Abstract
The extra sex combs (esc) and Enhancer of zeste [E(z)] proteins are members of the Drosophila Polycomb group (Pc-G) of transcriptional repressors. Here we present evidence for direct physical interaction between the esc and E(z) proteins using yeast two-hybrid and in vitro binding assays. In addition, coimmunoprecipitation from embryo extracts demonstrates association of esc and E(z) in vivo. We have delimited the esc-binding domain of E(z) to an N-terminal 33-amino-acid region. Furthermore, we demonstrate that site-directed mutations in the esc protein previously shown to impair esc function in vivo disrupt esc-E(z) interactions in vitro. We also show an in vitro interaction between the heed and EZH1 proteins, which are human homologs of esc and E(z), respectively. These results suggest that the esc-E(z) molecular partnership has been conserved in evolution. Previous studies suggested that esc is primarily involved in the early stages of Pc-G-mediated silencing during embryogenesis. However, E(z) is continuously required in order to maintain chromosome binding by other Pc-G proteins. In light of these earlier observations and the molecular data presented here, we discuss how esc-E(z) protein complexes may contribute to transcriptional silencing by the Pc-G.
The homeotic genes of the Drosophila Antennapedia and bithorax complexes encode transcription factors that assign developmental fates to cells along the anterior-posterior body axis (35, 45). Homeotic gene expression is confined to specific positions along this axis (9, 10, 34, 87). These expression patterns are established during embryogenesis and must be maintained throughout embryonic, larval, and pupal stages in order to continuously instruct cells about their segmental identities (44, 50, 75).
Transcription of homeotic genes begins at the blastoderm stage, at about 2 h of development. At this time, their expression is controlled by the products of the segmentation genes (27, 86). For example, the product of the gap gene hunchback (hb) represses the transcription of Ultrabithorax (Ubx), thereby delimiting its anterior border of expression (64, 86, 89). However, shortly after gastrulation, at merely 2 h after this pattern of Ubx expression is established, the hb protein decays (79). From that point onward, the maintenance of Ubx repression in anterior cells is provided by the Polycomb group (Pc-G) proteins (for recent reviews, see references 61 and 69). A second group of proteins, collectively known as the trithorax group (trx-G), is required to maintain the transcriptional activity of Ubx and other homeotic genes (see references 36 and 78 for reviews).
There are approximately 13 identified genes in the Pc-G. Additional genes whose products contribute to Pc-G-mediated repression have been postulated (32, 41). The mechanism by which this large collection of Pc-G proteins maintains the transcriptional repression of target genes is not known. Several models that involve packaging of target genes into a condensed and inaccessible conformation (56), modification of the local organization or structure of nucleosomes (48, 61, 62), formation of loop domains that interfere with local enhancer function (61, 62), or interference with specific factors that are needed for transcriptional activation (5, 48) have been considered.
Whichever mechanism underlies Pc-G-mediated repression, a growing body of evidence suggests that it requires the assembly of Pc-G proteins into heteromeric complexes. This model is based on immunohistochemical (8, 17, 46, 47, 59, 63, 65) and biochemical (3, 17, 21) studies that have provided evidence for the in vivo association of Pc-G proteins. However, the precise composition of Pc-G complexes and whether they exist as a single complex or a more heterogeneous variety of complexes are yet to be determined.
Pc-G-mediated silencing of homeotic genes begins early in embryogenesis and is continuously required throughout embryonic, larval, and pupal development. One member of the Pc-G, extra sex combs (esc), is unusual in that its activity is primarily required during early embryogenesis (70, 77). In agreement with this fact, esc is expressed most abundantly during early embryonic stages (22, 55, 66, 70). Also, whereas other Pc-G proteins participate in other examples of gene repression, such as the negative regulation of engrailed, knirps, and giant (49, 58), esc is not required for control of these additional target loci. Taken together, these results have led to the suggestion that esc may recognize the initial repressed state of homeotic genes and then recruit the binding of other Pc-G proteins (22, 66, 70).
Several lines of evidence are consistent with a molecular connection between esc and another member of the Pc-G, Enhancer of zeste [E(z)]. Females that are homozygous for temperature-sensitive E(z) alleles produce embryos at the restrictive temperature that display posteriorly directed homeotic transformations (30, 60). These phenotypes very closely resemble the phenotypes of embryos produced by esc mutant females (74). A complete lack of esc or E(z) activity, each of which is primarily contributed maternally, results in virtually identical patterns of ectopic homeotic gene expression in embryos (30, 71, 76). However, unlike esc, E(z) is required continuously throughout development in order to maintain homeotic gene repression (30, 60, 68). A more intimate relationship between esc and E(z), as opposed to other Pc-G proteins, was suggested by the observation that the esc maternal effect is exacerbated by either decreasing or increasing the zygotic dosage of E(z)+ (6). This finding suggests that a balance in the relative concentrations of the esc and E(z) proteins may be important for homeotic gene repression. In this report, we provide multiple lines of evidence that show a direct physical interaction between the Drosophila esc and E(z) proteins. Coimmunoprecipitation of the two proteins from fly extracts also demonstrates their close association in vivo. In addition, we show a direct interaction between human homologs of esc and E(z). The data suggest that the esc and E(z) proteins are direct partners in Pc-G-mediated repression and that this relationship has been evolutionarily conserved.
MATERIALS AND METHODS
Yeast two-hybrid constructs and assays.
pEG202-esc contains a 1.4-kb SfuI-DraI segment derived from the esc cDNA e2 (70) and carries a full-length esc protein fused to LexA at the normal esc start codon. pEG202-esc was constructed by inserting a 1.4-kb SalI-XbaI fragment from the e2 cDNA derivative pe2Sf (70) into the vector pEG202 (18). pJG4-5-esc was constructed by inserting the same 1.4-kb SalI-XbaI fragment into pJG4-5 (18).
pEG202-E(z) was constructed with a modified version of the E(z) cDNA e32 (31), Bg-e32, which contains a BglII site immediately 5′ to the E(z) translation start site. A 2.5-kb BglII-NotI fragment containing the entire E(z) coding region was isolated from Bg-e32 and inserted into BamHI-NotI-cut pBluescript to make pBS-Bg-e32. The 2.5-kb E(z) fragment was excised from pBS-Bg-e32 by EcoRI-NotI digestion and inserted into pEG202 to make pEG202-E(z). pJG4-5-E(z) was constructed by inserting the 2.5-kb EcoRI-XhoI fragment from pEG202-E(z) into pJG4-5.
The base yeast strain for two-hybrid tests was EGY48 (MATα his3 trp1 ura3 6lexAop-LEU2) (23). Activation of the LEU2 reporter was tested by scoring growth on minimal medium lacking histidine, tryptophan, uracil, and leucine and supplemented with 2% galactose and 1% raffinose. Activation of the lacZ reporter on pSH18-34 (18) was assayed by scoring blue color on galactose-raffinose minimal medium lacking histidine, tryptophan, and uracil and containing 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml.
Generation and testing of HA-esc germ line transformants.
The germ line transformation construct cep420 contains an influenza virus hemagglutinin (HA) epitope-tagged genomic copy of the esc gene. A double-stranded oligonucleotide encoding the HA epitope (88) was inserted into an SfuI site located 3 bp upstream of the normal esc ATG. A 1.1-kb PstI fragment containing the epitope tag was inserted into the context of a 4.2-kb genomic XbaI fragment containing the entire esc gene. This 4.2-kb fragment was then inserted into the pCaSper4 transformation vector (80) to make cep420, which is identical to the esc rescue construct E223-cas (70), except for the epitope tag insertion and elimination of the pCasper4 polylinker EcoRI site. Germ line transformants were generated in a y Df(1)w67c23 (y w) genetic background. Tests for the rescue of esc function were performed with HA-esc gene inserts on the X or third chromosomes as described previously for rescue with genomic esc constructs (70). All three independent HA-esc transformants tested showed rescue of viability to adulthood and produced embryos with normal patterns of abdA homeotic protein expression.
Immunoprecipitations and Western blots.
Preparation of embryo extracts and immunoprecipitations were performed as described previously (15) with modified immunoprecipitation buffer (10 mM HEPES [pH 7.5], 50 mM NaCl, 10% glycerol, 0.1% Triton X-100, 1 mM dithiothreitol [DTT], 1 μM ZnSO4, 1 mM phenylmethylsulfonyl fluoride [PMSF], 2 μg of leupeptin per ml, 2 μg of aprotinin per ml, 1 μg of pepstatin A per ml). Extracts were prepared from 0- to 12-h HA-esc transgenic embryos or y w embryos, which lack the transgene. Ten microliters of mouse monoclonal anti-HA antibody (HA.11 ascites; BAbCo) or 20 μl of rabbit polyclonal anti-E(z) antibody (8) was used to precipitate HA-esc or E(z), respectively, from 300 μg of embryo extract. Precipitates were recovered with protein G-Sepharose (Sigma) or protein A-agarose (Boehringer Mannheim Biochemicals) in combination with anti-HA or anti-E(z) antibody, respectively. Immunodetection of precipitated proteins on Western blots was done with HA.11 (1:10,000) and horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:20,000; Jackson Laboratories) or with anti-E(z) antibody (1:800) and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:50,000; Jackson). Signals were developed with an ECL detection kit (Amersham).
