Abstract
Background
Primary percutaneous coronary intervention (PPCI) and fibrinolysis have proved to be major discoveries regarding treatment of ST-segment elevation myocardial infarction (STEMI). The threshold at which PPCI becomes less favourable than fibrinolysis remains unclear and controversial. Trials have studied the impact of delayed reperfusion in relation to symptom onset, but to our knowledge, none have focused on the outcome of patients past the expected 120-minute window regarding first medical contact (FMC) in the concomitant era of PPCI and fibrinolysis.
Methods
STEMI patients who presented to a single PPCI-capable hospital, in the period from 2016 to 2020, and were treated with PPCI within 120 -240 minutes after FMC, and those who received fibrinolysis, were included. Outcomes of patients treated with delayed PPCI were compared to those of patients treated with fibrinolysis. The primary endpoint was a net adverse clinical event composite of all-cause mortality, myocardial re-infarction, ischemia-driven target-vessel revascularization, disabling stroke, and major bleeding at discharge.
Results
Inclusion criteria were met for 536 STEMI patients, 429 treated with PPCI and 107 treated with fibrinolysis. The primary endpoint (net adverse clinical events) was not significantly different between the 2 groups (2.8% vs 3.7%, P = 0.61). However, intracranial hemorrhage (0% vs 2.8%, P = 0.008) and bleeding (BARC 3 or 5) (0.9% vs 3.7%, P = 0.048) significantly favoured the PPCI group.
Conclusions
This retrospective study suggests that delayed PPCI may be a safer approach than fibrinolysis in patients with an FMC-to-balloon time of > 120 minutes, owing to reduction in the risk of intracranial and severe bleeding. These retrospective observations should be validated in larger randomized trials.
Résumé
Contexte
L’intervention coronarienne percutanée primaire (ICPP) et la fibrinolyse se sont avérées des découvertes majeures dans le traitement de l’infarctus du myocarde avec élévation du segment ST (STEMI). Le seuil où l’ICPP devient moins favorable que la fibrinolyse demeure ambigu et controversé. Des essais ont porté sur les répercussions d’une reperfusion tardive après l’apparition des symptômes, mais, à notre connaissance, aucun ne s’est penché sur l’issue des patients au-delà de la fenêtre attendue des 120 minutes après le premier contact avec les services médicaux (PCSM) dans le domaine concomitant de l’ICPP et de la fibrinolyse.
Méthodologie
Des patients ayant subi un STEMI qui s’étaient présentés à un seul hôpital en mesure de réaliser une ICPP durant la période allant de 2016 à 2020 et qui avaient été traités par une ICPP dans les 120 à 240 minutes suivant leur PCSM, ainsi que ceux qui avaient reçu une fibrinolyse, ont été inclus dans l’analyse. L’issue des patients traités au moyen d’une ICPP tardive a été comparée à celle des patients traités par fibrinolyse. Le principal critère d’évaluation était un paramètre composite d’événements indésirables nets comprenant la mortalité toutes causes confondues, la survenue d’un nouvel infarctus du myocarde, une revascularisation du vaisseau cible en raison d’une ischémie, un accident vasculaire cérébral invalidant et une hémorragie majeure au moment du congé de l’hôpital.
Résultats
Au total, 536 patients ayant subi un STEMI répondaient aux critères d’inclusion, soit 429 traités par une ICPP et 107, par une fibrinolyse. Le taux de survenue du principal critère d’évaluation (événements indésirables cliniques nets) n’était pas significativement différent entre les deux groupes de traitement (2,8 % vs 3,7 %; p = 0,61). Cependant, les taux d’hémorragies intracrâniennes (0 % vs 2,8 %; p = 0,008) et de saignements (de type 3 ou 5 selon la classification BARC [Bleeding Academic Research Consortium]) (0,9 % vs 3,7 %; p = 0,048) étaient significativement en faveur du groupe des patients traités par ICPP.
Conclusions
Cette étude rétrospective permet de présumer qu’une ICPP tardive pourrait être une option thérapeutique plus sûre que la fibrinolyse chez les patients dont le délai entre le PCSM et l’insertion du ballonnet est supérieur à 120 minutes, en raison de la réduction du risque d’hémorragie intracrânienne ou grave. Ces observations rétrospectives doivent toutefois être validées dans le cadre de plus vastes essais à répartition aléatoire.
