Abstract
Older adults share a growing burden of cancer morbidity and mortality. This is present across the spectrum of oncologic diagnoses and is particularly true with colorectal cancer (CRC), where older adults continue to share the burden of diagnoses. However, optimal cancer treatment decision making in older adults remains a significant challenge, as the majority of previous clinical trials shaping the current treatment landscape have focused on younger patients, often with more robust performance status and fewer medical comorbid conditions. The heterogeneous aging process of older adults with CRC necessitates a personalized treatment approach, as approximately three-quarters of older adults with CRC also have a concomitant geriatric syndrome and more than half of older adults with CRC are pre-frail or frail. Treatment decisions should be multifaceted, including consultation with the patient and their families regarding their wishes, with consideration of the patient’s quality of life, functional status, medical comorbid conditions, social support, and treatment toxicity risk. Geriatric assessment is a systematic and validated approach to assess an older adults’ potential strengths and vulnerabilities, which can in turn be used to assist with comprehensive cancer care planning and support. In this review, we will summarize current treatment approaches for older adults with CRC, with a particular focus on the incorporation of the geriatric assessment.
Keywords: Aging, Colon Cancer, Frailty, Geriatric oncology
Introduction
Cancer continues to be an increasing challenge to society and health care. In 2023, it is estimated that approximately 1.9 million new cancer cases and over 600,000 people will die from cancer in the US.1 Colorectal cancer (CRC) is predicted to account for 153,000 new diagnoses in 2023, and approximately 1 in 3 of these patients will die from CRC.1 Furthermore, cancer is a disease of aging. Currently, older adults account for 60% of all new cancer diagnoses and 70% of cancer-related deaths.2 This burden is expected to continue to increase. By 2040, 69% of the approximately 2.4 million new cancer diagnoses per year will occur in patients ≥ 65 years of age, and the proportion of cancer cases for those ≥ 85 years is expected to nearly double to 13% of all cancer cases.3 Despite the majority of cancer diagnoses occurring in older adults, and the anticipated increases over upcoming years, there remains a substantial evidence gap in the optimal management of older adults with cancer.4 Older adults remain under-represented in many clinical trials, and those enrolled frequently represent the healthiest subset of older patients.5,6 This is frequently a result of common eligibility criteria that either purposefully (strict upper age limits) or none purposefully (performance status or comorbidity restrictions) cause older adults to be ineligible for clinical trials. Therefore, oncology providers often have to extrapolate such data to the general older adult population.7 In addition, many oncologists’ lack sufficient knowledge and experience on how to systematically assess older patients and tailor traditional oncology treatment strategies to the care of older adults with cancer.
Unlike the developmental process, aging trajectories are heterogenous. Thus, older adults of the same chronologic age may have vastly different levels of functioning, geriatric syndromes, medical comorbid conditions, frailty, social needs, treatment toxicity, and related health factors and conditions that may vary greatly from those seen in younger populations.8, 9, 10 In a comprehensive study, Nguyen et al. found that health heterogeneity increases with chronological age.11 In this study, it was found that while some parameters showed increasing heterogeneity with aging, other parameters showed decreasing heterogeneity and some parameters showed no variability. Concepts of heterogeneity that increase with age included systolic blood pressure, bone mineral density, chair rise, timed up and go (TUG), Older Americans Resources and Services (OARS) and life space assessment assessing function and disability, number of chronic comorbidities, fragility index and laboratory parameters (hemoglobin, creatinine, glomerular filtration rate, total cholesterol, low density lipoprotein, thyroid stimulating hormone, hemoglobin A1c, ferritin, and vitamin D). This further highlights the complexity of the heterogeneity of aging.11 In this review we summarize evidence regarding assessment and treatment planning for older adults with colorectal cancer.
Geriatric Assessment
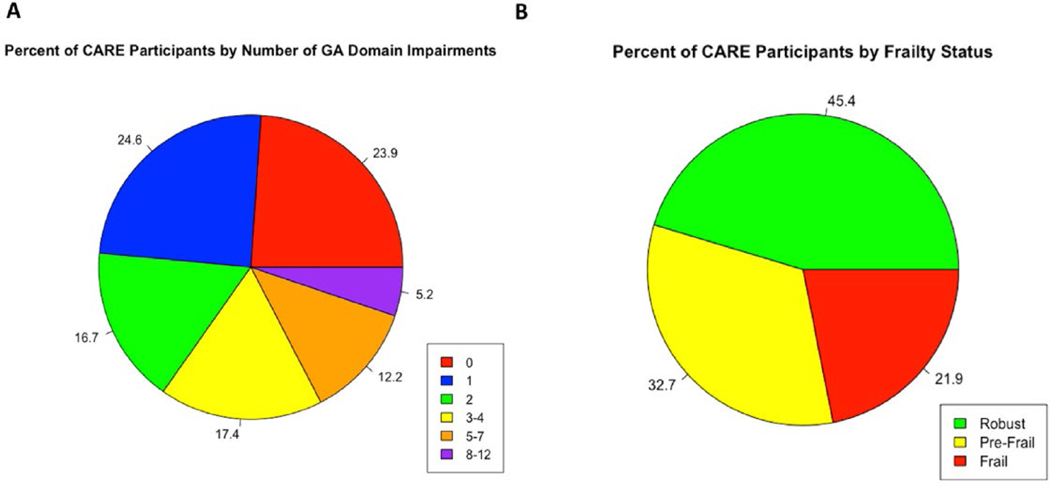
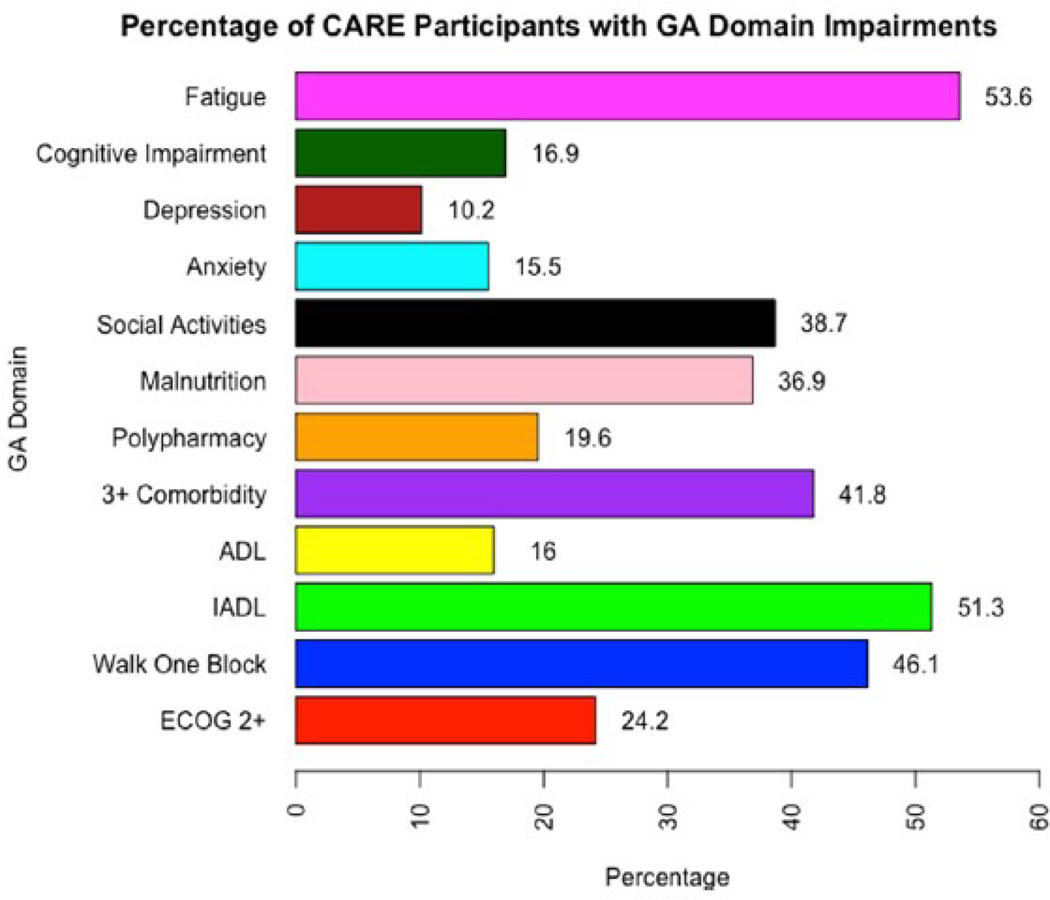
As chronological age alone is an insufficient marker of the aging process, a systematic assessment is required. Geriatric assessment (GA) is a multidomain-focused, systematic approach for assessing an older adult, particularly regarding potential vulnerabilities.12, 13, 14, 15 The GA assesses multiple aging-related domains of health, including functional status and capabilities, nutrition, comorbid medical conditions, medications and polypharmacy, cognition, social activities and support, and mental health.16,17 This allows identification of potential problems that may be missed by a standard history, physical examination, and simple assessment of performance status, as traditionally performed by the oncology team.10,18 Identification of GA impairments is common amongst older adults with cancer, including those with CRC. Based on prospective data of older adults with CRC (n = 460) from the ongoing Cancer and Aging Resilience Evaluation (CARE) Registry,76.1% (n = 350) had at least 1 identified GA impairment