Glutathione S-transferase (GST) fusion constructs.
pGEX-esc contains the full-length esc coding region and was made by inserting a 1.4-kb SfuI-DraI fragment from the e2 cDNA (70) into pGEX-2T (Pharmacia).
pGEX-E(z) constructs were made in the vector pGEX-BgR, which is a modified version of pGEX-2T that contains unique BglII and EcoRI polylinker sites (a gift from Mark Peifer). pGEX-E(z) contains the full-length E(z) coding region and was generated by inserting a 2.5-kb BglII-EcoRI fragment from the Bg-e32 cDNA into pGEX-BgR. Other pGEX-E(z) constructs, containing different portions of the E(z) protein, were made by PCR with a Bg-e32 template and primers that added terminal BglII and EcoRI restriction sites for insertion into pGEX-BgR.
pGEX-EZH1 constructs were made in the vector pGEX-BgRP3i. This vector was made by inserting the BglII-EcoRI polylinker from pPolyIIIi (42) into pGEX-BgR. The full-length EZH1 coding sequence was assembled from the MTO-159 and MTO-163 partial cDNAs (1) in pBC-KS (Stratagene). pGEX-EZH1 was constructed by inserting a 2.3-kb XhoI fragment which contains the entire EZH1 coding region into pGEX-BgRP3i. pGEX-EZH1(1-166) was made by inserting a 0.5-kb XhoI-XbaI fragment encoding residues 1 to 166 into pGEX-BgRP3i. EZH1 cDNAs were kindly provided by Ken Abel.
Expression and purification of GST fusion proteins.
Escherichia coli strains harboring parental or recombinant pGEX plasmids were grown overnight at 37°C in Luria-Bertani medium plus ampicillin. Cultures were diluted 1/20 into 100 ml of fresh medium and grown for 2 h at 37°C, followed by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to 0.5 mM. After induction overnight at 25°C, the cells were pelleted and resuspended in 2 ml of ice-cold lysis buffer (10 mM Tris [pH 8.0], 20% sucrose, 1 mM DTT, 1 mM PMSF), and lysozyme was added to 4 mg/ml. Cells were lysed by incubation for 30 min on ice, followed by the addition of EDTA to 25 mM. After an additional 10 min of incubation, the lysates were briefly sonicated and then centrifuged at 16,000 × g and 4°C for 20 min. GST-E(z) and GST-esc fusion proteins were affinity purified by incubating the supernatants with glutathione-agarose beads (Sigma) at 4°C. Bead-bound proteins were washed six times with 20 mM Tris (pH 7.5)–150 mM NaCl–1 mM DTT–0.1% Triton X-100–1 μM ZnSO4–1 mM PMSF–2 μg of leupeptin per ml, 2 μg of aprotinin per ml, 1 μg of pepstatin A per ml and then stored in this buffer at 4°C. For preparation of GST-E(z), GST-E(z)155-760, and GST-EZH1, N-laurylsarcosine was added to 2% before sonication in order to maximize fusion protein solubilization (16). The N-laurylsarcosine was present during the bead attachment step and was removed during the six subsequent washes prior to storage. The GST-E(z) fusion protein used for the binding assays shown in Fig. 6 was produced and purified by an alternative protocol as described previously (59). The concentration and quality of each affinity-purified protein preparation were assessed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and staining with Coomassie blue. Prior to use in GST pull-down assays, the protein concentrations were equalized by dilution with unbound beads.
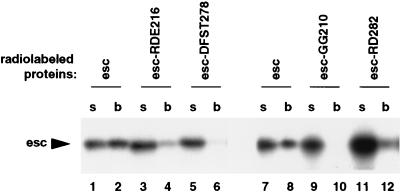
FIG. 6.
Effects of clustered alanine mutations in esc on binding to E(z) protein. Wild-type (esc) or mutant (RDE216, DFST278, GG210, and RD282) esc proteins were tested for binding to GST-E(z) fusion protein in vitro. The RDE216 mutation changes RDE to AAA, DFST278 changes DFST to AFAA, GG210 changes GG to AA, and RD282 changes RD to AA (54). Wild-type and mutant esc proteins were tested as radiolabeled, full-length proteins. Lanes labeled “s” contain 4% of the first supernatant removed after the binding reaction, and lanes labeled “b” contain bound radiolabeled protein after extensive washing. Lanes 1 and 2 and lanes 7 and 8 show two independent binding assays with wild-type esc.
GST pull-down assays.
Radiolabeled esc, E(z), and heed proteins were synthesized by in vitro transcription-translation with the TNT-coupled reticulocyte lysate system (Promega) and [35S]methionine. 35S-esc was produced from the e2 cDNA (70) by use of SP6 RNA polymerase. 35S-E(z) was produced from the cDNA clone e32-55.26 in the vector pBC-SK (Stratagene) by use of T7 RNA polymerase. e32-55.26 is a 5′ deletion derivative of the E(z) cDNA e32 (31) that contains the entire E(z) coding sequence and 46 bp of the 5′-untranslated region (29). 35S-heed was produced from a cDNA clone, hu-e2, which contains the entire heed coding region fused to the 5′- and 3′-untranslated regions of the Drosophila esc e2 cDNA. Radiolabeled heed was synthesized by use of SP6 RNA polymerase. The heed cDNA was kindly provided by Gloria Lee.
Glutathione-agarose-bound fusion proteins were preincubated for 1 h at 4°C in binding buffer (20 mM Tris [pH 7.5], 200 mM NaCl, 1 mM DTT, 0.1% Triton X-100, 1 μM ZnSO4, 1 mM PMSF, 2 μg of leupeptin per ml, 2 μg of aprotinin per ml, 1 μg of pepstatin A per ml, 0.25% bovine serum albumin [BSA]). After blocking, each bead sample was brought to a final volume of 250 μl in fresh buffer. Radiolabeled proteins were precleared by incubation with GST-bound glutathione-agarose beads for 30 min at 4°C. For binding assays, 5 μl of in vitro-translated reaction products was incubated with 250 μl of bead-bound GST-fusion protein for 2 h at 4°C on a rotator. After six washes with 450 μl of binding buffer (lacking BSA), bound radioactive proteins were resuspended in SDS sample buffer and separated by SDS-PAGE. Gels were dried, and radiolabeled proteins were detected by autoradiography. The binding assays shown in Fig. 6 were performed by using a similar protocol (59).
For GST pull-down assays with embryo extracts, glutathione-agarose-bound fusion proteins were equilibrated for 1 h at 4°C in buffer E (10 mM HEPES [pH 7.5], 50 mM NaCl, 0.1% Triton X-100, 10% glycerol, 1 mM DTT, 1 μM ZnSO4, 1 mM PMSF, 2 μg of leupeptin per ml, 2 μg of aprotinin per ml, 1 μg of pepstatin A per ml, 0.25% BSA) and brought to a final volume of 250 μl in fresh buffer E. Approximately 300 μg (15 to 40 μl) of HA-esc or y w embryo extract was added to 250 μl of bead-bound GST-fusion protein and incubated for 40 min at room temperature on a rotator. After six washes with 450 μl of buffer E (lacking BSA), bound proteins were eluted in SDS sample buffer and separated by SDS-PAGE. The HA-esc and E(z) proteins were detected on Western blots as described above for immunoprecipitates.
Site-directed mutagenesis.
Site-directed mutations in E(z) and esc were generated by use of the Altered Sites II in vitro mutagenesis system (Promega). A 2.5-kb KpnI-XbaI fragment from E(z) cDNA e32-55.26 was inserted into pALTER-1 (Promega) to construct pALTER-E(z), which was used as a template to make alanine substitutions. The mutagenic oligonucleotides were 5′-GAG GCGTGGATAAGAGCCTGGGACGAGCACAAC-3′ for the E(z)N40A mutation, 5′-CACAATGTACAGGATGCGTACTGCGAGTCGAAG-3′ for the E(z)L51A mutation, 5′-GATCTGTACTGCGCGTCGAAGGTTTGG-3′ for the E(z)E54A mutation, and 5′-CTGTACTGCGAGTCGGCGGTTTGGCAGGCTAAAC-3′ for the E(z)K56A mutation. Mutant clones were identified by DNA sequencing. Mutant pGEX-E(z) constructs were made by cutting pGEX-E(z) with Eco47III, which cuts within codon 26, and NotI, which cuts at a 3′ vector site, and replacing the wild-type E(z) sequence with the Eco47III-NotI fragments from the mutant pALTER-E(z) clones. Wild-type and mutant pGEX-E(z)1-218 constructs were made by cutting pGEX-E(z) with HindIII and EcoRI, blunting the ends with Klenow enzyme, and self-ligating the plasmids. The generation of alanine substitutions in esc is described elsewhere (54).
RESULTS
Interaction between esc and E(z) in the yeast two-hybrid system.
