Abstract
The GADD45 gene is a growth arrest-associated gene that is induced by certain DNA-damaging agents and other stresses, such as starvation, in all mammalian cells. In addition to a strong p53-binding element in an intronic sequence, we have recently found that p53, while not required or sufficient alone, may contribute to the stress responsiveness of the promoter. Much of the responsiveness was localized to a GC-rich motif in the proximal promoter which contains multiple Egr1 sites and a larger WT1 site; this 20-bp WT1 motif is identical to the WT1-binding site in the PDGF-A gene. In extracts from a human breast carcinoma cell line expressing p53 and WT1, which is known to associate with p53 in vivo, evidence was obtained that these proteins are in a complex that binds this 20-bp element. A combination of p53 and WT1 expression vectors strongly induced a GADD45-reporter construct, while mutation of the WT1-Egr1 site in the promoter prevented this induction. Abrogation of p53 function by a dominant-negative vector or abrogation of WT1 function by an antisense vector markedly reduced the induction of this promoter. Since p53 does not bind directly to the promoter, these results indicate that p53 can contribute to the positive regulation of a promoter by protein-protein interactions.
The tumor suppressor p53 plays an important and often central role in cellular responses to genotoxic stress and other adverse environmental cues. While the protein’s effects are probably mediated by both transcriptional and nontranscriptional mechanisms (reviewed in reference 32), its ability to regulate the expression of a variety of cellular genes is required for important functions. For example, sequence-specific transcriptional activation has been shown to be essential for growth suppression by p53 (45). The list of p53-inducible genes, which contain specific sequences that bind p53, is continually expanding, and it has been estimated that there may be several hundred such p53-binding sites in the human genome (54). In addition to its ability to up-regulate transcription by sequence-specific binding, p53 is also known to be able to down-regulate the expression of a number of genes (39), probably by interacting with the basal transcription machinery (32). p53 has been found to interact with a variety of cellular and viral proteins, including transcription factors, although transcriptional activation by protein-protein interactions in the absence of site-specific DNA binding by p53 has not been observed. If p53 transcriptional up-regulation occurs by such protein-protein interactions, then it could substantially broaden the known role of p53 in positive gene regulation.
There are a number of striking similarities between the responses of the gadd gene products and p53 activation. Like p53 protein, the products of the gadd genes are activated by stresses, such as those caused by DNA-damaging agents or starvation, that elicit growth arrest, and overexpression of these proteins suppresses cell growth (61). The five gadd genes were originally isolated in hamster cells on the basis of induction by UV radiation (12), but three, gadd45, gadd153, and gadd34, have been found to be stress inducible in a wide variety of mammalian cells (13). The regulation of these genes after stress is probably mediated by multiple mechanisms. For example, gadd153 contains a stress-responsive AP-1 site, but its deletion only partially abrogates gadd153 induction by DNA-damaging agents (35). In the case of gadd45, the induction of the gene by ionizing radiation (IR) is strictly p53 dependent while its induction by other stresses, such as treatment with the alkylating agent methyl methanesulfonate (MMS), has been observed in all mammalian cells examined to date (28). As discussed previously (23, 28), the p53-binding element in the third intron of gadd45 is probably required for IR responsiveness, since the transfected human gene, but not promoter-reporter constructs, was induced by IR. In addition, the promoter contains no identifiable p53-binding site and did not bind baculovirus p53 (28), and a human GADD45 promoter-reporter construct was not transactivated when introduced with a p53 expression vector (59).
While results of early studies of gadd45 suggest two distinct signaling pathways for IR-type damage and typical gadd gene-inducing agents like MMS, UV radiation, or medium starvation (referred to as MUM stresses), recent results indicate that p53 may have a contributory role in MUM-type gadd gene responses. Surprisingly, MUM-type stresses actually elicited stronger activation of p53 than did IR, as determined by induction of a promoter construct containing p53-binding sites (59). Compared to normal human keratinocytes, human papillomavirus-immortalized keratinocytes and an oral cancer cell line showed reduced induction of GADD45 and GADD153 mRNA after UV irradiation, as well as loss of p53 protein induction (16). In mouse embryo fibroblasts from p53-null mice and in human lines where p53 function was blocked with dominant-negative vectors, MUM stress responses, as measured by increased mRNA, were appreciably reduced for GADD45 and GADD153 (60). In the same study, this p53 effect was localized to the promoters of these genes, since a similar attenuation of induction was observed for promoter-reporter constructs. Considering that neither promoter contains detectable p53-binding sites, a reasonable explanation is that p53 is mediating its positive transcriptional effect indirectly by protein-protein interaction(s) rather than direct DNA binding.
Like many other growth control-related genes, human GADD45 contains GC-rich motifs that match the consensus sequence for various transcription factors, such as Egr1 and WT1 (47). The Egr1-WT1 family of transcription factors defines a group of related proteins that have been associated with a variety of cellular processes, including stress responses in the case of Egr1 (4, 31) and growth suppression for WT1. WT1 is of particular interest for several reasons: it is a tumor suppressor; like the Gadd proteins, it suppresses cell growth; and it has been found to associate in vivo with p53 (37). In the case of growth suppression, all four splice forms of WT1 suppressed growth in transient assays (17). In most studies, WT1 appears to function as a transcriptional suppressor (36); suppression by WT1 has been found with a variety of human promoters, including EGFR (9), CSF-1 (20), IGF-II (8), IGF-I-R (57), BCL2 and c-MYC (21), RAR-α1 (14), and TGF-β1 (6). Evidence has been presented that WT1 contains both a transcriptional repression domain and an activation domain and that interaction with another protein(s) may determine the effect of WT1 on transcription (55). In the case of p53, cotransfection of p53 and WT1 with a reporter construct containing a p53-binding site led to increased transcription, which suggests that WT1 can have a cooperative interaction with p53; when a reporter containing an Egr1-WT1 binding site was studied, cotransfection with p53 suppressed transcription by WT1 (37). Since much of the stress responsiveness of the human GADD45 promoter, as well as the p53-dependent effect on the promoter, was localized to the GC-rich motif in the proximal promoter, the roles of WT1 and Egr1 were investigated. p53 and WT1, but not Egr1, were found in a complex that associated with this GC-rich region. Expression vectors for WT1 and p53 in combination were found to induce the promoter, and suppression of either p53 or WT1 reduced this responsiveness. Since p53 does not bind directly to this promoter, these results indicate that p53 can contribute to the positive regulation of a promoter by protein-protein interactions.
MATERIALS AND METHODS
Plasmid clones.
The following cDNA clones were used: pHG45-CAT1, constructed by inserting the SalI-SmaI fragment of GADD45 spanning −2256 to +144 relative to the transcription start site into pCAT-Basic (Promega); pHG45-CAT2, similarly constructed by inserting the HindIII-SmaI fragment of this promoter from −909 to +144 into pCAT-Basic; and pHG45-CAT3, similarly constructed by using a SphI-SmaI fragment spanning −70 to +144. These restriction fragments were excised from pHG45HC, a 6-kb human genomic clone. pHG45-CAT2mut was derived from pHG45-CAT2 by replacement of the GC-rich WT1-EGR1 sites at positions −204 to −190; 5′ CGCCCCCCGCCCCCGC 3′ was replaced with 5′ TATTTTTATTTTTAT 3′. The other pHG45-CAT2 derivatives were constructed by PCR cloning, as described previously (61), and were cloned into the HindIII-SmaI site of pCAT-Basic. The wt1 construct NA is a murine WT1 protein expression vector in which the wt1 cDNA with splice form A was cloned into a pCMV vector (17, 18). pC53-SN3, which expresses wild-type (wt) p53 protein driven by a cytomegalovirus promoter, and pC53-SCX3, which expresses a dominant-negative mutant p53 protein containing a substitution of Ala for Val-143, were provided by B. Vogelstein (59). pCMV-EGR1, which expresses human Egr1 protein, was provided by F. J. Rauscher III. pCMV-E6 expresses human papillomavirus type 16 E6 and has been shown to be an effective dominant-negative expression vector that blocks p53 action (30, 52). c-fos–CAT was derived from a β-galactosidase reporter construct which consists of a c-fos gene where the reporter (followed by translation stop codons) was inserted into the ATG start site of c-fos (48); in c-fos–CAT the reporter was replaced with the open reading frame encoding chloramphenicol acetyltransferase (CAT). pCMV-ASWT1 was constructed by inserting a 497-bp XbaI-XhoI fragment from the 5′ end of a full-length human WT1 cDNA clone (38) into pCI-neo vector (Promega) in the antisense orientation. The Tac expression vector pcI Tac was provided by J. Ashwell (40).
Cells and cell treatment.
