Abstract
Obesity is a significant risk factor for health issues like type 2 diabetes and cardiovascular disease. It often proves resistant to traditional lifestyle interventions, prompting a need for more precise therapeutic strategies. This has led to a focus on signaling pathways and neuroendocrine mechanisms to develop targeted obesity treatments. Recent developments in obesity management have been revolutionized by introducing novel glucagon-like peptide-1 (GLP-1) based drugs, such as semaglutide and tirzepatide. These drugs are part of an emerging class of nutrient-stimulated hormone-based therapeutics, acting as incretin mimetics to target G-protein–coupled receptors like GLP-1, glucose-dependent insulinotropic polypeptide (GIP), and glucagon. These receptors are vital in regulating body fat and energy balance. The development of multiagonists, including GLP-1–glucagon and GIP–GLP-1–glucagon receptor agonists, especially with the potential for glucagon receptor activation, marks a significant advancement in the field. This review covers the development and clinical efficacy of various GLP-1-based therapeutics, exploring the challenges and future directions in obesity management.
Keywords: Glucagon-like peptide-1 receptor agonists, Glucose-dependent insulinotropic polypeptide, Glucagon, Multiagonist, Obesity
INTRODUCTION
Obesity, currently defined as a body mass index (BMI) of 30 kg/m2 or greater, affects 800 million people worldwide [1]. In the United States, approximately 42% of adults have obesity [2], and obesity-related costs are estimated at $173 billion annually [3]. Obesity causes organ dysfunction via several pathophysiological mechanisms, ranging from the physical impact of increased adipose tissue mass, the presence of ectopic fat within tissues and organs, metabolic effects, inflammatory mechanisms, and psychological consequences [4,5].
Obesity is associated with many disorders such as type 2 diabetes (T2D), cardiovascular (CV) disease, fatty liver, osteoarthritis, and obstructive sleep apnea, etc. [6,7]. Obesity arises from an imbalance between energy intake and expenditure, and this relationship can be summarized as the energy balance-feedback model, which provides the basis for drug treatment. Antiobesity medications have specific mode of action such as reducing dietary intake, modifying energy metabolism, and increasing energy expenditure or both [8,9].
Development of antiobesity medication has not been easy, because of unexpected adverse events. Reports of heart valve disease associated with fenfluramine, texfenfluramine, and phentermine raised concerns about drug treatment of obesity since 2000 [10,11]. These products were withdrawn by the U.S. Food and Drug Administration (FDA) in 2011. Additionally, an obstacle to drug treatment for obesity is that the drug is perceived as ineffective because the patient gains weight again when the drug is stopped.
Obesity is a chronic disease caused by various causes [12,13]. Therefore, a complete cure is rare, and therefore treatment should be aimed at palliation. When treating diseases such as high blood pressure or hyperlipidemia with drugs, we do not expect a cure, but we expect relief and are concerned about recurrence when drug treatment for these diseases is discontinued. Obesity treatment needs to be thought of the same way.
It is obvious that healthy lifestyle should be the mainstay of the treatment. Antiobesity medications can be used as an adjunct when lifestyle modification is not enough to reduce body weight or maintain the reduced body weight.
STANDARDS FOR EVALUATING THE EFFICACY OF OBESITY TREATMENT DRUGS
Various drugs that cause weight loss are being developed, but many drugs are often eliminated at the stage of obtaining final approval. Many countries in Europe and the United States have introduced strict policy for obesity drug approval mainly because of safety reasons.
Currently, the following drugs are approved for long-term treatment of obesity worldwide: Orlistat (Xenical, Xenical, Roche, Basel, Switzerland), 1999; Bupropion/Naltrexone (Contrave, Orexigen Therapeutics Inc., La Jolla, CA, USA), 2018; Liraglutide 3.0 mg (Saxenda, Novo Nordisk, Bagsvaerd, Denmark), 2018; Phentermine/Topiramate (Qsymia, Vivus, Mountain View, CA, USA), 2019; Semaglutide 2.4 mg (Wegovy, Novo Nordisk), 2021; Tirzepatide (Zepbound, Eli Lilly, Indianapolis, IN, USA), 2023.
Among these, glucagon-like peptide-1 receptor agonists (GLP-1RAs) and GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) coagonists draw much attention because these agents showed potent weight lowering efficacy. This approach, originally proposed by Professor Ernest Starling in the early 1900s, initially focused on gut hormones but was overshadowed by discoveries in insulin and leptin [14]. However, the introduction of GLP-1, a key incretin hormone, has revolutionized the treatment of obesity and T2D. More recently, the concept of nutrient-stimulated hormone-based therapeutics, involving novel combinations like GLP-1 with other gut hormones such as GIP and glucagon, highlights the growing importance and effectiveness of GLP-1 based therapies in managing these chronic conditions. In this manuscript, we discuss GLP-1 based antiobesity medication focusing on its mechanism, clinical trial results, and potential adverse events.
CURRENTLY AVAILABLE GLP-1RA BASED THERAPIES FOR OBESITY
The mechanisms of action and structural formulations of GLP-1RA-based therapies for obesity are detailed in Figs. 1, 2. Additionally, Table 1 comprehensively outlines their long-term impact on weight reduction and safety profiles [15-20].
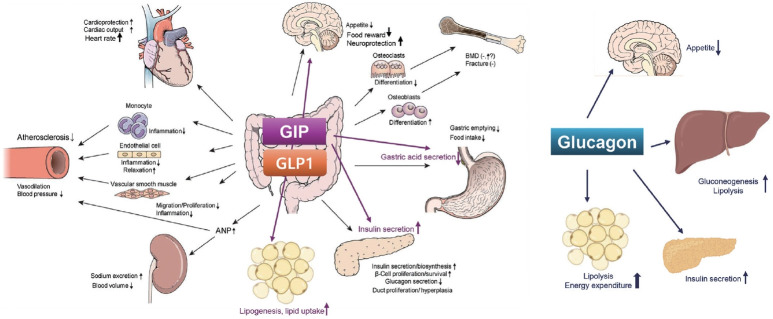
Fig. 1.
Effects of glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon on various vital organs and tissues. Modified from Lim et al. [37], with permission from Elsevier. ANP, atrial natriuretic peptide; BMD, bone mineral density.
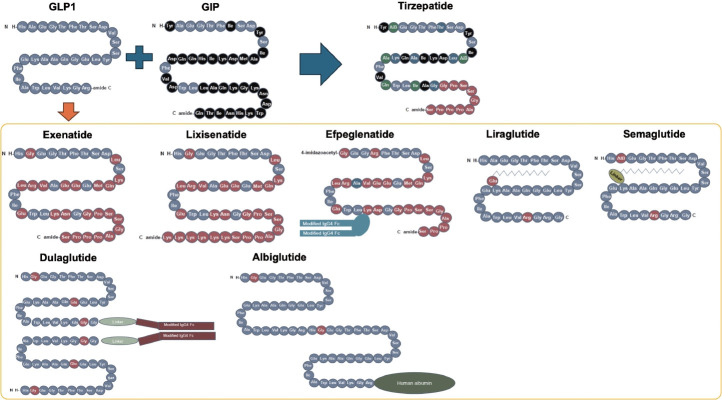
Fig. 2.
