Abstract
Introduction
Currently, age-related hearing loss has become prevalent, awareness and screening rates remain dismally low. Duing to several barriers, as time, personnel training and equipment costs, available hearing screening tools do not adequately meet the need for large-scale hearing detection in community-dwelling older adults. Therefore, an accurate, convenient, and inexpensive hearing screening tool is needed to detect hearing loss, intervene early and reduce the negative consequences and burden of untreated hearing loss on individuals, families and society.
Objectives
The study harnessed "medical big data" and "intelligent medical management" to develop a multi-dimensional screening tool of age-related hearing loss based on WeChat platform.
Methods
The assessment of risk factors was carried out by cross-sectional survey, logistic regression model and receiver operating characteristic (ROC) curve analysis. Combining risk factor assessment, Hearing handicap inventory for the elderly screening version and analog audiometry, the screening software was been developed by JavaScript language and been evaluated and verified.
Results
A total of 401 older adults were included in the cross-sectional study. Logistic regression model (univariate, multivariate) and reference to literature mention rate of risk factors, 18 variables (male, overweight/obesity, living alone, widowed/divorced, history of noise, family history of deafness, non-light diet, no exercising habit, smoking, drinking, headset wearer habit, hypertension, diabetes, hyperlipidemia, cardiovascular and cerebrovascular diseases, hyperuricemia, hypothyroidism, history of ototoxic drug use) were defined as risk factors. The area under the ROC curve (AUC) of the cumulative score of risk factors for early prediction of age-related hearing loss was 0.777 [95% CI (0.721, 0.833)]. The cumulative score threshold of risk factors was defined as 4, to classify the older adults into low-risk (< 4) and high-risk (≥ 4) hearing loss groups. The sensitivity, specificity, positive predictive value, and negative predictive value of the screen tool were 100%, 65.5%, 71.8%, and 100.0%, respectively. The Kappa index was 0.6.
Conclusions
The screening software enabled the closed loop management of real-time data transmission, early warning, management, whole process supervision of the hearing loss and improve self-health belief in it. The software has huge prospects for application as a screening approach for age-related hearing loss.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00405-023-08389-9.
Keywords: Age-related hearing loss, Screening software, Community, General practitioner, Loop management
Introduction
Age-related hearing loss
Age-related hearing loss (ARHL) is defined as hearing loss caused by aging and degeneration of auditory organs. The main risk factors include aging, environmental noise, smoking, drinking, genetic susceptibility, diabetes, hypertension, hyperuricemia, hyperlipidemia, hypothyroidism, ototoxic drugs, ear inflammation, etc. Although the pathogenesis of ARHL remains unclear it is widely thought to result from the joint action of multiple physiological mechanisms [1–3].
During early stages, the person's symptoms are binaural symmetrical high-frequency hearing loss, which can be accompanied by tinnitus in some cases, resulting in the decline of auditory recognition ability. However, the early subjective sensory speech recognition ability can meet daily communication needs, and the symptoms are easily ignored.
Current evidence suggests that hearing loss affects 33%, 45% and 63.1% of persons over 50, 60 [4]and 70 [5], respectively. Old adults with hearing loss are at significantly greater risk of incident dementia [5–7], falls [8, 9], depression [10, 11], social isolation [12], and loss of independence [13]. The latest projections suggest that hearing loss will be the 9th leading contributor to the global burden of disease worldwide in 2030 [14]. The medical costs of hearing loss range from $3.3 to $12.8 billion in the United States [15, 16] and $11.75 billion in Australia [17]. The serious adverse consequences of ARHL are often largely underestimated by society, older adults, healthcare professionals, and superintendents [18, 19]. It has been established that treating hearing loss may effectively reduce the adverse consequences. Nevertheless, the key to early treatment is identifying individuals with hearing loss as soon as possible [20, 21].
Screening of age-related hearing loss
The United States Centers for Disease Control and Prevention (CDC) reported that 97% of newborn babies underwent hearing screening in 2013. In China, the rate of newborn hearing screening reached more than 60% in 2021, and the completion rate in Shanghai was 100%. However, the screening of ARHL is still in its infancy, and there is no unified view of the data [22–26].
Globally, compared with developed countries (10–40%), less than 1% of hearing loss patients in developing countries receive hearing aid treatment. Although improvements have been made to treating hearing loss, the acceptance and use rates of hearing aids have not increased significantly in the past 50 years [27]. More than 95% of persons who can benefit from hearing aids do not use hearing aids. Currently, the cognition and willingness to seek the help of older adults are very low. Little emphasis has hitherto been placed on ARHL screening, with no effective referral management means available.
One of the gold standard for assessing hearing loss is pure tone audiometry (PTA); however, this method is not feasible for large-scale, population-based epidemiological screening projects, because it requires high-cost audiological equipment and trained specialists [28]. The other screening methods of ARHL include the subjective faces scale [29], whispering experiment 31, hearing handicap inventory for the elderly (HHIE) [31], and screening for ontological functional impairments (SOFI) [32]. It has been shown that despite the advent of screening tools for hearing loss, they exhibit limitations in sensitivity, specificity, positive predictive value, and negative predictive value [24]. Screening of hearing loss should be weighed according to the sensitivity, specificity, technological requirements, per capita labor cost and ease of operation to improve the effectiveness of screening and reduce ineffective referral.
Intelligent medicine and chronic disease
Smart medicine originated from the concept of smart earth and was put forward by Peng Mingsheng of IBM (International Business Machines Corporation) in the United States in November 2008. This project aimed to achieve medical information interconnection, medical technological innovation, scientific evidence-based diagnosis and big data of public health prevention and management through the Internet of Things technology from the perspective of information technology [33].
