Abstract
Global prevalence of hypertension is on the rise, burdening healthcare, especially in developing countries where infectious diseases, such as malaria, are also rampant. Whether hypertension could predispose or increase susceptibility to malaria, however, has not been extensively explored. Previously, we reported that hypertension is associated with abnormal red blood cell (RBC) physiology and anemia. Since RBC are target host cells for malarial parasite, Plasmodium, we hypothesized that hypertensive patients with abnormal RBC physiology are at greater risk or susceptibility to Plasmodium infection. To test this hypothesis, normotensive (BPN/3J) and hypertensive (BPH/2J) mice were characterized for their RBC physiology and subsequently infected with Plasmodium yoelii (P. yoelii), a murine-specific non-lethal strain. When compared to BPN mice, BPH mice displayed microcytic anemia with RBC highly resistant to osmotic hemolysis. Further, BPH RBC exhibited greater membrane rigidity and an altered lipid composition, as evidenced by higher levels of phospholipids and saturated fatty acid, such as stearate (C18:0), along with lower levels of polyunsaturated fatty acid like arachidonate (C20:4). Moreover, BPH mice had significantly greater circulating Ter119+ CD71+ reticulocytes, or immature RBC, prone to P. yoelii infection. Upon infection with P. yoelii, BPH mice experienced significant body weight loss accompanied by sustained parasitemia, indices of anemia, and substantial increase in systemic pro-inflammatory mediators, compared to BPN mice, indicating that BPH mice were incompetent to clear P. yoelii infection. Collectively, these data demonstrate that aberrant RBC physiology observed in hypertensive BPH mice contributes to an increased susceptibility to P. yoelii infection and malaria-associated pathology.
Keywords: red blood cell, blood pressure high, Plasmodium, reticulocyte, anemia, blood pressure
Graphical Abstract
Graphical Abstract.
Introduction
High blood pressure, or hypertension, remains a significant global health concern and is the leading cause of cardiovascular diseases. Hypertensive individuals often experience a range of other health anomalies, including anemia, stroke, kidney diseases, and metabolic disorders.1,2 Anemia is a common comorbidity of hypertension and can manifest in two forms: (1) normocytic anemia, characterized by low levels of hemoglobin and of red blood cells (RBC), or (2) microcytic anemia, where RBC are smaller, leading to a reduced hematocrit value.1,3,4 Alterations in RBC morphology are a critical contributing factor to hypertension, as RBC account for 50% of all blood cells and can adapt their shape to maintain blood flow.5 Additionally, RBC have the capacity to influence blood viscosity, thereby contributing to blood pressure (BP) regulation. Given their significance in circulation and BP regulation, understanding changes in RBC morphology and membrane composition is crucial to comprehend their role not only in hypertension but also in RBC specific infectious diseases.
Malaria is a severe and often life-threatening disease caused by the protozoan parasite Plasmodium spp. that infects humans through Anopheles mosquitoes as vectors.6,7 Once the parasite enters the bloodstream of a human host, it invades RBC as part of its blood-stage life cycle. Several RBC-related parameters have been shown to play a key role in determining susceptibility toward malaria, one of which is RBC morphology. For instance, the sickle cell trait that affects the shape of RBC and hemoglobin content is known to confer resistance against Plasmodium infection.8,9 Yet, it remains to be investigated whether individuals with alterations in RBC morphology, but without sickle cell trait nor other blood disorders, could be at a higher risk of malaria-associated pathology.
Researchers have been trying to study the link between hypertension and malaria, since several case studies suggest that hypertensive patients are more susceptible to malaria and it is now known that malaria can increase blood pressure.10–15 Further, angiotensin, a protein closely involved in regulation of BP, modulates the severity of malaria infection in mice. Stimulation of angiotensin I and deficiency of angiotensin II can increase the severity of cerebral malaria in mice.16–18 Antihypertensive drugs that inhibit angiotensin converting enzyme reduce systemic angiotensin II and, therefore, increase the risk of severe malaria in humans.12 Gallego-Delgado et al. showed that using chloroquine alone to treat cerebral malaria in mice caused death whereas combining chloroquine with an antihypertensive drug, such as irbesartan that blocks angiotensin II receptor, increased survival in these mice.16
In light of the published literature suggesting a potential association between hypertension and malaria pathogenesis, we set out to investigate whether there are any disparities in RBC properties and susceptibility to malaria between hypertensive and normotensive mice. To test this hypothesis, we used hypertensive (BPH/2J) mice and their normotensive counterparts (BPN/3J), which are well-established models of genetic hypertension. About 50 yr ago, Gunther Schlager cross-bred 8 strains, followed by sibling mating, to obtain hypertensive BPH/2 J, normotensive, BPN/3J, and hypotensive, BPL/1J mice. BPH mice are, thus, genetically hypertensive.19 Further, hypertension is sympathetically regulated in BPH mice wherein aberrant activation of sympathetic neurons is responsible for high BP.20 Our investigation herein revealed several abnormalities in RBC-related parameters in BPH mice. Firstly, BPH mice have microcytic anemia. Secondly, BPH RBC are resistant to hemolysis in hypotonic NaCl solutions. Thirdly, we observed increased phospholipid levels in the RBC membranes of BPH mice. After characterizing the RBC properties and lipid profile in both groups, we aimed to determine whether BPH mice are more susceptible to infection by a nonlethal strain of murine-specific Plasmodium yoelii (P. yoelii). We closely monitored parasitemia levels, body weight changes, and the overall course of infection to evaluate the impact of hypertension on malaria susceptibility. Our results demonstrated that BPH mice exhibit prolonged and sustained P. yoelii infection with severe anemia. Together, we aimed to shed light on the potential relationship between hypertension, RBC morphology, and susceptibility to malaria. Understanding the role of RBC in these processes could provide valuable insights into the pathogenesis of both hypertension and malaria and may have implications for the development of targeted therapies.
Materials and Methods
Mice
Hypertensive (BPH/2J, strain #:003005) and normotensive (BPN/3J, strain #003004) mice19–21 were procured from Jackson Laboratory and bred in-house in specific pathogen-free condition at the University of Toledo College of Medicine and Life Sciences, Department of Laboratory Animal Resources. Mice were housed in cages (n = 5 mice/cage) containing corn cob bedding (Bed-O-Cob; The Andersons Co.) and nestlets (Cat #CABFM00088; Ancare). The cages were housed at 23°C under a 12/12-h light/dark phase cycle and fed a grain-based laboratory chow diet (LabDiet 5001), ad libitum. The institutional animal care and use committee (IACUC) at the University of Toledo approved all the mouse experiments.
Measurement of Blood Pressure By Tail‐Cuff Method
The CODA 8 noninvasive blood pressure system for mice (Kent Scientific, Torrington, CT) was used for tail‐cuff measurements. This system uses volume pressure recording to detect blood pressure based on volume changes in the tail. CODA system was calibrated, and standard settings and recommendations were used to measure blood pressure in BPN and BPH mice as mentioned by Daugherty et al.22
Serum Collection
Blood from 8 to 10-wk-old BPN and BPH male and female mice was collected in BD microtainer tubes (BD Biosciences), and centrifuged at 9391 × g for 10 min. Hemolysis-free sera was collected and stored at −80°C until further analyses.
Serum Biochemical Analyses
Serum total bile acids (TBA) was measured using Diazyme total bile acids assay kit (Cat # DZ042A) as per manufacturer’s instructions. Further, serum total cholesterol, aspartate transaminase (AST), and alkaline phosphatase (ALP) were measured using respective assay kits from Randox (Cat # CH200, AS3804, and AP9764, respectively) as per manufacturer’s instructions.
Serum ELISA
Serum lipocalin 2 (Lcn2), serum amyloid A (SAA), keratinocyte-derived chemoattractant (KC, alias CXCL1), were measured in hemolysis-free serum via Duoset ELISA kits from R&D Systems (Cat # DY1857, DY2948-05, DY453, respectively).
Complete Blood Count
Blood was collected in EDTA-containing microtubes (Sarstedt Inc) to determine complete blood count using VETSCAN HM5 Hematology Analyzer (ABAXIS HM5C & VS2, Allied Analytic).
Osmotic Fragility Test
Blood was collected in EDTA-containing Vacuette (Greiner bio-one) tubes and then 10 µL of blood was transferred into a round bottom 96-well plate, corresponding to each of the concentrations of the salt solution [NaCl (%) 0, 0.30, 0.35, 0.40, 0.45, 0.48, 0.50, and 0.90]. The respective concentration of salt solution was added to the samples (300 µL/well). The plate was gently mixed and incubated for 30 min at room temperature (20°C–25°C), and then centrifuged at 333 × g for 10 min and supernatant was transferred into a 96-well flat bottom plate. Degree of hemolysis was measured spectrophotometrically at 540 nm by a Biotek Eon microplate spectrophotometer and normalized to the absorbance value of 0.0% salt solution (100% hemolysis).
