Abstract
Despite its reputation as the most widely used restorative dental material currently, resin-based materials have acknowledged shortcomings. As most systematic survival studies of resin composites and dental adhesives indicate, secondary caries is the foremost reason for resin-based restoration failure and life span reduction. In subjects with high caries risk, the microbial community dominated by acidogenic and acid-tolerant bacteria triggers acid-induced deterioration of the bonding interface and/or bulk material and mineral loss around the restorations. In addition, resin-based materials undergo biodegradation in the oral cavity. As a result, the past decades have seen exponential growth in developing restorative dental materials for antimicrobial applications addressing secondary caries prevention and progression. Currently, the main challenge of bioactive resin development is the identification of efficient and safe anticaries agents that are detrimental free to final material properties and show satisfactory long-term performance and favorable clinical translation. This review centers on the continuous efforts to formulate novel bioactive resins employing 1 or multiple agents to enhance the antibiofilm efficacy or achieve multiple functionalities, such as remineralization and antimicrobial activity antidegradation. We present a comprehensive synthesis of the constraints and challenges encountered in the formulation process, the clinical performance-related prerequisites, the materials’ intended applicability, and the current advancements in clinical implementation. Moreover, we identify crucial vulnerabilities that arise during the development of dental materials, including particle aggregation, alterations in color, susceptibility to hydrolysis, and loss of physicomechanical core properties of the targeted materials.
Keywords: dental caries, composite dental resin, nanotechnology, tooth remineralization, dentin-bonding agents
Introduction
Secondary Caries and Degradative Challenges inside the Mouth
Secondary caries develops in biofilm stagnation areas, such as gingival margins and interdental spaces, where the dental biofilm can develop relatively undisturbed (Paris et al. 2020). Secondary caries lesions at the gingival margin of class II and V composite restorations are twice more prevalent than in other regions. Its higher prevalence is related to increased plaque accumulation near the gingival margin and deficiencies in the adaptation of the restorative material compared to different restoration types (Fig. 1). The restoration replacement was the choice in 70% of cases according to a cross-sectional study (Nedeljkovic et al. 2020).
Figure 1.
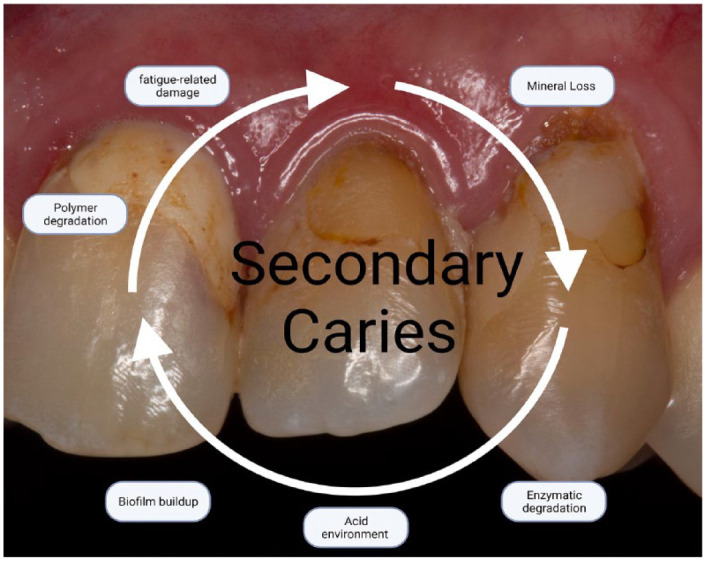
Clinical image of secondary caries around composite restorations and the vulnerability factors associated with secondary caries.
The long-term survival of direct dental restorations depends on a complex connection of influencing factors. The interactions between the restorative material and the substrate are critical, especially in patients with a high caries risk. In these patients, higher sugar intake, higher biofilm buildup, higher counts of mutans streptococci, and lower buffering capacity contribute significantly to the higher risk profiles and establish a challenging oral environment for resin-based restorations (Paris et al. 2020). In addition, eradicating certain key-pathogen biofilms, such as acidogenic bacteria, remains challenging due to the complex mechanisms of the pathogen in disrupting its host’s immune system and the barriers presented by dysbiotic biofilms.
The composite material and the dental bonding interface must resist the oral environment for years. In a harsh cariogenic environment, bacterial acid attacks and other bacterial virulences are pronounced due to the continuous biofilm activity (Astasov-Frauenhoffer and Kulik 2021). The increased and frequent acidification of the environment at the dental bonding interface increases the risk of bacterial microleakage, mineral loss, and polymer degradation (bonding and bulk composite) (Huang et al. 2018). In addition, factors such as a low degree of conversion of the resin, high polymerization shrinkage, poor cavity preparation, and degradation of the hybrid layer can promote marginal microleakage. Furthermore, fatigue-related damage, thermal stress, and enzymatic degradation of the collagen matrix contribute to the degradation of the resin-based material (Orrego et al. 2017). In patients with a high caries risk, the resin-based materials do not offer any endurance to the harsh cariogenic environment and are prone to premature failure. These challenges, together, highlight the demand for alternative and effective preventive strategies.
Practice-based studies have shown exciting data on the long-term performance of resin composites. A previous long follow-up during 29 y revealed a cumulative survival rate of 91.7% at 6 y, 81.6% at 12 y, and 71.4% at 19 y, with an annual failure rate of only 1.92% (Montag et al. 2018). However, the patient’s characteristics and behavior strongly impact the reliable outcomes of dental restorations. A previous meta-analysis indicated that patients with a high risk of caries had an annual failure rate of 4.6% in 10 y, while those with a low risk of caries showed 1.6% in the same period. In other words, the annual failure rate of patients with high caries risk was almost 3 times higher than those with low caries risk (Opdam et al. 2014). However, the success of using preventive measures against dental caries and secondary caries (SC) around resin-based restorations continues to impose significant challenges on oral health. It is the most prevailing reason for failure (Opdam et al. 2014).
Dental Resins—Transition from Bioinert to Bioactive
The term bioactive is a new expression for direct restorative materials, interpreted in many ways by dentists and researchers. Based on the need for materials that could provide benefits to prevent secondary caries, demanding investigations have been carried out into what represents the term bioactive resin materials. In 2022, the FDI issued a Policy Statement on this topic highlighting general characteristics of bioactive materials, such as beneficial/desired effects from dental restorative materials and local effects comprising biological reactions, intentionality, and repair (Schmalz et al. 2023). From a dental restorative perspective, “bioactivity” represents anticaries approaches at the material level, mostly involving modulation of the cariogenic biofilm, prevention of mineral loss, and the development of polymeric resistance to acidic, enzymatic, and bacterial decomposition (Melo et al. 2022).
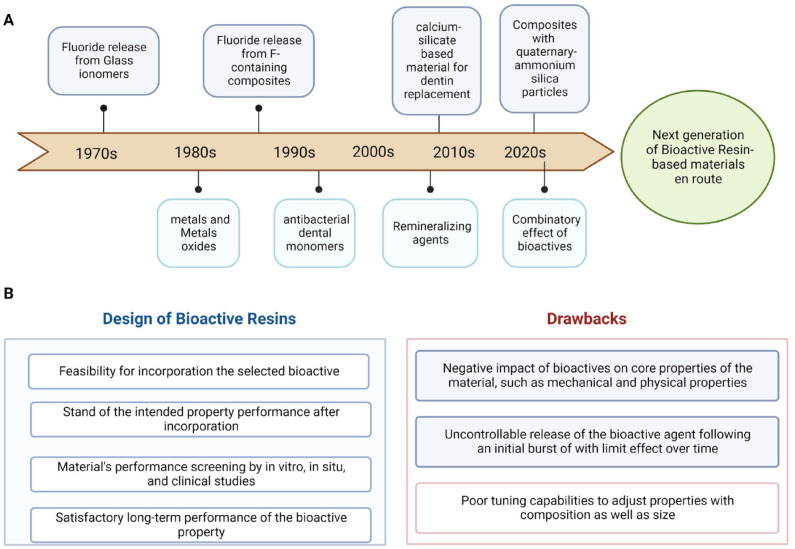
The investigations on bioactive materials in the past 2 decades have provided insightful information for furthering this topic. Such advances have driven and enabled new dental products to reach the market. Different bioactive agents have been incorporated into dental materials to achieve a multifunctional design (Fig. 2A).