Intelligent medicine represents a driving force for innovation in treating incurable diseases. With the emergence of various wearable medical devices, we can timely obtain the information indicators of various monitoring data fed back by medical devices to prevent and control the development of diseases. The application of big data and artificial intelligence enables us to obtain comprehensive information, understand medical records and even the details of medical processes, and harness high-speed logical operation to process and analyze problems in medical treatment and corresponding management. With the emergence of smartphones, various mobile health applications have been developed, providing strong help for managing chronic disease and improving health-related status [34].
A systematic review and meta-analysis on the intervention effect of hypertensive patients based on smartphone applications, which included eight studies involving 1657 subjects, suggested that smartphone program intervention could reduce the blood pressure of hypertensive patients and increase drug compliance [26]. A randomized controlled feasibility study showed that mHealth could help persons with COPD self-manage their physical activity levels [35].
The occurrence of ARHL is related to many behavioral factors and disease factors. Although the primary prevention of ARHL cannot be accurately implemented, we can refer to the management policy of chronic diseases to implement secondary prevention and tertiary prevention.
Materials and methods
Assessment of risk factors of ARHL
Participants
Five community health centers in Pudong New Area, Shanghai [urban (n = 3), urban–rural fringe (n = 1) and suburban (n = 1)] were selected by the stratified sampling method. Older adults who participated in the annual physical examination (National public health service project) in these community health service centers from January to December 2019 were included in the present study.
Data collection
1) General information:
The participants were asked to complete a questionnaire, including the following contents:
Socio-demographic variables (age, gender, residential area, whether living alone,, marital status, education background), lifestyle habits (history of smoking, history of drinking, exercising habit, diet, headphone-wearing), physiological variables (weight, height, BMI, blood pressure), history of chronic disease (hypertension, diabetes, cardiovascular disease, hyperlipidemia, hyperuricemia, history of chronic otitis media, history of noise exposure, family history of deaf, history of ototoxic drug use).
2) Sample size calculation:
According to Kendall's sample estimation method, the number of observations is at least 10 times the number of variables. The indicators involved in this project include 7 sociodemographic statistical variables, 5 physiological factor indicators, and 14 hearing assessments, totaling 26 statistical analysis variables. Approximately 260 older adults, considering invalid questionnaires and no response rates, assuming an effective sample recovery rate of 70%, the minimum sample size is 371.
3) Hearing handicap inventory for the elderly screening version (HHIE-s):
HHIE-s consists of 10 items involving 5 emotional and 5 situational problems with three options: yes, sometimes, never. The corresponding scores are 4, 2 and 0, respectively. The lowest score is 0, and the highest score is 40. The scale was completed within 5 min, with higher scores associated with more serious hearing loss. According to the American Speech–Language–Hearing Association hearing screening guidelines, 0–8, 10–22, and 24–40 points correspond to no obvious, mild to moderate, and severe hearing impairment, respectively [3].
4) Audiometric assessment:
Puretone audiometry (PTA) is a commonly used method for hearing monitoring. Audiometric pure tone testing was administered by two trained audiologists. The Madsen audiometer was used in the room, where the indoor noise was controlled within 40 dB. Air conduction data were conducted, and the average value of 0.5 kHz, 1 kHz, 2 kHz and 4 kHz pure tone air conduction threshold of the better ear was taken as audiometric results. ≤ 25 dB/HL, 26–40 dB/HL, 41–55 dB/HL, 56–70 dB/HL,71–90 dB/HL and ≥ 91 dB/HL correspond to normal, mild hearing loss, moderate hearing loss, moderately severe hearing loss, severe hearing loss and profound hearing loss, respectively [20, 36, 37].
5) Assessment of risk factors:
Based on the cross-sectional data, using conditional logic equations, assign values of 1 or 0 to whether they have risk factors, and obtain the cumulative scores. Using pure-tone audiometry as the reference, the cutoff value of the count of risk factors for predicting hearing loss was obtained by the receiver operating characteristic curve (ROC) analysis.
Analog audiometry
In the study by Min Zhang 2019 [18], using the two tones (2 kHz and 0.5 kHz), which were selected by the decision tree analysis was applied to screen hearing loss in a community-based geriatric population. First, the better ear was tested with 42 dB/HL audio of 2 kHz twice. If there was no perception of the stimulus sound or it was only heard once, it was directly judged as moderate or above hearing loss. If it was heard twice, we further tested with the 47 dB/HL audio of 0.5 kHz twice. If heard twice, it was judged as normal or mild hearing loss. If there was no perception of the stimulus sound or it was only heard once, it was judged as moderate or above hearing loss. The other ear was tested similarly. Normal and mild hearing loss were judged as "passed", and moderate and above hearing loss were judged as "failed". Accuracy, sensitivity and specificity of a simple two-step screening procedure was 91.20%, 95.35% and 86.85%.
According to the above procedure, the study adopted two professional analog audio sources (2 kHz 42 dB/HL and 0.5 kHz 47 dB/HL), and set up simulated audiometry in the screening software. The testing environment was supposed to be in a relatively quiet environment with maximum volume of the phone.
Screening software of ARHL
Design of screening software
From January to June 2020, screening software for hearing loss was developed on the WeChat platform and was written in JavaScript. The software collected the basic data of older adults via questionnaires. The three-step evaluation was as follows: assessment of risk factors, HHIE-s and analog audiometry, and output of the corresponding conclusions and suggestions.
Suitability evaluation of screening software
From February to May 2021,106 general practitioners from the community health centers were invited to evaluate the suitability of the procedure through an online questionnaire. The Likert scale questionnaire included the following responses: full agreement, basic agreement, general agreement, little agreement, and disagreement, corresponding to 5, 4, 3, 2 and 1 points, respectively. The contents include: basic information: age, gender, educational background, professional title and working years. Compulsory questions: ① are you willing to use the screening software for hearing loss? ② Do you think the structure of screening software is reasonable? ③ Do you think the screening software is suitable for older adults? ④ Do you think the screening software will help to raise the attention of older adults? ⑤ Do you think the screening software can assist in managing ARHL in community health service centers? ⑥ Will you use the screening software for older adults? ⑦ Do you think there are defects in the screening software? ⑧ Do you think the screening software will increase the work burden? Questions 1 to 6 are forward-scoring, while questions 7 and 8 are reverse-scoring.
