Abstract
Background
The incidence of hip fracture is increasing and it is more common with increasing age. Surgery is used for almost all hip fractures. Blood loss occurs as a consequence of both the fracture and the surgery and thus red blood cell transfusion is frequently used. However, red blood cell transfusion is not without risks. Therefore, it is important to identify the evidence for the effective and safe use of red blood cell transfusion in people with hip fracture.
Objectives
To assess the effects (benefits and harms) of red blood cell transfusion in people undergoing surgery for hip fracture.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (31 October 2014), the Cochrane Central Register of Controlled Trials (The Cochrane Library, 2014, Issue 10), MEDLINE (January 1946 to 20 November 2014), EMBASE (January 1974 to 20 November 2014), CINAHL (January 1982 to 20 November 2014), British Nursing Index Database (January 1992 to 20 November 2014), the Systematic Review Initiative's Transfusion Evidence Library, PubMed for e‐publications, various other databases and ongoing trial registers.
Selection criteria
Randomised controlled trials comparing red blood cell transfusion versus no transfusion or an alternative to transfusion, different transfusion protocols or different transfusion thresholds in people undergoing surgery for hip fracture.
Data collection and analysis
Three review authors independently assessed each study's risk of bias and extracted data using a study‐specific form. We pooled data where there was homogeneity in the trial comparisons and the timing of outcome measurement. We used GRADE criteria to assess the quality (low, moderate or high) of the evidence for each outcome.
Main results
We included six trials (2722 participants): all compared two thresholds for red blood cell transfusion: a 'liberal' strategy to maintain a haemoglobin concentration of usually 10 g/dL versus a more 'restrictive' strategy based on symptoms of anaemia or a lower haemoglobin concentration, usually 8 g/dL. The exact nature of the transfusion interventions, types of surgery and participants varied between trials. The mean age of participants ranged from 81 to 87 years and approximately 24% of participants were men. The largest trial enrolled 2016 participants, over 60% of whom had a history of cardiovascular disease. The percentage of participants receiving a red blood cell transfusion ranged from 74% to 100% in the liberal transfusion threshold group and from 11% to 45% in the restrictive transfusion threshold group. There were no results available for the smallest trial (18 participants). All studies were at some risk of bias, in particular performance bias relating to the absence of blinding of personnel. We judged the evidence for all outcomes, except myocardial infarction, was low quality reflecting risk of bias primarily from imbalances in protocol violations in the largest trial and imprecision, often because of insufficient events. Thus, further research is likely to have an important impact on these results.
There was no evidence of a difference between a liberal versus restricted threshold transfusion in mortality, at 30 days post hip fracture surgery (risk ratio (RR) 0.92, 95% confidence interval (CI) 0.67 to 1.26; five trials; 2683 participants; low quality evidence) or at 60 days post surgery (RR 1.08, 95% CI 0.80 to 1.44; three trials; 2283 participants; low quality evidence). Assuming an illustrative baseline risk of 50 deaths per 1000 participants in the restricted threshold group at 30 days, these data equate to four fewer (95% CI 17 fewer to 14 more) deaths per 1000 in the liberal threshold group at 30 days.
There was no evidence of a difference between a liberal versus restricted threshold transfusion in functional recovery at 60 days, assessed in terms of the inability to walk 10 feet (3 m) without human assistance (RR 1.00, 95% CI 0.87 to 1.15; two trials; 2083 participants; low quality evidence).
There was low quality evidence of no difference between the transfusion thresholds in postoperative morbidity for the following complications: thromboembolism (RR 1.15 favouring a restrictive threshold, 95% CI 0.56 to 2.37; four trials; 2416 participants), stroke (RR 2.40 favouring a restrictive threshold, 95% CI 0.85 to 6.79; four trials; 2416 participants), wound infection (RR 1.61 favouring a restrictive threshold, 95% CI 0.77 to 3.35; three trials; 2332 participants), respiratory infection (pneumonia) (RR 1.35 favouring a restrictive threshold, 95% CI 0.95 to 1.92; four trials; 2416 participants) and new diagnosis of congestive heart failure (RR 0.77 favouring a liberal threshold, 95% CI 0.48 to 1.23; three trials; 2332 participants). There was very low quality evidence of a lower risk of myocardial infarction in the liberal compared with the restrictive transfusion threshold group (RR 0.59, 95% CI 0.36 to 0.96; three trials; 2217 participants). Assuming an illustrative baseline risk of myocardial infarction of 24 per 1000 participants in the restricted threshold group, this result was compatible with between one and 15 fewer myocardial infarctions in the liberal threshold group.
Authors' conclusions
We found low quality evidence of no difference in mortality, functional recovery or postoperative morbidity between 'liberal' versus 'restrictive' thresholds for red blood cell transfusion in people undergoing surgery for hip fracture. Although further research may change the estimates of effect, the currently available evidence does not support the use of liberal red blood cell transfusion thresholds based on a 10 g/dL haemoglobin trigger in preference to more restrictive transfusion thresholds based on lower haemoglobin levels or symptoms of anaemia in these people. Future research needs to address the effectiveness of red blood cell transfusions at different time points in the surgical pathway, whether pre‐operative, peri‐operative or postoperative. In particular, such research would need to consider people who are symptomatic or haemodynamically unstable who were excluded from most of these trials.
Keywords: Aged, 80 and over; Humans; Anemia; Anemia/therapy; Erythrocyte Transfusion; Erythrocyte Transfusion/adverse effects; Erythrocyte Transfusion/methods; Erythrocyte Transfusion/mortality; Hemoglobin A; Hemoglobin A/analysis; Hip Fractures; Hip Fractures/blood; Hip Fractures/mortality; Hip Fractures/surgery; Postoperative Complications; Randomized Controlled Trials as Topic; Recovery of Function
Plain language summary
Red blood cell transfusion for people undergoing hip fracture surgery
Background and aims
Most people who break their hip (hip fracture) are over 65 years old. Almost all hip fractures require surgery. People with hip fracture often receive red blood cell transfusions that aim to correct their anaemia (low levels of haemoglobin in the blood; haemoglobin is an oxygen‐carrying molecule found within red blood cells) resulting from blood loss from their fracture or surgery. However, blood transfusion is not without risk. We aimed to look at the evidence for the use of red blood cell transfusion in people undergoing surgery for a broken hip. We wanted to find out whether and when blood transfusion is of benefit and whether there are better alternatives to transfusion for these people.
Results of the search
We searched medical databases up to 20 November 2014 for studies that compared red blood cell transfusion versus no transfusion or an alternative to transfusion, different transfusion protocols or different transfusion thresholds in people undergoing any type of surgery for hip fracture. We found six studies (2722 people), all of which compared two different 'transfusion thresholds' for a red blood cell transfusion. The trials compared a liberal red blood cell transfusion threshold (giving a transfusion when the haemoglobin concentration was less than 10 g/dL) with a restrictive red blood cell transfusion threshold (giving a transfusion only when the person had symptoms of anaemia or when the haemoglobin concentration was less than 8 g/dL). Five studies applied these thresholds after surgery. The average age of trial participants was over 80 years and around three‐quarters were women.
Key results
We found no difference between the two transfusion threshold groups in the number of people who had died at 30 and 60 days after their operation. We found similar numbers of people in the two groups were unable to walk 10 feet (3 metres) or across a room without help at 60‐day follow‐up.
We were interested in the number of major complications following surgery (thromboembolism (blood clots), stroke, chest and wound infection, and cardiovascular events (heart attacks, heart failure or abnormal heart rhythms)). There was little difference between the two transfusion threshold groups in the number of people experiencing any of these major complications. Although we found the risk of a heart attack was lower in people treated with the liberal red blood cell transfusion threshold than in people treated with the restrictive red blood cell transfusion threshold, we are very unsure of this finding.
Quality of the evidence
All of the studies had some aspects that could undermine the reliability of their results. We decided the evidence was of low quality for all outcomes. Thus, we have some uncertainty about these findings and further research may provide evidence that could change our conclusions.
Conclusions
The current evidence does not support the use of liberal red blood cell transfusion thresholds based on a 10 g/dL haemoglobin trigger in preference to more restrictive transfusion thresholds based on lower haemoglobin levels or symptoms of anaemia in people with a broken hip. Further research needs to address the use of red blood cell transfusions before, during and after hip fracture surgery, and the use of red blood cell transfusions especially in people who have symptoms that reflect impaired blood flow and function.
Summary of findings
Summary of findings for the main comparison. Liberal versus restrictive threshold transfusion for people undergoing hip fracture surgery.
| Liberal versus restrictive threshold transfusion for people undergoing hip fracture surgery | ||||||
|
Patient or population: people undergoing hip fracture surgery1
Settings: hospital
Intervention: liberal threshold red blood cell transfusion2 Comparison: restrictive threshold red blood cell transfusion3 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Restrictive threshold | Liberal threshold | |||||
| 30‐day mortality Follow‐up: mean 30 days | 50 per 10004 | 46 per 1000 (33 to 63) | RR 0.92 (0.67 to 1.26) | 2683 (5 studies) | ⊕⊕⊝⊝ low5,6 | ‐ |
| Inability to walk 10 feet (3 m; or across a room) without human assistance Follow‐up: mean 60 days | 283 per 10004 | 283 per 1000 (246 to 326) | RR 1.00 (0.87 to 1.15) | 2083 (2 studies) | ⊕⊕⊝⊝ low7,8 | ‐ |
| Thromboembolism (in hospital) | 20 per 10004 | 23 per 1000 (11 to 47) | RR 1.15 (0.56 to 2.37) | 2416 (4 studies) | ⊕⊕⊝⊝ low6,9 | ‐ |
| Stroke (in hospital) | 2 per 10004 | 5 per 1000 (2 to 14) | RR 2.4 (0.85 to 6.79) | 2416 (4 studies) | ⊕⊕⊝⊝ low6,9 | ‐ |
| Wound infection (in hospital) | 8 per 10004 | 13 per 1000 (6 to 27) | RR 1.61 (0.77 to 3.35) | 2332 (3 studies) | ⊕⊕⊝⊝ low6,10 | ‐ |
| Cardiovascular events ‐ myocardial infarction | 24 per 10004 | 14 per 1000 (9 to 23) | RR 0.59 (0.36 to 0.96) | 2217 (3 studies) | ⊕⊝⊝⊝ very low6,11 | ‐ |
| Respiratory infections (namely pneumonia) | 18 per 10004 | 24 per 1000 (17 to 35) | RR 1.35 (0.95 to 1.92) | 2416 (4 studies) | ⊕⊕⊝⊝ low6,12 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Although we included evidence for pre‐operative, peri‐operative and postoperative transfusion, the majority of the evidence applied to postoperative transfusion.
2. The liberal transfusion threshold was a haemoglobin concentration of about 10 g/dL in four trials, and 11.3 g/dL in one trial.
3. The restrictive transfusion threshold in four trials was a haemoglobin concentration of about 8 g/dL or symptoms of anaemia, and 9.7 g/dL in one trial.
4. The assumed risk was the median control risk across studies.
5. We downgraded the evidence one level because of risk of bias: 57% of the weighting for this outcome came from one trial in which there was a statistical difference (P value = 0.003) in the number of major protocol violations post randomisation between the two transfusion threshold groups.
6. We downgraded the evidence one level because of imprecision: generally because of the small number of events in the studies reporting data for this outcome has resulted in wide confidence intervals for these studies.
7. We downgraded the evidence one level because of risk of bias: 96% of the weighting for this outcome came from one trial in which there was a statistical difference (P value = 0.003) in the number of major protocol violations post randomisation between the two transfusion threshold groups.
8. We further downgraded the evidence one level because of risk of bias: both participants and study personnel "were aware of study group assignment after randomisation" in the two studies reporting data for this subjective outcome. Given that the participants themselves are involved in assessing this outcome, knowledge of treatment allocation may influence outcome measurement.
9. We downgraded the evidence one level because of risk of bias: 60% of the weighting for this outcome came from one trial in which there was a statistical difference (P value = 0.003) in the number of major protocol violations post randomisation between the two transfusion threshold groups.
10. We downgraded the evidence one level because of risk of bias: 70% of the weighting for this outcome came from one trial in which there was a statistical difference (P value = 0.003) in the number of major protocol violations post randomisation between the two transfusion threshold groups.
11. We downgraded the evidence two levels because of risk of bias: 94% of the weighting for this outcome came from one trial in which there was a statistical difference (P value = 0.003) in the number of major protocol violations post randomisation between the two transfusion threshold groups; and, although numbers were comparable between the two transfusion threshold groups in this trial, overall 265 (13%) participants had incomplete electrocardiographic results and in 355 (18%) participants there was no blood sample for troponin testing. Thus, attrition bias was a potential problem.
12. We downgraded the evidence one level because of bias: 93% of the weighting for this outcome came from one trial in which there was a statistical difference (P value = 0.003) in the number of major protocol violations post randomisation between the two transfusion threshold groups.
Background
Description of the condition
Hip fracture is the common term for fractures of the proximal end of the femur (upper end of the thigh bone). These fractures are often subdivided into intracapsular fractures, which are fractures located within the hip joint capsule, and extracapsular fractures, which are outside the hip joint capsule. These main types have different degrees of mean blood loss and require different fixation techniques. Most hip fractures occur in old and frail people (mean age about 80 years) and are usually the result of a low impact injury or a fall. The incidence of hip fracture is increasing in line with increases of the mean age of populations in many countries: the number of hip fractures worldwide has been estimated to rise from 1.7 million in 1990 to 6.3 million in 2050 (Gullberg 1997).
There is general agreement among orthopaedic surgeons that hip fracture requires surgery either to repair the fracture or to replace part or all of the hip joint (Egol 2009). About 5% to 10% of people with hip fracture die within one month of their fracture (Parker 2006). Many people who have hip fracture surgery do not recover fully after their hip fracture, being less mobile and less independent than before their injury (Egol 2009).
Description of the intervention
Alongside the need for surgery, many people with hip fracture will receive red blood cell transfusions because of anaemia or bleeding. Anaemia in these people (resulting in a deficiency in the oxygen‐carrying red blood cells) may be present prior to their fracture (Penninx 2006). It is well recognised that the incidence of anaemia in the general population rises with increasing age, often reflecting concurrent illnesses and comorbidities. Anaemia may also occur as a consequence of blood loss at the time of the fracture or during and after surgery (Foss 2006). It is now recognised that people with hip fracture may have large blood loss that occurs after the fracture and before surgery (Smith 2011). Mean blood loss has been calculated at 1.5 units of red blood cells in intracapsular fractures and 2 units in extracapsular fractures. People with hip fracture are often frail and have less reserve than younger people to cope with the resulting haemodynamic changes. In the UK, many people wait more than 24 hours for surgery although early operation was incentivised by the Department of Health in April 2010 through their 'best practice tariffs' scheme (Department of Health Payment by Results team 2012). Red blood cell transfusion is then used to improve the oxygen‐carrying capacity of the blood and is a key part of supportive management of people undergoing hip fracture surgery. People receiving blood transfusions require active monitoring of all vital signs including pulse, blood pressure and temperature to detect any acute adverse reactions.
The use, timing and quantity of red blood cell transfusion may depend on several factors, including the severity of anaemia. One retrospective cohort study of over 3000 people operated on for hip fracture reported that nearly 30% received a perioperative allogeneic (blood donated from other people) blood transfusion (Johnston 2006). Various studies of people with hip fracture have demonstrated substantial variability in the use of red blood cell transfusion among both physicians and hospitals (e.g. Hutton 2005). The decision for red blood cell transfusion is often based on a threshold haemoglobin concentration with a red blood cell transfusion being triggered should the haemoglobin fall below this threshold value. For instance, Foss 2006 reported that people with hip fracture were given red blood cell transfusion if the haemoglobin concentration fell below 10 g/dL at any point during their hospitalisation. The decision for transfusion may also be influenced by other factors such as participant age and co‐existing medical morbidity such as coronary or respiratory disease (Dillon 2005).
Other strategies for preventing or correcting anaemia should be considered in people with hip fracture, in addition to transfusion needs. Methods aimed at reducing the need for red blood cell transfusion include perioperative cell salvage or treatment with oral or intravenous iron, erythropoietin, or iron plus erythropoietin. Erythropoietin is a hormone that promotes the formation of red blood cells by the bone marrow.
How the intervention might work
It is generally accepted that red blood cell transfusion corrects any pre‐existing anaemia and replaces lost blood in people with hip fracture and improves or maintains the oxygen‐carrying capacity in the circulation. Improved tissue oxygenation may then help recovery during and after surgery. However, limited data indicate improved functional outcomes after surgery with higher haemoglobin concentrations in transfused people with hip fracture (Lawrence 2003). There are also well‐recognised risks associated with red blood cell transfusion as for any blood component for transfusion. Adverse effects of transfusion include transfusion of the wrong blood products (due to errors in the pathways of processing or administration) resulting in acute haemolytic transfusion reactions, transfusion‐transmitted infections, other types of haemolytic reactions, respiratory complications and allergic reactions (SHOT 2010). A 2‐unit transfusion of red blood cells represents a volume of over 500 mL and may result in transfusion‐associated circulatory overload in older people who may poorly tolerate infusion of even moderate volumes of fluid because of co‐morbidities such as cardiac disease.
Why it is important to do this review
Red blood cell transfusion is a frequently used clinical intervention with around two million red blood cell units issued by UK transfusion services per year (SHOT 2010). It is a costly and scarce resource and is associated with risks (see How the intervention might work) (Carson 1999; SHOT 2010). Many red blood cell transfusions are given to stable and non‐bleeding people where the evidence from clinical studies suggests no clear benefit (Carless 2010). Surgery for hip fracture is common and many people receive red blood cell transfusions. In these people, important outcomes also include postoperative functional recovery, mobility and quality of life (QoL) (Adunsky 2008; Foss 2008). Given the rising incidence of hip fracture, the wide variation in transfusion practice and risks of red blood cell transfusion, it is important to identify and appraise the evidence for its safe and effective use in order to inform practice. This review includes evidence from randomised controlled trials on the use of red blood cell transfusions (or alternatives) in people with hip fracture.
Objectives
To assess the effects (benefits and harms) of red blood cell transfusion in people undergoing surgery for hip fracture.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials or quasi‐randomised controlled trials (where the method of allocating participants to a treatment is not strictly random: e.g. by date of birth, hospital record number, alternation) assessing red blood cell transfusion for people undergoing hip fracture surgery.
Types of participants
People requiring or undergoing surgery for hip fracture (examples of surgery for hip fracture include sliding hip screw, intermedullary hip screw and arthroplasty).
