR/Z50354X/1), and the UK National Institute of Health and Care Research (NIHR) Bristol Biomedical Research Centre (NIHR 203315). GH and GDS are supported by the MRC (MC_UU_00032/01), and the NIHR Bristol Biomedical Research Centre (NIHR 203315). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care, UK.
Footnotes
Conflict of Interest
No conflicts of interest were reported.
Contributor Information
Naoise Mac Giollabhui, Depression Clinical & Research Program, Department of Psychiatry, Massachusetts General Hospital, USA..
Chloe Slaney, MRC Integrative Epidemiology Unit at the University of Bristol, UK; Centre for Academic Mental Health, Bristol Medical School, University of Bristol, Bristol, UK.
Gibran Hemani, MRC Integrative Epidemiology Unit at the University of Bristol, UK.
Éimear M. Foley, MRC Integrative Epidemiology Unit at the University of Bristol, UK; Centre for Academic Mental Health, Bristol Medical School, University of Bristol, Bristol, UK.
Peter J. van der Most, University of Groningen, University Medical Center Groningen, the Netherlands.
Ilja M. Nolte, University of Groningen, University Medical Center Groningen, the Netherlands.
Harold Snieder, University of Groningen, University Medical Center Groningen, the Netherlands..
George Davey Smith, MRC Integrative Epidemiology Unit at the University of Bristol, UK..
Golam Khandaker, MRC Integrative Epidemiology Unit at the University of Bristol, Bristol, UK; Centre for Academic Mental Health, Bristol Medical School, University of Bristol, Bristol, UK; NIHR Bristol Biomedical Research Centre, University Hospitals Bristol and Weston NHS Foundation Trust and University of Bristol, Bristol, UK; Avon and Wiltshire Mental Health Partnership NHS Trust, Bristol, UK..
Catharina A. Hartman, Interdisciplinary Center Psychopathology and Emotion Regulation, Department of Psychiatry, University Medical Center Groningen, University of Groningen, the Netherlands..
References
- 1.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. (2020): Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 396:1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Charlson FJ, Norman RE, Flaxman AD, Patten SB, Vos T, et al. (2013): The epidemiological modelling of major depressive disorder: application for the Global Burden of Disease Study 2010. PLoS One. 8:e69637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. (2018): Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor CA, Bouldin ED, McGuire LC (2018): Subjective Cognitive Decline Among Adults Aged ≥45 Years - United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 67:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janelsins MC, Kesler SR, Ahles TA, Morrow GR (2014): Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 26:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. (2022): Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 101:93–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Liu M, Lu Q, Farrell M, Lappin JM, Shi J, et al. (2020): Global prevalence and burden of HIV-associated neurocognitive disorder. A meta-analysis. 95:e2610–e2621. [DOI] [PubMed] [Google Scholar]
- 8.Barreira DP, Marinho RT, Bicho M, Fialho R, Ouakinin SRS (2018): Psychosocial and Neurocognitive Factors Associated With Hepatitis C - Implications for Future Health and Wellbeing. Front Psychol. 9:2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fava M, Mahableshwarkar AR, Jacobson W, Zhong W, Keefe RS, Olsen CK, et al. (2018): What is the overlap between subjective and objective cognitive impairments in MDD? Ann Clin Psychiatry. 30:176–184. [PubMed] [Google Scholar]
- 10.Keefe RS, Harvey PD (2012): Cognitive impairment in schizophrenia. Novel antischizophrenia treatments.11–37. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. (2006): Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 163:28–40. [DOI] [PubMed] [Google Scholar]
- 12.Ritchie K, Chan D (2021): The emergence of cognitive COVID. World Psychiatry. 20:52–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fesharaki-Zadeh A, Lowe N, Arnsten AFT (2023): Clinical experience with the α2A-adrenoceptor agonist, guanfacine, and N-acetylcysteine for the treatment of cognitive deficits in “Long-COVID19”. Neuroimmunology Reports. 3:100154. [Google Scholar]
- 14.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. (2010): A meta-analysis of cytokines in major depression. Biol Psychiatry. 67:446–457. [DOI] [PubMed] [Google Scholar]
- 15.Ironson G, Banerjee N, Fitch C, Krause N (2018): Positive emotional well-being, health Behaviors, and inflammation measured by C-Reactive protein. Soc Sci Med. 197:235–243. [DOI] [PubMed] [Google Scholar]
- 16.Felger JC, Treadway MT (2017): Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology. 42:216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB (2021): The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 26:3302–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014): Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA (2010): Association of C-reactive protein with cognitive impairment. Arch Neurol. 67:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baune B, Ponath G, Golledge J, Varga G (2008): Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol Aging. 29:937–944. [DOI] [PubMed] [Google Scholar]