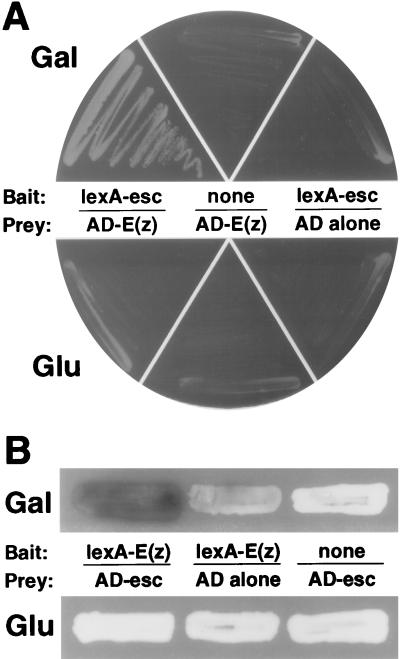
The full-length coding sequences from esc cDNA e2 (70) and E(z) cDNA e32 (31) were each inserted into both the pEG202 and the pJG4-5 vectors (18, 23) to produce LexA and activation domain (AD) fusions, respectively. The constructs were introduced into the yeast strain EGY48, which contains multiple LexA-binding sites in the promoter region of the chromosomal LEU2 gene (23). As shown in Fig. 1, yeast strains that harbor the pEG202-esc and pJG4-5-E(z) constructs were able to grow in the absence of exogenously supplied leucine. Growth was seen only on galactose medium, indicating that it required the expression of AD-E(z) from the galactose-inducible promoter in pJG4-5. Additional controls depicted in Fig. 1 show that growth required both the esc bait and the E(z) prey fusion proteins. These results suggest an interaction between the esc and E(z) proteins.
FIG. 1.
Tests for esc-E(z) interaction in the yeast two-hybrid system. (A) Growth tests with EGY48 cells containing the following combinations of plasmids are shown: left, pEG202-esc and pJG4-5-E(z), which express LexA-esc and AD-E(z), respectively; middle, pRFHMø, which does not produce a LexA protein, and pJG4-5-E(z); right, pEG202-esc and pJG4-5 (no insert). Yeast strains were streaked in parallel on media lacking leucine and containing galactose (top) or glucose (bottom). Expression of AD fusion proteins from pJG4-5 and its derivatives was induced on galactose medium but not on glucose medium. Yeast strains were grown for 4 days at 30°C. (B) X-Gal assays of yeast strains containing the following combinations of plasmids: left, pEG202-E(z) and pJG4-5-esc; middle, pEG202-E(z) and pJG4-5; right, pRFHMø and pJG4-5-esc. Yeast strains were grown for 48 h on X-Gal indicator medium containing galactose (top) or glucose (bottom).
The reciprocal two-hybrid test, with full-length E(z) in the bait configuration and esc in the prey plasmid, was also performed. However, the LexA-E(z) bait produced slow growth on Leu− medium by itself, indicating that LexA-E(z) can weakly activate transcription on its own. To circumvent this complication, we tested the LexA-E(z)–AD-esc combination for activation of the lacZ reporter on plasmid pSH18-34 (18). We found that yeast strains expressing LexA-E(z) and AD-esc and containing the lacZ reporter produced substantially more β-galactosidase than similar yeasts expressing only LexA-E(z) (Fig. 1B). Thus, the reciprocal two-hybrid test suggests a physical interaction between the full-length esc and E(z) proteins expressed in yeast. Independent tests were then pursued to assess the esc-E(z) interaction in the more biologically relevant environment of the Drosophila embryo.
esc and E(z) are associated in Drosophila embryos.
Association of the esc and E(z) proteins in Drosophila embryo extracts was tested by coimmunoprecipitation. To immunoprecipitate and detect E(z) protein from fly embryos, we used an affinity-purified rabbit polyclonal antibody (8). To immunoprecipitate and detect esc protein, we constructed transgenic lines that express an epitope-tagged version of esc. The tag is a single copy of the influenza virus HA epitope (88) inserted at the extreme esc N terminus. This HA-esc protein provides wild-type esc function in vivo, as demonstrated by rescue of lethality and restoration of normal homeotic gene expression in esc null mutant embryos (see Materials and Methods). Figure 2A shows that the anti-HA monoclonal antibody HA.11 detected HA-esc protein at the expected molecular mass (∼50 kDa) on immunoblots of HA-esc transformant embryo extracts (lane 1). Anti-HA antibody specificity was demonstrated by a failure to detect proteins in embryo extracts from the y w strain (Fig. 2A, lane 2), which served as the parental strain for generating the HA-esc transformants.
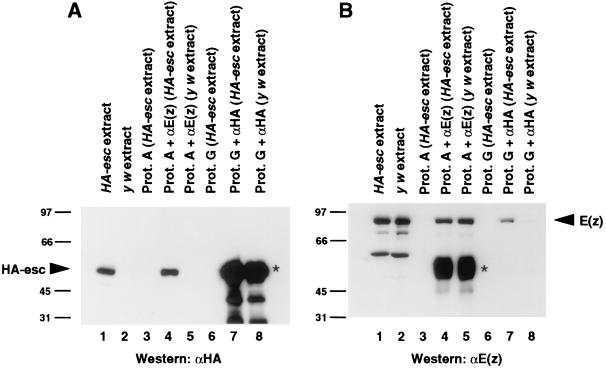
FIG. 2.
Coimmunoprecipitation of HA-esc and E(z) from Drosophila embryo extracts. Proteins were immunoprecipitated from embryo extracts with either anti-HA or anti-E(z) antibodies as indicated. Equal amounts of immunoprecipitates were separated on SDS gels, transferred to nitrocellulose filters, and incubated with anti-HA (A) or anti-E(z) (B) antibodies. Lanes: 1, 30 μg of extract from HA-esc embryos; 2, 30 μg of extract from y w embryos; 3, mock immunoprecipitation from HA-esc extract by protein (Prot.) A-Sepharose without antibody; 4, immunoprecipitation from HA-esc extract with anti-E(z) antibody; 5, immunoprecipitation from y w extract with anti-E(z) antibody; 6, mock immunoprecipitation from HA-esc extract by protein G-Sepharose without antibody; 7, immunoprecipitation from HA-esc extract with anti-HA antibody; 8, immunoprecipitation from y w extract with anti-HA antibody. Bands corresponding to HA-esc and E(z) are indicated by arrowheads. In panel B, lanes 1 and 2, the smaller species are E(z) degradation products. Signals indicated by asterisks in panel A, lanes 7 and 8, and panel B, lanes 4 and 5, are due to cross-reactivity between the secondary antibodies and the heavy chains of the antibodies used in immunoprecipitations. Numbers at left of panels are kilodaltons.
To test for coimmunoprecipitation, anti-E(z) antibodies were used to immunoprecipitate proteins from HA-esc embryo extracts. Equal amounts of the precipitated protein samples were electrophoresed on separate gels, immunoblotted, and probed with either anti-HA (Fig. 2A) or anti-E(z) (Fig. 2B) antibodies. Figure 2B, lanes 1 and 2, shows detection of the 89-kDa E(z) protein in the embryo extracts, and lane 4 shows that the E(z) protein was immunoprecipitated by anti-E(z) antibodies. Figure 2A, lane 4, shows that HA-esc was coimmunoprecipitated in this same sample. Neither protein was precipitated by protein A-Sepharose alone (Fig. 2, lanes 3). In addition, the band corresponding to HA-esc was not detected in anti-E(z) antibody-precipitated material from control y w extracts (compare lanes 5 in Fig. 2).
We also performed the reciprocal coimmunoprecipitation test for esc-E(z) association. Anti-HA antibodies were used to immunoprecipitate HA-esc from HA-esc embryo extracts, and the precipitated proteins were separately probed for the presence of HA-esc and E(z). Detection of HA-esc in this immunoprecipitate was obscured by signal from the immunoglobulin heavy chains, which migrated at a similar position (Fig. 2A, lane 7). However, coimmunoprecipitation of E(z) was clearly detected in this sample (Fig. 2B, lane 7). Controls show that this E(z) signal required precipitation with anti-HA antibodies (Fig. 2B, lane 6) and the use of HA-esc transformant embryo extract (Fig. 2B, lane 8). These reciprocal tests indicate that the esc and E(z) proteins are associated in vivo. We note that E(z) appeared less efficiently coimmunoprecipitated by anti-HA antibodies than was HA-esc by anti-E(z) antibodies (Fig. 2A, lanes 1 and 4; Fig. 2B, lanes 1 and 7). This result may reflect independence of a relatively greater proportion of E(z) from esc in vivo. Alternatively, it may simply be due to a lower efficiency of immunoprecipitation by anti-HA antibodies. The immunoglobulin signal (Fig. 2A, lane 7) precluded resolution of these alternatives.
esc and E(z) interact directly in vitro.
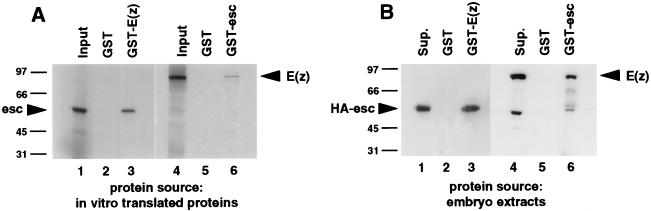
In vitro binding assays were performed to test for direct interactions between the esc and E(z) proteins (Fig. 3A). A full-length GST-E(z) fusion protein was purified from E. coli, immobilized on glutathione-agarose beads, and tested for binding to radiolabeled esc protein produced by in vitro translation. Radiolabeled esc was incubated with equal amounts of GST or GST-E(z) attached to beads and, after extensive washing, binding was assessed by SDS-PAGE analysis of the bead samples. Radiolabeled esc protein bound to GST-E(z) (Fig. 3A, lane 3) but not to GST alone (lane 2). In reciprocal pull-down experiments, radiolabeled E(z) protein bound to GST-esc (Fig. 3A, lane 6) but not to GST alone (lane 5). Thus, esc and E(z) are able to bind to each other in vitro in the absence of other cellular proteins.
