Abstract
Multisensory sensitivity (MSS), observed in some chronic pain patients, may reflect a generalized central nervous system sensitivity. While several surveys measure aspects of MSS, there remains no gold standard. We explored the underlying constructs of four MSS-related surveys (80 items in total) using factor analyses using REDCap surveys (N = 614, 58.7% with pain). Four core- and six associated-MSS factors were identified from the items assessed. None of these surveys addressed all major sensory systems and most included additional related constructs. A revised version of the Somatosensory Amplification Scale was developed, encompassing five core MSS systems: vision, hearing, smell, tactile, and internal bodily sensations: the 12-item Multisensory Amplification Scale (MSAS). The MSAS demonstrated good internal consistency (alpha = 0.82), test-retest reliability (ICC3,1 = 0.90), and construct validity in the original and in a new, separate cohort (R = 0.54 – 0.79, p < 0.0001). Further, the odds of having pain were 2 – 3.5 times higher in the highest sex-specific MSAS quartile relative to the lowest MSAS quartile, after adjusting for age, sex, BMI, and pain schema (p < 0.03). The MSAS provides a psychometrically comprehensive, brief, and promising tool for measuring the core-dimensions of MSS.
Keywords: Somatosensory amplification scale, Adolescent/adult sensory profile, Adult sensory questionnaire, Highly sensitive person scale, Sensory Hypersensitivity, Generalized sensory sensitivity
Introduction
Chronic pain is challenging to treat clinically. Mechanism-based approaches have been widely advocated,10 yet are limited in clinical practice by inadequate markers to reflect the underlying pathologies. However, emerging evidence suggests elevated multisensory sensitivity (MSS) may be a factor associated with chronic pain. While there is a lack of clear consensus on a definition of elevated MSS, it has been generally described as sensory hypersensitivity to multiple non-painful stimuli.53 People with a chronic pain condition have reported heightened sensitivity to normal daily sensations more frequently than healthy, pain-free individuals. For example, sensitivity to multiple sensations,11, 17, 24, 53 bright light, i.e., photophobia,14, 19, 52 or sound, i.e., phonophobia,8, 14 are noted in those with fibromyalgia, migraine, low back pain and other conditions. Furthermore, generalized sensory sensitivity is associated with the presence of chronic overlapping pain conditions.44
MSS has been suggested as a potential surrogate measure or a complementary tool to identify a generally sensitized central nervous system (CNS) state.17, 56 Similarly, central sensitization, or nociplastic pain, is a manifestation of synaptic plasticity in the central nervous system (CNS) resulting in pain hypersensitivity.10, 15 Central sensitization is thought to contribute to chronic pain, such as widespread pain or coexisting central sensitivity syndromes.15, 57, 58 Analogously, the Central Sensitization Inventory, a self-report instrument aimed at identifying central sensitization through assessment of multiple pain condition symptoms, includes several items evaluating aspects of MSS, such as, “I am sensitive to bright lights”.40 Accordingly, while MSS is not a measure of pain central sensitization specifically, it represents a related construct of generalized central sensitivity and/or risk factor for altered CNS processing.20, 56 That is, elevated MSS may be a trait-like factor35, 38 that could precede chronic pain. Thus, assessing MSS in people both with and without pain would be of value. However, compared to psychological factors, fatigue, and sleep disturbances, relatively little attention has been given to the predictive or concurrent relationships between MSS and pain.20
Self-reported questionnaires are the most common and efficient way to assess MSS, as opposed to psychophysical testing of multiple sensation domains. Aspects of sensory sensitivity have been assessed using multiple tools in psychology or neurology fields for decades, such as, the Adolescent/Adult Sensory Profile (AASP),9 the Somatosensory Amplification Scale (SSAS)6, 7, 39 and the Highly Sensitive Person Scale (HSPS).2, 35 These instruments were not originally developed with pain populations in mind, however, and often include additional constructs more relevant to autism spectrum disorder or psychological conditions. Studies relating MSS to pain have used various instruments as well as items combined from multiple surveys in an attempt to cover all major sensory systems not otherwise adequately addressed.53 However, most available tools are lengthy, do not include all major sensory domains, and/or require a fee. The degree to which different instruments provide similar MSS information is unknown, making comparison between studies challenging. Accordingly, there remains a need for a brief measure of MSS that includes all major sensory systems for clinical and research applications that is appropriate for use as a potential marker for altered CNS processing, as it relates to pain.
Therefore, the purposes of this study were two-fold: 1) to explore the domains assessed by four available measures involving sensory sensitivity: the SSAS, two subscales of the AASP, the HSPS, and one subscale of the Adult Sensory Questionnaire (ASQ); and 2) to modify a brief MSS assessment to better target five key sensory domains: vision, hearing, tactile, smell, and internal bodily perceptions in a general population of adults with and without pain.
Materials and Methods
This study consisted of two parts. A single cohort of adults, fluent in English, were recruited to address both study parts. Subjects were asked to complete a series of questionnaires using online REDCap (Research Electronic Data Capture) software, a secure, web-based software platform designed to support data capture for research studies.25, 26 The study was reviewed and approved by the local Institutional Review Board (IRB). Exempt informed consent was obtained online from all participants, as approved by the local IRB. Surveys were completed voluntarily, and participants had a 1 in 10 chance of receiving an Amazon gift card for their time.
Comparing multiple instruments to assess and compare their underlying content was completed as Part I. To do this, we characterized the latent domains observed across four MSS-related surveys to identify core-MSS from associated-MSS domains and evaluate which domains each MSS survey most represents. In Part II, we developed a modified version of the SSAS using the original primary study cohort, evaluating several psychometric properties. A second smaller cohort was also recruited for Part II to further validate this modified tool in a new sample.
Methods Part I – Comparison of MSS-related Surveys
Primary Cohort Subject Characteristics
Subjects for the primary study cohort were recruited in three ways from our community in an effort to obtain a more diverse cohort in terms of lifespan: 1) university-wide mass email to faculty, staff, and students; 2) online Facebook announcements with hyperlinks to target non-university and middle-aged individuals; and 3) Seniors Together in Aging Research (STAR) platform to target older individuals. Inclusion criteria was simply that participants were over 18 and fluent in English. Demographic information collected included: age, race, dichotomous sex, height, weight, education level, current employment status and marital status. Body mass index (BMI) was computed from height and weight. Current pain, average pain, and worst pain in the past seven days were measured using a 0–10 scale allowing 0.5 increments, where 0 = “no pain” and 10 = “maximal pain ever experienced or imagined.”
