Abstract
This study aims to investigate the potential impact of high-dose radiotherapy (RT) on brain structure, cognitive impairment, and the psychological status of patients undergoing brain tumor treatment. We recruited and grouped 144 RT-treated patients with brain tumors into the Low dose group (N = 72) and the High dose group (N = 72) according to the RT dose applied. Patient data were collected by using the HADS and QLQ-BN20 system for subsequent analysis and comparison. Our analysis showed no significant correlation between the RT doses and the clinicopathological characteristics. We found that a high dose of RT could aggravate cognitive impairment and deteriorate patient role functioning, indicated by a higher MMSE and worsened role functioning in the High dose group. However, the depression status, social functioning, and global health status were comparable between the High dose group and the Low dose group at Month 0 and Month 1, while being worsened in the High dose group at Month 3, indicating the potential long-term deterioration of depression status in brain tumor patients induced by high-dose RT. By comparing patient data at Month 0, Month 1, Month 3, Month 6, and Month 9 after RT, we found that during RT treatment, RT at a high dose could aggravate cognitive impairment in the short term and lead to worsened patient role functioning, and even deteriorate the overall psychological health status of patients in the long term.
Keywords: Radiotherapy, Glioma, Cognitive impairment, Psychological health status, Mini-mental state examination
Subject terms: Neuroscience, Neurology, Oncology, Risk factors
Introduction
Many patients around the world receive radiotherapy (RT) to treat primary and metastasized tumors in the brain1,2. As a fundamental therapy for most brain tumors, RT of the brain is divided into partial brain RT (PBRT) and whole brain RT (WBRT)3,4. Currently, more than 100,000 patients in the United States receive brain irradiation each year and more than 50% of these patients display cognitive problems, such as the affected ability of memory, learning, and attention2,5. The pathology of cognitive problems caused by radiation is complicated and involves multiple mechanisms. It was proposed that previously unnoticed but subtle indications of irradiation damage to the central nervous system may eventually lead to lasting abnormalities and long-term cognitive disability6,7.
The creation of new memory is linked to radiosensitive and mitotically active populations of neural stem cells found in the sub-granular region in the hippocampal dentate gyrus8. Damage to these neural stem cells may play a main role in the pathology of early cognitive issues induced by radiation9. Pre-clinical studies have also shown that a moderate dose of radiation can trigger a significant reduction in neurogenesis of the sub-granular area10. On top of that, the latest medical research has shown a correlation between the dose of radiation and the risk of impaired learning11.
Multiple mechanisms have been used to describe the impaired cognitive ability observed after brain RT12. Deoxyribonucleic acid damage caused by X-ray radiation may cause cell death and dysfunction. The dynamic cellular process related to oxidative stress and inflammation seems to cause long-term toxicity13,14. Moreover, studies have shown the exhaustion of endothelial cells as well as changes to micro-vascularization after brain RT15. As an important organ involved in cognition, the hippocampus can be injured during RT and its pathophysiology has been extensively researched16. For example, X-ray-induced inhibition of neurogenesis may cause changes in the performance of memory tests, eventually leading to a notable level of cognitive impairment in mice17–19. It was also shown that minocycline can prevent neuronal apoptosis induced by radiation during WBI to improve the cognitive function of rats receiving irradiation, clearly demonstrating the protective role of minocycline in neurons against cognitive defects as well as neuronal death induced by radiation20,21.
In a previous report on the routine first-line glioblastoma practice, a standard RT treatment did not worsen the cognitive function and psychological status of patients22. When observing children with posterior fossa tumors, the verbal comprehension scores were notably decreased in children treated with a higher radiation dose23. This study aimed to study the potential influence of high-dose RT on cognitive impairment and patient psychological status in the treatment of brain tumors.
Materials and methods
Patient recruitment
A total of 144 brain tumor patients who were subjected to RT were recruited and grouped according to the dosage of RT as the Low dose group (N = 72, patients who received an RT dose no more than 30 Gy) and the High dose group (N = 72, patients who received an RT dose more than 30 Gy). The research was approved in advance by the Ethics Committee of the Affiliated Hospital of Nantong University, and all procedures were carried out in strict compliance with the Declaration of Helsinki. Informed consent was obtained before the initiation of this study. Patient parameters including MMSE, depression, role functioning, social functioning, bladder control, global health status, itchy skin, and weakness of legs were collected from the patients in both groups before the application of RT (Month 0) and at Months 1, 3, 6, and 9 after the application of RT using the HADS and QLQ-BN20 system. All sets of questions were administered to the patients during the baseline assessment, during the visit conducted 2 weeks after the surgical operation, before the start of the concomitant treatment, and during the visits conducted at 1, 3, 6, and 9 months after the start of the concomitant treatment, or when the patients showed progressive disease.