“Time is muscle” is the least well-guarded secret in the medical community regarding the management of ST-segment elevation myocardial infarction (STEMI). Guidelines around the world agree that the faster perfusion is restored in a culprit artery in a STEMI patient, the better the outcome.1,2 Before the era of percutaneous coronary intervention (PCI), fibrinolysis was the mainstay of treatment.3 Since then, primary PCI has played a major role in the treatment of STEMI patients. Canadian Cardiology Society guidelines for STEMI management state that if primary PCI can be performed within 90 minutes from the first medical contact (FMC), for patients presenting to a PCI-capable centre, or within 120 minutes from a non-PCI-capable centre, PPCI should be the intervention of choice, as demonstrated in the Danish Trial in Acute Myocardial Infarction-2 (DANAMI-2 trial).4, 5, 6
Unfortunately, not all patients can be treated in that time span, for various reasons. Geographic location, necessity for stabilization before transportation, and transportation delay are the main ones. Therefore, fibrinolysis still plays a role in the population of patients who cannot reach the PPCI desired time interval, if it can be administered within 30 minutes of FMC and without contraindications. The Primary Angioplasty in Patients Transferred From General Community Hospitals to Specialized PTCA Units With or Without Emergency Thrombolysis (PRAGUE-2A) trial suggests that if the fibrinolysis approach is selected, it needs to be administered within 3 hours from symptom onset to optimize the chances of success, and that the longer the delay, the worse the prognosis.7 Fibrinolysis certainly can be beneficial if administered in a timely fashion, but it comes with a higher risk of major bleeding and can change the outcome of patients dramatically if the most-feared complication occurs. If fibrinolysis is initiated, most agree that the fibrinolysis-PCI combination (pharmacoinvasive approach) should be the intervention of choice in that scenario.
Patients who are close to the 120-minute window are a challenge for physicians, as data on this population are so scarce that the Canadian Cardiology Society guidelines even state that the maximum time from FMC to device, beyond which PPCI is inferior to the pharmacoinvasive approach, remains uncertain and controversial.8,9 The Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial showed no significant clinical benefit of fibrinolysis within 3 hours of symptom onset followed by PCI, compared to PPCI with delays longer than 60 minutes and an increase in intracranial hemorrhage.10 Therefore, this study focuses on the outcome of this specific population of patients that exceed the 120-minute window from FMC to reperfusion.
Material and Methods
Study design
This is a single-centre retrospective cohort study using electronic medical record analysis. Data were collected by chart review and validated by a single reviewer. Patients with an admission diagnosis of STEMI were screened for inclusion criteria. The study was approved by our local institutional review board.
Population
Suspected STEMI patients who presented to a single PPCI-capable centre, during the period from January 1, 2016 to December 31, 2020 and were treated with PPCI with an FMC to reperfusion time of 120-240 minutes, and those who received fibrinolysis, were included in this study. Patients had to be 18 years of age or older. Exclusion criteria included the following: (i) absolute contraindication to fibrinolysis (prior intracranial hemorrhage, arteriovenous malformations, primary brain tumours, metastases, suspected aortic dissection, uncontrolled hypertension (systolic blood pressure > 180 mm Hg), ischemic stroke in the preceding 3 months, uncontrolled bleeding, facial trauma in the preceding 3 months, and neurologic surgery in the preceding 2 months); (ii) cardiogenic shock; and (iii) cardiopulmonary resuscitation prior to arrival at the PPCI-capable hospital.
Endpoint
The primary endpoint is a net adverse clinical events (NACEs) composite of all-cause mortality, myocardial reinfarction, ischemia-driven target vessel revascularization, disabling stroke (Rankin score of 2 or higher), and major bleeding (Bleeding Academic Research Consortium [BARC] score of 3 or 5) at discharge from the index hospitalization at the “hub” hospital. Secondary endpoints included each single component of the NACE composite endpoint, cardiovascular mortality, intracranial hemorrhage, and severe non-intracranial hemorrhage (BARC score of 3a, 3b, or 5 not intracranial).
Statistical analysis
Study sample size
We used G∗Power version 3.1 software to estimate the sample size needed to achieve statistical significance at the P < 0.05 level with 80% power. We used data from the STREAM trial10 and the DANAMI-2 trial4 to estimate a NACE rate of 12.8% for the PPCI group, and 20.2% for the pharmacoinvasive group. The software calculation for the difference between 2 proportions estimated that 311 patients per group were needed to achieve statistical significance at the P < 0.05 level with 80% power.