Verification of the performance of the screening software of ARHL
From July 2020 to January 2021, 135 older adults from the community were recruited by general practitioners for pre-testing of screening software.
Statistical analysis
EpiData 3.0 was used to input data, and SPSS 25.0 software was used for statistical analysis. The description of normal distribution data was represented by X ± s and compared by T test. The description of non-normal distribution data was represented by M (P25, P75) and compared by Mann–Whitney U test. Categorical data were represented by frequency and rate. The Chi-square test was used to compare non-ordinal categorical variables, and the rank sum test was used to compare ordinal categorical variables. The related factors were analyzed by Pearson, Spearman, and Logistic regression model. P value < 0.05 was statistically significant.
Results
Assessment of risk factors of ARHL
1.1 Basic information of participants: a total of 401 older adults were enrolled in the cross-sectional study, including 182 (38.4%) males, 219 (54.6%) females, average age (71.0 ± 6.1) years. Take PTA detection as the gold indicator, The prevalence of hearing loss in males was 84.9%, while in females was 78.8%, with no statistically significant difference (χ2 = 2.691, P = 0.101). The constituent ratios of mild, moderate, moderately severe, and profound hearing loss were 48.5%, 23.4%, 7.8%, 1.3% and 0.7%, respectively. (Table 1).
Table 1.
Basic information of participants
| Variables | n (%) |
|---|---|
| Socio-demographic | |
| Age | 71.0 ± 6.1 |
| Gender | |
| Male | 182 (45.4%) |
| Female | 219 (54.6%) |
| Residential area | |
| Urban | 231(57.6%) |
| Urban–rural | 83(20.7%) |
| Rural | 87(21.7%) |
| Whether living alone | |
| Living alone | 30(7.5%) |
| Non-living alone | 371(92.5%) |
| Marital status | |
| Divorced | 6(1.5%) |
| Widowed | 36(9.0%) |
| Married | 359(89.5%) |
| Education level | |
| Primary school and below | 70(17.5%) |
| Secondary school | 288(71.8%) |
| Bachelor's degree or above | 42(10.5%) |
| Lifestyle | |
| History of smoking | |
| Smoking | 71(17.7%) |
| No-smoking | 330(82.3%) |
| History of drinking | |
| Drinking | 41(10.2%) |
| Non-drinking | 360(89.9%) |
| Exercising habit | |
| Have exercising habit | 209 ( 51.9%) |
| No exercising habit | 192 ( 48.1%) |
| Diet | |
| Light diet | 289 ( 72.1%) |
| Non-light diet | 112 (27.9%) |
| Headphone-wearing habit | 29 (7.2%) |
| Chronic otitis media | |
| Physiological | |
| Height(cm, x ) | 162.1 11.1 |
| Weight(kg, x ) | 64.1 10.1 |
| BMI(kg/m2, x ) | 24.45 8.9 |
| Overweight/obese | |
| Blood pressure | |
| HBP (mmHg, x | 136.4 17.4 |
| SBP (mmHg, x ) | 79.8 10.1 |
| History of chronic disease | |
| Hypertension | 258 (64.3%) |
| Diabetes | 123 (30.7%) |
| Cardiovascular disease | 98 (24.4%) |
| Hyperlipidemia | 37 (9.2%) |
| Hyperuricemia | 68 (17.0%) |
| History of chronic otitis media | 1 (0.2%) |
| Hearing loss (score > 25 dB/HL) | 320 (79.8%) |
| HHIE-s (score > 8) | 232(57.9%) |
Logistic regression model
With the hearing loss of older adults as the dependent variable and the 22 remaining variables as the independent variable, binary logistic regression analysis was carried out (the values of the variables were as follows: gender variables were female = 1, male = 2; age and BMI were continuous variables, other independent variables and dependent variables were assigned no = 0, yes = 1). The results of univariate logistic regression analysis showed that age, BMI, overweight/obesity, history of noise exposure, non-light diet, no exercising habit, hypertension, diabetes, hyperlipidemia, cardiovascular and cerebrovascular diseases, hyperuricemia, and hypothyroidism were significant factors affecting hearing loss in older adults (P < 0.05).
The univariate logistic regression model showed that age, overweight, widowed/divorced, history of noise exposure, non-light diet, no exercising habit, hypertension, diabetes mellitus, cardiovascular disease, hyperuricemia, and hypothyroidism could significantly promote ARHL (Table 2).
Table 2.