Patient data collection
For the assessment of cognitive functions and psychological status, the Cognitive Function and Psychological Status Scale, the QLQ-C30 questionnaire, the QLQ-BN20 questionnaire, and the assessment of the European Organization for Research and Treatment of Cancer (EORTC) were used. Furthermore, for the assessment of health-related quality of life (HRQOL), we selected two assessment approaches that were well-established, validated, and most frequently applied in the HRQOL assessment of brain cancer patients: QLQ-BN20 and EORTC QLQ-C30, to assess the status of HRQOL19,20. We also utilized the Mini-Mental State Examination (MMSE) to test the general cognitive function of the patients21 and the Hospital Anxiety and Depression Scale (HADS) to assess the levels of depression and anxiety of the patients22.
As a general multi-dimensional survey frequently applied in the assessment of cancer patients, the EORTC QLQ-C3018 questionnaire contains 30 test items in 5 functional scales (cognitive function, emotional function, physical function, role function, and social function), 3 symptom scales (symptom of fatigue, symptom of nausea and vomiting, and symptom of pain), 1 global health status scale, as well as 6 single item scales to assess additional symptoms like constipation, appetite loss, dyspnea, diarrhea, perceived financial impact, and sleep disturbance. As a questionnaire particularly designed for patients of brain cancer, the EORTC QLQ-BN2019 questionnaire features 20 test items in 11 groups of symptom scales, i.e., motor dysfunction, visual disorders, various disease symptoms such as seizures and headaches, communication deficit, future uncertainty, and treatment toxicities such as hair loss. In this study, the raw score of questionnaires was converted to a linear range ranging from 0 to 100, in which a higher rating indicates a higher functioning level or a greater symptom and problem. Finally, the statistical analysis took into consideration the scores from the six scales of the EORTC QLQ-C30 questionnaire, i.e., physical functioning, cognitive functioning, social functioning, role functioning, emotional functioning, and global health status, as well as all items of the QLQ-BN20 questionnaire.
The short MMSE21 questionnaire of 18 items was used to determine the severity and prognosis of cognitive impairment. In the MMSE scale, a score in the range from 0 to 30 was provided and the lower score represented a more severe level of cognitive impairment. The HADS22 questionnaire including 14 items was used to determine the level of depression and anxiety of the patients. The score of each item in the HADS22 questionnaire ranged from 0 to 3 points, while the overall score in each subscale ranged from 0 to 21 points, and a higher score indicated a higher level of symptomatology.
Statistical analysis
The statistical analyses of the scores of EORTC QLQ-C30, MMSE, BN20, as well as anxiety and depression were conducted by using mixed effects linear models, to study the improvements or deterioration of cognitive impairment over time between the two groups. In each mixed effects linear model, time analysis was used as a discrete variable, while clinical characteristics as well as the interaction among clinical characteristics for fixed effects were used besides a compound symmetry covariance structure to determine the random effect on the intercept. In addition, the age at the time of diagnosis, gender, and the location of tumors were treated as clinical variables. To compensate for the errors induced by multiple comparisons while minimizing type I errors, P = 0.01 was used as the statistical significance level. A difference of ≥ 10 points between the average EORTC scores compared or a difference of ≥ 1.5 between the average HADS scores compared was deemed clinically significant. In addition, patients with an MMSE score of ≤ 26 were deemed to suffer from cognitive function impairment. All statistical analyses were carried out by making use of the SAS version 9.2 statistical software (SAS, Cary, NC).
Ethics approval
The research was approved in advance by the Ethics Committee of Affiliated Hospital of Nantong University, and all procedures were carried out in strict compliance with the Declaration of Helsinki.
Consent to participate
Informed consent was received before the initiation of this study.
Results
MRI results for brain injury of different patient groups
Basic patient clinicopathological characteristics were collected and summarized in Table 1, and no significant differences in these parameters were observed between these two patient groups. Moreover, we also presented the MRI results for brain injury of patients (Fig. 1A) from the Low dose group before the RT (Month 0) and after the RT (Month 1), as well as the MRI results for brain injury of patients from the high dose group (Fig. 1B) before the RT (Month 0) and after the RT (Month 1).
Table 1.
