Abstract
Context:
Photobiomodulation therapy (PBMT) has been widely used to improve strength, fatigue resistance and increase muscle mass in healthy individuals. These effects could help critically ill patients admitted to intensive care units (ICUs) who show reduced mobility and muscle strength. ICU-acquired weakness lessens overall health and increases the patient’s length of stay in the ICU.
Objective:
This study evaluated the effects of PBMT using low intensity light-emitting diodes (LEDs) on the mobility and muscle strength (functional capacity) and length of stay of patients admitted to hospital ICU.
Methods:
This randomized, triple-blind, sham-controlled trial was conducted in a hospital ICU. Sixty patients were randomly assigned to two equal groups: (a) PBMT and (b) Sham. PBMT was applied daily to patients until their discharge from the ICU, using a flexible neoprene array of 264 LEDs (120 at 635 nm, 1.2 mW each; 144 at 880 nm, 15 mW each) for 90s (207.36 Joules) at each site. Ten sites were located bilaterally on the thighs, legs, arms, and forearms ventrally and dorsally, 15 min totaling 2,073.6 Joules per session. Outcomes were length of stay (in h) until discharge from the ICU, muscle strength by the Medical Research Council (MRC) score and handgrip dynamometry (HGD), patient mobility by Intensive Care Unit Mobility Scale (IMS) and the Simplified Acute Physiology Score 3 (SAPS 3) for predicting mortality of patients admitted to the ICU.
Results:
PBMT reduced the average length of stay in the ICU by ~30% (p = 0.028); increased mobility (IMS: 255% vs. 110% p = 0.007), increased muscle strength (MRC: 12% vs. −9% p = 0.001) and HGD (34% vs. −13% p < 0.001), and the SAPS3 score was similar (p > 0.05).
Conclusion:
The results suggest that daily PBMT can reduce the length of stay of ICU patients and increase muscle strength and mobility.
Keywords: handgrip, intensive care unit, light-emitting diode, low-level laser therapy, medical research council
Graphical Abstract

1 |. INTRODUCTION
Patients in intensive care unit (ICU) have a high incidence of muscle atrophy and contracture, loss of mobility, muscle weakness, and general reduction in fatigue resistance, which are proportional to the immobilization time and loss of function [1–3]. The first 7 days of bedrest can already lead to ~26% loss of muscle mass [4]. This generalized muscle weakness, which is unrelated to the patient’s acute disease or its treatment, is called “Intensive Care Unit Acquired Weakness” (ICUAW). ICUAW can affect both the peripheral and respiratory muscles and leads to important reductions in the functional capacity of ICU patients and is associated with a higher risk of death [1, 5]. Early and noninvasive assessment of these patients, such as the use of handgrip strength evaluation [6], muscle strength by Medical Research Council score (MRC) [7], Intensive Care Unit Mobility Scale (IMS) [8, 9], and quantification of length of stay in the ICU is essential to monitor these patients and for clinical decision making [4].
Therapeutic approaches that minimize the adverse effects of ICU admission have high clinical and economic relevance, because longer hospital stays increase the probability of complications, such as thromboembolic disease, insulin resistance, microvascular dysfunction, pressure ulcers, atelectasis, pneumonia, and delirium, as well as increased mortality rate and the hospital expenditure [1–3]. In this context, low-level laser (light) therapy, now known as photobiomodulation therapy (PBMT) [10] has been widely applied to skeletal muscle tissue immediately before or after an exercise session, or during training programs to increase muscle strength or improve fatigue resistance [11, 12]. These effects seen in healthy people could also be important in ICU patients, improving their muscle strength and mobility. However, to the best of our knowledge, no studies have investigated the effects of PBMT in ICU patients unrelated to the COVID-19 pandemic.
PBMT can be delivered by lasers or by LEDs (light-emitting diodes). LEDs have very similar effects to lasers when the light parameters are similar, wavelength (nm), energy (Joules), energy density (J/cm2), power density (mW/cm2), and treated area (cm2) [13]. PBMT with LEDs is relatively safer than lasers due to the divergent light beams, it is cheaper, more convenient, and can stimulate the same chromophores (light-absorbing molecules) that are located within the mitochondria [14–16]. Furthermore, cells with many mitochondria and high metabolic activity are particularly responsive to light, such as muscle tissue [15–18].
The effects of PBMT on muscle tissue have been widely investigated in healthy individuals, athletes and/or practitioners of various sport activities. These effects include increased protein synthesis (muscle hypertrophy), increased synthesis of the muscle energy sources ATP (adenosine triphosphate) and glycogen, and greater resistance to oxidative stress, which produce increased strength and fatigue resistance [11, 18]. However, few studies have investigated the effects of PBMT in populations in hospital or intensive care [6, 19–22]. Moreover, the literature does not contain properly controlled randomized trials of the effects of PBMT in critically ill patients or those admitted to ICUs. These patients develop immobility and reduced muscle function due to bed restriction, especially those requiring invasive mechanical ventilation (IMV) [1–3].
Considering the effects of PBMT on muscle tissue, and the complications that ICU patients suffer because of their extended immobilization, PBMT may be beneficial in the treatment of critically ill ICU patients. It is known that the irradiated muscle area is an important factor, because if the purpose is to improve the performance of an entire muscle group, the number of points or sites irradiated by PBMT must cover the greatest possible area [11, 15, 23]. There have been many types of equipment reported in the literature, such as focused laser beams, arrays of lasers and/or LEDs (clusters), flexible LED devices that cover large body areas (“blankets”), or even whole body light beds [11, 23–25]. The hypothesis of the present study was that PBMT applied to large muscle groups using LED “blankets” could improve muscle strength and mobility (better functional capacity), and also decrease the patients’ length of stay in the ICU.
2 |. OBJECTIVE
2.1 |. General purpose
This study evaluated the effects of PBMT with low intensity LEDs on the mobility, muscle strength, and length of stay of ICU patients.
2.2 |. Specific objectives
Primary outcome: length of stay in the ICU (in h);
Secondary outcome 1: mobility of the upper and lower limbs of these patients at admission and discharge from the ICU (IMS—Intensive Care Unit Mobility Scale),
Secondary outcome 2: handgrip strength of these patients at admission and discharge from the ICU (dynamometry);
Secondary outcome 3: assessment of the global muscle strength (Medical Research Council) of these patients at admission and discharge from the ICU.
3 |. MATERIALS AND METHODS
3.1 |. Study design
This was a randomized, triple-blind, and sham-controlled trial. The study design was chosen to ensure blinding in the allocation of patients to groups, blinding in the choice of PBMT or Sham treatment, blinding in data analysis, and to minimize the chance of distinguishing between Sham and PBMT.
3.2 |. Clinical trial record
The study was approved by local Ethics Committee in Human Research (approval 3.407.860) and registered in the Brazilian Clinical Trials Registry (ReBEC) platform (registration: RBR-8fffvv; UTN trial identification: U1111-1241-4974).
3.3 |. Participants, recruitment, and selection
The study was conducted in four ICUs of a philanthropic hospital in Brazil. Each ICU of the hospital had 10 beds. Sample size consisted of 60 adult patients (>18 years old) who were admitted to the ICUs of this hospital. The number of patients was estimated a priori from sample size calculation in GPower software (version 3.1) considering a medium effect size (0.25), study power of at least 80%, two groups and two repeated measurements (admission and discharge from ICU) for the IMS, MRC, and dynamometry assessment. In addition, two groups and one measurement were chosen for the outcome length of stay in the ICU, considering an effect size of 0.5. The data collection of the study had a duration of 7 months approximately.
