Abstract
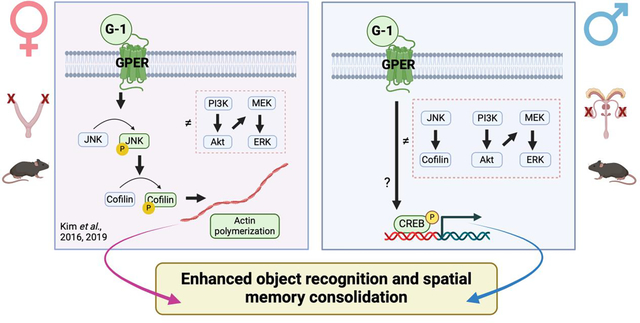
Studies in ovariectomized (OVX) female rodents suggest that G protein-coupled estrogen receptor (GPER) is a key regulator of memory, yet little is known about its importance to memory in males or the cellular mechanisms underlying its mnemonic effects in either sex. In OVX mice, bilateral infusion of the GPER agonist G-1 into the dorsal hippocampus (DH) enhances object recognition and spatial memory consolidation in a manner dependent on rapid activation of c-Jun N-terminal kinase (JNK) signaling, cofilin phosphorylation, and actin polymerization in the DH. However, the effects of GPER on memory consolidation and DH cell signaling in males are unknown. Thus, the present study first assessed effects of DH infusion of G-1 or the GPER antagonist G-15 on object recognition and spatial memory consolidation in gonadectomized (GDX) male mice. As in OVX mice, immediate post-training bilateral DH infusion of G-1 enhanced, whereas G-15 impaired, memory consolidation in the object recognition and object placement tasks. However, G-1 did not increase levels of phosphorylated JNK (p46, p54) or cofilin in the DH 5, 15, or 30 minutes after infusion, nor did it affect phosphorylation of ERK (p42, p44), PI3K, or Akt. Levels of phospho-cAMP-responsive element binding protein (CREB) were elevated in the DH 30 minutes following G-1 infusion, indicating that GPER in males activates a yet unknown signaling mechanism that triggers CREB-mediated gene transcription. Our findings show for the first time that GPER in the DH regulates memory consolidation in males and suggests sex differences in underlying signaling mechanisms.
Keywords: Mouse, object recognition, object placement, spatial memory, CREB, JNK, cofilin
Graphical Abstract
1. Introduction
Although 17β-estradiol (E2) has long been known to promote memory and synaptic plasticity (Frick, 2015; Rocks and Kundakovic, 2023; Sheppard et al., 2018; Taxier et al., 2020; Woolley et al., 1997), the cellular and molecular mechanisms driving these effects are not fully understood in either sex. Far more is known about these mechanisms in females. For example, among ovariectomized (OVX) female mice, bilateral infusion of E2 into the dorsal hippocampus (DH) before or immediately after training in object recognition, object placement, and social memory tasks enhances memory in a manner dependent on rapid phosphorylation of extracellular signal-regulated kinase (ERK) and phosphatidylinositol/Akt (PI3K/Akt) signaling in the DH, effects mediated by intracellular estrogen receptors ERα and ERβ binding at the plasma membrane to metabotropic glutamate receptor 1a (mGluR1a) (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Sheppard et al., 2023). The extranuclear interactions among ERα, ERβ, and mGluR1a at the membrane provide a non-classical mechanism to trigger the rapid downstream cell signaling events upon which memory consolidation relies.
Another non-classical mechanism through which E2 may influence memory consolidation is via the membrane estrogen receptor G protein-coupled estrogen receptor. In female rodents, GPER regulates hippocampal synaptic plasticity and memory mediated by the DH. For example, the GPER agonist G-1 increased excitatory postsynaptic potentials (EPSPs) in hippocampal slices from OVX ERα knockout and ERβ knockout mice (Kumar et al., 2015), and increased miniature excitatory synaptic current (mEPSC) frequency and amplitude in hippocampal slices from OVX rats (Oberlander and Woolley, 2016). Accordingly, chronic systemic administration of the GPER agonist G-1 to OVX rats enhanced hippocampal cholinergic function and improved spatial working memory in a delayed matching-to-position T-maze task, spatial reference memory in the Barnes maze, and object recognition memory in a novelty task, whereas systemic treatment with the GPER antagonists G-15 or G-36 impaired memory in these tasks (Bai et al., 2020; Hammond et al., 2012, 2011, 2009). In addition, chronic systemic G-1 injections improved contextual fear memory in gonadally intact mature adult (12 month-old) female mice (Xu et al., 2018). G-1 also has rapid effects on memory, as acute pre-training systemic G-1 injection in OVX mice facilitated object recognition, spatial, and social recognition memories, and these effects were associated with increased density of CA1 dendritic spines 40 min after G-1 administration (Gabor et al., 2015). Acute post-training systemic G-1 injection has also been shown to reverse the negative effects of neonatal iron treatment on memory in the object placement and inhibitory avoidance tasks among gonadally intact and OVX female rats (Machado et al., 2019). As such, data from systemic treatments suggest that GPER activation promotes memory acquisition and consolidation in female rodents, although the peripheral routes of administration used make it impossible to attribute these effects to a particular brain region.
To this end, our laboratory recently established that GPER activation in the DH is necessary and sufficient for the consolidation of long-term memories by showing that bilateral DH infusion of G-1 or G-15 immediately after training in the object recognition and object placement tasks enhanced and impaired, respectively, memory consolidation in OVX mice (Kim et al., 2016). DH G-1 infusion in OVX mice also increased CA1 apical dendritic spine density within 40 minutes (Kim et al., 2019), which is consistent with effects observed after DH infusion of E2 (Phan et al., 2015; Sheppard et al., 2023; Tuscher et al., 2018). Accordingly, DH infusion of E2 or G-1 also increased phosphorylation of the actin binding protein cofilin within 5 minutes (Kim et al., 2019), suggesting rapid effects of E2 and GPER activation on the actin cytoskeleton that support the observed increases in DH spinogenesis. Interestingly, however, the ability of G-1 to enhance memory consolidation in the object tasks or increase CA1 spine density was not dependent on DH ERK activation as with E2, but rather relied upon phosphorylation of c-Jun N-terminal kinase (JNK) (Kim et al., 2019, 2016). Moreover, E2’s effects on memory and cofilin phosphorylation were not blocked by G-15 or a JNK inhibitor (Kim et al., 2019, 2016), suggesting that cellular mechanisms through which GPER regulates memory consolidation and CA1 spine density differ from those underlying the effects of E2 and agonists of ERα and ERβ in OVX females.
Despite the progress made thus far in understanding the role of GPER in mediating memory in females, very little is known about its influence on memory in males. GPER is expressed in the hippocampus of both female and male rodents (Brailoiu et al., 2007), although its expression in rats differs between the sexes and across the estrous cycle. For example, GPER immunoreactivity in CA1-CA3 and the dentate gyrus of rats is higher in males and estrus females than in diestrus females (Llorente et al., 2020); however, ultrastructural analyses revealed reduced GPER expression in CA1 axons and glia in estrus females relative to males and diestrus females (Waters et al., 2015). Furthermore, systemic activation of GPER in gonadally intact male rodents suggests that this receptor facilitates hippocampus-dependent memory in males. For example, acute systemic G-1 treatment in young adult male rats enhanced memory consolidation in the object recognition and inhibitory avoidance tasks when injected immediately after training, but not 3 or 6 hours after training (de Souza et al., 2021). Accordingly, immediate post-training injection of G-15 impaired object recognition memory consolidation (de Souza et al., 2021), suggesting an important role for GPER in memory consolidation among males. Among mature adult (12-month-old) male mice, chronic systemic G-1 treatment enhanced spatial memory in the Morris water maze and contextual fear memory (Xu et al., 2018). Although these studies suggest a similar memory-promoting role for GPER in males as previously observed in females, the neural mechanisms underlying its regulatory effects are unknown, both in terms of brain regions and cell signaling pathways involved. Moreover, there is reason to believe that the cellular mechanisms mediating GPER’s effects on memory may differ in males and females. For example, G-1 increased mEPSC frequency and amplitude in hippocampal slices from OVX rats, but not gonadectomized (GDX) male rats (Oberlander and Woolley, 2016), indicating sex differences in the role of post-synaptic GPER activation in regulating hippocampal glutamate sensitivity. These findings echo sex differences in the mechanisms through which E2 influences glutamate neurotransmission and synaptic plasticity in rats, such that long-term potentiation (LTP) in OVX females, but not GDX males, depends on synaptic activity, cAMP-regulated protein kinase A (PKA) activation, and calcium-permeable AMPA receptors (Jain et al., 2018; Jain and Woolley, 2023). Furthermore, our laboratory previously reported that the memory-enhancing effects of E2 on object recognition and spatial memory consolidation depend on p42ERK phosphorylation in OVX mice, but not gonadally intact or GDX male mice (Koss et al., 2018). Although it remains unclear which signaling mechanisms mediate E2-induced memory enhancement in males, E2 did increase phosphorylation of the transcription factor CREB in both sexes (Koss et al., 2018), suggesting that E2 may sex-dependently regulate the activity of kinases upstream of CREB. Collectively, these data suggest key sex differences in the cellular mechanisms that underlie E2’s effects on hippocampal synaptic plasticity and memory and raise the possibility that the mechanisms regulating GPER’s effects on memory may also be sex specific.
Given the dearth of information about the role of GPER in mediating memory in males, as well as the neural mechanisms through which GPER may regulate memory, the goals of this study were to determine the extent to which dorsal hippocampal GPER regulates object recognition and spatial memory consolidation in GDX male mice and to identify cell signaling pathways, such as JNK, that might be involved. As in females, our data indicate that GPER in the DH is a key regulator of memory consolidation in males but suggest sex differences in underlying signaling mechanisms. These findings provide the first evidence that GPER in the DH modulates memory formation in males and suggest the intriguing possibility that this receptor may do so by triggering different signaling pathways in males and females.
2. Methods
2.1. Subjects
Male C57BL/6 mice (N=80) were purchased from Taconic Biosciences at 8 weeks of age and housed individually in shoebox cages in a room (22–23°C) with a 12/12-h light-dark cycle. Food (Teklad Rodent Diet 8604, Envigo) and water were provided ad libitum. Mice were handled for 30 s/day for three consecutive days before the start of behavioral testing to become accustomed to the experimenters. All procedures were conducted from 10:00 to 17:00 h in a quiet room and experimenters conducting behavioral testing were blind to treatment regimen. Mice were monitored regularly throughout the experiments for any sign of pain or distress. All experimental procedures were approved by the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Animals.
