Abstract
Using a yeast two-hybrid method, we identified a novel protein which interacts with glycogen synthase kinase 3β (GSK-3β). This protein had 44% amino acid identity with Axin, a negative regulator of the Wnt signaling pathway.We designated this protein Axil for Axin like. Like Axin, Axil ventralized Xenopus embryos and inhibited Xwnt8-induced Xenopus axis duplication. Axil was phosphorylated by GSK-3β. Axil bound not only to GSK-3β but also to β-catenin, and the GSK-3β-binding site of Axil was distinct from the β-catenin-binding site. Furthermore, Axil enhanced GSK-3β-dependent phosphorylation of β-catenin. These results indicate that Axil negatively regulates the Wnt signaling pathway by mediating GSK-3β-dependent phosphorylation of β-catenin, thereby inhibiting axis formation.
Axin, which is a product of the mouse Fused locus, has been identified as a negative regulator of the Wnt signaling pathway (45). Fused is a mutation that causes dominant skeletal and neurological defects and recessive lethal embryonic defects including neuroectodermal abnormalities (36). Two spontaneous alleles of Fused, called Kinky (FuKi) and Knobbly (FuKb), and a transgenic insertional allele, FuTg1, carry axis duplications and are lethal between 8 and 10 days postcoitus, suggesting that the Fused locus plays a role in the determination of the embryonic axis (9, 14, 33). The cDNA of this locus has been sequenced, and the Fused gene has been renamed Axin. Dorsal injection of wild-type Axin in Xenopus embryos blocks axis formation, and coinjection of Axin inhibits Wnt8-, dishevelled (Dsh)-, and kinase-negative glycogen synthase kinase 3β (GSK-3β)-induced axis duplication (45). These results suggest that Axin exerts its effects on axis formation by inhibiting the signal transduction in the Wnt signaling pathway. However, the molecular mechanism by which Axin regulates axis formation is not known.
Wnt and Wg signal many key developmental decisions, regulating anterior-posterior and dorsal-ventral patterns in both vertebrates and flies (22, 30, 31). In vertebrates, the Wnt signaling pathway consists of an intracellular cascade that includes frizzled, Dsh, GSK-3β, and β-catenin (5). The Wnts are a family of secreted polypeptides, whose receptors are believed to be members of the frizzled family (3). It has been suggested that Dsh acts downstream of frizzled (22, 30). GSK-3β is a constitutively active protein kinase and antagonizes downstream elements of the Wnt signaling pathway through changes in the β-catenin level (10). Wnt inactivates GSK-3β activity through Dsh, although by which mechanism is not known (6). In the presence of Wnt, there is a decrease in the phosphorylation of β-catenin and an increase in its stability, and β-catenin translocates to the nucleus (44). This translocation involves the association of β-catenin with the transcriptional enhancers of lymphocyte enhancer binding factor/T cell factor (LEF/TCF) family (2, 24). β-Catenin has a consensus sequence of a phosphorylation site for GSK-3β, and elimination of this possible phosphorylation site stabilizes β-catenin (22, 26, 44). It has been recently shown that the ubiquitination-proteasome pathway is involved in the degradation of β-catenin and that mutations in the GSK-3β consensus phosphorylation site of β-catenin prevent ubiquitination (1). It is well known that adenomatous polyposis coli gene product (APC) is required for the degradation of β-catenin, although the role of APC is not well understood (35). Furthermore, it has been shown that GSK-3β phosphorylates APC and that the phosphorylation enhances the binding of APC to β-catenin (37). Thus, it appears that GSK-3β is a key mediator in the Wnt signaling pathway to regulate β-catenin turnover and that the phosphorylation of β-catenin by GSK-3β is essential for this process. However, it is not clear how GSK-3β regulates the degradation of β-catenin since GSK-3β does not significantly phosphorylate β-catenin by the use of the mammalian purified proteins.
To obtain insights into the action of GSK-3β on the degradation of β-catenin and the specification of cell fate, we have tried to find a GSK-3β-interacting protein by using a yeast two-hybrid method. We isolated a protein which interacts with GSK-3β and found that this protein has 44% identity with Axin (45). We designated this protein Axil (Axin like). We show here that Axil inhibits Xwnt8-induced axis formation of Xenopus embryos like Axin. Further, we demonstrate that Axil makes a complex with both GSK-3β and β-catenin and that it promotes GSK-3β-dependent phosphorylation of β-catenin. These results suggest that Axil negatively regulates the Wnt signaling pathway by interacting with GSK-3β and β-catenin and by mediating the signal from GSK-3β to β-catenin, resulting in the regulation of axis formation.
MATERIALS AND METHODS
Materials and chemicals.
Yeast strain L40, plasmid vectors for two-hybrid screening, and a pGAD10-derived rat brain cDNA library; pBSSK/GSK-3β; pBSSK/β-catenin; a λZAP rat brain cDNA library; pEF-BOS; pGEX-KG; a peptide substrate of GSK-3 (GSK peptide 1); the anti-hemagglutinin 1 (HA) antibody; and the anti-glutathione-S-transferase (anti-GST) and anti-maltose binding protein (anti-MBP) antibodies were kindly supplied by Y. Takai and K. Tanaka (Osaka University, Suita, Japan), J. R. Woodgett (Ontario Cancer Institute, Toronto, Ontario, Canada), A. Nagafuchi and S. Tsukita (Kyoto University, Kyoto, Japan), Y. Hata (ERATO, Japan Science and Technology Corp., Kobe), S. Nagata (Osaka University) (23), F. Tamanoi (University of California, Los Angeles), C. W. Turck (University of California, San Francisco) (29), Q. Hu (Chiron Corp., Emeryville, Calif.), and M. Nakata (Sumitomo Electric Industries, Yokohama, Japan), respectively. The anti-Myc antibody was prepared from 9E10 cells. GST fused to GSK-3β (GST–GSK-3β) was purified from Escherichia coli as described previously (29). MBP fused to Axil (MBP-Axil), MBP fused to the Axil region including residues 265 to 483 [MBP-Axil(265-483)], MBP-Axil(265-412), MBP-Axil(412-483), GST–β-catenin, GST–β-catenin(1-423), and GST–β-catenin(423-781) were purified from E. coli according to the manufacturer’s instructions. The anti-GSK-3β and anti-β-catenin antibodies were purchased from Transduction Laboratories (Lexington, Ky.). [α-32P]dCTP and [γ-32P]ATP were obtained from Amersham Inc. (Buckinghamshire, United Kingdom). Other materials were from commercial sources.
Plasmid construction.
