Abstract
Objectives
To compare the efficacy and safety of bimekizumab 160 mg every 4 weeks, a selective inhibitor of IL-17F and IL-17A, with those of biologic/targeted synthetic DMARDs (b/tsDMARDs) in non-radiographic axial SpA (nr-axSpA) and AS.
Methods
A systematic literature review identified randomized controlled trials until January 2023 for inclusion in Bayesian network meta-analyses (NMAs), including three b/tsDMARDs exposure networks: predominantly-naïve, naïve, and experienced. Outcomes were Assessment of SpondyloArthritis international Society (ASAS)20, ASAS40 and ASAS partial remission (PR) response rates at 12–16 weeks. A safety NMA investigated discontinuations due to any reason and serious adverse events at 12–16 weeks.
Results
The NMA included 36 trials. The predominantly-naïve network provided the most comprehensive results. In the predominantly-naïve nr-axSpA analysis, bimekizumab had significantly higher ASAS20 response rates vs secukinumab 150 mg [with loading dose (LD)/without LD], and comparable response rates vs other active comparators. In the predominantly-naïve AS analysis, bimekizumab had significantly higher ASAS40 response rates vs secukinumab 150 mg (without LD), significantly higher ASAS-PR response rates vs secukinumab 150 mg (with LD) and comparable response rates vs other active comparators. Bimekizumab demonstrated similar safety to that of other b/tsDMARDs.
Conclusion
Across ASAS outcomes, bimekizumab was comparable with most b/tsDMARDs, including ixekizumab, TNF inhibitors and upadacitinib, and achieved higher response rates vs secukinumab for some ASAS outcomes in predominantly b/tsDMARD-naïve nr-axSpA and AS patients at 12–16 weeks. In a pooled axSpA network, bimekizumab demonstrated comparable safety vs other b/tsDMARDs.
Keywords: axial spondyloarthritis, systematic literature review, network meta-analysis, b/tsDMARDs, nr-axSpA, r-axSpA
Rheumatology key messages.
Bimekizumab achieves higher response rates than secukinumab for some ASAS outcomes in nr-axSpA and AS.
Bimekizumab is associated with similar response rates compared with other b/tsDMARDs across ASAS outcomes.
Bimekizumab demonstrates similar safety and tolerability to those of other b/tsDMARDs.
Introduction
Axial SpA (AxSpA) is a chronic inflammatory disease that predominantly affects the axial skeleton (SI joints and spine) [1, 2]. AxSpA comprises patients with evident radiographic damage to the SI joints [AS, also known as radiographic axSpA (r-axSpA)] and those without definitive radiographic sacroiliitis [non-radiographic axSpA (nr-axSpA)] [3, 4]. The age of disease onset is typically mid-twenties [5], with an estimated 10–40% of nr-axSpA patients progressing to AS over 2–10 years [6].
Historically, nr-axSpA emerged as a subclassification of axSpA; however, axSpA is now widely considered a single disease spectrum, encompassing both nr-axSpA and AS [7]. AS and nr-axSpA share a similar clinical presentation and disease burden [8–10]; both are associated with chronic back pain, fatigue, and morning stiffness, affecting mobility and the ability to perform daily activities [11, 12]. Many patients with axSpA also have peripheral musculoskeletal manifestations, with peripheral arthritis and enthesitis being most common (affecting an estimated 28–30% and 29–35% of patients, respectively) [13]. Some patients also present with extra-musculoskeletal manifestations, including acute anterior uveitis, psoriasis, and IBD [12, 14–16]. As such, axSpA has a considerable impact on quality of life [17–20].
The initial pharmacological treatment for axSpA is NSAIDs. For patients with active disease and an inadequate response, intolerance, or contraindication to NSAIDs, available therapies include biologic DMARDs (bDMARDs), comprising TNF inhibitors, IL-17A inhibitors, and targeted synthetic DMARDs (tsDMARDs), such as the recently approved Janus kinase (JAK) inhibitors [21, 22].
Despite the available treatments, many patients do not achieve a sufficient treatment response or partial remission, and some lose their clinical response to treatment over time [23, 24]. Furthermore, clinical response to second-line bDMARDs is lower than in bDMARD-naïve patients [25]; hence, there is a considerable need for treatment options achieving deep and sustained responses via a novel mechanism of action [23, 24].
Bimekizumab is a humanized monoclonal IgG1 antibody that recently received marketing authorization in the European Union and the UK, which selectively inhibits IL-17F in addition to IL-17A. IL-17A and IL-17F are pro-inflammatory cytokines and key mediators of inflammation and new bone formation, which leads to structural damage in axSpA [26–28]. Unlike IL-17A–specific inhibitors, bimekizumab enables neutralization of IL-17F/F in addition to IL-17A/A and IL-17A/F. Preclinical data demonstrate that dual blockade of IL-17A and IL-17F is required for optimal inhibition of downstream inflammatory and tissue remodelling responses [29]. In the Phase III trial program for axSpA, bimekizumab resulted in significant and rapid improvements in efficacy outcomes vs placebo [BE MOBILE 1 (NCT03928704) and BE MOBILE 2 (NCT03928743)] [30].
The aim of this analysis was to establish the comparative efficacy, safety, and tolerability of s.c. bimekizumab 160 mg every 4 weeks (Q4W) vs b/tsDMARDs in axSpA using a systematic literature review (SLR) and Bayesian network meta-analyses (NMA). The current analysis provides an up-to-date synthesis of the available evidence, including the BE MOBILE studies [30], which were published after the completion of previous SLRs/NMAs in axSpA. Although TNF inhibitors were included in the analysis, the relative efficacy of these is already well established [31, 32], so this NMA focuses on comparisons between recently approved IL-17A, IL-17A/F and JAK inhibitors.
Methods
Systematic literature review
A clinical SLR was initiated in May 2012 and updated eight times, most recently on 10 January 2023, to identify randomized controlled trial (RCT) evidence assessing bimekizumab and relevant b/tsDMARDs for the treatment of adult patients with AS or nr-axSpA with an inadequate response to, intolerance of, or contraindication to NSAID therapy (see Supplementary Data S1 for dates of all SLR updates, available at Rheumatology online). Studies were required to report outcome measurements after a minimum of 12 weeks of follow-up, but before switch/cross-over or early escape [33, 34]. Eligible interventions comprised IL-17A inhibitors, IL-17A/F inhibitors (i.e. bimekizumab), TNF inhibitors and JAK inhibitors. Eligible comparators comprised any of the aforementioned interventions, conventional DMARDs, NSAIDS, or placebo. The pre-specified population, intervention, comparator, outcomes, and study design (PICOS) elements used to assess study eligibility are presented in Supplementary Table S1, available at Rheumatology online.