2.2. General Experimental Design Overview
Fig. 1 illustrates a schematic overview of the general experimental design used in these studies. Male mice were first bilaterally gonadectomized and bilaterally implanted with stainless steel guide cannulae aimed at each DH within the same surgical session. They were then allowed a minimum of one week to recover before the start of habituation in the object recognition (OR) and object placement (OP) tasks. Although memory consolidation in both tasks depends on intact DH function (Tuscher et al., 2018), our prior work indicates somewhat differential roles of estrogen receptors in mediating OR and OP memory in OVX mice, as DH infusion of the ERα antagonist MPP selectively impaired OP memory, whereas the ERβ antagonist PHTPP impaired both OR and OP memory (Kim and Frick, 2017). Thus, both tasks were included here to determine the extent to which GPER might modulate both spatial and object recognition memories. Immediately after training in the OR and OP tasks, mice were restrained gently and infused bilaterally into the DH with vehicle, the GPER agonist G-1, or the GPER antagonist G-15 (to test effects of GPER antagonism). Two experiments were conducted; one in which mice were infused with vehicle or G-1 (4 or 8 ng/hemisphere) and another in which they were infused with vehicle or G-15 (1.85 or 7.4 ng/hemisphere). For G-1, memory consolidation was evaluated 48 h later in OR and 24 h later in OP. For G-15, memory was tested 24 and 4 hours later in OR and OP, respectively. These time points were selected because vehicle-treated gonadectomized mice of both sexes spend more time with the novel or moved objects in OR and OP testing, 24 h and 4 h, but not 48 h and 24 h, respectively, after training (Kim et al., 2019, 2016; Koss et al., 2018). Thus, the longer delays allowed us to assess the possible memory-enhancing effects of G-1, whereas the shorter delays permitted testing of potential memory-impairing effects of G-15. A minimum of 14 days separated OR and OP testing, the order of which was counterbalanced within a group (each cohort included 10–12 mice/group); this interval between test bouts allowed metabolic clearance of the drugs from the brain and for any acute effects of infusion on brain structure or function to dissipate prior to the next infusion. Finally, at least ten days after the final behavioral test, mice were infused again with G-1 and the DH was collected bilaterally 5, 15, or 30 min later to determine levels of phosphorylated JNK, cofilin, ERK, PI3K, Akt, and CREB via Western blotting.
Fig. 1.
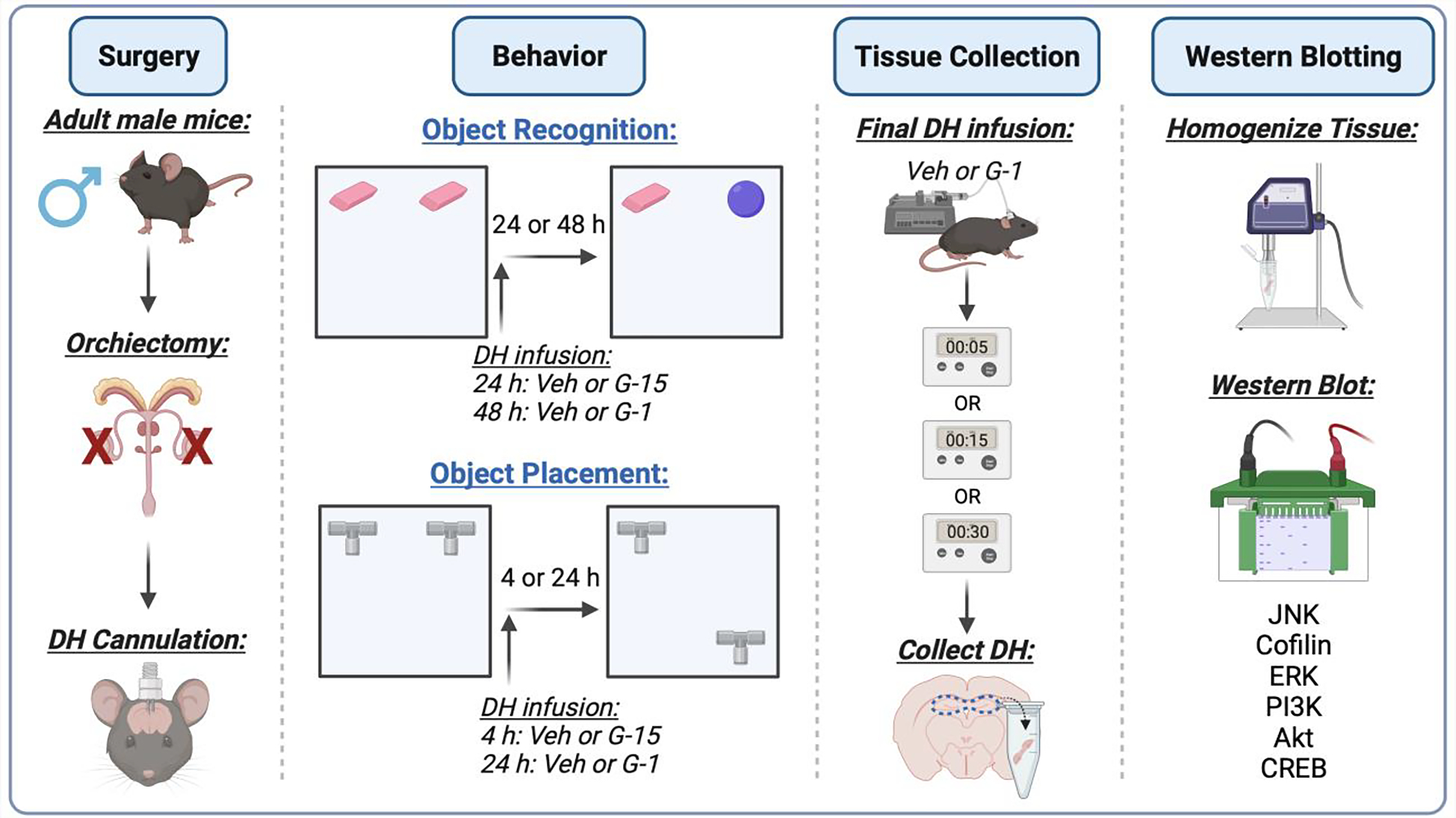
Schematic diagram illustrating the general experimental design. Male C57BL/6 mice (~8 weeks old) underwent bilateral orchiectomy and implantation of dorsal hippocampus (DH) cannulae, and were then given at least 7 days to recover before the start of behavioral testing. All mice were then trained in the object recognition (OR) and object placement (OP) tasks, infused with vehicle (4% or 16% DMSO), G-1 (4 or 8 ng/hemisphere), or G-15 (1.85 or 7.4 ng/hemisphere) immediately after training (upwards arrow), and then tested at the delays indicated in the figure (see text for additional detail). Two weeks separated testing in each task and the sequence of OR and OP testing was counterbalanced within each group. Two weeks after the final behavioral test, mice were infused again and DH tissue was collected bilaterally 5, 15, or 30 min later for homogenization and Western blotting to assess levels of phosphorylated c-Jun N-terminal kinase (JNK), cofilin, extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K), Akt, and cyclic-AMP binding protein (CREB). Figure created with BioRender.com.
2.3. Surgical Procedures
At least four days after arrival in the laboratory, mice underwent bilateral orchiectomy followed immediately by bilateral implantation of chronic indwelling stainless-steel guide cannulae into the DH as described previously (Kim et al., 2019, 2016; Koss et al., 2018). Briefly, mice were anesthetized with 5% isoflurane in 100% oxygen for induction and secured in a stereotaxic apparatus (Kopf Instruments). Anesthesia was maintained at 2–3% isoflurane throughout surgery and analgesia was provided via a 5 mg/kg subcutaneous injection of Rimadyl prior to surgery. For orchiectomy surgeries, a midline incision was made on the scrotal sac, and then the testes were isolated and carefully separated from the fat, tied off at the vas deferens, and removed. The incision was closed with monofilament sutures. Mice were then implanted with bilateral guide cannulae (22 gauge; C232G, P1 Technologies (formerly Plastics One Inc.)) aimed at each hemisphere of the DH (1.7 mm AP, ±1.5 mm ML, 2.3 mm DV). Dummy cannulae (C232DC, P1 Technologies) were inserted into each guide cannula to maintain patency. Cannulae were fixed to the skull with dental cement (Darby Dental Supply), which also served to close the wound. During post-operative recovery, mice were observed carefully for any sign of discomfort and received ¼ of a 2 mg Rimadyl tablet for pain relief on the first post-operative day, and as then needed.
2.4. Drugs and Infusions
Post-training drug infusions were performed as described previously (Kim et al., 2016, 2019; Koss et al., 2018) by gently restraining each mouse to remove the dummy cannulae, followed by placement of an infuser into each guide cannula (C3131; DH; 28 gauge, extending 0.8 mm beyond the 1.5 mm guide). The infuser was secured to PE50 polyethylene tubing attached to a 10 μl Hamilton syringe. Infusions were controlled by a micro infusion pump (KDS Legato 180, KD Scientific) and delivered at a rate of 0.5 μl/min for 1 min. Our unpublished data using AlexaFlour 384 indicates that the spread of infusions using this protocol is restricted to the DH. Infusers remained in place for 1 min after each infusion to avoid diffusion of drugs back up the cannula track.
The selective GPER agonist G-1 (1-[4-(6-bromobenzo[1,3]dioxol-5yl)-3a,4,5,9b-tetrahydro3Hcyclopenta [c]quinolin-8-yl]-ethanone; Azano Biotech) was dissolved in 16% dimethylsulfoxide (DMSO) and infused at doses of 4 or 8 ng/hemisphere into the DH as per our laboratory’s previous work (Kim et al., 2016; 2019). The GPER-selective antagonist G-15 ((3aS*,4R*, 9bR*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-3H-cyclopenta[c] quinolone; Azano Biotech) was dissolved in 2% DMSO and infused at doses of 1.85 or 7.4 ng/hemisphere as described previously (Kim et al., 2019, 2016). Vehicle controls for G-1 and G-15 were 16% and 2% DMSO, respectively.
2.5. Memory Assessment
The effects of G-1 and G-15 on memory consolidation were examined using the OP and OR tasks, which assess spatial and object recognition memory, respectively (Boulware et al., 2013; Fernandez et al., 2008; Tuscher et al., 2016). Both tasks were conducted in a white open field box (width, 60 cm; length, 60 cm; height, 47 cm) under dim lighting (~75 lx) provided by torchiere lights spaced around the box to provide equivalent light to the corners of the box. Before the start of behavioral training, mice were handled for 30 s/day for three consecutive days. On the second handling day, a single Lego Duplo block was placed in the home cage to acclimate mice to objects. Following handling, mice were habituated to the empty open field box for 5 min/day for two consecutive days. During habituation, mice could freely explore the apparatus without objects present.
Following habituation, mice underwent OP or OR training, during which mice were given up to 20 min to accumulate 30 s exploring two identical objects placed in the upper right and left corners of the open field box. Experimenters manually scored object exploration in real-time using ANYmaze tracking software (Stoelting). Exploration was counted when the mouse’s nose and/or front paws were directed towards and/or touching the objects. Time spent exploring the objects and time to accumulate 30 s of object exploration were recorded. Different objects were used for OP and OR, and all objects used were counterbalanced across mice and between sides of the box to account for any potential preferences for objects or locations. For OP testing, the least explored training object was moved to the box’s lower right or left corner. For OR testing, the least explored training object was replaced with a novel object. Immediately following training, mice were given bilateral DH infusions of vehicle, G-1, or G-15 as described above. These treatments were administered immediately post-training to pinpoint effects of G-1 and G-15 to the consolidation phase of spatial and object recognition memory formation. Mice that did not accumulate 30 s of exploration during training were re-trained 4–7 days later with different objects and were given up to three subsequent chances to successfully do so.
The interval between training and testing varied depending on the drug infused as described previously (Kim et al., 2019, 2016). For OR and OP, mice were tested using delays of 48 and 24 h, respectively, for G-1, and delays of 24 and 4 h for G-15. Longer time points were used for G-1 based on previous evidence that vehicle-treated gonadally intact male mice do not remember the location or identity of objects at these time points (Koss et al., 2018), thus allowing us to observe potential memory enhancing effects of G-1. On the other hand, gonadally intact male mice can remember object location and identity at the shorter delays (Frick and Gresack, 2003; Koss and Frick, 2019), permitting observation of potential memory-impairing effects of G-15. Mice that remember the location and identity of the training objects should spend more time than chance with the moved and novel objects. Chance is designated at 15 s because this value represents an equal exploration of both objects (Frick and Gresack, 2003).