Basic characteristics of brain tumor patients from the Low dose group and the High dose group
| Characteristics | Low dose (N = 72) | High dose (N = 72) | P value |
|---|---|---|---|
| Sex, male, n (%) | 43 (59.7) | 46 (63.9) | 0.6087 |
| Age, years | 55.8 ± 13.2 | 51.9 ± 16.7 | 0.1131 |
| Weight, kg | 69.8 ± 15.8 | 74.1 ± 12.3 | 0.0705 |
| Height, cm | 168.8 ± 11.9 | 165.4 ± 14.7 | 0.1294 |
| Right-handed, n | 58 (80.6) | 53 (73.6) | 0.3192 |
| Age at study entry, years | 8.5 ± 3.9 | 9.4 ± 5.1 | 0.2362 |
| Mean education, years | 9.8 ± 3.3 | 9.3 ± 4.2 | 0.4283 |
| Mean estimated verbal IQ | 95.2 ± 7.5 | 93.5 ± 8.4 | 0.2023 |
| Temporal D50% | 23.14 ± 12.26 | 36.32 ± 14.81 | < 0.0001 |
| Hippocampus D50% | 21.38 ± 11.93 | 34.52 ± 12.66 | < 0.0001 |
| Tumor type | |||
| Low grade glioma | 15 (20.8) | 12 (16.7) | 0.5300 |
| High grade glioma | 25 (34.7) | 27 (37.5) | 0.7275 |
| Primary CNS lymphoma | 26 (36.1) | 23 (31.9) | 0.5960 |
| Others | 6 (8.4) | 10 (13.9) | 0.2961 |
| Tumor location | |||
| Frontal/frontal–temporal/frontal-parietal | 35 (48.6) | 38 (52.8) | 0.6155 |
| Temporal/parietal/occipital | 16 (22.2) | 20 (27.8) | 0.4394 |
| Cortical/subcortical | 21 (29.2) | 14 (19.4) | 0.1719 |
| Predominant tumor side | |||
| Left | 21 (29.2) | 21 (29.2) | 1.0000 |
| Right | 26 (36.1) | 27 (37.5) | 0.8622 |
| Bilateral | 25 (34.7) | 24(33.3) | 0.8597 |
| Radiation field | |||
| Craniospinal + boost | 27 (37.5) | 31 (43.1) | 0.4948 |
| Focal | 45 (62.5) | 41 (56.9) | 0.4948 |
| KPS at baseline | |||
| 100 | 16 (22.2) | 15 (20.8) | 0.8385 |
| 90 | 5 (6.9) | 6 (8.4) | 0.7358 |
| 80 | 35 (48.6) | 38 (52.8) | 0.6155 |
| 70 | 16 (22.2) | 13 (18.0) | 0.5309 |
D50%: median dose, the dose of 50% volume of PTV (planning total volume).
Figure 1.
MRI results of brain injury were similar before and after RT. (A): Brain injury detected by MRI at Month 0; (B): Brain injury detected by MRI at Month 1.
High-dose RT deteriorates cognitive impairment in the short term
To predict the effect of different doses of RT on patient neurocognitive status, the MMSE score system was utilized. As shown in Fig. 2A, the cognitive impairment of the Low dose and High dose groups was similar before the treatment of RT (Month 0). However, at Months 1, 3, 6, and 9 after the treatment of RT, a significant reduction in the MMSE scores was observed in patients treated with high-dose RT compared with those treated with low-dose RT, indicating the potential acute negative effect of the higher dose of RT on patient cognitive impairment. Moreover, when analyzing the possible correlation between radiation dose and individual patient’s MMSE scores recorded at Month 1 or Month 9 after RT (Fig. 2B), we found a significant negative correlation between the high dose of radiation and cognitive impairment after the first month of RT, while no significant correlation was demonstrated between radiation dose and cognitive performance after the ninth month of RT (Fig. 2C). Therefore, it is suggested that high-dose RT may exhibit an acute adverse effect on cognitive performance.
Figure 2.