To construct pBTM116HA/GSK-3β, pBSSK/GSK-3β was digested with BclI and EcoRI and blunted with Klenow fragment. The 1.3-kb fragment was inserted into pBTM116HA, which was digested with BamHI and blunted with Klenow fragment. To construct pCGN/GSK-3βK85M, the 1.3-kb fragment encoding GSK-3βK85M with XbaI and SmaI sites synthesized by PCR was inserted into the XbaI- and SmaI-cut pCGN. pCGN/GSK-3β and pGEX-2T/GSK-3β were constructed as described before (29). To construct pEF-BOS-Myc, the fragment encoding Myc epitope synthesized by PCR was inserted into pEF-BOS, which was digested with XbaI and blunted with Klenow fragment. To construct pBSKS/Axil, the 70-base fragment encoding Axil(1-23) with an SmaI site at the 5′ end was synthesized by PCR. This fragment was digested with SmaI and SacII and inserted into the pBSKS containing the 3.2-kb fragment encoding Axil prepared from the library in pGAD10, which was digested with XbaI, blunted with Klenow fragment, and digested with SacII. To construct pEF-BOS-Myc/Axil, pBSKS/Axil was digested with SpeI and XbaI and the 3.2-kb fragment encoding Axil was inserted into the XbaI-cut pEF-BOS-Myc. To construct pEF-BOS-Myc/Axil(1-670), pBSKS/Axil was digested with SpeI and SmaI and the 2.0-kb fragment encoding Axil(1-670) was inserted into the XbaI- and SmaI-cut pEF-BOS-Myc. To construct pBSKS/Axil(682-838), pGAD10/Axil(682-838) was digested with EcoRI and the 0.48-kb fragment encoding Axil(682-838) was inserted into the EcoRI-cut pBSKS. To construct pBJ-Myc/Axil(682-838), pBSKS/Axil(682-838) was digested with ClaI and XbaI and blunted with Klenow fragment. The 0.48-kb fragment encoding Axil(682-838) was inserted into pBJ-Myc, which was digested with BamHI and blunted with Klenow fragment. To construct pEF-BOS/Myc-Axil(1-265), the fragment encoding Myc epitope-tagged Axil(1-265) with EcoRI and BamHI sites synthesized by PCR was digested with EcoRI and BamHI and blunted with Klenow fragment. The 0.85-kb fragment encoding Axil(1-265) was inserted into pEF-BOS, which was digested with XbaI and blunted with Klenow fragment. To construct pBSKS/Axil(265-483), the 0.66-kb fragment encoding Axil(265-483) with XbaI and BamHI sites synthesized by PCR was inserted into the XbaI- and BamHI-cut pBSKS. To construct pEF-BOS-Myc/Axil(265-483), pBSKS/Axil(265-483) was digested with XbaI and BamHI and the 0.66-kb fragment encoding Axil(265-483) was inserted into the XbaI- and BamHI-cut pEF-BOS-Myc. To construct pMAL-c2/Axil(265-483), pBSKS/Axil(265-483) was digested with XbaI and HindIII and the 0.66-kb fragment encoding Axil(265-483) was inserted into the XbaI- and HindIII-cut pMAL-c2. To construct pMAL-c2/Axil(265-412), the 0.45-kb fragment encoding Axil(265-412) with XbaI and HindIII sites was synthesized by PCR, digested with XbaI and HindIII, and inserted into the XbaI- and HindIII-cut pMAL-c2. To construct pMAL-c2/Axil(412-483), pMAL-c2/Axil(265-483) was digested with SacI, the 0.45-kb fragment encoding Axil(265-412) was removed, and the remaining pMAL-c2 containing the fragment encoding Axil(412-483) was self-ligated. To construct pMAL-c2/Axil, pBSKS/Axil was digested with SpeI and XbaI and the 3.2-kb fragment encoding Axil was inserted into the XbaI-cut pMAL-c2. To construct pGEX-2T/β-catenin, pBSSK/β-catenin was digested with XhoI and blunted with Klenow fragment and the 2.7-kb fragment encoding β-catenin was inserted into the SmaI-cut pGEX-2T. To construct pGEX-2T/β-catenin(1-423), pBSSK/β-catenin was digested with BamHI and EcoRI and the 1.3-kb fragment encoding β-catenin(1-423) was inserted into the BamHI- and EcoRI-cut pGEX-2T. To construct pGEX-KG/β-catenin(423-781), pBSSK/β-catenin was digested with EcoRI and XhoI, and the 1.4-kb fragment encoding β-catenin(423-781) was inserted into the EcoRI- and XhoI-cut pGEX-KG.
Yeast two-hybrid screening and molecular cloning of Axil.
A yeast strain L40 (MATa trp 1 leu2 his3 ade2 LYS2::lexA-HIS3 URA3::lexA-lacZ) carrying pBTM116HA/GSK-3β was transformed with a rat brain cDNA library constructed in pGAD10 (12, 42). Approximately 3.7 × 106 transformants were screened for growth on SD plate medium lacking tryptophan, leucine, and histidine as evidenced by transactivation of a lexA-HIS3 reporter gene and histidine prototrophy. His+ colonies were scored for β-galactosidase activity. Plasmids harboring cDNAs were recovered from positive colonies, and the nucleotide sequences of plasmid DNAs which conferred the LacZ+ phenotype on L40 containing pBTM116HA/GSK-3β were determined. To obtain a full-length cDNA of a GSK-3β-interacting protein, the clone isolated by the yeast two-hybrid method was labeled with random primers and [α-32P]dCTP and used to screen a λZAP rat brain cDNA library. A number of positive clones were isolated, and all clones, collectively spanning 3.2 kb, were sequenced.
Xenopus injections and analysis of phenotypes.
Axil, Xwnt8, and Xglobin cDNAs were individually subcloned into the BglII site of pSP64T (20). Sense mRNA was obtained by in vitro transcription of linearized templates by using the mCAP RNA capping kit (Stratagene, La Jolla, Calif.). Fertilized eggs were dejellied, and Axil mRNA (250 pg) was injected into dorsal or ventral blastomeres at the four-cell stage. Xglobin mRNA (250 pg) was injected into dorsal blastomeres at the four-cell stage. Xwnt8 mRNA (0.1 pg) was injected with or without Axil mRNA (250 pg) into ventral blastomeres at the four-cell stage. Injection was performed with 5% Ficoll in Steinberg’s solution, and embryos were cultured for 2 days in Steinberg’s solution. The phenotypes of the injected embryos were evaluated by the Dorso-Anterior Index (DAI) (15).
Interaction of GSK-3β and β-catenin with Axil.
To determine whether Axil interacts with GSK-3β and β-catenin in intact cells, COS cells (10-cm-diameter dish) were transfected with pCGN-, pBJ-, and pEF-BOS-derived plasmids and lysed as described previously (16). Axil and its deletion mutants were tagged with Myc epitope at their N termini. GSK-3β and GSK-3βK85M were tagged with HA epitope at their N termini. The lysates (500 to 1,000 μg of protein) were immunoprecipitated with the anti-Myc or anti-HA antibody, and then the precipitates were probed with the anti-Myc, anti-HA, anti-GSK-3β, and anti-β-catenin antibodies (11, 16, 28). To examine the interaction of β-catenin with Axil by using crude lysates in vitro, GST–β-catenin, GST–β-catenin(1-423), or GST–β-catenin(423-781) (50 pmol each) was incubated with the lysates (500 μg of protein) of COS cells expressing Myc-Axil for 2 h at 4°C. GST–β-catenin and its mutants were precipitated with glutathione Sepharose 4B, and the precipitates were probed with the anti-Myc antibody. To examine the interaction of GSK-3β and β-catenin with Axil by using the purified proteins in vitro, GST–GSK-3β or GST–β-catenin (8 pmol each) was incubated with MBP-Axil(265-483), MBP-Axil(265-412), or MBP-Axil(412-483) (2 pmol each) immobilized on amylose resin in 40 μl of reaction mixture (20 mM Tris-HCl [pH 7.5] and 1 mM dithiothreitol) for 2 h at 4°C. MBPs fused to proteins were precipitated by centrifugation, and the precipitates were probed with the anti-GSK-3β and anti-β-catenin antibodies.