- 21.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. (2001): Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 58:445–452. [DOI] [PubMed] [Google Scholar]
- 22.Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, et al. (2014): Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 83:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisan AF, Oancea C, Timar B, Fira-Mladinescu O, Crisan A, Tudorache V (2014): Cognitive impairment in chronic obstructive pulmonary disease. PLoS One. 9:e102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang YS, Guilleminault C, Hwang FM, Cheng C, Lin CH, Li HY, et al. (2016): Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine. 95:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Robertson CM, Yu X, Cheypesh A, Dinu IA, Li J (2014): Early postoperative systemic inflammatory response is an important determinant for adverse 2-year neurodevelopment-associated outcomes after the Norwood procedure. J Thorac Cardiovasc Surg. 148:202–206. [DOI] [PubMed] [Google Scholar]
- 26.Nightingale S, Dreyer AJ, Saylor D, Gisslén M, Winston A, Joska JA (2021): Moving on From HAND: Why We Need New Criteria for Cognitive Impairment in Persons Living With Human Immunodeficiency Virus and a Proposed Way Forward. Clin Infect Dis. 73:1113–1118. [DOI] [PubMed] [Google Scholar]
- 27.Mac Giollabhui N (2021): Inflammation and depression: Research designs to better understand the mechanistic relationships between depression, inflammation, cognitive dysfunction, and their shared risk factors. Brain Behav Immun Health. 15:100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krogh J, Benros ME, Jorgensen MB, Vesterager L, Elfving B, Nordentoft M (2014): The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav Immun. 35:70–76. [DOI] [PubMed] [Google Scholar]
- 29.Goldsmith DR, Haroon E, Woolwine BJ, Jung MY, Wommack EC, Harvey PD, et al. (2016): Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav Immun. 56:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mac Giollabhui N, Alloy LB, Hartman CA (2021): Investigating whether depressed youth exhibiting elevated C reactive protein perform worse on measures of executive functioning, verbal fluency and episodic memory in a large, population based sample of Dutch adolescents. Brain Behav Immun. 94:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro-Santos R, Lucio Teixeira A, Vinicius Salgado J (2014): Evidence for an immune role on cognition in schizophrenia: a systematic review. Curr Neuropharmacol. 12:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mac Giollabhui N, Swistun D, Murray S, Moriarity DP, Kautz MM, Ellman LM, et al. (2020): Executive dysfunction in depression in adolescence: the role of inflammation and higher body mass. Psychol Med. 50:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAfoose J, Baune BT (2009): Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 33:355–366. [DOI] [PubMed] [Google Scholar]
- 34.Carvalho AF, Miskowiak KK, Hyphantis TN, Kohler CA, Alves GS, Bortolato B, et al. (2014): Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol Disord Drug Targets. 13:1819–1835. [DOI] [PubMed] [Google Scholar]
- 35.Chesnokova V, Pechnick RN, Wawrowsky K (2016): Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun. 58:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldsmith D, Bekhbat M, Mehta ND, Felger JC (2023): Inflammation-related functional and structural dysconnectivity as a pathway to psychopathology. Biol Psychiatry. 93:405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010): Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 68:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD (2008): Peripheral Inflammation is Associated with Altered Substantia Nigra Activity and Psychomotor Slowing in Humans. Biol Psychiatry. 63:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison NA, Cercignani M, Voon V, Critchley HD (2015): Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 40:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davey Smith G, Ebrahim S (2003): ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 32:1–22. [DOI] [PubMed] [Google Scholar]
- 41.Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. (2022): Mendelian randomization. Nat Rev Methods Primers. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khandaker GM, Zuber V, Rees JMB, Carvalho L, Mason AM, Foley CN, et al. (2020): Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry. 25:1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perry BI, Upthegrove R, Kappelmann N, Jones PB, Burgess S, Khandaker GM (2021): Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: A bi-directional two-sample mendelian randomization study. Brain Behav Immun. 97:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly KM, Smith JA, Mezuk B (2021): Depression and interleukin-6 signaling: A Mendelian Randomization study. Brain Behav Immun. 95:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Z, Kappelmann N, Moser S, Smith GD, Burgess S, Jones PB, et al. (2021): Role of Inflammation in Depression and Anxiety: Tests for Disorder Specificity, Linearity and Potential Causality of Association in the UK Biobank. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunter CA, Jones SA (2015): IL-6 as a keystone cytokine in health and disease. Nat Immunol. 16:448–457. [DOI] [PubMed] [Google Scholar]