Data analysis
Descriptive statistics were treated as follows: Categorical variables are presented as frequency and percentages, and comparison tests used the χ2 test, or the Fisher exact test if cells had fewer than 5 values, as appropriate. Continuous variables with a normal distribution are displayed as means and standard deviations and were compared using the Student t test. Finally, continuous variables with an abnormal distribution are exhibited as medians and ranges and were compared using the Mann-Whitney U test. A P-value of < 5% was considered statistically significant. For inferential statistics for primary and secondary endpoints, we used multivariable logistic regression tests, which were considered statistically significant if the P-value was < 5%. Data analysis was done using SPSS software, version 28 (IBM, Armonk, NY).
Results
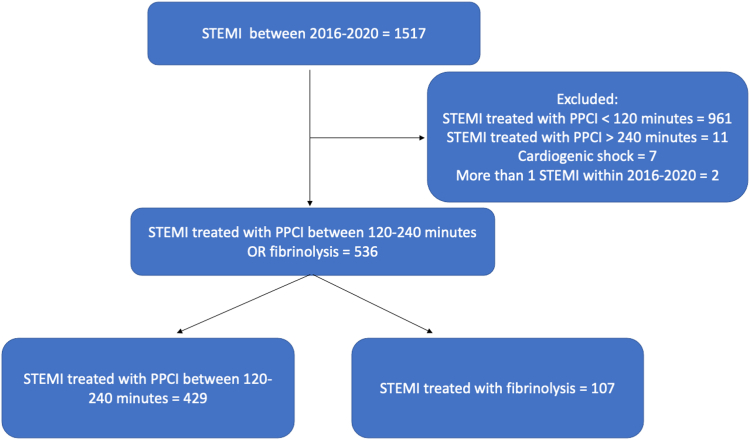
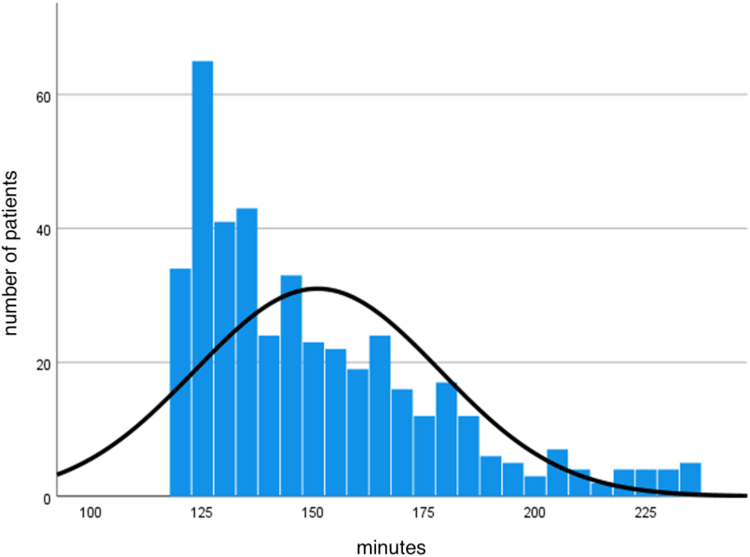
The inclusion criteria were met by 536 STEMI patients, and all were included in the analysis: 429 patients were treated with delayed PPCI, and 107 were treated with thrombolysis (Fig. 1). Baseline characteristics are presented in Table 1. Notably, PPCI patients were significantly older than the fibrinolysis patients (66 vs 62 years), and had more prior PCI (14.9% vs 7.5%), peripheral vascular disease (5.4% vs 0%), atrial fibrillation and/or flutter (7.7% vs 0.9%), and direct oral anticoagulant (DOAC) use (7.5% vs 0.9%) than the thrombolysis patients. The study population comprised 75% male patients (72.7% vs 75.7%), and was overweight, with a mean body mass index of about 28 kg/m2 (27.8 kg/m2 vs 28.1 kg/m2). The remainder of the demographic characteristics were similar for the 2 groups. Prior usage of aspirin was not significantly different between the 2 groups (19.6% vs 14.0%). The vast majority presented with a Killip 1 STEMI (85.3% vs 87.9%), and the right coronary artery (42.7% vs 44.1%) and left anterior descending artery (37.1% vs 40.2%) were 2 main culprit vessels. The median time from FMC to PPCI was 144 minutes. PPCI was achieved within 180 minutes from FMC in 85% of patients (Fig. 2). PCI with drug-eluting stent implantation was performed in 99.3% of patients in the PPCI group, and in 95.3% of the lytic group. Alternative diagnosis at discharge (such as pericarditis and stress cardiomyopathy) was more frequent in the thrombolysis group (0.7% vs 4.7%, P = 0.01).