3.4 |. Inclusion criteria
The patients (>18 years of age) were admitted to the ICU for reasons such as postoperative cardiovascular surgery, abdominal surgery, or acute respiratory failure. These patients were aware, oriented, and collaborative, without administration of any drugs that depress the central nervous system or preserve hemodynamic and respiratory stability. In addition, the patients were included when they could carry out 3 of 5 instructions: (1) open and close your eyes; (2) look at me; (3) open your mouth and show your tongue; (4) nod your head; and (5) raise your eyebrows when I count to five [26, 27].
3.5 |. Exclusion criteria
Patients with plegia, paresis, amputation, or congenital deformities in at least one of the limbs, diagnosed at musculoskeletal evaluation performed during initial anamnesis, were excluded. In addition, patients who progressed to sepsis, septic shock, hemodynamic or respiratory instability were excluded. Insufficient data in their medical records was also a reason for exclusion from the study.
3.6 |. Randomization and blinding
Sixty patients were recruited and randomly allocated into two balanced groups: (a) photobiomodulation therapy (PBMT) and (b) Sham. The treatments differed as to the doses of light (Joule—J) delivered. PBMT emitted red and infrared light (207.36 J per site), whereas the Sham device did not emit any light (zero J). Patients and therapists could not distinguish between PBMT and Sham devices because they both wore protective goggles, which blocked the light. Moreover, the devices did not cause any detectable rise in temperature. Randomization of therapies (PBMT and Sham) was performed by a single evaluator who was blinded to the study treatment protocol. Opaque envelopes contained either:
“S”: sham treatment (0 Joule—J);
“PBMT” photobiomodulation treatment (207.36 Joules—J).
4 |. EXPERIMENTAL PROCEDURES
4.1 |. Initial evaluation
All patients were enrolled in the study after their medical records had been consulted upon their entry to the ICU. Those who met the inclusion criteria received the informed consent form to read and sign. Caregivers were approached for those patients in the immediate postoperative period (day 1 in ICU), who were still under the influence of central nervous system depressant drugs but were only evaluated when fully conscious, oriented, and cooperative.
Information was collected from the patients’ medical records to complete the evaluation form such as hospitalization report, reason for hospitalization, medical diagnosis, age, gender, comorbidities, simplified acute physiology score 3 (SAPS 3), as well as outcome variables such as date and time of admission and discharge (length of stay in the ICU), IMS, MRC, and dynamometry.
The SAPS 3 is a prognostic system used at the time of admission to quantify the mortality risk of ICU patients. This important prognostic tool helps to anticipate how long individuals will stay in the ICU, as well as providing support for choosing therapies during the decision-making process. This score is divided into three groups with 20 variables for score compilation [28]. The higher SAPS 3 score, the worse the prognosis of the patient. Thus, the SAPS 3 score was used to assess the physiological condition of patients when admitted to the ICU by a specific medical team at the hospital without any input from the present study research team. The decision to discharge each patient from ICU was strictly determined by the same medical team without any influence from the research team.
4.2 |. Outcome variables
Outcome variables included patient mobility using the IMS; muscle strength measured by the Medical Research Council (MRC) score and hand grip dynamometry; and length of stay until discharge from the ICU. This information was recorded on the evaluation form.
Researchers not involved in the randomization and therapy application (PBMT or Sham) conducted all assessments for IMS, MRC, and dynamometry, and counted the number of hours until patient discharge from the ICU. Other researchers not blinded to randomization, and not involved in assessments, were in charge of delivering PBMT or Sham irradiation. Patients did not know which therapy they were receiving, ensuring triple blinding of the study: (1) researchers not involved in randomization assessed IMS, MRC, dynamometry, and hours until ICU discharge; (2) patients; (3) researchers in charge for data analysis and not involved with randomization or other assessments. The reassessments were performed daily during the day, while the intervention was applied every day until the patient was discharged from ICU at night. The study design is illustrated in Figure 1.
FIGURE 1.
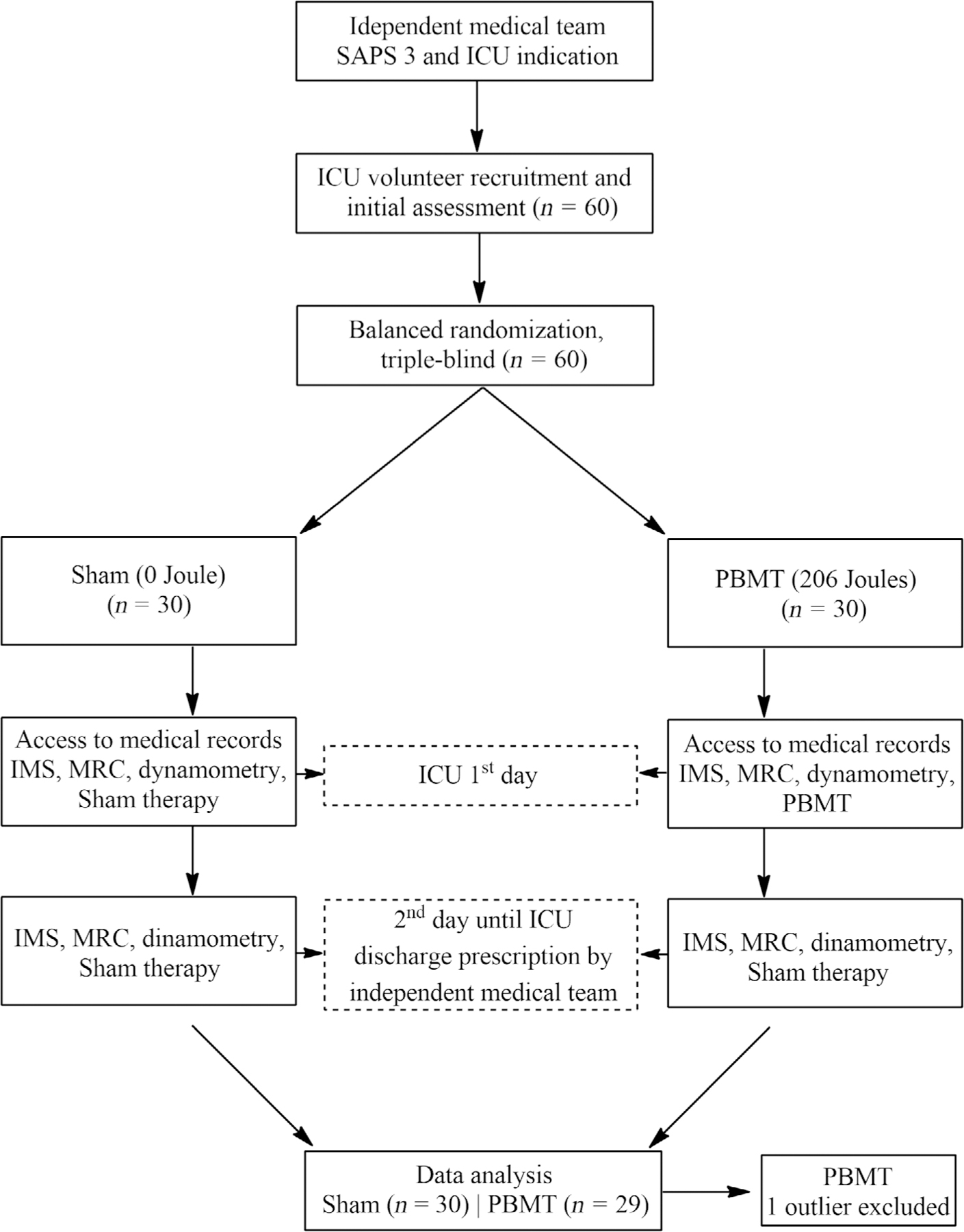
Flowchart of the study design. Photobiomodulation (PBMT) or Sham therapy applied to the patients’ lower and upper limb muscles. Intensive Care Unit Mobility Scale (IMS)—functionality scale; Simplified Acute Physiology Score 3 (SAPS 3)—score for physiological conditions; Medical Research Council (MRC)—degree of global muscle strength; and photobiomodulation therapy (PBMT).
