Abstract
Introduction
Unilateral pulmonary edema (UPE), a life-threatening complication of cardiac surgery, often occurs after prolonged cardiopulmonary bypass and lung collapse, especially in minimally-invasive cardiac surgery (MICS). The present study reported a young patient with severe UPE after the surgery as well as corresponding clinical treatments. In addition to the supportive treatment of extracorporeal membrane oxygenation (ECMO), monitoring changes in cardiopulmonary function and early clinical interventions are crucial.
Conclusion
By weighing the beneficial and detrimental effects of the treatment, it calls for early diagnosis and new therapeutic strategies for the complication.
Keywords: Case report, Lung re-expansion, Unilateral pulmonary edema, Minimally-invasive bentall surgery, Extracorporeal membrane oxygenation
1. Introduction
Unilateral pulmonary edema (UPE) is a severe complication in cardiac patients subjected to minimally invasive heart surgery [1]. Current treatments are focused on respiratory support after pulmonary edema, diagnosed by computer tomography. Nevertheless, the mortality of the complication is still high. The present case reported a young patient suffering from UPE after MICS, including his image examination, treatments, and prognosis.
1.1. Case presentation
A 37-year-old man complained of chest tightness, palpitation, and shortness of breath after regular exercise in the last four months, which was deteriorated for over one month. Transthoracic echocardiography reported an aneurysm (57 mm at the aortic root) localized at the aortic sinus that was extended to the proximal ascending aorta. Both left atrial (44 mm) and ventricle (54 mm in the end-systolic stage and 72 mm in the end-diastolic stage, respectively) were dilated with severe aortic valve regurgitation, and the value of the left ventricular ejection fraction was decreased (LVEF: 50 %). In line with the echocardiographic results, the electrocardiogram reported sinus rhythm, increased V1 Ptf, and high voltage in the left ventricle. The young man was admitted with the diagnosis of aortic aneurysm with severe aortic regurgitation and prepared for a minimally-invasive Bentall surgery.
After anesthetic induction with a left double-lumen endotracheal tube, the surgical procedure was performed through the right second anterolateral intercostal space. Single lung ventilation (left side) was used. Cardiopulmonary bypass (CPB) was established via the right femoral artery and vein after heparin was applied. An additional cannula for left atrial drainage was placed through the right superior pulmonary vein. Lung ventilation was paused, and constant supportive pressure was set to 4 mmHg. After direct aortic cross-clamping (ACC), antegrade cardioplegic solutions were perfused to induce cardiac arrest. The thoracic cavity was filled with carbon dioxide through the procedure. The CPB procedure took 198 minutes in the surgery, and the ACC time was 157 minutes. Double-lung ventilation was used at the end of the CPB since the patient's blood oxygen level was dropped under 90 %, accompanied by exudation in the right airway. Transesophageal echocardiography indicated cardiac contractility was decreased while both mitral and aortic valves functioned well. In support of epinephrine and noradrenaline, a fiber-optic bronchoscope was applied to drain away exudation.
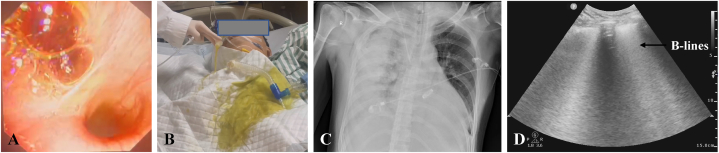
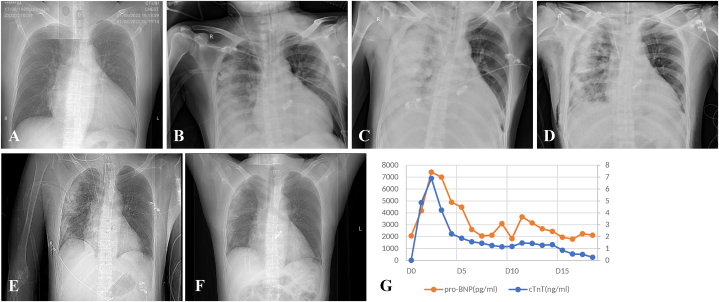
The patient had high positive end-expiratory ventilation pressure in the intensive care unit (ICU). Meanwhile, serous fluid was consistently observed in the right, but not the left bronchus (Fig. 1A–B). Thus, veno-arterial extracorporeal membrane oxygenation (ECMO) was used through the left femoral artery and vein since patient oxygen saturation was barely maintained at 88–92 % in support of high doses of epinephrine (0.2 μg/kg/min), norepinephrine (0.12 μg/kg/min) and pituitrin (6 IU/h). Transthoracic echocardiography showed decreased ventricular systolic activity and reduced tricuspid annular plane systolic excursion (12 mm). Stenosis in the pulmonary vein was excluded since bedside echocardiography showed that the flow velocity in the right upper pulmonary was about 0.69 m/s. The chest radiograph showed abundant B-lines in the right lung, indicating massive exudation (Fig. 1C–D). Therefore, treatments of diuresis and inotropic medicine were applied. The patient had the veno-arterial ECMO support for eight days and was extubated on Day 12 (Fig. 2A–G). Informed consent for the publication of the clinical details and images was provided by the patient.
