Abstract
Objectives
Clinical observations in patients with dermatomyositis (DM) and autoantibodies against the melanoma differentiation–associated protein 5 (MDA5) suggest that the autoantibodies contribute to the pathogenesis of MDA5(+) DM. To gain insight into the role of the anti-MDA5 autoantibodies, we aimed to identify their binding sites on the different domains of the MDA5 protein.
Methods
We developed an in-house ELISA to assess the reactivity against the MDA5 domains (conformational epitopes) in plasma (n = 8) and serum (n = 24) samples from MDA5(+) patients with varying clinical manifestations and disease outcomes. The reactivities were also assessed using western blot (linearized epitopes). An ELISA-based depletion assay was developed to assess cross-reactivity among the different MDA5 domains.
Results
All eight plasma samples consistently showed reactivity towards conformational and linearized epitopes on the helicase domains of the MDA5 protein. The ELISA-based depletion assay suggests that anti-MDA5 autoantibodies specifically target each of the three helicase domains. Twenty-two of the 24 serum samples showed reactivity in the in-house ELISA and all 22 displayed reactivity towards the helicase domains of the MDA5 protein.
Conclusions
Our data revealed that the main immunogenic targets of anti-MDA5 autoantibodies from MDA5(+) patients are the helicase domains. Considering that the helicase domains are responsible for the enzymatic activity and subsequent triggering of an inflammatory response, our findings suggest that binding of anti-MDA5 autoantibodies could alter the canonical activity of the MDA5 protein and potentially affect the downstream induction of a pro-inflammatory cascade.
Keywords: DM, lung diseases, interstitial, autoantibodies, melanoma differentiation-associated protein 5, MDA5, IFN-induced helicase, IFIH1
Graphical Abstract
Rheumatology key messages.
The reactivity of anti–melanoma differentiation–associated protein 5 (MDA5) autoantibodies from DM patients against the MDA5 protein was characterized.
In-house ELISA showed that the main binding sites are located on MDA5 helicase domains.
These findings suggest that binding of anti-MDA5 autoantibodies could alter MDA5 activity.
Introduction
Idiopathic inflammatory myopathies (IIM) or myositis are rare systemic autoimmune diseases, characterized by muscle weakness and extra-muscular manifestations such as arthritis, skin rash and interstitial lung disease (ILD). The majority of patients have at least one myositis-specific autoantibody and establishing an association between each myositis-specific autoantibody and a distinct set of clinical symptoms has greatly improved the diagnosis of IIM [1, 2]. Moreover, the recent addition of B cell–depleting therapies such as rituximab [3, 4] or antibody-replacement therapies like intravenous immunoglobulin (IVIG) to the treatment regimen have been shown to be beneficial [5]. These clinical observations suggest a potential role for the autoantibodies in the pathogenesis of the disease, but experimental evidence is largely missing.
One subgroup of particular interest in which to explore this hypothesis are patients with DM who have autoantibodies against the melanoma differentiation–associated protein 5 (MDA5). These patients often present with severe lung, skin and/or joint manifestations, but muscular manifestations can be mild or absent. Since the patients are at high risk of developing a rapidly progressing (and difficult to treat) form of ILD that can result in respiratory failure and death [6–9], elucidating the role of the autoantibodies in the disease process could influence therapeutic strategies in the future.
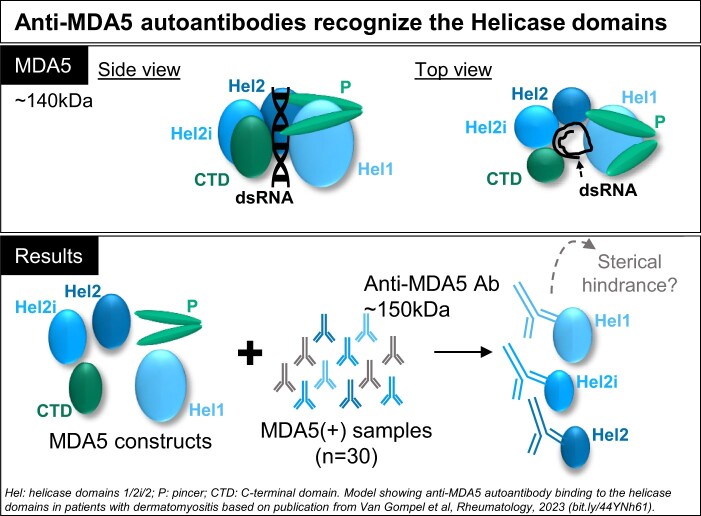
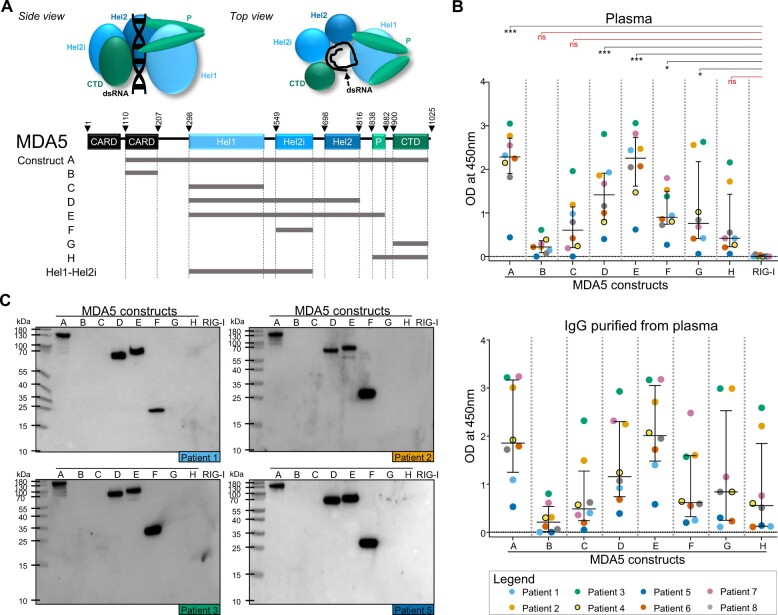
Under physiological circumstances, the MDA5 protein is an intracellular sensor of viral and endogenous dsRNA that upon binding potently induces the type I IFN pathway [10, 11]. The C-terminal domain (CTD) of MDA5 is responsible for RNA recognition and binding, the helicase (Hel) domains 1/2i/2 are responsible for RNA modification through ATP hydrolysis and the N-terminal caspase activation and recruitment domains (CARD) are responsible for intracellular signalling (Fig. 1A) [12, 13]. A recent study confirmed that overactivation of the type I IFN signalling pathway is a key feature in MDA5(+) DM [14]. However, the initial trigger for this mechanism remains unclear.
