Abstract
Background:
Studies of acute heart failure (AHF) outcomes suggest that there are different predictors of mortality depending on region, ethnicity, and gender.
Objective:
The purpose of this study was to identify predictors of six months’ post discharge outcome among AHF patients in a Nigerian tertiary hospital.
Methods and Materials:
This study was a prospective observational study conducted at the cardiology unit of the Department of Medicine at the University College Hospital Ibadan. One hundred and sixty AHF participants ≥ 18 years old were recruited.
Results:
The mean age of the cohort was 58.0±15.1 years and most were males (59.4%). The independent predictors for death outcome after six months of discharge for AHF and the adjusted hazard ratio) (95% CI) were male gender 2.77 (1.17 -6.56); p =0.020 ; systolic blood pressure (mmHg) 0.98 (0.96 - 0.99); p = 0.011 ; and the presence of hepatomegaly 2.58 (1.02 - 6.51); p = 0.045 . Independent predictors for readmission or rehospitalization within 6 months after discharge were presence of right abdominal pain adjusted HR (95% CI): 2.07(1.14 - 3.76), p=0.017; SBP 0.98(0.96 - 0.99), p=0.005. Independent predictors for composite endpoint were male gender: adjusted HR: 2.08 (1.16 - 3.72), p= 0.014 and pericardial effusion and tamponade: 5.31(1.79 - 15.74), p=0.003 .
Conclusion:
The study provided an insight into the factors contributing to outcomes six-month after admission in a tertiary centre in South-Western Nigeria, and it highlighted the predictive role of systolic blood pressure.
Keywords: Acute heart failure, Ibadan, Outcomes
INTRODUCTION
Acute heart failure (AHF) is a clinical syndrome characterised by the sudden or gradual onset of heart failure symptoms and/or signs severe enough to necessitate hospitalization or an emergency department visit.1 Interestingly, the understanding of its complexity and heterogeneity has evolved over the last few decades. Despite the increased understanding of clinical intervention and therapeutics on the condition, survival remains poor in terms of intrahospital mortality, readmission, or post-discharge mortality.2-4
Few literatures available suggest multiple independent factors are associated with AHF outcomes such as mortality, readmission, or all-cause events (mortality combined with readmission). These factors include sociodemographic, past medical history, clinical profile and comorbidities, laboratory parameters, electrocardiographic and echocardiographic features among others.5-7 In IN-HF Outcome Registry, predictors found for AHF outcome included age, low systolic blood pressure, anaemia, and renal dysfunction, signs of cerebral hypo-perfusion, low serum sodium, chronic obstructive pulmonary disease, and pulmonary oedema.8 There is a direct relationship between renal function and AHF outcomes, whether in the short or long term.5, 6
Although most AHF studies were conducted in non-black African populations, the first multinational study on the subject in Africa identified only few factors as predictors.7 In one study in Northern Nigeria, the identified risk factors included low left ventricular ejection fraction (VEF) (≤40%), renal impairment, cardiac rhythm abnormalities such as atrial fibrillation (AF) at six months, prolonged corrected QT interval (QTC) and complete left bundle branch block (LBBB), anaemia and advanced age.9, 10 While lower body mass index, low literacy, low serum sodium level, the presence of atrial fibrillation, renal dysfunction, and valvular dysfunction were predictors of readmission with a six-month follow-up in the Abeokuta HF registry study.11
Overall, there are diverse predictors of outcomes in AHF depending on the location of study.
This study explored a broad range of the variables, notably clinical variables and socio-demographic to identify predictors of 180-day outcomes among AHF patients presenting at the University College Hospital (UCH), Ibadan, Nigeria
METHODS AND MATERIALS
Study Design
The study was a prospective observational study.3
Study Location
The study was carried out at the Cardiology Unit of the Department of Medicine, University College Hospital (UCH) in Ibadan. The hospital is a 1000-bed Federal tertiary health care facility that serves the people of Oyo State and other Nigerian states.
Study Population
Acute heart failure patients were consecutively recruited if they fulfilled the inclusion criteria. The recruiting between 07/06/2018 and 07/01/2019.
Participants had to be at least 18 years old and have either de novo or acute decompensated chronic HF, regardless of the primary cause. Participants who declined enrollment and did not meet the inclusion criteria, pregnant women, and HIV-1 and HIV-2 patients were all excluded from the study.
Enrollment and Data Collection
Informed consent was obtained from all the participants. Each participant was interviewed in complete privacy. Interview and assessment sessions lasted approximately 40 minutes per patient. To facilitate follow-up, phone numbers and addresses of participants and their next-of-kin were collected. The interviewer read questionnaire questions to the participants and recorded their responses. Basic demographic information as well as clinical history were gathered. Cigarette smoking was assessed and recorded as ever, never, and current smoking and alcohol use were similarly assessed. Cardiovascular risks factors were assessed in the participants. This has also been described elsewhere.3 The causes and precipitating factors of AHF episodes were also documented. There were four interviews during the assessment process: during recruitment, one month, three months, and six months after discharge. All recruitment interviews were conducted in person, and all follow-up sessions were conducted in person as well. When the patient was unable or refused to meet the follow-up schedule, a phone interview was conducted. The purpose of the interview was to determine readmission, the presence of an event, or complications. For death outside of the study facility and the investigator does not have access to the records, a verbal autopsy was performed. The interviews with the participants were conducted by an interviewer who was fluent in the patients' language as well as English if the patient could adequately converse in any of these. For speakers of other Nigerian dialects who are unable to communicate in English, a clinical staff member who is fluent in that dialect was used as a translator.
Diagnosis of Acute AHF
Acute heart failure was diagnosed using the European Society of Cardiology (ESC) criteria and were classified as de novo AHF or acute decompensated chronic HF.12
Definition of AHF Terms:
De novo AHF (New-onset AHF) is meant to be AHF in patients with no prior history of HF
Acute decompensated chronic HF (CHF) is worsening HF features in a known HF patient, i.e., acutely decompensated CHF patients.
Procedures
Laboratory
Fasting blood glucose and fasting lipid samples were collected after 8-10hours overnight fasting.