Univariate logistic regression model
| Variable | β | SE | Wald X2 | P valve | Exp(B) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Age | 0.116 | 0.027 | 17.965 | 0.000 | 1.123 | 1.064 | 1.185 |
| Sex | 0.365 | 0.255 | 2.061 | 0.151 | 1.441 | 0.875 | 2.373 |
| BMI | 0.118 | 0.042 | 7.927 | 0.005 | 1.125 | 1.037 | 1.222 |
| Overweight/obesity | 0.551 | 0.259 | 4.516 | 0.034 | 1.734 | 1.044 | 2.881 |
| Living alone | 0.532 | 0.552 | 0.929 | 0.335 | 1.702 | 0.577 | 5.024 |
| Widowed/divorced | -0.923 | 0.542 | 2.902 | 0.088 | 0.397* | 0.137 | 1.149 |
| Educational background of primary school and below | 0.278 | 0.357 | 0.609 | 0.435 | 1.321 | 0.657 | 2.657 |
| History of noise exposure | 1.368 | 0.611 | 5.011 | 0.025 | 3.928 | 1.186 | 13.015 |
| Family history of deafness | 0.839 | 1.062 | 0.625 | 0.429 | 2.315 | 0.289 | 18.541 |
| Non-light diet | -1.201 | 0.358 | 11.219 | 0.001 | 0.301 | 0.149 | 0.608 |
| No exercising habit | -1.498 | -0.332 | 20.300 | 0.000 | 0.301 | 0.149 | 0.608 |
| Smoking | 0.262 | 0.344 | 0.580 | 0.446 | 0.769 | 0.392 | 1.511 |
| Drinking | 0.656 | 0.494 | 1.760 | 0.185 | 1.927 | 0.731 | 1.538 |
| Headset wearer | 19.924 | 7463.606 | 0.000 | 0.998 | 449,668,255.2 | - | - |
| History of chronic otitis media | 19.541 | 40,192.970 | 0.000 | 1.000 | 410,198,941.3 | - | - |
| Hypertension | 1.237 | 0.257 | 23.144 | 0.000 | 3.445 | 2.081 | 5.702 |
| Diabetes | 1.794 | 0.412 | 18.936 | 0.000 | 6.001 | 2.680 | 13.484 |
| Hyperlipidemia | 0.963 | 0.290 | 11.021 | 0.001 | 2.620 | 1.484 | 4.627 |
| Cardiovascular disease | 1.273 | 0.393 | 10.508 | 0.001 | 3.571 | 1.654 | 7.708 |
| Hyperuricemia | 1.571 | 0.532 | 8.738 | 0.003 | 4.812 | 1.698 | 13.640 |
| Hypothyroidism | 2.317 | 1.022 | 5.141 | 0.023 | 10.141 | 1.369 | 75.113 |
| History of ototoxic drug use | 19.845 | 17,974.837 | 0.000 | 0.999 | 415,407,816.7 | - | - |
1) Overweight/obesity is defined as BMI ≥ 24.0 kg/m2; History of noise is defined as continuous operation > 1 year in an environment > 85 dB; Smoking is defined as smoking > 1 piece/day for 6 consecutive or cumulative months; Drinking is defined as drinking more than once/week, continuous or cumulative 12 months; Exercise habit is defined as 0.5 h/time and > 3 times/week; headset wearer habit is defined as wearing > 3 days/week, continuous or cumulative 12 months
2) "-" indicates that there are too few positive cases, resulting in extreme OR value, so 95% CI is not displayed
Continue incorporating the variables (P < 0.2) from the univariate logistic regression model into the multivariate logistic model. It was found that age growth [OR = 1.100, 95% CI (1.037, 1.166)], history of noise exposure [OR = 3.886, 95% CI (1.077, 14.022)], non-light diet [OR = 2.445, 95% CI (1.127, 5.305)], hypertension [OR = 1.839, 95% CI (1.015, 3.330)], diabetes [OR = 4.310, 95% CI (1.817, 10.225)], and hyperuricemia [OR = 3.174, 95% CI (1.030, 9.779)] were independent risk factors for hearing loss in older adults (P < 0.05) (Table 3).
Table 3.
Multivariate logistic regression model
| Variable | β | SE | Wald X2 | P valve | Exp(B) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Age | 0.104 | 0.031 | 11.221 | 0.001 | 1.100 | 1.037 | 1.166 |
| Sex | − 0.056 | 0.322 | 0.030 | 0.863 | 0.946 | 0.503 | 1.779 |
| BMI | 0.059 | 0.075 | 0.609 | 0.435 | 1.060 | 0.915 | 1.228 |
| Overweight/obesity | − 0.081 | 0.468 | 0.030 | 0.836 | 0.922 | 0.368 | 2.310 |
| Widowed/divorced | 0.148 | 0.712 | 0.043 | 0.836 | 1.159 | 0.287 | 4.681 |
| History of noise | 1.357 | 0.655 | 4.298 | 0.038 | 3.886 | 1.077 | 14.022 |
| Non-light diet | 0.894 | 0.395 | 5.114 | 0.024 | 2.445 | 1..127 | 5.305 |
| No exercise habit | − 0.385 | -0.403 | 0.915 | 0.339 | 0.680 | 0.309 | 1.498 |
| Drinking | 0.741 | 0.635 | 1.359 | 0.244 | 2.098 | 0.604 | 7.287 |
| Hypertension | 0.609 | 0.303 | 4.038 | 0.044 | 1.839 | 1.015 | 3.330 |
| Diabetes | 1.461 | 0.441 | 10.985 | 0.001 | 4.310 | 1.817 | 10.225 |
| Hyperlipidemia | 0.246 | 0.355 | 0.479 | 0.489 | 1.278 | 0.637 | 2.564 |
| Cardiovascular disease | 0.362 | 0.463 | 0.613 | 0.434 | 1.437 | 0.580 | 3.558 |
| Hyperuricemia | 1.155 | 0.574 | 4.045 | 0.044 | 3.174 | 1.030 | 9.779 |
| Hypothyroidism | 1.645 | 1.066 | 2.379 | 0.123 | 5.181 | 0.641 | 41.897 |
Hierarchical evaluation system of risk factors of ARHL
According to the results of the logistic regression model, variables as non-light diet, hypertension, diabetes, hyperlipidemia, and history of noise exposure were defined as risk factor of age-related hearing loss. The univariate analysis tipped the overweight/obesity, living alone, divorce/widowhood, non-exercise habit, family history of deafness, smoking history, drinking history, hypothyroidism, hyperuricemia history of wearing headphones, history of ototoxic drug was with statistical significance. In combination with the mention rate of risk factors in literature and expert recommendations [21, 22, 27, 29] the above variables were also defined as risk factors. Population surveys [21, 22, 27, 29] suggested that the incidence of hearing loss in male was higher than female. In the study, there was sex difference in the incidence rate, with no statistical significance. Considering possible selection bias, male was included in the risk factors. Participants of the study were older adults, so age was not included in the risk factors. Finally, 18 risk factors were included in the list.