Four MSS-related Surveys
The AASP includes four, 15-item subscales: low registration, sensation seeking, sensory sensitivity, and sensation avoiding.9 Due to the specific interest in MSS and to reduce participant burden, only two subscales; sensory sensitivity and sensation avoiding, were included as separate subscales. Participants rated the frequency of each item (i.e., “I only eat familiar foods.”) using a four-point Likert scale. Each subscale score was the sum of the corresponding 15 items. The AASP has moderate to good internal consistency (Cronbach’s α = 0.64 to 0.78).9
The 10-item SSAS was originally designed to measure somatic sensory amplification, with application towards people with hypochondriasis.6, 7 Participants rate how true each statement is for them on a five-point Likert scale (1 = not at all true to 5 = extremely true). An example is, “Sudden loud noises really bother me.” The SSAS has good test-retest reliability (r = 0.79) and internal consistency (α = 0.82).7
The ASQ was originally described as a measure of sensory defensiveness with four subscales: sensitivity to sensory stimuli (14 items), social emotional behaviors (4 items), ability to self-regulate (4 items), and coping strategies (4 items).42 Only the sensitivity to sensory stimuli subscale were assessed (i.e., “I am sensitive or bothered by sounds that don’t seem to bother other people.”). However, due to a technical error, one item assessing “lights/contrasts/reflections” was not able to be scored. Thus, only 13 of the 14 original subscale items were analyzed in this study. The ASQ survey was designed as a true/false questionnaire but was rescaled to a five-point scale (1 = not at all true to 5 = extremely true) for the current study to more readily compare responses with the other four- and five-point survey items in the exploratory factor analysis. The original ASQ has demonstrated good test-retest reliability (r = 0.92).42
The 27-item HSPS was developed to measure individual differences in sensory processing sensitivity in psychological research.2 Participants indicate their level of agreement with each item on a seven-point scale (0 = “strongly disagree” to 7 = “strongly agree”). One example item asks, “Are you easily overwhelmed by strong sensory input?” The HSPS has good internal consistency (α = 0.87),38 and good content validity.2
Other Assessments
To assess for individual differences in pain schema, i.e., how an individual conceptualizes numeric pain intensity, the Pain Severity Questionnaire was assessed.21 Participants rate items described as ‘mild’, ‘moderate’, and ‘severe’ for six common pain conditions using a 0 to 10 numeric pain rating scale with 0.5 increments, i.e., a 21-pt scale. 29 The average pain rating across these conditions was computed as a general indicator of scale usage (i.e., “pain schema”) for use as a covariate, i.e., general tendency to rate pain intensities as high or low. The Pain Severity Questionnaire provides good internal consistency (α = 0.96) and test-retest reliability (r = 0.82).21
Self-perceived generalized pain sensitivity, i.e., subjects’ perceptions of how sensitive they are to pain, was assessed using the seven-item Generalized Pain Questionnaire49 for comparison to the MSS scales. Participants rated each item using a five-point Likert scale (0 = never noticed to 4 = very strongly noticed). The Generalized Pain Questionnaire has demonstrated good test-retest reliability (r = 0.90).49
Part I – Analyses
Only participants who completed all four MSS-related surveys (i.e., five scales/subscales) were included in the analyses for Part I. Completion was operationally defined as having 90% or more item responses, with any missing data (< 10% per survey) imputed as the average of the remaining items. A total of 80 MSS items were included. independent t tests with Bonferroni Corrections for multiple comparisons (adjusted p-value) were used to assess for sex differences in all variables, and effect sizes (Cohen’s d) computed. Statistics were computed using SAS 9.4 (SAS Institute, Cary, NC, USA) and SPSS v24, (IBM, New York, NY, USA) with significance set at p ≤ 0.05. Data were expressed as mean ± standard deviation (SD) or percentage (%) as appropriate. All surveys allowed participants to report sensitivity using a range of responses which does not inherently assume any linear stimulus-response curve.
Correlational Analyses of MSS surveys
Associations between the five MSS scale or subscale scores were assessed using Pearson’s correlation coefficients. In addition, the correlations between each MSS scale and self-perceived pain sensitivity were assessed as an indicator of construct validity for each, with adjustment for sex, age, BMI, and pain schema. Correlation strength was operationally defined as: r < 0.4 = weak; 0.4 – 0.7 = moderate; > 0.7 = strong.1 Internal consistency was computed using Cronbach’s alpha for each MSS scale and subscale.
Exploratory Factor Analyses
Exploratory factor analysis was used to identify the underlying latent factors assessed across all MSS surveys assessed (i.e., 80 MSS items total) using principal factor analysis with the Oblimin oblique rotation to allow factors to be correlated. The preferred number of retained factors was determined by examining the scree plot, considering rotated factor eigenvalues, avoiding dual-loadings, and considering the resulting clarity/interpretability of the rotated solutions.12 Only items with factor loadings of 0.30 or greater were considered as meaningfully contributing to any particular factor. The latent variable labels were identified based on highest-loading items. Based on prior psychometric structure findings of one survey, the HSPS,18 we anticipated the underlying factors would consist of both core- and associated-MSS constructs. We operationally defined core-MSS factors as those best interpreted to measure sensitivity to primary non-noxious senses including tactile, olfactory, visual, auditory and internal bodily sensations. We chose to not include vestibular/balance or taste as targets for core-MSS domains due to likelihood of underlying pathology (e.g. balance or vestibular dysfunction) or cultural/ personal taste preferences, respectively, as opposed to normal variation in sensitivity to common daily sensory inputs.
To further evaluate the four original MSS surveys, the proportion of items with loadings ≥ 0.30 on core-MSS factors was identified for each scale/subscale. The correlations between extracted core- and associated-MSS factor scores with each original MSS scale/subscale were also assessed.
Results - Part I
Of the 742 participants who initially clicked the survey link, 667 finished the first demographic survey (89.9%) and 614 completed all four MSS-related surveys required for Part I analyses (93.5% Caucasian; 436 F, 177 M, 1 sex unreported). Summary subject characteristics for this cohort (N=614) are presented in Table 1. Current pain was reported in 360 participants (58.7%), of whom the mean (SD) pain intensity was 2.4 (2.3), ranging from 0.5 to 10. Nearly all participants reported experiencing some pain in the prior week (93.2%) based on worst pain ratings, with an average of 3.6 (2.3) out of 10. Women did not differ from men on any participant characteristics, except current pain ratings (effect size d = 0.39, adjusted p = 0.02). However, women reported significantly higher MSS scores than men in four of the five scales/subscales (adjusted p ≤ 0.001) with consistently moderate effect sizes (Cohen’s d = 0.45 to 0.61, Table 1). The exception was the AASP sensory avoiding subscale (adjusted p = 0.06; Cohen’s d = 0.26). This dataset is publicly available.22
Table 1.
Summary characteristics and survey responses for primary study cohort (Mean (SD) or Proportion, %).
| Total (observed range) | All (n=614) | Women (n=436) | Men(n=177) | Raw-p† | Adjusted-p† | Effect size † (F–M) (95% CI) |
|---|---|---|---|---|---|---|
|
| ||||||
| Age (18–90 yrs) | 48.3 (20.4) | 47.5 (20.2) | 50.4 (20.8) | 0.11 | 1 | −0.14 (−0.32, 0.03) |
|
| ||||||
| BMI (16.5–57.3 kg/m2) | 27.0 (6.4) | 26.9 (6.7) | 27.5 (5.9) | 0.26 | 1 | −0.09 (−0.27, 0.08) |
|
| ||||||
| Race/Ethnicity | ||||||
| Caucasian (%) | 93.50% | 93.30% | 93.80% | 0.84 | 1 | −0.04 (−0.44, 0.36) |
| non-Hispanic (%) | 97.00% | 97.80% | 95.20% | 0.09 | 1 | 0.45 (−0.08, 0.98) |
|
| ||||||
| Marital status | ||||||
| Never married, (%) | 31.00% | 32.10% | 28.20% | 0.35 | 1 | 0.10 (−0.11, 0.31) |
|
| ||||||
| Current employment status | ||||||
| Employed, (%) | 57.80% | 58.90% | 54.80% | 0.35 | 1 | −0.09 (−0.10, 0.29) |
|
| ||||||
| Education level | ||||||
| College or higher, (%) | 82.40% | 81.70% | 84.20% | 0.46 | 1 | −0.10 (−0.36, 0.16) |
|
| ||||||
| Pain Status | ||||||
| Current Pain - incidence (%) | 58.60% | 56.90% | 63.30% | 0.17 | 1 | −0.15 (−0.35, 0.05) |
| Intensity‡ (mean, SD) | 2.4 (2.3) | 2.7 (2.5) | 1.8 (1.7) | 0.001 | 0.02 | 0.39 (0.17, 0.62) |
| Worst 7-day Pain - incidence (%) | 93.20% | 92.30% | 94.40% | 0.73 | 1 | −0.15 (−0.56, 0.25) |
| Intensity‡ (mean, SD) | 3.6 (2.3) | 3.7 (2.4) | 3.4 (2.2) | 0.22 | 1 | 0.13 (−0.05, 0.31) |
| Average 7-day Pain - incidence (%) | 78.50% | 76.60% | 83.10% | 0.19 | 1 | −0.22 (−0.47, 0.03) |
| Intensity‡ (mean, SD) | 2.0 (1.6) | 2.1 (1.7) | 1.7 (1.5) | 0.02 | 0.38 | 0.24 (0.05, 0.44) |
|
| ||||||
| Pain Schema, PSQ (1.6–8.2) | 4.7 (1.0) | 4.7 (0.9) | 4.5 (1.0) | 0.01 | 0.19 | 0.22 (0.04, 0.39) |
|
| ||||||
| MSS-related survey Scores: | ||||||
| SSAS (10–49) | 26.6 (6.1) | 27.6 (5.8) | 24.0 (6.0) | <0.001 | 0.02 | 0.61 (0.44, 0.79) |
|
| ||||||
| AASP sensory sensitivity (15–63) | 32.1 (8.9) | 33.5 (8.9) | 28.8 (7.9) | <0.001 | 0.02 | 0.55 (0.37, 0.72) |
|
| ||||||
| AASP sensory avoiding (16–68) | 34.1 (9.2) | 34.8 (9.2) | 32.4 (9.0) | 0.004 | 0.08 | 0.26 (0.09, 0.44) |
|
| ||||||
| ASQ subscale (13–54) | 23.9 (7.9) | 24.9 (8.2) | 21.4 (6.3) | <0.001 | 0.02 | 0.45 (0.28, 0.63) |
|
| ||||||
| HSPS (27–181) | 98.5 (27.8) | 102.9 (27.8) | 87.9 (24.7) | <0.001 | 0.02 | 0.56 (0.38, 0.73) |
Table 1 Notes: PSQ, Pain Severity Questionnaire; MSS, Multisensory Sensitivity; SSAS, Somatosensory Amplification Scale; AASP, Adolescent/Adult Sensory Profile; ASQ, Adult Sensory Questionnaire; HSPS, Highly Sensitive Person Scale.