Source: Author.
4.3 |. Intensive Care Unit Mobility Scale
Patients were evaluated using the IMS [29], which grades the general functioning of critically ill patients admitted to an ICU. This scale scores from 0 to 10 in a single domain, where a 0 score shows low mobility where the patient can perform only passive bed exercises. A score of 10 shows high mobility, for patients who can walk independently without assistance [8, 9].
4.4 |. Muscle strength (Medical Research Council—MRC score)
The MRC score is a simple and widely adopted technique to assess global muscle strength in critically ill patients using six specific movements of the upper and lower limbs. The rating of each limb movement ranges from 0 (plegia) to 5 points (normal strength) [7], and the score can total 60 points. All patients included in the study were positioned supine in bed with the head elevated at 45° for this evaluation.
4.5 |. Muscle strength (dynamometry)
Patients were assessed for overall muscle strength by measuring the maximal handgrip strength of the dominant upper limb, measured by a handgrip dynamometer [6]. The patients were positioned supine, head elevated at 45°, elbow flexion at 90°, and wrist in neutral position.
4.6 |. Length of stay in the ICU
Patients were monitored for the length of stay in the ICU (in h). For ICU discharge to be recommended, the reasons why the patients needed intensive care should have been reversed. The decision to discharge each patient from the ICU was determined by an independent specific medical team, without any influence from the research team. It is important to note that while patients remained in the ICU, they all received standard physical therapy care from hospital physical therapists (not from the research team).
4.7 |. PBMT using low-intensity LEDs
PBMT by low-intensity LEDs was performed using a flexible “blanket” (neoprene) containing 264 individual commercial LED light emitters (BODY 264 PAD RED, Inlight therapy™). This device featured 120 LEDs that emitted light in the red range (635 ± 10 nm) and 144 LEDs emitting in the infrared range (880 ± 20 nm) arranged in rows of 24 parallel LEDs with an irradiation surface area of 333.5 cm2 (11.5 × 29 cm) (Figure 2). Each red LED had 1.2 mW optical power, and each infrared LED had 15 mW both with 4.7 kHz pulse frequency.
FIGURE 2.

Light-emitting device. Light emitting device consisted of 264 low-intensity light-emitting diodes (LEDs) containing 120 red LEDs (5 rows of 24 LEDs) and 144 infrared LEDs (6 rows of 24 LEDs).
Table 1 lists the optical parameters of the light-emitting device as well as the photobiomodulation irradiation parameters which were calibrated and measured using a power and optical energy meter (PM100D Thorlabs®) equipped with an S130C sensor.
TABLE 1.
Optical parameters of red and infrared light-emitting diodes (LEDs) of the PBMT device.
| Parameters | Red | Infrared |
|---|---|---|
| Number of LEDs: 264 | 120 | 144 |
| Wavelength (nm) | 635 ± 10 | 880 ± 20 |
| Optical power—mW (each LED) | 1.2 | 15.0 |
| Area of each LED (cm2) | 0.2 | 0.2 |
| Irradiance—mW/cm2 (each LED) | 6 | 75 |
| Time of irradiation at site (s) | 90 | 90 |
| Energy—Joule (each LED) | 0.108 | 1.350 |
| Fluence—Joule/cm2 (each LED) | 0.54 | 6.75 |
| Total Energy per site (90 s): 207.36 J | 12.96 | 194.40 |
| Frequency (KHz) | 4.7 | 4.7 |
| Total power of the device (mW): 2,304 mW | 144 | 2,160 |
| Total Energy (per session—10 sites, 15 min): 2,073.6 Joules | 129.6 | 1,944 |
| Irradiation area: | 333.5 cm2 | |
| Application mode: | Contact with light pressure | |
PBMT was applied bilaterally to the thighs (quadriceps femoris and hamstring muscles), legs (tibialis anterior, gastrocnemius, and soleus), arms, and forearms ventrally and dorsally, totaling 10 sites, similar to previous studies [30, 31], while the patients were laying supine. The LED devices (“blankets”) were covered with thin plastic film and after the application was completed, the equipment and its cables were sanitized with 70% alcohol. Photobiomodulation was applied with an irradiation time of 90 seconds per site according to the study design and Table 1. The Sham device did not emit any light (0 J and 0 mW), while the device had the same therapy time programmed. During the irradiation, patients and therapist used protective glasses and could not see any light.
PBMT was applied by the same person (researcher 2) throughout the study until the patient was discharged, in a triple-blind, randomized, sham fashion. Neither patient nor therapist could tell which type of therapy they received (PBMT or Sham). Researcher 1 (responsible for assessment of IMS, MRC, dynamometry, and hours in ICU), and researcher 3 (responsible for data analysis) were also blinded to the PBMT. Finally, PBMT was applied daily at night until patient discharge from the ICU, which was determined by the responsible medical team (independent from the research team).
5 |. STATISTICAL ANALYSIS
Outcome variables were analyzed for normal distribution of data by the Shapiro–Wilk test. When normal, repeated-measures analysis of variance (ANOVA with Tukey post hoc test) was used to identify interaction between groups (PBMT and Sham) and time (admission and discharge from the ICU).
When the outcome variables had a non-normal distribution, ICU admission and discharge times were analyzed intragroup using the Wilcoxon test. The intergroup comparison used the Mann–Whitney U test to compare the ICU discharge time between the groups, followed by Bonferroni correction. Briefly, the Bonferroni correction is an adjustment made to P values when several dependent or independent statistical tests are being performed simultaneously on a single data set. This adjustment is used to reduce the chances of obtaining false-positive results (type I errors) when multiple pair wise tests are performed on a single set of data.
The percentage of change (%) was calculated as the difference between the values measured at ICU admission and ICU discharge in percentage for each outcome variable and compared between groups by T test for independent samples (if normal distribution), and Mann Whitney U (if not normal). Example: taking account a patient with score of 1 at ICU admission, and score of 4 at ICU discharge for IMS score, the difference from ICU discharge to admission is 3 (i.e., 4–1). The number 3 (the change) represents 300% of change taking account the score 1 (ICU admission) as 100%. For all outcome variables, mean ± standard deviation was calculated, and the 95% confidence interval (95% CI) for variables with normal distribution. The significance level was set at 5% and intention-to-treat analysis was applied.