The conditional logic equation was used to calculate the cumulative score of risk factors. Receiver operating characteristic curve (ROC) analysis was conducted to assess the predictive value of the cumulative score of risk factors on hearing loss in older adults was obtained. The results showed the AUC (area under the ROC curve) was 0.777 [95% CI (0.721, 0.833)], the maximum Youden index was 0.534, and the optimal cutoff value was 3.5. The sensitivity and specificity were 70.9% and 75.3%, respectively. The threshold of the cumulative score of risk factors was 4, and used to classify older adults into low-risk (< 4) and high-risk (≥ 4) groups. (Fig. 1).
Fig. 1.
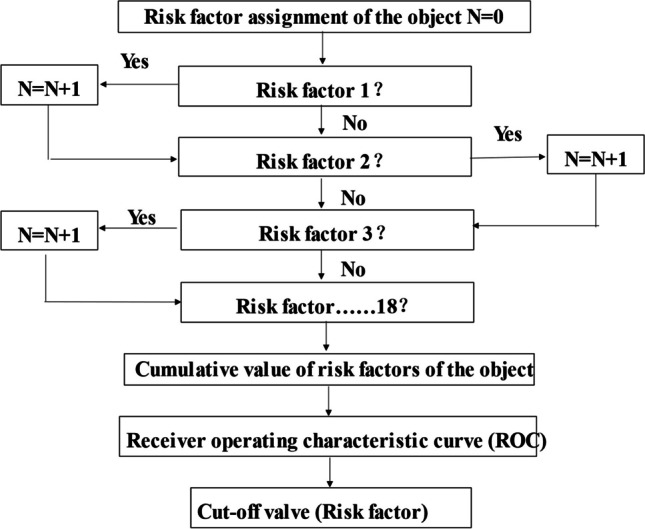
Hierarchical assessment system of risk factors of age-related hearing loss
Screening software of ARHL
Basic structure
The screening software was written in JavaScript language. The structure included three layers: foundation, display, and processing. The software provides dual-port management. Older adults used the WeChat platform terminal, and general practitioners used computer terminals. Older adults in the community could conduct dynamic self-assessments without geographical, time and space restrictions. The screening data could be updated in real time and synchronously to ensure that general practitioners could track and follow-up from time to time by the computer terminal.
Suitability setting
Each subject's WeChat login has a unique ID code, and the assessment could be repeated, suggesting it is suitable as a follow-up tool.
The program interface uses large font and simplified expression, suitable for older adults.
The program is easy to operate and does not occupy the mobile phone's storage space.
All questions were single-choice ones. The language was concise with no ambiguity, convenient for older adults to operate and provided accurate feedback for data collection.
The two-step analog audiometry adopts the standardized analog audio recognized in the industry and uses the measurement of maximizing the volume of the external amplifier. The requirements for listening equipment and listening environment are relatively low.
Five sections
1) Assessment of risk factors:
A cumulative score of risk factors was obtained from the questionnaire and divided testers into high-risk or low-risk groups.
2) HHIE-s:
Testers were divided into normal hearing and hearing loss groups.
3) Two-step analog audiometry:
The analog audio was played through the mobile phone terminal using the two tones (2 kHz 42 dB/HL and 0.5 kHz 47 dB/HL) selected by decision tree analysis. Normal and mild hearing loss were judged as "passed", and moderate and above hearing loss were judged as "failed" [18].
4) Conclusion
If the risk factor is assessed as low risk, the HHIE-s score is 0–8, and the two-step pure tone audiometry result was normal, the following suggestion would be given: conduct a hearing loss screening software self-assessment every 6 months.
If the risk factor is assessed as high risk, the score of HHIE-s was ≥ 10, and the two-step pure tone audiometry result was abnormal, the following suggestion would be given: conduct a hearing loss screening software self-assessment every 3 months.
5) Health education:
Participants received health education to prevent hearing loss and were regularly updated on educational programs on hearing loss.
Suitability evaluation of screening software of ARHL
1) Basic information:
106 general practitioners from community health centers had participated in the questionnaire, and the basic information as follows: male 30 (28.7%), average age 38.35 ± 7.98, graduate degree or above 10(9.4%), over 10 of working years 71 (57.7%), intermediate and above professional titles 81 (75.5%). The response rate of participants to the questionnaire was 100% (supplemental Table 1).
2) Questionnaire of suitability evaluation:
The score distribution suggests that general practitioners approved the structure, convenience, and value of the hearing loss screening software (Table 4).
Table 4.
Questionnaire for suitability evaluation of screening software of ARHL
| Score | Select percentage n (%) |
|
|---|---|---|
| Are you willing to use the screening software for age-related hearing loss? | 4 | 15 (14.2) |
| 5 | 90 (857) | |
| Do you think the structure of screening software is reasonable? | 3 | 1 (1.0) |
| 4 | 19 (18.1) | |
| 5 | 85 (81.0) | |
| Do you think the screening software is suitable for older adults? | 4 | 29 (27.6) |
| 5 | 76 (72.4) | |
| Do you think the hearing loss screening software will help raise the attention of older adults? | 3 | 9 (8.6) |
| 4 | 24 (22.9) | |
| 5 | 72 (68.6) | |
| Do you think the hearing loss screening software can assist in the management of age-related hearing loss? | 3 | 8 (7.6) |
| 4 | 26 (24.8) | |
| 5 | 71 (67.7) | |
| Will you use the hearing loss screening software for older adults? | 3 | 10 (9.5) |
| 4 | 33 (31.4) | |
| 5 | 62 (59.0) | |
| Do you think there are defects in the screening software? * | 3 | 17 (16.2) |
| 4 | 29 (27.7) | |
| 5 | 59 (56.2) | |
| Do you think using screening software will increase the work burden? * | 3 | 15 (14.3) |
| 4 | 28 (26.7) | |
| 5 | 62 (59.0) |
*Indicates a negative score
Verification of the performance of the screening software of ARHL
All 135 older adults in the community were randomly recruited by the general practitioner through the WeChat platform to participate in software testing and pure tone audiometry. Finally, 109 persons with a mean age of 69.17 ± 7.3 years and exhibiting male predominance (n = 72, 66.1%) completed the screening software test and pure tone audiometry, with a participation rate of 80.7%.