Sex-differences comparisons; Effect size is positive when values are higher for women than men.
Pain intensities of those reporting pain.
The five MSS scales and subscales were all significantly correlated, with moderate to strong associations (r = 0.49 – 0.77, p < 0.0001, Table 2). Each measure showed moderate-to-high internal consistency: AASP sensory sensitivity (α = 0.81) and sensory avoiding (α = 0.83) subscales, SSAS (α = 0.75), ASQ subscale (α = 0.82), and HSPS (α = 0.93). The MSS scales were correlated to self-perceived pain sensitivity, ranging from 0.39 to 0.49 (p < 0.0001).
Table 2.
Correlations† between measures of multisensory sensitivity (N=614).
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
|
| |||||
| 1. AASP sensory sensitivity | 1 | ||||
| 2. AASP sensory avoiding | 0.77 | 1 | |||
| 3. ASQ subscale | 0.71 | 0.62 | 1 | ||
| 4. HSPS | 0.69 | 0.70 | 0.60 | 1 | |
| 5. SSAS | 0.50 | 0.45 | 0.49 | 0.54 | 1 |
Notes: AASP, Adolescent/Adult Sensory Profile; ASQ subscale, Adult Sensory Questionnaire sensitivity to sensory stimuli; HSPS, Highly Sensitive Person Scale; SSAS, Somatosensory Amplification Scale.
Pearson correlation with adjustment for age, sex, BMI and mean pain rating schema assessed by pain severity questionnaire. All correlations were significant at p < 0.0001 level.
Four MSS Instrument Constructs
Using exploratory factor analysis, factor solutions ranging from 1 to 10 factors were considered based on the eigenvalues, the Scree plot, and latent factor loadings and interpretation. For instance, the high eigenvalue for a 1-factor solution indicates a single uniting commonality among all items, supporting the conceptual construct of MSS (for more details, see Online Resource 1, Table S1). However, a 1-factor solution only explains 26% of the total variance across all survey items, thus is an incomplete factor solution. A ten-factor solution was finally determined to best represent the 80 items from the four MSS-related surveys (Table 3). This solution was chosen for several reasons: all 10 eigenvalues were greater than 1; the scree plot demonstrated a reasonable “elbow” (i.e., point at which eigenvalues level off) at 10 factors (Online resource, Figure S1); the 10 factors were readily interpretable; more survey items loaded on one of the latent factors (84%) with fewer dual-loadings than the other solution options; and it explained a majority of the total variance observed across all 80 MSS items (51.1%). Despite the large number of items evaluated, only four factors, consisting of 25 items (31.3% of total), were clearly relevant to our operationally defined MSS core sensory domains: vision, hearing, tactile, smell, and internal bodily perceptions (See Table 3). These four factors were labeled as: Smell/Tactile Sensitivity; Sound/Startle Sensitivity; Internal Bodily Sensitivity; and Food Texture Sensitivity. One item loaded on both Smell/Tactile Sensitivity and Sound/Startle Sensitivity, thus is counted under both factors below. Of the 80 items from the five scales and subscales, very few items assessed light sensitivity, thus no light-specific factor emerged.
Table 3.
Ten latent factors explaining items in four MSS surveys (N=614).
| Latent Factors | Core vs Assoc. MSS | # Items † | Factor loading range | Item contents |
|---|---|---|---|---|
| Smell/Tactile Sensitivity | Core | 9 | (0.31, 0.64) | sensitivity or avoidance to smell or being bothered by clothing and tactile sensations |
| Sound/Startle Sensitivity | Core | 8 | (0.37, 0.76) | sensitivity to sounds or being easily startled by sound |
| Internal Body Sensitivity | Core | 6 | (0.30, 0.48) | sensitivity to somatic or body sensations |
| Food /Texture Sensitivity | Core | 3 | (0.41, 0.86) | sensitivity to food textures |
| Easily Stressed | Associated | 12 | (0.31, 0.62) | becoming easily mentally overwhelmed by life. |
| Avoids Crowds | Associated | 8 | (0.31, 0.67) | avoiding crowded situations |
| Easily Distracted | Associated | 6 | (0.53, 0.73) | impact of bothersome distractions, e.g. in workplace situations |
| Vestibular Sensitivity | Associated | 7 | (0.36, 0.78) | sensitivity to, or avoidance of, movement such as elevator or cars |
| Aesthetic Sensitivity | Associated | 6 | (0.40, 0.65) | aesthetic awareness, such as being “moved by arts and movies” |
| Fear | Associated | 3 | (0.31, 0.37) | Fear to heights, pain and violent videos |
Items with factor loadings ≥ 0.30 on the corresponding latent factor.
The remaining six non-core factors consisted of 42 items (53% of total), which focused primarily on psychological impacts and coping strategies (i.e., fear and avoidance behaviors); aesthetic sensitivity; or vestibular sensitivity (See Table 3). While correlated to the core-MSS factors (Table S2), these associated factors did not fall clearly within the scope of pre-determined sensory sensitivity domains. Only 13 items (16%) did not clearly load at a minimum of 0.30 with any of the ten latent factors identified. We did not categorize vestibular sensitivity as a core-MSS factor, but rather an associated-MSS factor as it could involve pathophysiological conditions, such as balance impairment, vertigo, or vestibulitis, often associated with a variety of neurological or vasovagal conditions and which may confound the assessment of generalized sensory sensitivity.48
When evaluating the proportion of items assessing core-MSS constructs within each survey, only 13% - 20% of AASP items loaded on any of the four core-MSS factors (Figure 1A). Similarly, the HSPS had 22.2% of items which loaded on two core factors: startle/sound and tactile/smell; yet each item included multiple sensory domains, e.g., “bright lights, strong smells, coarse fabrics, or sirens.” Conversely, 61.5% of ASQ subscale items loaded on three of the four core-MSS factors and 70% of the SSAS items loaded on two of the four core-MSS factors: startle/sound and internal bodily sensitivity.
Figure 1.