2.6. Western Blotting Analysis
Tissue collection and Western blotting were performed as described previously (Kim et al., 2019, 2016; Taxier et al., 2022) to measure effects of G-1 on cell signaling proteins. Briefly, mice were infused as described above and were then cervically dislocated and decapitated 5, 15, or 30 min later for bilateral dissection of the DH on an ice-cold plate. The overlying parietal, occipital, and temporal cortices were removed using a scalpel and forceps to expose the DH, and horizontal cuts were made at a 45° angle through each side of the DH at the level of the base of the superior colliculus to isolate and remove each DH. Tissue samples were immediately weighed and frozen on dry ice, and then stored at 80 °C until homogenization. DH tissues were resuspended to 50 μl/mg in lysis buffer and homogenized using a probe sonicator (Branson Sonifier 250) as described previously (Kim et al., 2019, 2016; Taxier et al., 2022). Proteins were electrophoresed on 10% Tris-HCl precast gels (Bio-Rad) and transferred to PVDF membranes (Bio-Rad). Blots were blocked with 5% skim milk and incubated with primary antibodies (phospho-ERK, phospho-Akt, phospho-PI3K, phospho-JNK, phospho-cofilin, phospho-CREB, 1:1000; Cell Signaling Technology) overnight at 4 °C. Blots were incubated for 1 h at room temperature with a rabbit HRP-conjugated secondary antibody (1:5000; Cell Signaling Technology) and developed using Clarity Max chemiluminescent substrate (BioRad). A ChemiDoc MP gel imager (Bio-Rad) detected signal correlating with protein expression and densitometry was performed using Image Lab Software (Bio-Rad, Image Lab version 6.0.1). Blots were then stripped with ReBlot commercial stripping buffer (Bio-Rad) and incubated with antibodies (total-ERK, total-Akt, total-PI3K, and total-JNK, total-cofilin, total-CREB, 1:1000; Cell Signaling Technology) for protein normalization. Data (n = 5–19/group) were represented as immunoreactivity relative to 5-minute vehicle-treated controls. Treatment effects were measured within single gels.
2.7. Statistical Analyses
Statistical analyses were conducted using GraphPad Prism 9 (La Jolla, CA). To assess learning within each group, separate one-sample t-tests were performed within each group to determine if the time spent with the novel or moved object differed from chance (15 s) (Boulware et al., 2013; Frick and Gresack, 2003; Taxier et al., 2022; Tuscher et al., 2016). To assess between-group treatment effects, one-way analyses of variance (ANOVAs) were conducted to assess potential main effects of Treatment, followed by Tukey’s posthoc tests (Kim et al., 2019, 2016). The time to accumulate 30 s of object exploration was analyzed using one-way ANOVA. Normalized Western blot data were analyzed using two-way ANOVAs with Treatment (vehicle, G-1) and Time (5, 15, 30 min) as independent variables. Tukey’s multiple comparisons tests were used to evaluate simple effects within columns (treatment) and rows (time) (Taxier et al., 2022). Statistical significance was determined at p ≤ 0.05. The Shapiro-Wilk Test was used to assess the normality of our samples (p ≥ 0.05), and all passed this normality test. Effect sizes were calculated as Cohen’s d for significant t-tests and eta squared (η2) for significant ANOVAs.
3. Results
3.1. Dorsal hippocampal GPER activation promotes memory consolidation in male mice
We first evaluated the extent to which immediate post-training DH infusion of G-1 (4 or 8 ng/hemisphere (ng/h)) could facilitate memory consolidation in GDX male mice tested 48 and 24 h after OR and OP training, respectively, to provide a direct comparison with our use of these G-1 doses and testing parameters as in OVX mice (Kim et al., 2019, 2016). Mice infused with either 4 or 8 ng G-1 spent significantly more time with the novel object than chance (4 ng/h: t(12) = 3.64, p = 0.003, d = 1.00; 8 ng/h: t(12) = 6.60, p < 0.0001, d = 1.83; Fig. 2A), suggesting that both doses enhanced OR memory consolidation in males. The main effect of Treatment was also significant in the one-way ANOVA (F(2,36) = 12.25, p < 0.0001, η2 = 0.405), and posthoc tests revealed that mice treated with 4 or 8 ng/hemisphere G-1 spent significantly more time with the novel object than those infused with vehicle (4 ng/h: p = 0.002; 8 ng/h: p = 0.0001; Fig. 2A). Elapsed time to accumulate 30 s of exploration did not differ among the groups (F(2,36) = 3.03, p > 0.05; vehicle = 425.2 ± 48.07; 4 ng/h G-1 = 600.8 ± 58.55; 8 ng/h G-1 = 580.2 ± 58; Fig. 2C). In OP (Fig. 2B), only mice infused with 8 ng/h G-1 explored the moved object significantly more than chance (t(11) = 2.3, p = 0.04, d = 0.67). Interestingly, the 4 ng/h G-1 group spent significantly less time than chance with the moved object (t(9) = 4.08, p = 0.002, d = 1.29). The main effect of Treatment was significant (F(2,31) = 5.99, p = 0.006, η2 = 0.279; Fig. 2B) due to differences between the 8 ng/h G-1 group and both the vehicle (p = 0.03) and 4 ng/h (p = 0.008) groups. As with OR, elapsed time to accumulate 30 s of exploration in OP during testing did not differ among the groups (F(2,31) = 0.98, p > 0.05; vehicle = 300.8 ± 23.78; 4 ng G-1 = 350.9 ± 34.75; 8 ng G-1 = 355.4 ± 34.39; Fig. 2D). Collectively, these data suggest that GPER activation dose-dependently enhances both OR and OP memory consolidation in GDX males, such that 8 ng/h G-1 enhanced memory in both tasks, whereas the effects of 4 ng/h G-1 were task-dependent.
Fig. 2.
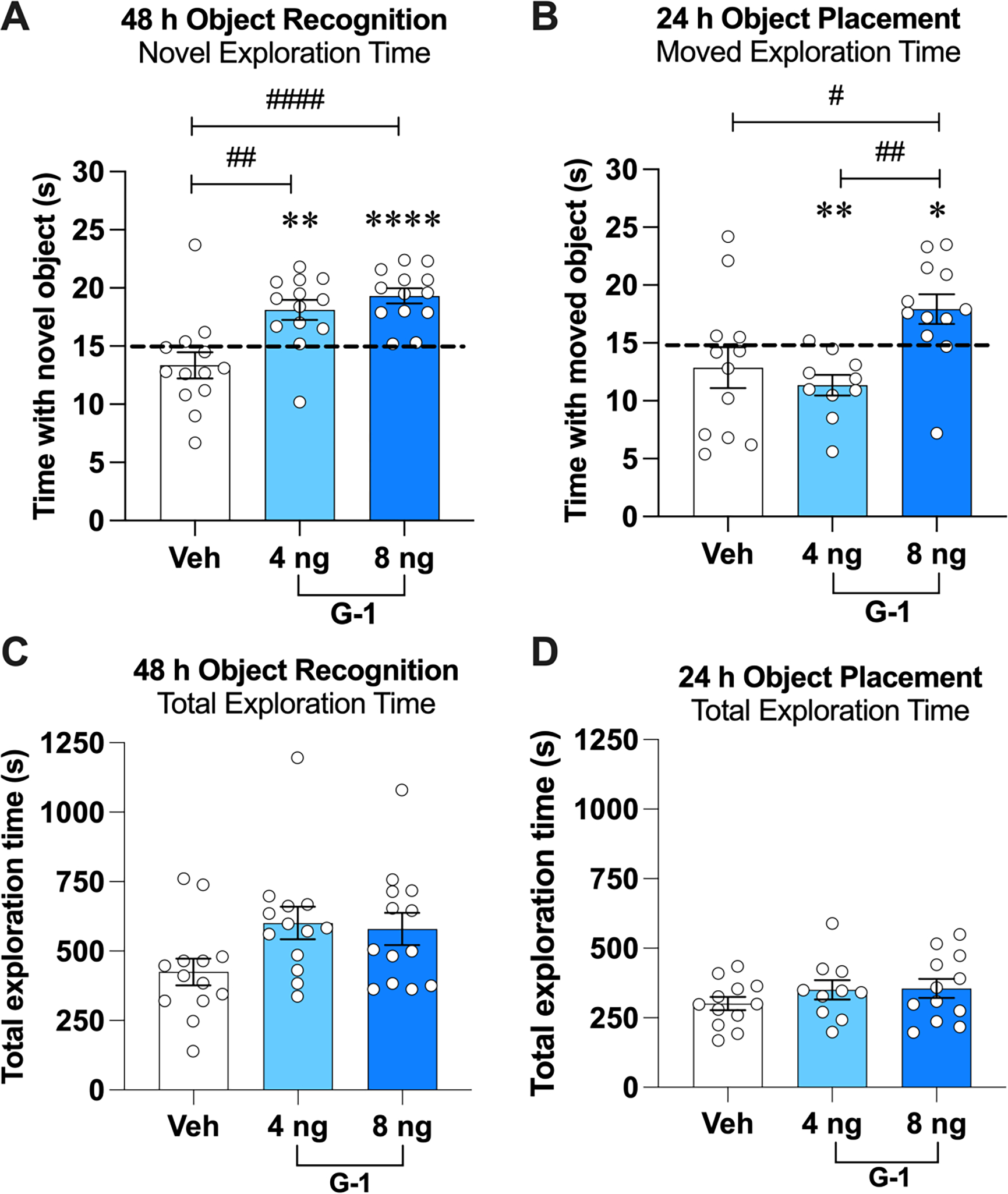
The GPER agonist G-1 enhanced memory consolidation in male mice. A) During OR testing, GDX mice receiving DH infusion of G-1 (4 or 8 ng/h) spent significantly more time with the novel object than chance (dashed line at 15 s). Both G-1 groups also spent significantly more time with the novel object than the vehicle group. B) During OP testing, mice receiving DH infusion of 8 ng/h G-1 spent significantly more time than chance with the moved object, whereas those treated with vehicle or 4 ng/h G-1 did not. The 8 ng/h G-1 group also spent more time with the moved object than the vehicle and 4 ng/h groups. C, D) The groups did not differ significantly in time to accumulate 30 s of exploration during testing in OP or OR tasks. Circles represent individual mice and each error bar represents the mean ± standard error of the mean (SEM) time (s) spent with the novel (OR) or moved (OP) object. (*p < 0.05, **p < 0.005, ****p < 0.0001 relative to chance; #p < 0.05, ##p < 0.01, ####p < 0.0001 relative to vehicle or 4 ng/h group) (n = 12–13 mice/group).