Serum creatinine level was used to determine the eGFR with the Cockcroft–Gault formula.13
Electrocardiography
A standard 12-lead electrocardiography studies were done for the participants using a commercially available CONTEC® Workstation Model, CONTEC EC8000G, ECG machine (Made in China) at a speed of 25mm/s and 1mV/cm calibration while they were supine and at rest.3 Various parameters such as PR intervals, QRS duration and axis, rate, rhythm, conduction abnormalities, types of arrhythmias, and QT intervals were assessed.3
Echocardiography
Transthoracic echocardiography were done for the participants based on the American Society of Echocardiography guidelines.14 The participants had the echocardiographic procedure within 72 hours of admission in the partial left lateral decubitus position using a Toshiba Xario (Toshiba Medical Systems Corp) with a 3.5 MHz transducer.3
Follow-up Details
Participants were followed-up for 6 months for major adverse cardiovascular events (MACEs) such as cardiac death, hospitalization for HF, and cardiovascular events. They were assessed at one, three and six months in the clinic. Medication intake were also recorded as types and either yes or no and those lost to follow up were documented. The composite all-cause mortality for HF during 6 months of follow up after discharge from hospital were documented. Deaths were further classified into the cardiovascular cause and non-cardiovascular cause. The ECG, echocardiographic, hematologic and biochemistry studies conducted for the patients at baseline were not repeated at follow up.
Ethical Considerations
Ethics approval was obtained from the institution's ethics review committee (approval number -(UI/EC/18/0004) and the study was conducted according to international and local ethics principles.
The study's questionnaires were kept secure, and the data generated was also kept safe from third parties.
Data Management and Statistical Analysis
Data was entered into a secured electronic database. International Business Machines (IBM) Corporation Statistical Product and Service Solutions (SPSS) for Windows version 23.0 was used for the statistical analysis (Armonk, NY: IBM Corp).
Sociodemographic, lifestyle, co-morbidities, risk factors, clinical symptoms at admission, vital signs at admission, anthropometry, laboratory results, ECG and echocardiographic results, precipitants of AHF, medication on admission and post-discharge status, and outcome made up the explanatory variables. Readmissions for HF, readmissions for non-HF cause, intrahospital deaths, and intrahospital cardiovascular complications were the study's primary outcome variables, while all-cause mortality, cardiac deaths, and cardiovascular events were its secondary outcomes. The normality test was performed on the data. Means, standard deviations, and other summary statistics for normally distributed continuous variables were compared with one-way analysis of variance (ANOVA).
The Mann-Whitney U test was used to compare the non-normally distributed data, which were summarized as median and interquartile range. Quantitative (categorical) variables were compared using the Pearson chi-square test and were expressed as frequencies and percentages. To identify the independent predictors of mortality, readmission, and all-cause events (composite events), Cox regression models were fitted. The models were also built with the time from admission to the first event in mind. For patients without the relevant event, the times were censored at the earlier of the relevant period of interest or the last date the patient was known to be alive. To find explanatory factors connected to the outcome variable that were statistically significant, Cox analysis was used. In order to have a strong mix of predictors to take into account, factors with a p-value of 0.1 or above were excluded from the multivariate model. Previously identified factors in the literature were also included. To identify potential risk factors for mortality six months after discharge, readmission six months after discharge, and all-cause events, multivariate Cox proportional hazards models were fitted. To determine every target outcome, all statistically significant univariate analysis variables were progressively fitted into the multivariate logistics regression model. Kaplan Meir survival analysis was used to analyse survival at six months. Two-sided p-values of 0.05 or less were regarded as significant for all tests.
RESULTS
The age range of participants was 18 to 90 years, with about half being older than 60 years. The mean ± standard deviation age was 58.0±15.1 years and most were males (59.4%) (Table 1). About four out of five participants were married. Hypertension (77.5%), kidney disease (34.4%), and diabetes mellitus (18.1%) were the most prevalent comorbidities. Alcohol consumption and smoking were the common behavioural cardiovascular risk factors. Reduced exercise tolerance, fatigue & tiredness, difficulty recovering from exercise, and dyspnoea were present in 88.1%, 83.8%, 83.8%, and 83.1% respectively (Table 2).
Table 1:
Sociodemographic characteristics of the 160 participants
| Variables | All (n=160) |
|---|---|
|
| |
| Age (mean ± SD) years | 58.0±15.1 |
| Gender | |
| Male n (%) | 95 (59.4) |
| Female n (%) | 65 (40.6) |
| Aged ≥60 years n (%) | 76 (47.5) |
| Marital Status n (%) | |
| Single | 6 (3.8) |
| Married | 138 (86.3) |
| Divorced | 1 (0.6) |
| Separated | 5 (3.1) |
| Widowed | 10 (6.3) |
| Educational level n (%) | |
| None | 15 (9.4) |
| Primary | 32 (20.0) |
| Secondary | 65 (40.6) |
| Tertiary | 45 (28.1) |
| Postgraduate | 3 (1.9) |
| Employment status n (%) | |
| Unemployed | 73 (45.6) |
| Employed | 87 (54.4) |
| Estimated monthly income n (%) | |
| ≤₦100.000 | 146 (93.1) |
| >₦100 000 | 14 (6.9) |
| Comorbidities n (%) | |
| Hypertension | 124 (77.5) |
| Kidney disease | 55 (34.4) |
| Diabetes Mellitus | 29 (18.1) |
| Obesity | 16 (10.0) |
| COPD | 8 (5.0) |
| Asthma | 3 (1.9) |
| Arthritis | 3 (5.1) |
