Abstract
Background
In patients with locally advanced breast cancer (LABC) receiving neoadjuvant chemotherapy (NAC), quantitative ultrasound (QUS) radiomics can predict final responses early within 4 of 16-18 weeks of treatment. The current study was planned to study the feasibility of a QUS-radiomics model-guided adaptive chemotherapy.
Methods
The phase 2 open-label randomized controlled trial included patients with LABC planned for NAC. Patients were randomly allocated in 1:1 ratio to a standard arm or experimental arm stratified by hormonal receptor status. All patients were planned for standard anthracycline and taxane-based NAC as decided by their medical oncologist. Patients underwent QUS imaging using a clinical ultrasound device before the initiation of NAC and after the 1st and 4th weeks of treatment. A support vector machine-based radiomics model developed from an earlier cohort of patients was used to predict treatment response at the 4th week of NAC. In the standard arm, patients continued to receive planned chemotherapy with the treating oncologists blinded to results. In the experimental arm, the QUS-based prediction was conveyed to the responsible oncologist, and any changes to the planned chemotherapy for predicted non-responders were made by the responsible oncologist. All patients underwent surgery following NAC, and the final response was evaluated based on histopathological examination.
Results
Between June 2018 and July 2021, 60 patients were accrued in the study arm, with 28 patients in each arm available for final analysis. In patients without a change in chemotherapy regimen (53 of 56 patients total), the QUS-radiomics model at week 4 of NAC that was used demonstrated an accuracy of 97%, respectively, in predicting the final treatment response. Seven patients were predicted to be non-responders (observational arm (n=2), experimental arm (n=5)). Three of 5 non-responders in the experimental arm had chemotherapy regimens adapted with an early initiation of taxane therapy or chemotherapy intensification, or early surgery and ended up as responders on final evaluation.
Conclusion
The study demonstrates the feasibility of QUS-radiomics adapted guided NAC for patients with breast cancer. The ability of a QUS-based model in the early prediction of treatment response was prospectively validated in the current study.
Clinical trial registration
clinicaltrials.gov, ID NCT04050228.
Keywords: breast cancer, radiomics, quantitative ultrasound (QUS), neoadjuvant chemotherapy, adaptive chemotherapy, artificial intelligence
Introduction
Breast cancer is a common malignancy in women associated with significant morbidity and mortality (1). Clinical outcomes are determined predominantly by the stage of disease during diagnosis, its molecular characteristics, and treatment-related factors (2). Locally advanced breast cancer (LABC) denotes advanced disease of the primary site or regional lymph nodes and is associated with higher chances of recurrence and poorer outcomes than early breast cancer (3, 4). Neoadjuvant chemotherapy (NAC) is the standard of care for patients with LABC, resulting in down-staging disease, increasing operability aiding in breast conservation, and has shown survival benefits in specific molecular subtypes. Chemotherapy regimens for NAC are typically administered in a set manner over a period of months, and final treatment response is determined through histopathological evaluation a few weeks after completion of scheduled chemotherapy and surgery. Neoadjuvant treatment also enables patients to be stratified according to pathological response for adjuvant therapies.
Radiomics involves quantitative analysis of imaging data usually coupled with machine learning classifiers to arrive at a meaningful link to clinical endpoints (5, 6). Radiomic analyses can be undertaken on different morphological and functional imaging modalities like ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) (7). Quantitative ultrasound (QUS) carries out direct analysis of the raw radiofrequency (RF) data from ultrasound imaging devices, as opposed to standard B-mode US, which involves transformed data leading to loss of information (8–10). Quantitative ultrasonography relies on the elastic properties of the tissues, with analysis of different spectral parameters highlighting various microstructural properties like acoustic scatterer size, shape, density, and organization which can be related to cellular morphology and arrangement. With cancer-directed therapy like chemotherapy or radiation, microscopic tissue changes are expected to start immediately after the initiation of treatment. However, typically response is appreciated only after months into treatment resulting from cumulative cell death and tumor size changes, the latter which can then be appreciated through standard imaging modalities, which lack sensitivity to detect microstructural changes during early phases of therapy. Cell death resulting from chemotherapy or RT leads to events like cell fragmentation, pyknosis, and formation of apoptotic bodies and cell death structures leading to changes in scatterer elastic properties which can be effectively determined by QUS imaging as demonstrated from preclinical and clinical studies.
Radiomic analysis of QUS imaging in accurate determination of treatment response to chemotherapy and RT in breast and head-neck malignancies had been demonstrated in prospective observational studies (11–14). The current phase 2 randomized study was undertaken to study the feasibility of using QUS-based response prediction for adaptive chemotherapy in patients with breast cancer receiving NAC. This is the first clinical study using a radiomics-guided approach for individualized treatment in oncology.