Preparation of RBC Ghosts
Red blood cell membranes (ghost) were prepared as described by Hanahan and Ekholm.23 Briefly, after separating plasma, RBC were suspended and mixed gently in isotonic Tris:HCl buffer (0.172 M, pH 7.6) on ice. The cell suspension was centrifuged at 1000 × g for 10 min at 4°C and the supernatant was discarded. RBC were hemolyzed in hypotonic Tris:HCl buffer (0.011 M, pH 7.6) and allowed to stand for 5 min on ice before centrifugation at 20 000 × g for 20 min at 4°C. The supernatant was carefully removed without disrupting the RBC ghost pellet. Membranes were repeatedly washed with 0.011 M Tris:HCl buffer until their color turned to creamy white. After the last wash, the supernatant was removed, and RBC ghosts were stored at −80°C until analyses.
Analyses of Phospholipids and Cholesterol From Serum and RBC Ghosts
Serum and RBC ghost lipid analyses was performed at mouse metabolic phenotyping centers (MMPC) Lipid Core Laboratory, Vanderbilt University, using tissue/cell lipid gas chromatography analysis. Briefly, lipids were extracted using the method of Folch-Lees.24 The extracts were filtered, and lipids were recovered in the chloroform phase. Individual lipid classes were separated by thin layer chromatography using Silica Gel 60 A plates developed in petroleum ether, ethyl ether, acetic acid (80:20:1) and visualized by rhodamine 6 G. Phospholipids, diglycerides, triglycerides, and cholesteryl esters were scraped from the plates and methylated using BF3/methanol as described by Morrison and Smith.25 The methylated fatty acids were extracted and analyzed by gas chromatography. Gas chromatographic analyses was carried out on an Agilent 7890A gas chromatograph equipped with flame ionization detectors and a capillary column (SP2380, 0.25 mm × 30 m, 0.20 µm film, Supelco, Bellefonte, PA). Helium was used as the carrier gas. The oven temperature was programmed from 160°C to 230°C at 4°C/min. Fatty acid methyl esters were identified by comparing the retention times to those of known standards. Inclusion of lipid standards with odd chain fatty acids permits quantitation of the amount of lipid in the sample. Dipentadecanoyl phosphatidylcholine (C15:0), diheptadecanoin (C17:0), trieicosenoin (C20:1), and cholesteryl eicosenoate (C20:1) were used as standards. Lipid concentration was normalized to total protein in RBC ghosts.
Flow Cytometric Analysis of Reticulocytes
To determine reticulocyte population in mice, 2 µL whole blood was collected via tail nick and suspended in 1 mL sterile PBS (pH 7.0). Cells were then centrifuged at 211 × g for 5 min, the supernatant was carefully removed, and cells were resuspended in 1 mL PBS containing 0.2% FBS. Cells were washed and centrifuged (211 × g for 5 min) twice with PBS containing FBS. Cells are then resuspended in 100 µL PBS + 0.2% FBS and stained with fluorophore-conjugated anti-mouse mAb from BD Biosciences directed against the following cell surface proteins: Ter119-PE, CD71-FITC/CD71-APC in staining buffer (PBS + 0.2% FBS) and incubated for 40 min at room temperature in the dark. Cells were then washed with PBS + 0.2% FBS and analyzed by Accuri C6 flow cytometer (BD Biosciences) with BD Accuri C6 Software (BD Biosciences).
Plasmodium yoelii Infection and Quantification of Parasitemia
Mice were infected with P. yoelii 17XNL:PyGFP by intraperitoneal injection of 1 × 105 in 100 µL sterile PBS prepared from frozen stock. Parasitemia was determined by flow cytometry between days 3 and 15 post-infection (p.i.) in blood collected from tails of infected mice. Parasitemia was defined as % GFP positivity in whole blood. Plasmodium yoelii subsp. yoelii, Strain 17XNL:PyGFP, MRA-817 was obtained from BEI Resources and contributed by Ana Rodriguez. After day 15 p.i., infected mice were euthanized, serum was collected, and organs were harvested and stored at −80°C until analyses.
Microscopy
For Giemsa staining, thin blood smears of uninfected and infected mice were fixed in methanol (Sigma-Aldrich) for 1-2 min and air dried. Slides were then submerged into Giemsa stain (1:20 in deionized water) (Sigma-Aldrich) for 15 min and then rinsed in deionized water and air-dried for imaging and analysis using a Leica DM2500 LED optical microscope. For fluorescence analysis, blood smears were fixed in methanol, and imaged and analyzed using a BioTek Cytation 5 Cell Imaging Multimode Reader (Agilent microscope). All images were captured at 40× magnification (Bright field and FITC).
Histology
Livers and spleen sections were fixed in 10% neutral buffered formalin (NBF), underwent paraffin-embedded sectioning (2 µm) and stained with Hematoxylin & Eosin (H&E) and Periodic acid-Schiff (PAS). Histological images were generated from VS120 Virtual Slide Microscope (Olympus) and OlyVIA software.
Statistical Analysis
The results are expressed as mean ± SEM. Statistical analyses for significance between 2 groups were determined using Student’s t-test (unpaired, two-tailed) with P < .05 considered as significant. Statistical analysis for significance between the means of 3 or more groups was performed using one-way ANOVA with pairwise multiple comparisons. All statistical analyses were performed using GraphPad Prism 9.0 program (GraphPad Software, Inc, La Jolla, CA).
Results
Hypertensive BPH Mice Exhibit Microcytic Anemia and RBC Abnormalities
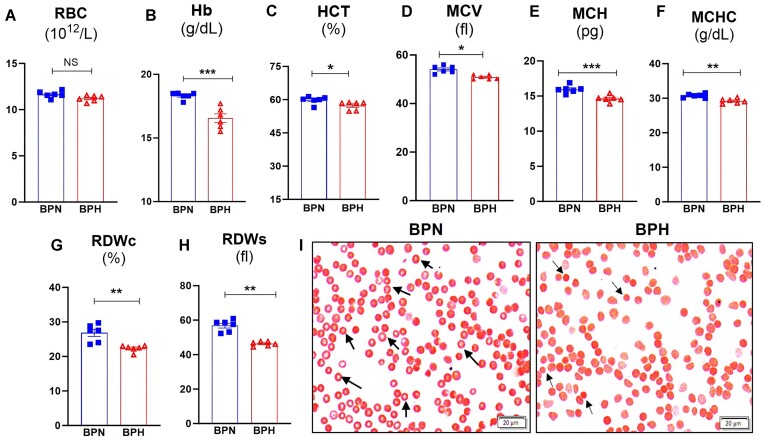
Anemia is associated with several comorbidities, including but not limited to chronic inflammation, cardiovascular diseases, and hypertension.26 It has been estimated that 16%–20% of hypertensive patients have normocytic anemia.27 Given the association between anemia and hypertension and the potential role of RBC dysfunction as a risk factor in hypertension, we investigated hematology parameters in BPH and BPN mice via complete blood count (CBC) analyses (Figure 1A). Although the number of RBC between the 2 strains is comparable (Figures 1A and S1A), BPH mice have significantly lower hemoglobin (Figures 1B and S1B) and hematocrit values (Figure 1C) compared to BPN mice. Moreover, we observed that BPH mice have abnormal RBC physiology as indicated by lower mean corpuscular volume (MCV; Figure 1D), mean corpuscular hemoglobin (MCH; Figure 1E), and mean corpuscular hemoglobin concentration (MCHC; Figure 1F). Intriguingly, RBC distribution width (RDW; a measure of variation in RBC size and volume) are significantly lower in BPH mice (Figure 1G and H). Further, to assess RBC morphology, we performed Giemsa staining and found that BPN RBC had a clear zone of central pallor (black arrows), which was absent in BPH RBC, rather they appear more flattened. Moreover, BPH RBC were smaller compared to BPN RBC, (Figure 1I) indicating microcytic anemia.
Figure 1.
Hypertensive mice exhibited microcytic anemia. Blood from BPN and BPH mice (8-wk-old males, n = 6) was collected (EDTA tubes) for complete blood count (CBC; via VetScan hematology analyzer). Results for (A) Erythrocytes, red blood cells (RBC), (B) Hemoglobin (Hb), (C) Hematocrit (HCT, % RBC), (D) Mean corpuscular volume (MCV, average size of RBC), (E) Mean corpuscular hemoglobin (MCH average amount of hemoglobin in RBC), (F) Mean corpuscular hemoglobin concentration (MCHC, average concentration of hemoglobin in a given volume of RBC) via CBC. RBC distribution width (RDW, variation of the size/volume), (G) RDWc (RDW coefficient of variation), and (H) RDWs (RDW standard deviation), and (I) Giemsa staining of RBC from BPN and BPH mice. Data represented as mean ± SEM from three independent experiments. *P < .05, **P < .01, and ***P < .001.