The human colorectal carcinoma cell line RKO and the human large-cell lung carcinoma cell line H1299 were grown in modified Ham’s F-12 medium supplemented with 10% fetal bovine serum; the human breast carcinoma cell line MCF-7 was grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. While a variety of different MCF-7 lines are in use in different laboratories, the line employed in this study has a wt p53 genotype and was shown to express relatively low levels of p53 in unstressed cells; with activation of p53, a substantial increase in nuclear p53 has consistently been seen (43). MCF7-E6#9, designated MCF7-E6, was selected on the basis of its high-level expression of E6 after stable transfection with pCMV-E6 and has been described previously (60). For MMS treatment, cells were exposed in medium to MMS (Aldrich) at 100 μg/ml for 4 h, after which the medium was replaced with fresh medium. For UV irradiation, 100-mm-diameter dishes were rinsed with buffered saline and irradiated with germicidal lamps at a dose rate of 2.1 J m−2 s−1 to 14 J m−2. The original medium was then replaced, and the cells were incubated at 37°C for the indicated time.
Transfection of cells was performed by a calcium-phosphate method as described previously (59). Cells stably expressing various CAT reporter constructs were developed as described previously (59); unless otherwise specified, experiments were conducted with pooled cultures containing >50 clonal isolates. For assays involving transient transfection of expression vectors, growing cells were seeded in 10-cm-diameter dishes at 60% confluence, with multiple plates per point in each experiment; 20 h later they were cotransfected with 5 μg of the indicated GADD45 CAT reporter construct and 5 μg of the indicated expression vector, pCMV-WT1 or pCMV-EGR1, plasmid DNA. Either pC53-SN3 or pC53-SCX3 (0.5 μg) was used. The amount of transfected DNA was kept constant by using pCMV-neo, which expresses the neomycin resistance factor, or the pCMV.3 vector alone.
For isolation of transiently transfected cells, 5 μg of either pCMV-ASWT1 or pCMV-neo and 0.5 μg of pcI Tac were cotransfected into MCF-7 cells. After 36 h, the cells were harvested and washed once with cold medium. Dyna-beads (Dynal, Lake Success, N.Y.) coated with anti-Tac antibody (provided by J. Ashwell) were added to the cells and incubated with gentle rotation at 4°C for 1 h. The Tac-expressing cells were isolated magnetically, washed once with buffered saline, and then lysed for protein analysis (40).
The osteosarcoma cell line U2OS (31), which contains a tetracycline-regulated WT1 expression vector, was grown in Dulbecco’s modified Eagle’s medium with 1 μg of tetracycline/ml. To examine GADD45 mRNA levels, cells were plated in 150-mm-diameter dishes in the presence of tetracycline; after 24 h the medium was replaced with fresh medium lacking tetracycline, and the cells were harvested 4 h later. Poly(A) RNA was isolated and analyzed by blot analysis as described previously (61); this included Northern-type analysis with labeled probes for GADD45 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as well as quantitative dot blot hybridization.
CAT assay.
Measurement of CAT activity was carried out as described previously (59). Briefly, cells were collected and resuspended in 0.25 M Tris (pH 7.8). The cells were then disrupted by three freeze-thaw cycles, and equivalent amounts of protein were used for each assay. The CAT reaction mixture was incubated at 37°C for 16 h, and the CAT activity was determined by measuring the acetylation of 14C-labeled chloramphenicol by thin-layer chromatography. Radioactivity was measured directly with a Betascope model 630 blot analyzer (Betagen Inc.). The specific CAT activity was calculated by determining the fraction of chloramphenicol that had been acetylated. The relative CAT activity was determined by normalizing the activity of the treated samples to that of the untreated sample. Each value presented was based on the average of at least three separate determinations from samples transfected separately. Results with this approach, where CAT activity was measured relative to protein content, were comparable to those with a second reporter as an internal control (53); in addition, the latter approach could be of concern in stress studies, where activated p53 may cause unexpected repression by interaction with the basal transcription machinery.
DNase protection assay.
A 183-bp HindIII-SphI restriction fragment was labeled on the antisense strand with α-32P-labeled deoxynucleoside triphosphate, using Klenow fragment. DNA binding and DNase I digestion were carried out as previously described (27) in a 50-μl volume with 0.5 ng (1 fmol) of labeled DNA, 1 μg of poly(dI-dC), and 60 μg of nuclear extracts (7) in a final buffer of 25 mM Tris-HCl (pH 7.9), 6.25 mM MgCl2, 0.5 mM EDTA, 50 mM KCl, 0.5 mM dithiothreitol, 2% polyvinyl alcohol, and 10% glycerol. After 15 min on ice followed by 1 min of digestion at 25°C with 1 to 2 μl of freshly diluted DNase I (5 μg/ml; Worthington), the reactions were terminated and the DNA fragments were extracted, and precipitated before being loaded on a 10% sequencing gel. Purine sequence ladders of DNA probes were prepared by the Maxim and Gilbert procedure.
EMSA.
Nuclear extracts were prepared, and an electrophoretic mobility shift assay (EMSA) was carried out as described previously (59). DNA binding reactions were performed for 20 min at room temperature in a buffer containing 20 mM HEPES (pH 7.8), 100 mM KCl, 1 mM dithiothreitol, 1 μg of poly(dI-dC) (Sigma, St. Louis, Mo.), 1 mM ZnCl2, 104 dpm of labeled probe, 10% glycerol, and 15 μg of nuclear protein extract in a volume of 30 μl. For immunodepletion prior to EMSA, the nuclear extract was incubated with the indicated antibodies on ice for 3 h, and then protein A-Sepharose was added to the protein mixture to remove antibody complex. The probe used was a 30-mer double-stranded synthetic oligonucleotide containing the sequence TCGGCACCGCCCCCGCCCCCGCCCCCTCGG, which corresponds to positions −211 to −182 of the human GADD45 promoter with the 20-bp binding region. In some experiments, a 30-mer oligonucleotide, TCGGCACTATTTTTATTTTTATCCCCTCGG, with the WT1 or EGR1 sequence replaced with an AT-rich motif (underlined) was used. Each strand was labeled separately with T4 polynucleotide kinase (New England Biolabs) and [γ-P32]ATP (3,000 Ci/mmol; Dupont), and then the strands were annealed. Unincorporated counts were separated on a Nick column (Pharmacia). The samples were analyzed on a nondenaturing 4% acrylamide gel.
Gel electrophoresis and immunoblot analysis.
For measurement of WT1, Egr1, and p53 protein levels, total cellular protein was prepared as described previously (59). One hundred micrograms of the protein was loaded onto sodium dodecyl sulfate–15% polyacrylamide denaturing gels; following electrophoresis, the protein was transferred electrophoretically to Immobilon membranes (Millipore, Bedford, Mass.). The membranes were then blocked for 30 min in 5% nonfat milk at room temperature. A monoclonal mouse antibody to p53 (pAb1801; Oncogene Science, Mineola, N.Y.) was used to measure the p53 protein level; for WT1 detection, the membrane was probed with the monoclonal antibodies H2 (50) and H7 (provided by F. Rauscher), mWT12 (provided by D. Haber), and WTc8, a polyclonal rabbit anti-mouse antibody (provided by D. Haber) (9, 10). For Egr1 (NGFI-A) detection, the membrane was probed with the anti-Egr1/NGFI-A monoclonal antibody 1H4, which was a gift of J. Milbrandt (5).
Biotin-streptavidin pull-down assay.
Oligonucleotides (Midland) containing biotin on the 5′ nucleotide of the sense strand consisted of TCGGCACCGCCCCCGCCCCCGCCCCCTCGG, which corresponds to positions −211 to −182 of the human GADD45 promoter, TCGGCACTATTTTTATTTTTATCCCCTCGG, and TCGGCACCGTTTCCGTTTCCGTTTCCTCGG. In the second oligonucleotide the GC-rich motif (underlined) was replaced with an AT-rich motif; in the third oligonucleotide, C bases, which are critical for binding to WT1 and Egr1 (46a), were replaced with T. These oligonucleotides were annealed to their respective complementary oligonucleotides, and 30-bp double-stranded oligonucleotides were gel purified and used. Cellular protein was extracted as described previously (11). One microgram of each oligonucleotide was incubated with 2 mg of cellular protein for 20 min at room temperature in binding buffer containing 12% glycerol, 12 mM HEPES (pH 7.9), 4 mM Tris (pH 7.9), 150 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, and 10 μg of poly(dI-dC) competitor. Following this reaction, 30 μl of streptavidin-agarose (Sigma) was added to the reaction and incubated at 4°C for 4 h. Prior to this step, 300 μl of the original streptavidin-agarose bead preparation was preabsorbed with 500 μg of bovine serum albumin, 50 μg of poly(dI-dC), and 50 μg of sheared salmon sperm DNA for 20 min at 25°C; the beads were washed three times and resuspended in 300 μl of the binding buffer. The protein-DNA–streptavidin-agarose complex was then washed three times with binding buffer and loaded onto a sodium dodecyl sulfate–polyacrylamide gel. After electrophoresis, the protein was transferred to Immobilon membranes (Millipore). The detection of p53, WT1, and Egr1 was performed as described in “Gel electrophoresis and immunoblot analysis” above. For immunodepletion prior to the DNA-protein binding reaction, the protein extract was incubated with the indicated antibodies on ice for 3 h and then protein A-Sepharose was added to the protein mixture to remove antibody complex.