Figure 1.
Flowchart of the study population. PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Table 1.
Demographic characteristics of primary percutaneous coronary intervention (PPCI) vs fibrinolysis
| Characteristic | PPCI (n = 429) | Fibrinolysis (n = 107) | P |
|---|---|---|---|
| Age, y | 65.7 ± 12.5 | 62.3 ± 12.9 | 0.013∗ |
| Sex, female | 117 (27.3) | 26 (24.3) | 0.5† |
| Body mass index, kg/m2 | 27.8 ± 5.2 | 28.1 ± 6.4 | 0.5∗ |
| Heart rate, beats/min | 75.2 ± 14.6 | 76.1 ± 16.3 | 0.6∗ |
| Systolic blood pressure, mm Hg | 124.9 ± 22.3 | 131.5 ± 23.8 | 0.008∗ |
| Hemoglobin, g/L | 136.7 ± 17.5 | 139.0 ± 16.0 | 0.2∗ |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 83.3 ± 21.3 | 83.1 ± 20.7 | 1.0∗ |
| Tobacco | 138 (32.2) | 39 (36.4) | 0.4† |
| Alcohol | 26 (6.1) | 5 (4.7) | 0.6† |
| Recreational drugs | 6 (1.4) | 3 (2.8) | 0.4‡ |
| Diabetes | 79 (18.4) | 12 (11.2) | 0.08† |
| Hypertension | 202 (47.1) | 49 (45.8) | 0.8† |
| Dyslipidemia | 181 (42.2) | 38 (35.5) | 0.2† |
| Prior left ventricular ejection fraction < 40% | 5 (1.2) | 0 (0.0) | 0.6‡ |
| Prior heart failure | 7 (1.6) | 0 (0.0) | 0.4‡ |
| Prior myocardial infarction | 50 (11.7) | 11 (10.3) | 0.7† |
| Prior percutaneous coronary intervention | 64 (14.9) | 8 (7.5) | 0.043† |
| Prior coronary artery bypass graft | 13 (3.0) | 2 (1.9) | 0.7‡ |
| Prior valve surgery | 1 (0.2) | 0 (0.0) | 1.0‡ |
| Prior implantable cardioverter defibrillator | 3 (0.7) | 1 (0.9) | 1.0‡ |
| Prior stroke | 15 (3.5) | 3 (2.8) | 1.0‡ |
| Peripheral vascular disease | 23 (5.4) | 0 (0.0) | 0.007‡ |
| Atrial fibrillation and/or flutter | 33 (7.7) | 1 (0.9) | 0.007‡ |
| Liver disease | 8 (1.9) | 0 (0.0) | 0.4‡ |
| Prior cancer | 31 (7.2) | 12 (11.2) | 0.2† |
| Coagulopathy | 3 (0.7) | 0 (0.0) | 1.0‡ |
| Prior transfusion | 0 (0.0) | 0 (0.0) | — |
| Prior hospitalization for bleeding | 6 (1.4) | 0 (0.0) | 0.6‡ |
| Prior aspirin use | 84 (19.6) | 15 (14.0) | 0.2† |
| Prior ticagrelor use | 8 (1.9) | 0 (0.0) | 0.4‡ |
| Prior clopidogrel use | 7 (1.6) | 0 (0.0) | 0.4‡ |
| Prior prasugrel use | 0 (0.0) | 0 (0.0) | — |
| Prior direct-acting oral anticoagulant use | 32 (7.5) | 1 (0.9) | 0.01‡ |
| Prior low-molecular-weight heparin use | 2 (0.5) | 0 (0.0) | 1.0‡ |
| Prior warfarin use | 2 (0.5) | 0 (0.0) | 1.0‡ |
| Killip class (1-2-3-4) | |||
| 1 | 366 (85.3) | 94 (87.9) | 0.8† |
| 2 | 41 (9.6) | 9 (8.4) | |
| 3 | 22 (5.1) | 4 (3.7) | |
| Culprit artery | |||
| Ramus artery | 4 (0.9) | 1 (1.0) | 0.9‡ |
| Circumflex artery | 77 (17.9) | 14 (13.7) | |
| Left anterior descending artery | 159 (37.1) | 41 (40.2) | |
| Left main artery | 2 (0.5) | 0 (0.0) | |
| Right coronary artery | 183 (42.7) | 45 (44.1) | |
| Saphenous veinous graft | 4 (0.9) | 1 (1.0) | |
| Alternative diagnosis at discharge | 3 (0.7) | 5 (4.7) | 0.01‡ |
Values are frequency (percentage) or mean ± standard deviation, unless otherwise indicated.