Methods
Study design and participants
This prospective randomized phase 2 study was conducted at a single institute, Sunnybrook Health Sciences Centre, Toronto, Canada. The study was approved by the institutional ethics committee and registered with the clinicaltrials.gov registry (NCT04050228). Women older than 18 years with a histologic diagnosis of primary breast malignancy with size of primary tumor ≥ 5 cm longest-dimension without distant metastasis, or smaller tumor (>2 cm diameter) with bulky axillary nodes, and eligible for NAC (normal blood counts, creatinine, liver function tests, and cardiac function) were considered eligible for the study. Contraindications included inflammatory breast cancer, previous history of connective tissue disease, past history of dermatologic disease involving breast, Eastern Cooperative Oncology Group (ECOG) performance status ≥ 3 and known sensitivity to components in ultrasound gel. Written informed consent form was obtained from all the study participants.
Patients enrolled in the study were randomly assigned through 1:1 allocation using the block randomization method to observational arm or experimental arm (adaptive chemotherapy for predicted non-responders), with hormone receptor status as a stratification factor (positive or negative). Study participants and investigators were not blinded to the allocation arm. The funding agencies had no role in the study design, analysis, or interpretation of the results.
Treatment procedures
Patients accrued in the study underwent QUS imaging before starting NAC (within 7 days) and after weeks 1 and 4 of NAC. QUS Data was acquired by experienced sonographers using a Sonix RP clinical system (Analogic Medical Corp.) with an L14-5/60 linear transducer (central frequency 6.5 MHz, bandwidth range 3.0-8.5 MHz) or GE LOGIC E9 system with ML6-15 linear transducer (central frequency 6.9 MHz, bandwidth range 4.5-9.9 MHz). The primary tumor was imaged at 1 cm intervals to encompass the entire span of the disease volumetrically. The region of interest (ROI) delineation corresponding to the tumor was carried out by the sonographers and individually verified by an expert breast radiologist and principal investigator. The raw radiofrequency data was extracted from the ROI. Then a fast Fourier transform (FFT)-based approach was applied, with data normalization carried out using a reference phantom approach, and various spectral parameters and texture features determined as described previously (10, 15). A QUS-radiomics model incorporating texture analysis based on a support vector machine-radial based function algorithm (SVM-RBF) developed from over 100 patient’s data was applied in order to monitor responses to chemotherapy for patients and classify them after 4 weeks of treatment as responders or non-responders (15).
Patients in the observation arm were planned for standard of care NAC with dose-dense AC-T or FEC-D regimens as decided by their treating medical oncologist. Typically, dose-dense AC-T chemotherapy consisted of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 weekly (AC) for 4 cycles, followed by paclitaxel 175 mg/m2 every two weeks (T) for 4 cycles. FEC-D included 5-FU 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 3 weekly (FEC) for 3 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 3 cycles. Use of growth factors and monitoring of hemogram, liver function tests, and renal function tests were done as per standard institutional practice. QUS Imaging was carried out before starting and during NAC at different experimental times, as mentioned earlier, with the treating medical oncologists blinded to results, and no changes in scheduled chemotherapy regimens were made.
The experimental arm involved the start of NAC either with AC-T or FEC-D regimens as planned by oncologists (as for the observational arm). QUS-Radiomics model prediction results at 4 weeks were made available to patients’ medical oncologists and were used in conjunction with clinical findings to potentially adapt treatments. Any treatment alterations were decided by the responsible oncologist. The use of the radiomics model to classify patients as a responder or non-responder was carried out within 48 hours of week 4 QUS data acquisition. Treatment changes decided by treating medical oncologists typically involved an early switch to taxane regimens, using alternative chemotherapy regimens, or planning early surgery.
In both the arms, patients underwent surgery with mastectomy or breast conservation surgery as decided by the breast surgeon. All patients were treated with adjuvant radiation and further maintenance targeted therapy or endocrinal therapy as appropriate, according to standard institutional practice. Patients that had HER2+ status received trastuzumab treatment and patients without pathological complete response received capecitabine adjuvantly.
Study outcomes
Being a phase 2 feasibility study, the primary objectives of the study included recruitment rate, refusal rates, the proportion of patients classified as non-responders, patient/physician acceptability of adaptive changes in response to QUS prediction, and proportion of patients randomized to experimental arm undergoing adaptive change to the chemotherapy arm. The recruitment rate was defined as the number of patients who underwent randomization divided by the study period (date of the last patient randomized – the date of the first patient randomized in months). The refusal rate was defined as 1 – (the number of patients who signed the informed consent for this study divided by the number of patients who were approached to enter this study). The proportion of patients classified as non-responders was defined as the number of patients classified as non-responders to their neoadjuvant chemotherapy by quantitative ultrasound divided by the number of patients randomized to the experimental arm. For response monitoring by QUS a score is determined mathematically which is a combination of calculated tumor QUS metrics for each patient individually. These are combined into a predictive score. Patients are classified as predicted responders if their predictive score is more than ** or as predicted non-responders if their QUS score is less than **. Response assessment was carried out based on histopathological evaluation by dedicated breast pathologists following surgery. For clinical response standard RECIST criteria are used based on tumor size initially using MRI (where available) or clinical assessment, or tumor size at the time of surgery. To be specific a modified RECIST score was used such that if on histopathology there was tumor chemotherapy response noted with remaining cellularity less than 1% patients were recognized to be responders. The few patients in this situation potentially had large radiological structures noted which on pathology were made of scarring from chemotherapy response with little to no viable cancer cells remaining on histopathology. This is consistent with previous work (12, 15, 16). Patient/physician acceptability rate: was defined as the number of patients who switched their chemotherapy regimen on the basis of quantitative ultrasound, divided by the number of patients who were classified as non-responders amongst those randomized to the experimental arm. The proportion of patients whose treatment was adaptively changed based on quantitative ultrasound was defined as the number of patients who had their neoadjuvant chemotherapy altered due to quantitative ultrasound divided by the number of patients randomized to the experimental arm.
