Abstract
In the last decade, organoid research has entered a golden era, signifying a pivotal shift in the biomedical landscape. The year 2023 marked a milestone with the publication of thousands of papers in this arena, reflecting exponential growth. However, amid this burgeoning expansion, a comprehensive and accurate overview of the field has been conspicuously absent. Our review is intended to bridge this gap, providing a panoramic view of the rapidly evolving organoid landscape. We meticulously analyze the organoid field from eight distinctive vantage points, harnessing our rich experience in academic research, industrial application, and clinical practice. We present a deep exploration of the advances in organoid technology, underpinned by our long-standing involvement in this arena. Our narrative traverses the historical genesis of organoids and their transformative impact across various biomedical sectors, including oncology, toxicology, and drug development. We delve into the synergy between organoids and avant-garde technologies such as synthetic biology and single-cell omics and discuss their pivotal role in tailoring personalized medicine, enhancing high-throughput drug screening, and constructing physiologically pertinent disease models. Our comprehensive analysis and reflective discourse provide a deep dive into the existing landscape and emerging trends in organoid technology. We spotlight technological innovations, methodological evolution, and the broadening spectrum of applications, emphasizing the revolutionary influence of organoids in personalized medicine, oncology, drug discovery, and other fields. Looking ahead, we cautiously anticipate future developments in the field of organoid research, especially its potential implications for personalized patient care, new avenues of drug discovery, and clinical research. We trust that our comprehensive review will be an asset for researchers, clinicians, and patients with keen interest in personalized medical strategies. We offer a broad view of the present and prospective capabilities of organoid technology, encompassing a wide range of current and future applications. In summary, in this review we attempt a comprehensive exploration of the organoid field. We offer reflections, summaries, and projections that might be useful for current researchers and clinicians, and we hope to contribute to shaping the evolving trajectory of this dynamic and rapidly advancing field.
Graphical abstract
Public summary
-
•
The landscape of organoids history: organoids mark a new and efficient model in tissue and organ level.
-
•
Multiple applications of organoids in biomedicine and life healthcare.
-
•
Organoids benefit to drug discovery, disease study, prevention, control, and therapy.
-
•
Synthetic biology, artificial intelligence and automation integration broaden the role of organoids.
Introduction
Organoid technology, the use of three-dimensional cultures derived from stem cells that closely mimic the architecture and functionality of native organs, represents a seminal advance in biomedical science, offering a revolutionary perspective on human physiology and pathology.1 Organoid technology has profoundly impacted various fields, notably oncology and regenerative medicine, demonstrating unparalleled adaptability and precision.2 Organoids, essentially miniature versions of organs, offer a highly physiologically relevant model for understanding human biology. This relevance is particularly critical in drug development, an area in which traditional models often fall short.3 Compared to two-dimensional cell cultures or animal models, organoids provide a more accurate representation of human tissues, enabling more reliable and efficient drug screening and functional validation.4 This feature is particularly valuable in the context of cancer research, where organoids can mimic the tumor microenvironment, providing insights into tumor-immune interactions and host-pathogen dynamics.5 The clinical fidelity of organoids is higher than that of conventional models, as they can replicate the complex biological processes of human organs in vitro. This attribute enables the rapid functional testing of drugs, increasing the efficiency of the pathway from discovery to clinical application.6 Additionally, organoids present a novel platform for in vitro gene-editing therapies. By leveraging CRISPR-Cas9 and other gene-editing tools, researchers can use organoids to model genetic diseases and test therapeutic strategies, significantly advancing the field of personalized medicine.7,8 Our research findings emphasize the transformative potential of organoids in biomedical sciences. In the future, we expect organoids to play a pivotal role in advancing our understanding of human biology, evolving from cellular self-organization to integration with technologies such as synthetic biology and artificial intelligence (AI), offering substantial potential to revolutionize fields such as drug discovery, disease modeling, and personalized medicine. Progress in organoid technology continues to break barriers, offering insights once deemed unattainable. Accordingly, this review not only explores the current landscape of organoid technology but also offers a forward-looking perspective on the profound anticipated impact of organoids on future biomedical research.
Organoid history
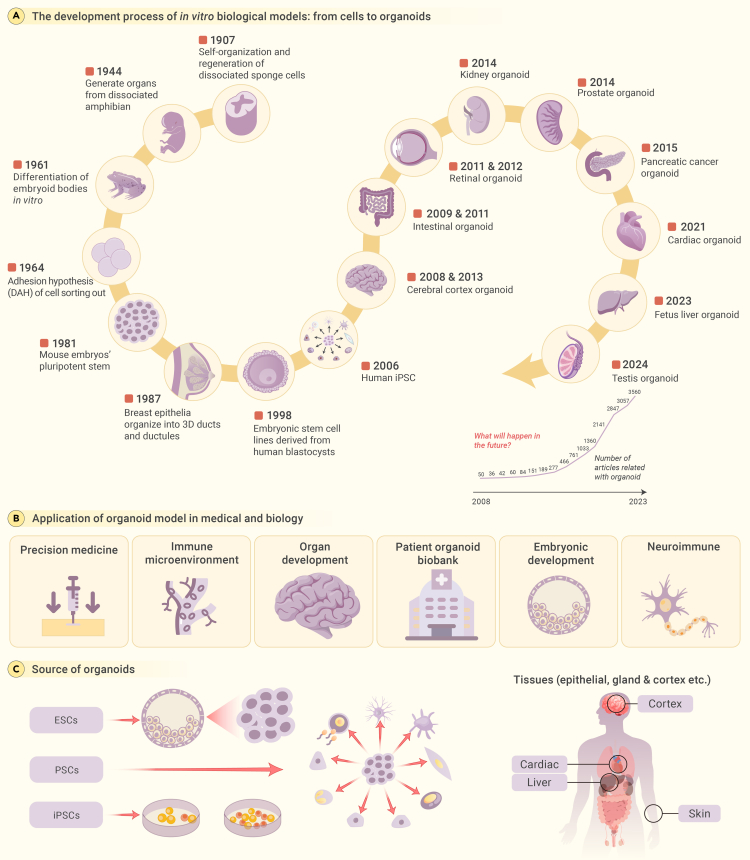
Organoid research, originating in the early 20th century, explores the self-organizing properties of cells (Figure 1A). The initial studies involved cultivating mechanically dissociated sponge cells to create functional organisms in vitro. H.V. Wilson’s observations in 1907 marked the inception of organoid research, revealing the self-organization and regeneration of dissociated sponge cells.9,10,11 In subsequent decades, several groups performed dissociation-reaggregation experiments to generate different types of organs from dissociated amphibian pronephros and chick embryos,12,13,14 demonstrating the ability of cells to self-organize and form organ-like structures in vitro.
Figure 1.
Unlocking organoids: A tale of historical discoveries and advanced taxonomies
(A) The chronological history of the development of organoids.
(B) Numerous novel applications of organoids have surfaced in the realm of life science research in recent years.
(C) Further refinement is required in classification as a result of the diversification of organoid sources.
In 1981, pluripotent stem cells (PSCs) were isolated from mouse embryos, a milestone in stem cell research.15,16,17,18 In November 1998, human embryonic stem cells (ESCs) were derived from blastocysts, a breakthrough demonstrating human PSC derivation.19 The development of human induced pluripotent stem cell (iPSC) technology further revolutionized the field.20,21 The modern era of organoid technology truly began in the 2000s with the development of iPSCs by Shinya Yamanaka’s team at Kyoto University22,23,24,25: their pioneering work showed that mouse fibroblasts could be reprogrammed into a pluripotent state by introducing four transcription factors (Oct4, Sox2, Klf4, and c-Myc), generating the first iPSCs.18,22
In 2009, Hans Clevers introduced the term “organoid,” emphasizing self-organization, particularly in cancer research.26 The term “organoid” draws inspiration from the ancient Greek lexicon, where “organon” is the origin of “organ” and the suffix “-oid” denotes resemblance or likeness. Therefore, “organoid” refers to something that resembles an organ. Researchers such as Lancaster and Knoblich expanded upon this seminal work.27 They opined that the true essence of organoids lies not solely in their structural replication but in their ability to mirror the cellular diversity, specific functionalities, and even intricate cellular arrangements of the organs they represent.28 Subsequently, the field of organoid technology advanced rapidly, ushering in a new era in scientific innovation.29,30 Hans Clevers’ intestinal organoids paved the way for new research on intestinal biology and disease mechanisms31 and continue to be a valuable resource for investigating the molecular mechanisms underlying intestinal development and disease progression.26,32 Cerebral organoids, created by Lancaster et al. in 2013, mimic human brain development and aid in understanding developmental brain disorders.33
In 2014, groundbreaking advances occurred in prostate organoid research, providing a robust platform for studying prostate cancer dynamics and therapeutic drug testing.34,35 In 2015, kidney organoids derived from iPSCs provided insights into kidney development and disease modeling.36,37 Subsequent years saw the burgeoning of organoid technology across various organ systems, including the pancreas. The development of pancreatic cancer organoids has illuminated the pathophysiological mechanisms that underlie pancreatic ductal adenocarcinoma, fostering advances in personalized medicine and targeted therapeutic strategies.38,39 A surge in methodological refinements in retinal organoid cultivation have occurred since 2018. These organoids recapitulate the functionality of retinal cells, representing a significant leap forward from earlier models that lacked structural and functional fidelity.40,41 Most recently, cardiac organoids emerged, aiding in modeling heart diseases.42,43
Organoids have revolutionized biomedical research, enhancing drug assessments and personalized medicine.44 In oncology, organoids have provided critical insights into the tumor immune microenvironment, propelling advancements in immunotherapy.45 These three-dimensional cultures serve as pivotal tools in deciphering the complexities of organ and embryonic development, spotlighting essential elements of morphogenesis and the inception of life.46 Moreover, the establishment of extensive organoid biobanks has supported personalized research approaches, offering a plethora of tissue-specific samples for in-depth study. In neuroimmune research, organoids have been instrumental in elucidating the intricate interplay between the nervous and immune systems, leading to new insights into a myriad of associated disorders.47,48 The remarkable ability of organoids to replicate organ-level functions within controlled environments is responsible for their indispensable role in precision medicine, disease modeling, and regenerative medicine, enabling a transformative phase in modern medical research (Figure 1B).
The field of organoid development utilizes a variety of stem cell sources, each with unique characteristics. Among these are the well-known ESCs and the revolutionary iPSCs, both of which fall under the broader category of PSCs (Figure 1C). The versatile mesenchymal stem cells also play a crucial role in this domain.49,50,51,52,53,54,55 Additionally, the world of clinical medicine offers a treasure trove of sources in the form of tissue specimens, both from individuals battling diseases and from healthy donors.56,57 This range includes surgical specimens and other less obvious sources such as nasopharyngeal swabs, which have recently gained prominence.58
In conclusion, organoid research has introduced a new era in biomedical sciences, enhancing our understanding of life, disease, and therapies.
Organoids outshine conventional biomedical models
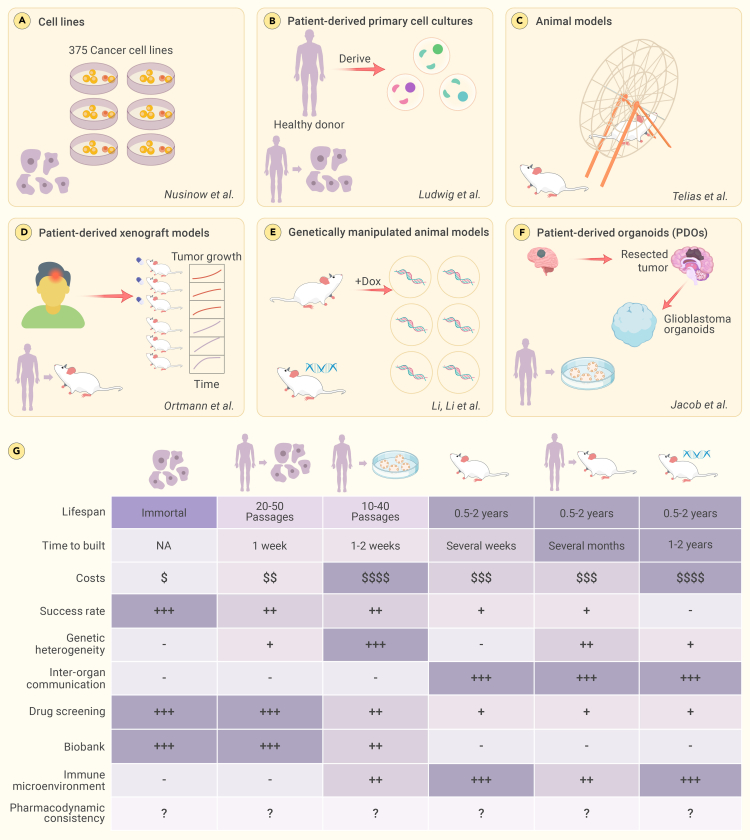
Biomedical researchers, in relentless pursuit of understanding life’s complexity, have long sought models to mirror human physiology and pathology (Figure 2). Animal models, once pivotal in research,59 present challenges due to interspecies differences and translational limitations.60,61,62 As the limitations of animal models grew more apparent, the use of cell lines became prevalent.63 However, cell lines, though scalable, have the disadvantages of genetic homogeneity and two-dimensional constraints.64,65,66 In this complex scenario, the scientific community witnessed the emergence of organoids, three-dimensional structures derived from stem cells. These scaled-down models of organs are designed to bridge the gap between the simplicity of cell lines and the complexity of in vivo organisms, providing a clearer perspective on human biology.57,67
Figure 2.
Organoids represent significant improvements over existing biomedical models
Organoids, the miniaturized facsimiles of organs, have redefined the landscape of biomedical models, presenting a more physiologically relevant alternative. Compared to other models, organoids not only boast a shorter construction cycle and a higher success rate but also excel in preserving individualized tissue characteristics of patients.