Structures of formulations of glucagon-like peptide-1 receptor agonists (GLP-1RAs) and glucose-dependent insulinotropic polypeptide/ GLP-1RA. Modified from Tschop et al. [36]. GIP, glucose-dependent insulinotropic polypeptide.
Table 1.
Efficacy and Safety of GLP-1 Based Treatments for Obesity Management
| Drug | Phase | Year of approval for obesity | Trial (study duration) | No. of participants | Maximum dosage | Mean weight loss from baseline | Proportion of patients losing >15%/>10% of baseline weight | Most common adverse events (nausea) | Discontinuation rate |
|---|---|---|---|---|---|---|---|---|---|
| GLP-1RAs | |||||||||
| Liraglutide [15] | Phase 3 | 2018 | SCALE (56 weeks) | 3,731 | 3.0 mg, qd | 8.0% | 14.4%/26.1% | 40.2% | 9.9% |
| Semaglutide [16] | Phase 3 | 2021 | STEP1 (68 weeks) | 1,961 | 2.4 mg, qw | 14.9% | 50.5%/69.1% | 44.2% | 7.0% |
| Efpeglenatide [17] | Phase 2 | - | NCT02075281 (20 weeks) | 297 | 6 mg, qw | 7.3% | -/27.1% | 59.3% | 19.0% |
| GIP/GLP-1RA | |||||||||
| Tirzepatide [18] | Phase 3 | 2023 | SURMOUNT1 (72 weeks) | 2,539 | 15 mg, qw | 20.9% | 70.6%/83.5% | 31.0% | 6.2% |
| GLP-1/GCGRA | |||||||||
| Survodutide (BI 456906) [20] | Phase 2 | NCT04667377 (46 weeks) | 387 | 4.8 mg, qw | 14.9% | 68.8%/54.7% | - | 24.6% | |
| GIP/GLP-1/GCGRA | |||||||||
| Retatrutide (LY3437943) [19] | Phase 2 | - | NCT04881760 (48 weeks) | 338 | 12 mg, qw | 17.5% | 93%/83% | 45.0% | 16.0% |
GLP-1, glucagon-like peptide-1; GLP-1RA, glucagon-like peptide-1 receptor agonist; SCALE, Satiety and Clinical Adiposity-Liraglutide; qd, once a day; STEP1, Semaglutide Treatment Effect in People with obesity 1; qw, once weekly; GIP, glucose-dependent insulinotropic polypeptide; GCGRA, glucagon receptor agonist.
GLP-1RA
In 1964, it was discovered that when glucose was administered orally, more insulin was secreted than when it was injected intravenously, confirming the existence of incretin, a gastrointestinal factor that promotes insulin secretion [21]. There are several incretins including GLP-1 and GIP. Compared to GIP, which has normal secretion but poor response in patients with T2D, GLP-1, which has low secretion but preserved response, is an important target in the development of antidiabetic drugs. However, the half-life of GLP-1 is too short at 1 to 2 minutes, making it difficult to use as a drug [21].
Since the mid-2000s, the structure of dipeptidyl peptidase-4 (DPP-4, CD26), an enzyme that decomposes GLP-1, has been discovered. Based on this finding, two incretin-based medications were developed: (1) DPP-4 inhibitors that increase the concentration of GLP-1 by inhibiting it [22], and (2) GLP-1RAs, which act directly on the GLP-1 receptor [23]. In contrast with DPP-4 inhibitors, GLP-1RAs have attracted attention because a weight loss effect appears when the dose of this drug is increased [23].
Mechanism of action and physiological action
GLP-1 is secreted by L cells in the ileum upon meal stimulation [24]. The most important effect of GLP-1 is to increase insulin secretion after a meal, and this effect is dependent on glucose concentration, so despite promoting insulin secretion, the occurrence of hypoglycemia is rare [25].
On the other hand, GLP-1 suppresses glucagon secretion from pancreatic α-cells [26]. This action is linked to the concentration of glucose in the blood, so glucagon secretion is suppressed in hyperglycemia conditions, but when hypoglycemia occurs, glucagon secretion increases.
GLP-1 also suppresses appetite and gastric emptying [23]. Therefore, administration of this drug causes gastrointestinal side effects such as nausea or weight loss. In some animal experiments, it has been reported that GLP-1 may be effective in improving inflammation [27,28], postprandial hyperlipidemia [29,30], endothelial cell dysfunction [31], and oxidative stress [32], as well as protecting β-cells [33,34].
Pharmacokinetics and drug metabolism
GLP-1RA was developed to be resistant to the degradation of DPP-4. Several different types of GLP-1RAs are currently available. Exenatide, a twice-daily formulation (exenatide twice daily), was developed based on exendin-4, which is derived from the saliva of the American poisonous lizard (Gila monster) and shows 53% homology to human GLP-1 [35]. Human GLP-1 based GLP-1RA includes liraglutide, albiglutide, dulaglutide, efpeglenatide, and semaglutide.
They can be classified by their duration of action: short-acting GLP-1RAs and long-acting GLP-1RAs. Their main characteristics are shown in Fig. 2 [36]. Short-acting GLP-1RAs generally lower postprandial blood glucose by delaying gastric emptying time, while long-acting GLP-1RAs reduce fasting glucagon or increase fasting insulin concentration [37]. Commonly, long-acting ones show a more powerful glucose lowering effect as it lowers not only postprandial glucose levels but also fasting glucose levels [23].
Weight loss effects of GLP-1RAs
Liraglutide
Liraglutide (3.0 mg, Saxenda), a GLP-1RA that activates the GLP-1 receptor, was approved by the FDA in December 2014 for adult obesity treatment [38] and has recently shown efficacy in adolescents aged 12 to 18 years. Liraglutide shares 97% similarity with GLP-1 and is characterized by its prolonged action in the body [39]. Administered via subcutaneous injection, it crosses the blood-brain barrier to act on various regions of the hypothalamus, including the arcuate nucleus. It directly stimulates pro-opiomelanocortin/cocaine-and-amphetamine-regulated transcript (POMC/CART) neurons and indirectly inhibits neuropeptide-Y/agouti-related protein (NPY/AgRP) neurons through GABA neurotransmission, reducing appetite and promoting weight loss [40].
Liraglutide 3.0 mg’s efficacy in obesity was assessed in the Satiety and Clinical Adiposity-Liraglutide (SCALE) Obesity and Prediabetes and SCALE-diabetes trials [15,41]. In these trials, significant weight loss was demonstrated with liraglutide 3.0 mg therapy, compared to placebo (8.0% in SCALE obesity and prediabetes, 6.0% in SCALE-diabetes). The SCALE-maintenance study investigated weight loss maintenance with liraglutide 3.0 mg. In this study, 422 participants who lost over 5% of initial body weight on a low-calorie diet were randomized to liraglutide 3.0 mg or placebo for 56 weeks. Liraglutide 3.0 mg resulted in an additional 6.2% weight loss from the initial average of 6.0% [42]. The drug’s efficacy in obese adolescents was also notable, with significant reductions in BMI and higher proportions achieving over 5% and 10% weight loss compared to placebo [38].
A real-world study with 769 Korean people with obesity, body weight and BMI decreased significantly after initiation of liraglutide 3.0 mg treatment: –2.94 kg and –1.08 kg/m2 at 2 months; –4.23 kg and –1.55 kg/m2 at 4 months; and –5.14 kg and –1.89 kg/m2 at 6 months [43].