We next evaluated the extent to which GPER antagonism could impair memory consolidation in GDX mice. OR and OP memory consolidation were tested 24 and 4 h after training, delays at which vehicle-infused OVX mice show intact memory for the identity and location of the training objects (Kim et al., 2016, 2019). Accordingly, vehicle-infused GDX males spent significantly more time with the novel (t(11) = 4.36, p = 0.0011; d = 1.26, Fig. 3A) and moved (t(11) = 2.553, p = 0.0268, d = 0.73; Fig. 3B) objects than chance. In contrast, mice infused with 7.4 ng/h G-15 spent chance amounts of time with the novel (t(12) = 0.4481, p = 0.6621) and moved (t(12) = 2.16, p = 0.0517) objects. The 1.85 ng/h dose of G-15 impaired OP (t(11) = 0.2936, p = 0.7745), but had no detrimental effect on memory in OR (t(13) = 4.248, p = 0.001, d = 1.13). These treatment effects were reflected in one-way ANOVAs, such that the main effects of Treatment were significant for both OR (F(2,36) = 3.704, p = 0.0345, η2 = 0.171) and OP (F(2,34) = 5.785, p = 0.0069, η2 = 0.254). Posthoc analyses indicated that the 7.4 ng/h group spent significantly less time with the novel (p = 0.0332) and moved (p = 0.0056) objects than the vehicle group. The 1.85 ng/h group did not differ from vehicle in either task. Elapsed time to accumulate 30 s of exploration did not differ among the groups for either OR (F(2,36) = 2.15, p > 0.05; vehicle = 457.2 ± 46.15; 1.85 ng G-15 = 493.9 ± 39.17; 7.4 ng G-15 = 622.2 ± 81.76; Fig. 3C) or OP (F(2,34) = 0.04, p > 0.05; vehicle = 475.5 ± 49.08; 1.85 ng G-15 = 464.4 ± 75.08; 7.4 ng G-15 = 453.4 ± 44.94; Fig. 3D). Together, these data indicate that G-15 dose-dependently impaired OR and OP memory consolidation in males and suggest that spatial memory consolidation in GDX males may be more susceptible than in OVX females to the memory-impairing effects of GPER antagonism.
Fig. 3.
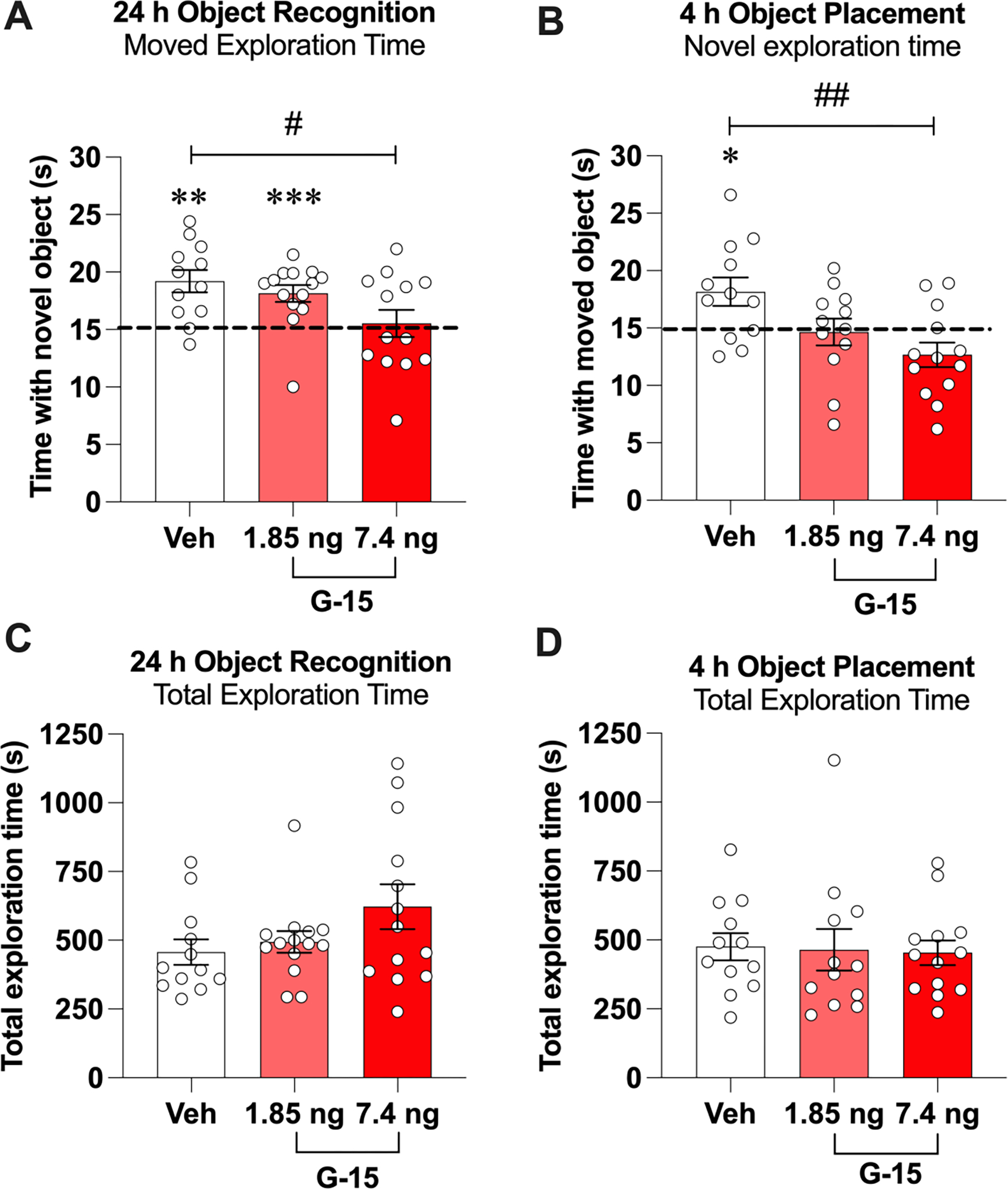
The GPER antagonist G-15 impaired memory consolidation in male mice. A) In the OR task, GDX mice receiving DH infusion of 7.4 ng/h G-15 spent significantly less time than chance (dashed line) or than the vehicle group with the novel object during testing. B) During OP testing, mice receiving DH infusion of G-15 (1.85 or 7.4 ng/h) spent significantly less time than chance with the moved object, but only the group that received 7.4 ng/h of G-15 spent significantly less time with the moved object than vehicle. C, D) There were no significant differences among groups in time to accumulate 30 s of exploration during OP or OR testing. Circles represent individual mice and each error bar represents the mean ± SEM time (s) spent with the novel (OR) or moved (OP) object. (*p < 0.05, **p < 0.01, ***p < 0.001 relative to chance; #p < 0.05, ##p < 0.01 relative to vehicle) (n = 12–14 mice/group).
3.2. Neither JNK nor cofilin were phosphorylated by GPER activation in the male DH within 30 minutes
We next sought to identify downstream cellular mechanisms underlying GPER-induced enhancements in object recognition and spatial memory consolidation. Thus, we evaluated the impact of DH G-1 infusion on the phosphorylation of signaling kinases known to regulate the effects of GPER or E2 on memory consolidation in OVX mice. In our previous work with OVX mice, levels of phospho-JNK(p46), phospho-JNK(p54), and phospho-cofilin were elevated 5 min after DH G-1 infusion, and inhibitors of JNK or actin polymerization blocked the memory-enhancing effects of G-1 (Kim et al., 2019, 2016). As such, we first examined the extent to which bilateral DH infusion of G-1 (8 ng/h) in GDX mice affected phosphorylation of the 46kD and 54kD isoforms of JNK and the actin regulatory protein cofilin. Surprisingly, G-1 did not increase DH phosphorylation of p46 or p54 JNK at any time point (Fig. 4A,B), as indicated by null effects of Treatment (p46: F(1,59) = 0.99, p = 0.32; p54: F(1,59) = 0.88, p = 0.35), Time (p46: F(2,59) = 2.86, p = 0.07; p54: F(2,59) = 2.51, p = 0.09), and Treatment × Time (p46: F(2,59) = 0.48, p = 0.62; p54: F(2,59) = 1.47, p = 0.23).
Fig. 4.
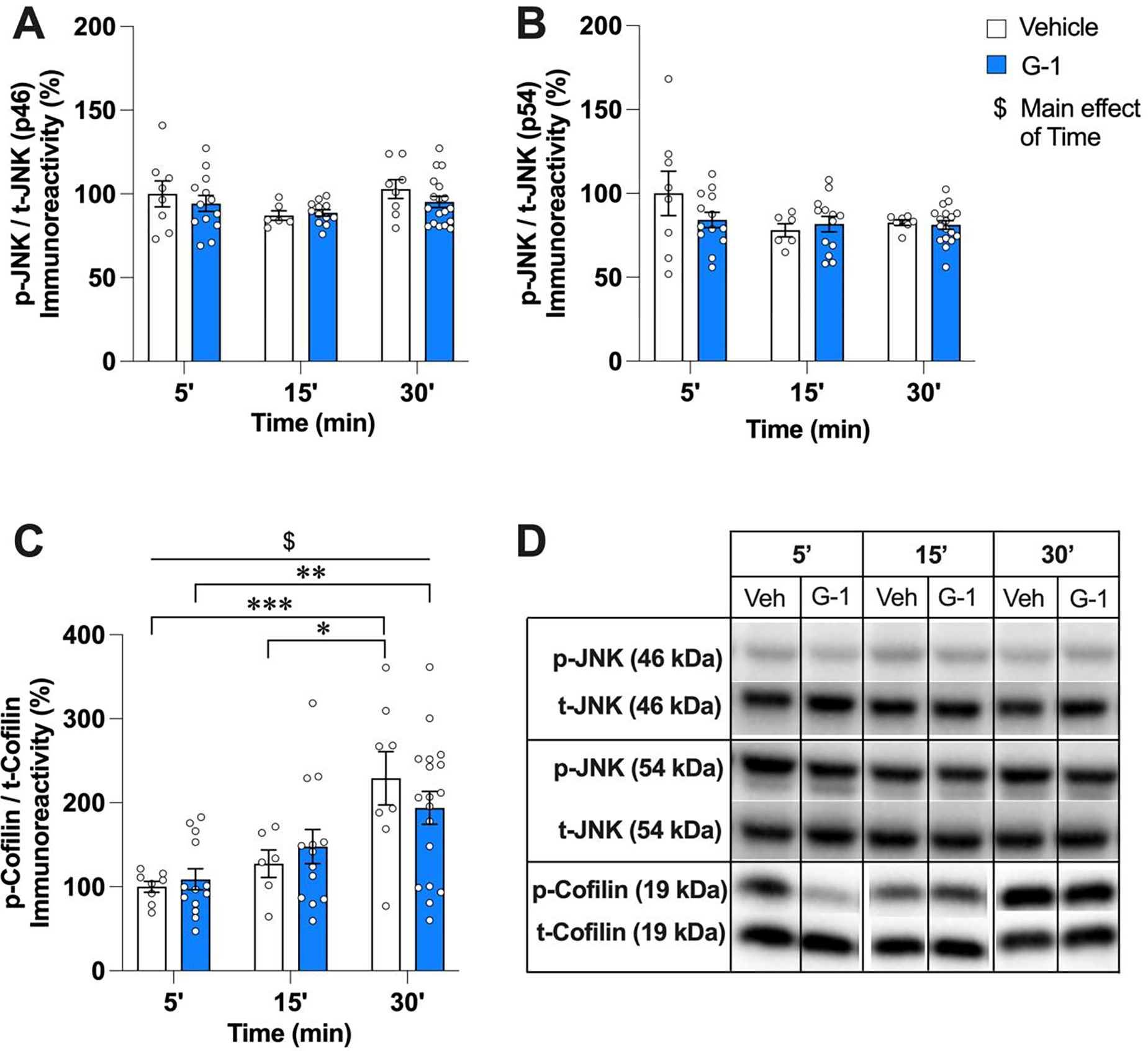
G-1 did not affect JNK or cofilin phosphorylation in the DH of male mice within 30 min of infusion. A, B) Infusion of 8 ng/h G-1 did not alter levels of phospho-p46 or phospho-p54 JNK at the 5, 15, or 30 min timepoints. C) Levels of phospho-cofilin were significantly higher 30 min after either vehicle or G-1 infusion, suggesting non-specific effects of infusion rather than a specific increase by GPER activation. Circles represent individual mice and each error bar represents the mean ± SEM % change from vehicle controls ($ represents a significant main effect of Time. Significant simple main effects of Time within treatment are represented by *p < 0.05, **p < 0.01, ***p < 0.001) (n = 6–18 mice/group). D) Images of representative blots at each time point for phosphorylated and total forms of each protein.