- 47.Rose-John S, Jenkins BJ, Garbers C, Moll JM, Scheller J (2023): Targeting IL-6 trans-signalling: past, present and future prospects. Nat Rev Immunol. 23:666–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crick DC, Khandaker GM, Halligan SL, Burgner D, Mansell T, Fraser A (2023): Comparison of the stability of glycoprotein acetyls and high sensitivity C-reactive protein as markers of chronic inflammation. Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connelly MA, Otvos JD, Shalaurova I, Playford MP, Mehta NN (2017): GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med. 15:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, et al. (2015): GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin Chem. 61:714–723. [DOI] [PubMed] [Google Scholar]
- 51.Ritchie Scott C, Würtz P, Nath Artika P, Abraham G, Havulinna Aki S, Fearnley Liam G, et al. (2015): The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Systems. 1:293–301. [DOI] [PubMed] [Google Scholar]
- 52.Kappelmann N, Arloth J, Georgakis MK, Czamara D, Rost N, Ligthart S, et al. (2020): Dissecting the Association Between Inflammation, Metabolic Dysregulation, and Specific Depressive Symptoms: A Genetic Correlation and 2-Sample Mendelian Randomization Study. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crick DCP, Sanderson E, Jones H, Goulding N, Borges MC, Clayton G, et al. (2023): Glycoprotein acetyls and depression: Testing for directionality and potential causality using longitudinal data and Mendelian randomization analyses. J Affect Disord. 335:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bekkevold O-J, Damås JK, Brumpton BM, Åsvold BO (2023): The causal role of C-reactive protein and interleukin-6 on anxiety and depression symptoms and life satisfaction: Mendelian randomisation analyses in the HUNT study. Psychol Med.1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galan D, Perry BI, Warrier V, Davidson CC, Stupart O, Easton D, et al. (2022): Applying Mendelian randomization to appraise causality in relationships between smoking, depression and inflammation. Sci Rep. 12:15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slaney C, Sallis HM, Jones HJ, Dardani C, Tilling K, Munafo MR, et al. (2023): Association between inflammation and cognition: Triangulation of evidence using a population-based cohort and Mendelian randomization analyses. Brain Behav Immun. 110:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pagoni P, Korologou-Linden RS, Howe LD, Davey Smith G, Ben-Shlomo Y, Stergiakouli E, et al. (2022): Causal effects of circulating cytokine concentrations on risk of Alzheimer’s disease and cognitive function. Brain Behav Immun. 104:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleshner M, Frank M, Maier SF (2017): Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology. 42:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slavich GM (2020): Social safety theory: a biologically based evolutionary perspective on life stress, health, and behavior. Annu Rev Clin Psychol. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy K, Weaver C (2017): Janeway’s Immunobiology (9th Edition). New York, New York: Garland Science, Taylor & Francis Group. [Google Scholar]
- 61.Kiecolt-Glaser JK, Derry HM, Fagundes CP (2015): Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 172:1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller AH, Raison CL (2016): The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slavich GM, Irwin MR (2014): From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 140:774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM, et al. (2015): Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 44:1172–1180. [DOI] [PubMed] [Google Scholar]
- 65.Sijtsma A, Rienks J, van der Harst P, Navis G, Rosmalen JGM, Dotinga A (2021): Cohort Profile Update: Lifelines, a three-generation cohort study and biobank. Int J Epidemiol. 51:e295–e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ross TP (2014): The reliability and convergent and divergent validity of the Ruff Figural Fluency Test in healthy young adults. Arch Clin Neuropsychol. 29:806–817. [DOI] [PubMed] [Google Scholar]
- 67.Ruff RM, Light RH, Evans RW (1987): The Ruff Figural Fluency Test: a normative study with adults. Dev Neuropsychol. 3:37–51. [Google Scholar]
- 68.Kuiper JS, Oude Voshaar RC, Verhoeven FE, Zuidema SU, Smidt N (2017): Comparison of cognitive functioning as measured by the Ruff Figural Fluency Test and the CogState computerized battery within the LifeLines Cohort Study. BMC psychology. 5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gulpers B, Lugtenburg A, Zuidersma M, Verhey F, Voshaar RO (2018): Anxiety disorders and figural fluency: A measure of executive function. J Affect Disord. 234:38–44. [DOI] [PubMed] [Google Scholar]