Figure 2.
Timeline of bioactive restorative materials. (A) In the upper section, the mileposts in developing dental materials commercialized in the market with claimed bioactivity toward secondary caries prevention. In the lower section, the mileposts of the innovative approaches and investigations for developing new bioactive resin-based materials. (B) Some highlighted steps in the design and faced drawbacks in the development of new resin-based formulations proposed to convey bioactivity to dental resin materials, such as resin composites, adhesives, and luting agents.
Bioactive resins have the potential to boost research in the next few years, eventually enabling the development of restorative materials that can assist in the prevention of secondary caries (Lee, Kim, et al. 2020). First, it is paramount to address the identification of efficient and safe anticaries agents. When incorporated, these agents should not cause any detrimental effect on the materials’ physicochemical properties. Furthermore, they should present a satisfactory long-term performance (Fig. 2B). Another significant bioactive resin assessment shortcoming is the limited remineralization efficacy models. In vitro models, although more controllable (i.e., pH, ions concentration, temperature), broadly differ when compared with the dynamic biological system of the oral cavity in vivo (i.e., saliva–bacteria interactions). In addition, the long-term efficacy of these materials and their effect on the bond strength require further investigation.
We are fully addressing these challenges, and the development of bioactive resins presents some limits. New approaches in this context should aim to understand better the design and tuning of materials’ properties and implement strategies for modifying dysbiotic biofilms. This review focuses on recent developments in resin-based materials that show bioactivity toward caries prevention and mineral loss. We then conclude with a forward-looking perspective about the existing challenges and future directions for designing the next-generation bioactive restorative materials.
Pathways to Impart Bioactivity to Dental Resins
Bioactive Metalic Materials
Zinc oxide (ZnO) (Gutiérrez et al. 2019), titanium dioxide (TiO2) (Stürmer et al. 2021), copper oxide (CuO) (Gutiérrez et al. 2019), iron oxide (Fe2O3), and silver nanoparticles (Balhaddad et al. 2021) can be highlighted among the metal/metal oxide nanostructures most used to develop bioactive dental resins. In addition, several nanostructures have demonstrated inherent broad-spectrum antibacterial properties against Gram-positive and Gram-negative bacteria. Some were also tested to provide activity against matrix metalloproteinases (MMPs) or to confer mineral deposition in the collagen fibers of demineralized dentin (Osorio et al. 2014).
ZnO is the most tested metal oxide for bioactive purposes in dental resins. ZnO stands out because it is biocompatible and radiopaque, inhibits the catalytic activity of MMPs, and is bioactive and antimicrobial. However, although widely used, ZnO presents a reduced antibacterial performance when challenged against mature dental biofilms derived from human saliva. Therefore, the bioactive agent/dental resin needs to be tuned, and a higher concentration of this oxide in the resin is required to obtain a satisfactory biofilm reduction (Garcia et al. 2021). Increasing concentration to reach a substantial antibacterial effect, compromising the flexural strength, modulus of elasticity, and degree of conversion, reduces the potential for clinical translation of these materials.
TiO2 is abundant in nature, is cost-effective, and has demonstrated interesting outcomes in dental materials. The mechanisms responsible for the antibacterial activities of various metal–oxides are attributed to dissolved ions on the surfaces of nanostructures and the formation of free radicals from oxidative stress (Fig. 3A–C). For TiO2 nanostructures, its photocatalytic activity results in the release of hydroxyl radicals (OH−) and the subsequent formation of superoxide radicals (O2−), leading to DNA damage (López de Dicastillo et al. 2020).
Figure 3.
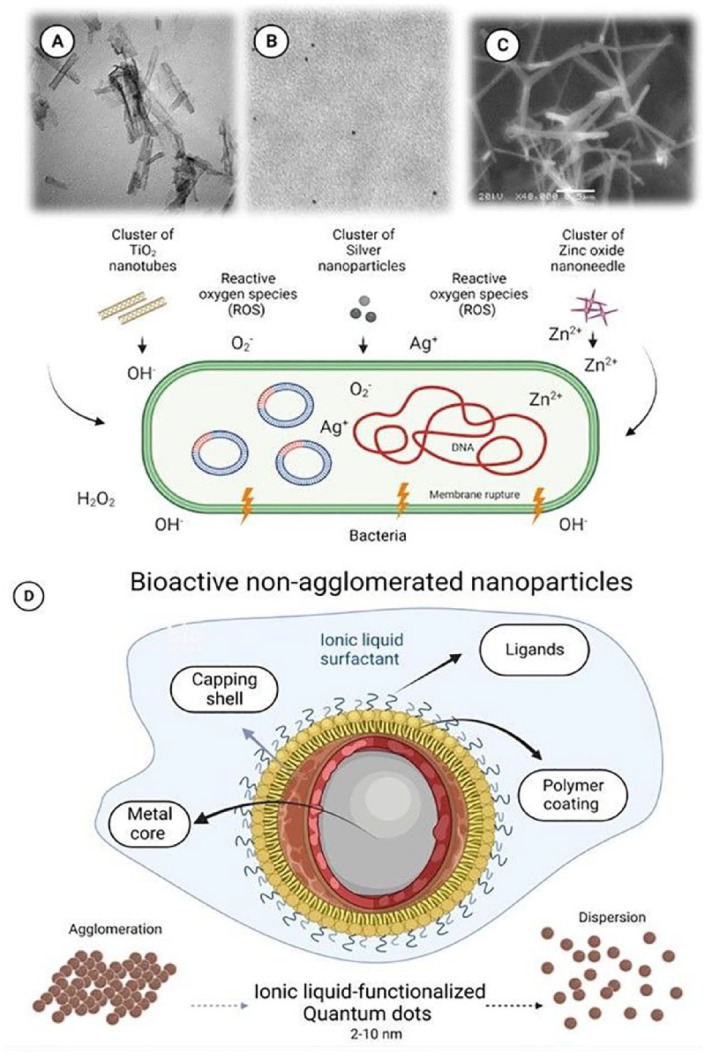
Illustrative transmission electron microscopy images of clusters of nanostructures and their respective mechanisms responsible for the antibacterial activities. (A) Titanium dioxide (TiO2) used as nanotubes (Stürmer et al. 2021), and (B) silver nanoparticles and (C) zinc oxide (ZnO) used as nanoneedles. A variety of metal–oxide nanostructures release metal ions on the surfaces of nanostructures and/or promote nanostructure-induced oxidative stress. (D) Illustration of the structures of ionic liquid–functionalized quantum dots of metal oxides investigated to overcome the drawbacks of agglomeration often presented by several nanostructures.
