Abstract
Purpose of Review
This review focuses on the challenges of diagnosing and treating spontaneous intracranial hypotension (SIH), a condition caused by spinal CSF leakage. It emphasizes the need for increased awareness and advocates for early and thoughtful use of empirical epidural blood patches (EBPs) in suspected cases.
Recent Findings
SIH diagnosis is hindered by variable symptoms and inconsistent imaging results, including normal brain MRI and unreliable spinal opening pressures. It is crucial to consider SIH in differential diagnoses, especially in patients with connective tissue disorders. Early EBP intervention is shown to improve outcomes.
Summary
SIH remains underdiagnosed and undertreated, requiring heightened awareness and understanding. This review promotes proactive EBP use in managing suspected SIH and calls for continued research to advance diagnostic and treatment methods, emphasizing the need for innovative imaging techniques for accurate diagnosis and timely intervention.
Introduction
Spontaneous intracranial hypotension (SIH) is a neurologic disorder caused by noniatrogenic leakage of CSF through a spinal dural defect, ruptured meningeal diverticulum, or CSF-venous fistula (CVF). SIH profoundly degrades health-related quality of life and severely affects ability to maintain partner relationships.1,2 One report noted that SF-36 quality-of-life scores were lower than those reported for patients with multiple sclerosis, measures of spiritual well-being were worse than those reported among patients with cancer or AIDS, and nearly a quarter of patients with SIH symptoms described having wished they were dead.2 This is a true crisis in the life of those afflicted. In addition, patients with chronic SIH are at risk of long-term complications including cerebral venous sinus thrombosis, superficial siderosis, and bibrachial amyotrophy.3-5 Prompt diagnosis and treatment dramatically improves the quality of life and health outcomes of these patients.
Recognition and diagnosis of SIH remain challenging because of both imaging and clinical factors. At least 19% of patients with SIH have normal-appearing brain MRI, and brain MRI changes in chronic SIH may disappear over time, despite persistence of the leak.6,7 Low opening pressure (OP) is diagnostically unreliable because most of the patients with SIH have OP that is within the normal range.8,9 Even if suspected, localizing spinal CSF leaks and CVF require sophisticated imaging techniques that are not widely available.10
Epidural blood patches (EBPs) are widely available, effective treatments of SIH with minimal associated risk.6,11 However, because of lack of familiarity with SIH or uncertainty regarding the risk vs benefits in patients without a certain diagnosis, many clinicians who perform EBP may be reluctant to do so in patients who do not have documented low OP, a spinal epidural fluid collection, or obvious imaging features of SIH.
This review was written by a panel of experts derived from the fields of neurology, neurosurgery, and neuroradiology who have extensive experience in the treatment of SIH and are privy to the current obstacles to care. This experience was synthesized with current literature regarding diagnosis and treatment of SIH to both advocate for the patient with known or suspected SIH and provide the reader with a panoramic view of evidence-based best practices for the management of this condition.
Epidemiology and Risk Factors
SIH is mischaracterized as “rare” and may thus not routinely be considered in the differential diagnosis of a patient with headache. However, recent data indicate that SIH occurs more frequently than previously thought, with an incidence ranging from 3.8 to 5 per 100,000.12,13 For perspective, the incidence of esophageal cancer and spontaneous subarachnoid hemorrhage are 4.6 and 8 per 100,000, respectively. These data indicate that SIH occurs with a frequency such that its presentation in a busy medical center should be expected and the diagnosis routinely considered.
SIH is reported more frequently in women and individuals with a hereditary disorder of connective tissue (HDCT) such as Marfan syndrome, Ehlers-Danlos syndrome, or joint hypermobility syndrome.14 Because HDCTs are a risk factor, further assessment for these can help inform clinical suspicion of SIH, especially in those with atypical symptoms.
Precipitating Factors
Most patients with SIH cannot identify any definite precipitating event. In a study of 80 consecutive patients with SIH, only 28 indicated an event coinciding with the onset of symptoms, and most of these were atraumatic.15 Table 1 lists common inciting events.
Table 1.
Inciting Events
| Stretching |
| Yoga and Pilates |
| Valsalva maneuver associated with constipation |
| Weightlifting |
| Protracted coughing |
| Vomiting |
| Intercourse |
| Bending over to pick up something heavy |
| Chiropractic manipulation |
| Roller-coaster rides |
| Whiplash |
Challenges in the Clinical Diagnosis of SIH
Correctly diagnosing SIH is challenging because of both clinical and imaging factors. In a study of 18 patients with positional headaches and SIH confirmed by CT myelogram, only one was diagnosed with SIH by the first physician consulted.16
Non-Orthostatic Headache
Nearly all patients with SIH experience positional head pain at some point during their course, typically initially. However, headaches experienced with chronic SIH often lack a clear “orthostatic” pattern and may be accompanied by other symptoms.6,17 One study of 90 patients with SIH found that 1% had no headache, 24% had non-positional headache, and 16% had a headache that was orthostatic but took between 1/4 and 2 hours of being upright to manifest. Only 59% had an orthostatic headache within 15 minutes of sitting or standing.18 Thus, orthostatic head pain is useful when present, but does not exclude SIH when absent.19 Further complicating the clinical picture are reports of SIH associated with headaches described as non-positional, exertional, cough-triggered, thunderclap onset, and even paradoxical (i.e., headache worse when flat).20,21 Patients with otherwise compelling clinical circumstances should not be precluded from an evaluation or empiric treatment on the basis of a non-orthostatic headache.