Statistical analysis
Since the current study was primarily designed as a phase 2 feasibility study, sample size calculation was based on convenience without formal statistical analysis. A total of 120 patients was decided with 60 patients allocated equally to the observational and experimental arms. After the accrual of half of the patients (60), an unplanned interim analysis was carried out, and reported here, since the rate of accrual was slowed down due to the ongoing COVID-19 pandemic. Descriptive analysis was performed to study the patient, disease, treatment-related factors and response rates. Image preprocessing, feature extraction, and radiomics model development were carried out using MATLAB R2016a (MathWorks). Other statistical tests were performed using IBM SPSS version 22 (IBM Corporation). Standard statistical methods were used to calculate test performance (16, 17) and computed in combined group patients (Observational Arm and Experimental Arm-Non-Adapted).
Results
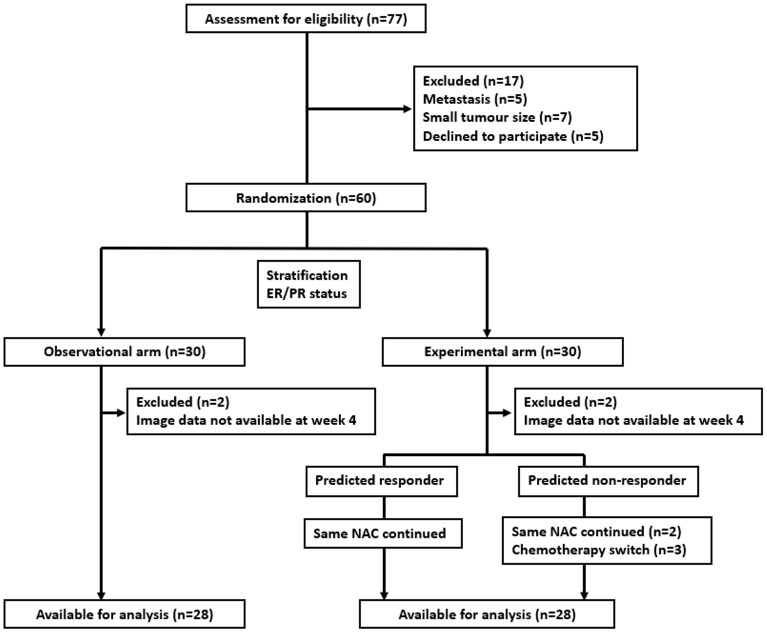
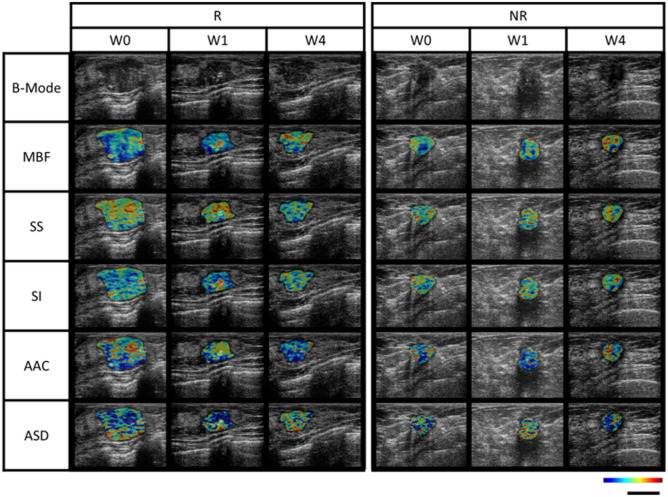
A total of 77 patients were screened for study eligibility between June 2018 and July 2021, with 60 accrued and randomized 1:1 with 30 patients each in both arms. In each arm, 2 patients were ineligible, resulting in 56 patients available for analysis, as presented in the consort diagram in Figure 1 . Baseline features were comparable in both arms, as summarized in Table 1 . The median age for patients in the observational arm and experimental arm was 49 years and 50 years, respectively. The median primary tumor size was 3.6 cm and 3.8 cm in the observational and experimental arms, respectively. The majority of patients (71%) received AC-T chemotherapy, while FEC-D was used in the remaining. Trastuzumab was used in 27% of the patients. B-mode Ultrasound images along with representative QUS parametric maps before treatment and after 1st and 4th week of NAC for one patient each from the responder and non-responder group are presented in Figure 2 . Patient characteristics are presented in Table 1 and Supplementary Tables 1-3 . Specifically, amongst responders 19% were HR (hormonal receptor including ER/PR) +//Her2+, 47% were HR+Her2-, 8% HR-/Her2+ and 26% HR-/HER2-. Specifically, amongst non-responders 67% were HR+Her2-, and 33% HR-/HER2-. In our cohort, complete response rate was seen in 31%, partial response rate in 63%, and patients with stable or progressive disease made up 6% of all patients.