- 70.Mac Giollabhui N, Alloy LB, Schweren LJS, Hartman CA (2021): Investigating whether a combination of higher CRP and depression is differentially associated with worse executive functioning in a cohort of 43,896 adults. Brain Behav Immun. 96:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Overbeek T, Schruers K, Griez E (1999): MINI: Mini international neuropsychiatric interview, Dutch version 5.0. 0 (DSM-IV). Maastricht: University of Maastricht. [Google Scholar]
- 72.Hergueta T, Weiller E (2017): MINI-S for DSM-5, Dutch version. [Google Scholar]
- 73.Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonora I, Sheehan K, et al. (1997): The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur Psychiatry. 12:224–231. [Google Scholar]
- 74.Wanders RB, van Loo HM, Vermunt JK, Meijer RR, Hartman CA, Schoevers RA, et al. (2016): Casting wider nets for anxiety and depression: disability-driven cross-diagnostic subtypes in a large cohort. Psychol Med. 46:3371–3382. [DOI] [PubMed] [Google Scholar]
- 75.Watson D, Clark LA, Tellegen A (1988): Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 54:1063. [DOI] [PubMed] [Google Scholar]
- 76.Crawford JR, Henry JD (2004): The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 43:245–265. [DOI] [PubMed] [Google Scholar]
- 77.Team RC (2021): R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing,. [Google Scholar]
- 78.Hemani G, Elsworth B, Palmer T, Rasteiro R (2024): ieugwasr: Interface to the ‘OpenGWAS’ Database API. R package version 0.2.2–9000. https://github.com/MRCIEU/ieugwasr. [Google Scholar]
- 79.Said S, Pazoki R, Karhunen V, Võsa U, Ligthart S, Bodinier B, et al. (2022): Genetic analysis of over half a million people characterises C-reactive protein loci. Nat Commun. 13:2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collaboration IRGCERF, Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, et al. (2012): Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, et al. (2012): The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosa M, Chignon A, Li Z, Boulanger MC, Arsenault BJ, Bosse Y, et al. (2019): A Mendelian randomization study of IL6 signaling in cardiovascular diseases, immune-related disorders and longevity. NPJ Genom Med. 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Project IOG (2023): Glycoprotein acetyls Dataset: met-d-GlycA. [Google Scholar]
- 84.Prins BP, Abbasi A, Wong A, Vaez A, Nolte I, Franceschini N, et al. (2016): Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study. PLoS Med. 13:e1001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swerdlow DI, Kuchenbaecker KB, Shah S, Sofat R, Holmes MV, White J, et al. (2016): Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol. 45:1600–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Machiela MJ, Chanock SJ (2015): LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 31:3555–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myers TA, Chanock SJ, Machiela MJ (2020): LDlinkR: An R Package for Rapidly Calculating Linkage Disequilibrium Statistics in Diverse Populations. Frontiers in Genetics. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. (2007): PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aulchenko YS, De Koning D- J, Haley C (2007): Genomewide rapid association using mixed model and regression: a fast and simple method for genomewide pedigree-based quantitative trait loci association analysis. Genetics. 177:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kleiber C, Zeileis A (2008): Applied econometrics with R. Springer Science & Business Media. [Google Scholar]
- 91.Burgess S, Thompson SG (2011): Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 40:755–764. [DOI] [PubMed] [Google Scholar]
- 92.Slavish DC, Jones DR, Smyth JM, Engeland CG, Song S, McCormick NM, et al. (2020): Positive and Negative Affect and Salivary Markers of Inflammation Among Young Adults. Int J Behav Med. 27:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brouwers C, Mommersteeg PM, Nyklíček I, Pelle AJ, Westerhuis BL, Szabó BM, et al. (2013): Positive affect dimensions and their association with inflammatory biomarkers in patients with chronic heart failure. Biol Psychol. 92:220–226. [DOI] [PubMed] [Google Scholar]
- 94.Sepah SC, Bower JE (2009): Positive affect and inflammation during radiation treatment for breast and prostate cancer. Brain Behav Immun. 23:1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stellar JE, John-Henderson N, Anderson CL, Gordon AM, McNeil GD, Keltner D (2015): Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion. 15:129. [DOI] [PubMed] [Google Scholar]