Types of interventions
We set out to compare the following:
red blood cell transfusion versus no transfusion;
red blood cell transfusion versus alternative methods such as cell salvage, iron supplements or erythropoietin (we excluded studies evaluating use of tranexamic acid, as this is the subject of an another Cochrane review (Perel 2013));
red blood cell transfusion protocol A versus red blood cell transfusion protocol B;
an example of this would be a comparison of a red blood cell transfusion given according to criteria detailed in one protocol (e.g. volume of transfusion, rate of transfusion) with a red blood cell transfusion given according to criteria detailed in another protocol (e.g. with a different volume of transfusion or different rate of transfusion);
red blood cell transfusion threshold A versus red blood cell transfusion threshold B. Trials using different measures, such as haematocrit (a measure of the percentage of red blood cells to the total blood) for setting thresholds were also eligible;
an example of this would be a comparison of a liberal versus a restrictive haemoglobin concentration threshold, whereby a person would only be eligible to receive a red blood cell transfusion when their haemoglobin concentration fell below a given liberal or restrictive transfusion threshold.
Types of outcome measures
We included the following outcomes. We did not specify in advance the time points for the measurement of these outcomes, as we were interested in recording all measures that had been made per outcome. Overall, however, we were interested in outcomes reported in the immediate postoperative period through to outcomes reported during the follow‐up for the trial. We have reported the time points at which each outcome was measured alongside the analysis of data.
Primary outcomes
Mortality.
Mobility and functional recovery.
Postoperative morbidity, including medical complications (e.g. wound infections (in hospital), thromboses (in hospital), stroke, myocardial infarction and other cardiovascular events) and respiratory infections (including pneumonia).
Secondary outcomes
Postoperative or discharge haemoglobin.
Quality of life (QoL).
Length of stay in hospital.
Adverse effects of transfusion (including haemolytic transfusion reaction, inappropriate blood component transfusion, allergic reactions and transfusion‐transmitted infections).
Search methods for identification of studies
Electronic searches
The Systematic Review Initiative's Information Specialist (CD) formulated the search strategies in collaboration with the Cochrane Heart Group.
Bibliographic databases
We searched the following databases:
Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (31 October 2014);
Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 10);
MEDLINE (Ovid) (1946 to 20 November 2014);
EMBASE (Ovid) (1974 to 20 November 2014);
PubMed (searched for e‐publications ahead of print on 20 November 2014);
CINAHL (NHS Evidence) (1982 to 20 November 2014);
British Nursing Index Database (NHS Evidence) (1992 to 20 November 2014);
Transfusion Evidence Library (1980 to 20 November 2014);
LILACS (1982 to 20 November 2014);
IndMed (1985 to 20 November 2014);
KoreaMed (1997 to 20 November 2014);
PakMediNet (1995 to 20 November 2014);
Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (Thomson Reuters, 1990 to 20 November 2014).
Online databases of ongoing trials
ClinicalTrials.gov (20 November 2014).
ISRCTN Registry (20 November 2014).
World Health Organization International Clinical Trials Registry Search Platform (WHO ICTRP) (20 November 2014).
Appendix 1 shows all search strategies used. We combined searches in MEDLINE with the Cochrane Highly Sensitive RCT Search Filter as detailed in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We combined searches in EMBASE and CINAHL with adaptations of the relevant Scottish Intercollegiate Guidelines Network (SIGN) RCT filters. We applied no restrictions on language or publication status.
Searching other resources
We checked reference lists of relevant articles to identify any eligible studies missed through the electronic searching.
Data collection and analysis
Selection of studies
Three review authors (SB, SM and AS) screened all titles and abstracts of papers identified via the electronic searches. We excluded only clearly irrelevant references at this first stage. We retrieved the full‐texts of the remaining references and three review authors independently assessed them for inclusion using a review‐specific eligibility form. We resolved any screening disagreements by discussion.
Pairs of review authors from four authors (SB, SM, AS and SS) undertook the screening of the references identified in the three search periods ending 21 February 2012, 8 October 2013 and 20 November 2014.
Data extraction and management
Two review authors (AS and SM) independently undertook data extraction using a piloted study‐specific data extraction form. We extracted data on the setting of the trial, the methods and statistical assumptions made, the characteristics of participants and interventions, details of the outcomes measured and timing of the outcome assessments. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
Two review authors (AS and SM) independently assessed risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved disagreements by discussion and through consultation with a third review author (SB) as required. For each of the included trials, we assessed the risk of bias as low risk, high risk or unclear risk for the following domains.
Generation of random sequence (selection bias).
Concealment of treatment allocation schedule (selection bias).
Blinding of participants to treatment allocation (performance bias or detection bias).
Blinding of personnel (person(s) delivering the treatment) to treatment allocation (performance bias).
Blinding of outcome assessors to treatment allocation (detection bias).
Completeness of the outcome data (including checks for possible attrition bias through withdrawals, loss to follow‐up and protocol violations).
Selective reporting of outcome (reporting bias).
Other sources of bias (other bias). We assessed whether each trial was free of problems not identified via the above domains.
Measures of treatment effect
We calculated risk ratios (RR) for dichotomous outcomes. We expressed treatment effects for continuous data outcomes as mean differences (MD). We used 95% confidence intervals (CI) throughout. Where reported, we described non‐parametric measures such as medians and interquartile ranges in the text and tables.
Unit of analysis issues
We did not anticipate finding or find cross‐over or cluster randomised trials. Should either of these have been found, we would have made appropriate adjustments according to the advice in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
Where possible, we sought missing data directly from the author(s) of the individual trial(s). For all included trials, we noted levels of attrition. If data had been available, we would have undertaken sensitivity analysis to examine the impact of losses on dichotomous outcomes.
Assessment of heterogeneity
Assessment of clinical heterogeneity included consideration of participant characteristics (e.g. underlying morbidity, type of fracture and surgery), trial design and risk of bias, care programmes provided to trial participants such as method of anaesthesia, and outcome definition and measurement.
We assessed statistical heterogeneity of treatment effects between trials using a Chi² test with a significant level at P value < 0.1. We used the I² statistic to quantify the percentage of variability that was due to heterogeneity (where I² > 40% indicated moderate heterogeneity and I² > 75% indicated considerable heterogeneity).
Assessment of reporting biases
We did not formally assess reporting biases in this review. We made every effort to identify unpublished studies through the search activities identified earlier in this report. If there had been sufficient numbers of studies (over 10) included in individual meta‐analysis, we would have used funnel plots to assess possible reporting biases.
Data synthesis
Meta‐analysis was undertaken using Review Manager 5 where there were sufficient data (RevMan 2011). We used a fixed‐effect model for combining data in all instances, as we observed no substantial heterogeneity. If substantial heterogeneity had been identified in a fixed‐effect meta‐analysis, we would have noted this and repeated the analysis using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
If there had been sufficient data, we would have undertaken subgroup analyses of type of hip fracture (intracapsular vs. extracapsular), type of surgery (hip fracture fixation vs. hip replacement) and gender to examine for significant differences in treatment effects. We did not have appropriate data to substantiate this type of analysis within the review, but we intend to undertake these subgroup analyses in future updates of the review as data allow. We would use the test for subgroup differences provided in Review Manager 5 to establish whether the subgroups are statistically significantly different from one another (RevMan 2011).
Sensitivity analysis
We planned to undertake sensitivity analyses exploring aspects of trial and review methodology. These would have included exploring the effects of removing trials at high or unclear risk of selection bias (reflecting lack of confirmation of random sequence generation and allocation concealment); detection bias (reflecting lack of assessor blinding) or attrition bias, such as from high levels of missing data. There were not enough data to enable these analyses to be undertaken. In future updates of this review, we will perform these sensitivity analyses as data allow.
However, we undertook two post‐hoc sensitivity analyses. In the first, we removed the largest trial from all pooled analyses where it reported data to examine the result of pooling the other smaller trials. In the second, we removed the trial that randomised and transfused participants perioperatively to determine whether the timing of the study eligibility for transfusion (perioperative or postoperatively) affected the pooled estimates for those outcomes where it had reported data. We provided details of these sensitivity analyses in the Effects of interventions.
'Summary of findings' tables
We constructed a 'Summary of findings' table for this review; this included using the GRADE approach to assess the quality of evidence related to the primary outcomes listed in the Types of outcome measures (Schünemann 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search strategy identified 1086 references of which we excluded 208 in the first screening for being either a duplicate or clearly irrelevant to the scope of this review. Of the remaining 878 references, we excluded 844: 537 did not meet the inclusion criteria for participants, interventions, or both and 307 were not randomised controlled trials.
We obtained the full text of the remaining 34 references. We contacted, by email, the authors of seven identified trials for further information about their trials (Carson 2011; Foss 2009; Gregersen 2015; Matot 2012; Nielsen 2012; Palmer 1998; Parker 2013). All the authors responded to our enquiries and we incorporated the information and data they provided into this review.
Of the 34 references subject to full‐text screening, we deemed six trials (reported in 22 references) to be eligible for inclusion (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Palmer 1998; Parker 2013), and one as a trial awaiting assessment (ChiCTR‐TRC‐10000822). We excluded 11 studies for not meeting the eligibility criteria of this review (Gampopoulou 2004; Izuel‐Rami 2005; Izuel‐Rami 2006; Jans 2011; Matot 2012; Moghaddam 2009; Muir 1995; Nielsen 2012; Prasad 2009; Serrano Trenas 2011; Zufferey 2010). We identified no ongoing trials. Full details are reported in the PRISMA flow diagram (Figure 1).
1.
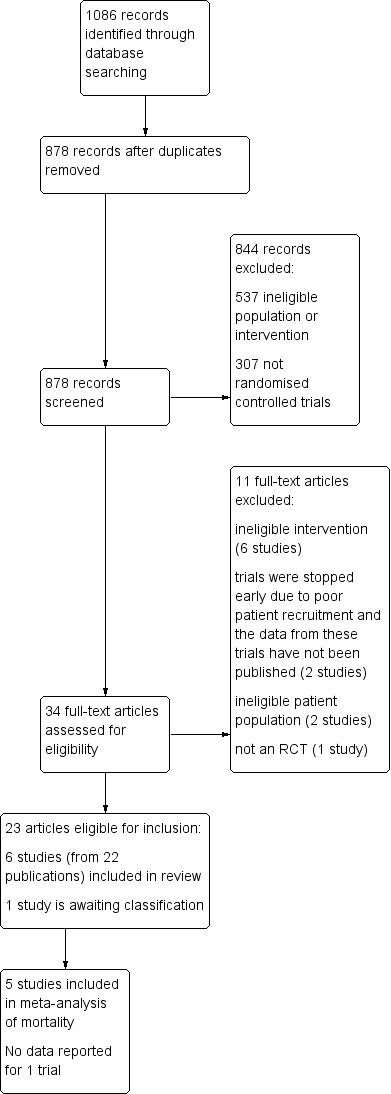
Study flow diagram
Included studies
Five of the six included studies were published in full (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013), whereas Palmer 1998 was published only in a conference abstract and presented no outcome data. All six studies compared red blood cell transfusion threshold A versus red blood cell transfusion threshold B. Thus, no included studies investigated our other three listed comparisons: red blood cell transfusion versus no transfusion, red blood cell transfusion versus an alternative method to red blood cell transfusion or red blood cell transfusion protocol A versus red blood cell transfusion protocol B.
Carson 1998 acted as a pilot trial for the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) trial (Carson 2011), but otherwise these were conducted as separate trials with no overlap in recruitment. Some overlap occurred between Carson 1998 and Palmer 1998; four participants were reported as being enrolled at the Edinburgh site in one month "at the end of recruitment" in Carson 1998 and included in the analysis. A further account found in Table 25.2 of McClelland 2009 linked the two trials and stated that "two patients included in this series [Palmer 1998] are included among the 80 patients in the US study [Carson 1998]". The absence of results for Palmer 1998 means that any overlap (whether two or four participants) does not impact on the results of this review and we have, for convenience but at the risk of some very limited double counting, treated Carson 1998 and Palmer 1998 as separate trials.
Sample sizes
The six trials randomised 2722 participants. The numbers of participants randomised into each trial were 18 (Palmer 1998), 84 (Carson 1998), 120 (Foss 2009), 200 (Parker 2013), 284 (Gregersen 2015), and 2016 (Carson 2011). The total number of participants included per outcome is detailed in the Effects of interventions section and ranged from 107 to 2683 participants.
Setting
Two trials were multicentre with participants from across the USA (Carson 1998) or from both the USA and Canada (Carson 2011). As described above, Carson 1998 also performed the study in Edinburgh (UK) for one month only. The four single‐centre trials were from Denmark (Foss 2009; Gregersen 2015), and the UK (Palmer 1998; Parker 2013).
Participants
All trials included people requiring surgery for a hip fracture. Table 2 details the types of surgical procedures and the types of hip fracture in the trials. Palmer 1998 did not report any further demographic data for their randomised participants. Table 3 describes age, gender, cardiac and red blood cell transfusion history in the trials.
1. Type of surgical procedure and type of fracture.
| Study ID | Type of surgical procedure | Type of hip fracture |
| Carson 1998 | Multiple screws/plates (n = 59) Hemiarthroplasty (n = 12) Bipolar hemiarthroplasty (n = 13) |
Femoral neck (n = 30)* Intertrochanteric (n = 55) Subtrochanteroc (n = 6) |
| Carson 2011 | Not stated | Femoral neck (n = 854)# Intertrochanteric (n = 1034) Subtrochanteroc (n = 183) Reverse oblique (n = 21) |
| Foss 2009 | Screws/pins (n = 10) Arthroplasty (n = 46) Sliding hip screw (n = 46) Intermedullary hip screw (n = 18) |
Not stated |
| Gregersen 2015 | Internal fixation (n =221) Arthroplasty (n = 57) Other (n = 6) |
Not stated |
| Palmer 1998 | Not stated | Not stated |
| Parker 2013 | Not stated Percutaneous screw fixation was an exclusion criterion |
Not described in full Intracapsular (n = 68) |
* Seven participants (five in the liberal and two in the restrictive transfusion threshold groups) in the trial had more than one type of hip fracture.
# Several participants had more than one type of hip fracture: there was an excess of 41 fractures in the liberal group and 39 in the restricted group (data not available for four participants).
2. Baseline characteristics of randomised participants.
| Study ID | Liberal transfusion threshold | Restrictive transfusion threshold |
| Carson 1998 | Number of participants reporting these data: 42 Age: mean (SD): 81.3 (8.1) years; range: 50‐94 years Gender: number (%) men: 9 (21.4) Number (%) people withcardiac conditions: Any: 19 (45.2) Coronary artery disease: 12 (28.6) Congestive heart failure: 6 (14.3) Transfusion history: Number of red blood cell transfusions received before randomisation, mean (SD): 0.5 (1.0) Last preoperative haemoglobin concentration, mean (SD): 11.7 (1.6) g/dL Randomisation haemoglobin concentration, mean (SD): 9.1 (0.6) g/dL |
Number of participants reporting these data: 42 Age: mean (SD): 83.3 (10.8) years; range: 32‐95 years Gender: number (%) men: 11 (26.2) Number (%) people withcardiac conditions: Any: 19 (45.2) Coronary artery disease: 12 (28.6) Congestive heart failure: 6 (14.3) Transfusion history: Number of red blood cell transfusions received before randomisation, mean (SD): 0.3 (0.6) Last preoperative haemoglobin concentration, mean (SD): 11.6 (1.0) g/dL Randomisation haemoglobin concentration, mean (SD): 9.1 (0.6) g/dL |
| Carson 2011 | Number of participants reporting these data: 1007 Age: mean (SD): 81.8 (8.8) years Gender: number (%) men: 250 (24.8) Number (%) people withcardiac conditions: Any: 637 (63.3) Coronary artery disease: 402 (39.9) Congestive heart failure: 184 (18.3) Hypertension: 824/1003 (82.2) Transfusion history: transfusions before randomisation: number/total number (%) of participants receiving 0 red blood cell transfusions: 754/1006 (75.0) number/total number (%) of participants receiving ≥ 1 red blood cell units: 252/1006 (25.0) Haemoglobin concentration before surgery, mean (SD): 11.3 (1.5) g/dL Haemoglobin concentration during eligibility screening, mean (SD): 9.0 (0.8) g/dL |
Number of participants reporting these data: 1009 Age: mean (SD): 81.5 (9.0) years Gender: number (%) men: 239 (23.7) Number (%) people withcardiac conditions: Any: 631 (62.5) Coronary artery disease: 403 (39.9) Congestive heart failure: 167 (16.6) Hypertension: 821/1005 (81.7) Transfusion history: transfusions before randomisation: number/total number (%) of participants receiving 0 red blood cell transfusions: 720/1008 (71.4) number/total number (%) of participants receiving ≥ 1 red blood cell units: 288/1008 (28.6) Haemoglobin concentration before surgery, mean (SD): 11.3 (1.5) g/dL Haemoglobin concentration during eligibility screening, mean (SD): 9.0 (0.8) g/dL |
| Foss 2009 | Number of participants reporting these data: 60 Age: mean (SD): 81 (6.8) years Gender: number (%) men: 14 (23) Number (%) people withcardiac conditions: Hypertension: 14 (23) Atrial fibrillation: 3 (5) Congestive heart failure: 5 (8) Ischemic heart disease: 4 (7) Cardiovascular disease: 21 (35) Transfusion history: not stated Haemoglobin concentration on admission: reported graphically and not all the data were readily interpretable, but mean was clearly 13.0 g/dL |
Number of participants reporting these data: 60 Age: mean (SD): 81 (7.3) years Gender: number (%) men: 14 (23) Number (%) people withcardiac conditions: Hypertension: 20 (33) Atrial fibrillation: 3 (5) Congestive heart failure: 3 (5) Ischemic heart disease: 10 (17) Cardiovascular disease: 28 (47) Transfusion history: not stated Haemoglobin concentration on admission: reported graphically and not all the data were readily interpretable, but mean was between 13.0 and 14.0 g/dL |
| Gregersen 2015 | Number of participants reporting these data: 140 Age: mean (SD): 88 (6.9) years Gender: number (%) men: 34 (24) Number (%) people withcardiac conditions: Cardiovascular disease: 25 (18) Transfusion history: not stated Number (%) of people with anaemia at baseline: 68 (49) Postoperative haemoglobin levels between 9.7 g/dL and 11.3 g/dL during the first 6 postoperative days Repeated measurements of haemoglobin concentration levels showed maintained mean haemoglobin concentration levels of 12.2 g/dL (95% CI 12.2 to 12.3) |
Number of participants reporting these data: 144 Age: mean (SD): 86 (6.8) years Gender: number (%) men: 36 (25) Number (%) people withcardiac conditions: Cardiovascular disease: 34 (24) Transfusion history: not stated Number (%) of people with anaemia at baseline: 70 (49) Postoperative haemoglobin levels between 9.7 g/dL and 11.3 g/dL during the first 6 postoperative days Repeated measurements of haemoglobin concentration levels showed maintained mean haemoglobin concentration levels of 11.3 g/dL (95% CI 11.3 to 11.4) |
| Palmer 1998 | No data reported | No data reported |
| Parker 2013 | Number of participants reporting these data: 100 Age: mean (range): 84.4 years (60‐104) Gender: number (%) men: 17 (17) Number (%) of people withcardiac conditions: Any cardiac disease: 37 (37) Hypertension: 42 (42) Angina: 7 (7) Previous myocardial infarction: 4 (4) Previous congestive cardiac failure: 5 (5) Other cardiac disease: 21 (21) Transfusion history: not stated Haemoglobin concentration (mean) on admission: 11.5 g/dL Haemoglobin concentration (mean) after surgery (time point post surgery not defined): 8.7 g/dL |
Number of participants reporting these data: 100 Age: mean (range): 84.2 years (60‐97) Gender: number (%) men: 15 (15) Number (%) of people with cardiac conditions: Any cardiac disease: 50 (50) Hypertension: 46 (46) Angina: 9 (9) Previous myocardial infarction: 9 (9) Previous congestive cardiac failure: 3 (3) Other cardiac disease: 29 (29) Transfusion history: not stated Haemoglobin concentration (mean) on admission: 11.8 g/dL Haemoglobin concentration (mean) after surgery (time point post surgery not defined): 8.9 g/dL |
SD: standard deviation.