Figure 1.
Consort diagram of the study.
Table 1.
Patient, disease, and treatment characteristics for patients in observational and experimental arm.
| Characteristic | Observational Arm (n=28) | Experimental Arm (n=28) |
|---|---|---|
| Age (years) | ||
| Median (Range) | 49 (29-73) | 50 (27-80) |
| Initial tumor size (cm) | ||
| Median (Range) | 3.6 (2.0-12.0) | 3.8 (2.1-10.7) |
| Molecular Markers | ||
| ER+ | 19 | 18 |
| PR+ | 13 | 13 |
| HER2+ | 7 | 8 |
| TNBC | 6 | 8 |
| Histological Type | ||
| IDC | 26 | 25 |
| IMC/Other | 2 | 3 |
| Chemotherapy | ||
| AC-T | 19 | 21 |
| FEC-D | 9 | 7 |
| Trastuzumab | 7 | 8 |
| Treatment Response | ||
| Responder | 26 | 23 (27) |
| Non-Responder | 2 | 5 (1) |
| Response Rate | 93% | 82% (96%) |
Figure 2.
B-mode imaging and corresponding QUS-parametric maps at different time points (pretreatment or week 0, and week 1 and 4 of neoadjuvant chemotherapy) for 1 patient from responder and non-responder group. MBF range was from -9.6 dB to 34.0 dB, SS range was from -5.7 dB/MHz to 1.6 dB/MHz, SI range from -7.3 dB to 49.0 dB, AAC range was from 20.2 db/cm3 to 81.6 db/cm3, ASD range was from 40 μm to 171 μm. The scale bar represents a length of 2 cm. MBF, mid-band fit; SS, spectral slope; SI, spectral intercept; AAC, average acoustic concentration; ASD, average spectral diameter.
With a total of 60 patients accrued over a period of 38 months, the recruitment rate was faster than anticipated (1.5 patients/month) but then affected by the COVID-19, else, the recruitment rate was >2.5 patients/month before the onset of the pandemic. The refusal rate for study participation was 8% mostly due to patients wishing to be allocated to the experimental arm. The proportions of non-responders were 7% and 18% in the observational arm and experimental arm, respectively.
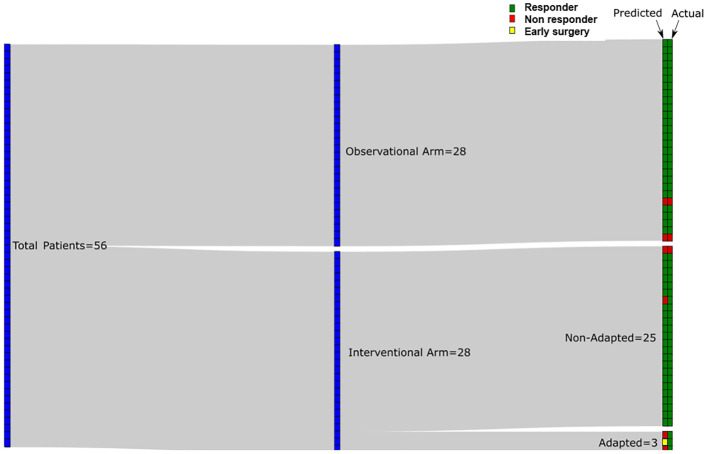
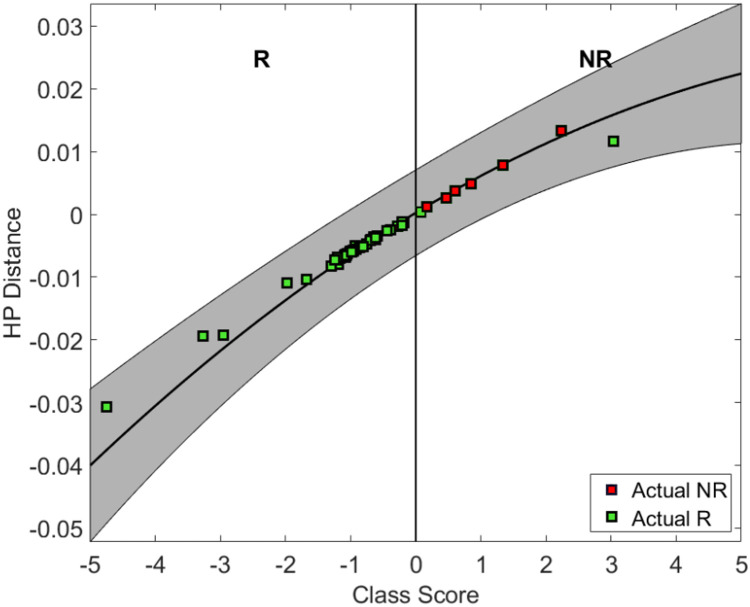
Using a QUS-radiomics prediction model at week 4 of NAC, a total of 7 patients were predicted to be non-responders based on surgical pathology (2 in the observational arm and 5 in the experimental arm). Figures 3 and 4 demonstrate the prediction at 4 weeks on an individual patient basis with the final response across the two treatment arms. Of the 5 non-responders in the experimental arm, 3 patients (60%) were considered for adaptive chemotherapy based on physician decision and patient acceptance, whereas others (2 patients) continued on initial original planned NAC regimen. Changes made to chemotherapy are presented in Figure 5 . All 2 predicted non-responders in the observational arm and 1 of 2 in the experimental arm (non-adapted branch) were true non-responders based on final evaluation following surgery. Considering patients who continued on an unchanged neoadjuvant regimen (Observation Arm and Experimental Arm Non-adapted), the sensitivity, specificity, and accuracy of the QUS-radiomics model at 4 weeks were 98%, 80%, and 97%, respectively ( Table 2 ). All the three patients in whom adaptive chemotherapy was considered started with AC regimens, which were switched to taxane chemotherapy. Two of them continued receiving taxane (weekly taxane in one patient and dose-dense treatment in another patient), after which they were taken for surgery. The patient treated with weekly taxane also received trastuzumab. In the other patient, early surgery was considered after one cycle of taxane since the primary disease appeared to progress clinically.