RBC From Hypertensive Mice are Resistant to Osmosis-Induced Hemolysis
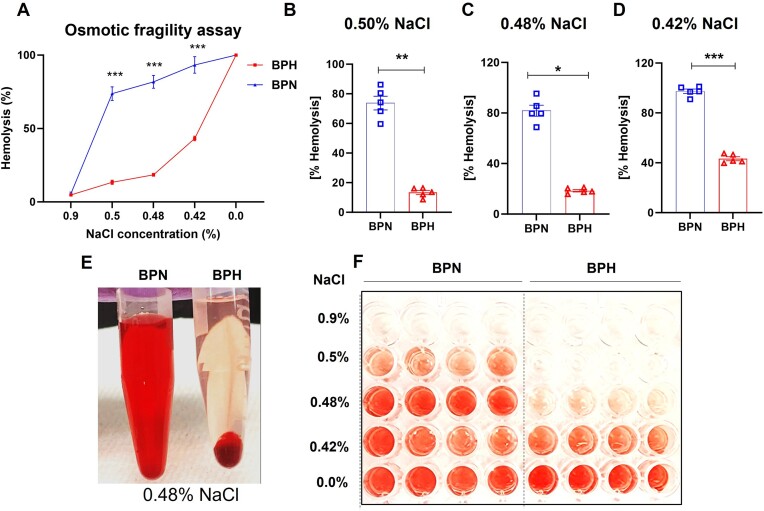
Biomembranes are responsible for various tasks that are essential for the proper functioning of the cell such as maintaining osmotic balance within the cell. RBC must prudently regulate their internal environment to ensure proper shape, stability, and overall functionality. Hence, we asked whether BPH mice with microcytic anemia (Figures 1 and S1) also exhibit changes in RBC membrane integrity. To this end, we subjected whole blood from BPN and BPH mice to varying concentrations of NaCl (0.0% to 0.9%). Interestingly, we observed that RBC from BPH mice were strikingly resistant to hemolysis at NaCl concentrations of 0.50%, 0.48%, and 0.42% compared to RBC from BPN mice (Figure 2A-D). Further, we observed a stark contrast in the color of the supernatant of BPH and BPN RBC exposed to hypotonic 0.48% NaCl solution (Figure 2E-F). Compared to the bright red color in the BPN supernatant, the hemolytic resistance in BPH mice corresponded merely to a red tint that was near colorless (Figure 2E-F). Likewise, RBC from female BPH mice show similar resistance to osmolysis compared to BPN mice (Figure S2). Collectively, these results emphasize that RBC from BPH mice are resistant to osmotic hemolysis.
Figure 2.
Red blood cell from hypertensive mice were resistant to osmotic hemolysis. Uncoagulated blood from 8-wk-old male BPN and BPH mice (n = 5) was collected, and RBC were subjected to increasing osmotic pressure (A) (from 0.90% to 0.00% NaCl) and calculated as % hemolysis. (B-D) Bar graphs represent osmotic fragility test of BPN and BPH mice RBCs in 0.50%, 0.48%, and 0.42% NaCl. (E) Supernatant from RBC incubated in 0.48% NaCl. (F) Supernatant from RBC incubated in 0.90% to 0.00% NaCl. Data represented as mean ± SEM from 3 independent experiments. *P < .05, **P < .01, and ***P < .001.
Hypertensive Mice Are Hypolipidemic But Have Higher Saturated Fatty Acids in Their RBC Membranes
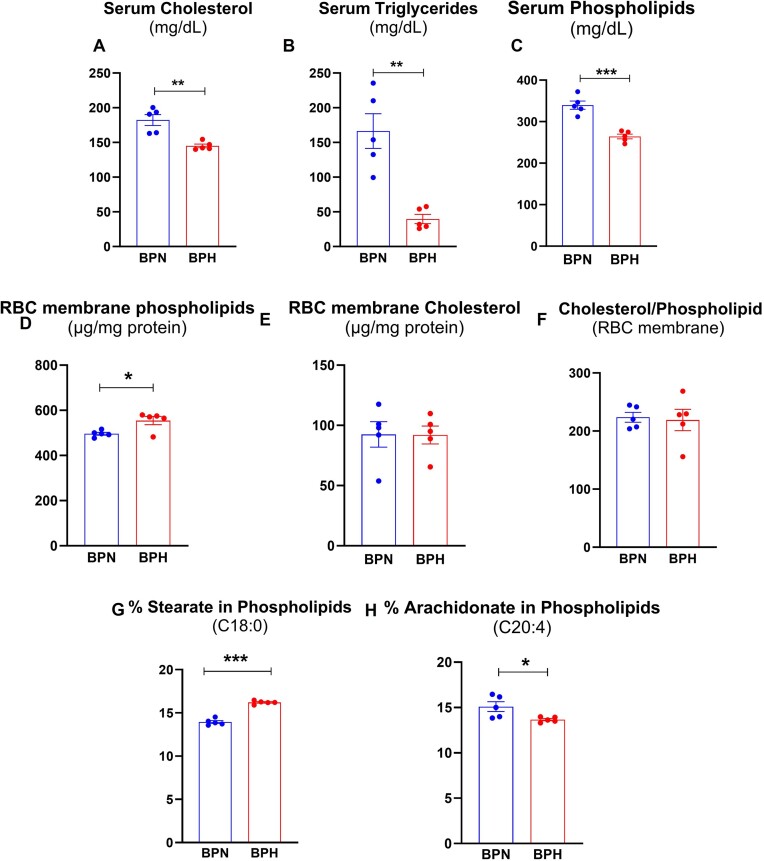
Circulating lipids, specifically serum cholesterol, triglycerides, and phospholipids play a significant role in cardiovascular health. We initially expected that BPH might suffer from hyperlipidemia, one of the major risk factors for cardiovascular diseases. However, this was not the case as BPH mice were found to be hypolipidemic instead, as evident by their significantly lower serum cholesterol, triglycerides, and phospholipids, compared to BPN mice (Figure 3A-C). Several studies have shown that lipoproteins exchange lipids, especially cholesterol, with blood cells such as erythrocytes, which is crucial for cholesterol metabolism in the blood. This led us to ask whether hypolipidemia in BPH mice is associated with alterations in membrane cholesterol and phospholipids in a manner that alters membrane composition.28–32 Interestingly, RBC from BPH mice had higher membrane phospholipids compared to those from BPN mice (Figure 3D). However, we found no significant differences in RBC membrane cholesterol between the 2 strains (Figure 3E). Likewise, the cholesterol-to-phospholipid ratio was comparable between the 2 groups (Figure 3F). Further profiling of fatty acids in RBC membrane phospholipids revealed that BPH RBC had elevated stearate (C18:0) (Figure 3G). Conversely, they had lower mono-unsaturated fatty acids such as palmitoleate, oleate, and vaccenic acid (data not shown), and lower poly-unsaturated fatty acids, specifically arachidonate (Figure 3H). These compositional differences, that is, higher saturated and lower unsaturated fatty acid in RBC from BPH mice may be accountable for observed resistance to osmotic fragility. However, there are no changes in the expression of RBC membrane proteins, Na+K+-ATPase, or ferroportin in BPH and BPN mice (data not shown). In this study, however, the individual lipoproteins were not measured and therefore it would be interesting for future study to address whether there is any association between serum lipids and lipoprotein levels.
Figure 3.
Hypertensive mice were hypolipidemic and their RBC membrane had altered phospholipid content. Serum was collected from 8-wk-old male BPN and BPH mice (n = 5) for measurement of (A) serum total cholesterol, (B) triglycerides, and (C) phospholipids. Uncoagulated RBC from 8-wk-old male BPN and BPH mice (n = 5) was collected, and RBC ghost membranes were prepared to be analyzed for (D) phospholipids and (E) cholesterol; (F) Cholesterol and Phospholipid ratio (Chol/PL); and (G) % Stearate and (H) % Arachidonate in phospholipids. Data represented as mean ± SEM from 2 independent experiments. *P < .05, **P < .01, and ***P < .001.