In summary, the mean age of participants ranged from 81 to 87 years and the trials included far fewer men than women: overall 16% of the participants were men in Parker 2013, 23% were men in Foss 2009, 24% were men in both Carson 1998 and Carson 2011, and 25% were men in Gregersen 2015. Five trials included people with baseline cardiac conditions (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013). The percentage of people with any cardiac condition at baseline was reported by four trials and was 21% in Gregersen 2015, 43.5% in Parker 2013, 45.2% in Carson 1998, and 62.9% in Carson 2011. Foss 2009 reported the number of people with any of five pre‐existing chronic cardiac conditions and was the only trial explicitly excluding participants with "acute cardiac or other acute severe medical conditions".
Timing of randomisation varied, with four trials randomising participants when their haemoglobin concentration dropped postoperatively: in Carson 1998 and Carson 2011, when haemoglobin concentration fell to less than 10 g/dL within the first three days post operation; in Palmer 1998 when haemoglobin concentration dropped to between 8 and 10 g/dL within the first two days post operation and in Parker 2013 when haemoglobin concentration measured between 8 and 9.5 g/dL on their first or second day postoperatively. Foss 2009 randomised participants preoperatively at admission to hospital. Gregersen 2015 randomised participants after surgery.
Two trials reported details of the number of red cell transfusions prior to study randomisation (Carson 1998; Carson 2011). Carson 1998 reported the mean (standard deviation (SD)) number of red blood cell transfusions received before randomisation, which were similar between groups at 0.5 (SD 1.0) in the liberal transfusion threshold group and 0.3 (SD 0.6) in the restrictive transfusion threshold group. Carson 2011 reported the number of participants receiving at least 1 unit of red blood cells: 252 (25%) in the liberal transfusion threshold group and 288 (28.6%) in the restrictive transfusion threshold group.
Interventions
In all trials, the intervention groups were a liberal haemoglobin concentration threshold for red blood cell transfusion versus a restrictive haemoglobin concentration threshold for red blood cell transfusion. The liberal transfusion threshold was receipt of 1 unit of packed red blood cells at the time of random assignment and as much blood as necessary to maintain the haemoglobin concentration greater than 10 g/dL (Carson 1998; Carson 2011; Palmer 1998; Parker 2013), receipt of a red blood cell transfusion when haemoglobin concentration fell to below 10.0 g/dL at any time between admittance "to the post‐anaesthesia care unit" and the fifth postoperative day (Foss 2009), and receipt of 1 or 2 units of red blood cells when the haemoglobin threshold was at or below 11.3 g/dL within the first three weeks following surgery (Gregersen 2015).
The restrictive transfusion threshold was receipt of a red blood cell transfusion if participants showed symptoms of anaemia or if their haemoglobin dropped to less than 8 g/dL in Carson 1998 and Carson 2011, when participants were symptomatic of anaemia in Parker 2013, when perceived necessary by the physicians (Palmer 1998), when the haemoglobin concentration was 8 g/dL or less (with transfusion not based on symptoms or presence of clinical anaemia) in Foss 2009, and receipt of 1 or 2 units of red blood cells when the haemoglobin threshold was at 9.7 g/dL or less within the first three weeks following surgery (Gregersen 2015).
Outcomes
The follow‐up periods for outcomes identified as primary outcomes by individual trials ranged from three days to one year post operation. Of our primary outcomes, mortality data were available at 30 days for five trials (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013), at 60 days for three trials (Carson 1998; Carson 2011; Parker 2013), at 90 days for two trials (Gregersen 2015; Parker 2013), and at 120 and 365 days for one trial (Parker 2013). Five trials reported on postoperative function or mobility. Two trials reported on inability to walk 10 feet (3 m; or across a room) without human assistance at 60 days (Carson 1998; Carson 2011). Foss 2009 reported on the gaining of functional independence during hospitalisation and the cumulated ambulation score (CAS) on the first three postoperative days. Parker 2013 assessed mobility at eight weeks from discharge using a commonly used mobility score. Gregersen 2015 measured physical ability before and at 90 days after surgery and reported the data as physical recovery. Four studies reported data on complications (Carson 1998; Carson 2011; Foss 2009; Parker 2013), as detailed in the Characteristics of included studies table. Gregersen 2015 only reported on complications as the main causes of death. Palmer 1998 did not report what outcomes were measured (primary or secondary) within their study.
Excluded studies
For the full list of excluded studies, see Characteristics of excluded studies.
We excluded 11 studies from this review following assessment of the full text or correspondence with the authors. We excluded six studies because of ineligible interventions (Izuel‐Rami 2005; Izuel‐Rami 2006; Moghaddam 2009; Prasad 2009; Serrano Trenas 2011; Zufferey 2010); two studies because their study population was ineligible (Gampopoulou 2004; Nielsen 2012); one study was not a randomised clinical trial (Muir 1995); and two studies, which were only reported in trial registrars, were stopped at the early stages due to poor recruitment (Jans 2011; Matot 2012).
Ongoing trials and studies awaiting classification
We assessed one study as awaiting assessment (ChiCTR‐TRC‐10000822; see Characteristics of studies awaiting classification). We identified no ongoing trials.
Risk of bias in included studies
Figure 2 presents a summary of the risk of bias assessments by risk of bias domain and by trial. In the following, we provide details only on the risk of bias in the five fully reported trials (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013). Reflecting the lack of detailed information on methods and absence of results, we judged that Palmer 1998 was at unclear risk of bias for all domains.
2.
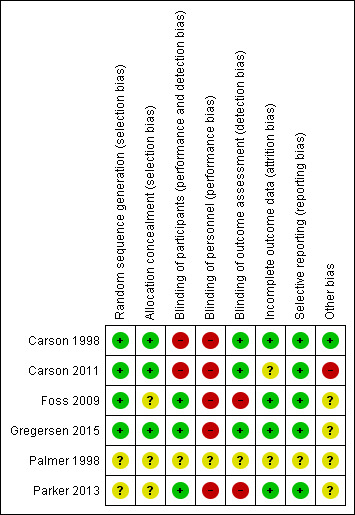
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Four trials reported details of the randomisation sequence and used methods that we assessed as being at low risk of bias (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015). The methods used were automated telephone randomisation systems (Carson 1998; Carson 2011), a computer‐generated list by a person not affiliated with the project (Foss 2009), or a web‐based clinical trial support system (Gregersen 2015). As Parker 2013 did not report details of their generation of the randomisation sequence method, we assessed this as having an unclear risk of bias for this domain.
Concealment of treatment allocation
We deemed the method of randomisation (as described above) adequate (low risk of bias) to conceal treatment allocation in three trials (Carson 1998; Carson 2011; Gregersen 2015), with allocation being concealed using data co‐ordinating centres at central locations in Carson 1998 and Carson 2011, and use of a web‐based system in Gregersen 2015.
In two trials, we considered the methods used to conceal treatment allocation to be at unclear risk of bias (Foss 2009; Parker 2013). Neither trial used 'sequentially, numbered, opaque, sealed envelopes', which are believed to be the most robust way of concealing treatment allocation (Higgins 2011a): one trial used 'sealed envelopes' (Foss 2009); the other trial used 'numbered, opaque, sealed envelopes' (Parker 2013).
Blinding
We reported details of who was blinded to treatment allocation separately for participants, study personnel and outcome assessors.
Blinding of participants
Only Foss 2009 and Gregersen 2015 described the blinding of trial participants. We judged Foss 2009, Gregersen 2015, and Parker 2013 to be at low risk of bias as the participants were not involved in outcome assessment in any of these trials and so any participant knowledge of treatment allocation would have limited impact on the data measured by the study. There was no blinding in the other two trials, which we judged to be at high risk of bias as the participants were involved in assessing outcomes and knowledge of treatment allocation may have an influence on their outcome measurement (Carson 1998; Carson 2011). There was insufficient information reported in Gregersen 2015 to judge whether there was any blinding of participants to treatment allocation.
Blinding of study personnel
In these trials (all comparing a liberal with a restrictive haemoglobin concentration threshold for red blood cell transfusion), the blinding of clinicians would have been difficult as the clinicians themselves determined whether a participant met the requirements for a red blood cell transfusion. Thus, we classified five trials to be at high risk of performance bias relating to lack of blinding of study personnel (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013).
Blinding of outcome assessors
Five trials reported that there was blinding of outcome assessors to treatment allocation (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013). In all these trials, the methods used to enact this blinding were described. In three trials, we deemed the methods used to be adequate (low risk of bias) as the manuscripts reported that outcome assessors were blinded to treatment allocation (Carson 1998; Carson 2011; Gregersen 2015).
In two trials, we deemed the methods used to blind outcome assessors to treatment allocation to be inadequate (high risk of bias) (Foss 2009; Parker 2013). In one trial, details of the participant's treatment allocation were not securely stored and could have been seen by outcome assessors (Foss 2009). In the other trial, the outcome assessor for one outcome (change in mobility score) was blinded to treatment allocation: while other outcome assessments were made by clinicians aware of treatment allocation, which may have influenced outcome measurement (Parker 2013).
Incomplete outcome data
We deemed four trials to have a low risk of attrition bias: two trials included all randomised participants in the analysis of outcome data and did not lose any participants during follow‐up (Carson 1998; Parker 2013), and two trials reported missing data for some outcome analyses but documented the level and reason of attrition per treatment group (Foss 2009; Gregersen 2015). Carson 2011 reported the numbers of participants not included in the 60‐day follow‐up and reasons why, but did not provide details of the reasons for attrition at the 30‐day follow‐up period and had 13% missing data for each group on one key outcome. Although this small variability may not have affected event rates (pooled or individual study), we downgraded the risk of bias to unclear for Carson 2011.
Selective reporting
In five trials, all pre‐specified outcomes were reported on in their results section and we deemed them to be at low risk of reporting bias (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013).
Other potential sources of bias
Other potential sources of bias (significant difference in protocol violations and baseline imbalance) were observed in three trials (Carson 2011; Foss 2009; Parker 2013). In Carson 2011, there was a statistical difference in the number of major protocol violations between the two transfusion threshold groups. In Foss 2009, there was baseline imbalance in the type of surgery received and the American Society of Anesthesiologists (ASA) classification between the liberal and liberal transfusion threshold groups, and, in Parker 2013, the baseline imbalance was in the proportion of participants presenting with cardiac disease between the interventions groups. We rated the impact that the protocol violations had as high in Carson 2011, and the impact of the baseline imbalances on the outcome measurements as unclear in Foss 2009.
Effects of interventions
See: Table 1
The six trials investigated only one of the four comparisons outlined in the Types of interventions section.
Red blood cell transfusion threshold A (liberal) versus red blood cell transfusion threshold B (restrictive)
Five trials, randomising 2704 participants, presented outcome data on red blood cell transfusion threshold A (liberal) versus red blood cell transfusion threshold B (restrictive) (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013). No outcome results were available for 18 participants of the sixth included study (Palmer 1998). Throughout, the denominator numbers we have used and reported were the number of participants included in each particular outcome analysis in the trial reports; this does not always correspond to the number of participants randomised into the respective trial groups.
One study randomised participants at hospital admission and participants' eligibility for the trial was assessed perioperatively (Foss 2009), while the other trials randomised and assessed eligibility post surgery (see Characteristics of included studies table). As there is a difference between assessing haemoglobin concentration (and thus study eligibility) perioperatively and postoperatively, we undertook a sensitivity analysis (by temporarily removing Foss 2009 from each meta‐analysis in which it was included) and if the pooled result of the sensitivity analysis did not differ from the overall pooled result, we have not reported the results of the sensitivity analysis in this section.
Carson 2011 was a large study, contributing 75% of the total number of participants randomised across the four trials that reported outcome data. We undertook sensitivity analyses for all outcomes that included Carson 2011 by removing these data from each pooled result. If the pooled result of the sensitivity analysis did not differ from the overall pooled result, we have not reported the results of the sensitivity analysis in this section.
Table 4 presents data on the number of participants receiving a red blood cell transfusion and on the quantity of red blood cells received in the two transfusion threshold groups. The percentage of participants receiving a red blood cell transfusion ranged from 74% to 100% in the liberal transfusion threshold group and from 11% to 45% in the restrictive transfusion threshold group. Gregersen 2015 did not report data on the percentage of participants receiving a red blood cell transfusion.
3. Quantity of red blood cell units received (post randomisation).
| Study ID | Liberal transfusion threshold | Restrictive transfusion threshold | ||
| Number (%) of participants transfused | Quantity of red blood cell units transfused | Number (%) of participants transfused | Quantity of red blood cell units transfused | |
| Carson 1998 | 41 (98) | Median number of red blood cell units transfused: 2, maximum of 4 (interquartile range 1 to 2) | 19 (45) | Median number of red blood cell units transfused: 0, maximum of 6 (interquartile range 0 to 2) 45% (n = 19) participants received a red blood cell transfusion |
| Carson 2011 | 970 (97) | Median number of red blood cell units transfused: 2 (interquartile range 1 to 2) 96.6% (n = 970) participants received a red blood cell transfusion 3.3% (n = 33) participants received 0 units of red blood cells 41.9% (n = 420) participants received 1 unit of red blood cells 34.5% (n = 346) participants received 2 units of red blood cells 13.2% (n = 132) participants received 3 units of red blood cells 7.2% (n = 72) participants received ≥ 4 units of red blood cells |
413 (41) | Median number of red blood cell units transfused: 0 (interquartile range 0 to 1) 41% (n = 413) participants received a red blood cell transfusion 59.0% (n = 594) participants received 0 units of red blood cells 24.4% (n = 246) participants received 1 unit of red blood cells 12.6% (n = 127) participants received 2 units of red blood cells 2.4% (n = 24) participants received 3 units of red blood cells 1.6% (n = 16) participants received ≥ 4 units of red blood cells |
| Foss 2009 | 44 (74) | Median number of red blood cell units transfused: 2 (interquartile range 1 to 2) 74% (n = 44) participants received a red blood cell transfusion |
22 (37) | Median number of red blood cell units transfused: 1 (interquartile range 1 to 2) 37% (n = 22) participants received a red blood cell transfusion |
| Gregersen 2015 | Not stated | Median number of red blood cell units per patient was 3.0 (interquartile range 2 to 5) | Not stated | Median number of red blood cell units per patient was 1.0 (interquartile range 1 to 2) |
| Palmer 1998 | 9 (100)** | Not reported | 1 (11)** | Not reported |
| Parker 2013 | 100 (100) | A mean of 1.9 units of red blood cells transfused 16 participants received 1 unit of red blood cells 92 participants received 2 units of red blood cells 1 participant received 3 units of red blood cells 4 participants received 4 units of red blood cells |
11 (11) | Participants received either 1 or 2 units of red blood cells: no further details reported |
* 4 of these 42 participants received a transfusion in violation of the protocol (i.e. they did not have symptoms of anaemia or a haemoglobin of < 8 g/dL).
** Data taken from Table 25.2 (McClelland 2009).
Carson 1998 reported five protocol violations (6.2%): one participant in the liberal transfusion threshold group did not receive a transfusion and four in the restrictive transfusion threshold group received a transfusion but did not have symptoms of anaemia or haemoglobin less than 8 g/dL. Carson 2011 reported "major protocol violations" in 147 participants (7.3%): 91 participants (9.0%) in the liberal threshold group of whom 30 did not receive a transfusion and 61 were discharged with a haemoglobin level less than 10 g/dL; and 56 participants (5.6%) in the restricted threshold group who received transfusion despite not having symptoms or rapid bleeding. Gregersen 2015 reported eight deviations from protocol in each group, either not receiving or receiving transfusion outside the group threshold; and a further four drop‐outs in each group, seven of whom refused transfusion. There was no mention of protocol violations relating to transfusion in the other trials.
Primary outcomes
Mortality
All studies measured mortality as an outcome. Five trials reported mortality at 30 days (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013). There was no evidence of a difference in mortality at 30 days post operation between the liberal and restrictive transfusion threshold groups (RR 0.92, 95% CI 0.67 to 1.26; I² = 38%; 2683 participants; event rate 68/1337 in the liberal transfusion threshold group vs. 75/1346 in the restrictive transfusion threshold group; Analysis 1.1).
1.1. Analysis.
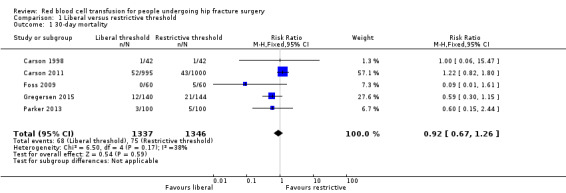
Comparison 1 Liberal versus restrictive threshold, Outcome 1 30‐day mortality.
Three studies also reported 60‐day mortality (Carson 1998; Carson 2011; Parker 2013). There was no evidence of a difference in mortality at 60 days between the liberal and restrictive transfusion threshold groups (RR 1.08, 95% CI 0.80 to 1.44; I2 = 0%; 2283 participants; event rate 87/1140 in the liberal transfusion threshold group vs. 81/1143 in the restrictive transfusion threshold group; Analysis 1.2).