For cofilin phosphorylation (Fig. 4C), the main effect of Time was significant (F(2,60) = 13.90, p < 0.0001, η2= 0.317), but not the main effect of Treatment (F(1,60) = 0.012, p = 0.91) or the Treatment × Time interaction (F(2,60) = 0.95, p = 0.40). Tukey’s multiple comparison tests to evaluate simple main effects of Time within each treatment indicated that phospho-cofilin levels were significantly higher among vehicle-treated mice 30 min after infusion relative to 5 and 15 min (30 vs 5 min: adjusted p = 0.0009; 30 vs 15 min: adjusted p = 0.019), and among G-1-treated mice 30 min after infusion relative to 5 min (30 vs 5 min: adjusted p = 0.0028), suggesting likely non-specific effects of the infusion procedure on phospho-cofilin levels.
Together, these data indicate that GPER activation in the DH of GDX males does not rapidly trigger JNK signaling or actin polymerization, and therefore, suggest that the effects of GPER on memory consolidation in GDX males do not depend on these processes as previously demonstrated in OVX females (Kim et al., 2019, 2016). Thus, we next assessed activation of classical intracellular pathways associated with E2-induced memory consolidation in OVX mice.
3.3. G-1 did not activate signaling pathways in the DH of males associated with E2-induced memory enhancement in females
In OVX mice, the ability of E2 to enhance memory consolidation in the OR and OP tasks depends on rapid activation of p42 ERK (but not p44 ERK) and PI3K/Akt (Fernandez et al., 2008; Fortress et al., 2013; Frick, 2015; Koss et al., 2018). However, G-1 in OVX mice does not activate these signaling pathways (Kim et al., 2016), suggesting that the memory-enhancing effects of E2 and GPER involve different signaling pathways in OVX females. Nevertheless, it is possible that these pathways could be involved in the memory-enhancing effects of G-1 in GDX males. Thus, levels of phosphorylated p42 ERK, p44 ERK, PI3K, and Akt were measured in the DH of the vehicle- and G-1-infused mice.
As in OVX mice (Kim et al., 2016), G-1 had no effects on p42 or p44 ERK phosphorylation at any timepoint in GDX males, as illustrated by null effects of Treatment (p42 ERK: F(1,61) = 1.24, p = 0.27; p44 ERK: F(1,60) = 0.08, p = 0.78), Time (p42 ERK: F(2,61) = 0.50, p = 0.60; p44 ERK: F(2,60) = 2.00, p = 0.14), and Treatment × Time (p42 ERK: F(2,61) = 1.89, p = 0.16; p44 ERK: F(2,60) = 2.37, p = 0.10) (Fig. 5A,B). Also similar to OVX mice, G-1 did not affect levels of phospho-PI3K (Treatment: F(1,59) = 0.03, p = 0.87; Time: F(2,59) = 0.20, p = 0.81; Treatment × Time: F(2,59) = 0.03, p = 0.96) or phospho-AKT (Treatment: F(1,47) = 1.54, p = 0.22; Time: F(2,47) = 1.12, p = 0.34; Treatment × Time: F(2,47) = 1.94, p = 0.15) (Fig. 5C,D). These findings suggest that the effects of G-1 on memory in GDX males do not involve ERK or PI3K/Akt signaling.
Fig. 5.
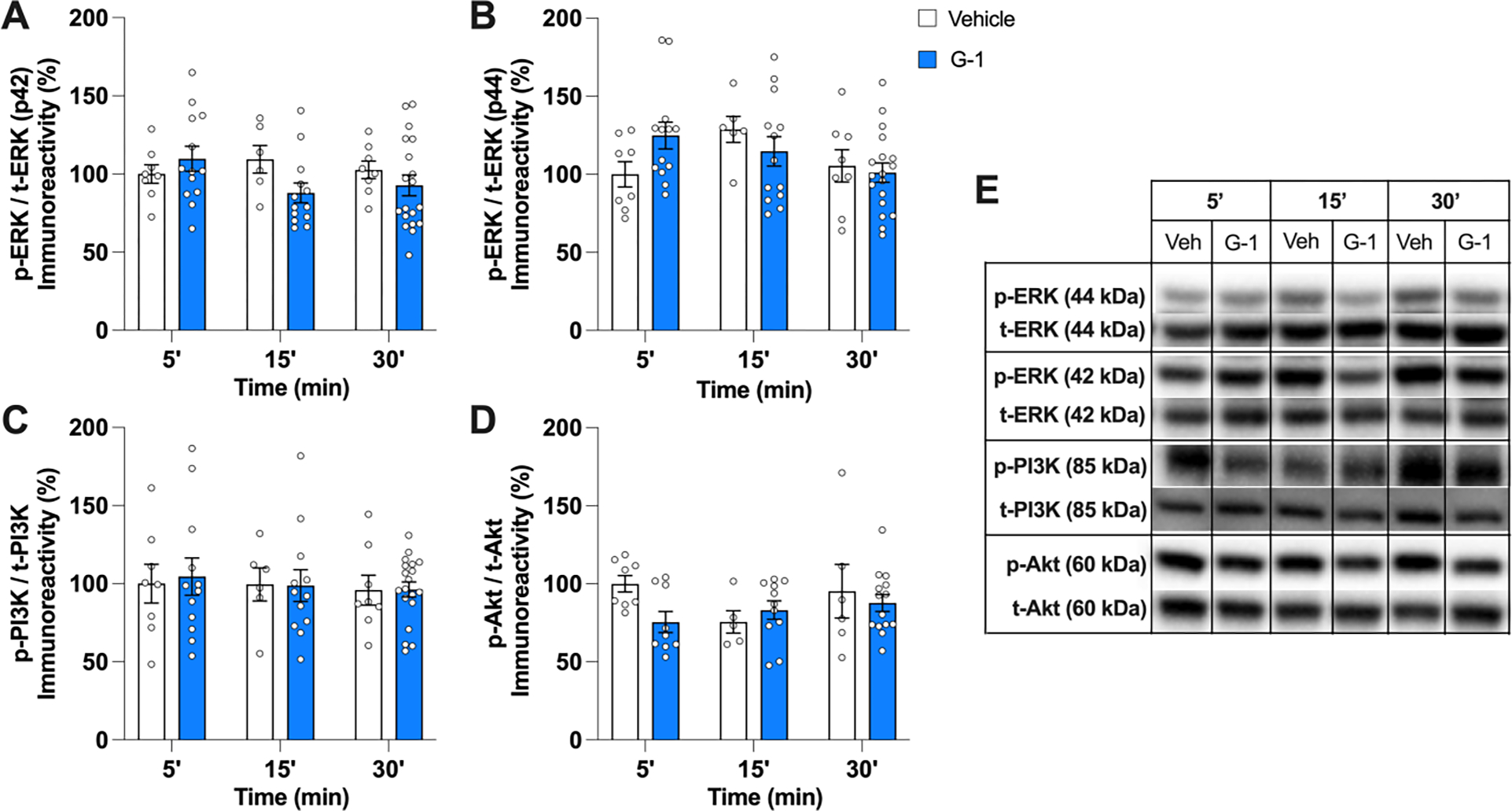
G-1 did not activate ERK/PI3K/Akt pathways in the DH of male mice within 30 min of infusion. A, B) Infusion of 8 ng/h G-1 did not alter levels of p42 ERK or p44 ERK relative to vehicle 5, 15, or 30 min after infusion. C, D) G-1 also did not affect PI3K or Akt phosphorylation relative to vehicle at any time point. Circles represent individual mice; each error bar represents the mean ± SEM % change from vehicle controls (n = 5–19 mice/group). E) Images of representative blots at each time point for phosphorylated and total forms of each protein.
3.4. G-1 increased CREB phosphorylation 30 minutes after DH infusion
CREB is a primary transcription factor required for the formation of long-term memories and for synaptic plasticity in the hippocampus and other cognitive brain regions (Barco et al., 2003; Bernabeu et al., 1997; Bevilaqua et al., 1997; Koss et al., 2018). Systemic administration of G-1 in OVX rats increased phospho-CREB levels after 3 h (Machado et al., 2019), and DH infusion of E2 increased DH phospho-CREB levels within 5 min in OVX mice and both gonadally-intact and GDX male mice (Koss et al., 2018). Thus, we next examined effects of 8 ng/h G-1 on DH CREB phosphorylation. G-1 significantly increased phospho-CREB levels in a time-dependent manner (Fig. 6A), as illustrated by main effects of Treatment (F(1,57) = 5.08, p = 0.03, η2= 0.082) and Time (F(2,57) = 31.53, p < 0.001, η2= 0.525), although the Treatment × Time interaction (F(2,57) = 2.77, p = 0.07) was not significant. Tukey’s multiple comparison tests to evaluate simple effects of Time within each treatment demonstrated that levels of phospho-CREB among G-1-infused mice were significantly elevated 30 min after infusion relative to the 5 and 15 min time points (adjusted p < 0.0001 for both comparisons). Interestingly, the 30 min vehicle group also exhibited significantly elevated levels of phospho-CREB relative to the 5 min vehicle group (adjusted p = 0.003), although the significant main effect of Treatment suggests a greater increase at this time point after G-1 infusion.
Fig. 6.