Hypertensive BPH Mice Have a Deficiency in Clearing Malarial Parasites
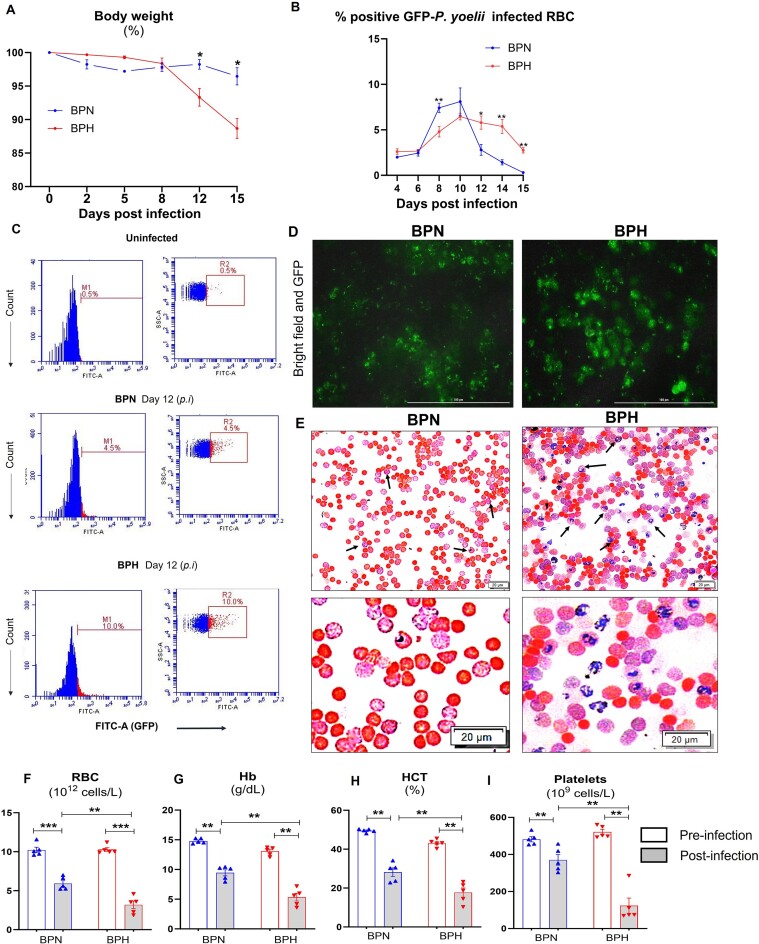
Red blood cells play a crucial role as hosts for blood-stage malarial parasites, which depend on RBC for many metabolites including cholesterol.33 Having observed hypolipidemia and alterations in membrane phospholipids and fatty acid composition of RBC from BPH mice, we next investigated to what extent these factors could promote or demote Plasmodium infection and its associated pathology. First, we confirmed that the BPH mice in our colony have higher BP compared with BPN mice (mean arterial BP was 93.52 ± 1.94 mmHg vs 110.6 ± 3.45 mmHg, respectively, for BPN and BPH mice at 10 wk old). Next, we infected both strains with green fluorescent protein (GFP)-tagged-P. yoelii, a nonlethal strain of Plasmodium to study malaria pathogenesis in rodents. After infection, mice were monitored for their body weights for 15 days and we observed that BPH mice lost significant body weight compared to similarly infected BPN mice (Figure 4A). On day 15 p.i., BPH mice exhibited hepatomegaly, splenomegaly, and cardiomegaly, but smaller kidneys and lungs (Figure S3A-S3E). Flow cytometric analysis for % GFP-positivity in RBC (ie, P. yoelii-infected RBC), as a measure of parasitemia, revealed a distinctive pattern of P. yoelii infection in BPH mice. As the infection progressed, the parasitemia in BPH mice gradually increased (Figure 4B). Notably, after day 10 p.i., BPN mice showed a reduction in parasitemia, and by day 15 p.i., they were able to clear the infection substantially. However, in BPH mice, parasitemia was still present on day 15 p.i., indicating a delayed clearance of P. yoelii (Figure 4B-C). Likewise, fluorescent microscopic analysis showed a greater number of GFP-positive RBC in P. yoelii-infected BPH mice than similarly infected BPN mice (Figure 4D). To further validate this observation, we performed Giemsa staining (Figure 4E), which confirmed higher parasitemia in BPH mice. Collectively, these findings indicate that parasite burden was higher in BPH mice.
Figure 4.
Hypertensive mice had sustained P. yoelii infection and severe anemia. Ten-week-old BPN and BPH females (n = 5) were infected with P. yoelii. (A) Body weight loss P. yoelii post-infection (day 15). (B) % Parasitemia, that is, GFP-positive RBC measured by flow cytometry during the infection. Quantification of parasitemia (% GFP+ RBC, ie, GFP-P. yoelii-infected RBC. (C) Flow cytometry representation of % GFP-P. yoelii-infected RBC in histogram and dot plots after day 12. (D) Representative images of GFP-P. yoelii-infected RBC visualized under fluorescence microscope in BPN and BPH mice 12 d p.i. (E) Representative images of infected RBC (Giemsa staining) after day 12. Scale bar = 100 µm; magnification = 40× (Bright field and FITC). Blood from BPN and BPH mice (control and P. yoelii infected, 10-wk-old females, n = 5) was collected (EDTA tubes) for complete blood count (CBC; via VetScan hematology analyzer) after 15 d of infection. Results for (F) RBC, (G) hemoglobin (Hb), (H) hematocrit (HCT, % RBC), and (I) platelets. Data represented as mean ± SEM from 3 independent experiments. *P < .05, **P < .01, and ***P < .001.
Red blood cells infected by Plasmodium spp. eventually lyse or rupture to release the schizont stage of the parasites into the bloodstream to infect new RBC. The destruction of a substantial number of RBC through this process can lead to severe anemia, a hallmark symptom of malaria. Given this context, we performed CBC for both strains on days 0 and 15 p.i. with P. yoelii. We noticed that P. yoelii-infected BPH mice demonstrated notably lower RBC population and hemoglobin (Figure 4F-G), as well as reduced hematocrit values (Figure 4H), when compared to BPN mice. Additionally, platelet counts were significantly lower in P. yoelii-infected BPH mice (Figure 4I). These results collectively point to the presence of severe anemia in P. yoelii-infected BPH mice, which reflect the prolonged duration of their infection.
Plasmodium yoelii-Infected Hypertensive Mice Suffered From Severe Hepatic Injury
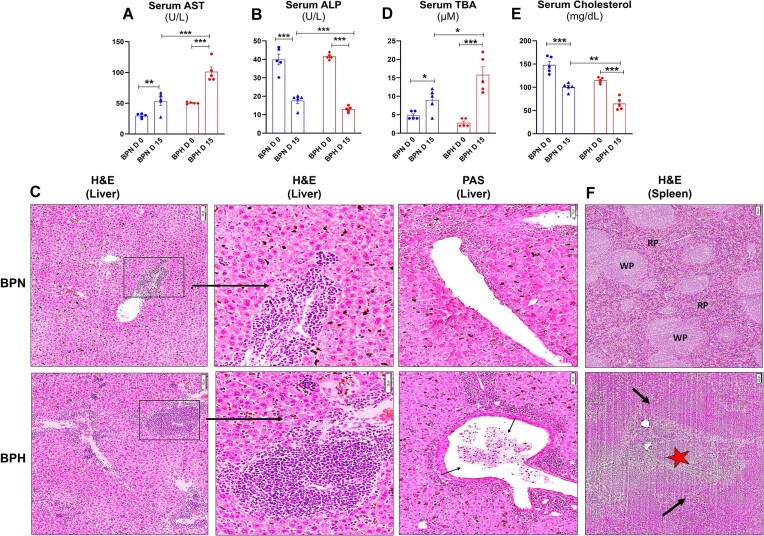
Hemolysis in malaria is a major cause of liver damage in both animal models34 and human patients.35,36 Hepatocyte dysfunction35 and death during malaria occur because of accumulation of free heme that stimulates the proinflammatory response and neutrophil infiltration in the liver,34 which further aggravates liver damage. We noticed that marker of multiple organ damage, aspartate transaminase (AST), and liver injury marker alkaline phosphatase (ALP), were higher in serum from P. yoelii-infected BPH mice compared to similarly treated BPN mice (Figure 5A-B).
Figure 5.
Plasmodium yoelii-infected hypertensive mice showed severe liver dysfunction. Ten-week-old BPN and BPH females (n = 5) were infected with P. yoelii. Serum samples were collected after 15 d p.i. and analyzed for (A) serum aspartate aminotransferase (AST), (B) serum alkaline phosphatase (ALP), and (C) liver histology: hematoxylin-eosin (H&E) and Periodic acid-Schiff (PAS) staining. (D) Serum total bile acid (TBA), (E) serum cholesterol. (F) Spleen histology—H&E. Data represented as mean ± SEM from 3 independent experiments. *P < .05, **P < .01, and ***P < .001.