RESULTS
Mapping of a stress-responsive element in the proximal GADD45 promoter.
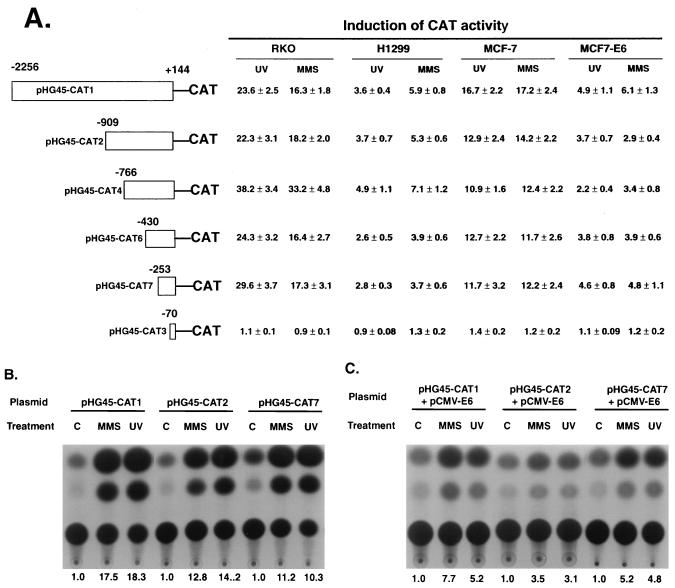
As discussed earlier, the GADD45 promoter does not contain a p53-binding site and is not induced by overexpression of p53 or by ionizing radiation in cells with functional p53. However, responses to stresses, such as MMS or UV radiation, are stronger in wt p53 cells and p53 dominant-negative vectors reduce the stress responsiveness of this promoter. In order to localize the control elements involved in this response, 5′-deletion analysis of the human GADD45 promoter was undertaken. As shown in Fig. 1, the longest promoter region (pHG45-CAT1) was strongly responsive in the two wt p53 lines, RKO and MCF-7. This was seen for stably integrated reporter constructs (RKO) as well as transiently transfected constructs (MCF-7). In contrast, the p53-deficient H1299 line was appreciably less responsive. In the MCF7-E6 subline, where much of the p53 action has been blocked by E6, induction was also substantially less than that in the parent MCF-7 line and was comparable to that of H1299 (Fig. 1A). When the E6 expression vector was cotransfected into MCF-7 cells with the reporter constructs shown in Fig. 1C, induction was also less than that in cells transfected with a control expression vector (Fig. 1B). With progressive 5′ deletions, induction did not change substantially until the last construct, pHG45-CAT3 (Fig. 1), which extended 5′ only to −70 relative to the transcription start site. With this minimal promoter, treatment of cells with MMS or UV radiation had little effect. These studies indicate that the first 253 bp of the GADD45 promoter contain the major control regions required for responsiveness to these agents. However, a similar pattern, albeit of lower magnitude, was also observed in the p53-deficient lines. Thus, both the p53-dependent and -independent stress responsiveness mapped to the proximal promoter.
FIG. 1.
GADD45 promoter deletion analysis in wt p53 and p53-deficient human cells. (A) Summary of results for reporter constructs containing the indicated regions of the GADD45 promoter linked to the CAT reporter that were stably integrated in RKO (wt p53) and H1299 (p53-null) cell lines. The cells were treated with 100 μg of MMS/ml for 4 h or with 14 J m−2 of UV radiation; the cells were harvested 24 h after treatment, and CAT assays were carried out as described in Materials and Methods. Results are also summarized for MCF-7 and MCF7-E6 (stably expressing E6) cells that were transfected with 5 μg of the indicated reporter constructs 20 h prior to treatment and harvested 24 h after treatment. The values represent the relative expression with standard deviations compared to that of the untreated controls. (B and C) Five micrograms of the indicated reporter constructs was cotransfected into MCF-7 cells with 5 μg of the indicated expression vectors 20 h prior to treatment with MMS or UV radiation; the cells were harvested 24 h later. The reporter constructs were cotransfected with control plasmid, pCMV-neo (B), or with the E6 expression vector, pCMV-E6 (C). The values below the panels indicate the relative induction compared to that of untreated control cells (lanes C).
While the basal expression of the various GADD45 promoter constructs was not appreciably affected by expression of E6 (Fig. 1C), the basal expression in untreated cells did vary with progressive 5′ deletions. Relative to that for pHG45-CAT1, the values for basal expression for pHG45-CAT2, -4, -6, -7, and -3 were 1.1, 2.4, 2.3, 7.2, and 0.3, respectively, in MCF-7 cells; in the case of stably transfected H1299 cells the values, relative to that for pHG45-CAT1 in this line, were 5.2, 11.5, 9.3, 16.5, and 0.2. In both cases, it appears that one or more negative regulatory elements exist in the upstream promoter that controls basal expression. With 5′ deletion to −70 in pHG45-CAT3, basal expression, as well as stress responsiveness (see above), was markedly reduced.
Identification of a GC-rich site with binding proteins in MCF-7 but not H1299 cells.
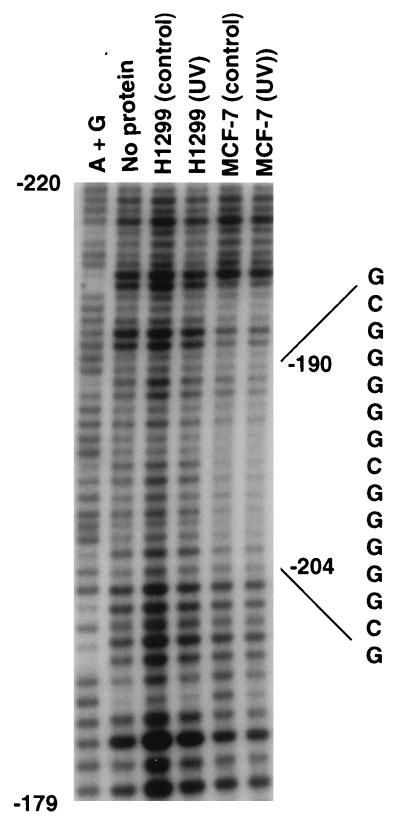
In an initial attempt to distinguish possible protein-binding sites that may differ between strongly and weakly responsive cells, in vitro DNase I footprinting was carried out with nuclear extracts from MCF-7 and H1299 cells. The most prominent difference was a strongly protected area in a GC-rich region that was observed only in the MCF-7 extract; the region containing this protected area is shown in Fig. 2. This region contained a continuous run of either G or C from −186 to −205. Strong protection could be seen from −195 to −202, and weaker, less prominent protection could be seen in flanking regions from −189 to −194 and from −203 to −212. The protected region was similar in extracts from either UV-irradiated or untreated MCF-7 cells. Even though the fourth lane, H1299 (control), was somewhat overloaded, there was a clear difference discernible in this GC-rich region in the MCF-7 extracts compared to the other lanes. Interestingly, this region contains a novel 15-bp symmetrical motif that consists of two perfect overlapping EGR1-WT1 consensus sequences, CGCCCCCGC (plus-strand sequence) (47).
FIG. 2.
DNA footprint analysis of the GADD45 promoter. A labeled fragment from the proximal promoter (see Materials and Methods) was analyzed by in vitro footprinting with DNase I. Extracts were prepared from either untreated growing H1299 (control) or MCF-7 (control) cells or cells harvested 4 h after UV irradiation [H1299 (UV) and MCF-7 (UV)]. The sequence of the sense strand is shown for the two overlapping WT1-EGR1 sites. Purine sequence ladders of the antisense strand (A+G) were prepared by the Maxim and Gilbert technique.
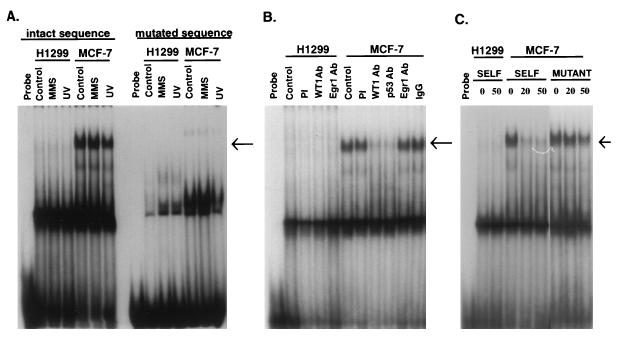
Using a labeled double-stranded oligonucleotide of this region, we detected several DNA-protein complexes by EMSA with extracts from MCF-7 cells (Fig. 3A). A prominent slowly migrating band was seen in MCF-7 cells, while it was much less intense or undetectable in H1299 extracts. In addition, this band was also substantially less intense in MCF-7 cells stably expressing E6 (data not shown). When this GC-rich motif was replaced with another sequence (Fig. 3A, “mutated sequence”) this prominent band disappeared. In competition experiments (Fig. 3C) the MCF-7-specific band was effectively competed away with wt but not with mutant sequence. The identity of the prominent faster-migrating band that was common to H1299 and MCF-7 is unknown, but its failure to change in the competition experiment would argue that its binding is not specific to this sequence.