| Family history of hypertension n (%) | 16 (10.0) |
| Family history of heart failure n (%) | 1 (0.6) |
| History of atrial fibrillation n (%) | 17 (10.7) |
| History of stroke n (%) | 2 (1.3) |
| History of thyroid disease n (%) | 1 (0.6) |
| Smoking n (%) | |
| Never | 139 (86.9) |
| Previous | 18 (11.3) |
| Current | 3 (1.9) |
| Alcohol intake n (%) | |
| Never | 108 (67.5) |
| Previous | 41 (25.6) |
| Current | 11 (6.9) |
SD= standard deviation
Table 2:
Heart failure symptoms and frequencies of previous AHF admissions among the 160 AHF participants
| Variables | All (n=160) |
|---|---|
|
| |
| Symptoms at presentation/recruitment n (%) | |
| Reduced exercise tolerance | 141 (88.1) |
| Fatigue & tiredness | 134 (83.8) |
| Difficulty to recover after exercise | 134 (83.8) |
| Dyspnoea | 133 (83.1) |
| Paroxysmal nocturnal dyspnea (PND) | 127 (79.4) |
| Leg swelling | 126 (78.8) |
| Cough | 118 (73.8) |
| Orthopnoea | 117 (73.1) |
| Easy Satiety | 76 (47.5) |
| Right abdominal pain | 57 (35.6) |
| Palpitation | 50 (31.3) |
| Abdominal swelling | 50 (31.3) |
| Chest pain | 43 (26.9) |
| Loss of appetite | 30 (18.8) |
| Nausea | 24 (15.0) |
| Vomiting | 23 (14.4) |
| Headache | 21 (13.1) |
| Confusion | 9 (5.6) |
| Wheezing | 8 (5.0) |
| Bloated feeling | 5 (3.1) |
| Tachypnoea | 158 (98.8) |
| Physical signs and New York Heart Association (NYHA) functional class of the participants | |
| Laterally displaced apical impulse (Displaced Apex) | 128 (80.0) |
| Third heart sound (gallop rhythm) | 118 (73.8) |
| Pulmonary crepitations | 118 (73.1) |
| Peripheral oedema (ankle, sacral, scrotal) | 117 (73.1) |
| Elevated jugular venous pressure (JVP) | 109 (68.1) |
| Hepatomegaly | 97 (60.6) |
| Ascites | 96 (90.0) |
| Narrow pulse pressure | 84 (52.5) |
| Tachycardia | 73 (45.6) |
| Systolic murmur | 59 (36.9) |
| Irregular pulse | 52 (32.5) |
| Cold extremities | 46 (28.8) |
| Pallor | 30 (18.8) |
| Reduced breath sound and dullness to percussion at lung bases | 26 (16.3) |
| Fever | 18 (11.3) |
| Oliguria | 12 (7.5) |
| Jaundice | 10 (6.3) |
| Central cyanosis | 10 (6.3) |
| Cheyne–Stokes respiration | 9 (5.6) |
| Diastolic murmur | 7 (4.4) |
| Finger clubbing | 5 (3.1) |
| New York Heart Association Class n (%) | |
| I | 1 (0.6) |
| II | 25 (15.6) |
| III | 103 (64.4) |
| IV | 31 (19.4) |
The commonest physical finding at baseline evaluation were tachypnoea, ascites, laterally displaced apical impulse and the presence of third heart sound with the following frequencies 98.8%, 90.0% and 73.8%, respectively (Table 2). About 80% were in NYHA class III/IV. Majority were either overweight (63.1%) or obese (10.0%) (Table 2). About half of the AHF was precipitated by infection (51.3%). Other common precipitants were arrhythmias (25.0%) and poor medication adherence (18.1%). Physical stress and emotional excesses (0.6%) and fluid overload (0.1%) were the least common precipitants. (Table 2).
The biophysical profile and laboratory findings are presented in Table II. The packed cell volume (PCV) mean± SD was 40.1±6.3%. Only 156 (97.5%) of the AHF patients in this study were on loop diuretics at the time of recruitment/admission. Similarly, spironolactone (93.1%) and ACE inhibitor (83.8%) were prescribed in high proportions (Table 3).
Table 3:
Biophysical profile, laboratory results and use of medications of the 160 participants
| Variables | All (n=160) |
|---|---|
|
| |
| Biophysical profile | |
| Weight median (IQR) Kg | 69.8 (62.78-76.82) |
| Height median (IQR) metre | 1.63 (1.36-1.90) |
| BMI median (IQR) Kg/m2 | 26.5 (24.66-28.44) |
| BMI Category n (%) | |
| <18.5kg/m2 | 5 (3.1) |
| 18.5-24.9kg/m2 | 38 (23.8) |
| 25-29.9kg/m2 | 101 (63.1) |
| ≥30Kg/m2 | 16 (10.0) |
| Pulse median (IQR) cycles/min | 90.0 (70.0-110.0) |
| Respiratory rate median (IQR) beats/min | 28.0 (20.0-36.0) |
| SBP median (IQR) mmHg | 110.0 (80.0-140.0) |
| DBP median (IQR) mmHg | 80.0 (51.5-108.5) |
| Heart rate mean± SD beats/min | 97.9±16.3 |
| Tachycardia n (%) | 52 (32.5) |
| Pulse Pressure median (IQR) mmHg | 38.5 (22.5-54.5) |
| Body surface area (mean ± SD) m2 | 1.74±0.12 |
| Laboratory results | |
| PCV mean± SD % | 40.1±6.3 |
| WBC median (IQR) /10mm3 | 7.5 (4.2-10.8) |
| Platelet median (IQR) /mm3 | 220.5 (121.7-319.3) |
| Sodium (median ± IQR) | 137.0 (130.0-144.0) |
| Potassium (median ± IQR) | 3.8 (3.8-4.0) |
| Urea median (IQR) mg/dl | 45.4 (8.6-82.2) |
| Creatinine median (IQR) mg/dl | 1.30 (0.5-2.1) |
| eGFR median (IQR) mL/min/1.73 m2 | 53.1 (19.9-86.3) |
| Total cholesterol mean± SD mg/dl | 153.6 ± 30.6 |
| LDL mean ± SD mg/dl | 94.4 ± 24.9 |
| HDL mean ± SD mg/dl | 40.4 ± 15.1 |
| Triglyceride mean ± SD mg/dl | 99.0 ± 28.7 |
| Use of medications at presentation | |
| Loop diuretics | 156 (97.50) |
| Mineralocorticoid receptor inhibitors | 149 (93.1) |
| ACEi/ARB | 138 (86.3) |
| Anticoagulant agents | 126 (78.8) |
| Beta-blocker | 90 (56.3) |
| Digoxin | 34 (21.3) |
| CCBs (Amlodipine) | 21 (31.1) |
| Amiodarone | 10 (6.3) |
| Aspirin | 7 (4.4) |
| Anti-glycaemic agent | 5 (3.1) |
| Thiazide | 3 (1.9) |
| Centrally acting | 1 (0.6) |
Kg- kilogramme IQR-Interquartile range SBP-Systolic Blood Pressure DBP-Diastolic Blood Pressure BMI- Body Mass Index PCVPacked cell volume WBC-White blood cells IQR-Interquartile range SD-Standard deviation eGFR- estimated glomerular filtration rate LDL-low-density lipoproteins cholesterol HDL-high-density lipoprotein cholesterol LMWH= Low Molecular Weight Heparin ACEi= Angiotensin-converting enzyme inhibitor ARB=Angiotensin II Receptor Blockers CCBs-Calcium channel blockers Anticoagulant agent (LMWH, Warfarin, Traditional heparin) Anti-glycaemic agent (Insulin and oral hypoglycaemic agent)
The electrocardiographic results of the study participants were also displayed in Table 4. Approximately two out of every five people had arrhythmias. The prevalence of left ventricular systolic dysfunction is high (75.0%). (Table 4). Mitral incompetence (84.4%) and tricuspid incompetence (78.1%) were the most prevalent valvular abnormalities (Table 4).