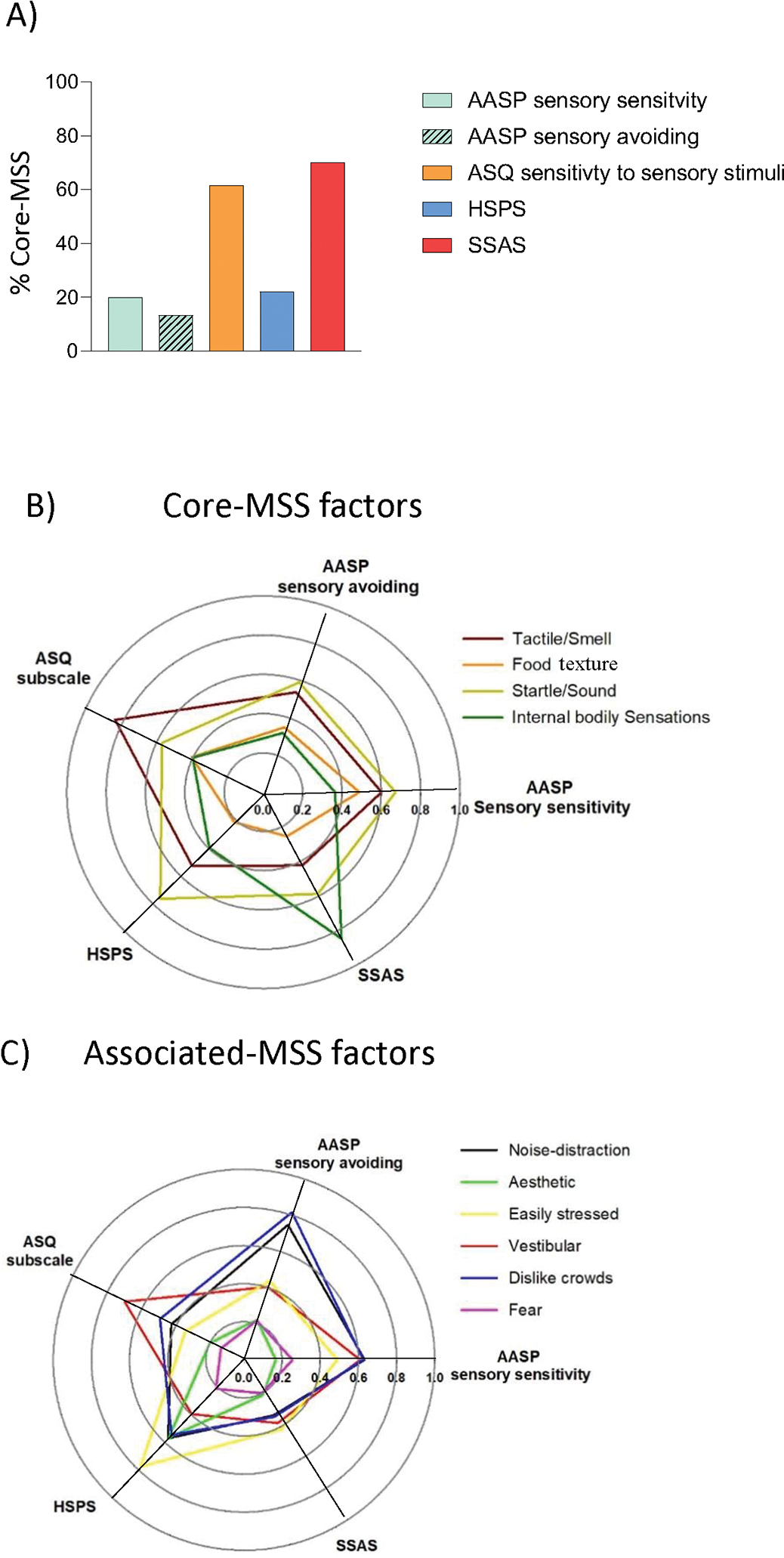
Graphical characterization of the original MSS surveys: A) Proportion of MSS core items per survey, assessed as the number of items loading ≥ 0.30 on core-MSS factors relative to total number of survey items assessed; B) Polar plot showing correlations between each survey and the 4 identified core-MSS latent factors, where the center = no correlation and the outermost radius equals a maximum correlation (1.0); and C) Polar plots showing the correlations between each survey and the 6 associated-MSS latent factors. AASP, Adolescent/adult sensory profile; ASQ, Adult sensory questionnaire; HSPS, Highly sensitive person scale; SSAS, Somatosensory amplification scale.; MSS, multisensory sensitivity.
All MSS scales/subscales displayed significant correlations with both core- and associated-MSS factors (p < 0.0001), but to varying degrees. Figure 1B and 1C show these correlation magnitudes as polar plots to graphically represent these relationships. For example, the AASP sensory sensitivity subscale was moderately correlated to seven of the 10 factors (r = 0.52 – 0.67, 27% - 45% shared variance) but less so with the other three: internal bodily sensitivity, aesthetic sensitivity and fear (r = 0.17 – 0.41, 3% -17% shared variance). Whereas the AASP sensory avoiding subscale had moderate to strong correlations with only four factors: startle/sound, easily stressed, easily distracted, and avoids crowds (r = 0.50 – 0.81, 25% - 66% shared variance). The ASQ subscale was particularly related to tactile sensitivity (r = 0.80, 64% shared variance); the SSAS was strongly correlated with internal bodily sensitivity (r = 0.86, 74% shared variance). No survey strongly correlated with all core-MSS domains identified, and not all key sensory domains were represented by the latent factors from the 80 items.
Methods Part II – Modified MSS Scale
Identifying items
To develop a brief MSS assessment, which incorporates all primary non-noxious sensory domains, we aimed to modify one of the four MSS surveys assessed. The SSAS is the shortest, is freely available, and uses a simple Likert-type scale, making it a strong candidate for modification to represent multiple non-noxious sensory domains. Further, we obtained written permission from the original SSAS author to modify the scale for the purpose of generating a MSS measure.
To identify possible candidate items, first our research team developed a list of sensory domains to potentially supplement those already assessed by the SSAS, following an extensive literature review to identify core MSS domains. Because only limited information was available for any of surveys found, we then drafted more than 20 candidate items to cover our a priori defined range of core MSS domains: vision, hearing, tactile, smell, and internal bodily perceptions, as well as a few items assessing related sensory domains involving vestibular and taste. These items centered on potentially missing MSS domains from the SSAS and were written as statements matching the existing SSAS format and response scale (1 = not at all true to 5 = extremely true). After edit and review by our immediate research team (PhD pain researcher, PhD student, and PT students), these candidate items were then reviewed for face validity by a team of 3 additional PhD pain researchers. Collectively, 11 items were qualitatively deemed to be “most clear” and “related to sensory sensitivity” of the original 20, thus were included for consideration in the primary study cohort surveys. The goal was to identify a combination of 10 – 12 items in total, from the original SSAS items and the possible 11 new items, for use as a modified MSS survey. These initial steps are consistent with previous recommended steps for questionnaire development, e.g. literature review and expert opinion.31
As no single validated MSS gold standard exists, for comparative purposes we extracted the 25 items from the four original MSS surveys representing the core-MSS factors (in Part I) as our gold standard, solely for the purposes of this item selection phase. Item-by-item correlational analyses of these 25 plus the 11 new items (36 in total) were conducted to assess the correlations between each single item with the total. We aimed for significant, moderate to strong item-to-total correlations, sufficiently high to demonstrate commonality with core-MSS domains, without being so high as to be repetitive or redundant and thereby represent unique aspects of MSS. Further, as not all core domains were well represented in the 25 items used for comparison, items assessing missing domains (e.g., light sensitivity) were specifically targeted for inclusion. No more than 3 items were retained that assessed a similar core domain for brevity.
Additional Assessments – Second Cohort
A second cohort was recruited to assess two-week test-retest reliability16 and construct validity of the revised MSS survey relative to a separate measure of MSS not used in the original development phase. Inclusion criteria included: ages 18 and over and being fluent in English. Recruitment consisted of University-wide mass emails to faculty, staff and students. Exempt informed consent was approved by the local IRB, and all responses were collected electronically using REDCap.
The Generalized Sensory Sensitivity-8 survey includes 8 yes/no items asking individuals to indicate whether they have experienced various sensitivity symptoms for at least three months in the past year (e.g., sensitivity to sound). These items were identified as measuring generalized sensory sensitivity through analysis of the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network, extracted from the larger, 41-item Complex Medical Symptom Inventory (CMSI), originally intended to assess common symptomology in fibromyalgia.44, 55 Five items provide the external sensory sensitivity subscale (light, sound, smell, taste, and touch) producing a subscale, GSS-5, score ranging from 0 – 5. The other three items assess interoception, i.e., balance, nausea, and rapid heart rate, which when summed altogether create the total GSS-8 score (range: 0 – 8).