FIG. 3.
EMSAs with DNA containing the GADD45 WT1-EGR1 site. (A) Nuclear extracts from H1299 or MCF-7 cells, which were prepared from untreated cells (Control) or cells 4 h after treatment with MMS or UV radiation, were incubated with a labeled 30-bp probe corresponding to this region (intact sequence) or to one containing mutated WT1-EGR1 sites (mutated sequence) as described in Materials and Methods. (B) EMSA was carried out in the same manner except that extracts were immunodepleted prior to analysis with preimmune serum (PI); antibody (Ab) against WT1 (WTc8), Egr1 (NGF-1), or p53 (PAB421; Oncogene Science); or nonspecific IgG prior to analysis. Control, no immunodepletion. (C) EMSA was carried out as for panel A, but with a 20- or 50-fold excess of either the wt (SELF) or the same mutated sequence (MUTANT) as in A. Probe, no nuclear extract. The arrows indicate specific bands in MCF-7 cell extract.
Evidence for a complex containing WT1 and p53 that binds to the GADD45 GC-rich promoter element.
Even though the MCF-7-specific bandshift shown in Fig. 3 did not show any change in UV-irradiated cells, it may still represent binding by proteins important in the stress responsiveness of wt p53 cells. Constitutive binding is not without precedent for promoter elements important in stress responsiveness. For example, the β-polymerase promoter contains an ATF-CREB site that was required for mediating induction by alkylating agents, and the EMSA bandshift was similar for treated and untreated cells (29). In an initial effort to identify which proteins bind the GADD45 element, supershift-type experiments were conducted with antibodies to WT1, Egr1, and p53. However, no convincing mobility shifts were seen with these antibodies (data not shown). It has previously been reported (9) that anti-WT1 and -Egr1 antibodies failed to supershift a complex containing a typical Egr1-binding site motif. An alternative approach was undertaken in the experiment shown in Fig. 3B, where nuclear cell extracts were immunodepleted prior to the EMSA. As controls, immunodepletion with preimmune serum or nonspecific immunoglobulin G (IgG) had no effect on the MCF-7-specific band, and no effect was seen with any antibody with the H1299 extract. In contrast, this band was markedly reduced by immunodepletion with anti-WT1 antibody, but anti-Egr1 antibody had no effect. To confirm that these antibodies were effective in depleting the extracts, aliquots of the extracts before and after immunodepletion were analyzed by immunoblotting, and WT1 and Egr1 were found to be markedly reduced in their respective immunodepleted extracts (data not shown). In addition, the p53 antibody was also effective in reducing this band, as shown in Fig. 3B. In order to determine if p53 binds directly to this sequence, EMSA-type experiments (see reference 49 for the methodology) were undertaken with baculovirus-produced p53; no binding was detected with this oligonucleotide, while strong binding occurred with an oligonucleotide probe containing the GADD45 intronic p53-binding site (data not shown).
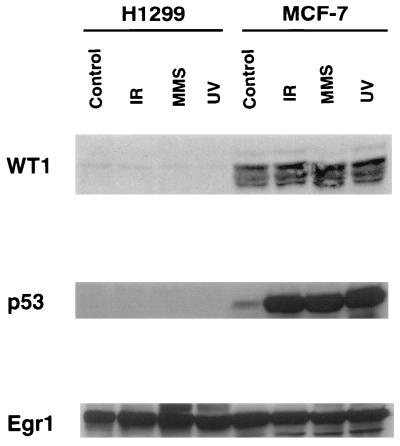
WT1 plays an important role in renal cells, but there is increasing evidence that it is expressed in a variety of tissues and cell types (3, 44). As shown in Fig. 4, immunoblot analysis with the H2 antibody demonstrated that this protein was expressed at appreciable levels in MCF-7 cells, while a negligible effect was detected in H1299 cells. In order to confirm that this was in fact WT1, a battery of three other anti-WT1 antibodies was tested, including WTc8, mWT12, and H7. In each case, the same-size protein band was detected in MCF-7 cells (data not shown), although the multiple WT1 isoforms, ranging from 46 to 65 kDa, were best visualized with the H2 antibody. In the case of Egr1, similar expression was detected in both MCF-7 and H1299 cells. Although EGR1 has been found to be IR inducible in human fibroblasts and kidney epithelial cells (19) and UV inducible in NIH 3T3 cells (25), it was expressed at the same appreciable level in untreated control cells as in both MCF-7 and H1299 cells treated with IR, UV radiation, or MMS. No evidence for WT1 induction was observed in either of these cell lines, as shown in Fig. 4. In the case of p53, no expression was detected in H1299, which is known to have deletions in both p53 alleles (59), and typical induction was seen for all three damaging agents in MCF-7 cells, as seen previously in this line (11, 43). Taken together with the EMSA results, these studies suggest that in MCF-7 cells both WT1 and p53 could well play roles in the regulation of GADD45 by interaction with this element, i.e., WT1-EGR1.
FIG. 4.
Expression of WT1, p53, and Egr1 in H1299 and MCF-7 cells. The cells were treated as described in the legend to Fig. 3 or were irradiated with 20 Gy of γ rays and harvested 1 h later (IR). Cellular protein was analyzed by immunoblotting with antibodies to the indicated proteins. The visualized bands are shown; their estimated masses were 46 to 65 kDa for WT1, 53 kDa for p53, and 69 kDa for Egr1.
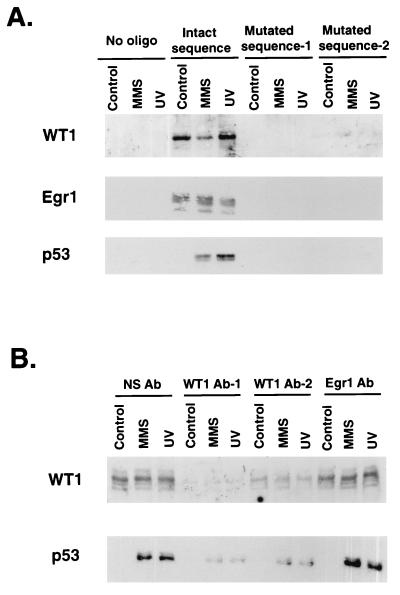
The results shown in Fig. 3 and 4 suggest several possible interpretations for our EMSA results. In particular, p53 levels were quite low in untreated MCF-7 cells but the bandshifts showed no difference for either untreated or treated cells. This could indicate that the association of p53 with the WT1-containing complex is disrupted during the EMSA, or perhaps p53 constitutively binds to this complex. In addition, any conclusion is dependent on the assumption that the anti-p53 or -WT1 antibodies specifically depleted only p53- or WT1-containing complexes, respectively, in the gels shown in Fig. 3B. To address these issues, a biotin-avidin pull-down procedure was employed to isolate proteins that bind to the GADD45 GC-rich motif. As shown in Fig. 5A, protein complexes containing WT1 and Egr1 specifically bound to the GC-rich motif while mutation of bases known to be involved in Egr1 and WT1 DNA binding ablated this association. Interestingly, p53 was barely detected in the extract from untreated cells but showed substantial binding with the extract from MMS- and UV-treated cells. Non-sequence-specific binding by p53 was prevented by the inclusion of excess carrier DNA (see Materials and Methods). While both WT1 and p53 bound to the GC-rich motif, this experiment cannot distinguish between binding to the same or separate DNA molecules in the association assay. To address this issue, the cell extracts were immunodepleted with various antibodies prior to addition of the biotin-labeled DNA (Fig. 5B). As demonstrated in the figure, two different anti-WT1 antibodies depleted both WT1- and p53-containing complexes while an anti-Egr1 antibody had no effect. This occurred in spite of the fact that the last antibody removed much of the Egr1 from the extract, as determined by immunoblotting (data not shown). Interestingly, the majority of the p53 protein, which bound to this DNA, was removed by WT1 immunodepletion. Considering that WT1 is known to bind strongly to this sequence (56) while p53 does not directly bind to it (see earlier comments), these results indicate that p53 is associated with WT1-containing complexes but not with EGR1-containing complexes.
FIG. 5.