Table 4:
Electrocardiographic profile of the participants
| Variables | All (n=160) |
|---|---|
|
| |
| Electrocardiographic results | |
| Heart rate mean ± SD/min | 97.9±16.3 |
| Sinus rhythm n (%) | 98 (61.3) |
| Normal ECG axis n (%) | 110 (68) |
| Atrial fibrillation n (%) | 23 (14.4) |
| Atrial flutter n (%) | 4 (2.5) |
| Premature Ventricular Contractions (PVCs) n (%) | 28 (17.5) |
| Premature Supraventricular Contractions n (%) | 2 (1.3) |
| Atrial chamber enlargement n (%) | |
| Left | 24 (15.0) |
| Right | 15 (9.4) |
| Bi-atrial | 6 (3.8) |
| None | 115 (71.9) |
| Ventricular Chamber hypertrophy | |
| Left Ventricular hypertrophy (LVH) n (%) | 48 (30.0) |
| Left Ventricular hypertrophy (LVH) with strain n (%) | 37 (23.1) |
| Right Ventricular hypertrophy (LVH) n (%) | 13 (8.1) |
| Right Ventricular hypertrophy (LVH) with strain n (%) | 8 (5.0) |
| Conduction abnormalities n (%) | 22 (13.8) |
| First degree AV block n (%) | 9 (5.6) |
| Second degree AV block | 3 (1.9) |
| Complete heart block | 0 |
| Right Bundle Branch Block (RBBB) n (%) | 3 (1.9) |
| Left Bundle Branch Block (LBBB) n (%) | 6 (3.8) |
| Intermediate interventricular block n (%) | 1 (0.6) |
| Presence of infarction | 2 (1.3) |
| QT interval mean± SD ms | 369.7±71.5 |
| QTc interval mean± SD ms | 415.6±65.3 |
| QRS mean± SD ms | 112.7±39.7 |
| Echocardiographic Results | |
| Aortic valve opening (mean ± SD) (cm) | 2.01 ± 0.38 |
| Aortic root diameter (mean ± SD) (cm) | 2.92 ± 0.57 |
| Left atrial diameter (mean ± SD) (cm) | 4.47 ± 7.28 |
| IVST(d) mean ± SD (cm) | 1.06 ± 0.25 |
| IVST(d) mean ± SD cm | 1.16±0.28 |
| LVPWT(d) mean ± SD (cm) | 1.07 ± 0.27 |
| LVPWT(s) mean ± SD (cm) | 1.32 ± 0.33 |
| LVID(d) mean ± SD (cm) | 5.87 ± 1.07 |
| LVID(s) mean ± SD (cm) | 4.68 ± 1.16 |
| Pulsed wave Doppler study of trans-mitral inflow | |
| Mitral E wave velocity (mean ± SD) m/s | 0.95±0.33 |
| Mitral A wave velocity (mean ± SD) m/s | 0.57± 0.47 |
| E/A wave ratio median (IQR) | 1.67(0.80,2.54) |
| DT of Mitral E mean ± SD ms | 202.8 ±112.9 |
| IVRT (mean ± SD) (ms) | 122.7±31.1 |
| LV filling pattern n (%) | |
| Normal | 102(63.8) |
| Impaired relaxation | 23(14.4) |
| Pseudo normalization | 5(3.1) |
| Restrictive pattern | |
| Reversible | 4(2.5) |
| Fixed | 26(16.3) |
| LV geometry n (%) | |
| Normal geometry | 19(11.9) |
| Concentric remodeling | 10(6.3) |
| Concentric geometry | 109(68.1) |
| Eccentric geometry | 22(13.8) |
| EDV (mean ± SD) (ml) | 187.1 ± 71.4 |
| ESV (mean ± SD) (ml) | 116.0 ± 51.6 |
| Fractional shortening (mean ± SD) | 19.8 ± 8.7 |
| Ejection fraction (EF) (mean ± SD) | 38.7 ± 13.8 |
| EF Category n (%) | |
| Reduced EF | 114(71.3) |
| Mid-range EF | 21(13.1) |
| Preserved EF | 25(15.6) |
| Relative wall thickness (RWT)(mean ± SD) (cm) | 0.37 ± 0.10 |
| TAPSE mean ± SD cm | 1.63 ± 0.46 |
| LV mass (mean ± SD) g | 258.0 ± 84.5 |
| LV mass indexed to BSA (mean ± SD) g | 149.1 ± 50.8 |
| LV mass indexed to height2.7 (mean ± SD) g | 70.0 ± 25.7 |
| Valvular Abnormalities n (%) | |
| Mitral incompetence | 135 (84.4) |
| Tricuspid incompetence | 125 (78.1) |
| Aortic incompetence | 28 (17.5) |
| Pulmonary incompetence | 12 (7.5) |