Part II – Analyses
Confirmatory Factor Analysis
To confirm all core sensory domains were represented by the revised MSS survey, confirmatory factor analysis with maximum likelihood estimations was used to fit the resulting items to a five-factor model consisting of the primary sensory domains: vision, hearing, tactile, smell, and internal bodily perceptions. Each item was assigned to one of the five domains to ensure all domains were adequately represented. It should be noted, however, that the sum of all items provides the MSAS score, an index of MSS, which does not consider which domain each item was conceptually assigned. The goodness of fit was determined using several common model fit indices, including: the comparative fit index (CFI), the non-normed fit index (NNFI), the root mean square error of approximation (RMSEA), and the standardized root-mean square residual (SRMR). It is generally accepted that a confirmatory factor model has a good fit if the fit indices are high: CFI and NNFI ≥ 0.90, and the error indices are low: RMSEA and SRMR ≤ 0.06.30 In addition, inter-item correlations of the revised MSS were also computed.
Psychometric Assessments
Multiple psychometric assessments of the revised MSS measure were performed, as suggested for questionnaire validation.31 The MSS score was extracted as the total sum of the final retained items. Internal consistency of the revised MSS scale was computed using both Cronbach’s alpha and McDonald’s omega. Construct validity of the revised MSS scale was assessed using Pearson Correlation Coefficients with 1) the four original MSS-related subscales in the primary study cohort, and 2) the GSS-5 subscale and GSS-8 total scores in the new cohort. Convergent validity was assessed in the primary cohort using: 1) independent t-tests for sex differences; 2) the Pearson partial correlation between the revised scale and self-reported pain sensitivity, adjusting for age, sex, BMI and pain schema; and 3) odds of having pain relative to no pain between those with high versus low MSS (i.e., by quartiles) assessed using logistic regression. Finally, two-week, test-retest reliability of the revised MSS scale was assessed using the intraclass correlation coefficient for absolute agreement (ICC3,1)47. It is generally accepted that ICCs between 0.5 and 0.75 indicate moderate reliability and ICCs larger than 0.75 indicate good reliability.33
Results - Part II
Of the 682 who finished the first demographic survey in the primary cohort, a total of 647 respondents completed the original SSAS and the additional candidate MSS items required for Part II (19 more participants than met criteria for Part I inclusion). A summary of the participant characteristics with these additional subjects are provided in the Online Resource, Table S3.
Based on the item-to-total core-MSS score correlations and the goal of addressing the five non-painful sensory domains, 12 items were chosen for the revised MSS scale. Five items from the original SSAS and 7 of the 11 candidate items were retained, with 2–3 items conceptually representing each of the five MSS domains (see Table 3). The correlations of each item with the total core-MSS score ranged from 0.35 to 0.56 (p < 0.0001 for all, Table 4). Confirmatory factor analysis revealed that all 12 items significantly loaded on one of the five sensory factors, as expected (p < 0.0001, Figure 2), supporting the construct validity of the revised scale. Despite a significant Chi-square statistic, X2 (44, N = 647) = 130.06, p < 0.0001, the fit of the CFA model was good, based on the four Goodness-of-Fit metrics: the high fit indices, CFI = 0.95, NNFI = 0.92; and low error indices, SRMR = 0.04, RMSEA = 0.055. Using the factor scores, the resulting five factors of the MSS scale were significantly intercorrelated, R= 0.48 to 0.79 (p < 0.001 for all, Figure 2), supporting the use of a generalized MSS measure. The individual items showed sufficient variance, supporting the value of retaining all 12 items (Online Resource 1, Table S4). We refer to this revised version of the SSAS as the Multisensory Amplification Scale (MSAS), as it now represents more than the somatosensory domain.
Table 4.
Psychometric Characteristics of the Multisensory Amplification Scale (N=647).
| Multisensory Amplification Scale | Mean | SD | Correlation with Total | |||
|---|---|---|---|---|---|---|
|
| ||||||
| # | Item | Origin† | r | p | ||
|
| ||||||
| 1 | I can’t stand smoke, smog, or pollutants in the air. | SSAS | 3.35 | 1.17 | 0.38 | <0.0001 |
| 2 | I am often aware of various things happening within my body. | SSAS | 3.43 | 1.04 | 0.38 | <0.0001 |
| 3 | Sudden loud noise really bothers me. | SSAS | 2.95 | 1.23 | 0.49 | <0.0001 |
| 4 | I hate to be too hot or too cold. | SSAS | 3.37 | 1.12 | 0.52 | <0.0001 |
| 5 | I am quick to sense the hunger contractions in my stomach. | SSAS | 2.75 | 1.11 | 0.36 | <0.0001 |
| 6 | Strong perfumes or colognes really bother me. | New | 2.88 | 1.31 | 0.42 | <0.0001 |
| 7 | I can’t stand certain food textures (e.g., pudding, cottage, cheese or oysters). | New | 1.99 | 1.17 | 0.35 | <0.0001 |
| 8 | Wearing certain fabrics can really bother me (e.g., wool, silk, nylon). | New | 2.19 | 1.18 | 0.50 | <0.0001 |
| 9 | I can’t stand bright lights. | New | 2.46 | 1.20 | 0.56 | <0.0001 |
| 10 | I am often bothered by background sound like a dripping faucet or passing train. | New | 2.23 | 1.18 | 0.50 | <0.0001 |
| 11 | I hate to have the sun shine directly in my eyes. | New | 3.44 | 1.28 | 0.39 | <0.0001 |
| 12 | I am easily irritated by certain types of clothing (e.g., ties, turtlenecks, socks, or waistbands). | New | 2.24 | 1.22 | 0.55 | <0.0001 |
The first 5 items are from the original Somatosensory Amplification Scale (SSAS).
Figure 2.
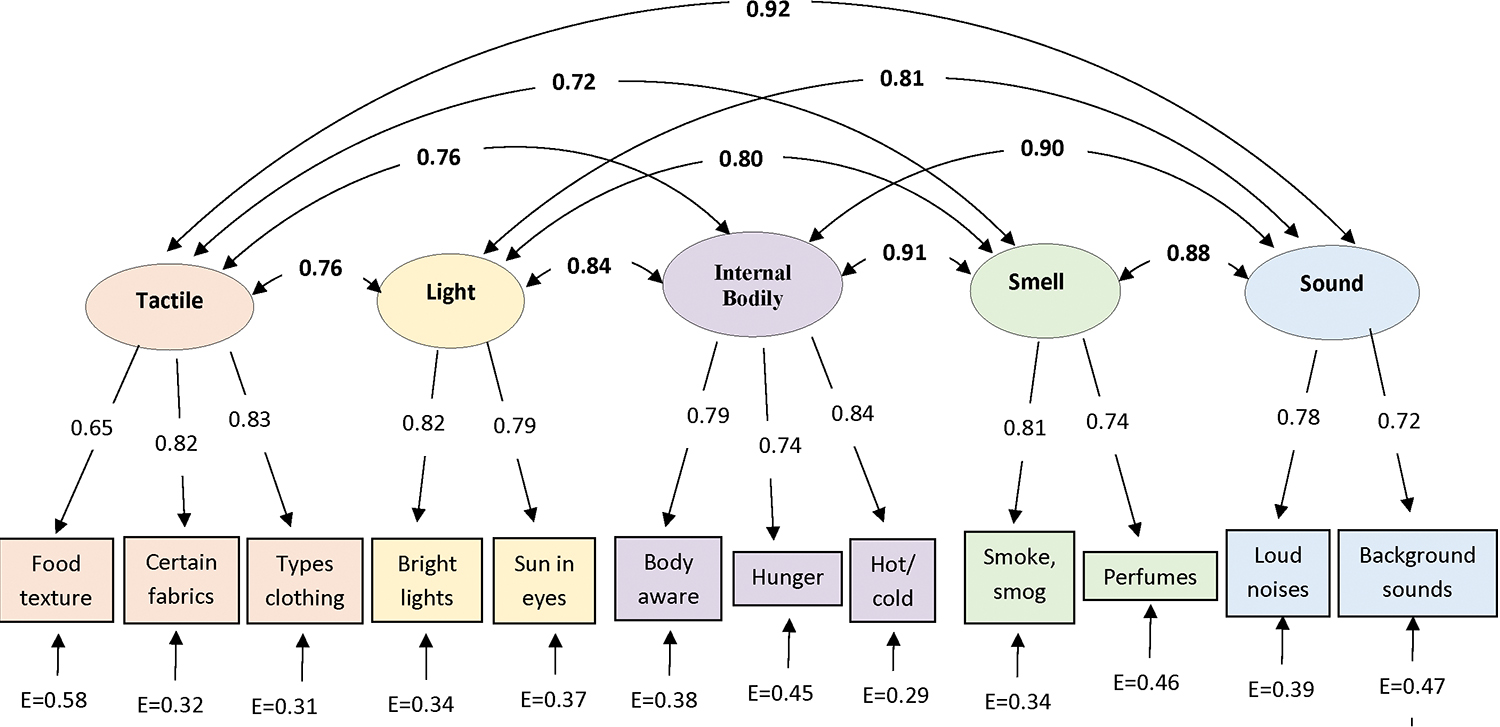
Standardized parameters of the five factors proposed for the Multisensory Amplification Scale (MSAS) model, demonstrating the confirmatory factor solution. Note the value between each factor and the individual items represent the loading coefficients; the values between factors indicates the strong correlations observed between factors (0.72 – 0.91), consistent with a shared construct, multisensory sensitivity. E represents the error variance per item, all lower than the corresponding factor loadings. All parameters were significant, p < 0.0001.
