Abstract
Saccharomyces cerevisiae BUB1 encodes a protein kinase required for spindle assembly checkpoint function. In the presence of spindle damage, BUB1 is required to prevent cell cycle progression into anaphase. We have identified a dominantly acting BUB1 allele that appears to activate the spindle assembly checkpoint pathway in cells with undamaged spindles. High-level expression of BUB1-5 did not cause detectable spindle damage, yet it delayed yeast cells in mitosis at a stage following bipolar spindle assembly but prior to anaphase spindle elongation. Delayed cells possessed a G2 DNA content and elevated Clb2p mitotic cyclin levels. Unlike cells delayed in mitosis by spindle damage or MPS1 kinase overexpression, hyperphosphorylated forms of the Mad1p checkpoint protein did not accumulate. Similar to cells overexpressing MPS1, the BUB1-5 delay was dependent upon the functions of the other checkpoint genes, including BUB2 and BUB3 and MAD1, MAD2, and MAD3. We found that the mitotic delay caused by BUB1-5 or MPS1 overexpression was interdependent upon the function of the other. This suggests that the Bub1p and Mps1p kinases act together at an early step in generating the spindle damage signal.
Successful mitotic cell division requires the coordination of numerous complex processes. Since loss of coordination would cause drastic consequences, cells utilize surveillance feedback mechanisms, referred to as “checkpoints,” to enforce the proper sequence of cell division events (3, 9). Checkpoint mechanisms monitor aspects of normal division processes and can act to halt cell cycle progression should defects be detected. The spindle assembly (or mitotic) checkpoint acts to ensure that replicated chromosomes are properly attached to a functioning mitotic spindle (reviewed in references 3, 21, and 30). In the presence of spindle damage, this mechanism prevents cells from entering anaphase, the chromosome segregation stage of mitosis.
Studies of the budding yeast Saccharomyces cerevisiae have revealed seven genes (BUB1, BUB2, BUB3, MAD1, MAD2, MAD3, and MPS1) whose functions are required to properly arrest cell cycle progression following spindle damage. While the normal response to spindle damage (i.e., as caused by microtubule-depolymerizing compounds) is to arrest in M phase, loss of function of these checkpoint genes causes cells to prematurely exit from mitosis, rebud, and reinitiate DNA replication (10, 15, 29). Loss of BUB or MAD function also causes precocious sister chromatid disjunction under microtubule-depolymerizing conditions (26).
The studies reported in this article are directed toward understanding the role of the BUB1 product and its relationships to the products of the other checkpoint genes. BUB1 and MPS1 encode protein kinases, suggesting that they function in transduction of the spindle damage signal (14, 20). MAD1 encodes a phosphoprotein that is believed to be a substrate of the Mps1p kinase in vivo (6, 7). Bub3p is a substrate of Bub1p in vitro (20). Studies of vertebrate cells have revealed that unattached kinetochores are a major source of the signal indicating spindle malfunction (17). Interestingly, vertebrate homologs of the Bub1p protein kinase and Mad2p are physically associated with unattached prometaphase kinetochores (2, 16, 27). In addition, physical associations of S. cerevisiae Bub1p with Bub3p (20) and Mad1p with Mad2p (cited in reference 21) have been demonstrated. Therefore, it seems possible that many of the checkpoint gene products participate in a spindle damage-signaling complex at kinetochores.
Although the cellular site of action of the Mps1p protein kinase has not been determined, it is believed to function at an early step in the generation of the spindle damage signal. Overexpression of MPS1 is able to delay cell cycle progression into anaphase in a manner similar to checkpoint activation by spindle damage (7). Both treatments cause cellular accumulation of hyperphosphorylated forms of Mad1p (6, 7). The delay caused by overexpressed MPS1 is dependent upon the functions of the six MAD and BUB gene products, suggesting that they act downstream from, or in concert with, Mps1p. Unlike the MAD and BUB genes, MPS1 is essential for cell viability; it is required for the essential process of spindle pole body duplication (31).
Loss of BUB1 function is recessive and causes inappropriate cell cycle progression through mitosis. In this study, we identified the dominantly acting BUB1-5 allele and demonstrated that it blocks yeast cell cycle progression in mitosis at a stage prior to anaphase onset. The mitotic delay resembled that caused by spindle damage or MPS1 overexpression by a few criteria but differed in that hyperphosphorylated forms of Mad1p were not detected. Similar to the mitotic delay caused by overexpressed MPS1, the BUB1-5 delay was dependent upon the functions of the other checkpoint genes. In addition, we found that the delay caused by BUB1-5 or MPS1 overexpression was interdependent upon the function of the other. This suggests that these kinases act together at an early step in generating the spindle damage signal.
MATERIALS AND METHODS
Yeast strains and media.
The yeast strains used in these experiments are listed in Table 1. bub1Δ and bub3Δ frequently become aneuploid and suppress their slow-growth phenotype. Therefore, bub1Δ and bub3Δ strains were routinely generated by sporulation of heterozygous diploids (MAY2517 and MAY2068, respectively). MAD gene-deleting DNA constructs were gifts from K. Hardwick and A. W. Murray. All deletions were verified by Southern blotting or PCR, as well as by benomyl sensitivity.
TABLE 1.