The CV safety of a lower dose of liraglutide (1.8 mg, Victoza, Novo Nordisk) in people with T2D was established in the Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results (LEADER) study, showing a 13% reduction in major CV events over an average of 3.8 years [44].
The primary side effects of liraglutide are gastrointestinal, including nausea, diarrhea, constipation, and vomiting, mitigated by gradual dose escalation. There are concerns about acute pancreatitis, thus it’s recommended to discontinue liraglutide upon symptom onset and avoid use in individuals with a history of pancreatitis. Due to potential risks of medullary thyroid carcinoma and multiple endocrine neoplasia, it’s not recommended for individuals with a personal or family history of these conditions [45]. Liraglutide improves blood pressure and lipid profiles but increased heart rate by an average of 2 beats per minute in the SCALE-diabetes study [41]. Women of childbearing age should undergo pregnancy testing before use and use contraception during treatment. Its safety in individuals over 75 years of age is not established. Similar to adults, serious side effects in pediatric studies were not significant.
Semaglutide
Semaglutide shares 94% structural homology with GLP-1 and is administered as a weekly subcutaneous injection [46]. The initial dosing starts at 0.25 mg per week and is gradually increased at 4-week intervals to 0.5, 1.0, 1.7 mg, and finally to a maximum dose of 2.4 mg per week. In June 2021, semaglutide (2.4 mg, Wegovy) received U.S. FDA approval as a treatment for obesity or overweight in adults.
The Semaglutide Treatment Effect in People with obesity (STEP) program, a phase III randomized clinical trial (RCT), was conducted in patients with T2D, obesity, and various metabolic disorders. In the STEP1 involved 1,961 participants with obesity or overweight, 68-week treatment of semaglutide 2.4 mg demonstrated an average weight reduction of 14.9% [16]. In the semaglutide group, 86% achieved at least a 5% weight loss, and 69% lost over 10% of their baseline weight [16]. In the STEP2 focusing on 1,210 patients with obesity or overweight and T2D, average weight loss with semaglutide 2.4 mg therapy was 6.2% compared to the placebo [47]. The STEP3 trial involved 611 patients with obesity or overweight, semaglutide 2.4 mg treatment demonstrated an average weight loss of 10.3%, with 86.6% of participants achieving over 5% weight reduction (vs. 47.6% in the control group) [48].
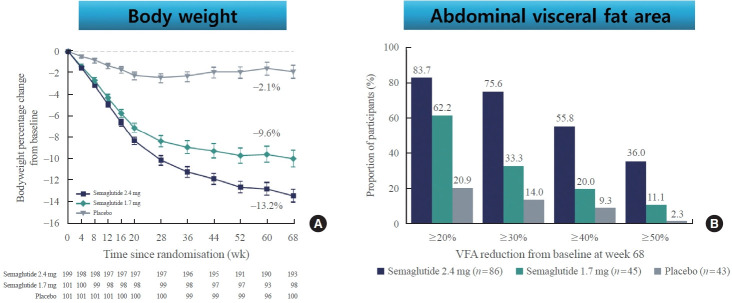
Recently, STEP6 phase IIIa RCT assessed the effect of semaglutide 2.4 and 1.7 mg in an east Asian population (Fig. 3) [49]. In this study, mean bodyweight was reduced between baseline and week 68 in the semaglutide 2.4 mg (13.2%) and semaglutide 1.7 mg (9.6%) groups when compared with the placebo group. Additionally, abdominal visceral fat area, measured by computed tomography scan, was also significantly reduced in the semaglutide groups compared with the placebo group. Cardiometabolic risk factors, such as C-reactive protein, lipids, and blood pressure, were also reduced with semaglutide treatment compared with placebo [49].
Fig. 3.
Comparison of bodyweight (A) and abdominal visceral fat (B) from baseline to week 68 for semaglutide 2.4 mg, semaglutide 1.7 mg, and placebo in Janpanese and Korean people with obesity. Adapted from Kadowaki et al. [49], with permission from Elsevier. VFA, visceral fat area.
In these STEP trials, most participants experienced mild to moderate symptoms, with the incidence of severe side effects being 9.8% in the semaglutide treatment group and 6.4% in the placebo group. Severe adverse reactions included serious gastrointestinal disorders, hepatobiliary disorders, gallbladder disorders, and mild acute pancreatitis. Additionally, an increase in the average heart rate of 1 to 4 beats per minute was observed in the semaglutide treatment group [16].
In addition to discussing the weight loss efficacy of semaglutide, the recently published results of CV outcome trials will be addressed later in this paper.
Efpeglenatide
Efpeglenatide is a long-acting GLP-1RA, which was developed for the management of hyperglycemia in patients with T2D [50]. Efpeglenatide is composed of a modified exendin molecule attached to a fragment of human immunoglobulin 4 using a special technology called long-acting peptide/protein [49].
Efpeglenatide is a drug in which an exendin-4 analog is linked to a human immunoglobulin G4 fragment through a polyethylene glycol (PEG linker), allowing for Fc receptor of neonate (FcRn)-mediated recycling and reduced excretion through the kidneys [50]. Based on this mechanism, the half-life was observed up to 166 hours, and based on this, a once a week dosage was developed.
In a phase II trial, adults with a BMI of ≥30 or ≥27 kg/m² with comorbidities were randomized to receive efpeglenatide at doses of 4 mg weekly, 6 mg weekly, 6 mg biweekly, or 8 mg biweekly, or a placebo, all combined with a hypocaloric diet [17]. The main goal was to assess the change in body weight over 20 weeks.
The study found that all efpeglenatide dosages notably decreased body weight, showing reductions between –6.3 and –7.2 kg compared to the placebo. Participants receiving efpeglenatide more frequently achieved weight loss of ≥5% or ≥ 10%. Additionally, efpeglenatide significantly improved blood sugar levels and lipid profiles. The rate of discontinuation due to adverse events varied from 5% to 19%, predominantly due to gastrointestinal side effects [17].
Moreover, efpeglenatide therapy reduced the risk of CV disease by about 27% compared to the placebo in the Effect of Efpeglenatide on Cardiovascular Outcomes (AMPLITUDE-O) study [51]. Recently, phase III clinical trials for obesity with efpeglenatide are ongoing.
Multifaceted benefits of GLP-1RAs beyond weight loss
GLP-1RAs have various metabolic benefits including glucose lowering, CV benefits, renal benefits, and improvement in fatty liver (Fig. 1) [37,52].
Glucose lowering effect
GLP-1RAs improve hyperglycemia by enhancing insulin secretion through increases in β-cell cytoplasmic cyclic adenosine monophosphate [53], which activates protein kinase A (PKA) and, thus, increases in intracellular calcium and triggers insulin exocytosis in a glucose-dependent manner [54]. While GIP has lost most of its insulinotropic activity in patients with T2D, GLP-1 has retained enough of its insulinotropic activity to stimulate insulin secretory responses even in patients with T2D [55]. In addition, GLP-1RAs have the potential to preserve or improve pancreatic β-cell function [33,34]. In high-fat diet-induced diabetes mice, administration of liraglutide activated pancreatic and duodenal homeobox-1 (Pdx-1), an essential regulator of proliferation and function of pancreatic β-cells [56]. In a recent study, administration of exendin-4, a long-acting GLP-1RA, stimulated β-cell proliferation in juvenile human islets by inducing gene expressions of calcineurin/nuclear factor of activated T cells (NFAT) signaling, a proliferating factor such as NFATC1, and a cell cycle regulator such as cyclin A1 [57].