Correlation analysis showed that the spearman coefficient between PTA and the results of HHIE-s, the cumulative score of risk factors, and two-step analog audiometry was 0.704, 0.672, and 0.710, respectively (P < 0.001). Taking PTA as the reference, the conclusion of screening software was evaluated from three aspects: authenticity, reliability, and predictive value. The sensitivity, specificity, positive predictive value, and negative predictive value were 100%, 65.5%, 71.8%, and 100.0%, respectively. The Kappa index was 0.6.
26 elderly persons did not complete the screening software evaluation for reasons listed as follows (selection ratio from high to low): "Test is of little significance" 14 (54.8%), "No further willingness to test" 10 (38.7%), "Test procedure is too troublesome" 4 (15.4%), "Worried about personal privacy disclosure" 4 (15.4%), "Too many test sections" 3 (11.5%), "Some questions are not understood" 2 (7.7%), "Test environment is not suitable" 1 (3.8%), "Unexpected signal interruption during test" 1 (3.8%) (supplemental Table 2).
Discussion
Hierarchical assessment system of risk factors of ARHL
Based on the theoretical model of health risk factors, risk factors were divided into four levels (biological factors, environmental factors, disease factors, behavior, and lifestyle factors). Logistic regression analysis was applied to the data of 401 elderly persons, and the interaction effects were assessed. The risk factors were: (1) biological factors: gender, family history of deafness, (2) environmental factors: living alone, marital status, noise exposure history, (3) disease factors: high blood pressure, diabetes, hyperlipidemia, cardiovascular disease, hypothyroidism, hyperuricemia, ototoxic drug use history, and (4) behavior and lifestyle factors: being overweight, diet, exercise, smoking history, drinking history, headset wearing history.
Although the current literature suggests that regional distribution and education level are risk factors for hearing loss, this finding was not observed in our study. Accordingly, these two factors were not included. Variables mentioned in the literature, including gender, education background, family history of deafness, smoking, drinking, headset wearer habit, history of chronic otitis media, and history of ototoxic drug use, did not exhibit statistical significance. These findings may be attributed to the following reasons. Since convenience sampling was adopted, there may be a bias in the included elderly persons, lacking certain representativeness. Besides, variables such as smoking and drinking had no significant difference, which may be attributed to the imbalanced gender ratio. Moreover, the actual number of cases was too small for some parameters (headset wearer, history of chronic otitis media and history of ototoxic drug use), leading to inaccurate results.
Compared with diabetes, hypertension, and other chronic diseases, ARHL has a high prevalence and brings many adverse events and a heavy disease burden. Although primary prevention of ARHL is challenging, it is widely thought that 72.2% (13/18) of these factors are preventable, controllable, delayable, and improvable.
Development of hearing loss screening software based on the WeChat platform
The US Preventive Services Task Force summarized and analyzed 20 screening tests, including 4 from primary health care and 16 from residents. The results showed that no hearing screening tool has sufficient reliability and positive and negative predictive values [7]. Although ARHL does not belong to the chronic non-communicable diseases (NCD) category, its incidence is related to various behavioral factors. Hearing loss can cause serious damage to health, which is often irreversible. General practitioners play an important role in preventing and treating ARHL, especially in older adults. However, it remains unclear how to establish an effective and economic screening model, emphasizing the need for further research.
The advantages of the hearing test software include simulated multi-frequency pure-tone audiometry and professional test results, including listening age, listening bulletin, audiogram, and language area. Finally, relatively complete conclusions can be obtained. However, it should be borne in mind that the development team consists mostly of hearing experts, and the test focuses more on specialized knowledge of hearing. Given that the operation and conclusion are too complex, subjects cannot understand them independently. Moreover, this approach does not consider high-risk factors, self-management ability, diagnosis, and treatment compliance. The methods can realize the online service function of specialist doctors, but it cannot realize the closed-loop management of hearing loss screening, and cannot promote the formation of self-health management beliefs of high-risk groups of hearing loss.
In the study, the hierarchical assessment system of risk factors, HHIE-s, two-step pure analog audiometry and the WeChat platform were optimized and integrated to construct the self-assessment software for screening of ARHL, yielding the following advantages and effects. For the operation section, the interface is friendly and in line with the usage habits of older adults. WeChat programs do not occupy the storage space of mobile phones and have low requirements for mobile phone configuration. At present, intelligent elderly mobile phones can meet the test requirements. For the function section, the WeChat platform terminal is required for elderly residents and a computer terminal for general practitioners. Older adults can be tested from time to time, regardless of time, space, and geographical restrictions. General practitioners can inspect the test data at predefined intervals, and the ID number can be dynamically compared. To implement self-health management, the software realizes real-time data transmission, early warning, management, whole process supervision and closed-loop management process. For the screening methods, the software was set as a three-layer framework: (1) the conditional logic equation was adopted to assign and count the risk factors of the included individuals. Through the boundary value, the hierarchical assessment of risk factors was obtained; 2) the HHIE-s scale was adopted to evaluate the hearing loss and speech communication situation by simulating daily life scenes, reflecting the extent of hearing loss and communication barriers of the elderly, and forming a multidimensional and multi-level evaluation; and 3) the program adopts the two-step pure tone audiometry based on the decision tree intelligent algorithm, which breaked through the bottleneck that the previous clinical standard pure tone range audiometry cannot take into account the accuracy, time cost and economic cost, and being difficult to carry out effective development among the large population in the coverage area of the grass-roots community health center 16, the method could realize the sensitivity of 95.4% for the recognition of moderate and above deafness within 30 s. The analog audio signals used were recognized by the industry, and subjects could obtain their own hearing loss measurement results more quickly and easily. According to the results of the above three steps, the general practitioner can monitor in real time on the PC terminal, find the high-risk population of ARHL in time, and implement effective screening and timely intervention. The software sets up regular health education content and real-time updated special education content for hearing loss, which can promote the perceived susceptibility, perceived severity, perceived benefits and cue to action of the elderly to hearing loss based on health belief mode.