Patient-derived organoids, which resemble the original tissues, hold promise in biomedical research. These models are established from patient samples, retain their genetic complexity, and offer a rapid and cost-effective way to study diseases.68 Patient-derived organoids maintain interpatient heterogeneity, making them valuable for personalized medicine, especially in cancer research; however, their applications extend beyond oncology. For example, endometrial organoids have been successfully established from both normal and diseased human endometrium, including tissue samples representing conditions such as endometriosis, endometrial hyperplasia, and endometrial cancer.69 These organoids maintain tissue characteristics and genetic stability, which can facilitate the understanding of disease heterogeneity. For instance, organoids from patients with Mayer-Rokitansky-Küster-Hauser syndrome have revealed unique gene-expression patterns, highlighting their potential for personalized medicine.70 This emphasizes the potential of endometrial organoids to model individual patient pathologies and contribute to personalized medicine. Endometrial organoids have shown promise in high-throughput drug screening: they can be used to test the efficacy and toxicity of new therapeutic agents in a controlled environment that closely mimics in vivo conditions.69 By testing drugs on patient-specific organoids, researchers can identify the most effective treatments for individual patients.69 This personalized strategy has the potential to transform endometrial disease treatment by customizing therapies to each patient’s specific needs, thereby advancing precision medicine.71,72
In contrast, although patient-derived primary cell cultures closely resemble the original tumor,73 they have a finite lifespan and limited replicative capacity. Their establishment can be challenging due to low success rates and slow growth, restricting the duration of drug-response testing.74 Patient-derived xenograft models, which can take several months to establish and are costly to maintain, also exhibit genetic heterogeneity.75 Moreover, the success rate of establishing these models can vary widely, making them less predictable than patient-derived organoids.76 Genetically manipulated animal models are another alternative but present their own set of challenges.77 These models take months or even years to develop, impose high maintenance costs, and exhibit genetic and cellular heterogeneity depending on the specific genetic modifications made.78,79,80 The rigidity and complexity of these models make them less adaptable than the more versatile and representative patient-derived organoids.
Organoid technology has advanced beyond other in vitro techniques to become a leading approach within the field of personalized medicine.81 Central to this advancement are iPSC-derived patient-specific organoids. These models have revolutionized our approach to understanding complex diseases by closely mirroring the genetic, cellular, and functional characteristics of a patient’s tissue.82,83,84,85 iPSC-derived organoids offer unique advantages in disease modeling, particularly due to their patient-specific nature.84 They allow personalized disease modeling, which is crucial in understanding complex diseases such as cancers, neurological disorders, and rare genetic diseases. Moreover, iPSCs are amenable to genetic editing using CRISPR-Cas9, enabling the study of disease mechanisms and the development of targeted therapies.82,83 iPSC-derived organoids have filled a gap in research opportunities by providing a more accurate, human-specific model, thereby revolutionizing the scope and methods of disease research. In Alzheimer’s disease research, organoids replicate key disease features such as β-amyloid accumulation and tau protein buildup,86,87 offering insights into molecular mechanisms and genetic factors such as APOE4.83 Similarly, Parkinson’s disease research benefits from the use of midbrain-specific organoids in exploring interactions involving dopamine-producing neurons.88,89,90,91 The pathology of amyotrophic lateral sclerosis has been successfully mimicked in cerebral organoids using patient-derived protein extracts, illustrating TDP-43 aggregation and disease progression.92 This model has also demonstrated the time-dependent spread of pathogenic TDP-43, inducing astrogliosis, cellular apoptosis, and DNA double-strand breaks in the recipient cerebral organoids. Accordingly, this model enables the in-depth exploration of genetic mechanisms and the development of targeted gene therapies for amyotrophic lateral sclerosis.93,94,95
The pharmaceutical industry, ever in search of therapeutic innovations, has embraced organoids as a powerful tool.96,97,98 Patient-specific organoids, with their personalized genetic tapestries, have proven to be robust platforms for drug testing, providing a path for individualized therapeutic blueprints. Their high accuracy, consistency, reproducibility, and fidelity in drug-screening paradigms support their potential as vanguards of drug discovery.99,100
Furthermore, the convenience of subculturing and the potential for extended storage render organoids a favored option for extensive biobanking, accommodating a wide range of patient specimens.101 Because of their differences from and synergies with other models, organoids illuminate a path toward personalized medicine, drug discovery, and a more profound understanding of life itself.
Organoids solve clinical difficulties in oncology
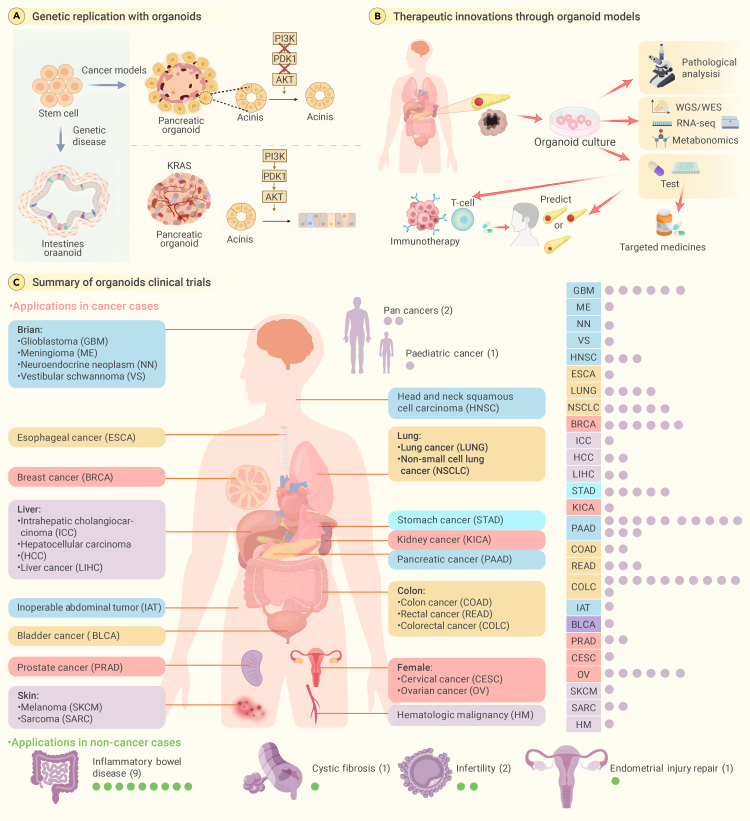
Organoid technology introduces a new generation of detailed and accurate simulations, capturing the genetic traits and heterogeneity inherent in complex diseases such as cancer.102,103,104 The ability of organoids to mimic diverse patient-specific responses is instrumental in dissecting the multifaceted nature of tumors, paving the way for targeted therapeutic interventions (Figure 3A). The detailed insights offered by these models are fostering new developments in biomedical research and pharmaceuticals, providing a novel approach to address complex clinical challenges, such as pancreatic cancer,102,105 tumor drug resistance, recurrence, and metastasis.103,106,107
Figure 3.
Organoid solves clinical difficulties in oncology: Pioneering models, therapies, and global trials
(A) Utilizing organoids for genetic replication in a groundbreaking manner. Organoids are an innovative method that successfully generates genetically homogeneous models for cancers, specifically those that demonstrate resistance to medicine or repeated spread to other parts of the body.
(B) Therapeutic innovations through organoid models. Utilizing the organoid-based genetic models, the scientific community is embarking on a journey of exploring and validating novel therapeutic regimens for tumors previously deemed untreatable.
(C) Organoids at the forefront of global clinical endeavors. The global initiation of clinical trials that harness the capabilities of organoids testifies to their transformative potential.
Pancreatic cancer presents a significant challenge in oncology, as it is characterized by late diagnosis and limited treatment options, an aggressive nature, a dense stroma, high heterogeneity, and a lack of effective screening techniques. These factors contribute to its poor prognosis as a clinically incurable disease.108,109,110 The development of organoids, which are three-dimensional cell cultures replicating organ structure and function, introduces new possibilities in addressing this challenging cancer.102 Patient-derived organoids have been successfully derived from pancreatic ductal adenocarcinoma patients, demonstrating the ability to test the efficacy of various chemotherapy drugs.111 In some cases, organoid drug responses have correlated with patient clinical responses.102 Promising results have been observed in drug-screening experiments, where kinase inhibitors, which are drugs that block certain enzymes called kinases, have been shown to decrease the viability of patient-derived organoids, with CHK1 inhibitors exhibiting exceptional growth inhibition.104 Organoids also show potential for treating drug-resistant tumors by maintaining key characteristics of primary tumors even after long-term passaging.103,112 They facilitate the exploration of resistance mechanisms related to cancer stem cells and can be used to study drug-resistance mechanisms, personalized medicine, and high-throughput drug-screening methodologies. Organoids have been successfully established from multiple human tumor types, such as breast, pancreatic, gastrointestinal, lung, prostate, ovarian, and bladder cancers.106 They can be used to target key genes and cancer stem cells to reverse drug resistance in cancer and have been shown to accurately predict patient responses to targeted therapies and chemotherapies (Figure 3B).113
Organoid models have emerged as a crucial asset in the field of immunotherapy, an innovative type of cancer therapy that leverages the body’s immune system to identify and eradicate cancer cells.114,115,116 Organoid models have been instrumental in the generation and proliferation of tumor-reactive T cells derived from patient peripheral blood lymphocytes. Notably, these T cells exhibit a unique ability to target and annihilate autologous tumor organoids, emphasizing the promising role of organoids in the advancement of tailored immunotherapies.117 Moreover, organoids have proven crucial in forecasting individualized responses to immunotherapeutic interventions. Because they preserve the genetic integrity of the original tumor samples, organoids offer a highly representative model for assessing the effectiveness of various immunotherapeutic agents and their synergistic combinations.117,118 Research utilizing cancer organoids has shown that combination therapies markedly outperform monotherapy in terms of drug-response rates. This finding highlights the importance of organoids in refining treatment protocols and exploring novel therapeutic combinations.117 Furthermore, the cocultivation of organoids with immune cells, including tumor-infiltrating lymphocytes and peripheral blood mononuclear cells, has yielded valuable insights into the tumor microenvironment and its intricate interactions with the immune system. This methodology is vital for the evaluation of immunotherapies and for elucidating the dynamics of tumor-immune interplay.118 Advanced methodologies such as organoid-on-a-chip and three-dimensional bioprinting techniques have also been developed, enabling the creation of more elaborate cancer models.119 These sophisticated models enable precise manipulation of the tumor microenvironment and effectively simulate multiorgan metastases in cancer, providing a more accurate testing ground for immunotherapeutic agents.117 In summary, organoid models stand in the vanguard of cancer research and immunotherapy evaluation. This evolving technology is poised to become an essential instrument in the global battle against cancer, paving the way for more efficacious and personalized treatment for patients worldwide.120,121,122
Organoid-based clinical trials are gaining momentum internationally, with significant contributions from countries including China and the United States (Figure 3C).1 Clinical trials, prospective observations, and randomized controlled trials using organoids cover a wide range of diseases, including cystic fibrosis and various types of cancers such as breast cancer, colorectal cancer, lung cancer, esophageal cancer, and liver cancer. These trials are at the forefront of translating laboratory findings into clinical therapies to address some of the most daunting challenges in modern medicine.123 Numerous applications of organoid technology are currently undergoing rigorous clinical evaluations and hold promise for a new era of personalized and effective treatment strategies.124 These trials highlight the potential of organoids to provide tangible solutions to some of the most perplexing medical challenges of our time.125 The integration of organoid technology into mainstream healthcare could revolutionize treatment modalities and present unprecedented opportunities to improve patient outcomes. As these clinical trials progress, they pave the way for the next generation of medical breakthroughs, powered by the sophisticated simulations that only organoid technology can provide.
The intersection of synthetic biology and organoid technology
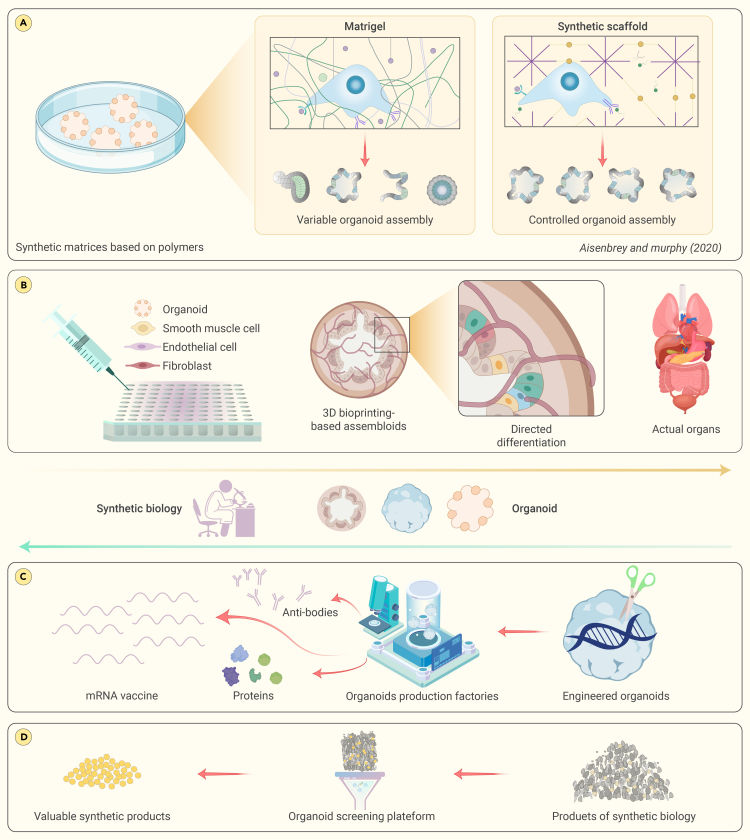
The path to realizing the full potential of organoid technology is fraught with complexities and inconsistencies. These challenges, along with technical difficulties in creation and analysis, have somewhat impeded widespread adoption. Synthetic biology, however, is a field poised to surmount these hurdles. Synthetic biology is introducing a new era by connecting innovative methodologies to the multifaceted world of organoids.126 This nexus between synthetic biology and organoid research is a rapidly growing field that is expected to overhaul our comprehension of biological systems and inaugurate unique therapeutic strategies.127
Genetically encoded fluorescent biosensors are revolutionizing real-time monitoring in organoid development,128 enabling the measurement of protein kinase activity, pH levels, and lactate levels.129 From studying cancer formation and progression by monitoring specific biochemical markers to investigating neurological disorders through precise measurements, these tools offer powerful insights into disease at a molecular level.128,130 In gastrointestinal organoid culture, bioengineered sensors or biosensors have been integrated to enable measurements specific to gastrointestinal organoids.131
Advances in synthetic biology include the creation of tunable synthetic matrices using materials such as polyethylene glycol and hyaluronic acid to enhance organoid growth and functionality.132 Bioengineered hydrogels, such as those that emulate the esophageal microenvironment or support ovarian follicle culture, play crucial roles in promoting cell differentiation and organoid-like structure formation (Figure 4A). Synthetic biology, driven by advances in biomaterials such as hydrogels, is crucial for the development of organoid technology.133 Cruz-Acuña et al. developed a bioengineered hydrogel system that mimics the esophageal microenvironment, promoting the differentiation of PSCs into esophageal epithelial cells.134 Nason-Tomaszewski et al. created fibrous hydrogels to support organoid-like structure formation and oocyte survival in ovarian follicle culture.135 Nguyen et al. utilized tryptophan zipper peptides to craft bioactive hydrogels with antimicrobial properties, promoting mammalian cell growth and inducing polarity changes in human intestinal organoids.136 The concept of “assembloids” represents a further advance in bottom-up construction.137 These organoids, derived from spatially organized multiple cell types, mimic the complexity of actual organs. By combining different cellular components, assembloid technology provides a high-throughput platform for generating functional human organoids. For example, researchers have created multilayer bladder assembloids that mimic the interactions between epithelium and stroma found in mature adult bladders.138 These assembloids not only exhibit functional characteristics but also enable the modeling of specific conditions, such as urinary tract infections and urothelial carcinoma (Figure 4B).