Student t test.
χ2test.
Fisher’s exact test.
Figure 2.
Distribution of time from first medical contact to device, for patients in the primary percutaneous coronary intervention group. For patients with no percutaneous coronary intervention, artery puncture time was used instead of device time.
The primary endpoint of NACEs was not significantly different between the 2 groups (2.8% vs 3.7%, P = 0.61), with a numerical absolute reduction of 0.9% in favour of the PPCI group (Table 2). All-cause mortality, cardiovascular mortality, myocardial infarction (MI), revascularization, major adverse cardiovascular events, and non-intracranial hemorrhage were not significantly different between the 2 groups. However, intracranial hemorrhage (0% vs 2.8%, P = 0.008), major bleeding (BARC score of 3 or 5; 0.9% vs 3.7%, P = 0.048), and disabling stroke (0.2% vs 2.8%, P = 0.03) significantly favoured the PPCI group. Rescue PCI occurred in 39.3% of patients in the fibrinolysis group.
Table 2.
Primary and secondary outcomes
| Outcome | PPCI (n = 429) | Fibrinolysis (n = 107) | Odds ratio (95% CI) | P |
|---|---|---|---|---|
| Primary | ||||
| NACE | 12 (2.8) | 4 (3.7) | 1.35 (0.43–4.27) | 0.6 |
| Secondary | ||||
| All-cause mortality | 4 (0.9) | 3 (2.8) | 3.06 (0.68–13.90) | 0.1 |
| Cardiovascular mortality | 4 (0.9) | 0 (0.0) | — | 0.6∗ |
| Myocardial infarction | 4 (0.9) | 0 (0.0) | — | 0.6∗ |
| Revascularization | 4 (0.9) | 0 (0.0) | — | 0.6∗ |
| Disabling stroke | 1 (0.2) | 3 (2.8) | 12.35 (1.27–119.90) | 0.03 |
| MACE | 8 (1.9) | 3 (2.8) | 1.52 (0.40–5.82) | 0.5 |
| Intracranial hemorrhage | 0 (0.0) | 3 (2.8) | — | 0.008∗ |
| Non-intracranial hemorrhage | 4 (0.9) | 1 (0.9) | 1.00 (0.11–9.06) | 1.0 |
| Bleeding (BARC score 3 or 5) | 4 (0.9) | 4 (3.7) | 4.13 (1.01–16.77) | 0.048 |
Values are n (%), unless otherwise indicated.
BARC, Bleeding Academic Research Consortium; CI, confidence interval; MACE, major adverse cardiovascular events; NACE, net adverse clinical event; PPCI, primary percutaneous coronary intervention.
Fisher’s exact test cannot be calculated when one group does not have any event.
Discussion
This real-life study comparing the outcomes of patients receiving delayed PPCI, with an FMC time to reperfusion of 120-240 minutes, compared to those receiving thrombolytic therapy, demonstrated the following: (i) no statistical difference occurred in the rate of NACEs between the 2 groups; (ii) the rate of intracranial hemorrhage, disabling stroke, and severe bleeding was significantly increased for patients receiving thrombolytic therapy; and (iii) the increase in (ii) occurred despite this group being younger, with fewer comorbidities and a lower bleeding risk.
Overall, the rate of NACEs was low in the 2 groups, which is appealing, given that STEMI patients who are not in cardiogenic shock upon arrival have a reasonably good prognosis, even though they fall outside the recommended 2-hour window for PPCI. A point worth noting is that 85% of the PPCI group had their culprit artery reopened within 180 minutes, consistent with the geographic location of 6 of our major referring centres. These centres have a 50-70 minute needed driving time for transfer, and over years, have had a median FMC to balloon time of 120-140 minutes. For over 40% of patients who received fibrinolysis, a right coronary artery was the culprit artery. Older lytic data suggested that the outcomes of isolated inferior MI were not improved with lytic therapy, compared to no reperfusion.