Symptom Heterogeneity
Heterogeneity in the type and severity of symptoms may confound the diagnosis of SIH. While headache is by far the most common presenting manifestation of SIH, some patients with radiologically proven SIH present without headache but have other neurologic symptoms (Table 2).6 Non-headache symptoms may not be recognized as orthostatic or may only become prominent with prolonged upright time. Given the wide-ranging nature of symptoms, SIH should be considered in the differential diagnosis for persistent headaches and other constellations of symptoms.
Table 2.
Non-Headache Neurologic Symptoms in SIH
| Neck or interscapular pain |
| Nausea |
| Vestibulocochlear dysfunction (tinnitus, dizziness, vertigo, imbalance, or hearing changes) |
| Aural fullness/ear “popping” |
| Blurred vision |
| Photophobia |
| Elevated prolactin and galactorrhea (from traction on the pituitary stalk) |
| Cognitive difficulties (ranging from “brain fog” to frontotemporal dementia) |
| Tremor |
| Dysarthria |
| Dysphagia |
| Parkinsonism (gait disturbance, tremor, bradykinesia) |
| Fatigue |
Situations in Which SIH Should Be Considered in the Differential Diagnosis
Postural orthostatic tachycardia syndrome (POTS) is characterized by orthostatic intolerance, a high prevalence of headache (both orthostatic and non-orthostatic), and connective tissue disease as a predisposing factor.22 Headache, typically migrainous, is the most common comorbidity in POTS.23 Tilt-table testing is used in clinical practice to diagnose POTS.23 However, the overlapping positional/orthostatic components and similar results during tilt-table testing in both syndromes may cause misdiagnosis. It is of interest that one study comparing postural heart rate change among patients with POTS with patients with SIH found no difference in postural increase in heart rate in those conditions.22 This deserves further research, and patients presenting with clinical features of POTS should also have SIH considered in the appropriate context.
SIH should also be considered in patients with chronic headaches refractory to treatment. A significant percentage of patients with SIH also have comorbid chronic migraine, and SIH is a secondary cause of new daily persistent headache.24,25
SIH is frequently misdiagnosed as Chiari I malformation because they can share a similar imaging finding of cerebellar tonsillar ectopia.26 However, in SIH, the cerebellar tonsils and posterior fossa structures caudally migrate because of a CSF leak, whereas in a Chiari I malformation, displacement of the cerebellar tonsils through the foramen magnum is due to an abnormally small posterior fossa. These imaging features are easily confused, although they can often be distinguished by careful neuroradiologic scrutiny of brain MRI.26 Thus, SIH should be considered in patients before undergoing suboccipital craniectomy because a posterior fossa decompression will not fix and may worsen SIH. Finally, the manifestations of SIH are similar to those of post-concussion syndrome or mild traumatic brain injury with cervical involvement. It is important to distinguish these conditions after a head injury as trauma may precipitate a CSF leak.
The Role of Lumbar Puncture: Opening Pressure and CSF Analysis
Lumbar Puncture Opening Pressure
Lumbar puncture (LP) OP does not reliably distinguish patients with SIH from those without, despite its presence in the International Headache Society ICHD-3 diagnostic criteria.8,9 A low OP should indeed prompt an evaluation of SIH as should a normal or elevated OP when the clinical features support the diagnosis. Several studies have shown OP to be within the normal range in most patients with a confirmed leak, particularly those with a longer duration of symptoms.8,9,19 Combined with the possibility of worsening an ongoing leak by performing the procedure, we advise against LP solely to measure OP or relying on OP to guide diagnosis or treatment.
Radiologic Assessment of the Brain and Spine
Brain Imaging
Contrast-enhanced brain MRI should be obtained in patients with suspected SIH. Brain MRI findings in SIH include smooth pachymeningeal enhancement, brain sagging, subdural fluid collections, venous distension, and pituitary enlargement (Figure 1). A recent meta-analysis estimated that approximately 80% of patients with SIH have at least one MRI brain abnormality within a month of symptom onset.6 However, pachymeningeal enhancement, a prominent brain MRI finding in SIH, may resolve over time.7 This, and the lack of a gold standard against which to compare imaging, may explain why estimates of brain imaging sensitivity vary widely. A study of 250 patients with suspected CSF leak found that 186 patients (74%) showed evidence of CSF leak on radionuclide cisternography. Among those, a sagging brain was observed in only 21 (13%) of the 159 patients with sagittal MRIs, and only 1 of the 101 patients with gadolinium contrast-enhanced images demonstrated pachymeningeal enhancement.27
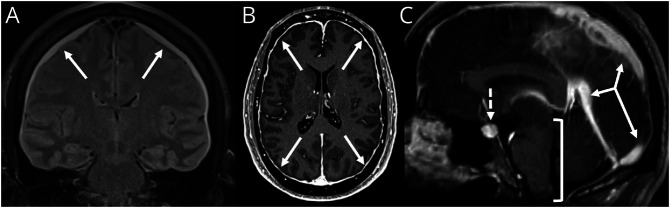
Figure 1. Classic “SEEPS” MRI Findings in Spontaneous Intracranial Hypotension.
(A) Coronal T2 FLAIR image demonstrates bilateral subdural fluid collections (arrows). (B) Axial T1 postcontrast MRI demonstrates smooth circumferential pachymeningeal enhancement. (C) Sagittal T1 postcontrast MRI demonstrates venous engorgement (solid arrows), pituitary engorgement (dashed arrow), and sagging of the brainstem (bracket).