6 |. RESULTS
Sixty patients were included in the study, 32 women (PBMT: 15; Sham: 17) and 28 men (PBMT: 15; Sham: 13). Participant’s anthropometric characteristics are presented in Table 2. The reasons for ICU admission are listed in Table 3:
TABLE 2.
Anthropometric characteristics of the participants.
| PBMT | Sham | p-value | |
|---|---|---|---|
| Age (years) | 69.76 ± 12.17 | 67.06 ± 14.91 | 0.445 |
| Body mass (kg) | 67.15 ± 16.59 | 67.87 ± 8.98 | 0.872 |
| Height (m) | 1.60 ± 0.10 | 1.58 ± 0.97 | 0.570 |
| BMI (kg/m2) | 25.84 ± 4.49 | 27.31 ± 3.57 | 0.293 |
TABLE 3.
Reasons for hospitalization of ICU patients.
| Reason for admission to ICU | PBMT (n = 30) | Sham (n = 30) |
|---|---|---|
| Myocardial revascularization | 8 | 5 |
| Acute breathing insufficiency | 4 | 5 |
| Angioplasty | 3 | 3 |
| Acute myocardial infarction | 2 | 3 |
| Chronic obstructive pulmonary disease | 1 | 2 |
| Severe digestive bleeding | 2 | 0 |
| Postoperative heart valve replacement | 1 | 1 |
| Unstable angina | 1 | 1 |
| Postoperative cholecystectomy | 0 | 2 |
| Decompensated congestive heart failure | 1 | 1 |
| Postoperative gastroduodenopancreatectomy | 0 | 1 |
| Catheterization | 1 | 0 |
| Postoperative prostatectomy | 1 | 0 |
| Postoperative laparotomy | 1 | 0 |
| Asthma | 0 | 1 |
| Acute pulmonary edema | 0 | 1 |
| Esophagogastrectomy postoperative | 1 | 0 |
| Postoperative meningioma resection | 0 | 1 |
| Postoperative aortic aneurysm | 0 | 1 |
| Postoperative cranial-hydrocephalus drainage | 1 | 0 |
| Postoperative aneurysm embolization | 0 | 1 |
| Postoperative pancreas resection | 1 | 0 |
| Postoperative rectosigmoidectomy | 1 | 0 |
| Postoperative duodenopancreatectomy | 0 | 1 |
6.1 |. Length of stay in the ICU
All patients admitted into the ICU were eventually discharged. First, any outliers were investigated in both groups for the primary outcome: length of stay in the ICU. The criterion established to identify an outlier for the upper limit was: the group mean (30 patients per group) + 3* the group standard deviation value (30 patients per group). Thus, for the PBMT group, one patient was excluded from analysis because he remained in the ICU for 231.5 h, that is, this was higher than the value of 211.15 h calculated for the upper limit [average of 84.12 + (3*42.34 of standard deviation) = 211.15 h]. For the Sham group, no outlier was identified [average of 111.72 + (3*71.68 of standard deviation) = 326.76 h]. The lower limit was not considered in the analysis as a negative value (in h) for both groups.
After the initial analysis, the 29 remaining patients in the PBMT group had an average length of stay (counted from ICU admission until discharge) of 79.04 ± 32.47 h (95% CI: 69.88–98.35). The sum of all hours of hospitalization, that is, the total of hours that patients stayed in ICU for PBMT group was 2292.16 h. On the other hand, the Sham group had an average length of stay in the ICU of 111.72 ± 71.68 h (95% CI: 80.30–143.13), and the sum of hours of hospitalization in the ICU was 3351.66 h. The average length of stay in the PBMT group was 29.25% shorter than the control group and this was statistically significant (p = 0.028). Figure 3 shows the average and total length of stay (hours of hospitalization) of both groups.
FIGURE 3.

Length of stay in intensive care unit (ICU) of patients treated with PBMT or Sham groups. *Statistical significance (p = 0.028) t-test for independent samples.
6.2 |. Simplified acute physiology score 3 (SAPS 3) prognostic system
The SAPS 3 prognostic system aims to establish a mortality prediction for ICU patients. The mean SAPS 3 score was not significantly different between groups (p = 0.179) using a t-test for independent samples (data with normal distribution). The PBMT group had a mean score of 48.52 ± 13.38 (95% CI: 42.66–54.39), while the Sham group had a mean score of 42.52 ± 13.64 (95% CI: 36.39–48.66). These results are shown in Figure 4.
FIGURE 4.
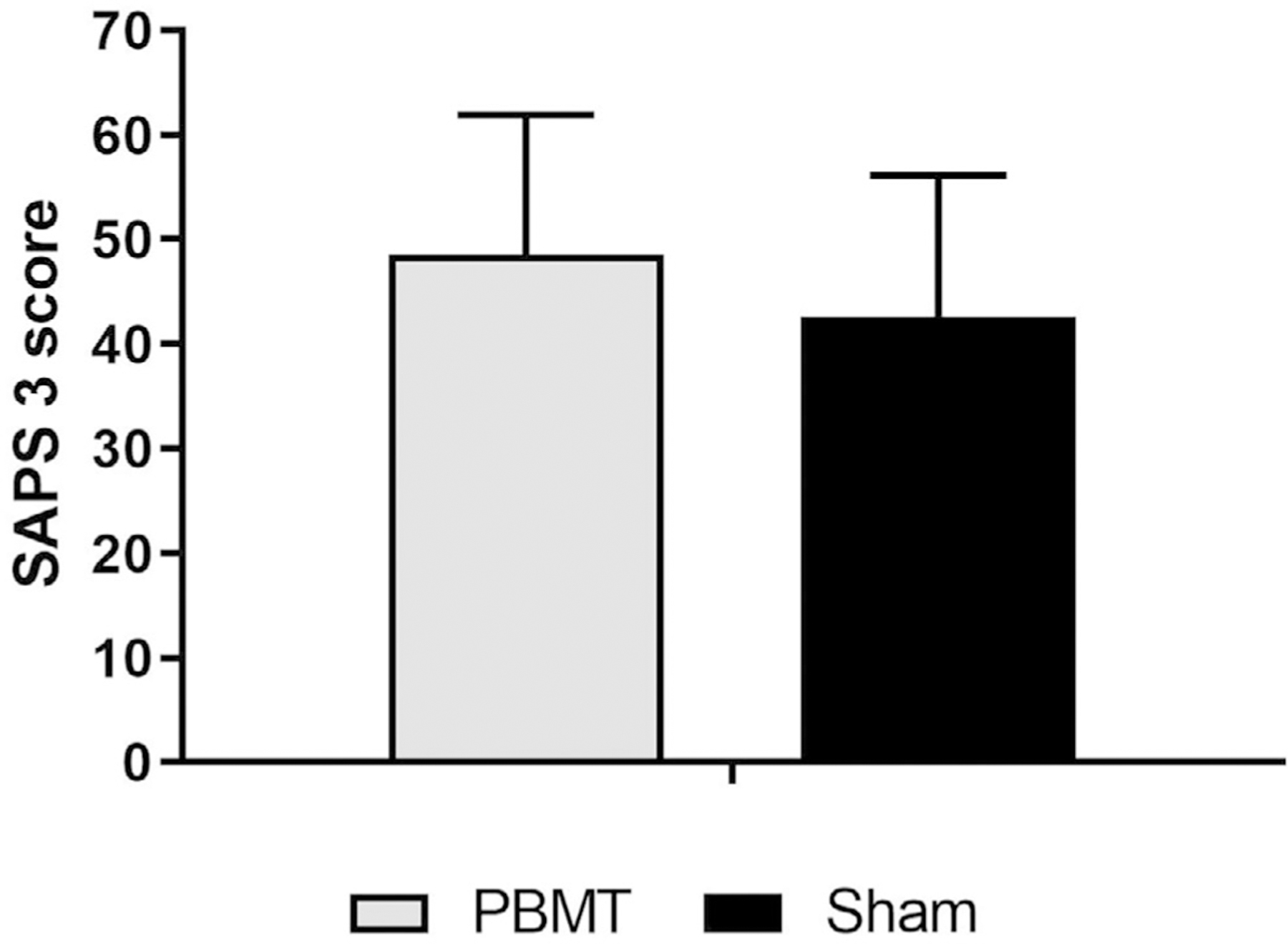
Simplified Acute Physiology Score 3 (SAPS 3) for the Photobiomodulation Therapy (PBMT) and Sham groups. The t-test for independent samples did not find any statistical difference between groups (p = 0.179).