and nearly one-third had 3 or more impairments (Figure 1A).19 The most commonly identified impairments included fatigue (53.6%), limitations in instrumental activities of daily living (51.3%), limitations in walking 1 block (46.1%), and the presence of 3 of more medical comorbid conditions (41.8%) (Figure 2). These GA results can also be utilized to identify prefrail or frail older adults who may be at increased risk of adverse effects of their cancer therapy, such a by using a deficit accumulation approach.20, 21, 22 For example, based upon the CARE Frailty Index, 32.7% of older adults with CRC in the CARE Registry were found to be prefrail and 21.9% were found to be frail (Figure 1B). Furthermore, GA identified impairments are potentially strong predictors of cancer outcomes and can assist in informing cancer treatment decisions and guiding targeted interventions to areas of deficit.23, 24, 25, 26, 27
Figure 1.
Prevalence of geriatric assessment identified impairments and frailty results for older adults with colorectal cancer from the CARE Registry (n = 460).
Figure 2.
Prevalence of specific geriatric assessment impairments in older adults with colorectal cancer from the CARE Registry (n = 460).
A seminal study by Geriatric Oncology pioneer and visionary leader, Dr. Arti Hurria, led to the creation of the currently available Cancer and Aging Research Group (CARG) toxicity score tool to assist in predicting chemotherapy toxicity in older adults with cancer.24 Based upon assessment of 500 older adults receiving chemotherapy, it was found that age ≥72 years, a diagnosis of gastrointestinal or genitourinary cancer, provision of standard-dose chemotherapy, utilization of multiple chemotherapy agents (polychemotherapy), low hemoglobin, reduced renal function, reduced hearing, limitations in walking 1 block, presence of falls over the past 6 months, the requirement of assistance with medication administration (an instrumental activity of daily living [IADL] impairment), and decreased social activity were independent predictors of chemotherapy toxicity. The CARG score categorizes patients into 3 risk groups for severe, grade 3 to 5 chemotherapy toxicity, as per the National Cancer Institute Terminology Criteria for Adverse Events.28 This includes patients with a low-risk score of 0 to 5 and an estimated 25% to 32% risk of severe toxicity, those with medium-risk scores of 6 to 9 and a 50% to 54% risk of severe toxicity, and those with high-risk scores of 10 to 19, with a 77% to 89% risks of severe chemotherapy toxicity.24,28 This chemotherapy toxicity predictor has been externally validated and later adapted specifically for use in older adults with breast cancer.29,30 In another study further evaluating the CARG toxicity score, Nishjima et al. conducted a prospective, observational study of 50 patients with gastrointestinal cancer (noncolorectal). According to the CARG score, patients were grouped as high risk (CARG score ≥10) versus low risk (CARG score <10), as well as standard treatment and reduced treatment according to treatment intensity. Patients identified as high-risk for chemotherapy receiving standard dose chemotherapy had a higher incidence of chemotherapy toxicities (88% vs. 40%, P < .01) and hospitalizations (50% vs 15%, P = .03), than those treated with reduced intensity treatment.23 These results suggest that the CARG score is a practical and useful clinical decision support tool for the evaluation of older adults with cancer. Of note, however, is that the CARE score was studied for patients receiving more traditional cytotoxic chemotherapy agents. Modification may be needed for current and future targeted cancer therapies, such as for patients with BRAF-mutated colorectal cancer, and immunotherapies, such as checkpoint inhibitor therapy for patients with MMR-deficient colorectal cancer, as these therapies are playing a growing role in cancer care for patients with colorectal cancer.
While the use of GA may have a predictive role in identifying older adults at risk for adverse outcomes of cancer therapy, there is also significant potential benefit from the GA in assisting with guiding interventions for identified impairments. For example, for an older adult with CRC identified to have a recent fall, limitations in IADLs, and malnutrition, the GA can assist with risk-adapted cancer treatment adjustment, as well as directing the patient to a physical and occupational therapist and the nutrition team. For a list of recommendations based on identified impairments from the CARE tool, see Table 1.19 Using the GA to inform cancer treatment decisions and guide such targeted interventions is critical to optimizing care.
Table 1.
The CARE Tool Suggested Intervention Based on Identified Impairments
| Domain | Measure* | Items | Definition of Impairments- List Impairments | Recommendation If Impaired |
|---|---|---|---|---|
|
| ||||
| Physical function | Falls | Single item of falls in last 6 months | ≥1 falls |
Weigh risks and benefits of treatment options incorporating information about the patient’s physical performance
Consider Physical Therapy (outpatient or home-based depending on eligibility for home care): request gait/assistive device evaluation, strength, and balance training Consider Occupational Therapy (if eligible for home care, referral for safety evaluation): request evaluation and treatment |
| Self-rated ECOG performance status | Single item | ≥3 | If falls specifically - check orthostatic blood pressure and decrease or eliminate blood | |
| Physical function | Walking 1 block and climbing 1 flight of stairs | Any limitation (a little or lot) |
pressure medications if blood pressure is low or low normal.