Phosphorylation of Axil by GSK-3β.
To examine the phosphorylation of full-length Axil by GSK-3β, the lysates (250 μg of protein) of COS cells expressing Myc-Axil were immunoprecipitated with the anti-Myc antibody. The precipitates were washed and incubated with or without GST–GSK-3β (100 ng of protein) in 30 μl of kinase reaction mixture (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 50 μM [γ-32P]ATP [500 to 2,000 cpm/pmol]) for 20 min at 30°C. When kinetics for the phosphorylation of Axil by GST–GSK-3β was examined, MBP-Axil(265-483) purified from E. coli was used as a substrate. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography. Where specified, the radioactivities of phosphorylated Axil were counted.
Phosphorylation of β-catenin by GSK-3β in the presence of Axil.
GST–β-catenin or GST–β-catenin(1-423) (2 μg of protein each) was incubated with GST–GSK-3β (400 or 600 ng of protein) in the presence of several deletion mutants of MBP-Axil (200 ng of each protein) or MBP-Axil (160 ng of protein) in 30 μl of kinase reaction mixture for 30 min at 30°C. The samples were subjected to SDS-PAGE followed by autoradiography. When the amounts of phosphate incorporated into GST–β-catenin were determined, the radioactivities of phosphorylated GST–β-catenin were counted.
Other assays.
Northern blot analysis was performed as described previously (21). Protein concentrations were determined with bovine serum albumin as a standard (4). When the effect of Axil on the GSK-3β activity was examined, GST–GSK-3β (400 ng of protein) was incubated with 50 μM GSK peptide 1 in the presence of MBP-Axil(265-483) or MBP-Axil in 30 μl of kinase reaction mixture for 10 min at 30°C. The reaction mixture was then spotted on phosphocellulose filters and washed with phosphoric acid (29).
Nucleotide sequence accession number.
The GenBank accession number for rat Axil cDNA is AF017757.
RESULTS
Isolation of GSK-3β-interacting protein.
To identify proteins that physically interact with GSK-3β, we conducted a rat brain cDNA library screening by the yeast two-hybrid method. We identified four partial cDNA clones that specifically interact with GSK-3β. Among these clones, two were found to encode a sequence of 94% identity with the sequence of mouse Axin, and we designated this protein rAxin for rat Axin (13). Since another clone had a high percentage of GC base pairs (62%), we did not characterize it further. The remaining clone encoded a novel protein. Therefore, we will focus on this clone in this report.
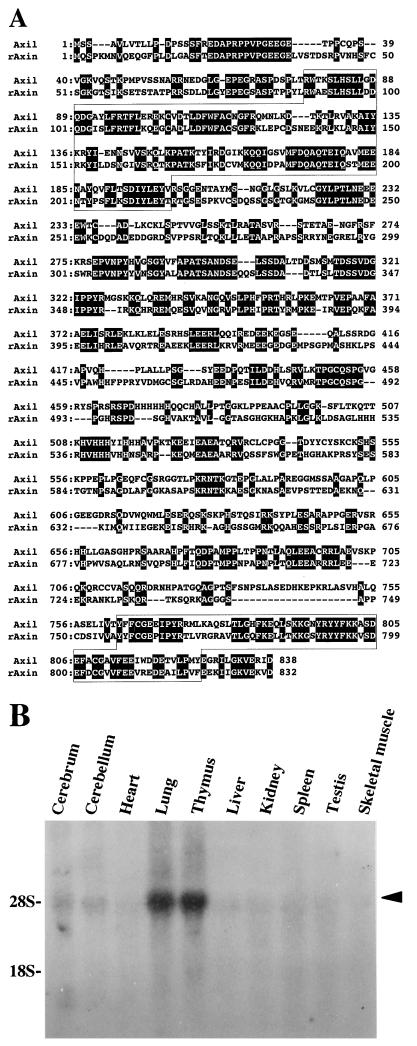
A full-length cDNA of this GSK-3β-interacting protein was isolated from a rat brain cDNA library. This clone spanned a distance of 3.2 kb and contained an uninterrupted open reading frame of 2,514 bp, encoding a predicted protein of 838 amino acids with a calculated Mr of 92,946 (Fig. 1A). The first ATG was preceded by stop codons in all three reading frames and the 5′-noncoding region had a high percentage of GC base pairs (86%). The neighboring sequence of the first ATG was consistent with the translation initiation start proposed by Kozak (19). This protein was found to have 44% amino acid identity with rAxin, so we designated this GSK-3β-interacting protein Axil (Axin like) (Fig. 1A). Like Axin, Axil had two domains which are homologous to regulators of G protein signaling (RGS) and Dsh. The N-terminal region of Axil, residues 77 to 200, had 29% amino acid identity with residues 58 to 178 of RGS4, which has been identified as a GTPase-activating protein (GAP) for heterotrimeric GTP-binding protein (G protein) (8). The C-terminal region, residues 763 to 826, had 35% amino acid identity with residues 8 to 73 of the mouse Dsh homolog, DVL-1 (41). A single band of 4.9-kb Axil mRNA was detected in various rat tissues and was highly expressed in lung and thymus (Fig. 1B).
FIG. 1.
Structure of Axil. (A) Amino acid sequence of Axil. Identical residues in Axil and rAxin are denoted by a black background. The RGS homologous and Dsh homologous domains are boxed. (B) Northern blot analysis of Axil. Total RNAs (20 μg/lane) of various rat tissues were probed with cDNA encoding Axil(1-148). The positions of 28S and 18S ribosomal RNAs are indicated. The arrowhead indicates the positions of Axil mRNA. The results shown are representative of two independent experiments.
Effect of Axil on axis formation of Xenopus embryos.
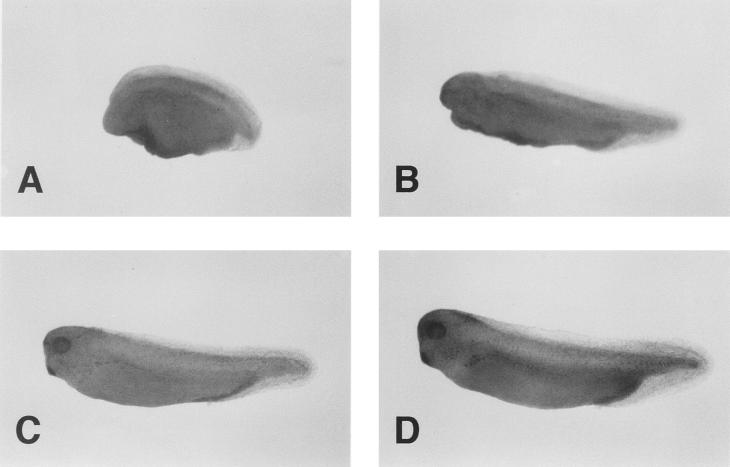
It has been demonstrated that Axin regulates axis formation of Xenopus embryos (45). Therefore, we examined the effect of Axil on embryonic axis formation. When Axil mRNA was injected into the dorsal blastomeres at the four-cell stage, these embryos showed various ventralized phenotypes such as loss of head and microcephaly (Fig. 2A and B; Table 1). However, embryos injected ventrally with Axil mRNA developed normally (Fig. 2C; Table 1). Control injection of Xglobin mRNA had no effect (Fig. 2D; Table 1). Xwnt8 has been shown to induce a secondary dorsal axis when injected into the ventral side of the embryos (40). Coinjection of Axil mRNA blocked the Xwnt8 mRNA-induced secondary axis formation (Table 2). These results suggest that Axil inhibits either normal or secondary dorsal axis formation in Xenopus embryos by interfering with the Wnt signaling pathway. Therefore, it appears that Axil has the same activity to regulate axis formation as Axin.