Microscopic analysis of hematoxylin-eosin (H&E)-stained liver sections from day 15 post P. yoelii infection revealed significant accumulation of malarial parasite pigment, hemozoin, in BPH mice. Importantly, immune cell infiltration was prominently observed in BPH mice, in comparison to BPN mice. The portal tract exhibited congestion with a substantial number of parasitized erythrocytes and hemozoin crystals in BPH mice. In addition, Periodic acid-Schiff (PAS) staining confirmed the portal vein in sections from infected BPH mice displayed congestion with P. yoelii-infected RBC, macrophages, and lymphocytes (Figure 5C). Such pathology in BPH mice corroborated with higher serum total bile acids, indicating severe cholestasis, relative to BPN mice (Figure 5D). Next, we measured serum total cholesterol, considering that Plasmodium has been shown to interrupt cholesterol biosynthesis by hepatocytes.37 Serum cholesterol levels were significantly reduced in P. yoelii-infected BPH mice compared to infected BPN mice, indicating that BPH mice exhibited a more pronounced liver dysfunction p.i. (Figure 5E). Histological assessment (H&E) of spleen from P. yoelii-infected BPN and BPH mice revealed distinct features. In BPH spleens, a more prominent loss of central germinal structure was observed, compared to BPN spleens. While P. yoelii-infected BPN mice spleens exhibited distinct regions of red pulp (RP) and white pulp (WP), these regions were absent in P. yoelii-infected spleens from BPH mice. Notably, splenic infarction (black arrows) and the loss of central germinal structure (red star) were mostly evident in P. yoelii-infected BPH mice spleens (Figure 5F). These histologic observations highlight significant differences in the pathologic changes induced by P. yoelii-infected BPH and BPN mice and confirms that P. yoelii infection caused severe liver and spleen damage in BPH mice compared to BPN mice.
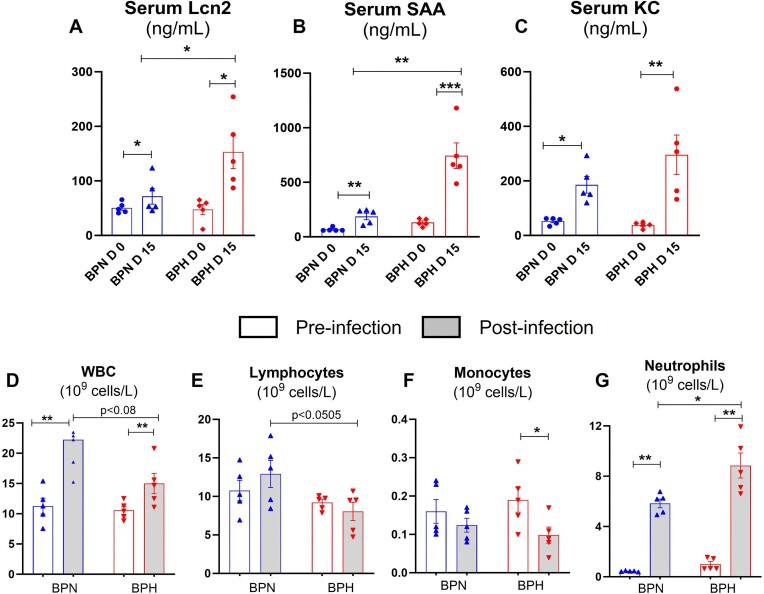
Plasmodium yoelii-Infected Hypertensive Mice Displayed Heightened Inflammatory Responses
Next, we asked whether the reduced clearance of P. yoelii in BPH mice could be due to the lack of appropriate innate immune response. Therefore, we investigated circulating inflammatory markers/mediators on day 15 p.i. P. yoelii-infected BPH mice showed significantly elevated levels of acute phase proteins, serum lipocalin-2 and serum amyloid A, and cytokine, keratinocyte chemoattractant (CXCL1, ie, KC) (Figure 6A-C). Hematological alterations represent some of the most prevalent complications in malaria and are pivotal in malaria pathogenesis. These changes encompass various major cell types, including RBC, white blood cells (leucocytes), lymphocytes, and platelets. Given this context, we analyzed CBC in both BPN and BPH mice. These analyses were performed both before, and 15 d p.i. with P. yoelii. Our findings reveal significant differences between the 2 groups. We found that BPH mice displayed a reduction in leucocyte count, largely due to diminished lymphocyte and monocyte populations (Figure 6D-F). Though neutrophilia was observed in both BPN and BPH p.i., a higher neutrophil population was observed in BPH mice, indicating a more robust neutrophilic inflammatory response (Figure 6G). These results indicate that there is an elevated and sustained inflammatory response in P. yoelii-infected BPH mice after day 15 p.i. These results suggest that the immune system of BPH mice was able to respond appropriately to the increase in parasitemia, albeit the heightened inflammatory responses may be detrimental for the host.
Figure 6.
Plasmodium yoelii-infected hypertensive mice display stark inflammatory responses. Ten-week-old BPN and BPH females (n = 5) were infected with P. yoelii. Serum samples were collected after 15 d p.i. and analyzed for (A-C) Serum cytokines, Lipocalin 2 (Lcn2), serum amyloid A (SAA), and KC (CXCL 1) measured by ELISA. Blood from BPN and BPH mice (control and P. yoelii infected, 10-wk-old females, n = 5) was collected (EDTA tubes) for complete blood count (CBC; via VetScan hematology analyzer) after 15 d of infection. Results for (D) white blood cells (WBC), (E) lymphocytes, (F) monocytes, and (G) neutrophils. Data represented as mean ± SEM from 3 independent experiments. *P < .05, **P < .01, ***P < .001.
Plasmodium yoelii Infection Substantially Reduced Circulating Ter119+ CD71+ Reticulocytes in Hypertensive Mice
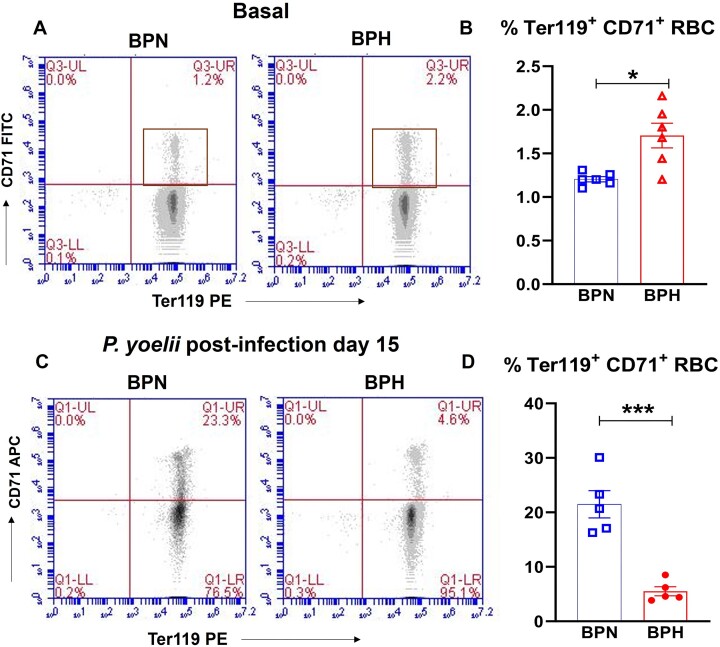
In bone marrow, erythropoiesis is a precisely regulated process that transforms hematopoietic stem cells into mature erythrocytes38,39 via reticulocytes (aka immature RBC, CD71+ Ter119+) that undergo complex changes, including shedding of several membrane proteins like the transferrin receptor, CD71. These reticulocytes then enter circulation and further mature into erythrocytes (mature RBC, CD71− Ter119+).40 Reticulocytes are known targets for malarial parasites, including P. yoelii.41 Given the prolonged and consistent P. yoelii infection in BPH mice, we assessed the baseline % reticulocyte population in BPN and BPH mice and found that BPH mice exhibit a higher abundance of Ter119+ and CD71+ reticulocytes (∼1.5-fold) (Figure 7A-B). This finding suggests that higher parasite burden in BPH mice could be due to higher abundance of reticulocytes, which favors P. yoelii infection.
Figure 7.
Reduced circulating reticulocytes in P. yoelii-infected hypertensive mice. Flow cytometry analysis for Ter119+ CD71+ cells in blood from 10-wk-old female BPN, BPH mice (n = 6). Representative (A) gating plots and (B) bar graphs for Ter119+ CD71+ reticulocytes in BPN and BPH mice. After 15 d of P. yoelii, blood from 10-wk-old female BPN, BPH mice (n = 5) was collected and analyzed for reticulocytes by flow cytometry. Representative (C) gating plots and (D) bar graphs for Ter119+ CD71+ reticulocytes in P. yoelii-infected BPN and BPH mice. Data represented as mean ± SEM from 3 independent experiments. *P < .05 and ***P < .001.