Figure 4.
The intersection of synthetic biology and organoid
(A) Synthetic biology aids in creating various auxiliary matrices and scaffolds. These structures provide an optimized environment, enhancing the growth and development of organoids.
(B) By combining different cellular components, assembloids mimic the complexity of actual organs.
(C) The utilization of organoid technology is establishing a distinct and specialized role within the realm of synthetic biology product manufacture.
(D) Organoids, which are three-dimensional multicellular constructs that replicate the activity of actual organs, offer a distinct prospect for enhanced and streamlined screening procedures of synthetic drugs.
Organoids also present a unique advantage in the field of synthetic biology, namely, the production of a myriad of biological products.139 The unique milieu of these miniature organ structures mimics physiological conditions within the human body, facilitating the ex vivo production of complex molecules with high biocompatibility.140 For example, organoid technology shows great promise in antibody production by mimicking immune system components to produce specific antibodies against pathogens in vitro. This accelerates therapeutic development and enables research into immune response.141 In another area of synthetic biology, organoid technology has revolutionized toxin production for medical research. The cultivation of organoids capable of producing snake venom is an important advance that offers potential for antivenom development, pharmacological research, and therapeutic applications. Organoids derived from snake venom gland cells provide an in vivo-like model for venom production.142 This study represents a significant stride in venom research, as these new organoids can serve as a viable platform that not only fosters a deeper understanding of venom biology but also propels the development of antivenoms and other therapeutic agents.143 In summary, organoid technology is carving a new niche in the production of synthetic biology products and is expected to propel the development of innovative drugs and therapeutic strategies in the future (Figure 4C). Recently, Kim et al. reported another significant advance in the field of synthetic biology and organoid technology.144,145 Researchers created human enamel organoids from iPSCs to mimic tooth development. These organoids, which resemble ameloblasts, formed enamel in response to the presence of calcium and interacted with dental mesenchyme to enable mineralization. They also displayed differentiation potential and regenerative abilities. This advance in synthetic biology introduces new possibilities for dental treatments and research.144,145
Synthetic biology, an evolving field, shows promise for the generation of diverse biochemical compounds, which is crucial for therapeutic development. The main challenge is efficiently identifying compounds with high biological activity and therapeutic value, and an emerging solution is using organoids in high-throughput screening systems. Organoids, which mimic the functions of real organs in three-dimensional structures, provide a platform for more accurately evaluating synthetic compounds. For instance, a study by Yang et al. emphasizes that organoids provide a three-dimensional platform suitable for high-throughput drug screening, potentially bridging the gap between basic research and clinical practice.146 The integration of compound production by synthetic biology and the screening capabilities of organoids could revolutionize therapeutic development. The synergy between generating diverse biological compounds and efficiently screening them for the most promising candidates could significantly expedite drug discovery, bringing effective treatments to patients more quickly and reliably (Figure 4D).
In conclusion, the fusion of synthetic biology and organoid research represents a monumental advance in biomedicine, presenting new avenues for studying and manipulating organoids. This intersection is poised to reshape our understanding of human biology and disease, paving the way for transformative clinical applications and developments in biomedical research.
Organoids: Revolutionizing the landscape of toxicology research
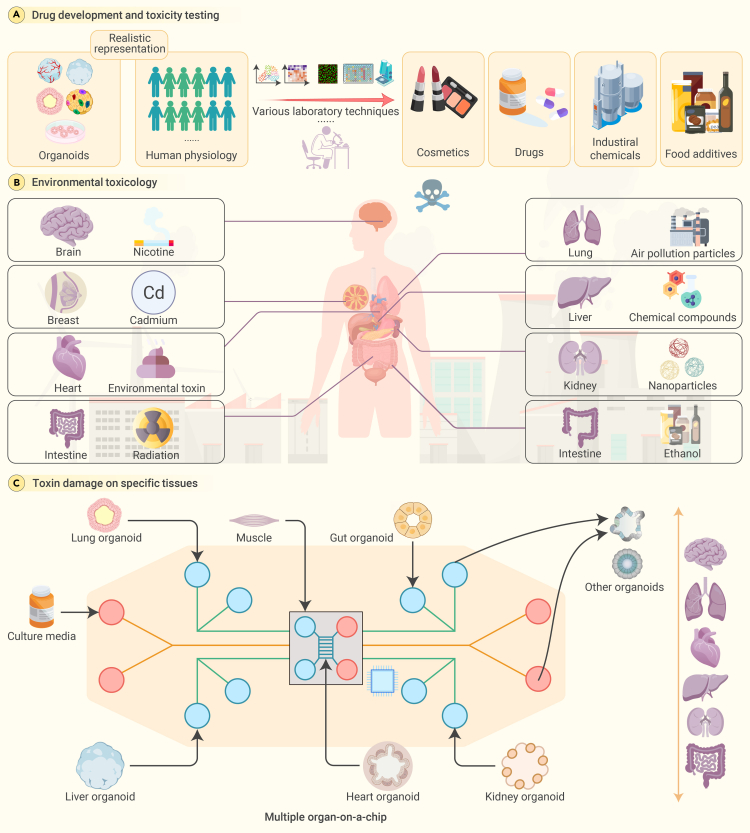
Toxicology research, which is instrumental in evaluating the safety of pharmaceutical drugs, industrial chemicals, cosmetics, and food additives, has traditionally relied on animal models and two-dimensional cell cultures. The application of organoids in toxicology allows the exploration of tissue-specific responses to toxicants, which was hitherto impossible with the above traditional methods.147 The primary use of organoid models in toxicology lies in drug development and toxicity testing.148 Conventional in vitro and animal models frequently fail to precisely forecast toxicity in humans, causing late-stage drug failures and unforeseen adverse effects after market release. However, the ability to generate organoids that mimic the complexity and functionality of human organs presents new opportunities for understanding toxicity mechanisms at a cellular and molecular level. The significant contributions of organoids to toxicology include their use in drug-toxicity testing, where liver organoids have aided in predicting drug-induced liver injuries—a major cause of drug withdrawal from the market.149
Liver organoids have proven to be a valuable in vitro tool for drug testing, including toxicity assessment.150 Another study discusses the potential of using human PSC-derived organoids for high-fidelity drug-induced liver injury screening.151 Moreover, organoids derived from pluripotent or tissue-resident adult stem cells can self-organize into three-dimensional structures, closely mirroring the in vivo architecture and functionality of various organs.152 This ability allows organoids to mimic the effects of toxin damage on specific tissues, providing platforms for the specialized study of toxicity pathways. For instance, brain organoids comprising various neuronal and glial subtypes facilitate the analysis of the neurodevelopmental and neurodegenerative effects of drug toxicity.153 Likewise, intestinal organoids have been utilized to study gastrointestinal toxicity, such as the mucosal damage induced by compounds like non-steroidal anti-inflammatory drugs.154 In addition, the creation of patient-specific organoids exhibits tremendous potential in personalized medicine.148 These organoids can help define individual susceptibility to various toxins, which could transform toxicity testing by facilitating personalized risk assessment and intervention strategies (Figure 5A).
Figure 5.
Organoids: Transforming the field of toxicology research
(A) Organoids represent a significant advancement in accurately predicting human reactions to common items, surpassing the reliability of conventional models. Stem cell-derived organoids provide a more precise reproduction of human tissue structure and physiological reactions, making them suitable for assessing the safety of common consumer goods.
(B) The environmental sentinel: organoids detecting global toxic threats. Organoids serve as powerful tools for detecting environmental toxins, including those affecting the brain.
(C) Toward a comprehensive human model: multiorgan chips for effective toxicology. Microfluidic platforms and multiorganoid systems are at the forefront of simulating complex human physiology.
The potential application of organoid models extends further to the field of environmental toxicology. Organoids facilitate research on how environmental toxins affect human health.148 For instance, lung organoids have been used to study the impact of air-pollution particles on lung tissues, providing a realistic representation of human lung exposure to environmental hazards (Figure 5B).155
Technological advances, including microfluidics and high-throughput screening systems, promise a bright future for organoids in toxicology. Microfluidic platforms can provide increased control over the organoid microenvironment,156 fostering the examination of dynamic processes and cell interactions (Figure 5C). High-throughput screening systems can expedite the testing of numerous compounds, enabling efficient and cost-effective toxicological assessments. The incorporation of multiorganoid systems also shows great promise. By amalgamating different types of organoids, researchers can construct more intricate and interconnected models to simulate the interaction among organs in the human body.157,158 The system was able to demonstrate drug metabolism, interorgan crosstalk, and adverse drug reactions, offering a comprehensive assessment beyond what single-organ models can provide.159 This strategy can enrich our understanding of systemic toxicity and the effects of toxins on multiple organs. Moreover, organoid engineering can help overcome some of the limitations of current organoid models. By incorporating additional cell types, such as immune or vascular cells, researchers can increase the physiological relevance of organoids and simulate the responses of human tissue to toxicants.147 However, organoid models pose substantial challenges due to their complexity and variability. The differentiation of organoids into multiple cell types can fluctuate, leading to inconsistent study results.160,161 Additionally, the lack of vascularization in organoids can impede toxin delivery, skewing the interpretation of toxicological results.147
In conclusion, organoid models in toxicology present immense potential. Addressing the challenges of standardization, scalability, physiological relevance, and ethical considerations will be pivotal to fully exploiting organoids in toxicology research. With continued technological advancement and interdisciplinary collaborations, organoid models present an opportunity to revolutionize toxicology, leading to more accurate and efficient testing of harmful substances and thereby significantly increasing human health and safety.
The organoid epoch: A paradigm shift in drug development
Organoid technology has played a pivotal role in the advancement and evaluation of new pharmaceuticals. While organoids are not directly involved in the creation of novel drugs, they are essential for facilitating the discovery of promising drug candidates via drug screening and testing.162,163 Specifically, organoids derived from colorectal cancer patients have been instrumental in assessing responses to inhibitors of tankyrases, enzymes that are crucial in Wnt signaling and are frequently disrupted in colorectal cancer. This testing has led to the identification of new drug candidates targeting the Wnt pathway.68 Moreover, organoids from breast and lung cancer patients with certain mutations have been valuable in predicting sensitivity to PARP inhibitors, drugs that focus on DNA-repair mechanisms,68,164,165 contributing significantly to the development of individualized treatment plans involving PARP inhibitors. An additional study revealed that organoids possessing ARID1A missense mutations exhibited particular sensitivity to ATR inhibitors, which affect the DNA-damage response. This discovery holds significant promise for the development of new ATR-targeting drugs for cancers with ARID1A mutations.166 Furthermore, organoids from endometrial and metastatic tumors responded to STAT3 inhibitors, hinting at the potential for novel drugs that target STAT3 signaling in endometrial adenocarcinoma.167 Organoid lines from mesothelioma patients retained biomarkers and histological features of the patients’ tumors.168 In drug testing with cisplatin and pemetrexed, the organoids mirrored the patients’ responses, demonstrating the potential of organoids in predicting treatment outcomes for mesothelioma. Finally, organoids from appendiceal carcinoma were used in immunotherapy trials, paving the way for the creation of new immunotherapeutic agents tailored to the unique characteristics of appendiceal tumors.167 In every facet of pharmaceutical development, organoids manifest indispensable significance, providing robust support and verification from foundational research to clinical trials and all the way to the guidance of personalized medication, facilitating all-encompassing success in drug innovation.
The emergence of organoids in high-throughput drug screening has transformed drug discovery by enabling the targeted identification of effective molecules from large compound libraries.162,163 Recent advances in automation and systematic platforms have made organoids a gold standard in drug screening with the potential to streamline drug-discovery pipelines.169,170,171 Their ability to efficiently identify the most effective molecules from large compound libraries is transformative, and organoids are poised to become an integral part of drug-discovery pipelines. A large-scale organoid-based screening platform was used to test 1,172 Food and Drug Administration-approved compounds on pancreatic cancer organoids, and 22 drugs with anticancer effects were identified. This effort underscored the importance of three-dimensional culture-based platforms for drug screening, as some drugs that were effective in organoids did not show the same effects in two-dimensional cultures.166 One of the most critical aspects of drug development is the early identification of toxicological profiles. Traditional animal models often fail to capture the full spectrum of human-specific toxicities, leading to costly failures in late-stage clinical trials. Organoids, because of their human-derived cells and tissue-specific architectures, constitute a more accurate model for assessing drug toxicity.172,173 Kidney organoids,174,175 for instance, have been used to evaluate nephrotoxicity, which is a significant concern in human pharmacology. Organoids are crucial and powerful tools not only for testing compound efficacy and toxicity but also for understanding disease mechanisms and validating therapeutic targets.176 In cancer research, patient-derived tumor organoids play an important role in validating oncogenes and tumor-suppressor genes, thereby offering insights into drug mechanisms. In a study by Hirt et al.177 reported by Mainardi and Bernards,166 a newly derived pancreatic organoid biobank comprising 31 three-dimensional cell lines derived from tumors and nine from healthy tissue was used to investigate novel drug-gene interactions with potential translational value. The evaluation of drug responses in a context that mimics human physiology is crucial for the success of any therapeutic intervention. The advantages of organoids in drug responses can even be applied to clinical trials of new drugs.81,178 Clinical trials are the bottleneck of drug development, with significant time and resource investments followed by high failure rates. Organoids have the potential to increase the success rate of clinical trials by providing a more accurate representation of patients’ diseased tissue and predicting their responses to specific therapies.179 Organoids derived from patients enrolled in a trial could be used in parallel to evaluate drug responses, helping to adapt or modify the trial’s course in real time. This personalized approach to clinical trials holds great promise for improving patient outcomes and reducing the time and cost associated with drug development.180 The aim of personalized medicine is to tailor treatments to individual patients based on their genetic and physiological characteristics. In oncology, patient-derived tumor organoids can be used to screen for effective chemotherapy agents, thereby personalizing treatment plans.180,181,182,183 The ability to evaluate drug responses in patient-specific organoids holds great promise for improving treatment outcomes and reducing adverse effects. Immunotherapies have revolutionized cancer treatment, but their efficacy varies among patients. Organoids constitute a powerful platform for studying the immune microenvironment and developing novel immunotherapies.184,185 Researchers can assess the efficacy of immunotherapeutic treatments by coculturing organoids with immune cells, such as tumor-infiltrating lymphocytes.184 The study of tumor-infiltrating lymphocytes within organoids has also introduced new avenues for personalized immunotherapies tailored to the specific immunological landscape of each patient’s tumor.186,187 The ability to model complex diseases ex vivo has been significantly advanced by the advent of organoid technology and gene-editing techniques. For instance, CRISPR-Cas9 has been instrumental in creating lung cancer models by introducing specific mutations into lung organoids.188 Such models offer a multifaceted approach to understanding complex diseases and have therefore been particularly useful in studying developmental and genetic diseases (Figures 6A and 6B).