GLP-1RAs significantly reduced endoplasmic reticulum (ER) stress-associated β-cell death and recovered rat insulinoma cell line (INS-1) cells from ER stress-mediated translational repression in a PKA-dependent manner [34]. Moreover, ccontinuous infusion of GLP-1 upregulated islet Pdx-1 gene expression and induced β-cell proliferation and neogenesis in rats [58]. Similarly, treatment of GLP-1RAs induced proliferation and neogenesis of pancreatic β-cell in animal models [59].
GLP-1RA therapy inhibits glucagon secretion, at least transiently delays gastric emptying, and reduces appetite and food intake [60,61]. Treatment with 3 mg liraglutide for 20 weeks reduced appetite and energy intake, resulting in a significant weight loss (7.2 kg) in a large, RCT in people with obesity [15].
The effect of lowering fasting glucose level and glycated hemoglobin is generally greater with long-acting agents, and the effect of lowering postprandial glucose level is more evident with short-acting agents. This is due to the gastric emptying delaying effect of short-acting agents, and the gastric emptying delaying effect of long-acting agents gradually decreases due to tachyphylaxis, thereby reducing the effect of postprandial glycemic control. Compared to short-acting exenatide, long-acting ones such as dulaglutide, liraglutide, and exenatide once-weekly reduced hemoglobin A1c (HbA1c) by 0.51%, 0.45%, and 0.38% respectively [62]. Similarly, compared with lixisenatide, dulaglutide, liraglutide, exenatide once-weekly, and albiglutide decreased HbA1c by 0.66%, 0.60%, 0.53%, and 0.39%, respectively [63].
In several RCTs, GLP-1RAs, when used alone or in combination with other hypoglycemic agents including metformin, sulfonylurea, DPP-4 inhibitor, thiazolidinedione, and sodium-glucose cotransporter 2 inhibitor, showed superior or non-inferior blood glucose lowering ability compared to insulin treatment [64,65].
CV system protection effect
GLP-1RAs improve risk factors for CV disease, including weight loss, blood glucose control, blood pressure reduction, and lipid improvement. It also has a direct effect on the heart and endothelial cells, preventing the progression of atherosclerosis through mechanisms such as increasing nitric oxide and decreasing endothelin-1, reactive oxygen species, and inflammatory cytokines. It is known that the CV protection effect of GLP-1RAs have been proven through eight large-scale clinical studies, including Evaluation of LIXisenatide in Acute coronary syndrome (ELIXA) [66], LEADER [44], Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN)-6 [67], Exenatide Study of Cardiovascular Event Lowering (EXSCEL) [68], Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes) [69], Researching Cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) [70], Peptide Innovation for Early Diabetes Treatment (PIONEER) 6 [67,71], and AMPLITUDE-O [51]. Among these, liraglutide in the LEADER study, semaglutide in the SUSTAIN-6, albiglutide in the Harmony Outcomes, dulaglutide in the REWIND, and efpeglenatide in the AMPLITUDE-O exhibited significant beneficial effects on 3-point major adverse cardiac events (3P-MACE: death from CV disease, non-fatal myocardial infarction, and non-fatal stroke), the primary end point of each study, compared to the placebo group, respectively.
In the ELIXA [66], EXSCEL [68], and PIONEER 6 [67,71] studies, treatments of exenatide twice-daily, exenatide once-weekly, and oral semaglutide groups were non-inferior to placebo in terms of CV disease safety, respectively. In a meta-analysis of major RCTs, GLP-1RAs reduced composite major CV events by 14% (hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.80 to 0.93), myocardial infarction by 13% (HR, 0.87; 95% CI, 0.77 to 0.98), stroke by 18% (HR, 0.82; 95% CI, 0.73 to 0.92), and death from CV disease by 13% (HR, 0.87; 95% CI, 0.81 to 0.94) [52].
Recently, semaglutide treatment demonstrated a 1.5% lower risk in absolute risk in the 3P-MACE compared to placebo (6.5% vs. 8.0%, P<0.05) in nondiabetic people with obesity in the Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial [72]. This result suggests that semaglutide has cardioprotective effect independent of any substantial glucose lowering properties.
Given the relatively smaller reduction in body weight (9.4%) in the SELECT trial compared to previous RCTs: 14.9% in STEP1 (BMI 37.8±6.7 kg/m2) [16] and 13.2% STEP6 (BMI 31.9 kg/m2) [49], pleiotropic effects of semaglutide may contribute to the overall CV risk reduction [25,37]. These include not only effects of weight loss, but also improvements in endothelial function, reductions in low-grade inflammation (evidenced by a significant decrease in high-sensitivity C-reactive protein levels), and potential plaque stabilization [73].
GLP-1RAs also reduce hepatic steatosis, circulating triacylglycerol, and low-density lipoprotein cholesterol concentrations and effectively reduce blood pressure [25]. A combination of effects on CV risk factors and direct effect on the process of atherosclerosis are supported by preclinical studies where GLP-1RA showed anti-atherosclerotic and anti-inflammatory effects and improved endothelial function [73].
Kidney protection effect
In some of the large-scale clinical studies evaluating the safety of GLP-1RAs against CV disease, the effect on kidneys was evaluated as a secondary endpoint. LEADER (liraglutide) [44], SUSTAIN-6 (semaglutide) [67], and REWIND (dulaglutide) [70] study showed beneficial renal outcomes, mainly reducing the occurrence of proteinuria. A meta-analysis [74] including the ELIXA [66], LEADER [44], SUSTAIN-6 [67], EXSCEL [68], REWIND [70], and AMPLITUDE-O [51] studies found that GLP-1RAs were associated with better renal outcomes: a 30% or 40% or more decrease in glomerular filtration rate, need for renal replacement therapy, end-stage renal failure, or death from kidney disease by 21%.
GLP-1RAs increase natriuresis by inhibiting sodium reabsorption by acting on the Na/H exchanger in the proximal tubule of the kidney [75]. Accordingly, hyperfiltration decreases according to tubular glomerular feedback due to increased Na in the distal tubule. In addition, reduction of inflammatory factors, reduction of angiotensin II, and improvement of blood sugar and blood pressure by GLP-1RAs have been suggested as mechanisms of kidney protection effect. Most GLP-1RAs do not require drug dose adjustment according to renal function, but lixisenatide and exenatide are not recommended for use in chronic kidney disease (CKD) or end-stage renal disease with a glomerular filtration rate <30 mL/min/1.73 m2.
In contrast, a pooled analysis of clinical trials reporting separate CV events rates in patients with T2D and CKD did not find GLP1-RA to be associated with a reduction in composite CV event rates [76]. More data are needed in this group.