Consider falls prevention handout. |
|
| Functional status | OARS IADL | 6-items IADL items (walking, transportation, meals, housework, medicines, money) | Any 2 IADL items with “some help” or “unable” | Consider the following potential treatment modifications, particularly in the palliative treatment setting: 1) consider single agent rather than doublet therapy if appropriate 2) modify dosage (eg, 20% dose reduction with escalation as tolerated) 3) |
| OARS ADL | 3-items ADL items (in/out of bed, dressing, bath/shower) | Any ADL items with “some help” or “unable” |
modify treatment schedule if appropriate.
Consider frequent toxicity checks (weekly or every other week) Consider Physical Therapy (outpatient or home-based depending on eligibility for home care): request gait/assistive device evaluation, strength, and balance training Consider Occupational Therapy (outpatient or home-based depending on eligibility for home care): request evaluation and treatment for functional impairment |
|
| Global health | Fatigue | Single item none to very severe | Moderate, severe, very severe |
Provide energy conservation handout
Consider providing exercise prescription Consider Occupational Therapy referral for energy conservation and activity management Consider Physical Therapy referral for structured exercise and/or physical activity program |
| Pain | Single item 0–10 | ≥4 pain level |
Consider initiation of pain medication(s) if not already prescribed
Consider referral to palliative care if already on pain medications which are not adequate |
|
| Nutrition | PG-SGA of nutrition short form | Significant weight loss defined Overall PG-SGA scoring | Weight loss: 3% within 3 months or 6% within 6 months PG-SGA scoring, <6, ≥6 |
Discuss concerns related to nutrition and how potential treatment may impact nutrition
Consider recommendations and/or handouts for nutritional supplements, small frequent meals, and/or high protein/high calorie snacks Consider referral to 1) nutritionist/dietician, 2) dentist if poor dentition or denture issues, 3) speech referral if difficulty with swallowing, 4) meals-on-wheels Use caution with highly emetogenic regimens and utilize aggressive anti-emetic therapy |
| Social support | Medical outcomes survey (MOS) social support 8 item | Instrumental items 1–4 | Any item with none, a little, or some of the time |
Discuss adequacy of social support at home
Discuss who the patient can call in case of an emergency Confirm documented health care proxy is in the medical record Consider referral or information on 1) social worker 2) visiting nurse service or home health aide (if meets criteria) |
| Psychological | PROMIS anxiety 4-item | Summed 4–20 raw score | Raw score: ≥11 | Discuss history of mood issues and treatment history |
| PROMIS depression 4-item | Summed 4–20 raw score | Raw score: ≥11 |
Consider referral to 1) psycho-oncology for counseling, 2) psychiatry if severe symptoms or if already on medications which are not adequate, 3) spiritual counseling or Chaplaincy services, 4) palliative care if other physical and/or cancer symptoms present
Consider initiating pharmacologic therapy if appropriate in conjunction with PCP Provide linkage to community resources (such as support groups and local/national buddy or volunteer programs) Assess suicide risk and/or elder abuse if appropriate |
|
| Cognitive function | PROMIS cognitive abilities 4-item | Summed 4–20 raw score | Raw score: ≤11 |
Provide explicit and written instruction for appointments,
medications, and treatments Elicit input and perspectives from caregiver(s) about patient’s cognition Assess decision-making capacity and elicit health care proxy information and input if the patient lacks decision-making capacity Consider referral to cognitive specialist (e.g., neurologist or geriatrician) Consider Occupational Therapy referral for cognitive rehabilitation If dementia is suspected, consider neuropsychological testing |
| Comorbidity | OARS comorbidity | No/yes summed (0–13) Interference for each |
≥3 conditions Or any condition with a great deal of interference Specific for any history of diabetes, heart disease, or liver/kidney disease |
Initiate direct communication (written, electronic, or phone) with patient’s PCP about the plan for the patient’s cancer
Discuss how comorbidities affect risks and benefits of treatments choices Modify dosage or schedule if there is concern about how the patient will tolerate therapy or if there is a concern about worsening of comorbidities If history of diabetes (of any level)- avoid neurotoxic agents if another option is equivalent If history of heart disease (of any level)- consider minimizing volume of agents and/or administer at slower infusion rate If history of chronic liver or kidney disease (of any level)- adjust medication dose as appropriate |
| Polypharmacy | # of daily medications | Meds ≥9 |
Ask patient to bring in prescribed, over-the-counter medications, and supplements to review at the next visit
Consider medication review: minimize psychoactive medications including those used for supportive care, minimize duplicative medications, and reduce medicines solely used for hypertension or diabetes if appropriate Consider having pharmacist meet with patient to evaluate drug interactions and medication counseling Recommend pillbox and/or medication calendar |
|
| Hearing | Single item | fair/poor/deaf | Ensure wearing hearing aids if indicated and consider hearing specialist referral | |
| Vision | Single item | fair/poor/blind | Ensure wearing glasses if indicated and consider vision specialist referral | |
| Social determinants of health | Financial distress | Single item from Patient Satisfaction Questionnaire (PSQ-18) | Strongly agree or agree |
Take into consideration cost of necessary medications and/or infusions including insurance coverage and out-of-pocket costs
Assess for barriers to acquiring medications Consider referral to social worker and/or financial counselor regarding insurance coverage and costs of medical care |
| Health literacy Health numeracy | Two items from brief health literacy screening | Somewhat, a little, not at all Hard, very hard |
Simplify communication using plain language and make use of graphics/pictures as appropriate
Confirm comprehension of treatment plan to minimize risk of miscommunication Provide explicit and written instruction for appointments, medications, and treatments Provide written instructions (at the sixth grade level) to patient/caregiver regarding the cancer treatment and supportive care plan(s) as well as when initiating new medications Consider assessment of medication adherence |
|
| Transportation | Two items: How much trouble to get transportation? Missed appointments? | Some or a lot of trouble Yes |
Provide information on transportation services and ride assistance programs
Consider referral to social worker |
|
| Housing | Two items: What is your housing situation? | I do not have housing Yes, worried about losing housing |
Provide information on community housing resources as available
Consider referral to social worker for assessment of living |
|
| Worried about losing housing? | environment and potential housing alternatives | |||
| Food, utilities, clothing… | Six items addressing specific needs | Yes to any of the following: food, utilities, medicine or health care, clothing |
Provide information on community resources and assistance programs (e.g., food stamps, meal delivery, energy assistance, cash assistance) as available
Consider referral to social worker for assistance in identifying assistance programs and medical insurance advising (Medicaid) as appropriate |
|
Abbreviations: ADL = activity of daily living; ECOG = Eastern Cooperative Oncology Group; IADL = instrumental activity of daily living; OARS = Older Americans Resources and Services; PG-SGA = patient-generated subjective global assessment; PROMIS = Patient-Reported Outcomes Measurement Information System.