FIG. 3.
In vitro binding of the esc and E(z) proteins. (A) Autoradiographs of SDS gels. Radiolabeled esc protein (lanes 1 to 3) and radiolabeled E(z) protein (lanes 4 to 6) were tested for binding to GST or GST fusion proteins as indicated. The input lanes (1 and 4) contain 4% the amount of radiolabeled protein used in the binding assays. (B) Western blots. Proteins from Drosophila HA-esc embryo extracts were tested for binding to GST or GST fusion proteins. Proteins that bound to the indicated GST or GST fusion proteins were separated by SDS-PAGE, transferred to nitrocellulose, and incubated with anti-HA (lanes 1 to 3) or anti-E(z) (lanes 4 to 6) antibodies. Lanes labeled “Sup.” contain 4% the unbound material recovered after incubation of the HA-esc extracts with the GST-E(z) (lane 1) or GST-esc (lane 4) fusion proteins. Numbers at left of panels are kilodaltons.
Interaction between purified fusion proteins and embryonic proteins.
We wished to assess if the direct esc-E(z) interactions seen with proteins produced in vitro accurately reflect the binding properties of their in vivo counterparts. To do this, GST-esc and GST-E(z) were tested for binding to their respective partners from Drosophila embryo extracts. GST-esc and GST-E(z) were attached separately to glutathione-agarose beads and incubated with HA-esc embryo extracts, and immunoblots of the bead-associated proteins were probed with anti-HA or anti-E(z) antibodies. Figure 3B shows that GST-E(z) and GST-esc bound to the HA-esc and E(z) proteins, respectively, present in embryonic extracts (lanes 3 and 6). Based upon the experiments shown in Fig. 3, we conclude that the esc and E(z) proteins are direct binding partners.
The esc-interacting domain of E(z) lies within an N-terminal 33-amino-acid region.
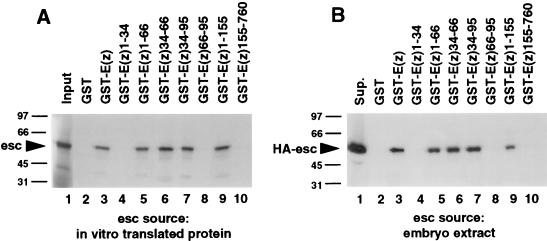
The portion of E(z) that is responsible for the interaction with esc was mapped by testing multiple deletion-derivative GST-E(z) fusion proteins for esc binding in vitro. As shown in Fig. 4A, the ability of GST-E(z)1-155 to bind to radiolabeled esc (lane 9) and the failure of GST-E(z)155-760 to bind to esc (lane 10) localized the interaction domain to an N-terminal region of E(z). The interaction domain was further delimited by subdividing this N-terminal region. The smallest fusion protein that bound to radiolabeled esc was GST-E(z)34-66 (Fig. 4A, lane 6). Fusion proteins that do not include this 33-amino-acid region did not interact with esc (Fig. 4A, lanes 4, 8, and 10).
FIG. 4.
Localization of the E(z) domain that mediates binding to esc protein. GST, GST-E(z) (full length, amino acids 1 to 760), and GST-E(z) deletion derivatives were tested for binding to radiolabeled esc protein produced by in vitro translation (A) or HA-esc protein from embryo extracts (B). The GST fusion proteins used are indicated above the lanes; the numbers refer to E(z) amino acids included in each fusion protein. (A) Autoradiograph of SDS gel. (B) Western blot probed with anti-HA antibodies. Lanes labeled “Input” and “Sup.” are as described in the legend to Fig. 3. Numbers at left of panels are kilodaltons.
Figure 4B shows tests of the same truncated GST-E(z) fusion proteins for binding to HA-esc from Drosophila embryo extracts. GST fusion proteins were immobilized on beads and incubated with HA-esc embryo extracts, and the bead-bound proteins were immunoblotted and probed with anti-HA antibodies. The results obtained for binding to embryonic HA-esc paralleled the binding seen for in vitro-translated esc (compare Fig. 4B and A). In particular, GST-E(z)34-66 bound to HA-esc from extracts (Fig. 4B, lane 6) with about the same efficiency as did the full-length GST-E(z) fusion protein (lane 3), and HA-esc binding correlated with the presence of E(z) amino acids 34 to 66 in the fusion protein used. Taken together, these results indicate that the esc-interacting domain of E(z) maps to the interval from amino acids 34 to 66.
Effects of E(z) point mutations on the esc interaction.
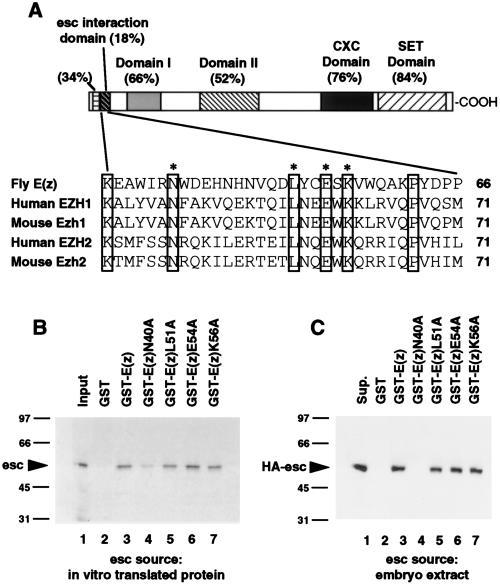
Figure 5A shows the sequence of the pertinent E(z) 33-amino-acid region and its location relative to identified homology domains (24, 25, 31, 39). We were surprised to find that only 6 of 33 amino acids in this region are identical among fly E(z), the murine homologs Ezh1 (39) and Ezh2 (25), and the human homologs EZH1 (1) and EZH2 (11, 24, 39). The amino acid sequences among these E(z) homologs are much more highly conserved in domains outside this region.
FIG. 5.
Effects of E(z) point mutations on binding to esc protein. (A) Diagram of E(z) protein and amino acid sequence of the esc-interacting domain. (Top) Evolutionarily conserved E(z) regions are depicted as shaded or hatched boxes. Names of these domains and/or percent identities between fly E(z) and mammalian homologs are indicated. (Bottom) Alignment of the esc-interacting domain (amino acids 34 to 66) with homologous regions of the human EZH1 and EZH2 proteins and mouse Ezh1 and Ezh2 proteins (amino acids 39 to 71). Residues that are identical in all five proteins are boxed. Asterisks indicate E(z) residues that were substituted by alanine in this study. (B) Effects of E(z) alanine substitutions on binding to in vitro-translated esc protein. GST fusion proteins containing E(z) amino acids 1 to 218 and the indicated substitution mutations were incubated with radiolabeled esc protein. Equal amounts of bound protein samples were electrophoresed on an SDS gel. Relative band intensities on the autoradiograph were measured with the spot densitometry feature of the AlphaImager 2000 gel documentation system (Alpha Innotech). (C) Effects of E(z) alanine substitutions on binding to HA-esc from embryo extracts. Mutant GST fusion proteins were incubated with HA-esc embryo extracts. Bound proteins were separated by SDS-PAGE and transferred to nitrocellulose, and HA-esc protein was detected with anti-HA antibodies. Lanes labeled “Input” and “Sup.” are as described in the legend to Fig. 3. Numbers at left of panels B and C are kilodaltons.
To identify individual residues required for the interaction with esc, site-directed point mutations that substitute evolutionarily conserved E(z) residues with alanine were introduced. The mutant proteins were purified from E. coli as GST fusion proteins that included E(z) amino acids 1 to 218. GST pull-down assays were performed to test for in vitro binding to radiolabeled esc protein (Fig. 5B). The L51A, E54A, and K56A mutations had no detectable effect on esc binding (Fig. 5B, lanes 5 to 7). However, the N40A mutation reduced binding to radiolabeled esc by approximately 3.5-fold (Fig. 5B, lane 4).
The same mutant fusion proteins were also tested for binding to HA-esc from embryo extracts (Fig. 5C). HA-esc interacted about as well with wild-type E(z) (Fig. 5C, lane 3) as with the L51A, E54A, and K56A mutants (lanes 5 to 7), paralleling the results obtained with radiolabeled esc (Fig. 5B). However, the reduction in HA-esc binding seen with the N40A mutant (Fig. 5C, lane 4) was even more dramatic than the reduction in binding to radiolabeled esc (Fig. 5B, lane 4). Thus, of the four highly conserved residues tested, only N40 appears to be critically needed for binding to esc. Presumably other residues, which are not identical in E(z) homologs and which were not altered in this study, also contribute to the interaction.