Fig. 1.
(A) Massive fluid accumulation in the middle and lower lobe of the right lung under Bronchoscopy. (B) Light yellow fluid gushing from the endotracheal tube.(C) Unilateral pulmonary edema in X-ray. (D) Massive Bline (black arrow) in lung ultrasound.
Fig. 2.
Radiographical images in a time-dependent manner. Image taken before the surgery(A), Day 0(B), Day 1(C), Day 5(D), Day 8(E), Day 18(F). Changes of Myocardial markers (pro-BNP, cTNT) in a time-dependent manner(G).
2. Discussion
Unilateral pulmonary edema (UPE) incidence is about 2.1 % in patients with chronic heart failure, pulmonary hypertension, high body mass index, and prolonged cardiopulmonary bypass [1]. Nevertheless, the incidence of the complication reaches 25 % in patients subjected to MICS [2], accompanied by intraoperative collapse, which further leads to an increase in ECMO support in ICU (2 %) and mortality (4–33 %) [3,4]. It is known that lung re-expansion induces reperfusion injury, resulting in increased vascular permeability in pulmonary microcirculation [5]. Ipsilateral pulmonary edema is induced by aspiration, re-expansion, contusion, misplaced central line, vein occlusive disease, prolonged decubitus positioning, and mitral regurgitation, while contralateral pulmonary edema is probably caused by pulmonary embolism, lobectomy, inflammation, and denervation [6]. In the present report, the young muscular patient had an unexcepted complication of pulmonary edema after the surgery. The incidence of embolism was ruled out since heparin was applied in the surgery and the patient did not have previously reported abnormalities of pulmonary venous. The procedure of right lung re-expansion aggravated ipsilateral pulmonary edema, which teamed with uncompromised cardiac function in a vicious cycle. The veno-arterial, but not veno-venous ECMO, was used to support cardiopulmonary function for the patients since veno-venous ECMO supports pulmonary functions, while veno-arterial ECMO supports cardiopulmonary functions. Early use of ECMO improved oxygen contracts while reducing the amount of cardiac work.
Point-of-care lung ultrasonography outweighs traditional chest radiography, with increased sensitivities (87.6 %) and specificities (96.2 %) [7,8] since increased counts of B-line in the ultrasonography are correlated with high serum levels of pro-brain natriuretic peptide and increased incidences of major adverse cardiovascular events [9]. Thus, monitoring cardiopulmonary function by transthoracic ultrasonography is highly recommended for ICU patients undergoing thoracic surgeries [10].
Treatments for pulmonary edema include mechanical ventilation and supportive medicine, such as diuretics, inotropes, and steroids [11]. It has been established that ECMO is an important salvage therapy in UPE treatments, although some literature report that the application of ECMO weakens cardiac function [12,13]. Current therapeutic strategies or early interventions for UPE include intermittent ventilation of the collapsed lung, restoration of bilateral ventilation, pretreatment of mannitol, and induction of mild hypothermia during ACC [14]. Of note, a recent meta-analysis suggests some protective effects of neutrophil inhibitors and endothelial barrier modulators, including protease-activated receptor 1 and tyrosine-protein kinase receptor 2 for UPE patients [15].
In brief, clinicians have to weigh the beneficial and detrimental effects of the treatments. It is crucial to monitor cardiopulmonary function in the progression of the disease and apply for ECMO support when conventional treatments fail to take effect.
3. Limitation
There are some concerns and lessons. In the present case, pulmonary exudate could be caused by cardiac failure and/or lung re-expansion. We considered re-expansion pulmonary edema as the initial factor followed by cardiac failure, resulting in the pulmonary exudate, although direct evidence was absent. The surgical decision of minimally invasive Bentall surgery was made on the patient's cardiac function, which was underestimated. Compared with MICS, traditional sternotomy approaches expose a larger cardiac surgical field, shorten the CPB circuits, and probably avoid lung collapse during the surgery. Thus, it would be better to fully evaluate the patient's cardiac function and make decisions on the surgical option. Furthermore, we highly recommend an early application of ECMO for patients with severe UPE.