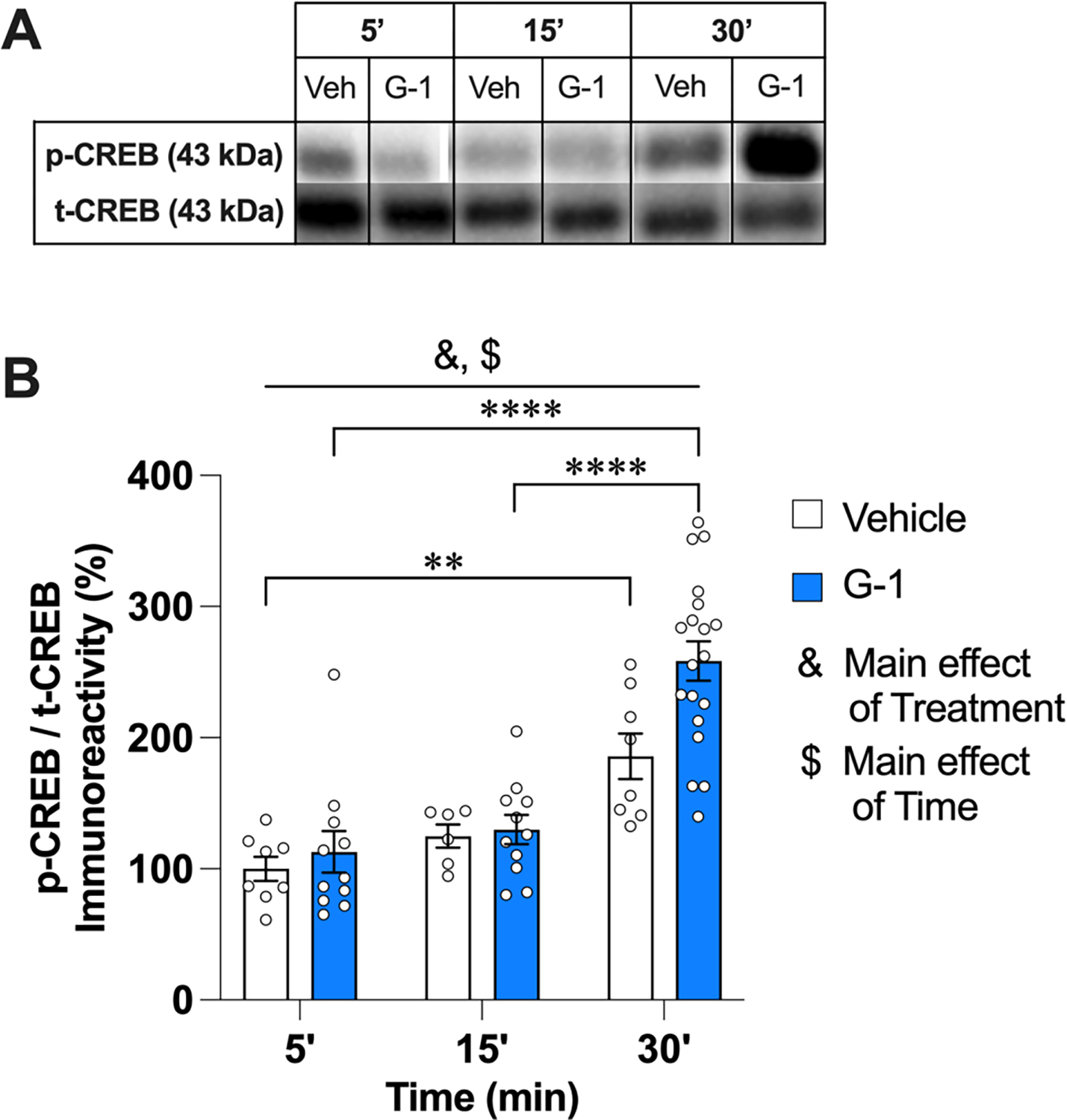
G-1 increased phospho-CREB levels in the DH 30 min after infusion. A) Images of representative blots at each time point for phosphorylated and total forms of each protein. B) Infusion of either vehicle or 8 ng/h G-1 increased CREB phosphorylation in the male DH 30 min later, although the significant Treatment effects suggests that the increase in the G-1 group was greater than that of vehicle. Circles represent individual mice; each error bar represents the mean ± SEM % change from vehicle controls (& represents a significant main effect of Treatment; $ represents a significant main effect of Time. Significant simple main effects of Time within treatment are represented by *p < 0.05, **p < 0.01, ****p < 0.0001) (n = 6–19 mice/group).
4. Discussion
The molecular and cellular mechanisms through which estrogens regulate memory remain unclear, but substantial progress has been made in recent years to pinpoint key molecules and neural processes underlying estrogenic memory modulation in females. Considerably less work has focused on males, despite the presence of E2, estrogen receptors, and de novo estrogen synthesis in the male brain (Cornil et al., 2012; Frick et al., 2018; Hutson et al., 2019; Koss and Frick, 2019; Maney and Pinaud, 2011). As such, the current study was designed to provide new insights into estrogenic regulation of memory in males by specifically focusing on the membrane estrogen receptor GPER, whose activation in OVX females enhances memory by rapidly increasing JNK signaling, actin polymerization, and CA1 dendritic spine density in the DH (Kim et al., 2019, 2016). Consistent with the data from OVX females, the present findings demonstrate that pharmacological activation of dorsal hippocampal GPER by G-1 enhances object recognition and spatial memory consolidation in GDX male mice. Additionally, antagonism of GPER by G-15 impaired the consolidation of both types of memory, as observed in females (Kim et al., 2019, 2016). Unlike in previous studies of males in which G-1 and GPER antagonists were injected systemically (de Souza et al., 2021; Xu et al., 2018), G-1 and G-15 were infused here into the DH, establishing specific involvement of GPER in this brain region in males. However, the cellular mechanisms within the DH that regulate the effects of GPER in males are unclear. Unlike in females (Kim et al., 2019, 2016), DH infusion of G-1 did not enhance the phosphorylation of JNK or cofilin within 30 minutes, nor did it activate the ERK or PI3K/Akt pathways as E2 does in OVX females (Fernandez et al., 2008; Fortress et al., 2013). G-1 did increase the levels of phospho-CREB in the DH 30 minutes after infusion, indicating downstream effects on gene transcription, but the cell signaling events leading to this increase remain unclear at the present time. Together, our findings indicate that GPER activation in the DH is a key regulator of object recognition and spatial memory consolidation in GDX male mice, although the intracellular signaling pathways involved appear to be distinct from those described previously in OVX female mice. As such, these data underscore the importance of investigating sex differences in the neural mechanisms underlying hormonal regulation of memory.
4.1. A role for DH GPER activation on spatial and object recognition memory consolidation in males
We first demonstrated that the bilateral DH infusion of G-1 immediately after training enhanced object recognition and spatial memory consolidation in GDX male mice in a manner dependent on dose and task. The beneficial effects of G-1 on memory consolidation in the OR and OP tasks are consistent with previous studies in which systemic administration of G-1 enhanced spatial and contextual memory in male and female rodents (Bai et al., 2020; de Souza et al., 2021; Hammond et al., 2012, 2009; Hawley et al., 2014; Lymer et al., 2017; Machado et al., 2019; Xu et al., 2018). However, the use of intrahippocampal infusions in this study allowed us to specifically pinpoint the memory-enhancing effects of G-1 to GPER in the DH. Here, the 4 ng/h dose of G-1 facilitated object recognition memory consolidation only, whereas 8 ng/h G-1 enhanced consolidation in both tasks. These data suggest that spatial memory consolidation is less sensitive to the beneficial effects of G-1 in males, in that consolidation in the OP task was not facilitated by the lower 4 ng/h dose. These findings also suggest potentially important sex differences in the dose-response to G-1, given previous work from our laboratory demonstrating that DH infusion of 4 ng/h G-1 improved memory consolidation in both the OP and OR tasks among OVX mice (Kim et al., 2016). As such, the sensitivity of spatial memory to GPER activation may differ somewhat between female and male mice. It should be noted, however, that the 8 ng/h G-1 dose was not tested in our previous studies with OVX mice, so the response of females to this dose is unknown. A previous study in which 1 or 5 μg G-1 was injected subcutaneously for 15 consecutive days reported that both doses improved spatial memory in the Morris water maze in mature adult intact male mice, suggesting that the dose sensitivity of spatial memory to G-1 could be specific to the OP task or related to age or the loss of gonadal hormones (Xu et al., 2018). Future work directly comparing effects of G-1 on consolidation in OP and other spatial tasks among young adult male and female mice (with and without gonads) will support more definitive conclusions about the potential reduction in OP sensitivity to GPER activation.
We next showed that immediate post-training DH infusion of G-15 impaired memory consolidation in both the OP and OR tasks. The doses of G-15 used (1.85 and 7.4 ng/h) were based on our laboratory’s previous work with OVX mice in which post-training DH infusion of 7.4 ng/h, but not 1.85 ng/h, impaired memory consolidation in OP and OR (Kim et al., 2016). As in OVX females, we found that 7.4 ng/h G-15 impaired consolidation in both tasks, suggesting that activation of GPER is necessary for memory consolidation in males as it is in females. However, as with G-1, effects of the lower dose were task-dependent, in that 1.85 ng/h G-15 impaired memory consolidation in OP but not OR. The memory-impairing effect of 1.85 ng/h in OP was surprising, given that this dose had no effects on OP in female mice (Kim et al., 2016), and suggest that spatial memory in males may be more dependent on GPER activation than in females. However, as with G-1, direct comparisons within the same study will be necessary to support conclusions about the differential sensitivity of males and females tested in OR and OP to low and high doses of G-15. Nevertheless, the detrimental effects of DH 7.4 ng/h G-15 infusion on memory consolidation observed here are consistent not only with our own DH infusions in OVX mice, but also with those of previous systemic studies in which acute post-training G-15 injection impaired object recognition memory consolidation in gonadally-intact male rats (de Souza et al., 2021) and chronic minipump administration of G-15 impaired spatial memory in a T-maze among female OVX rats (Hammond et al., 2012).
Together, these data suggest that activation of GPER in the DH is necessary for the formation of recognition and spatial memories in both sexes, yet the effects of G-15 in this and other studies beg the question of what signal G-15 inhibits in OVX and castrated mice with low circulating sex steroid levels. In males, estrogens are mainly produced in the testis (Hess, 2003) and the brain, including the hippocampus (Hojo et al., 2004; Prange-Keil et al., 2003; Hernández-Vivanco et al., 2022). Local E2 synthesis is necessary for maintaining hippocampal spine synapses (Kretz et al., 2004) and hippocampal synaptic plasticity (Zhou et al., 2010). In male zebra finches, social interactions with females or exposure to other male’s songs increase forebrain E2 synthesis (Remage-Healey et al., 2008), suggesting that learning triggers brain E2 synthesis. Our work with OVX females suggests that object learning increases DH E2 synthesis, and that infusion of the aromatase inhibitor letrozole into the DH blocks both this increase and memory consolidation in the OR and OP tasks (Tuscher et al., 2016). Thus, we hypothesize that the signal inhibited by G-15 in the DH is induced by object learning. This hypothesis is supported by our previous work in GDX male mice showing that DH letrozole infusion blocks memory consolidation in the OR and OP tasks (Hernández-Vivanco et al., 2022; Hojo et al., 2004; Prange-Kiel et al., 2003), as well as data from hippocampal cultures showing that treatment with NMDA increases E2 synthesis (Fester et al., 2016). Because our findings from OVX females suggest that E2 and GPER regulate memory consolidation via divergent cell signaling pathways (Kim et al., 2016), it is unclear whether E2 is the ligand whose actions are blocked by G-15, or whether other estrogens or sex steroids are the critical signal(s). This issue should be addressed in future studies.