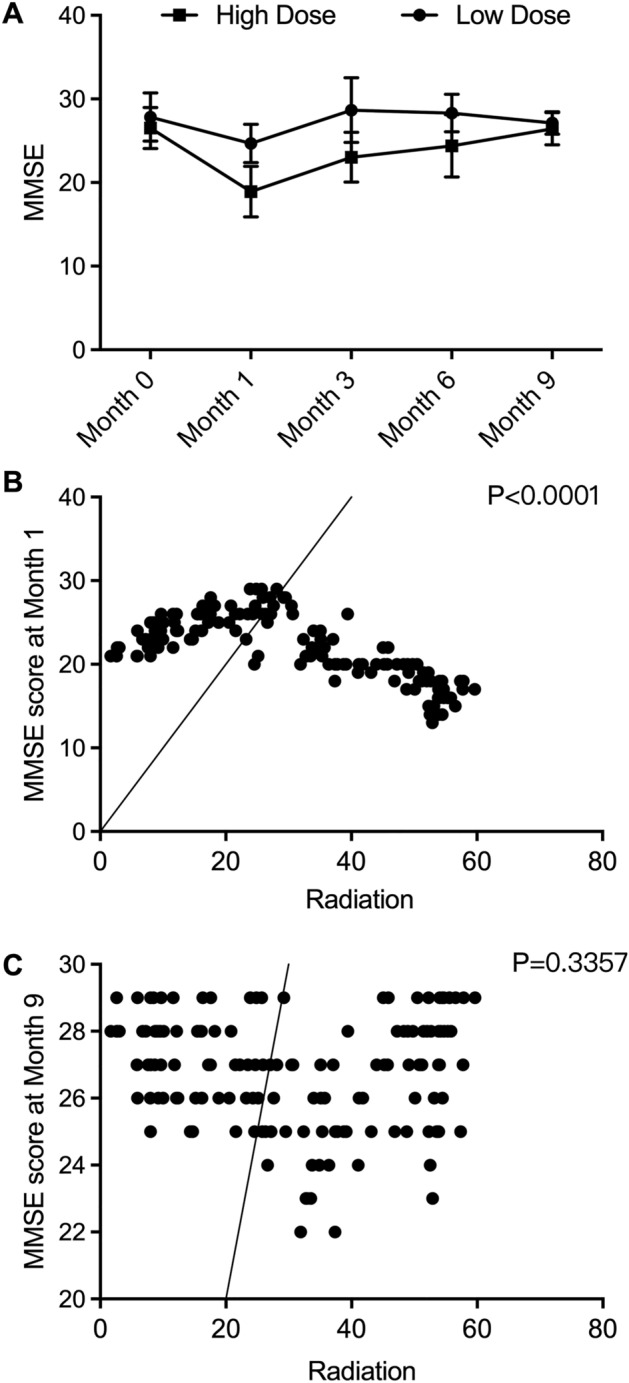
High-dose RT aggravated cognitive impairment in the short term. and aggravated depression in the long term (* P value < 0.05 compared with low dose group). (A): MMSE score of High dose group and Low dose group recorded before RT (Month 0), Month 1, Month 3, Month 6 and Month 9 indicated acute adverse effect of high-dose RT on cognitive impairment; (B): Correlation analysis between radiation dose and MMSE score at Month 1; (C): Correlation analysis between radiation dose and MMSE score at Month 9.
High-dose RT aggravates depression, deteriorates patient role functioning, and obstructs social functioning in the long term
Meanwhile, as indicated by Fig. 3A, depression status, as observed by HADS between the High dose group and the Low dose group, showed interesting patterns. The differences between the Low dose and High dose groups were insignificant at Months 0, 3, 6, and 9, while the depression status was worsened in the High dose group compared with the Low dose group at Month 1, indicating the potential long-term deterioration of depression status of patients induced by high-dose RT.
Figure 3.
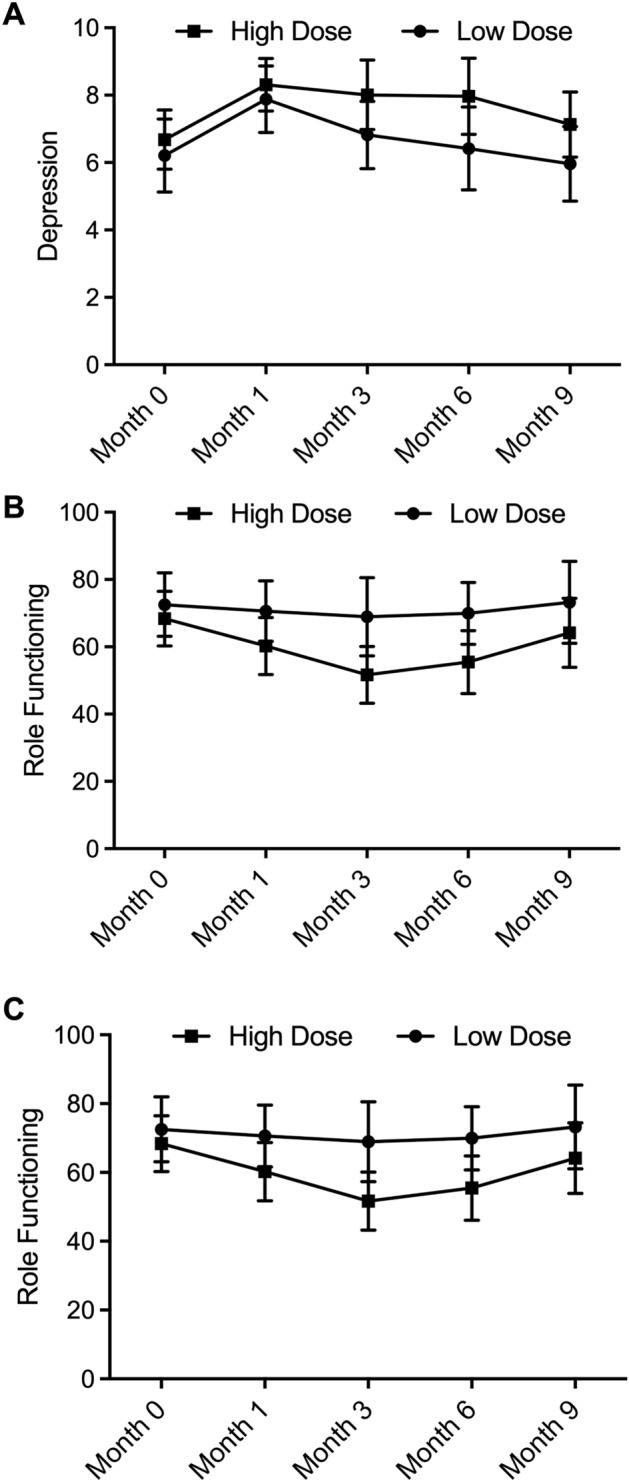
High-dose RT aggravated depression, deteriorated patient role functioning and obstructed social functioning in the long term (* P value < 0.05 compared with low dose group). (A): Depression score of the High dose and the Low dose group recorded before RT (Month 0), Month 1, Month 3, Month 6 and Month 9 indicated long-term adverse effect of high-dose RT on depression; (B): The role functioning was deteriorated by high-dose RT in the long term according to role functioning score recorded before RT (Month 0), Month 1, Month 3, Month 6 and Month 9; (C): The social functioning was obstructed by high-dose RT in the long term according to role functioning score recorded before RT (Month 0), Month 1, Month 3, Month 6 and Month 9.
As shown in Fig. 3B, the role functioning of both patient groups measured by QLQ-BN20 showed a similar tendency as the MMSE scores. At Month 0, the role functioning was comparable in both patient groups, while the patients in the High dose group showed a lower role functioning score at Months 1, 3, 6, and 9, indicating that high-dose RT could deteriorate the role functioning of brain tumor patients in the long term.