Figure 3.
Sankey diagram for predicted response at week 4 using QUS-radiomics model with the final response on an individual patient basis. Red tiles indicate non-response (predicted or actual) and green tiles indicate response (predicted or actual). In the experimental arm 25/28 patients were not adapted based on information provided to their oncologist whereas 3/28 were adapted. Patient with early surgery is indicated with a yellow tile.
Figure 4.
Individual patient predictions based on predictor class scores at week 4 for patient response. R indicates the zone (negative class score) for predicted response and NR indicates the zone (+ve class score) for non-response.
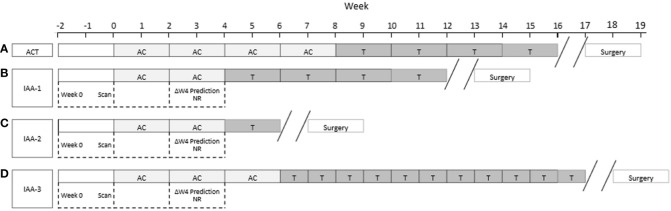
Figure 5.
(A) Schematic diagram for the administration of standard AC-T chemotherapy. Weeks are shown from left to right. Typical durations are illustrated. (B-D) indicate the three patients in the Experimental Arm that were adapted (IAA-1, IIA-2, and IIA-3) in Figure 3 . In (B) AC was shortened to move to T. In (C) the AC and T were shortened to move to surgery and in (C) AC was shortened and T was intensified,.
Table 2.
Classifier performance of QUS-radiomics model at week 4 of neoadjuvant chemotherapy.
| Parameter | Value (95% confidence interval) |
|---|---|
| Sensitivity | 98% (88-100%) |
| Specificity | 80% (28-100%) |
| Positive predictive value | 98% (89-99%) |
| Negative predictive value | 80% (36-97%) |
| Accuracy | 97% (88-99%) |
Confidence intervals for sensitivity, specificity, and accuracy are "exact" Clopper-Pearson confidence intervals. Confidence intervals for predictive values are standard logit confidence intervals.
In the experimental non-adapted arm (n=25 of 28) there were 2 patients who were predicted non-responders and were actual non-responders at the end of their therapy. In the experimental adapted arm (n=3 of 28) there were 3 patients who were predicted non-responders but due to intervention ended up converting to responders. More specifically, for the three patients two had the first phase of their chemotherapy shortened and went on to the second phase of their chemotherapy (taxane) sooner and responded to that phase of chemotherapy. In one patient treatment was intensified in terms of frequency of taxane administration. An additional (third) patient had the second phase of their chemotherapy shortened due to a continued lack of response and went to surgery promptly removing all tumor - and was therefore considered a responder to salvage surgical treatment.
In the observational arm all patients predicted to be responders (n=26) and non-responders (n=2) ended up responding and not responding to their treatment, respectively ( Table 3 ).
Table 3.
Patient predicted and actual reponses to neoadjuvant chemotherapy.
| Response | Study Arm | |||
|---|---|---|---|---|
| Predicted | Actual | Observational | Experimental - Non-Adapted | Experimental - Adapted |
| Non-Responder | Non-Responder | 2 | 1 | 0 |
| Non-Responder | Responder | 0 | 1 | 3 |
| Responder | Responder | 26 | 23 | 0 |
| Responder | Non-Responder | 0 | 0 | 0 |
Discussion
Radiomics involving quantitative imaging analysis has led to promises in serving to generate prognostic and predictive biomarkers in oncology over the past decade (7, 17, 18). Imaging can aid in the non-invasive assessment of treatment response since histopathological or molecular characterization is often precluded during treatment due to the need for associated invasive procedures to obtain tissue and limited tissue sampling volumetrically (19, 20). Traditional morphological imaging can have limitations in determining response early in the course of treatment since the measurable effect of tumor size change is often manifested from cumulative cell death only after several weeks or months of treatment. Driven by encouraging results of QUS-radiomics in determining early responses in patients with breast cancer receiving NAC, the current phase 2 randomized controlled trial here was initiated to study the feasibility of adaptive chemotherapy for non-responders guided by QUS.