Pull-down assay with biotin-labeled GADD45 GC-rich motif. Nuclear extracts were prepared from MCF-7 cells as explained in the legend to in Fig. 4 and incubated with biotin-labeled 30-bp DNA; proteins binding to this DNA were isolated with streptavidin-agarose and detected by immunoblot analysis (see Materials and Methods). (A) Extracts were incubated with one of the following: no oligonucleotide (No oligo); mutated sequence-1, in which the GC-rich motif was replaced with an AT-rich motif; or mutated sequence-2, in which critical C nucleotides were replaced by T. Binding proteins were then detected by immunoblotting to the proteins designated to the left of the blots. (B) Extracts were immunodepleted with the antibodies (Ab) indicated at the top of the panel prior to addition of the oligonucleotide with the intact GC-rich motif. NS Ab, nonspecific IgG; WT1 Ab-1, WTc8; WT1 Ab-2, mWT12. Analysis was then carried out as described for panel A with mWT12 and PAb421 for immunoblot detection of WT1 and p53, respectively.
Induction of the GADD45 promoter-reporter construct by WT1 and p53 expression vectors.
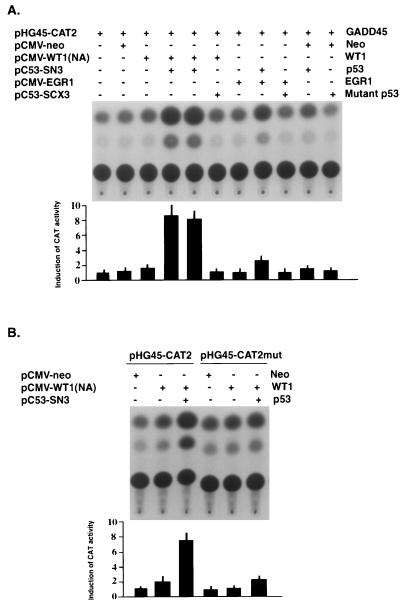
Even though the GADD45 promoter does not contain a p53-binding site (28) and is not induced by overexpression of p53 (59), the contribution of p53 to the responsiveness of this promoter could be mediated by protein-protein interactions with other factors, such as WT1, which has been shown to interact with p53 (37) and to bind to the same consensus sequence as Egr1. In order to test this possibility, H1299 cells were transfected with the GADD45 reporter construct and various expression vectors (Fig. 6). Since high-level expression of p53 with the pC53-SN3 expression vector has been shown to repress a variety of promoter constructs, including GADD45 (59), the amount of p53 expression vector was reduced. As shown in Fig. 6A, cotransfection of the reporter with p53 had no appreciable effect on expression. Likewise, cotransfection with an expression vector for WT1 had at most a minimal effect. In contrast, the combination of p53 and WT1 resulted in a consistent large increase in expression of the CAT reporter. This effect required wt p53, since no increased expression was seen with mutant p53 (143 Val→Ala) transfected either with reporter alone or with WT1 and the reporter. In the case of Egr1, a similar but substantially weaker effect was seen when it was cotransfected with p53; the increase in expression was less than 25% of that seen with WT1 and p53. Again, Egr1 alone or in combination with mutant p53 had no effect. In order to determine if the GC-rich motif in the GADD45 promoter was required for this effect, similar experiments were carried out (Fig. 6B) with a reporter construct containing a mutated WT1-EGR1 site. When this binding site was mutated in pHG45-CATmut, induction was markedly reduced and more than 80% of the effect was lost compared to that of the reporter with the wt WT1-EGR1 site.
FIG. 6.
Induction of the GADD45 promoter by cotransfection with WT1 and p53 expression vectors. (A) H1299 cells were transfected with the GADD45 promoter-reporter construct pHG45-CAT2 and the indicated pCMV expression vectors; pCMV-neo was included as a control. Plasmids used are shown to the left with simplified names to the right. Vector, pCMV.3, was included in some cases to keep the total amount of transfected DNA constant. CAT assays were carried out as described earlier. (B) The experiment was carried out in a similar manner with the exception that the reporter was driven by the normal GADD45 promoter or by a similar construct, pHG45-CAT2mut (143 Val→Ala), where the WT1-EGR1 site had been mutated (see Materials and Methods). Results from multiple separate determinations of relative expression are shown at the bottom of each panel as bar graphs with standard deviations.
The WT1-EGR1-binding site and WT1 contribute to GADD45 responsiveness in MCF-7 cells.
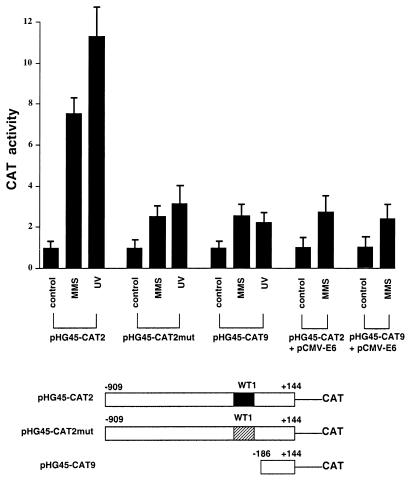
Since expression by exogenous p53 and WT1 resulted in induction of the GADD45 promoter, the role of the WT1-EGR1 site and cellular WT1 on the responsiveness of this promoter was studied in the wt p53 MCF-7 line. Reporter constructs driven by either the normal promoter sequence or one where the WT1-EGR1 site had been mutated were transfected into cells, and responsiveness to MMS or UV radiation was measured (Fig. 7). As shown in earlier figures, the normal promoter showed strong induction by these stresses, while the reporter driven by the promoter with the mutated WT1-EGR1 site was markedly less responsive, even though levels of basal expression in the untreated controls were similar (data not shown). In fact, the induction of the mutated construct was comparable to that of a deletion construct, pHG45-CAT9, where the WT1-EGR1 site and all upstream sequence had been deleted. Interestingly, this residual induction in the latter two constructs is comparable to that seen with the normal promoter in MCF-7 cells expressing E6 (Fig. 1 and 7). In addition, expression of E6 had no appreciable effect on the MMS induction of pHG45-CAT9, as shown in Fig. 7, or of pHG45-CAT2mut (data not shown). Presumably, the residual induction of constructs, such as pHG45-CAT2mut and pHG45-CAT9, is due to one or more additional stress-responsive control elements between −186 and −70 because pHG45-CAT3 (−70 to +144) (Fig. 1) showed no responsiveness. Since this line expresses both WT1 and p53, these findings are consistent with roles for these proteins in mediating stress responsiveness through the WT1-EGR1 site.
FIG. 7.
Role of the WT1-EGR1 site in the stress responsiveness of the GADD45 promoter. CAT reporter constructs, shown diagrammatically at the bottom of the figure, were transiently transfected into MCF-7 cells and then treated with MMS or UV radiation and analyzed in the same manner as described in the legend to in Fig. 1. In the case of pPH45-CAT2mut, the WT1-EGR1 site (WT1) was replaced with an unrelated sequence (see Materials and Methods). The two righthand pairs of bars show pHG45-CAT2 and pHG45-CAT9 cotransfected with the E6 expression vector as described in the legend to Fig. 1B. The average inductions (with standard deviations) relative to that of untreated cells for repeated experiments are shown.
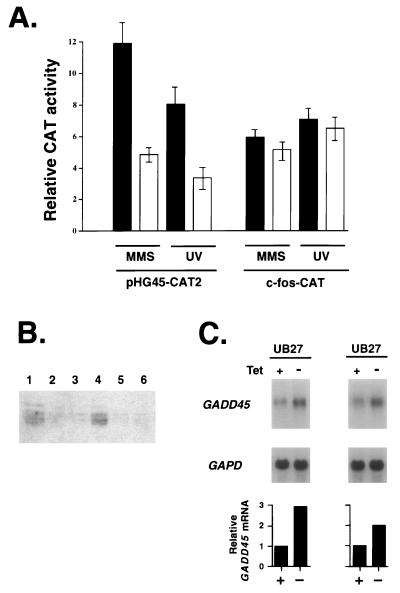
In order to further examine the role of cellular WT1 in regulation of the GADD45 gene, MCF-7 cells were transfected with the normal GADD45 promoter and an expression vector encoding human WT1 cDNA in the antisense orientation. As shown in Fig. 8A, induction in the presence of antisense WT1 was significantly reduced for the GADD45 promoter. To rule out some general attenuation of cellular stress responsiveness in the presence of antisense WT1, an unrelated stress gene, c-fos, which has been shown to be inducible by a wide variety of DNA-damaging agents (24), was also tested. In contrast to GADD45, antisense WT1 had no significant effect on the induction of c-fos. It should also be noted that antisense WT1 had no appreciable effect on the basal expression of these promoters in untreated cells: the basal expression of CAT2 transfected with pCMV-ASWT1 was 93% of that with pCMV-neo, and c-fos-CAT basal expression with pCMV-ASWT1 was 84% of that with pCMV-neo. As an additional control, cells transiently expressing pCMV-ASWT1 were isolated, as described in Materials and Methods, and WT1 protein levels were shown to be markedly reduced by immunoblot analysis with either the H2 (Fig. 8B) or WTc8 (data not shown) antibody. Thus, abrogation of either cellular p53 (such as by E6 [Fig. 1 and 7]) or WT1 expression (Fig. 8) in this line resulted in reduced stress responsiveness of the human GADD45 promoter.