6.3 |. Intensive care unit mobility scale (IMS)
The IMS data had a non-normal distribution; therefore, a Wilcoxon test (nonparametric for dependent samples) was used to verify intragroup differences. The IMS scores at admission versus discharge times in the ICU for both groups. The intergroup comparison was made by Mann–Whitney U test (nonparametric for independent samples) considering the discharge ICU moment with Bonferroni correction. Finally, the percentage of change (%) in IMS of both groups was compared using the Mann–Whitney U test.
The PBMT group experienced an increased mean IMS score from admission to discharge (1.31 ± 1.07 to 4.24 ± 3.03; p < 0.001), with a mean percentage of change (%) of 255.55 ± 248.66. The Sham group also increased the mean IMS score from 1.90 ± 1.70 to 3.10 ± 2.64 (p = 0.026), with an average percentage of change (%) of 110.80 ± 214.95. There was no difference between groups measured at the ICU discharge moment (p = 0.254) after Bonferroni correction. The difference in the percentage of change (%) between the groups was significant (p = 0.007). These results are summarized in Figure 5.
FIGURE 5.

Intensive Care Unit Mobility Scale (IMS) score for the Photobiomodulation Therapy (PBMT) and Sham groups. There was a statistical difference between the admission and discharge times in the intensive care unit (ICU), which was significantly different (*p < 0.001) for PBMT and Sham groups (*p = 0.026) (Wilcoxon test). The percentage of change (%) (Mann–Whitney U test) showed a significant difference between groups (#p = 0.007).
6.4 |. Muscle strength measured by the MRC (medical resource council) score
The MRC data had a non-normal distribution, and the analysis was the same as used for the IMS. The PBMT group showed an increase in the overall muscle strength according to the mean MRC score (45.55 ± 7.34 to 50.75 ± 8.21) but without statistical significance (p = 0.107), with an average percentage of change (%) of 12.66 ± 16.65. The Sham group showed a decrease in overall muscle strength according to the mean MRC score (44.70 ± 8.92 to 40.33 ± 16.10) with statistical significance (p = 0.001), with an average percentage of change (%) of −9.29 ± 38.38. The ICU discharge moment was compared between groups, showing a significantly higher muscle strength (p = 0.012) for the PBMT group compared to the Sham group after Bonferroni correction. The difference in the percentage of change (%) between groups was also significant (p = 0.001). These results are summarized in Figure 6.
FIGURE 6.
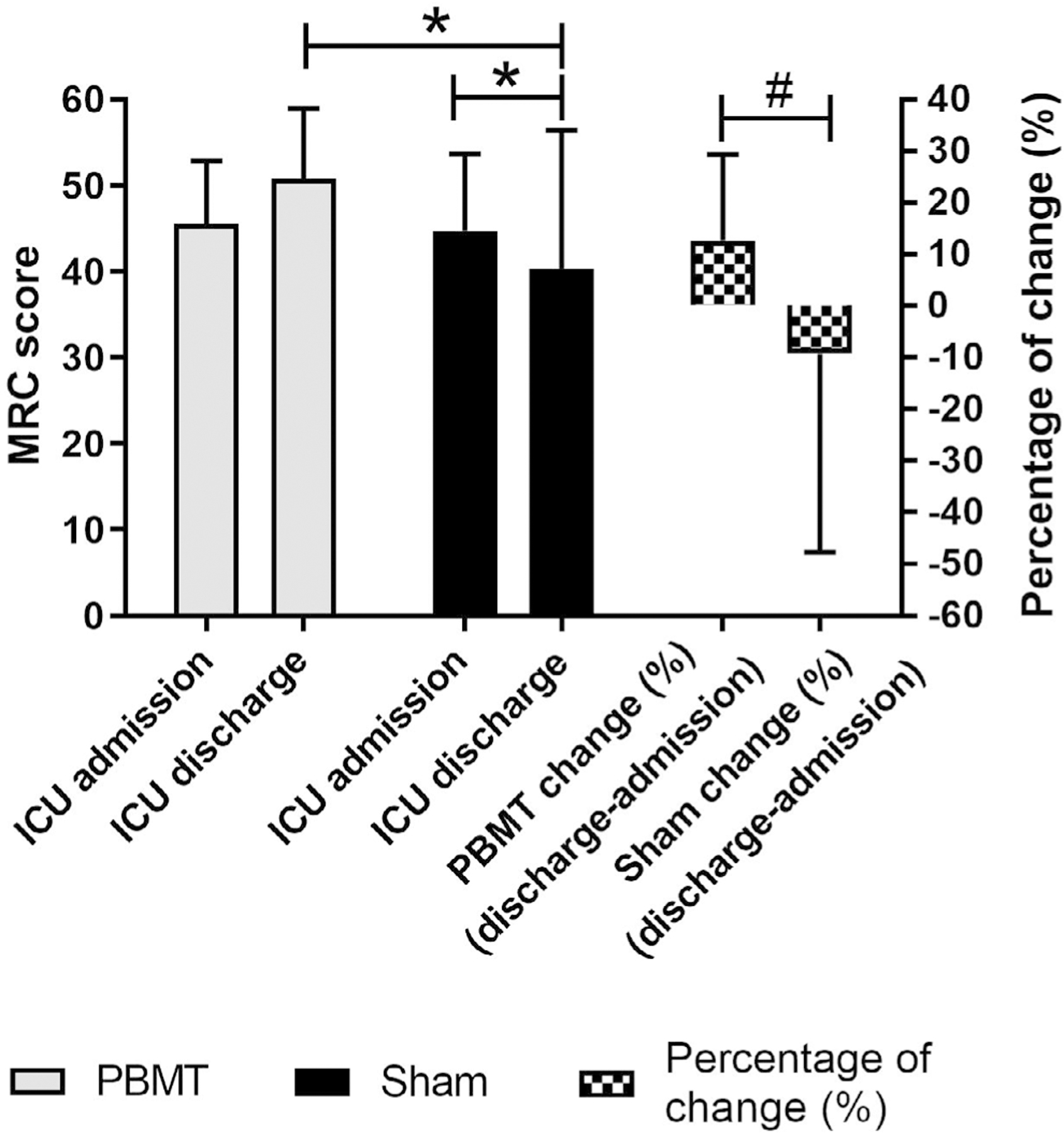
Muscle strength by the MRC (Medical Resource Council) score. The Sham group showed a statistical difference between the admission and discharge moments in the intensive care unit (ICU), *p = 0.001 (Wilcoxon test). The ICU discharge moment had a significant difference between groups (*p = 0.012) (Mann–Whitney U test). The percentage of change (%) was also significantly different between groups (#p = 0.001) (Mann–Whitney U test).