Randomized Trials Using GA
Recent randomized trials have demonstrated reductions in chemotherapy toxicities with a GA-informed approach to cancer therapy. The GAIN study by Li et al. sought to determine whether a Geriatric Assessment-Driven Intervention (GAIN) could reduce the incidence of severe chemotherapy toxicity in older adults with cancer.31 In this 2:1 randomized controlled trial of 605 patients 65 years of age and older, 410 patients were enrolled in the GAIN arm and 203 patients were enrolled in the standard of care (SOC) arm. Patients were followed until completion of their chemotherapy protocol or for up to 6 months of therapy. In the GAIN arm, 50.5% of patients developed severe chemotherapy toxicities, while 60.6% of patients developed severe toxicity in the SOC arm, a statistically significant reduction.31 Another major study addressing GA is the GAP70+ (Geriatric Assessment for Patients 70 years and older) study.32 The GAP70+ study was a cluster randomized trial of 718 patients with advanced solid tumors or lymphoma, 70 years of age and older, with at least 1 impaired GA measure. Patients randomized to the intervention arm received a GA summary and recommendations for possible interventions, as opposed to the usual standard of care (SOC) with no GA information provided. There was a significant difference in the development of severe chemotherapy toxicity, at 50.7% in the intervention arm, compared to 71.3% in the SOC arm. Empiric chemotherapy dose reductions occurred in 48.7% of patients in the intervention arm and 35% of patients receiving SOC. There was no statistically significant difference in 6-month and 12-month survival between the 2 arms (adjusted hazard ratio 1.13 [95% CI, 0.85–1.50] and 1.05 [95% CI, 0.85–1.29]), respectively. After adjusting for covariates, the GA-guided treatment approach reduced the risk of toxicity by 26% (adjusted risk ratio [aRR]) 0.74; [95% CI, 0.64–0.86].32,36 Another important study is the Integrated Geriatric Evaluation and Treatment Effectiveness (INTEGERATE) study.33 INTEGERATE was a prospective, 1:1 randomized controlled trial of 154 patients aged 70 years and older with solid tumors or diffuse large B-cell lymphoma. The primary endpoint was health-related quality of life (HRQOL) using the Elderly Functional Index (ELFI (ELFI is a 12-item composite measure of the functionality of cancer patients)33) at baseline, week 12, week 18, and week 24. The adjusted ELFI change score in the integrated oncogeriatric care group was better than the usual care group at 24 weeks, with a maximum between-group difference at week 18. No statistically significant difference in overall survival was observed between the 2 groups (absolute difference at 12 months was 9.9% [95% CI, 5.2–25.1]).33 In the analysis of treatment modification, there was no evidence of significant differences between the 2 groups in reducing, increasing, or delaying treatment. However, participants in the integrated oncogeriatric care group were less likely to discontinue their current treatment early after 24 weeks from the start of follow-up (33% versus 53%).33 See Table 2 for a summary of the design and findings of these studies.
Table 2.
Highlighted Outcomes From GAP70+, GAIN, and INTEGRATE
| Empty Cell | GAP70+ | GAIN | INTEGERATE |
|---|---|---|---|
|
| |||
| Age | ≥ 70 Mean 77.2 (70–96) | ≥ 65 Median 71 (65–91) | ≥ 70 Median 75.5 (72–79) |
| Number of patients | 718 | 605 | 154 |
| Diagnosis of GI malignancy | 246 (34%) | 202 (33%) | 44 (29%) |
| Primary endpoint | Any Grade 3–5 toxicity | Any Grade 3–5 toxicity | HRQOL |
| Endpoint result | GAP70+ arm 50.7% Standart arm 71% | GAIN arm 50.5% Standart arm 60.6% | ELFI index change INTERGERATE arm −6.9 Standart arm −12.5 |
| Overall Survival | GAP70+ arm 71.6% Standard arm 74.5% P:.38 | Gain arm 66% Standard arm 64% P:.55 | INTERGERATE arm 68.9% Standard arm 59% P:.79 |
Abbreviations; ELFI = Elderly Functional Index, GI =Gastrointestinal; GAIN = Geriatric assessment-driven intervention, HRQOL = Health-Related Quality of Life; GAP70+ = Geriatric Assessment for Patients 70 years and older; INTEGRATE = Integrated Geriatric Assessment and Treatment Effectiveness.
Barriers to GA Implementation
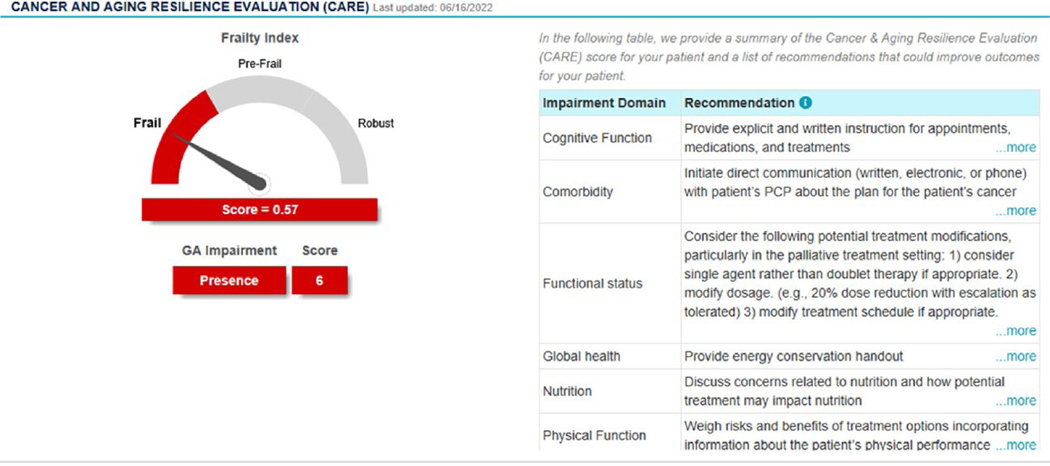
Although a GA-driven treatment approach in older adults with cancer improves cancer outcomes (as summarized above), and it is now recommended by multiple cancer treatment guidelines,26,34 barriers exist to the routine incorporation of GA in clinical practice. In a recent survey of oncology provides, only 21% routinely utilized a GA in clinical practice.35 Insufficient time, lack of supportive staff, and lack of training or knowledge were the primary identified barriers to GA utilization.35 Moreover, only 5% of community oncology practices within the Unites States have access to outpatient geriatric specialty care services, and therefore, such specialized assessments must be done by the oncology team.36 Given these identified barriers and lack of geriatric specialty care, cancer-specific GAs have been developed that are either predominantly or completely patient-reported, such as the CARG GA and the CARE tool.19,37 Practical GA options, such as the CARE tool, can be completed by older adults (or their care team) in as little as 10 minutes,19 and the results can then be used to guide management using toxicity risk assessment and suggested interventions, as highlighted in Table 1.19 The recently updated ASCO Geriatric Assessment guidelines suggest the use of a practical GA.15,38 Furthermore, electronic GA data capture allows for the creation of an integrated dashboard that can immediately synthesize and provide a simplified dashboard of the results. As illustrated in Figure 3 from the web-enabled CARE (We-CARE) tool, an integrated GA dashboard can provide overall results from the GA with related suggested interventions in a simple glance by the oncology provider. Similar practical tools have been developed by the American Society of Clinical Oncology (ASCO) for use in a community-based oncology practice.15
Figure 3.