The current study prospectively validates the ability of QUS-radiomics to predict tumor response after 4 weeks of treatment during 4-5 months of NAC. Cell death starts immediately at the microscopic level within a few hours of initiating therapies in the form of chemotherapy or radiotherapy (21, 22). Quantitative ultrasound has been promising in predicting final treatment response as early as 24 hours of starting treatment for breast and head-neck malignancies. The cascade of events associated with cell death like nuclear fragmentation, pyknosis, or apoptosis leads to changes in tissue architecture and elastic properties, which can be detected by QUS. The results are better depicted when quantitative image analysis of QUS data is undertaken along with machine learning algorithms. In a multicenter study involving 59 patients with breast cancer, the sensitivity, specificity at week 4 of NAC in the prediction of final response was 80% and 82%, respectively (12). In a different study of 100 patients a better performing classifier was developed with an accuracy of 90% (15) which was used here. Better classification performance results using that classifier were obtained from the current study (98% accuracy). It is well known that the performance matrices of machine learning algorithms improve with an increase in the magnitude of data (patient number). The radiomics model used in the study was developed with QUS and QUS-texture features using an SVM-RBF classifier from 100 patients’ data, explaining the better results using the current model when applied to the patients in this study. These performance indices using QUS-radiomics provide robustness when used in a clinical setting early in the course of neoadjuvant therapy, where the window of possible treatment modifications can be considered based on individual patient responses. In recent work, it has been demonstrated that the inclusion of higher-order imaging features in the form of texture derivatives has further improved the performance of classifier models. Although such features were not included in the current model (study here initiated in 2018), the future inclusion of a larger number of patients (currently data available for >300 patients) and higher-order features will lead to further increased reliability of the model for future applications.
The classifier here was based on a response-monitoring model using a SVM-RBF algorithm to predict treatment responses. That algorithm has previously demonstrated cross-validated accuracies of 90% (sensitivity 90%, specificity 90%). The classifier used is based on 4 texture features from QUS parametric images generated from data at 4-weeks after the start of neoadjuvant chemotherapy from 100 patients (15) separate from those (n=56) in this study. The performance in the totally separate data set here was very good (accuracy 98%).
The response to NAC in patients with breast cancer can be varied, with the majority achieving partial response, approximately 15-40% having pathological complete response, and 20-30% have no significant response to the treatment. Identifying non-responders early in their course of therapy can provide an opportunity to either switch to a different chemotherapy regimen or early consideration of surgery rather than continuing an ineffective treatment for the next few months. The current work is the first study to use a QUS radiomics-guided approach for considering a treatment switch. Biological imaging like PET has been used to predict response early in NAC. In the AVATAXHER phase 2 randomized trial, fluorodeoxyglucose (FDG) PET was done before the second cycle of NAC for patients with HER 2 positive breast cancer (23). Predicted non-responders were randomly allocated to therapy intensification with the addition of bevacizumab or continuing the same regimen of docetaxel and trastuzumab-based treatment. The pathological complete response rates were higher (44% versus 24%) in patients receiving additional treatment with bevacizumab. In another study (PHERGain), chemotherapy de-escalation was considered for patients with HER 2 positive cancers having a positive response on FDG-PET after 2 cycles (24). Pathological complete response rate was seen in 38% of PET predicted responders receiving chemotherapy-free dual HER2 blockade with trastuzumab and pertuzumab, with survival data pending. Another approach using circulating tumor DNA (ctDNA) collected during the course of NAC has demonstrated a lack of ctDNA clearance to be a significant predictor of poor response and metastatic relapse (25). QUS can be used as a simple portable imaging modality in the clinic with excellent prediction accuracies (more than 90%), as demonstrated by the study here with the added advantage of lesser cost and lack of technical challenges associated with PET or liquid biopsy.
The current study was a randomized phase 2 trial primarily designed to assess the feasibility of QUS radiomics-guided adaptive chemotherapy approach and the acceptance of patients and physicians. Given the first step towards NAC modification, the change of chemotherapy was not considered mandatory for all predicted non-responders - QUS-based predictive information was provided to oncologists to incorporate into their practice according to their judgement. With prospective validation of the radiomics model in predicting non-responders, the robustness of the model has been established, which can be made for decision-making in future with a higher degree of confidence. In the current study, all the patients where switching chemotherapy was carried out in non-responders finally ended up being responders or had tumor removed sooner. In contrast, almost all patients in the observational arm or patients who were not adapted in the experimental arm, who were non-responders ended up as non-responders This suggests that the role of treatment escalation or switching therapies with consideration of more intense chemotherapy regimens might help improve response rates. It is important to note that the patient population reported here nevertheless is relatively small, and that treatment modification choices may benefit from identifying partial responders as well. Previous work has demonstrated the capability of QUS radiomics to be used for such a purpose.