4.2. GPER activation in the DH of males did not affect phosphorylation of JNK, cofilin, ERK, PI3K, or Akt within 30 minutes
Given the consistent memory-enhancing effects of 8 ng/h G-1 in both tasks, we next used this dose to determine which cell signaling pathways might mediate the memory-enhancing effects of GPER activation in males. In OVX mice, we previously found that G-1 increased levels of phospho-JNK in the DH 5 minutes after DH infusion and increased DH cofilin phosphorylation 5 and 15 minutes after G-1 infusion (Kim et al., 2019, 2016). Activation of both pathways in the DH was necessary for G-1 to promote memory consolidation in the OR and OP tasks, and cofilin-dependent actin polymerization was necessary for G-1 to increase CA1 dendritic spine density (Kim et al., 2019, 2016). Thus, our initial hypothesis here was that JNK and cofilin phosphorylation would be increased by G-1 in male mice. Surprisingly, even though most of the behavioral effects of G-1 mirrored our previous findings in females, G-1 in this study did not affect levels of phospho-JNK in males at any time point after DH infusion. Interestingly, DH phospho-cofilin levels were significantly increased in both the vehicle and G-1 groups 30 minutes after infusion, suggesting a non-specific effect of the infusion procedure on cofilin at this time point. Although unclear what might have caused an increase in cofilin that was not observed for other signaling proteins, one possibility is related to our vehicle solution. Here, our vehicle was 16% DMSO, and earlier in vitro studies showed that four days of constant exposure to 2% DMSO was associated with progressive reorganization of the cytoskeleton of B16 melanoma cells and an increase in the cellular content of the membrane cytoskeletal protein vinculin (Lampugnani et al., 1987; Sousa-Squiavinato et al., 2019). The concentration of DMSO is an important determinant of its effects on cell membranes, as DMSO in low concentrations (<10 mol %) is associated with a significant reduction in membrane thickness, such that concentrations between 10–20 mol % are associated with increased water pore formation, and concentrations >20 mol % are associated with desorption of individual lipid molecules and disintegration of the lipid bilayer membrane structure (Gurtovenko and Anwar, 2007). It is important to note that although these effects are particularly important for the diffusion of hydrophilic molecules, they could potentially mediate the enhancement observed in phospho-cofilin levels in our vehicle group, as caveolae structural changes in the phospholipid membrane can lead to kinase activation (Mineo et al., 1998). Alternatively, the elevated levels of phospho-cofilin in the vehicle group could be associated with the infusion itself, as infusion-associated intracerebral bleeding or disruption of neurons and glia can lead to increased cofilin levels (Almarghalani et al., 2023; Van Troys et al., 2008). Regardless, the current data suggest that JNK activation and cofilin phosphorylation are not associated with the memory-enhancing effects of GPER in GDX male mice. However, future studies in which G-1 is co-infused with the JNK inhibitor SP600125 or actin polymerization inhibitor latrunculin-A will be necessary to rule out involvement of these pathways more definitively.
The unexpected JNK findings suggest key sex differences in the mechanisms regulating GPER-induced memory modulation. This is not, however, the first time that our laboratory has observed sex differences in the signaling mechanisms underlying estrogenic regulation of memory consolidation; several years ago, we found that the memory-enhancing effects of E2 depend on activation of p42 ERK in female, but not male, mice (Koss et al., 2018). Other reports have demonstrated sex differences in the role of PKA in mediating synaptic potentiation (Jain et al., 2018; Jain and Woolley, 2023), and that the effects of E2 on glutamatergic sensitivity depend on post-synaptic GPER in female, but not male, rats (Oberlander and Woolley, 2016). Thus, we next explored other cell signaling mechanisms involved in estrogenic memory regulation.
Although work from our laboratory and others calls into question the extent to which the rapid effects of E2 mediated by ERα and ERβ overlap with those mediated by GPER activation (Arterburn and Prossnitz, 2023; Kim et al., 2019, 2016; Luo and Liu, 2020; Prossnitz and Barton, 2023), we next turned to the ERK and PI3K/Akt pathways because the phosphorylation of p42 ERK, PI3K, and Akt is necessary for E2 to enhance object recognition and spatial memory consolidation in OVX mice (Boulware et al., 2013; Fan et al., 2010; Fernandez et al., 2008; Koss et al., 2018). However, we thought it unlikely that G-1 would increase phosphorylation of these kinases in males because G-1 failed to activate them in OVX females (Kim et al., 2016). Yet given previous sex differences in E2-induced kinase activation, we thought these kinases were worth examining. As expected, G-1 did not affect phosphorylation of either ERK isoform, PI3K, or Akt. The lack of effects is consistent with our previous findings in OVX females (Kim et al., 2016), and supports our previous conclusions that the ability of DH GPER to enhance memory consolidation does not involve activation of the ERK or PI3K/Akt pathways. The fact that GPER does not activate the same signaling pathways as E2 in either sex may be advantageous in considering future use of GPER agonists to promote memory formation in clinical practice, as systemic G-1 administration is not associated with cell proliferation in tissues like the uterus (Machado et al., 2019).
4.3. GPER activation enhanced CREB phosphorylation in the DH 30 minutes after infusion
Despite the inability of G-1 to activate the JNK, ERK, and PI3K/Akt pathways in the DH 5, 15, or 30 min after infusion, it did increase CREB phosphorylation in the DH of GDX males 30 minutes after infusion. Literature on the effects of GPER activation on CREB phosphorylation in the brain is scarce, but one previous study did find that CREB phosphorylation was significantly increased in the hippocampus of OVX rats 3 hours after acute systemic G-1 injection, and this effect was abolished by systemic PKA inhibitor administration (Machado et al., 2019). In cumulus cells from mouse oocytes, G-1 incubation was also associated with elevated phospho-CREB levels, and this effect was blocked by G-15 (Zhang et al., 2020). Thus, there is some precedence for increased CREB after G-1 treatment, however, the upstream signaling kinases involved are not clear. Here, G-1 did not activate ERK, PI3K/Akt, or JNK signaling in the DH, suggesting that these kinase pathways did not mediate the effects of G-1 on CREB. A previous study from our laboratory evaluating E2 effects on memory consolidation in gonadally intact and GDX male mice found that the levels of CREB phosphorylation in the DH were increased 5 minutes after E2 infusion, with no significant effect on phospho-ERK or phospho-Akt levels (Koss et al., 2018). Interestingly, CREB was increased by G-1 30 minutes after infusion instead of 5 minutes. At the present time, it is unclear how G-1 leads to CREB phosphorylation, and the delayed effect on CREB relative to that of E2 perhaps suggests additional mechanisms aside from cell signaling. For example, E2 activates ERK-dependent histone acetylation in the DH 30 minutes after bilateral DH infusion in OVX females (Zhao et al., 2010), so the effects of E2 on CREB may involve other processes as well. Immediate early genes, such as c-fos, Egr-1, arc, and AP-1, could also play a role. Additional work will be necessary in future studies to determine the mechanisms governing G-1-induced CREB phosphorylation in males.
Finally, we did not expect the levels of phospho-CREB to be significantly elevated in the vehicle group 30 minutes following DH infusion. As described before, vehicle mice received 16% DMSO, which has been previously used by our and other laboratories (Hoeger-Bement and Sluka, 2003) for intracranial administrations. In other work, incubation of pancreatic rat cells with 2% DMSO did not increase cAMP levels, PKA activity, or phosphorylated levels of CREB, CRE-modulator, and activating transcription factor-1 (ATF-1) (Kemp and Habener, 2002). Another in vitro study incubated Chinese hamster ovary cells with 98% DMSO for 72 h and did not find a significant increase in the levels of phospho-CREB, suggesting that DMSO does not increase the phosphorylation of CREB (Hu et al., 2010). Thus, it remains unclear why the 30-minute vehicle group displayed increased CREB phosphorylation. Importantly, however, treatment with G-1 significantly increased phospho-CREB levels beyond that of vehicle at this timepoint, supporting the conclusion that GPER agonism increases CREB phosphorylation 30 minutes after infusion.
4.4. Conclusions
In sum, the present study demonstrated for the first time that acute GPER activation in the dorsal hippocampus regulates memory consolidation in GDX male mice. The GPER agonist G-1 enhanced spatial memory and object recognition consolidation in the OP and OR tasks, respectively, whereas the GPER antagonist G-15 blocked the formation of both types of memory when infused directly into the dorsal hippocampus immediately post-training. Effects of both compounds were dose-dependent, with object placement differentially sensitive to lower doses of the drugs compared with OVX females. These findings provide new insights relative to previous studies of males that used systemic injections, in that bilateral dorsal hippocampal infusions allowed us to pinpoint the role of GPER in memory formation to the dorsal hippocampus. In addition, the data add to a growing literature showing that different cellular mechanisms underlie the effects of E2 and estrogen receptors on hippocampal function. Unlike in OVX females, we found that G-1 does not affect JNK or cofilin in the dorsal hippocampus within 30 minutes, suggesting that these signaling kinases do not mediate the memory-enhancing effects of G-1 in GDX male mice. However, G-1 also did not activate the ERK or PI3K/Akt pathways, which is consistent with findings in OVX mice. Similar to E2 in OVX mice (Koss et al., 2018), we found that G-1 increased CREB phosphorylation in the dorsal hippocampus of GDX male mice 30 minutes after infusion. As such, the ability of GPER to regulate memory may be related to activation of this transcription factor. Together, our findings provide novel insights about the role of GPER in mediating cognitive function and suggest intriguing new sex differences in cell signaling pathways that underlie estrogenic regulation of memory. Future studies should seek to better understand the neural mechanisms through which GPER influences memory in males.
Highlights.
GPER agonism enhanced spatial and object recognition memory consolidation in males
GPER antagonism impaired spatial and object recognition memory formation in males
Dorsal hippocampal GPER promotes memory formation like in ovariectomized females
GPER agonism did not activate dorsal hippocampal JNK, cofilin, ERK, PI3K, or Akt
GPER agonism increased hippocampal CREB phosphorylation 30 minutes after infusion
Acknowledgements
This work was supported by National Institutes of Health R01MH107886 and UWM Discovery and Innovation Grant 101X418 to KMF, a Dr. Robert Cialdini and Bobette Gordon Graduate Fellowship to GDBM, and a UWM Support for Undergraduate Research Fellowship (SURF) to ALS.