FIG. 8.
The effect of antisense WT1 expression on the stress responsiveness of the GADD45 promoter. (A) MCF-7 cells were transfected with 5 μg of either the GADD45 (pHG45-CAT2) or c-fos (c-fos-CAT) reporter and 5 μg of either the WT1 antisense expression vector (solid bars) or pCMV-neo (open bars); 20 h later they were treated with the indicated damaging agent and then harvested and analyzed 24 h after the start of treatment in the same manner as described in the legend to Fig. 1. (B) MCF-7 cells were transfected with 5 μg of pCMV-ASWT1 plus 0.5 μg of pcI Tac and either the WT1 antisense expression vector (lanes 2, 3, 5, and 6) or pCMV-neo (lanes 1 and 4). Tac-expressing cells were isolated (see Materials and Methods), and cell lysates (40 μg of protein) were analyzed by immunoblotting with the H2 anti-WT1 antibody. Results are shown for two separate experiments (lanes 1 to 3 vs. lanes 4 to 6) done on different days. (C) The U2OS line UB27, containing tetracycline-inducible WT1, was analyzed by blot analysis of poly(A) RNA (1 μg) isolated from cells containing tetracycline (Tet +) and cells where the tetracycline had been removed 4 h earlier (Tet −); to show equivalent loading, the same blot was hybridized with GAPDH probe. The two pairs of samples were prepared separately; only the hybridizing bands of 1.4 kb for GADD45 and 1.3 kb for GAPD are shown. The relative GADD45 expression, shown in the bar graph, was determined on the same samples by quantitative hybridization.
To further strengthen the evidence for the role of WT1 in the regulation of the GADD45 gene, wt p53 osteosarcoma cells containing a tetracycline-inducible WT1 expression vector, which were developed by Englert et al. (9), were studied for changes in GADD45 expression. Removal of tetracycline consistently led to a rapid and significant increase in GADD45 expression in multiple independent experiments. As shown in Fig. 8C, the GADD45 mRNA level increased severalfold. These results demonstrate that increased GADD45 expression can occur in vivo when WT1 levels are increased in cells with wt p53.
DISCUSSION
Studies reported here and earlier (60) have shown that abrogation of either p53 function or WT1 expression reduces the stress responsiveness of the GADD45 promoter in spite of the fact that p53 does not bind to the promoter directly, while transient expression of p53 and WT1 could induce this promoter without typical direct p53 binding to DNA. An important implication of these studies is that the role of p53 in mediating positive transcriptional responses is broader than induction by direct binding to p53-binding sites alone and could involve many genes that lack p53-binding sites. Transcriptional repression by p53 protein-protein interactions has been demonstrated by numerous investigators (discussed in reference 32), and the present studies indicate that protein-protein interactions can also contribute to transcriptional up-regulation. In the case of both GADD45 and GADD153, abrogation of p53 function reduced the responsiveness to MUM-type (i.e., MMS, UV radiation, and medium starvation) agents in spite of the fact neither promoter contains a p53-binding site (60). This MUM-type response differed from typical p53 activation in that p53 alone was neither required nor sufficient; e.g., overexpression of p53 alone did not induce the GADD45 promoter (59), while abrogation of p53 function, such as in p53−/− mouse embryo fibroblasts (60), reduced but did not prevent induction. The p53 effect was mapped to the proximal promoter of GADD45, and deletion or replacement of the GC-rich motif with unrelated sequence removed the p53 responsiveness; e.g., induction of pHG45-CAT9 by MMS was similar in MCF-7 and MCF7-E6 cells. By DNase footprinting and EMSA, protein binding to this region was detected in wt p53 cells, and prior immunodepletion by antibodies to p53 or WT1 substantially reduced this binding, while an anti-Egr1 antibody had no effect. This same band in the EMSA was much less intense or absent in H1299 cells, which do not express appreciable levels of p53 or WT1, and in MCF-7 cells expressing E6. The biotinylated-DNA pull-down experiments indicate that one or more complexes containing both WT1 and p53 can bind to the GC-rich motif, while Egr1-binding complexes do not contain appreciable p53. The combination of p53 and WT1 expression vectors induced the pHG45-CAT2 reporter construct in transient assays, while the effect with Egr1 plus p53 was much less. While the argument could be made that such expression vectors produce abnormally high levels of protein that may not be physiologically relevant, attenuation of cellular WT1 expression by an antisense vector reduced the stress responsiveness of the GADD45 promoter in transfected MCF-7 cells. In addition, increased expression of WT1 in vivo (Fig. 8B) caused a rapid increase in GADD45 expression in a wt p53 cell line. WT1 was expressed at appreciable levels in MCF-7 cells (Fig. 4), and it also has been found to be expressed in various human wt p53 tumor cell lines (unpublished data) and in a variety of tissues and cell types, including tumor cells in vivo (3, 44). This may indicate that WT1 plays a role in p53-mediated stress responses in certain cell types, such as MCF-7, while other p53 protein-protein interactions could also contribute to stress responsiveness in other cases.
The GC-rich motif required for the p53 effect on the GADD45 promoter contains consensus sequences for multiple transcription factors in addition to WT1. In particular, it contains three overlapping Egr1 sites, two of which are perfect matches. In fact, this 9-bp motif has been shown to bind Egr1 more strongly than WT1, while for certain related sites WT1 binding was stronger (41, 47). However, p53 has been shown to interact with WT1 in vivo by binding involving the first two Zn fingers of WT1, while p53 does not bind Egr1 (5, 37). In the same study, WT1 was found to stabilize p53 and modulate its transactivational properties. In combination with p53, induction of the GADD45 promoter-reporter construct was much weaker for Egr1 than WT1 (Fig. 6) and expression vectors for Egr2 or Egr3 had no effect (data not shown). In addition, no footprint in this area was detected with extract from H1299 cells (Fig. 2) in spite of the fact that this line showed a level of Egr1 expression similar to that of MCF-7 cells (Fig. 4). The argument for stronger WT1 binding to the GADD45 site is strengthened by the fact that this region defines a larger WT1 consensus sequence that matches the 20-bp site in the PDGF-A promoter (GenBank accession no. HUMPDGFA1), which strongly binds WT1. In combination with a second site in the PDGF-A gene, this site has been shown to mediate transcriptional repression by WT1, while reporter constructs containing only one of the two sites resulted in induction by WT1 in transient assays (56). All four WT1 splice variants were found to bind to this site, or to the site and several adjacent nucleotides, in the PDGF-A gene (56), and expression vectors for the four different splice forms in combination with p53 caused appreciable induction of pHG45-CAT2, as shown in Fig. 6 for splice form A. A BLAST search of the GenBank database revealed a surprising number of matches for this relatively long (20-bp) consensus sequence: there were 12 perfect matches, which were all mammalian sequences, and >40 matches with one mismatch, which were predominantly mammalian sequences. In several cases, homology extended beyond 20 bp, such as for JUN-D (25 bp) and MDR3 (26 bp). A simple approximation demonstrates that the chances for such matches (10−12 to 10−15) are very remote in a genome of 109 bp. Considering that this motif is in the proximal promoters of multiple human genes (such as GADD45, PDGF-A, JUN-D, and MDR3)—a much smaller target than the entire genome—their presence certainly has functional significance. Unlike PDGF-A and MDR3, only JUN-D is in the same orientation as GADD45 relative to the transcription start site. It is of interest that JUN-D is also an immediate-early gene that is induced by stresses such as hypoxia (46), which have been shown to induce p53 protein and the GADD45 gene (discussed in reference 32).
The role of p53 in induction of the gadd gene promoters exhibits some important differences from its role with previously described p53-regulated genes. In addition to lacking p53-binding sites, neither the GADD45 promoter nor the GADD153 gene is inducible by IR in most wt p53 cells (23, 58), in contrast to genes containing p53-binding sites. As discussed previously (22), IR damage is qualitatively and quantitatively different from MUM-type stresses and probably activates p53 by DNA strand breakage (42). The regulation of GADD45 is complex, since the IR response is probably mediated by the strong p53-binding site in the third intron (23, 28), while most of the MUM-type responses are localized to the promoter. This highlights the multifactorial aspect of stress gene regulation, which often involves multiple sites and interacting proteins. Another important difference from other p53-regulated genes is that p53 alone is neither required nor sufficient for activation of the GADD45 promoter. Promoter constructs lacking the WT1 site were still inducible by MUM-type stresses, albeit at lower levels, and such stresses caused induction in p53-deficient cells. A reasonable interpretation of these results could be that MUM-type stresses activate adjacent control elements, such in pHG45-CAT9, that interact with proteins binding to the WT1 site and also with proteins of the basal transcription machinery.