Conserved regions in the esc protein are required for E(z) interaction.
The esc protein is 425 amino acids long and consists largely of multiple WD repeats, which occupy amino acids 68 to 425 (22, 66, 70). The crystal structure of another WD repeat protein, the G protein β subunit, has revealed that WD repeats form a circular structure, termed a β propeller (72, 85). The β propeller is built from β-strand stacks, called β blades, that are arranged side by side, like slices of a pie (see reference 53 for a review); each β blade in the tertiary structure corresponds approximately to a WD repeat in the primary sequence. Homology modeling predicts that the esc protein folds into a seven-bladed β propeller (54).
We wished to identify portions of the esc protein that contribute to the interaction with E(z). However, simple deletion analysis of esc was unlikely to prove useful; since the β propeller requires physical interactions between elements encoded by disparate parts of the primary sequence, simple unidirectional or internal deletions seemed likely to disrupt the overall protein fold. Instead, we tested clustered alanine substitutions in regions of the esc protein predicted to form loops that extend above the plane of the β propeller toroid (54). Loop residues were chosen for mutagenesis based upon two criteria: (i) they are likely to be accessible on the protein surface and thus available for partner contact, and (ii) particular loop regions display high evolutionary conservation (54) and thus are likely important for function.
Four esc mutants with clustered alanine substitutions, RDE216AAA, DFST278AFAA, GG210AA, and RD282AA, were tested for binding to E(z). Beginning with amino acid 216, the residues RDE map within the loop that connects β blades 3 and 4; the mutation replaces all three residues with alanine. The GG210 mutation alters residues in the same predicted loop. DFST278 and RD282 are located in a different loop that connects β blades 4 and 5. The mutant esc proteins were synthesized and radiolabeled by in vitro translation and then tested for interaction with GST-E(z) fusion proteins in vitro. As shown in Fig. 6, all four esc mutants showed reduced binding to full-length GST-E(z) protein as compared to the wild type (lanes 2, 4, 6, 8, 10, and 12). We conclude that residues in two conserved esc loop regions contribute to the E(z) interaction in vitro. In agreement with this conclusion, these same mutations reduced esc function in vivo (54). However, it remains possible that other parts of esc also participate in E(z) binding.
In vitro interaction between human eed and EZH1.
A mouse homolog with 55% amino acid sequence identity to fly esc, called eed, was recently identified (67). Mutations in eed cause posterior transformations in mouse embryos, indicating that esc and eed are functional homologs. A human eed gene that encodes a protein 100% identical to mouse eed also has been identified (43). The mammalian eed proteins bear extensive homology to fly esc in the loop regions targeted by the mutations described above. In particular, 12 of 12 consecutive amino acids are identical in the predicted loop between β blades 3 and 4, and 16 of 16 residues are identical in the loop between β blades 4 and 5. Thus, all of the residues altered in the four esc mutants described above are identical in these mammalian proteins.
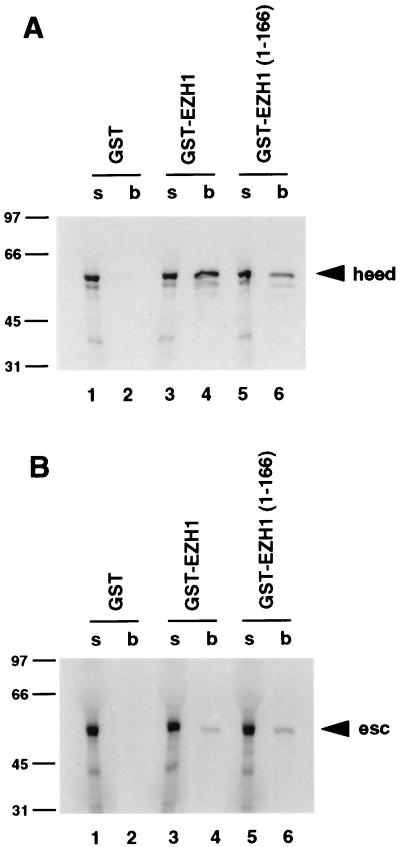
In contrast, the esc-interacting region of E(z) is not very well conserved (Fig. 5A). Given these differences in conservation, we wondered if the mammalian homologs of E(z) and esc are able to directly interact. To test this, we performed in vitro binding assays with human eed (heed) and one of the two human E(z) homologs, EZH1 (1). GST-EZH1, which includes the full-length EZH1 polypeptide, was attached to glutathione-agarose beads and incubated with radiolabeled heed. As shown in Fig. 7A, heed bound to GST-EZH1 (lane 4) but not to GST alone (lane 2). Radiolabeled heed also bound to a GST fusion protein that contains EZH1 residues 1 to 166 (lane 6); this protein contains the region (EZH1 residues 39 to 70) that corresponds to the esc-interacting domain of E(z). Both GST-EZH1 and GST-EZH1(1-166) also bound to radiolabeled Drosophila esc, but with a lower efficiency than to the human protein (Fig. 7B, lanes 4 and 6). This reduced binding is consistent with the divergence of the EZH1 sequence in the esc-interacting region. This result suggests that amino acids other than those that are evolutionarily conserved between E(z) and EZH1 participate in the interaction with esc. It also suggests that esc residues outside the absolutely conserved portions of the loops connecting β blades 3, 4, and 5 are involved in binding to E(z) and EZH1. Additional studies will be required to further map the interacting domains of heed and EZH1 and to demonstrate their in vivo association.
FIG. 7.
Tests for in vitro binding of human EZH1 to human eed (heed) and Drosophila esc proteins. Autoradiographs of SDS gels are shown. (A) Radiolabeled, full-length heed was tested for binding to GST alone, full-length human EZH1 (GST-EZH1), or an N-terminal portion of EZH1 [GST-EZH1(1-166)]. (B) Radiolabeled, full-length Drosophila esc protein was tested for binding to the same GST fusion proteins as in panel A. Lanes labeled “s” and “b” contain unbound supernatant and bound protein samples, respectively, as described in the legend to Fig. 6. Numbers at left of panels are kilodaltons.
DISCUSSION
Evolutionary conservation of the esc-E(z) relationship.
We have presented evidence for direct physical interaction between the fly esc and E(z) proteins in vitro and for the association of these proteins in fly embryonic extracts. We have also presented evidence for binding between human esc and E(z) homologs in vitro. These results suggest that esc and E(z) are molecular partners in Pc-G repression and that the two proteins may have coevolved to maintain their interaction. Indeed, there is evidence that the biological functions of both esc and E(z) have been conserved in their mammalian homologs. Mouse embryos that are homozygous for mutant alleles in the esc homolog, eed, display posteriorly directed homeotic transformations of axial skeletal structures (67). As has been shown for the corresponding fly mutants (71, 76), this phenotype likely reflects derepression of Hox genes along the anterior-posterior axis. Since mouse and human eed proteins are 100% identical (43), it is likely that heed plays a similar role in human embryogenesis. Mice with mutations in the E(z) homologs Ezh1 and Ezh2 have not been described. However, overexpression of either fly E(z) or human EZH2 in Drosophila reduces the expression of a copy of the white gene, which exhibits position effect variegation due to its juxtaposition with heterochromatin (39). This finding suggests that at least some of the biochemical activities of E(z) homologs have also been conserved.
Genetic analyses of vertebrate homologs have revealed the functional conservation of several additional Pc-G proteins. Knockout mutations of the mouse bmi1 and mel-18 genes, which are homologs of the Drosophila Posterior sex combs (Psc) and Suppressor 2 of zeste [Su(z)2] genes (4, 33, 84), produce posterior homeotic transformations (2, 83). Homeotic phenotypes are also produced by a null mutation in mouse M33, which is a homolog of fly Pc (14). Furthermore, the expression of mouse M33 protein in Drosophila partially rescues Pc mutant phenotypes (51). Given the functional conservation of multiple components, it is likely that the Drosophila Pc-G proteins and their mammalian counterparts use similar biochemical mechanisms for transcriptional repression of homeotic genes.
Evolutionary conservation of the esc-E(z) partner relationship is supported by recent studies of two Caenorhabditis elegans maternal-effect sterile genes, mes-2 and mes-6. Mutant alleles of either mes-2 or mes-6 produce grandchildless phenotypes that result from the limited proliferation and death of germ cells (7, 57). MES-2 and MES-6 share sequence similarity with E(z) and esc, respectively, across extensive portions of these proteins (26, 37a). However, unlike E(z) and esc functions, mes-2 and mes-6 functions appear to be restricted to the germ line, and thus far, there is no evidence for their involvement in Hox gene control. Thus, the developmental roles of MES-2 and MES-6 in worms are distinct from those of their E(z) and esc homologs in flies and mammals. Nevertheless, there is evidence that the basic biochemical partnership between these proteins has been conserved. The spatial and temporal patterns of MES-2 and MES-6 accumulation in nuclei are identical, and mutations in either gene disrupt the spatial distribution or stability of the other protein (26, 37a). One of these mutations, mes-6bn66, substitutes a residue at a position that aligns with the predicted loop region of esc that is required for binding to E(z) protein (37a, 54) (Fig. 6). Taken together, these results are consistent with a direct physical interaction between MES-2 and MES-6 in vivo. The conservation of these worm homologs, together with the evolutionary divergence of their developmental functions, may reflect a common biochemical role in chromatin that has been adapted for use in different cell lineages in worms and flies.