| Aortic stenosis | 4 (2.5) |
| Mitral stenosis | 1 (0.6) |
| Tricuspid stenosis | 0 |
| Pulmonary stenosis | 0 |
| Presence of wall motion abnormality n (%) | 48(30.0) |
| Pericardium pericardial effusion n (%) | 84(52.5) |
| Size of Pericardial Effusion n (%) | |
| Nil | 84 (52.5) |
| Negligible | 59 (36.9) |
| Less than 1cm | 8 (5.0) |
| Between 1-2cm | 4 (2.5) |
| More than 2cm | 5 (3.1) |
| Spontaneous echo contrast n (%) | 9(5.6) |
| Intra-cardiac clots n (%) | 2(1.3) |
SD-standard deviation, QTc -Corrected QT IVST(d)- Interventricular septal thickness(diastole) IVST(s)- Interventricular septal thickness(systole) LVPWT(d)- Left Ventricular posterior wall thickness(diastole) LVPWT(s)- Left Ventricular posterior wall thickness(systole) LVID (d)- Left Ventricular internal diameter-diastole LVID (s)- Left Ventricular internal diameter-systole IQRInterquartile range DT- Deceleration time IVRT- Isovolumetric relaxation time TAPSE-Tricuspid annulus plane systolic excursion
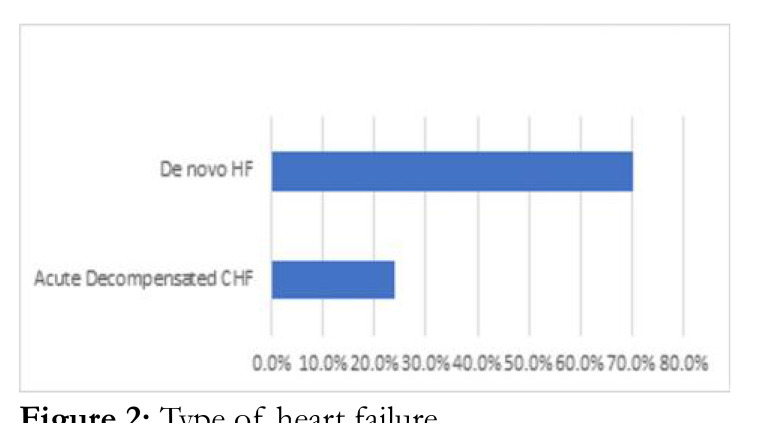
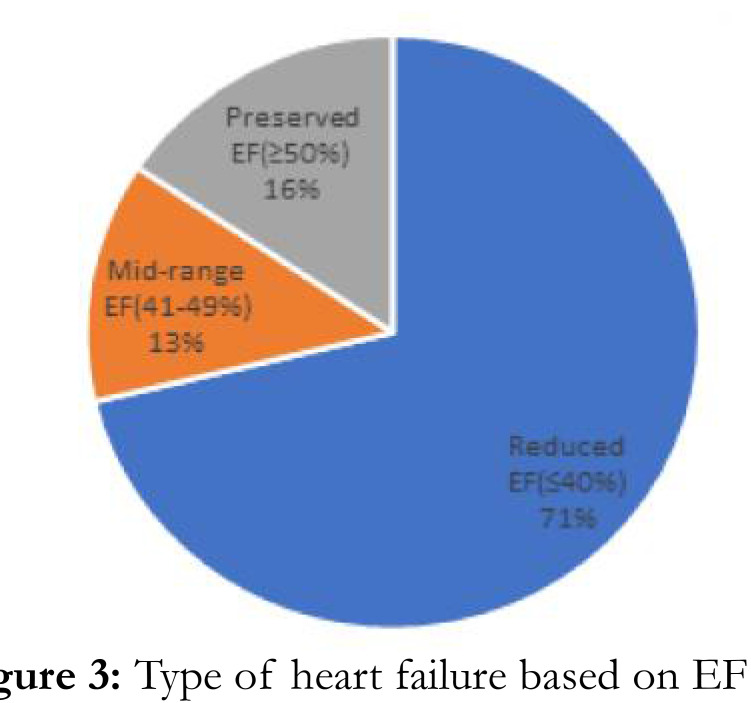
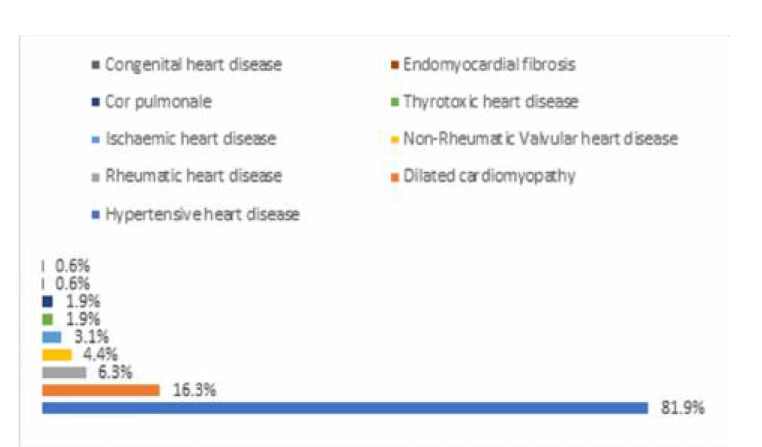
Hypertensive heart disease was the most frequent risk factor in AHF (131 (84.2%)). (Figure 2). Only 26 people (16.3%) and 10 people (6.3%), respectively, were found to have dilated cardiomyopathy and rheumatic heart disease (Figure 2). Majority of participants had de novo AHF (75.6%) compared to 24.4% for ADCHF, p=0.350. De novo AHF and ADCHF had mean SD of 38.6 12.9% and 39.1 16.6, respectively, with a pvalue of 0.830. 51 (67.1%), 10 (13.2%), and 15 (19.2%) fall into the categories of reduced, mid-range, and preserved ejection AHF, respectively. De novo HF and ADCHF had 59 (36.1%) and 17 (10.6%) more people over the age of 60, respectively (p=0.929) (Figure 3).
Figure. 2:
Type of heart failure
Figure. 3:
Type of heart failure based on EF
The survivors that were followed up during 6 months' post-discharge were 150(93.8%) with 16(10.0%) of the total study population lost to follow up. A total of 41(25.6%) of the total study population died during the study period.
Most deaths were after 8 days of stay for both genders while more males died on admission compared to females HR (95% confidence interval (CI)), p-value; 1.51(0.86,2.62),0.151. More of the death at post-discharge 6 months' follow-up were within the first 3 months of discharge (70.7%).
The various categories of the variables assessed showed that the following were significantly different between the dead and survivors after six months of follow-up, sociodemographic crude hard ratio (HR) (95% confidence interval (CI) gender: 2.40(1.03 to 5.56); p=0.042), physical signs (elevated jugular venous pressure: 1.96(1.16 to 3.32); p= 0.012), hepatomegaly: 1.76(1.12 to 2.74; p=0.013), ascites :1.56(1.04 to 2.12; p=0.029), and systolic blood pressure: 0.94(0.96 to 0.99); p-value=0.006) (Table 5). Others are laboratory results; hyponatremia:1.49(1.04 to 2.12); p=0.026), Echocardiographic finding: presence of pericardial effusion/tamponade: 2.78(1.52(1.52 to 5.06); p=0.001, presence of co-morbidities: diabetes mellitus: 1.61(1.03 to 2.51); p=0.037.