The SLR was performed in accordance with best practice guidelines from the Cochrane Collaboration, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and the Centre for Reviews and Dissemination (CRD) [35–37]. The Ovid platform was used to search EMBASE, MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Cochrane Database of Systematic Reviews (CDSR) on 10 January 2023. Electronic database search strings were developed for EMBASE, then translated for the other databases to account for differences in syntax and subject headings (Supplementary Table S2–S4, available at Rheumatology online). Title/abstract screening and full-text screening were both performed by two independent reviewers. Any conflicts regarding eligibility were resolved through discussion; where necessary, arbitration was provided by a third reviewer. Hand-searching of conference proceedings, health technology assessment (HTA) submissions, clinical trial registries, and the reference lists of relevant SLRs/NMAs were used as supplementary measures to ensure all relevant studies were captured (Supplementary Data S2, available at Rheumatology online). Relevant unpublished clinical study reports for bimekizumab were also eligible for inclusion. Data extraction and risk-of-bias assessments were performed by one reviewer, with all data points and risk-of-bias judgments checked by a second independent reviewer. Risk of bias was assessed using the CRD 7-item checklist for RCTs, currently recommended by the National Institute for Health and Care Excellence (NICE) [38].
NMA feasibility assessment
Additional eligibility criteria (Supplementary Table S5, available at Rheumatology online) were applied to identify studies suitable for inclusion in the NMA. Licensed b/tsDMARDs were the comparators of primary interest. Reasons for exclusion from the NMA included atypical axSpA classification criteria, biosimilar studies, and treatments with limited licensing or for which the development program has been terminated. While secukinumab can be increased from 150 mg to 300 mg in clinical practice, the 300 mg dose could not be included in the NMA due to insufficient trial data on the approved s.c. 300 mg dose [39]. Although the MEASURE 3 trial reports data for secukinumab 300 mg s.c. Q4W at week 16, loading was by i.v. infusion, which is currently not approved.
Network meta-analysis
For efficacy outcomes, separate Bayesian NMAs were performed for patients with nr-axSpA and AS. Analyses were performed for three subpopulations, defined by patients’ prior b/tsDMARD exposure:
Predominantly (>50%) b/tsDMARD-naïve network: Studies where >50% of the enrolled patients were b/tsDMARD-naïve or where it can be assumed that >50% of patients were b/tsDMARD-naïve.
100% b/tsDMARD-naïve network: Studies where either 100% of the enrolled patients were b/tsDMARD-naïve or where studies reported separate data for a b/tsDMARD-naïve subgroup.
100% b/tsDMARD-experienced network: Studies where either 100% of the enrolled patients were b/tsDMARD-experienced or where studies reported separate data for a b/tsDMARD-experienced subgroup.
Based on the time point at which the included studies reported primary and secondary efficacy results, the time point used for the NMA was 12–16 weeks; for inclusion in the NMA, studies had to report outcomes after a minimum of 12 weeks of follow-up [40], but before switch/cross-over or early escape. ASAS-defined improvement criteria (used in clinical trials) of interest for the efficacy NMA were ASAS20, ASAS40 and ASAS-PR (Table 1).
Table 1.
Outcomes included in the NMA
| Outcome | Definition |
|---|---|
| ASAS20 and ASAS40 |
|
| ASAS-PR |
|
ASAS20: Assessment of Spondyloarthritis international Society improvement of ≥20%; ASAS40: Assessment of Spondyloarthritis international Society improvement of ≥40%; ASAS-PR: Assessment of Spondyloarthritis international Society partial remission; axSpA: axial SpA; NMA: network meta-analysis.
Two tolerability and safety outcomes at weeks 12–16 were also analysed: discontinuation due to any reason, and serious adverse events (SAEs). These analyses were conducted in a combined nr-axSpA and AS population irrespective of previous TNF exposure, due to the small number of patients experiencing events, and because patient characteristics and dose exposure of treatments were similar across the two indications.
Bimekizumab was compared with both placebo and active comparators. Doses for bDMARD comparators included in the NMA were:
IL-17A inhibitors: ixekizumab 80 mg Q4W s.c., secukinumab 150 mg Q4W s.c. [some patients may first receive initial loading doses (LDs) of secukinumab 150 mg s.c. at weeks 0, 1, 2, 3 and 4 followed by Q4W thereafter; herein referred to as ‘with LD’ or ‘without LD’, respectively)].
IL-17A/F inhibitors: bimekizumab 160 mg Q4W.
TNF inhibitors: adalimumab 40 mg twice-weekly (Q2W) s.c., certolizumab pegol 200 mg Q2W or 400 mg Q4W s.c., etanercept 25 mg BIW or 50 mg QW s.c., golimumab 50 mg Q4W s.c. or 2 mg/kg Q8W i.v., infliximab 5 mg i.v. Q6W. TNF inhibitors were pooled as a single treatment class because the relative efficacy of TNF inhibitors in axSpA is already well established [46, 47]. Moreover, HTA publications, such as NICE TA383, conclude that TNF inhibitors should be considered as a single class with broadly similar effects [48].
Doses for tsDMARD comparators were:
JAK inhibitors: upadacitinib 15 mg once-daily (QD) oral, tofacitinib 5 mg twice-daily (BID) oral (AS only).
Statistical analysis
A Bayesian framework was chosen for the NMAs, as Bayesian analysis is a standard approach that has been extensively used by researchers due to the fact that, as described in the NICE Decision Support Unit (DSU) guidance, ‘simulation from a Bayesian posterior distribution supplies both statistical estimation and inference, and a platform for probabilistic decision making under uncertainty’ [49]. The NMAs were conducted using standard methods for clinical data synthesis in WinBUGs using validated model code for binomial outcomes, using a binomial model with logit link, available from the NICE DSU [49–57]. The WinBUGs models were run for a minimum burn-in of 10 000 iterations to maximize convergence. Subsequently, three chains of at least 1000 samples (3000 simulations) were drawn from the posterior distributions. Both random and fixed effect, and unadjusted and placebo-adjusted models were fitted to the data, with the mean residual deviance and the deviance information criteria (DIC) used to estimate how well the predicted values fitted the observed dataset. An alternative form of the DIC was also estimated (total residual deviance + posterior variance) as, for some analyses, the number of effective parameters was estimated to be negative [58]. However, this parameter did not always differentiate between models, and other factors, such as poor convergence or unrealistically large credible intervals (CrIs), would eliminate a particular model. Fixed-effect (nr-axSpA; combined axSpA) and fixed-effect placebo-adjusted (AS) models offered preferred model fit. The results are expressed as odds ratios (ORs). Significant differences between treatments were based on the 95% CrI for the OR crossing 1. Surface under the cumulative ranking curve (SUCRA) values were calculated for each treatment, with higher values representing higher-ranked treatments [59]. Note that SUCRA values should not be considered in isolation but interpreted alongside OR point estimates and CrIs [59].