PPCI and fibrinolysis have both clearly demonstrated their own benefits, depending on the expected reperfusion delays. PPCI is beneficial when STEMI patients can have a time-to-device delay of less than 2 hours. On the other hand, fibrinolysis is valuable for patients who clearly fall outside the 2-hour reperfusion window because they live further away and longer transportation times are expected. The optimal treatment for STEMI patients who fall just outside the 2-hour window is less clear. Hence, the cutoff time at which the fibrinolysis benefit exceeds that of PPCI is difficult to know with certainty, and guidelines have used extrapolation of data to determine the cutoff of 120 minutes. In the recent past, 2 randomized trials have failed to demonstrate a benefit of lytic therapy over PPCI. One of them was the combination of using fibrinolysis while in transit to a PCI-capable centre—known as facilitated fibrinolysis. Unfortunately, the Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) trial resulted in more harm with the facilitated fibrinolysis approach than with PPCI, and the trial was ended abruptly.11 Following this, the STREAM trial showed that patients treated with fibrinolysis within 3 hours of symptom onset, followed by PCI, for whom access to PPCI was not possible within 60 minutes had similar clinical outcomes than those receiving PPCI, at the cost of an increase in intracranial hemorrhage (1% vs 0.2%).10 After the amendment of the protocol to exclude full-dose fibrinolysis to patients aged > 75 years, this increase in intracranial bleeding was negated. This strategy was further evaluated in the Strategic Reperfusion in Elderly Patients Early After Myocardial Infarction (STREAM-2) trial,12 which compared half-dose lytics in patients aged ≥ 60 years to PPCI outside the 60-minute window. Intracranial hemorrhage was seen in 1.5% of patients treated with lytics and none of those treated with PPCI.
The results of our study mirror what has been found in the STREAM trials—lytics provide no benefit in terms of mortality, with an increase in life-threatening bleeding. The major difference between our study and the STREAM trial is that our reperfusion times were even longer than those in the STREAM trial for PPCI. Given that > 85% of our study population was treated within 180 minutes of FMC, our observation may not provide good representation of those with longer delays. With these data, we believe that an aggressive strategy of usage of thrombolytic therapy for most patients with an estimated reperfusion time of 2-3 hours with PPCI should be discouraged. The decision to go ahead with fibrinolysis should not be made lightly, especially for patients who have an equivocal electrocardiogram (ECG), who could be facing catastrophic repercussions. Regardless of the risks of fibrinolysis, referring physicians must estimate efficiently the expected delay in time-to-device before electing to go with PPCI or fibrinolysis, to yield the best possible outcome for their patients. This estimation is dependent on multiple variables, such as medication availability, intravenous access, weather, traffic, and ambulance availability. The variability in transfer times is inevitable. Nevertheless, results from this study should reassure physicians in “spoke” centres that redirecting an ambulance or transferring a STEMI patient to a “hub” centre within a reasonable distance for PPCI is not deleterious, even if the arbitrary 2-hour reperfusion window has recently expired.
Most, if not all, STEMI patients who are treated with thrombolysis will inevitably need a coronary angiogram and hence will be transferred to a “hub” centre that has more expertise and resources to treat STEMI patients and their inherent complications. At our institution, all patients are shipped within minutes after their thrombolytic treatment. Moreover, 39.3% of fibrinolysis patients required a rescue PCI, and valuable time was lost by delivering fibrinolysis rather than transferring them rapidly for PPCI.
By favouring a more liberal approach to delayed PPCI, we also negate the risk of bleeding associated with fibrinolysis when patients have false-positive ECGs. In our study, 5% of patients treated with lytics ended up having an alternative diagnosis. The risk associated with a normal angiogram is far less than that associated with receiving fibrinolysis, especially when the patients does not have a true-positive ECG. Additionally, physicians at “spoke” centers often reach out to the “hub” centres within their network to review the clinical care, including investigations, and the criteria for fibrinolysis administration in cases that may have challenging ECGs or equivocal clinical criteria. This process sometimes takes time that could be avoided by initiating an early transfer to a “hub” centre, where a decision could be made upon arrival. A liberal approach to delayed PPCI brings these patients to an experienced physician who has the best knowledge to treat them. The delay associated with PPCI could allow results from blood drawn at the “spoke” hospital to become available before arrival for PPCI, and help the overall care of patients by creating a clearer picture of their kidney, liver, and coagulation functions.