Yeast strains and plasmids
| Strain or plasmid |
Genotype |
|---|---|
| Strains | |
| AS131-2d | MATa ura3-52 his leu2-3,112 ade2-101 mps1-3796 |
| KH34 | MATa ura3-1 his3-11 leu2-3,112 ade2-1 trp1-1 can1-100 PGAL>MPS1URA3 |
| MAY589 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 |
| MAY2055 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 bub2::URA3 |
| MAY2068 | MATa/MATα ura3-52/ura3-52 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 ade2-101/ADE2 lys2-801/LYS2 bub3::LEU2/BUB3 |
| MAY2517 | MATa/MATα ura3-52/ura3-52 his3-Δ200/his3-Δ200 leu2-3,112/leu2-3,112 ade2-101/ADE2 lys2-801/LYS2 bub1::HIS3/BUB1 |
| MAY3728 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 mad1::HIS3 |
| MAY4428 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 mad2::URA3 |
| MAY4537 | MATa ura3-52 his3-Δ200 leu2-3,112 lys2-801 bub1::HIS3 |
| MAY4574 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 lys2-801 bub3::LEU2 |
| MAY4612 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 mad3::URA3 |
| MAY4955 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 bub1::HIS3 cin8::HIS3 (pKF44) |
| MAY5035 | MATa ura3-52 his3-Δ200 leu2-3,112 ade2-101 bub1::HIS3 cin8::HIS3 (pKF48) |
| Plasmids | |
| p413 | PGAL (vector) HIS3 CEN |
| pBM272 | PGAL (vector) URA3 CEN |
| pKF11 | BUB1 URA3 2μm |
| pKF14 | PGAL>BUB1-5 URA3 CEN |
| pKF16 | PGAL>BUB1-5 HIS3 CEN |
| pKF17 | PGAL>BUB1 HIS3 CEN |
| pKF44 | BUB1 URA3 CEN |
| pKF48 | BUB1-5 URA3 CEN |
| pKF51 | PGAL>bub1-K733R URA3 CEN |
| pKF55 | BUB1-5 URA3 2μm |
| pKF60 | PGAL>bub1-5-K733R URA3 CEN |
| pRS316 | URA3 CEN |
| pSM217 | URA3 2μm |
| pTR134 | PGAL>BUB1 URA3 CEN |
Rich and minimal synthetic media were as described previously (23). To derepress galactose-inducible genes, cells were grown in 2% raffinose synthetic medium at 26°C for 24 h prior to induction by the addition of galactose to 2%. Derepression on solid medium was accomplished with glycerol-lactate plates (3% and 2%, respectively) prior to transfer to galactose medium. Cells were synchronized in G1 phase by the addition of α-factor (Bachem) to liquid medium, pH 4.0, to 4 to 6 μg/ml. Cells were synchronized in S phase by the addition of hydroxyurea (Sigma) to liquid medium, pH 5.8, to 0.1 M. To inhibit microtubule function in liquid cultures, nocodazole (Sigma) was added to 10 μg/ml. To inhibit microtubule function on solid media, benomyl (DuPont) was added to 10 μg/ml.
Isolation of BUB1-5.
Ten micrograms of plasmid pTR134 (PGAL>BUB1 [BUB1 overexpressed from promoter PGAL] URA3 CEN) was mutagenized (25) with hydroxylamine (Sigma) at 70°C for 1 h (1 M hydroxylamine, 50 mM sodium pyrophosphate [pH 7.0], 100 mM sodium chloride, and 2 mM EDTA) and then concentrated with Microcon filters (Amicon). To assess the degree of mutagenesis, treated pTR134 DNA was transformed into the pyrF Escherichia coli strain KC8. Uracil auxotrophs were found at a frequency of 4.7%. Mutagenized plasmids, amplified in E. coli DH5α, were transformed into the wild-type yeast strain MAY589 and plated onto solid minimal medium missing uracil. Developed colonies were then replica transferred to glycerol-lactate medium (missing uracil) and after 24 h were transferred again to minimal medium (missing uracil) containing either galactose or glucose. Mutants that exhibited poor growth on galactose, but not glucose, were chosen for further study. Only the BUB1-5 plasmid caused slow growth on galactose upon retesting.
DNA manipulations.
Standard DNA manipulation techniques were utilized to construct the various plasmids described in Table 1 (22). The BUB1-5 mutation was mapped by exchanging fragments of the wild-type and mutant BUB1 genes. Specifically, NotI-XbaI, XbaI-KpnI, and KpnI-BstXI fragments were used for this experiment. When exchanged into the wild-type PGAL>BUB1 plasmid pKF28, the 1.6-kb KpnI-BstXI 3′ region from BUB1-5 was found to confer slow growth on galactose medium. The reciprocal exchange of the wild-type KpnI-BstXI fragment into the PGAL>BUB1-5 plasmid pKF30 resulted in normal growth on galactose. The KpnI-BstXI region of BUB1-5 was sequenced, revealing a single mutation of G to A at nucleotide position 2352 (relative to the A in the initiation codon).
The bub1-5 K733R double mutant was created via a PCR-based method. Briefly, a BUB1 region downstream from K733R was amplified with primer A (CTACGCCAGTCAAAGTACGGTCTTGGATT), containing the BUB1-5 mutation, and 3′-located primer B (GTATGACGCCTGCTAATCC). The 421-bp product of this reaction was used as a primer along with 5′-located primer C (CGTTCACCTACAGTAACAGCT) to amplify a fragment that includes the bub1-K733R mutation.
Cytological techniques.
To observe DNA, cells were fixed with 70% ethanol on ice for 30 min and stained with DAPI (4′,6-diamidino-2-phenylindole). Samples were scored microscopically for cell and nuclear morphology. Cells were considered large budded if the diameter of the bud was about three-fourths of the diameter of the mother or larger. For immunofluorescence microscopy of tubulin structures, cells were fixed with formaldehyde and stained with the antitubulin antibody YOL1/34 (Harland Bioproducts) as described previously (19). Cells were observed with a Zeiss Axiovert inverted microscope with a 100× objective. A cooled, slow-scan CCD camera was used to capture digital images. Samples were fixed for electron microscopy in 2% phosphate-buffered glutaraldehyde at 26°C for 30 min, as described previously (1). Thin sections were analyzed with a JEOL 100S electron microscope. Flow-cytometric analysis of DNA content was performed as described previously (11) with an EPICS 752 flow cytometer.
Protein analysis.
Yeast protein extracts were prepared by beating cells with glass beads (diameter, 425 to 600 μm) in 50 mM Tris-HCl (pH 6.8) plus 0.6 mM phenylmethylsulfonyl fluoride. Cells were vortexed four times at the maximum speed for 1 min, with 1 min on ice in between. Lysate was separated from cell debris and glass beads via centrifugation for 5 min in the cold. An aliquot was reserved for protein concentration determination by the BCA assay (Pierce) to ensure equal loading of samples. One volume of 2× sodium dodecyl sulfate sample buffer was added to the lysate, which was then boiled. Samples were run on sodium dodecyl sulfate-polyacrylamide gels, and the proteins were transferred to polyvinylidene difluoride Immobilon-P (Millipore) membranes by standard techniques (22). Reagents for immunoblot analysis were obtained from Tropix. The manufacturer’s instructions were followed for the detection of proteins. Mad1p antibody was kindly provided by K. Hardwick and A. W. Murray. Clb2p antibody was a generous gift of D. Kellogg. The Bub1p antibody has been described elsewhere (20). Goat anti-rabbit secondary antibody was obtained from Jackson ImmunoResearch.
RESULTS
Identification of the dominant BUB1-5 allele.
The Bub1p protein kinase may be involved in generating or transmitting a signal indicating spindle damage. Defects in BUB1 or other spindle assembly checkpoint genes lead to inappropriate cell cycle progression in the presence of spindle damage. This implies that the spindle assembly checkpoint has the capacity to inhibit cycle progression when it senses damage. To gain a better understanding of this inhibitory mechanism we screened for a dominant BUB1 allele that would constitutively block progression through mitosis.