1.2. Analysis.
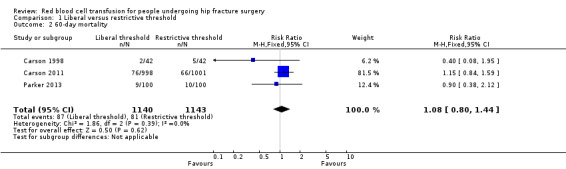
Comparison 1 Liberal versus restrictive threshold, Outcome 2 60‐day mortality.
Two trials reported mortality at 90 days (Gregersen 2015; Parker 2013), and Parker 2013 reported 120‐day and 365‐day mortality. At all time points there was no evidence of a difference in mortality between the liberal and restrictive threshold groups, with an RR of 0.88 (95% CI 0.55 to 1.16) at 90 days (484 participants; event rate 40/240 in the liberal transfusion threshold group vs. 51/244 in the restrictive transfusion threshold group); an RR of 1.18 (95% CI 0.56 to 2.51) at 120 days (200 participants; event rate 13/100 in the liberal transfusion threshold group vs. 11/100 in the restrictive transfusion threshold group); and an RR of 1.04 (95% CI 0.65 to 1.65) at 365 days (200 participants; event rate 27/100 in the liberal transfusion threshold group vs. 26/100 in the restrictive transfusion threshold group; Analysis 1.3).
1.3. Analysis.
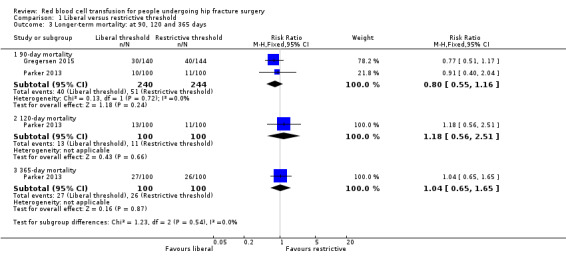
Comparison 1 Liberal versus restrictive threshold, Outcome 3 Longer‐term mortality: at 90, 120 and 365 days.
Our post‐hoc sensitivity analysis, which excluded Foss 2009, found similar results but heterogeneity was significantly reduced (I² = 0%). The second sensitivity analyses undertaken, which excluded Carson 2011, also found a similar lack of difference between the two groups. We have not presented these results in the review.
Mobility and functional recovery
All five studies reported on mobility and functional recovery. However, because the trials used a variety of measures to assess functional recovery, only limited meta‐analysis was possible.
Carson 2011 measured this outcome as the inability to walk 10 feet (3 m; or across a room) without human assistance at 30‐day follow‐up. There was little difference in this measurement of mobility between the liberal and restrictive transfusion threshold groups (RR 0.93, 95% CI 0.84 to 1.03; 1995 participants; event rate 407/995 in the liberal transfusion threshold group vs. 438/1000 in the restrictive transfusion threshold group; Analysis 1.4). Two trials measured the same outcome at 60‐day follow‐up (Carson 1998; Carson 2011). There was no difference in this measurement of mobility between the liberal and restrictive transfusion threshold groups (RR 1.00, 95% CI 0.87 to 1.15; I² = 26%; 2083 participants; event rate 292/1040 in the liberal transfusion threshold group vs. 292/1043 in the restrictive transfusion threshold group; Analysis 1.4).
1.4. Analysis.
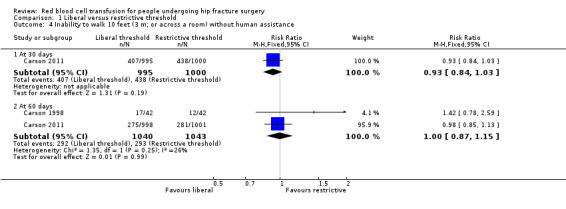
Comparison 1 Liberal versus restrictive threshold, Outcome 4 Inability to walk 10 feet (3 m; or across a room) without human assistance.
Foss 2009 measured the number of participants who "regained functional independence during hospitalisation" and found very little difference between the liberal and restrictive transfusion threshold groups (RR 0.95, 95% CI 0.67 to 1.34; 107 participants; event rate 29/54 in the liberal transfusion threshold group vs. 30/53 in the restrictive transfusion threshold group; Analysis 1.5). Foss 2009 also reported CAS (CAS is a composite score evaluating independence in walking or getting up from the chair, with scores ranging from 0 to 18; higher scores indicate better mobility). Foss 2009 reported there was no statistically significant difference (reported P value = 0.46) between the liberal and restrictive transfusion threshold groups in the cumulative CAS values over three days (median CAS (interquartile range): 9 (9 to 15) in the liberal transfusion threshold group vs. 9 (9 to 13.5) in the restrictive transfusion threshold group).
1.5. Analysis.
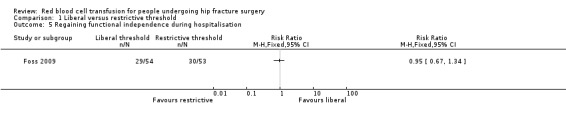
Comparison 1 Liberal versus restrictive threshold, Outcome 5 Regaining functional independence during hospitalisation.
Gregersen 2015 reported data using the New Mobility Score and the CAS and measured the independence/dependence of participants in transferring from bed to chair and their independence/dependence with regards to their walking ability. Gregersen 2015 reported there was no statistically significant difference (reported P value = 0.49) between the liberal and restrictive transfusion threshold groups in the New Mobility Score 10 days after hip fracture surgery (median (interquartile range): 1 (0 to 1) in the liberal transfusion threshold group vs. 1 (0 to 1) in the restrictive transfusion threshold group). Gregersen 2015 reported the numbers of participants who were able to walk, perform sit‐to‐stand‐to‐sit or were bedridden based on CAS categories at 10 days after hip fracture surgery. They found no evidence of a difference between the liberal and restrictive transfusion threshold groups in people unable to walk (RR 1.05, 95% CI 0.96 to 1.16; 284 participants; event rate 124/140 in the liberal transfusion threshold group vs. 121/144 in the restrictive transfusion threshold group) or people who were bedridden (RR 0.92, 95% CI 0.68 to 1.24; 284 participants; event rate 50/140 in the liberal transfusion threshold group vs. 56/144 in the restrictive transfusion threshold group) (Analysis 1.6).
1.6. Analysis.
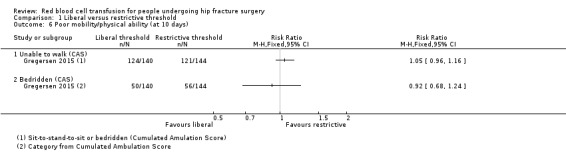
Comparison 1 Liberal versus restrictive threshold, Outcome 6 Poor mobility/physical ability (at 10 days).
Parker 2013 assessed mobility at eight weeks from discharge using a self developed but commonly used mobility score (where 0 represented a bed‐bound person and 9 represented full mobility indoors and outdoors without walking aids). They found very little difference between the liberal and restrictive transfusion threshold groups (MD 0.40, 95% CI ‐0.43 to 1.23; 106 participants; Analysis 1.7).
1.7. Analysis.
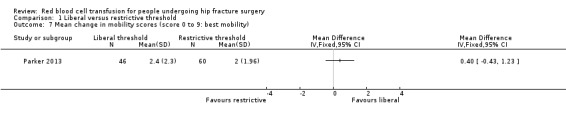
Comparison 1 Liberal versus restrictive threshold, Outcome 7 Mean change in mobility scores (score 0 to 9: best mobility).
Postoperative morbidity
The four trials assessed postoperative morbidity using different events; we reported data for each event separately.
Four studies reported thromboembolism (inpatient) (Carson 1998; Carson 2011; Foss 2009; Parker 2013). There was little difference in the incidence of thromboembolism between the liberal and restrictive transfusion threshold groups (RR 1.15, 95% CI 0.56 to 2.37; I² = 0%; 2416 participants; event rate 15/1207 in the liberal transfusion threshold group vs. 13/1209 in the restrictive transfusion threshold group; Analysis 1.8).
Four studies reported stroke (inpatient) (Carson 1998; Carson 2011; Foss 2009; Parker 2013). There was little difference in the incidence of stroke between the liberal and restrictive transfusion threshold groups (RR 2.40, 95% CI 0.85 to 6.79; I² = 0%; 2416 participants; event rate 11/1207 in the liberal transfusion threshold group vs. 4/1209 in the restrictive transfusion threshold group; Analysis 1.9).
Three studies reported wound infection (both superficial and deep wound infections) (Carson 2011; Foss 2009; Parker 2013). There was little difference in the incidence of wound infection between the liberal and restrictive transfusion threshold groups (RR 1.61, 95% CI 0.77 to 3.35; I² = 31%; 2332 participants; event rate 18/1165 in the liberal transfusion threshold group vs. 11/1167 in the restrictive transfusion threshold group; Analysis 1.10).
Five studies reported on cardiovascular events (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013), but often using different terminology. We selected two types: myocardial infarction and congestive heart failure. The incidence of myocardial infarction was reduced in the liberal transfusion threshold group in comparison with the restrictive transfusion threshold group (RR 0.59, 95% CI 0.36 to 0.96; I² = 0%; three trials; 2217 participants; event rate 23/1107 in the liberal transfusion threshold group vs. 40/1110 in the restrictive transfusion threshold group; Analysis 1.11). This result was dominated by data from Carson 2011; where, although numbers were comparable in both groups, 13% (135 vs. 130) of participants had incomplete electrocardiographic results and in 18% (180 vs. 175), there was no blood sample for troponin testing. A sensitivity analysis removing Carson 2011 resulted in little difference between the two groups in the incidence of myocardial infarction for the remaining two trials (0/102 in the liberal transfusion group vs. 2/102 in the restrictive transfusion group; RR 0.33, 95% CI 0.04 to 3.15; I² = 0%; 204 participants).
There was little difference in the number of new‐onset congestive heart failure events between the two transfusion threshold groups (RR 0.77, 95% CI 0.48 to 1.23; I² = 0%; three trials; 2332 participants; event rates: +29/1165 in the liberal transfusion threshold group vs. 38/1167 in the restrictive transfusion threshold group; Analysis 1.11). Once again, the findings of Carson 2011 dominated these results.
Five studies reported respiratory infections (Carson 1998; Carson 2011; Foss 2009; Gregersen 2015; Parker 2013). Pooled data showed a slightly increased incidence of respiratory infection (namely pneumonia) in the liberal transfusion threshold group compared with the restrictive transfusion threshold group (RR 1.35, 95% CI 0.95 to 1.92; I² = 0%; 2416 participants; event rate 69/1207 in the liberal transfusion threshold group vs. 51/1209 in the restrictive transfusion threshold group; Analysis 1.12).
1.8. Analysis.
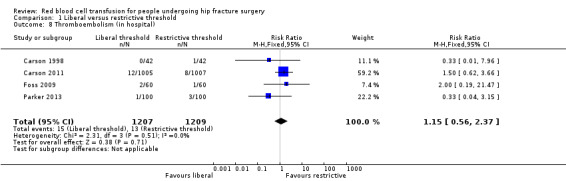
Comparison 1 Liberal versus restrictive threshold, Outcome 8 Thromboembolism (in hospital).
1.9. Analysis.
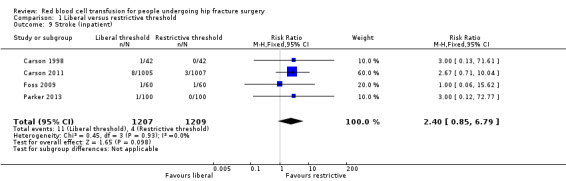
Comparison 1 Liberal versus restrictive threshold, Outcome 9 Stroke (inpatient).
1.10. Analysis.
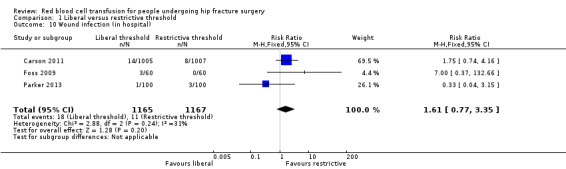
Comparison 1 Liberal versus restrictive threshold, Outcome 10 Wound infection (in hospital).
1.11. Analysis.
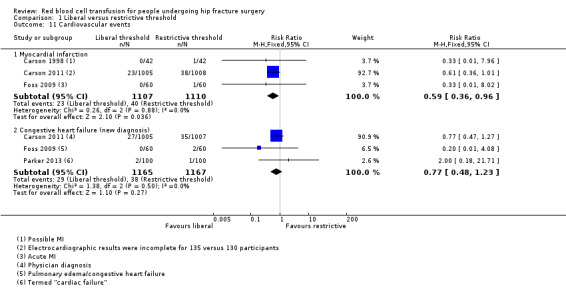
Comparison 1 Liberal versus restrictive threshold, Outcome 11 Cardiovascular events.
1.12. Analysis.
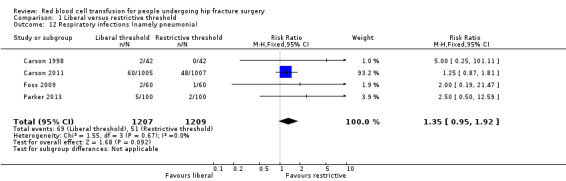
Comparison 1 Liberal versus restrictive threshold, Outcome 12 Respiratory infections (namely pneumonia).
Aside from myocardial infarction, our other sensitivity analyses excluding Carson 2011 did not show important differences in effect. The same applied for sensitivity analyses in which Foss 2009 was excluded for all five postoperative morbidity events.
Gregersen 2015 reported only on complications as the main cause of death of the 70 participants who had died during 90 days post operation (Table 5). Although these are incomplete data in terms of complications for the whole trial population, we observed that the greater incidence of stroke and lower incidence of heart failure in the liberal transfusion group compared with the restrictive threshold group were consistent with the patterns found in Analysis 1.9 for stroke and Analysis 1.11 for heart failure, whereas the opposite was the case for pneumonia (Analysis 1.12).
4. Main cause of death (Gregersen 2014).
| Cause of death | Liberal threshold | Restrictive threshold |
| 30 deaths | 40 deaths | |
| Stroke | 8 (27%) | 2 (5%) |
| Heart failure | 3 (10%) | 11 (28%) |
| Pneumonia | 8 (27%) | 18 (45%) |
| Sepsis | 3 (10%) | 5 (12%) |
| Dementia | 3 (10%) | 4 (10%) |
| Liver failure | 5 (17%) | 0 (0%) |
Percentages do not add up to 100 in the liberal threshold column because of rounding errors
Secondary outcomes
Postoperative or discharge haemoglobin
Postoperative haemoglobin level data, split by treatment group, at days one, two, four and seven post operation were presented graphically in Carson 2011; at days one, two, three and seven post operation in Foss 2009; and at days three, 10, 17, 24 and 30 post operation in Gregersen 2015. Exact figures (means and SDs) were not available for these trials. It was clear that the haemoglobin levels were higher in the liberal transfusion threshold groups in all three trials. Carson 2011 reported the mean haemoglobin level before transfusion was 1.3 g/dL higher in the liberal transfusion threshold group.
Quality of life
Carson 2011, Foss 2009, and Gregersen 2015 reported QoL data using very different outcome measures. Foss 2009 provided no data for pooling.
Carson 2011 used three measurement scales to assess QoL at 30 and 60 days post randomisation: the Lower‐extremity Physical Activities of Daily Living scale, the Instrumental Activities of Daily Living scale and the Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐Fatigue) scale. Appendix 2 presents details of the components of each scale and how the scale is scored. There was a substantial drop in the numbers of participants contributing these data. There was minimal or no difference in the mean change in score between the liberal and restrictive transfusion threshold groups for any scale at any measurement time point: Lower‐extremity Physical Activities of Daily Living scale at 30 days post randomisation (MD ‐0.10, 95% CI ‐0.60 to 0.40; 979 participants; Analysis 1.13) and at 60 days post randomisation (MD 0.00, 95% CI ‐0.51 to 0.51; 1076 participants; Analysis 1.13); the Instrumental Activities of Daily Living scale at 30 days post randomisation (MD 0.00, 95% CI ‐0.06 to 0.06; 887 participants; Analysis 1.14) and at 60 days post randomisation (MD 0.00, 95% CI ‐0.12 to 0.12; 800 participants; Analysis 1.14); and the FACIT‐Fatigue scale at 30 days post randomisation (MD 0.10, 95% CI ‐0.89 to 1.09; 915 participants; Analysis 1.15) and at 60 days post randomisation (MD ‐0.50, 95% CI ‐1.38 to 0.38; 1069 participants; Analysis 1.15).
1.13. Analysis.
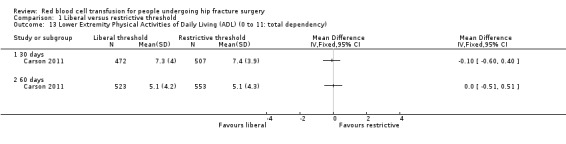
Comparison 1 Liberal versus restrictive threshold, Outcome 13 Lower Extremity Physical Activities of Daily Living (ADL) (0 to 11: total dependency).
1.14. Analysis.
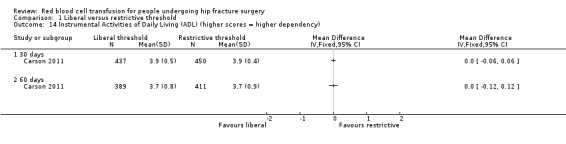
Comparison 1 Liberal versus restrictive threshold, Outcome 14 Instrumental Activities of Daily Living (ADL) (higher scores = higher dependency).
1.15. Analysis.
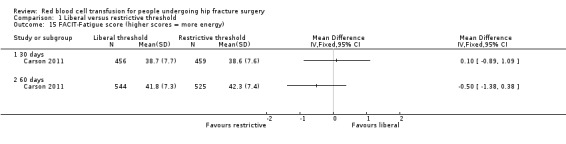
Comparison 1 Liberal versus restrictive threshold, Outcome 15 FACIT‐Fatigue score (higher scores = more energy).
Foss 2009 (107 participants) assessed QoL by recording ambulation scores on days one to three post operation and symptoms of anaemia (fatigue and dizziness) during physiotherapy (on days one to three post operation) and reported the data as medians and interquartile ranges. Table 6 presents full details. These indicated that the only measure with a statistically significant difference (i.e. P value < 0.05) between the two transfusion threshold groups was the fatigue score at day two post operation whereby the liberal transfusion threshold group reported lower levels of fatigue than the restrictive transfusion threshold group (reported P value = 0.04).