Figure 1.
MDA5(+) samples from plasma exchange (PLEX) show reactivity to helicase-bearing MDA5 constructs. (A) Schematic representation of the MDA5 protein (side and top view) and the MDA5 protein constructs. (B) ELISA to assess the reactivity of MDA5(+) samples from PLEX (n = 8, upper panel) and the corresponding purified IgG (n = 8, lower panel) against conformational epitopes on the MDA5 constructs A–H and the negative control (RIG-I). (C) Western blot analysis to assess the reactivity of purified IgGs against linearized epitopes on the MDA5 constructs A–H and RIG-I (n = 4 representative blots). In (B), statistical analysis was performed using Kruskal–Wallis test with Dunn’s correction: *P-value < 0.05, ***P-value < 0.001, nsP-value > 0.05. Dots represent individual subjects, lines/bars represent median (± interquartile range). MDA5: melanoma differentiation–associated protein 5; OD: optical density; CARD: N-terminal caspase activation and recruitment domains; Hel: helicase domains 1/2i/2; P: pincer; CTD: C-terminal domain; RIG-I: retinoic acid–inducible protein I
Key to understanding the antigen specificity of the autoimmune response is to identify the binding sites on the antigen, or epitopes, that are recognized by the autoantibodies. These epitopes can be linear or conformational. Linear epitopes are present in the primary structure of the protein and consist of a continuous sequence of amino acids. Conformational epitopes are present in higher-order protein structures and consist of discontinuous sequences of amino acids that are only brought together upon protein folding. Therefore, conformational epitopes are lost upon protein denaturation [15].
To better understand the nature of the polyclonal anti-MDA5 autoantibody response in patients, we aimed to identify the antibody binding sites on the different domains of the MDA5 protein.
Patients and methods
Subjects and clinical evaluation
The first sample collection consisted of plasma from plasma exchange (PLEX) that was performed on MDA5(+) patients from Sorbonne Université (France, n = 7) and from Karolinska Institutet (Sweden, n = 1) as part of the treatment strategy.
The second sample collection consisted of sera from 24 patients diagnosed between 1999 and 2021 at Karolinska University Hospital (Sweden) and found to be MDA5(+) by a commercially available lineblot assay (Euroimmun, Lübeck, Germany) and/or immunoprecipitation combined with ELISA [16] kindly performed by Dr T. Mimori (Kyoto, Japan). Patients were classified according to the 2017 EULAR/ACR IIM classification criteria [17]. Sera (n = 100) from age [mean (s.d.) 56.0 (15.0) years] and sex-matched (40% male) healthy subjects with no suspicion of autoimmune disease were used to determine the cut-off for MDA5(+) in the in-house ELISA. Serum and plasma samples were stored at –80°C.
Amyopathic DM was defined by the absence of patient-reported muscle weakness and laboratory signs of myopathy. Clinically amyopathic DM was defined by the absence of patient-reported muscle weakness with some signs of myopathy. Myositis-associated ILD was defined as radiological observations of inflammation or scarring (fibrosis) and/or abnormal pulmonary function tests (total lung capacity; forced vital capacity; diffusing capacity for carbon monoxide). Rapidly progressing ILD was defined as rapid worsening of pulmonary manifestations within 3 months after the diagnosis of ILD. Disease duration was defined as the time between patient-reported onset of symptoms and diagnosis. Ethnicity was reported by the physician. Demographic, laboratory and clinical data at diagnosis were retrieved from the SweMyoNet register, the international MYONET (former Euromyositis) register and clinical records.
The study complied with the Declaration of Helsinki and the ethical permits were issued by the Regional Ethics Committee in Stockholm and the Swedish Ethical Review Authority (DNR 2005/792-31/4, amendment 2020-06900) and by the Comité de Protection des Personnes ‘Ile-de-France V’ in Paris, France (NCT05454527). All subjects included in this study provided written informed consent for the use of the samples for research purposes. Patients were not actively involved in this research project, but results from our research are regularly communicated to the patient representatives at the Division of Rheumatology at Karolinska Institutet and at (inter)national conferences with patient participation.