Nonalcoholic fatty liver disease
GLP-1RAs are attractive candidates for the treatment of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) because they can reduce body weight and improve insulin secretion [77]. In a meta-analysis with six studies (three liraglutide studies, three exenatide studies), treatment of GLP-1RAs produced significant improvements in liver histology such as lobular inflammation, hepatocellular ballooning, and fibrosis [78]. In another phase II trial with 52 people with clinical evidence of NASH, liraglutide 1.8 mg treatment led to histological improvement of NASH [79]. Additionally, the risk of progression to fibrosis was reduced by 80%. Furthermore, semaglutide treatment resulted in a significantly higher percentage of patients with NASH resolution than placebo in the phase II trial involving patients with NASH [80]. However, there was no significant improvement in fibrosis stage by semaglutide therapy.
Thus, liraglutide and semaglutide have proven its effectiveness in treating fatty liver disease in patients with biopsy-proven NASH, although the study number was small [81]. Emerging evidence suggests that treatment with the dual GLP-1 and GIP receptor agonist, tirzepatide, might prove even more potent than a GLP-1RA alone, to ameliorate NAFLD.
Safety and tolerability
The most common side effects of GLP-1RAs are gastrointestinal side effects, including nausea, vomiting, and diarrhea. Among these, nausea mostly disappears after a few weeks of administration, and can be minimized by starting with a low dose and gradually increasing the dose. While nausea and vomiting are more common with short-acting agents, side effects such as itching or nodules at the injection site are more common with long-acting agents and usually disappear in 3 to 4 weeks. Although the risk of hypoglycemia is very low due to its glucose-dependent mechanism of action, the risk of hypoglycemia may increase when used in combination with medications that can cause hypoglycemia.
Animal model studies have suggested that GLP-1RAs may cause pancreatitis and exocrine dysplasia, but in the large RCTs and a meta-analysis, GLP-1RAs did not increase the risk of pancreatitis or pancreatic cancer [82,83]. Currently, the U.S. FDA and European Medicines Agency (EMA) have concluded that there is no direct evidence for the association of the use with GLP-1RAs with pancreatitis or pancreatic cancer.
In rodent animal models, GLP-1RAs increased thyroid paracellular cell (C-cell) proliferation and the incidence of tumors. This is possibly due to the fact that rodent C cells express more GLP-1Rs than humans. In a long-term evaluation of the LEADER trial with 9,340 patients with T2D over a 3.5- to 5-year period, there was no evidence of C-cell malignancies occurred in the liraglutide group [84]. Although there is no data in humans, GLP-1RAs should not be used in patients with a past or family history of medullary thyroid cancer or in patients with multiple endocrine neoplasia type 2.
Dual peptides for the treatment of obesity
GIP/GLP‐1 receptor co‐agonists: tirzepatide
Tirzepatide, a novel dual GIP and GLP-1RA, offers significant insights into its mechanism of action, therapeutic benefits, and clinical applications for the management of T2D and obesity in general practice. Structurally, it is a 39-amino acid modified peptide, attached to a C20 fatty diacid moiety, which facilitates albumin binding. This modification extends its half-life, making it suitable for a once-weekly subcutaneous injection formulation [85,86].
Historically, the role of GIP in obesity treatment was overlooked due to its potential to cause weight gain. However, weight reduction effect of tirzepatide have renewed interest in the GIP role within dual agonists for GLP-1 and GIP receptors. Recent hypotheses suggest that GIP induces appetite suppression through independent receptors in the brain, mitigates gastrointestinal side effects of GLP-1 action, and prevents desensitization of GLP-1 receptors, thereby acting as a biased GLP-1RA [87].
The starting dose of tirzepatide is 2.5 mg once a week, and after 4 weeks, the dose is increased to the therapeutic dose of 5 mg and administered subcutaneously in patients with T2D. If additional glycemic control is needed, the dose can be increased by 2.5 mg every 4 weeks, and the maximum dose of this drug is 15 mg once a week.
Across the SURPASS phase 3 programme versus placebo and active comparators, including semaglutide, tirzepatide has shown superior dose-dependent reductions in HbA1c levels up to 2.5% and body weight up to 13%, compared with all comparators [88-91]. Thus, tirzepatide has demonstrated significant efficacy in lowering HbA1c levels and body weight in individuals with T2D, often surpassing the results seen with other antidiabetic agents. Its weight-reducing effects are particularly notable, offering a promising option for patients with concomitant obesity, a common comorbidity in T2D.
In the SURMOUNT-1 study, a phase 3 clinical trial in obese patients, randomized participants in a 1:1:1:1 ratio to tirzepatide (5, 10, 15 mg) or placebo over 72 weeks, significant weight reductions were observed at 72 weeks: –15.0% for 5 mg, –19.5% for 10 mg, –20.9% for 15 mg, compared to –3.1% for placebo [18]. Of note, more than 50% of participants in the tirzepatide 10 and 15 mg groups experienced weight reductions of over 20%. The most common side effects were gastrointestinal, mostly mild to moderate, and tended to improve after initial dose escalation. Treatment discontinuation due to side effects occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants in the 5, 10, 15 mg, and placebo groups, respectively [18].
In the SURMOUNT-2 study, which involved 938 adults with obesity or overweight and T2D, tirzepatide showed significant weight loss effects of 13.4% and 15.7% for the 10 and 15 mg doses, respectively, with over 80% of participants experiencing at least a 5% weight loss [92]. Moreover, tirzepatide led to significant improvements in HbA1c and other CV metabolic risk factors [92].
In the SURMOUNT-3 and SURMOUNT-4 studies, participants experienced significant weight losses with tirzepatide treatment, marking a mean weight loss of 26.6% and 26.0% over 84 and 88 weeks respectively [93,94].
In summary, tirzepatide is an innovative therapeutic agent with a dual mechanism of action that provides a potent tool for managing T2D and obesity. Its introduction into clinical practice offers a new horizon for improving metabolic outcomes in patients, with a favorable impact on CV risk factors.
Amylin/GLP‐1 receptor co‐agonists: CagriSema (cagrilintide+semaglutide)
Cagrilintide+semaglutide (CagriSema) is a combination of 2.4 mg of long-acting GLP-1RA semaglutide and amylin analog cagrilintide administered once a week. Amylin is a peptide hormone composed of 37 amino acids and secreted by pancreatic β-cells following a meal [95]. It exerts various physiological effects by acting on the area postrema in the brainstem. Amylin’s physiological roles include inhibition of glucagon secretion, reduction in food intake, delay in gastric emptying, activation of brown adipose tissue via the sympathetic nervous system, and increased energy expenditure, ultimately leading to decreased blood glucose levels and weight loss [95]. Conversely, antagonizing amylin receptors leads to an increase in adipose tissue [95].
Pramlintide, a synthetic amylin analog modified with three proline substitutions, is approved for treating type 1 diabetes and T2D in the United States. It effectively reduces weight in diabetic and nondiabetic obese individuals [96]. The drug’s efficacy is further enhanced when combined with agents like metreleptin, phentermine, and sibutramine. However, its short action duration necessitates frequent injections [97].
To overcome the short half-life and frequent subcutaneous administration of pramlintide, new amylin receptor agonists like cagrilintide have been developed. Cagrilintide, with six amino acid substitutions in human amylin and a dual carboxylic fatty acid chain similar to semaglutide attached to the first amino acid, lysine, exhibits an extended half-life, allowing for a once-weekly subcutaneous administration regimen [97,98].