Table 5:
Univariate analysis results of variables with significant differences between dead and survivors, between readmission after discharge and those not admitted and all composite endpoint 6 months after discharge and those no event
| Dead and survivors | ||||
|---|---|---|---|---|
| Variables | Dead | Survivors | Univariate analysis | |
|
| ||||
| Crude HR (95% CI) | P-value | |||
|
| ||||
| Gender n (%) | 2.40 (1.03 to 5.56) | 0.042** | ||
| Male | 28 (68.3) | 56 (54.4) | ||
| Female | 13 (31.7) | 47 (45.6) | ||
| SBP (mmHg) | 107.2 ± 22.1 | 119.7 ± 22.9 | 0.94 (0.96 to 0.99) | 0.006** |
| Elevated JVP n (%) | 1.96 (1.16 to 3.32) | 0.012** | ||
| Yes | 37 (90.2) | 64 (62.1) | ||
| No | 4 (9.8) | 39 (37.9) | ||
| Hepatomegaly n (%) | 1.76 (1.12 to 2.74) | 0.013** | ||
| Yes | 33 (80.5) | 58 (56.3) | ||
| No | 8 (19.5) | 45 (43.7) | ||
| Ascites n (%) | 1.56 (1.04 to 2.33) | 0.031** | ||
| Yes | 33 (80.5) | 56 (54.4) | ||
| No | 8 (19.5) | 47 (45.6) | ||
| Hyponatraemia n (%) | 1.49 (1.04 to 2.12) | 0.029** | ||
| Yes | 18 (43.9) | 26 (25.2) | ||
| No | 23 (56.1) | 77 (74.8) | ||
| Pericardial effusion/tamponade n (%) | 2.78 (1.52 to 5.06) | 0.001** | ||
| Yes | 4 (9.8) | 0 | ||
| No | 37 (90.2) | 103 (100.0) | ||
| Diabetes Mellitus n (%) | 1.61 (1.03 to 2.51) | 0.037** | ||
| Yes | 11 (26.8) | 8 (7.8) | ||
| No | 30 (73.2) | 95 (92.2) | ||
|
| ||||
| Readmission after discharge and those not admitted | ||||
| Variables | Readmitted | Not admitted | Univariate analysis | |
|
| ||||
| Crude HR (95% CI) | P-value | |||
|
| ||||
| Right abdominal pain n (%) | ||||
| Yes | 24 (63.2) | 80 (72.1) | 1.35 (1.02 to 1.80) | 0.039** |
| No | 14 (36.8) | 31 (27.9) | ||
| SBP mean ± SD mmHg | 110.1 ± 22.9 | 121.1 ± 22.7 | 0.98 (0.96 to 0.99) | 0.004** |
| LAD mean ± SD cm | 4.7 ± 0.8 | 4.4 ± 0.7 | 1.46 (1.01 to 2.10) | 0.045** |
| LVPWT(d) cm | 1.1 ± 0.2 | 1.0 ± 0.3 | 2.94 (1.05 to 8.18) | 0.040** |
| Pericardial effusion/Tamponade n (%) | 2.17 (1.07 to 4.41) | 0.032** | ||
| Yes | 1 (4.3) | 3 (2.0) | ||
| No | 26 (68.4) | 87 (78.4) | ||
| Valvular heart disease n (%) | ||||
| Yes | 4 (10.5) | 1 (0.9) | 0.32 (0.32 to 0.80) | 0.015** |
| No | 34 (89.5) | 110 (99.1) | ||
| All composite endpoint 6 months after discharge and those no event | ||||
|
| ||||
| Variables | Presence of event | Absence of event | Univariate analysis | |
|
| ||||
| Crude HR (95% CI) | P-value | |||
|
| ||||
| Elderly n (%) | ||||
| Yes | 32 (57.1) | 44 (42.5) | 1.68 (1.01 to 2.81) | 0.047** |
| No | 24 (42.9) | 60 (57.7) | ||
| Gender n (%) | ||||
| Male | 43 (76.8) | 52 (50.0) | 0.53 (0.30 to 0.92) | 0.025** |
| Female | 13 (23.2) | 52 (50.0) | ||
| SBP mean ± SD mmHg | 111.1 ± 22.8 | 121.2 ± 22.8 | 0.98 (0.97 to 1.00) | 0.007** |
| Narrow pulse pressure n (%) | ||||
| Yes | 33 (58.9) | 51 (49.0) | 1.32 (1.02 to 1.72) | 0.033** |
| No | 23 (41.1) | 53 (51.0) | ||
| LVPWT(d) mean ± SD cm | 1.1 ± 0.3 | 1.0 ± 0.3 | 2.88 (1.81 to 7.04) | 0.020** |
| Pericardial effusion/tamponade n (%) | ||||
| Yes | 3 (5.4) | 2 (1.9) | 2.55 (1.53 to 4.24) | <0.0001** |
| No | 53 (94.6) | 102 | ||
| Uncontrolled hypertension n (%) | ||||
| Yes | 2 (5.6) | 15 (14.4) | 0.48 (0.24 to 0.97) | 0.042** |
| No | 54 (94.4) | 89 (85.6) | ||
Significant p-value Â0.05 SBP- Systolic Blood Pressure JVP- Jugular Venous Pressure
During univariate analysis the presence of right abdominal pain: crude HR 1.35(1.02 to 1.80), p-value- 0.039, systolic blood pressure 0.98(0.96 to 0.99), p=0.004, echocardiographic left atrial diameter 1.46(1.01 to 2.10),p=0.045, Ventricular Posterior Wall Thickness (diastole): 2.94(1.05 to 8.18), p=0.040, presence of pericardial effusion/tamponade during echocardiography: 2.17(1.07 to 4.41), p=0.032 and presence of valvular heart disease: 0.32(0.32 to 0.80), p=0.015 were different among survivors (Table 5).
Univariate analysis of composite endpoints(death and readmissions) were been elderly crude HR (95% CI): 1.68(1.01 to 2.81), p= 0.04&, male gender: 0.53(0.30 to 0.92), p=0.025, systolic blood pressure: 0.98(0.97 to 1.00), p=0.007, presence of narrow pulse pressure: 1.32(1.02 to 1.72), p=0.033, ventricular posterior wall thickness (diastole): 2.88(1.81 to 7.04), p=0.020, presence of pericardial effusion/tamponade: 2.55(1.53 to 4.24), p=<0.0001 and presence of uncontrolled hypertension: 0.48(0.24 to 0.97), p=0.042 Systolic Blood Pressure (mmHg) (Table 5).
Multivariate analysis showed that the independent predictors for death outcome after 6 months of discharge for AHF and the adjusted hazard ratio (95% CI) are gender(male) 2.77(1.17 to 6.56); p=0.020, systolic Blood Pressure (mmHg) 0.98(0.96 to 0.99); p=0.011, the presence of hepatomegaly 2.58(1.02 to 6.51); p=0.045 (Table 6).