Affinity purification of human anti-MDA5 autoantibodies
Anti-MDA5 autoantibodies were purified from plasma samples (at least 150 ml per sample) using two-step affinity chromatography following a standard protocol [18, 19]. First, the IgG fraction, hereafter referred to as ‘purified IgG’, was isolated from the plasma using a Protein G affinity column. Next, anti-MDA5 autoantibodies (hereafter referred to as ‘purified anti-MDA5 autoantibodies’) were purified from the IgG fraction using an in-house generated MDA5-coupled column and used to generate a standard curve to determine the anti-MDA5 autoantibody concentration in serum samples. The IgG concentration of the purified fractions was measured by NanoDrop (Fisher Scientific GTF AB, Gothenburg, Sweden). Experimental details of the purification process (yield, purity and specificity) are described in Supplementary Data S1, Supplementary Table S1 and Supplementary Fig. S1 (available at Rheumatology online).
Enzyme-linked immunosorbent assay
An in-house ELISA was developed to determine the reactivity levels [in optical density (OD) and µg/ml] of samples against the MDA5 protein and to identify the epitopes on the different domains of the MDA protein. Eight constructs were designed based on the crystal structure of the MDA5 protein and the available literature [11] and contained one domain or a combination of domains of the MDA5 protein as presented in Fig. 1A (Supplementary Data S1.1 and Supplementary Table S2, available at Rheumatology online) [20, 21]. A retinoic acid–inducible protein I (RIG-I) construct, part of the same protein family as MDA5, served as a negative control. Briefly, plates coated with streptavidin (0.5 µg/ml in PBS) were blocked with 1% BSA in PBS 0.05% Tween20 (PBST) and incubated for 1 h with biotinylated MDA5 constructs at 0.25 µg/ml (in PBST 0.1% BSA). Plates were incubated for 1.5 h with samples diluted in PBST 0.1% BSA. A secondary horseradish peroxidase (HRP)-conjugated F(ab′)2 Fragment Goat Anti-Human IgG (Jackson ImmuResearch Europe Ltd, Ely, UK) was diluted 1:10 000 (in PBST 0.1% BSA) and incubated for 30 min. The assay was developed by incubating the plates with 3,3′,5,5′-tetramethylbenzidine substrate for 5 min and the reaction was stopped by adding 0.1 M H2SO4. The OD was measured at 450 nm using the SpectraMax Plus 384 microplate reader. Anti-MDA5 autoantibody levels in sera were estimated by interpolating the OD values from a sigmoidal 4PL log10(x) standard curve, which consisted of a serial dilution of anti-MDA5 autoantibodies purified from five plasma samples from PLEX that were pooled in equal parts. Samples included plasma (and corresponding purified IgG) and sera from patients with MDA5(+) DM and sera from healthy subjects. MDA5(+) plasma samples were diluted 1:5000 and the corresponding purified IgGs were incubated at 1 µg/ml. MDA5(+) sera were diluted from 1:500 to 1:20 000 to fit in the linear range of the standard curve. Healthy control sera were diluted to 1:500. Receiver operating characteristic analysis was performed to determine the cut-off of the in-house ELISA.
To evaluate the cross-reactivity of samples towards different Hel-bearing constructs, an ELISA-based depletion assay was developed [22]. Briefly, wells coated with a Hel-bearing construct were incubated with purified IgGs from MDA5(+) plasma for 30 min, after which the supernatant was transferred to a next well that was coated with the same construct. This step was repeated four times to deplete the reactivity. After depletion, the supernatant was transferred to wells coated with another Hel-bearing construct and the residual reactivity was assessed. The reactivity levels were expressed as relative reactivity index (%), the ratio between the residual reactivity and the original reactivity towards that construct (in OD). The depletion efficiency is expressed as the difference in relative reactivity index before and after depletion [23, 24]. Additional technical details are described in Supplementary Data S1.5, available at Rheumatology online.
Western blot
Western blot was carried out to identify linearized epitopes within the eight MDA5 constructs. The constructs (250 ng/well) were denatured and separated by electrophoresis as described in Supplementary Data S1.4, available at Rheumatology online. The denatured constructs were transferred to a PVDF membrane using the iBlot® Gel Transfer Device and iBlot® Transfer Stacks (both Fisher Scientific GTF AB, Gothenburg, Sweden). The membrane was air-dried and washed with PBS 0.1% Tween 20 (PBST) and blocked at room temperature for 1 h in PBST 5% milk (blocking buffer). The membranes were incubated overnight at 4°C with purified IgG fractions (n = 8) at 1 µg/ml in blocking buffer. After washing, membranes were incubated at room temperature for 1 h with HRP-conjugated F(ab′)2 Fragment Goat Anti-Human IgG (diluted 1:10 000 in blocking buffer, Jackson ImmuResearch Europe Ltd, Ely, UK) and washed again. Finally, the membranes were subjected for 5 min to SuperSignal™ West Pico Chemiluminescent Substrate (Fisher Scientific GTF AB, Gothenburg, Sweden) for development. Western blots were visualized using the ChemiDoc Imaging System and analysed using Image Lab (both Bio-Rad, Solna, Sweden).
Statistical analyses
Descriptions of continuous variables are expressed as mean and s.d. or median and interquartile range and categorical variables are expressed as frequencies (%). The Kruskal–Wallis test with Dunn’s correction was used to compare autoantibody levels. Two-tailed P-values <0.05 were considered statistically significant. GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA) and R (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis and visualization.
