Abstract
Objectives
Pulmonary hypertension (PH) is a leading cause of death in patients with SSc. The purpose of this study was to determine the prognostic significance of pericardial effusion in patients with SSc-PH.
Methods
Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) is a prospective multicentre registry which enrolled patients with newly diagnosed SSc-PH from 2005 to 2016. The prognostic impact of pericardial effusion status, including those who ever or never had pericardial effusion, and those who had persistent or intermittent pericardial effusion, was analysed. Kaplan–Meier survival analyses, log-rank test, and multivariable Cox proportional hazards regression were performed.
Results
Of the 335 patients with SSc-PH diagnosed by right heart catheterization and documentation of pericardial effusion presence or absence on echocardiogram, 166 (50%) ever had pericardial effusion. Ever having pericardial effusion was not predictive of survival (log-rank test P = 0.49). Of the 245 SSc-PH patients who had at least two echocardiograms, 44% had a change in pericardial effusion status over an average of 4.3 years of follow up. Having a persistent pericardial effusion was an independent predictor of survival [adjusted hazard ratio (aHR)=2.34, 95% CI 1.20, 4.64, P = 0.002], while intermittent pericardial effusion was not a predictor of survival (aHR = 0.89, 95% CI 0.52, 1.56, P = 0.68), in a multivariable-adjusted analysis.
Conclusion
Persistent pericardial effusion, but not ever having had pericardial effusion or intermittent pericardial effusion, was independently associated with poorer survival. Incorporating information from serial echocardiograms may help clinicians better prognosticate survival in their SSc-PH patients.
Keywords: SSc, pulmonary hypertension, pericardial effusion, prospective cohort
Rheumatology key messages.
Temporal variation of pericardial effusion status is frequent in patients with SSc-PH.
Persistent pericardial effusion is independently associated with increased mortality in patients with SSc-PH.
Clinicians may better prognosticate survival in SSc-PH patients by incorporating information from serial echocardiograms.
Introduction
SSc is a systemic autoimmune rheumatic disease characterized by immune dysregulation, vasculopathy and organ fibrosis. Pulmonary hypertension (PH) is a severe manifestation of SSc which leads to significant morbidity and mortality [1, 2].
Pericardial effusion is a common finding in both SSc and SSc-associated pulmonary hypertension (SSc-PH) [3–5]. Although pericardial effusion has been consistently associated with a poorer prognosis in patients with pulmonary arterial hypertension (PAH) in general [6–8], its prognostic significance in SSc-PH has been conflicting in previous studies [2, 5, 9, 10]. Given that pericardial effusion can originate from primary pericardial involvement of SSc [11], it is plausible that its prognostic role in SSc-PH could be different from that in idiopathic PAH. Moreover, the presence of pericardial effusion can change over time in patients with PAH [12, 13], although the temporal variation of pericardial effusion status in SSc-PH is unknown. As patients with SSc-PH will often be monitored with serial echocardiograms, incorporating serial assessments of pericardial effusion status is potentially more informative for clinicians.
The objective of this study was to determine the prognostic significance of ever having had a pericardial effusion and of having a persistent pericardial effusion over time in patients with SSc-PH enrolled in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) registry.
Patients and methods
The PHAROS registry is a prospective, multicentre, observational study of SSc patients who have or are at high risk of developing PH. Each of the 19 participating centres obtained Institutional Review Board approval (Supplementary Table S1, available at Rheumatology online). All the study participants have given written informed consent. Participants were enrolled and followed from 2005 to 2016.
All enrolled participants in the PHAROS registry were adults >18 years who met 1980 American College of Rheumatology criteria for SSc [14] or the LeRoy definitions of limited cutaneous or diffuse cutaneous SSc [15]. Two subgroups of adults with SSc were enrolled and followed in the PHAROS registry: (I) those within 6 months of PH diagnosis confirmed by right heart catheterization (RHC) using the 4th World Symposium of Pulmonary Hypertension’s definition of PH [16] and (II) those with high risk of developing PH if they meet any one of the following criteria: (i) diffusing capacity for carbon monoxide (DLCO) <55% predicted without severe interstitial lung disease (ILD); (ii) forced vital capacity (FVC) %predicted/DLCO %predicted ratio ≥1.6; and (iii) estimated right ventricular systolic pressure (RVSP) >40 mmHg on Doppler echocardiography. Details of the study protocol and population have been reported previously [17].
Participants from the PHAROS registry were included in this study if they were (i) diagnosed with World Health Organization (WHO) Groups 1 (PAH), 2 (PH due to left heart disease) or 3 (PH due to lung disease or hypoxemia) PH during their initial study visit or during follow-up visits (for those who were initially enrolled in the ‘high risk of developing PH’ subgroup) and (ii) had at least one measurement of pericardial effusion status by echocardiogram at or after PH diagnosis. Of note, in PHAROS, group 3 PH only included SSc patients with PH due to ILD.
During the study period in which the PHAROS registry was enrolling participants (2005–2016), PH was defined as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg based on criteria from the 4th World Symposium of Pulmonary Hypertension in 2009 [16]. We therefore used this hemodynamic definition of PH for our primary analysis. In 2018, the hemodynamic definition of PH was updated to an mPAP >20 mmHg. [18] To be classified as pre-capillary PH (WHO group 1 or 3) according to this updated definition, patients also had to have a pulmonary vascular resistance (PVR) ≥3 Woods units and a pulmonary capillary wedge pressure ≤15 mmHg [18]. We performed a sensitivity analysis using this updated PH definition to confirm the robustness of the study findings.