Figure 6.
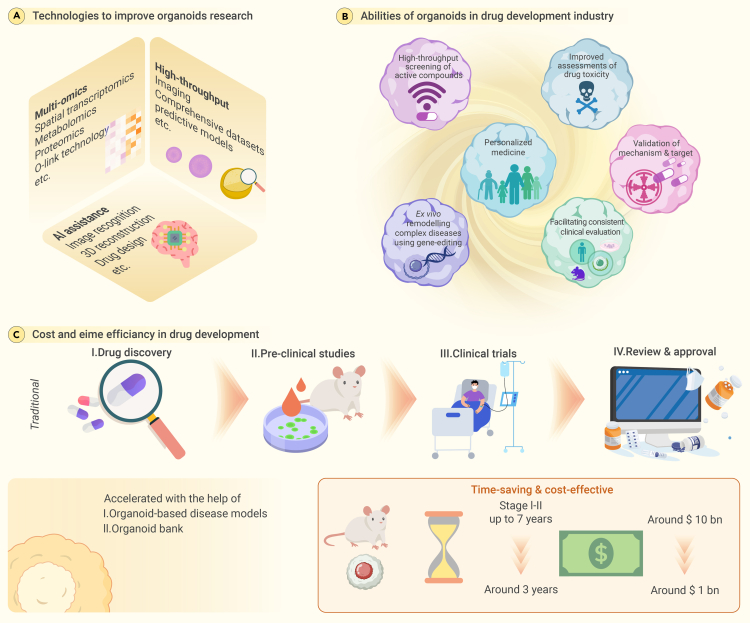
The organoid epoch: A paradigm shift in drug development
(A) Organoid technology: advancing drug development across all stages. Organoids are crucial in influencing the drug-development sector, providing extensive assistance throughout the entire process.
(B) The various applications of organoid models in biological research. Organoids play a pivotal role in the drug-development industry, offering a wide range of applications that streamline and enhance the process.
(C) Comparing the cost and time efficiency of drug development: traditional methods with organoid-based approaches. Organoids represent a fundamental change in medication development, providing notable benefits compared to conventional approaches.
Ex vivo modeling of complex diseases in organoids, aided by advanced gene-editing techniques, represents a cost-effective and ethical alternative to traditional drug-development methods. Organoids accurately replicate human physiology, thereby reducing the time and costs associated with drug discovery (Figure 6C).189 Their utility in academic research and clinical application is significant, offering the potential to revolutionize the field and pave the way for more efficient therapeutic advancements.
Organoids advance biomedical fields across the board
The field of biomedical research has achieved a significant leap forward with the advent of organoid technology. Their versatility and adaptability make them a valuable tool for a wide range of research applications, with the potential to reshape therapeutic approaches to a range of clinical challenges (Figure 7A). The promise of organoids is particularly palpable in the realm of diabetes treatment. Researchers are abuzz regarding the creation of islet-like organoids that respond to glucose, which herald a potential paradigm shift in diabetes management.190 These organoids house a diverse array of cells: insulin-producing β cells, glucagon-producing α cells, somatostatin-producing δ cells, and pancreatic polypeptide-producing γ cells.191 Their efficacy was tested by transplantation into diabetic mice,192 where these organoids mimicked native pancreatic β cells, releasing insulin in response to blood glucose fluctuations and stabilizing glucose levels. Despite the promise of this approach, challenges remain, such as scaling up organoid production and maintaining their long-term functionality.
Figure 7.
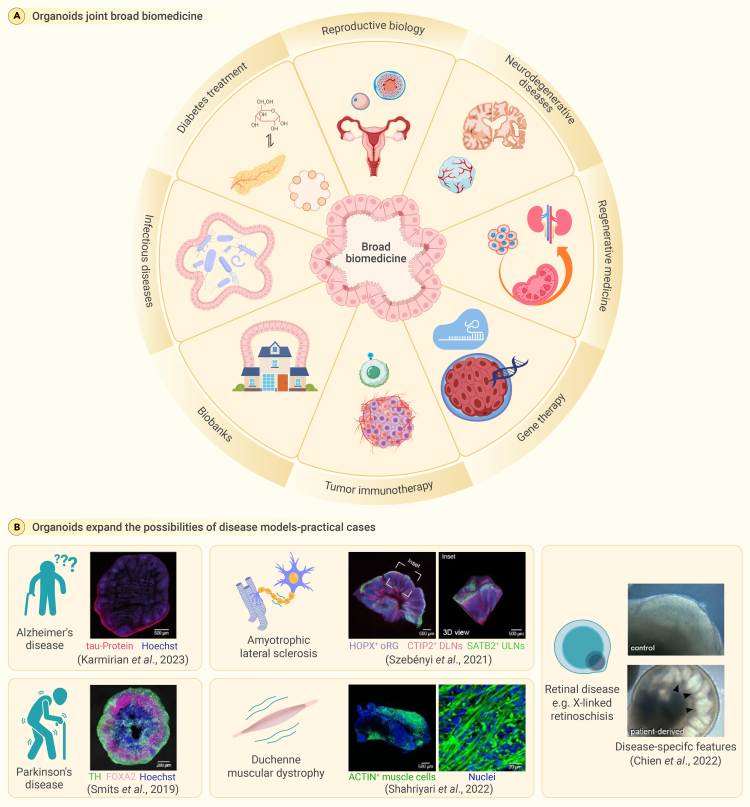
The multifaceted impact of organoid technology in biomedical science
(A) The domain of biomedical research has experienced a substantial advancement with the introduction of organoid technology. The valuable attributes of versatility and adaptability possessed by these tools render them highly useful in a diverse array of research applications. Consequently, they hold great potential for revolutionizing therapeutic strategies aimed at addressing a wide range of clinical difficulties.
(B) The application of organoids has greatly expanded the scope of disease inquiry. Technological advancements have made it possible to explore scientific realms that were previously inaccessible, especially when with regard to situations that are unique to humans.
Regenerative medicine, which is synonymous with restoration and healing, has found in organoids a robust and versatile ally.125 These structures are pioneering advances in tissue repair,50,53,193,194 transplantation, and overall regenerative strategies.53 In inflammatory bowel disease research, organoids are providing insights into tissue-regeneration mechanisms, paving the way for innovative treatments.125 Organoid transplantation is also presenting new possibilities. A prime example is the transplantation of human intestinal organoids into mice,195 which resulted not only in the successful integration of the organoids but also in physiological improvements in the recipient mice. Similarly, research into human kidney organoids transplanted into rats has shown promising results,196,197 including enhanced vascularization and function. Such studies hint at a future in which organ transplant waiting lists could be significantly reduced, with organoids serving as a viable alternative. In biobanking, organoids are revolutionizing the collection, preservation, and utilization of normal and pathological specimens, improving disease modeling and drug screening.198 Organoids also enable the preservation and study of viruses or microorganisms that are difficult to culture in traditional cell lines, providing more accurate models for research on tropism and immunity induction.199,200,201
The intersection of organoids and infectious disease research is also particularly compelling. Organoids offer a dynamic platform for studying host-pathogen interactions, paving the way for deeper insights and innovative treatments.202,203,204 For instance, organoids have been pivotal in the study of respiratory viruses.205 Their application in research related to influenza and respiratory syncytial virus is just the tip of the iceberg.204 However, research on SARS-CoV-2 is where the potential of organoids is truly showcased.206,207,208,209 Researchers have used lung and intestinal organoids to gain a nuanced understanding of how the virus invades human cells.210,211,212,213 These studies are revealing vital data about viral entry, replication, and spread. Furthermore, organoids provide a platform for testing potential drugs against the virus, accelerating the drug-discovery process.214,215 Beyond viral infection, organoids have a role to play in bacterial and parasitic infection research.202,216 Their three-dimensional structure, mirroring that of human tissue, offers a unique environment to study the dynamics of bacterial infections at a cellular level. Additionally, in parasitic research, organoids are illuminating host-helminth interactions,217 unveiling the intricate relationships between parasites and their human hosts.
Moreover, organoid models are a powerful tool for studying female fertility and reproductive biology. These organoids, derived from tissues in the female reproductive tract, enable research on normal reproductive processes and pathologies such as endometriosis, endometrial cancer, and ovarian cancer.156,218,219,220 Ovarian organoids facilitate the investigation of oocyte growth and maturation along with hormonal stimulation responses.220 Fallopian tube, endometrial, and cervical organoids are utilized to study various aspects of female reproductive health, including the development and function of the fallopian tubes, the menstrual cycle, endometriosis, endometrial hyperplasia, cervical clear cell carcinoma, and the impact of infections such as Chlamydia trachomatis and human papillomavirus. Additionally, organoids derived from pathological tissues have aided in understanding reproductive disorders and have been valuable for drug screening.156,218,220 Organoids also model early developmental events, such as implantation, and can be used to investigate interactions between different cell types such as trophoblast cells and the maternal decidua. Recent studies have also involved the development and characterization of trophoblast organoids and decidual organoids from human placental samples, which serve as models to study the maternal-fetal interface.221 These organoids have been used to investigate innate immune signaling and the differential antiviral defenses present at the maternal-fetal interface. Moreover, organoids have been employed to study the effects of environmental toxicants on female fertility and reproductive health.219 They offer a physiologically relevant and controlled system for the examination of intricate cellular processes within a contextually appropriate environment, offering the potential to revolutionize the field of reproductive medicine.
Duchenne muscular dystrophy, a debilitating genetic disorder, is also the subject of promising advances due to organoids. Using gene-editing technologies, researchers have been able to correct genetic mutations in iPSC-derived muscle cells, which constitutes a monumental advance in the development of effective treatments for Duchenne muscular dystrophy (Figure 7B).82,83 Organoid-based research has also enabled the development of innovative therapeutic strategies for retinal diseases, which have historically been challenging to treat.84,85 Leveraging organoids, scientists have been able to explore the potential of gene augmentation therapy using systems such as CRISPR-Cas9. One notable success has been the promise shown in treating Leber congenital amaurosis, a severe form of inherited blindness: targeted gene therapies have demonstrated potential in restoring vision.83
In summary, the versatile applications of organoids are undeniably setting the stage for groundbreaking advancement in biomedical research. As we continue to harness their potential, the bridge between laboratory-based discoveries and real-world clinical applications is becoming more robust. The future promises a new era of personalized medicine with organoids at its core, offering hope for myriad clinical challenges.
Tech-infused organoids: The new vanguard of biomedicine
Recent technological advances are further empowering organoid systems and enabling the derivation of new biological insights. New tools in single-cell omics, imaging, genetics, and AI are being integrated with organoid platforms. Single-cell RNA sequencing (scRNA-seq) and other omics tools enable the high-resolution dissection of organoid composition and function (Figure 8).222 Advanced imaging techniques allow the dynamic visualization of organoid morphogenesis and cell-cell interactions.223 AI and machine learning provide computational power to extract complex patterns and build predictive models from multidimensional organoid data.224 The integration of organoids with these emerging technologies has created a new generation of “organs-on-chips” that recapitulate tissue- and organ-level physiology in unprecedented detail.225,226
Figure 8.
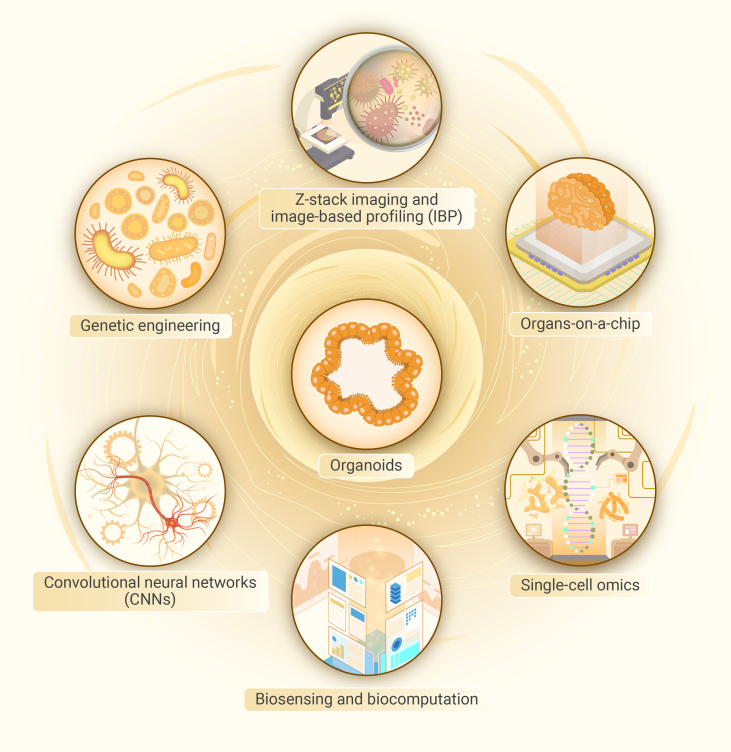
Tech-infused organoids: The cutting-edge advancement in biomedicine
Organoids are now being enhanced with cutting-edge technical advancements. The integration of several disciplines in joint efforts has led to the development of organoids that are equipped with cutting-edge technology. This advancement holds the potential to bring about a significant transformation in the field of biomedicine.