Ethics statement
The patient agreed to use all images, clinical data, and other data for scientific research, and provided written informed consent for the publication of the clinical details and images.
Funding
This work was supported by the Nature Science Foundation of Shanghai (No 22ZR1411600 to YS).
Data availability statement
There was no data sharing for the present case report.
CRediT authorship contribution statement
Yan Hu: Writing – original draft. Kefang Guo: Project administration. Yi Shi: Writing – review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29911.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Kesavuori R.I., et al. Unilateral pulmonary oedema after minimally invasive and robotically assisted mitral valve surgery. Eur. J. Cardio. Thorac. Surg. 2020;57(3):504–511. doi: 10.1093/ejcts/ezz271. [DOI] [PubMed] [Google Scholar]
- 2.Tutschka M.P., et al. Unilateral postoperative pulmonary edema after minimally invasive cardiac surgical procedures: a case-control study. Ann. Thorac. Surg. 2015;99(1):115–122. doi: 10.1016/j.athoracsur.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 3.Moss E., et al. Prevention of unilateral pulmonary edema complicating robotic mitral valve operations. Ann. Thorac. Surg. 2017;103(1):98–104. doi: 10.1016/j.athoracsur.2016.05.100. [DOI] [PubMed] [Google Scholar]
- 4.Keyl C., et al. Unilateral pulmonary oedema after minimally invasive cardiac surgery via right anterolateral minithoracotomy. Eur. J. Cardio. Thorac. Surg. 2015;47(6):1097–1102. doi: 10.1093/ejcts/ezu312. [DOI] [PubMed] [Google Scholar]
- 5.Sivrikoz M.C., et al. The role of tissue reperfusion in the reexpansion injury of the lungs. Eur. J. Cardio. Thorac. Surg. 2002;22(5):721–727. doi: 10.1016/s1010-7940(02)00447-5. [DOI] [PubMed] [Google Scholar]
- 6.Kanner C., Hardy S.M. An unusual cause of unilateral pulmonary edema. Ann. Intern. Med. 2013;158(8):639–640. doi: 10.7326/0003-4819-158-8-201304160-00020. [DOI] [PubMed] [Google Scholar]
- 7.Maw A.M., et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw. Open. 2019;2(3) doi: 10.1001/jamanetworkopen.2019.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekgoz B., et al. BLUE protocol ultrasonography in Emergency Department patients presenting with acute dyspnea. Am. J. Emerg. Med. 2019;37(11):2020–2027. doi: 10.1016/j.ajem.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Platz E., et al. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail. 2019;7(10):849–858. doi: 10.1016/j.jchf.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo P.H., et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019;45(9):1200–1211. doi: 10.1007/s00134-019-05725-8. [DOI] [PubMed] [Google Scholar]
- 11.Haga T., Kurihara M., Kataoka H. Risk for re-expansion pulmonary edema following spontaneous pneumothorax. Surg. Today. 2014;44(10):1823–1827. doi: 10.1007/s00595-013-0726-y. [DOI] [PubMed] [Google Scholar]
- 12.Viox D., et al. Unilateral pulmonary edema after robotically assisted mitral valve repair requiring veno-venous extracorporeal membrane oxygenation. J. Cardiothorac. Vasc. Anesth. 2022;36(1):321–331. doi: 10.1053/j.jvca.2021.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Gray W.H., et al. Successful rescue therapy with venovenous extracorporeal membrane oxygenation for re-expansion pulmonary oedema in a patient with one lung. Eur. J. Cardio. Thorac. Surg. 2019;55(3):582–584. doi: 10.1093/ejcts/ezy262. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K., et al. Preventive strategy for reexpansion pulmonary edema after minimally invasive cardiac surgery. Ann. Thorac. Surg. 2020;109(5):e375–e377. doi: 10.1016/j.athoracsur.2019.10.073. [DOI] [PubMed] [Google Scholar]
- 15.Dekker N., et al. Pharmacological interventions to reduce edema following cardiopulmonary bypass: a systematic review and meta-analysis. J. Crit. Care. 2020;56:63–72. doi: 10.1016/j.jcrc.2019.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There was no data sharing for the present case report.