We investigated two primary research questions: (i) whether ever having had pericardial effusion at or after PH diagnosis was associated with poorer prognosis and (ii) whether having a persistent pericardial effusion on serial echocardiograms at and after PH diagnosis was associated with worse survival. When investigating the prognostic significance of persistent pericardial effusion, the study population was restricted to those who had at least two measures of pericardial effusion status after PH diagnosis during the study period. We classified these SSc-PH patients into three groups: persistent pericardial effusion, intermittent pericardial effusion, and never pericardial effusion. Persistent pericardial effusion was defined as having at least two echocardiograms positive for pericardial effusion, with no interim resolution of the pericardial effusion at any study visit. Intermittent pericardial effusion was defined as having interim resolution of pericardial effusion at least once, or only one echocardiogram positive for pericardial effusion. Never pericardial effusion was defined as the persistent absence of pericardial effusion on all echocardiograms. The primary outcome was all-cause mortality. The secondary outcomes were PH-specific and non-PH mortality. Cause of death was determined by the investigator at each site.
Statistical analyses
We compared baseline characteristics between participants who ever had pericardial effusion and those who were persistently negative for pericardial effusion. The baseline visit was defined as the study visit when participants first met the hemodynamic definition of PH. Fisher’s exact test was used to compare categorical variables, and the Mann–Whitney U test was used to compare continuous variables.
For our primary outcome, Kaplan–Meier curves were used to visualize the survival trajectory in patients by pericardial effusion status. The study duration was defined as time from the study visit when participants first met the hemodynamic definition of PH to death or last study visit. The log-rank test was performed for univariate survival analysis. We performed multivariable Cox proportional hazards regression to determine whether pericardial effusion status was an independent predictor of mortality. Covariates were selected based on literature review of significant prognostic predictors in SSc-PH and included age, sex, SSc subtype (diffuse cutaneous SSc, limited cutaneous SSc or other), New York Heart Association (NYHA) functional class, WHO Group of PH, 6-min walk test (6MWT) distance, DLCO, systolic peroxidase–antiperoxidase on echocardiogram, and mPAP and PVR on RHC [2, 19]. For these covariates, 44% of the cohort had at least one missing value at baseline. Missing values of covariates were handled by carry over if values were recorded in any other study visits within 6 months, while multiple imputation was performed for the remaining variables with missing proportion >10%. Twenty imputed datasets were generated, and pooled results were reported for multivariable analyses performed on the imputed datasets. The test of proportionality was performed to test the proportional hazards assumption for Cox regression models [20].
For our secondary outcomes of cause-specific mortality, the Kaplan–Meier estimate is not appropriate because the assumption of noninformative censoring is violated in the presence of a competing risk of death. We therefore used the Cumulative Incidence Function (CIF), which does not require the noninformative censoring assumption, to estimate PH-specific and non-PH mortality. We used Gray’s test [21] to assess the association between pericardial effusion status and cause-specific death. Subgroup analyses were performed in those with WHO group 1, WHO group 2 and WHO group 3 PH because these are clinically and biologically distinct subgroups of SSc-PH.
We performed sensitivity analyses to strengthen the robustness of our findings. We performed sensitivity analyses using the 2018 updated hemodynamic definition of PH [18]. We also performed additional sensitivity analyses to assess whether there was heterogeneity among SSc-PH patients with intermittent pericardial effusion. Patients with intermittent pericardial effusion included those who had pericardial effusion at the first echocardiogram which then resolved, as well as those who did not have pericardial effusion at the first echocardiogram but later developed one. To examine whether these two groups were different, we compared their baseline characteristics and survival.
Statistical significance was set at a two-sided alpha of 0.05. All analyses were performed using R version 4.2.1 (Vienna, Austria).