Genetic engineering in organoids is a rapidly advancing field that combines organ-like complexity with molecular precision. Methods such as viral (retroviral, lentiviral, adenoviral) and nonviral (electroporation, lipofection) delivery systems enable gene manipulation in organoids,227 and CRISPR-Cas9 is revolutionizing precise gene editing.228 RNA interference is also used to modulate gene expression.227
Organoids are vital in high-throughput screening for assessing cell viability and metabolic activity. Enzyme-linked immunosorbent assays quantify biomarkers, while image-based profiling techniques such as epifluorescence and confocal microscopy are used to analyze organoid morphology and functional changes after treatment.163,229 High-content analysis dissects developmental processes and disease progression at the cellular level, integrating omics data and multidimensional imaging for a comprehensive view of organoid responses to drug treatments. z-stack imaging provides a complete cross-sectional view of three-dimensional organoids, increasing the accuracy of drug efficacy and toxicity assessments.229 Computational methods model cellular interactions and biomechanical properties in organoids, facilitating the optimization of culture conditions and the prediction of drug-treatment outcomes.230 Human liver organoids derived from PSCs have been successfully used to model drug-induced liver injury, aiding in antifibrosis drug screening and the identification of compounds with significant antifibrotic effects, such as SD208 and imatinib.230
The integration of single-cell omics and organoid technologies has significantly advanced our understanding of organ development, disease mechanisms, and potential therapeutic approaches. This synergy has been especially effective in the field of neurobiology and brain organoid research. Fleck et al. investigated the gene-regulatory network underlying human cerebral organoid development and used multiomics single-cell data to develop an algorithm called Pando to infer the gene-regulatory network, thereby revealing the involvement of different transcription factors at various stages of cerebral organoid development. Their research emphasizes the conservation of developmental programs across species and the predictive capability of multiregion human cerebral organoids as model systems.231 Vento-Tormo emphasized the revolutionary upgrade that organoid technology,232 combined with single-cell profiling, has brought to the field of human development. This combination allows a deeper understanding of cell-cell communication and cellular heterogeneity, as evidenced by the work of Camp et al. in studying human liver organoids using scRNA-seq.232 Yin et al. further elaborated on the strengths of combining scRNA-seq and organoid technologies, highlighting applications such as the discovery of novel cell types and gene markers, the recapitulation of cellular heterogeneity, the delineation of cell-differentiation pathways, and the modeling of diseases.233 Single-cell omics technologies also enable the high-resolution dissection of cellular heterogeneity within organoid systems. scRNA-seq has been transformative in decoding organoid composition and identifying novel cell states. For example, scRNA-seq was used to map the cell lineages and transitional states in intestinal organoids, reconstructing the differentiation trajectories from stem cells to all intestinal epithelial cell types.222 In patient-derived organoids, a recent study adopted an integrative approach combining scRNA-seq, epigenomic single-nucleotide polymorphism (SNP)-to-gene mapping, and genome-wide association study summary statistics to deduce the cellular subtypes and molecular pathways by which genetic variants affect disease phenotypes.234 In summary, the integration of single-cell omics with organoid technologies provides a robust platform for modeling organ development and diseases. This combination has proven effective in revealing the cellular and molecular intricacies of organogenesis and disease pathogenesis.235 These advancements not only deepen our understanding of human biology but also pave the way for the development of novel therapeutic strategies.
The field of single-cell omics faces challenges in data interpretation due to the complexity of merging high-dimensional single-cell data with spatial information. The integration of AI and deep-learning algorithms can help streamline this process, enabling the identification of intricate patterns and interactions that are not easily discernible through traditional methods. Combining organoids with AI and deep learning enables innovative approaches to analyzing complex biological datasets and constructing predictive models. Organoids produce extensive multidimensional data, including cellular imaging, omics profiles, phenotypic readouts, and time series, and AI offers the computational power to extract meaningful patterns from this data overload.236
One major application is automated image analysis using convolutional neural networks, which can segment and classify cells in organoid imaging data orders of magnitude faster than human annotation.237 AI also enables phenotypic profiling from images. A deep-learning model was trained to predict the emergence of retinal tissue morphology and cell types in optic cup organoids over time.238 In the absence of reliable markers for assessing organoid vitality, researchers recently devised an innovative approach using AI algorithms to analyze a spectrum of phenotypic parameters. Remarkably, the AI-driven method can ascertain organoid aging solely through bright-field imaging. This approach eliminates the cumbersome steps of preparing single-cell suspensions and performing senescence-associated β-galactosidase staining, thereby streamlining quality control processes and making biobank operations more efficient.224 Beyond merely visualizing images, AI can concurrently reconstruct multiple parameters from multiomics data in organoids. These data are then integrated with complementary datasets, such as functional metrics and patient records, all derived from the same experimental conditions. The resulting repository is a large multimodal database. AI algorithms sift through this complex data to identify patterns and correlations, elucidating connections within diverse multiparametric information that may be challenging for human analysts to interpret. Overall, the integration of organoids with AI and deep learning constitutes an exciting new frontier.
The integration of organoids with microfluidics and sensors has enabled the development of “organs-on-chips” that recapitulate key aspects of human tissue physiology in vitro. These biomimetic systems incorporate organoids into microengineered devices with tissue-tissue interfaces, fluid flow, and real-time monitoring to model organ function and disease.225 One example is the gut-on-a-chip, which combines intestinal organoids with a microfluidic device lined by endothelial and immune cells to model the intestinal epithelium. This system faithfully reproduces the microenvironment of the living intestine, including peristalsis-like motions and cyclic strain from breathing. Using this model, researchers showed that cyclic strain promotes intestinal stem cell differentiation and epithelial permeability. Thus, the gut-on-a-chip provides a powerful platform to study intestinal absorption, inflammation, gut-microbe interactions, and more.239,240,241 Another study by Campisi et al. presents a three-dimensional self-organized microvascular model of the blood-brain barrier using human iPSC-derived endothelial cells, brain pericytes, and astrocytes in a fibrin gel. This microfluidic system replicates in vivo neurovascular organization, exhibiting lower permeability than conventional in vitro models, and thus provides a physiologically relevant and robust platform for drug discovery and the prediction of neurotherapeutic transport efficacy.242 Hajal et al. developed a human blood-brain barrier model within microfluidic devices by incorporating endothelial cells, brain pericytes, and astrocytes in a three-dimensional gel matrix. This model forms three-dimensional vessel architectures that resemble the natural blood-brain barrier, with gene-expression profiles and permeability values comparable to those observed in vivo.243 Accordingly, this blood-brain barrier model facilitates the quantitative assessment of molecular permeabilities and is suitable for use in both academic and industrial research to predict transport across the blood-brain barrier. These studies exemplify significant advances in organ-on-a-chip technology, specifically in modeling the blood-brain barrier.244 This progress underscores the potential of organ-on-a-chip technology to revolutionize biomedical research and therapeutic development.
Another exciting emerging application of organoids is the modeling of neural networks for biocomputation. Like their in vivo counterparts, the organized neuronal networks generated within brain organoids can perform specialized information processing.245 Researchers now plan to develop brain organoids that can serve as “biocomputers” for tasks such as pattern recognition and classification.246 In one pioneering study, a proof of concept of using brain organoids for biosensing and biocomputation was demonstrated. A recent publication in the journal Neuron revealed groundbreaking research conducted by Brett Kagan and his team at Cortical Labs, demonstrating that organoid neural networks cultured in vitro can learn to play the classic arcade game Pong.247 These so-called mini-brains, composed of interconnected neural cells, exhibit the ability to perceive and interact with their surrounding environment. Utilizing a computer interface, the neural networks were trained to detect the position of the game’s digital sphere and manipulate a virtual paddle accordingly. Remarkably, the assemblage of brain cells acquired this skill within 5 min. Biocomputing with brain organoids could potentially be faster, more efficient, and more powerful than silicon-based computing and AI, requiring only a fraction of the energy.248 This technology could enable brain-inspired AI and powerful models for studying neural information processing.
Looking forward, the integration of organoids with new technologies has immense potential to transform biomedicine and human health. Such advances could power a new generation of preclinical disease models for drug screening, enable regenerative therapies using laboratory-grown tissues, and provide platforms for precision medicine. Realizing this potential will require collaborative multidisciplinary efforts at the intersection of stem cell biology, bioengineering, and data science. An exciting future lies ahead as organoids and emerging technologies combine to elucidate human biology in unprecedented detail.
Conclusion and outlook
The advent of organoid technology marks a watershed in biomedical research, transcending previous boundaries of scientific inquiry. This innovation has revolutionized our understanding of human biology and introduced unprecedented avenues in disease management, addressing obstacles once considered insurmountable. At the cusp of this burgeoning field, the possibilities seem limitless, with organoid technology poised to reshape healthcare, research methodologies, and ethical considerations concerning human life.
Throughout this review, we have highlighted the multifaceted impact of organoids, emphasizing their utility in personalized medicine, drug discovery, and disease modeling. The bespoke nature of organoid systems, particularly patient-derived organoids, has introduced a new era of precision in biomedical research. By closely mimicking the intricate architecture and functionality of human organs, organoids offer a more representative model for human physiology and pathology, surpassing traditional in vitro and animal models. The capacity of organoids to replicate complex biological processes, such as the tumor immune microenvironment and host-pathogen interactions, positions them as a vanguard in further elucidating disease mechanisms and therapeutic responses.
Looking to the future, the integration of organoids with cutting-edge technologies such as synthetic biology, AI, and gene editing heralds a transformative phase in biomedical innovation. This synergy holds promise for unlocking new therapeutic pathways, advancing drug development, and providing profound new insights into complex diseases. The potential of organoids to serve as platforms for rapid drug testing and functional experimentation, coupled with their compatibility with gene-editing techniques, signifies a quantum leap in medical research.
In summary, organoid technology stands at the forefront of a revolution in the biomedical field. Its impact on human society will be profound, and its future applications appear boundless. As researchers and clinicians continue to harness the power of this technology, we anticipate a future replete with groundbreaking discoveries and innovative treatments that will ultimately reshape our approach to understanding and curing human diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82373719, 82173662, and 32200581), the National Key R&D Program of China (2023YFC3605702 and 2023YFC2308002), and the Extraordinary 2025 Elite Project of Fudan University.
Author contributions
X. Han, C.D., X. He, and X.C. provided direction and guidance throughout the preparation of this manuscript. X. Han, C.C., and W.D. collected and interpreted studies and were major contributors to writing and editing of the manuscript. B.F., Y.S., L.L., C.W., J.Z., M.R., J.L., H.H., and X.L. participated in discussions, language editing, and manuscript revision. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published Online: April 1, 2024
Contributor Information
Xinxin Han, Email: xxhan@sibs.ac.cn.
Chuxia Deng, Email: cxdeng@um.edu.mo.
Xiao He, Email: hexiao@ihep.ac.cn.
Xin Cao, Email: caox@fudan.edu.cn.
Lead contact website
References
- 1.Yang S., Hu H., Kung H., et al. Organoids: The current status and biomedical applications. MedComm. 2023;4(3) doi: 10.1002/mco2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbuzano J. Organoids: A new window into disease, development and discovery. Harvard Stem Cell Institute News. 2017 https://hsci.harvard.edu/organoids [Google Scholar]
- 3.Melzer M.K., Resheq Y., Navaee F., et al. The application of pancreatic cancer organoids for novel drug discovery. Expert Opin. Drug Discov. 2023;18(4):429–444. doi: 10.1080/17460441.2023.2194627. [DOI] [PubMed] [Google Scholar]
- 4.Taverna J.A., Hung C.N., Williams M., et al. Ex vivo drug testing of patient-derived lung organoids to predict treatment responses for personalized medicine. Lung Cancer. 2024;190:107533. doi: 10.1016/j.lungcan.2024.107533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai S., McOlash L., Palen K., et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer. 2018;18(1):335. doi: 10.1186/s12885-018-4238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S.S., Sreedharan S.N., Patil S., et al. The potential role of organoids in pathology and oncology research. Pathol. Oncol. Res. 2020;26(2):1353–1354. doi: 10.1007/s12253-019-00642-z. [DOI] [PubMed] [Google Scholar]
- 7.Driehuis E., Clevers H. CRISPR/Cas 9 genome editing and its applications in organoids. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312(3):G257–G265. doi: 10.1152/ajpgi.00410.2016. [DOI] [PubMed] [Google Scholar]
- 8.Duan J., Penzes P., Gejman P. From genetic association to disease biology: 2d and 3d human iPSC models of neuropsychiatric disorders and Crispr/Cas9 genome editing. Eur. Neuropsychopharmacol. 2019;29:S763–S764. doi: 10.1016/j.euroneuro.2017.06.120. [DOI] [Google Scholar]
- 9.Eerkes-Medrano D., Feehan C.J., Leys S.P. Sponge cell aggregation: checkpoints in development indicate a high level of organismal complexity. Invertebr. Biol. 2015;134(1):1–18. doi: 10.1111/ivb.12072. [DOI] [Google Scholar]
- 10.Wilson H.V. Development of sponges from dissociated tissue cells. Bulletin of the Bureau of Fisheries. 1910;30:1–35. [Google Scholar]
- 11.Wilson H.V. A new method by which sponges may be artificially reared. Science. 1907;25(649):912–915. doi: 10.1126/science.25.649.912. [DOI] [PubMed] [Google Scholar]
- 12.Corrò C., Novellasdemunt L., Li V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020;319(1):C151–C165. doi: 10.1152/ajpcell.00120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okabayashi K., Asashima M. In vitro organogenesis using amphibian pluripotent cells. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006;82(7):197–207. doi: 10.2183/pjab.82.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwilling E. Development of fragmented and of dissociated limb bud mesoderm. Dev. Biol. 1964;9(1):20–37. doi: 10.1016/0012-1606(64)90012-0. [DOI] [PubMed] [Google Scholar]