An example of a geriatric assessment dashboard from the web-enabled CARE tool.
Evidence of Chemotherapy in Older Adults With Colorectal Cancer
Although older adults share a growing burden of cancer diagnoses, there remains significant under-representation in clinical trial research. For example, it is estimated that less than 10% of patients 70 years of age and older with cancer participate in National Cancer Institute (NCI) sponsored clinical trials.6 Additionally, less than a quarter of patients participating in cancer clinical trials that are registered with the United States Food & Drug Administration (FDA) are 70 years of age and older.6 As a consequence, clinical trial data from younger, often more fit patients, with significant physiologic and pathology differences compared to older adults, are being used to guide cancer care treatment approaches for older patients. Inadequate representation of the older patient population may lead to both over-treatment and undertreatment with cancer care.6 Only a handful of select studies have specifically focused on the care of older adults with CRC.
Colon Cancer—Metastatic Malignancy Therapy in Older Adults
One of these important studies is the FOCUS2 trial, which investigated low-dose chemotherapy options in frail older patients with metastatic CRC.39 This study specifically evaluated colorectal cancer therapy for older adults who were selected by oncologist assessment as being “unsuitable” for standard chemotherapy. The median age of the patient population in this 4-arm randomized trial of 459 patients was 74 years. Of these patients, 43% were 75 years of age or older and 13% were 80 years of age or older. About 71% of the patients were included in this study because of frailty and 68% because of advanced age. In Group A (fluorouracil monotherapy arm), the median overall survival (OS) was 10.1 months and the rate of improvement in quality of life between weeks 12% to 14 was 60%. In Group B (fluorouracil plus oxaliplatin arm), the median OS was 10.7 months and the quality-of-life improvement was 52%. In Group C (capecitabine arm), median OS was 11 months and the quality-of-life improvement was 65%. In Group D (capecitabine plus oxaliplatin arm), the median OS was 12.4 months and quality of life improvement was 47%. Detailed results are included in Table 3. The addition of oxaliplatin had a trend towards improved PFS and OS, although this was not statistically significant. These results provide strong evidence that vulnerable older adults with colorectal cancer may benefit from dose-modified chemotherapy treatment decisions. The FOCUS2 trial also emphasized that older adults, even those who are deemed to be at higher risk of adverse effects of treatment, can participate in appropriately designed cancer clinical trials.39
Table 3.
Summarized Results From the FOCUS2 Trial in Frail Older Adults With Metastatic CRC
| Fluorouracil | Fluorouracil + Oxaliplatin | Capecitabine | Capecitabine + Oxaliplatin | |
|---|---|---|---|---|
|
| ||||
| Disease control rate (week 12–14) | 46% | 71% | 50% | 65% |
| Median progression free survival | 3.5 mo (2.8–6.2) | 5.8 mo (3.2–7.6) | 5.2 mo (2.8–6.7) | 5.8 mo (3.3–7.4) |
| Median overall survival | 10.1 mo (5.1–17.3) | 10.7 mo (5.7–17.2) | 11.0 mo (5.4–18.0) | 12.4 mo (5.8–18.0) |
| Improved quality of life (week 12–14) | 60% | 52% | 65% | 47% |
| Good overall treatment utility (week 12) | 35% | 54% | 37% | 41% |
AVEX is another important study for older adults with CRC, and was a randomized phase III study of patients receiving first-line cancer therapy for metastatic CRC.40 AVEX was 1:1 randomized trial of 280 patients of 70 years of age and older (median age 76), and compared single agent capecitabine to capecitabine plus bevacizumab. Median progression-free survival (PFS) was longer in the combination therapy arm, at 9.1 months versus 5.1 months, P < .0001. Of note, the incidence of grade 3 or higher treatment-related adverse events was 40% in the combination arm compared to 22% in the single-agent capecitabine arm.40
Colon Cancer—Adjuvant Therapy in Older Adults
As discussed previously, chronological age alone is a poor predictor of treatment toxicity and outcomes for older adults. However, ongoing work is needed to clarify the optimal adjuvant therapy planning for older adults with resected colon cancer.41 In general, it is believed that there is potential benefit to adjuvant systemic cancer therapy for older adults with colon cancer (typically in the form of fluorouracil or capecitabine monotherapy), although there is ongoing debate regarding incorporation of oxaliplatin.42 There are multiple previous studies that been analyzed, seeking to address this question, with some potentially conflicting results. In a subset analysis of 315 adults 70 to 75 years of age in the MOSAIC study, the addition of oxaliplatin compared to fluorouracil and leucovorin (FOLFOX) in the adjuvant setting did not lead to statistically-significant differences in disease-free recurrence (hazard ratio 0.93, 95% confidence interval 0.64–1.35) or time to recurrence (HR 0.72, 95% CI, 0.47–1.11), and may been associated with a trend towards decreased overall survival (HR 1.10, 95% CI, 0.73–1.65, P = .661).43 As another example, in a subset analysis of 396 older adults age ≥70 years in the NSABP C-07 study, the addition of oxaliplatin to fluorouracil and leucovorin (FLOX) in the adjuvant setting similarly did not appear to lead to improvement in survival (HR 1.18, 95% CI, 0.86–1.62, P = .30).44 Ongoing research is needed to assist with adjuvant cancer therapy decisions for older adults with colon cancer. At this time, a shared decision-making process should be utilized. The potential risks versus benefits of adjuvant cancer therapy decisions should be carefully considered in older adults, and more intensive adjuvant treatment may be helpful for older adults who are active, fit, without significant medical comorbidities, and have longer anticipated life expectancy.41
Checkpoint Inhibitor Therapy in Older Adults With Colorectal Cancer
Checkpoint inhibitor therapy has moved to the forefront of public interest in colorectal cancer care, and there is now tumor agnostic approval by the United States Food and Drug Administration for patients with MMR-deficient (mismatch repair deficient) or MSI-high (microsatellite instability high) advanced malignancy. There is also interest in the first-line utilization of checkpoint inhibitor therapy for patients with advanced colorectal cancer. For example, the KEYNOTE-177 compared first-line pembrolizumab to fluorouracil-based chemotherapy for 307 patients with MMR-deficient / MSI-high metastatic colorectal cancer.45 Pembrolizumab therapy led to improvements in objective response rate (ORR) (43.8% vs. 33.1%) and PFS (16.5 months versus 8.2 months), with a reduction in grade 3+ treatment-related adverse events (22% vs. 66%). The CheckMate 142 trial also included a cohort of 45 patients receiving first-line nivolumab with low-dose ipilimumab for the treatment of advanced colorectal cancer, with an objective response rate (ORR) of 69% with grade 3 to 4 treatment-related adverse events in 22%.46 However, there is a need for ongoing data specifically for older adults with CRC, as the balance of treatment efficacy and potential adverse events plays an even larger role in cancer therapy decisions.