Results
Anti-MDA5 reactivity profile in plasma samples from PLEX
Reactivity against the different domains of the MDA5 protein (Fig. 1A) was first determined using plasma samples that originated from PLEX, carried out on MDA5(+) patients with ILD at high risk of mortality due to respiratory distress (Table 1). PLEX was performed 2 months (in 6/8 cases), 9 months or 40 months after diagnosis of DM. All plasma samples showed significant reactivity in the ELISA towards the MDA5 constructs A, D, E, F and G compared with the negative control (RIG-I), whereas no significant difference in reactivity was measured towards constructs B, C and H (Fig. 1B, upper panel). Similar reactivity profiles were observed in the IgG purified from these plasma samples (Fig. 1B, lower panel, Supplementary Data S1, available at Rheumatology online). The reactivities towards constructs A, D, E and F (which all contain at least one helicase domain) were confirmed by western blot analysis (n = 8, Fig. 1C) [19].
Table 1.
Demographic and disease characteristics of the MDA5(+) patients
| PLEX | Serum | |
|---|---|---|
| n = 8 | n = 22a | |
| Demographics | ||
| Age at diagnosis (years), mean (s.d.) | 51.1 (10.7) | 43.7 (12.9) |
| Gender: male, n (%) | 5 (62.5) | 14 (63.6) |
| Ethnicity | ||
| Caucasian | 7 (87.5) | 21 (95.5) |
| African | 0 (0) | 0 (0) |
| Asian | 1 (12.5) | 1 (4.6) |
| Diagnosis | ||
| PM, n (%) | 0 (0) | 3 (13.6) |
| DM, n (%) | 5 (62.5) | 19 (86.4) |
| ADM, n (%) | 0 (0) | 1 (5.3) |
| CADM, n (%) | 3 (37.5) | 1 (5.3) |
| Myositis-associated autoantibodies | ||
| Anti-SSA/Ro52 positive, n (%) | 2 (25) | 11 (50.0) |
| Clinical symptoms at diagnosis | ||
| Muscular manifestations | ||
| Muscle weakness, n (%) | 3 (50)c | 8 (47.1)e |
| MMT8, mean (s.d.) | NA | 75.07 (6.7)g |
| Elevated muscle enzymes, n (%) | NA | 11 (68.8)f |
| Joint manifestations | ||
| Arthritis/arthralgia, n (%) | 3 (50)c | 9 (50.0)d |
| Dermatological manifestations | ||
| Heliotrope rash, n (%) | 4 (50) | 10 (55.6)d |
| Gottron’s papules/sign, n (%) | 7 (87.5) | 15 (83.3)d |
| Respiratory manifestations | ||
| TLC <80%, n (%) | NA | 12 (70.6)e |
| FVC <80%, n (%) | NA | 12 (70.6)e |
| DLCO <75%, n (%) | NA | 9 (69.2)h |
| ILD, n (%) | 8 (100) | 16 (88.9)d |
| RP-ILD, n (%) | 6 (75) | 3 (16.7)d |
| Disease course | ||
| Disease duration (months), median (IQR) | 2.0 (0.7–3.3) | 2.0 (1.0–3.0) |
| Immunosuppressive treatment, n (%) | 6 (100)c | 16 (72.7) |
| Time to plasmapheresis <2 months, n (%) | 6 (75) | NA |
| Time to plasmapheresis >2 months, n (%) | 2 (25) | NA |
| Deceased, n (%) | 4 (57.1)b | 2 (11.1) |
| Respiratory failure, n (%) | 4 (100) | 2 (100) |
| Radiographic lesions, n (%) | NA | 16 (88.9)d |
One patient was included in both collections. Missing data: b1, c2, d4, e5, f6, g8, h9. MDA5: melanoma differentiation–associated protein 5; PLEX: plasma exchange; ADM: amyopathic DM; CADM: clinically amyopathic DM; MMT8: Manual Muscle Test-8; TLC: total lung capacity; FVC: forced vital capacity; DLCO: diffusing capacity for carbon monoxide; ILD: interstitial lung disease; RP-ILD: rapidly progressing ILD; IQR: interquartile range; NA: not available/applicable.
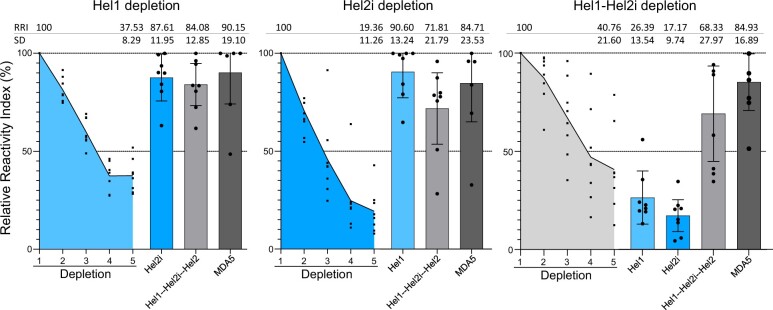
A depletion ELISA was developed to assess cross-reactivity of the purified IgGs among Hel1 (construct C), Hel2i (constructs F) and Hel2 (part of construct D) domains (Fig. 2 and Supplementary Fig. S2, available at Rheumatology online). After depleting the reactivity towards Hel1 [depletion efficiency 62.47% (±8.52)], there was residual reactivity against Hel2i. After depleting the reactivity towards Hel2i [depletion efficiency 80.64% (±11.71)], there was residual reactivity against Hel1. After depleting the reactivity against a construct that contained both Hel1 and Hel2i [depletion efficiency 59.24% (±20.20)], the reactivity against Hel1 and Hel2i substantially decreased (respectively –73.64% and –82.83% on average) while residual reactivity against Hel2 (69.12%) remained. Altogether, these data indicate that MDA5(+) plasma samples from PLEX mainly show reactivity towards conformational and linearized epitopes on the MDA5 helicase domains and suggest that MDA5 autoantibodies can target each of the helicase domains.