5. Quality of life outcome data (Foss 2009).
| Quality of life dimension1 |
Liberal transfusion threshold (n = 54) |
Restrictive transfusion threshold (n = 53) |
P value (as reported by study) |
| Ambulation score (0‐6)2 | |||
| Day 1 | 3 (3 to 4) | 3 (2.25 to 3.75) | 0.75 |
| Day 2 | 3 (3 to 5) | 3 (3 to 4.75) | 0.35 |
| Day 3 | 3 (3 to 6) | 3 (3 to 5) | 0.67 |
| Fatigue (0‐4)3 | |||
| Day 1 | 1 (1 to 2) | 1 (1 to 2) | 0.37 |
| Day 2 | 1 (1 to 2) | 2 (1 to 3) | 0.04 |
| Day 3 | 1 (1 to 2) | 2 (1 to 2) | 0.11 |
| Cumulated fatigue score (0‐12)4 | 4 (3 to 6) | 5 (3 to 7) | 0.46 |
| Dizziness score (0‐4)3 | |||
| Day 1 | 0 (0 to 1) | 0 (0 to 1) | 0.94 |
| Day 2 | 0 (0 to 1) | 0 (0 to 1) | 0.48 |
| Day 3 | 0 (0 to 1) | 0 (0 to 1) | 0.82 |
| Cumulated dizziness score (0‐12)4 | 0 (0 to 1) | 0 (0 to 1) | 0.64 |
1 Values reported as medians, with 25 to 75 percentiles. 2 Ambulation score was a composite score evaluating independence in walking or getting up from a chair; higher scores = better mobility. 3 Via a verbal rating scale with scores (0‐4); higher scores = worse outcome (4 = very severe). 4 Cumulated scores were the cumulated values from days 1‐3.
Gregersen 2015 used the Modified Barthel Index to assess the range of independence or dependence (substantial or complete) a person had in their activities of daily living 10 days after hip fracture surgery. There was no evidence of a difference between the liberal and restrictive transfusion threshold groups in people who were dependent (RR 1.05, 95% CI 0.92 to 1.20; 284 participants; event rate 108/140 in the liberal transfusion threshold group vs. 106/144 in the restrictive transfusion threshold group) or completely dependent (RR 0.94, 95% CI 0.69 to 1.27; 284 participants; event rate 50/140 in the liberal transfusion threshold group vs. 55/144 in the restrictive transfusion threshold group) (Analysis 1.16).
1.16. Analysis.
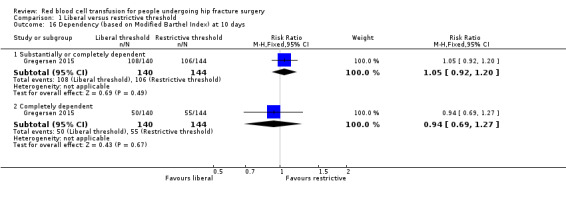
Comparison 1 Liberal versus restrictive threshold, Outcome 16 Dependency (based on Modified Barthel Index) at 10 days.
Length of stay in hospital
Although four studies provided length of hospital stay data, we decided not to pool the four trials because length of stay in hospital is usually protocol‐based and can vary between countries.
Two studies (1304 participants) reported length of stay data from participants hospitalised in the USA (Carson 1998; Carson 2011). There was little evidence of a difference in length of hospital stay between the liberal and restrictive transfusion threshold groups (MD ‐0.27 days, 95% CI ‐0.66 to 0.12; I² = 0%; Analysis 1.17). Carson 2011 also reported length of stay data from 791 participants hospitalised in Canada and found little evidence of a difference in the mean length of stay between the liberal and restrictive transfusion threshold groups (MD ‐0.67 days, 95% CI ‐1.98 to 0.64). Likewise, there was little evidence of a difference in the mean length of hospital stay between the liberal and restrictive transfusion threshold groups in Foss 2009 based in Denmark (MD 1.80 days, 95% CI ‐3.23 to 6.83; 107 participants) or Parker 2013 based in the UK (MD ‐1.50 days, 95% CI ‐7.81 to 4.81; 200 participants).
1.17. Analysis.
Comparison 1 Liberal versus restrictive threshold, Outcome 17 Length of stay in hospital (days).
Length of hospital stay was reported to be similar in the two transfusion groups of Gregersen 2015, but dependent more on whether the participants were from nursing homes (median two days length of hospital stay in both groups) or sheltered housing median 10 vs. 11 days; reported P value = 0.35).
Adverse effects of transfusion
Only Gregersen 2015 (284 participants) and Parker 2013 (200 participants) commented on adverse effects of transfusion. Gregersen 2015 reported that no complications were observed during or after transfusion and Parker 2013 reported that no participant had an adverse reaction to transfusion.
Discussion
Summary of main results
The objective of this systematic review was to assess the effects (benefits and harms) of red blood cell transfusion in people undergoing surgery for hip fracture. All six included trials compared 'liberal' versus 'restricted' red blood cell transfusion thresholds. Hence, we found no trials comparing red blood cell transfusion with no transfusion, red blood cell transfusion versus alternative methods or different protocols for red blood cell transfusion administration.
Table 1 shows the main findings of the review. With the exception of myocardial infarction, we judged the quality of the evidence for each outcome to be low, which means that we consider that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate for all outcomes presented in this table. We judged the evidence for myocardial infarction to be very low indicating greater uncertainty regarding these results.
The low quality evidence from the data pooled from five trials (2683 participants) provided no evidence of a difference between the two transfusion thresholds in mortality at 30 days' follow‐up. Assuming an illustrative baseline risk at 30 days of 50 per 1000 people in the restricted threshold group, the result was compatible with 17 fewer deaths and 13 more deaths in the liberal threshold group. A similar conclusion applied for the low quality evidence on mortality at 60 days from three trials (2283 participants). The low quality evidence from the data pooled from the two trials (2083 participants) reporting inability to walk 10 feet (3 m; or across a room) without human assistance at 60 days showed little difference between liberal versus restricted threshold transfusion at 60 days' follow‐up. Assuming an illustrative baseline risk at 60 days of 283 per 1000 people unable to walk 10 feet unsupported in the restricted threshold group, this result was compatible with 37 fewer and 43 more people with this outcome at 60 days in the liberal threshold group.
Table 1 presents postoperative morbidity for the following complications: thromboembolism (four trials; 2416 participants); stroke (four trials; 2416 participants); wound infection (three trials; 2332 participants); cardiovascular events, measured as myocardial infarction (three trials; 2217 participants) and new‐onset congestive heart failure (three trials; 2332 participants); respiratory infection, measured as pneumonia (four trials; 2416 participants) and risk of developing congestive heart failure (three trials; 2332 participants). In all, we pooled low quality evidence and we found no evidence of a difference between the two transfusion thresholds in all of these postoperative morbidities except myocardial infarction. We found some low quality evidence for a reduction in the incidence of myocardial infarction in the liberal transfusion threshold group in comparison with the restrictive transfusion threshold group. Assuming an illustrative baseline risk of myocardial infarction of 24 per 1000 people in the restricted transfusion threshold group, this result was compatible with between 1 and 15 fewer myocardial infarctions in the liberal transfusion threshold group.
Overall completeness and applicability of evidence
This review only identified trials that compared red blood cell transfusion thresholds (liberal vs. restrictive). One of these trials, which randomised 18 participants, did not report any outcome data; therefore, the quantitative findings of the review are based on data from five trials. In the five trials,1349 participants were randomised to the liberal transfusion threshold group and 1355 participants were randomised to the restrictive transfusion threshold group. The mean age of participants was similar across all the trials, ranging from 81 to 87 years and far fewer men than women were included in the trials: the percentage of men in each trial ranged from 16% in Parker 2013 to 25% in Gregersen 2015. In both these characteristics, these trials are comparable with the typical epidemiology of people with a hip fracture.
The timing of randomisation varied, with one trial randomising people preoperatively at admission to hospital (Foss 2009), one trial randomising participants after surgery (Gregersen 2015), and four trials randomising participants when their haemoglobin concentration dropped postoperatively (between one and three days post surgery) (Carson 1998; Carson 2011; Palmer 1998; Parker 2013). Thus, the majority of evidence applied to postoperative transfusion.
A key factor that should be acknowledged when interpreting the findings of this review is the dominance of the results by those of one relatively large study (Carson 2011), which randomised 2016 participants. Our post‐hoc sensitivity analyses exploring the impact this trial on the pooled effect estimates for each outcome found the only outcome affected was postoperative morbidity cardiovascular events; without Carson 2011, there was no longer a difference in the risk of a myocardial infarction between the two threshold groups (only two events occurred in the two other trials with this outcome).
There was heterogeneity in how the trials defined restrictive and liberal thresholds, while, there was some consistency in how the trials defined a liberal threshold for transfusion: receipt of 1 unit of packed red blood cells at the time of random assignment and as much blood as necessary to maintain the haemoglobin concentration greater than 10 g/dL (Carson 1998; Carson 2011; Palmer 1998; Parker 2013), when the haemoglobin concentration was less than 10 g/dL (Foss 2009), or receipt of 1 or 2 units of red blood cells when the haemoglobin threshold was at or below 11.3 g/dL within the first three weeks following surgery (Gregersen 2015). There was less consistency across the restrictive transfusion threshold groups. The restrictive transfusion threshold was receipt of a red blood cell transfusion if participants showed symptoms of anaemia or if their haemoglobin dropped to less than 8 g/dL in Carson 1998 and Carson 2011; when the haemoglobin concentration was 8 g/dL or less (with transfusion not based on symptoms or presence of clinical anaemia) in Foss 2009; when the haemoglobin threshold was at 9.7 g/dL or less within the first three weeks following surgery in Gregersen 2015; when perceived necessary by the physicians in Palmer 1998; and when participants were symptomatic of anaemia in Parker 2013. While such heterogeneity reflects variability in clinical practice, it introduced a limitation when we pooled outcome data across these studies. We did not explore the impact that this heterogeneity brought to the pooled effect estimates and overall findings in this review, but it may be an important consideration in future updates. With the exception of Gregersen 2015, these restrictive thresholds are broadly consistent with recommendations in many guidelines for red blood cell transfusion; although practice is increasingly advocating lower thresholds (Goodnough 2014; Rohde 2014).
There were differences in participants' cardiac history between the trials, with the largest trial only including participants with cardiovascular or cerebrovascular history (or both) or cardiovascular risk factors (Carson 2011). Three trials did not list pre‐existing cardiac disease as an eligibility criterion (Carson 1998; Gregersen 2015; Parker 2013), and one trial excluded people with acute cardiac conditions (Foss 2009). This is important to consider when interpreting the findings of postoperative morbidity in terms of cardiovascular events.
The timing of the intervention differed between the trials. In Foss 2009, the study protocol required blood tests daily from admission to the fifth postoperative day with any indication for transfusion acted on immediately. In Gregersen 2015, participants were randomised after surgery. In the restrictive transfusion threshold group in Carson 1998 and in the other two trials (Carson 2011; Parker 2013), both the monitoring of haemoglobin concentration and transfusion of red blood cells started postoperatively. In the liberal transfusion threshold group in Carson 1998, participants received a red blood cell transfusion at the time of randomisation and as much additional blood as needed thereafter to keep the haemoglobin concentration above 10 g/dL. These differences in the timing of the intervention may be relevant if there are significant acute factors, particularly perioperative haemodynamic instability, which may relate to blood loss or to other common factors such as inadequate volume replacement, effects of medication and anaesthesia. A transfusion given in the immediate perioperative period (e.g. in theatre recovery) may be associated with more intensive surveillance and attention to other variables such as electrocardiograph monitoring, electrolytes or blood pressure monitoring compared with the clinical setting of a transfusion given up to four to five days later.
Quality of the evidence
The key methodological limitations of these studies that places these at risk of bias lie in the lack of blinding of study personnel, the baseline imbalance observed in two trials (Foss 2009 due to either type of surgery received or ASA classification and in Parker 2013 the proportion of participants presenting with cardiac disease) and the protocol violations of Carson 2011. Minimising these limitations in any further studies in this area would certainly improve the quality of the available trial evidence.
We assessed the quality of the evidence for each of our primary outcomes using the GRADE approach (GRADE 2011), see Table 1. With the exception of myocardial infarction, all outcomes were graded as of low quality of evidence, meaning that we have limited confidence in the reliability of the outcome findings. For most outcomes, the grading of low quality was as a result of imprecision (typically small number of outcome events resulting in wide CI values) and concerns over risk of bias, in particular resulting from a statistically significant difference (P value = 0.003) in the number of major protocol violations post randomisation between the two transfusion threshold groups in the largest trial (Carson 2011). There was no consistency between the trials in how functional recovery was measured by the trials. Although this outcome was measured by all of the five principal studies (2672 participants) in the review, the varied way in which it was measured prevented a pooling of outcome data for an outcome of critical importance to this participant population. While we acknowledge that the blinding of all involved in the study to treatment allocation is a difficulty with these interventions, the noted existence of performance bias for what is an often subjective outcome is a further limitation to the quality of the evidence for this outcome; which was represented by the inability to walk 10 feet (3 m; or across a room) unsupported in the Table 1. We downgraded the evidence for myocardial infarction a further level to very low quality because of the additional risk of attrition bias from incomplete electrocardiographic results in 13% of the trial population and lack of blood samples for troponin tests in 18% of the trial population of Carson 2011.
Potential biases in the review process
The strengths of this review lie in the robust and comprehensive methodology employed to find and assess all relevant trials. We have followed standard Cochrane Collaboration methods for data extraction and analysis with reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), and have referred to a statistician where necessary. We have had a clinician and methodologist working on all stages, independently of each other to control any bias that ensues due to clinical or systematic review methodology knowledge. When we were limited by a lack of reporting data to allow inclusion and assessment of trials, we contacted authors directly to obtain the necessary data.
In the protocol, we had planned the transfusion protocol and threshold comparisons to be restrictive versus liberal. We changed this order to bring it in line with the ordering of the comparisons used in the included studies and to minimise the risk of transcription errors. Given that both thresholds have been considered the control intervention in the literature, with variation in current practice, other authors may have chosen the reverse order as presented in our protocol. These changes were made during the preparation of data for analysis and have been documented in the Differences between protocol and review. We do not consider this change to have biased the findings of our review.
Agreements and disagreements with other studies or reviews
Evidence for the safety and efficacy of red blood cell transfusion thresholds has been published across a variety of surgical settings (Carson 2012; Carson 2013; Hajjar 2010; Shokoohi 2012a; So‐Osman 2013; Villanueva 2013; Walsh 2013).
One Cochrane meta‐analysis of 19 randomised controlled trials compared clinical outcomes of higher versus lower red blood cell transfusion triggers in over 6000 people and found no evidence for benefit of liberal red blood cell transfusion policies (Carson 2012). Two trials reported evidence of harm with liberal red blood cell policies, with postoperative infection incidence being higher in the liberal transfusion group in comparison with the restrictive group in elective orthopaedic knee and hip replacement surgery (So‐Osman 2013); and rate of further bleeding, mortality at 45 days and overall rate of complications being lower in the restrictive group in a trial in people with acute upper gastrointestinal bleeding (Villanueva 2013). Two pilot feasibility trials have enrolled people with symptomatic coronary artery disease (Carson 2013), and older mechanically ventilated critically ill people (Walsh 2013), but neither were sufficiently powered to demonstrate a difference in clinical outcomes. One retrospective audit of practice in an elderly population (919 people followed, of which 313 received a red blood cell transfusion) undergoing surgery for hip fracture found that receiving a red blood cell transfusion was not associated with changes in mortality, but, unlike this review, reported an association with an increased rate of postoperative infection in the transfused group (Shokoohi 2012a).
Authors' conclusions
Implications for practice.
This review presents only the findings of a comparison of two thresholds for red blood cell transfusion: a 'liberal' versus a 'restrictive' strategy. We found no trials comparing red blood cell transfusion with no transfusion; red blood cell transfusion with alternative methods such as iron supplements or comparing different red blood cell transfusion protocols (e.g. based on the volume or rate of a transfusion).
This review found low quality evidence of no difference in mortality, functional recovery and several key postoperative complications between a liberal versus a restrictive transfusion threshold. Although there was some evidence of an increased incidence of myocardial infarctions in participants randomised to receive a restrictive red blood cell transfusion threshold, this was very low quality evidence and we are very uncertain of this finding. Overall, the currently available evidence does not support the use of liberal red blood cell transfusion thresholds based on a 10 g/dL haemoglobin trigger in preference to more restrictive transfusion thresholds based on lower haemoglobin levels or symptoms of anaemia in people undergoing surgery for hip fracture.
Implications for research.
Further research would be justified to evaluate transfusion thresholds in the immediate perioperative period: both preoperatively and including the first 24 hours post operation. In particular, such research would need to consider people who were symptomatic or haemodynamically unstable who were excluded from most of these trials. In clinical practice, this presentation in a frail older person with a hip fracture, often with a degree of cognitive impairment, and frequently with one or more vascular risk factors in addition to age, may pose a clinical dilemma for the surgeon, anaesthetist and physician. The effects of the transfusion itself need to be separated from the possible effects of increased monitoring and medical input, and a description of the wider management protocol and service would be useful in new trials. Future trials should more clearly report on causes of fracture (e.g. fragility or trauma), should consider including a measure for cognitive impairment (e.g. delirium) and should consider standardised assessments of health‐related quality of life, adapted for use in an elderly population, or validated for completion by the participant's relative or carer. In addition, new research is needed to manage better anaemia identified preoperatively, including appropriate use of iron as part of the broader initiatives of patient blood management (Goodnough 2014).
Acknowledgements
We would like to acknowledge the advice and support of Marialena Trivella during the preparation of the review. We also thank Matt Costa, Helen Handoll, Mario Lenza and Laura MacDonald for their comments on the review, and Joanne Elliott for her help with the searches of the Bone, Joint and Muscle Trauma Group Specialised Register.
We would like to thank all the trialists who responded to email enquiries and especially Jeffrey Carson, Nicolai Foss and Martyn Parker for providing additional study data.