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
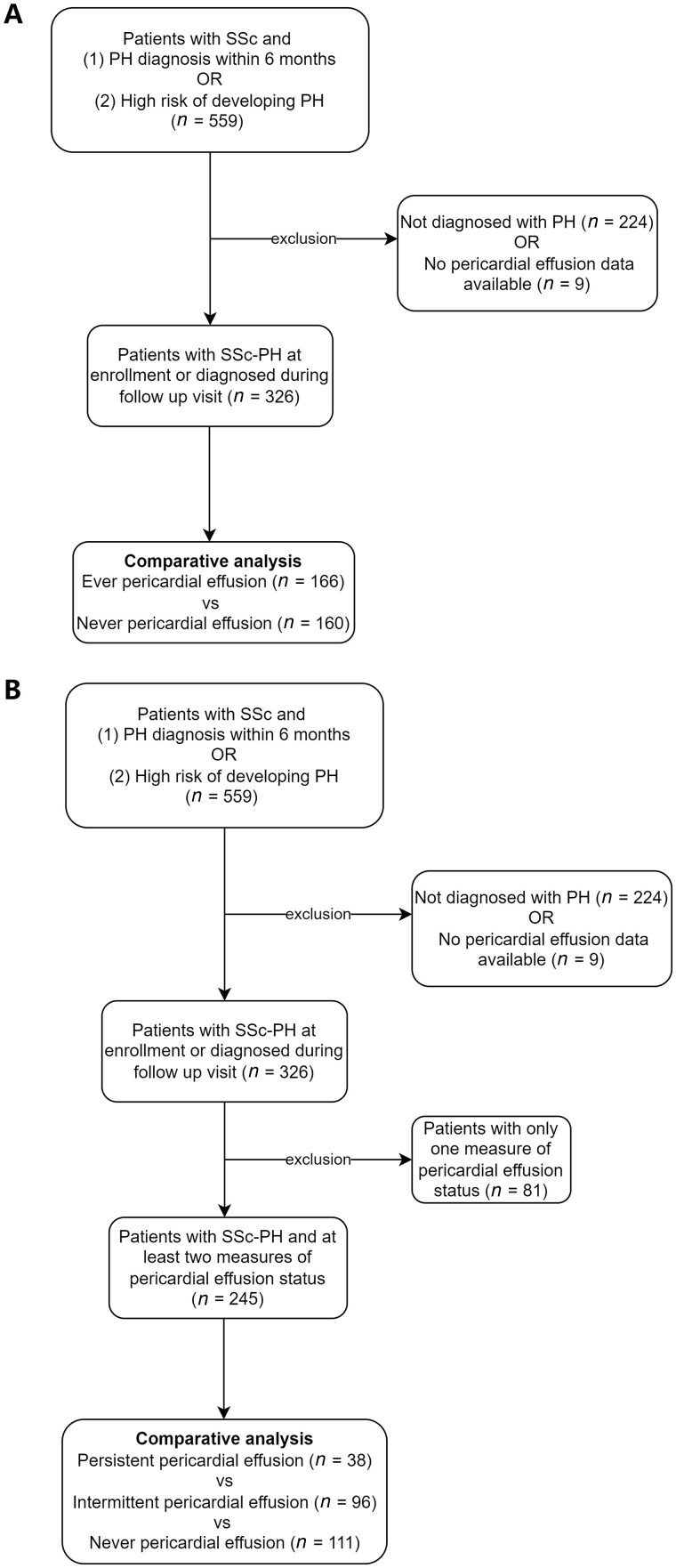
Among a total of 559 SSc patients enrolled in PHAROS, 335 were classified as having PH based on mPAP ≥25 mmHg either at initial or follow-up study visits. Nine patients were excluded because pericardial effusion status was missing at all study visits. A total of 326 patients were therefore included in this study. A flowchart of the included patients was summarized in Fig. 1.
Figure 1.
Flowchart of patient inclusion for the study. (A) Outlines the flowchart for patients included in the comparative analysis between the ever vs never pericardial effusion groups. (B) Outlines the flowchart for patients included for the comparative analysis among the persistent vs intermittent vs never pericardial effusion groups. PH: pulmonary hypertension
Of the 326 included SSc-PH patients, 166 (50.9%) had at least one echocardiogram positive for pericardial effusion at or after PH diagnosis, while 160 (49.1%) were persistently negative for pericardial effusion. The demographic and clinical characteristics of the SSc-PH patients with and without pericardial effusion are presented in Table 1. The mean (s.d.) age was 58 (11) years, and the majority were female (82%) and Caucasian (74%). A greater proportion of patients who ever had pericardial effusion were anti-centromere antibody positive (33% with ever pericardial effusion vs 24% with never pericardial effusion), while a greater proportion of patients who never had a pericardial effusion were Scl-70 antibody positive (19% with never pericardial effusion vs 9% with ever pericardial effusion, P = 0.03). A similar proportion of patients in each group had the diffuse cutaneous subtype (33% with ever pericardial effusion vs 35% with never pericardial effusion, P = 0.51). A greater proportion of patients who ever had pericardial effusion had PAH (WHO group 1), while a greater proportion of patients who never had a pericardial effusion had PH due to SSc-ILD (WHO group 3) (PAH: 70% with ever pericardial effusion vs 58% with never pericardial effusion, PH due to SSc-ILD: 24% with never pericardial effusion vs 14% with ever pericardial effusion, P = 0.049). A smaller proportion of patients in the ever pericardial effusion group were treated with mycophenolate during the study period than those in the never pericardial effusion group (13% vs 23%, P = 0.03). Patients who ever had pericardial effusion also had higher baseline mPAP compared with those who never had pericardial effusion (35 vs 31 mmHg, P < 0.01). There were no statistically significant differences in age, sex, race/ethnicity, disease duration, baseline functional status or other hemodynamic measures between those with and without pericardial effusions.
Table 1.