The new cohort included a total of 158 adults who completed the MSAS survey twice in a two-week interval; out of 259 adults (61% completion rate) who initially registered to begin the study. Summary characteristics for this independent new cohort are presented in more detail in the Online Resource, Table S5; briefly 89.2% were Caucasian, 138 were women, and age ranged from 18 – 70, with an average of 25.9 (11.8 SD) years. Pain was reported in 65.8% participants and pain intensity in those with pain averaged 2.3 (2.1 SD). Women did not differ from men on age or pain ratings (adjusted p = 1.0).
The internal consistency of the MSAS was excellent in the primary (α= 0.80 Table S4) and new cohorts (α= 0.82, ω = 0.90). Evidence of construct validity included significant correlations between the MSAS with 1) the original four scales/subscales (range: r = 0.54 to 0.79, p < 0.001) in the primary cohort and 2) the GSS-5 and GSS-8 in the new cohort (r = 0.63 and 0.59, respectively, p < 0.0001). Convergent validity of the MSAS was further supported by a moderate correlation with self-reported pain sensitivity (r = 0.48, p < 0.001) and expected sex differences, with women reporting significantly higher MSAS total scores than men (p < 0.0001; Cohen’s d = 0.52). This is consistent with the original surveys from Part I and prior reports.2, 17. Because MSS is greater in women than men, sex-specific MSAS quartiles for the primary cohort were identified (Table 5) and used to assess odds of reporting pain (Table 6). Relative to those with the lowest quartile MSAS scores (Q1), adults with above average (Q3) or the highest quartile (Q4) MSAS scores were approximately 2 times more likely to report having mild intensity pain (< 3/10) and approximately 3.5 times more likely to report having pain > 3/10 than to be pain-free (p ≤ 0.03, Table 6), even after adjusting for covariates. The MSAS demonstrated excellent reliability, with a two-week test-retest absolute agreement ICC3,1 = 0.91 in the new cohort. Lastly, MSAS declined slightly with age; there was a weak but significant correlation between age and MSAS total score (r = -0.11, p = 0.005) in the original cohort indicating age explains roughly 1% of variability in MSAS.
Table 5.
Multisensory Sensitivity Amplification Scale (MSAS) sex-specific quartiles (n=647).
| MSAS SEX SPECIFIC QUARTILES |
||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
|
|
||||
| MEN | ≤ 24 | 25 – 30 | 31 – 35 | ≥ 36 |
| WOMEN | ≤ 29 | 30 – 34 | 35 – 39 | ≥ 40 |
Note: MSAS score = sum of 12 items, range 12 – 60.
Table 6.
Odds (ORs [95% CI]) of having mild pain (<3/10) or pain ≥3/10 between sex-specific MSAS quartiles (Q2 – Q4) relative to low MSAS (Q1), after adjusting for age, BMI, sex, and pain schema.
| Reported pain level | MSAS Sex-Specific Quartiles |
|||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
|
| ||||
| Pain ≥ 3 | Ref | 1.3 [0.6, 2.7] | 3.7 [1.8, 7.6] | 3.5 [1.7, 7.1] |
| Mild pain (< 3) | Ref | 1.4 [0.9, 2.3] | 1.9 [1.1, 3.2] | 2.3 [1.3, 3.9] |
| No pain (=0) | Ref | Ref | Ref | Ref |
Note: Bold indicates significant odds relative to referent groups.
Discussion
Despite increasing interest in and evidence for MSS as a predictor of centrally mediated pain, there has been little assessment of MSS tools. Our findings reveal that multiple existing scales or subscales assess potentially related, yet non-core MSS constructs, such as avoidance or other coping strategies, distress, fear, and aesthetic preference. Further, several MSS surveys are lacking one or more primary non-noxious sensory domains. To address these limitations, a revised version of the SSAS was developed, the MSAS, following recommended psychometric survey development standards. This 12-item MSAS provides a brief, valid, and simple tool to measure core MSS domains, including: light, smell, sound, tactile, and internal bodily sensations with minimal confounding from other related constructs. It further provides a means to assess a wide range of MSS variability, thus has the potential to assess both high and low sensory sensitivity, which may prove useful as a biomarker for risk or resilience to pain development.
Evidence has been available for some time supporting common central processing across sensory modalities, based on observed interactions between sensory domains, e.g. visual and sound or tactile stimuli.46 In addition, cross-species meta-analyses suggest common cortical and subcortical regions are involved in aversive stimuli regardless of whether it is painful or not.27, 28 Further, dopamine may be a means of generally influencing sensory signaling in brain stem nuclei, potentially contributing to commonalities in response to aversive stimuli.50 Thus, the premise that a generalized sensitivity across multiple sensory domains may be present and measurable, and that the aversive nature of heightened sensory sensitivity could be related to the aversive nature of pain, is biologically plausible.
Although interest in MSS as a factor related to pain has gained traction in the last several years, there is no consensus regarding the operational definition of MSS. For example, some propose that MSS refers to an exaggerated behavioral response (e.g., covering ears in noisy environments),3 whereas others describe it as discomfort from multiple sensory systems,32 with personality-like trait properties.2 Analogously, there is a wide range of terminology used in the literature to describe this phenomenon, including: MSS or multisensory hypersensitivity;51, 53 sensory-processing sensitivity;2 sensory over-responsiveness;4 sensory defensiveness;54 sensory amplification;7 generalized sensory sensitivity;44 and others. This high degree of terminology variance likely reflects the varied fields converging on this construct from different perspectives. We prefer multisensory sensitivity as the construct name, as it does not inherently imply normality or pathology of sensory response (high versus low sensitivity), but broadly and clearly encompasses its most basic components: sensory sensitivity to multiple stimuli, without assumptions regarding the underlying mechanisms or impacts.
Previous studies have successfully identified relationships between MSS and pain using various instruments, including the AASP, SSAS, or GSS or simply several select items related to sensory sensitivity, despite not all sensory domains being equally represented.34, 41, 44, 45, 53 This suggests some degree of flexibility with MSS assessment, likely due to the generalized nature of MSS, where adequate representation of a few core sensory domains, even if incomplete, may still be capable of detecting heightened CNS processing of sensory signals. However, more complete, and psychometrically sound measures likely provide better markers for future clinical and research applications. The MSAS is the first measure of MSS to be developed based on predetermined targets of sensory domains, as well as apply confirmatory factor analysis to confirm those targets, as suggested for optimal questionnaire development and validation,31 to ensure the items adequately fit the conceptual model. Finally, the MSAS demonstrated sound psychometric properties considering multiple different validity and reliability assays, yet efficiently with only 12 items due to minimal extraneous item assessment.