It is important to emphasize the fact that it is meaningful to predict non-responders early before they are recognized clinically as non-responders. Relying only on clinical observations may result in loss of the opportunity to modify systemic treatment when ineffective to an effective different chemotherapy regimen. Proceeding to surgical management directly in such a scenario is also not ideal since it truncates systemic therapy which could potentially impact systemic relapse risk as well.
Pathological complete response matters at individual patient level given that it directs care and subsequent therapies, even if found not to be strong surrogate for event free survival or overall survival at trial level (26). For certain phenotypes of breast cancer (ER- PR- HER2-/HER2+), it is still very important to maximize pathological complete response as it not only prognosticates but predicts need for post operative therapies (27, 28). The protocol used here suggests that one can tailor a “personalized” approach to neoadjuvant therapy in order to maximize this individual patient benefit.
At present a QUS model is being developed to demarcate between pathological complete responders versus partial responder, which might have a more significant impact on outcomes, as a complete response has been shown to impact survival positively. Partial responders could also be considered for additional treatment with such a QUS tool when able to differentiate responders from partial responders (29). Future work will involve a phase 3 randomized controlled trial of chemotherapy intensification, where all predicted non-responders will be considered for more intensive chemotherapy.
Conclusion
The current study was the first demonstration of the feasibility of QUS-radiomics guided adaptive neoadjuvant chemotherapy for patients with breast cancer, leading the way toward a phase 3 randomized controlled trial. The ability of QUS-radiomics model to predict non-responders was validated prospectively in this study with sensitivity, specificity, and accuracy of 98%, 80%, and 97%, respectively. Patients who were non-responders had their chemotherapy adapted based on QUS-radiomic monitoring of therapy response leading to improved response rates.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Sunnybrook Health Sciences Centre Research Ethics Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AD: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DD: Formal analysis, Investigation, Methodology, Writing – review & editing, Software. LS: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing, Software. SG: Data curation, Investigation, Methodology, Writing – review & editing. RP: Data curation, Investigation, Methodology, Writing – review & editing. AE: Data curation, Investigation, Methodology, Writing – review & editing. EW: Data curation, Investigation, Methodology, Writing – review & editing. FW: Data curation, Investigation, Methodology, Writing – review & editing. NL-H: Data curation, Investigation, Methodology, Writing – review & editing. AS-N: Data curation, Investigation, Methodology, Writing – review & editing. BC: Data curation, Investigation, Methodology, Writing – review & editing. MK: Methodology, Writing – review & editing, Data curation, Investigation. MT: Data curation, Investigation, Methodology, Writing – review & editing. GC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was received from the Terry Fox Foundation and Canadian Institutes of Health Research (awarded to GC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1273437/full#supplementary-material
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. (2019) 321:288–300. doi: 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- 3. Specht J, Gralow JR. Neoadjuvant chemotherapy for locally advanced breast cancer. Semin Radiat Oncol. (2009) 19:222–8. doi: 10.1016/j.semradonc.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 4. Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2013) 24 Suppl 6:vi7–23. doi: 10.1093/annonc/mdt284 [DOI] [PubMed] [Google Scholar]
- 5. Kuo MD, Jamshidi N. Behind the numbers: Decoding molecular phenotypes with radiogenomics–guiding principles and technical considerations. Radiology. (2014) 270:320–5. doi: 10.1148/radiol.13132195 [DOI] [PubMed] [Google Scholar]
- 6. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. (2015) 278:563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer. (2012) 48:441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czarnota GJ, Kolios MC, Abraham J, Portnoy M, Ottensmeyer FP, Hunt JW, et al. Ultrasound imaging of apoptosis: high-resolution non-invasive monitoring of programmed cell death in vitro, in situ and in vivo . Br J Cancer. (1999) 81:520–7. doi: 10.1038/sj.bjc.6690724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolios MC, Czarnota GJ, Lee M, Hunt JW, Sherar MD. Ultrasonic spectral parameter characterization of apoptosis. Ultrasound Med Biol. (2002) 28:589–97. doi: 10.1016/S0301-5629(02)00492-1 [DOI] [PubMed] [Google Scholar]
- 10. Sannachi L, Tadayyon H, Sadeghi-Naini A, Tran W, Gandhi S, Wright F, et al. Non-invasive evaluation of breast cancer response to chemotherapy using quantitative ultrasonic backscatter parameters. Med Image Anal. (2015) 20:224–36. doi: 10.1016/j.media.2014.11.009 [DOI] [PubMed] [Google Scholar]