FIG. 2.
Effect of Axil on axis formation of Xenopus embryos. (A and B) Dorsal injection of Axil mRNA. The embryo has no head region (DAI = 1) (A) or has microcephaly (DAI = 3) (B). (C) Ventral injection of Axil mRNA. (D) Control injection of Xglobin mRNA. Embryos were evaluated at the tail bud stage, and examples are shown.
TABLE 1.
Frequency and extent of ventralization by dorsal injection of Axil mRNAa
| mRNA and region | No. (%) of embryos with phenotype
|
No. (%) of dead | No. of specimens | % Ventralized embryos | Avg DAI | ||
|---|---|---|---|---|---|---|---|
| Normal | Microcephaly | Headless | |||||
| Axil | |||||||
| Dorsal blastomere | 4 (9.3) | 11 (25.6) | 21 (48.8) | 7 (16.3) | 43 | 74.4 | 1.9 |
| Ventral blastomere | 27 (90) | 0 | 2 (6.7) | 1 (3.3) | 30 | 6.7 | 4.7 |
| Xglobin: dorsal blastomere | 48 (90.6) | 4 (7.5) | 0 | 1 (1.9) | 53 | 7.5 | 4.7 |
All mRNAs were injected into the subequatorial region of two dorsal or ventral blastomeres of four-cell embryos. The Dorso-Anterior Index (DAI) is a measure of axial development, where 5 is normal and 0 is completely ventralized (15).
TABLE 2.
Frequency of axis duplication by ventral injection of Axil and Xwnt8 mRNAa
| mRNA and region | No. of embryos with phenotype
|
No. of dead | No. of speci- mens | % of specimens with axis duplication | |
|---|---|---|---|---|---|
| Normal | Axis dupli- cation | ||||
| Xwnt8: ventral blastomere | 18 | 30 | 1 | 49 | 61.2 |
| Axil + Xwnt8: ventral blastomere | 36 | 11 | 1 | 48 | 22.9 |
All mRNAs were injected into the subequatorial region of two ventral blastomeres of four-cell embryos.
Interaction of Axil with GSK-3β.
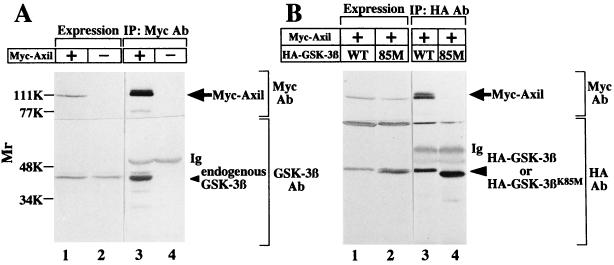
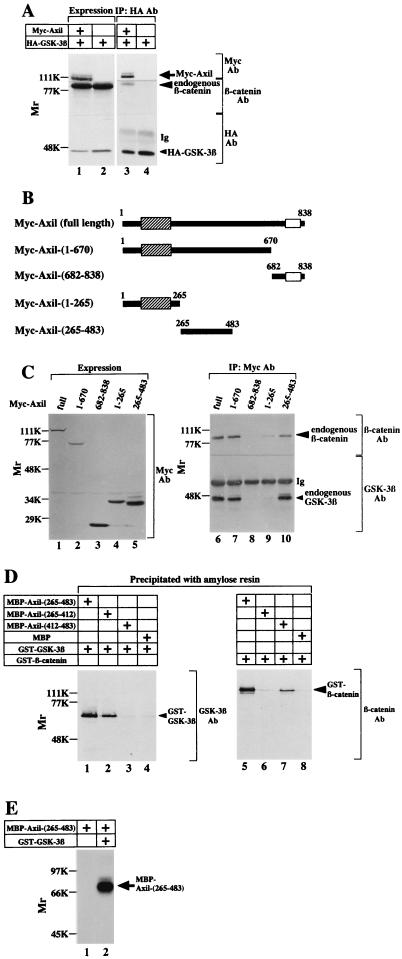
Previously, we found that GSK-3β is expressed in COS cells (29). To examine whether Axil interacts with endogenous GSK-3β in intact cells, we expressed Myc-Axil in COS cells (Fig. 3A). When the lysates expressing Myc-Axil were immunoprecipitated with the anti-Myc antibody, endogenous GSK-3β was detected in the Myc-Axil immune complex (Fig. 3A). Neither Myc-Axil nor GSK-3β was immunoprecipitated from the lysates expressing Myc-Axil with nonimmune immunoglobulin (data not shown). GSK-3βK85M, in which the ATP binding site is mutated, is known to be a catalytically inactive mutant. Cotransfection of Myc-Axil with wild-type HA–GSK-3β or HA–GSK-3βK85M did not alter the level of expression of transfected Myc-Axil as assessed by immunoblot analysis (Fig. 3B). When these lysates were immunoprecipitated with the anti-HA antibody, Myc-Axil was coprecipitated with wild-type HA–GSK-3β but not with HA–GSK-3βK85M (Fig. 3B). These results demonstrate that Axil makes a complex with GSK-3β in intact cells and that the kinase activity of GSK-3β is necessary for its complex formation with Axil.
FIG. 3.
Interaction of Axil with GSK-3β. (A) Interaction of Axil with endogenous GSK-3β in COS cells. The lysates (20 μg of protein) of COS cells expressing Myc-Axil were probed with the anti-Myc and anti-GSK-3β antibodies (lane 1). The same lysates (500 μg of protein) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-Myc and anti-GSK-3β antibodies (lane 3). The lysates of COS cells transfected with empty vectors were used as controls (lanes 2 and 4). (B) Requirement of kinase activity of GSK-3β for its interaction with Axil. Myc-Axil was coexpressed with wild-type HA–GSK-3β (lanes 1 and 3) or HA–GSK-3βK85M (lanes 2 and 4) in COS cells, and the lysates were probed with the anti-Myc and anti-HA antibodies (lanes 1 and 2) or immunoprecipitated with the anti-HA antibody. The immunoprecipitates were then probed with the anti-Myc and anti-HA antibodies (lanes 3 and 4). IP, immunoprecipitation; Ab, antibody; Ig, immunoglobulin; WT, wild type HA–GSK-3β; 85M, HA–GSK-3βK85M. The arrows, small arrowhead, and large arrowhead indicate the positions of Myc-Axil, endogenous GSK-3β, and HA–GSK-3β or HA–GSK-3βK85M, respectively. The results shown are representative of three independent experiments.
Phosphorylation of Axil by GSK-3β.