There has also been a growing interest in the utilization of neoadjuvant checkpoint inhibitor therapy for patients with localized or resectable MMR-deficient / MSI-high CRC, including with nivolumab + ipilimumab, pembrolizumab, and dostarlimab.47, 48, 49 However, ongoing research is needed regarding the optimal checkpoint inhibitor therapy utilization for older adults with CRC.
A recent meta-analysis of 30 studies with a total 17,476 patients with various subtypes of malignancy sought be explore potential differences between outcomes in older adults and younger adults (<65 years of age). In this meta-analysis, 42% were 65 years of age or older and 58% were under 65 years of age, and similar treatment efficacy was found in both age groups (HR for OS 0.77 in both arms).50 In a study by Lawrence et al. of 19 trials of ICI monotherapy evaluating reduction in the risk of death patients ≥65 years versus < 65 years, the HR was 0.73 (95% CI, 0.65–0.81) (P < .001) among those ≥65 years and 0.79 (95% CI, 0.72–0.85) (P < .001) among those <65 years, suggesting ICI similarly improved overall survival in both older and younger groups.51 Although ongoing work is needed, given these previous results, checkpoint inhibitor appears to be an effective option in older adults independent of chronological age, despite some initial concerns regarding the potential consequences of aging-related immune senescence.
Dosing and Treatment Patterns for Older Adults With Cancer
The primary goals palliative systemic cancer therapy for patients with advanced malignancy are to improve quality of life, with maintance of functional status and improvement in cancer-related symptoms, and overall survival, with control or reduction in the burden of malignancy. Along this line, there is always of balance of the potential benefits of cancer therapy with potential toxicity. Therefore, as mentioned above, determining the optimal dose is crucial, particularly for older, vulnerable or frail patients who at an even higher risk of adverse events than the patients when the therapeutic regimens are typically studied in clinical trials. There is ongoing research exploring dosing patterns and the best methods for initial treatment dosing in older adults. For example, in a study by Jimenez et al, of 367 oncologists, more than half of oncologists utilized up-front dose reductions in at least 10% of their patients.52 These rates increased to 72% amongst gastrointestinal oncologists. In this subgroup of patients, almost 90% reviewed the potential risks of toxicity with the potential benefits of treatment effect with their patients. Approximately two-thirds of surveyed oncologists agreed with the principle of starting with dose modifications of cancer therapy to decrease potential adverse events even in the setting of possible reductions in treatment benefits. There was also strong support for additional research to determine the “optimally effective” dose of cancer therapy for patients.52
A study by Bradley et al. explored treatment patterns, outcomes, and costs for older adults with advanced colon cancer (n = 16,117) and advanced rectal cancer (n = 4008) over a 10-year period from 2000 to 2009, utilizing the Surveillance, Epidemiology, and End Results (SEER) database.53 During this time, there was a substantial increase in the number of older adults receiving 3 or more systemic cancer agents, from approximately 3% at the beginning to approximately 75% by the end of the study period for patients with colon cancer, age 65 to 74 years; a similar trend was noted from 2% at the beginning to 53% at the end for patients age 75 years and older. There was also an improvement in median overall survival of approximately 6 months for patients age 65 to 74 years over this time period, although this improvement was only approximately 1 month of for adults 75 years and older.53
Another study by Gigli et al, reviewed treatment patterns for 1396 patients from the Veneto-Tuscany Cancer Registry (VTCR) database in Italy, as well 18,438 patients from the United States SEER-Medicare database, 66 years of age and older, with newly diagnosed colon or rectal cancer in years 2000 to 2001.54 Adjuvant chemotherapy was administered for management of stage III colon cancer in approximately 61% of patients in the SEER-Medicare database and 45% in VTCR database. Chemotherapy was utilized in approximately 57% and 45% of patients with metastatic colon cancer, respectivly.54
Since the completion of these studies, practice patterns have certainly continued to evolve, as new regimens are utilized for colorectal cancer care, including changes in the approach to adjuvant therapy, as well as newer targeted therapy options and checkpoint inhibitor therapies for patients. However, these studies further highlight the need for ongoing research into the optimal care of older adults with CRC, and the importance of weighing potential risks versus benefits of cancer therapy decisions, particularly for older subgroups of patients, where there appears to be relatively lower added survival benefit in the adjuvant and palliative-intent treatment setting compared to younger adults. Clearly ongoing work is needed to refine treatment approaches for older adults with colorectal cancer.
Management Amongst Older Adults With Colorectal Cancer
Based on the growing available data, and the need to tailor cancer care to older adults, current cancer management guidelines from the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the International Society of Geriatric Oncology (SIOG), recommend that all older adults with cancer should undergo a GA, particularly those considering chemotherapy.26,34,55 This evaluation should at a minimum, including a review of functional status, falls, medical comorbid conditions, mental health/depression, cognition, and nutritional status.26 This information can in turn be incorporated into additional tools, such as a frailty index, chemotherapy toxicity score, noncancer life expectancy assessment depending on the relevant cancer situation, which can play a crucial role in cancer therapy decisions for older adults.22,24,56 Additionally, specific deficits or areas for improvement identified on the GA can be identified, potentially improving quality of life, functionality, and cancer care with targeted interventions, such as cancer rehabilitation.57,58
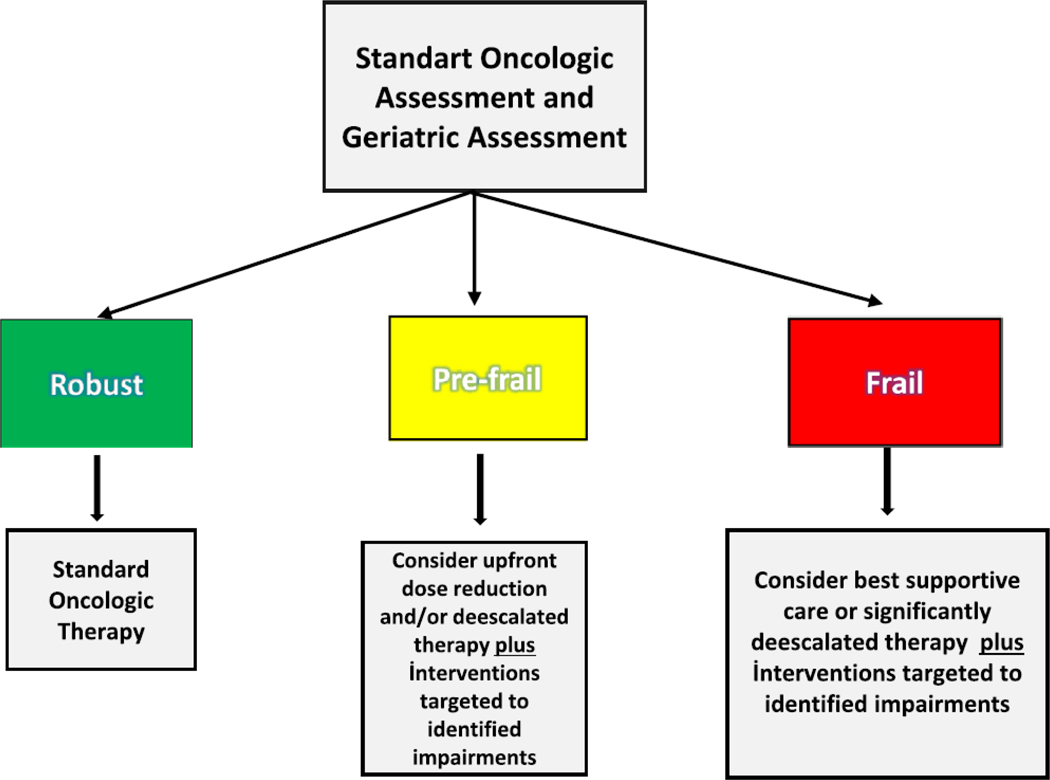