Table 6:
Independent predictors of surviving after 6 months after discharge, readmission after discharge and composite endpoint 6 months after discharge using multivariate analyses among the participants
| Independent predictors of surviving after 6 months after discharge using multivariate analyses among the participants | |||||
|
| |||||
| Variables | B | SE | Wald | Adjusted HR (95.0% CI) | P-value |
|
| |||||
| Gender(Male) | 1.020 | 0.439 | 5.391 | 2.77(1.17 to 6.56) | 0.020** |
| Systolic Blood Pressure (mmHg) | -0.026 | 0.010 | 6.497 | 0.98(0.96 to 0.99) | 0.011** |
| Elevated jugular venous pressure (JVP) | 0.962 | 0.544 | 3.133 | 2.62 (0.90 to 7.60) | 0.077 |
| Hepatomegaly | 0.946 | 0.473 | 4.006 | 2.58(1.02 to 6.51) | 0.045** |
| Ascites | 0.475 | 0.427 | 1.238 | 1.61(0.70 to 3.71) | 0.266 |
| Hyponatraemia | 0.356 | 0.385 | 0.855 | 1.43(0.67 to 3.04) | 0.355 |
|
| |||||
| Independent predictors for readmission after discharge using multivariate analysis | |||||
|
| |||||
| Variables | B | SE | Wald | Adjusted HR (95.0% CI) | Sig. |
| Right abdominal pain | 0.727 | 0.305 | 5.706 | 2.07(1.14 to 3.76) | 0.017** |
| Systolic Blood Pressure (mmHg) | -0.023 | 0.008 | 7.989 | 0.98(0.96 to 0.99) | 0.005** |
| Left atrial diameter (cm) | 0.268 | 0.191 | 1.977 | 1.31((.90 to 1.90) | 0.160 |
| LVPWT(d) | 0.834 | 0.565 | 2.177 | 2.30(0.76 to 6.98) | 0.140 |
| Pericardial effusion/tamponade | 1.591 | 0.962 | 2.735 | 4.91(0.75 to 32.35) | 0.098 |
| VHD | 0.089 | 0.683 | 0.017 | 1.09(0.29 to 4.17) | 0.896 |
|
| |||||
| Independent predictors for composite endpoint 6 months after discharge by multivariate analysis | |||||
|
| |||||
| Variables | B | SE | Wald | Adjusted HR (95.0% CI) | p-value |
| Elderly | 0.377 | 0.266 | 2.016 | 1.46(0.87 to 2.46) | 0.156 |
| Gender (Male) | 0.732 | 0.297 | 6.087 | 2.08(1.16 to 3.72) | 0.014** |
| Systolic Blood Pressure (mmHg) | -0.011 | 0.007 | 2.578 | 1.00(0.98 to 1.00) | 0.108 |
| Narrow pulse pressure | 0.274 | 0.285 | 0.924 | 1.32(0.75 to 2.30) | 0.336 |
| LVPWT(d) | 0.891 | 0.466 | 3.648 | 2.44(0.98 to 6.08) | 0.056 |
| Pericardial effusion/tamponade | 1.669 | 0.555 | 9.045 | 5.31(1.79 to 15.74) | 0.003** |
| Uncontrolled hypertension | -1.007 | 0.737 | 1.866 | 0.37(0.09 to 1.55) | 0.172 |
Significant p-value <0.05 SBP- Systolic blood pressure LAD- Left atrial diameter LVPWT(d)- Left Ventricular posterior wall thickness(diastole) VHD-Valvular heart disease
Independent predictors for readmission or rehospitalization within 6months after discharge were presence of right abdominal pain during admission adjusted HR (95% CI): 2.07(1.14 to 3.76), p=0.017, systolic blood pressure 0.98(0.96 to 0.99), p= 0.005 (Table 6).
Independent predictors from multivariate analysis are male gender: adjusted hazard ratio (HR): 2.081.16 to 3.72, p= 0.014 and pericardial effusion and tamponade: 5.31(1.79 to 15.74, p=0.003(Table 6).
DISCUSSION
Overall, this study showed the importance of systolic blood pressure (SBP) as an independent predictor of survival six months after admission and readmission after baseline hospital discharge. Although, it had no effect on the composite endpoints (mortality and readmission) six months after discharge. Furthermore, this study showed that variables such as hepatomegaly, right abdominal pain, and pericardial effusion/tamponade associated with congestion also play an important role in predicting the outcome of AHF after 180 days of discharge.
Low SBP, male gender, and hepatomegaly were independent predictors of AHF survival six months after discharge. The predictive role of SBP, a readily accessible vital sign at admission and on AHF post-discharge during clinic visits has been previously noted.15, 16 It has also been noted to play a great role in morbidity and mortality outcomes among black patients with AHF.15 Elevated blood pressure at admission is associated with the likelihood of better response to treatment during admission.15
Systolic blood pressure was also an independent factor for readmission/rehospitalization in this study population similar to previous studies.15, 16 SBP was also found to be statistically significant during univariate analysis in the Romanian Acute Heart Failure Registry (RO-AHF); however, this was not statistically significant after multivariate analysis.17 Similar finding was documented in Abeokuta Heart failure registry and THESUS-HF registry.18 The role of elevated SBP in readmission calls for better post-discharge care among patients with AHF, especially through the provision of HF home care and continuous optimization of therapy cannot be underemphasized.
Lower survival in males with AHF have been highlighted in previous studies.19-21 The explanation for better survival among females after the onset of heart failure is probably because they have a better ventricular function and more frequently suffer from preserved HF compare to males.15 Females in this study also had a higher mean EF, and a greater proportion had preserved EF compared to males.3, 22 Other explanations for better survival in females with HF found in this study include a lower burden of AF and ventricular arrhythmias.3, 22 Notwithstanding that our study included a similar proportion of males and females.3 Furthermore, females with non-ischaemic HF are noted to have an attenuated sympathetic activation and parasympathetic withdrawal compared with men with its attendant prognostic implications.22
Evidence or markers of congestion, such as right abdominal pain, were independent predictors of survival, readmission/rehospitalization, and all-cause events. These are not unconnected with the key pathophysiology associated with AHF, which is congestion and the major reason for admission or readmission.23, 24 Furthermore, systemic congestion in AHF predispose to multi-organ dysfunction and activation of neurohormonal system which is already activated above the physiological baseline level early during disease progression in patients with chronic HF.16 This role of congestion suggests that adequate physical examination among AHF especially attempt to identify all HF features that maybe congestive in nature cannot be underestimated.25
Longer follow-up studies on prognosis in this setting are required to provide additional insights into patient prognosis. Given the limited contribution of ECG and echocardiography to prognosis determination in AHF, the importance of exploring the roles of biomarkers, particularly cardiac biomarkers, such as cystatin, natriuretic peptides, and troponin cannot be overemphasized.
The results of this study will aid in the efficient management of AHF, particularly in low-resource environments, such as Nigeria and black subjects. Additionally, knowledge of the clinical and laboratory predictors would help in the creation of prevention, outcome improvement, and adverse outcome reduction strategies for AHF in Nigeria. Finally, knowing the prognosis at six months would add to the knowledge about enhancing clinical outcomes for AHF among black patients and improving survival strategies.