Suitability evaluation of screening software of ARHL
106 general practitioners from the community health service centers were invited to evaluate the suitability of screening software for hearing loss through an online questionnaire. The majority had a bachelor's degree or above (96.2%), worked for more than 10 years (57.6%), and had intermediate or above professional titles (75.5%). The questionnaire adopted a 5-point Likert subscale with 8 single-choice questions, focusing on the evaluation of using intention, convenience, framework structure, and significance. For question 1: "Are you willing to use the screening software of ARHL?", 85.8% chose full agreement and 14.4% chose basic agreement. Question 2: "Do you think the structure of screening software is reasonable?", 99.1% of general practitioners basically or generally agreed. Question 4: "Do you think the use of hearing loss screening software will help to raise the attention of older adults?" 92.5% of general practitioners agreed with this view. Therefore, from the perspective of grass-roots general practitioners, the promotion and use of elderly hearing loss screening software are feasible. Importantly, our screening software, based on the intelligent medical terminal, enables online data transmission in real time, which provides the platform to help hearing loss screening among the older adults.
Verification of screening software of ARHL
135 older adults were selected for the pre-test. 109 participants were finally included, with a participation rate of 80.7%. The correlation analysis showed that the HHIE-s, assessment of risk factors and two-step analog audiometry were moderately correlated with PTA. Moreover, the sensitivity, specificity, positive predictive value, and negative predictive value were 100%, 65.5%, 71.8%, and 100.0%, respectively.
26 participants did not complete the pre-test for the following reasons: "the test is of little significance" (54.8%), "no further willingness to test" (38.7%), "test procedure is too troublesome" (15.4%), "worried about personal privacy disclosure" (15.4%), and "too many screening items" (11.5%).
The main reasons for not completing the screening software evaluation were low willingness and lack of health knowledge. With the help of general practitioners, such as strengthening health education on hearing loss, fostering the use of screening software, and seeking the assistance of family members, we can improve the awareness of older adults on hearing loss and the acceptance of screening.
Conclusion
The screening software for age-related hearing loss documented in this study enables early screening and intervention through real-time data transmission to achieve self-health management among elderly residents. At the same time, from the perspective of primary care and general practitioners, our screening software meet the need for large-scale hearing detection in community-dwelling older adults, provides a new approach to hearing loss control which can be easily implemented.
Declaration
Conflict of interest
There are no conflicts of interest to declare.
Ethics statement
Ethics approval and consent to participate Ethics approval by the Academic Ethics Committee of Shanghai East Hospital (wf2021094) was acquired prior to the current study, which did not involve any ethical issues.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The work was supported by National Key Research and Development Program of China (No. 2022YFC3601504), Pudong New Area Health Commission Discipline Leader Training Program Project (PWRd2020-12), National research project on general practice education and teaching (B-YXgp20210301-03).
Author contributions
All authors listed have met all the International Committee of Medical Journal Editors (ICMJE) criteria for authorship and have contributed equally to this work. HJ and XMS led the statistical analysis. JLG drafted and redrafted the whole report. YYY was responsible for data analysis. YQZ, XC and HZL collected and interpreted the patient data and analysis. All authors contributed to reading and revising this paper for important intellectual content.
Data availability
Data description: cross section data,no missing data in the dataset. Data usage: Data can be referenced by similar studies. By providing this data availability statement, we aim to promote transparency and reproducibility in scientific research. We believe that making this data available will contribute to a better understanding of the research topic and facilitate further investigations.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaoming Sun, Email: xm_sun2003@aliyun.com.
Hua Jiang, Email: Huajiang2013@tongji.edu.cn.
References
- 1.Tavanai E, Mohammadkhani G. Role of antioxidants in prevention of age-related hearing loss: a review of literature. Eur Arch Otorhinolaryngol. 2017;274(4):1821–1834. doi: 10.1007/s00405-016-4378-6. [DOI] [PubMed] [Google Scholar]
- 2.Nieman CL, Oh ES. Hearing Loss. Ann Intern Med. 2020 Dec1;173(11):ITC81-ITC96. [DOI] [PubMed]
- 3.Shahidipour Z, Geshani A, Khosravifard E, et al. Auditory memory deficit in elderly people with hearing loss. Iran J Otorhinolaryngol. 2013;25(72):169–176. [PMC free article] [PubMed] [Google Scholar]
- 4.Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. Am J Public Health. 2016;106(10):e1. doi: 10.2105/AJPH.2016.303299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin FR, Thorpe R, Gordonsalant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol. 2011;66(5):582. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 7.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criter RE, Honaker JA. Audiology patient fall statistics and risk factors compared to non-audiology patients. Int J Audiol. 2016;55(10):564–570. doi: 10.1080/14992027.2016.1193235. [DOI] [PubMed] [Google Scholar]
- 9.Criter RE, Honaker JA. Fall risk screening protocol for older hearing clinic patients. Int J Audiol. 2017;56(10):1–8. doi: 10.1080/14992027.2017.1329555. [DOI] [PubMed] [Google Scholar]
- 10.Brewster KK, Ciarleglio A, Brown PJ, Chen C, Kim HO, Roose SP, et al. Age- related hearing loss and its association with depression in later life. Am J Geriatr Psychiatry. 2018;26(7):788–796. doi: 10.1016/j.jagp.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation, and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. 2014;150(3):378–384. doi: 10.1177/0194599813518021. [DOI] [PubMed] [Google Scholar]
- 13.Gopinath B, Schneider J, Mcmahon CM, Teber E, Leeder SR, Mitchell P. Severity of age-related hearing loss is associated with impaired activities of daily living. Age Ageing. 2012;41(2):195–200. doi: 10.1093/ageing/afr155. [DOI] [PubMed] [Google Scholar]
- 14.Mathers C, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huddle MG, Goman AM, Kernizan FC, Foley DM, Price C, Frick KD, et al. The economic impact of adult hearing loss: a systematic review. JAMA Otolaryngol Head Neck Surg. 2017;143(10):1040–1048. doi: 10.1001/jamaoto.2017.1243. [DOI] [PubMed] [Google Scholar]
- 16.Foley DM, Frick KD, Lin FR. Association between hearing loss and healthcare expenditures in older adults. J Am Geriatr Soc. 2014;62(6):1188–1189. doi: 10.1111/jgs.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Economics BA. Listen hear ! The economic impact and cost of hearing loss in Australia. Access Economics. 2006.