Here we highlight a clinical example of how the GA can directly positively impact cancer care decisions for older adults. An 81-year-old man initially presented with fatigue, with laboratory evaluation concerning for anemia. On colonoscopy, he is found to have a sigmoid mass with adenocarcinoma and further staging imaging reveals concern for metastatic disease. At his initial medical oncology visit, GA revealed concern for 2 falls in the past 6 months, limited ability to walk 1 block, limitations in taking his own medications and completing housework (IADL impairments), and weight loss of more than 5% in the past 3 months. He was deemed to have been at high risk for chemotherapy toxicity, given his evaluation above and correspondingly high CARG toxicity score and prefrail status by the CARE Frailty Index. After discussing these results with the patient and his wife, the decision was made to give single agent capecitabine combined with bevacizumab (based on the AVEX study), with an upfront dose reduction and plans to augment systemic cancer therapy as tolerated. In addition, the patient was referred to physical therapy and occupational therapy, as well as the nutrition team. See Figure 4 for a simplified treatment approach for older adults with CRC.
Figure 4.
Simplified geriatric assessment informed treatment algorithm.
Conclusion
As older adults are projected to share a growing burden of cancer diagnoses and mortality, and as the goal of providing personalized cancer care continues to evolve, the GA will play an expanding role in precision cancer care for older adults. The list of benefits of GA-informed cancer treatment planning are also increasing, as the GA has already demonstrated the ability to reduce severe chemotherapy toxicities and improve health-related quality of life for older adults.31, 32, 33 Although only a few therapeutic studies have focused exclusively on older adults with CRC, this group of patients has a high prevalence of GA impairments and deserves a GA informed treatment paradigm to improve all aspects of cancer care. In the future, cancer clinic trials should be more representative of real-world populations seen in routine oncology practice and incorporate GA as part of the study, with specific studies conducted in older, vulnerable or frail populations to help to continue to refine tailored, comprehensive cancer care and support.
Clinical Practice Points.
Management of older adults with CRC is complicated by the heterogeneous aging process and requires a personalized treatment approach
3/4s of older adults with CRC have a geriatric assessment identified impairment and over half are prefrail/frail
Geriatric assessment is a systematic and validated approach to assess an older patient’s potential vulnerabilities and strengths to guide treatment choices and interventions
Acknowledgment
Supported in part by the National Cancer Institute of the National Institutes of Health (K08CA234225, G. Williams, PI) and the Doris Duke Charitable Foundation CARES program at UAB. M. Fowler receives support from the Agency for Healthcare Research and Quality (5T32HS013852, M. Mugavero, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Williams GR, Outlaw D, Harvey DR, Lichtman MS, Zamboni CW, Giri S. Chemotherapy dosing in older adults with cancer: one size does NOT fit all. J Geriatr Oncol. 2023;14(1):101363. doi: 10.1016/j.jgo.2022.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Garner BW, Smith DB, Ludmir BE, et al. Predicting future cancer incidence by age, race, ethnicity, and sex. J Geriatr Oncol. 2023;14(1):101393. doi: 10.1016/j.jgo.2022.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Hurria A, Levit AL, Dale W, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33(32):3826–3833. doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]
- 5.Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30(17):2036–2038. doi: 10.1200/JCO.2012.41.6727. [DOI] [PubMed] [Google Scholar]
- 6.Sedrak SM, Freedman AR, Cohen JH. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71(1):78–92. doi: 10.3322/caac.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocque GB, Williams GR. Bridging the data-free zone: decision making for older adults with cancer. J Clin Oncol. 2019;37(36):3469–3471. doi: 10.1200/JCO.19.02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2017;72(7):877–884. doi: 10.1093/gerona/glw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giri S, Al-Obaidi M, Weaver A, et al. Association between chronologic age and geriatric assessment-identified impairments: findings from the CARE registry. J Natl Compr Canc Netw. 2021;19(8):922–927. doi: 10.6004/jnccn.2020.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolly AT, Deal MA, Nyrop AK, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20(4):379–385. doi: 10.1634/theoncologist.2014-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen DQ, Moodie ME, Forget FM, Desmarais P, Keezer RM, Wolfson C. Health heterogeneity in older adults: exploration in the Canadian longitudinal study on aging. J Am Geriatr Soc. 2021;69(3):678–687. doi: 10.1111/jgs.16919. [DOI] [PubMed] [Google Scholar]
- 12.DuMontier C, Sedrak MS, Soo WK, et al. Arti Hurria and the progress in integrating the geriatric assessment into oncology: Young International Society of Geriatric Oncology review paper. J Geriatr Oncol. 2020;11(2):203–211. doi: 10.1016/j.jgo.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams GR. Geriatric assessment: precision medicine for older adults with cancer. J Oncol Pract. 2018;14(2):97–98. doi: 10.1200/JOP.18.00010. [DOI] [PubMed] [Google Scholar]
- 14.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: current evidence and potential mechanisms for future research. J Geriatr Oncol. 2016;7(4):242–248. doi: 10.1016/j.jgo.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale W, Klepin HD, Williams GR. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline update. J Clin Oncol. 2023;17:JCO2300933. doi: 10.1200/JCO.23.00933. [DOI] [PubMed] [Google Scholar]
- 16.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol. 2014;5(3):245–251. doi: 10.1016/j.jgo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Gajra A, Loh KP, Hurria A, et al. Comprehensive geriatric assessment-guided therapy does improve outcomes of older patients with advanced lung cancer. J Clin Oncol. 2016;34(33):4047–4048. doi: 10.1200/JCO.2016.67.5926. [DOI] [PubMed] [Google Scholar]
- 18.Guerard EJ, A Deal AM, Williams GR, Jolly TA, Nyrop KA, Muss HB. Falls in older adults with cancer: evaluation by oncology providers. J Oncol Pract. 2015;11(6):470–474. doi: 10.1200/JOP.2014.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: the cancer and aging resilience evaluation (CARE). J Geriatr Oncol. 2020;11(2):270–273. doi: 10.1016/j.jgo.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerard EJ, Deal AM, Chang YK, et al. Frailty index developed from a cancerspecific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw. 2017;15(7):894–902. doi: 10.6004/jnccn.2017.0122. [DOI] [PubMed] [Google Scholar]