- 15.Axelrod H.R. Embryonic stem cell lines derived from blastocysts by a simplified technique. Dev. Biol. 1984;101(1):225–228. doi: 10.1016/0012-1606(84)90133-7. [DOI] [PubMed] [Google Scholar]
- 16.Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 17.Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 20.Chang E.A., Jin S.W., Nam M.H., et al. Human induced pluripotent stem cells : clinical significance and applications in neurologic diseases. J. Korean Neurosurg. Soc. 2019;62(5):493–501. doi: 10.3340/jkns.2018.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chhabra A. Derivation of human induced pluripotent stem cell (iPSC) lines and mechanism of pluripotency: historical perspective and recent advances. Stem Cell Rev. Rep. 2017;13(6):757–773. doi: 10.1007/s12015-017-9766-9. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K., Tanabe K., Ohnuki M., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig T.E., Kujak A., Rauti A., et al. 20 Years of human pluripotent stem cell research: it all started with five lines. Cell Stem Cell. 2018;23(5):644–648. doi: 10.1016/j.stem.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Ochi M. Shinya Yamanaka’s 2012 Nobel Prize and the radical change in orthopedic strategy thanks to his discovery of iPS cells. Acta Orthop. 2013;84(1):1–3. doi: 10.3109/17453674.2013.765642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omole A.E., Fakoya A.O.J. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ. 2018;6 doi: 10.7717/peerj.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato T., Vries R.G., Snippert H.J., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194) doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 28.Purnell B.A. The making of bodies part by part. Science. 2014;345(6194):280. doi: 10.1126/science.345.6194.280-j. [DOI] [Google Scholar]
- 29.Bender E. Q&A: Hans Clevers. Nature. 2015;521(7551):S15. doi: 10.1038/521S15a. [DOI] [PubMed] [Google Scholar]
- 30.A gutsy approach to stem cells and signalling: an interview with Hans Clevers. Dis Model Mech. 2013;6(5):1053–1056. doi: 10.1242/dmm.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha G. The organoid architect. Science. 2017;357(6353):746–749. doi: 10.1126/science.357.6353.746. [DOI] [PubMed] [Google Scholar]
- 32.Pleguezuelos-Manzano C., Puschhof J., Van Den Brink S., et al. Establishment and culture of human intestinal organoids derived from adult stem cells. Curr. Protoc. Immunol. 2020;130(1) doi: 10.1002/cpim.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancaster M.A., Renner M., Martin C.A., et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prostate cancer organoids make debut. Cancer Discov. 2014;4(11):1248. doi: 10.1158/2159-8290.CD-NB2014-143. [DOI] [PubMed] [Google Scholar]
- 35.Karthaus W.R., Iaquinta P.J., Drost J., et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159(1):163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takasato M., Er P.X., Chiu H.S., et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526(7574):564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 37.Morizane R., Lam A.Q., Freedman B.S., et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015;33(11):1193–1200. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boj S.F., Hwang C.I., Baker L.A., et al. Model organoids provide new research opportunities for ductal pancreatic cancer. Mol. Cell. Oncol. 2016;3(1) doi: 10.1080/23723556.2015.1014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang L., Holtzinger A., Jagan I., et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell– and patient-derived tumor organoids. Nat. Med. 2015;21(11):1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fligor C.M., Langer K.B., Sridhar A., et al. Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xian B., Luo Z., Li K., et al. Dexamethasone provides effective immunosuppression for improved survival of retinal organoids after epiretinal transplantation. Stem Cells Int. 2019;2019 doi: 10.1155/2019/7148032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis-Israeli Y.R., Wasserman A.H., Gabalski M.A., et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021;12(1):5142. doi: 10.1038/s41467-021-25329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards D.J., Li Y., Kerr C.M., et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020;4(4):446–462. doi: 10.1038/s41551-020-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L., Yu L., Li Z., et al. Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J. Transl. Med. 2021;19(1):40. doi: 10.1186/s12967-020-02677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominijanni A., Mazzocchi A., Shelkey E., et al. Bioengineered tumor organoids. Curr. Opin. Biomed. Eng. 2020;13:168–173. doi: 10.1016/j.cobme.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosseini Z.F., Nelson D.A., Moskwa N., et al. Generating embryonic salivary gland organoids. Curr. Protoc. Cell Biol. 2019;83(1):e76. doi: 10.1002/cpcb.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X.Y., Ju X.C., Li Y., et al. Generation of vascularized brain organoids to study neurovascular interactions. Elife. 2022;11 doi: 10.1101/2022.01.04.474960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagerlund I., Dougalis A., Shakirzyanova A., et al. Microglia-like cells promote neuronal functions in cerebral organoids. Cells. 2021;11(1):124. doi: 10.3390/cells11010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen K.B., Little M.H. Organoids are not organs: sources of variation and misinformation in organoid biology. Stem Cell Rep. 2023;18(6):1255–1270. doi: 10.1016/j.stemcr.2023.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turhan A.G., Hwang J.W., Chaker D., et al. iPSC-derived organoids as therapeutic models in regenerative medicine and oncology. Front. Med. 2021;8 doi: 10.3389/fmed.2021.728543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCauley H.A., Wells J.M. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development. 2017;144(6):958–962. doi: 10.1242/dev.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe R.G., Daley G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019;20(7):377–388. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim W., Gwon Y., Park S., et al. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact. Mater. 2023;19:50–74. doi: 10.1016/j.bioactmat.2022.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee C.T., Bendriem R.M., Wu W.W., et al. 3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017;24(1):59. doi: 10.1186/s12929-017-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z.A., Shang J., Xiang S., et al. Articular tissue-mimicking organoids derived from mesenchymal stem cells and induced pluripotent stem cells. Organoids. 2022;1(2):135–148. doi: 10.3390/organoids1020011. [DOI] [Google Scholar]
- 56.Azar J., Bahmad H.F., Daher D., et al. The use of stem cell-derived organoids in disease modeling: an update. Int. J. Mol. Sci. 2021;22(14):7667. doi: 10.3390/ijms22147667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J., Koo B.K., Knoblich J.A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020;21(10):571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nio Y., Takebe T. In: Medical Applications of iPS Cells: Innovation in Medical Sciences. Current Human Cell Research and Applications. Inoue H., Nakamura Y., editors. Springer; 2019. Organoid models of development and disease towards therapy; pp. 149–168. [DOI] [Google Scholar]
- 59.Li L., Bowling S., McGeary S.E., et al. A mouse model with high clonal barcode diversity for joint lineage, transcriptomic, and epigenomic profiling in single cells. Cell. 2023;186(23):5183–5199.e22. doi: 10.1016/j.cell.2023.09.019. [DOI] [PubMed] [Google Scholar]
- 60.Marshall L.J., Bailey J., Cassotta M., et al. Poor translatability of biomedical research using animals — A narrative review. Altern. Lab. Anim. 2023;51(2):102–135. doi: 10.1177/02611929231157756. [DOI] [PubMed] [Google Scholar]
- 61.Domínguez-Oliva A., Hernández-Ávalos I., Martínez-Burnes J., et al. The importance of animal models in biomedical research: current insights and applications. Animals. 2023;13(7) doi: 10.3390/ani13071223. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10093480/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barroca N.C.B., Della Santa G., Suchecki D., et al. Challenges in the use of animal models and perspectives for a translational view of stress and psychopathologies. Neurosci. Biobehav. Rev. 2022;140 doi: 10.1016/j.neubiorev.2022.104771. [DOI] [PubMed] [Google Scholar]
- 63.Nusinow D.P., Szpyt J., Ghandi M., et al. Quantitative proteomics of the cancer cell line encyclopedia. Cell. 2020;180(2):387–402.e16. doi: 10.1016/j.cell.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorsch J.R., Collins F.S., Lippincott-Schwartz J. Fixing problems with cell lines: technologies and policies can improve authentication. Science. 2014;346(6216):1452–1453. doi: 10.1126/science.1259110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaur G., Dufour J.M. Cell lines: valuable tools or useless artifacts. Spermatogenesis. 2012;2(1):1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J., Settivari R.S., LeBaron M.J. Genetic instability of in vitro cell lines: implications for genetic toxicity testing. Environ. Mol. Mutagen. 2019;60(6):559–562. doi: 10.1002/em.22280. [DOI] [PubMed] [Google Scholar]
- 67.Mead B.E., Karp J.M. All models are wrong, but some organoids may be useful. Genome Biol. 2019;20(1):66. doi: 10.1186/S13059-019-1677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rae C., Amato F., Braconi C. Patient-derived organoids as a model for cancer drug discovery. Int. J. Mol. Sci. 2021;22(7):3483. doi: 10.3390/ijms22073483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu Z.Y., Jia S.Z., Liu S., et al. Endometrial organoids: A new model for the research of endometrial-related diseases. Biol. Reprod. 2020;103(5):918–926. doi: 10.1093/biolre/ioaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brucker S.Y., Hentrich T., Schulze-Hentrich J.M., et al. Endometrial organoids derived from Mayer-Rokitansky-Küster-Hauser syndrome patients provide insights into disease-causing pathways. Dis. Model. Mech. 2022;15(5) doi: 10.1242/dmm.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen P., Zhang X., Ding R., et al. Patient-derived organoids can guide personalized-therapies for patients with advanced breast cancer. Adv. Sci. 2021;8(22) doi: 10.1002/advs.202101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boretto M., Maenhoudt N., Luo X., et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019;21(8):1041–1051. doi: 10.1038/s41556-019-0360-z. [DOI] [PubMed] [Google Scholar]
- 73.Ludwig L.S., Lareau C.A., Ulirsch J.C., et al. Lineage tracing in humans enabled by mitochondrial mutations and single-cell genomics. Cell. 2019;176(6):1325–1339.e22. doi: 10.1016/j.cell.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richter M., Piwocka O., Musielak M., et al. From donor to the lab: a fascinating journey of primary cell lines. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.711381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X., Shen C., Wei Z., et al. Patient-derived non-small cell lung cancer xenograft mirrors complex tumor heterogeneity. Cancer Biol. Med. 2021;18(1):184–198. doi: 10.20892/j.issn.2095-3941.2020.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ortmann J., Rampášek L., Tai E., et al. Assessing therapy response in patient-derived xenografts. Sci. Transl. Med. 2021;13(620):eabf4969. doi: 10.1126/scitranslmed.abf4969. [DOI] [PubMed] [Google Scholar]
- 77.Telias M., Sit K.K., Frozenfar D., et al. Retinoic acid inhibitors mitigate vision loss in a mouse model of retinal degeneration. Sci. Adv. 2022;8(11) doi: 10.1126/sciadv.abm4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oh B.Y., Hong H.K., Lee W.Y., et al. Animal models of colorectal cancer with liver metastasis. Cancer Lett. 2017;387:114–120. doi: 10.1016/j.canlet.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 79.Tagaya H., Ishikawa K., Hosokawa Y., et al. A method of producing genetically manipulated mouse mammary gland. Breast Cancer Res. 2019;21(1):1. doi: 10.1186/s13058-018-1086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arends M.J., White E.S., Whitelaw C.B.A. Animal and cellular models of human disease. J. Pathol. 2016;238(2):137–140. doi: 10.1002/path.4662. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi T. Organoids for drug discovery and personalized medicine. Annu. Rev. Pharmacol. Toxicol. 2019;59(1):447–462. doi: 10.1146/annurev-pharmtox-010818-021108. [DOI] [PubMed] [Google Scholar]
- 82.Costamagna G., Comi G.P., Corti S. Advancing drug discovery for neurological disorders using iPSC-derived neural organoids. Int. J. Mol. Sci. 2021;22(5):2659. doi: 10.3390/ijms22052659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J., Fu Y., Yamazaki Y., et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat. Commun. 2020;11(1):5540. doi: 10.1038/s41467-020-19264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olgasi C., Cucci A., Follenzi A. iPSC-derived liver organoids: A Journey from drug screening, to disease modeling, arriving to regenerative medicine. Int. J. Mol. Sci. 2020;21(17):6215. doi: 10.3390/ijms21176215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blaszkiewicz J., Duncan S.A. Advancements in disease modeling and drug discovery using iPSC-derived hepatocyte-like cells. Genes. 2022;13(4):573. doi: 10.3390/genes13040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bubnys A., Tsai L.H. Harnessing cerebral organoids for Alzheimer’s disease research. Curr. Opin. Neurobiol. 2022;72:120–130. doi: 10.1016/j.conb.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Karmirian K., Holubiec M., Goto-Silva L., et al. In: Chun J., editor. Vol. 2561. Springer US; 2023. Modeling Alzheimer’s disease using human brain organoids; pp. 135–158. (Alzheimer’s Disease). [DOI] [PubMed] [Google Scholar]
- 88.Galet B., Cheval H., Ravassard P. Patient-derived midbrain organoids to explore the molecular basis of Parkinson’s disease. Front. Neurol. 2020;11:1005. doi: 10.3389/fneur.2020.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McComish S.F., MacMahon Copas A.N., Caldwell M.A. Human brain-based models provide a powerful tool for the advancement of Parkinson’s disease research and therapeutic development. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.851058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smits L.M., Reinhardt L., Reinhardt P., et al. Modeling Parkinson’s disease in midbrain-like organoids. Npj Park Dis. 2019;5(1):5. doi: 10.1038/s41531-019-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smits L.M., Schwamborn J.C. Midbrain organoids: a new tool to investigate Parkinson’s disease. Front. Cell Dev. Biol. 2020;8:359. doi: 10.3389/fcell.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tamaki Y., Ross J.P., Alipour P., et al. Spinal cord extracts of amyotrophic lateral sclerosis spread TDP-43 pathology in cerebral organoids. Myers A.J., editor. PLoS Genet. 2023;19(2) doi: 10.1371/journal.pgen.1010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szebényi K., Wenger L.M.D., Sun Y., et al. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat. Neurosci. 2021;24(11):1542–1554. doi: 10.1038/s41593-021-00923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du H., Huo Z., Chen Y., et al. Induced pluripotent stem cells and their applications in amyotrophic lateral sclerosis. Cells. 2023;12(6):971. doi: 10.3390/cells12060971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghasemi M. Gene therapy in amyotrophic lateral sclerosis. MDPI. 2022;11(13):2066. doi: 10.3390/cells11132066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miranda C.C., Cabral J.M.S. Organoids for Cell Therapy and Drug Discovery. Instituto Superior Técnico. 2020;1:461–471. https://linkinghub.elsevier.com/retrieve/pii/B9780128191781000459 [Google Scholar]
- 97.Choo N., Ramm S., Luu J., et al. High-throughput imaging assay for drug screening of 3D prostate cancer organoids. SLAS Discov. 2021;26(9):1107–1124. doi: 10.1177/24725552211020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rauth S., Karmakar S., Batra S.K., et al. Recent advances in organoid development and applications in disease modeling. Biochim. Biophys. Acta. Rev. Cancer. 2021;1875(2) doi: 10.1016/j.bbcan.2021.188527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ortolano N. Organoids bring drug discovery and development to the culture hood. 10x Genomics. 2022 https://www.10xgenomics.com/blog/organoids-bring-drug-discovery-and-development-to-the-culture-hood [Google Scholar]
- 100.Urs S. Organoid technology: a reliable tool for drug screening. Crown Biosci Blog. 2023 https://blog.crownbio.com/organoid-technology-a-reliable-tool-for-drug-screening [Google Scholar]