We are also grateful to Sally Hopewell, and Bill Gillespie, Helen Handoll and Martyn Parker for helpful comments during the development of the protocol.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR or the UK National Health Service or Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (Wiley Online Library)
#1 MeSH descriptor: [Hip Fractures] explode all trees #2 (hip* or intertrochanteric or subtrochanteric or trochanteric or pertrochanteric or peritrochanteric or femur or femoral or acetabul*) near/6 fracture* #3 #1 or #2 #4 MeSH descriptor: [Blood Transfusion] explode all trees #5 MeSH descriptor: [Erythrocytes] this term only #6 transfus* or posttransfus* or post‐transfus* or retransfus* or hypertransfus* or hemotransfus* or haemotransfus* or red cell* or red blood cell* or RBC* or erythrocyte* #7 ((allogeneic next blood) or (unit* near/2 blood) or (allogenic next blood) or (blood near/2 exposure) or (blood near/3 management) or (blood next product*) or (blood next component*) or (donor* near/2 blood) or (donat* near/2 blood)) #8 blood sparing or cell salvage or cell saver* or (blood near/2 salvag*) or blood support or (blood near/2 requir*) or (blood near/2 replac*) or autotransfus* #9 (blood near/1 need*) or hemotherap* or haemotherap* #10 (leukodeplet* or leukoreduc* or leucodeplet* or leucoreduc* or leukofiltrat* or leucofiltrat*):ti #11 ((leukocyte* or leucocyte*) near/2 (remov* or deplet* or reduc* or poor or filtrat*)):ti #12 MeSH descriptor: [Anemia] explode all trees #13 (anemi* or anaemi*):ti #14 ((haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) near/3 (level* or low* or below or concentration* or cutoff or rais* or increas*)) #15 #4 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 #16 #3 and #15
MEDLINE (Ovid)
1. exp Hip Fractures/ 2. ((hip* or intertrochanteric or subtrochanteric or trochanteric or femur or femoral or acetabul* or collum or pertrochanteric or subcapital or peritrochanteric) adj6 fracture*).ti,ab. 3. or/1‐2 4. exp Blood Transfusion/ 5. Erythrocytes/ 6. (transfus* or posttransfus* or post‐transfus* or retransfus* or hypertransfus* or hemotransfus* or haemotransfus* or red cell* or red blood cell* or RBC* or erythrocyte*).ti,ab. 7. ((allogeneic adj blood) or (unit* adj2 blood) or (allogenic adj blood) or (blood adj2 exposure) or (blood adj3 management) or (blood adj product*) or (blood adj component*) or (donor* adj2 blood) or (donat* adj2 blood)).ti,ab. 8. (blood sparing or cell salvage or cell saver* or (blood adj2 salvag*) or blood support or (blood adj2 requir*) or (blood adj2 replac*) or autotransfus*).ti,ab. 9. ((blood adj1 need*) or hemotherap* or haemotherap*).ti,ab. 10. (leukodeplet* or leukoreduc* or leucodeplet* or leucoreduc* or leukofiltrat* or leucofiltrat*).ti. 11. ((leukocyte* or leucocyte*) adj2 (remov* or deplet* or reduc* or poor or filtrat*)).ti. 12. exp Anemia/ 13. (anemi* or anaemi*).ti. 14. ((haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) adj3 (level* or low* or below or concentration* or cutoff or rais* or increas*)).tw. 15. or/4‐14 16. 3 and 1
EMBASE (Ovid)
1. exp Hip Fracture/ 2. ((hip* or intertrochanteric or subtrochanteric or trochanteric or femur or femoral or acetabul* or collum or pertrochanteric or subcapital or peritrochanteric) adj6 fracture*).ti,ab. 3. or/1‐2 4. exp Blood Transfusion/ 5. Erythrocyte/ 6. (transfus* or posttransfus* or post‐transfus* or retransfus* or hypertransfus* or hemotransfus* or haemotransfus* or red cell* or red blood cell* or RBC* or erythrocyte*).ti,ab. 7. ((allogeneic adj blood) or (unit* adj2 blood) or (allogenic adj blood) or (blood adj2 exposure) or (blood adj3 management) or (blood adj product*) or (blood adj component*) or (donor* adj2 blood) or (donat* adj2 blood)).ti,ab. 8. (blood sparing or cell salvage or cell saver* or (blood adj2 salvag*) or blood support or (blood adj2 requir*) or (blood adj2 replac*) or autotransfus*).ti,ab. 9. ((blood adj1 need*) or hemotherap* or haemotherap*).ti,ab. 10. (leukodeplet* or leukoreduc* or leucodeplet* or leucoreduc* or leukofiltrat* or leucofiltrat*).ti. 11. ((leukocyte* or leucocyte*) adj2 (remov* or deplet* or reduc* or poor or filtrat*)).ti. 12. exp Anemia/ 13. (anemi* or anaemi*).ti. 14. ((haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) adj3 (level* or low* or below or concentration* or cutoff or rais* or increas*)).tw. 15. or/4‐14 16. 3 and 15
PubMed (US National Library of Medicine)
#1 (blood or erythrocyte* or red cell* or red blood cell* or RBC*) AND (transfus* or infus* or hypertransfus* or retransfus*) #2 (transfus* or retransfus*) AND (trigger* or level* or threshold* or rule* or restrict*) #3 transfusion* AND (management or practice* or policy or policies or strateg* or guideline* or indication* or protocol* or criteri* or autologous) #4 (red cell* management or red cell* sparing or red cell* support or red cell* requirement*) #5 transfus*[ti] or posttransfus*[ti] or post‐transfus*[ti] or retransfus*[ti] or hypertransfus*[ti] or red cell*[ti] or red blood cell*[ti] or RBC*[ti] or erythrocyte*[ti] #6 ("blood management" OR blood sparing OR cell salvage OR blood salvage OR blood support OR blood requirement* OR blood product* OR blood component* OR “need for blood”[ti] OR whole blood[ti] OR “use of blood”[ti]) #7 (leukodeplet*[ti] OR leukoreduc*[ti] OR leucodeplet*[ti] OR leucoreduc*[ti] OR leukofiltrat*[ti] OR leucofiltrat*[ti] OR ((leukocyte*[ti] OR leucocyte*[ti]) AND (remov*[ti] OR deplet*[ti] OR reduc*[ti] OR poor[ti] OR filtrat*[ti])) OR hemotransfus*[ti] OR haemotransfus*[ti]) #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #9 (hip* or intertrochanteric or subtrochanteric or trochanteric or pertrochanteric or peritrochanteric or femur or femoral or acetabul*) AND fracture* #10 #8 AND #9 #11 (random* OR blind* OR trial OR allocat* OR assign* OR "control group" OR intervention*) #12 #10 AND #11 #13 publisher[sb] NOT pubstatusnihms #14 #12 AND #13
CINAHL (EBSCO)
S1 (MH "Hip Fractures+") S2 TI ( (hip* or intertrochanteric or subtrochanteric or trochanteric or pertrochanteric or peritrochanteric or femur or femoral or acetabul*) N6 fracture* ) OR AB ( (hip* or intertrochanteric or subtrochanteric or trochanteric or pertrochanteric or peritrochanteric or femur or femoral or acetabul*) N6 fracture* ) S3 S1 OR S2 S4 (MH "Blood Transfusion+") S5 MH Erythrocytes S6 TI (transfus* or posttransfus* or post‐transfus* or retransfus* or hypertransfus* or red cell* or red blood cell* or RBC* or erythrocyte*) OR AB (transfus* or posttransfus* or post‐transfus* or retransfus* or hypertransfus* or red cell* or red blood cell* or RBC* or erythrocyte*) S7 TI ((blood N2 management) or blood sparing or cell salvage or (blood N2 salvag*) or blood support or (blood N2 requirement*) or autotransfus*) OR AB ((blood N2 management) or blood sparing or cell salvage or (blood N2 salvag*) or blood support or (blood N2 requirement*) or autotransfus*) S8 TI ((blood N1 need*) or whole blood or blood product* or blood component*) OR AB ((blood N1 need*) or whole blood or blood product* or blood component*) S9 TI (leukodeplet* or leukoreduc* or leucodeplet* or leucoreduc* or leukofiltrat* or leucofiltrat*) S10 TI ((leukocyte* or leucocyte*) N2 (remov* or deplet* or reduc* or poor or filtrat*)) S11 TI (hemotransfus* or haemotransfus*) OR AB (hemotransfus* or haemotransfus*) S12 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 S13 (MH "Anemia+") S14 TI (anaemi* or anemi*) S15 TI ((haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) N3 (level* or low* or below or concentration* or cutoff or rais* or increas*)) OR AB ((haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) N3 (level* or low* or below or concentration* or cutoff or rais* or increas*)) S16 S13 OR S14 OR S15 S17 S12 OR S16 S18 S3 AND S17
British Nursing Index Database (BNID) (NHS Evidence)
1. exp FRACTURES/ AND HIP JOINT/ 2. ((hip* or intertrochanteric or subtrochanteric or trochanteric or femur or femoral or acetabul*) adj5 fracture*).ti,ab 3. 1 OR 2 4. exp Blood Transfusion/ 5. (transfus* or posttransfus* or post‐transfus* or retransfus* or red cell* or red blood cell* or RBC* or erythrocyte*).ti,ab 6. 4 OR 5 7. 3 and 6
Transfusion Evidence Library, LILACS, IndMed, KoreaMed, PakMediNet, Web of Science, WHO ICTRP, ClinicalTrials.gov and ISRCTN Registry
We searched these databases using a combination of the following terms:
blood OR erythrocyte* OR red cell* OR RBC* OR transfus* OR retransfus* AND hip* or intertrochanteric or subtrochanteric or trochanteric or pertrochanteric or peritrochanteric or femur or femoral or acetabul* AND fracture*
Appendix 2. Details of the functional recovery and quality of life scales
The details below were taken from the trials using these scales and are provided as information.
Cumulated Ambulation Score: provides a daily score ranging from 0 to 6 (6 = best) and assesses getting in and out of bed, sitting‐to‐standing‐to‐sitting and walking ability with an appropriate aid.
Cumulated Fatigue and Cumulated Dizziness: symptoms were evaluated by a 5‐point verbal rating score (ranging from 0 = symptoms to 5 = very severe symptoms prohibiting person from rising from bed) for 'dizziness on standing' and 'feeling of general fatigue' separately. These scores were recorded on days 1, 2 and 3 post operation; a cumulated fatigue score and dizziness score (as cumulated values from days 1 to 3, both ranging from 0 to 12) were also presented.
Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐Fatigue score): includes 13 items with scores ranging from 0 to 4, with higher scores indicating a greater energy level. "Missing items were imputed as the mean of item scores within the same scale" (Carson 2011 page 2460, Table 3).
Instrumental activities of daily living (ADL): scores on the instrumental ADL scale ranged from 0 to 4 with higher scores indicating greater dependency. Scores were calculated by totalling the number of dependencies with respect to four advanced activities. Participants who reported that they needed assistance or were unable to perform a task for health reasons were considered to be dependent with respect to that activity.
Lower‐extremity physical activities of daily living (ADL): Scores on lower‐extremity ADL scale ranged from 0 to 11 with higher scores indicating greater dependency. Scores were calculated by totalling the numbers of dependencies with respect to 11 basic activities. Participants who reported that they had any human assistance in an activity or that they did not perform the activity for a health reason were considered to be dependent with respect to that activity.
Modified Barthel Index: measures the basic self care Activities of Daily Living (ADL) performance ranging from 0 to 100 points (100 = best), assessing 10 domains: eating, transferring, personal care, toileting, bathing, walking, managing stairs, dressing, bowel control and bladder control.
New Mobility Score: ranges from 0 to 9 points (9 = best) and assess both indoor and outdoor walking abilities and the ability to go shopping.
Data and analyses
Comparison 1. Liberal versus restrictive threshold.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐day mortality | 5 | 2683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.26] |
| 2 60‐day mortality | 3 | 2283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.80, 1.44] |
| 3 Longer‐term mortality: at 90, 120 and 365 days | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 90‐day mortality | 2 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.16] |
| 3.2 120‐day mortality | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.56, 2.51] |
| 3.3 365‐day mortality | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.65, 1.65] |
| 4 Inability to walk 10 feet (3 m; or across a room) without human assistance | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 30 days | 1 | 1995 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 4.2 At 60 days | 2 | 2083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 5 Regaining functional independence during hospitalisation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Poor mobility/physical ability (at 10 days) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Unable to walk (CAS) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Bedridden (CAS) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mean change in mobility scores (score 0 to 9: best mobility) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Thromboembolism (in hospital) | 4 | 2416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.56, 2.37] |
| 9 Stroke (inpatient) | 4 | 2416 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.85, 6.79] |
| 10 Wound infection (in hospital) | 3 | 2332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.77, 3.35] |
| 11 Cardiovascular events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Myocardial infarction | 3 | 2217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.96] |
| 11.2 Congestive heart failure (new diagnosis) | 3 | 2332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.48, 1.23] |
| 12 Respiratory infections (namely pneumonia) | 4 | 2416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.95, 1.92] |
| 13 Lower Extremity Physical Activities of Daily Living (ADL) (0 to 11: total dependency) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 30 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 60 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Instrumental Activities of Daily Living (ADL) (higher scores = higher dependency) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 30 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 60 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 FACIT‐Fatigue score (higher scores = more energy) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 30 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 60 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Dependency (based on Modified Barthel Index) at 10 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Substantially or completely dependent | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.92, 1.20] |
| 16.2 Completely dependent | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| 17 Length of stay in hospital (days) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 USA | 2 | 1304 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.66, 0.12] |
| 17.2 Canada | 1 | 791 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.98, 0.64] |
| 17.3 Denmark | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐3.23, 6.83] |
| 17.4 UK | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐7.81, 4.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carson 1998.
| Methods | Randomised controlled trial (pilot study) Number of centres: 4 in the USA Dates enrolled: March 1996 to March 1997 Follow‐up: 60 days |
|
| Participants | 84 participants; of which 24% were men Inclusion criteria: presenting for hip fracture repair, and if haemoglobin < 10 g/dL in the immediate postoperative period (defined as time from end of anaesthesia to 11:59 p.m. 3 days after surgery counted from 12:00 midnight on first day after surgery) Exclusion criteria: people who refused transfusion because of religious beliefs, had multiple trauma or had symptoms of anaemia Mean (SD) age in the liberal transfusion threshold: 81.3 (8.1) years; range 50‐94 years Mean (SD) age in the restrictive transfusion threshold: 83.3 (10.8) years; range 32‐95 years Number of men per intervention: 9/11 |
|
| Interventions | Liberal transfusion threshold versus restrictive transfusion threshold. Participants were randomised when their postoperative haemoglobin fell to < 10 g/dL within the first 3 days post operation Liberal transfusion threshold (classified as 'Threshold arm' in the trial): participants received 1 unit of packed red blood cells at the time of randomisation and as much additional blood as necessary (not further defined) to keep the haemoglobin concentration > 10 g/dL during hospitalisation Restrictive transfusion threshold (classified as 'Symptomatic arm' in the trial): transfusion delayed until person developed symptoms or experienced consequence of anaemia (chest pain thought to be cardiac in origin, myocardial infarction, congestive cardiac failure, unexplained tachycardia/hypotension, decreased urine output unresponsive to fluid replacement; poor rehabilitation: inability to get out of bed for rehabilitation by third day post operation). When a transfusion was given for symptoms of anaemia, enough red blood cells were transfused to relieve symptoms Transfusion was also permitted if haemoglobin fell < 8 g/dL. If the haemoglobin fell < 8 g/dL, enough red blood cells were to be transfused to get the haemoglobin concentration > 9 g/dL Number of people randomised per intervention: 42/42 Number of people included in the analysis of the primary outcome: 42/42 |
|
| Outcomes |
Primary outcomes: Mortality Mobility and functional recovery Postoperative morbidity Secondary outcomes: Length of stay in hospital |
|
| Notes | Email communication with the main author on 7 March 2013 was successful in identifying the time point when the respiratory infections developed in the two people who reported respiratory infections: postoperatively In the trial, haemoglobin was checked daily for first 3 days but people only included in the trial if the haemoglobin < 10 g/dL Based on data that suggested that people with cardiovascular disease may not tolerate anaemia as well as people without cardiovascular disease, randomisation schedules were stratified by clinical site and cardiovascular disease status This was a pilot study for the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) trial included as Carson 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation undertaken by "contacting the data co‐ordinating centre's 24 hour automated telephone service" (page 524 of manuscript) |
| Allocation concealment (selection bias) | Low risk | Randomisation undertaken at a central location by contacting the data co‐ordinating centres 24‐hour automated telephone service (manuscript page 524) |
| Blinding of participants (performance and detection bias) | High risk | The manuscript did not state whether the participants were blinded to their treatment allocation. If able, the participants were the reporters of their status at 60 days. Given that the participants themselves were involved in assessing outcomes (quality of life, functional mobility), knowledge of treatment allocation may have influenced outcome measurement, hence an assignment of high risk of bias |
| Blinding of personnel (performance bias) | High risk | There was no report of blinding of clinicians within the manuscript. However, the blinding of clinicians would have been difficult as the clinicians themselves determined whether a participant met the requirements for a red blood cell transfusion. 5 protocol violations were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Study nurses, blind to the transfusion status of the patient, obtained information from patients or proxies on survival, place of residence and functional status...With a telephone interview 60 days after (operation)" (page 524 of manuscript) Subjective outcomes were measured by the study nurse following discharge (at 60 days after randomisation) with additional information being provided by the participant's physician as needed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data: all randomised participants were followed up and reported to have completed the study |
| Selective reporting (reporting bias) | Low risk | The protocol was not available, but all (expected) outcomes specified in the methods section were reported in the results |
| Other bias | Low risk | No obvious other biases were noted Participants were stratified by cardiovascular disease status (present or not) and site to prevent an imbalance per treatment group based on these factors Baseline characteristics were similar between the 2 treatment groups |
Carson 2011.