- 21.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receives chemotherapy. Cancer. 2016;122(24):3865–3872. doi: 10.1002/cncr.30269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giri S, Al-Obaidi M, Harmon C, et al. Patient-reported geriatric assessment-based frailty index among older adults with gastrointestinal malignancies. J Am Geriatr Soc. 2023;71(1):136–144. doi: 10.1111/jgs.18054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishijima TF, Deal AM, Williams GR, Sanoff HK, Nyrop KA, Muss HB. Chemotherapy toxicity risk score for treatment decisions in older adults with advanced solid tumors. Oncologist. 2018;23(5):573–579. doi: 10.1634/theoncologist.2017-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams GR, Dai C, Giri S, et al. Geriatric assessment predictors of 1-year mortality in older adults with GI malignancies: a survival tree analysis. JCO Clin Cancer Inform. 2022;6:e2200065. doi: 10.1200/CCI.22.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohile SG, Dale W, Somerfield MR, Hurria A. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. doi: 10.1200/JOP.18.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams GR, Dunham L, Chang YK, et al. Geriatric assessment predicts hospitalization frequency and long-term care use in older adult cancer survivors. J Oncol Pract. 2019;15(5):e399–e409. doi: 10.1200/JOP.18.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366–2371. doi: 10.1200/JCO.2015.65.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnuson A, Sedrak MS, Grosset CP, et al. Development and validation of a risk tool for predicting severe toxicity in older adults receiving chemotherapy for early-stage breast cancer. J Clin Oncol. 2021;39(6):608–618. doi: 10.1200/JCO.20.02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Sun CL, Kim H, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158. doi: 10.1001/jamaoncol.2021.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70 +): a cluster-randomised study. Lancet. 2021;398(10314):1894–1904. doi: 10.1016/S0140-6736(21)01789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soo WK, King MT, Pope A, Parente P, D ¯arzi ¸n š P, Davis ID. Integrated Geriatric Assessment and Treatment Effectiveness (INTEGERATE) in older people with cancer starting systemic anticancer treatment in Australia: a multicentre, open label, randomised controlled trial. Lancet Healthy Longev. 2022;3(9):e617–e627.400 [DOI] [PubMed] [Google Scholar]
- 34.Dotan E, Walter LC, Browner IS, et al. NCCN guidelines(R) insights: older adult oncology, version 1.2021. J Natl Compr Canc Netw. 2021;19(9):1006–1019. doi: 10.6004/jnccn.2021.0043. [DOI] [PubMed] [Google Scholar]
- 35.Dale W, Williams GR, MacKenzieet AR, et al. How is geriatric assessment used in clinical practice for older adults with cancer? A survey of cancer providers by the American Society of Clinical Oncology. JCO Oncol Pract. 2021;17(6):336–344. doi: 10.1200/OP.20.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams GR, Weaver KE, Lesser GJ, et al. Capacity to provide geriatric specialty care for older adults in community oncology practices. Oncologist. 2020;25(12):1032–1038. doi: 10.1634/theoncologist.2020-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 38.Williams GR, Hopkins JO, Klepin HD, et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline questions and answers. JCO Oncol Pract. 2023;17. doi: 10.1200/OP.23.00263. [DOI] [PubMed] [Google Scholar]
- 39.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open label, randomised factorial trial. Lancet. 2011;377(9779):1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(11):1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 41.Williams GR, Sanoff HK. Adjuvant chemotherapy in older adults with colon cancer. Am J Hematol Oncol. 2015;11(3):5–10. [Google Scholar]
- 42.Ramsdale E, Sanoff H, Muss H. Approach to the older patient with stage II/III colorectal cancer: who should get curative-intent therapy? Am Soc Clin Oncol Educ Book. 2013:163–168. doi: 10.14694/EdBook_AM.2013.33.163. [DOI] [PubMed] [Google Scholar]
- 43.Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the multicenter international study of oxaliplatin, fluorouracil, and leucovorin in the adjuvant treatment of colon cancer trial. J Clin Oncol. 2012;30(27):3353–3360. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 44.Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.André T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite-instabilityhigh advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 46.Lenz HJ, Cutsem EV, Limonet ML, et al. First-line nivolumab plus lowdose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol. 2022;40(2):161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 47.Chalabi M, Fanchi LF, Dijkstraet KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 48.Ludford K, Ho WJ, Thomas JV, et al. Neoadjuvant pembrolizumab in localized microsatellite instability high/deficient mismatch repair solid tumors. J Clin Oncol. 2023;41(12):2181–2190. doi: 10.1200/JCO.22.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386(25):2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim CM, Lee JB, Shin SJ, Ahn JB, Lee M, Kim HS. The efficacy of immune checkpoint inhibitors in elderly patients: a meta-analysis and meta-regression. ESMO Open. 2022;7(5):100577. doi: 10.1016/j.esmoop.2022.100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kasherman L, Siu DHW, Lee KWC, et al. Efficacy of immune checkpoint inhibitors in older adults with advanced stage cancers: a meta-analysis. J Geriatr Oncol. 2020;11(3):508–514. doi: 10.1016/j.jgo.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Jimenez RB, Schenkel C, Levit LA, et al. Oncologists’ perspectives on individualizing dose selection for patients with metastatic cancer. JCO Oncol Pract. 2022;18(11):e1807–e1817. doi: 10.1200/OP.22.00427. [DOI] [PubMed] [Google Scholar]
- 53.Bradley CJ, Yabroff KR, Warren JL, Zeruto C, Chawla N, Lamont EB. Trends in the treatment of metastatic colon and rectal cancer in elderly patients. Med Care. 2016;54(5):490–497. doi: 10.1097/MLR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 54.Gigli A, Warren JL, Yabroff KR, et al. Initial treatment for newly diagnosed elderly colorectal cancer patients: patterns of care in Italy and the United States. J Natl Cancer Inst Monogr. 2013;2013(46):88–98. doi: 10.1093/jncimonographs/lgt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wildiers H, Heeren P, Puts M, et al. International Society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5- year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24(10):1115–1122. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pergolotti M, Lyons KD, Williams GR. Moving beyond symptom management towards cancer rehabilitation for older adults: answering the 5W’s. J Geriatr Oncol. 2018;9(6):543–549. doi: 10.1016/j.jgo.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Pergolotti M, Williams GR, Campbell C, Munoz LA, Muss HB. Occupational therapy for adults with cancer: why it matters. Oncologist. 2016;21(3):314–319. doi: 10.1634/theoncologist.2015-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]