Anemia is a well-known consequence of Plasmodium infection, leading to stress hematopoiesis that may generate pathologic CD71+ reticulocytes. Considering the severe anemia observed in BPH mice, we measured the reticulocyte population in P. yoelii-infected BPN and BPH mice 15 d p.i at which BPN cleared infection but not BPH. Surprisingly, we found that the percentage of reticulocytes was significantly lower in P. yoelii-infected BPH mice, reduced by ∼4.5 times compared to P. yoelii-infected BPN mice (Figure 7C-D). These findings suggest that while P. yoelii-infected BPN mice were able to undergo stress erythropoiesis as evident by the increased reticulocytes count, such erythropoiesis pathway was compromised in P. yoelii-infected BPH mice. Further, our histological examination of spleen, which is a primary organ for stress erythropoiesis,42 confirmed that P. yoelii-infected BPN mice had more prominent splenic red pulp (RP) and white pulp (WP) areas than P. yoelii-infected BPH mice (Figure 5F). These results reinforce our understanding of severe anemia observed in P. yoelii-infected BPH mice.
Discussion
Identifying risk factors and understanding how they impact susceptibility to diseases/infections is of paramount importance for designing targeted prophylactics and therapeutics. Of note, RBC invasion is integral to the life cycle and survival of Plasmodium spp., thus representing not only a determinant of malaria susceptibility and severity, but also a target for interventions. Indeed, certain genetic traits that affect RBC morphology (eg, sickle cell) have been shown to confer resistance against malaria. However, it remains unclear whether RBC morphology altered by physiological factors, such as anemia and hypertension, could promote malaria pathogenesis. This query is clinically important, considering that anemia and hypertension are prevalent health conditions that affect a significant number of individuals worldwide. Notably, research suggests that ∼20% of individuals with hypertension also suffer from normocytic anemia.1,3,27 Moreover, studies have identified lower hemoglobin concentrations in patients with uncontrolled hypertension compared to those with controlled hypertension, indicating an increased risk of cardiovascular complications.27 In our study, we observed that BPH mice suffer from microcytic anemia and exhibit abnormal RBC. Specifically, these mice displayed lower hematocrit and hemoglobin levels, alongside reduced MCV and RDW, when compared to their normotensive counterparts, BPN mice. This led us to question how altered RBC characteristics might affect the susceptibility to blood-borne infections, particularly those that target RBC, such as malaria.
A direct correlation has been observed between hypertension and significant alterations in the structural and functional properties of both animal and human RBC membranes.43,44 Of note, individuals with essential hypertension exhibit modified lipid composition of RBC membranes, as is evident through a notably increased cholesterol/phospholipid ratio in comparison to normotensive individuals.45 Furthermore, studies have unveiled that individuals with hypertension exhibit a decrease in RBC deformability, signifying an increase in RBC membrane rigidity, thus, greater blood viscosity.46,47 Intriguingly, a seminal study on individuals with the Dantu blood group has shown that their increase in RBC membrane tension, along with changes in surface expression of membrane transporters affecting RBC hydration, could substantially increase resistance to malaria.48 These findings prompted our inquiry into whether the observed differences in RBC morphology also influence RBC function in hypertensive mice. Our investigations unveiled that RBC from BPH mice exhibited greater resistance to hemolysis in hypotonic solution containing as little as 0.42% NaCl compared to their normotensive BPN counterparts. To provide a more comprehensive understanding of why BPH RBC exhibit greater resistance to hemolysis, we examined their RBC membrane composition. Specifically, we observed that RBC membranes from BPH mice contained higher phospholipids. However, they had notably lower poly-unsaturated fatty acids (PUFA), such as arachidonate, which is a major fatty acid esterified to these phospholipids. In addition, the analysis indicated higher saturated fatty acids (SFA), such as stearate, attached to the phospholipids in BPH RBC membranes when compared to BPN RBC. Altered composition of RBC membranes can alter membrane properties, and thus, result in difference in osmotic hemolysis.
While studies and case reports have suggested a potential link between malaria and hypertension, definitive connections have not yet been established.10 Plasmodium parasite enters the bloodstream and eventually infects the host RBC, leading to hemolysis and anemia in severe cases. To study malaria in mice, a nonlethal strain of the malaria parasite, P. yoelii, was employed. In our study, the use of GFP-tagged P. yoelii allowed tracking of parasitemia using flow cytometry. A compelling observation emerged from our research—BPH mice displayed a distinct pattern of malaria infection. This pattern was characterized by a gradual, yet prolonged parasitemia progression compared to their normotensive counterparts, BPN mice. An important finding was that after 15 d of P. yoelii infection, severe anemia was detected in BPH mice. This anemia was marked by reduced RBC count and decreased hemoglobin levels, as well as reduced hematocrit, in comparison to normotensive mice along with heightened levels of proinflammatory cytokines and liver injury markers (ie, ALT, ALP). These observations suggest that altered RBC characteristics in BPH mice might contribute to longer duration of parasite burden. Our study underscores the intricate relationship between RBC characteristics, susceptibility to malaria, and the potential impact of hypertension. It is important to note that we cannot completely rule out the possibility that P. yoelii infection and the associated severe anemia might play a role in the pathophysiology of hypertension. Moreover, RBC membrane cholesterol plays an important source of nutrition to the Plasmodium and is therefore involved in modulating severity of malaria in mice.49,50 However, we could not perform RBC membrane analysis after infection with P. yoelii since it requires highly pure RBC membrane ghosts. During high parasitemia obtaining pure RBC membranes without parasite membrane lipids is technically challenging. A more in-depth study on this subject is warranted to better understand the relationship between RBC membrane cholesterol, malaria, anemia, and hypertension, and shed light on any potential interactions between these factors.
Erythrocyte tropism is a crucial aspect of Plasmodium pathogenesis, and numerous studies have aimed to characterize merozoite preferences for erythrocytes.51–53 For instance, P. falciparum can invade RBC at all stages,54 while P. vivax prefers reticulocytes54–56; such RBC tropism has been considered as one of the potential reasons why P. falciparum tends to be more virulent than P. vivax. Such disparity can be observed among the murine-specific Plasmodium spp. Specifically, P. yoelii prefers reticulocytes over erythrocytes by approximately 2.5 times,41 while P. chabaudi and P. vinckei exhibit no specific tropism.54 Given the prolonged and consistent P. yoelii infection observed in BPH mice, our findings indicate that BPH mice have a higher abundance of reticulocytes expressing erythroid lineage marker, TER119, and transferrin receptor, CD71, at basal levels. This may explain the heightened susceptibility of BPH mice to P. yoelii infections possibly attributed to a preference for reticulocytes. We speculate that higher reticulocyte population is due to extramedullary hematopoiesis that results from anemia.57,58 Certainly, the presence of elevated immature RBC in BPH mice raises the possibility of extramedullary hematopoiesis as a compensatory response to their anemic condition.
Indeed, the study conducted by Shimizu et al. in the Japanese population has provided valuable insights into the correlation between hypertension and reticulocyte population.59 The positive association observed in their cross-sectional study underscores the interplay between these 2 factors, suggesting that reticulocyte levels could be influenced by the presence of hypertension, possibly linked to anemia among hypertensive patients. Interestingly, these findings parallel our own observations in BPH mice. Our study has revealed that BPH mice, characterized by their microcytic anemia, demonstrate a significantly higher basal reticulocyte population. This phenomenon is in line with the concept that microcytic anemia triggers an elevation in reticulocyte count. This, in turn, aligns with the heightened susceptibility of BPH mice to Plasmodium infection, as their already elevated basal reticulocyte population provides a more favorable environment for P. yoelii infection, given the parasite’s known predilection for reticulocytes.
Anemia is a significant clinical manifestation of malaria, arising from both increased RBC lysis and diminished RBC production due to defective erythropoiesis. The mechanisms contributing to suppressed erythropoiesis are intricate and multifaceted, and gaining a deeper understanding of them could offer a comprehensive view of malarial pathophysiology. Ineffectual erythropoiesis encompasses inhibition of erythroid cell proliferation and division,60–62 leading to a notable decrease in reticulocyte count, particularly evident in patients infected with Plasmodium spp. such as, P. falciparum, P. vivax,63–65 and in animal models of infection.62,66–68
In the context of our study, we observed a significant reduction in the reticulocyte population of BPH mice post-infection, while it increased (∼4.5-fold) in BPN mice during their recovery phase 15 d after infection. This observation points toward a scenario of ineffective erythropoiesis, or “dyserythropoiesis,” in BPH mice, likely due to the prolonged P. yoelii infection. In contrast, the recovery of BPN mice seems to reflect a relatively more efficient erythropoietic response, evident from the higher percentage of reticulocytes and lesser anemic condition. Further, we speculate that this diminished reticulocyte count might be indicative of spleen damage, as the spleen is involved in extramedullary hematopoiesis aimed at combating severe anemia during recovery in BPN mice that manifests as splenomegaly and changes in spleen histology.