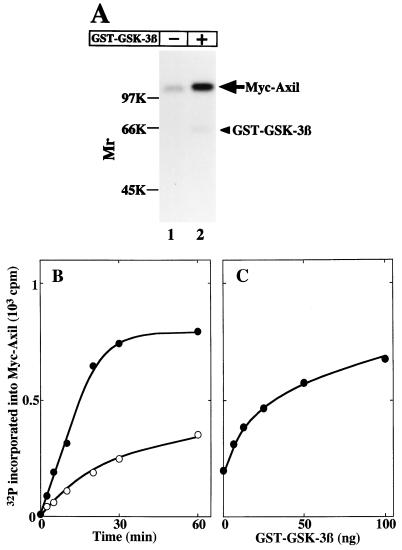
To determine whether Axil is a substrate for GSK-3β, Myc-Axil immunoprecipitated from COS cell lysates was incubated with or without GST–GSK-3β. Myc-Axil was phosphorylated without GST–GSK-3β, and this phosphorylation was enhanced by GST–GSK-3β (Fig. 4A). GST–GSK-3β phosphorylated Myc-Axil in time- and dose-dependent manners (Fig. 4B and C). In Fig. 3, we showed that endogenous GSK-3β is coprecipitated with Myc-Axil. Therefore, the phosphorylation of Myc-Axil without GST–GSK-3β might be due to the associated endogenous GSK-3β. However, we cannot rule out the possibility that GSK-3β phosphorylates and activates other protein kinases which interact with and phosphorylate Myc-Axil. Alternatively, other protein kinases which interact with Myc-Axil might phosphorylate it independently of GSK-3β.
FIG. 4.
Phosphorylation of Axil by GSK-3β. (A) Autoradiography. Myc-Axil immunoprecipitated from COS cell lysates (250 μg of protein) was incubated with (lane 2) or without (lane 1) GST–GSK-3β (100 ng of protein) for 20 min, and the samples were subjected to SDS-PAGE followed by autoradiography. The arrow and arrowhead indicate the positions of Myc-Axil and GST–GSK-3β, respectively. (B) Time course. Myc-Axil immunoprecipitated from COS cell lysates was incubated with (•) or without (○) GST–GSK-3β (100 ng of protein) for the indicated periods of time. (C) Dose dependency. Myc-Axil was incubated with the indicated amounts of GST–GSK-3β for 20 min. The results shown are representative of four independent experiments.
Interaction of Axil with β-catenin.
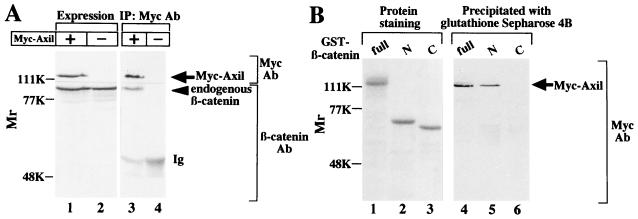
It has been demonstrated that Axin negatively regulates the Wnt signaling pathway downstream of GSK-3β and upstream of β-catenin (45). As shown in Fig. 2, we have found that Axil also inhibits the Wnt signaling pathway to induce axis duplication like Axin. Therefore, we next examined the relationship between Axil and β-catenin. When the lysates expressing Myc-Axil were immunoprecipitated with the anti-Myc antibody, endogenous β-catenin was coprecipitated with Myc-Axil (Fig. 5A). Neither Myc-Axil nor β-catenin was immunoprecipitated from the lysates expressing Myc-Axil with nonimmune immunoglobulin (data not shown). To determine the region of β-catenin which interacts with Axil, GST–N-terminal β-catenin(1-423) and GST–C-terminal β-catenin(423-781) were purified from E. coli. Myc-Axil was coprecipitated with GST–N-terminal β-catenin but not with GST–C-terminal β-catenin (Fig. 5B). These results indicate that Axil makes a complex with the N-terminal region of β-catenin in intact cells.
FIG. 5.
Interaction of Axil with β-catenin in intact cells (A) and in vitro (B). (A) The lysates (20 μg of protein) of COS cells expressing Myc-Axil were probed with the anti-Myc and anti-β-catenin antibodies (lane 1). The same lysates (500 μg of protein) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-Myc and anti-β-catenin antibodies (lane 3). The lysates of COS cells transfected with empty vectors were used as controls (lanes 2 and 4). (B) GST–β-catenin(full length) (lane 1), GST–N-terminal β-catenin (lane 2), and GST–C-terminal β-catenin (lane 3) (10 pmol each) were subjected to SDS-PAGE followed by Coomassie brilliant blue staining. After the lysates (500 μg of protein) of COS cells expressing Myc-Axil were incubated with GST–β-catenin (lane 4), GST–N-terminal β-catenin (lane 5), and GST–C-terminal β-catenin (lane 6) (50 pmol each), β-catenin and its deletion mutants were precipitated with glutathione Sepharose 4B. The precipitates were probed with the anti-Myc antibody. IP, immunoprecipitation; Ab, antibody; Ig, immunoglobulin; full, GST–β-catenin(full length); N, GST–N-terminal β-catenin; C, GST–C-terminal β-catenin. The arrows and arrowhead indicate the positions of Myc-Axil and endogenous β-catenin, respectively. The results shown are representative of three independent experiments.
Complex formation of GSK-3β, Axil, and β-catenin.
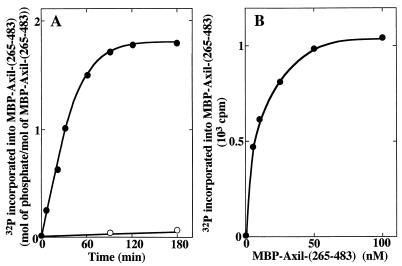
From the results of Fig. 3 and 5, we found that Axil makes a complex with both GSK-3β and β-catenin. Therefore, we examined whether these three proteins make a ternary complex. When Myc-Axil and HA–GSK-3β were coexpressed in COS cells and HA–GSK-3β was immunoprecipitated with the anti-HA antibody, both Myc-Axil and endogenous β-catenin were detected in the HA–GSK-3β immune complex (Fig. 6A). These results suggest that GSK-3β, Axil, and β-catenin make a ternary complex in intact cells, although caution is necessary in interpreting these results until a gel filtration experiment shows that all three components are present in a large complex. To examine which region of Axil interacts with GSK-3β and β-catenin, several deletion mutants of Myc-Axil were expressed in COS cells (Fig. 6B). When these Myc-Axil mutants were immunoprecipitated with the anti-Myc antibody, Myc-Axil(1-670) and Myc-Axil(265-483) made a complex with both GSK-3β and β-catenin like Myc-Axil(full length) did (Fig. 6C). Neither GSK-3β nor β-catenin was coprecipitated with Myc-Axil(682-838) (Fig. 6C). Although GSK-3β was not detected in the Myc-Axil(1-265) immune complex, β-catenin was faintly coprecipitated (Fig. 6C). These results demonstrate that the region containing residues 265 to 483 of Axil is responsible for making a complex with both GSK-3β and β-catenin. To characterize this region, MBP-Axil(265-483), MBP-Axil(265-412), and MBP-Axil(412-483) were purified from E. coli. MBP-Axil(265-483) bound to both GST–GSK-3β and GST–β-catenin. Furthermore, MBP-Axil(265-412) and MBP-Axil(412-483) bound to GST–GSK-3β and GST–β-catenin, respectively (Fig. 6D). These results clearly show that GSK-3β and β-catenin directly interact with different sites on Axil. Sequence S/TXXXS/T is known to be a consensus sequence for a GSK-3β phosphorylation site (34). In residues 265 to 483 of Axil, there are four possible phosphorylation sites for GSK-3β: SFKRS277, SANDSELSSDALT308, SMSMT315, and SMTDS317 (S and T mean the possible phosphorylation residues by GSK-3β). Indeed, MBP-Axil(265-483) was directly phosphorylated by GST–GSK-3β (Fig. 6E). GST–GSK-3β phosphorylated MBP-Axil(265-483) in a time-dependent manner, and 1.8 mol of phosphate was maximally incorporated into 1 mol of MBP-Axil(265-483) (Fig. 7A). The Km and Vmax values for the phosphorylation of Axil(265-483) by GSK-3β were calculated to be 6.4 nM and 580 pmol/min/mg, respectively (Fig. 7B). Since the Km value of Axil(265-483) is much lower than those of other known substrates such as inhibitor-2, myelin basic protein, and κ-casein (43), Axil could be a good substrate for GSK-3β, although the Km value of full-length Axil for GSK-3β remains to be clarified.