A phase II trial focusing on individuals with overweight or obesity, coupled with hypertension or dyslipidemia but without T2D, found that cagrilintide (2.4 mg) alongside diet and exercise, led to a 10% body weight reduction compared to 3% with placebo over 26 weeks [99]. Additionally, in a phase I study, treatment of cagrilintide (up to 4.5 mg) combined with semaglutide (2.4 mg) resulted in greater body weight reduction than semaglutide (17% vs. 10%) over 20 weeks [100]. This evidence suggests that using these treatments together, leveraging their complementary actions, could enhance their effectiveness [101].
In a recently published phase II study conducted on 92 T2D patients with a BMI ≥27 kg/m2, a 2.18% decrease in HbA1c and a 15.6% reduction in body weight were confirmed in the group administered CagriSema once a week [102]. This weight reduction was significantly greater than 5.1% by semaglutide and 8.1% by cagrilintide alone therapy. CagriSema is the next in a series of gut hormone analogs with the potential to herald a new era in treating obesity and preventing diabesity.
Glucagon/GLP‐1 receptor co‐agonists: survodutide
The mechanisms of most currently used obesity treatments predominantly focus on appetite suppression. However, a more effective approach for weight loss and maintenance would involve strategies that both suppress appetite and promote energy expenditure. Although substances that increase energy expenditure have existed, their clinical use has been limited due to safety concerns.
Recent attention in the field of glucagon research has highlighted its potential for inducing weight loss in obese patients through mechanisms like lipolysis, thermogenesis, and enhanced energy metabolism [103]. Glucagon is emerging as a hormone with dual facets in metabolic diseases, characterized by its contrasting physiological responses of inducing hyperglycemia and promoting weight loss through increased energy metabolism [104]. Consequently, therapeutic strategies that either inhibit or activate glucagon are being actively researched in clinical settings [104].
As demonstrated by oxyntomodulin, a weak endogenous agonist for GLP-1 receptor and glucagon receptor, a strategy that simultaneously stimulates both GLP-1 and glucagon receptors can reduce food intake while increasing energy expenditure, synergistically enhancing weight loss [105-107]. Activating both receptors can mitigate the undesirable effects of glucagon and improve obesity and metabolic complications [106].
Recent advances in peptide engineering have moved beyond the combination of multiple agents, as seen with compounds like tirzepatide, which act on several receptors simultaneously with a single peptide, emulating the effects of combination therapy. BI 456906 (survodutide), developed as a dual agonist for GLP-1 and glucagon receptors for the treatment of obesity and NASH, is structurally modified from glucagon [109]. In humans, this drug exhibits full activation of GLP-1 receptor while only partial activation of glucagon receptor [109]. As a potent acylated peptide, BI 456906 incorporates a C18 fatty acid to prolong its half-life and maintains stability against DPP-4 proteolytic degradation through C-terminal amidation and substitution at the second amino acid [109].
Survodutide (BI 456906) was evaluated in two phase I studies involving healthy volunteers and individuals with overweight/obesity. The studies showed that survodutide therapy led to a maximum placebo-corrected body weight loss of 13.8% by week 16, demonstrating its potential for significant weight loss [110]. However, increased doses were associated with more adverse events, primarily gastrointestinal and cardiac or vascular in nature. A phase II study result showed that administering a high dose of survodutide, up to 1.8 mg twice weekly, resulted in over 50% of T2D participants achieving more than 5% body weight reduction, and over 25% experiencing a weight loss exceeding 10% [111]. Phase III studies are ongoing to further investigate the efficacy and safety of survodutide in obesity management.
Triple peptides for the treatment of obesity
GIP/GLP‐1/glucagon receptor triple co‐agonists: retatrutide
Retatrutide, a single peptide linked to a fatty diacid moiety, acts as a triple-agonist targeting three receptors: GIP, GLP-1, and glucagon. Compared to endogenous receptor ligands, retatrutide exhibits a stronger affinity for the GIP receptor than for the glucagon and GLP-1 receptors [112]. Its action on these three receptors suggests a complementary mechanism where glucagon-induced gluconeogenesis is inhibited by GLP-1/GIP, and glucagon’s enhancement of energy expenditure is augmented by the appetite-suppressing effects of GLP-1/GIP, thereby promoting weight loss [112]. The pharmacokinetics of retatrutide are dose-proportional, with a half-life of approximately 6 days, making weekly dosing feasible [113].
In a phase II trial, participants with obesity were randomly assigned to various doses of retatrutide from 1–4 , 8, or 12 mg or a placebo for 48 weeks [19]. After 48 weeks, the 4 mg retatrutide group led to weight reductions of more than 5%, 10%, and 15% in 92%, 75%, and 60% of participants, respectively; for the 8 mg dose, these figures were 100%, 91%, and 75%; for the 12 mg dose, 100%, 93%, and 83% [19].
Another phase II study assessed the safety and efficacy of varying doses of retatrutide, included participants with a BMI of 25 to 50 kg/m2 and an HbA1c of 7.0% to 10.5% [114]. After 36 weeks, the 0.5 mg retatrutide group showed a 3.19% body weight reduction, the 4 mg group showed a 10.37% decrease, the 8 and 12 mg groups demonstrated decreases of 16.81% and 16.94% respectively, which were significantly greater than those observed with placebo (3.00%) and 1.5 mg dulaglutide (2.02%) [114]. Gastrointestinal side effects were mostly mild to moderate, with the highest incidence in the 8 mg fast escalation group [114].
The most common side effects in the retatrutide group were gastrointestinal symptoms, which were dose-dependent and mostly mild to moderate in severity. Notably, a dose-dependent increase in heart rate, peaking at 24 weeks, subsequently decreased [19]. This increase in heart rate is hypothesized to result from glucagon’s activation of the sympathetic nervous system.