Noble elements such as the gold/silver/palladium (Au/Ag/Pd) nanostructures are the most promising emerging trend in designing anticaries resin-based materials. Gold is appropriate for several biological purposes (drug delivery, cell tracking, and bioimaging), while platinum has an antioxidant effect due to its strong catalytic activity. In addition, both stand out against MMP due to the ability to chelate Zn2+ in the active sites of the human MMP-8 and MMP-9 (Hashimoto et al. 2015). Silver has also shown great activity against bacteria, including Streptococcus mutans. However, these metallic nanostructures have the disadvantage of changing the resin color, impairing the material’s esthetic properties, and decreasing light availability through the resin in the photoactivation process (Balhaddad et al. 2021). To surpass this issue, core-shell nanoparticles can be an exciting approach. The core still releases the ions from the metal (gold, platinum, and silver), and the shell (silicon oxide) hides the dark color of the metal.
Despite improving dental resins’ physicochemical and biological properties, metal and metal oxide nanostructures have significant shortcomings defying the bioactive materials’ development progress. Nanostructures have a high area/volume ratio, which increases the material’s surface energy and decreases its stability. Due to this reason, nanoparticles tend to agglomerate. The agglomerates lead to lower mechanical properties and jeopardize the homogeneous distribution of bioactive nanostructures through the dentin collagen and hybrid layer (Garcia et al. 2018).
Two strategies have been used in dental materials development to overcome this issue. One relates to the prior functionalization of the nanoparticles with typical surfactants, such as polyethylene glycol. The other approach is to synthesize quantum dots. Quantum dots are inorganic nanoparticles that measure 1 to 10 nm. These “nanocrystals” are usually made from semiconductor materials, and their electrons are confined within the dot in 3-dimensional states, leading to a “quantum confinement effect” (Garcia et al. 2018). These small structures have been used as promising nonagglomerated nanoparticles. They were already used “naked,” as ZnO (Garcia et al. 2018), or functionalized with imidazolium ionic liquids, as TiO2 (Garcia et al. 2019) and tantalum oxide (Ta2O5) (Garcia et al. 2020) (Fig. 3D). In this context, quantum dots of ZnO, tantalum oxide (Ta2O5), and TiO2 were already tested as bioactive nonagglomerated nanoparticles into dental adhesives. The quantum dots of Ta2O5 and TiO2 were kept nonagglomerated due to ionic liquids surfactants surrounding their surface (Garcia et al. 2019, 2020). ZnO quantum dots were probably nonagglomerated because of the cationic charge around them, causing them to be repelled (Garcia et al. 2018).
Bioactive Polymeric Materials
Antibacterial monomers can be directly incorporated into the resin matrix formulation or used to synthesize platforms to carry bioactive agents. The first method is the most prevailing, with several investigations assessing the performance of dental resins containing quaternary ammonium monomers (QAMs) and cross-linked quaternary ammonium polyethyleneimine (QPEI) nanoparticles (Zaltsman et al. 2017). Nevertheless, their direct use or incorporation into dental resins is precluded by chemical instability with increased water sorption/solubility and potential toxicity to human cells, with particular attention to those QAMs lacking cross-linking capabilities released in the oral environment. In addition, other QAMs presenting 1 or 2 methacrylates can covalently bond with other monomers in the resin formulations, decreasing the probability of leaching and losing therapeutic effects over time (Li et al. 2013). For example, 12-methacryloyloxydodecyl pyridinium bromide (MDPB) is the only antimicrobial monomer used in a commercial resin. It is synthesized by combining a quaternary ammonium compound with a methacrylate group (Imazato et al. 1995). Still, the potential of a resin polymer network to copolymerize with QAM is restricted. Recently, Wang, Wu, et al. (2019) have successfully investigated new QAM-containing tetra-functional methacrylate groups where the heterogeneity and cross-link density had increased, and strong antibacterial properties were observed.
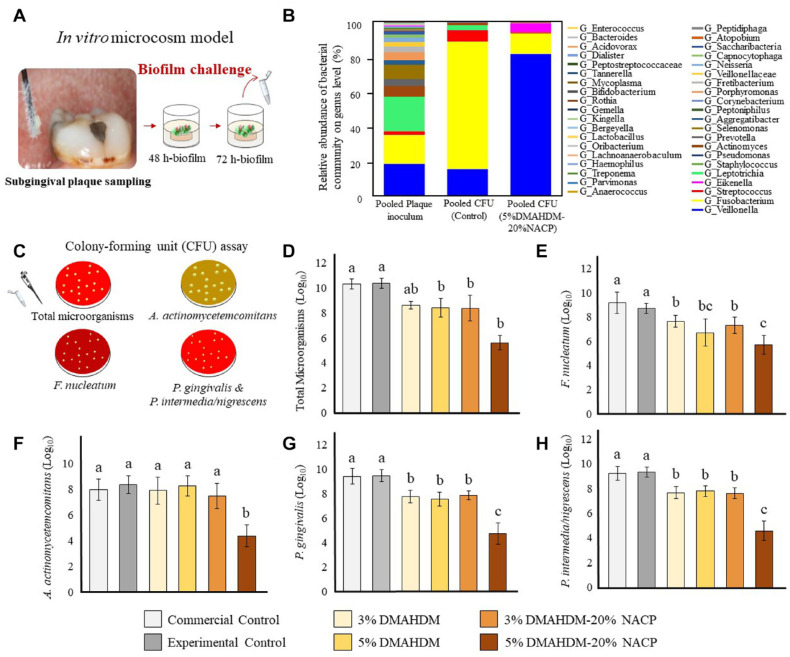
The antibacterial performance of this class of polymers varies according to several impelling factors. To maximize antibacterial effectiveness against pathogenic oral biofilms with minimum undesired cytotoxicity, researchers are trying to identify the significant determinants of QAMs’ design. The effect of the chain length, cationic group, backbone composition, charge density, hydrophobic group, molecular weight (MW) end group, and counterion has been associated with the desired outcome of bacterial reduction (Pham et al. 2023). Dimethylaminohexadecyl methacrylate (DMAHDM) and dimethylaminododecyl methacrylate (DMADDM) have shown robust antibacterial activity against oral pathogenic biofilms derived from dental plaque (Balhaddad et al. 2021) (Fig. 4).
Figure 4.
Microbiological Experiments. (A) The plaque samples isolated from the subgingival area were used to initiate plaque-derived microcosm biofilms in vitro. (B) A color-coded stack barplot graph shows the average bacterial relative abundance on genus level in both groups of pooled plaque inoculum. Pooled plaque inoculum refers to the bacterial composition used to initiate the plaque-derived biofilms. The pooled colony-forming unit (CFU) control refers to the CFU isolates derived from the control composites. The pooled CFU 5% dimethylaminohexadecyl methacrylate (DMAHDM)–20% nanosized amorphous calcium phosphate (NACP) refers to the CFU isolates derived from the biofilm grown over 5% DMAHDM–20% NACP composites. (C) Schematic illustration for the nonselective and selective agar plates used in the CFU assay to count (D) total microorganisms, (E) Fusobacterium nucleatum, (F) Aggregatibacter actinomycetemcomitans, (G) Porphyromonas gingivalis, and (H) Prevotella intermedia/nigrescens. Values indicated by different letters are statistically different (P < 0.05). Reproduced from Balhaddad et al. (2021).
QPEI nanoparticles’ antimicrobial activity depends on the number of quaternary amines in their structure. They have already been tested against many important bacteria for dental caries in adhesives, resin composites, sealers, and cements. During QPEI synthesis, it is recommended to boost the alkylation with alkyl halides of several lengths before the methylation process, improving the degree of amino group substitution (Chrószcz and Barszczewska-Rybarek 2020).