FIG. 6.
Complex formation of GSK-3β, Axil, and β-catenin. (A) Complex formation in intact cells. The lysates (20 μg of protein) of COS cells expressing HA–GSK-3β and Myc-Axil (lanes 1 and 3) and HA–GSK-3β alone (lanes 2 and 4) were probed with the anti-Myc, anti-β-catenin, and anti-HA antibodies (lanes 1 and 2). The same lysates (500 μg of protein) were immunoprecipitated with the anti-HA antibody, and the immunoprecipitates were probed with the anti-Myc, anti-β-catenin, and anti-HA antibodies (lanes 3 and 4). IP, immunoprecipitation, Ab, antibody; Ig, immunoglobulin. The arrow, large arrowhead, and small arrowhead indicate the positions of Myc-Axil, endogenous β-catenin, and HA–GSK-3β, respectively. (B) Deletion mutants of Axil. The hatched and empty boxes indicate the RGS and Dsh homologous domains, respectively. (C) Expression of Axil deletion mutants and their interaction with GSK-3β and β-catenin. The lysates (20 μg of protein) of COS cells expressing Myc-Axil(full length) (lane 1), Myc-Axil(1-670) (lane 2), Myc-Axil(682-838) (lane 3), Myc-Axil(1-265) (lane 4), and Myc-Axil(265-483) (lane 5) were probed with the anti-Myc antibody (left panel). The same lysates (500 to 1,000 μg of protein) (lanes 6 to 10) were immunoprecipitated with the anti-Myc antibody, and the immunoprecipitates were probed with the anti-GSK-3β and anti-β-catenin antibodies (right panel). The small and large arrowheads indicate the positions of endogenous GSK-3β and β-catenin, respectively. (D) Different binding sites of Axil for GSK-3β and β-catenin. After GST–GSK-3β (lanes 1 to 4) and GST–β-catenin (lanes 5 to 8) (8 pmol each) were incubated with MBP-Axil(265-483) (lanes 1 and 5), MBP-Axil(265-412) (lanes 2 and 6), MBP-Axil(412-483) (lanes 3 and 7), or MBP (lanes 4 and 8) (2 pmol each) immobilized on amylose resin, MBPs fused to proteins were precipitated by centrifugation. The precipitates were probed with the anti-GSK-3β and anti-β-catenin antibodies. The small and large arrowheads indicate the positions of GST–GSK-3β and GST–β-catenin, respectively. (E) Phosphorylation of Axil(265-483) by GSK-3β. MBP-Axil(265-483) (200 ng of protein) was incubated with (lane 2) or without (lane 1) GST–GSK-3β (100 ng of protein) for 30 min. The arrow indicates the position of MBP-Axil(265-483). The results shown are representative of three independent experiments.
FIG. 7.
Kinetics for the phosphorylation of Axil(265-483) by GSK-3β. (A) Time course. MBP-Axil(265-483) (200 ng of protein) purified from E. coli was incubated with (•) or without (○) GST–GSK-3β (100 ng of protein) for the indicated periods of time. (B) Dose dependency. The indicated concentrations of MBP-Axil(265-483) were incubated with GST–GSK-3β (100 ng of protein) for 20 min. The results shown are representative of three independent experiments.
Phosphorylation of β-catenin by GSK-3β in the presence of Axil.
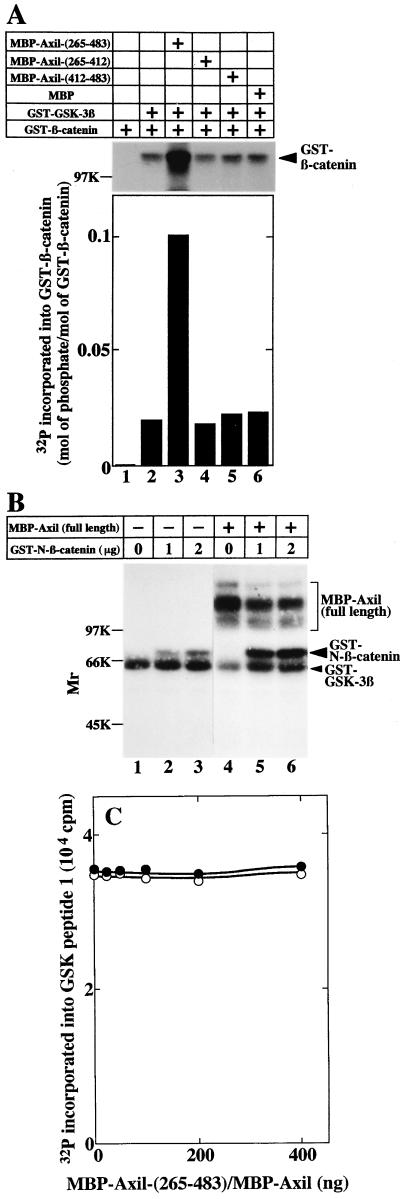
It has been shown that β-catenin has a consensus sequence of the phosphorylation site for GSK-3β and that β-catenin mutants lacking this site are more stable than the wild type (26, 44). Therefore, it is thought that the phosphorylation of β-catenin by GSK-3β regulates the stability of β-catenin. Compared with the phosphorylation of GST–β-catenin by GST–GSK-3β in the absence of MBP-Axil(265-483), MBP-Axil(265-483) increased GST–GSK-3β-dependent phosphorylation of GST–β-catenin fivefold (Fig. 8A). Approximately 0.02 and 0.1 mol of phosphates were incorporated into 1 mol of GST–β-catenin in the absence and presence of MBP-Axil(265-483), respectively. However, neither MBP-Axil(265-412) nor MBP-Axil(412-483) affected the phosphorylation (Fig. 8A). These results demonstrate that Axil promotes GSK-3β-dependent phosphorylation of β-catenin and that the enhancement of GSK-3β-dependent phosphorylation of β-catenin by Axil requires both the GSK-3β- and β-catenin-binding sites of Axil. We also examined the effect of full-length Axil on GSK-3β-dependent phosphorylation of β-catenin. It was difficult to purify MBP-Axil from E. coli, and degradation products were observed on SDS-PAGE (data not shown). Since the Mrs of GST–β-catenin and MBP-Axil were similar, GST–N-terminal β-catenin [GST–β-catenin(1-423)], which contains the phosphorylation site for GSK-3β, was used in this experiment. As expected, MBP-Axil enhanced the phosphorylation of GST–N-terminal β-catenin by GST–GSK-3β (Fig. 8B). Since neither MBP-Axil(265-483) nor MBP-Axil affected the GST–GSK-3β activity to phosphorylate GSK peptide 1 under the condition that Axil promoted GSK-3β-dependent phosphorylation of β-catenin (Fig. 8C), it is unlikely that Axil activates GSK-3β kinase.