Approximately 90% of the C. elegans genome has been sequenced. Database searches so far have failed to reveal homologs for Pc-G genes besides E(z) and esc (37a). Thus, these E(z) and esc homologs may function independently from the rest of the Pc-G in C. elegans. If this idea is correct, it may imply a biochemical function of esc-E(z) complexes in Drosophila that is at least partially independent of other Pc-G members. It has been suggested, based upon genetic interactions in Drosophila, that esc and E(z) may belong to a subset of the Pc-G that shares common functions distinct from those of other Pc-G members (6, 12).
Recently, an Arabidopsis gene called curly leaf (clf) was shown to share sequence similarity with E(z) (20). Strikingly, clf is required for stable repression of agamous, a floral homeotic gene. Like E(z), clf is needed to maintain silencing of the target gene, rather than to initiate the repressed state. Thus, the role of E(z)-related proteins in the maintenance of transcriptional repression appears to have been evolutionarily conserved across kingdoms. To date, other Pc-G homologs in plants have not been characterized. However, a cDNA that encodes a protein with sequence similarity to fly esc has been identified in an Arabidopsis expressed sequence tag database (19), suggesting that at least these two partners may coexist in plants.
The esc-E(z) interaction and Pc-G-mediated silencing.
Evidence is accumulating that Pc-G proteins function as components of heteromeric complexes. Originally, proposals about Pc-G complexes were based upon the similar phenotypes produced by mutations in different Pc-G genes and the phenotypic enhancement seen with double and triple Pc-G mutant combinations (6, 12, 32, 37, 41). Cytological evidence for Pc-G complexes derives from the extensive colocalization at sites on polytene chromosomes of all Pc-G proteins analyzed to date (8, 17, 46, 47, 59, 65). Biochemical evidence for Pc-G protein associations is provided by coimmunoprecipitation of Pc with ph (17) and esc with E(z) (Fig. 2) from embryonic extracts. Furthermore, mouse homologs of Psc and ph coimmunoprecipitate with each other and with mouse Pc (3), and human homologs of Psc and ph coimmunoprecipitate and cofractionate on sucrose gradients (21).
The availability of temperature-sensitive E(z) mutations (30, 60) has allowed assessment of the role of E(z) in Pc-G complexes in polytene nuclei. Heat inactivation of temperature-sensitive E(z) proteins abolishes polytene chromosome binding by Psc, Su(z)2, ph, and Pc at most loci (63, 65). These observations implicate E(z) in the formation and/or stabilization of Pc-G complexes on chromosomes.
Less is known about the possible roles of esc in complexes of Pc-G proteins. Unlike E(z), esc is primarily required early in embryogenesis (70, 77), overlapping the time when Pc-G-mediated repression of homeotic genes is first established. E(z) is also required at this early stage and is then continuously needed during embryonic, larval, and pupal development to maintain repression (30, 60, 68). An intriguing possibility is that esc may help recognize the initial repressed state of homeotic genes, mediated by the segmentation gene products, and may play a role in the transition to repression by the Pc-G (22, 66, 70). In this scenario, E(z) could be recruited through its direct interaction with esc and could help assemble other Pc-G proteins onto the target gene. Once a silencing complex is assembled, the stable association of E(z) may involve interactions with other Pc-G proteins, and esc may no longer be required. To evaluate this and other models, it will be necessary to determine which Pc-G components exist together in stable complexes.
Functional domains in the E(z) and esc proteins.
Sequence alignments of E(z) and its homologs (1, 11, 20, 24, 25, 39) (Fig. 5) indicate that the E(z) protein is composed of multiple functional domains. Genetic and molecular analyses of E(z) mutant alleles (8, 31) show that several of these homology domains are required for E(z) function. For example, heat inactivation of the temperature-sensitive E(z)28 or E(z)61 proteins causes the mutant E(z) protein, as well as other Pc-G proteins, to dissociate from chromosomes. The E(z)28 and E(z)61 mutations map to the conserved domain II and CXC domain, respectively (8), suggesting that these domains may bind to other Pc-G proteins and that these associations may be mutually required for stable chromosomal binding. In addition, E(z) contains a highly conserved C-terminal SET domain, which is present in many other proteins (39, 73, and references therein). All characterized SET domain proteins are chromosomal proteins, and they have been implicated in aspects of transcriptional regulation that may involve chromatin structure modification (8, 13, 38, 81, 82). Although the biochemical activity of the SET domain has yet to be defined, its conservation in functionally related proteins suggests an interaction with a common protein partner or substrate in chromatin. In vitro tests indicate that the domain lacks DNA-binding activity (28).
In contrast to E(z), the esc protein appears to be composed of a single major domain. Aside from a short N-terminal region, which includes a PEST sequence (70), most of the esc protein is occupied by WD repeats, which are predicted to fold into a β-propeller structure (54, 85). In the vast majority of cases, the biochemical function of WD repeat domains is to bind to other proteins (52). In addition, some β propellers simultaneously contact multiple binding partners (85). Thus, the esc protein may act as a scaffold for protein contacts that contribute to the assembly or organization of a multiprotein complex. Our analysis indicates that one of these contacts is to an N-terminal domain in the E(z) protein (Fig. 4). Given its limited domain organization, the main role of the esc protein in the context of esc-E(z)-containing complexes may be to position or tether the E(z) functional domains. These domains could then act by binding other Pc-G proteins or recruiting other proteins with enzymatic roles.
Regulatory roles of the E(z) protein that are independent from esc.
We have provided in vitro and in vivo evidence for a functional partnership between the E(z) and esc proteins. However, several lines of evidence indicate that Drosophila E(z) and esc are not obligate partners at all loci and during all developmental stages. First, although both proteins display uniform spatial distributions in nuclei of blastoderm and early gastrulation-stage embryos, the esc protein is much more limited than E(z) in mid-to-late-stage embryos (8, 22). Second, there are target genes other than homeotic genes that require E(z), but not esc, for repression during development (49, 58). These examples show that the E(z) protein can be recruited to and function at target loci without assistance from the esc protein. Presumably, E(z) action at these loci involves alternative binding partners. The ability of E(z) to sometimes act independently of esc is one possible explanation for the differential efficiencies of reciprocal coimmunoprecipitations seen in Fig. 2.
It has been suggested that, besides its role in Pc-G repression, E(z) may also be involved in the maintenance of transcriptional activity by trx-G proteins (40). In contrast, the known role of the esc protein is limited to repression. A dual role for E(z) could reflect participation in more than one type of protein complex. For example, when E(z) is bound to esc, it is a component of silencing complexes. However, when E(z) is associated with other, as-yet-unidentified proteins, it may contribute to trx-G-mediated transcriptional activation. In support of this idea, heat inactivation of temperature-sensitive E(z) proteins reduces chromosomal binding by the trx protein (38). To assess these possibilities, it will be necessary to define the number and constituents of E(z)-containing complexes isolated from fly embryos.
ACKNOWLEDGMENTS
We thank Susan Strome for valuable discussions, comments on the manuscript, and sharing information prior to publication. We thank Gloria Lee for providing heed cDNA and for sharing information prior to publication, Ken Abel for providing EZH1 cDNAs, and Justin Goodrich for sharing unpublished information. We thank Yong Ma, Ellen Miller, and Chris Schwartz for generating plasmid constructs used in this work. We thank Doug Bornemann for input on GST pull-down experiments and Andre Silvanovich and Tom Hays for advice on epitope tag reagents. We thank Judy Berman for comments on the manuscript.
This work was supported by NIH grant GM49850 to J.S. and NIH grant GM46567 to R.S.J.