- 11. Sannachi L, Gangeh M, Tadayyon H, Sadeghi-Naini A, Gandhi S, Wright FC, et al. Response monitoring of breast cancer patients receiving neoadjuvant chemotherapy using quantitative ultrasound, texture, and molecular features. PloS One. (2018) 13:e0189634. doi: 10.1371/journal.pone.0189634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quiaoit K, DiCenzo D, Fatima K, Bhardwaj D, Sannachi L, Gangeh M, et al. Quantitative ultrasound radiomics for therapy response monitoring in patients with locally advanced breast cancer: Multi-institutional study results. PloS One. (2020) 15:e0236182. doi: 10.1371/journal.pone.0236182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tran WT, Suraweera H, Quiaoit K, DiCenzo D, Fatima K, Jang D, et al. Quantitative ultrasound delta-radiomics during radiotherapy for monitoring treatment responses in head and neck Malignancies. Future Sci OA. (2020), FSO624. doi: 10.2144/fsoa-2020-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fatima K, Dasgupta A, DiCenzo D, Kolios C, Quiaoit K, Saifuddin M, et al. Ultrasound delta-radiomics during radiotherapy to predict recurrence in patients with head and neck squamous cell carcinoma. Clin Transl Radiat Oncol. (2021) 28:62–70. doi: 10.1016/j.ctro.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sannachi L, Gangeh M, Tadayyon H, Gandhi S, Wright FC, Slodkowska E, et al. Breast cancer treatment response monitoring using quantitative ultrasound and texture analysis: comparative analysis of analytical models. Trans Oncol. (2019) 12:1271–81. doi: 10.1016/j.tranon.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DiCenzo D, Quiaoit K, Fatima K, Bhardwaj D, Sannachi L, Gangeh M, et al. Quantitative ultrasound radiomics in predicting response to neoadjuvant chemotherapy in patients with locally advanced breast cancer: Results from multi-institutional study. Cancer Med. (2020) 9:5798–806. doi: 10.1002/cam4.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. (2017) 14:749–62. doi: 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 18. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. (1934) 26:404–13. doi: 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 19. O’Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. (2017) 14:169–86. doi: 10.1038/nrclinonc.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. deSouza NM, Achten E, Alberich-Bayarri A, Bamberg F, Boellaard R, Clément O, et al. Validated imaging biomarkers as decision-making tools in clinical trials and routine practice: current status and recommendations from the EIBALL* subcommittee of the European Society of Radiology (ESR). Insights Imaging. (2019) 10:87. doi: 10.1186/s13244-019-0764-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Czarnota GJ, Kolios MC, Vaziri H, Benchimol S, Ottensmeyer FP, Sherar MD, et al. Ultrasonic biomicroscopy of viable, dead and apoptotic cells. Ultrasound Med Biol. (1997) 23:961–5. doi: 10.1016/s0301-5629(97)00067-7 [DOI] [PubMed] [Google Scholar]
- 22. Vlad RM, Brand S, Giles A, Kolios MC, Czarnota GJ. Quantitative ultrasound characterization of responses to radiotherapy in cancer mouse models. Clin Cancer Res. (2009) 15:2067–75. doi: 10.1158/1078-0432.CCR-08-1970 [DOI] [PubMed] [Google Scholar]
- 23. Coudert B, Pierga J-Y, Mouret-Reynier M-A, Kerrou K, Ferrero J-M, Petit T, et al. Use of [18F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [18F]-FDG PET-predicted non-responders (AVATAXHER): an open-label, randomised phase 2 trial. Lancet Oncol. (2014) 15:1493–502. doi: 10.1016/S1470-2045(14)70475-9 [DOI] [PubMed] [Google Scholar]
- 24. Pérez-García JM, Gebhart G, Ruiz Borrego M, Stradella A, Bermejo B, Schmid P, et al. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. (2021) 22:858–71. doi: 10.1016/S1470-2045(21)00122-4 [DOI] [PubMed] [Google Scholar]
- 25. Radovich M, Jiang G, Hancock BA, Chitambar C, Nanda R, Falkson C, et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast cancer: preplanned secondary analysis of the BRE12-158 randomized clinical trial. JAMA Oncol. (2020) 6:1410–5. doi: 10.1001/jamaoncol.2020.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Squifflet P, Saad ED, Loibl S, van Mackelenbergh MT, Untch M, Rastogi P, et al. Re-evaluation of pathologic complete response as a surrogate for event-free and overall survival in human epidermal growth factor receptor 2-positive, early breast cancer treated with neoadjuvant therapy including anti-human epidermal growth factor receptor 2 therapy. J Clin Oncol. (2023) 41:2988–97. doi: 10.1200/JCO.22.02363 [DOI] [PubMed] [Google Scholar]
- 27. Derks MGM, van de Velde CJH. Neoadjuvant chemotherapy in breast cancer: more than just downsizing. Lancet Oncol. (2018) 19:2–3. doi: 10.1016/S1470-2045(17)30914-2 [DOI] [PubMed] [Google Scholar]
- 28. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res. (2020) 26:2838–48. doi: 10.1158/1078-0432.CCR-19-3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sannachi L, Gangeh M, Tadayyon H, Sadeghi-Naini A, Gandhi S, Wright FC, et al. Response monitoring of breast cancer patients receiving neoadjuvant chemotherapy using quantitative ultrasound, texture, and molecular features. PloS One. (2018) 13. doi: 10.1371/journal.pone.0189634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.