Results
Systematic literature review
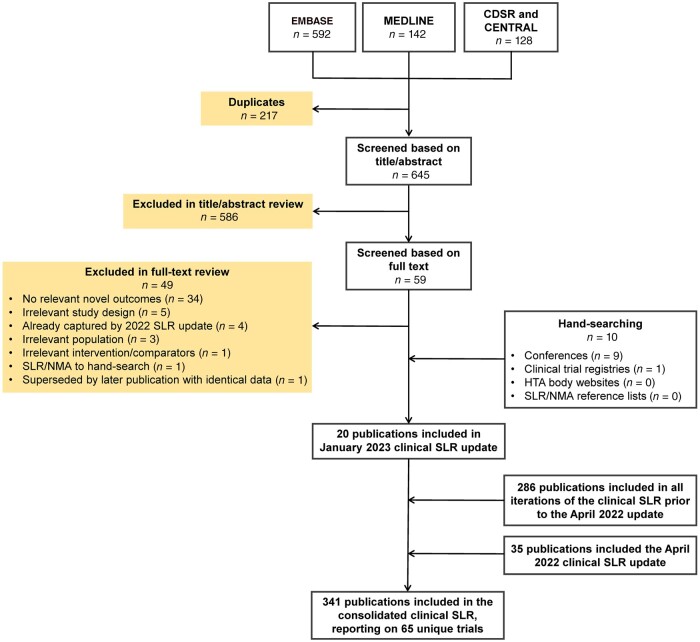
Overall, 341 publications reporting on 65 unique trials were included in the SLR (Fig. 1 and Supplementary Figs S1 and S2, available at Rheumatology online); the feasibility assessment determined that 36 trials were suitable for inclusion in this NMA, comprising 10 in nr-axSpA, and 27 in AS [note, one reported separate data for both populations (RAPID-axSpA [10])]. Baseline patient and disease characteristics of the included studies are provided in Supplementary Tables S6–S9, available at Rheumatology online. Reasons for exclusion of the 29 remaining trials are provided in Supplementary Table S10, available at Rheumatology online. Trials were broadly similar in terms of their main baseline characteristics (where reported). Enrolment of patients with nr-axSpA was based on meeting the 2009 ASAS axSpA classification criteria [60], with the presence of objective signs of inflammation defined by bone marrow oedema on MRI and/or elevated CRP. For most AS trials (24 of 27), enrolment of patients was based on the 1984 modified New York (mNY) criteria [61]. However, the entry criteria for three studies (COAST-V, COAST-W and Xue 2022) restricted patients to those meeting the more recent ASAS classification criteria [60], which uses the imaging criterion of the mNY criteria for radiographic axSpA plus additional criteria (>1 feature of axSpA). Agreement between the mNY and ASAS r-axSpA criteria is reported to be very high [4], and these populations are likely to have considerable clinical overlap, despite differences in classification. In BE MOBILE 2, patients were enrolled with mNY criteria, and met ASAS criteria for the classification of r-axSpA. It was therefore assumed that the trial populations were sufficiently similar to be directly compared in the NMA.
Figure 1.
PRISMA flow diagram of SLR study selection. CDSR: Cochrane Database of Systematic Reviews; CENTRAL: Cochrane Central Register of Controlled Trials; HTA: health technology assessment; NMA: network meta-analysis; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SLR: systematic literature review
The risk of bias assessment for individual trials included in the NMA is provided in Supplementary Table S11, available at Rheumatology online. Overall, the included RCTs had a low risk of bias, with some elements of the assessment ranking unclear due to missing reporting. One area of weakness was that a small number of baseline characteristics differed across the randomized treatment groups within five AS trials and one nr-axSpA trial [47, 62–66]. However, no studies were deemed unsuitable for inclusion in the NMA based on concerns regarding risk of bias.
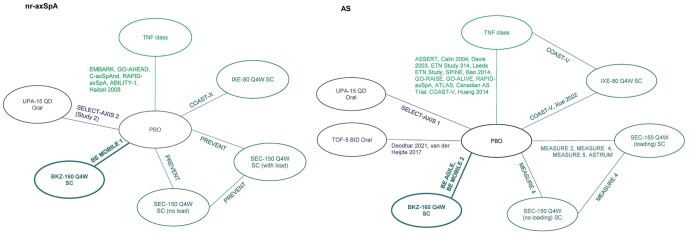
The network diagram for the predominantly b/tsDMARD-naïve networks (in nr-axSpA and AS) are presented in Fig. 2. The 100% b/tsDMARD-naïve, experienced (for efficacy) and combined axSpA population (tolerability and safety) networks are provided in Supplementary Figs S3–S5, available at Rheumatology online.
Figure 2.
Network diagrams in nr-axSpA and AS (predominantly b/tsDMARD-naïve network).a,b,c aPresented network diagrams are all studies that are included in one or more of the predominantly b/tsDMARD-naïve analyses. bSee Supplementary Tables S12 and S13, available at Rheumatology online, for lists of studies that report ASAS20, ASAS40 or ASAS-PR outcomes. cNetwork diagrams for 100% b/tsDMARD-naïve and experienced networks are provided in Supplementary Figs S3–S5, available at Rheumatology online. ASAS20: Assessment of Spondyloarthritis international Society improvement of ≥20%; ASAS40: Assessment of Spondyloarthritis international Society improvement of ≥40%; ASAS-PR: Assessment of Spondyloarthritis international Society partial remission; BID: twice-daily; BKZ: bimekizumab; b/tsDMARD: biologic/targeted synthetic DMARD; IXE: ixekizumab; nr-axSpA: non-radiographic axial SpA; PBO: placebo; Q4W: every 4 weeks; QD: once daily; SEC: secukinumab; TOF: tofacitinib; UPA: upadacitinib
Network meta-analysis
Predominantly (>50%) b/tsDMARD-naïve network
The predominantly b/tsDMARD-naïve network provided the most comprehensive results across outcomes and comparators comprising all 10 nr-axSpA studies and 25 out of 27 AS studies. Across the network, ∼90% of patients were b/tsDMARD-naïve (67–100% naïve in nr-axSpA and 61–100% naïve in AS).