REFERENCES
- 1.Abel K J, Brody L C, Valdes J M, Erdos M R, McKinley D R, Castilla L H, Merajver S D, Couch F J, Friedman L S, Ostermeyer E A, Lynch E D, King M-C, Welcsh P L, Osborne-Lawrence S, Spillman M, Bowcock A M, Collins F S, Weber B L. Characterization of EZH1, a human homolog of Drosophila Enhancer of zeste near BRCA1. Genomics. 1996;37:161–171. doi: 10.1006/geno.1996.0537. [DOI] [PubMed] [Google Scholar]
- 2.Akasaka T, Kanno M, Balling R, Antonio Mieza M, Taniguchi M, Koseki H. A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development. 1996;122:1513–1522. doi: 10.1242/dev.122.5.1513. [DOI] [PubMed] [Google Scholar]
- 3.Alkema M J, Bronk M, Verhoeven E, Otte A, van’t Veer L J, Berns A, van Lohuizen M. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian Polycomb complex. Genes Dev. 1997;11:226–240. doi: 10.1101/gad.11.2.226. [DOI] [PubMed] [Google Scholar]
- 4.Brunk B P, Martin E C, Adler P N. Drosophila genes Posterior Sex Combs and Suppressor two of zeste encode proteins with homology to the murine bmi-1 oncogene. Nature. 1991;353:351–353. doi: 10.1038/353351a0. [DOI] [PubMed] [Google Scholar]
- 5.Bunker C A, Kingston R E. Transcriptional repression by Drosophila and mammalian Polycomb group proteins in transfected mammalian cells. Mol Cell Biol. 1994;14:1721–1732. doi: 10.1128/mcb.14.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell R B, Sinclair D A R, Couling M, Brock H W. Genetic interactions and dosage effects of Polycomb group genes of Drosophila. Mol Gen Genet. 1995;246:291–300. doi: 10.1007/BF00288601. [DOI] [PubMed] [Google Scholar]
- 7.Capowski E E, Martin P, Garvin C, Strome S. Identification of grandchildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics. 1991;129:1061–1072. doi: 10.1093/genetics/129.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrington E C, Jones R S. The Drosophila Enhancer of zeste gene encodes a chromosomal protein: examination of wild-type and mutant protein distribution. Development. 1996;122:4073–4083. doi: 10.1242/dev.122.12.4073. [DOI] [PubMed] [Google Scholar]
- 9.Carroll S B, Laymon R A, McCutcheon M A, Riley P D, Scott M P. The localization and regulation of the Antennapedia protein expression in Drosophila embryos. Cell. 1986;47:113–122. doi: 10.1016/0092-8674(86)90372-7. [DOI] [PubMed] [Google Scholar]
- 10.Celniker S E, Keelan D J, Lewis E B. The molecular genetics of the bithorax complex of Drosophila: characterization of the product of the Abdominal-B domain. Genes Dev. 1989;3:1424–1436. doi: 10.1101/gad.3.9.1424. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Rossier C, Antonarakis S E. Cloning of a human homolog of the Drosophila Enhancer of zeste gene (EZH2) that maps to chromosome 21q22.2. Genomics. 1996;38:30–37. doi: 10.1006/geno.1996.0588. [DOI] [PubMed] [Google Scholar]
- 12.Cheng N N, Sinclair D A R, Campbell R B, Brock H W. Interaction of Polyhomeotic with Polycomb group genes of Drosophila melanogaster. Genetics. 1994;138:1151–1162. doi: 10.1093/genetics/138.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinwalla V, Jane E P, Harte P J. The Drosophila trithorax protein binds to specific chromosomal sites and is co-localized with Polycomb at many sites. EMBO J. 1995;14:2056–2065. doi: 10.1002/j.1460-2075.1995.tb07197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Core N, Bel S, Gaunt S J, Aurrand-Lions M, Pearce J, Fisher A, Djabali M. Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development. 1997;124:721–729. doi: 10.1242/dev.124.3.721. [DOI] [PubMed] [Google Scholar]
- 15.Dingwall A K, Beek S J, McCallum C M, Tamkun J W, Kalpana G V, Goff S P, Scott M P. The Drosophila snr1 and brm proteins are related to yeast SWI/SNF proteins and are components of a large protein complex. Mol Biol Cell. 1995;6:777–791. doi: 10.1091/mbc.6.7.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangioni J V, Neel B G. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- 17.Franke A, DeCamillis M, Zink D, Cheng N, Brock H W, Paro R. Polycomb and polyhomeotic are constituents of a multimeric protein complex in chromatin of Drosophila melanogaster. EMBO J. 1992;11:2941–2950. doi: 10.1002/j.1460-2075.1992.tb05364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golemis E A, Gyuris J, Brent R. Two hybrid systems/interaction traps. In: Ausubel F M, Brent R, Kingston R, Moore D, Seidman J, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 13.14.1–13.14.17. [Google Scholar]
- 19.Goodrich, J. Personal communication.
- 20.Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz E M, Coupland G. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997;386:44–51. doi: 10.1038/386044a0. [DOI] [PubMed] [Google Scholar]
- 21.Gunster M J, Satijn D P, Hamer K M, den Blaauwen J L, de Bruijn D, Alkema M J, van Lohuizen M, van Driel R, Otte A P. Identification and characterization of interactions between the vertebrate polycomb-group protein BMI1 and human homologs of polyhomeotic. Mol Cell Biol. 1997;17:2326–2335. doi: 10.1128/mcb.17.4.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutjahr T, Frei E, Spicer C, Baumgartner S, White R A H, Noll M. The Polycomb-group gene, extra sex combs, encodes a nuclear member of the WD-40 repeat family. EMBO J. 1995;14:4296–4306. doi: 10.1002/j.1460-2075.1995.tb00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 24.Hobert O, Jallal B, Ullrich A. Interaction of Vav with ENX-1, a putative transcriptional regulator of homeobox gene expression. Mol Cell Biol. 1996;16:3066–3073. doi: 10.1128/mcb.16.6.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobert O, Sures I, Ciossek T, Fuchs M, Ullrich A. Isolation and developmental expression analysis of Enx-1, a novel mouse Polycomb group gene. Mech Dev. 1996;55:171–184. doi: 10.1016/0925-4773(96)00499-6. [DOI] [PubMed] [Google Scholar]
- 26.Holdeman, R., S. Nehrt, and S. Strome. The Polycomb group MES-2, a maternal protein essential for viability of the germline in C. elegans, is homologous to a Drosophila Polycomb group protein. Submitted for publication. [DOI] [PubMed]
- 27.Ingham P W, Martinez-Arias A. The correct activation of Antennapedia and bithorax complex genes requires the fushi tarazu gene. Nature. 1986;324:592–597. doi: 10.1038/324592a0. [DOI] [PubMed] [Google Scholar]
- 28.Jones, C. A., and R. S. Jones. Unpublished data.
- 29.Jones, R. S. Unpublished data.
- 30.Jones R S, Gelbart W M. Genetic analysis of the Enhancer of zeste locus and its role in gene-regulation in Drosophila melanogaster. Genetics. 1990;126:185–199. doi: 10.1093/genetics/126.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones R S, Gelbart W M. The Drosophila Polycomb-group gene Enhancer of zeste contains a region with sequence similarity to trithorax. Mol Cell Biol. 1993;13:6357–6366. doi: 10.1128/mcb.13.10.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurgens G. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature. 1985;316:153–155. [Google Scholar]
- 33.Kanno M, Hasegawa M, Ishida A, Isono K, Taniguchi M. mel-18, a Polycomb-group-related mammalian gene, encodes a transcriptional negative regulator with tumor suppressive activity. EMBO J. 1995;14:5672–5678. doi: 10.1002/j.1460-2075.1995.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karch F, Bender W, Weiffenbach B. abdA expression in Drosophila embryos. Genes Dev. 1990;4:1573–1587. doi: 10.1101/gad.4.9.1573. [DOI] [PubMed] [Google Scholar]
- 35.Kaufman T C, Lewis R, Wakimoto B. Cytogenetic analysis of chromosome 3 in Drosophila melanogaster: the homeotic gene complex in polytene interval 84A-B. Genetics. 1980;94:115–133. doi: 10.1093/genetics/94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennison J A. Transcriptional activation of Drosophila homeotic genes from distant regulatory elements. Trends Genet. 1993;9:75–79. doi: 10.1016/0168-9525(93)90227-9. [DOI] [PubMed] [Google Scholar]
- 37.Kennison J A, Tamkun J W. Dosage-dependent modifiers of Polycomb and Antennapedia mutations in Drosophila. Proc Natl Acad Sci USA. 1988;85:8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Korf, I., R. Holdeman, Y. Fan, and S. Strome. The Polycomb group in C. elegans and maternal control of germline development. Submitted for publication. [DOI] [PubMed]
- 38.Kuzin B, Tillib S, Sedkov Y, Mizrokhi L, Mazo A. The Drosophila trithorax gene encodes a chromosomal protein and directly regulates the region-specific homeotic gene fork head. Genes Dev. 1994;8:2478–2490. doi: 10.1101/gad.8.20.2478. [DOI] [PubMed] [Google Scholar]
- 39.Laible G, Wolf A, Dorn R, Reuter G, Nislow C, Lebersorger A, Popkin D, Pillus L, Jenuwein T. Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. EMBO J. 1997;16:3219–3232. doi: 10.1093/emboj/16.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaJeunesse D, Shearn A. E(z): a polycomb group gene or a trithorax group gene? Development. 1996;122:2189–2197. doi: 10.1242/dev.122.7.2189. [DOI] [PubMed] [Google Scholar]
- 41.Landecker H L, Sinclair D A R, Brock H W. Screen for enhancers of Polycomb and Polycomblike in Drosophila melanogaster. Dev Genet. 1994;15:425–434. doi: 10.1002/dvg.1020150505. [DOI] [PubMed] [Google Scholar]
- 42.Lathe R, Vilotte J L, Clark A J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57:193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- 43.Lee, G. Personal communication.