A nuanced, probabilistic scoring system (Bern Score) has demonstrated predictive ability to subsequently identify a leak or CVF on myelography.8,28,29 Using values assigned to both qualitative imaging features and quantitative measurements, this score categorizes a patient as having low, moderate, or high probability of finding a CSF leak or CVF (Table 3). One study found a significant number of patients with SIH whose MRI reports initially indicated no evidence of SIH actually had either moderate or high probability according to the Bern Score.30 Therefore, we encourage routine application of this score to brain MRIs in patients who are suspected of having SIH. However, even when the Bern Score indicates low probability, a patient should not be denied appropriate workup and treatment if there is a sufficiently high clinical suspicion because leaks and CVF occur in patients with “normal” brain MRI (Figure 2). One study documented that 10% of patients with an orthostatic headache and normal brain and spine MRI have a fixable CVF detected with specialized lateral digital subtraction myelography (DSM).31
Table 3.
Bern Score
| Pachymeningeal enhancement | Venous engorgement | Suprasellar narrowing (≤4 mm) | Subdural collection | Prepontine narrowing (≤5 mm) | Mamillopontine narrowing (≤6.5 mm) |
| 2 points | 2 points | 2 points | 1 point | 1 point | 1 point |
Each finding is assigned a point value. The sum of the points reflects the probability to localize a CSF leak on subsequent myelography. 2 or less points is low probability, 3–4 points is moderate probability, and 5 or more points is high probability.
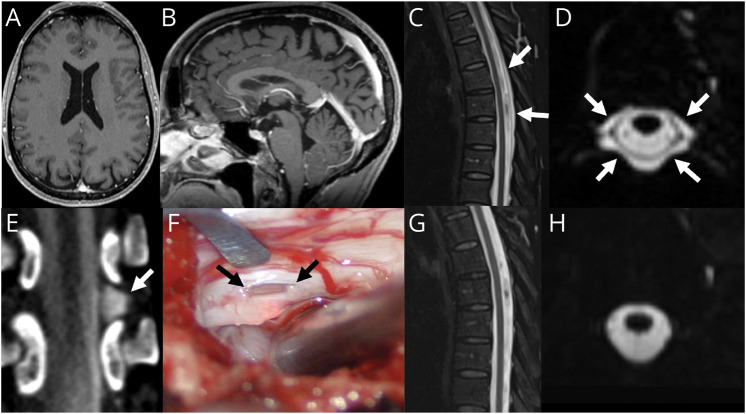
Figure 2. “Normal” Brain MRI in a Patient With SIH.
(A) Axial T1 postcontrast MRI demonstrates no pachymeningeal enhancement or subdural collections. (B) Sagittal T1 MRI demonstrates no significant sagging of the brainstem. (C) Sagittal T2 STIR MRI demonstrates a posterior epidural fluid collection. (D) Axial 3D T2 fat-saturated image demonstrates abnormal epidural fluid surrounding the dura in the midthoracic spine. (E) Dynamic decubitus myelography demonstrates extravasation of subarachnoid contrast into the lateral epidural space at T9-T10 (arrow). (F) Intraoperative photograph during surgical repair demonstrates a lateral dural defect at T9-T10 along the axilla of the left T9 nerve root (arrows). Postoperative T2 fat-saturated imaging in the sagittal (G) and axial (H) planes shows resolution of the epidural fluid collection.
Finally, head CT is not a sufficient evaluation for signs of SIH. This is important because it is typically the default imaging study ordered in the emergency department (ED) when patients present with a new severe headache.
Spine Imaging
Spine MRI and “Non-Contrast Myelography”
With rare exceptions, CSF leaks that result in SIH originate in the spine. The workup of patients with suspected SIH should include spinal imaging.32 A spine MRI can be helpful before dynamic myelography because the presence or absence of an epidural fluid collection guides the subsequent myelography technique.33 “Conventional” spine MRI protocols, which are designed to characterize a wide range of pathology, can identify a dorsal or ventral epidural fluid collection but do not adequately assess the lateral epidural space because they lack axial T2 fat-saturated sequences, and both fat and CSF are hyperintense on T2-non–fat-saturated imaging. Instead, “non-contrast myelography”, referring to heavily T2-weighted spine MRI with thin slice 3D acquisition, allows for superior detection of subtle fluid signal, assessment of the lateral epidural space, and characterization of meningeal nerve root sleeve diverticula. Studies have demonstrated noninferiority of this type of MRI compared with “conventional” CT myelography.34 In addition, this sequence includes minimization of CSF flow artifacts that can mimic epidural fluid collections.
Predisposing Spinal Anatomy: Meningeal Diverticula and Spinal Disk Osteophytes
Meningeal diverticula, or aneurysmal dilatations of the thecal sac where the exiting spinal nerves pass through the dura, are points of relative dural weakness where both CSF leaks and CVFs often occur. Their presence should be noted on diagnostic studies of the spine in patients with suspected spinal CSF leak. While meningeal diverticula are present in 44% of healthy patients, these can rupture causing CSF leak, and diverticula are associated with twice the rate of CVF among patients with orthostatic headache.31
Degenerative spinal disks can calcify, perforate the ventral dura, and become the source of a spontaneous CSF leak.35 These diskogenic spurs are typically difficult to distinguish on MRI and are best appreciated by careful examination of spinal CT.