Numerous ‘associated’ factors are observed in several of the original instruments assessed as either non-sensory assessments (e.g., “I prefer fine things”) or as consequences of and/or avoidance behaviors due to the bothersome sensations. While many of these associated factors may be of interest, we propose that they represent a notably separate construct from MSS. An analogy for the distinction between core- and associated-MSS factors may be pain intensity versus pain impact domains. For example, for migraine, there are separate tools to assess severity43 from its functional impact.23 Thus, some of the identified associated-MSS factors may have value in terms of the impact of MSS on psychological and behavioral components. Yet, being able to assess primarily core-MSS domains may prove to be a clearer, albeit indirect, marker of generalized CNS sensory processing. That is, we suggest that how an individual responds to, or copes with, heightened sensory sensitivity is distinct from whether they indeed exhibit sensory sensitivity, thus warrant separate assessment. Future studies may explore the development of a separate MSS-impact questionnaire.
While MSS may be a construct of interest to multiple populations, the use of instruments not originally developed for assessment relative to pain or pain sensitivity may not be optimal for such a purpose. That is, several notable differences between the varied latent factors contained within the four original sensory sensitivity surveys likely reflect the varied original intention and priorities for each instrument. For instance, the SSAS heavily assesses internal, somatic sensations, appropriate for its original proposed application as a correlate of hypochondriasis.7 Whereas the HSPS focuses on stress and aesthetic sensitivity, consistent with its intended purpose of assessing a personality-like trait of being “highly sensitive,” where these authors conceptualized “sensitivity” as being more broadly interpreted than the stated goals here.2 Finally, as a measure of altered sensory processing originally developed for people with autism spectrum disorder, it follows that a large majority (87%) of items from the two AASP subscales assess coping strategies and psychological impacts.13, 37 Thus, while the importance of elevated sensory sensitivity has been noted across a range of fields and conditions, this current study reveals that not all surveys assessing aspects of MSS provide equivalent insights into core sensory sensitivity and may thus be influenced by their original intended populations.
MSS may be useful as an indirect measure of generalized CNS sensitivity and has been proposed as a potential proxy for central sensitization or quantitative sensory testing (QST).17, 56 While MSS and QST likely both provide insights regarding CNS processing, they may not be fully interchangeable. First, MSS has been associated with mechanical but not heat QST assessments, in otherwise pain-free individuals in one study,51 but in another study involving knee OA patients, no relationship between MSS and QST was observed.32 However, this area has not been well explored, leaving the relationships between MSS and QST poorly understood. Although MSS may not provide equivalent information as QST, additional evidence supports MSS as a relevant measure for CNS processing, including: reports of elevated MSS in chronic pain cohorts compared to pain-free adults;5, 24, 53 higher number of pain comorbidities in those with heightened MSS;44 moderate correlations between MSS and the Central Sensitivity Index in patients with low back pain;11 and altered brain imaging in response to normal sensory stimuli in fibromyalgia patients.36 Accordingly, MSS assessment may represent the overall status of CNS sensory processing.
Several limitations of the current study should be considered when interpreting the results. First, not every possible sensory survey currently available was assessed to reduce subject burden; others may assess core MSS domains better or worse than the four considered. Second, the sample did not equally recruit all races and ethnicities, and had more women than men, thus, may not be fully generalizable across all populations. Third, the population was not isolated to pain patients, but rather represented a cross-sectional sample of adults. Although chronic pain is a prevalent problem and many reported having pain, the nature of their pain was not assessed. Fourth, the ASQ responses were rescaled from dichotomous to a Likert-type range to better align items with the remaining instruments for the purposes of identifying MSS factors in the current study. However, this is a departure from its original design thus does not necessarily reflect typical ASQ scores. Lastly, the exclusion of vestibular, taste, proprioception or other possible sensory signals from our targeted core domains may result in missed MSS-relevant domains. However, the inclusion of five core domains is more than included in the original measures considered.
Conclusions
This study uniquely examined and compared the underlying constructs in multiple sensory sensitivity surveys revealing a mix of core- and associated-MSS factors. Although correlated, each survey targeted different aspects of MSS and its impact. This knowledge highlights the complexity of MSS assessment and suggests the necessity of increasing awareness for differentiating core-MSS versus associated-MSS constructs. Accordingly, we modified the SSAS to create the 12-item MSAS as a promising tool for measuring five core-dimensions of MSS that is freely available and potentially useful as a pragmatic tool with good psychometric properties. This measure could help clinicians and researchers assess for heightened MSS as a potential risk factor for, or low MSS as a resilience factor to, altered CNS processing of sensory inputs, including pain. Future research is needed to verify and differentiate MSAS profiles in chronic pain populations and evaluate its relationships with psychological factors, such as depression and anxiety.
Supplementary Material
Perspective.
Multiple multisensory sensitivity (MSS) tools are used, but without exploration of their underlying domains. We found several measures lacking core MSS domains, thus we modified an existing scale to encompass five core MSS domains: light, smell, sound, tactile, and internal bodily sensations using only 12 items, with good psychometric properties.
Acknowledgements.
Research reported in this publication (REDCap) was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002537. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Funding:
NIH Award Number UL1TR002537 to support REDCap through the University of Iowa, CTSA.
Abbreviations:
- AASP
Adolescent/Adult Sensory Profile
- ASQ
Adult Sensory Questionnaire
- HSPS
Highly Sensitive Person Scale
- MSAS
Multisensory Amplification Scale
- MSS
Multisensory Sensitivity
- SSAS
Somatosensory Amplification Scale
- GSS
Generalized Sensitivity Scale
Footnotes
Disclosures:
Conflicts of Interest: All authors declare that they have no conflicts of interest to declare.
Ethics Approval: This study was approved by the University of Iowa Biomedical Institutional Review Board (#201902722), with exempt consent.
Ethics Approval: This study was approved by the University of Iowa Biomedical Institutional Review Board, with exempt consent.
Availability of data:
Frey Law, Laura; Wang, Dan, 2021, "Multisensory Sensitivity Community Sample", https://doi.org/10.7910/DVN/LXFXXT, Harvard Dataverse, V1, UNF:6:gCAtUcDbkHfJw8ILejO0YQ== [fileUNF]
References
- 1.Akoglu H: User's guide to correlation coefficients. Turkish journal of emergency medicine 18:91–93, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron EN and Aron A: Sensory-processing sensitivity and its relation to introversion and emotionality. J Pers Soc Psychol 73:345–368, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Aykan S, Vatansever G, Doğanay-Erdoğan B and Kalaycıoğlu C: Development of Sensory Sensitivity Scales (SeSS): Reliability and validity analyses. Research in Developmental Disabilities 100:103612, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Bar-Shalita T, Seltzer Z, Vatine JJ, Yochman A and Parush S: Development and psychometric properties of the Sensory Responsiveness Questionnaire (SRQ). Disabil Rehabil 31:189–201, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bar-Shalita T, Livshitz A, Levin-Meltz Y, Rand D, Deutsch L and Vatine JJ: Sensory modulation dysfunction is associated with Complex Regional Pain Syndrome. PLoS One 13:e0201354, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barsky AJ, Goodson JD, Lane RS and Cleary PD: The amplification of somatic symptoms. Psychosom Med 50:510–519, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Barsky AJ, Wyshak G and Klerman GL: The somatosensory amplification scale and its relationship to hypochondriasis. J Psychiatr Res 24:323–334, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Blomhoff S, B. JM, S. S, A. D and Malt UF: Perceptual Hyperreactivity to Auditory Stimuli in Patients with Irritable Bowel Syndrome. Scandinavian Journal of Gastroenterology 35:583–589, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Brown C and Dunn W: Adult/adolescent sensory profile: User’s manual. San Antonio, Texas: Psychological Corporation, 2002 [Google Scholar]
- 10.Chimenti RL, Frey-Law LA and Sluka KA: A Mechanism-Based Approach to Physical Therapist Management of Pain. Physical therapy 98:302–314, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark JR, Nijs J, Yeowell G, Holmes P and Goodwin PC: Trait Sensitivity, Anxiety, and Personality Are Predictive of Central Sensitization Symptoms in Patients with Chronic Low Back Pain. Pain Pract 19:800–810, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Comrey AL and Lee HB: A First Course in Factor Analysis, Taylor & Francis, 2013, [Google Scholar]
- 13.Crane L, Goddard L and Pring L: Sensory processing in adults with autism spectrum disorders. Autism 13:215–228, 2009 [DOI] [PubMed] [Google Scholar]
- 14.de Klaver MJ, van Rijn MA, Marinus J, Soede W, de Laat JA and van Hilten JJ: Hyperacusis in patients with complex regional pain syndrome related dystonia. J Neurol Neurosurg Psychiatry 78:1310–1313, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Boer C, Dries L, Terluin B, van der Wouden JC, Blankenstein AH, van Wilgen CP, Lucassen P and van der Horst HE: Central sensitization in chronic pain and medically unexplained symptom research: A systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res 117:32–40, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Diehr P and Patrick DL: Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials 12:142s–158s, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Dixon EA, Benham G, Sturgeon JA, Mackey S, Johnson KA and Younger J: Development of the Sensory Hypersensitivity Scale (SHS): a self-report tool for assessing sensitivity to sensory stimuli. J Behav Med 39:537–550, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ershova RV, Yarmotz EV, Koryagina TM, Semeniak IV, Shlyakhta DA and Tarnow E: A psychometric evaluation of the highly sensitive person scale: the components of sensory-processing sensitivity. Electronic Journal of General Medicine 15, 2018 [Google Scholar]
- 19.Evans RW and Digre KB: Light sensitivity in migraineurs. Headache 43:917–920, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Fleming KC and Volcheck MM: Central sensitization syndrome and the initial evaluation of a patient with fibromyalgia: a review. Rambam Maimonides medical journal 6:e0020–e0020, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frey-Law LA, Lee JE, Wittry AM and Melyon M: Pain rating schema: three distinct subgroups of individuals emerge when rating mild, moderate, and severe pain. J Pain Res 7:13–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey Law L and Wang D (2021). Multisensory Sensitivity Community Sample, Harvard Dataverse.