6.5 |. Muscle strength measured by handgrip dynamometry (HGD)
The HGD data had a normal distribution; therefore, repeated measures analysis of variance (ANOVA) with Tukey post hoc test was used to verify the intragroup and intergroup differences, between the ICU admission versus discharge moments. The intergroup comparison of the percentage of change (%) in HGD used a Mann–Whitney U test (data with non-normal distribution).
The PBMT group showed a significant increase in maximum muscle strength with the mean HGD increasing from 15.85 ± 8.56 (95% CI: 12.09–19.60) to 18.57 ± 9.54 (95% CI: 14.39–22.76) (p = 0.010), with an average percentage of change (%) of 34.49 ± 99.05. The Sham group showed a decrease in muscle strength with the mean HGD decreasing from 14.69 ± 6.91 (95% CI: 11.66–17.72) to 12.57 ± 6.91 (95% CI: 9.53–15.60) but without statistical significance (p = 0.052), with an average percentage of change (%) of −13.11 ± 38.91. The HGD measured at the ICU discharge time compared between groups showed no significant difference (p = 0.162). The difference between the percentage of change (%) between groups was significant (p < 0.001). These results are summarized in Figure 7.
FIGURE 7.

Maximum muscle strength measured by hand grip dynamometry (HGD) for the Photobiomodulation Therapy (PBMT) and Sham groups. The PBMT group showed a statistical difference (*p = 0.010) between the ICU admission and discharge moments (repeated measures ANOVA with Tukey post hoc test). There was a significant difference between groups (#p < 0.001) for the percentage of change (%) (Mann–Whitney U test).
7 |. DISCUSSION
The results of the present study show, for the first time in the literature, that PBMT applied daily to the muscles of the upper and lower limbs was able to increase the overall muscle strength and mobility (functional capacity), as well as decrease the length of stay in the ICU of critically ill hospitalized patients.
In the last decade, the scientific literature has shown the wide applicability of PBMT, especially for pain control [32, 33], and the healing of various types of tissue, such as epithelial, bone [34, 35], and muscle [15]. In addition, PBMT has been shown to provide specific benefits to muscle tissue leading to increased performance, either by reducing fatigue, or by increasing strength in young, healthy individuals or athletes [11, 15, 23, 25].
In the present study, we showed for the first time that PBMT was also able to increase muscle strength in critically ill ICU patients, who are likely to develop ICU-acquired muscle weakness (ICUAW) [1]. Decreased muscle strength appears to start in the early days of ICU admission, and patients may experience increasing sarcopenia (loss of muscle mass) of ~2% per day. The incidence of ICU sarcopenia can affect 70% of hospitalized patients, depending on the patient’s age and comorbidity [3], while the prevalence of ICUAW can reach 43% [5]. Therefore, early and noninvasive therapeutic interventions that can reduce or prevent muscle weakness, such as PBMT, are important for these patients [36], as well as for wider public health interests.
The PBMT application significantly increased the maximum handgrip strength measured by dynamometry (HGD) by ~34% (from 95% CI: 12.09–19.60 to 95% CI: 14.39–22.76), while the sham group HGD decreased by approximately −13% 95% CI: 11.66–17.72 to 95% CI: 9.53–15.60. The literature suggests that values of HGD < 11 kgf in men and <7 kgf in women are indicative of ICUAW [37]. Due to the pioneering nature of the present study, we found only one similar published study that also showed an increase in PBMT-mediated handgrip strength in critically ill patients with chronic kidney disease undergoing hemodialysis [6]. In that double-blind, randomized, placebo-controlled study, 15 patients underwent 4 sessions of PBMT (550-diode laser cluster, 1 W, 20 sec, 3 forearm regions, total 60 Joules/session) or placebo (sham therapy), had their handgrip strength measured before and after the PBMT. An increase of approximately 5% in the average strength of these patients was found in the PBMT group [6].
In agreement with the handgrip muscle strength results, PBMT also provided a higher percentage increase (~12%) in overall muscle strength measured by the MRC score compared to a decrease after Sham therapy (~ −9%). Some studies classify a total MRC score lower than 48 as indicating ICUAW [4, 38], this classification has been correlated with a longer hospital stay and overall functional impairments. Therefore, the increased MRC score in the PBMT group in our study confirmed that the ICUAW classification no longer applied, and the patients could leave the ICU (45.55 ± 7.34 to 50.75 ± 8.21).
In a published study that included 45 patients with 18 having established criteria for a ICUAW diagnosis, the hand grip strength (HGD) and the global muscle strength (MRC score) showed good agreement with each other (100% accuracy; Kappa coefficient = 1; p < 0.001) [39]. De Marchi et al. [21] also reported interesting results when they applied PBMT combined with a static magnetic field (PBMT-sMF) in ICU patients. Their focus was on severe COVID-19 patients requiring intubation, and their results showed some differences to our study, because they performed the therapy once a day until either ICU discharge or death. The authors applied PBMT with a cluster of 20 diodes on six sites (lower thoracic/upper abdominal region and the neck area), delivering a total energy of 252 J. Unlike our study, they did not find a significant difference in the length of stay in the ICU for PBMT-sMF group when compared to the placebo group (16.26 vs. 23.06 days, respectively). Interestingly, the authors found an increased thickness of the diaphragm, which may have prevented the muscle atrophy related to ICU stay, something we could also observe with our muscle strength assessments.
Regarding the length of stay in the ICU, PBMT applied over large areas of the body reduced significatively by ~30% the patients’ length of stay in ICU compared to sham. In addition, this result induced an important reduction (but without statistical significance) in the sum of total hours of ICU hospitalization in PBMT group (Figure 3). There was at least 1,000 h less length of stay in ICU in PBMT group compared to sham. These results together may have a very positive impact over patients’ health, decreasing the chance of hospital complications as infections and ICUAW, and suggest an economy (lower costs) to patients and health insurance plans.
The patients’ functional capacity (mobility), measured by the IMS, indicated that both groups (PBMT and sham) had higher scores at discharge compared to those at ICU admission. However, the percentage gain of ~255% in functional capacity for the PBMT group was significantly larger than the gain of ~214% in the sham group. It is important to note that the minimal clinically important difference in the IMS score has been set at 1.4 to 3 points [40]. Thus, despite the Sham group increasing the IMS score from 1.90 ± 1.70 to 3.10 ± 2.64 (p = 0.026), this did not achieve the level of a minimal important clinical difference [40]. On other hand, the PBMT group achieved the minimal clinically important difference, increasing from 1.31 ± 1.07 to 4.24 ± 3.03 points in the IMS score. The IMS score shows a strong correlation with muscle mass, global muscle strength (MRC score), and handgrip strength, as shown in a previous study that evaluated 25 patients undergoing cardiopulmonary bypass oxygenation [41]. This study also measured the decrease in femoral quadriceps cross-sectional area using ultrasound. Thus, the improved functional outcome of the patients treated with PBMT agrees with the results of the handgrip strength and the global muscle strength (MRC score).