Unlike role functioning, social functioning (Fig. 3C) measured by QLQ-BN20 was similar between the Low dose group and the High dose group before RT and at 1 month after RT. However, since the third month after RT, the social functioning of patients receiving high-dose RT decreased compared with that of patients receiving low-dose RT, thus indicating that high-dose RT could obstruct patient social functioning in the long term.
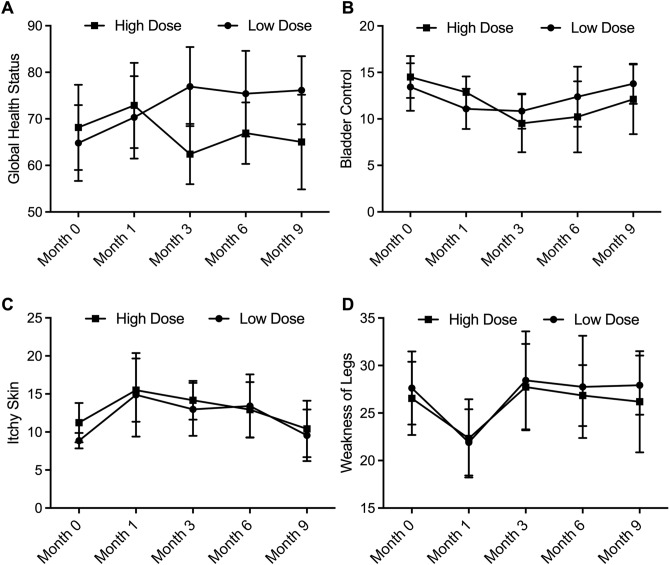
High-dose RT deteriorated global health status in the long term while exhibiting no effect on patient bladder control, itchy skin, and weakness of legs
As shown in Fig. 4A, the global health status of brain tumor patients was recorded before and after RT. At Months 0 and 1, the global health status of the Low dose group and the High dose group was comparable. However, since the third month after RT, the score for global health status decreased in patients receiving high-dose RT. Meanwhile, other parameters including patient bladder control, itchy skin, and weakness of legs were compared between the Low dose group and the High dose group before RT (Month 0) and at Months 1, 3, 6, and 9 after RT. As indicated by the results, no statistical significance was found between patients treated with high-dose RT and low-dose RT concerning patient bladder control (Fig. 4B), itchy skin (Fig. 4C), or weakness of legs (Fig. 4D) at all time points, thus demonstrating that the dose of RT was not statistically correlated with itchy skin and weakness of legs.
Figure 4.
High-dose RT deteriorated global health status in the long term, while RT exhibited no effect on patient bladder control, itchy skin or weakness of legs (* P value < 0.05 compared with low dose group). (A): Patient global health status was comparable between the the Low dose group and the High dose group at Month 0 and Month 1, while being deteriorated in the High dose group since Month 3; (B): Patient bladder control conditions were comparable between the Low dose group and the High dose group at Month 0, Month 1, Month 3, Month 6 and Month 9; (C): Patient itchy skin conditions were comparable between the Low dose group and the High dose group at Month 0, Month 1, Month 3, Month 6 and Month 9; (D): Patient weakness of legs status was comparable between the Low dose group and the High dose group at Month 0, Month 1, Month 3, Month 6 and Month 9.