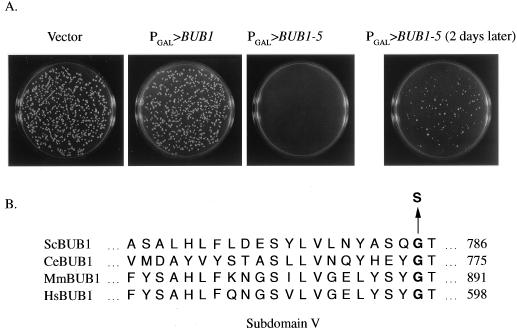
Overexpression of BUB1 from the galactose-inducible GAL1 promoter (PGAL>BUB1) caused only mild phenotypes (20) but did not block cell cycle progression or reduce growth rates (Fig. 1A and 2A). We mutagenized a PGAL>BUB1 centromere-containing plasmid (see Materials and Methods) and identified a mutant (designated BUB1-5) that caused slow growth of wild-type cells specifically on galactose-containing medium (Fig. 1A). Cells harboring the PGAL>BUB1-5 plasmid also exhibited reduced plating efficiency on galactose, relative to glucose, medium (44% for PGAL>BUB1-5 versus 100% for PGAL>BUB1 [Table 2]). A restriction fragment exchange experiment (see Materials and Methods) demonstrated that a mutation within a 1.6-kb 3′ region of BUB1 caused the slow-growth effect. Sequencing of this entire region revealed a single base pair mutation causing a change from glycine to serine at amino acid residue 785 within the Bub1p kinase domain (Fig. 1B). This position is located within protein kinase subdomain V, a region of weakly conserved amino acid sequence implicated in ATP binding (5). Gly785 is conserved in Caenorhabditis elegans, mouse, and human Bub1p homologs but not in other protein kinases.
FIG. 1.
BUB1-5 slows cell growth when overexpressed. (A) A wild-type strain (MAY589) was transformed with a PGAL plasmid vector (pBM272) or plasmids containing PGAL>BUB1 (pTR134) or PGAL>BUB1-5 (pKF14) and plated on galactose-containing medium. The plates in the left three panels were incubated at 26°C for 4 days. The panel on the right is the PGAL>BUB1-5 plate photographed after another two days at 26°C. (B) The BUB1-5 mutation changes a glycine to serine within the kinase domain. This position occurs within the kinase domain region designated as subdomain V and is conserved among known Bub1p homologs. Sc, S. cerevisiae; Ce, C. elegans; Mm, Mus musculus; Hs, Homo sapiens.
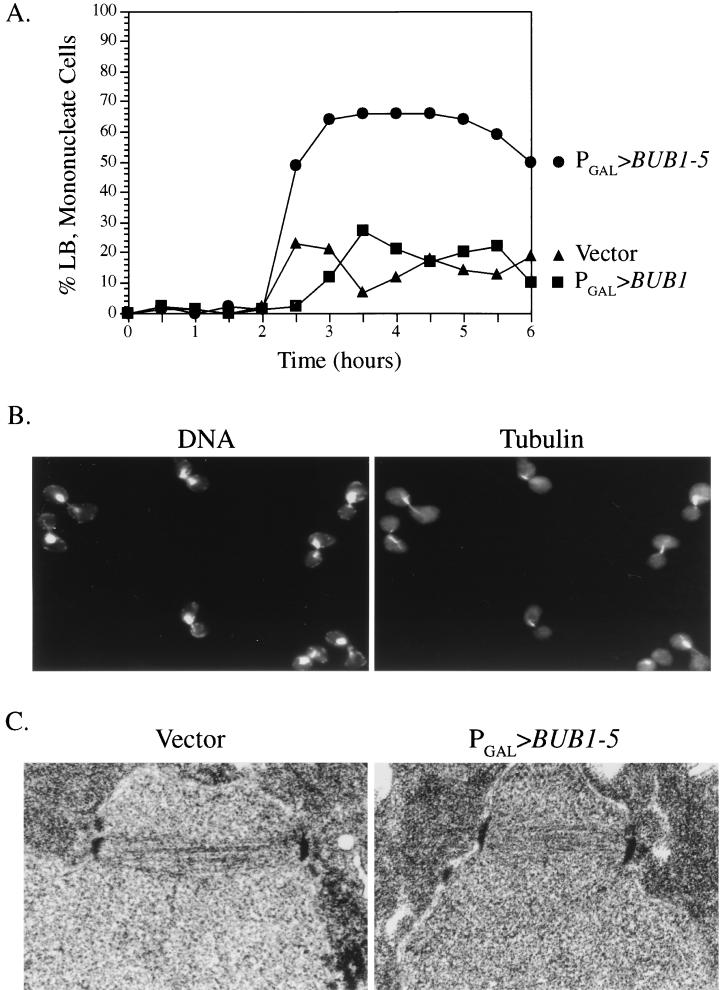
FIG. 2.
BUB1-5 overexpression causes a mitotic delay. (A) Wild-type cells carrying the indicated plasmid construct were synchronized with α-factor and released into galactose-containing medium at 26°C. At specific time points, samples were fixed, stained with DAPI, and observed by microscopy. The percentages of total cells with large-budded (LB) morphologies (diameter of bud cell, ≥75% of diameter of mother cell) and single nuclei are displayed. This morphology is characteristic of mitotic cells. ▴, vector plasmid; ▪, PGAL>BUB1 plasmid; •, PGAL>BUB1-5 plasmid. (B) Images of PGAL>BUB1-5 cells prepared as described for panel A and stained for DNA with DAPI and antitubulin with a specific antibody. (C) Thin-section electron micrograph of PGAL vector (left) and PGAL>BUB1-5 (right) cells fixed after growth in galactose for 3 h.
TABLE 2.
Plating efficiency of cells overexpressing BUB1-5 or BUB1
| Genotype | Plating efficiencya (%)
|
|
|---|---|---|
| BUB1-5 | BUB1 | |
| Wild type | 44 ± 2 | 100 ± 7 |
| mad1Δ | 10 ± 0.7 | 102 ± 0.7 |
| mad2Δ | 22 ± 6 | 98 ± 3 |
| mad3Δ | 38 ± 5 | 99 ± 1 |
| bub1Δ | 76 ± 0.7 | 111 ± 7 |
| bub2Δ | 26 ± 2 | 99 ± 1 |
| bub3Δ | 86 ± 0.7 | 101 ± 1 |
| mps1-3796 | 16 ± 3 | 104 ± 2 |
Values are derived as follows: (colonies on galactose medium/colonies on glucose medium) × 100 ± standard deviation at 26°C. Each value is the average of two or three trials.