ASAS20
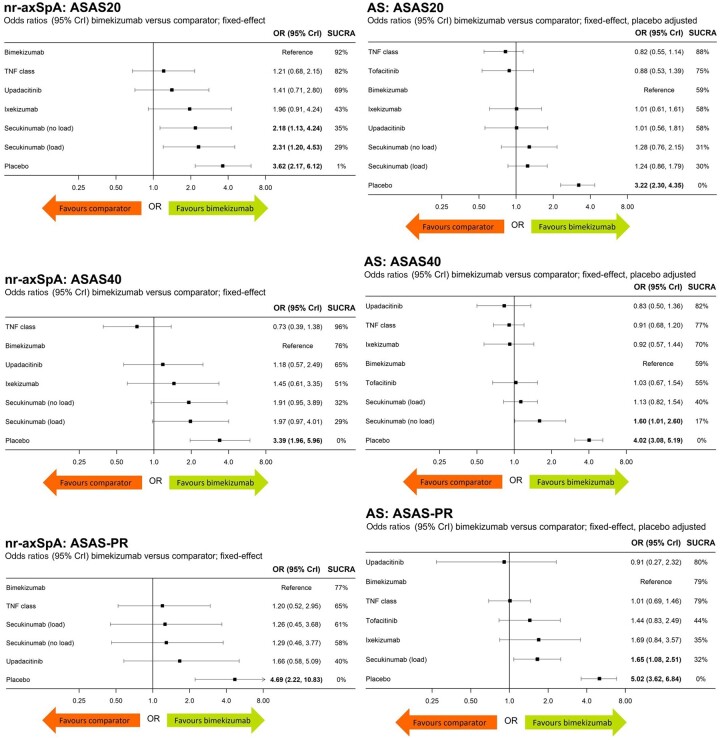
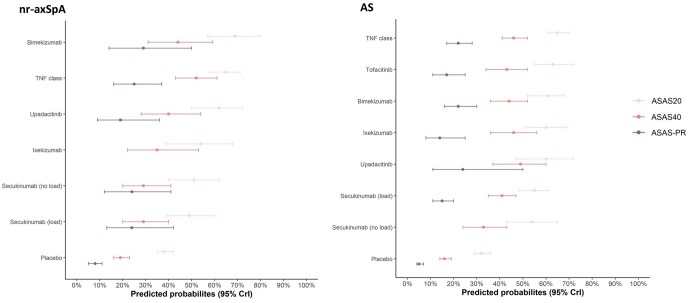
Ten nr-axSpA studies and 24 AS studies reported ASAS20 (Supplementary Tables S12 and S13, available at Rheumatology online). In nr-axSpA, bimekizumab was associated with significantly higher ASAS20 vs secukinumab 150 mg Q4W without LD (OR 2.18, 95% CrI: 1.13, 4.24) and secukinumab 150 mg Q4W with LD (OR 2.31, 95% CrI: 1.20, 4.53). Bimekizumab was comparable with all other active comparators; no further significant differences between bimekizumab and active comparators were observed. In AS, bimekizumab was associated with similar ASAS20 response rates compared with other active treatments in AS (Fig. 3). Predicted probabilities for ASAS20 response by treatment are presented in Fig. 4, and relative risk (RR) estimates for bimekizumab compared with other b/tsDMARDs are presented in Supplementary Table S14, available at Rheumatology online.
Figure 3.
ASAS20, ASAS40 and ASAS-PR outcomes in predominantly b/tsDMARD-naïve patients with nr-axSpA and AS.aaResults expressed as ORs; higher ORs indicate better outcomes for bimekizumab. Bold denotes significance based on 95% CrI. ASAS: Assessment in Spondyloarthritis International Society; b/tsDMARD: biologic/targeted synthetic DMARD; CrI: credible interval; nr-axSpA: non-radiographic axial SpA; OR: odds ratio; PR: partial remission; SUCRA: surface under the cumulative ranking curve
Figure 4.
Predicted probabilities of response in nr-axSpA and AS (predominantly b/tsDMARD-naïve network), ranked by ASAS20. ASAS: Assessment in Spondyloarthritis International Society; b/tsDMARD: biologic/targeted synthetic DMARD; CrI: credible interval; nr-axSpA: non-radiographic axial SpA; PR: partial remission
ASAS40
Ten nr-axSpA studies and 22 AS studies reported ASAS40 (Supplementary Tables S12 and S13, available at Rheumatology online). In nr-axSpA, bimekizumab was associated with similar (95% CrI crosses 1.0) ASAS40 response rates compared with those for other active treatments. In AS, the ASAS40 response rate was significantly higher for bimekizumab in AS vs secukinumab 150 mg Q4W without LD (OR 1.60, 95% CrI: 1.01, 2.60). No other significant differences between bimekizumab and active comparators were observed (Fig. 3). Predicted probabilities for ASAS40 response by treatment are presented in Fig. 4, and RR estimates for bimekizumab compared with other b/tsDMARDs are presented in Supplementary Table S14, available at Rheumatology online.
ASAS-PR
Nine nr-axSpA studies and 16 AS studies reported ASAS-PR (Supplementary Tables S12 and S13, available at Rheumatology online). In nr-axSpA, bimekizumab was associated with similar ASAS-PR response rates compared with other active treatments. In AS, bimekizumab was associated with significantly higher ASAS-PR compared with secukinumab 150 mg with LD (OR 1.65, 95% CrI: 1.08, 2.51) and similar ASAS-PR response rates compared with the other active treatments in AS (Fig. 3). Predicted probabilities for ASAS-PR response by treatment are presented in Fig. 4, and RR estimates for bimekizumab compared with other b/tsDMARDs are presented in Supplementary Table S14, available at Rheumatology online.
No other significant differences between bimekizumab and active comparators were observed in the predominantly b/tsDMARD-naïve network. League tables of pairwise comparisons for all treatments in the predominantly b/tsDMARD-naïve network are presented in Supplementary Tables S15–S20, available at Rheumatology online.
100% b/tsDMARD-naïve network
Results of the 100% b/tsDMARD-naïve network were broadly consistent with the results of the predominantly b/tsDMARD-naïve network; no significant differences between bimekizumab and active comparators were observed for any outcome (Supplementary Table S21, available at Rheumatology online).
100% b/tsDMARD-experienced network
Two AS studies enrolled 100% b/tsDMARD-experienced patients [COAST-W and SELECT-AXIS 2 (Study 1)], and a further seven AS studies reported data for the subgroup of b/tsDMARD-experienced patients enrolled in the trial (Supplementary Table S13, available at Rheumatology online). These nine AS studies were included in the 100% b/tsDMARD-experienced analysis for ASAS20 and ASAS40. Seven of these studies also reported ASAS-PR for this subgroup; however, the analysis did not converge as there were too few patients in the subgroup and zero events in some of the placebo-control arms. No significant differences between bimekizumab and active comparators were observed for any outcome (Table 2). A b/tsDMARD-experienced network was not feasible in nr-axSpA, as too few studies reported data for b/tsDMARD-experienced patients.