FIG. 8.
Phosphorylation of β-catenin by GSK-3β in the presence of Axil. (A) Effect of MBP-Axil(265-483) on GSK-3β-dependent phosphorylation of β-catenin. GST–β-catenin (2 μg of protein) was incubated with GST–GSK-3β (600 ng of protein) in the presence of MBP-Axil(265-483) (lane 3), MBP-Axil(265-412) (lane 4), MBP-Axil(412-483) (lane 5), or MBP (lane 6) (200 ng of protein each) for 30 min. As a control, GST–β-catenin was incubated with (lane 2) or without (lane 1) GST–GSK-3β. (Upper panel) Autoradiography is shown. (Lower panel) The radioactivities incorporated into GST–β-catenin were counted and the stoichiometry of the phosphorylation was calculated. (B) Effect of full-length Axil on GSK-3β-dependent phosphorylation of β-catenin. The indicated amounts of GST–N-terminal β-catenin were incubated with GST–GSK-3β (400 ng of protein) in the presence (lanes 4 to 6) and absence (lanes 1 to 3) of MBP-Axil (160 ng of protein). GST–N-β-catenin, GST–N-terminal β-catenin. (C) Effect of Axil on GSK-3β activity. GST–GSK-3β (400 ng of protein) was incubated with 50 μM GSK peptide 1 in the presence of the indicated amounts of MBP-Axil(265-483) (•) or MBP-Axil (○). The results shown are representative of five independent experiments.
DISCUSSION
In a search for targets of GSK-3β-mediated determination of cell fate, we have identified Axil, an Axin homolog, as a GSK-3β-interacting protein. Axil displays the characteristics expected of a factor regulating axis formation in Xenopus embryos. Injection of Axil induces ventralization, and coinjection with Xwnt8 blocks Xwnt8-induced secondary axis formation. These results indicate that Axil has a function similar to that of Axin. The recent identification of Axin has provided an important insight into the Wnt-induced axis formation (45). Axin induces strong axis defects, a phenotype characteristic of completely ventralized embryos. Dorsal injection of Axin reduces the expression of the dorsal markers, Siamois, Goosecoid, and Chordin. Although Xwnt8, Xdsh, and kinase-negative GSK-3β induce a secondary axis, coinjection of Axin inhibits their activities. However, Axin did not affect secondary axis formation by β-catenin or Siamois. Thus, Axin regulates axis formation in Xenopus embryos by blocking the signaling through the Wnt pathway downstream of GSK-3β and upstream of β-catenin. Although the level at which Axil acts to inhibit the Wnt signaling pathway is not clear from our experiments using Xenopus embryos, the observations that Axil directly interacts with both GSK-3β and β-catenin and enhances GSK-3β-dependent phosphorylation of β-catenin suggest that Axil inhibits the Wnt signaling pathway by mediating the signal from GSK-3β to β-catenin. Taken together with the structural homology, it is possible that Axil and Axin have the same mode of action to regulate axis formation.
We have shown that Axil interacts with GSK-3β in COS cells and that the region containing residues 265 to 483 of Axil is responsible for the interaction. Axil interacts with the wild type but not a catalytically inactive mutant of GSK-3β. These results suggest that the interaction of GSK-3β with Axil requires the kinase activity of GSK-3β. We have demonstrated that Myc-Axil immunoprecipitated from COS cells and MBP-Axil(285-483) purified from E. coli are phosphorylated by GST–GSK-3β. The observations that Axil(265-483) binds to and is phosphorylated by GSK-3β directly indicate that the phosphorylation of Axil by GSK-3β requires their physical interaction.
Our results have shown that Axil interacts not only with GSK-3β but also with β-catenin and that the region containing residues 265 to 483 of Axil is also responsible for its binding to both proteins. In residues 265 to 483 of Axil, residues 265 to 412 and 412 to 483 are responsible for the binding to GSK-3β and β-catenin, respectively. Therefore, it is clear that GSK-3β and β-catenin bind to separate sites on Axil. Taken together with the observation that β-catenin is immunoprecipitated with GSK-3β in the presence of Axil, these results suggest that Axil, GSK-3β, and β-catenin make a ternary complex. However, to prove definitively this possibility, we have to demonstrate that all three components are present in a large complex in a gel filtration experiment. Furthermore, we have found that Axil promotes GSK-3β-dependent phosphorylation of β-catenin. The mechanism may involve the formation of a protein complex in which Axil brings together GSK-3β and β-catenin. Alternatively, Axil increases the affinity between GSK-3β and β-catenin. The stoichiometry of the phosphorylation of β-catenin by GSK-3β is still low even in the presence of Axil. Although we do not know the exact reason for this low stoichiometry, it might be due to the fact that Axil competes with β-catenin for the phosphorylation by GSK-3β since both proteins are substrates or that bacterially expressed proteins are used. It is well known that the phosphorylation of β-catenin is essential for its degradation (22). Therefore, Axil may cause the degradation of β-catenin by interacting with both GSK-3β and β-catenin and by enhancing the phosphorylation of β-catenin. This model is consistent with the observation that Axil negatively regulates the Wnt signaling pathway in development of Xenopus embryos. It has been shown that APC makes a complex with β-catenin and that the phosphorylation of APC by GSK-3β increases the binding of APC to β-catenin (37). Although it is not known whether APC promotes GSK-3β-dependent phosphorylation of β-catenin, APC and Axil may regulate the degradation of β-catenin by different mechanisms. At present, we do not know the physiological significance of the phosphorylation of Axil by GSK-3β for its functional interaction with GSK-3β and β-catenin.
Axil possesses the RGS homologous domain, as does Axin. Recently, a family of RGS proteins has been identified in eukaryotic species ranging from yeast to mammals (7). The RGS domain of this family member binds to the GTP-bound form of Gα and stimulates GTP hydrolysis of Gα. It has been shown that Wnt stimulates the phosphatidylinositol signaling pathway via G protein and that Wg-induced GSK-3 inactivation involves protein kinase C (6, 39). Since the phosphatidylinositol turnover may be important in Wnt-induced degradation of β-catenin, it is intriguing to speculate that the RGS domain of Axil is a functional Gα GAP and thereby inhibits the Wnt signaling pathway. Alternatively, it may have an additional activity to transmit the signal by interacting with another protein(s). It has been shown that ΔRGS, a mutant of Axin in which the RGS domain is deleted, produces a secondary axis and that Axin blocks the axis-inducing activity of ΔRGS (45). These results indicate that ΔRGS acts through a dominant-negative mechanism to inhibit an endogenous Axin activity and that it competes for binding to a protein with which Axin normally interacts. Although we have not yet examined the effects of an RGS domain deletion mutant of Axil on axis formation of Xenopus embryos, our observation that the RGS domain of Axil is distinct from the GSK-3β- and β-catenin-binding sites suggests that the RGS domain deletion mutant of Axil also acts as a dominant negative form in the axis formation. Recently, we have found that the RGS domain of rAxin directly interacts with APC and that rAxin stimulates the degradation of β-catenin (17). Our result that Axil(1-265) containing the RGS domain makes a complex with β-catenin suggests that the RGS domain of Axil also interacts with APC which binds to β-catenin. Therefore, Axil and APC may also cooperatively regulate the stabilization of β-catenin. Besides the RGS domain, Axil has a domain homologous to the N-terminal region of Dsh. Since the function of this region of Dsh is not known, the role of the Dsh homologous domain in the action of Axil remains to be clarified.