Dynamic Myelography: Fluoroscopic and CT Techniques
“Conventional” CT myelography (CTM) refers to a procedure whereby contrast is injected into the CSF (usually with fluoroscopic guidance) and the patient is imaged under CT after a substantial delay to allow diffusion and mixing of contrast material. While excellent at delineating the subarachnoid space, various limitations of this procedure may prohibit detecting and/or localizing a CSF leak. For example, in the case of a large epidural fluid collection, by the time the patient is imaged, contrast has diffused throughout the fluid collection, obviating localization of the leak. Furthermore, CVFs are generally not detectable by conventional CTM (Figure 3).
Figure 3. Conventional vs Decubitus Myelography in a Patient With CSF Venous Fistula.
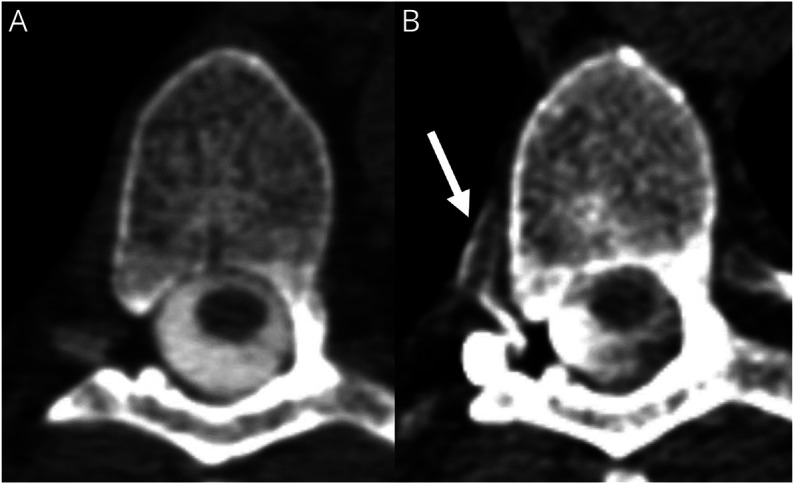
(A) Conventional myelogram at T6-7 demonstrates no evidence of CSF leak or CVF. (B) Dynamic decubitus CT myelography performed in the same patient demonstrates a CSF-venous fistula at T6-7 (arrow).
Dynamic myelography is the only reliable method for detecting CVFs, which completely escaped medical detection until 2014. As dynamic myelography techniques have advanced, recent reports suggest CVFs may cause as many as half of all cases of SIH.12,36 Dynamic myelography can be performed with fluoroscopic DSM or CT techniques.37,38 These differ from CTM in that imaging is obtained immediately after contrast injection, using subsequent CT or DSM to identify CSF loss into adjacent veins or the epidural space. Lateral decubitus positioning is critical to identifying CVF.10 Various provocative maneuvers such as resisted inspiration and pressure augmentation may enhance detection of small CVF.39,40
The Role of Epidural Blood Patches
EBPs are the first-line treatment of SIH. The mechanism of leak closure has been hypothesized to be through direct interaction of injected blood and procoagulant components of CSF emanating from a dural defect, allowing a buttress upon which the integrity of the dura can be restored, and/or through cephalad displacement of CSF removing pressure from the leak site.41,42 EBP may be “non-targeted” or “targeted”.6,11 Non-targeted EBPs for SIH are generally performed using a lumbar midline approach and by injecting a high volume (20 mL or more) of autologous blood. Targeted patching may be performed when a leak is localized and may be performed in the cervical or thoracic spine under imaging guidance, often incorporating fibrin sealant. When a ventral leak is identified, a transforaminal approach may facilitate direct patching of the leak site. While there are no randomized controlled trials investigating the optimal technique for EBP, more blood (greater than 20 mL) is likely better than less, and earlier patching is likely better than later.6,11,17,43 Some studies suggest that multifocal patches, in experienced hands, may be more efficacious.11 Both targeted and non-targeted patches can be effective, probably because there is extensive spread of epidural blood even from a single spinal needle site.44
The effectiveness of EBPs often declines over time, and the need for repeat patches is expected.45 However, even a single non-directed lumbar EBP can be a meaningful and often life-changing intervention for a patient with SIH and has been reported to have cure rates above 70% when performed early in the disease course.46
Patients With SIH With Abnormal Brain MRI
In patients with positive brain imaging but no epidural fluid collection visualized on appropriate spinal imaging, a CVF is suspected and 3/4 will be found to have a CVF as the cause of SIH with skilled lateral decubitus myelography.10 However, the inability to perform dynamic myelography should not preclude offering the patient a non-targeted patch because there remains potential for substantial clinical benefit.
Insisting on long trials of conservative treatment such as bed rest, hydration, caffeine, or an abdominal binder is not appropriate for several reasons: (1) There are no high-quality data showing efficacy of this approach; (2) a small but important minority of CSF leaks will progress to life-threatening complications; (3) some data suggest that earlier treatment with EBP is associated with a higher likelihood of success; and (4) over time, the headache arising from an ongoing leak is likely to become less orthostatic, pachymeningeal enhancement may resolve resulting in a false-negative brain MRI, and opening pressure is less likely to be low.7,9,17,19,32 All of these factors reduce confidence in diagnosis over time and reduce the likelihood a patient will ever receive accurate diagnosis and/or successful treatment.
Patients With Suggestive Symptoms and Signs but “Normal” MRI
More challenging is how to proceed with patients who have symptoms of SIH without convincing neuroimaging findings. This population represents a poorly quantified composite of patients with imaging-negative CSF leak (false negatives) and patients who do not actually have a leak (true negatives).
The following considerations deserve careful attention in such patients.