- 18.Grandori F, Lerch M, Tognola G, Paglialonga A, Smith P. Adult hearing screening: an undemanded need. Eur Geriatr Med. 2013;4(1):S4–5. doi: 10.1016/j.eurger.2013.07.059. [DOI] [Google Scholar]
- 19.GBD 2019 Hearing Loss Collaborators, Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019, Lancet, 2021 Mar 13; 397(10278): 996–1009. [DOI] [PMC free article] [PubMed]
- 20.Zhang M, Bi Z, Fu X, et al. A parsimonious approach for screening moderate-to-profound hearing loss in a community-dwelling geriatric population based on a decision tree analysis. BMC Geriatr. 2019;19(1):214. doi: 10.1186/s12877-019-1232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed NS, Altan A, Deal JA, et al. Trends in Health Care Costs and Utilization Associated With Untreated Hearing Loss Over 10 Years. JAMA Otolaryngol Head Neck Surg. 2019;145(1):27–34. doi: 10.1001/jamaoto.2018.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castiglione A, Casa M, Gallo S, et,al. Correspondence between cognitive and audiological evaluations among the elderly: A preliminary report of an audiological screening model of subjects at sisk of cognitive decline with slight to moderate hearing loss. Front Neurosci. 2019 Dec 10;13:1279. [DOI] [PMC free article] [PubMed]
- 23.Man J, Chen H, Zhang T, et al. Global, regional, and national burden of age-related hearing loss from 1990 to 2019. Aging (Albany NY) 2021;13(24):25944–25959. doi: 10.18632/aging.203782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feltner C, Wallace IF, Kistler CE, et al. Screening for Hearing Loss in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(12):1202–1215. doi: 10.1001/jama.2020.24855. [DOI] [PubMed] [Google Scholar]
- 25.Labanca L, Guimarães FS, Costa-Guarisco LP, et al. Screening of hearing in elderly people: assessment of accuracy and reproducibility of the whispered voice test. Cien Saude Colet. 2017;22(11):3589–3598. doi: 10.1590/1413-812320172211.31222016. [DOI] [PubMed] [Google Scholar]
- 26.Dedhia K, Graham E, Park A. Hearing Loss and Failed Newborn Hearing Screen. Clin Perinatol. 2018;45(4):629–643. doi: 10.1016/j.clp.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein BE. Screening for Otologic Functional Impairments in the Elderly: Whose Job is it Anyway? [J] Audiol Res. 2011;1(E12):42–48. doi: 10.4081/audiores.2011.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gates GA, Murphy MRees TS, Fraher A (2003) Screening for handicapping hearing loss in the elderly. J Fam Pract 52(1):56–62 [PubMed]
- 29.Costa-Guarisco LP, Dalpubel D, Labanca L, Chagas MHN (2017) Perception of hearing loss: use of the subjective faces scale to screen hearing among the elderly [J]. Cien Saude Colet 22(11):3579–3588 [DOI] [PubMed]
- 30.Reed NS, Altan A, Deal JA, et al (2019) Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngol Head Neck Surg 145(1):27–34 [DOI] [PMC free article] [PubMed]
- 31.Wang Y, Mo LY, Li YG, et al (2016) Analyzing use of the Chinese HHIE-S for hearing screening of elderly in a northeastern industrial area of China. Int Audiol 56(4):242–247 [DOI] [PubMed]
- 32.Weinstein BE. Tool Kit for Screening Otologic Function of Older Adults. Am J Audiol. 2013;22(1):179–182. doi: 10.1044/1059-0889(2013/12-0057). [DOI] [PubMed] [Google Scholar]
- 33.Kim S-H, Chung K. Emergency monitoring service using context motion tracking of chronic disease patients[J] Clust Comput. 2015;18(2):747–759. doi: 10.1007/s10586-015-0440-1. [DOI] [Google Scholar]
- 34.Tentori M, Hayes GR, Reddy M. Pervasive computing for hospital, chronic, and preventive care. Found Trends Human-Comput Interaction. 2012;5(1):1–95. doi: 10.1561/1100000024. [DOI] [Google Scholar]
- 35.Bentley CL, Powell L, Potter S, Parker J, Mountain GA, Bartlett YK, Farwer J, O'Connor C, Burns J, Cresswell RL, Dunn HD, Hawley MS. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR Mhealth Uhealth. 2020;8(6):e16203. doi: 10.2196/16203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H, Ha J, Park HY. Pure Tone Audiometry Threshold Changes for 10 Years in the Same Individuals of General Population: A Retrospective Cohort Study [J] Ann Otol Rhinol Laryngol. 2019;128(5):433–440. doi: 10.1177/0003489419826142. [DOI] [PubMed] [Google Scholar]
- 37.Göthberg H, Rosenhall U, Tengstrand T, et al. Cross-sectional assessment of hearing acuity of an unscreened 85-year-old cohort - Including a 10-year longitudinal study of a sub-sample [J] Hear Res. 2019;382:107797. doi: 10.1016/j.heares.2019.107797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data description: cross section data,no missing data in the dataset. Data usage: Data can be referenced by similar studies. By providing this data availability statement, we aim to promote transparency and reproducibility in scientific research. We believe that making this data available will contribute to a better understanding of the research topic and facilitate further investigations.