- 44.Lewis E B. Genetic control and regulation of developmental pathways. In: Locke M, editor. Role of chromosomes in development. New York, N.Y: Academic Press, Inc.; 1964. pp. 231–252. [Google Scholar]
- 45.Lewis E B. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 46.Lonie A, D’Andrea R, Paro R, Saint R. Molecular characterization of the Polycomblike gene of Drosophila melanogaster, a trans-acting negative regulator of homeotic gene expression. Development. 1994;120:2629–2636. doi: 10.1242/dev.120.9.2629. [DOI] [PubMed] [Google Scholar]
- 47.Martin E C, Adler P N. The Polycomb group gene Posterior sex combs encodes a chromosomal protein. Development. 1993;117:641–655. doi: 10.1242/dev.117.2.641. [DOI] [PubMed] [Google Scholar]
- 48.McCall K, Bender W. Probes for chromatin accessibility in the Drosophila bithorax complex respond differently to Polycomb-mediated repression. EMBO J. 1996;15:569–580. [PMC free article] [PubMed] [Google Scholar]
- 49.Moazed D, O’Farrell P H. Maintenance of the engrailed expression pattern by Polycomb group genes in Drosophila. Development. 1992;116:805–810. doi: 10.1242/dev.116.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morata G, Botas J, Kerridge S, Struhl G. Homeotic transformations of the abdominal segments of Drosophila caused by breaking or deleting a central portion of the bithorax complex. J Embryol Exp Morphol. 1983;78:319–341. [PubMed] [Google Scholar]
- 51.Muller J, Gaunt S, Lawrence P A. Function of the Polycomb protein is conserved in mice and flies. Development. 1995;121:2847–2852. doi: 10.1242/dev.121.9.2847. [DOI] [PubMed] [Google Scholar]
- 52.Neer E J, Schmidt C J, Nambudripad R, Smith T F. The ancient regulatory-protein family of WD repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- 53.Neer E J, Smith T F. G protein heterodimers: new structures propel new questions. Cell. 1996;84:175–178. doi: 10.1016/s0092-8674(00)80969-1. [DOI] [PubMed] [Google Scholar]
- 54.Ng J, Li R, Morgan K, Simon J. Evolutionary conservation and predicted structure of the Drosophila extra sex combs repressor protein. Mol Cell Biol. 1997;17:6663–6672. doi: 10.1128/mcb.17.11.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng, J., K. Morgan, and J. Simon. Unpublished data.
- 56.Paro R, Hogness D S. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci USA. 1991;88:263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paulsen J E, Capowski E E, Strome S. Phenotypic and molecular analysis of mes-3, a maternal-effect gene required for proliferation and viability of the germ line in C. elegans. Genetics. 1995;141:1383–1398. doi: 10.1093/genetics/141.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelegri F, Lehmann R. A role of Polycomb group genes in the regulation of gap gene expression in Drosophila. Genetics. 1994;136:1341–1353. doi: 10.1093/genetics/136.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson A J, Kyba M, Bornemann D, Morgan K, Brock H W, Simon J. A domain shared by the Polycomb group proteins Scm and ph mediates heterotypic and homotypic interactions. Mol Cell Biol. 1997;17:6683–6692. doi: 10.1128/mcb.17.11.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips M D, Shearn A. Mutations in polycombeotic, a Drosophila polycomb-group gene, cause a wide range of maternal and zygotic phenotypes. Genetics. 1990;125:91–101. doi: 10.1093/genetics/125.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pirrotta V. PcG complexes and chromatin silencing. Curr Opin Genet Dev. 1997;7:249–258. doi: 10.1016/s0959-437x(97)80135-9. [DOI] [PubMed] [Google Scholar]
- 62.Pirrotta V, Rastelli L. white gene expression, repressive chromatin domains, and homeotic gene regulation in Drosophila. Bioessays. 1994;16:549–556. doi: 10.1002/bies.950160808. [DOI] [PubMed] [Google Scholar]
- 63.Platero J S, Sharp E J, Adler P N, Eissenberg J C. In vivo assay for protein-protein interactions using Drosophila chromosomes. Chromosoma. 1996;104:393–404. doi: 10.1007/BF00352263. [DOI] [PubMed] [Google Scholar]
- 64.Qian S, Capovilla M, Pirrotta V. Molecular mechanisms of pattern formation by the BRE enhancer of the Ubx gene. EMBO J. 1993;12:3865–3877. doi: 10.1002/j.1460-2075.1993.tb06065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rastelli L, Chan C S, Pirrotta V. Related chromosome binding sites for zeste, suppressors of zeste, and Polycomb group proteins in Drosophila and their dependence on Enhancer of zeste function. EMBO J. 1993;12:1513–1522. doi: 10.1002/j.1460-2075.1993.tb05795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sathe S S, Harte P J. The Drosophila extra sex combs protein contains WD motifs essential for its function as a repressor of homeotic genes. Mech Dev. 1995;52:77–87. doi: 10.1016/0925-4773(95)00392-e. [DOI] [PubMed] [Google Scholar]
- 67.Schumacher A, Faust C, Magnuson T. Positional cloning of a global regulator of anterior-posterior patterning in mice. Nature. 1996;383:250–253. doi: 10.1038/383250a0. [DOI] [PubMed] [Google Scholar]
- 68.Shearn A, Hersperger G, Hersperger E. Genetic analysis of two allelic temperature-sensitive mutants of Drosophila melanogaster both of which are zygotic and maternal-effect lethals. Genetics. 1978;89:341–353. doi: 10.1093/genetics/89.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simon J. Locking in stable states of gene expression: transcriptional control during Drosophila development. Curr Opin Cell Biol. 1995;7:376–385. doi: 10.1016/0955-0674(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 70.Simon J, Bornemann D, Lunde K, Schwartz C. The extra sex combs product contains WD40 repeats and its time of action implies a role distinct from other Polycomb group products. Mech Dev. 1995;53:197–208. doi: 10.1016/0925-4773(95)00434-3. [DOI] [PubMed] [Google Scholar]
- 71.Simon J, Chiang A, Bender W. Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development. 1992;114:493–505. doi: 10.1242/dev.114.2.493. [DOI] [PubMed] [Google Scholar]
- 72.Sondek J, Bohm A, Lambright D G, Hamm H E, Sigler P B. Crystal structure of a GA protein βγ dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 73.Stassen M J, Bailey D, Nelson S, Chinwalla V, Harte P J. The Drosophila trithorax proteins contain a novel variant of the nuclear receptor type DNA binding domain and an ancient conserved motif found in other chromosomal proteins. Mech Dev. 1995;52:209–223. doi: 10.1016/0925-4773(95)00402-m. [DOI] [PubMed] [Google Scholar]
- 74.Struhl G. A gene product required for correct initiation of segmental determination in Drosophila. Nature. 1981;293:36–41. doi: 10.1038/293036a0. [DOI] [PubMed] [Google Scholar]
- 75.Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci USA. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Struhl G, Akam M. Altered distributions of Ultrabithorax gene of transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 1985;4:3259–3264. doi: 10.1002/j.1460-2075.1985.tb04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Struhl G, Brower D. Early role of the esc+ gene product in the determination of segments in Drosophila. Cell. 1982;31:285–292. doi: 10.1016/0092-8674(82)90428-7. [DOI] [PubMed] [Google Scholar]
- 78.Tamkun J W. The role of brahma and related proteins in transcription and development. Curr Opin Genet Dev. 1995;5:473–477. doi: 10.1016/0959-437x(95)90051-h. [DOI] [PubMed] [Google Scholar]
- 79.Tautz D. Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centres. Nature. 1988;332:281–284. doi: 10.1038/332281a0. [DOI] [PubMed] [Google Scholar]
- 80.Thummel C S, Pirrotta V. Technical notes: new pCaSpeR P-element vectors. Dros. Inf. Newsl. http://flybase.bio.indiana.edu/docs/news/DIN/dinvol2.txt. 1991. [Google Scholar]
- 81.Tripoulas N, Lajeunesse D, Gildea J, Shearn A. The Drosophila ash1 gene product, which is localized at specific sites on polytene chromosomes, contains a SET domain and a PHD finger. Genetics. 1996;143:913–928. doi: 10.1093/genetics/143.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tschiersch B, Hofmann A, Krauss V, Dorn R, Korge G, Reuter G. The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3-9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 1994;13:3822–3831. doi: 10.1002/j.1460-2075.1994.tb06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Lugt N M T, Domen J, Linders K, van Roon M, Robanus-Maandag E, Riele H T, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M, Berns A. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- 84.van Lohuizen M, Frasch M, Wientjens E, Berns A. Sequence similarity between the mammalian bmi-1 proto-oncogene and the Drosophila regulatory genes Psc and Su(z)2. Nature. 1991;353:353–355. doi: 10.1038/353353a0. [DOI] [PubMed] [Google Scholar]
- 85.Wall M A, Coleman D E, Lee E, Iniguez-Lluhi J A, Posner B A, Gilman A G, Sprang S R. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 86.White R A H, Lehmann R. A gap gene, hunchback, regulates the spatial expression of Ultrabithorax. Cell. 1986;47:311–321. doi: 10.1016/0092-8674(86)90453-8. [DOI] [PubMed] [Google Scholar]
- 87.White R A H, Wilcox M. Distribution of Ultrabithorax proteins in Drosophila. EMBO J. 1985;4:2035–2043. doi: 10.1002/j.1460-2075.1985.tb03889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson I A, Niman H L, Houghten R A, Cherenson A R, Connolly M L, Lerner R A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 89.Zhang C-C, Bienz M. Segmental determination in Drosophila conferred by hunchback (hb), a repressor of the homeotic gene Ultrabithorax (Ubx) Proc Natl Acad Sci USA. 1992;89:7511–7515. doi: 10.1073/pnas.89.16.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]