First, the important predictive properties of brain and spine MRIs, such as the false-negative rate, are not well established among patients with orthostatic headache. A recent meta-analysis concluded that based on published aggregated cohort data that the rate of negative brain MRI findings in patients with SIH was 19%.6 Other studies have found that 24% of patients with spine MRI evidence of CSF leak had false-negative brain MRIs.32 Conversely, while spine MRI was initially reported to have 90% sensitivity for CSF leaks, we now know it misses a whole class of leaks (CVFs) that are the cause of up to 50% of all cases of SIH.12,36 In short, the false-negative rate of screening MRI studies is poorly quantified but is high enough that such negative results should be carefully weighed against the patient's history including predisposing conditions (HDCT), inciting events, orthostatic headache, additional symptoms of SIH (e.g., tinnitus), and risks and benefits of EBP.
Second, the outcomes of EBP for patients with orthostatic headache who have negative imaging are beginning to emerge and favor offering an EBP. A recent cohort study reported 90% of such patients have 50% improvement 3 months after EBP, with 52% reporting complete remission.47 In another cohort of 86 patients, three-quarters of patients who had clinical suspicion of SIH and subsequent resolution of symptoms after EBP did not satisfy ICHD-3 criteria (low OP or positive imaging).48
Third, the harm of failing to patch an imaging-negative patient with true SIH should be weighed carefully against the potential harm of patching a patient in whom there is actually no SIH. The harm of failing to patch a patient who has an imaging-negative true SIH arises from the continued adverse impact of SIH on quality of life.
Over half of patients with SIH have to leave work for more than 3 months, and one-quarter remain unable to return to work, which has substantial adverse financial impacts on these patients and their families.1 Ability to maintain partner relationships is degraded, spiritual well-being is worse than among patients with AIDS, and suicidal thoughts are common. In patients whose SIH is treated effectively, quality-of-life scores improve to that comparable with the general population.2
The issues discussed above were anticipated by the International Headache Society when proposing the ICHD-3 diagnostic criteria for low-pressure headache. The same ICHD-3 diagnostic criteria for SIH that codify reliance on either positive imaging localization or low spinal OP also explicitly recognize a role for offering treatment in the absence of a visible leak with the following codicil: “In patients with typical orthostatic headache and no apparent cause, and after exclusion of POTS, it is reasonable in clinical practice to provide autologous lumbar EBP.”45 This is echoed by a recent systematic review and meta-analysis that noted that SIH should not be excluded on the basis of a non-orthostatic headache, normal neuroimaging findings, or normal OP.6
We strongly recommend that patients for whom there is high clinical suspicion of SIH be offered an EBP even in the face of “negative” imaging when this can be done safely and with appropriate informed consent regarding the uncertainty of the diagnosis. When patients are felt to have borderline cases for patching, we find it useful to convene a multidisciplinary review with neurologists, radiologists, and interventionalists to discuss the patient's history, symptoms, impairment, exposure to previous treatments, imaging, and therapeutic options.33
Post-Patch Management
We recommend having patients spend 2 hours in recumbency after patching, but no trials have assessed different durations of recumbency after EBP for SIH (as distinguished from postdural puncture headache). Longer periods may prove useful in those at low risk of deep venous thrombosis from extended bed rest. We also recommend that patients avoid bending, lifting, and twisting for periods of up to 6 weeks after EBP. While the specific durations for these recommendations have not been rigorously evaluated, it is sensible to limit activity for some period of time to limit the risk of disrupting physiologic leak repair initiated by the EBP.
Rebound intracranial hypertension is a common complication of EBP in patients with SIH, in which repair of the leak results in a subsequent symptomatic increase in intracranial pressure. Typically presenting within 24–28 hours, the headache may worsen in recumbency and be more frontal in location. It should be discussed as a possibility with patients and treated with acetazolamide or other carbonic anhydrase inhibitors.49 Preexisting intracranial hypertension should be considered in patients with refractory rebound intracranial hypertension.50
Complications of Epidural Patching
The most common adverse effect of EBP is self-limiting back pain, with nearly all cases reported to resolve within 4 weeks after patching.42,51 More serious adverse effects of EBP including epidural or subdural hematoma, venous thrombosis, infection, or cauda equina syndrome have been reported and are considered rare.42,52,53 Accidental dural puncture during attempted EBP can lead to worsened headache, and unintended unrecognized intrathecal blood injection is a likely cause of the rare cases of arachnoiditis reported after EBP.54 Back pain and radicular pain during the procedure are common and frequently limit the amount of blood that can be administered. There is no meaningful data on whether the risks of EBP differ in patients with orthostatic headache and true-negative imaging without CSF leak.
Conclusion
Clinicians should be prepared to care for patients with the symptoms of SIH in the absence of a recent dural puncture or obvious imaging findings. Such leaks occur surprisingly commonly and often without a precipitating event. Although the most common symptom is severe orthostatic headache, there is wide variability in clinical presentations which can make the diagnosis challenging. The decision to trial EBP should be considered in the appropriate clinical context.
TAKE-HOME POINTS
→ Spontaneous intracranial hypotension (SIH) is often difficult to diagnose because of variable symptoms and inconsistent imaging results, including normal brain MRIs and unreliable spinal opening pressures. It is particularly important to consider SIH in patients with connective tissue disorders.
→ Epidural blood patches (EBPs) are a crucial low-risk treatment of SIH. It is recommended for use even in suspected cases of SIH, despite the challenges in diagnosis.