Demographic and clinical characteristics of SSc-PH patients who ever or never had pericardial effusion
| Total (n = 326) | Ever pericardial effusion (n = 166) | Never pericardial effusion (n = 160) | P-value | |
|---|---|---|---|---|
| Age, mean (s.d.) (317) | 58 (11) | 59 (11) | 58 (12) | 0.50 |
| Female sex (317) | 259 (82) | 139 (85) | 120 (78) | 0.11 |
| Race/Ethnicity (315) | 0.20 | |||
| Caucasian | 234 (74) | 118 (73) | 116 (76) | |
| African-American | 52 (17) | 8 (5) | 22 (14) | |
| Hispanic | 19 (6) | 30 (19) | 11 (7) | |
| Asian/Pacific Islander | 4 (1) | 4 (2) | 0 0 | |
| Other | 6 (2) | 2 (1) | 4 (3) | |
| Diffuse cutaneous SSc subtype (323) | 109 (34) | 54 (33) | 55 (35) | 0.51 |
| Autoantibodies (317) | 0.03 | |||
| Anti Scl-70 | 43 (14) | 14 (9) | 29 (19) | |
| Anti-centromere | 91 (29) | 54 (33) | 37 (24) | |
| Anti-RNA polymerase III | 14 (4) | 4 (2) | 10 (6) | |
| Isolated nucleolar pattern of ANA | 78 (25) | 45 (28) | 33 (21) | |
| Anti-U1RNP | 12 (4) | 5 (3) | 7 (5) | |
| Negative | 19 (6) | 12 (7) | 7 (5) | |
| Mixed or other | 60 (19) | 29 (18) | 31 (20) | |
| Pulmonary hypertension group (326) | 0.05 | |||
| Group 1 | 209 (64) | 116 (70) | 93 (58) | |
| Group 2 | 56 (17) | 27 (16) | 29 (18) | |
| Group 3 | 61 (19) | 23 (14) | 38 (24) | |
| Disease duration (from first non-RP symptom), median, Q1–Q3 (305) | 7.4 (3.5–13.3) | 8.0 (3.8–14.4) | 6.9 (2.8–9.4) | 0.40 |
| Creatine Kinase, U/L, median, Q1–Q3 (171) | 65 (46–101) | 65 (41–98) | 68 (44–104) | 0.27 |
| NYHA functional class (309) | 0.84 | |||
| Class I or II | 166 (54) | 82 (52) | 84 (56) | |
| Class III or IV | 143 (46) | 77 (48) | 66 (44) | |
| 6MWD, meter, median, Q1–Q3 (233) | 360 (259–435) | 338 (250–426) | 365 (269–450) | 0.25 |
| Home oxygen dependent (309) | 106 (34) | 54 (35) | 52 (34) | 1.00 |
| DLCO %predicted, median, Q1–Q3 (279) | 37 (29–48) | 37 (30–47) | 37 (28–48) | 0.73 |
| Right heart catheterization, median, Q1–Q3 | ||||
| Mean peroxidase–antiperoxidase, mmHg (325) | 32 (28–42) | 35 (29–44) | 31 (27–38) | <0.01 |
| Pulmonary arterial wedge pressure, mmHg (325) | 11 (8–15) | 11 (8–15) | 11 (8–15) | 0.66 |
| Pulmonary vascular resistance, WU (320) | 328 (227–565) | 365 (241–633) | 303 (219–506) | 0.07 |
| Cardiac output, L/min (320) | 5.1 (4.0–6.0) | 5.1 (4.0–6.2) | 5.0 (4.0–5.9) | 0.39 |
| Therapeutics (ever, 326) | ||||
| Mycophenolate | 58 (18) | 22 (13) | 36 (23) | 0.03 |
| Cyclophosphamide | 20 (6) | 10 (6) | 10 (6) | 1.00 |
| Phosphodiesterase type 5 (PDE5) inhibitors | 144 (44) | 78 (47) | 66 (41) | 0.32 |
| Endothelin receptor antagonist | 80 (25) | 41 (25) | 39 (24) | 1.00 |
| Prostacyclin | 20 (6) | 12 (7) | 8 (5) | 0.49 |
Values in parentheses are number of patients with data available for each variable at baseline.
Data presented as mean (s.d.), frequency (percentage) and median (interquartile range).
6MWD: 6-min walk test; DLCO: diffusing capacity for carbon monoxide; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; peroxidase–antiperoxidase: pulmonary arterial pressure; PH: pulmonary hypertension; WU: Wood units.
A total of 129 patients died during a mean (s.d.) follow-up time of 3.6 (2.4) years after PH diagnosis. Ever having pericardial effusion was not predictive of mortality (log-rank test P = 0.49) (Fig. 2).
Figure 2.