BUB1-5 causes a delay in mitosis.
To determine the cause of the slow-growth phenotype, we examined the morphology of cells expressing the BUB1-5 allele. Cells harboring PGAL>BUB1-5, PGAL>BUB1, or PGAL vector only were synchronized in G1 with α-factor and released into galactose-containing medium to induce expression from PGAL. At intervals, samples were fixed, stained with DAPI, and observed by microscopy for cellular and nuclear morphology (Fig. 2A and B). The morphologies of all three strains were indistinguishable up until about 2.5 h postinduction. At this time, PGAL>BUB1-5 cells with large-budded (diameter of bud cell, ≥75% diameter of mother cell) morphologies and undivided nuclei began to accumulate in the culture. Cells with this morphology, characteristic of a mitotic block, peaked at around 67% of total. In contrast, large-budded mononucleate cells did not accumulate in the PGAL>BUB1 or PGAL vector only cultures, indicating that these cells were able to pass through mitosis unhindered. Antitubulin immunofluorescence microscopy revealed that the large-budded mononucleate PGAL>BUB1-5 cells possessed short bipolar spindles characteristic of cells blocked in mitosis prior to the onset of anaphase (Fig. 2B). As judged from images generated by thin-section electron microscopy, these spindles were indistinguishable from the short spindles found in cells not expressing BUB1-5 (Fig. 2C). Within the resolution limits of electron microscopy, therefore, BUB1-5 expression delayed cells at a point in mitosis following the assembly of normal-appearing bipolar spindles but prior to anaphase.
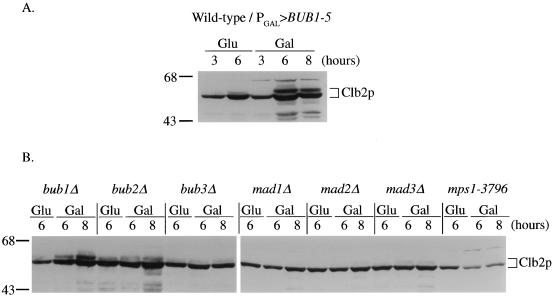
Cells in metaphase contain high amounts of the mitotic cyclin proteins, required for the activation of cyclin-dependent kinases (13). Entry into anaphase is accompanied by rapid degradation of these cyclins mediated by the anaphase-promoting complex (12). Induced PGAL>BUB1-5 cells were found to contain elevated levels of the mitotic cyclin Clb2p relative to uninduced cells, as determined by Western blot analysis (Fig. 3A). In particular, a slower-migrating form of Clb2p greatly accumulated in PGAL>BUB1-5 cells during incubation in galactose. This slower-migrating Clb2 form, specific to cells blocked in mitosis, has been observed by others (7). Cells overexpressing BUB1 did not exhibit elevated levels of Clb2p (data not shown).
FIG. 3.
BUB1-5 overexpression affects Clb2p mitotic cyclin levels. (A) Wild-type cells carrying the PGAL>BUB1-5 construct were grown in raffinose-containing medium and then switched to glucose (Glu) or galactose (Gal) medium for the indicated number of hours. Protein extracts were prepared and subjected to polyacrylamide gel electrophoresis and Western blot analysis with an antibody specific for the mitotic cyclin Clb2p. (B) The indicated checkpoint mutant cells, carrying the PGAL>BUB1-5 plasmid, were grown in galactose for the indicated number of hours and examined for Clb2p levels as in panel A.
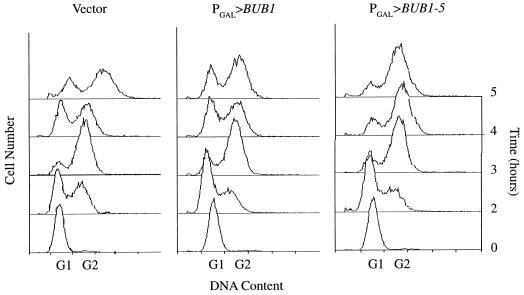
Flow-cytometric analysis of DNA content also demonstrated that PGAL>BUB1-5 cells were delayed in mitosis (Fig. 4). Following release from α-factor synchronization in late G1, PGAL>BUB1-5, PGAL>BUB1, and PGAL vector cells replicated their DNA. By 4 to 5 h after release, the PGAL>BUB1 and PGAL vector cells had undergone mitosis and cytokinesis, as indicated by the reappearance of cells with a G1 DNA content. In contrast, most of the PGAL>BUB1-5 cells still maintained their G2 DNA content, characteristic of a block to mitosis. Therefore, by three criteria—cell morphology, mitotic cyclin content, and DNA content—overexpression of BUB1-5 caused a block or delay to cell cycle progression in mitosis.
FIG. 4.
BUB1-5 overexpression causes arrest after DNA synthesis. Wild-type cells carrying the indicated construct were synchronized with α-factor and released into galactose-containing medium at 26°C. At the indicated time points, samples were prepared for flow-cytometric analysis of DNA content. Displayed is cell number as a function of DNA content. G1 and G2 roughly indicate the positions of cells with unreplicated and replicated DNA, respectively.
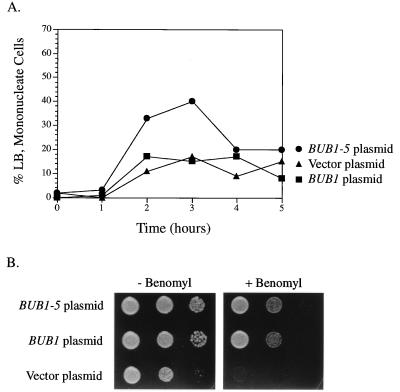
The phenotypes described above required that the BUB1-5 mutant form be expressed from the high-level galactose-inducible promoter. Expression of BUB1-5 from its normal promoter did not slow colony growth of wild-type cells (data not shown). This was observed for both low-copy (centromere-based) and high-copy (2μm-based) constructs. However, we did detect a slight delay in mitosis for cells expressing BUB1-5 from its normal promoter (on a centromere plasmid). Following synchronization with α-factor and release, more cells harboring a BUB1-5 plasmid with a mitotic morphology (large-budded and mononucleate) accumulated than did BUB1 or vector plasmid cells (Fig. 5A).
FIG. 5.
Normally expressed BUB1-5 causes a slight mitotic delay and complements bub1Δ. (A) Wild-type cells carry the indicated constructs that express BUB1 alleles from its normal promoter. These cells were synchronized with α-factor, released into galactose-containing medium, and examined for mitotic cells as described for Fig. 2A. A second trial of this experiment yielded a similar increase in the number of large-budded (LB) mononucleate cells for the BUB1-5 strain only. ▴, vector plasmid; ▪, BUB1 plasmid; •, BUB1-5 plasmid. (B) A bub1Δ strain was transformed with the indicated construct expressing BUB1 alleles from its normal promoter. These cells were spotted onto rich medium and the same medium containing 10 μg of benomyl per ml. Each spot to the right is a 1:50 dilution of the corresponding spot on the left. Plasmids used: BUB1, pKF44; BUB1-5, pKF48.