Table 2.
| Treatment | ASAS20 |
ASAS40 |
ASAS-PR |
|||
|---|---|---|---|---|---|---|
| OR (95% CrI) | SUCRA | OR (95% CrI) | SUCRA | OR (95% CrI) | SUCRA | |
| Bimekizumab | Reference | 66% | Reference | 65% | Analysis did not converge—too few b/tsDMARD-experienced patients/zero events in control arm | |
| TNF inhibitor classd | 1.30 (0.11, 6.50) | 52% | 0.70 (0.17, 2.35) | 83% | ||
| Upadacitinib | 0.82 (0.28, 2.95) | 81% | 0.85 (0.40, 1.78) | 78% | ||
| Tofacitinib | 1.85 (0.07, 11.39) | 35% | 1.71 (0.50, 5.21) | 35% | ||
| Ixekizumab | 1.50 (0.35, 4.75) | 40% | 1.94 (0.83, 4.43) | 28% | ||
| Secukinumab (load) | 1.13 (0.36, 3.93) | 58% | 0.86 (0.39, 1.87) | 77% | ||
| Secukinumab (no load) | 1.05 (0.28, 4.51) | 62% | 1.87 (0.65, 5.30) | 31% | ||
| Placebo | 2.91 (0.93, 8.51) | 6% | 3.46 (1.67, 6.88) | 2% | ||
Results expressed as ORs; higher ORs indicate better outcomes for bimekizumab. Bold denotes significance based on 95% CrI.
Network not feasible for nr-axSpA.
NMA based on three chains of 1000 simulations.
Includes certolizumab pegol only. ASAS20: Assessment of Spondyloarthritis international Society improvement of ≥20%; ASAS40: Assessment of Spondyloarthritis international Society improvement of ≥40%; ASAS-PR: Assessment of Spondyloarthritis international Society partial remission; b/tsDMARD: biologic/targeted synthetic DMARD; Crl: credible interval; NMA: network meta-analysis; OR: odds ratio; SUCRA: surface under the cumulative ranking curve; nr-axSpA: non-radiographic axial SpA.
Combined axSpA population safety network
The combined axSpA population network for discontinuation due to any reason and for SAEs included 25 and 24 studies, respectively (Supplementary Table S22, available at Rheumatology online). Bimekizumab demonstrated comparable discontinuations due to any reason and comparable SAEs, relative to all active treatments in the network (Supplementary Tables S23 and S24, available at Rheumatology online).
Discussion
This SLR and NMA provides an up-to-date synthesis of the available evidence for determining the relative efficacy, tolerability, and safety of bimekizumab compared with b/tsDMARDs in adult patients with nr-axSpA or AS with an inadequate response to, intolerance of, or contraindication to NSAID therapy. Separate analyses were undertaken across three ASAS efficacy outcomes in the following patient subpopulations: predominantly (>50%) b/tsDMARD-naïve, 100% b/tsDMARD-naïve, and 100% b/tsDMARD-experienced. Tolerability and safety analyses were conducted in a combined axSpA population.
Prior to the approval of IL-17A and JAK inhibitors for axSpA, treatment options were relatively limited, with TNF inhibitors being the only available targeted therapies [21, 22]. Today, a wider range of treatments are available. The comparative efficacy of available b/tsDMARDs is of great interest to patients, clinicians, and payers alike; it is important to understand the relative efficacy of the available therapies to determine best practices. Several placebo-controlled RCTs have demonstrated the efficacy of recently available therapies, but head-to-head trials are lacking, and so NMA can assist in assessing their comparative effectiveness [67]. While previous NMAs in axSpA evaluated the relative efficacy of some therapies [46, 68–76], this is the first analysis that incorporates upadacitinib and bimekizumab.
The predominantly bDMARD-naïve network provided the most complete set of results across outcomes and comparators. In nr-axSpA, bimekizumab was associated with significantly higher ASAS20 response rates vs secukinumab 150 mg (in both the with and without LD comparisons), and in AS, ASAS40 and ASAS-PR response rates were significantly higher with bimekizumab vs secukinumab 150 mg without LD and secukinumab 150 mg with LD, respectively. Apart from these comparisons, no significant differences were found; bimekizumab was associated with similar response rates to those of all other b/tsDMARDs.
Inclusion of recent Phase III trials of bimekizumab (BE MOBILE 1 and BE MOBILE 2 [77, 78]) enabled analysis of 100% b/tsDMARD-naïve and experienced subpopulations. Broadly, conclusions of the 100% b/tsDMARD-naïve network were consistent with those of the predominantly b/tsDMARD-naïve network, so the latter can be used as a proxy for the former [79]. Importantly, the predominantly-naïve network used a more robust dataset (intention-to-treat/full analysis set) than an analysis using subgroup data, allowing more comparators to be included in the networks. In the 100% b/tsDMARD-experienced network, analyses were possible in AS for ASAS20 and ASAS40, and no significant differences between bimekizumab and active comparators were observed. These b/tsDMARD-experienced analyses are a novel addition to the literature, albeit with low trial and patient numbers, and should be interpreted with caution. Additionally, the safety and tolerability of bimekizumab at weeks 12–16 were comparable with those of all active comparators in the combined axSpA population.
To the best of our knowledge, this research represents the most recent and comprehensive SLR/NMA for axSpA, building upon previous meta-analyses in b/tsDMARD-naïve patients [48, 70, 74–78]. A 2018 NMA published by Deodhar et al. compared the efficacy of TNF, IL-17 and JAK inhibitors (tofacitinib only) in AS [70]. The authors concluded that tofacitinib, golimumab i.v. and infliximab had the highest SUCRA values for efficacy; however, the differences in efficacy were not significant, and the analyses were based on one small Phase II study for tofacitinib [70]. The current analysis includes Phase III trials for bimekizumab [60] and JAK inhibitors [79–81] which were not available at the time of the previous NMA, as well as data for nr-axSpA. The present analysis also includes new outcomes relative to the Deodhar study, including ASAS40, ASAS-PR and safety/tolerability outcomes. For ASAS20, the only outcome included in both studies, results in the predominantly-naïve network were consistent with those previously published, with bimekizumab now featuring amongst the most efficacious treatments.