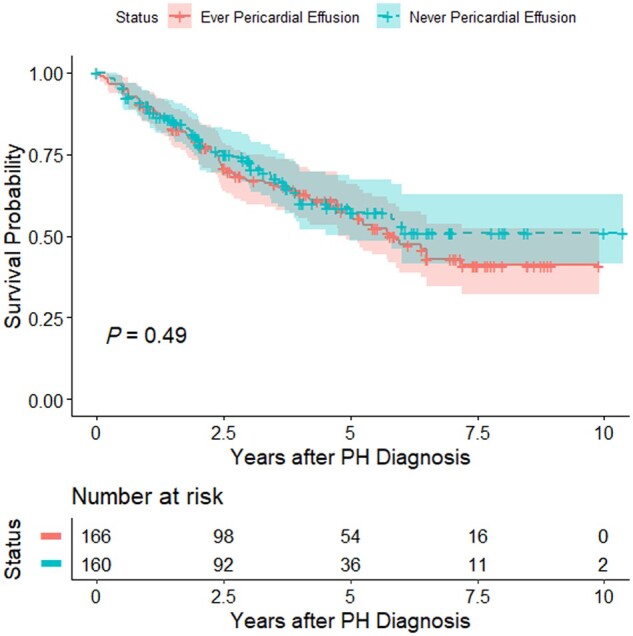
Kaplan–Meier survival curves for SSc-PH patients enrolled in the PHAROS registry who ever or never had pericardial effusion. Log-rank test P-value = 0.49. PH: pulmonary hypertension
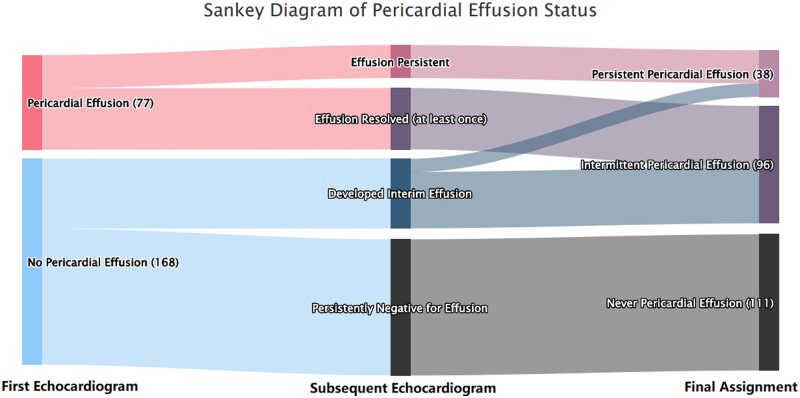
Of the 326 included SSc-PH patients, 245 (75%) had at least two measures of pericardial effusion status. The mean (s.d.) number of measures was 3.8 (2.1) over an average of 4.3-year follow up. Seventy-seven (31%) patients had a pericardial effusion on their first echocardiogram. Among these 77 patients, 27 (35%) were persistently positive for pericardial effusion, while 50 (65%) had resolution of their pericardial effusion at least once during their follow-up echocardiograms. Among the 168 patients with no pericardial effusion on their first echocardiogram, 111 (66%) remained negative for pericardial effusion during their subsequent echocardiograms, while 57 (34%) developed a new pericardial effusion during their follow-up echocardiograms. Of these 57 patients with interim development of pericardial effusions, 24 (42%) had resolution of their pericardial effusion at least once during their subsequent echocardiograms. Overall, 107 out of 245 patients (44%) had changes in their pericardial effusion status after PH diagnosis. The dynamic changes in pericardial effusion status are shown in Fig. 3.
Figure 3.
Sankey diagram of pericardial effusion status changes over time in patients with SSc-PH enrolled in the PHAROS registry
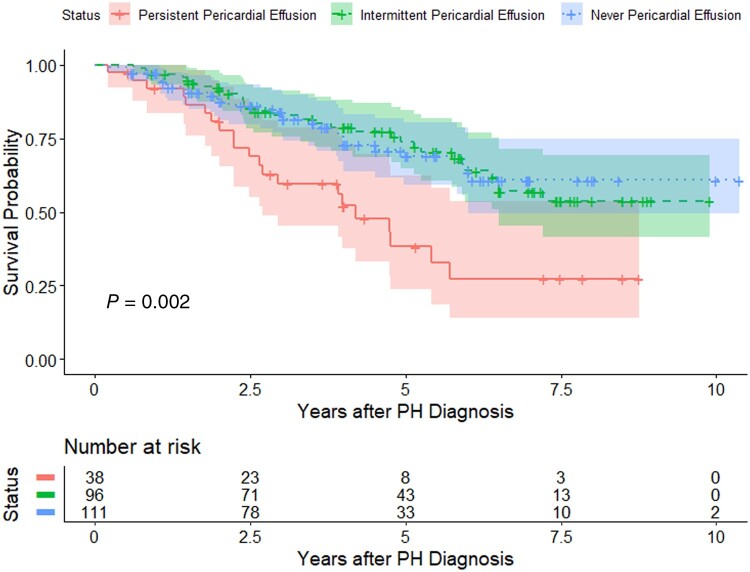
SSc-PH patients with a persistent pericardial effusion had statistically significantly worse survival than those with intermittent or never pericardial effusion (10-year survival 45% vs 69% vs 74% for persistent, intermittent and never pericardial effusion, respectively; log-rank test P = 0.002). In a multivariable Cox proportional hazards regression model, persistent pericardial effusion was an independent predictor of mortality [adjusted hazard ratio (aHR)=2.34, 95% CI 1.20, 4.64, P = 0.002, with the ‘never pericardial effusion’ group as the reference group], while intermittent pericardial effusion was not a predictor of mortality (aHR = 0.89, 95% CI 0.52, 1.56, P = 0.68, with the ‘never pericardial effusion’ group as the reference group) (Fig. 4).
Figure 4.