Figure 2.
Depletion ELISA shows anti-MDA5 autoantibodies specifically target the helicase domains of the MDA5 protein. Relative Reactivity Index (RRI, residual reactivity as a fraction of the original reactivity, in %) of IgG purified from MDA5(+) plasma samples (n = 8) during and after depletion in ELISA. Dots represent individual subjects, lines/bars represent mean (± s.d.). MDA5: melanoma differentiation–associated protein 5; Hel: helicase domains 1/2i/2
Anti-MDA5 reactivity profile in serum samples
The cut-off for MDA5 positivity against construct A in the in-house ELISA was determined based on the reactivity in serum samples from healthy subjects and using receiver operating characteristic analysis set at 1.07 µg/ml (specificity 91.67%, sensitivity 94.95%, likelihood ratio 18.15, Fig. 3A). Twenty-two of 24 serum samples from patients with MDA5(+) DM had anti-MDA5 autoantibody levels >1.07 µg/ml with our in-house ELISA, while two serum samples had levels <1.07 µg/ml and were excluded from further analysis (Fig. 3B). Demographic, clinical and laboratory data at time of DM diagnosis for the MDA5(+) patients are summarized in Table 1. At the time of sampling [<14 days (18/22) or >1 year after diagnosis (4/22)], 72.7% of patients were on immunosuppressive treatment.
Figure 3.
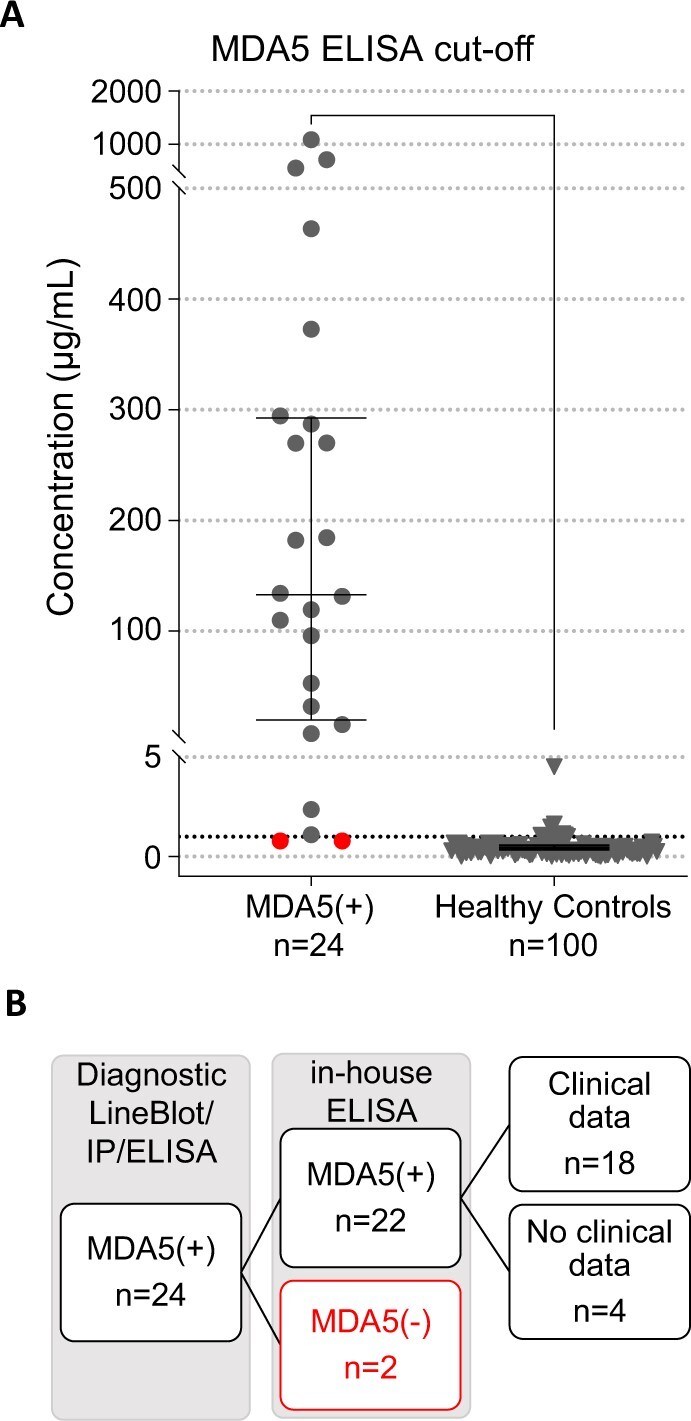
Receiver operating characteristic curve analysis to determine the cut-off for positivity of the in-house MDA5 ELISA. (A) The cut-off for reactivity against MDA5 construct A in the in-house MDA5 ELISA was determined based on subjects diagnosed with MDA5(+) DM (n = 24) and healthy control subjects (n = 100) using receiver operating characteristic analysis and set at 1.07 µg/ml (dotted line). Red dots indicate samples below cut-off. Dots represent individual patients, lines/bars represent median (± interquartile range). (B) Flow diagram of in-/excluded patients with MDA5(+) DM. MDA5: melanoma differentiation–associated protein 5; IP: immunoprecipitation
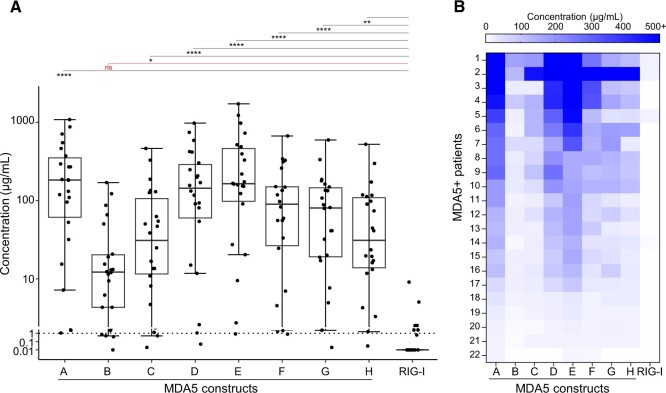
We next assessed the reactivity profiles of the MDA5(+) serum samples (n = 22) in ELISA. The median anti-MDA5 autoantibody level was 158.0 µg/ml (interquartile range 47.5–313.9 µg/ml). All sera showed reactivity in ELISA towards conformational epitopes on constructs D and E (Fig. 4A, Supplementary Table S3 and Supplementary Figs S3 and S4, available at Rheumatology online) and the reactivity towards the negative control (RIG-I) was significantly lower compared with construct A. Overall, patients that showed high/low reactivity towards construct A respectively showed high/low reactivity towards constructs C, D, E, F, G and H (Fig. 4B). These results confirm that the MDA5-helicase domains are the main target of anti-MDA5 autoantibodies.
Figure 4.
MDA5(+) sera show reactivity to multiple MDA5 constructs, with the main reactivity directed towards helicase-bearing constructs. (A) Reactivity profile of MDA5(+) serum samples (n = 22) in in-house ELISA, reported as anti-MDA5 autoantibody levels calculated based on a standard curve consisting of a serial dilution of purified anti-MDA5 autoantibodies. The black dotted line represents the cut-off for reactivity against construct A. Statistical analysis was performed using Kruskal–Wallis test with Dunn’s correction: *P-value < 0.05, **P-value < 0.01, ****P-value < 0.0001, nsP-value > 0.05. Dots represent individual patients, lines/bars represent median (± interquartile range). (B) Heatmap showing the reactivity profiles in individual patient samples, ranked from highest to lowest reactivity against construct A. MDA5: melanoma differentiation–associated protein 5