| Methods | Randomised controlled trial Number of centres: 47 in Canada and USA and 1 centre in UK (see Palmer 1998) Dates enrolled: 19 July 2004 to 28 February 2009 Follow‐up: 60 days |
|
| Participants | 2016 participants, of which 24% were men Inclusion criteria: people aged ≥ 50 years who were undergoing primary surgical repair of a hip fracture and who had clinical evidence of a history of ischaemic heart disease, ECG evidence of previous myocardial infarction, a history or presence of congestive heart failure or peripheral vascular disease, or a history of stroke or transient ischaemic attack) or risk factors for cardiovascular disease (see below) were eligible if they had a haemoglobin concentration of < 10 g/dL within 3 days after surgery After December 2005, people with any of the following cardiovascular criteria were eligible regardless of haemoglobin concentration:
Exclusion criteria:
Mean (SD) age in the liberal transfusion threshold: 81.8 (8.8) years Mean (SD) age in the restrictive transfusion threshold: 81.5 (9.0) years Number of men per intervention: 250/239 |
|
| Interventions | Liberal transfusion threshold vs. restrictive transfusion threshold. Participants were randomised when their postoperative haemoglobin fell to < 10 g/dL within the first 3 days post operation Liberal transfusion threshold: "Patients ..... received 1 unit of packed red cells and additional blood as needed to maintain a haemoglobin level of 10g or more per deciliter. An assessment of the haemoglobin level after transfusion was required and an additional unit of blood was transfused if the patients blood was below 10 g per deciliter" (page 2454 of manuscript) Restrictive transfusion threshold: "Patients ... were permitted to receive transfusions if symptoms or signs of anaemia developed or at the discretion of their physicians if the haemoglobin level fell below 8 g per deciliter" (page 2454 of manuscript) Number of people randomised per intervention: 1007/1009 Number of people included in the analysis of the primary outcome: 995/1000 |
|
| Outcomes |
Primary outcomes: Mortality Mobility and functional recovery Postoperative morbidity Secondary outcomes: quality of life Length of stay in hospital |
|
| Notes | Email communication with the main author on 7 March 2013 was successful in identifying the discharge protocols for the US and Canadian participants and the time points for the reported postoperative morbidity. This trial changed its inclusion criteria in 2005 from including people with a history of cardiovascular disease to people with a history of cardiovascular disease OR risk factors as outlined. An ancillary study (the FOCUS Cognitive Ancillary Study), with enrolment in the last few months of the trial (April 2008 to February 2009) of 139 participants, that collected delirium outcomes was reported in Gruber‐Baldini 2013. Our reading of the articles for the ancillary study was that the population of this study was a subgroup of that of FOCUS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study used an "automated telephone randomisation system" (page 2454 of manuscript) with randomisation schedules for each site using randomly ordered block sizes of 2, 4, 6 and 8 |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken at a central location "staff members at the data co‐ordinating centre" (page 2454 of manuscript) |
| Blinding of participants (performance and detection bias) | High risk | "Patients, clinical‐site staff members & clinicians were aware of study group assignment after randomisation" (page 2454 of manuscript) Given that the participants themselves were involved in assessing outcomes (quality of life, functional mobility), knowledge of treatment allocation may influence outcome measurement, hence an assignment of high risk of bias |
| Blinding of personnel (performance bias) | High risk | "Patients, clinical‐site staff members & clinicians were aware of study group assignment after randomisation" (page 2454 of manuscript). The blinding of clinicians would have been difficult anyway as the clinicians themselves determined whether a participant met the requirements for a red blood cell transfusion. 147 protocol violations were reported. In addition, given that the clinicians themselves were involved in assessing outcomes (postoperative morbidity), knowledge of treatment allocation may have influenced outcome measurement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Study investigators who classified cardiovascular events and those who did follow‐up telephone assessments (to assess outcomes [quality of life, mobility & functional recovery] after hospital discharge) were unaware of study group assignments" (page 2455 of manuscript) Subjective outcomes were measured following discharge (at 30 and 60 days after randomisation) and by people blinded to treatment assignment, hence an assignment of low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A small number of participants were not included in most analyses: mortality and mobility at 30 (n = 21) and 60 days (n = 17) and postoperative morbidity (n = 3). The manuscript reported the number of withdrawals and losses to follow‐up, but did not state when these happened. ECG was used to measure myocardial infarction incidence: ECG results (after randomisation) were incomplete in 13.4% (n = 135) in the liberal transfusion threshold group and 12.9% (n = 130) in the restrictive transfusion threshold group of participants. Of note also is that there was no blood sample for troponin testing in 17.9% (n=180) in the liberal transfusion threshold group and in 17.3% (n=175) in the restrictive transfusion threshold group of participants. A large number of people were not included in the quality of life assessments at 30 days (45.6% in the liberal transfusion threshold group vs. 45.9% in the restrictive transfusion threshold group missing) and at 60 days (54% vs. 52% missing). At each time point, and for each assessment tool, the percentage of participants not included in the analysis was similar between the 2 transfusion threshold groups. The manuscript documented the reasons for participants not being included in these analyses as either inability to perform the physical assessment or an incomplete data set per assessment |
| Selective reporting (reporting bias) | Low risk | Study protocol was available (on clinical trials.gov) and all of the study's pre‐specified primary, secondary and composite outcomes are reported in the pre‐specified way |
| Other bias | High risk | There was a statistical difference in the number of major protocol violations post randomisation between the 2 transfusion threshold groups: 9% in the liberal transfusion threshold and 5.6% in the restrictive transfusion threshold group (P value = 0.003) The difference between the 2 transfusion threshold groups in the number of transfusions received before randomisation was not significant (P value = 0.07) but may be of interest in the overall context of this review. Full details are available in Table 3 |
Foss 2009.
| Methods | Randomised clinical trial Single‐centre (Hvidovre University Hospital) study, Denmark Dates enrolled: February 2004 to July 2006 Follow‐up period: 3 days (to record CAS) and for 30 days (from Danish civil registry) for record of 30‐day mortality |
|
| Participants | 120 participants of which 23% were men People with hip fracture admitted to the hip fracture unit at the department of orthopaedics at Hvidovre University Hospital were screened for inclusion Inclusion criteria:
Exclusion criteria:
Mean (SD) age in the liberal transfusion threshold: 81 (6.8) years Mean (SD) age in the restrictive transfusion threshold: 81 (7.3) years Number of men per intervention: 14/14 |
|
| Interventions | 120 people were randomly assigned to 2 groups of 60 (liberal vs. restrictive transfusion policy). Participants were randomised at admission to hospital and transfused by protocol thereafter Liberal transfusion threshold: "... patients received transfusion when their Hb [haemoglobin] level decreased to below 10.0 g per dL" (page 228 of manuscript) at any time between admittance to "the post‐anaesthesia care unit" and the fifth postoperative day: 8.8 g/dL < haemoglobin concentration < 10 g/dL ⇒ 1 unit of red blood cells 7.2 g/dL < haemoglobin concentration < 8.8 g/dL ⇒ 2 units of red blood cells Haemoglobin concentration < 7.2 g/dL ⇒ 3 units of red blood cells Restrictive transfusion threshold: "patients ... received transfusion when their Hb level decreased to below 8.0 g per dL" (page 228 of manuscript): 7.2 g/dL < haemoglobin concentration < 8 g/dL ⇒ 1 units of red blood cells 5.6 g/dL < haemoglobin concentration < 7.2 g/dL ⇒ 2 units of red blood cells Haemoglobin concentration < 5.6 g/dL ⇒ 3 units of red blood cells Number of participants randomised per intervention: 60/60 Number of participants included in the analysis of the primary outcome: 54/53 |
|
| Outcomes |
Primary outcome: CAS as registered on the first 3 postoperative days Secondary outcomes: Length of stay Cardiac complications (defined as acute myocardial infarction, pulmonary oedema/acute congestion or new‐onset arrhythmia) Infectious complications (pneumonia, sepsis or wound infection) Mortality |
|
| Notes | Email communication with the author on 7 September 2014 was successful in confirming the exact timing of the randomisation: at hospital admission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was done by a computer‐generated list by a person not affiliated with the project" (page 228 of manuscript) |
| Allocation concealment (selection bias) | Unclear risk | The details of the randomisation were placed in a "sealed envelope", which was then placed in the participant's notes for use when needed. To minimise bias, the study should have used sequentially numbered, opaque, sealed envelopes, hence the assignment of unclear risk of bias |
| Blinding of participants (performance and detection bias) | Low risk | The manuscript stated that the participants were blinded to treatment allocation However, it would have been difficult for the participants to be aware of treatment allocation and even if they were, participants were not involved in the assessment of their outcome measures in the study, hence the assignment of low risk of bias |
| Blinding of personnel (performance bias) | High risk | There was no blinding of the clinicians who determined whether a participant met the requirements for a red blood cell transfusion. (There was no reporting of protocol violations) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was done by physiotherapists (for the primary outcome of mobility) and clinicians. Although the physiotherapists were blinded, clinicians rating the primary outcomes of this review did not appear to be, hence the assignment of high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up. Missing outcome data balanced in numbers across intervention groups, with similar reasons reported for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The protocol was available (on clinicaltrials.gov') and all pre‐specified outcomes that were of interest to this review have been reported (in the study publication) in the pre‐specified way |
| Other bias | Unclear risk | This study had baseline imbalances, one of which was reported to be statistically significant. The statistically significant imbalance is in the proportion of participants in each ASA scale between the treatment groups (P value = 0.02). Clinically, it is known that participants with a higher ASA score are more likely to die. Another baseline imbalance was noted in the per‐protocol analysis where there were imbalances in the surgical procedures between the intervention groups, with 2 of the procedures (sliding hip screw and intermedullary hip screw procedures) being indicative of higher blood loss. However, the impact of this imbalance to the outcomes in the review were unclear, hence the assignment of unclear risk of bias |
Gregersen 2015.
| Methods | Randomised controlled trial Single‐centre study (Departments of Geriatrics and Orthopaedics, Aarhus University Hospital, Aarhus), Denmark Dates enrolled: 18 January 2010 to 6 June 2013 Follow‐up period: 90 days (record of mortality and physical ability) |
|
| Participants | 284 participants of which 25% were men Inclusion criteria:
Exclusion criteria:
Mean (SD) age in the liberal transfusion threshold: 88 (6.9) years Mean (SD) age in the restrictive transfusion threshold: 86 (6.8) years Number of men per intervention: 34/36 |
|
| Interventions |
Liberal transfusion strategy:
Restrictive transfusion strategy:
Haemoglobin concentration was measured daily during the first 3 postoperative days then at least once during the following 4‐6 days and at least once weekly for the subsequent 3 weeks Number of participants randomised per intervention: 140/144 Number of participants included in the analysis of the primary outcome: 140/144 |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Original details of the trial were taken from the protocol uploaded to clinicaltrials.gov; the first reports of the trial following trial completion were presented in conference format Email communication with the author on 11 December 2014 resulted in us receiving a copy of the final (unpublished) version of the manuscript for this trial with outcome data and information that allowed us to complete a risk of bias assessment. We added the data and information to the text and tables for this review. Subsequently the manuscript for this trial was published in Acta Orthopaedica; currently online (March 2015) 88% of participants in the restrictive transfusion threshold group and 87% of participants in the liberal transfusion threshold group received iron supplementation while on the trial The trial stratified participants at the time of randomisation between people who were living in sheltered housing and people living in a nursing home. Subgroup analyses were conducted on place of residence within the trial. Except for some observations on length of hospital stay, we reported the analysis by intervention alone within this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was provided by an allocation concealment process in the web based clinical trial support system." Participants were allocated to the trial's intervention groups by the project manager entering the participant's civil registration number into the computer program (page 5 of unpublished trial manuscript) |
| Allocation concealment (selection bias) | Low risk | "Randomization was provided by an allocation concealment process in the web based clinical trial support system" (page 5 of unpublished trial manuscript) |
| Blinding of participants (performance and detection bias) | Low risk | The central computer programme allocated each participant to one of the trial's two intervention groups. The project manager entered the patient's civil registration number into the computer programme and passed on the randomisation result to the electronic patient record which was available to the hospital staff in the Orthopaedic and Geriatric wards: the wards in which the transfusions were to be administered The trial stated that the participants, their relatives and the outcome assessors were blinded to the result of the randomisation and to information on the participant's haemoglobin concentration levels (page 5 of unpublished trial manuscript) |
| Blinding of personnel (performance bias) | High risk | The trial stated that the participants, their relatives and the outcome assessors were blinded to the result of the randomisation and to information on the participant's haemoglobin concentration levels. No information was reported as to whether study personnel were blinded to treatment allocation However, there were 8 (3%) deviations from protocol in each intervention group In the liberal transfusion threshold group, there were 4 protocol deviations due to inattention to haemoglobin concentrations and in 4 cases physicians prescribed more blood In the restrictive transfusion threshold group, there were 6 protocol deviations due to inattention to haemoglobin concentrations and in 2 cases physicians refused to prescribe red blood cell transfusion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physical and cognitive outcomes were assessed by 2 occupational therapists blinded to treatment allocation Dates (and causes) of deaths up to 90 days post surgery were obtained from the Danish Civil Registration System (and from death certificates from the Danish Health and Medicine Authority) (page 7 of unpublished trial manuscript) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat and per‐protocol analyses conducted. Similar numbers (12 in each group) were dropped from the per‐protocol analysis. All participants included in the primary analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined as being of interest to the trial in the trial protocol on clinicaltrials.gov were reported on in the unpublished trial manuscript |
| Other bias | Unclear risk | Insufficient data to assess low or high risk of bias No evidence of baseline imbalance between the 2 trial groups and no details were reported as to any protocol violations |
Palmer 1998.
| Methods | Randomised controlled trial Single‐centre study, UK Dates enrolled: June to July 1997 Follow‐up: 4 months |
|
| Participants | 18 participants, number of men not given Inclusion criteria:
Exclusion criteria:
Age not given |
|
| Interventions | Liberal transfusion threshold vs. restrictive transfusion threshold. Participants were randomised when their postoperative haemoglobin concentration dropped to between 8 and 10 g/dL within the first 2 days post operation Liberal transfusion threshold:
Restrictive transfusion threshold:
Number of participants randomised per intervention: 9/9 Number of participants included in the analysis of the primary outcome: not stated |
|
| Outcomes | None reported ‐ authors contacted, none available | |
| Notes | We tried to contact any of the authors of this conference abstract and received an email response in January 2013 from one of the authors explaining that this was a pilot trial and that the authors were unsuccessfully in obtaining a grant to finish the trial. The study was only presented as a conference abstract. There was a small overlap of trial participants (either 2 or 4) with Carson 1998 (McClelland 2009) This study was not included in the analysis of data due to a lack of data reported in the manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient data to assess low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data to assess low or high risk of bias |
| Blinding of participants (performance and detection bias) | Unclear risk | Insufficient data to assess low or high risk of bias |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient data to assess low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient data to assess low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to assess as no outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess as no outcome data reported |
| Other bias | Unclear risk | Insufficient data to assess low or high risk of bias |
Parker 2013.
| Methods | Randomised controlled trial Single‐centre (Peterborough City Hospital) study, UK Dates enrolled: April 2002 to March 2011 Follow‐up: 1 year |
|
| Participants | 200 participants, of which 16% were men Inclusion criteria:
Exclusion criteria:
Mean age in the liberal transfusion threshold: 84.4 years; range 60‐104 years Mean age in the restrictive transfusion threshold: 84.2 years; range 60‐97 years |
|
| Interventions | Liberal transfusion threshold vs. restrictive transfusion threshold. Participants were randomised when their haemoglobin measured between 8 and 9.5 g/dL on their first or second day postoperatively Liberal transfusion threshold (classified as 'Transfusion group' in the trial) received at least 1 unit of blood to raise haemoglobin concentration to ≥ 10 g/dL Restrictive transfusion threshold (classified as 'No transfusion group' in the trial) were transfused when definite symptoms of anaemia were found (recurrent vasovagal episodes on mobilisation, chest pain of cardiac origin, congestive cardiac failure, unexplained tachycardia, hypotension or dyspnoea that was considered to be due to anaemia. Decreased urine output unresponsive to fluid replacement and any other symptoms as indicated and felt appropriate by the medical staff caring for the participant) Number of participants randomised per intervention: 100/100 Number of participants included in the analysis of the primary outcome: 100/100 Number of men per intervention: 17/15 |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Email communication with author on 14 March 2013 provided an update on the status of the trial. The trial was subsequently published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient data to assess low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | "...numbered, sealed opaque envelopes, that were prepared before the start of the study" were used. No details were reported as to whether these were sequential, hence the assignment of unclear risk of bias |
| Blinding of participants (performance and detection bias) | Low risk | Additional email correspondence with the author identified that no participant was blinded to treatment allocation. There were no subjective outcomes that this participant knowledge could influence, hence the assignment of low risk of bias |
| Blinding of personnel (performance bias) | High risk | There was no blinding of the clinicians who determined whether a participant met the requirements for a red blood cell transfusion (there was no reporting of protocol violations) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The nurse making the assessment of change in mobility scores was blinded to treatment allocation (detail from additional email correspondence with study author) Other assessments were made by clinicians aware of treatment allocation. This awareness may have influenced outcome measurement, hence the assignment of high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data: all randomised participants were included in the outcome analyses |
| Selective reporting (reporting bias) | Low risk | The protocol for this trial was available and all pre‐specified outcomes in the protocol were addressed and reported in the manuscript in the pre‐specified way |
| Other bias | Unclear risk | There was some baseline imbalance between the treatment groups for the number of participants with any cardiac disease at baseline. 37% of participants in the liberal transfusion group and 50% of participants in the restrictive transfusion group had cardiac disease. The study reported that this imbalance was not statistically significant. Clinically, in this population there was a high level of cardiac disease both diagnosed and undiagnosed, but it is unclear what impact this imbalance could have on the findings of the study or this review, hence an assignment of unclear risk of bias |
ASA: American Society of Anesthesiologists; CAS: Cumulated Ambulation Score; ECG: electrocardiography; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gampopoulou 2004 |
Ineligible participants The participants were not acute admissions and did not receive surgery for a hip fracture |
| Izuel‐Rami 2005 |
Ineligible intervention No abstract was available from the search strategy: on obtaining the full‐text article, it was clear that none of the interventions included red blood cell transfusion |
| Izuel‐Rami 2006 |
Ineligible intervention No abstract was available from the search strategy: on obtaining the abstract, it was clear that none of the interventions included red blood cell transfusion |
| Jans 2011 | Details on the ClinicalTrials.gov register (accessed 14 March 2013) identified that the study had been terminated early due to a lack of enrolment. The trial record on ClinicalTrials.gov had been updated with this information on 12 September 2012 |
| Matot 2012 | Email contact with the author revealed that the trial had closed in "very early stages due to difficulties in patients' recruitment" and thus it should be listed as an excluded study |
| Moghaddam 2009 |
Ineligible intervention No abstract was available from the search strategy: on obtaining the abstract, it was clear that none of the interventions included red blood cell transfusion |
| Muir 1995 |
Ineligible study type No abstract was available from the search strategy: on obtaining the full‐text article, it was clear that this was a question and answer paper and not a randomised controlled trial |
| Nielsen 2012 |
Ineligible participants Study was identified from ClinicalTrials.gov on 25 July 2012. It was not clear from the ClinicalTrials.gov record whether the study would be eligible for this review. Email communication with the study contact (Kamilla Nielsen) in March 2013 identified that their study participants "underwent replacement of total hip arthroplasty" and we were interested in people requiring or undergoing surgery for hip fracture |
| Prasad 2009 |
Ineligible intervention No abstract was available from the search strategy: on obtaining the full‐text article, it was clear that none of the interventions included red blood cell transfusion |
| Serrano Trenas 2011 |
Ineligible intervention A comparison of the use of perioperative intravenous iron therapy in addition to red blood cell transfusion. The intervention groups were red blood cell transfusion with iron sucrose compared with red blood cell transfusion alone |
| Zufferey 2010 |
Ineligible intervention No abstract was available from the search strategy: on obtaining the full‐text article, it was clear that none of the interventions included red blood cell transfusion |
Characteristics of studies awaiting assessment [ordered by study ID]
ChiCTR‐TRC‐10000822.
| Methods | Randomised controlled trial |
| Participants | Older people undergoing orthopaedic surgery who meet the following criteria:
|
| Interventions |
Group 1:
Group 2:
Group 3:
Group 4:
Group 5:
|
| Outcomes |
|
| Notes | The trial was identified on the Chinese Clinical Trial Registry, but the record has not been updated since first added in 2010. We have been unable to find contact details for the named applicant (checked again 14 March 2013). The trial record states that the trial is due to close by 1 May 2015 |
Hct: haematocrit.