Kaplan–Meier survival curves for SSc-PH patients enrolled in the PHAROS registry with persistent, intermittent and never pericardial effusion. Log-rank test P-value = 0.002. PH: pulmonary hypertension
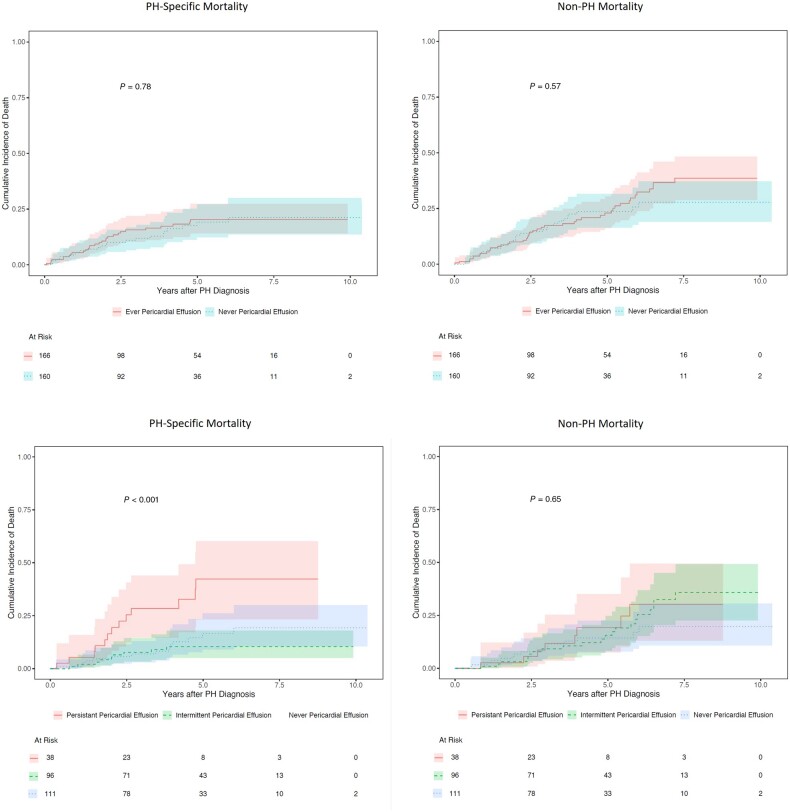
Of the 129 deaths, 53 (41%) were due to PH. Ever having pericardial effusion was not predictive of PH-specific or non-PH mortality (Gray’s test P = 0.78 for PH-specific mortality, P = 0.57 for non-PH mortality). Eighty of the participants who died had at least two measures of pericardial effusion status; 36 (45%) of these participants died from PH. Having a persistent pericardial effusion was predictive of PH-specific mortality (Gray’s test P < 0.001) but not of non-PH mortality (Gray’s test P = 0.57) (Fig. 5).
Figure 5.
Cumulative incidence function curves of PH-specific and non-PH mortality for SSc-PH patients enrolled in the PHAROS registry with different pericardial effusion status. P-values were calculated by Gray’s test. PH: pulmonary hypertension
Subgroup and sensitivity analyses
When evaluating WHO Groups 1, 2 and 3 separately, ever having pericardial effusion remained not predictive of survival (Supplementary Figs S1–S3, available at Rheumatology online). Persistent pericardial effusion remained a significant predictor of mortality in SSc-PAH (WHO group 1) in both univariate (log-rank test P = 0.02) and multivariable models (aHR = 2.54, 95% CI 1.27, 5.04, P = 0.01, with the combined ‘intermittent pericardial effusion’ and ‘never pericardial effusion’ group as the reference group) (Supplementary Fig. S4, available at Rheumatology online). The sample size for WHO Groups 2 and 3 PH was too small to perform meaningful statistical testing, but patients with persistent pericardial effusion had a numerically lower survival rate at the end of their follow-up than those with either intermittent or never pericardial effusion in both of these WHO groups (group 2 PH: survival in the persistent pericardial effusion group was 56% compared with 81% in the combined intermittent/never pericardial effusion group; group 3 PH: survival in the persistent pericardial effusion group was 0% compared with 72% in the combined intermittent/never pericardial effusion group).
Our results were consistent when using the updated hemodynamic definition of PH to define our study population (Supplementary Figs S5 and S6, available at Rheumatology online). In SSc-PH patients with intermittent pericardial effusion, there was no difference in survival between those with and without pericardial effusion at their first echocardiogram in univariate (log-rank test P = 0.82) or multivariable (aHR = 1.06, 95% CI 0.50, 2.22, P = 0.88) analyses (Supplementary Table S2 and Fig. S7, available at Rheumatology online).
Discussion
In this large US multicentre prospective cohort study of patients with SSc-PH and at high risk for PH, we observed that the presence of pericardial effusion often changes over time. Moreover, we found that a persistent pericardial effusion, but not an ever pericardial effusion, was an independent predictor of death in SSc-PH.
We found that compared with SSc-PH patients who never had a pericardial effusion, those who ever had a pericardial effusion had unique clinical features, such as a lower proportion of patients with the Scl-70 antibody and PH due to ILD and a lower percentage of patients treated with mycophenolate. Although the exact cause for these differences is unclear, it is possible that the immunosuppressive therapies used to treat a subset of SSc-PH patients suppressed the development of pericardial effusion in the setting of primary pericardial involvement of SSc. Pericardial abnormalities in SSc may manifest as fibrinous or fibrous pericarditis, pericardial adhesions, or pericardial effusion, and rarely as pericardial tamponade or constrictive pericarditis [4]. Symptomatic pericarditis was observed in up to 20% of SSc patients in the period before modern immunosuppressive therapies were available [22]. In three cross-sectional studies using cardiac magnetic resonance imaging (MRI), pericardial effusion was present in 10 out of 52 (19%) unselected SSc patients [23], as well as in 22 out of 50 (44%) and 9 out of 20 (45%) SSc patients with symptomatic cardiac involvement [24, 25]. In a small study reporting eight SSc patients with symptomatic pericardial disease requiring pericardial biopsies, all had inflammatory or fibrotic changes [26]. Similar pathological findings were reported in 53% of the 58 SSc patients who underwent autopsy in a 1969 study [27]. Thus, pericardial effusions in SSc are multifactorial in aetiology and not necessarily due to severe PH.