Discussion
Despite emerging clinical insight, anti-MDA5 autoantibody–associated disease remains difficult to treat. This highlights the need to gain more mechanistic insights in the role of the anti-MDA5 autoantibodies and the MDA5 as an autoantigen. Here we aimed to identify the anti-MDA5 autoantibody binding sites on the different domains of the MDA5 protein. We observed reactivity against conformational epitopes on the helicase, pincer and CTD domains in all the MDA5(+) samples, with the highest reactivity against the three helicase domains. Moreover, the depletion experiments suggest that there are specific autoantibodies against each helicase domain. We only observed reactivity against linearized epitopes within the Hel2i domain in MDA5(+) plasma samples from PLEX. In ELISA, there was no difference in the reactivity profile between samples from PLEX and serum samples. Altogether, we propose that the helicase domains are the main immunogenic domains of the MDA5 protein.
Myositis-specific autoantibodies often target intracellular endogenous proteins that have nucleic acid binding capacities, like histidyl-tRNA synthetase (HisRS, the target of Jo1 autoantibodies) or tripartite motif containing-21 (TRIM21, the target of Ro52 autoantibodies). MDA5 is an intracellular sensor of viral/endogenous RNA and is a potent inducer of the pro-inflammatory type I IFN cascade [11, 13]. After binding RNA through the CTD and modifying the RNA through the helicase domains, the CARD domains are responsible for downstream signalling. Our findings that the main target of the anti-MDA5 autoantibodies are located in the enzymatically active helicase domains [12] suggest that the autoantibodies potentially affect the canonical function of the MDA5 protein as an RNA sensor. This would be in line with previous reports that show autoantibody binding may (indirectly) inhibit the enzymatic activity of the HisRS and TRIM21 proteins, of which the downstream effects potentially contribute to the pathogenesis [25–27]. As a consequence of anti-MDA5 autoantibody binding there could be a local dysregulation of the IFN pathway, which we hypothesize may drive the severe lung inflammation that is observed in these patients [14, 28]. However, whether or how the autoantibodies can access their intracellular antigenic target is still under debate and additional functional experiments are needed to explore these hypotheses.
The finding that serum samples taken close to diagnosis showed reactivity towards several domains of the MDA5 protein and that these reactivities were found against each of the helicase domains, might indicate that the process of epitope spreading had occurred already before diagnosis. Under physiological circumstances the mechanism of epitope spreading is important for the diversification of the adaptive immune response. In autoimmune diseases, however, this diversification process exists against self-antigens and its pathogenic role has been reported in several animal models [29] and in autoimmune bullous disease [3, 30, 31]. Since epitope spreading can occur long before the onset of symptoms, this process greatly complicates the serological identification of the immunodominant epitopes. Our results may indicate a longstanding autoimmune reaction, which is in contrast to the acute onset or worsening of pulmonary manifestations in MDA5(+) patients.