Moreover, polymers with therapeutic properties can be used to produce nanocapsules and microspheres that can contain agents in their core (Genari et al. 2018) or on their polymeric walls (microspheres) (Dornelles et al. 2018). For this intent, nanospheres or microspheres do not contain oil in the composition, and a polymer forms the core. The loaded drug by spheres will be retained in the polymer, adsorbed, or dispersed. Some examples of antimicrobial agents already incorporated into these polymeric particles are triclosan (Genari et al. 2018) and chlorhexidine, which is widely used as a broad-spectrum antibacterial agent.
Bioactive Mineralizing Materials
Bioactive resins have the potential to carry bioactive agents capable of releasing critical ions that aid in restoring the chemical imbalance that leads to tooth mineral loss. The main goal of bioactive resins with remineralizing properties is to replenish the eroded minerals from dental tissues and neutralize acids and low pH levels of microenvironments (Lee et al. 2019; Lee, Seo, et al. 2020). In addition, this approach aims to prevent the bonded interface’s degradation and extend the restorations’ durability (Huang 2011). The most common strategies to impart bioactivity to dental resins include the addition of bioactive glasses (Tezvergil-Mutluay et al. 2017) and polyanionic polymers (Kepa et al. 2015).
Mineralizing nanofillers can release calcium (Ca2+) and phosphate (PO43−) ions when in contact with saliva. These ions can diffuse into the subsurface of the enamel lesions or at the margins of dental restorations. Due to their chemical similarity to the inorganic component of teeth and bone, calcium phosphate (CaP) nanofillers are added to dental resins in different phases, including hydroxyapatite (HAp), amorphous calcium phosphate (ACP), and mono-, di-, and tetracalcium phosphates (Dorozhkin 2013). For example, Yu et al. (2021) fabricated a dental adhesive incorporated with nanoparticles of ACP to evaluate its remineralization effect. The dental adhesive filled with ACP inhibited dentin demineralization, evidenced by sustained dentin hardness and decreased lactic acid production after a 10-d biofilm challenge compared to the adhesive without nanofillers.
Among leaching compounds, fluoride is the principal remineralizing agent (Kim et al. 2021). Fluoride stabilizes the tooth’s cyclic demineralization and remineralization processes. Fluoride ions (F−), released from the resins, replace OH ions in the tooth HAp, forming fluorapatite or fluorhydroxyapatite. These minerals are more resistant to demineralization (Fernández et al. 2016). Mitwalli et al. (2020) fabricated a dental fluoride–releasing composite containing DMAHDM, 2 methacryloyloxyethyl phosphorylcholine (MPC), and nanoparticles of calcium fluoride (nCaF2) for extended longevity. The nCaF2 + DMAHDM + MPC composite had the lowest biofilm colony-forming units (CFU) and the greatest ion release; however, its mechanical properties were lower than the commercial control composite. However, when only CaF2 + DMAHDM were incorporated into the composite, the biofilm reduction was similar to nCaF2 + DMAHDM + MPC, with mechanical properties matching commercial control composite (Mitwalli et al. 2020).
Fluoride-releasing dental materials have also demonstrated antibacterial effects in preventing secondary caries (Wiegand et al. 2007). Evolution and new formulations of these materials have allowed the development of ion-releasing resins with pleasing aesthetics, stability, mechanical properties, and bond strength to the dental tissue that are currently used in the clinic (e.g., Activa BioActive Restorative, Cention N, Fuji IX, Z250, among others). However, a significant limitation of ion-releasing bioactive resins is the loss of mechanical stability, short-term efficacy (<2 y) of the remineralization properties due to the quick depletion of the ions, and the difficulty of recharging (Ionescu et al. 2022).
Polyanionic polymers are biomaterials that hold negatively charged ions. These materials can be grafted into dental resins to mimic noncollagenous protein (NCP)–crystal interactions during mineralization (Liang et al. 2019). Examples of these biomaterials include elastin-like polypeptides, poly(allylamine hydrochloride), poly(L-aspartic acid), and poly(amidoamine) (PAMAM). Polyanionic polymers can suppress bulk mineral crystallization, stabilize the amorphous phase of ACP, and achieve intrafibrillar mineralization (Liu et al. 2013). For example, the polymer-induced liquid precursor (PILP) process uses poly(L-aspartic acid) to assist in the achievement of mineralizing elements into collagen fibrils after ACP deposition to create apatite (Chien et al. 2017). Using polyanionic polymers in combination with amorphous nanoparticles of ACP, a remineralization synergy is achieved by increased nucleation, acid neutralization capabilities, and higher ionic release (i.e., Ca and P ions) (Liang et al. 2019). Significant disadvantages of these remineralizing materials are the low mechanical properties of the mineral formed compared to natural tissue and the inability to fully re-create the complexity of the mineral–organic interactions and hierarchy of tissues like dentin (Liu et al. 2013).
Bioactive glasses (BGs) are highly reactive surfaces formed by melt or sol–gel techniques (Boyan et al. 2017). When in contact with physiological fluids (i.e., saliva), BGs trigger ion–exchange reactions on their surface, resulting in the formation of a layer of carbonated HAp that favors its biocompatibility and its integration into surrounding bone and hard dental tissues (Mocquot et al. 2020). For dentin and enamel remineralization, BGs of different sizes (micro/nano) and compositions (conventional silicates, phosphate based, borate based, alkali free, sodium free, and phosphate free) have been incorporated into dental resins (Mai et al. 2022). Despite their excellent bioactive properties, the clinical applications of BGs are mainly related to no-load-bearing applications due to low mechanical strength (i.e., flexural strength) compared to other bioactive materials (Kaur et al. 2019).
Multimodal or Combinatory: Multifunctional Bioactive Materials
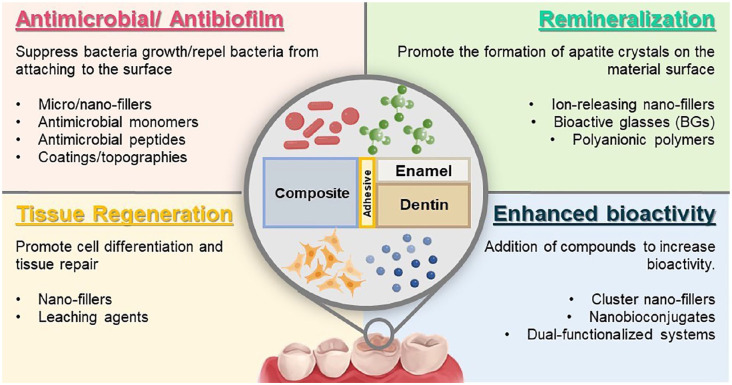
Numerous innovative approaches have emerged as the skill to engineer multifunctional dental materials continually advances. In this scenario, multimodal or multifunctional biomaterials offer different bioactive therapies integrated into 1 material system (Montoya et al. 2023). A typical example is a dental resin that combines tissue regeneration and mineralization effects (Wang, Xia, et al. 2019). A dental resin was designed containing metformin (a bioactive agent). This multimodal system stimulated odontogenic differentiation and promoted mineral synthesis of dental pulp stem cells to treat caries and severe dental injury (Wang, Xia, et al. 2019). Standard multifunctional therapies include antimicrobial/remineralization, antimicrobial/tissue regeneration, and enhanced bioactivity (Fig. 5).
Figure 5.
Schematic illustration of multimodal or combinatory bioactive materials. These materials can present multiple properties, such as antibacterial, remineralization, and tissue regeneration. The combinatorial approach can also be used to enhance the cited properties.