- 23.Hareendran A, Skalicky A, Mannix S, Lavoie S, Desai P, Bayliss M, Thach AV, Mikol DD and Buse DC: Development of a New Tool for Evaluating the Benefit of Preventive Treatments for Migraine on Functional Outcomes - The Migraine Functional Impact Questionnaire (MFIQ). Headache 58:1612–1628, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harriott AM and Schwedt TJ: Migraine is associated with altered processing of sensory stimuli. Curr Pain Headache Rep 18:458, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N and Conde JG: Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J and Duda SN: The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95:103208, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes DJ and Northoff G: Identifying a network of brain regions involved in aversion-related processing: a cross-species translational investigation. Front Integr Neurosci 5:49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes DJ and Northoff G: Common brain activations for painful and non-painful aversive stimuli. BMC Neurosci 13:60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herr KA, Spratt K, Mobily PR and Richardson G: Pain Intensity Assessment in Older Adults: Use of Experimental Pain to Compare Psychometric Properties and Usability of Selected Pain Scales With Younger Adults. The Clinical Journal of Pain 20:207–219, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lt Hu and Bentler PM: Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal 6:1–55, 1999 [Google Scholar]
- 31.Jensen MP: Questionnaire Validation: A Brief Guide for Readers of the Research Literature. The Clinical Journal of Pain 19:345–352, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Kmiecik MJ, Tu FF, Silton RL, Dillane KE, Roth GE, Harte SE and Hellman KM: Cortical Mechanisms of Visual Hypersensitivity in Women at Risk for Chronic Pelvic Pain. medRxiv:2020.2012.2003.20242032, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo TK and Li MY: A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of chiropractic medicine 15:155–163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Borgne M, Boudoukha AH, Petit A and Roquelaure Y: Chronic low back pain and the transdiagnostic process: How do cognitive and emotional dysregulations contribute to the intensity of risk factors and pain? Scand J Pain 17:309–315, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Listou Grimen H and Diseth Å: Sensory Processing Sensitivity: Factors of the Highly Sensitive Person Scale and Their relationships to Personality and Subjective Health Complaints. Percept Mot Skills 123:637–653, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Sola M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodriguez O, Monfort J, Garcia-Fructuoso F and Deus J: Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol 66:3200–3209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marco EJ, Hinkley LB, Hill SS and Nagarajan SS: Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res 69:48r–54r, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montoya-Pérez KS, Ortega JIM, Montes-Delgado R, Padrós-Blázquez F, de la Roca Chiapas JM and Montoya-Pérez R: Psychometric Properties Of The Highly Sensitive Person Scale In Mexican Population. Psychol Res Behav Manag 12:1081–1086, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakao M and Barsky AJ: Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med 1:17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG and Gatchel RJ: The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. The journal of pain : official journal of the American Pain Society 14:438–445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peña M, Barallat L, Vilarrasa J, Vicario M, Violant D and Nart J: Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: a triple-blind randomized clinical trial. Clin Oral Investig 23:1673–1683, 2019 [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer B and Kinnealey M: Treatment of sensory defensiveness in adults. Occupational Therapy International 10:175–184, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Sajobi TT, Amoozegar F, Wang M, Wiebe N, Fiest KM, Patten SB and Jette N: Global assessment of migraine severity measure: preliminary evidence of construct validity. BMC Neurology 19:53, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrepf A, Williams DA, Gallop R, Naliboff BD, Basu N, Kaplan C, Harper DE, Landis JR, Clemens JQ, Strachan E, Griffith JW, Afari N, Hassett A, Pontari MA, Clauw DJ and Harte SE: Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP Research Network study. Pain 159:2002–2011, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeder S, Gerlach AL and Martin A: Implicit affective evaluation of somatosensory sensations in patients with noncardiac chest pain. J Behav Ther Exp Psychiatry 45:381–388, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Shimojo S and Shams L: Sensory modalities are not separate modalities: plasticity and interactions. Curr Opin Neurobiol 11:505–509, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Shrout PE and Fleiss JL: Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428, 1979 [DOI] [PubMed] [Google Scholar]
- 48.Solan HA, Shelley-Tremblay J and Larson S: Vestibular function, sensory integration, and balance anomalies: A brief literature review. Optometry and Vision Development 38:13, 2007 [Google Scholar]
- 49.van Bemmel PF, Voshaar MAO, Klooster PMT, Vonkeman HE and van de Laar MA: Development and preliminary evaluation of a short self-report measure of generalized pain hypersensitivity. J Pain Res 12:395–404, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vander Weele CM, Siciliano CA, Matthews GA, Namburi P, Izadmehr EM, Espinel IC, Nieh EH, Schut EHS, Padilla-Coreano N, Burgos-Robles A, Chang CJ, Kimchi EY, Beyeler A, Wichmann R, Wildes CP and Tye KM: Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563:397–401, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Merkle SL, Lee JE, Sluka KA, Rakel B, Graven-Nielsen T and Frey-Law LA: Multisensory Sensitivity is Related to Deep-Tissue but Not Cutaneous Pain Sensitivity in Healthy Individuals. Journal of Pain Research 13:2493–2508, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watson NF, Buchwald D, Goldberg J, Noonan C and Ellenbogen RG: Neurologic signs and symptoms in fibromyalgia. Arthritis Rheum 60:2839–2844, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilbarger JL and Cook DB: Multisensory hypersensitivity in women with fibromyalgia: implications for well being and intervention. Arch Phys Med Rehabil 92:653–656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilbarger P and Wilbarger JL: Sensory defensiveness in children aged 2–12: An intervention guide for parents and other caretakers, Therapro, 1991,
- 55.Williams DA and Schilling S: Advances in the assessment of fibromyalgia. Rheumatic diseases clinics of North America 35:339–357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams DA: Phenotypic Features of Central Sensitization. J Appl Biobehav Res 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woolf CJ: Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yunus MB: Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 37:339–352, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Frey Law, Laura; Wang, Dan, 2021, "Multisensory Sensitivity Community Sample", https://doi.org/10.7910/DVN/LXFXXT, Harvard Dataverse, V1, UNF:6:gCAtUcDbkHfJw8ILejO0YQ== [fileUNF]