- 101.Jacob F., Salinas R.D., Zhang D.Y., et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180(1):188–204.e22. doi: 10.1016/j.cell.2019.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sereti E., Papapostolou I., Dimas K. Pancreatic cancer organoids: an emerging platform for precision medicine? Biomedicines. 2023;11(3):890. doi: 10.3390/biomedicines11030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pernik M.N., Bird C.E., Traylor J.I., et al. Patient-derived cancer organoids for precision oncology treatment. J. Pers. Med. 2021;11(5):423. doi: 10.3390/jpm11050423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watanabe S., Yogo A., Otsubo T., et al. Establishment of patient-derived organoids and a characterization-based drug discovery platform for treatment of pancreatic cancer. BMC Cancer. 2022;22(1):489. doi: 10.1186/s12885-022-09619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi J.I., Jang S.I., Hong J., et al. Cancer-initiating cells in human pancreatic cancer organoids are maintained by interactions with endothelial cells. Cancer Lett. 2021;498:42–53. doi: 10.1016/j.canlet.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 106.Xu H., Jiao D., Liu A., et al. Tumor organoids: applications in cancer modeling and potentials in precision medicine. J. Hematol. Oncol. 2022;15(1):58. doi: 10.1186/s13045-022-01278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagle P.W., Plukker J.T.M., Muijs C.T., et al. Patient-derived tumor organoids for prediction of cancer treatment response. Semin. Cancer Biol. 2018;53:258–264. doi: 10.1016/j.semcancer.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Bazeed A.Y., Day C.M., Garg S. Pancreatic cancer: challenges and opportunities in locoregional therapies. Cancers. 2022;14(17):4257. doi: 10.3390/cancers14174257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeng S., Pöttler M., Lan B., et al. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019;20(18):4504. doi: 10.3390/ijms20184504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liot S., Balas J., Aubert A., et al. Stroma involvement in pancreatic ductal adenocarcinoma: an overview focusing on extracellular matrix proteins. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.612271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piro G., Agostini A., Larghi A., et al. Pancreatic cancer patient-derived organoid platforms: a clinical tool to study cell- and non-cell-autonomous mechanisms of treatment response. Front. Med. 2021;8 doi: 10.3389/fmed.2021.793144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harada K., Sakamoto N. Cancer organoid applications to investigate chemotherapy resistance. Front. Mol. Biosci. 2022;9 doi: 10.3389/fmolb.2022.1067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Su C., Olsen K.A., Bond C.E., et al. The efficacy of using patient-derived organoids to predict treatment response in colorectal cancer. Cancers. 2023;15(3):805. doi: 10.3390/cancers15030805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luckett K.A., Ganesh K. Engineering the immune microenvironment into organoid models. Annu. Rev. Cancer Biol. 2023;7(1):171–187. doi: 10.1146/annurev-cancerbio-061421-040659. [DOI] [Google Scholar]
- 115.Sun C.P., Lan H.R., Fang X.L., et al. Organoid models for precision cancer immunotherapy. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.770465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J., Chen C., Wang L., et al. Patient-derived tumor organoids: new progress and opportunities to facilitate precision cancer immunotherapy. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.872531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan H., Demirci U., Chen P. Emerging organoid models: leaping forward in cancer research. J. Hematol. Oncol. 2019;12(1):142. doi: 10.1186/s13045-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Magré L., Verstegen M.M.A., Buschow S., et al. Emerging organoid-immune co-culture models for cancer research: from oncoimmunology to personalized immunotherapies. J. Immunother. Cancer. 2023;11(5) doi: 10.1136/jitc-2022-006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saiding Q., Ma J., Ke C., et al. From “organs on a chip” to “patient on a chip.”. Innovation. 2022;3(5) doi: 10.1016/j.xinn.2022.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Subtil B., Iyer K.K., Poel D., et al. Dendritic cell phenotype and function in a 3D co-culture model of patient-derived metastatic colorectal cancer organoids. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1105244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu Y., Kang E., Wilson M., et al. 3D tumor spheroid and organoid to model tumor microenvironment for cancer immunotherapy. Organoids. 2022;1(2):149–167. doi: 10.3390/organoids1020012. [DOI] [Google Scholar]
- 122.Miebach L., Berner J., Bekeschus S. In vivo model in cancer research and tumor immunology. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1006064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao X., Jiang Y., Liu C., et al. Organoid technology and clinical applications in digestive system cancer. Engineering. 2022;9:123–130. doi: 10.1016/j.eng.2021.04.017. [DOI] [Google Scholar]
- 124.Takebe T., Wells J.M., Helmrath M.A., et al. Organoid center strategies for accelerating clinical translation. Cell Stem Cell. 2018;22(6):806–809. doi: 10.1016/j.stem.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi W.H., Bae D.H., Yoo J. Current status and prospects of organoid-based regenerative medicine. BMB Rep. 2023;56(1):10–14. doi: 10.5483/BMBRep.2022-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Glykofrydis F., Elfick A. Exploring standards for multicellular mammalian synthetic biology. Trends Biotechnol. 2022;40(11):1299–1312. doi: 10.1016/j.tibtech.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 127.Sorrentino G., Rezakhani S., Yildiz E., et al. Mechano-modulatory synthetic niches for liver organoid derivation. Nat. Commun. 2020;11(1):3416. doi: 10.1038/s41467-020-17161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ovechkina V.S., Zakian S.M., Medvedev S.P., et al. Genetically encoded fluorescent biosensors for biomedical applications. Biomedicines. 2021;9(11):1528. doi: 10.3390/biomedicines9111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yousafzai M.S., Hammer J.A. Using biosensors to study organoids, spheroids and organs-on-a-chip: a mechanobiology perspective. Biosensors. 2023;13(10):905. doi: 10.3390/bios13100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin W., Mehta S., Zhang J. Genetically encoded fluorescent biosensors illuminate kinase signaling in cancer. J. Biol. Chem. 2019;294(40):14814–14822. doi: 10.1074/jbc.REV119.006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim G.A., Ginga N.J., Takayama S. Integration of sensors in gastrointestinal organoid culture for biological analysis. Cell. Mol. Gastroenterol. Hepatol. 2018;6(1):123–131.e1. doi: 10.1016/j.jcmgh.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Poudel H., Sanford K., Szwedo P.K., et al. Synthetic matrices for intestinal organoid culture: implications for better performance. ACS Omega. 2022;7(1):38–47. doi: 10.1021/acsomega.1c05136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y., Li D., Liu Y., et al. 3D-bioprinted anisotropic bicellular living hydrogels boost osteochondral regeneration via reconstruction of cartilage–bone interface. Innovation. 2024;5(1) doi: 10.1016/j.xinn.2023.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cruz-Acuña R., Kariuki S.W., Sugiura K., et al. Engineered hydrogel reveals contribution of matrix mechanics to esophageal adenocarcinoma and identifies matrix-activated therapeutic targets. J. Clin. Invest. 2023;133(23) doi: 10.1172/JCI168146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nason-Tomaszewski C.E., Thomas E.E., Matera D.L., et al. Extracellular matrix-templating fibrous hydrogels promote ovarian tissue remodeling and oocyte growth. Bioact. Mater. 2024;32:292–303. doi: 10.1016/j.bioactmat.2023.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nguyen A.K., Molley T.G., Kardia E., et al. Hierarchical assembly of tryptophan zipper peptides into stress-relaxing bioactive hydrogels. Nat. Commun. 2023;14(1):6604. doi: 10.1038/s41467-023-41907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang R., Shang L., Zhao Y. Biomimic organ architectures and functions by assembling organoid models. Sci. Bull. 2021;66(9):862–864. doi: 10.1016/j.scib.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 138.Kim E., Choi S., Kang B., et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature. 2020;588(7839):664–669. doi: 10.1038/s41586-020-3034-x. [DOI] [PubMed] [Google Scholar]
- 139.Mukherjee A., Sinha A., Maibam M., et al. In: Organoid Bioengineering - Advances, Applications and Challenges. 13. Paul M.K., editor. 2022. Organoids and commercialization. [DOI] [Google Scholar]
- 140.Hernandez-Gordillo V., Kassis T., Lampejo A., et al. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials. 2020;254 doi: 10.1016/j.biomaterials.2020.120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wagar L.E., Salahudeen A., Constantz C.M., et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 2021;27(1):125–135. doi: 10.1038/s41591-020-01145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sang Y., Miller L.C., Nelli R.K., et al. Harness organoid models for virological studies in animals: a cross-species perspective. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.725074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Puschhof J., Post Y., Beumer J., et al. Derivation of snake venom gland organoids for in vitro venom production. Nat. Protoc. 2021;16(3):1494–1510. doi: 10.1038/s41596-020-00463-4. [DOI] [PubMed] [Google Scholar]
- 144.Kim K.H., Kim E.J., Kim H.Y., et al. Fabrication of functional ameloblasts from hiPSCs for dental application. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1164811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alghadeer A., Hanson-Drury S., Patni A.P., et al. Single-cell census of human tooth development enables generation of human enamel. Dev. Cell. 2023;58(20):2163–2180.e9. doi: 10.1016/j.devcel.2023.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang J., Huang S., Cheng S., et al. Application of ovarian cancer organoids in precision medicine: key challenges and current opportunities. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.701429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li M., Gong J., Gao L., et al. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol. Environ. Saf. 2022;235 doi: 10.1016/j.ecoenv.2022.113429. [DOI] [PubMed] [Google Scholar]
- 148.Caipa Garcia A.L., Arlt V.M., Phillips D.H. Organoids for toxicology and genetic toxicology: applications with drugs and prospects for environmental carcinogenesis. Mutagenesis. 2022;37(2):143–154. doi: 10.1093/mutage/geab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Matsui T., Shinozawa T. Human organoids for predictive toxicology research and drug development. Front. Genet. 2021;12 doi: 10.3389/FGENE.2021.767621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haaker M.W., Kruitwagen H.S., Vaandrager A.B., et al. Identification of potential drugs for treatment of hepatic lipidosis in cats using an in vitro feline liver organoid system. J. Vet. Intern. Med. 2020;34(1):132–138. doi: 10.1111/jvim.15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shinozawa T., Kimura M., Cai Y., et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell–derived organoids. Gastroenterology. 2021;160(3):831–846.e10. doi: 10.1053/j.gastro.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Truskey G.A. Human microphysiological systems and organoids as in vitro models for toxicological studies. Front. Public Health. 2018;6:185. doi: 10.3389/FPUBH.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seo Y., Bang S., Son J., et al. Brain physiome: a concept bridging in vitro 3D brain models and in silico models for predicting drug toxicity in the brain. Bioact. Mater. 2022;13:135–148. doi: 10.1016/j.bioactmat.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shin W., Kim H.J. 3D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell culture insert. Nat. Protoc. 2022;17(3):910–939. doi: 10.1038/s41596-021-00674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Winkler A., Santo N., Madaschi L., et al. Lung organoids and microplastic fibers: a new exposure model for emerging contaminants. bioRxiv. 2021 doi: 10.1016/j.envint.2022.107200. https://www.biorxiv.org/content/10.1101/2021.03.07.434247v2 Preprint at. [DOI] [PubMed] [Google Scholar]
- 156.Heidari-Khoei H., Esfandiari F., Hajari M.A., et al. Organoid technology in female reproductive biomedicine. Reprod. Biol. Endocrinol. 2020;18(1):64. doi: 10.1186/s12958-020-00621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lichou F., Trynka G. Functional studies of GWAS variants are gaining momentum. Nat. Commun. 2020;11(1):6283. doi: 10.1038/s41467-020-20188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Menon R., Richardson L. Organ-on-a-chip for perinatal biology experiments. Placenta Reprod. Med. 2022;1:98. [PMC free article] [PubMed] [Google Scholar]
- 159.Ingber D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022;23(8):467–491. doi: 10.1038/s41576-022-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Espinosa Angarica V., Del Sol A. Modeling heterogeneity in the pluripotent state: a promising strategy for improving the efficiency and fidelity of stem cell differentiation. Bioessays. 2016;38(8):758–768. doi: 10.1002/bies.201600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hemeryck L., Hermans F., Chappell J., et al. Organoids from human tooth showing epithelial stemness phenotype and differentiation potential. Cell. Mol. Life Sci. 2022;79(3):153. doi: 10.1007/s00018-022-04183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang S., Shen J., Wang X., et al. Integration of organoids in peptide drug discovery: Rise of the high-throughput screening. VIEW. 2023;4 doi: 10.1002/viw.20230010. [DOI] [Google Scholar]
- 163.Lampart F.L., Iber D., Doumpas N. Organoids in high-throughput and high-content screenings. Front. Chem. Eng. 2023;5 doi: 10.3389/fceng.2023.1120348. [DOI] [Google Scholar]
- 164.Campaner E., Zannini A., Santorsola M., et al. Breast cancer organoids model patient-specific response to drug treatment. Cancers. 2020;12(12):3869. doi: 10.3390/CANCERS12123869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim M., Mun H., Sung C.O., et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019;10(1):3991. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mainardi S., Bernards R. A large-scale organoid-based screening platform to advance drug repurposing in pancreatic cancer. Cell Genom. 2022;2(2) doi: 10.1016/j.xgen.2022.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang R., Yu Y. Patient-derived organoids in translational oncology and drug screening. Cancer Lett. 2023;562 doi: 10.1016/j.canlet.2023.216180. [DOI] [PubMed] [Google Scholar]
- 168.Ito F., Kato K., Yanatori I., et al. Matrigel-based organoid culture of malignant mesothelioma reproduces cisplatin sensitivity through CTR1. BMC Cancer. 2023;23(1):487. doi: 10.1186/s12885-023-10966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dhakal B., Li C.M.Y., Li R., et al. The antianginal drug perhexiline displays cytotoxicity against colorectal cancer cells in vitro: a potential for drug repurposing. Cancers. 2022;14(4):1043. doi: 10.3390/cancers14041043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Boehnke K., Iversen P.W., Schumacher D., et al. Assay establishment and validation of a high-throughput screening platform for three-dimensional patient-derived colon cancer organoid cultures. SLAS Discov. 2016;21(9):931–941. doi: 10.1177/1087057116650965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Renner H., Grabos M., Becker K.J., et al. A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids. Elife. 2020;9 doi: 10.7554/eLife.52904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Karabicici M., Akbari S., Ertem O., et al. Human liver organoid models for assessment of drug toxicity at the preclinical stage. Endocr., Metab. Immune Disord.: Drug Targets. 2023;23(14):1713–1724. doi: 10.2174/1871530323666230411100121. [DOI] [PubMed] [Google Scholar]