The susceptibility of hypertensive patients to Plasmodium infection raises important public health concerns. The potential for sustained infection and severe anemia could indeed pose life-threatening risks, particularly in regions where malaria is endemic. Furthermore, even in areas where malaria is not a major concern, other blood-borne pathogens such as Bartonella, Toxoplasma, and Hepatitis B can significantly affect populations.69 The interaction between hypertension, anemia, and susceptibility to various infections highlights the complexity of health outcomes and emphasizes the importance of research in this area. The fact that our study holds significance not only in the context of malaria-endemic regions but also in countries like the United States, where lifestyle disorders including hypertension are on the rise, underscores the global relevance of our findings. Our study has the potential to shed light on how hypertensive patients might be more susceptible to various infections due to their anemic condition, a factor that should be considered in patient care. However, it is important to note that hypertension in BPH mice is not accompanied by metabolic disorders since it is sympathetically regulated.20,70 Therefore, it is important to conduct these studies in the future with other rodent models of hypertension, such as the spontaneously hypertensive rats, Dahl salt-sensitive rats, angiotensin II-deficient mice, and DOCA salt-sensitive mice.71 We are positive that our results can be reproduced in other mouse models of hypertension such as the angiotensin-converting-enzyme (Ace) I and II deficient mice since they suffer from normocytic anemia, similar to hypertensive humans, and have greater reticulocyte population compared to Ace sufficient mice.72–73 By addressing these limitations and recognizing the broader implications of this study, we believe that this study will contribute to the advancement of our understanding of the interplay between hypertension, anemia, and RBC infection susceptibility.
Supplementary Material
Contributor Information
Mrunmayee R Kandalgaonkar, Center for Hypertension and Precision Medicine, Department of Physiology and Pharmacology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA.
Beng San Yeoh, Center for Hypertension and Precision Medicine, Department of Physiology and Pharmacology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA.
Bina Joe, Center for Hypertension and Precision Medicine, Department of Physiology and Pharmacology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA.
Nathan W Schmidt, Ryan White Center for Pediatric Infectious Diseases and Global Health, Herman B. Wells Center for Pediatric Research, and Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Matam Vijay-Kumar, Center for Hypertension and Precision Medicine, Department of Physiology and Pharmacology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA.
Piu Saha, Center for Hypertension and Precision Medicine, Department of Physiology and Pharmacology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA.
Author Contributions
P.S.: conceptualization and formal analysis; P.S. and M.V-K.: funding acquisition; M.R.K., B.S.Y., M.V-K., and P.S.: investigation and methodology; P.S.: project administration; P.S. and M.V-K.: resources; P.S.: supervision; M.R.K., B.S.Y., B.J., N.W.S., M.V-K., and P.S.: visualization; M.R.K. and P.S.: writing—original draft; and M.R.K., B.S.Y., B.J., N.W.S., M.V-K., and P.S.: writing—review & editing.
Funding
This work was supported by grants from the Crohn’s and Colitis Foundation (CCF) and American Heart Association (AHA) Career Development Award [854385 and 855256, respectively] to P.S.; grant from the National Institutes of Health (NIH) to M.V-K. [CA219144] and B.J. [HL143082]; and AHA Postdoctoral Fellowship [831112] to B.S.Y.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Mozos I. Mechanisms linking red blood cell disorders and cardiovascular diseases. Biomed Res Int. 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iqbal AM, Jamal SF. Essential hypertension. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2023. [Google Scholar]
- 3. Marketou M, Patrianakos A, Parthenakis F et al. Systemic blood pressure profile in hypertensive patients with low hemoglobin concentrations. Int J Cardiol. 2010;142(1):95–96. [DOI] [PubMed] [Google Scholar]
- 4. Vyssoulis G, Karpanou E, Kyvelou SM, Tzamou V, Theodosiadis G, Stefanadis C. Ambulatory blood pressure profile in anemic hypertensive patients. Int J Cardiol. 2010;145(2):301–302. [DOI] [PubMed] [Google Scholar]
- 5. Cicco G, Pirrelli A. Red blood cell (RBC) deformability, RBC aggregability and tissue oxygenation in hypertension. Clin Hemorheol Microcirc. 1999;21(3-4):169–177. [PubMed] [Google Scholar]
- 6. Adams JH, Mueller I. The biology of plasmodium vivax. Cold Spring Harb Perspect Med. 2017;7(9):a025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Srivastava A, Creek DJ, Evans KJ et al. Host reticulocytes provide metabolic reservoirs that can be exploited by malaria parasites. PLoS Pathog. 2015;11(6):e1004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Depetris-Chauvin E, Weil DN. Malaria and early African development: evidence from the sickle cell trait. Econ J (London). 2018;128(610):1207–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luzzatto L. Sickle cell anaemia and malaria. Mediterr J Hematol Infect Dis. 2012;4(1):e2012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etyang AO, Smeeth L, Cruickshank JK, Scott JA. The malaria-high blood pressure hypothesis. Circ Res. 2016;119(1):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eze IC, Bassa FK, Esse C et al. Epidemiological links between malaria parasitaemia and hypertension: findings from a population-based survey in rural Cote d'Ivoire. J Hypertens. 2019;37(7):1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallego-Delgado J, Rodriguez A. Malaria and hypertension. Another co-evolutionary adaptation?. Front Cell Infect Microbiol. 2014;4:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffmeister B, Aguilar Valdez AD. Hypertension is associated with an increased risk for severe imported falciparum malaria: a tertiary care hospital based observational study from Berlin, Germany. Malar J. 2019;18(1):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiwari A, De A, Sinha A. Increasing blood pressure: could malaria have a role?. Lancet Glob Health. 2023;11(11):e1697. [DOI] [PubMed] [Google Scholar]
- 15. Verdecchia P, Angeli F, Reboldi G. Does malaria cause hypertension?. Circ Res. 2016;119(1):7–9. [DOI] [PubMed] [Google Scholar]
- 16. Gallego-Delgado J, Basu-Roy U, Ty M et al. Angiotensin receptors and beta-catenin regulate brain endothelial integrity in malaria. J Clin Invest. 2016;126(10):4016–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silva LS, Silva-Filho JL, Caruso-Neves C, Pinheiro AA. New concepts in malaria pathogenesis: the role of the renin-angiotensin system. Front Cell Infect Microbiol. 2015;5:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De A, Tiwari A, Pande V, Sinha A. Evolutionary trilogy of malaria, angiotensin II and hypertension: deeper insights and the way forward. J Hum Hypertens. 2022;36(4):344–351. [DOI] [PubMed] [Google Scholar]
- 19. Schlager G. Selection for blood pressure levels in mice. Genetics. 1974;76(3):537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson KL, Head GA, Gueguen C, Stevenson ER, Lim K, Marques FZ. Mechanisms responsible for genetic hypertension in schlager BPH/2 Mice. Front Physiol. 2019;10:1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta V, Kapopara PR, Khan AA et al. Functional promoter polymorphisms direct the expression of cystathionine gamma-lyase gene in mouse models of essential hypertension. J Mol Cell Cardiol. 2017;102:61–73. [DOI] [PubMed] [Google Scholar]
- 22. Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp. 2009;(27):1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanahan DJ, Ekholm JE. The preparation of red cell ghosts (membranes). Methods Enzymol. 1974;31:168–172. [DOI] [PubMed] [Google Scholar]
- 24. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 25. Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—methanol. J Lipid Res. 1964;5(4):600–608. [PubMed] [Google Scholar]
- 26. Anderson J, Glynn LG, Newell J, Iglesias AA, Reddan D, Murphy AW. The impact of renal insufficiency and anaemia on survival in patients with cardiovascular disease: a cohort study. BMC Cardiovasc Disord. 2009;9(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paul B, Wilfred NC, Woodman R, Depasquale C. Prevalence and correlates of anaemia in essential hypertension. Clin Exp Pharma Physio. 2008;35(12):1461–1464. [DOI] [PubMed] [Google Scholar]