CONCLUSIONS
Obesity is a disease that can be treated with targeted pharmacotherapy in addition to lifestyle modification and behavioral interventions. Previous antiobesity medications showed modest efficacy with limited long-term safety data and limited benefits beyond weight loss. Liraglutide 1.8 mg and semaglutide 2.4 mg are established GLP1-RAs for obesity treatment. Moreover, drugs with greater efficacy are being developed by combining two or three of GLP-1 and GIP or glucagon or amylin. Tirzepatide, a GLP-1 and GIP receptor coagonist, was also approved for treating T2D and obesity. The integration of GLP-1 based treatments with other gut hormone actions offers a promising future in managing obesity and its associated diseases more effectively. Drugs adopting this strategy produced body weight reduction more than 20%. Furthermore, multiagonists activating multiple gut hormone receptors could potentially replace surgical interventions, offering similar metabolic outcomes.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Elmaleh-Sachs A, Schwartz JL, Bramante CT, Nicklas JM, Gudzune KA, Jay M. Obesity management in adults: a review. JAMA. 2023;330:2000–15. doi: 10.1001/jama.2023.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 3.Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16:e0247307. doi: 10.1371/journal.pone.0247307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluher M. Metabolically healthy obesity. Endocr Rev. 2020;41:bnaa004. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320:C375–91. doi: 10.1152/ajpcell.00379.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576:51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 8.Loffler MC, Betz MJ, Blondin DP, Augustin R, Sharma AK, Tseng YH, et al. Challenges in tackling energy expenditure as obesity therapy: from preclinical models to clinical application. Mol Metab. 2021;51:101237. doi: 10.1016/j.molmet.2021.101237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller TD, Bluher M, Tschop MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21:201–23. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oury C, Marechal P, Donis N, Hulin A, Hego A, Tridetti J, et al. Dexfenfluramine and pergolide cause heart valve disease via valve metabolic reprogramming and ongoing matrix remodeling. Int J Mol Sci. 2020;21:4003. doi: 10.3390/ijms21114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–8. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 12.Loos RJ, Yeo GS. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120–33. doi: 10.1038/s41576-021-00414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooqi SI. Genetic, molecular and physiological mechanisms involved in human obesity: Society for Endocrinology Medal Lecture 2012. Clin Endocrinol (Oxf) 2015;82:23–8. doi: 10.1111/cen.12588. [DOI] [PubMed] [Google Scholar]
- 14.Edie ES. Further observations on the treatment of diabetes mellitus by acid extract of duodenal mucous membrane. Biochem J. 1906;1:446–54. doi: 10.1042/bj0010446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 16.Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 17.Pratley RE, Kang J, Trautmann ME, Hompesch M, Han O, Stewart J, et al. Body weight management and safety with efpeglenatide in adults without diabetes: a phase II randomized study. Diabetes Obes Metab. 2019;21:2429–39. doi: 10.1111/dom.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 19.Jastreboff AM, Kaplan LM, Frias JP, Wu Q, Du Y, Gurbuz S, et al. Triple-hormone-receptor agonist retatrutide for obesity: a phase 2 trial. N Engl J Med. 2023;389:514–26. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 20.Le Roux CA, Steen O, Lucas KJ, Startseva E, Unseld A, Hennige AM. 51-OR: a phase 2, randomized, double-blind, placebo-controlled, dose-finding study of BI 456906 in people with overweight/obesity. Diabetes. 2023;72(Supplement 1):51–OR. [Google Scholar]
- 21.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 22.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–56. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Muller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. 2023;20:463–74. doi: 10.1038/s41569-023-00849-3. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, Muller TD. Incretin hormones and type 2 diabetes. Diabetologia. 2023;66:1780–95. doi: 10.1007/s00125-023-05956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 28.Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425:304–8. doi: 10.1016/j.bbrc.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–52. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo M, Rizvi AA, Patti AM, Nikolic D, Giglio RV, Castellino G, et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovasc Diabetol. 2016;15:162. doi: 10.1186/s12933-016-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E12 09–15. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 32.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 33.Salehi M, Aulinger BA, D’Alessio DA. Targeting beta-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins. Endocr Rev. 2008;29:367–79. doi: 10.1210/er.2007-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Parkes DG, Mace KF, Trautmann ME. Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov. 2013;8:219–44. doi: 10.1517/17460441.2013.741580. [DOI] [PubMed] [Google Scholar]
- 36.Tschop M, Nogueiras R, Ahren B. Gut hormone-based pharmacology: novel formulations and future possibilities for metabolic disease therapy. Diabetologia. 2023;66:1796–808. doi: 10.1007/s00125-023-05929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim S, Kim KM, Nauck MA. Glucagon-like peptide-1 receptor agonists and cardiovascular events: class effects versus individual patterns. Trends Endocrinol Metab. 2018;29:238–48. doi: 10.1016/j.tem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382:2117–28. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 39.Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–88. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol. 2016;310:R885–95. doi: 10.1152/ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314:687–99. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 42.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443–51. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 43.Park JH, Kim JY, Choi JH, Park HS, Shin HY, Lee JM, et al. Effectiveness of liraglutide 3mg for the treatment of obesity in a real-world setting without intensive lifestyle intervention. Int J Obes (Lond) 2021;45:776–86. doi: 10.1038/s41366-021-00739-z. [DOI] [PubMed] [Google Scholar]
- 44.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallo M. Thyroid safety in patients treated with liraglutide. J Endocrinol Invest. 2013;36:140–5. doi: 10.1007/BF03346749. [DOI] [PubMed] [Google Scholar]
- 46.Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne) 2019;10:155. doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies M, Faerch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–84. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 48.Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 Randomized Clinical Trial. JAMA. 2021;325:1403–13. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10:193–206. doi: 10.1016/S2213-8587(22)00008-0. [DOI] [PubMed] [Google Scholar]
- 50.Ha JH, Kim JE, Kim YS. Immunoglobulin Fc heterodimer platform technology: from design to applications in therapeutic antibodies and proteins. Front Immunol. 2016;7:394. doi: 10.3389/fimmu.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with Efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385:896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400:1803–20. doi: 10.1016/S0140-6736(22)01655-5. [DOI] [PubMed] [Google Scholar]
- 53.Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4:525–36. doi: 10.1016/S2213-8587(15)00482-9. [DOI] [PubMed] [Google Scholar]
- 54.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–9. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 55.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao T, Zhang H, Li S, Tian H. Glucagon-like peptide 1 receptor agonist ameliorates the insulin resistance function of islet β cells via the activation of PDX-1/JAK signaling transduction in C57/BL6 mice with high-fat diet-induced diabetes. Int J Mol Med. 2017;39:1029–36. doi: 10.3892/ijmm.2017.2910. [DOI] [PubMed] [Google Scholar]
- 57.Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J Clin Invest. 2017;127:3835–44. doi: 10.1172/JCI91761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–5. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- 59.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–6. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 60.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–16. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19:524–36. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
- 63.Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18:317–32. doi: 10.1111/dom.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh S, Wright EE, Jr, Kwan AY, Thompson JC, Syed IA, Korol EE, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2017;19:228–38. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19:216–27. doi: 10.1111/dom.12804. [DOI] [PubMed] [Google Scholar]