In addition to the regulation of development of Xenopus lavies and Drosophila melanogaster, β-catenin plays a role in promoting tumor formation in mammalian cells (32). A potential role for β-catenin in human cancer is supported by the findings that APC deletion mutants in colon cancer fail to downregulate levels of free β-catenin (26, 27). Furthermore, it has been shown that there are mutations of serine in the possible phosphorylation site of β-catenin for GSK-3β in melanoma and colon cancer that have normal APC protein (18, 25, 38). Thus, there are at least two ways by which levels of free β-catenin increase due to mutations in APC and β-catenin itself. Therefore, mutations in GSK-3β- and β-catenin-binding sites on Axil may cause human cancer. Further investigations are necessary to fully elucidate the functions of the Axin family in cellular proliferation and differentiation.
ACKNOWLEDGMENTS
We are grateful to Y. Takai, K. Tanaka, A. Nagafuchi, S. Tsukita, S. Nagata, J. R. Woodgett, Y. Hata, F. Tamanoi, C. W. Turck, Q. Hu, and M. Nakata for donating plasmids, cDNA libraries, GSK peptide 1, and antibodies. We thank the Research Center for Molecular Medicine, Hiroshima University School of Medicine, for the use of their facilities.
This work was supported by a grant-in-aid for scientific research (B) from the Ministry of Education, Science, and Culture, Japan (1996, 1997), and by grants from the Yamanouchi Foundation for Research on Metabolic Disorders (1996, 1997), the Fukuyama Transporting Shibuya Longevity and Health Foundation (1996), the Tsuchiya Foundation (1996), and the Kato Memorial Bioscience Foundation (1997).
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens J, von Kries J P, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 3.Bhanot P, Brink M, Samos C H, Hsieh J C, Wang Y, Macke J P, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Cadigan K M, Nusse R. Wnt Meeting 1996. Biochim Biophys Acta. 1997;1332:R1–R5. doi: 10.1016/s0304-419x(96)00037-6. [DOI] [PubMed] [Google Scholar]
- 6.Cook D, Fry M J, Hughes K, Sumathipala R, Woodgett J R, Dale T C. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 1996;15:4526–536. [PMC free article] [PubMed] [Google Scholar]
- 7.Dohlman H G, Thorner J. RGS proteins and signaling by heterotrimeric G proteins. J Biol Chem. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- 8.Druey K M, Blumer K J, Kang V H, Kehrl J H. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature. 1996;379:742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- 9.Gluecksohn-Schoenheimer S. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J Exp Zool. 1949;110:47–76. doi: 10.1002/jez.1401100105. [DOI] [PubMed] [Google Scholar]
- 10.He X, Saint-Jeannet J P, Woodgett J R, Varmus H E, Dawid I B. Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374:617–622. doi: 10.1038/374617a0. [DOI] [PubMed] [Google Scholar]
- 11.Hinoi T, Kishida S, Koyama S, Ikeda M, Matsuura Y, Kikuchi A. Post-translational modifications of Ras and Ral are important for the action of Ral GDP dissociation stimulator. J Biol Chem. 1996;271:19710–19716. doi: 10.1074/jbc.271.33.19710. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda M, Ishida O, Hinoi T, Kishida S, Kikuchi A. Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral. J Biol Chem. 1998;273:814–821. doi: 10.1074/jbc.273.2.814. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent β-catenin phosphorylation. EMBO J. 1988;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs-Cohen R J, Spiegelman M, Cookingham J C, Bennett D. Knobbly, a new dominant mutation in the mouse that affects embryonic ectoderm organization. Genet Res. 1984;43:43–50. doi: 10.1017/s0016672300025702. [DOI] [PubMed] [Google Scholar]
- 15.Kao K R, Elinson R P. The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus lavies embryos. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi A, Williams L T. Regulation of interaction of ras p21 with RalGDS and Raf-1 by cyclic AMP-dependent protein kinase. J Biol Chem. 1996;271:588–594. doi: 10.1074/jbc.271.1.588. [DOI] [PubMed] [Google Scholar]
- 17.Kishida, S., H. Yamamoto, S. Ikeda, M. Kishida, I. Sakamoto, S. Koyama, and A. Kikuchi. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with APC and regulates the stabilization of β-catenin. J. Biol. Chem., in press. [DOI] [PubMed]
- 18.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 19.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreig P A, Melton D A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 22.Miller J R, Moon R T. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 23.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 25.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 26.Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence stabilizes β-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murai H, Ikeda M, Kishida S, Ishida O, Okazaki-Kishida M, Matsuura Y, Kikuchi A. Characterization of Ral GDP dissociation stimulator-like (RGL) activities to regulate c-fos promoter and the GDP/GTP exchange of Ral. J Biol Chem. 1997;272:10483–10490. doi: 10.1074/jbc.272.16.10483. [DOI] [PubMed] [Google Scholar]
- 29.Murai H, Okazaki M, Kikuchi A. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 1996;392:153–160. doi: 10.1016/0014-5793(96)00806-x. [DOI] [PubMed] [Google Scholar]
- 30.Nusse R. A versatile transcriptional effector of Wingless signaling. Cell. 1997;89:321–323. doi: 10.1016/s0092-8674(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 31.Nusse R, Varmus H E. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 32.Peifer M. β-Catenin as oncogene: the smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- 33.Perry W L, III, Vasicek T J, Lee J J, Rossi J M, Zeng L, Zhang T, Tilghman S M, Costantini F. Phenotypic and molecular analysis of a transgenic insertional allele of the mouse Fused locus. Genetics. 1995;141:321–332. doi: 10.1093/genetics/141.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plyte S E, Hughes K, Nikolakaki E, Pulverer B J, Woodgett J R. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 35.Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–F147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 36.Reed S C. The inheritance and expression of Fused, a new mutation in the house mouse. Genetics. 1937;22:1–13. doi: 10.1093/genetics/22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 38.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 39.Slusarski D C, Corces V G, Moon R T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signaling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 40.Sokol S, Christian J L, Moon R T, Melton D A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- 41.Sussman D J, Klingensmith J, Salinas P, Adams P S, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 42.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q M, Fiol C J, DePaoli-Roach A A, Roach P J. Glycogen synthase kinase-3β is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994;269:14566–14574. [PubMed] [Google Scholar]
- 44.Yost C, Torres M, Miller J R, Huang E, Kimelman D, Moon R T. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 45.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T J, Perry III W L, Lee J J, Tilghman S M, Gumbiner B M, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]