- 28. Niesor EJ, Nader E, Perez A et al. Red blood cell membrane cholesterol may be a key regulator of sickle cell disease microvascular complications. Membranes. 2022;12(11):1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohkawa R, Low H, Mukhamedova N et al. Cholesterol transport between red blood cells and lipoproteins contributes to cholesterol metabolism in blood. J Lipid Res. 2020;61(12):1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hung KT, Berisha SZ, Ritchey BM, Santore J, Smith JD. Red blood cells play a role in reverse cholesterol transport. ATVB. 2012;32(6):1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gold JC, Phillips MC. Effects of membrane lipid composition on the kinetics of cholesterol exchange between lipoproteins and different species of red blood cells. Biochim Biophys Acta Biomembr. 1990;1027(1):85–92. [DOI] [PubMed] [Google Scholar]
- 32. Quarfordt SH, Hilderman HL. Quantitation of the in vitro free cholesterol exchange of human red cells and lipoproteins. J Lipid Res. 1970;11(6):528–535. [PubMed] [Google Scholar]
- 33. Turner S, Voogt J, Davidson M et al. Measurement of reverse cholesterol transport pathways in humans: in vivo rates of free cholesterol efflux, esterification, and excretion. JAHA. 2012;1(4):e001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dey S, Bindu S, Goyal M et al. Impact of intravascular hemolysis in malaria on liver dysfunction: involvement of hepatic free heme overload, NF-kappaB activation, and neutrophil infiltration. J Biol Chem. 2012;287(32):26630–26646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kochar DK, Agarwal P, Kochar SK et al. Hepatocyte dysfunction and hepatic encephalopathy in Plasmodium falciparum malaria. QJM. 2003;96(7):505–512. [DOI] [PubMed] [Google Scholar]
- 36. Premaratna R, Gunatilake AK, de Silva NR, Tilakaratne Y, Fonseka MM, de Silva HJ. Severe hepatic dysfunction associated with falciparum malaria. Southeast Asian J Trop Med Public Health. 2001;32(1):70–72. [PubMed] [Google Scholar]
- 37. Labaied M, Jayabalasingham B, Bano N et al. Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver. Cell Microbiol. 2011;13(4):569–586. [DOI] [PubMed] [Google Scholar]
- 38. Zivot A, Lipton JM, Narla A, Blanc L. Erythropoiesis: insights into pathophysiology and treatments in 2017. Mol Med. 2018;24(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orkin SH. Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet. 2000;1(1):57–64. [DOI] [PubMed] [Google Scholar]
- 40. Martin-Jaular L, Elizalde-Torrent A, Thomson-Luque R et al. Reticulocyte-prone malaria parasites predominantly invade CD71hi immature cells: implications for the development of an in vitro culture for Plasmodium vivax. Malar J. 2013;12(1):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landau I, Killick-Kendrick R. Rodent plasmodia of the Republique Centrafricaine: the sporogony and tissue stages of Plasmodium chabaudi and P. berghei yoelii. Trans R Soc Trop Med Hyg. 1966;60(5):633–649. [DOI] [PubMed] [Google Scholar]
- 42. Grzywa TM, Nowis D, Golab J. The role of CD71(+) erythroid cells in the regulation of the immune response. Pharmacol Ther. 2021;228:107927. [DOI] [PubMed] [Google Scholar]
- 43. Orlov SN, Gulak PV, Litvinov IS, Postnov Yu V. Evidence of altered structure of the erythrocyte membrane in spontaneously hypertensive rats. Clin Sci (Lond). 1982;63(1):43–45. [DOI] [PubMed] [Google Scholar]
- 44. Montenay-Garestier T, Aragon I, Devynck MA, Meyer P, Helene C. Evidence for structural changes in erythrocyte membranes of spontaneously hypertension rats. A fluorescence polarization study. Biochem Biophys Res Commun. 1981;100(2):660–665. [DOI] [PubMed] [Google Scholar]
- 45. Fu YF, Dong YZ, Li H, Lu ZM, Wang W. Erythrocyte membrane lipid composition fluidity in patients with essential hypertension. Chin Med J (Engl). 1992;105(10):803–808. [PubMed] [Google Scholar]
- 46. Odashiro K, Saito K, Arita T, Maruyama T, Fujino T, Akashi K. Impaired deformability of circulating erythrocytes obtained from nondiabetic hypertensive patients: investigation by a nickel mesh filtration technique. Clin Hypertens. 2015;21(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaczmarska M, Fornal M, Messerli FH, Korecki J, Grodzicki T, Burda K. Erythrocyte membrane properties in patients with essential hypertension. Cell Biochem Biophys. 2013;67(3):1089–1102. [DOI] [PubMed] [Google Scholar]
- 48. Kariuki SN, Marin-Menendez A, Introini V et al. Red blood cell tension protects against severe malaria in the Dantu blood group. Nature. 2020;585(7826):579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maier AG, van Ooij C. The role of cholesterol in invasion and growth of malaria parasites. Front Cell Infect Microbiol. 2022;12:984049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahiya AI, Bhatnagar S, Morrisey JM, Beck JR, Vaidya AB. Dramatic consequences of reducing erythrocyte membrane cholesterol on Plasmodium falciparum. Microbiol Spectr. 2022;10(1):e0015822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Imwong M, Madmanee W, Suwannasin K et al. Asymptomatic natural human infections with the simian malaria parasites Plasmodium cynomolgi and Plasmodium knowlesi. J Infect Dis. 2019;219(5):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh B, Kim Sung L, Matusop A et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet North Am Ed. 2004;363(9414):1017–1024. [DOI] [PubMed] [Google Scholar]
- 54. Leong YW, Russell B, Malleret B, Renia L. Erythrocyte tropism of malarial parasites: the reticulocyte appeal. Front Microbiol. 2022;13:1022828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chan LJ, Dietrich MH, Nguitragool W, Tham WH. Plasmodium vivax reticulocyte binding proteins for invasion into reticulocytes. Cell Microbiol. 2020;22(1):e13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Malleret B, Li A, Zhang R et al. Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood. 2015;125(8):1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schubert TE, Obermaier F, Ugocsai P et al. Murine models of anaemia of inflammation: extramedullary haematopoiesis represents a species specific difference to human anaemia of inflammation that can be eliminated by splenectomy. Int J Immunopathol Pharmacol. 2008;21(3):577–584. [DOI] [PubMed] [Google Scholar]
- 59. Shimizu Y, Kawashiri SY, Yamanashi H et al. Reticulocyte levels have an ambivalent association with hypertension and atherosclerosis in the elderly: a cross-sectional study. Clin Interv Aging. 2019;14:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gruszczyk J, Kanjee U, Chan LJ et al. Transferrin receptor 1 is a reticulocyte-specific receptor for Plasmodium vivax. Science. 2018;359(6371):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nanda N. Ultrastructural study on the erythrocytic schizogony of Plasmodium vivax. Indian J Malariol. 1990;27(1):15–23. [PubMed] [Google Scholar]
- 62. Barnwell JW, Ingravallo P, Galinski MR, Matsumoto Y, Aikawa M. Plasmodium vivax: malarial proteins associated with the membrane-bound caveola-vesicle complexes and cytoplasmic cleft structures of infected erythrocytes. Exp Parasitol. 1990;70(1):85–99. [DOI] [PubMed] [Google Scholar]
- 63. Joice R, Nilsson SK, Montgomery J et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Sci Transl Med. 2014;6(244):244re245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mantel PY, Hoang AN, Goldowitz I et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13(5):521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Regev-Rudzki N, Wilson DW, Carvalho TG et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153(5):1120–1133. [DOI] [PubMed] [Google Scholar]
- 66. Nantakomol D, Dondorp AM, Krudsood S et al. Circulating red cell-derived microparticles in human malaria. J Infect Dis. 2011;203(5):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Campos FM, Franklin BS, Teixeira-Carvalho A et al. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J. 2010;9(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wickramasinghe SN, Looareesuwan S, Nagachinta B, White NJ. Dyserythropoiesis and ineffective erythropoiesis in Plasmodium vivax malaria. Br J Haematol. 1989;72(1):91–99. [DOI] [PubMed] [Google Scholar]
- 69. McCullough J. RBCs as targets of infection. Hematology Am Soc Hematol Educ Program. 2014;2014(1):404–409. [DOI] [PubMed] [Google Scholar]
- 70. Jackson KL, Nguyen-Huu TP, Davern PJ, Head GA. Energy metabolism in BPH/2 J genetically hypertensive mice. Hypertens Res. 2014;37(5):413–421. [DOI] [PubMed] [Google Scholar]
- 71. Jama HA, Muralitharan RR, Xu C et al. Rodent models of hypertension. British J Pharmacology. 2022;179(5):918–937. [DOI] [PubMed] [Google Scholar]
- 72. Cole J, Ertoy D, Lin H et al. Lack of angiotensin II-facilitated erythropoiesis causes anemia in angiotensin-converting enzyme-deficient mice. J Clin Invest. 2000;106(11):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cole JM, Corvol P, Bernstein KE. Evaluation of anemia in angiotensin converting enzyme (ACE) knockout mice. Hypertension. 2000;36(1):693–693. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.