The primary strategy for developing multifunctional dental materials combines 2 or more bioactive agents that usually act independently. Another approach is to add different antimicrobial agents to enhance and extend the range of coverage of multiple microbes to improve efficacy. For example, adding silver nanoparticles and ciprofloxacin into a dental resin enhanced the antimicrobial response against microbial strains, including Enterococcus faecalis, S. mutans, and saliva microcosm (Arif et al. 2022). In other studies, the augmentation of the antimicrobial effects of dental resins was achieved by the addition of MPC (i.e., a protein-repellent monomer that reduces bacterial adhesion) and quaternary ammonium DMAHDM (i.e., contact killing agent) into dental resins (Mitwalli et al. 2020). This multiple-agent approach’s limitations are the increased complexity of the formulations, the limited tunability of the biomaterial properties for clinical applications, increased cost, and potential collateral damages.
A more sophisticated strategy to enable multifunctional dental materials is using a single agent that offers combined therapies. For example, recent work showed that incorporating piezoelectric nanoparticles into dental resins resulted in a biomaterial offering both antimicrobial and remineralization effects (Montoya et al. 2021). Furthermore, activation of the piezoelectric charges by an external force (mastication) triggered a reduction in biofilm growth (up to 90%) (Montoya et al. 2021).
In addition, several metal/metal oxide nanofillers used as antimicrobial agents may have a bioactive effect regarding possible dental tissue remineralization. For example, the high concentration of hydroxyl groups (OH−) on ZnO surfaces is the main reason for the initial calcium and phosphate ions deposition on ZnO’s surface. This result was observed on the surface of ZnO-doped adhesives, which favored a mineral formation on dental surfaces (Osorio et al. 2014). Other oxides such as TiO2 (Kokubo and Takadama 2006), niobium pentoxide (Nb2O5) (Leitune et al. 2013), and zirconium oxide (ZrO2) (Provenzi et al. 2018) also have the excess of OH− on their surface necessary for ion deposition and mineral formation. When exposed to simulated body fluid, these oxides can induce mineral deposition on adhesive surfaces. However, most of these particles were also not tested for true biomimetic remineralization, evidencing a critical subject to be addressed in future investigations. In biomimetic remineralization, these oxides could induce the formation of mineral deposits along the dental tubules and increase minerals on specific sites of demineralized collagen fibers, assisting in the recovery of affected dentin after caries removal.
Another strategy to create antimicrobial/remineralizing systems is tethering different bioactive peptides into dental resins. For example, Yuca et al. (2021) fabricated peptide–polymer conjugates composed of a dental adhesive, an antimicrobial peptide (AMPM7), and a HAp-binding peptide (HABP). Evaluation of the adhesive system demonstrated both potent metabolic inhibition of S. mutans and localized calcium phosphate remineralization (Yuca et al. 2021). However, the bond strength’s long-term efficacy and clinical durability require further studies. Table summarizes of Bioactive Agents Commonly Incorporated into Dental Resins and Their Corresponding Features.
Table.
Summary of Bioactive Agents Commonly Incorporated into Dental Resins and Their Corresponding Features.
| Incorporated agent | Parent Material | Main Findings on Physical and Chemical Properties | Main Results on Biological Properties | Author, Year |
|---|---|---|---|---|
| 2-methacryloyloxyethyl phosphorylcholine (MPC) | Surface prereacted glass-ionomer (SPRG)–filled resin composite | The addition of MPC decreased the flexural strength and wettability properties. The addition of MPC decreased the release of ions. |
The addition of MPC improved acid-neutralizing effects. The addition of MPC reduced the adsorbed bovine serum albumin and proteins. Adding MPC reduced the attachment of Streptococcus mutans, Actinomyces naeslundii, Veillonella parvula, and Porphyromonas gingivalis. |
(Lee et al. 2019) |
| Amoxicillin-loaded microspheres | Endodontic resin sealers | The microsphere addition did not jeopardize the physical and chemical properties of the sealer. | The microspheres have reduced the viability of Enterococcus faecalis. | (Dornelles et al. 2018) |
| Amphiphilic peptoids | Pretreatment of carious dentin | — | A class of peptide-like polymers that enhanced the ordering and mineralization of collagen and induced functional remineralization of dentin lesions. | (Chien et al. 2017) |
| Bioactive glass and MPC | Resin-based sealant | Adding bioactive glass and MPC increased the wettability, water sorption, solubility, viscosity, and release of multiple ions. The addition of bioactive glass and MPC did not change the flexural strength. |
The addition of bioactive glass and MPC reduced protein and bacteria adhesion and suppressed multispecies biofilm attachment. | (Lee, Kim, et al. 2020) |
| Ciprofloxacin-loaded silver nanoparticles (CIP-AgNPs) | Resin composite | The CIP-AgNP group has higher compressive strength than the control groups (unmodified resin composites and AgNP-resin composites). | The CIP-AgNP group showed higher antibacterial activity compared to the control groups. The CIP-AgNP group showed lower cytotoxicity compared to AgNP-resin composites. |
(Arif et al. 2022) |
| Dimethylaminohexadecyl methacrylate (DMAHDM) and nanosized amorphous calcium phosphate (NACP) | Resin composite | DMAHDM and NACP addition decreased the surface-free energy of the resin composites. | DMAHDM and NACP addition provided antibacterial activity against bacteria from the subgingival margin. | (Balhaddad et al. 2021) |
| DMAHDM, MPC, and nanoparticles of calcium fluoride (nCaF2) | Resin composite | The addition of nCaF2 + DMAHDM + MPC decreased the flexural strength and elastic modulus. The addition of nCaF2 + DMAHDM did not change the flexural strength and elastic modulus. |
The addition of nCaF2 + DMAHDM + MPC decreased the biofilm formation. The addition of nCaF2 + DMAHDM + MPC improved the ion release. The addition of nCaF2 + DMAHDM decreased biofilm formation compared to the control group. |
(Mitwalli et al. 2020) |
| Indomethacin- and triclosan-loaded nanocapsules (NCs) | Primer Adhesive resin |
The higher the concentration of NCs, the lower the microtensile bond strength after aging. Adding up to 5 wt.% of NCs in the adhesive and no addition in the primer showed the best physical and chemical properties. |
These NCs had shown substantial antibacterial and anti-inflammatory effects. | (Genari et al. 2018) |
| Micrometer-sized particles of Bioglass 45S5 (BAG) or fluoride-containing phosphate-rich bioactive glass (BAG-F) | Adhesive resin | — | The addition of BAG-F showed the most significant remineralizing effect on the stiffness of the completely demineralized dentin. BAG-F showed a better ability to decrease enzyme activity compared to BAG particles. |
(Tezvergil-Mutluay et al. 2011) |
| NACP | Adhesive resin | — | Adding NACP in the adhesive neutralized the acids, increased the pH to above 5, and released large amounts of calcium and phosphorus ions. Adding NACP in the adhesive decreased lactic acid production, inhibited dentin demineralization, and sustained the dentin hardness in the S. mutans biofilm-challenged environment. |
(Yu et al. 2021) |
| Nanostructured zirconium dioxide (ZrO2) | Adhesive resin | The higher the ZrO2 content, the higher the softening after immersion in a solvent. The addition of ZrO2 did not change the microtensile bond strength. Adding a small concentration (1%) increased the degree of conversion. |
ZrO2 promoted mineral deposition. | (Provenzi et al. 2018) |
| Nano-zinc oxide (ZnONP) | Adhesive resin | Up to 7.5 wt.%, the adhesives’ properties were still according to ISO recommendations despite this concentration changing the physicochemical performance of the adhesives. | Adding ZnONP at a high concentration (7.5 wt.%) reduced the viability of bacteria in microcosms derived from saliva. | (Garcia et al. 2021) |