Footnotes
Declaration of Interest
Dr. Frick is a co-founder and the Chief Scientific Officer of Estrigenix Therapeutics, Inc., a company which aims to improve women’s health by developing safe, clinically proven treatments for the mental and physical effects of menopause. The rest of the authors have no conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almarghalani DA, Sha X, Mrak RE, Shah ZA, 2023. Spatiotemporal cofilin signaling, microglial activation, neuroinflammation, and cognitive impairment following hemorrhagic brain injury. Cells 12, 1153. 10.3390/cells12081153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arterburn JB, Prossnitz ER, 2023. G Protein–coupled estrogen receptor GPER: Molecular pharmacology and therapeutic applications. Annu. Rev. Pharmacol. Toxicol. 63, 295–320. 10.1146/annurev-pharmtox-031122-121944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N, Zhang Q, Zhang W, Liu B, Yang F, Brann D, Wang R, 2020. G-protein-coupled estrogen receptor activation upregulates interleukin-1 receptor antagonist in the hippocampus after global cerebral ischemia: Implications for neuronal self-defense. J. Neuroinflammation 17, 45. 10.1186/s12974-020-1715-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Pittenger C, Kandel ER, 2003. CREB, memory enhancement and the treatment of memory disorders: Promises, pitfalls and prospects. Expert Opin. Ther. Targets 7, 101–114. 10.1517/14728222.7.1.101 [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH, 1997. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. 94, 7041–7046. 10.1073/pnas.94.13.7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua L, Ardenghi P, Schörder N, Bromberg E, Schmitz PK, Schaeffer E, Quevedo J, Bianchin M, Walz R, Medina JH, Izquierdo I, 1997. Drugs acting upon the cyclic adenosine monophosphate/protein kinase A signalling pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behav. Pharmacol. 8, 331–338. 10.1097/00008877-199708000-00006 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM, 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci. 33, 15184–15194. 10.1523/JNEUROSCI.1716-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ, 2007. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 193, 311–321. 10.1677/JOE-07-0017 [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J, 2012. Rapid control of male typical behaviors by brain-derived estrogens. Front. Neuroendocrinol. 33, 425–446. 10.1016/j.yfrne.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza LO, Machado GDB, de Freitas BS, Rodrigues SLC, Severo MPA, Molz P, da Silva JAC, Bromberg E, Roesler R, Schröder N, 2021. The G protein-coupled estrogen receptor (GPER) regulates recognition and aversively–motivated memory in male rats. Neurobiol. Learn. Mem. 184, 107499. 10.1016/j.nlm.2021.107499 [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM, 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci. 30, 4390–4400. 10.1523/JNEUROSCI.4333-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci. 28, 8660–8667. 10.1523/JNEUROSCI.1968-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Brandt N, Windhorst S, Pröls F, Bläute C, Rune GM, 2016. Control of aromatase in hippocampal neurons. J. Steroid Biochem. Mol. Biol. 160, 9–14. 10.1016/j.jsbmb.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM, 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem. 20, 147–155. 10.1101/lm.026732.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, 2015. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav. 74, 4–18. 10.1016/j.yhbeh.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE, 2003. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci. 117, 1283–1291. 10.1037/0735-7044.117.6.1283 [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Koss WA, 2018. Estradiol and hippocampal memory in female and male rodents. Curr. Opin. Behav. Sci. 23, 65–74. 10.1016/j.cobeha.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor C, Lymer J, Phan A, Choleris E, 2015. Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol. Behav. 149, 53–60. 10.1016/j.physbeh.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Gurtovenko AA, Anwar J, 2007. Modulating the structure and properties of cell membranes: The molecular mechanism of action of dimethyl Sulfoxide. J. Phys. Chem. B 111, 10453–10460. 10.1021/jp073113e [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB, 2009. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm. Behav. 56, 309–314. 10.1016/j.yhbeh.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB, 2011. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 36, 182–192. 10.1016/j.psyneuen.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB, 2012. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm. Behav. 62, 367–374. 10.1016/j.yhbeh.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Moody NM, Dohanich GP, Vasudevan N, 2014. Activation of G-protein-coupled receptor 30 is sufficient to enhance spatial recognition memory in ovariectomized rats. Behav. Brain Res. 262, 68–73. 10.1016/j.bbr.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Hernández-Vivanco A, Cano-Adamuz N, Sánchez-Aguilera A, González-Alonso A, Rodríguez-Fernández A, Azcoitia Í, De La Prida LM, Méndez P, 2022. Sex-specific regulation of inhibition and network activity by local aromatase in the mouse hippocampus. Nat. Commun. 13, 3913. 10.1038/s41467-022-31635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, 2003. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 1, 52. 10.1186/1477-7827-1-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger-Bement MK, Sluka KA, 2003. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J. Neurosci. 23, 5437–5445. 10.1523/JNEUROSCI.23-13-05437.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii H, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S, 2004. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. 101, 865–870. 10.1073/pnas.2630225100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Zhang R, Zhang Y, Xia Z, Hu Y, 2010. Role of CREB in the regulatory action of sarsasapogenin on muscarinic M 1 receptor density during cell aging. FEBS Lett. 584, 1549–1552. 10.1016/j.febslet.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Hutson DD, Gurrala R, Ogola BO, Zimmerman MA, Mostany R, Satou R, Lindsey SH, 2019. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol. Sex Differ. 10, 4. 10.1186/s13293-019-0219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Woolley CS, 2023. Mechanisms that underlie expression of estradiol-induced excitatory synaptic potentiation in the hippocampus differ between males and females. J. Neurosci. 43, 1298–1309. 10.1523/JNEUROSCI.2080-19.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Zhe Huang G, Woolley CS, 2018. Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci. 1897–18. 10.1523/JNEUROSCI.1897-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DM, Habener JF, 2002. Synergistic effect of dimethyl sulfoxide on glucagon-like peptide 1 (GLP-1)-stimulated insulin secretion and gene transcription in INS-1 cells: characterization and implications. Biochem. Pharmacol. 64, 689–697. 10.1016/S0006-2952(02)01212-1 [DOI] [PubMed] [Google Scholar]
- Kim J, Frick KM, 2017. Distinct effects of estrogen receptor antagonism on object recognition and spatial memory consolidation in ovariectomized mice. Psychoneuroendocrinology 85, 110–114. 10.1016/j.psyneuen.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Kim J, Schalk JC, Koss WA, Gremminger RL, Taxier LR, Gross KS, Frick KM, 2019. Dorsal hippocampal actin polymerization is necessary for activation of G-protein-coupled estrogen receptor (GPER) to increase CA1 dendritic spine density and enhance memory consolidation. J. Neurosci. 39, 9598–9610. 10.1523/JNEUROSCI.2687-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, Frick KM, 2016. 17β-estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms. J. Neurosci. 36, 3309–3321. 10.1523/JNEUROSCI.0257-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Frick KM, 2019. Activation of androgen receptors protects intact male mice from memory impairments caused by aromatase inhibition. Horm. Behav. 111, 96–104. 10.1016/j.yhbeh.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Haertel JM, Philippi SM, Frick KM, 2018. Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-estradiol. eNeuro 5, ENEURO.0267–18.2018. 10.1523/ENEURO.0267-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM, 2004. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci. 24, 5913–5921. 10.1523/JNEUROSCI.5186-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bean LA, Rani A, Jackson T, Foster TC, 2015. Contribution of estrogen receptor subtypes, ERα, ERβ, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice: EB effects in estrogen receptor knockout mice. Hippocampus 25, 1556–1566. 10.1002/hipo.22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Pedenovi M, Niewiarowski A, Casali B, Donati MB, Corbascio GC, Marchisio PC, 1987. Effects of dimethyl sulfoxide (DMSO) on microfilament organization, cellular adhesion, and growth of cultured mouse B16 melanoma cells. Exp. Cell Res. 172, 385–396. 10.1016/0014-4827(87)90396-X [DOI] [PubMed] [Google Scholar]
- Llorente R, Marraudino M, Carrillo B, Bonaldo B, Simon-Areces J, Abellanas-Pérez P, Rivero-Aguilar M, Fernandez-Garcia JM, Pinos H, Garcia-Segura LM, Collado P, Grassi D, 2020. G protein-coupled estrogen receptor immunoreactivity fluctuates during the estrous cycle and show sex differences in the amygdala and dorsal hippocampus. Front. Endocrinol. 11, 537. 10.3389/fendo.2020.00537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Liu D, 2020. Does GPER really function as a G protein-coupled estrogen receptor in vivo? Front. Endocrinol. 11, 148. 10.3389/fendo.2020.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymer J, Robinson A, Winters BD, Choleris E, 2017. Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice. Psychoneuroendocrinology 77, 131–140. 10.1016/j.psyneuen.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Machado GDB, Freitas BS, Florian LZ, Monteiro RT, Gus H, Schröder N, 2019. G protein-coupled oestrogen receptor stimulation ameliorates iron- and ovariectomy-induced memory impairments through the CAMP / PKA / CREB signalling pathway. J. Neuroendocrinol. 31. 10.1111/jne.12780 [DOI] [PubMed] [Google Scholar]
- Maney D, Pinaud R, 2011. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front. Neuroendocrinol. 32, 287–302. 10.1016/j.yfrne.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C, Ying Y-S, Chapline C, Jaken S, Anderson RGW, 1998. Targeting of protein kinase Cα to caveolae. J. Cell Biol. 141, 601–610. 10.1083/jcb.141.3.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Woolley CS, 2016. 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci. 36, 2677–2690. 10.1523/JNEUROSCI.4437-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CDC, Kow L-M, MacLusky NJ, Pfaff DW, Choleris E, 2015. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc. Natl. Acad. Sci. 112, 16018–16023. 10.1073/pnas.1522150112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM, 2003. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus 13, 226–234. 10.1002/hipo.10075 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M, 2023. The G protein-coupled oestrogen receptor GPER in health and disease: an update. Nat. Rev. Endocrinol. 19, 407–424. 10.1038/s41574-023-00822-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks D, Kundakovic M, 2023. Hippocampus-based behavioral, structural, and molecular dynamics across the estrous cycle. J. Neuroendocrinol. 35, e13216. 10.1111/jne.13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard PAS, Chandramohan D, Lumsden A, Vellone D, Denley MCS, Srivastava DP, Choleris E, 2023. Social memory in female mice is rapidly modulated by 17β-estradiol through ERK and Akt modulation of synapse formation. Proc. Natl. Acad. Sci. 120, e2300191120. 10.1073/pnas.2300191120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard PAS, Koss WA, Frick KM, Choleris E, 2018. Rapid actions of oestrogens and their receptors on memory acquisition and consolidation in females. J. Neuroendocrinol. 30, e12485. 10.1111/jne.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Squiavinato ACM, Rocha MR, Barcellos-de-Souza P, de Souza WF, Morgado-Diaz JA, 2019. Cofilin-1 signaling mediates epithelial-mesenchymal transition by promoting actin cytoskeleton reorganization and cell-cell adhesion regulation in colorectal cancer cells. Biochim. Biophys. Acta BBA - Mol. Cell Res. 1866, 418–429. 10.1016/j.bbamcr.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Taxier LR, Gross KS, Frick KM, 2020. Oestradiol as a neuromodulator of learning and memory. Nat. Rev. Neurosci. 21, 535–550. 10.1038/s41583-020-0362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxier LR, Philippi SM, Fleischer AW, York JM, LaDu MJ, Frick KM, 2022. APOE4 homozygote females are resistant to the beneficial effects of 17β-estradiol on memory and CA1 dendritic spine density in the EFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 118, 13–24. 10.1016/j.neurobiolaging.2022.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM, 2016. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm. Behav. 83, 60–67. 10.1016/j.yhbeh.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Taxier LR, Fortress AM, Frick KM, 2018. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol. Learn. Mem. 156, 103–116. 10.1016/j.nlm.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C, 2008. Ins and outs of ADF/cofilin activity and regulation. Eur. J. Cell Biol. 87, 649–667. 10.1016/j.ejcb.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Patel P, Gonzales AD, Ye H. (Zhiyu), Filardo EJ., Clegg DJ., Gorecka J., Akama KT., McEwen BS., Milner TA., 2015. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J. Neurosci. 35, 2384–2397. 10.1523/JNEUROSCI.1298-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA, 1997. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: Correlation with dendritic spine density. J. Neurosci. 17, 1848–1859. 10.1523/JNEUROSCI.17-05-01848.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Cao J, Zhou Y, Wang L, Zhu G, 2018. GPR30 activation improves memory and facilitates DHPG-induced LTD in the hippocampal CA3 of middle-aged mice. Neurobiol. Learn. Mem. 149, 10–19. 10.1016/j.nlm.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Zhang H, Lu S, Xu R, Tang Y, Liu J, Li C, Wei J, Yao R, Zhao X, Wei Q, Ma B, 2020. Mechanisms of estradiol-induced EGF-like factor expression and oocyte maturation via G protein-coupled estrogen receptor. Endocrinology 161, bqaa190. 10.1210/endocr/bqaa190 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM, 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. 107, 5605–5610. 10.1073/pnas.0910578107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Fester L, Von Blittersdorff B, Hassu B, Nogens H, Prange-Kiel J, Jarry H, Wegscheider K, Rune GM, 2010. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 151, 1153–1160. 10.1210/en.2009-0254 [DOI] [PubMed] [Google Scholar]