BUB1-5 provides BUB1 function.
As a first step toward determining the nature of the BUB1-5 defect, we examined its ability to provide normal BUB1 function. For these tests, we used a centromere plasmid that contained BUB1-5 expressed from its normal promoter. Cells deleted for BUB1 exhibit slow growth and sensitivity to the antimicrotubule agent benomyl. The BUB1-5 plasmid was able to rescue these phenotypes just as well as a BUB1 plasmid (Fig. 5B). bub1Δ is also lethal in combination with a deletion of CIN8, which encodes a nonessential kinesin-related mitotic motor (4). By standard genetic techniques, we were able to construct strains that were deleted for BUB1 and CIN8 but were viable due to the presence of the BUB1-5 plasmid (i.e., strain MAY5035). Therefore, by three criteria, BUB1-5 expressed from its normal promoter was able to provide BUB1-complementing activity.
Kinase activity is necessary for the BUB1-5 phenotype.
Bub1p is a protein kinase with the ability to phosphorylate itself, Bub3p, and possibly other proteins in vitro (20). The kinase activity of Bub1p is required for its checkpoint function and its ability to support a normal growth rate and benomyl resistance. The ability of BUB1-5 to complement bub1Δ indicated that it could provide some kinase activity. We investigated whether kinase activity was required for the Bub1-5p effect.
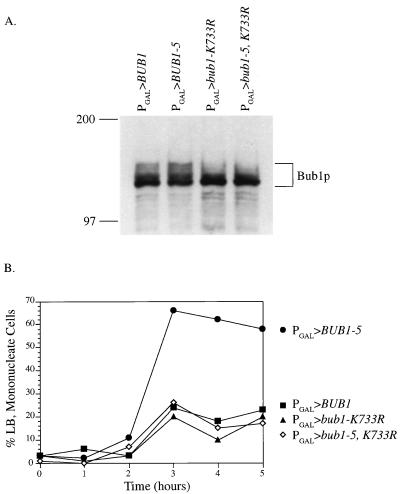
Lysine733 of Bub1p corresponds to an active-site residue conserved among the protein kinases. A Bub1p mutant form in which this lysine was changed to arginine (bub1-K733R) exhibited no kinase activity in vitro and a checkpoint defect in vivo (2a, 20). To examine the requirement for kinase function for the Bub1-5p effect, we created the bub1-5-K733R double mutant. When expressed from the galactose promoter, the amount of double mutant protein that accumulated in cells was similar to that of the wild type and either single mutant (Fig. 6A). Following α-factor synchronization and release into galactose medium, PGAL>BUB1-5 caused a delay in mitosis (Fig. 6B). In contrast, galactose-driven BUB1, bub1-K733R and bub1-5, K733R cells were not blocked. Therefore, a functional kinase domain is required for Bub1-5p to effect a cell cycle delay.
FIG. 6.
The BUB1-5 mitotic delay requires a functional kinase domain. (A) Wild-type cells carrying the indicated construct were induced in galactose for 4 h. Protein extracts were then prepared and subjected to gel electrophoresis and immunoblotting with Bub1p antibody. As previously described (20), multiple forms of Bub1p were observed. These have been determined to be due to different phosphorylation states (3b). (B) Wild-type cells carrying the indicated construct were synchronized with α-factor, released into galactose-containing medium, and examined for mitotic cells as described for Fig. 2A. The K733R mutation inactivates kinase function. ▪, PGAL>BUB1 plasmid; •, PGAL>BUB1-5 plasmid; ▴, PGAL>bub1-K733R plasmid; ◊, PGAL>bub1-5, K733R plasmid.
Mad1p protein does not become hyperphosphorylated when BUB1-5 is overexpressed.
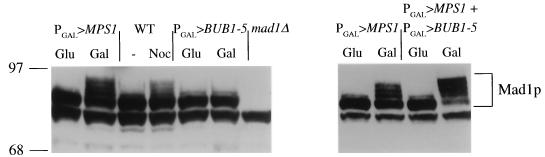
Activation of the spindle assembly checkpoint by microtubule disruption or mutational inactivation of spindle pole body duplication or kinetochore structure results in the appearance of hyperphosphorylated forms of the Mad1p checkpoint protein (3a, 6, 7). This modification is dependent upon BUB1, BUB3, and MAD2 and is believed to be mediated by the protein kinase product of MPS1 (6, 7). Using Mad1p-specific antibodies and Western blot analysis, we examined whether overexpression of BUB1-5 would lead to the appearance of hyperphosphorylated forms of Mad1p. Extracts from cells overexpressing BUB1-5 or MPS1, as well as wild-type cells treated with the microtubule-disrupting agent nocodazole, were compared (Fig. 7). The hyperphosphorylated forms of Mad1p migrate more slowly in polyacrylamide gels (6). Slower-migrating forms of Mad1p were clearly induced in PGAL>MPS1 cells treated with galactose or wild-type cells treated with nocodazole. These slower-migrating forms were not evident in extracts from PGAL>BUB1-5 cells, however. The MAD1-specific bands were unchanged in comparisons of PGAL>BUB1-5 cells grown in glucose versus those grown in galactose. Therefore, unlike other treatments that block S. cerevisiae cells in mitosis, the mitotic delay mediated by BUB1-5 did not result in a detectable elevation in Mad1p phosphorylated forms. To rule out the possibility that BUB1-5 overexpression inhibited the phosphorylation of Mad1p, PGAL>MPS1 cells were transformed with the PGAL>BUB1-5 plasmid. Overexpression of both MPS1 and BUB1-5 resulted in the appearance of hyperphosphorylated Mad1p forms. Under these conditions, more Mad1p reproducibly appeared to be shifted to the slower-migrating forms than resulted from overexpression of MPS1 only. Therefore, BUB1-5 overexpression enhanced but did not inhibit Mad1p phosphorylation mediated by overexpression MPS1.
FIG. 7.
BUB1-5 overexpression does not lead to Mad1p hyperphosphorylation. Wild-type cells carrying the indicated construct(s) were grown in glucose (Glu)- or galactose (Gal)-containing medium for 5 h or treated with 10 μg of nocodazole per ml (in glucose medium) for 4 h. Protein extracts were analyzed by gel electrophoresis and Western blotting probed with an antibody specific for Mad1p. To help identify Mad1p-specific bands, a mad1Δ strain was also examined. We detected no difference in Mad1p forms between PGAL>BUB1-5-containing cells grown in glucose or galactose even when the film was greatly overexposed.