The relationship between muscle strength, patient functional performance, ICUAW, and length of stay in the ICU has also been recently explored [39, 42, 43]. These previous studies reveal that a decrease in muscle strength and loss of functionality may increase the incidence of ICUAW, which consequently increases the length of stay of ICU patients, as well as raising the possibility of additional hospitalization within 6 months. Taken together, all the results of the present study suggested that PBMT significantly increased specific muscle strength (handgrip), overall muscle strength (MRC score) and mobility (IMS), culminating in improved patient functionality and an average 30% shorter length of stay in the ICU compared to sham therapy. Also, an extended length of stay in the ICU is a risk factor for ICUAW [4], showing that this relationship goes in both directions. Note that both groups of patients had the same score (without any statistical difference) for the SAPS 3 prognostic system, which aims to establish a mortality prediction for patients admitted to the ICU [28]. This finding suggests that both groups had the same physiological condition at baseline (ICU admission). Therefore, we assumed that the shorter ICU stay (~ 30%) for the group treated with PBMT was not influenced by any difference in their physiological condition at baseline.
The mechanisms of action of PBMT on muscle tissue are not yet fully elucidated [15]. However, it is known that PBMT can modulate muscle energy metabolism, increase the activity of cytochrome c oxidase (CCO) enzyme, increase the synthesis of adenosine triphosphate (ATP) to meet energy demands, and improve the defense against oxidative stress. The PBMT effects depend on the light dose (dose response) and the time after application (time response) [16–18]. PBMT can produce gains in muscle mass (protein synthesis) while decreasing muscle atrophy, muscle damage, and delayed-onset muscle soreness [11, 25, 44].
The mechanisms of action of PBMT to promote increased muscle strength, improve functional capacity, and consequently reduce the length of stay in the ICU, could not be fully explored in this study. However, it is well known that ICU patients eventually develop muscle atrophy, sarcopenia, and generalized loss of muscle strength, which leads to significant impairment of functional capacity due to the immobility of prolonged bed rest [1–3]. This produces a generalized state of tissue stress, especially in the musculoskeletal tissue. In this context, PBMT seems to show better effects on metabolically stressed or injured cells or tissues, because these cells and tissues are more likely to suffer hypoxia and higher inhibitory concentrations of nitric oxide [14, 45, 46]. This makes it more difficult to transfer electrons from complexes I, II, and III, and finally to complex IV along the electron transport chain catalyzed by the enzyme cytochrome c oxidase (CCO), resulting in lower cellular respiration and less energy synthesis (ATP). Thus, PBMT might reverse this tissue stress, improve ATP synthesis [16, 17], and promote the gain of muscle strength and function without any adverse effects.
PBMT is a safe, inexpensive, and easy to apply intervention that may decrease the length of stay in the ICU and increase muscle strength, therefore preventing the onset of ICUAW, improving functionality, and reducing costs in the healthcare system. However, there is a need for additional studies to confirm our findings.
As one limitation to our study, we did not perform any post-discharge follow up, so we do not know if the PBMT group had a better overall recovery than the sham group after their ICU discharge. The literature shows that ICUAW is associated with lower MRC, functional ability and quality of life in the post-discharge period, and with higher mortality risks [5], so future studies should include a longer follow-up period to test whether PBMT has any long-term benefits in this population. In addition, other limitations of the present study were not assessing skin phototypes, or tone, and the physical activity level of the patients before their hospitalization. These assessments could help understand better how the skin phototype or tone [47] and physical activity level (i.e., patients with background in sports or strength conditioning) [48] could interact with photobiomodulation therapy to promote better response.
8 |. CONCLUSION
The results of the present study confirmed the hypothesis that PBMT could improve muscle tissue characteristics such as global and specific muscle strength, and thus could improve the functional capacity of ICU patients. In addition, PBMT significantly reduced the length of stay in the ICU (primary outcome). Thus, further randomized, double- or triple-blind, sham-controlled studies are needed to confirm these potential benefits of PBMT, so it could be used as a non-invasive, non-pharmacological, inexpensive adjunct therapy in patients admitted to the ICU for a wide variety of reasons.
ACKNOWLEDGMENTS
We would like to thank the Piauiense Association to Fight Cancer (APCC), Sao Marcos Hospital, and Federal University of São Carlos.
FUNDING INFORMATION
Michael R Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700.
US NIH Grants, Grant/Award Numbers: R21AI121700, R01AI050875
Footnotes
CONFLICT OF INTEREST STATEMENT
Michael R. Hamblin declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; Hologenix Inc. Santa Monica, CA; Vielight, Toronto, Canada; JOOVV Inc, Minneapolis-St. Paul, MN; Sunlighten, Kansas City, MO; Consulting; USHIO Corp, Japan; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; Klox Asia, Guangzhou, China. Stockholding: Niraxx Light Therapeutics, Inc, Irvine CA; JelikaLite Corp, New York, NY. The other authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- [1].Hermans G, Van den Berghe G, Crit. Care 2015, 19, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yosef-Brauner O, Adi N, Ben Shahar T, Yehezkel E, Carmeli E, Clin. Respir. J 2015, 9, 1. [DOI] [PubMed] [Google Scholar]
- [3].Farhan H, Moreno-Duarte I, Latronico N, Zafonte R, Eikermann M, Anesthesiology 2016, 124, 207. [DOI] [PubMed] [Google Scholar]
- [4].Raurell-Torredà M, Arias-Rivera S, Martí JD, Frade-Mera MJ, Zaragoza-García I, Gallart E, Velasco-Sanz TR, San José-Arribas A, Blazquez-Martínez E, Aust. Crit. Care 2021, 34, 435.33663950 [Google Scholar]
- [5].García-Pérez-de-Sevilla G, Pinto BS-P, Intensive Crit. Care Nurs 2023, 74, 103333. [DOI] [PubMed] [Google Scholar]