- 66.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 67.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 68.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 70.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 71.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 72.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–32. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 73.Rakipovski G, Rolin B, Nohr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844–57. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sattar N, Lee MM, Kristensen SL, Branch KR, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–62. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 75.Tonneijck L, Muskiet MH, Blijdorp CJ, Smits MM, Twisk JW, Kramer MH, et al. Renal tubular effects of prolonged therapy with the GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2019;316:F231–40. doi: 10.1152/ajprenal.00432.2018. [DOI] [PubMed] [Google Scholar]
- 76.Kelly M, Lewis J, Rao H, Carter J, Portillo I, Beuttler R. Effects of GLP-1 receptor agonists on cardiovascular outcomes in patients with type 2 diabetes and chronic kidney disease: a systematic review and meta-analysis. Pharmacotherapy. 2022;42:921–8. doi: 10.1002/phar.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moon JS, Hong JH, Jung YJ, Ferrannini E, Nauck MA, Lim S. SGLT-2 inhibitors and GLP-1 receptor agonists in metabolic dysfunction-associated fatty liver disease. Trends Endocrinol Metab. 2022;33:424–42. doi: 10.1016/j.tem.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Dong Y, Lv Q, Li S, Wu Y, Li L, Li J, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in nonalcoholic fatty liver disease: a systematic review and metaanalysis. Clin Res Hepatol Gastroenterol. 2017;41:284–95. doi: 10.1016/j.clinre.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 80.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 81.Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in nonalcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2023;8:179–91. doi: 10.1016/S2468-1253(22)00338-7. [DOI] [PubMed] [Google Scholar]
- 82.Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab. 2017;19:1233–41. doi: 10.1111/dom.12926. [DOI] [PubMed] [Google Scholar]
- 83.Nreu B, Dicembrini I, Tinti F, Mannucci E, Monami M. Pancreatitis and pancreatic cancer in patients with type 2 diabetes treated with glucagon-like peptide-1 receptor agonists: an updated meta-analysis of randomized controlled trials. Minerva Endocrinol (Torino) 2023;48:206–13. doi: 10.23736/S2724-6507.20.03219-8. [DOI] [PubMed] [Google Scholar]
- 84.Hegedüs L, Sherman SI, Tuttle RM, von Scholten BJ, Rasmussen S, Karsbol JD, et al. No evidence of increase in calcitonin concentrations or development of C-cell malignancy in response to liraglutide for up to 5 years in the LEADER Trial. Diabetes Care. 2018;41:620–2. doi: 10.2337/dc17-1956. [DOI] [PubMed] [Google Scholar]
- 85.Forzano I, Varzideh F, Avvisato R, Jankauskas SS, Mone P, Santulli G. Tirzepatide: a systematic update. Int J Mol Sci. 2022;23:14631. doi: 10.3390/ijms232314631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sinha R, Papamargaritis D, Sargeant JA, Davies MJ. Efficacy and safety of Tirzepatide in type 2 diabetes and obesity management. J Obes Metab Syndr. 2023;32:25–45. doi: 10.7570/jomes22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boer GA, Hay DL, Tups A. Obesity pharmacotherapy: incretin action in the central nervous system. Trends Pharmacol Sci. 2023;44:50–63. doi: 10.1016/j.tips.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398:1811–24. doi: 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 89.Rosenstock J, Wysham C, Frias JP, Kaneko S, Lee CJ, Fernandez Lando L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–55. doi: 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 90.Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–15. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 91.Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, et al. Effect of subcutaneous Tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 Randomized Clinical Trial. JAMA. 2022;327:534–45. doi: 10.1001/jama.2022.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402:613–26. doi: 10.1016/S0140-6736(23)01200-X. [DOI] [PubMed] [Google Scholar]
- 93.Wadden TA, Chao AM, Machineni S, Kushner R, Ard J, Srivastava G, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. 2023;29:2909–18. doi: 10.1038/s41591-023-02597-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aronne LJ, Sattar N, Horn DB, Bays HE, Wharton S, Lin WY, et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 Randomized Clinical Trial. JAMA. 2024;331:38–48. doi: 10.1001/jama.2023.24945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mathiesen DS, Bagger JI, Knop FK. Long-acting amylin analogues for the management of obesity. Curr Opin Endocrinol Diabetes Obes. 2022;29:183–90. doi: 10.1097/MED.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 96.Ryan GJ, Jobe LJ, Martin R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin Ther. 2005;27:1500–12. doi: 10.1016/j.clinthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother. 2011;12:1439–51. doi: 10.1517/14656566.2011.581663. [DOI] [PubMed] [Google Scholar]
- 98.Dehestani B, Stratford NR, le Roux CW. Amylin as a future obesity treatment. J Obes Metab Syndr. 2021;30:320–5. doi: 10.7570/jomes21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lau DC, Erichsen L, Francisco AM, Satylganova A, le Roux CW, McGowan B, et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398:2160–72. doi: 10.1016/S0140-6736(21)01751-7. [DOI] [PubMed] [Google Scholar]
- 100.Enebo LB, Berthelsen KK, Kankam M, Lund MT, Rubino DM, Satylganova A, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2.4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397:1736–48. doi: 10.1016/S0140-6736(21)00845-X. [DOI] [PubMed] [Google Scholar]
- 101.Becerril S, Fruhbeck G. Cagrilintide plus semaglutide for obesity management. Lancet. 2021;397:1687–9. doi: 10.1016/S0140-6736(21)00944-2. [DOI] [PubMed] [Google Scholar]
- 102.Frias JP, Deenadayalan S, Erichsen L, Knop FK, Lingvay I, Macura S, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2.4 mg with once-weekly semaglutide 2.4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402:720–30. doi: 10.1016/S0140-6736(23)01163-7. [DOI] [PubMed] [Google Scholar]
- 103.Wewer Albrechtsen NJ, Holst JJ, Cherrington AD, Finan B, Gluud LL, Dean ED, et al. 100 years of glucagon and 100 more. Diabetologia. 2023;66:1378–94. doi: 10.1007/s00125-023-05947-y. [DOI] [PubMed] [Google Scholar]
- 104.Haedersdal S, Andersen A, Knop FK, Vilsboll T. Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases. Nat Rev Endocrinol. 2023;19:321–35. doi: 10.1038/s41574-023-00817-4. [DOI] [PubMed] [Google Scholar]
- 105.Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142:4244–50. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 106.Scott R, Minnion J, Tan T, Bloom SR. Oxyntomodulin analogue increases energy expenditure via the glucagon receptor. Peptides. 2018;104:70–7. doi: 10.1016/j.peptides.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holst JJ, Albrechtsen NJ, Gabe MB, Rosenkilde MM. Oxyntomodulin: actions and role in diabetes. Peptides. 2018;100:48–53. doi: 10.1016/j.peptides.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 108.Zhihong Y, Chen W, Qianqian Z, Lidan S, Qiang Z, Jing H, et al. Emerging roles of oxyntomodulin-based glucagon-like peptide-1/glucagon co-agonist analogs in diabetes and obesity. Peptides. 2023;162:170955. doi: 10.1016/j.peptides.2023.170955. [DOI] [PubMed] [Google Scholar]
- 109.Zimmermann T, Thomas L, Baader-Pagler T, Haebel P, Simon E, Reindl W, et al. BI 456906: discovery and preclinical pharmacology of a novel GCGR/GLP-1R dual agonist with robust anti-obesity efficacy. Mol Metab. 2022;66:101633. doi: 10.1016/j.molmet.2022.101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jungnik A, Arrubla Martinez J, Plum-Morschel L, Kapitza C, Lamers D, Thamer C, et al. Phase I studies of the safety, tolerability, pharmacokinetics and pharmacodynamics of the dual glucagon receptor/glucagon-like peptide-1 receptor agonist BI 456906. Diabetes Obes Metab. 2023;25:1011–23. doi: 10.1111/dom.14948. [DOI] [PubMed] [Google Scholar]
- 111.Bluher M, Rosenstock J, Hoefler J, Manuel R, Hennige AM. Dose-response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial. Diabetologia. 2024;67:470–82. doi: 10.1007/s00125-023-06053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coskun T, Urva S, Roell WC, Qu H, Loghin C, Moyers JS, et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: from discovery to clinical proof of concept. Cell Metab. 2022;34:1234–47. doi: 10.1016/j.cmet.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 113.Urva S, Coskun T, Loh MT, Du Y, Thomas MK, Gurbuz S, et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: a phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Lancet. 2022;400:1869–81. doi: 10.1016/S0140-6736(22)02033-5. [DOI] [PubMed] [Google Scholar]
- 114.Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402:529–44. doi: 10.1016/S0140-6736(23)01053-X. [DOI] [PubMed] [Google Scholar]