One limitation of the current study is the limited number of patient samples. We tried to overcome this limitation by combining samples from different cohorts, but still with too few patients to be able to analyse whether different epitope reactivity is associated with specific clinical manifestations. The finding that we could only confirm MDA5(+) in 30 of 32 samples in our in-house ELISA compared with commercially available or standardized lineblot/immunoprecipitation/ELISA indicates there might be a discrepancy between the different tests, which in the case of MDA5(+) DM could impact the diagnosis and treatment approach. Since the majority of autoantibody epitopes are believed the be conformational [32], this strategy to identify both conformational and linearized anti-MDA5 autoantibody binding sites on the helicase domains could therefore ameliorate diagnostic assays and potentially lay the basis to study whether anti-MDA5 autoantibodies are pathogenic and why/how the MDA5 protein becomes an autoantigen.
Conclusions
By studying the antigen binding sites of the polyclonal anti-MDA5 autoantibody response, we gained some insight in the interaction between the MDA5 autoantigen and the anti-MDA5 autoantibodies. We established that the main binding sites of the anti-MDA5 autoantibodies in samples from patients with MDA5(+) DM are located on the enzymatically active helicase domains. This knowledge could improve the currently available diagnostic tests and will help to elucidate the role of anti-MDA5 autoantibodies in the pathogenesis of DM. Whether the autoantibodies can affect the endogenous function of the MDA5 protein and why/how the MDA5 protein becomes antigenic still needs to be explored.
Supplementary Material
Acknowledgements
We thank Dr Tsuneyo Mimori (Kyoto, Japan) for performing the immunoprecipitation and ELISA assays and Prof. Johan Rönnelid (Uppsala, Sweden) for performing the lineblot assay for diagnosis. We thank Qiongfei Zhou for helping with the code to visualize the results in Fig. 4A and Sepehr Sarrafzadeh Zargar for help with the experiments. This work was previously presented at EULAR2021, ECI2021 and ACR2021.
Contributor Information
Eveline Van Gompel, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden; Laboratory of Tissue Homeostasis and Disease, Skeletal Biology and Engineering Research Center, Department of Development and Regeneration, KU Leuven, Leuven, Belgium.
Deniz Demirdal, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Department of Gastro, Dermatology and Rheumatology, Karolinska University Hospital, Stockholm, Sweden.
Catia Fernandes-Cerqueira, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden.
Begum Horuluoglu, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden.
Angeles Galindo-Feria, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden; Department of Gastro, Dermatology and Rheumatology, Karolinska University Hospital, Stockholm, Sweden.
Edvard Wigren, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden; Structural Genomics Consortium, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden.
Susanne Gräslund, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden; Structural Genomics Consortium, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden.
Ellen De Langhe, Laboratory of Tissue Homeostasis and Disease, Skeletal Biology and Engineering Research Center, Department of Development and Regeneration, KU Leuven, Leuven, Belgium; Division of Rheumatology, University Hospitals Leuven, Leuven, Belgium.
Olivier Benveniste, Centre de Recherche en Myologie, Unité Mixte de Recherche Scientifique 974, Sorbonne Université, INSERM, Paris, France; Département de Médecine Interne et Immunologie Clinique, Centre de Référence Maladies Neuro-Musculaires, Assistance Publique-Hôpitaux de Paris, Groupe Hospitalier Pitié-Salpêtrière, Paris, France.
Antonella Notarnicola, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Department of Gastro, Dermatology and Rheumatology, Karolinska University Hospital, Stockholm, Sweden.
Karine Chemin, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden.
Ingrid E Lundberg, Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden; Department of Gastro, Dermatology and Rheumatology, Karolinska University Hospital, Stockholm, Sweden.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Contribution statement
Study concept and design: E.V.G., C.F.-C., K.C., I.E.L. Acquisition of data: E.V.G., D.D., A.G.-F., E.W., S.G., B.H., O.B., A.N., K.C., I.E.L. Statistical analysis: E.V.G, A.G.-F. Analysis and interpretation of data: E.V.G., K.C., B.H., I.E.L. Drafting of manuscript: E.V.G., K.C., I.E.L. Funding: E.D.L., I.E.L. Supervision: B.H., A.N., K.C., E.D.L., I.E.L. All authors critically reviewed the work and gave final approval.
Funding
This work was supported by grants from The Swedish Research Council [grant number 2020-01378], the Swedish Rheumatism Association, King Gustaf V 80 Year Foundation, Konung Gustaf V: soch Drottning Victorias Frimurarestiftelse, Region Stockholm (ALF project) and Heart and Lung Foundation [grant number 20200379] awarded to I.E.L. E.V.G.’s work is supported by a bursary from the Fonds Joël Hurlet. The sponsors had no role in study design, in writing the report or in the decision to submit the article for publication.
Disclosure statement: K.C. has received a research grant from Janssen, Cilag. I.E.L. has received consulting fees from Corbus Pharmaceuticals, Inc. and research grants from AstraZeneca, has served on the advisory board for Corbus Pharmaceutical, EMD Serono Research & Development Institute, Argenx, Octapharma, Kezaar, Orphazyme and Boehringer Ingelheim, has received speaker fees from Pfizer and Janssen, and has stock shares in Roche and Novartis. The other authors declare no competing interests.