- 96.Lin C, Michopoulos V, Powers A, Wingo AP, Schwartz A, Bradley B, et al. (2018): Affect, inflammation, and health in urban at-risk civilians. J Psychiatr Res. 104:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boylan JM, Lewis TT, Coe CL, Ryff CD (2015): Educational Status, Anger, and Inflammation in the MIDUS National Sample: Does Race Matter? Ann Behav Med. 49:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. (2021): Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. 26:7393–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Milaneschi Y, Lamers F, Berk M, Penninx B (2020): Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biol Psychiatry. 88:369–380. [DOI] [PubMed] [Google Scholar]
- 100.Felger JC, Haroon E, Miller AH (2018): What’s CRP got to do with it? Tackling the complexities of the relationship between CRP and depression. Brain Behav Immun. 73:163–164. [DOI] [PubMed] [Google Scholar]
- 101.Mac Giollabhui N, Ellman LM, Coe CL, Byrne ML, Abramson LY, Alloy LB (2020): To exclude or not to exclude: Considerations and recommendations for C-reactive protein values higher than 10 mg/L. Brain Behav Immun. 87:898–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG (2013): Elevated C-Reactive Protein Levels, Psychological Distress, and Depression in 73 131 Individuals. JAMA Psychiatry. 70:176. [DOI] [PubMed] [Google Scholar]
- 103.Khandaker GM, Zuber V, Rees JM, Carvalho L, Mason AM, Foley CN, et al. (2019): Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry.1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Consortium I-RMRA (2012): The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. The Lancet. 379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Collaboration CRPCHDG (2011): Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foley ÉM, Slaney C, Donnelly NA, Kaser M, Ziegler L, Khandaker GM (2024): A novel biomarker of interleukin 6 activity and clinical and cognitive outcomes in depression. Psychoneuroendocrinology. 164:107008. [DOI] [PubMed] [Google Scholar]
- 107.Zainal NH, Newman MG (2022): Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 52:3625–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mac Giollabhui N, Foster S, Lowry CA, Mischoulon D, Raison CL, Nyer M (2022): Interleukin-6 receptor antagonists in immunopsychiatry: Can they lead to increased interleukin-6 in the central nervous system (CNS) and worsening psychiatric symptoms? Brain Behav Immun. 103:202–204. [DOI] [PubMed] [Google Scholar]
- 109.Boogert NJ, Madden JR, Morand-Ferron J, Thornton A (2018): Measuring and understanding individual differences in cognition. Philos Trans R Soc Lond B Biol Sci. 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mac Giollabhui N, Mischoulon D, Dunlop BW, Kinkead B, Schettler PJ, Liu RT, et al. (2023): Individuals with depression exhibiting a pro-inflammatory phenotype receiving omega-3 polyunsaturated fatty acids experience improved motivation-related cognitive function: Preliminary results from a randomized controlled trial. Brain Behav Immun Health. 32:100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klein SL, Flanagan KL (2016): Sex differences in immune responses. Nat Rev Immunol. 16:626–638. [DOI] [PubMed] [Google Scholar]
- 112.Ferrucci L, Fabbri E (2018): Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vogelzangs N, Beekman ATF, de Jonge P, Penninx BWJH (2013): Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 3:e249–e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Costello H, Gould RL, Abrol E, Howard R (2019): Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ open. 9:e027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glaus J, von Känel R, Lasserre AM, Strippoli MPF, Vandeleur CL, Castelao E, et al. (2018): The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: results from a large longitudinal population-based study. Depress Anxiety. 35:360–371. [DOI] [PubMed] [Google Scholar]
- 116.O’Donovan A, Slavich GM, Epel ES, Neylan TC (2013): Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev. 37:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raison CL, Miller AH (2017): Pathogen–host defense in the evolution of depression: insights into epidemiology, genetics, bioregional differences and female preponderance. Neuropsychopharmacology. 42:5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klijs B, Scholtens S, Mandemakers JJ, Snieder H, Stolk RP, Smidt N (2015): Representativeness of the LifeLines Cohort Study. PLoS One. 10:e0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. (2020): Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 396:1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freeman MP, Cheng LJ, Moustafa D, Davies A, Sosinsky AZ, Wang B, et al. (2017): Vortioxetine for major depressive disorder, vasomotor, and cognitive symptoms associated with the menopausal transition. Ann Clin Psychiatry. 29:249–257. [PubMed] [Google Scholar]
- 121.Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS (2015): A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 40:2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olver JS, Ignatiadis S, Maruff P, Burrows GD, Norman TR (2008): Quetiapine augmentation in depressed patients with partial response to antidepressants. Hum Psychopharmacol. 23:653–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.