Discussion
While effective in treating cancer, radiotherapy (RT) also exerts side effects, such as cognitive impairment. Factors including the location of RT treatment, the dosage and duration of RT, the age and health status of patients receiving RT, and even the types of radiation may influence the onset of its side effects24. For radiation directed toward the brain or surrounding tissue areas, the risk of cognitive impairment is increased. Also, higher doses and longer durations of RT treatment can increase the risk of cognitive side effects. Aged adults with existing cognitive conditions were also found to be more susceptible to cognitive impairment as a side effect of RT treatment25. Proton therapy, compared with other types of radiation, is associated with a lower risk of cognitive impairment due to its greater precision in sparing surrounding healthy tissues26. In this study, we recruited brain tumor patients who received RT. By comparing the MRI results before RT and 1 month after RT, we found no significant structural changes. However, significant effects on cognitive functions were observed. After RT, the MMSE scores of the High dose group were lower than those of the Low dose group at Month 1 and Month 3, indicating that a higher dose of RT could have a negative effect on the patients' cognitive functions. Role functioning exhibited a similar tendency as that of the MMSE scores between the High dose group and the Low dose group at Months 0, 1, 3, 6, and 9, demonstrating that RT at a higher dose could deteriorate the role functioning of brain tumor patients. In addition, the depression status measured by HADS, as well as the social functioning and global health status measured by QLQ-BN20, were comparable between the High dose group and the Low dose group at Months 0 and 1. Meanwhile, the depression status, social functioning, and global health status all worsened in the High dose group at Month 3, indicating the potential long-term deteriorative effect of high-dose RT on the depression status of brain tumor patients.
Since brain tumors may result in isolated deficiencies, global scores of patient functionality may not show clinically crucial prognostic information about the patients if the deficiencies in motor or cognitive domains have various implications for survival. It was shown that neuropsychological examinations had a prognostic value in pooled samples of patients with glioblastoma and recurring anaplastic astrocytoma27,28. Various other studies also assessed the link between Mini-Mental Condition Examination (MMSE) scores and patient survival, and some revealed that MMSE impairment is linked with much shorter survival in individuals with newly diagnosed glioblastoma or low-grade glioma29,30.
A previous study showed that the apoptosis of neurons in the hippocampus in rodent brain tissues may cause delayed issues of cognitive deficits after RT31–33. Another research also showed that the number of viable neurons in the hippocampal CA1 domain was reduced after WBRT. In this study, it is noteworthy that the hippocampus D50% parameter was significantly higher in the High dose group (34.52 ± 12.66) compared with the Low dose group (21.38 ± 11.93). As a critical structure within the brain's medial temporal lobe, the hippocampus plays a critical role in memory formation, organization, and retrieval, as well as in spatial navigation. Conditions such as Alzheimer's disease, epilepsy, and depression are associated with hippocampal dysfunction, leading to memory deficits and other cognitive impairments. The impacts of hippocampal dysfunction on cognitive functions have been extensively studied in previous investigations34,35. Since the hippocampus D50% was higher for the High dose group, the hippocampal function may be potentially influenced by RT treatment as well. However, parameters including bladder control, itchy skin, and weakness of legs were not influenced by the dose of RT. The bladder control, itchy skin, and weakness of legs in the Low dose group were similar to those in the High dose group at Month 0, Month 1, Month 3, Month 6, and Month 9, demonstrating that the dose of RT was not statistically correlated with bladder control, itchy skin and weakness of legs.
Microglia and astrocytes can react to the irradiation of the brain by producing certain factors to cause neuro-inflammation as well as influence the differentiation and function of cells in the CNS5,6. Pro-inflammatory cytokines including tumor death factor-α, interleukin-1β, interleukin-6 as well as interleukin-18 were assayed in the brain's particular regions after radiation14. Inflammatory biomarkers like GFAP, NF-κb, as well as intercellular adhesion molecule-1 were additionally characterized in brain tissues after radiation36.
Apart from the extent of resection, the differences in functioning levels between different patient groups may also be attributed to other key factors. It has been demonstrated that there was a correlation between the subtotal resection procedure and the worse baseline functioning37. In another previous study on nasopharyngeal carcinoma patients, the correlation between cognitive features, lesion volume, and lesion site was identified. A strong correlation was shown between the site of radio necrosis and the forms of cognitive impairment38. Currently, the combination of RT and surgical treatment is the approach offering significant advantages of survival39,40. Temozolomide has also been added to RT to present a substantial advantage in OS and progression-free survival41. Most of the above studies showed a detrimental impact of RT on cognitive performance. The tests of executive function, memory, and motor coordination also showed a correlation between tumor volume and cognitive performance.