Limitations
While some baseline characteristics differed between studies, no studies were deemed unsuitable for inclusion in the NMA based on differences in baseline characteristics, and the overall risk of bias was low. However, for 100% b/tsDMARD-naïve and experienced analyses, some evidence comes from trial subgroup data for which baseline characteristics are not available; this introduces uncertainty regarding how balanced the study arms are. The results are based on fixed-effect (nr-axSpA; combined axSpA) and fixed-effect placebo-adjusted (AS) models, which may underestimate the uncertainty in the treatment effects. However, these results estimated some wide 95% CrIs for the relative treatment effects, even using the fixed-effect models, and thus additional clinical study data would be beneficial for reducing the uncertainty in the findings. Any additional study data would also help to enable a more rigorous assessment of between-study heterogeneity and placebo-effects adjustment. There is a paucity of RCT data for b/tsDMARD-experienced patients, and this network was not feasible in nr-axSpA. Nine studies reported data for this subpopulation in AS, leading to a network of eight treatments (including placebo). For the b/tsDMARD-experienced network, the comparisons against bimekizumab were based on subgroup data from BE MOBILE 2; however, the trial randomization was not designed to enrol a sufficient number of bDMARD-experienced patients to detect a difference between bimekizumab and placebo in this subgroup [30]. The placebo arm of BE MOBILE 2, which connects the trial to the rest of the network, contained just 17 patients, and so the analysis is subject to uncertainty. The safety analyses are also associated with increased uncertainty due to the small number of events. Further limitations of the NMA include the different ages of the included studies [published 2002 (infliximab) to 2022 (ixekizumab) [82, 83]], short-term efficacy analyses (12–16 weeks), and a lack of published data on 300 mg secukinumab, which prevented inclusion of this higher secukinumab dose in the NMA.
Conclusion
Across ASAS outcomes, bimekizumab demonstrated comparable efficacy with that of most b/tsDMARDs, including ixekizumab, TNF inhibitors and upadacitinib, and achieved higher response rates compared with secukinumab at 12–16 weeks for ASAS20 in predominantly b/tsDMARD-naïve nr-axSpA patients, and for ASAS40 and ASAS-PR in predominantly b/tsDMARD-naïve AS patients. In a pooled axSpA network, bimekizumab demonstrated comparable safety with that of other b/tsDMARDs. Overall, the present analyses provide evidence for bimekizumab being an efficacious option in the management of both b/tsDMARD-naïve and experienced patients across the axSpA spectrum, with similar safety and tolerability to existing treatments.
Supplementary Material
Acknowledgements
The authors are grateful to C. Menckeberg, an employee of UCB Pharma, for providing insights that assisted the preparation of the manuscript. The authors also thank A. Paine, H. Pilkington, N. Webb and D. Stewart, who are employees of Source Health Economics and contributed to the systematic literature review and network meta-analysis (funded by UCB Pharma). Third-party writing assistance was provided by P. White and D. Partridge of Source Health Economics, and was funded by UCB Pharma in accordance with Good Publication Practice (GPP 2022). Manuscript review management was provided by Shimaila Siddiqui of Costello Medical, and was funded by UCB Pharma.
Contributor Information
Atul Deodhar, Division of Arthritis and Rheumatic Diseases, Oregon Health & Science University, Portland, OR, USA.
Pedro M Machado, Centre for Rheumatology & Department of Neuromuscular Diseases, University College London, London, UK.
Michael Mørup, UCB Pharma, Copenhagen, Denmark.
Vanessa Taieb, UCB Pharma, Colombes, France.
Damon Willems, UCB Pharma, Brussels, Belgium.
Michelle Orme, ICERA Consulting Ltd, Swindon, UK.
David Pritchett, Source Health Economics, London, UK.
Lianne S Gensler, Department of Medicine/Division of Rheumatology, University of California, San Francisco, San Francisco, CA, USA.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by UCB Pharma.
Disclosure statement: D.P. and M.O. are employees of Source Health Economics and ICERA Consulting Ltd, respectively, the consultancy companies that conducted the systematic literature review and network meta-analysis (funded by UCB Pharma). M.M., V.T. and D.W. are employees of UCB Pharma. D.W. and V.T. are shareholders in UCB Pharma. A.D. has received honoraria from AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Eli Lilly, Janssen, MoonLake, Novartis, Pfizer, and UCB; and research grants from AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Galvani, Janssen, MoonLake, Novartis, Pfizer, and UCB. P.M.M. has received honoraria from AbbVie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche, and UCB, all unrelated to this manuscript, and is supported by the National Institute for Health Research (NIHR), University College London Hospitals (UCLH) and the Biomedical Research Centre (BRC). L.S.G. has received honoraria from AbbVie, Acelyrin, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and research grants support from Novartis and UCB, all unrelated to this manuscript.
References
- 1. Sieper J, Braun J, Dougados M, Baeten D.. Axial spondyloarthritis. Nat Rev Dis Primers 2015;1:15013. [DOI] [PubMed] [Google Scholar]
- 2. Navarro-Compán V, Sepriano A, El-Zorkany B, van der Heijde D.. Axial spondyloarthritis. Ann Rheum Dis 2021;80:1511–21. [DOI] [PubMed] [Google Scholar]
- 3. Robinson PC, Sengupta R, Siebert S.. Non-radiographic axial spondyloarthritis (nr-axSpA): advances in classification, imaging and therapy. Rheumatol Ther 2019;6:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boel A, Molto A, van der Heijde D. et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? Results from eight cohorts. Ann Rheum Dis 2019;78:1545–9. [DOI] [PubMed] [Google Scholar]
- 5. Boel A, Lopez-Medina C, van der Heijde D, van Gaalen FA.. Age at onset in axial spondyloarthritis around the world: data from the Assessment in SpondyloArthritis international Society Peripheral Involvement in Spondyloarthritis study. Rheumatology (Oxford) 2022;61:1468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Protopopov M, Poddubnyy D.. Radiographic progression in non-radiographic axial spondyloarthritis. Expert Rev Clin Immunol 2018;14:525–33. [DOI] [PubMed] [Google Scholar]
- 7. Michelena X, Lopez-Medina C, Marzo-Ortega H.. Non-radiographic versus radiographic axSpA: what’s in a name? Rheumatology (Oxford) 2020;59:iv18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopez-Medina C, Ramiro S, van der Heijde D. et al. Characteristics and burden of disease in patients with radiographic and non-radiographic axial Spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open 2019;5:e001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao SS, Ermann J, Xu C. et al. Comparison of comorbidities and treatment between ankylosing spondylitis and non-radiographic axial spondyloarthritis in the United States. Rheumatology (Oxford) 2019;58:2025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landewe R, Braun J, Deodhar A. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Axial Spondyloarthritis Society (NASS). What is axial SpA (AS)? https://nass.co.uk/about-as/what-is-as/ (1 June 2023, date last accessed).