This study has some limitations. First, because this study was conducted at a single tertiary center, the results may not be applicable to primary and secondary institutions in the same region, and it may not have adequately captured the rural patient population, although the institution is a major tertiary institution in south-western Nigeria. This limitation may have reduced the generalizability of the results to some extent. Secondly, the study could be influenced by unmeasured confounding variables, such as socioeconomic status, patient preference, and even post-hospital care, despite the fact that efforts were made to investigate a broad range of sociodemographic, bio-humoral or laboratory, electrocardiographic, echocardiographic, and therapeutic variables. Finally, BNP testing was not included in the HF diagnosis and follow-up.
CONCLUSION
Overall, the study provided an understanding of the factors contributing to HF admissions in a population in South-Western, Nigeria. A better understanding of AHF will help with the development of the protocol for trials to improve the prognostic outcome of AHF. The study also highlighted the relevance of clinical profile, particularly systolic blood pressure, has shown in previous studies.
Figure. 1:
Diagnosis of heart failure
ACKNOWLEDGEMENT
The authors will like to acknowledge Miss Iyanu Adufe who helped with data collection and entry duties during this research work.
Statements and Declarations
The authors have no financial or non-financial interests that were directly or indirectly related to the work submitted for publication to declare.
REFERENCES
- 1.McDonagh TA, Metra M, Adamo M. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Pang PS. Acute Heart Failure Syndromes. Journal of the American College of Cardiology. 2009;53(7):557–573. doi: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 3.Adebayo O, Ogah O, Adebiyi A. Clinical Characteristics, Management, and Six-Month Outcomes after Discharge of Patients Admitted for Acute Heart Failure in Ibadan, Nigeria. West African Journal of Medicine. 2023;40(1) [PubMed] [Google Scholar]
- 4.Chapman B, DeVore AD, Mentz RJ, Metra M. Clinical profiles in acute heart failure: an urgent need for a new approach. ESC heart failure. 2019;6(3):464–474. doi: 10.1002/ehf2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanfear DE, Peterson EL, Campbell J. Relation of worsened renal function during hospitalization for heart failure to long-term outcomes and rehospitalization. American Journal of Cardiology. 2011;107(1):74–78. doi: 10.1016/j.amjcard.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogunmodede JA, Kolo P, Dele-Ojo B. Rehospitalization rate and predictors of rehospitalization in heart failure patients in north central Nigeria. The Tropical Journal of Health Sciences. 2022;29(1):36–41. [Google Scholar]
- 7.Sliwa K, Davison BA, Mayosi BM. Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. European Heart Journal. 2013;34(40):3151–3159. doi: 10.1093/eurheartj/eht393. [DOI] [PubMed] [Google Scholar]
- 8.Oliva F, Mortara A, Cacciatore G. Acute heart failure patient profiles, management and in hospital outcome: results of the Italian Registry on Heart Failure Outcome. European journal of heart failure. 2012;14(11):1208–1217. doi: 10.1093/eurjhf/hfs117. [DOI] [PubMed] [Google Scholar]
- 9.Karaye KM, Sani MU. Factors associated with poor prognosis among patients admitted with heart failure in a Nigerian tertiary medical centre: a cross-sectional study. BMC Cardiovascular Disorder. 2008;8:16. doi: 10.1186/1471-2261-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karaye KM, Sani MU. Electrocardiographic abnormalities in patients with heart failure. Cardiovascular Journal of Africa. 2008;19(1):22–25. [PMC free article] [PubMed] [Google Scholar]
- 11.Ogah OS, Stewart S, Onwujekwe OE. Economic burden of heart failure: investigating outpatient and inpatient costs in Abeokuta, Southwest Nigeria. PLoS One. 2014;9(11):e113032. doi: 10.1371/journal.pone.0113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiologia Polska (Polish Heart Journal) 2016;74(10):1037–1147. doi: 10.5603/KP.2016.0141. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell C, Rahko PS, Blauwet LA. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2019;32(1):1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M, Abraham WT, Albert NM. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. Jama. 2006;296(18):2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 16.Arrigo M, Jessup M, Mullens W. Acute heart failure. Nature Reviews Disease Primers. 2020;6(1):16. doi: 10.1038/s41572-020-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chioncel O, Vinereanu D, Datcu M. The Romanian acute heart failure syndromes (ROAHFS) registry. American heart journal. 2011;162(1):142–153. doi: 10.1016/j.ahj.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Ogah OS, Stewart S, Falase AO. Short-term outcomes after hospital discharge in patients admitted with heart failure in Abeokuta, Nigeria: data from the Abeokuta Heart Failure Registry. Cardiovasc J Afr. 2014;25(5):217–223. doi: 10.5830/CVJA-2014-040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Lawati JA, Sulaiman KJ, Al-Zakwani I. Systolic blood pressure on admission and mortality in patients hospitalized with acute heart failure: Observations from the Gulf Acute Heart Failure Registry. Angiology. 2017;68(7):584–591. doi: 10.1177/0003319716672525. [DOI] [PubMed] [Google Scholar]
- 20.Ogah OS, Stewart S, Falase AO. Predictors of rehospitalization in patients admitted with heart failure in Abeokuta, Nigeria: data from the Abeokuta heart failure registry. J Card Fail. 2014;20(11):833–840. doi: 10.1016/j.cardfail.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 21.AlHabib KF, Elasfar AA, Alfaleh H. Clinical features, management, and short and long term outcomes of patients with acute decompensated heart failure: phase I results of the HEARTS database. European journal of heart failure. 2014;16(4):461–469. doi: 10.1002/ejhf.57. [DOI] [PubMed] [Google Scholar]
- 22.Strömberg A, Mårtensson J. Gender differences in patients with heart failure. European Journal of Cardiovascular Nursing. 2003;2(1):7–18. doi: 10.1016/S1474-5151(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 23.Gheorghiade M, Follath F, Ponikowski P. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. European journal of heart failure. 2010;12(5):423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 24.Mentz RJ, Kjeldsen K, Rossi GP. Decongestion in acute heart failure. European journal of heart failure. 2014;16(5):471–482. doi: 10.1002/ejhf.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvaraj S, Claggett B, Pozzi A. Prognostic implications of congestion on physical examination among contemporary patients with heart failure and reduced ejection fraction: PARADIGM-HF. Circulation. 2019;140(17):1369–1379. doi: 10.1161/CIRCULATIONAHA.119.039920. [DOI] [PubMed] [Google Scholar]