However, the findings of this study must be interpreted within the context of several limitations. First, the relatively small sample size could limit the statistical power of our analysis, suggesting that future studies with larger cohorts are necessary to validate our findings. Additionally, the lack of chemotherapy information and comorbidities from our patients’ data represents a significant limitation as these factors may influence cognitive functions or interact with RT to exacerbate cognitive decline, thereby confounding our results. Future research should aim to incorporate these parameters to provide a more comprehensive understanding of the multifactorial nature of cognitive impairment in brain tumor patients. Thirdly, the absence of information regarding the brain areas and volumes that received RT treatment further complicates the interpretation of our results, limiting the precision of our conclusions. Moreover, the lack of resection extent in each patient group also raises questions about the potential confounding effects of surgical variables on our findings, since this factor combined with significant differences in hippocampal dose between the two groups may suggest that our data may not solely reflect the impact of radiotherapy dose but also the influence of surgical outcomes and individual patient differences. With respect to these limitations, our study highlights the need for further research that employs larger, more diverse patient cohorts, including comprehensive treatment and comorbidity data, and detailed dosimetry analysis.
Conclusion
In conclusion, by measuring the cognitive impairment and other psychological health-related parameters at Month 0, Month 1, Month 3, Month 6, and Month 9 after RT, we found that during the treatment of brain tumor, RT at a high dose could aggravate cognitive impairment in the short term and lead to worsened patient role functioning, and even deteriorate the overall psychological health status of patients in the long term.
Acknowledgements
Not applicable.
Abbreviations
- PBRT
Partial brain radiotherapy
- WBRT
Whole brain radiotherapy
- KPS
Karnofsky performance status
- MMSE
Mini-mental state examination
- HADS
Hospital anxiety and depression scale
- EORTC
European organization for research and treatment of cancer
- HRQOL
Health-related quality of life
Author contributions
J.M. designed the study, T.L. collected the supporting literature, J.M., H.C., D.H., and W.W performed the experiments, T.L. analyzed the data, J.M and T.L. wrote the main manuscript text, H.C. and D.H. prepared the figures. All authors reviewed the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Park K, Bae GH, Kim WK, Yoo CJ, Park CW, Kim SK, et al. Radiotherapy for brain metastasis and long-term survival. Sci. Rep. 2021;11(1):8046. doi: 10.1038/s41598-021-87357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, et al. Current approaches to the treatment of metastatic brain tumours. Nat. Rev. Clin. Oncol. 2014;11(4):203–222. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2018;1(1):Cd003869. doi: 10.1002/14651858.CD003869.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst. Rev. 2017;9(9):Cd006121. doi: 10.1002/14651858.CD006121.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017;13(1):52–64. doi: 10.1038/nrneurol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attia A, Page BR, Lesser GJ, Chan M. Treatment of radiation-induced cognitive decline. Curr. Treat. Options Oncol. 2014;15(4):539–550. doi: 10.1007/s11864-014-0307-3. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong CL, Gyato K, Awadalla AW, Lustig R, Tochner ZA. A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol. Rev. 2004;14(1):65–86. doi: 10.1023/b:nerv.0000026649.68781.8e. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 9.Gondi V, Tome WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother. Oncol. 2010;97(3):370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 11.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(2):348–354. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J. Neurooncol. 2008;87(3):279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 13.Jiao Y, Cao F, Liu H. Radiation-induced cell death and its mechanisms. Health Phys. 2022;123(5):376–386. doi: 10.1097/hp.0000000000001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int. J. Radiat. Biol. 2010;86(2):132–144. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tregub PP, Averchuk AS, Baranich TI, Ryazanova MV, Salmina AB. Physiological and pathological remodeling of cerebral microvessels. Int. J. Mol. Sci. 2022;23(20):12683. doi: 10.3390/ijms232012683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Son Y, Yang M, Wang H, Moon C. Hippocampal dysfunctions caused by cranial irradiation: A review of the experimental evidence. Brain Behav. Immun. 2015;45:287–296. doi: 10.1016/j.bbi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 18.