| Peptides | Adhesive resin | The peptide addition did not change the adhesive resin’s conversion degree. | The addition of some specific peptides induced a “peptide-mediated remineralization process.” The peptide’s addition provided an antibacterial activity to the adhesive. |
(Yuca et al. 2021) |
| Piezoelectric nanoparticles of barium titanate (BaTiO3) | Resin composite | Adding BaTiO3 did not change the microtensile bond strength in simultaneous attacks from bacteria and cyclic mechanical loading operating in synergy. | The addition of BaTiO3 provided antibacterial activity and mineral deposition. | (Montoya et al. 2021) |
| Quantum dots of tantalum oxide with an imidazolium ionic liquid (Ta2O5QDS) | Adhesive resin | The addition of Ta2O5QDS maintained the degree of conversion of the adhesive resin. | Ta2O5QDS provided substantial antibacterial activity against S. mutans biofilms. | (Garcia et al. 2020) |
| Quaternary ammonium methacrylates (QAMs) with various alkyl chain lengths (CLs) | Adhesive resin | Adding the QAMs with different CLs did not affect the microtensile bond strength. | The higher the CL, the higher the antibacterial activity. The adhesives with QAMs did not affect the viability of human gingival fibroblasts or odontoblast-like MDPC-23 mouse cells. |
(Li et al. 2013) |
| Quaternary ammonium polyethyleneimine (QPEI) nanoparticles | Resin composite | The addition of QPEI nanoparticles did not affect the degree of conversion and shear bond strength. | The addition of QPEI nanoparticles did not change the viability of mammalian cells. The addition of QPEI nanoparticles provided an antibacterial activity to the resins. |
(Zaltsman et al. 2017) |
| Tetrafunctional methacrylate quaternary ammonium salt monomer (TMQA) | Resin composite | The composites with TMQA showed a similar degree of conversion and contact angle compared to the control. The addition of TMQA reduced the water sorption and solubility and increased the crosslink density. |
The addition of TMQA provided antibacterial activity against S. mutans. | (Wang et al. 2019) |
| Titanium dioxide nanotubes (nt-TiO2) or titanium dioxide nanotubes with triazine-methacrylate monomer (nt-TiO2:TAT) | Adhesive resin | The addition of nanotubes decreased the softening after immersion in a solvent. The addition of nanotubes increased the Knoop hardness. The groups with nanotubes showed higher microtensile bond strength after aging compared to the control group. |
The addition of the nanotubes maintained high cell viability. The addition of nt-TiO2:TAT displays antibacterial activity. |
(Stürmer et al. 2021) |
| Zinc oxide and copper nanoparticles (ZnO/CuNP) | Adhesive resin | The addition of ZnO/CuNP decreased the nanoleakage. Adding ZnO/CuNP maintained the ultimate tensile strength, degree of conversion, and microtensile bond strength or improved these properties depending on the adhesive added. |
ZnO/CuNP demonstrated anti–matrix metalloproteinase activity. The addition of ZnO/CuNP provided antibacterial activity against S. mutans strains. |
(Gutiérrez et al. 2019) |
| Zinc oxide quantum dots (ZnOQDs) | Adhesive resin | ZnOQDs were tested for physical and chemical properties in the previous study published on this subject. | ZnOQDs provided antibacterial activity against S. mutans, with no change in biocompatibility. | (Garcia et al. 2018) |
| Zinc-doped phosphate-based glass (Zn-PBG) | Flowable resin composite | The flexural strength of the control was significantly higher than those of Zn-PBG samples. The higher the Zn-PBG content, the lower the microhardness. |
The higher the Zn-PBG content, the higher the release of phosphorus, calcium, sodium, and zinc ions. The higher the Zn-PBG content, the higher the antibacterial activity against S. mutans. |
(Lee, Seo, et al. 2020) |
| Zinc oxide (ZnO) or zinc chloride (ZnCl2) | Adhesive resin | — | ZnO-doped adhesives induced calcium and phosphorus deposition. ZnCl2-doped adhesives induced mineral deposition in a more amorphous phase than ZnO-doped adhesives. |
(Osorio et al. 2014) |
The agents and their features are listed in alphabetical order for easy reference. Antibacterial methacrylate molecules, remineralization bioglasses, and dual-function oxides are the most frequently studied additives.
The appropriate evaluation of these multifunctional dental materials, both in vitro and in vivo, is challenged by the replication of the complexity of the oral environment. Potential synergistic effects are overlooked when the multifunctional material operates in complex microenvironments. As limitations, the negative impact on the core properties of materials can be challenging to quantify, particularly when testing their performance in vitro. When exposed to certain conditions, bioactive resins can experience changes in their physical and chemical properties, affecting their ability to perform their intended function. Furthermore, the bioactive effects of biomaterials can occur over a wide range of time frames, from minutes to months or even years. This can make it difficult to evaluate the performance of biomaterials under simultaneous in vitro conditions, as the time frames required to observe their effects may be much longer than the duration of a typical in vitro study.
Moreover, the deficiency of standardized models limits comparing outcomes among studies (Kreth et al. 2019). Future efforts could be directed toward developing organs-on-a-chip technologies that overcome the challenges of current testing methods (França et al. 2020). Tooth-on-a-chip systems highly mimic tissue functionalities while allowing the control of external parameters that are difficult to study in vivo (França et al. 2020). Finally, a topic that should be further explored is the possible microbial resistance to restorative materials with antimicrobial agents. Few studies have evaluated this topic in vitro. Generally, the analysis uses chlorhexidine (an agent known to induce microbial resistance) as one of the control groups (Wang et al. 2018). In these analyses, microorganisms face a high dose of contact with the antimicrobial agent, being tested with a probable low relationship with in vivo behaviors. In addition, it is worth remembering that the concentration of antimicrobial agents added to the materials is small to maintain good physicochemical properties and modify only the microenvironment around the polymer. Therefore, attention should be paid to whether we will have problems with microbial resistance when using restorative materials with antimicrobials and how we can test these materials so that the results are closer to the oral environment.
Summary
This critical review has provided comprehensive insight into developing bioactive resins for dental restorations—from target clinical problem, concept, and formulation designs to the most recent advances to progress the clinical translation of these materials. Optimizing the use of metal oxides and antibacterial polymers with strategies that minimize the ongoing drawbacks coupled with high-quality preclinical assessments could address the ongoing challenge of the recurrence of carious lesions around resin composite restorations and significantly impact the clinical translation of these materials.
Author Contributions
M.A.S. Melo, I.M. Garcia, L. Mokeem, M.D. Weir, H.H.K. Xu, C. Montoya, S. Orrego, substantially contributed to conception or design, data acquisition, analysis, or interpretation of data, drafted and critically revised the manuscript. All authors gave their final approval and agreed to be accountable for all aspects of the work.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute of Dental and Craniofacial Research (NIDCR) Awards R21DE030564 (PI: S. Orrego) and R03DE030562-01A1 (PI: M.A.S. Melo/S. Orrego). This work was partly supported by the Temple University Maurice Kornberg School of Dentistry start-up fund by the Office of Provost at Temple University strategic fund and the University of Maryland School of Dentistry departmental funds (M.A.S. Melo).