- [6].Macagnan FE, Baroni BM, Cristofoli É Z, Godoy M, Schardong J, Plentz RDM, Lasers Med. Sci 2019, 34, 835. [DOI] [PubMed] [Google Scholar]
- [7].Nordon-Craft A, Moss M, Quan D, Schenkman M, Phys. Ther 2012, 92, 1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, Young P, Zanni J, Buhr H, Higgins A, Presneill J, Berney S, Heart Lung 2014, 43, 19. [DOI] [PubMed] [Google Scholar]
- [9].Tipping CJ, Bailey MJ, Bellomo R, Berney S, Buhr H, Denehy L, Harrold M, Holland A, Higgins AM, Iwashyna TJ, Needham D, Presneill J, Saxena M, Skinner EH, Webb S, Young P, Zanni J, Hodgson CL, Ann. Am. Thorac. Soc 2016, 13, 887. [DOI] [PubMed] [Google Scholar]
- [10].Anders JJ, Lanzafame RJ, Arany PR, Photomed. Laser Surg 2015, 33, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferraresi C, Huang YY, Hamblin MR, J. Biophotonics 2016, 9, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vanin AA, Verhagen E, Barboza SD, Costa LOP, Leal-Junior ECP, Lasers Med. Sci 2018, 33, 181. [DOI] [PubMed] [Google Scholar]
- [13].Heiskanen V, Hamblin MR, Photochem. Photobiol. Sci 2018, 17, 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang YY, Chen AC, Carroll JD, Hamblin MR, Dose Response 2009, 7, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ferraresi C, Hamblin MR, Parizotto NA, Photonics Lasers Med 2012, 1, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferraresi C, Kaippert B, Avci P, Huang YY, de Sousa MV, Bagnato VS, Parizotto NA, Hamblin MR, Photochem. Photobiol 2015, 91, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ferraresi C, de Sousa MV, Huang YY, Bagnato VS, Parizotto NA, Hamblin MR, Lasers Med. Sci 2015, 30, 1259. [DOI] [PubMed] [Google Scholar]
- [18].Ferraresi C, Parizotto NA, Pires de Sousa MV, Kaippert B, Huang YY, Koiso T, Bagnato VS, Hamblin MR, J. Biophotonics 2016, 9, 976. [DOI] [PubMed] [Google Scholar]
- [19].de Souza GHM, Ferraresi C, Moreno MA, Pessoa BV, Damiani APM, Filho VG, Dos Santos GV, Zamunér AR, Lasers Med. Sci 2020, 35, 1055. [DOI] [PubMed] [Google Scholar]
- [20].Francisco C. d. O., Beltrame T, Hughson RL, Milan-Mattos JC, Ferroli-Fabricio AM, Benze BG, Ferraresi C, Parizotto NA, Bagnato VS, Borghi-Silva A, Porta A, Catai AM, Complement Ther. Med 2019, 42, 178. [DOI] [PubMed] [Google Scholar]
- [21].De Marchi T, Frâncio F, Ferlito JV, Weigert R, de Oliveira C, Merlo AP, Pandini DL, Pasqual-Júnior BA, Giovanella D, Tomazoni SS, Leal-Junior EC, J. Inflammation Res 2021, 14, 3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scontri C, de Castro Magalhaes F, Damiani APM, Hamblin MR, Zamuner AR, Ferraresi C, J. Biophotonics 2023, 16, e202300083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferraresi C, Photobiomodul. Photomed. Laser Surg 2020, 40, 810. [DOI] [PubMed] [Google Scholar]
- [24].Beltrame T, Ferraresi C, Parizotto NA, Bagnato VS, Hughson RL, Lasers Med. Sci 2018, 33, 1065. [DOI] [PubMed] [Google Scholar]
- [25].Ferraresi C, Bertucci D, Schiavinato J, Reiff R, Araujo A, Panepucci R, Matheucci E Jr., Cunha AF, Arakelian VM, Hamblin MR, Parizotto N, Bagnato V, Am. J. Phys. Med. Rehabil 2016, 95, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphaël JC, Outin H, Bastuji-Garin S, JAMA 2002, 288, 2859. [DOI] [PubMed] [Google Scholar]
- [27].Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, Casaer MP, Wouters P, Gosselink R, Van Den Berghe G, Muscle Nerve 2012, 45, 18. [DOI] [PubMed] [Google Scholar]
- [28].Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR, Intensive Care Med 2005, 31, 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kawaguchi YMF, Nawa RK, Figueiredo TB, Martins L, Pires-Neto RC, Jornal Brasileiro de Pneumologia 2016, 42. [DOI] [PMC free article] [PubMed]
- [30].Ferraresi C, Beltrame T, Fabrizzi F, do Nascimento ES, Karsten M, Francisco Cde O, Borghi-Silva A, Catai AM, Cardoso DR, Ferreira AG, Hamblin MR, Bagnato VS, Parizotto NA, Physiother. Theory Pract 2015, 31, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ferraresi C, Dos Santos RV, Marques G, Zangrande M, Leonaldo R, Hamblin MR, Bagnato VS, Parizotto NA, Lasers Med. Sci 2015, 30, 1281. [DOI] [PubMed] [Google Scholar]
- [32].Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM, Lancet 2009, 374, 1897. [DOI] [PubMed] [Google Scholar]
- [33].de Sousa MVP, Kawakubo M, Ferraresi C, Kaippert B, Yoshimura EM, Hamblin MR, J. Biophotonics 2018, 11, e201700370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hamblin MR, J. Neurosci. Res 2018, 96, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamblin MR, Huang Y-Y, Ferraresi C, Carroll JD, de Freitas LF, Low-Level Light Therapy: Photobiomodulation, SPIE Press, Bellingham: 2018. [Google Scholar]
- [36].Hodgson C, Bellomo R, Berney S, Bailey M, Buhr H, Denehy L, Harrold M, Higgins A, Presneill J, Saxena M, Skinner E, Young P, Webb S, Crit. Care 2015, 19, 81.25715872 [Google Scholar]
- [37].Parry SM, Berney S, Granger CL, Dunlop DL, Murphy L, El-Ansary D, Koopman R, Denehy L, Crit. Care 2015, 19, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Klawitter F, Ehler J, Bajorat R, Patejdl R, Int. J. Mol. Sci 2023, 24, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bragança RD, Ravetti CG, Barreto L, Ataíde T, Carneiro RM, Teixeira AL, Nobre V, Heart Lung 2019, 48, 532. [DOI] [PubMed] [Google Scholar]
- [40].Tipping CJ, Holland AE, Harrold M, Crawford T, Halliburton N, Hodgson CL, Heart Lung 2018, 47, 497. [DOI] [PubMed] [Google Scholar]
- [41].Hayes K, Holland AE, Pellegrino VA, Mathur S, Hodgson CL, J. Crit. Care 2018, 48, 1. [DOI] [PubMed] [Google Scholar]
- [42].Ali NA, O’Brien JM Jr., Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, Connors AF Jr, Marsh CB, Am. J. Respir. Crit. Care Med 2008, 178, 261. [DOI] [PubMed] [Google Scholar]
- [43].Lee JJ, Waak K, Grosse-Sundrup M, Xue F, Lee J, Chipman D, Ryan C, Bittner EA, Schmidt U, Eikermann M, Phys. Ther 2012, 92, 1546. [DOI] [PubMed] [Google Scholar]
- [44].Baroni BM, Rodrigues R, Freire BB, Franke Rde A, Geremia JM, Vaz MA, Eur. J. Appl. Physiol 2015, 115, 639. [DOI] [PubMed] [Google Scholar]
- [45].Sommer AP, Oron U, Kajander EO, Mester AR, J. Proteome Res 2002, 1, 475. [DOI] [PubMed] [Google Scholar]
- [46].Hamblin MR, Photochem. Photobiol 2018, 94, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Souza-Barros L, Dhaidan G, Maunula M, Solomon V, Gabison S, Lilge L, Nussbaum EL, Lasers Surg. Med 2018, 50, 291. [DOI] [PubMed] [Google Scholar]
- [48].Dellagrana RA, Rossato M, Orssatto LBR, Sakugawa RL, Baroni BM, Diefenthaeler F, Photobiomodul. Photomed. Laser Surg 2020, 38, 734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