Differences between protocol and review
Minor edits to 'Background' section.
In 'Types of interventions', the protocol referred to threshold A as being restrictive and threshold B as being liberal the comparisons of "Red blood cell transfusion protocol A versus red blood cell transfusion protocol B" and "Red blood cell transfusion threshold A versus red blood cell transfusion threshold B". We switched these round in the review, labelling threshold A as liberal and threshold B as restrictive. Over the last years, both a 'liberal transfusion threshold' and a 'restrictive transfusion threshold' have been seen as normal practice (and both equally as novel practice); therefore, the ordering of these interventions could anyway be viewed as interchangeable. As all the included studies ordered the interventions as liberal versus restrictive and so as to avoid any transcription errors, we changed the ordering of these interventions, ahead of the analysis of data, to liberal versus restrictive in the review.
Risk of performance bias was assessed separately for participants and personnel.
Two post‐hoc sensitivity analyses were conducted: one removing the largest trial and the other removing the trial that had specifically excluded people with pre‐existing cardiac conditions.
Contributions of authors
All review authors contributed to the development of the protocol and the final review manuscript. AS and SM prepared the first drafts of the manuscript and thereafter incorporated comments from all other authors through the preparation of the final manuscript.
Ali Shokoohi: undertook the screening, data extraction and risk of bias assessments, analysed the data and entered the data into Review Manager. He also provided haematology and transfusion medicine content expertise and acted as guarantor for the review.
Sarah Millette: undertook data extraction and risk of bias assessments, analysed the data and entered the data into Review Manager. She provided hip fracture geriatrician content expertise.
Susan Brunskill: undertook the screening of references, designed the data extraction forms and acted as project manager for the review. She also provided methodological expertise and helped to resolve disagreements between the review authors.
Claire Pulford: provided hip fracture geriatrician content expertise.
Carolyn Dorée: developed the search strategies, advised on resources to be searched for the review, ran the search strategies and did a first sift of the references removing duplicates and clearly irrelevant references.
Mike Murphy: provided transfusion medicine content expertise.
Simon Stanworth: undertook the screening of references and provided transfusion medicine content expertise.
Sources of support
Internal sources
NHS Blood & Transplant, Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
Ali Shokoohi: none. Sarah L Millette: none. Susan J Brunskill: none. E Claire Pulford: none. Carolyn Dorée: none. Michael F Murphy: none. Simon Stanworth: none.
New
References
References to studies included in this review
Carson 1998 {published data only}
- Brunskill SJ [per comm]. Pilot to the FOCUS trial. Email to: J Carson 7 March 2013.
- Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin‐level‐driven red blood cell transfusions following hip fracture. Transfusion 1998;28(6):522‐9. [DOI] [PubMed] [Google Scholar]
Carson 2011 {published data only}
- Barr PJ, Bailie KEM. Transfusion thresholds in FOCUS. New England Journal of Medicine 2011;365(26):2532‐3. [DOI] [PubMed] [Google Scholar]
- Brunskill SJ [per comm]. FOCUS trial. Email to: J Carson 7 March 2013.
- Carson J. FOCUS: transfusion triggers after fractured hip repair. Transfusion Medicine 2011;21:14. [Google Scholar]
- Carson J. Safety and effectiveness of two blood transfusion strategies in surgical patient with cardiovascular disease (FOCUS). clinicaltrials.gov/show/NCT00071032 (accessed 21 July 2014).
- Carson J. Transfusion triggers in orthopedic patients. Transfusion Alternatives in Transfusion Medicine 2011;12(1):9. [Google Scholar]
- Carson JL. FOCUS study: transfusion trigger in patients with cardiovascular disease undergoing hip fracture surgery. Transfusion Alternatives in Transfusion Medicine 2010;11:7. [Google Scholar]
- Carson JL, Terrin ML, Chaitman B, Magaziner J, Sanders D. Impact of transfusion triggers on postoperative myocardial infarction or death. American Heart Association's Scientific Sessions 2009 2010;120(21):2155. [Google Scholar]
- Carson JL, Terrin ML, Magaziner J, Chaitman BR, Apple FS, Heck DA, et al. Transfusion trigger trial for Functional Outcomes in Cardiovascular patients Undergoing Surgical hip fracture repair (FOCUS). Transfusion 2006;46(12):2192‐206. [DOI] [PubMed] [Google Scholar]
- Carson JL, Terrin ML, Magaziner J, Sanders D, Cook DR, Hildebrand K. Transfusion trigger trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical hip fracture repair (FOCUS): the principal results. Blood 2009;114:22. [DOI] [PubMed] [Google Scholar]
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. New England Journal of Medicine 2011;365(26):2453‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber‐Baldini A, Marcantonio ER, Orwig D, Magaziner J, Terrin M, Carson J, et al. FOCUS cognitive ancillary study: randomized clinical trial of blood transfusion thresholds on delirium severity. Journal of the American Geriatrics Society 2010;58:S86. [Google Scholar]
- Gruber‐Baldini AL, Marcantonio E, Orwig D, Magaziner J, Terrin M, Barr E, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. Journal of the American Geriatrics Society 2013;6(8):1286‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber‐Baldini AL, Marcatonio E, Orwig D, Magaziner J, Terrin M, Carson J. Blood transfusion and delirium at 45‐days post‐hip fracture: long term outcomes from the focus cognitive ancillary study. Journal of the American Geriatrics Society 2011;59:S113. [Google Scholar]
Foss 2009 {published data only}
- Brunskill SJ [pers comms]. Cochrane review transfusion in hip fracture ‐ request for information. Email to N Foss 4 September 2014.
- Foss N. The effect of liberal vs. restrictive transfusion strategies on rehabilitation after hip fracture surgery. clinicaltrials.gov/show/NCT00162617 (accessed 21 July 2014).
- Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion 2009;49(2):227‐34. [DOI] [PubMed] [Google Scholar]
- Pulford EC [pers comms]. Cochrane review transfusion in hip fracture ‐ request for information. Email to: Nocolai Foss 3 May 2013.
Gregersen 2015 {published data only}
- Brunskill SJ [pers comms]. Cochrane review transfusion in hip fracture ‐ request for information. Email to M Gregersen 11 December 2014.
- Gregersen M. Postoperative blood transfusion for frail elderly with hip fracture. clinicaltrials.gov/show/NCT01102010 (accessed 21 July 2014).
- Gregersen M, Borris LC, Damsgaard EM. A liberal blood transfusion strategy after hip fracture surgery does not increase the risk of infection in frail elderly. European Geriatric Medicine 2012;3 Suppl:S74. [Google Scholar]
- Gregersen M, Borris LC, Damsgaard EM. A liberal blood transfusion strategy improves survival in nursing home residents with hip fracture. European Geriatric Medicine 2013;4 Suppl:S106. [Google Scholar]
- Gregersen M, Borris LC, Damsgarrd EM. Postoperative blood transfusion strategy in frail anemic elderly with hip fracture: the TRIFE randomised controlled trial. Acta Orthopaedica 2015 (Posted online on January 14, 2015);86(3):1‐10 (online version numbering). [DOI: 10.3109/17453674.2015.1006980] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen MG, Damsgaard EM, Borris LC. Blood transfusion strategies in recovery from physical disability and overall quality of life in frail elderly with hip fracture. European Geriatric Medicine 2014;5 Suppl:S228. [Google Scholar]
Palmer 1998 {published data only}
- Brunskill SJ [pers comm]. Conference abstract ‐ British Blood Transfusion Society meeting 1998 ‐ Hip fracture and transfusion trial (HATT). Email to: B McClelland 24 January 2013.
- Palmer JB, Maciver CR, Scott R, Picken MG, McClelland DBL, Keating JF, et al. Hip fracture and transfusion trial (HATT). Transfusion Medicine 1998;8 Suppl:Abstract P52. [Google Scholar]
Parker 2013 {published data only}
- Brunskill SJ [pers comm]. Randomised trial of a blood transfusion policy after fracture of the proximal femur (hip fracture). Email to: M Parker 14 March 2013.
- Parker M. Randomised trial of a blood transfusion policy after fracture of the proximal femur (hip fracture). www.controlled‐trials.com/ISRCTN61328173 (accessed 21 July 2014).
- Parker M. Randomised trial of blood transfusion versus a restrictive transfusion policy after hip fracture surgery. Injury 2013;44(12):1916‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gampopoulou 2004 {published data only}
- Gampopoulou Z, Vretou V, Mauromati P, Chatzieleftheriou A, Vasilaki M, Papadimos M, et al. Red cell transfusion with and without leukocyte depletion for orthopedic surgery. Transfusion Alternatives in Transfusion Medicine 2004;6 Suppl:P20. [Google Scholar]
Izuel‐Rami 2005 {published data only}
- Izuel‐Rami M, Cuenca Espirrez J, Garcia‐Erce JA, Gomez‐Barrera M, Carceln Andreas J, Rabanaque Herníndez MJ. Perioperative anaemia in geriatric patients with hip fracture [Efectividad de distintas pautas de tratamiento de la anemia perioperatoria en pacientes ancianos con fractura de cadera]. Farmacia Hospitalaria 2005;29(4):250‐7. [DOI] [PubMed] [Google Scholar]
Izuel‐Rami 2006 {published data only}
- Izuel‐Rami M, Garcia‐Erce JA, Cuenca J, Gomez‐Barrera M, Villar I, Rabanaque MJ. Recovery from postoperative anaemia after hip fracture replacement surgery. Is there a role for erythropoietin and intravenous iron?. Transfusion Alternatives in Transfusion Medicine 2006;8 Suppl:P53. [Google Scholar]
Jans 2011 {published data only}
- Jans O. Liberal versus restrictive transfusion during symptomatic moderate anemia after hip arthroplasty. clinicaltrials.gov/show/NCT01452581 (accessed 21 July 2014).
Matot 2012 {published data only}
- Brunskill SJ [pers comm]. NCT01491308, TASMAC‐11‐IM‐0449‐11‐CTIL, Restrictive versus liberal red cell transfusion strategy in orthopedic‐oncology patients undergoing surgery ‐ a randomized controlled study. Email to: Idit Matot 14 March 2013.
- Matot I. Restrictive versus liberal red cell transfusion strategy in orthopedic‐oncology patients undergoing surgery ‐ a randomized controlled study. clinicaltrials.gov/show/NCT01491308 (accessed 21 July 2014).
Moghaddam 2009 {published data only}
- Moghaddam MJ, Razavi SS, Momenzadeh S, Radfar A. The effects of tranexamic acid on hemorrhage in femoral fracture surgery. Journal of the Faculty of Medicine 2009;33(2):3. [Google Scholar]
Muir 1995 {published data only}
- Muir L. Blood transfusion requirement in femoral neck fracture. Annals of the College of Surgeons of England 1995;77(6):453‐6. [PMC free article] [PubMed] [Google Scholar]
Nielsen 2012 {published data only}
- Brunskill SJ [pers comm]. Establishment of optimal transfusion threshold after major orthopedic surgery. Email to: K Neilsen 14 March 2013.
- Nielsen K. Establishment of optimal transfusion threshold after major orthopedic surgery. clinicaltrials.gov/ct2/show/NCT00906295 (accessed 21 July 2014).
Prasad 2009 {published data only}
- Prasad N, Rajamani V, Hullin D, Murray JM. Post‐operative anaemia in femoral neck fracture patients: does it need treatment? A single blinded prospective randomised controlled trial. Injury 2009;40(10):1073‐6. [DOI] [PubMed] [Google Scholar]
Serrano Trenas 2011 {published data only}
- Serrano‐Trenas JA, Ugalde PF, Cabello LM, Chofles LC, Lázaro PS, Benítez PC. Role of perioperative intravenous iron therapy in elderly hip fracture patients: a single‐center randomized controlled trial. Transfusion 2011;51(1):97‐104. [DOI] [PubMed] [Google Scholar]
Zufferey 2010 {published data only}
- Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, et al. Tranexamic acid in hip fracture surgery: a randomized controlled trial. British Journal of Anaesthesia 2010;104(1):23‐30. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR‐TRC‐10000822 {published data only}
- Wu Q. Clinical research of restrictive transfusion in elderly patients undergoing orthopedic surgery. apps.who.int/trialsearch/Trial.aspx?TrialID=ChiCTR‐TRC‐10000822 (accessed 21 July 2014).
Additional references
Adunsky 2008
- Adunsky A, Arad M, Blumstein T, Weitzman A, Mizrahi EH. Discharge hemoglobin and functional outcome of elderly hip fractured patients undergoing rehabilitation. European Journal of Physical and Rehabilitation Medicine 2008;44(4):417‐22. [PubMed] [Google Scholar]
Carless 2010
- Carless PA, Henry DA, Carson JL, Hebert PPC, McClelland B, Ker K. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD002042.pub2] [DOI] [PubMed] [Google Scholar]
Carson 1999
- Carson JL, Altman DG, Duff A, Noveck H, Weinstein MP, Sonnenberg FA, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion 1999;39(7):694‐700. [DOI] [PubMed] [Google Scholar]
Carson 2012
- Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD002042.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2013
- Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. American Heart Journal 2013;165(6):964‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Department of Health Payment by Results team 2012
- Department of Health Payment by Results team. A simple guide to Payment by Results. www.gov.uk/government/publications/simple‐guide‐to‐payment‐by‐results (accessed 21 July 2014).
Dillon 2005
- Dillon MF, Collins D, Rice J, Murphy PG, Nicholson P, Mac Elwaine J. Preoperative characteristics identify patients with hip fractures at risk of transfusion. Clinical Orthopaedics and Related Research 2005;439:201‐6. [DOI] [PubMed] [Google Scholar]
Egol 2009
- Egol KA, Strauss EJ. Perioperative considerations in geriatric patients with hip fracture: what is the evidence?. Journal of Orthopaedic Trauma 2009;23(6):386‐94. [DOI] [PubMed] [Google Scholar]
Foss 2006
- Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. Journal of Bone & Joint Surgery ‐ British Volume 2006;88(8):1053‐9. [DOI] [PubMed] [Google Scholar]
Foss 2008
- Foss NB, Kristensen MT, Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age and Aging 2008;37(2):173‐8. [DOI] [PubMed] [Google Scholar]
Goodnough 2014
- Goodnough LT, Murphy MF. Do liberal blood transfusions cause more harm than good?. BMJ 2014;349:g6897. [DOI] [PubMed] [Google Scholar]
GRADE 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Gruber‐Baldini 2013
- Gruber‐Baldini AL, Marcantonio E, Orwig D, Magaziner J, Terrin M, Barr E, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. Journal of the American Geriatrics Society 2013;6(8):1286‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gullberg 1997
- Gullberg B, Johnell O, Kanis JA. World‐wide projections for hip fracture. Osteoporosis International 1997;7:407‐13. [DOI] [PubMed] [Google Scholar]
Hajjar 2010
- Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304(14):1559‐67. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hutton 2005
- Hutton B, Fergusson D, Tinmouth A, McIntyre L, Kmetic A, Hebert PC. Transfusion rates vary significantly amongst Canadian medical centres. Canadian Journal of Anaesthesia 2005;52(6):581‐90. [DOI] [PubMed] [Google Scholar]
Johnston 2006
- Johnston P, Wynn‐Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture?. Journal of Orthopaedic Trauma 2006;20(10):675‐9. [DOI] [PubMed] [Google Scholar]
Lawrence 2003
- Lawrence VA, Silverstein JH, Cornell JE, Pederson T, Noveck H, Carson JL. Higher Hb level is associated with better early functional recovery after hip fracture repair. Transfusion 2003;43(12):1717‐22. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6.4.11: Search filters. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
McClelland 2009
- McClelland B, Walsh T. Good blood management: the effective and safe use of blood components. In: Murphy MF, Pamphilon DH editor(s). Practical Transfusion Medicine. 3rd Edition. Oxford: Wiley‐Blackwell, 2009:265‐84. [Google Scholar]
Parker 2006
- Parker M, Johansen A. Hip fracture. BMJ 2006;333(7557):27‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninx 2006
- Penninx BW, Pahor M, Woodman RC, Guralnik JM. Anemia in old age is associated with increased mortality and hospitalization. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 2006;61(5):474‐9. [DOI] [PubMed] [Google Scholar]
Perel 2013
- Perel P, Ker K, Morales Uribe CH, Roberts I. Tranexamic acid for reducing mortality in emergency and urgent surgery. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010245.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rohde 2014
- Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care‐associated infection after red blood cell transfusion: a systematic review and meta‐analysis. JAMA 2014;312(19):2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shokoohi 2012a
- Shokoohi A, Stanworth S, Mistry D, Lamb S, Staves J, Murphy MF. The risks of red cell transfusion for hip fracture surgery in the elderly. Vox Sanguinis 2012;103(3):223‐30. [DOI] [PubMed] [Google Scholar]
SHOT 2010
- Taylor C (Ed), Cohen H, Mold D, Jones H, et al on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2009 annual SHOT report. www.shotuk.org/shot‐reports/report‐and‐summary‐2009/ (accessed 21 July 2014).
Smith 2011
- Smith GH, Tsang J, Molyneux SG, White TO. The hidden blood loss after hip fracture. Injury 2011;42(2):133‐5. [DOI] [PubMed] [Google Scholar]
So‐Osman 2013
- So‐Osman C, Nelissen R, Brand R, Faber F, Slaa RT, Stiggelbout A, et al. The impact of a restrictive transfusion trigger on post‐operative complication rate and well‐being following elective orthopaedic surgery: a post‐hoc analysis of a randomised study. Blood Transfusion 2013;11(2):289‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villanueva 2013
- Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez‐Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New England Journal of Medicine 2013;268(1):11‐21. [DOI] [PubMed] [Google Scholar]
Walsh 2013
- Walsh TS, Boyd JA, Watson D, Hope D, Lewis S, Krishman A, et al. RELIEVE Investigators. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Critical Care Medicine 2013;41(10):2354‐63. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shokoohi 2012b
- Shokoohi A, Stanworth S, Doree C, Hopewell S, Brunskill SJ, Murphy MF. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009699] [DOI] [PMC free article] [PubMed] [Google Scholar]