We identified changes in pericardial effusion status over time in SSc-PH. Almost half of the SSc patients in this study had changes in their pericardial effusion status after PH diagnosis over an average of 4.3 years. Changes in pericardial effusion status have been described in the broader PAH population [12, 13]. For example, two US-based studies found that 13–16.5% of patients with PAH had changes in pericardial effusion status over 12 months [12, 13]. Thus, our results demonstrated greater variation of pericardial effusion status with longer follow-up time in SSc-PH than in previous studies of a broader PAH population. Our results also suggest that a one-time measure of pericardial effusion in SSc-PH may have limited clinical significance and reliability compared with serial measurements.
We found that persistent pericardial effusion, rather than ever having or intermittent pericardial effusion, was associated with poorer prognosis in SSc-PH. Our findings were consistent whether we used the previous or updated hemodynamic definition of PH. Our results are consistent with those of a previous study in PAH which found that persistence of pericardial effusion in both baseline and follow-up echocardiograms was associated with worse outcome over a shorter one-year follow-up [12]. Our study supports that in those who had interim resolution of their pericardial effusion, the pericardial effusion was due either to their PH which was responsive therapeutics, or to other reversible causes. In our cause-specific survival analysis, persistent pericardial effusion was associated with PH-specific mortality but not with other causes of mortality. This finding supports the theory that persistent pericardial effusions were likely a marker of a more severe spectrum of pulmonary hypertension. Indeed, patients with persistent pericardial effusions had a higher mean (s.d.) baseline mPAP than those with intermittent/never pericardial effusions (42.8 [12.3] vs 34.8 [9.5], P < 0.01). It is also worth noting that among SSc patients who were at risk for developing PH but did not develop PH in the PHAROS registry, baseline pericardial effusion was not associated with the development of new PH but was found to be an independent predictor of poorer survival [28]. This indicates that certain pericardial effusions not due to PH may still contribute to poor prognosis in SSc.
There are some limitations of this study. We did not capture the severity of pericardial effusion nor whether participants were clinically diagnosed with pericarditis. In addition, although different WHO Groups of PH represent aetiologically distinct entities, the statistical power of our subgroup analysis was only adequate for those with SSc-PAH. Nonetheless, the numerical effect size was consistent across all three WHO Groups. Finally, this study was conducted from 2005 to 2016, which may limit its generalizability to the current era when newer PAH-targeted therapies are available for the treatment of SSc-PH.
There are several strengths of our study. We used a large, multicentre, well-characterized cohort with prospectively collected data and long follow-up duration. We included not just SSc-PAH but also WHO Groups 2 and 3 SSc-PH. All patients in PHAROS were enrolled and followed from the time of their incident PH diagnosis, which ensured a more homogeneous observation starting time. Finally, ours is the first study to characterize the dynamic changes of pericardial effusion and its prognostic significance in SSc-PH.
In summary, approximately half of the patients with SSc-PH in this study ever had a pericardial effusion and half of these patients had a change in their pericardial effusion status over time. Persistent pericardial effusion, rather than ever having or intermittent pericardial effusion, was independently associated with mortality. The results of our study may help clinicians better prognosticate survival for their SSc-PH patients by incorporating information from serial echocardiograms.
Supplementary Material
Acknowledgements
PHAROS Investigators: Mary E Csuka, Chris T Derk, Robyn T Domsic, Aryeh Fischer, Tracy Frech, Avram Z Goldberg, Faye Hant, Monique Hinchcliff, Evelyn Horn, Laura Hummers, Vivien Hsu, Susanna Kafaja, Dinesh Khanna, Jerry Molitor, Lesley Saketkoo, Lee Shapiro, Rick Silver, Robert Simms.
Contributor Information
Yiming Luo, Division of Rheumatology, Department of Medicine, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, NY, USA.
Jessica K Gordon, Division of Rheumatology, Department of Medicine, Hospital for Special Surgery, New York, NY, USA.
Jiehui Xu, Division of Biostatistics, Department of Population Health, New York University Grossman School of Medicine, New York, NY, USA.
Kathleen D Kolstad, Division of Rheumatology, Department of Medicine, University of California, Los Angeles School of Medicine, Los Angeles, CA, USA.
Lorinda Chung, Division of Immunology and Rheumatology, Department of Medicine, Stanford University School of Medicine and Palo Alto VA Health Care System, Palo Alto, CA, USA.
Virginia D Steen, Division of Rheumatology, Georgetown University Medical Center, Washington, DC, USA.
Elana J Bernstein, Division of Rheumatology, Department of Medicine, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, NY, USA.
PHAROS Investigators:
Mary E Csuka, Chris T Derk, Robyn T Domsic, Aryeh Fischer, Tracy Frech, Avram Z Goldberg, Faye Hant, Monique Hinchcliff, Evelyn Horn, Laura Hummers, Vivien Hsu, Susanna Kafaja, Dinesh Khanna, Jerry Molitor, Lesley Saketkoo, Lee Shapiro, Rick Silver, and Robert Simms
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
Data collected for the study will not be made publicly available. Data will be made available to qualified researchers upon reasonable request, subject to scientific review. For codes related to this study, please contact Dr Yiming Luo: yl3232@cumc.columbia.edu.