While a variety of proteins are known to directly associate with WT1 or p53, results to date indicate that WT1 and p53 do not directly associate with each other. In MCF-7 cells we have confirmed previous results in other cells (37) showing that these two proteins associate in vivo, as determined by immunoprecipitation (data not shown); our results (Fig. 5) also demonstrate this association. While in vitro-translated WT1 has been to shown to bind to DNA containing WT1-binding sites (41) and to directly interact with another transcription factor, Par-4 (26), we consistently failed to see an in vitro association between baculovirus-synthesized p53 and in vitro-translated WT1 (data not shown); others (16a) also have not detected an in vitro association of these two proteins. A weak, possibly indirect interaction between the proteins is suggested by our EMSA results. In particular, the bandshift shown in Fig. 3 did not change with p53 induction by stress while p53 binding in the pull-down studies shown in Fig. 5 markedly increased after stress; a likely interpretation is that the p53 binding was disrupted during the electrophoresis in the EMSA. A less likely scenario is that p53 could conceivably compete with another protein for binding to the WT1 complex; however, it would be somewhat surprising to see no change in the footprint shown in Fig. 2 if this were the case. While the argument could be made that posttranslational changes are needed for the proteins to associate, a more likely explanation is that the WT1-p53 association is indirect and involves one or more intervening proteins in the binding complex. It has been suggested that WT1 contains both a repression domain, residues 85 to 124 in the protein, and an activation domain, residues 181 to 250, and that a second interactive protein is required for repression (55). Interacting proteins, such as p53, could affect this second interactive protein in the complex binding the GADD45 site following stress. Recently, p53 has been found to bind to the large transcriptional coactivator proteins CBP and p300 (2, 15, 34); these proteins function by interaction with a number of cellular activators, and probably with multiple components of the transcriptional machinery, and modulate p53 transcriptional activity. Thus, a number of known directly interacting proteins, such as CBP, p300, and Par-4, or perhaps as-yet-unknown interacting proteins, may be required for p53 to associate with proteins like WT1.
The physiologic consequences of WT1 interaction with p53 are uncertain but could very well contribute to growth control after stress. Overexpression of WT1 has been shown to arrest cells in G1 and to down-regulate Cdk kinase activities (33). In this study increased expression of any of the four WT1 splice forms reduced colony yield and also blocked entry into S phase after serum stimulation. This G1 arrest was blocked by increased expression of cyclin-cdk complexes (9). Interestingly, similar results have been found after increased expression of Gadd45: reduced colony survival (61), prevention of S-phase progression after serum stimulation (51), and rescue of Gadd45 growth suppression by cotransfection with cyclin expression vectors (57a). In addition, overexpression of Gadd45 has been found to trigger a G2-M arrest in wt p53 cells, and reduced Gadd45 expression resulted in reduced G2-M arrest after certain stresses (54a). As discussed earlier, EGR1, a member of the same gene family as WT1, has been found to be stress responsive in some cell types (4, 31), and recent evidence indicates that it may contribute to G1 arrest after IR exposure in melanoma cells (1). Since development has been found to be relatively normal in p53−/− mice, the primary role of p53 is probably in mediating various stress responses to damage produced either endogenously or by external agents. Our finding that p53 can contribute to induction by stress in the absence of direct p53-DNA binding indicates a possible role for the interacting proteins, such as WT1, in the maintenance of genomic stability.
ACKNOWLEDGMENTS
We thank D. A. Haber for providing cell lines, anti-WT1 antibodies, and cDNA clones; F. J. Rauscher III for providing anti-WT1 antibodies and cDNA clones; J. Milbrandt for providing the anti-Egr1 antibody; and J. Ashwell for providing the Tac expression vector and antibody.
REFERENCES
- 1.Ahmed M M, Sells S F, Venkatasubbarao K, Fruitwala S M, Muthukkumar S, Harp C, Mohiuddin M, Rangnekar V M. Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR-1. J Biol Chem. 1997;272:33056–33061. doi: 10.1074/jbc.272.52.33056. [DOI] [PubMed] [Google Scholar]
- 2.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 3.Buckler A J, Pelletier J, Haber D A, Glaser T, Housman D E. Isolation, characterization, and expression of the murine Wilms’ tumor gene (WT1) during kidney development. Mol Cell Biol. 1991;11:1707–1712. doi: 10.1128/mcb.11.3.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datta R, Taneja N, Sukhatme V P, Qureshi S A, Weichselbaum R, Kufe D W. Reactive oxygen intermediates target CC(A/T)6GG sequences to mediate activation of the early growth response 1 transcription factor gene by ionizing radiation. Proc Natl Acad Sci USA. 1993;90:2419–2422. doi: 10.1073/pnas.90.6.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day M L, Fahrner T J, Aykent S, Milbrandt J. The zinc finger protein NGFI-A exists in both nuclear and cytoplasmic forms in nerve growth factor-stimulated PC12 cells. J Biol Chem. 1990;265:15253–15260. [PubMed] [Google Scholar]
- 6.Dey B R, Sukhatme V P, Roberts A B, Sporn M B, Rauscher III F J, Kim S J. Repression of the transforming growth factor-beta 1 gene by the Wilms’ tumor suppressor WT1 gene product. Mol Endocrinol. 1994;8:595–602. doi: 10.1210/mend.8.5.8058069. [DOI] [PubMed] [Google Scholar]
- 7.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond I A, Madden S L, Rohwer-Nutter P, Bell G I, Sukhatme V P, Rauscher F J D. Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992;257:674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- 9.Englert C, Hou X, Maheswaran S, Bennett P, Ngwu C, Re G G, Garvin A J, Rosner M R, Haber D A. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 1995;14:4662–4675. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englert C, Vidal M, Maheswaran S, Ge Y, Ezzell R M, Isselbacher K J, Haber D A. Truncated WT1 mutants alter the subnuclear localization of the wild-type protein. Proc Natl Acad Sci USA. 1995;92:11960–11964. doi: 10.1073/pnas.92.26.11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan S, Smith M L, Rivet II D J, Duba D, Zhan Q, Kohn K W, Fornace A J, Jr, O’Connor P M. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res. 1995;55:1649–1654. [PubMed] [Google Scholar]
- 12.Fornace A J, Jr, Alamo I J, Hollander M C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci USA. 1988;85:8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fornace A J, Jr, Nebert D W, Hollander M C, Luethy J D, Papathanasiou M, Fargnoli J, Holbrook N J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989;9:4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodyer P, Dehbi M, Torban E, Bruening W, Pelletier J. Repression of the retinoic acid receptor-alpha gene by the Wilms’ tumor suppressor gene product, wt1. Oncogene. 1995;10:1125–1129. [PubMed] [Google Scholar]
- 15.Gu W, Shi X L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 16.Gujuluva C N, Baek J H, Shin K H, Cherrick H M, Park N H. Effect of UV-irradiation on cell cycle, viability and the expression of p53, gadd153 and gadd45 genes in normal and HPV-immortalized human oral keratinocytes. Oncogene. 1994;9:1819–1827. [PubMed] [Google Scholar]
- 16a.Haber, D. A. Personal communication.