→ Early intervention with EBP is important in improving patient outcomes
→ There is a need for heightened awareness and understanding of SIH among clinicians and advocates for ongoing research to advance diagnostic and treatment methods.
Appendix. Authors
| Name | Location | Contribution |
| Andrew L. Callen, MD | Department of Radiology, University of Colorado Anschutz Medical Campus | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Deborah I. Friedman, MD | Adjunct Faculty, Department of Neurology, Thomas Jefferson University | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Simy Parikh, MD | Department of Neurology, Thomas Jefferson University | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jill C. Rau, MD, PhD | Bob Bové Neuroscience Institute, Honorhealth Neurology, Scottsdale, AZ | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Wouter I. Schievink, MD | Department of Neurosurgery, Cedars Sinai | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Jeremy K. Cutsforth-Gregory, MD | Department of Neurology, Mayo Clinic Rochester | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Timothy J. Amrhein, MD | Department of Radiology, Duke University | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Elena Haight, MD | Department of Anesthesia, UCSF | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Robert P. Cowan, MD | Department of Neurology, Stanford University | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Meredith J. Barad, MD | Department of Neurology, Stanford University | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Jennifer M. Hah, MD | Department of Anesthesiology, Stanford University | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Tracy Jackson, MD | Department of Anesthesiology, Vanderbilt University | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Connie Deline, MD | Spinal CSF Leak Foundation | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Andrea J. Buchanan | Spinal CSF Leak Foundation | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Ian Carroll, MD, MS | Department of Anesthesiology, Stanford University | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Jesse CM, Häni L, Fung C, et al. The impact of spontaneous intracranial hypotension on social life and health-related quality of life. J Neurol. 2022;269(10):5466-5473. doi: 10.1007/s00415-022-11207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liaw V, McCreary M, Friedman DI. Quality of life in patients with confirmed and suspected spinal CSF leaks. Neurology. 2023;101(23):e2411-e2422. doi: 10.1212/WNL.0000000000207763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berroir S, Grabli D, Héran F, Bakouche P, Bousser M-G. Cerebral sinus venous thrombosis in two patients with spontaneous intracranial hypotension. Cerebrovasc Dis. 2004;17(1):9-12. doi: 10.1159/000073892 [DOI] [PubMed] [Google Scholar]
- 4.Schievink WI, Maya M, Moser F, Nuño M. Long-term risks of persistent ventral spinal CSF leaks in SIH: superficial siderosis and bibrachial amyotrophy. Neurology. 2021;97(19):e1964-e1970. doi: 10.1212/WNL.0000000000012786 [DOI] [PubMed] [Google Scholar]
- 5.Schievink WI, Maya MM, Harris J, Galvan J, Taché RB, Nuño M. Infratentorial superficial siderosis and spontaneous intracranial hypotension. Ann Neurol. 2023;93(1):64-75. doi: 10.1002/ana.26521 [DOI] [PubMed] [Google Scholar]
- 6.D'Antona L, Jaime Merchan MA, Vassiliou A, et al. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: a systematic review and meta-analysis. JAMA Neurol. 2021;78(3):329-337. doi: 10.1001/jamaneurol.2020.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kranz PG, Amrhein TJ, Choudhury KR, Tanpitukpongse TP, Gray L. Time-dependent changes in dural enhancement associated with spontaneous intracranial hypotension. AJR Am J Roentgenol. 2016;207(6):1283-1287. doi: 10.2214/AJR.16.16381 [DOI] [PubMed] [Google Scholar]
- 8.Callen A, Pattee J, Thaker AA, et al. Relationship of bern score, spinal elastance, and opening pressure in patients with spontaneous intracranial hypotension. Neurology. 2023;100(22):e2237-e2246. doi: 10.1212/WNL.0000000000207267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia. 2016;36(13):1209-1217. doi: 10.1177/0333102415623071 [DOI] [PubMed] [Google Scholar]
- 10.Schievink WI, Maya MM, Moser FG, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine. 2019:902-905. doi: 10.3171/2019.6.SPINE19487 [DOI] [PubMed] [Google Scholar]
- 11.Pagani-Estévez GL, Cutsforth-Gregory JK, Morris JM, et al. Procedural predictors of epidural blood patch efficacy in spontaneous intracranial hypotension. Reg Anesth Pain Med. 2019:rapm-2018-000021. doi: 10.1136/rapm-2018-000021 [DOI] [PubMed] [Google Scholar]
- 12.Pradeep A, Madhavan AA, Brinjikji W, Cutsforth-Gregory JK. Incidence of spontaneous intracranial hypotension in Olmsted County, Minnesota: 2019-2021. Interv Neuroradiol. 2023:159101992311654. doi: 10.1177/15910199231165429 [DOI] [PubMed] [Google Scholar]