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004;188(2):316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Huang P, Chen H, Tan W, Lu J, Liu W, et al. The inhibitory effect of minocycline on radiation-induced neuronal apoptosis via AMPKalpha1 signaling-mediated autophagy. Sci. Rep. 2017;7(1):16373. doi: 10.1038/s41598-017-16693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Li K, Sun R, Zhang Y, Ji J, Huang P, et al. Minocycline ameliorates cognitive impairment induced by whole-brain irradiation: An animal study. Radiat. Oncol. 2014;9:281. doi: 10.1186/s13014-014-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardi G, Bergo E, Del Bianco P, Bellu L, Pambuku A, Caccese M, et al. Quality of life perception, cognitive function, and psychological status in a real-world population of glioblastoma patients treated with radiotherapy and temozolomide: A single-center prospective study. Am. J. Clin. Oncol. 2018;41(12):1263–1271. doi: 10.1097/COC.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 23.Grill J, Renaux VK, Bulteau C, Viguier D, Levy-Piebois C, Sainte-Rose C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int. J. Radiat. Oncol. Biol. Phys. 1999;45(1):137–145. doi: 10.1016/s0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 24.Turnquist C, Harris BT, Harris CC. Radiation-induced brain injury: current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol. Adv. 2020;2(1):vdaa57. doi: 10.1093/noajnl/vdaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnuson A, Mohile S, Janelsins M. Cognition and cognitive impairment in older adults with cancer. Curr. Geriatr. Rep. 2016;5(3):213–219. doi: 10.1007/s13670-016-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohan R. A Review of proton therapy - current status and future directions. Precis. Radiat. Oncol. 2022;6(2):164–176. doi: 10.1002/pro6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson DR, Wefel JS. Relationship between cognitive function and prognosis in glioblastoma. CNS Oncol. 2013;2(2):195–201. doi: 10.2217/cns.13.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liouta E, Koutsarnakis C, Komaitis S, Kalyvas AV, Drosos E, García-Gómez JM, et al. Preoperative neurocognitive function as an independent survival prognostic marker in primary glioblastoma. Neurooncol. Pract. 2023;10(6):527–535. doi: 10.1093/nop/npad027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero-Ortuno R, Cogan L, Fan CW, Kenny RA. Intolerance to initial orthostasis relates to systolic BP changes in elders. Clin. Auton. Res. 2010;20(1):39–45. doi: 10.1007/s10286-009-0040-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim N, Chang JS, Wee CW, Kim IA, Chang JH, Lee HS, et al. Validation and optimization of a web-based nomogram for predicting survival of patients with newly diagnosed glioblastoma. Strahlenther Onkol. 2020;196(1):58–69. doi: 10.1007/s00066-019-01512-y. [DOI] [PubMed] [Google Scholar]
- 31.Huo K, Sun Y, Li H, Du X, Wang X, Karlsson N, et al. Lithium reduced neural progenitor apoptosis in the hippocampus and ameliorated functional deficits after irradiation to the immature mouse brain. Mol. Cell Neurosci. 2012;51(1–2):32–42. doi: 10.1016/j.mcn.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99(1):33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 33.Hassan HA, Hafez HS, Goda MS. Mentha piperita as a pivotal neuro-protective agent against gamma irradiation induced DNA fragmentation and apoptosis: Mentha extract as a neuroprotective against gamma irradiation. Cytotechnology. 2013;65(1):145–156. doi: 10.1007/s10616-012-9470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30(39):13005–13015. doi: 10.1523/jneurosci.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bettio LEB, Rajendran L, Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017;79:66–86. doi: 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Ballesteros-Zebadua P, Chavarria A, Celis MA, Paz C, Franco-Perez J. Radiation-induced neuroinflammation and radiation somnolence syndrome. CNS Neurol. Disord. Drug Targets. 2012;11(7):937–949. doi: 10.2174/1871527311201070937. [DOI] [PubMed] [Google Scholar]
- 37.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children's adaptive functioning following conformal radiation therapy for localized ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(1):217–223. doi: 10.1016/j.ijrobp.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan YL, Leung SF, King AD, Choi PH, Metreweli C. Late radiation injury to the temporal lobes: Morphologic evaluation at MR imaging. Radiology. 1999;213(3):800–807. doi: 10.1148/radiology.213.3.r99dc07800. [DOI] [PubMed] [Google Scholar]
- 39.Fernandes C, Costa A, Osorio L, Lago RC, Linhares P, Carvalho B, et al. Current Standards of Care in Glioblastoma Therapy. In: De Vleeschouwer S, et al., editors. Glioblastoma. Codon Publications; 2017. [PubMed] [Google Scholar]
- 40.Kirste S, Treier M, Wehrle SJ, Becker G, Abdel-Tawab M, Gerbeth K, et al. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: A prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer. 2011;117(16):3788–3795. doi: 10.1002/cncr.25945. [DOI] [PubMed] [Google Scholar]
- 41.Salah AO, Salameh AD, Bitar MA, Zyoud SH, Alkaiyat AS, Al-Jabi SW. Complementary and alternative medicine use in coronary heart disease patients: A cross-sectional study from Palestine. BMC Complement Med. Ther. 2020;20(1):231. doi: 10.1186/s12906-020-03028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.