- 12. Sieper J, Poddubnyy D.. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]
- 13. de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL.. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 2016;18:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R.. Quality of life in patients with psoriasis. Health Qual Life Outcomes 2006;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell R, Kremer A, Westwood N, Younge L, Ghosh S.. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohn’s Colitis 2009;3:1–3. [DOI] [PubMed] [Google Scholar]
- 16. Mielants H, Van den Bosch F.. Extra-articular manifestations. Clin Exp Rheumatol 2009;27:S56–61. [PubMed] [Google Scholar]
- 17. Ward MM. Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res 1999;12:247–55. [PubMed] [Google Scholar]
- 18. Boonen A, van der Linden SM.. The burden of ankylosing spondylitis. J Rheumatol 2006;78:4–11. [PubMed] [Google Scholar]
- 19. Cooksey R, Husain MJ, Brophy S. et al. The cost of ankylosing spondylitis in the UK using linked routine and patient-reported survey data. PLoS One 2015;10:e0126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Machado P, Landewé R, Braun J. et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis 2010;69:1465–70. [DOI] [PubMed] [Google Scholar]
- 21. Ramiro S, Nikiphorou E, Sepriano A. et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis 2023;82:e206. [DOI] [PubMed] [Google Scholar]
- 22. Ward MM, Deodhar A, Gensler LS. et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res 2019;71:1285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strand V, Singh JA.. Patient burden of axial spondyloarthritis. J Clin Rheumatol 2017;23:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poddubnyy D, Sieper J.. Current unmet needs in spondyloarthritis. Curr Rheumatol Rep 2019;21:43. [DOI] [PubMed] [Google Scholar]
- 25. Navarro-Compán V, Plasencia-Rodríguez C, de Miguel E. et al. Switching biological disease-modifying antirheumatic drugs in patients with axial spondyloarthritis: results from a systematic literature review. RMD Open 2017;3:e000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sieper J, Poddubnyy D, Miossec P.. The IL-23–IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol 2019;15:747–57. [DOI] [PubMed] [Google Scholar]
- 27. Glatt S, Baeten D, Baker T. et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018;77:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah M, Maroof A, Gikas P. et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 2020;6:e001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeremenko N. Out of the shadow of interleukin-17A: the role of interleukin-17F and other interleukin-17 family cytokines in spondyloarthritis. Curr Opin Rheumatol 2021;33:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Heijde D, Deodhar A, Baraliakos X. et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis 2023;82:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poddubnyy D, Sieper J.. What is the best treatment target in axial spondyloarthritis: tumour necrosis factor α, interleukin 17, or both? Rheumatology 2018;57:1145–50. [DOI] [PubMed] [Google Scholar]
- 32. Rios Rodriguez V, Poddubnyy D.. Tumor necrosis factor-α (TNFα) inhibitors in the treatment of nonradiographic axial spondyloarthritis: current evidence and place in therapy. Ther Adv Musculoskelet Dis 2017;9:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Heijde D, Ramiro S, Landewe R. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 34. van der Heijde D, Sieper J, Maksymowych WP. et al. ; Assessment of SpondyloArthritis International Society. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:905–8. [DOI] [PubMed] [Google Scholar]
- 35. Higgins J, Thomas J, Chandler J. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022. www.training.cochrane.org/handbook (1 June 2023, date last accessed).
- 36. Page MJ, McKenzie JE, Bossuyt PM. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination, University of York; 2009.
- 38. National Institute for Health and Care Excellence. Single technology appraisal: User guide for company evidence submission template. 2015. https://www.nice.org.uk/process/pmg24/chapter/instructions-for-companies (1 June 2023, date last accessed).
- 39. European Medicines Agency. Secukinumab European public assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/cosentyx (1 June 2023, date last accessed).
- 40. Ramiro S, Nikiphorou E, Sepriano A. et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis 2023;82:19–34. [DOI] [PubMed] [Google Scholar]
- 41. Anderson JJ, Baron G, Van Der Heijde D, Felson DT, Dougados M.. Ankylosing spondylitis assessment group preliminary definition of short‐term improvement in ankylosing spondylitis. Arthritis Rheum 2001;44:1876–86. [DOI] [PubMed] [Google Scholar]
- 42. Brandt J, Listing J, Sieper J. et al. Development and preselection of criteria for short term improvement after anti-TNF alpha treatment in ankylosing spondylitis. Ann Rheum Dis 2004;63:1438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landewé R, van Tubergen A.. Clinical tools to assess and monitor spondyloarthritis. Curr Rheumatol Rep 2015;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Braun J, Sieper J.. Remission and possible discontinuation of biological therapy in axial spondyloarthritis. Clin Exp Rheumatol 2013;31:S33–6. [PubMed] [Google Scholar]
- 45. Cruz-Machado AR, Rodrigues-Manica S, Silva JL. et al. Effect of biologic disease-modifying anti-rheumatic drugs targeting remission in axial spondyloarthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2020;59:3158–71. [DOI] [PubMed] [Google Scholar]
- 46. Ho A, Younis I, Le QA.. Impact of biologics on health-related quality of life in patients with Ankylosing spondylitis: a systematic review and meta-analysis of randomized controlled trials. Semin Arthritis Rheum 2022;54:151996. [DOI] [PubMed] [Google Scholar]
- 47. Huang Y, Chen Y, Liu T. et al. Impact of tumor necrosis factor alpha inhibitors on MRI inflammation in axial spondyloarthritis assessed by Spondyloarthritis Research Consortium Canada score: a meta-analysis. PLoS ONE 2020;15:e0244788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. NICE. TNF-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis. Technology appraisal guidance [TA383]. 2016. https://www.nice.org.uk/guidance/ta383/documents/ankylosing-spondylitis-and-axial-spondyloarthritis-nonradiographic-adalimumab-etanercept-infliximab-and-golimumab-inc-rev-ta143-and-ta233-id694-committee-papers2 (1 June 2023, date last accessed).
- 49. Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011.https://www.ncbi.nlm.nih.gov/books/NBK310366/pdf/Bookshelf_NBK310366.pdf (1 June 2023, date last accessed). [PubMed]
- 50. Ades AE, Sculpher M, Sutton A. et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. PharmacoEconomics 2006;24:1–19. [DOI] [PubMed] [Google Scholar]
- 51. Sutton A, Ades AE, Cooper N, Abrams K.. Use of indirect and mixed treatment comparisons for technology assessment. PharmacoEconomics 2008;26:753–67. [DOI] [PubMed] [Google Scholar]
- 52. Sutton AJ, Abrams KR.. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res 2001;10:277–303. [DOI] [PubMed] [Google Scholar]
- 53. Caldwell DM, Ades AE, Higgins JP.. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Br Med J 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu G, Ades AE.. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24. [DOI] [PubMed] [Google Scholar]
- 55. Hoaglin DC, Hawkins N, Jansen JP. et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011;14:429–37. [DOI] [PubMed] [Google Scholar]
- 56. Bayesian inference using Gibbs Sampling (BUGS). WinBUGS with DoodleBUGS version 1.4. 2003. –7. https://www.mrc-bsu.cam.ac.uk/wp-content/uploads/manual14.pdf (1 June 2023, date last accessed).