ORCID iDs: M.A.S. Melo  https://orcid.org/0000-0002-0007-2966
https://orcid.org/0000-0002-0007-2966
L. Mokeem  https://orcid.org/0000-0003-1071-1925
https://orcid.org/0000-0003-1071-1925
S. Orrego  https://orcid.org/0000-0003-3683-6750
https://orcid.org/0000-0003-3683-6750
Data Availability: The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
- Arif W, Rana NF, Saleem I, Tanweer T, Khan MJ, Alshareef SA, Sheikh HM, Alaryani FS, Al-Kattan MO, Alatawi HA, et al. 2022. Antibacterial activity of dental composite with ciprofloxacin loaded silver nanoparticles. Molecules. 27(21):7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astasov-Frauenhoffer M, Kulik EM. 2021. Cariogenic biofilms and caries from birth to old age. Monogr Oral Sci. 29:53–64. [DOI] [PubMed] [Google Scholar]
- Balhaddad AA, Garcia IM, Mokeem L, Alsahafi R, Collares FM, Sampaio de Melo MA. 2021. Metal oxide nanoparticles and nanotubes: ultrasmall nanostructures to engineer antibacterial and improved dental adhesives and composites. Bioengineering (Basel). 8(10):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhaddad AA, Garcia IM, Mokeem L, Ibrahim MS, Collares FM, Weir MD, Xu HHK, Melo MAS. 2021. Bifunctional composites for biofilms modulation on cervical restorations. J Dent Res. 100(10):1063–1071. [DOI] [PubMed] [Google Scholar]
- Boyan BD, Cohen DJ, Schwartz Z. 2017. 7.17 Bone tissue grafting and tissue engineering concepts. In: Ducheyne P, editor. Comprehensive biomaterials II. Oxford: Elsevier. p. 298–313. [Google Scholar]
- Chien Y-C, Tao J, Saeki K, Chin AF, Lau JL, Chen C-L, Zuckermann RN, Marshall SJ, Marshall GW, De Yoreo JJ. 2017. Using biomimetic polymers in place of non-collagenous proteins to achieve functional remineralization of dentin tissues. ACS Biomater Sci Eng. 3(12):3469–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrószcz M, Barszczewska-Rybarek I. 2020. Nanoparticles of quaternary ammonium polyethylenimine derivatives for application in dental materials. Polymers (Basel). 12(11):2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornelles NB, Collares FM, Genari B, de Souza Balbinot G, Samuel SMW, Arthur RA, Visioli F, Guterres SS, Leitune VCB. 2018. Influence of the addition of microsphere load amoxicillin in the physical, chemical and biological properties of an experimental endodontic sealer. J Dent. 68:28–33. [DOI] [PubMed] [Google Scholar]
- Dorozhkin SV. 2013. Calcium orthophosphates in dentistry. J Mater Sci Mater Med. 24(6):1335–1363. [DOI] [PubMed] [Google Scholar]
- Fernández CE, Tenuta LMA, Cury JA. 2016. Validation of a cariogenic biofilm model to evaluate the effect of fluoride on enamel and root dentine demineralization. PLoS One. 11(1):e0146478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, Ferracane JL, Bertassoni LE. 2020. The tooth on-a-chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip. 20(2):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia IM, Balhaddad AA, Ibrahim MS, Weir MD, Xu HHK, Collares FM, Melo MAS. 2021. Antibacterial response of oral microcosm biofilm to nano-zinc oxide in adhesive resin. Dent Mater. 37(3):e182–e193. [DOI] [PubMed] [Google Scholar]
- Garcia IM, Leitune VCB, Visioli F, Samuel SMW, Collares FM. 2018. Influence of zinc oxide quantum dots in the antibacterial activity and cytotoxicity of an experimental adhesive resin. J Dent. 73:57–60. [DOI] [PubMed] [Google Scholar]
- Garcia IM, Souza VS, Hellriegel C, Scholten JD, Collares FM. 2019. Ionic liquid-stabilized titania quantum dots applied in adhesive resin. J Dent Res. 98(6):682–688. [DOI] [PubMed] [Google Scholar]
- Garcia IM, Souza VS, Scholten JD, Collares FM. 2020. Quantum dots of tantalum oxide with an imidazolium ionic liquid as antibacterial agent for adhesive resin. J Adhes Dent. 22(2):207–214. [DOI] [PubMed] [Google Scholar]
- Genari B, Leitune VCB, Jornada DS, Aldrigui BR, Pohlmann AR, Guterres SS, Samuel SMW, Collares FM. 2018. Effect on adhesion of a nanocapsules-loaded adhesive system. Braz Oral Res. 32:e008. [DOI] [PubMed] [Google Scholar]
- Gutiérrez MF, Bermudez J, Dávila-Sánchez A, Alegría-Acevedo LF, Méndez-Bauer L, Hernández M, Astorga J, Reis A, Loguercio AD, Farago PV, et al. 2019. Zinc oxide and copper nanoparticles addition in universal adhesive systems improve interface stability on caries-affected dentin. J Mech Behav Biomed Mater. 100:103366. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sasaki JI, Yamaguchi S, Kawai K, Kawakami H, Iwasaki Y, Imazato S. 2015. Gold nanoparticles inhibit matrix metalloproteases without cytotoxicity. J Dent Res. 94(8):1085–1091. [DOI] [PubMed] [Google Scholar]
- Huang B, Sadeghinejad L, Adebayo OIA, Ma D, Xiao Y, Siqueira WL, Cvitkovitch DG, Finer Y. 2018. Gene expression and protein synthesis of esterase from Streptococcus mutans are affected by biodegradation by-product from methacrylate resin composites and adhesives. Acta Biomater. 81:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GTJ. 2011. Dental pulp and dentin tissue engineering and regeneration–advancement and challenge. Front Biosci. 3:788–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazato S, Russell RRB, McCabe JF. 1995. Antibacterial activity of MDPB polymer incorporated in dental resin. J Dent. 23(3):177–181. [DOI] [PubMed] [Google Scholar]
- Ionescu AC, Degli Esposti L, Iafisco M, Brambilla E. 2022. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci Rep. 12(1):5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Kumar V, Baino F, Mauro JC, Pickrell G, Evans I, Bretcanu O. 2019. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: state-of-the-art review and future challenges. Mater Sci Eng C Mater Biol Appl. 104:109895. [DOI] [PubMed] [Google Scholar]
- Kepa K, Coleman R, Grøndahl L. 2015. In vitro mineralization of functional polymers. Biosurf Biotribol. 1(3):214–227. [Google Scholar]
- Kim M-J, Lee M-J, Kim K-M, Yang S-Y, Seo J-Y, Choi S-H, Kwon J-S. 2021. Enamel demineralization resistance and remineralization by various fluoride-releasing dental restorative materials. Materials (Basel). 14(16):4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo T, Takadama H. 2006. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 27(15):2907–2915. [DOI] [PubMed] [Google Scholar]
- Kreth J, Ferracane JL, Pfeifer CS, Khajotia S, Merritt J. 2019. At the interface of materials and microbiology: a call for the development of standardized approaches to assay biomaterial-biofilm interactions. J Dent Res. 98(8):850–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-J, Kim J-Y, Seo J-Y, Mangal U, Cha J-Y, Kwon J-S, Choi S-H. 2020. Resin-based sealant with bioactive glass and zwitterionic material for remineralisation and multi-species biofilm inhibition. Nanomaterials (Basel). 10(8):1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-J, Kwon J-S, Kim J-Y, Ryu J-H, Seo J-Y, Jang S, Kim K-M, Hwang C-J, Choi S-H. 2019. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent Mater. 35(9):1331–1341. [DOI] [PubMed] [Google Scholar]