The BUB1-5 mitotic delay depends upon the presence of other mitotic checkpoint genes.
The ability of overexpressed BUB1-5 to mimic aspects of a spindle assembly checkpoint-induced cell cycle delay allowed us to examine where in this pathway the Bub1p kinase acts. We would expect that loss of function of a checkpoint pathway component that acts downstream of Bub1p, or in concert with Bub1p, would eliminate the ability of Bub1-5p to delay the cell cycle in mitosis. However, a component that acts upstream or in a different pathway from Bub1p would not be required for the Bub1-5p effect.
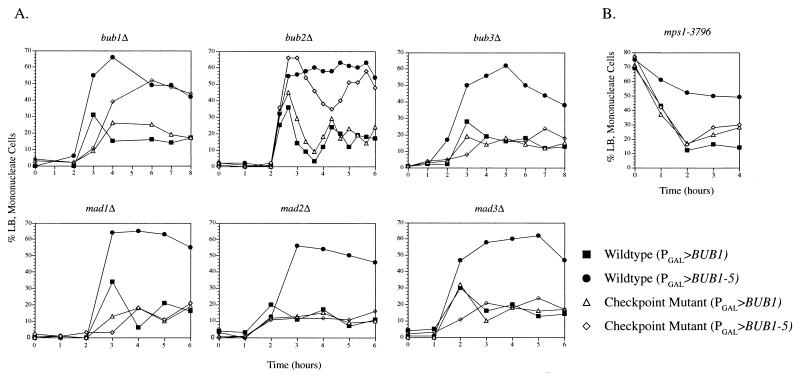
We transformed the PGAL>BUB1-5 and PGAL>BUB1 plasmids into strains deleted for either BUB1, BUB2, or BUB3 or MAD1, MAD2, or MAD3. Following α-factor synchronization and release into galactose-containing medium, we compared the effects of BUB1 and BUB1-5 overexpression between each mutant and a wild-type strain (Fig. 8A). As we previously observed for the wild type, overexpression of BUB1 did not cause a mitotic delay in any of the mutants. We found that deletion of the chromosomal BUB1 gene did not eliminate the Bub1-5p-induced delay, demonstrating that wild-type Bub1p was not required for the effect caused by Bub1-5p. In contrast, deletion of BUB3, MAD1, MAD2, and MAD3 eliminated the cell cycle delay caused by overexpressed BUB1-5. The effect of deleting BUB2 was intermediate between those of the wild type and the BUB3 and MAD deletions. Although bub2Δ (PGAL>BUB1-5) cells accumulated in mitosis at a high level, the peak was not sustained over time as was observed for wild-type cells. The peak of mitotic cells fluctuated down and then back up again during the 6-h observation period. This bub2Δ fluctuation effect was replicated in seven different experiments. In the example displayed in Fig. 8A, samples were taken at closely spaced time points in order to better document this behavior. This finding suggests that although BUB2 may not be required for the initial delay caused by BUB1-5, it may be required for normal maintenance of the delay.
FIG. 8.
The BUB1-5 mitotic delay requires the other spindle assembly checkpoint genes. (A) Cells with the indicated checkpoint mutation and carrying the PGAL>BUB1 or PGAL>BUB1-5 construct were synchronized with α-factor, released into galactose-containing medium, and examined for mitotic cells, as described for Fig. 2A. (B) The temperature-sensitive mps1-3796 mutant was transformed with the PGAL>BUB1 or PGAL>BUB1-5 construct. These cells were synchronized in S phase with hydroxyurea at 26°C and were switched to galactose-containing medium (plus hydroxyurea) for 3 h to induce BUB1 expression. This was followed by release from the hydroxyurea block by transfer into the same medium at 33°C. Passage of the cells through mitosis was evidenced by the reduction of large-budded (LB) mononucleate cells in the culture. The experiments in this figure were repeated 2 (mad2Δ), 3 (bub1Δ, mad1Δ), 4 (mad3Δ), 5 (bub3Δ, mps1-3796), and 10 (bub2Δ) times, always yielding the same results. Mutant strains: bub1Δ, MAY4537; bub2Δ, MAY2055; bub3Δ, MAY4574; mad1Δ, MAY3728; mad2Δ, MAY4428; mad3Δ, MAY4612; mps1-3796Δ, AS131-2d.
MPS1 is an essential gene. To test the requirement for MPS1 in the BUB1-5-induced mitotic delay, we transformed the PGAL>BUB1-5 and PGAL>BUB1 plasmids into a temperature-sensitive mps1-3796 mutant. The Mps1p kinase has two roles, a spindle pole duplication function and a spindle assembly checkpoint function. In this experiment, we synchronized the cells with the DNA synthesis inhibitor hydroxyurea. This inhibitor blocks the S. cerevisiae cell cycle after the execution point for the Mps1p spindle pole duplication function but before its execution point for checkpoint function (29). Hydroxyurea-arrested cells (at 26°C) were switched to galactose-containing medium (plus hydroxyurea) for 3 h to induce BUB1 expression. This was followed by release from the hydroxyurea block by transfer into the same medium but at a temperature nonpermissive for mps1-3796 (33°C). Passage of the cells through mitosis was evidenced by the reduction of large-budded mononucleate cells in the culture (Fig. 8B). Under these conditions, PGAL>BUB1-5 was able to delay progression through mitosis in the wild-type cells but not the mps1-3796 mutant cells. Once again, overexpression of BUB1 had no effect in either genetic background. Therefore, MPS1 function is required for the mitotic delay caused by overexpressed BUB1-5.
In the PGAL>BUB1-5 experiments described in this section, we also monitored levels of the mitotic cyclin Clb2p as an independent assay for mitotic delay (Fig. 3B). In strains for which we observed a BUB1-5-induced delay (wild type and bub1Δ), a corresponding increase in Clb2p levels was observed after the switch to galactose medium. In strains that were immune to the BUB1-5-induced delay (bub3Δ, mad1Δ, mad2Δ, and mad3Δ), Clb2p levels remained low and unchanged during galactose incubation. The fluctuating BUB1-5-induced delay we observed for the bub2Δ strain was accompanied by Clb2p accumulation that was somewhat lower than that observed for the wild-type and bub1Δ strains.