- 173.Dilz J., Auge I., Groeneveld K., et al. A proof-of-concept assay for quantitative and optical assessment of drug-induced toxicity in renal organoids. Sci. Rep. 2023;13(1):6167. doi: 10.1038/s41598-023-33110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karp S., Pollak M., Subramanian B. Disease modeling with kidney organoids. Micromachines. 2022;13(9):1384. doi: 10.3390/mi13091384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Astashkina A.I., Mann B.K., Prestwich G.D., et al. Comparing predictive drug nephrotoxicity biomarkers in kidney 3-D primary organoid culture and immortalized cell lines. Biomaterials. 2012;33(18):4712–4721. doi: 10.1016/j.biomaterials.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 176.Shamshirgaran Y., Jonebring A., Svensson A., et al. Rapid target validation in a Cas9-inducible hiPSC derived kidney model. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-95986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hirt C.K., Booij T.H., Grob L., Simmler P., Toussaint N.C., Keller D., Taube D., Ludwig V., Goryachkin A., Pauli C. Drug screening and genome editing in human pancreatic cancer organoids identifies drug-gene interactions and candidates for off-label treatment. Cell Genom. 2022;2:100095. doi: 10.1016/j.xgen.2022.100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou Z., Cong L., Cong X. Patient-derived organoids in precision medicine: drug screening, organoid-on-a-chip and living organoid biobank. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.762184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grossman J.E., Muthuswamy L., Huang L., et al. Organoid sensitivity correlates with therapeutic response in patients with pancreatic cancer. Clin. Cancer Res. 2022;28(4):708–718. doi: 10.1158/1078-0432.CCR-20-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vlachogiannis G., Hedayat S., Vatsiou A., et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Acklin-Wehnert S., Dayanidhi D., Czito B.G., et al. Feasibility of establishing and drug screening patient-derived rectal organoid models from pretreatment rectal cancer biopsies. J. Clin. Oncol. 2023;41(4_suppl):176. doi: 10.1200/jco.2023.41.4_suppl.176. [DOI] [Google Scholar]
- 182.Zhang Y., Houchen C.W., Li M. Patient-derived organoids pharmacotyping guides precision medicine for pancreatic cancer. Clin. Cancer Res. 2022;28(15):3176–3178. doi: 10.1158/1078-0432.CCR-22-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gerhards N.M., Rottenberg S. New tools for old drugs: functional genetic screens to optimize current chemotherapy. Drug Resist. Updat. 2018;36:30–46. doi: 10.1016/j.drup.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Neal J.T., Li X., Zhu J., et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(7):1972–1988.e16. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu C., Qin T., Huang Y., et al. Drug screening model meets cancer organoid technology. Transl. Oncol. 2020;13(11) doi: 10.1016/j.tranon.2020.100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zou F., Tan J., Liu T., et al. The CD39 positive hepatitis B virus surface protein (HBVs)-targeted CAR-T and personalized tumor-reactive CD8+ T cells exhibit potent anti-hepatocellular carcinoma (HCC) activity. Mol. Ther. 2021;29(5):1794–1807. doi: 10.1016/j.ymthe.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sahin U. Studying tumor-reactive T cells: a personalized organoid model. Cell Stem Cell. 2018;23(3):318–319. doi: 10.1016/j.stem.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 188.Weidinger P. University of Applied Sciences Technikum, Wien; 2017. Modeling Lung Tumorigenesis Using CRISPR/Cas9-Based Genome Editing in Ex Vivo 3D Organoids.https://www.marshallplan.at/images/All-Papers/MP-2017/Weidinger+Pia_747.PDF PhD Thesis. [Google Scholar]
- 189.Weeber F., Ooft S.N., Dijkstra K.K., et al. Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem. Biol. 2017;24(9):1092–1100. doi: 10.1016/J.CHEMBIOL.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 190.Yoshihara E., O’Connor C., Gasser E., et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 2020;586(7830):606–611. doi: 10.1038/s41586-020-2631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tahbaz M., Yoshihara E. Immune protection of stem cell-derived islet cell therapy for Treating Diabetes. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.716625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou J., Huang X. Scientists target human stomach cells for diabetes therapy. WCM Newsroom. 2023 https://news.weill.cornell.edu/news/2023/05/scientists-target-human-stomach-cells-for-diabetes-therapy [Google Scholar]
- 193.Arjmand B., Rabbani Z., Soveyzi F., et al. Advancement of organoid technology in regenerative medicine. Regen. Eng. Transl. Med. 2023;9(1):83–96. doi: 10.1007/s40883-022-00271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Qian S., Mao J., Liu Z., et al. Stem cells for organoids. Smart Med. 2022;1(1) doi: 10.1002/SMMD.20220007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Watanabe S., Kobayashi S., Ogasawara N., et al. Transplantation of intestinal organoids into a mouse model of colitis - Nature Protocols. Nat. Protoc. 2022;17(3):649–671. doi: 10.1038/s41596-021-00658-3. [DOI] [PubMed] [Google Scholar]
- 196.Grassi L., Alfonsi R., Francescangeli F., et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases - Cell Death & Disease. Cell Death Dis. 2019;10 doi: 10.1038/s41419-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reviewed by Emily Henderson B.S. World’s first-in-human research using organoid transplantation yields good results. News-Medicalnet. 2022 https://www.news-medical.net/news/20220822/Worlds-first-in-human-research-using-organoid-transplantation-yields-good-results.aspx [Google Scholar]
- 198.Boers S.N., Van Delden J.J., Clevers H., et al. Organoid biobanking: identifying the ethics. EMBO Rep. 2016;17(7):938–941. doi: 10.15252/embr.201642613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sridhar A., Simmini S., Ribeiro C.M.S., et al. A perspective on organoids for virology research. Viruses. 2020;12(11):1341. doi: 10.3390/v12111341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lensink M.A., Boers S.N., Jongsma K.R., et al. Organoids for personalized treatment of cystic fibrosis: professional perspectives on the ethics and governance of organoid biobanking. J. Cyst. Fibros. 2021;20(3):443–451. doi: 10.1016/j.jcf.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 201.Kim M.B., Hwangbo S., Jang S., et al. Bioengineered co-culture of organoids to recapitulate host-microbe interactions. Mater. Today. Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Roodsant T., Navis M., Aknouch I., et al. A human 2D primary organoid-derived epithelial monolayer model to study host-pathogen interaction in the small intestine. Front. Cell. Infect. Microbiol. 2020;10:272. doi: 10.3389/fcimb.2020.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pellegrino E., Gutierrez M.G. Human stem cell-based models for studying host-pathogen interactions. Cell Microbiol. 2021;23(7):e13335. doi: 10.1111/cmi.13335. [DOI] [PubMed] [Google Scholar]
- 204.Dutta D., Clevers H. Organoid culture systems to study host–pathogen interactions. Curr. Opin. Immunol. 2017;48:15–22. doi: 10.1016/j.coi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hong K.J., Seo S.H. Organoid as a culture system for viral vaccine strains. Clin. Exp. Vaccine Res. 2018;7(2):145–148. doi: 10.7774/cevr.2018.7.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang J., Gong Y., Zhang C., et al. Co-existence and co-infection of influenza a viruses and coronaviruses: public health challenges. Innovation. 2022;3(5) doi: 10.1016/j.xinn.2022.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li X.F., Cui Z., Fan H., et al. A highly immunogenic live-attenuated vaccine candidate prevents SARS-CoV-2 infection and transmission in hamsters. Innovation. 2022;3(2) doi: 10.1016/j.xinn.2022.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu W., Jia J., Dai Y., et al. Delineating COVID-19 immunological features using single-cell RNA sequencing. Innovation. 2022;3(5) doi: 10.1016/j.xinn.2022.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li T., Huang T., Guo C., et al. Genomic variation, origin tracing, and vaccine development of SARS-CoV-2: A systematic review. Innovation. 2021;2(2) doi: 10.1016/j.xinn.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ramírez-Flores C.J., Knoll L.J. Human intestinal enteroids as a model for SARS-CoV-2 infection and antiviral drug screening. Evans M.J., editor. PLoS Pathog. 2021;17(11) doi: 10.1371/journal.ppat.1010080. [DOI] [Google Scholar]
- 211.Bartfeld S. Organoids as host models for infection biology – a review of methods. Exp. Mol. Med. 2021;53:1471–1482. doi: 10.1038/s12276-021-00629-4. https://www.nature.com/articles/s12276-021-00629-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang Y., Huang Z., Tang Z., et al. Research progress, challenges, and breakthroughs of organoids as disease models. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.740574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Blutt S.E., Estes M.K. Organoid models for infectious disease. Annu. Rev. Med. 2022;73(1):167–182. doi: 10.1146/annurev-med-042320-023055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Masmoudi F., Santos-Ferreira N., Pajkrt D., et al. Evaluation of 3D human intestinal organoids as a platform for EV-A71 antiviral drug discovery. Cells. 2023;12(8) doi: 10.3390/cells12081138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ramezankhani R., Solhi R., Chai Y.C., et al. Organoid and microfluidics-based platforms for drug screening in COVID-19. Drug Discov. Today. 2022;27(4):1062–1076. doi: 10.1016/j.drudis.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bartfeld S. Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev. Biol. 2016;420(2):262–270. doi: 10.1016/j.ydbio.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 217.Duque-Correa M.A., Maizels R.M., Grencis R.K., et al. Organoids – new models for host–helminth interactions. Trends Parasitol. 2020;36(2):170–181. doi: 10.1016/j.pt.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Thompson R.E., Bouma G.J., Hollinshead F.K. The roles of extracellular vesicles and organoid models in female reproductive physiology. Int. J. Mol. Sci. 2022;23(6):3186. doi: 10.3390/ijms23063186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.S18.3: Building organoid and microfluidic models to identify toxicant risks to female reproduction. Am. J. Reprod. Immunol. 2022;87(S1):72–73. doi: 10.1111/aji.90_13546. [DOI] [Google Scholar]
- 220.Alzamil L., Nikolakopoulou K., Turco M.Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021;28(1):35–51. doi: 10.1038/s41418-020-0565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang L., Semmes E.C., Ovies C., et al. Innate immune signaling in trophoblast and decidua organoids defines differential antiviral defenses at the maternal-fetal interface. BioRxiv. 2021 doi: 10.1101/2021.03.29.437467. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brazovskaja A., Treutlein B., Camp J.G. High-throughput single-cell transcriptomics on organoids. Curr. Opin. Biotechnol. 2019;55:167–171. doi: 10.1016/j.copbio.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 223.Hof L., Moreth T., Koch M., et al. Long-term live imaging and multiscale analysis identify heterogeneity and core principles of epithelial organoid morphogenesis. BMC Biol. 2021;19(1):37. doi: 10.1186/s12915-021-00958-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yang R., Du Y., Kwan W., et al. A quick and reliable image-based AI algorithm for evaluating cellular senescence of gastric organoids. Cancer Biol. Med. 2023;20:519–536. doi: 10.20892/j.issn.2095-3941.2023.0099. Published Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Leung C.M., De Haan P., Ronaldson-Bouchard K., et al. A guide to the organ-on-a-chip. Nat Rev Methods Primer. 2022;2(1):33. doi: 10.1038/s43586-022-00118-6. [DOI] [Google Scholar]
- 226.Guenat O.T., Geiser T., Berthiaume F. Clinically relevant tissue scale responses as new readouts from organs-on-a-chip for precision medicine. Annu. Rev. Anal. Chem. 2020;13(1):111–133. doi: 10.1146/annurev-anchem-061318-114919. [DOI] [PubMed] [Google Scholar]
- 227.Teriyapirom I., Batista-Rocha A.S., Koo B.K. Genetic engineering in organoids. J. Mol. Med. 2021;99(4):555–568. doi: 10.1007/s00109-020-02029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lim S.W., Fang X., Cui S., et al. CRISPR-Cas9-Mediated correction of SLC12A3 gene mutation rescues the Gitelman’s disease phenotype in a patient-derived kidney organoid system. Int. J. Mol. Sci. 2023;24(3):3019. doi: 10.3390/ijms24033019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Li X., Fu G., Zhang L., et al. Assay establishment and validation of a high-throughput organoid-based drug screening platform. Stem Cell Res. Ther. 2022;13(1):219. doi: 10.1186/s13287-022-02902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu X., Jiang D., Yang Y., et al. Modeling drug-induced liver injury and screening for anti-hepatofibrotic compounds using human PSC-derived organoids. Cell Regen. 2023;12(1):6. doi: 10.1186/s13619-022-00148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fleck J.S., Jansen S.M.J., Wollny D., et al. Inferring and perturbing cell fate regulomes in human cerebral organoids. BioRxiv. 2021 doi: 10.1101/2021.08.24.457460. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vento-Tormo R. Single-cell omics meets organoid cultures. Nat. Rev. Genet. 2023;24(8):492. doi: 10.1038/s41576-023-00622-9. [DOI] [PubMed] [Google Scholar]
- 233.Yin Y., Liu P.Y., Shi Y., et al. In: Reviews of Physiology, Biochemistry and Pharmacology. Pedersen S.H.F., editor. Springer International Publishing; 2021. Single-cell sequencing and organoids: a powerful combination for modelling organ development and diseases; pp. 189–210. [DOI] [PubMed] [Google Scholar]
- 234.Jagadeesh K.A., Dey K.K., Montoro D.T., et al. Identifying disease-critical cell types and cellular processes by integrating single-cell RNA-sequencing and human genetics. Nat. Genet. 2022;54(10):1479–1492. doi: 10.1038/s41588-022-01187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jin F., Liu M., Zhang D., et al. Translational perspective on bone-derived cytokines in inter-organ communications. Innovation. 2023;4(1) doi: 10.1016/j.xinn.2022.100365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Du X., Chen Z., Li Q., et al. Organoids revealed: morphological analysis of the profound next generation in-vitro model with artificial intelligence. Biodes. Manuf. 2023;6(3):319–339. doi: 10.1007/s42242-022-00226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kassis T., Hernandez-Gordillo V., Langer R., et al. OrgaQuant: human intestinal organoid localization and quantification using deep convolutional neural networks. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-48874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kegeles E., Naumov A., Karpulevich E.A., et al. Convolutional neural networks can predict retinal differentiation in retinal organoids. Front. Cell. Neurosci. 2020;14:171. doi: 10.3389/fncel.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bein A., Shin W., Jalili-Firoozinezhad S., et al. Microfluidic organ-on-a-chip models of human intestine. Cell. Mol. Gastroenterol. Hepatol. 2018;5(4):659–668. doi: 10.1016/j.jcmgh.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Valiei A., Aminian-Dehkordi J., Mofrad M.R.K. Gut-on-a-chip models for dissecting the gut microbiology and physiology. APL Bioeng. 2023;7(1) doi: 10.1063/5.0126541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kasendra M., Tovaglieri A., Sontheimer-Phelps A., et al. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 2018;8(1):2871. doi: 10.1038/s41598-018-21201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Campisi M., Shin Y., Osaki T., et al. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–129. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hajal C., Offeddu G.S., Shin Y., et al. Engineered human blood–brain barrier microfluidic model for vascular permeability analyses. Nat. Protoc. 2022;17(1):95–128. doi: 10.1038/s41596-021-00635-w. [DOI] [PubMed] [Google Scholar]
- 244.Tian M., Ma Z., Yang G.Z. Micro/nanosystems for controllable drug delivery to the brain. Innovation. 2024;5(1) doi: 10.1016/j.xinn.2023.100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pereira A., Garcia J.W., Muotri A. Neural stimulation of brain organoids with dynamic patterns: a sentiomics approach directed to regenerative neuromedicine. NeuroSci. 2023;4(1):31–42. doi: 10.3390/neurosci4010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Serruya M.D. Connecting the brain to itself through an emulation. Front. Neurosci. 2017;11:373. doi: 10.3389/fnins.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kagan B.J., Kitchen A.C., Tran N.T., et al. In vitro neurons learn and exhibit sentience when embodied in a simulated game-world. Neuron. 2022;110(23):3952–3969.e8. doi: 10.1016/j.neuron.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Smirnova L., Caffo B.S., Gracias D.H., et al. Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish. Front. Sci. 2023;1 doi: 10.3389/fsci.2023.1017235. [DOI] [Google Scholar]