- 13.Schievink WI, Maya MM, Moser F, Tourje J, Torbati S. Frequency of spontaneous intracranial hypotension in the emergency department. J Headache Pain. 2007;8(6):325-328. doi: 10.1007/s10194-007-0421-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinstein E, Pariani M, Bannykh S, Rimoin DL, Schievink WI. Connective tissue spectrum abnormalities associated with spontaneous cerebrospinal fluid leaks: a prospective study. Eur J Hum Genet. 2013;21(4):386-390. doi: 10.1038/ejhg.2012.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schievink WI, Louy C. Precipitating factors of spontaneous spinal CSF leaks and intracranial hypotension. Neurology. 2007;69(7):700-702. doi: 10.1212/01.wnl.0000267324.68013.8e [DOI] [PubMed] [Google Scholar]
- 16.Schievink WI. Misdiagnosis of spontaneous intracranial hypotension. Arch Neurol. 2003;60(12):1713-1718. doi: 10.1001/archneur.60.12.1713 [DOI] [PubMed] [Google Scholar]
- 17.Mea E, Chiapparini L, Savoiardo M, Franzini A, Bussone G, Leone M. Clinical features and outcomes in spontaneous intracranial hypotension: a survey of 90 consecutive patients. Neurol Sci. 2009;30(suppl 1):S11-S13. doi: 10.1007/s10072-009-0060-8 [DOI] [PubMed] [Google Scholar]
- 18.Mea E, Chiapparini L, Savoiardo M, et al. Application of IHS criteria to headache attributed to spontaneous intracranial hypotension in a large population. Cephalalgia. 2009;29(4):418-422. doi: 10.1111/j.1468-2982.2008.01747.x [DOI] [PubMed] [Google Scholar]
- 19.Häni L, Fung C, Jesse CM, et al. Insights into the natural history of spontaneous intracranial hypotension from infusion testing. Neurology. 2020;95(3):e247-e255. doi: 10.1212/WNL.0000000000009812 [DOI] [PubMed] [Google Scholar]
- 20.Kong D-S, Park K, Nam D-H, et al. Atypical spontaneous intracranial hypotension (SIH) with nonorthostatic headache. Headache. 2007;47(2):199-203. doi: 10.1111/j.1526-4610.2006.00687.x [DOI] [PubMed] [Google Scholar]
- 21.Jesse CM, Schär RT, Goldberg J, et al. Patient-reported symptomatology and its course in spontaneous intracranial hypotension—beware of a chameleon. Clin Neurol Neurosurg. 2024;236:108087. doi: 10.1016/j.clineuro.2023.108087 [DOI] [PubMed] [Google Scholar]
- 22.Graf N, Fernandes Santos AM, Ulrich CT, et al. Clinical symptoms and results of autonomic function testing overlap in spontaneous intracranial hypotension and postural tachycardia syndrome: a retrospective study. Cephalalgia Rep. 2018;1:251581631877377. doi: 10.1177/2515816318773774 [DOI] [Google Scholar]
- 23.Mueller BR, Robinson-Papp J. Postural orthostatic tachycardia syndrome and migraine: a narrative review. Headache. 2022;62(7):792-800. doi: 10.1111/head.14365 [DOI] [PubMed] [Google Scholar]
- 24.Callen AL, Lennarson P, Carroll IR. A causative role for remote dural puncture and resultant arachnoid bleb in new daily persistent headache: a case report. Headache. 2023;63(7):981-983. doi: 10.1111/head.14584 [DOI] [PubMed] [Google Scholar]
- 25.Goadsby PJ. New daily persistent headache: a syndrome, not a discrete disorder. Headache. 2011;51(4):650-653. doi: 10.1111/j.1526-4610.2011.01872.x [DOI] [PubMed] [Google Scholar]
- 26.Houk JL, Amrhein TJ, Gray L, Malinzak MD, Kranz PG. Differentiation of Chiari malformation type 1 and spontaneous intracranial hypotension using objective measurements of midbrain sagging. J Neurosurg. 2021:1-8. [DOI] [PubMed] [Google Scholar]
- 27.Ohwaki K, Yano E, Shinohara T, et al. Spinal cerebrospinal fluid leaks detected by radionuclide cisternography and magnetic resonance imaging in patients suspected of intracranial hypotension. Adv Med Sci. 2014;59(2):196-199. doi: 10.1016/j.advms.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 28.Dobrocky T, Grunder L, Breiding PS, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol. 2019;76(5):580-587. doi: 10.1001/jamaneurol.2018.4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DK, Carr CM, Benson JC, et al. Diagnostic yield of lateral decubitus digital subtraction myelogram stratified by brain MRI findings. Neurology. 2021;96(9):e1312-e1318. doi: 10.1212/WNL.0000000000011522 [DOI] [PubMed] [Google Scholar]
- 30.Turner R, Zander D, Thaker AA, Timpone VM, Callen AL. Structured reporting for findings of spontaneous intracranial hypotension on brain MRI. AJR Am J Roentgenol. 2023;221(2):282-283. doi: 10.2214/AJR.23.29144 [DOI] [PubMed] [Google Scholar]
- 31.Schievink WI, Maya M, Prasad RS, et al. Spontaneous spinal cerebrospinal fluid-venous fistulas in patients with orthostatic headaches and normal conventional brain and spine imaging. Headache. 2021;61(2):387-391. doi: 10.1111/head.14048 [DOI] [PubMed] [Google Scholar]
- 32.Tsai P-H, Fuh J-L, Lirng J-F, Wang S-J. Heavily T2-weighted MR myelography in patients with spontaneous intracranial hypotension: a case-control study. Cephalalgia. 2007;27(8):929-934. doi: 10.1111/j.1468-2982.2007.01376.x [DOI] [PubMed] [Google Scholar]
- 33.Callen AL, Timpone VM, Schwertner A, et al. Algorithmic multimodality approach to diagnosis and treatment of spinal csf leak and venous fistula in patients with spontaneous intracranial hypotension. AJR Am J Roentgenol. 2022;219(2):292-301. doi: 10.2214/AJR.22.27485 [DOI] [PubMed] [Google Scholar]