- Lee M-J, Seo Y-B, Seo J-Y, Ryu J-H, Ahn H-J, Kim K-M, Kwon J-S, Choi S-H. 2020. Development of a bioactive flowable resin composite containing a zinc-doped phosphate-based glass. Nanomaterials (Basel). 10(11):2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitune VCB, Collares FM, Takimi A, de Lima GB, Petzhold CL, Bergmann CP, Samuel SMW. 2013. Niobium pentoxide as a novel filler for dental adhesive resin. J Dent. 41(2):106–113. [DOI] [PubMed] [Google Scholar]
- Li F, Weir MD, Xu HHK. 2013. Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res. 92(10):932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Wang S, Tao S, Xiao S, Zhou H, Wang P, Cheng L, Zhou X, Weir MD, Oates TW. 2019. Dental remineralization via poly (amido amine) and restorative materials containing calcium phosphate nanoparticles. Int J Oral Sci. 11(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Luo D, Kou X-X, Wang X-D, Tay FR, Sha Y-L, Gan Y-H, Zhou Y-H. 2013. Hierarchical intrafibrillar nanocarbonated apatite assembly improves the nanomechanics and cytocompatibility of mineralized collagen. Adv Funct Mater. 23(11):1404–1411. [Google Scholar]
- López de Dicastillo C, Guerrero Correa M, Martínez FB, Streitt C, Galotto MJ. 2020. Ch. 5. Antimicrobial effect of titanium dioxide nanoparticles. In: Mareș M, Lim SHE, Lai K-S, Cristina R-T, editors. Antimicrobial resistance. Rijeka: IntechOpen. doi: 10.5772/intechopen.90891 [DOI] [Google Scholar]
- Mai S, Zhang Q, Liao M, Ma X, Zhong Y. 2022. Recent advances in direct adhesive restoration resin-based dental materials with remineralizing agents. Front Dent Med. 3. doi: 10.3389/fdmed.2022.868651 [DOI] [Google Scholar]
- Melo MAS, Mokeem L, Sun J. 2022. Bioactive restorative dental materials-the new frontier. Dent Clin North Am. 66(4):551–566. [DOI] [PubMed] [Google Scholar]
- Mitwalli H, Balhaddad AA, AlSahafi R, Oates TW, Melo MAS, Xu HHK, Weir MD. 2020. Novel CaF2 nanocomposites with antibacterial function and fluoride and calcium ion release to inhibit oral biofilm and protect teeth. J Funct Biomater. 11(3):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocquot C, Attik N, Pradelle-Plasse N, Grosgogeat B, Colon P. 2020. Bioactivity assessment of bioactive glasses for dental applications: a critical review. Dent Mater. 36(9):1116–1143. [DOI] [PubMed] [Google Scholar]
- Montag R, Dietz W, Nietzsche S, Lang T, Weich K, Sigusch BW, Gaengler P. 2018. Clinical and micromorphologic 29-year results of posterior composite restorations. J Dent Res. 97(13):1431–1437. [DOI] [PubMed] [Google Scholar]
- Montoya C, Jain A, Londoño JJ, Correa S, Lelkes PI, Melo MA, Orrego S. 2021. Multifunctional dental composite with piezoelectric nanofillers for combined antibacterial and mineralization effects. ACS Appl Mater Interfaces. 13(37):43868–43879. [DOI] [PubMed] [Google Scholar]
- Montoya C, Roldan L, Yu M, Valliani S, Ta C, Yang M, Orrego S. 2023. Smart dental materials for antimicrobial applications. Bioact Mater. 24:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeljkovic I, De Munck J, Vanloy A, Declerck D, Lambrechts P, Peumans M, Teughels W, Van Meerbeek B, Van Landuyt KL. 2020. Secondary caries: prevalence, characteristics, and approach. Clin Oral Investig. 24(2):683–691. [DOI] [PubMed] [Google Scholar]
- Opdam NJM, van de Sande FH, Bronkhorst E, Cenci MS, Bottenberg P, Pallesen U, Gaengler P, Lindberg A, Huysmans MCDNJM, van Dijken JW. 2014. Longevity of posterior composite restorations: a systematic review and meta-analysis. J Dent Res. 93(10):943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego S, Melo MA, Lee S-H, Xu HHK, Arola DD. 2017. Fatigue of human dentin by cyclic loading and during oral biofilm challenge. J Biomed Mater Res B Appl Biomater. 105(7):1978–1985. [DOI] [PubMed] [Google Scholar]
- Osorio R, Cabello I, Toledano M. 2014. Bioactivity of zinc-doped dental adhesives. J Dent. 42(4):403–412. [DOI] [PubMed] [Google Scholar]
- Paris S, Banerjee A, Bottenberg P, Breschi L, Campus G, Doméjean S, Ekstrand K, Giacaman RA, Haak R, Hannig M, et al. 2020. How to intervene in the caries process in older adults: a joint ORCA and EFCD expert Delphi consensus statement. Caries Res. 54(5–6):1–7. [DOI] [PubMed] [Google Scholar]
- Pham P, Oliver S, Boyer C. 2023. Design of antimicrobial polymers. Macromol Chem Phys. 224(3):2200226. doi: 10.1002/macp.202200226 [DOI] [Google Scholar]
- Provenzi C, Collares FM, Cuppini M, Samuel SMW, Alves AK, Bergmann CP, Leitune VCB. 2018. Effect of nanostructured zirconium dioxide incorporation in an experimental adhesive resin. Clin Oral Investig. 22(6):2209–2218. [DOI] [PubMed] [Google Scholar]
- Schmalz G, Hickel R, Price RB, Platt JA. 2023. Bioactivity of dental restorative materials: FDI policy statement. Int Dent J. 73(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer M, Garcia IM, Souza VS, Visioli F, Scholten JD, Samuel SMW, Leitune VCB, Collares FM. 2021. Titanium dioxide nanotubes with triazine-methacrylate monomer to improve physicochemical and biological properties of adhesives. Dent Mater. 37(2):223–235. [DOI] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. 2011. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 90(4):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezvergil-Mutluay A, Seseogullari-Dirihan R, Feitosa VP, Cama G, Brauer DS, Sauro S. 2017. Effects of composites containing bioactive glasses on demineralized dentin. J Dent Res. 96(9):999–1005. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang H, Ren B, Li X, Wang L, Zhou H, Weir MD, Zhou X, Masri RM, Oates TW, et al. 2018. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci Rep. 8(1):5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xia Y, Ma T, Weir MD, Ren K, Reynolds MA, Shu Y, Cheng L, Schneider A, Xu HHK. 2019. Novel metformin-containing resin promotes odontogenic differentiation and mineral synthesis of dental pulp stem cells. Drug Deliv Transl Res. 9(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wu F, Zhang G, Zhu S, Ban J, Wang L. 2019. Preparation of a highly cross-linked biosafe dental nanocomposite resin with a tetrafunctional methacrylate quaternary ammonium salt monomer. RSC Adv. 9(71):41616–41627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand A, Buchalla W, Attin T. 2007. Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 23(3):343–362. [DOI] [PubMed] [Google Scholar]
- Yu Z, Tao S, Xu HHK, Weir MD, Fan M, Liu Y, Zhou X, Liang K, Li J. 2021. Rechargeable adhesive with calcium phosphate nanoparticles inhibited long-term dentin demineralization in a biofilm-challenged environment. J Dent. 104:103529. [DOI] [PubMed] [Google Scholar]
- Yuca E, Xie S-X, Song L, Boone K, Kamathewatta N, Woolfolk SK, Elrod P, Spencer P, Tamerler C. 2021. Reconfigurable dual peptide tethered polymer system offers a synergistic solution for next generation dental adhesives. Int J Mol Sci. 22(12):6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltsman N, Kesler Shvero D, Polak D, Weiss EI, Beyth N. 2017. Antibacterial orthodontic adhesive incorporating polyethyleneimine nanoparticles. Oral Health Prev Dent. 15(3):245–250. [DOI] [PubMed] [Google Scholar]