Funding
PHAROS was funded in part by investigator-initiated grants from Actelion and Gilead, the Mackley Foundation of Sibley Hospital and the Scleroderma Foundation. Y.L.’s work was supported by the Rheumatology Research Foundation Scientist Development Award. E.J.B’s work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant K23-AR-075112), the National Heart, Lung, and Blood Institute (grant R01-HL-164758), and the Department of Defense (grant W81XWH2210163).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Tyndall AJ, Bannert B, Vonk M. et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 2. Lefevre G, Dauchet L, Hachulla E. et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum 2013;65:2412–23. [DOI] [PubMed] [Google Scholar]
- 3. Gottdiener JS, Moutsopoulos HM, Decker JL.. Echocardiographic identification of cardiac abnormality in scleroderma and related disorders. Am J Med 1979;66:391–8. [DOI] [PubMed] [Google Scholar]
- 4. Lambova S. Cardiac manifestations in systemic sclerosis. World J Cardiol 2014;6:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung L, Farber HW, Benza R. et al. Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest 2014;146:1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLaughlin VV, Archer SL, Badesch DB. et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573–619. [DOI] [PubMed] [Google Scholar]
- 7. Austin C, Burger C, Kane G. et al. High-risk echocardiographic features predict mortality in pulmonary arterial hypertension. Am Heart J 2017;189:167–76. [DOI] [PubMed] [Google Scholar]
- 8. Batal O, Khatib OF, Dweik RA. et al. Comparison of baseline predictors of prognosis in pulmonary arterial hypertension in patients surviving </=2 years and those surviving >/=5 years after baseline right-sided cardiac catheterization. Am J Cardiol 2012;109:1514–20. [DOI] [PubMed] [Google Scholar]
- 9. Campo A, Mathai SC, Le Pavec J. et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 2010;182:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Launay D, Humbert M, Berezne A. et al. Clinical characteristics and survival in systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease. Chest 2011;140:1016–24. [DOI] [PubMed] [Google Scholar]
- 11. Vandecasteele EH, De Pauw M, Brusselle G. et al. The heart and pulmonary arterial hypertension in systemic sclerosis. Acta Clin Belg 2016;71:1–18. [DOI] [PubMed] [Google Scholar]
- 12. Batal O, Dardari Z, Costabile C. et al. Prognostic value of pericardial effusion on serial echocardiograms in pulmonary arterial hypertension. Echocardiography 2015;32:1471–6. [DOI] [PubMed] [Google Scholar]
- 13. Benza RL, Miller DP, Foreman AJ. et al. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant 2015;34:356–61. [DOI] [PubMed] [Google Scholar]
- 14. Preliminary criteria for the Classification of Systemic Sclerosis (Scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 15. LeRoy EC, Black C, Fleischmajer R. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988;15:202–5. [PubMed] [Google Scholar]
- 16. Badesch DB, Champion HC, Gomez Sanchez MA. et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54: S55–S66. [DOI] [PubMed] [Google Scholar]
- 17. Hinchcliff M, Fischer A, Schiopu E, Steen VD, Investigators P; PHAROS Investigators. Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS): baseline characteristics and description of study population. J Rheumatol 2011;38:2172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simonneau G, Montani D, Celermajer DS. et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolstad KD, Li S, Steen V, Chung L, Investigators P; PHAROS Investigators. Long-term outcomes in systemic sclerosis-associated pulmonary arterial hypertension from the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma Registry (PHAROS). Chest 2018;154:862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patricia M, Grambsch TMT.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. [Google Scholar]
- 21. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- 22. Janosik DL, Osborn TG, Moore TL. et al. Heart disease in systemic sclerosis. Semin Arthritis Rheum 1989;19:191–200. [DOI] [PubMed] [Google Scholar]
- 23. Hachulla AL, Launay D, Gaxotte V. et al. Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 2009;68:1878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krumm P, Mueller KA, Klingel K. et al. Cardiovascular magnetic resonance patterns of biopsy proven cardiac involvement in systemic sclerosis. J Cardiovasc Magn Reson 2016;18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meduri A, Di Molfetta DV, Natale L, Manfredi R.. Cardiac magnetic resonance in systemic sclerosis patients with cardiac symptoms. Eur Rev Med Pharmacol Sci 2017;21:4797–803. [PubMed] [Google Scholar]
- 26. Kitchongcharoenying P, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Nanagara R.. Pericardial fluid profiles of pericardial effusion in systemic sclerosis patients. Asian Pac J Allergy Immunol 2013;31:314–9. [DOI] [PubMed] [Google Scholar]
- 27. D'Angelo WA, Fries JF, Masi AT, Shulman LE.. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969;46:428–40. [DOI] [PubMed] [Google Scholar]
- 28. Hsu VM, Chung L, Hummers LK. et al. Risk factors for mortality and cardiopulmonary hospitalization in systemic sclerosis patients at risk for pulmonary hypertension, in the PHAROS registry. J Rheumatol 2019;46:176–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for the study will not be made publicly available. Data will be made available to qualified researchers upon reasonable request, subject to scientific review. For codes related to this study, please contact Dr Yiming Luo: yl3232@cumc.columbia.edu.