References
- 1. Betteridge Z, McHugh N.. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med 2016;280:8–23. [DOI] [PubMed] [Google Scholar]
- 2. McHugh NJ, Tansley SL.. Autoantibodies in myositis. Nat Rev Rheumatol 2018;14:290–302. [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal R, Oddis CV, Goudeau D. et al. Autoantibody levels in myositis patients correlate with clinical response during B cell depletion with rituximab. Rheumatology (Oxford) 2016;55:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moghadam-Kia S, Aggarwal R, Oddis CV.. Biologics for idiopathic inflammatory myopathies. Curr Opin Rheumatol 2017;29:645–51. [DOI] [PubMed] [Google Scholar]
- 5. Aggarwal R, Charles-Schoeman C, Schessl J. et al. ; ProDERM Trial Group. Trial of intravenous immune globulin in dermatomyositis. N Engl J Med 2022;387:1264–78. [DOI] [PubMed] [Google Scholar]
- 6. Sato S, Hirakata M, Kuwana M. et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005;52:1571–6. [DOI] [PubMed] [Google Scholar]
- 7. Sato S, Hoshino K, Satoh T. et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009;60:2193–200. [DOI] [PubMed] [Google Scholar]
- 8. Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R.. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016;68:689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sato S, Masui K, Nishina N. et al. ; JAMI Investigators. Initial predictors of poor survival in myositisassociated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford) 2018;57:1212–21. [DOI] [PubMed] [Google Scholar]
- 10. Dias Junior AG, Sampaio NG, Rehwinkel J.. A balancing act: MDA5 in antiviral immunity and autoinflammation. Trends Microbiol 2019;27:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu B, Peisley A, Richards C. et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 2013;152:276–89. [DOI] [PubMed] [Google Scholar]
- 12. Bamming D, Horvath CM.. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J Biol Chem 2009;284:9700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rehwinkel J, Gack MU.. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol 2020;20:537–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Y, Chen Z, Jiang S. et al. Single-cell profiling reveals distinct adaptive immune hallmarks in MDA5+ dermatomyositis with therapeutic implications. Nat Commun 2022;13:6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forsström B, Bisławska Axnäs B, Rockberg J. et al. Dissecting antibodies with regards to linear and conformational epitopes. PLoS One 2015;10:e0121673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato S, Murakami A, Kuwajima A. et al. Clinical utility of an enzyme-linked immunosorbent assay for detecting anti-melanoma differentiation-associated gene 5 autoantibodies. PLoS One 2016;11:e0154285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lundberg IE, Tjärnlund A, Bottai M. et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page M, Thorpe R.. Purification of IgG by precipitation with sodium sulfate or ammonium sulfate. In: Walker JM, ed. The protein protocols handbook. Totowa, NJ: Humana Press, 2009: 1749–51. [Google Scholar]
- 19. Ossipova E, Cerqueira CF, Reed E. et al. Affinity purified anti-citrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint. Arthritis Res Ther 2014;16:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gräslund S, Sagemark J, Berglund H. et al. The use of systematic N- and C-terminal deletions to promote production and structural studies of recombinant proteins. Protein Expr Purif 2008;58:210–21. [DOI] [PubMed] [Google Scholar]
- 21. Notarnicola A, Preger C, Lundström SL. et al. Longitudinal assessment of reactivity and affinity profile of anti-Jo1 autoantibodies to distinct HisRS domains and a splice variant in a cohort of patients with myositis and anti-synthetase syndrome. Arthritis Res Ther 2022;24:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou JJ, Wang F, Xu Z. et al. Secreted histidyl-tRNA synthetase splice variants elaborate major epitopes for autoantibodies in inflammatory myositis. J Biol Chem 2014;289:19269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mamillapalli A, Sunil S, Diwan SS. et al. Polymorphism and epitope sharing between the alleles of merozoite surface protein-1 of Plasmodium falciparum among Indian isolates. Malar J 2007;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sutton PL, Clark EH, Silva C, Branch OH.. The Plasmodium falciparum merozoite surface protein-1 19 KD antibody response in the Peruvian Amazon predominantly targets the non-allele specific, shared sites of this antigen. Malar J 2010;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Espinosa A, Hennig J, Ambrosi A. et al. Anti-Ro52 autoantibodies from patients with Sjögren’s syndrome inhibit the Ro52 E3 ligase activity by blocking the E3/E2 interface. J Biol Chem 2011;286:36478–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raben N, Nichols R, Dohlman J. et al. A motif in human histidyl-tRNA synthetase which is shared among several aminoacyl-tRNA synthetases is a coiled-coil that is essential for enzymatic activity and contains the major autoantigenic epitope. J Biol Chem 1994;269:24277–83. [PubMed] [Google Scholar]
- 27. Mathews MB, Bernstein RM.. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature 1983;304:177–9. [DOI] [PubMed] [Google Scholar]
- 28. Hall JC, Rosen A.. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol 2010;6:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. di Zenzo G, Calabresi V, Olasz EB, Zambruno G, Yancey KB.. Sequential intramolecular epitope spreading of humoral responses to human BPAG2 in a transgenic model. J Invest Dermatol 2010;130:1040–7. [DOI] [PubMed] [Google Scholar]
- 30. Didona D, di Zenzo G.. Humoral epitope spreading in autoimmune bullous diseases. Front Immunol 2018;9:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. di Zenzo G, Thoma-Uszynski S, Calabresi V. et al. Demonstration of epitope-spreading phenomena in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol 2011;131:2271–80. [DOI] [PubMed] [Google Scholar]
- 32. Van Regenmortel MV. Mapping epitope structure and activity: from one-dimensional prediction to four-dimensional description of antigenic specificity. Methods 1996;9:465–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.