While checkpoint gene mutations were able to eliminate the mitotic delay caused by PGAL>BUB1-5, none were able to rescue the slow growth caused by PGAL>BUB1-5 (although the BUB1 and BUB3 deletions caused slow growth on their own). In some cases, the plating efficiency of PGAL>BUB1-5 cells on galactose was reduced further by the checkpoint mutations (Table 2). A strong effect was caused by mad1Δ and mad2Δ, which, interestingly, also most strongly reduced the viability of cells overexpressing MPS1 (7). The reasons for these plating efficiency differences are not clear and may reflect complex interactions. For example, some of the checkpoint gene products may act in more than one functional pathway. Overexpression of BUB1 had no effect on the plating efficiency of any checkpoint mutant or of the wild type.
DISCUSSION
Eukaryotic cells that have incurred mitotic spindle damage are delayed or arrested prior to anaphase onset by the actions of the spindle assembly checkpoint. Recessive loss of function of the S. cerevisiae checkpoint gene BUB1 eliminates this arrest. We have identified a dominantly acting BUB1 allele that produces a phenotype that appears to be the opposite of loss of function. High-level expression of BUB1-5 delayed yeast cells in mitosis at a stage following bipolar spindle assembly but prior to anaphase spindle elongation. Characteristic of cells blocked at this stage, the intracellular level of the Clb2p mitotic cyclin was elevated. Continued high-level expression of BUB1-5 caused a reduction in growth rate but not lethality. These phenotypes required both a change of a conserved amino acid residue within the Bub1p kinase domain (glycine785) and high-level expression of the gene product. The single changes individually produced only subtle phenotypic effects, while the combination elicited a strong response.
The finding of a BUB1 allele that can delay cells in mitosis lends strong support to the hypothesis that Bub1p participates in spindle assembly checkpoint function. Our previous conclusions regarding BUB1 were based upon loss-of-function mutants that inappropriately disjoin sister chromatids, rebud, and reinitiate DNA replication in the presence of spindle damage (10, 20, 26). However, the apparent aberrant cycling of these mutants is not dramatic. For example, rebudding occurs efficiently only once and with slow kinetics. In addition, bub1 mutant cells grow poorly and are difficult to study due to aneuploidy. The conclusion that BUB1 encodes a checkpoint function was tempered by these problems. Our findings here indicate that the Bub1p kinase has the capacity to inhibit cell cycle progression in mitosis prior to anaphase onset, precisely the role expected for a spindle assembly checkpoint function.
A possible explanation for the BUB1-5 effect is that it encodes an overactive kinase. Overphosphorylation of a Bub1p target may mimic the spindle assembly checkpoint activation normally caused by spindle damage. Consistent with this hypothesis was our finding that the BUB1-5 phenotypes were eliminated by a second mutation that kills kinase function. Therefore, kinase activity is required for the BUB1-5 effect. Nevertheless, we have not observed elevated kinase activity for Bub1-5p in vitro. Immune complexes precipitated from cells overexpressing BUB1-5 were actually reduced in the ability to transfer labeled phosphate to both Bub1-5p and Bub3p compared to complexes from BUB1-overexpressing cells (3a). The significance of this in vitro finding with respect to the in vivo activity of Bub1p is not clear, however. We can conclude that altered kinase function is probably responsible for the BUB1-5 effect but are unsure of the nature of this change.
We found that the mitotic delay induced by BUB1-5 required the activities of other identified spindle assembly checkpoint genes (BUB2, BUB3, MAD1, MAD2, MAD3, and MPS1). Therefore, two possibilities, which are not mutually exclusive, may account for the BUB1-5-induced mitotic delay. (i) High levels of Bub1-5p may activate the spindle assembly checkpoint. The requirement for the remaining checkpoint components may indicate that Bub1p acts upstream or in a manner dependent upon their functions (see below). (ii) High levels of Bub1-5p may cause mitotic spindle damage. The remaining spindle assembly checkpoint components may therefore be required to respond to this damage. It is currently impossible to completely rule out one of these explanations. Nonetheless, we favor the checkpoint activation hypothesis for the following reasons. (i) Observations of spindles in PGAL>BUB1-5 cells by immunofluorescence and electron microscopy did not reveal any obvious defects. Of course, we cannot rule out damage that was not detectable by microscopy. (ii) Although PGAL>BUB1-5 greatly reduced cell growth rates, colony-forming ability was high (only reduced by a factor of 2). In addition, we found that PGAL>BUB1-5 cells maintained high viability following as much as 6 h in galactose-containing medium (3a). This indicates that any damage caused by Bub1-5p must be either reversible or not severe enough to disrupt the fidelity of chromosome segregation required for viability. (iii) We failed to observe the hyperphosphorylated forms of Mad1p that are characteristic of damaged spindles (6).
Components of the spindle assembly checkpoint may act in a pathway or complex.
The preanaphase delay caused by overexpression of either BUB1-5 or MPS1 requires the functions of all of the other spindle assembly checkpoint gene products (reference 7 and this study). This supports, but does not prove, the simple hypothesis that they all work in a common pathway. The common pathway hypothesis is complicated by the finding that loss of function of these checkpoint genes causes different phenotypes. For example, loss of BUB1 or BUB3 causes very slow growth while loss of BUB2 and the three MAD genes causes little change in the growth rate (10, 15, 20). In addition, MPS1 performs a function essential for spindle pole body duplication beyond its checkpoint role (31). Therefore, multiple roles or pathway participation for some of these gene products seems likely.
A simple linear pathway relationship that can explain some of the findings concerning the spindle assembly checkpoint gene products has been proposed (30). However, not all observations concerning this checkpoint (references 6 and 7 and this study) are easily accommodated by this linear model. An equally likely possibility is that many of the checkpoint proteins are commonly involved in a protein complex whose function depends upon the presence of all complex members. Vertebrate homologs of Mad2p and Bub1p are localized to kinetochores (2, 16, 27). In S. cerevisiae, Mad1p physically interacts with Mad2p and is phosphorylated by Mps1p (7, 21) and Bub3p physically interacts with Bub1p (20). Therefore, a kinetochore-localized spindle damage-signaling complex, constructed of many of the spindle assembly checkpoint proteins, seems reasonable.
Bub1p and Mps1p appear to act at a closely related early step in the checkpoint mechanism. First, we note the similarities in the effects caused by overexpression of BUB1-5 and MPS1. Both caused a preanaphase delay dependent upon the other checkpoint genes. Cells delayed with either treatment exhibited elevated Clb2p levels. Both caused increased lethality when overexpressed in mad1Δ and mad2Δ cells. A major difference, however, is that overexpression of MPS1 led to Mad1p hyperphosphorylation while overexpression of BUB1-5 did not. Most significantly, we found that the mitotic delay caused by overexpressed BUB1-5 was dependent upon MPS1 function. Conversely, it has been reported that the mitotic delay caused by overexpressed MPS1 is dependent upon BUB1 function (7). We verified this finding by monitoring the effect of overexpression of MPS1 in BUB1 and bub1Δ cells. In agreement with Hardwick et al., we found that deletion of BUB1 eliminated the delay caused by high levels of MPS1 (corroborative data not shown). These findings indicate that the functions of Bub1p and Mps1p are interdependent (at least with respect to mitotic delay). This may mean that Bub1p and Mps1p actions are both required for the same step in checkpoint activation or that the function of one activates the function of the other.