Our study has some limitations. The study is retrospective, was conducted at a single centre, and patients were identified from a database. Our study is underpowered because of a lower-than-expected number of patients in the lytic group. Patients were significantly different between groups, and the low number of events prevented a propensity-score analysis. However, NACEs were more common in the lower-risk group, and an adjustment most likely would have favoured the PPCI group. Our endpoint was limited to the index hospitalization at the “hub” hospital. Although our practice is not to send unstable patients to their referring hospitals, events before home discharge could have been missed in our study. Readmission for heart failure, an endpoint that may be related to faster reperfusion, was not evaluated in our study. Also, FMC to thrombolysis delays were not collected and could have impacted clinical outcomes. However, such delay is unlikely to be related to major or intracranial hemorrhage. These single-centre observations may not be generalizable.
Conclusion
In this real-life retrospective study of STEMI patients with expected PPCI delay of 120-240 minutes from FMC, no significant difference occurred in the rate of NACEs between the PPCI and the fibrinolysis groups. However, the risk of intracranial hemorrhage, disabling stroke, and major bleeding was significantly higher in the fibrinolysis group. The results of this study are in line with those of the STREAM trial10 and support a strategy favouring PPCI over fibrinolysis for patients who have an expected time from FMC to mechanical reperfusion of < 240 minutes. These observations should be validated in larger studies.
Acknowledgements
The authors acknowledge Dre Pier-Anne Gaulin and Lysianne Letarte, for data collection, and Catherine Allard and Samuel Lemaire-Paquette, for statistical analysis.
Ethics Statement
The study was approved by our local institutional review board.
Patient Consent
This is a retrospective study using de-identified data; therefore, the IRB did not require consent from patients.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 637 for disclosure information.
References
- 1.Scholz K.H., Maier S.K.G., Maier L.S., et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. 2018;39:1065–1074. doi: 10.1093/eurheartj/ehy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fath-Ordoubadi F., Huehns T.Y., Al-Mohammad A., Beatt K.J. Significance of the thrombolysis in myocardial infarction scoring system in assessing infarct-related artery reperfusion and mortality rates after acute myocardial infarction. Am Heart J. 1997;134:62–68. doi: 10.1016/s0002-8703(97)70107-8. [DOI] [PubMed] [Google Scholar]
- 3.Boden W.E., Eagle K., Granger C.B. Reperfusion strategies in acute ST-segment elevation myocardial infarction: a comprehensive review of contemporary management options. J Am Coll Cardiol. 2007;50:917–929. doi: 10.1016/j.jacc.2007.04.084. [DOI] [PubMed] [Google Scholar]
- 4.Andersen H.R., Nielsen T.T., Rasmussen K., et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Eng J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 5.Wong G.C., Welsford M., Ainsworth C., et al. Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology guidelines on the acute management of ST-elevation myocardial infarction: focused update on regionalization and reperfusion. Can J Cardiol. 2019;35:107–132. doi: 10.1016/j.cjca.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Van De Werf F., Adgey J., Ardissino D., et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–722. doi: 10.1016/s0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 7.Widimský P., Budešínský T., Voráč D., et al. Long distance transport for primary angioplasty vs. immediate thrombolysis in acute myocardial infarction: final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J. 2003;24:94–104. doi: 10.1016/s0195-668x(02)00468-2. [DOI] [PubMed] [Google Scholar]
- 8.O’Gara P.T., Kushner F.G., Ascheim D.D., et al. ACC/AHA guideline for the management of ST-elevation myocardial infarction: executive summary—a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 9.Ibánez B., James S., Agewall S., et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol. 2017;70:1082. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong P.W., Gershlick A.H., Goldstein P., et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Eng J Med. 2013;368:1279–1287. doi: 10.1056/NEJMoa1301092. [DOI] [PubMed] [Google Scholar]
- 11.Stone G.W., Gersh B.J. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomized trial. Lancet. 2006;367:569–578. doi: 10.1016/S0140-6736(06)68147-6. [DOI] [PubMed] [Google Scholar]
- 12.Van de Werf F., Ristic A.D., Averkov O.V., et al. STREAM-2: half-dose tenecteplase or primary percutaneous coronary intervention in older patients with ST-segment myocardial infarction: a randomized, open-label trial. Circulation. 2023;148:753–764. doi: 10.1161/CIRCULATIONAHA.123.064521. [DOI] [PubMed] [Google Scholar]