- 34.Tay AS-MS, Maya M, Moser FG, Nuño M, Schievink WI. Computed tomography vs heavily T2-weighted magnetic resonance myelography for the initial evaluation of patients with spontaneous intracranial hypotension. JAMA Neurol. 2021;78(10):1275-1276. doi: 10.1001/jamaneurol.2021.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck J, Ulrich CT, Fung C, et al. Diskogenic microspurs as a major cause of intractable spontaneous intracranial hypotension. Neurology. 2016;87(12):1220-1226. doi: 10.1212/WNL.0000000000003122 [DOI] [PubMed] [Google Scholar]
- 36.Mamlouk MD, Shen PY, Dahlin BC. Headache response after CT-guided fibrin glue occlusion of CSF-venous fistulas. Headache. 2022;62(8):1007-1018. doi: 10.1111/head.14379 [DOI] [PubMed] [Google Scholar]
- 37.Mamlouk MD, Ochi RP, Jun P, Shen PY. Decubitus CT myelography for CSF-venous fistulas: a procedural approach. AJNR Am J Neuroradiol. 2021;42(1):32-36. doi: 10.3174/ajnr.A6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DK, Brinjikji W, Morris PP, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol. 2020;41(1):21-28. doi: 10.3174/ajnr.A6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mark IT, Amans MR, Shah VN, et al. Resisted inspiration: a new technique to aid in the detection of CSF-venous fistulas. AJNR Am J Neuroradiol. 2022;43(10):1544-1547. doi: 10.3174/ajnr.A7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caton MT, Laguna B, Soderlund KA, Dillon WP, Shah VN. Spinal compliance curves: preliminary experience with a new tool for evaluating suspected CSF venous fistulas on CT myelography in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2021;42(5):986-992. doi: 10.3174/ajnr.A7018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signorelli F, Caccavella VM, Giordano M, et al. A systematic review and meta-analysis of factors affecting the outcome of the epidural blood patching in spontaneous intracranial hypotension. Neurosurg Rev. 2021;44(6):3079-3085. doi: 10.1007/s10143-021-01505-5 [DOI] [PubMed] [Google Scholar]
- 42.Shin HY. Recent update on epidural blood patch. Anesth Pain Med (Seoul). 2022;17(1):12-23. doi: 10.17085/apm.21113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J-W, Hseu S-S, Fuh J-L, et al. Factors predicting response to the first epidural blood patch in spontaneous intracranial hypotension. Brain. 2017;140(2):344-352. doi: 10.1093/brain/aww328 [DOI] [PubMed] [Google Scholar]
- 44.Giess R, Landwehr P, Heidenreich F. MRI after thoracic epidural blood patch. Neurology. 2003;61(10):1449. doi: 10.1212/01.wnl.0000082655.33469.f2 [DOI] [PubMed] [Google Scholar]
- 45.Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 46.Berroir S, Loisel B, Ducros A, et al. Early epidural blood patch in spontaneous intracranial hypotension. Neurology. 2004;63(10):1950-1951. doi: 10.1212/01.wnl.0000144339.34733.e9 [DOI] [PubMed] [Google Scholar]
- 47.Choi SY, Seong M, Kim EY, et al. Outcome of epidural blood patch for imaging-negative spontaneous intracranial hypotension. Cephalalgia. 2023;43(2):3331024221140471. doi: 10.1177/03331024221140471 [DOI] [PubMed] [Google Scholar]
- 48.Davies MJ, Davies MA, Sharpe R, Cordato D, Schwartz R. Epidural blood patch as a diagnostic and therapeutic intervention in spontaneous intracranial hypotension: a novel approach to management. World Neurosurg. 2020;137:e242-e250. doi: 10.1016/j.wneu.2020.01.163 [DOI] [PubMed] [Google Scholar]
- 49.Kranz PG, Amrhein TJ, Gray L. Rebound intracranial hypertension: a complication of epidural blood patching for intracranial hypotension. AJNR Am J Neuroradiol. 2014;35(6):1237-1240. doi: 10.3174/ajnr.A3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sulioti G, Gray L, Amrhein TJ. Popping the balloon: abrupt onset of a spinal CSF leak and spontaneous intracranial hypotension in idiopathic intracranial hypertension, a case report. Headache. 2022;62(2):208-211. doi: 10.1111/head.14264 [DOI] [PubMed] [Google Scholar]
- 51.Ferrante E, Trimboli M, Rubino F. Spontaneous intracranial hypotension: review and expert opinion. Acta Neurol Belg. 2020;120(1):9-18. doi: 10.1007/s13760-019-01166-8 [DOI] [PubMed] [Google Scholar]
- 52.Rettenmaier LA, Park BJ, Holland MT, et al. Value of targeted epidural blood patch and management of subdural hematoma in spontaneous intracranial hypotension: case report and review of the literature. World Neurosurg. 2017;97:27-38. doi: 10.1016/j.wneu.2016.09.076 [DOI] [PubMed] [Google Scholar]
- 53.Davidson B, Nassiri F, Mansouri A, et al. Spontaneous intracranial hypotension: a review and introduction of an Algorithm for management. World Neurosurg. 2017;101:343-349. doi: 10.1016/j.wneu.2017.01.123 [DOI] [PubMed] [Google Scholar]
- 54.Villani LA, Digre KB, Cortez MM, Bokat C, Rassner UA, Ozudogru SN. Arachnoiditis, a complication of epidural blood patch for the treatment of low-pressure headache: a case report and systematic review. Headache. 2021;61(2):244-252. doi: 10.1111/head.14076 [DOI] [PubMed] [Google Scholar]