The hyperphosphorylated forms of Mad1p that appear after spindle damage suggested that Mad1p modification may be an important step in activating the spindle assembly checkpoint (6). While it is possible that hyperphosphorylated forms of Mad1p may be a good indicator of spindle damage, Mad1p hyperphosphorylation is not always correlated with mitotic delay. We found that mitotic delay could be induced in cells by BUB1-5 overexpression without resulting in detectable Mad1p hyperphosphorylation. The opposite situation has also been observed: Mad1p can be hyperphosphorylated in cells that nevertheless are not delayed in mitosis (i.e., in bub1Δ cells in which MPS1 is overexpressed [7]). The ability of BUB1-5 to cause mitotic delay dependent upon MAD1 but without leading to Mad1p hyperphosphorylation may indicate that hyperphosphorylated Mad1p is not an intermediate in spindle damage signal transduction.
The relationships of Bub2p and Mad3p with the other checkpoint proteins is still somewhat unclear. bub2 and mad3 mutants exhibit less-severe phenotypes than the other checkpoint mutants (10, 15, 18, 28). Neither is required for Mad1p hyperphosphorylation, although both are required for the normal mitotic delay caused by BUB1-5 or MPS1 overexpression. It is possible that these perform a downstream function or act in a different pathway that is nonetheless required for normal response to spindle damage. Unlike the other checkpoint mutants, we found that the bub2Δ mutant was still delayed by overexpression of BUB1-5. However, unlike wild-type cells, bub2Δ cells were unable to maintain the delay. In the bub2Δ cells, the delay appeared to be relieved and then cyclically reinstated. Therefore, it is possible that Bub2p is not required for the establishment of the activated checkpoint delay but is required to properly maintain the delay (also see reference 18).
In summary, we have found a way to activate the spindle assembly checkpoint in cells with apparently normal spindles. Just as ectopic checkpoint activation has aided our definition of the S. cerevisiae spindle assembly checkpoint mechanism, so could similar approaches be used to study checkpoint regulation in mammalian cells. Loss of checkpoint regulation has been correlated with oncogenic transformation (3, 8, 24). Ectopic checkpoint activation, therefore, might also be exploited to prevent unrestrained proliferation of cancer cells.
ACKNOWLEDGMENTS
We gratefully thank Kevin Hardwick, Doug Kellogg, Andrew Murray, and Mark Winey for strains, DNA constructs, and antibodies; Mike Sepanski for assistance with electron microscopy; and Frank Cottingham, Cindy Dougherty, Emily Hildebrandt, and Doug Koshland for comments on the manuscript.
This study was supported by National Institutes of Health grant GM49363 to M.A.H.
REFERENCES
- 1.Byers B, Goetsch L. Preparation of yeast cells for thin-section electron microscopy. Methods Enzymol. 1991;194:602–608. doi: 10.1016/0076-6879(91)94044-d. [DOI] [PubMed] [Google Scholar]
- 2.Chen R, Waters J C, Salmon E D, Murray A W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- 2a.Dougherty, C., and M. A. Hoyt. Unpublished observations.
- 3.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 3a.Farr, K. A., and M. A. Hoyt. Unpublished observations.
- 3b.Farr, K. A., C. Dougherty, and M. A. Hoyt. Unpublished observations.
- 4.Geiser J R, Schott E J, Kingsbury T J, Cole N B, Totis L J, Bhattacharyya G, He L, Hoyt M A. S. cerevisiae genes required in the absence of the CIN8-encoded spindle motor act in functionally diverse mitotic pathways. Mol Biol Cell. 1997;8:1035–1050. doi: 10.1091/mbc.8.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanks S K, Hunter T. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 6.Hardwick K G, Murray A W. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol. 1995;131:709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardwick, K. G., E. Weiss, F. C. Luca, M. Winey, and A. W. Murray. 1996. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. 273:953–956. [DOI] [PubMed]
- 8.Hartwell L H, Kastan M B. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 9.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 10.Hoyt M A, Totis L, Roberts B T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 11.Hutter K J, Eipel H E. Flow cytometric determinations of cellular substances in algae, bacteria, molds and yeast. Antonie Leeuwenhoek J Microbiol Serol. 1978;44:269–282. doi: 10.1007/BF00394305. [DOI] [PubMed] [Google Scholar]
- 12.King R W, Deshaies R J, Peters J, Kirschner M W. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 13.King R W, Jackson P K, Kirschner M W. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 14.Lauzé E, Stoelcker B, Luca F C, Weiss E, Schutz A R, Winey M. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO J. 1995;14:1655–1663. doi: 10.1002/j.1460-2075.1995.tb07154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R, Murray A W. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 17.Nicklas R B. How cells get the right chromosomes. Science. 1997;275:623–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 18.Pangilinan F, Spencer F. Abnormal kinetochore structure activates the spindle assembly checkpoint in budding yeast. Mol Biol Cell. 1996;7:1195–1208. doi: 10.1091/mbc.7.8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pringle J R, Adams A E M, Drubin D G, Haarer B K. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- 20.Roberts B T, Farr K A, Hoyt M A. The Saccharomyces cerevisiae checkpoint gene BUB1 encodes a novel protein kinase. Mol Cell Biol. 1994;14:8282–8291. doi: 10.1128/mcb.14.12.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudner A D, Murray A W. The spindle assembly checkpoint. Curr Opin Cell Biol. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1983. [Google Scholar]
- 24.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 25.Sikorski R S, Boeke J D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 26.Straight A, Belmont A, Robinett C C, Murray A W. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–1608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- 27.Taylor S S, McKeon F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell. 1997;89:725–735. doi: 10.1016/s0092-8674(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Burke D J. Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6838–6844. doi: 10.1128/mcb.15.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss E, Winey M. The Saccharomyces cerevisiae Spindle Pole Body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells W A E. The spindle-assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]
- 31.Winey M, Goetsch L, Baum P, Byers B. MPS1 and MPS2: novel yeast genes defining distinct steps of Spindle Pole Body duplication. J Cell Biol. 1991;114:745–754. doi: 10.1083/jcb.114.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]