- 57. Dias S, Sutton AJ, Welton NJ, Ades A. NICE DSU technical support document 3: heterogeneity: subgroups, meta-regression, bias and bias-adjustment. 2011. https://www.ncbi.nlm.nih.gov/books/NBK395886/pdf/Bookshelf_NBK395886.pdf (1 June 2023, date last accessed).
- 58. The BUGS project. DIC: Deviance Information Criterion.1996–2012. https://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-dic/ (1 June 2023, date last accessed).
- 59. Mbuagbaw L, Rochwerg B, Jaeschke R. et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev 2017;6:79–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rudwaleit M, Landewe R, van der Heijde D. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 61. van der Linden S, Valkenburg HA, Cats A.. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheumatol 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 62. Yang A, Pechlivanoglou P, Aoyama K.. Interpreting and assessing confidence in network meta-analysis results: an introduction for clinicians. J Anesth 2022;36:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aparicio M, Guillén-Astete CA, López-Medina C, Sastre C, Rodríguez Martínez FJ.. Evidence for the use of secukinumab in patients with radiographic and non-radiographic axial spondyloarthritis in the last 5 years. Rheumatol Ther 2022;9:73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Azadeh H, Alizadeh-Navaei R, Rezaiemanesh A, Rajabinejad M.. Immune-related adverse events (irAEs) in ankylosing spondylitis (AS) patients treated with interleukin (IL)-17 inhibitors: a systematic review and meta-analysis. Inflammopharmacology 2022;30:435–51. [DOI] [PubMed] [Google Scholar]
- 65. Keeling S, Maksymowych WP.. JAK inhibitors, psoriatic arthritis, and axial spondyloarthritis: a critical review of clinical trials. Expert Rev Clin Immunol 2021;17:701–15. [DOI] [PubMed] [Google Scholar]
- 66. Li S, Li F, Mao N, Wang J, Xie X.. Efficacy and safety of Janus kinase inhibitors in patients with ankylosing spondylitis: a systematic review and meta-analysis. Eur J Intern Med 2022;102:47–53. [DOI] [PubMed] [Google Scholar]
- 67. Man S, Hu L, Ji X. et al. Risk of malignancy and tuberculosis of biological and targeted drug in patients with spondyloarthritis: systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 2021;12:705669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pinto AS, Farisogullari B, Machado PM, editors. Predictors of remission in people with axial spondyloarthritis: a systematic literature review. Semin Arthritis Rheum 2022;56:152078. [DOI] [PubMed] [Google Scholar]
- 69. Tański W, Świątoniowska-Lonc N, Dudek K, Jankowska-Polańska B.. Benefit of biological drugs for quality of life in patients with ankylosing spondylitis: a systematic review and meta-analysis of clinical trials. Best Pract Health Care 2020;1335:63–78. [DOI] [PubMed] [Google Scholar]
- 70. Deodhar A, Chakravarty S, Cameron C. et al. A systematic review and network meta-analysis of current and investigational treatments for active ankylosing spondylitis. Clinical Rheumatology 2020;39:2307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.UCB. Confidential data on file. BE MOBILE 2 (AS0011) Clinical Study Report: Week 24 Interim Analysis, Efficacy and Safety of Bimekizumab in Ankylosing Spondylitis (March 2022).
- 72.UCB. Confidential data on file. BE MOBILE 1 (AS0010) Clinical Study Report: Week 24 Interim Analysis, Efficacy and Safety of Bimekizumab in Non-Radiographic axSpA (March 2022).
- 73. Sieper J, Rudwaleit M, Baraliakos X. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Annals of the Rheumatic Diseases 2009;68(Suppl 2):ii1–44. [DOI] [PubMed] [Google Scholar]
- 74.NICE. Ixekizumab for treating axial spondyloarthritis. Technology appraisal guidance [TA718]. 2021. https://www.nice.org.uk/guidance/ta718/documents/committee-papers (1 June 2023, date last accessed).
- 75. NICE. Secukinumab for active ankylosing spondylitis after treatment with non-steroidal anti-inflammatory drugs or TNF-alpha inhibitors. Technology appraisal guidance [TA407]. 2016. https://www.nice.org.uk/guidance/ta407/documents/committee-papers (1 June 2023, date last accessed).
- 76. Ungprasert P, Erwin PJ, Koster MJ.. Indirect comparisons of the efficacy of biological agents in patients with active ankylosing spondylitis: a systematic review and meta-analysis. Clinical Rheumatology 2017;36:1569–77. [DOI] [PubMed] [Google Scholar]
- 77. Wang S, He Q, Shuai Z.. Risk of serious infections in biological treatment of patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Clinical Rheumatology 2018;37:439–50. [DOI] [PubMed] [Google Scholar]
- 78. Kiri S, Kim M, Betts M. et al. eds. Network meta-analysis of long-term efficacy (ASAS40) of biologic disease-modifying anti-rheumatic drugs (bDMARDs) in bDMARD-Naïve patients with non-radiographic axial spondyloarthritis. Arthritis Rheumatol 2020;72(suppl 10).
- 79. Deodhar A, Van den Bosch F, Poddubnyy D. et al. OP0016 efficacy and safety of upadacitinib in patients with active non-radiographic axial spondyloarthritis: a double-blind, randomized, placebo-controlled phase 3 trial. Ann Rheum Dis 2022;81:9–10. [Google Scholar]
- 80. van der Heijde D, Song IH, Pangan AL. et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 2019;394:2108–17. [DOI] [PubMed] [Google Scholar]
- 81. Van der Heijde D, Baraliakos X, Sieper J. et al. POS0306 efficacy and safety of upadacitinib in patients with active ankylosing spondylitis refractory to biologic therapy: a double-blind, randomized, placebo-controlled phase 3 trial. Ann Rheum Dis 2022;81:402–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Braun J, Brandt J, Listing J. et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93. [DOI] [PubMed] [Google Scholar]
- 83. Xue Y, Hu J, Liu D. et al. Efficacy and safety of ixekizumab in chinese patients with radiographic axial spondyloarthritis: 16-week results from a phase 3 study [abstract]. Arthritis Rheumatol 2022;74(suppl 9). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.