- 17.Haber D A, Park S, Maheswaran S, Englert C, Re G G, Hazen-Martin D J, Sens D A, Garvin A J. WT1-mediated growth suppression of Wilms tumor cells expressing a WT1 splicing variant. Science. 1993;262:2057–2059. doi: 10.1126/science.8266105. [DOI] [PubMed] [Google Scholar]
- 18.Haber D A, Sohn R L, Buckler A J, Pelletier J, Call K M, Housman D E. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci USA. 1991;88:9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallahan D E, Sukhatme V P, Sherman M L, Virudachalam S, Kufe D, Weichselbaum R R. Protein kinase C mediates x-ray inducibility of nuclear signal transducers EGR1 and JUN. Proc Natl Acad Sci USA. 1991;88:2156–2160. doi: 10.1073/pnas.88.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington M A, Konicek B, Song A, Xia X L, Fredericks W J, Rauscher F J. Inhibition of colony-stimulating factor-1 promoter activity by the product of the Wilms’ tumor locus. J Biol Chem. 1993;268:21271–21275. [PubMed] [Google Scholar]
- 21.Hewitt S M, Hamada S, McDonnell T J, Rauscher III F J, Saunders G F. Regulation of the proto-oncogenes bcl-2 and c-myc by the Wilms’ tumor suppressor gene WT1. Cancer Res. 1995;55:5386–5389. [PubMed] [Google Scholar]
- 22.Holbrook N J, Fornace A J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991;3:825–833. [PubMed] [Google Scholar]
- 23.Hollander M C, Alamo I, Jackman J, McBride O W, Fornace A J., Jr Sequence conservation and DNA damage-responsiveness of the mammalian gadd45 gene. J Biol Chem. 1993;268:24385–24393. [PubMed] [Google Scholar]
- 24.Hollander M C, Fornace A J., Jr Induction of fos RNA by DNA-damaging agents. Cancer Res. 1989;49:1687–1692. [PubMed] [Google Scholar]
- 25.Huang R P, Adamson E D. A biological role for Egr-1 in cell survival following ultra-violet irradiation. Oncogene. 1995;10:467–475. [PubMed] [Google Scholar]
- 26.Johnstone R W, See R H, Sells S F, Wang J, Muthukkumar S, Englert C, Haber D A, Licht J D, Sugrue S P, Roberts T, Rangnekar V M, Shi Y. A novel repressor, par-4, modulates transcription and growth suppression functions of the Wilms’ tumor suppressor WT1. Mol Cell Biol. 1996;16:6945–6956. doi: 10.1128/mcb.16.12.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones K A, Yamamoto K R, Tjan R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985;42:559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- 28.Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr A mammalian cell cycle checkpoint utilizing p53 and GADD45 is defective in ataxia telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 29.Kedar P S, Widen S G, Englander E W, Fornace A J, Jr, Wilson S H. The ATF/CREB transcription factor-binding site in the polymerase beta promoter mediates the positive effect of N-methyl-N′-nitro-N-nitrosoguanidine on transcription. Proc Natl Acad Sci USA. 1991;88:3729–3733. doi: 10.1073/pnas.88.9.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessis T D, Slebos R J, Nelson W G, Kastan M B, Plunkett B S, Han S M, Lorincz A T, Hedrick L, Cho K R. Human papillomavirus 16 E6 expression disrupts the p53 mediated cellular response to DNA damage. Proc Natl Acad Sci USA. 1993;90:3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khachigian L M, Lindner V, Williams A J, Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996;271:1427–1431. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- 32.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 33.Kudoh T, Ishidate T, Moriyama M, Toyoshima K, Akiyama T. G1 phase arrest induced by Wilms tumor protein WT1 is abrogated by cyclin/CDK complexes. Proc Natl Acad Sci USA. 1995;92:4517–4521. doi: 10.1073/pnas.92.10.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 35.Luethy J D, Fargnoli J, Park J S, Fornace A J, Jr, Holbrook N J. Isolation and characterization of the hamster gadd153 gene. Activation of promoter activity by agents that damage DNA. J Biol Chem. 1990;265:16521–16526. [PubMed] [Google Scholar]
- 36.Madden S L, Cook D M, Morris J F, Gashler A, Sukhatme V P, Rauscher F J., III Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991;253:1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- 37.Maheswaran S, Park S, Bernard A, Morris J F, Rauscher F J D, Hill D E, Haber D A. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci USA. 1993;90:5100–5104. doi: 10.1073/pnas.90.11.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris J F, Madden S L, Tournay O E, Cook D M, Sukhatme V P, Rauscher F J., III Characterization of the zinc finger protein encoded by the WT1 Wilms’ tumor locus. Oncogene. 1991;6:2339–2348. [PubMed] [Google Scholar]
- 39.Murphy M, Hinman A, Levine A J. Wild-type p53 negatively regulates the expression of a microtubule-associated protein. Genes Dev. 1996;10:2971–2980. doi: 10.1101/gad.10.23.2971. [DOI] [PubMed] [Google Scholar]
- 40.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 41.Nakagama H, Heinrich G, Pelletier J, Housman D E. Sequence and structural requirements for high-affinity DNA binding by the WT1 gene product. Mol Cell Biol. 1995;15:1489–1498. doi: 10.1128/mcb.15.3.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson W G, Kastan M B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994;14:1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor P M, Jackman J, Bae I, Myers T G, Fan S, Mutoh M, Scudiero D, Monks A, Sausville E A, Weinstein J N, Friend S, Fornace A J, Jr, Kohn K W. Characterization of the p53 tumor suppressor pathway in cell lines of the NCI anticancer drug screen and correlations with the growth inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 44.Park S, Bernard A, Bove K E, Sens D A, Hazen-Martin D J, Garvin A J, Haber D A. Inactivation of WT1 in nephrogenic rests, genetic precursors to Wilms’ tumour. Nat Genet. 1993;5:363–367. doi: 10.1038/ng1293-363. [DOI] [PubMed] [Google Scholar]
- 45.Pietenpol J A, Tokino T, Thiagalingam S, el-Deiry W S, Kinzler K W, Vogelstein B. Sequence-specific transcriptional activation is essential for growth suppression by p53. Proc Natl Acad Sci USA. 1994;91:1998–2002. doi: 10.1073/pnas.91.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prabhakar N R, Shenoy B C, Simonson M S, Cherniack N S. Cell selective induction and transcriptional activation of immediate early genes by hypoxia. Brain Res. 1995;697:266–270. doi: 10.1016/0006-8993(95)00994-2. [DOI] [PubMed] [Google Scholar]
- 46a.Rauscher, F. Personal communication.
- 47.Rauscher F J, III, Morris J F, Tournay O E, Cook D M, Curran T. Binding of the Wilms’ tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990;250:1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- 48.Robertson L M, Kerppola T K, Vendrell M, Luk D, Smeyne R J, Bocchiaro C, Morgan J I, Curran T. Regulation of c-fos expression in transgenic mice requires multiple interdependent transcription control elements. Neuron. 1995;14:241–252. doi: 10.1016/0896-6273(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 49.Schiestl R H, Khogali F, Carls N. Reversion of the mouse pink-eyed unstable mutation induced by low doses of x-rays. Science. 1994;266:1573–1576. doi: 10.1126/science.7985029. [DOI] [PubMed] [Google Scholar]
- 50.Shiao R T, Miglietta L, Khera S Y, Wolfson A, Freter C E. Dexamethasone and suramin inhibit cell proliferation and interleukin-6-mediated immunoglobulin secretion in human lymphoid and multiple myeloma cell lines. Leuk Lymphoma. 1995;17:485–494. doi: 10.3109/10428199509056862. [DOI] [PubMed] [Google Scholar]
- 51.Smith M L, Chen I, Zhan Q, Bae I, Chen C, Gilmer T, Kastan M B, O’Connor P M, Fornace A J., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 52.Smith M L, Chen I T, Zhan Q, O’Connor P M, Fornace A J., Jr Involvement of the p53 tumor suppressor in repair of UV-type DNA damage. Oncogene. 1995;10:1053–1059. [PubMed] [Google Scholar]
- 53.Smith M L, Kontny H U, Zhan Q, Sreenath A, O’Connor P M, Fornace A J., Jr Antisense GADD45 expression results in decreased DNA repair and sensitizes cells to UV-irradiation or cisplatin. Oncogene. 1996;13:2255–2263. [PubMed] [Google Scholar]
- 54.Tokino T, Thiagalingam S, el-Deiry W S, Waldman T, Kinzler K W, Vogelstein B. p53 tagged sites from human genomic DNA. Hum Mol Genet. 1994;3:1537–1542. doi: 10.1093/hmg/3.9.1537. [DOI] [PubMed] [Google Scholar]
- 54a.Wang, X. W., et al. Unpublished data.
- 55.Wang Z Y, Qiu Q Q, Gurrieri M, Huang J, Deuel T F. WT1, the Wilms’ tumor suppressor gene product, represses transcription through an interactive nuclear protein. Oncogene. 1995;10:1243–1247. [PubMed] [Google Scholar]
- 56.Wang Z Y, Qiu Q Q, Huang J, Gurrieri M, Deuel T F. Products of alternatively spliced transcripts of the Wilms’ tumor suppressor gene, wt1, have altered DNA binding specificity and regulate transcription in different ways. Oncogene. 1995;10:415–422. [PubMed] [Google Scholar]
- 57.Werner H, Shen-Orr Z, Rauscher III F J, Morris J F, Roberts C T, Jr, LeRoith D. Inhibition of cellular proliferation by the Wilms’ tumor suppressor WT1 is associated with suppression of insulin-like growth factor I receptor gene expression. Mol Cell Biol. 1995;15:3516–3522. doi: 10.1128/mcb.15.7.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Zhan, Q., et al. Unpublished data.
- 58.Zhan Q, Bae I, Kastan M B, Fornace A J., Jr The p53-dependent γ-ray response of GADD45. Cancer Res. 1994;54:2755–2760. [PubMed] [Google Scholar]
- 59.Zhan Q, Carrier F, Fornace A J., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993;13:4242–4250. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhan Q, Fan S, Smith M L, Bae I, Yu K, Alamo I, Jr, O’Connor P M, Fornace A J., Jr Abrogation of p53 function affects the response of gadd genes to DNA base damaging agents and medium starvation. DNA Cell Biol. 1996;15:805–815. doi: 10.1089/dna.1996.15.805. [DOI] [PubMed] [Google Scholar]
- 61.Zhan Q, Lord K A, Alamo I, Jr, Hollander M C, Carrier F, Ron D, Kohn K W, Hoffman B, Liebermann D A, Fornace A J., Jr The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]